Abstract
The index 2014 International Society for the Advancement of Spine Surgery Policy Statement—Minimally Invasive Surgical Sacroiliac Joint Fusion—was generated out of necessity to provide an International Classification of Diseases, Ninth Revision (ICD-9)-based background and emphasize tools to ensure correct diagnosis. A timely ICD-10-based 2016 update provided a granular threshold selection with improved level of evidence and a more robust and relevant database (Appendix Table A1). As procedures and treatment options have evolved, this 2020 update reviews and analyzes the expanding evidence base and provides guidance relating to differences between the lateral and dorsal surgical procedures for minimally invasive surgical sacroiliac joint fusion.
Keywords: sacroiliac joint, minimally invasive surgery, pelvis, diagnosis
OVERVIEW OF SACROILIAC JOINT ANATOMY, PAIN ETIOLOGY AND EPIDEMIOLOGY, DIAGNOSIS, AND NONSURGICAL INTERVENTIONS
Anatomy and Innervation
The sacroiliac joints (SIJs) are diarthrodial articulations of the sacrum and ilium with a joint capsule, synovial membrane, and opposing articular surfaces covered in hyaline cartilage.1 The SIJ serves as the biomechanical mediator between the spine and pelvis.2,3 The subchondral bone, capsule, and surrounding ligaments of the SIJ are innervated by spinal nerves.4 The joint moves (allowing both translational motion and rotation of the sacrum on the ilium), albeit only a small amount. SIJ pain is not correlated to the amount of joint motion.5 SIJ dysfunction or pain may be associated with altered laxity or stiffness of the SIJ, with increased or decreased joint translations, a new joint position, and/or exaggerated or reduced joint compression.6 The SIJ complex consists of an anterior portion (the articular portion of the joint) and a posterior extra-articular portion made up of the interosseous and dorsal ligamentous structures. The anterior portion of the joint is innervated anteriorly via branches of the ventral rami of the spinal nerves and ultimately from branches of the gluteal nerves, the obturator nerve, and the lumbosacral trunks4,7–11 and posteriorly by the lateral branches of the S1–S3 dorsal rami and fibers of the L5 dorsal ramus in some cases.7,10–17 Pain from the SIJ complex may arise from the posterior extra-articular elements in addition to, or separate from, the intra-articular elements.18 Frequent sources of extra-articular pain are ligamentous and muscular injuries, and enthesopathy.19
SIJ Pain Etiology and Epidemiology
SIJ pain is an important cause of acute and chronic low back pain. In some studies, the proportion of chronic low back pain attributable to the SIJ is 15%–30%.20–24 SIJ dysfunction or pain is frequently (up to 40% in some studies) implicated in patients with new or ongoing low back pain after lumbar fusion.25–28 Lumbar fusion is likely a risk factor for SIJ pain as biomechanical studies have shown significant stress transfer from the lumbar spine to the SIJ after 1- and 2-level lumbar fusion.29 Adjacent-segment degeneration after lumbar fusion is well described and well accepted.30,31 Several authors have documented the association of SIJ (the adjacent segment caudal to a lumbosacral fusion) pain with multilevel lumbar fusion.32–36 Unoki et al37 have shown increased incidence of postoperative SIJ pain with increased number of spinal levels fused. Sagittal alignment is also known to affect adjacent-segment degeneration after spinal fusion.38 Several authors have examined lumbar sagittal balance as a contributor to post–lumbar fusion SIJ pain suggesting that failure to restore lumbar lordosis (leaving the patient with a residual pelvic incidence–lumbar lordosis mismatch) is associated with post–lumbar fusion SIJ pain.39–41
Impact of SIJ Pain
The spectrum of pain and disability from SIJ dysfunction is wide. Patients may be affected mildly or may have substantial functional impairment (eg, cannot sit or stand for more than 5 minutes, cannot perform normal activities of daily living, cannot walk up or down stairs, may require a wheelchair). Patients with chronic SIJ dysfunction seeking surgical treatment have marked impairment of quality of life (QOL),42 similar to that observed in other spinal conditions commonly treated surgically.43
SIJ Pain Diagnosis
Convergence of the sensory pathway from the hip, the SIJ, and the lumbar spine may result in overlap of pain patterns from dysfunction of these structures. As such, proper SIJ pain diagnosis is key to appropriate patient management.
SIJ pain diagnosis is typically accomplished via a clinical diagnostic algorithm involving history, physical exam, diagnostic block, and ruling out other pain contributors. Patients with SIJ pain typically report pain in the buttock(s), with possible radiation into the groin or upper legs. Position and range of motion testing of the SIJ are not reliable.44–47 Specific physical examination tests that stress the SIJ (eg, distraction test, compression test, thigh thrust, Flexion ABduction External Rotation [FABER aka Patrick] test, Gaenslen maneuver) are typically performed in the physician's office; in combination, these tests are thought to be predictive of a positive response to intra-articular SIJ block and likely indicative of SIJ pain.48–51 Other authors have presented conflicting data and results stating physical exam maneuvers to identify intra-articular SIJ pain did not demonstrate diagnostic value when compared with the reference standard of an intra-articular anesthetic block.22,45,52 With an increasing number of positive physical examination maneuvers, the diagnosis of SIJ pain is more likely.53 A recent systematic review determined there was significant evidence to constitute a clinical diagnostic rule for SIJ pain based upon 3 of 5 positive tests.54
Imaging of the SIJ may be helpful in cases of inflammatory sacroiliitis55 and acute trauma. However, no specific imaging modality has demonstrated acceptable sensitivity and specificity in the diagnosis of noninflammatory, nontraumatic SIJ pain,2,19,56 including plain radiographs,57 computed tomography (CT),58 magnetic resonance imaging,59 and radionucleotide studies.60,61 In many cases, imaging may demonstrate nonspecific findings in the SIJ.62–64 Pelvic and spine imaging is used to ensure that the patient does not have alternative diagnoses that could mimic SIJ pain (eg, hip osteoarthritis, occasionally L5-S1 spine degeneration).
The diagnosis of SIJ pain is confirmed by performing an image-guided percutaneous intra-articular SIJ block with local anesthetic (eg, lidocaine). The SIJ has a large surface area but has a very small joint capacity which ranges from 0.8 to 2.5 mL in asymptomatic individuals, and from 1.0 to 2.5 mL in symptomatic individuals.65,66 It is generally advised to inject no more than 2.5 mL during an intra-articular diagnostic injection.67,68 Extravasation of injectate (local anesthetic) onto nearby neural structures theoretically compromises the specificity of the diagnostic injection.16 Periarticular SIJ block69,70 is not considered a reference standard for the diagnosis of pain coming from the articular SIJ. A periarticular block will likely anesthetize soft tissues in the dorsal aspect of the SIJ and sacrum. Pain arising from these soft tissues may or may not respond to surgical fusion of the SIJ. An acute reduction in typical pain following an intra-articular block indicates a positive test, suggesting that the injected joint is a pain generator. A study of patients undergoing blinded injection of saline or local anesthetic showed markedly high responses to the latter, validating the test.71
Occasionally, bilateral SIJ pain can occur. Diagnosis of bilateral SIJ pain should be made on the basis of typical history (bilateral symptoms), physical examination showing positive responses to SIJ-stressing maneuvers bilaterally, and bilateral acute pain relief upon bilateral, image-guided SIJ block.
Higher Response to Diagnostic Block Does Not Predict Higher Response to Definitive Treatment
While a marked response to SIJ block might reassure the physician that treatment will produce larger responses to anatomic-based intervention, published data suggest little, if any, relationship. In 2 large prospective clinical trials of lateral transiliac MIS SIJ fusion (SIJF) with transfixing devices, patients with suspected SIJ pain were included only if intra-articular SIJ block resulted in a 50% or greater acute pain relief within 60 minutes after the block. The amount of improvement at 12 and 24 months after SIJF was unrelated to the amount of acute pain relief during the block.72 In a retrospective analysis of predictors of outcome success after radiofrequency (RF) ablation of lateral branches of the sacral nerve roots in patients with SIJ pain, no relationship was observed between response to lateral branch block or SIJ anesthesia and response to RF ablation.73 Randomized trials of RF ablation of lateral branches of the sacral nerve roots excluded patients with <75% pain reduction after lateral-branch block (1 block in Cohen et al74 and 2 blocks in Patel et al75), leaving open the question of whether the selected threshold was appropriate. Application of an overly stringent selection criteria (ie, 75% response) has no basis in evidence and is likely to result in the withholding of a beneficial procedure from a substantial number of patients with significant pain and functional impairment.72
Nonsurgical Treatment
Multiple nonsurgical treatments for SIJ pain are available, including pain medications (eg, nonsteroidal anti-inflammatory drugs [NSAIDs]), physical therapy (PT), steroid injections into the SIJ, and RF ablation of the sacral nerves. NSAIDs in combination with icing and activity modification may be helpful in reducing pain in acute or subacute SIJ pain.76 However, NSAIDS have not been shown to impact the underlying disease process. Opioids have not been shown to be a safe and effective treatment for chronic SIJ pain and addiction remains an important public health concern.
The effectiveness of PT for treating chronic SIJ dysfunction and pain has not been demonstrated. There is a paucity of high-level literature secondary to the great variability in the functional biomechanical deficits in patients with SIJ pain, and that standard practice is to apply more than one form of treatment at a time. In addition, much of the literature evaluates patients with acute or subacute SIJ pain. A significant portion of this patient population would be expected to improve with time, with or without intervention. Generally, the approach to therapeutic exercise is linked to balancing muscle length, strength, and appropriate motor control in order to efficiently absorb and transmit force from the ilium to the sacrum.77 PT treatments (eg, exercise, manipulation) may provide benefit in some patients and are a reasonable option with few risks.19,78 It is reasonable to prescribe a short course (4–6 weeks) of individualized, supervised therapeutic exercises focused on strengthening of the core muscles (local stabilizers) progressing to strengthening of the global stabilizers as tolerated.79,80 Exercise programs should be individually tailored based on clinical findings, physical capacity, and anticipated compliance. It is not reasonable to continue with a therapeutic exercise program if it is painful for the patient or in cases where the patient fails to improve after a reasonable course of treatment (4–6 weeks).
Therapeutic exercise in SIJ pain patients is typically focused on core strengthening. One randomized, controlled trial (RCT) performed in peripartum women (acute and subacute SIJ pain onset during pregnancy or within 3 weeks of delivery) showed significant improvement in pain and function at 12 months. The therapy program consisted of an individualized, supervised program of stabilization exercises lasting 20 weeks.80 A second RCT in a similar patient population showed minimal improvement in pain or function with an 8-week program consisting of stabilization exercises that were not individualized and not supervised.81 A study comparing 4 weeks of therapeutic exercise to laser treatment in patients with acute and subacute SIJ pain (<3 months duration) showed improvements in pain and function at 12 months in the exercise group.82 Another small study (51 patients with SIJ-related leg pain [duration >4 weeks but less than 1 year] without confirmatory diagnostic block) compared therapeutic exercise (6 weeks) to manual PT and steroid injection.83 Only 20% of the patients in the PT cohort had improvement that met minimal clinically important difference (MCID) (2-point improvement on visual analog scale [VAS]) at 12 weeks. In the nonsurgical treatment cohorts of the iFuse Implant System (SI-BONE, Inc) RCTs, the percentage of patients (all of whom were symptomatic for at least 6 months) that met MCID for pain improvement at 6 months were 24% in INSITE (Investigation of Sacroiliac Fusion Treatment)84 (mean number of PT visits: 14) and 22% in iMIA (iFuse Implant Minimally Invasive Arthrodesis)85 (mean number of PT visits: 26). In the large multicenter MINT (Cost-Effectiveness of Minimal Interventional Procedures for Patients with Chronic Low Back Pain) RCT, patients with chronic SIJ pain were randomized to PT (3-month standardized exercise program and psychological support if needed) versus RF ablation. Fifty-five percent of the PT group patients had at least a 2-point improvement on VAS compared to 58% of the RF ablation group at 12 months.86
Manual medicine techniques (manual therapy, osteopathic manual treatment, chiropractic adjustments) are often part of the treatment of SIJ pain. Several poorly controlled studies of these treatments have demonstrated clinical improvement of SIJ pain.87–90 In a recent review of PT interventions, Al-Subahi et al91 identified 4 studies that met inclusion criteria and concluded there is some evidence that SIJ manipulation may lead to improvements in pain and function. These studies suffered from small sample size, lack of a control group or use of an unproven treatment as a control, or from inclusion based upon unreliable physical examination tests.92–95 SIJ bony asymmetries have been clinically shown in uncontrolled studies to resolve with manipulation.90,96 SIJ position and range of motion testing has been shown in well-controlled studies to be unreliable.44–47 Tullberg et al, 97 using radio stereophotogrammetric analysis, showed no change in SIJ bony position after SIJ manipulation and Dreyfuss et al45 showed no significant correlation between “joint motion” and response to intra-articular injection of local anesthetic. If an SIJ appears to require recurrent joint mobilization, a significant muscle imbalance may still exist or the patient may have joint hypermobility as a result of conditions such as Ehlers-Danlos syndrome. Some of this may be related to patient compliance or an inadequate neuromotor control and strength-directed program, or may be related to the patient's inherent collagen makeup that allows increased joint mobility and/or tissue laxity. Caution should be used with repetitive manipulation in the latter group.77 In spite of the low level of evidence to support manipulation and other manual treatments, the low risks of these interventions warrant consideration by trained professionals.98
Intra-articular SIJ steroid injections are commonly provided interventions worldwide.99,100 There is no high-level evidence supporting the short- or long-term effectiveness of this treatment option. Three pragmatic RCTs of SIJ steroid injection randomized against radiofrequency (RF) ablation have been published.101–103 None of these studies demonstrated improvement in pain or function beyond 1 month with injections. Another study of SIJ steroid injection versus prolotherapy showed no significant improvement in the steroid cohort beyond 2 weeks.104 In the absence of definitive data supporting corticosteroid injections as superior to placebo, the value of trials with corticosteroid injections as an active control group is questionable. Repeated steroid injections have shown association with accelerated cartilage degeneration in the hip and the knee.105,106 The cost-effectiveness of SIJ steroid injections has not been determined. No studies have demonstrated long-term pain relief, nor have studies confirmed the benefit of repeating this procedure multiple times. Other interventions to treat pain arising from the SIJ complex include prolotherapy and injection of platelet-rich plasma or other substances. There is no high-level literature to support these other interventions.
RF ablation is another commonly performed intervention to treat pain arising from the SIJ complex. Four explanatory randomized trials (RF ablation versus sham) have been published. Two studies showed that RF ablation of lateral branches of sacral nerve roots can temporarily reduce SIJ pain.74,75 One-year follow-up from one RF ablation randomized trial showed modest pain reduction.107 Mehta et al,108 in a small trial (n = 30) of RF ablation strip lesioning versus sham, showed significant improvement in VAS and EuroQOL-5D scores at 3 months. A more recent study of heated RF ablation versus sham demonstrated no significant difference in pain level or patient satisfaction at 1 or 3 months.109 There are 3 pragmatic RCTs of RF ablation randomized against SIJ steroid injection. Salman et al101 showed 53% of patients had at least 50% pain improvement with heated RF ablation at 6 months. Cánovas et al103 showed modest improvement (2 points on VAS at 12 months with bipolar palisade) and better results (4 points VAS improvement at 12 months) with a modified bipolar palisade technique. In a small series (30 patients), Dutta et al102 showed improvement in VAS in the RF ablation group of 3.8 points at 6 months with pulsed RF ablation. In a much larger study of SIJ RF ablation randomized against PT, the authors demonstrated no significant differences in pain level or patient satisfaction at 3, 6, 9, or 12 months.86 RF ablation was not considered to be cost-effective from a societal perspective for patients with chronic pain originating from the SIJ in the Dutch healthcare setting.110 Interpretation of the RF ablation literature is limited by variability in patient selection criteria, the specific nerves targeted for ablation, and the types of RF ablation technology and technique utilized.18
COVERAGE RATIONALE FOR OPEN AND MIS SIJ FUSION
Given the absence of published outcomes data supporting long-term pain relief from nonsurgical treatment, patients with a diagnosis of chronic SIJ pain who experience pain for a minimum of 6 months, and who do not respond to an appropriate course of nonsurgical treatment, may be considered for SIJF.
Open SIJF Procedures
Open fusion of the SIJ, first reported in the early 1900s,111 can provide pain relief but recovery times are long and complication rates may be high.112–117 Intraoperative times, bleeding, and hospital length of stay are more prominent compared to MIS SIJF,117–120 and recovery times are long and may require prolonged postoperative rehabilitation. The open surgical procedure, whether from an anterior, a posterior, or a lateral approach, requires a large incision (8 cm or greater) and extensive surgical dissection. Open procedures are associated with increased surgical time and correspondingly increased patient morbidity. In certain cases, such as acute trauma, tumor, infection, or for SIJF in conjunction with pelvic fixation in spinal deformity surgery, open SIJF is indicated.
Anterior Approach
The anterior surgical approach to the SIJ was described in 1941.121 The anterior approach to the SIJ for open reduction and internal fixation in cases of SIJ trauma was popularized in the 1980s.122,123 More recently, the anterior approach has been utilized for elective SIJF and several series have been reported.124–127 Most series describe a surgical approach that follows the anterior iliac crest deep to the iliacus muscle (subiliacus approach). This approach is associated with significant morbidity including possible injuries to the vascular, gastrointestinal, and genitourinary structures, L5 nerve root, and lateral femoral cutaneous nerve. Postoperative hip flexor weakness has also been described.128 Murakami et al129 have described a para-rectus retroperitoneal anterior approach to the SIJ with less patient morbidity. After the joint is accessed, the anterior joint capsule is removed and the articular SIJ is debrided with a burr and curettes. The articular joint is bone-grafted and the joint is stabilized with a short plate and multiple screws. The size of the plate is limited by the local bony anatomy and close proximity of the L5 nerve root.
Lateral Approach
The lateral approach was described by Smith-Petersen and colleagues in the 1920s.130–132 In this approach, the gluteus musculature is elevated from the lateral aspect of the ilium and a bone window is created through the ilium providing access to the SIJ. The interosseous ligament and the articular cartilage are debrided, the joint is grafted and the bone window is replaced. Several variations of the procedure have been described.116,133,134 Concurrent permanent fixation of the SIJ with screws, either from lateral or posterior approach, is described by most authors.
Posterior Approach
The posterior approach to the SIJ was first described in 1908.111 Early reports described debridement of posterior soft tissues and grafting of the dorsal recess. Postoperatively the patient was placed into a hip spica cast for several months to immobilize the joint. As fixation methods improved, a variety of posterior fusion procedures were described including application of posterior bars and iliac plates.113,135 The most recent description of the open posterior procedure describes an osteotomy of the medial aspect of the posterior iliac crest to allow surgical access to the articular SIJ. This open procedure includes excision or debridement of the dorsal ligaments, dorsal joint capsule, interosseous ligament, and osteoclasis of the articular SIJ cartilaginous surfaces, which lie ventral to the ligamentous portion of the joint. Surgical access to these structures is not possible without an osteotomy of the overlying iliac crest. Bone graft and spacers or cages are typically placed into the joint. The joint is then stabilized with fixation that includes a pedicle screw into the ilium, and a pedicle screw into the sacrum connected with a spinal rod.114,117
Open posterior SIJF is frequently performed as part of long spinal deformity fusion procedures that include pelvic fixation (sacroalar iliac [S2AI] or iliac screws or bolts).136 As described below, SIJF has been proposed in response to concerns of implant failure and postoperative SIJ pain. Long spinal fusion with pelvic fixation is associated with an elevated risk of new-onset SIJ pain32,33,35,36 and increased incidence of SIJ pain and an increased incidence of SIJ pain is correlated with increased number of levels fused.37 Pelvic fixation with both S2AI and iliac screws is associated with a significant risk of screw loosening (0%–39%) and screw failure (0%–24%).137 Pelvic fixation likely alters the biomechanics of the SIJ but clinical implications have not been defined.1 Continued SIJ motion after pelvic fixation may contribute to screw loosening and/or failure138 and may also contribute to postoperative lower back pain.139 Screw loosening is common even with solid fusion at L5–S1, indicating that the SIJ remains mobile.140,141 S2AI screws have been shown to violate the articular cartilage of the SI joint in 40% of cases.142 Some surgeons now advocate for 2 points of fixation in conjunction with SIJF in pelvic fixation cases in order to minimize SIJ rotation.143–145 Recent biomechanical studies have shown that placement of titanium triangular implants (TTI) in conjunction with an S2AI screw decreases SIJ motion by 30% and lowers strain on the adjacent S1 and S2AI screws.146 This approach is currently under study.
MIS SIJF Procedures
MIS principles and techniques have been applied to SIJF. New devices allow stabilization and fusion of the SIJ with small incisions and minimal soft tissue damage. As with other MIS procedures, MIS SIJF has shown decreased operative time, decreased blood loss, and decreased hospital stay compared to the open procedure.117,119,120 SIJF is most commonly performed via an MIS procedure.147 Two different MIS SIJF procedures have been described.
Lateral Transiliac MIS Procedure
The lateral transiliac MIS procedure involves placement of devices that transfix the SIJ (ie, a device that traverses the ilium and the SIJ and ends in the sacrum). The surgical approach is from the lateral or posterolateral ilium with device placement through the ilium, across the SIJ, and into the sacrum. Approximately 25 devices are currently cleared by the US Food and Drug Administration (FDA) for this procedure* and the FDA indication statement typically describes use in patients with chronic SIJ pain and/or disruption (eg, related to trauma or overuse) and degenerative sacroiliitis (osteoarthritic degeneration).
Posterior (Dorsal) MIS Procedure
The posterior (dorsal) MIS procedure involves placement of bone allograft products or devices into the ligamentous portion of the joint via dissection of the multifidus muscle and removal of a portion of the ligaments covering the outer posterior surface of the joint. The stabilization strategy with these products is ligamentotaxis and the fusion strategy is distraction arthrodesis. The majority of the products used for this procedure are unclassified allograft bone products (human cell and tissue products) and therefore do not carry an indication statement specific to SIJF. There are 2 FDA-cleared medical devices used for posterior MIS procedures: Catamaran SIJ Fixation System (Tenon Medical, Inc)† and NADIA (Ilion Medical, Inc).‡
LATERAL TRANSILIAC MIS PROCEDURES
Procedure Description
Lateral transiliac MIS SIJF has become progressively more common and more accepted147 as the physician community becomes more aware of the condition and better versed in the diagnosis and management (both surgical and nonsurgical) of the condition. Nearly all such procedures follow the lateral trajectory approach initially published by Routt et al148 for use in iliosacral fixation to treat traumatic injuries of the posterior pelvic ring.
Several reports describe lateral SIJF using iliosacral fixation screws with supplemental bone grafting from posterior or lateral approaches.116,149 In the United Kingdom, Mohanty popularized lateral MIS SIJF using a cage filled with bone in an effort to avoid supplemental bone grafting and thus lessen the morbidity and cost of the procedure. Three different series describe use of a lateral approach with placement of these hollow modular anchor cages from Aesculap.150–152 (This device is not FDA-cleared for use in the United States for this indication.)
In 2008, SI-BONE, Inc, received FDA clearance to market a TTI with a porous surface for SIJF. Subsequent to this, at least 25 different lateral transiliac transfixing devices have received FDA clearance for lateral MIS SIJF.§ The clinical evidence base for use of these devices has grown substantially over the past decade; however, most high-level clinical evidence supporting the safety, effectiveness, durability, and economic benefit of lateral MIS SIJF is derived from use of iFuse. Physician work and resource utilization studies have been published on the lateral MIS SIJF procedure.153–155
Clinical Evidence
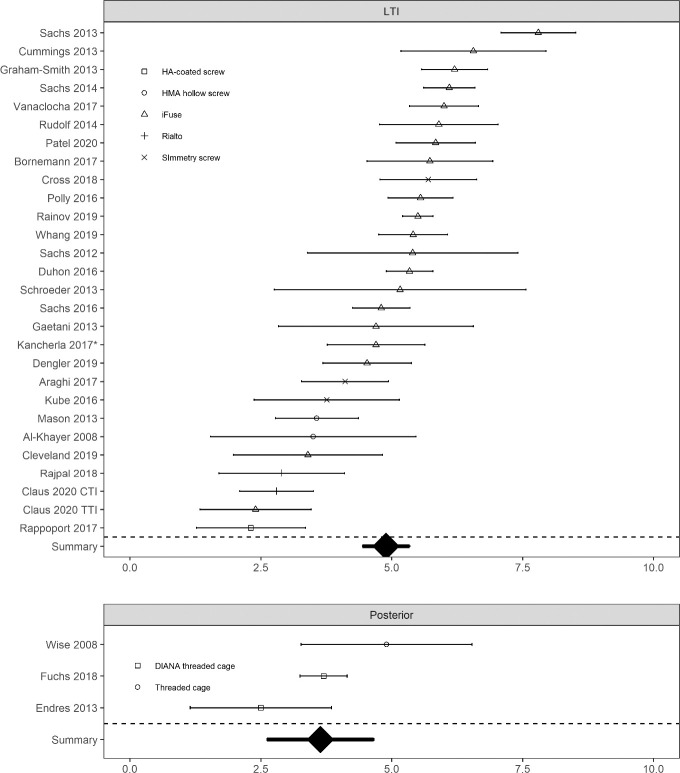
Clinical evidence for lateral transiliac MIS SIJF is now substantial and includes 2 RCTs and 5 multicenter prospective studies. These studies provide substantial evidence that lateral transiliac MIS SIJF with placement of lateral transfixing devices is safe and effective. Studies demonstrate consistent improvements in pain, function, and QOL. In both randomized trials, pain relief, disability reduction, and improvement in QOL were markedly higher in SIJF subjects compared to nonsurgically treated subjects. Results of lateral transiliac MIS SIJF with transfixing TTIs are consistent across patient populations and geographies, and preliminary data from non-TTI lateral transfixing devices appear to provide similar responses (Figure 1 and Figure 2).
Figure 1.
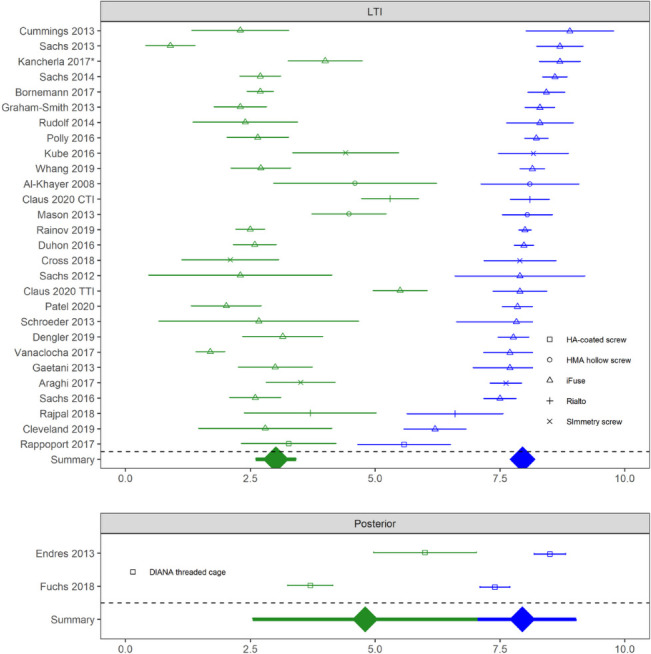
Baseline (blue) and last follow-up (green) visual analog scale or numeric rating scale sacroiliac joint (SIJ) pain scores in cohorts of patients treated with lateral transiliac (LTI) SIJ fusion (SIJF) or posterior SIJF. Horizontal bracketed bars denote confidence limit. Device type shown by shape. Kancherla is primarily titanium triangular implants but includes a small number of cases with a screw. CTI indicates cylindrical titanium implant.
Figure 2.
Change in visual analog scale or numeric rating scale sacroiliac joint pain score by study for devices placed by lateral transiliac (LTI) approach or posterior approach. Larger positive values mean more improvement. Device type shown by shape. Horizontal bracketed bars denote confidence limit. Note that Wise 2008 reported change scores but not population means at baseline and follow-up.
Level I Studies Summary
Two prospective, multicenter RCTs have yielded numerous (Level I) published studies evaluating lateral transiliac MIS SIJF using TTI (iFuse Implant System, SI-BONE, Inc). These studies demonstrate the immediate and sustained pain relief experienced by patients treated with lateral transiliac MIS SIJF.
INSITE
In the INSITE trial, a multicenter, prospective RCT conducted in the United States,156 subjects treated with iFuse had early (1 month) and sustained (24 months) improvement in pain and function. VAS SIJ pain improved from 82.3 to 26.1 (55.8 points, P < .0001). Mean changes in the nonsurgical group were not clinically significant (mean 12 points by month 6) and many subjects crossed over to surgical treatment (which was successful in most cases). Similarly, in the SIJF group, mean Oswestry Disability Index (ODI) scores decreased from 57.2 to 28.5 (28.5 points, P < .0001). The 6-month improvement in ODI in the nonsurgical group was 4.6 points (substantially less than the 15-point MCID threshold used in the study). Similar rapid and sustained clinically important improvements were seen in QOL measures (SF-36 PCS [Short Form-36 Physical Component Summary] and EuroQOL-5D [European Quality of Life Scale-Five Dimension]) in the surgical group. There was no statistical difference in number of adverse events per subject between the iFuse and the nonsurgical groups. There were 3 revision surgeries (3%) within the 24-month follow-up.
iMIA
In iMIA, a multicenter, prospective RCT conducted in Europe,157 similar results were seen as in the INSITE trial. The 2-year results were published in The Journal of Bone and Joint Surgery in 2019. Mean VAS low back pain improvement was 45 points in subjects undergoing SIJF with iFuse implants and 11 points for those treated with conservative management (primarily PT). Disability scores (ODI) improved by a mean of 26 points in the surgical group versus 8 points in the conservative management group. Statistically significant and clinically important improvements were also documented in VAS leg pain, EQ-5D TTO, and Zung Depression score. Patient-reported improvement in walking distance and improvement in work status were also documented.
Level II Studies Summary
Five prospective, multicenter (level II) studies evaluating lateral transiliac MIS SIJF using 3 different lateral transfixing devices have been published. These studies confirm the results of the level I studies in different practice settings, and confirm the homogeneity of the outcomes of the lateral transiliac MIS SIJF procedure.
Sacroiliac Joint Fusion with iFuse Implant System (SIFI)
In SIFI, a prospective, multicenter trial conducted in the United States, 172 subjects underwent SIJF with TTI (iFuse Implant System). Mean SIJ pain improved from 79.8 to 26.0 at 24 months.158 Mean ODI improved from 55.2 to 30.9 at 24 months. The SIJ revision rate was 4.7% (8 subjects) by 24 months.
Long Term Outcomes from INSITE and SIFI (LOIS)
In LOIS, subjects participating in the above-described SIFI and INSITE studies agreed to long-term follow-up.159 At year 5, improvements from baseline in pain, disability and QOL were durable. From baseline to 5 years there was a 54-point mean improvement in SIJ pain, a 26-point mean improvement in ODI, and a 0.29 (0–1 scale) improvement in EuroQOL-5D scores. There was no deterioration of results between year 2 and year 5. A total of 3 of 103 subjects (3%) had a surgical revision by 5 years. Independent radiographic analysis with thin-cut CT scans at 5 years demonstrated bridging bone across the articular SIJ adjacent or distant to the implants in 85% of patients.
Study of Bone Growth in the Sacroiliac joint after Minimally Invasive Surgery with Titanium Implants (SALLY)
In SALLY, a prospective multicenter clinical trial of a 3D-printed version of iFuse TTI (iFuse-3D), nearly identical improvements in pain, disability, and QOL were observed in 51 patients at 1 year after SIJF.160 ODI improved from 52.8 at baseline to 27.9 and SIJ pain improved from 78 preoperatively to 21 at 12 months (0–100 scale, P = .0001). There were 2 revision surgeries (3.9%): 1 revision to reposition a symptomatic malpositioned implant, and 1 revision after a motor vehicle accident. CT scans at 6 months and 1 year showed a high and increasing rate of bridging bone.
Evolusion
In Evolusion, a prospective, multicenter trial evaluating placement of the SImmetry implant (RTI Surgical), similar results were seen in the first 50 patients at 6 months' follow-up.161 Clinical results of 50 patients at 24 months showed improvement in VAS back pain (75.2 to 30.1) and improvement in ODI (55.2 to 26.9). One revision surgery to treat a symptomatic malposition was reported.¶
SI-LOK Study
Rappoport et al162 reported a prospective, multicenter trial of use of SI-LOK (Globus, Inc), a hydroxyapatite-coated screw, for SIJF in 32 subjects.162 VAS low back pain decreased from 55.8 at baseline to 28.5 at 12 months; ODI improved from 55.6 to 34.6. Two revision surgeries were reported in the first 12 months. Two-year results of 50 patients were recently presented. Improvements were noted in VAS back pain (30.1 points) and VAS leg pain (27.0 points) at 24 months.‖
A pooled analysis of patient-level data from the 3 prospective trials of TTI (2 level I and 1 level II studies) was published in Spine.163 This analysis showed similar improvements in pain, function, and QOL across studies and geographies (United States and Europe). Among patients undergoing SIJF, smokers had slightly lower but still clinically important improvements in pain, disability, and QOL scores. Similarly, patients taking opioids at baseline had somewhat lower (but still clinically important) improvements. Of the 326 patients undergoing SIJF with these lateral transfixing devices, 1.2% (n = 4) had an early (<1 month) surgical revision and 2.8% (n = 9) had a late (>1 month) surgical revision. Large observed effect sizes suggest a true underlying treatment effect. The authors found no predictors of outcomes in the nonsurgical groups.
Level III Studies
Several comparative retrospective case series of lateral transiliac MIS SIJF have been published. In these studies, operative measures (duration of surgery, blood loss, length of stay) were lower in MIS SIJF with TTI compared to open anterior119,120 or open posterior SIJF procedures.117 Implant survivorship (ie, freedom from surgical revision) at 4 years was superior in patients undergoing SIJF with TTI versus those treated with iliosacral fixation screws.164 Vanaclocha et al165 reported superior outcomes of patients treated with TTI compared to both conservative care and RF ablation of the sacral nerve root branches out to 6 years. Finally, Claus et al166 reported a single-center experience using either cylindrical threaded implants (CTI) (Rialto, Medtronic, Inc), or TTI. Surgical time was slightly less with TTI compared to CTI. There was no significant difference in patient-reported outcomes for VAS back, VAS leg, ODI, or SF-12 at 6 or 12 months between the 2 cohorts. The revision rate for CTI was higher than for TTI (6.1% versus 2.4%, P = .11).
Level IV Studies
Several case series add to the literature supporting lateral transiliac MIS SIJF with placement of transfixing device(s).167–185 These studies demonstrate generally consistent results across device types within this group and among geographies. Long-term studies with 5-178 and 6-year165 follow-up were similar to results of a prospective 5-year study of iFuse.159
Reviews
Several reviews (narrative, systematic, and meta-analysis) have been published focusing on lateral transiliac MIS SIJF with transfixing devices.186–193 These reviews support the previously stated conclusions, including that lateral MIS SIJF is beneficial (proven improvements in pain and function) in appropriately selected patients with SIJ pain.
Safety Studies
Complications
The prospective clinical trials of lateral transiliac MIS SIJF consistently demonstrate the safety and effectiveness of the approach. Adverse events in the clinical trials and in other studies have been consistently low (see Appendix Table A2). Schoell et al194 published an analysis of the Humana insurance claims database evaluating MIS SIJF for the time period 2007–2014. They reported an overall complication rate of 13.2% at 90 days and a 16.4% at 6 months. Most complications were wound and urinary tract infections (4.1%). As described in the paper, the authors did not have access to individual patient records; it is likely that this type of analysis captured patients who did not have lateral transiliac MIS SIJF procedures and/or patients who had a complication that was unrelated to the SIJF procedure. Thus, these numbers are likely overstated.
Revisions
Surgical revision rates in prospective studies of TTI and other devices are relatively low, ranging from 1%–5%.157,158 SI-BONE, Inc (manufacturer of iFuse TTI) reported lower complication rates in an analysis of spontaneously reported events.195 The complaint rate of 3.8% likely understates the true rate as all events are likely not captured through the described reporting mechanism. A second study focusing on implant survivorship showed a 96.5% survivorship at 4 years in over 11 000 procedures performed in the United States.196 The 4-year cumulative revision rate (3.5%) did not vary by age (<65 or >65) or sex. A third study by the same manufacturer compared survivorship between first-generation (11 070 cases) and second-generation (3D-printed, 3140 cases) TTI.197 There were no significant differences in complaints between the 2 generations of devices with an overall complaint rate of 1.3%. The cumulative 1-year probability of revision was 1.5% for the first-generation device and 1.0% for the 3D-printed device (P = .0408).
Biomechanical Studies
Two recent reviews provide summary information on SIJ biomechanics and the effects of lateral transfixing devices.198,199 Several other studies have evaluated the effectiveness of specific lateral transfixing devices,200–206 and have shown that these devices immediately stabilize the SIJ. Studies have shown that fusing the SIJ results in minimal stress transfer to adjacent joints. Lateral MIS SIJF results in minimal increase or decrease in motion or stress at the contralateral SIJ,207 minimal increase in motion at the L4–L5 or L5–S1 motion segment,208 and limited (5%) increase in stress at the hip joint.209 The amount of stress transfer to adjacent segments after lateral transiliac SIJF is far less than the stress transfer to the SIJ after a lumbar fusion, namely a 52% increase in SIJ motion after a L5–S1 fusion and 168% after fusion from L4 to the sacrum.29
Economic Analyses
Cost-Effectiveness
Multiple authors have evaluated the economic aspects of lateral transiliac MIS SIJF with joint-transfixing devices. In INSITE, the US-based randomized trial of TTI for lateral transiliac MIS SIJF, embedded healthcare utilization collection informed a Markov process cost-utility model, which showed a very modest incremental cost-effectiveness ratio of $13 300/QALY (Quality Adjusted Life Year) compared to nonsurgical treatment.210 SIJF with TTI was determined to be cost-effective in the short term and likely cost-saving in the longer term. The incremental cost-effectiveness ratio for lateral MIS SIJF with TTI ($13 300/QALY) is similar to that of hip ($10 000/QALY) and knee ($13 000/QALY) arthroplasty. Ackerman et al have shown the high cost and medical utilization in both the US Medicare211 and commercial payor populations.212 Publications by the same authors showed cost savings over time in both the Medicare213 and commercial payer214 populations when a patient is treated with lateral MIS SIJF versus continued nonsurgical management.
Physician Work and Resource Utilization
A retrospective comparative study utilizing Navicare data showed that operative time for lateral MIS SIJF Current Procedural Terminology (CPT) code 27279 was not statistically different from open microdiscectomy CPT 63030 (112 minutes versus 119 minutes (P = .135)).154 Postoperative work was found to be greater for MIS SIJF than for open microdiscectomy. A work-intensity study comparing CPT 27279 to CPT 63030 showed preoperative, intraoperative, and postoperative workload for MIS SIJF with lateral transfixing device was higher than for open microdiscectomy CPT 63030.153 In 2015, the International Society for the Advancement of Spine Surgery (ISASS) conducted a study consisting of a Rasch analysis of 2 separate surveys of surgeons to assess work and assigned relative value units (RVU) for CPT 27279 MIS SIJF with a lateral transfixing device.155 The regression analysis of the results of the 2 studies indicates a work RVU for CPT 27279 of 14.23.
POSTERIOR (DORSAL) MIS PROCEDURES
Procedure Description
The posterior (dorsal) MIS SIJF procedure is a recognized and well-described distinct surgical procedure.192,193,215 In the dorsal approach, allograft bone products or devices are placed into the ligamentous portion of the joint via dissection of the multifidus muscle and removal of a portion of the ligaments covering the dorsal, posterior aspect of the joint. A portion of the interosseous SIJ ligament is also typically removed.192,193,215
The posterior (dorsal) MIS SIJF procedure is distinct from lateral transiliac MIS SIJF using transfixing devices (CPT code 27279) in several fundamental ways:
The surgical anatomy, being distinct, likely carries different risks that, to this point, are not well studied.
The surgeon's work effort is distinct with the dorsal procedure requiring less surgical dissection, and the procedure generally taking much less time.
Initial stabilization is not achieved via transfixion with a laterally placed device, but rather by ligamentotaxis (tensioning of the ligaments supporting the SIJ via placement of a bone graft or device into the ligamentous SIJ).
Long-term stabilization or fusion is achieved via distraction arthrodesis rather than by integration of the surrounding bone of the ilium and sacrum into or onto the transfixing implants with eventual bridging bone across the SIJ adjacent to the implants. Distraction arthrodesis of the SIJ consists of placement of an implant or bone allograft into the ligamentous portion of the SIJ, thus “distracting the joint.” This places the supporting ligaments under tension, theoretically stabilizing the joint. Bone graft and/or recombinant human bone morphogenetic protein (rhBMP) are then utilized to achieve bone fusion. Distraction arthrodesis has also been described in the lumbar spine using stand-alone bone allograft216 and stand-alone fusion cages in the early 2000s.217,218 The results and clinical experience of ligamentotaxis and distraction arthrodesis are well described in the lumbar spine literature. These treatment strategies have fallen out of favor secondary to problems with both allografts (pseudarthrosis secondary to graft fracture or resorption) and cages (migration and subsidence, loss of lumbar lordosis, high pseudarthrosis rates, and the established need for supplemental spinal fixation219). Currently, only 3 low-quality studies address the safety and effectiveness of MIS posterior SIJF with distraction arthrodesis.220–222 There is no safety or effectiveness literature supporting the use of the latest generation of bone allograft products for posterior MIS SIJF.
The majority of the products (bone allograft) used in the dorsal MIS SIJF procedure are not medical devices cleared through the FDA Center for Devices and Radiological Health Premarket Notification process enumerated in section 510(k) of the federal Food, Drug, and Cosmetic Act. Rather, these allograft bone products are typically regulated through the FDA Center for Biologics Evaluation and Research, responsible for regulating human cell and tissue products. Certain human cell and tissue products are regulated under section 361 of the Food, Drug, and Cosmetic Act. These products must meet the criteria for “minimally manipulated tissue” outlined in 21 CFR 1271.109(a) and are not required to be licensed, cleared or approved by the FDA. No equivalence testing is required, and these are unclassified products per FDA regulations. Their labeling must reflect the manufacturer's intent that they be used only for homologous use (nonspecific to any product claim).
The level of support in the clinical literature for the posterior (dorsal) MIS SIJF procedure is far lower than for the lateral MIS SIJF procedure.
Due to these differences, data from studies of lateral MIS transiliac SIJF with transfixing devices are likely not generalizable to these posterior (dorsal) MIS SIJF procedures.
Clinical Evidence
There is limited published clinical evidence supporting the safety and effectiveness of posterior (dorsal) MIS SIJF. There is 1 prospective multicenter study,222 no comparative studies, and only a small number of case series.112,221 All published studies describe placement of bone grafts or devices within the ligamentous SIJ.
Fibular Allograft
McGuire et al112 published the results of a retrospective series of 37 patients where fibular strut allografts were placed dorsally into the SIJ. Results presented in this paper are likely not generalizable to the current posterior MIS SIJF procedure performed with allograft products. This is primarily due to the procedure being studied. While labeled as MIS by the authors, the procedure included a large incision over the posterior iliac crest with subperiosteal exposure of the medial and lateral aspects of the posterior crest. Authors then performed an osteotomy of the posterior iliac crest to gain access to the articular SIJ after debridement of the interosseous ligament. These steps are not typical of the procedural steps described for current posterior (dorsal) MIS SIJF with contemporary allograft products or devices. These steps would be more typical of an open posterior SIJF procedure.114,115,117 Autograft from the osteotomized iliac crest was placed into the articular joint and the fibular struts were placed from the dorsal aspect of the joint across the debrided ligamentous portion of the joint to the ventral articular portion of the SIJ. It is not possible to access the articular SIJ from this trajectory without an osteotomy of the overlying iliac crest. The fibular struts were greater than 10–12 mm in diameter and were several centimeters in length. Autogenous bone grafting of the dorsal sacrum and adjacent ilium bones was also part of this procedure. The results of the procedure showed, at 52 months (range 24–62), improvement in VAS of 4 points, and a fusion rate of 89%. There were 4 (11%) nonunions treated with open revision fusion.
The current posterior MIS SIJF procedure and products differ greatly from that described by McGuire et al.112 The current graft products used in the dorsal procedure are much smaller than the fibular grafts. They are placed into a different portion of the joint (primarily into the ligamentous portion of the joint). These procedures are not considered to be “open” procedures, and are performed with no osteotomy of the iliac crest and no debridement of the dorsal or interosseous ligaments. They are typically performed without autogenous bone grafting of the articular joint, the dorsum of the sacrum, and the medial ilium. These newer allograft products are placed through a small incision(s) in a true MIS or even percutaneous manner. The physician work, length of surgical procedure, and resource utilization are much less with the newer products and the MIS dorsal procedure.
Fusion Cages
One FDA-cleared medical device for posterior MIS SIJF (NADIA, Ilion Medical, Inc) employs similar stabilization (ligamentotaxis) and fusion (distraction arthrodesis) strategies. NADIA is an iteration of the DIANA fusion cage** (Signus, available only in Europe). No published studies document the safety and effectiveness of NADIA. A small retrospective case series reported 13-month results in 19 patients treated with the DIANA cage.221 Improvement from baseline to final follow-up in pain scores (8.5 to 6) and disability (ODI, 64 to 57) appears to be less than that observed with other approaches or devices. Estimated blood loss was less than 150 mL in all cases and length of stay was 7.3 days. Bone fusion described as bridging bone across the SIJ and absence of loosening around the implant was present in 79% of patients. A prospective, multicenter cohort using the same device in 171 patients showed somewhat larger 2-year improvements in SIJ pain (74 to 37) and ODI (51 to 33).222 Bone fusion described as bridging bone across the SIJ as evaluated on CT scan at 2 years was present in 31% of patients. This is less than bridging bone fusion of lateral transiliac MIS approaches with lateral transfixing devices shown in prospective trials of TTI (51% at 2 years and 85% at 5 years).159
Other reports of posterior MIS SIJF with distraction arthrodesis using cylindrical threaded cages filled with bone morphogenetic protein have cited higher fusion rates. Wise and Dall220 reported a prospective series of 13 patients treated with threaded fusion cages and rhBMP (notably, neither device is FDA-cleared for SIJF). VAS low back pain improved 4.9 points and VAS leg pain improved 2.8 points. There was 1 revision surgery for symptomatic pseudarthrosis. The fusion rate assessed on CT at 6 months was 79%. Other reported series also reported a higher fusion rate with use of rhBMP. Stark et al223 presented a series of SIJF cases using the DIANA cage with rhBMP reporting a fusion rate of 92%. Freeman reported a fusion rate of 89% at 24 months in a series of 38 patients undergoing SIJF using DIANA cages and rhBMP, many performed in conjunction with concurrent instrumented lumbar fusion.††
One additional device (Catamaran SIJ Fixation System, Tenon Medical, Inc) is FDA-cleared for MIS SIJF using a posterior approach. Rather than relying on ligamentotaxis and distraction arthrodesis, this device is placed across the posterior aspect of the inferior limb of the articular SIJ with one “outrigger” placed into the ilium and one into the sacrum. A scaffold connects the 2 outriggers. Biomechanical studies suggest that SIJ stabilization relies on lateral transfixion and avoiding disruption of the posterior interosseus and dorsal SIJ ligaments.203 Several other biomechanical studies have shown the effectiveness of various lateral transfixing devices for stabilizing the SIJ.200–202,204–207 As the Catamaran device is placed perpendicular to the axis of rotation (as opposed to placement parallel to the axis of rotation), the device does not engage the dense bone of the lateral iliac cortex. No biomechanical or clinical studies, to date, provide supportive evidence for this device.
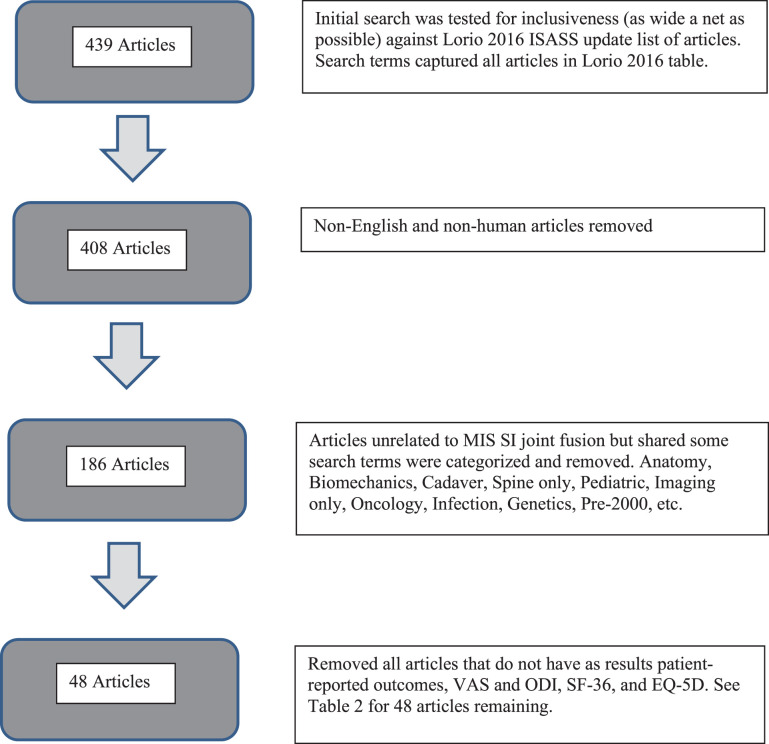
LITERATURE SEARCH
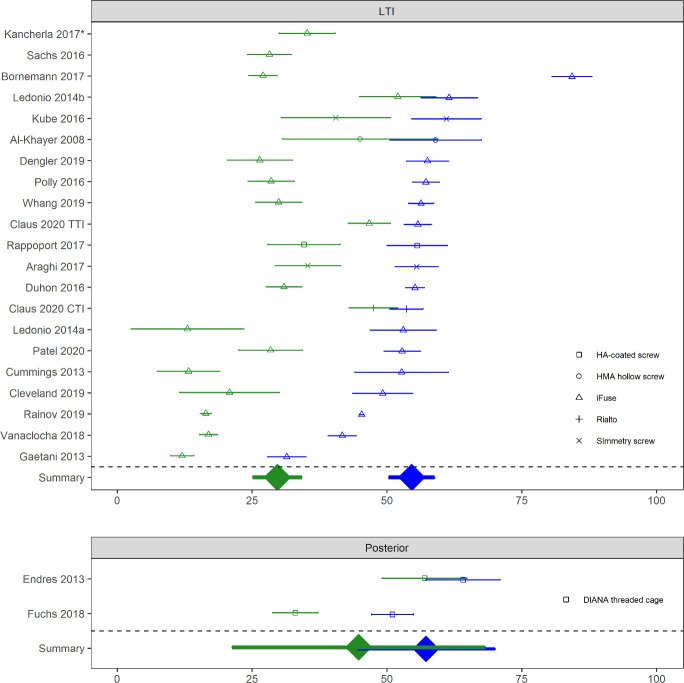
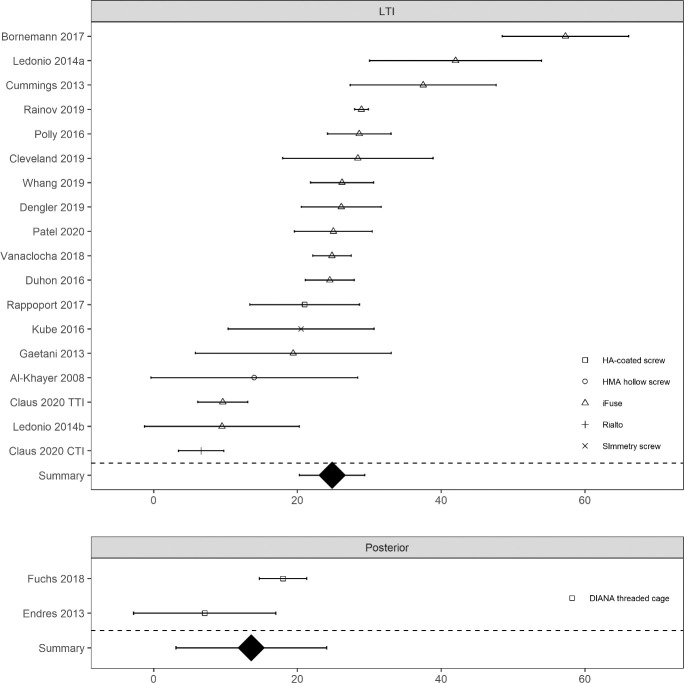
A literature search was performed using the terms “sacroiliac joint” and “fusion or arthrodesis” and the PubMed interface to Medline; 439 hits were identified and abstracts reviewed. Relevant articles describing clinical results reported in prospective trials, retrospective case series, case reports and review articles are presented in Appendix, Table A2. See Appendix, Figure A1, Literature Search Exclusions Flow Chart, for the literature search strategy. Further numeric analysis was performed as follows. For each article reporting either a case series or prospective study, data were abstracted for n, mean and SD of SIJ pain scores and ODI at both baseline and final follow-up. Change scores (from baseline to last follow-up) were also collected. Where sample sizes at final follow-up were low due to incomplete follow-up, an earlier time point was selected. Some articles did not report some SD values. Missing SD values were imputed conservatively using values derived from graphical analysis of SD as a function of study sample size. Data were entered into Excel and analyzed using R and the metafor package.‡‡ Each plot shows the mean observed value and 95% confidence limits per study at baseline and last follow-up (VAS pain: Figure 1 and Figure 2) or the mean change from baseline and last follow-up and 95% confidence limits (ODI: Figure 3 and Figure 4). Summary values with 95% confidence limits derived from random effects meta-analysis are shown at the bottom of each panel. Random effects meta-regression was performed comparing score improvements across surgical approaches (lateral transiliac versus posterior).
Figure 3.
Baseline (blue) and last follow-up (green) ODI scores in cohorts of patients treated with lateral transiliac (LTI) sacroiliac joint fusion (SIJF) or posterior SIJF. Two studies did not report baseline ODI scores. Device type shown by shape. Horizontal bars denote confidence limit.
Figure 4.
Change in Oswestry Disability Index by study for devices placed by lateral transiliac (LTI) procedure and posterior procedure. Larger positive values mean more improvement. Device type shown by shape. Horizontal bracketed bars denote confidence limit.
Numeric Synthesis
Meta-analysis was performed; graphical results are provided below.
SIJ pain scores were consistently and substantially lower at follow-up compared to baseline across studies and surgical approaches. Meta-analytic summaries showed similar baseline pain scores across procedure types but slightly lower scores at follow-up in studies using devices placed by a lateral transiliac approach (Figure 1). Score improvements were slightly larger for lateral transiliac studies (by 1.2 points [0–10 scale], P = .1497, Figure 3). There were some differences in outcomes between the lateral transfixing devices. Rialto and hydroxyapatite-coated screws appeared to show smaller changes.
For ODI scores, a similar pattern was observed with substantially lower scores at follow-up compared to baseline across studies. Final follow-up ODI scores appeared lower (better) for the lateral transiliac procedure; see Figure 2. ODI improvement from baseline was slightly larger (by 12 points, P = .1722) in studies using the lateral transiliac approach compared to the posterior approach. Analysis was limited by the small number of studies reporting score improvements after the posterior procedure. For the lateral transfixing devices, the only published Rialto study showed smaller ODI improvements (but changes in the TTI group in the same study were also relatively small).
INDICATIONS AND LIMITATIONS OF COVERAGE
Patients who have all of the following criteria may be eligible for lateral transiliac MIS SIJF with placement of lateral transfixing devices:
Chronic SIJ pain (pain lasting at least 6 months).
Significant SIJ pain that impacts QOL or significantly limits activities of daily living.
SIJ pain confirmed with at least 3 physical examination maneuvers that stress the SIJ (see list provided above) and reproduce the patient's typical pain.
Confirmation of the SIJ as a pain generator with ≥50% acute decrease in pain upon fluoroscopically guided diagnostic intra-articular SIJ block using a small volume (≤2.5 mL) of local anesthetic. Prospective trials have shown that patients with SIJ pain responses of 50%–75% respond to MIS SIJF as well as those with 75%–100% acute responses.72
Failure to respond to nonsurgical treatment consisting of NSAIDs and a reasonable course (4–6 weeks) of PT. Failure to respond means continued pain that interferes with activities of daily living and/or results in functional disability.
Intra-articular SIJ steroid injection may be considered but is not required as there is no high-quality evidence supporting the safety and effectiveness of this intervention and there are potential risks and significant costs associated with this intervention. Treatment with repeat intra-articular steroid injection is not recommended.
RF ablation of SIJ lateral branch nerves may be considered but is not required as there is only modest evidence supporting the safety and effectiveness of the procedure. There are currently no standardized patient selection algorithms, no standardized technology, and no standardized techniques. There are significant costs associated with the procedure. Treatment with repeat SIJ RF ablation is not recommended.
Additional or alternative diagnoses that could be responsible for the patient's ongoing pain or disability have been considered. Physicians should consider that patients can have multiple pain generators and addressing just one pain generator may not relieve all disability or all back pain.
MIS SIJF is not indicated for patients with the following:
Less than 6 months of SIJ pain and/or functional impairment.
Failure to pursue conservative treatment of the SIJ (unless contraindicated).
Pain not confirmed with a diagnostic SIJ block.
Presence of other pathology that would substantially prevent the patient from deriving benefit from SIJF.
MIS posterior (dorsal) SIJF is not recommended.
Bilateral SIJ pain is not uncommon. Diagnosis of bilateral SIJ pain must be made on the basis of a history of bilateral pain, bilateral elicitation of pain on physical examination maneuvers that stress each SIJ, and acute bilateral decrease in pain upon fluoroscopically guided intra-articular SIJ block with local anesthetic. Bilateral SIJF is probably best performed serially as successful treatment of one side may improve pain/disability to a degree acceptable to the patient. SIJF of the contralateral side may be necessary if contralateral SIJ pain continues and disability is significant for the patient. If bilateral fusion is performed at the same operative session, the surgeon must document both medical necessity and why serial fusion is not indicated in the patient.
It is expected that a person would not undergo more than 1 SIJF per side per lifetime except in the rare case that a revision is needed.
CODING
Open SIJF
The American Medical Association (AMA) recommends open SIJF be coded using CPT code 27280. Open SIJF may be performed from an anterior, posterior, or lateral transiliac (Smith-Petersen) approach, and all are appropriately reported via CPT 27280. The February 2014 AMA CPT Panel meeting revised CPT 27280 to include the word “open” and in addition to the anterior subiliacus approach found within the vignette, clarified further an anterior retroperitoneal approach. A second vignette describing a lateral transiliac (Smith-Petersen) approach was added. The open procedure, regardless of approach (anterior, lateral, posterior) includes extensive surgical dissection and access to the SIJ that allows visualization and excision of the dorsal and intraosseous ligaments in the posterior approach, excision of the intraosseous ligament in the lateral approach, and excision of the anterior joint capsule in an anterior approach with osteoclasis and grafting of the articular SIJ as a procedural component in all 3 approaches. Use of stabilizing fixation is typically performed in all 3 approaches. Fixation (regardless of type) is included as part of 27280. The American Medical Association (AMA) relative value scale (RVS) Update Committee (RUC) assigned intraservice time for the open procedure of 120 minutes; additionally, 2 postoperative in-hospital evaluation and management visits are associated with this code.
Lateral Transiliac MIS SIJF
The AMA recommends minimally invasive lateral transiliac SIJF with transfixing devices be coded using CPT code 27279. CPT 27279 includes bone grafting when performed and placement of transfixing devices. For bilateral procedures, report 27279 with modifier −50.
Posterior (Dorsal) MIS SIJF
Minimally invasive posterior (dorsal) SIJF should be coded using 22899 (unlisted procedure, spine) or 27299 (unlisted procedure, pelvis or hip joint).224 This includes placement of any device or product type which does not involve placement of a lateral transiliac transfixing device extending through the ilium, across the SIJ, and into the sacrum.
Hybrid: Lateral MIS with Dorsal Placement of Bone Graft
Procedures utilizing an MIS trajectory for placement of lateral transiliac transfixing devices, as well as for posterior placement of bone graft or other devices or products secondary to the primary (lateral MIS) procedure, should consider the primary CPT code for lateral MIS SIJF procedures (CPT 27279). Add-on CPT coding may be appropriate to report the addition of structural or osteopromotive bone allograft material placed posterior (dorsal) into the SIJ (eg, +20930, +20931).
Surgeons performing an open approach to the SIJ with associated surgical dissection to perform excision of the dorsal and interosseous ligament tissue and osteoclasis and grafting of the articular joint should consider CPT 27280 as well as any add-on CPT coding that may be appropriate to report services or procedures performed.
SIJF in Conjunction with Multi-Segment Spinal Fusion (Including Pelvic Fixation)
Pelvic fixation (eg, rods and connectors in a Galveston technique configuration) is commonly reported with add-on CPT code 22848. This may include placement of bolts and screws to fixate the pelvis. This may also be performed in conjunction with open or MIS SIJF, reported via CPT codes 27280 or 27279, respectively. TTIs (iFuse Implant System, SI-BONE, Inc) and some other devices are FDA-cleared to augment immobilization and stabilization of the SIJ as part of a lumbar or thoracolumbar fusion involving pelvic fixation.
Revision/Removal Procedures
Revision and/or removal of the SIJ implant would typically be coded using 22899 (unlisted procedure, spine) or 27299 (unlisted procedure, pelvis or hip joint) depending on the type of approach and procedure performed, whether within the global period of the fusion, or not.
DOCUMENTATION REQUIREMENTS
A complete history and physical documenting the likely existence of SIJ pain
Performance of a fluoroscopically-guided intra-articular SIJ block on the affected side (or both sides, see discussion above) which shows at least a 50% acute reduction in pain
A course of conservative treatment to include use of NSAIDs and one of the following: (1) an adequate period of rest and activity modification, (2) an adequate course of physical therapy wherein the physical therapist specifically documents lack of response to treatment
SIJ pain has continued for a minimum of 6 months
Other diagnoses that could be causing the patient's pain have been considered and the physician believes that SIJF is clinically required
SURGEON QUALIFICATIONS
MIS SIJF is a surgical procedure performed by orthopedic or neurologic surgeons who have successfully completed a residency in that specialty as well as at least 1 specialized training course in the procedure. Training should include device placement in cadavers or other anatomic training models under supervision of a surgeon experienced in the procedure.
Surgeons performing MIS SIJF should be specifically credentialed and/or privileged by at least 1 hospital to perform the procedure.
COVERAGE/CONCLUSION
Lateral MIS transiliac procedures for SIJF have become a recognized safe, predictable, and preferred surgical method for the management of intractable, debilitating primary or secondary SIJ pain disorders.215 The lateral procedure should be coded with CPT code 27279. The posterior (dorsal) MIS SIJF procedure is significantly distinct from the lateral procedure and is, as of yet, unproven. The posterior MIS procedure, regardless of device or product, should be coded with an unlisted procedure code.
The ISASS policy does not endorse any specific MIS SIJ system. There are numerous devices available that have received FDA 510(k) clearance for use in MIS or percutaneous lateral SIJF stabilization. The instrumentation utilized in a MIS SIJ procedure is the purview of surgeon preference.
APPENDIX
Figure A1.
Literature search exclusions flow chart. The flow chart excludes the following 8 articles; narrative, systematic reviews, and meta-analyses: Shamrock – Global Spine Journal – 2019: The safety profile of percutaneous minimally invasive sacroiliac joint fusion.191; Whelan – Techniques in Orthopaedics – 2019: The evidence for sacroiliac joint surgery.190; Tran – Pain Physician - 2019: Sacroiliac joint fusion methodology—minimally invasive compared to screw-type surgeries: a systematic review and meta analysis.188; Zaidi – J Neurosurg Spine 2015: Surgical and clinical efficacy of sacroiliac joint fusion: a systematic review of the literature.189; Heiney – Int J Spine Surg 2015: A systematic review of minimally invasive sacroiliac joint fusion utilizing a lateral transarticular technique.186; Lingutla – Eur Spine J 2016: Sacroiliac joint fusion for low back pain: a systematic review and meta-analysis.187; Yson - PM R 2019: Sacroiliac joint fusion: approaches and recent outcomes. Published online.192; Martin – Int J Spine Surg. 2020: Minimally invasive sacroiliac joint fusion: the current evidence.193 Also excludes: Mao A - Orthop Rev (Pavia) 2018: A Consideration for the utility of the post-operative Oswestry Disability Index for measuring outcomes after sacroiliac joint fusion, as this article was simply an evaluation of ODI as an outcome measure.182
Table A1.
ICD-10-CM diagnosis codes and descriptions.
| ICD-10-CM Diagnosis Code |
Code Descriptor |
| M46.1 | Sacroiliitis, not elsewhere classified |
| M53.2x8 | Spinal instabilities, sacral and sacrococcygeal region |
| M53.3 | Disorders of sacrum |
| S33.2xxA | Dislocation of sacroiliac and sacrococcygeal joint |
| S33.6xxA | Sprain of sacroiliac joint |
| 099.89 | Other specified diseases and conditions complicating pregnancy, childbirth, and the puerperium |
| 094 | Sequelae of complication of pregnancy, childbirth, and the puerperium |
Abbreviation: ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification.
Table A2.
Published literature on minimally invasive surgical sacroiliac joint fusion. Inclusion criteria included the following: indexed in PubMed, English language, fusion of the sacroiliac joint described as minimally invasive surgery or percutaneous, and clinical outcomes available. Exclusion criteria included single-patient case reports, imaging studies, and technique reports with no clinical outcomes are excluded. The table also excludes Mao et al,182 as this article was simply an evaluation of the Oswestry Disability Index as an outcome measure, and the following 8 articles because they are narratives, systematic reviews, or meta-analyses: Shamrock et al,191 Whelan and Duhon,190 Tran et al,188 Zaidi et al,189 Heiney et al,186 Lingutla et al,187 Yson et al,192 and Martin et al.193
|
Author and Year |
Study Design |
N |
Level of Evidence |
Follow- Up, mo |
Implant Type |
Technique |
BMP, DBM, Graft Used |
Demographics |
Results |
Complications |
Revisions |
| Dengler et al 2019157 | Prospective, multicenter, RCT (n = 52 iFuse, n = 51 CM) 2-y results | 103 | I | 24 | iFuse | Lateral | No | NSM (n = 51) Age: 46.7 y (23–69 y) Female: 37 (72.5%) 38 Pain duration: 4.5 y (0.45–23 y) BMI (kg/m2) 27.6 (16–44) SIJF (n = 52) Age: 49.4 y (27–70 y) 0.210 Female: 73.1% 0.999 Pain duration: 4.9 y (0.58–44 y) BMI 26.5 (18–42) | VAS improved by 45 points (37–54 points) after SIJF and 11 points (2–20 points) after CM, with a mean difference between groups of 34 points; ODI improved by 26 points (21–32 points) after SIJF and 8 points (2–14 points) after CM with a mean difference between groups of 18 points. EQ-5D at 6 mo (0.37 point) and 24 months (0.39 point) and smaller changes in the CM group at 6 mo (0.09 point) and 24 mo (0.15 point). The mean Zung Depression Scale score showed no improvement in the CM group and a 5.3-point improvement in SIJF group at 6 mo, and at 24 mo. | Within first 200 d, 20 AEs in the iFuse group and 17 AEs in the conservative group. No difference in rate between groups (p=0.664) By 24 months, 39 severe events in iFuse group 4 related to study device or procedure. In conservative group, 27 severe events 1 related to a study procedure. | 3.8% (2 of 52 iFuse patients within 2 y) |
| Dengler et al 2017225 | 1-y, prospective, multicenter randomized controlled trial | 103 | I | 12 | iFuse | Lateral | No | NSM (n = 51) Age: 46.7 y (23–69) Female: 37 (72.5%) Pain duration: 4.5 y (0.45–23 y) BMI (kg/m2) 27.6 (16–44) SIJF (n = 52) Age: 49.4 y (27–70 y) Female: 73.1% Pain duration: 4.9 y (0.58–44 y) BMI 26.5 (18–42) | VAS improved by 41.6 points in the SIJF group vs. 14.0 points in the CM group. ODI in the SIJF group improved from 57.5 points at bsseline to 32.1 points at month 12. EQ-5D TTO improved from 0.35 to 0.74 EQ-5D VAS improved from 48.1 to 53.5 Zung Depression Scale improved from 45.7 to 39.6 at month 12 | Two SIJF patients had recurrent pain attributed to possible device loosening and one had postoperative hematoma. In the CM group, 1 crossover patient had recurrent pain requiring a revision surgery. | 1 revision |
| Sturesson et al 201785 | Prospective, multicenter, RCT (n = 52 iFuse, n = 51 CM) 6-mo results | 103 | I | 6 | iFuse | Lateral | No | NSM (n = 51) Age: 46.7 y (23–69 y) Female: 37 (72.5%) 38 Pain duration: 4.5 y (0.45–23 y) BMI (kg/m2) 27.6 (16–44) SIJF (n = 52) Age: 49.4 y (27–70 y) 0.210 Female: 73.1% 0.999 Pain duration: 4.9 y (0.58–44 y) BMI 26.5 (18–42) | VAS: At 6 mo, mean LBP improved by 43.3 points in SIJF group and 5.7 points in the CM group (difference of 38.1 points). ODI improved by 26 points in the SIJF group and 6 points in CM group. EQ-5D changes of 0.15–0.46 reported | 2 procedure-related AEs | 0 revisions |
| Dengler et al 2017225 | 2-y, pooled patient- level analysis of 2 multicenter RCTs and 1 multicenter single- arm prospective trial | 423 | I | 12 | iFuse | Lateral | No | Mean (SD) age: 50.4 (11.2) y Female: 70.4% Pain duration: averaged 5.5 y (SD 6.7, table 2). Mean baseline SIJ pain: 80 points (SD 12.5) and ODI scores, 56 points (SD 12.7) were high. QOL was diminished (mean EQ-5D index TTO: 0.43, (SD 0.20) and mean SF-36 PCS: 31 (SD 5.9) | VAS 37.9 points larger in the SIJF group than in the NSM group. ODI 18.3 points larger. In NSM, no predictors of outcome. EQ-5D TTO index was 0.24 points larger for SIJF vs NSM. | Signs of wound infection occurred in 8 subjects overall, including deep wound infection requiring surgical washout (n = 1), drainage from wound treated with antibiotics (n = 3), redness treated with antibiotics (n = 3), and slow healing treated with antibiotics (n = 1) | Of the 326 patients undergoing SIJF, 1.2% (n = 4) underwent early surgical revision. Late revision surgery (>1 mo), performed in 2.8% (n = 9) |
| Polly et al 2016156 | Prospective, multicenter, RCT 2-y results | 148: 102 iFuse, 46 NSM | I | 24 | iFuse | Lateral | No | Mean age: 51.3 y; 12.2% (n = 18) were 65 y or older. 95.3% Caucasian 103 (69.6%) female Subjects were highly debilitated by SIJ pain as indicated by high baseline pain ratings (mean 82.3 on the 0–100 scale) and ODI scores (mean 56.8). | VAS improvement of 55.4 points at month 24. 83.1% and 82.0% received either clinical improvement or substantial clinical benefit in VAS SIJ pain score. ODI: 68.2% and 65.9% received clinical improvement or substantial clinical benefit at month 24. EQ-5D = 0.28 at 24 mo SF-36 = 11.2 points at 24 mo | Within first 180 d: 1.5 per iFuse subject 1.3 per NSM subject | 3% (3 of 102 iFuse patients within 2 y) |
| Polly et al 2015226 | Prospective, multicenter, RCT 12-mo results | 148: 102 iFuse, 46 NSM | I | 12 | iFuse | Lateral | No | Mean age: 51.3 y; 12.2% (18 subjects) were 65 years of age. 94.6% white 103 (69.6%) female Subjects were highly debilitated by SIJ pain, as indicated by high baseline pain ratings (mean, 82.3 on the 0–100 scale) and ODI scores (mean, 56.8) | VAS = 28.3 at the 12-mo follow-up (54.2-point improvement). ODI decreased from 57.2 at baseline to 28.1 at month 12 (improvement, 27.4 and 29.3 points, respectively. EQ-5D = improvement of 0.74 (0.20 points) SF-36 = 43.1 (10.3 points) | 12 mo of follow-up, a total of 42 severe AEs occurred | 1 revision |
| Whang et al 201584 | Prospective, multicenter, RCT 6-mo results | 148: 102 iFuse, 46 NSM | I | 6 | iFuse | Lateral | No | Mean age: 51 y and 18 (12.2%) were 65 y or older. 94.6% Caucasian Approximately two-thirds female. Subjects were highly debilitated by SIJ pain as indicated by high baseline pain ratings (mean 82.3 on the 0–100 scale) and ODI scores (mean 61.9). | 6-mo follow-up was obtained in 97.3%. By 6 mo, success rates were 81.4% in the surgical group vs 23.9% in the NSM group. ODI improvement at 6 mo occurred in 75% of surgery subjects vs 27.3% of NSM subjects. At 6 mo, QOL improved more in the surgery group and satisfaction rates were high. | At 6-mo follow-up, 181 AEs were reported (133 in the surgery group and 48 in the NSM group). The mean number of events per subject was slightly higher in the surgery group (1.3 vs 1.0 events, P = .1857). | 1 revision: implant-related impingement on a sacral nerve root requiring immediate revision |
| Whang et al 2019159 | Prospective, multicenter 5-y results | 103 | II | 60 | iFuse | Lateral | No | Mean age: 51 y; 97% Caucasian 73% female Subjects had high preoperative pain scores (mean [SD] of 81.5 [12.7]) and high levels of disability (ODI score 56.3 [12.1]). Duration of pain prior to enrollment averaged 5.7 y. EQ-5D at baseline was 0.45 (0.17), indicating a very poor QOL. 77% of subjects were taking opioids for back or SIJ pain preoperatively and 45% had a history of lumbar fusion, and concomitant spine and hip disease was common. | VAS pain scores at 5 y decreased by a mean of 54 points ODI decreased by 26 points EQOL-5D increased by 0.29 points | 3 AEs were rated as device-related (1, intermittent hip and gluteal pain) and procedure-related (2, SI joint pain) | 0 |
| Darr and Cher 2018227 | Prospective, multicenter 4-y results | 103 | II | 48 | iFuse | Lateral | No | NA | VAS pain scores in 91 subjects (88.3%) decreased by 54 points from baseline ODI decreased by 26 points EuroQOL-5D improved by 0.3 points | No new device- or procedure-related AEs during follow-up year 4 | <1% (1 subject underwent revision at year 3.8) |
| Darr et al 2018228 | Prospective, multicenter (n = 103) 3-y results | 103 | II | 36 | iFuse | Lateral | No | Mean (SD) preoperative SIJ pain score was 81.5 (12.6), and mean (SD) preoperative ODI was 56.3 (12.1) | At 3 y, VAS decreased to 26.2 (a 55-point improvement from baseline) and mean ODI was 28.2 (a 28-point improvement from baseline) EuroQol-5D (EQ-5D) improved by 0.30 points | No new device- or procedure-related AEs during follow-up year 3 | <1% |
| Patel et al 2020160 | Prospective, multicenter | 51 | II | 12 | iFuse | Lateral | No | Mean (SD) age: 53.2 y Female: 39 (76.5%) Mean (SD) BMI: 31.3 (7.6) Nonwhite race: 3 (5.9%) Hispanic: 2 (3.9%) ODI: 56.3 | ODI decreased from 52.8 at baseline to 27.9 at 12 months. VAS pain scores improved from 78 preoperatively to 21 at 12-mo follow-up 96% experienced an improvement of 20 points or more in VAS SIJ pain by month 12 EuroQOL-5D improved from 0.47 at baseline to 0.74 at month 12 | 112 AEs were reported in 43 subjects. Of these, 1 was related to the device. | 1 revision due to implant malposition and 1 revision after a vehicle accident. Revision rates consistent with those observed in postmarket surveillance for iFuse-3D. |
| Patel et al 2019229 | Prospective, multicenter | 28 | II | 6 | iFuse | Lateral | No | Mean age: 52 y (SD 15) Female: 23 (82%) Mean BMI: 30.7 (SD 5.0) Nonwhite race: 1 (3.6%) Hispanic: 2 (7.1%) | VAS decreased by 51 points and ODI decreased by 23.6 points | NA | NA |
| Duhon et al 2016158 | Prospective, multicenter, single-arm, clinical trial 2-y results | 172 | II | 24 | iFuse | Lateral | No | Average age: 50.9 y 96.5% Caucasian 69.8% femal Baseline SIJ pain (mean 79.8 points, 0–100 scale) ODI, mean 55.2 Average pain for 5.1 y (0.43–41.08 y). QOL was substantially diminished (mean EQ-5D TTO of 0.43 and mean SF-36 PCS of 31.7) | VAS decreased from 79.8 at baseline to 26.0 at 24 mo ODI decreased from 55.2 at baseline to 30.9 at 24 mo | 2.9% probably or definitely device-related 12.2% probably or definitely procedure-related | 4.7% (8 of 172 patients) |
| Duhon et al 2016230 | Prospective, multicenter, single-arm, clinical trial 1-y results | 172 | II | 12 | iFuse | Lateral | No | Mean age: 50.9 y 96.5% Caucasian 69.8% female Baseline pain ratings (mean 79.8) and (ODI mean 55.2). Mean pain duration 5.1 y (0.43–41 y); 84.3% had pain for >1 y and 64.5% had pain for >2 y. | VAS improved from 79.8 at baseline to 30.0 and 30.4 at 6 and 12 mo, respectively (mean improvements of 49.9 and 49.1 points) Mean ODI improved from 55.2 at baseline to 32.5 and 31.4 at 6 and 12 mo (improvements of 22.7 and 23.9 points) SF-36 PCS improved from 31.7 at baseline to 40.2 and 40.3 at 6 and 12 mo Mean EQ-5D TTO index improved from 0.43 at baseline to 0.69 at 6 mo and 0.71 at 12 mo, increases of 0.25 and 0.27 points, respectively | 5 AEs related to device: 2 Neuropathy related to malposition: 1 SIJ pain after fall associated with inadequate device placement: 1 Hip pain related to periosteal bone: growth around implant, 1 Mild buttock pain. 21 AEs related to procedure: 5 wound infection or drainage: 5 Buttocks or SIJ pain: 3 Postoperative nausea/vomiting: 2 Neuropathy related to malposition: 1 Staple irritation: 1 Numbness around Surgical wound: 1 Gluteal artery bleeding: 1 Urinary retention: 1 Fall causing SIJ pain: | 4 cases (2.3%). |
| Duhon et al 2013231 | Prospective, multicenter, single-arm, clinical trial 6-mo results | 94 | II | 6 | iFuse | Lateral | No | Mean age: 51 y (n = 94, safety cohort) 66% female Subjects were highly debilitated at baseline (mean VAS pain score 78, mean ODI score 54). | VAS improved from a baseline score of 76 (±16.2) to a 6-mo score of 29.3 (±23.3, an improvement of 49 points) ODI improved from 55.3 (±10.7) to 38.9 (±18.5, an improvement of 15.8 points) SF-36 PCS improved from 30.7 (±4.3) to 37.0 (±10.7, an improvement of 6.7 points) EQ-5D TTO index improved from 43 at baseline to 65 at follow-up, a 23-point improvement. EQ-5D improved from 54 to 65 (an increase of 10 points) | 53 AEs occurred in 34 subjects | 0 revisions |
| Capobianco et al 2015232 | Prospective, multicenter trial 12-mo results | 172 | II | 12 | iFuse | Lateral | No | 52 males, 100 females without PPGP and 20 females had PPGP. PPGP subjects were significantly younger (43.3 y, vs. 52.8 y for females without PPGP and 50.5 y for men. | Averaged VAS across 3 groups: 29.7 (31.4, 32.7, 25.0) Averaged ODI across 3 groups: 31.8 (32.8,30.8, 31.9). Women with PPGP experienced a significant improvement in pain (51 mm on VAS), function (20.6 points on ODI) and QOL (SF-36 PCS +10.4, MCS +7.2, EQ-5D +0.31) at 12 mo | 283 AEs occurred between enrollment and the 12-mo visit: 37 in women with PPGP, 158 in women without PPGP and 88 in men | Revision rate was 5% in PPGP subjects, 2% in women without PPGP, and 1.9% in men. The revision rate for all subjects in SIFI was 2.3% (4/172) |
| Cross et al 2018184 | Prospective multicenter | 19 | II | 24 | Threaded implant | Lateral | Autograft used | Mean age: 60.1 y 15 female BMI: 29.1 | VAS reduced to 2.2/10 12 mo postoperatively and 2.1/10 24 mo postoperatively 72% and 73% reduction in LBP, respectively. Reduction in LBP at both 12 and 24 mo, with large effect sizes of −3.5 and −2.9, respectively. At 12 months, 19/19 (100%) of patients met the MCID of ≥2/10 improvement on the NPS; at 24 mo follow-up 17/18 (94%) met the MCID. | No procedural complications, nor any device or procedure-related serious AEs | 0 revisions |
| Kube and Muir 2016185 | Prospective | 18 | II | 12 | Threaded implant | Lateral | Autograft used | Mean age: 47.2 y (SD 14.2) Mean BMI: 29.4 (SD 5.3), 56% female | VAS back pain improved from 81.7 to 44.1 points and VAS leg pain from 63.6 to 27.7 points. ODI improved from 61.0 to 40.5 (P = .009). | 4 procedural complications. | 0 revisions |
| Rappoport et al 2017162 | Prospective | 32 | II | 12 | Hydroxyapatite- coated screw | Lateral | Autograft used | Mean age: 55.2 y 62.5% female | VAS decreased from 55.8 mm preoperatively to 28.5 mm, 31.6 mm, and 32.7 mm, respectively, at 3, 6, and 12 mo postoperatively Mean ODI scores decreased from 55.6 to 33.3, 33.0, and 34.6, respectively, at 3, 6, and 12 mo postoperatively | No procedural complications | 2 revisions |
| Araghi et al 2017161 | Prospective multicenter | 50 | II | 6 | Threaded implant | Lateral | Autograft used | Average age at baseline: 61.5 y 58% female | VAS pain reduction at 6 mo from 76.2 at baseline to 35.1 Mean ODI improved from 55.5 to 35.3 at 6 mo | 2 serious AEs reported for 2 patients | 1 revision (2%) |
| Vanaclocha et al 2018165 | Comparative case series with 6-y follow-up | 137: 63 CM, 47 SI denervation, 27 SIJF | III | 72 | iFuse | Lateral | No | SIJF group (n = 27): Mean age: 48 y Female: 19 BMI: 29.5 | SIJF group: VAS mean pain 6 mo, 2.2; 12 mo, 1.9; 24 mo, 1.8; 36 mo, 1.8; 48 mo, 1.9; 60 mo, 2.0; 72 mo, 2.0 ODI: 6 mo, 26; 12 mo, 20; 24 mo, 19; 36 mo, 18; 48 mo, 17; 60 mo, 20; 72 mo, 22 | No SAEs or procedural complications | 0 revisions |
| Ledonio et al 2014119 | Retrospecive study | 44: 22 open, 22 MIS | III | 12 | iFuse | Lateral | Autograft used | MIS group only (n = 22): Average age: 47.9 y, BMI: 30.5, 17 female | MIS group only: preop ODI, 61.5; postop ODI, 52.0. | MIS group only: 3 complications | MIS group: 2 revisions |
| Claus et al 2020166 | Retrospective review: comparison of patient-reported outcomes | 156: 74 with CTIs, 82 with TDIs | III | 12 | Cylindrical threaded implants and iFuse | Contralateral posterior superior iliac spine; iFuse: lateral | Allograft used | Average age: 58.4 y, (23– 82 y) 54 female (73%) Triangular dowel implant cohort: 82 patients; 73.2% female (60 patients) Average age: 55.7 y (27–85 y) | Average both MIS VAS and ODI scores for Rialto and iFuse. VAS: −2.8, −2.4 = −2.6 subtract from baseline 8.0 = 5.4 ODI: −8.3, −6.2 = −7.25, baseline 54.65 = 47.4 Rialto (n=74): VAS back −2.8(3.3), VAS leg −1.7 (4.4), ODI −8.3 (14.9), SF-12 2.5 (5.7) iFuse: (n = 82) VAS back −2.4 (4.9), VAS leg −1.9 (4.6), ODI −6.2 (13.3), SF-12 −0.5 (9.9) | Complication rates have been seen as high as 16% in the iFuse system and as high as 52% in the CTI system. | 6.1% revision rate in the CTI cohort was observed compared to a 2.4% revision rate in iFuse |
| Rudolf 2012176 | Retrospective review: comparison of patient-reported outcomes | 50 | IV | 24 | iFuse | Lateral | No | Average age: 54 y (24–85 y), 34 female (68%), 16 male (32%), prior lumbar fusion 22 (44%) | NRS 1–10 reported pain measures. Reported in reductions from baseline. QOL measures, but not ODI. Pain scores improved by an average of 5.9 points (n = 45) | 10 peri-operative complications. | 8% at 3 y |
| Ledonio et al 2014120 | Comparative retrospective chart review | 39: 22 open fusion, 17 MIS | IV | 12 | 3-hole reconstruction plate vs iFuse | Open anterior vs MIS l ateral | Autograft on open SIJ surgery, not in MIS SIJF. | 39 patients (22 open, median age 66: 17 MIS, median age 51) who underwent SIJF with minimum 1-y follow-up | ODI scores improved significantly, from a median of 64 (44–78) to a median of 46 (10–80) in the open group and from a median of 53 (14–84) to a median of 13 (0–30) in the MIS group | ||
| Sachs and Capobianco 2012233 | Retrospective study | 11 | IV | 12 | iFuse | Lateral | Autograft used | Age: 65 y (45–82); 10 female (91%), 1 male (9%); prior lumbar spine surgery 3 (27%) | NRS 1–10 mean baseline pain score (SD) was 7.9 (± 2.2). Mean pain score at the 12-mo follow-up interval was 2.3 (±3.1). Mean (SD) change in pain score was 5.4 (3.4) points | ||
| Bornemann et al 2017179 | Retrospective 2-y | 24 | IV | 24 | iFuse | Lateral | No | Mean age: 54.9 y (18–76 y) Male: 2; female: 22 | VAS scores and ODI improved significantly directly after surgery from 84.3 ± 9.2 mm to 40.7 ± 9.2 mm and 19 from 76.8 ± 9.2% to 40.7 ± 9.2%. ODI improved further to 31 ± 5.4% after 24 mo, VAS 20 improved until the 3-mo examination and then stayed constant between 27.7 mm and 26.5 mm to 27 ± 6.6 mm at 24 mo | None | 0 revisions |
| Bornemann et al 2016234 | Retrospective 1-y | 24 | IV | 12 | iFuse | Lateral | No | Mean age: 54.9 y (18–76 y) Male: 2; female: 22 | VAS 58 ± 11 mm (68% ± 7%) ODI median decrease of 44 percentage points 57% | None | 0 revisions |
| Rudolf 2013235 | Retrospective review | 40 | IV | 24 | iFuse | Lateral | No | Mean age: 54 y NF: 18 (12 female, 6 male) PF: 11 female. 4 male LP: 7 (3 female, 4 male) | NRS scale mean change from baseline: NF cohort (n = 18) −5.94 (3.30), PF cohort (n = 15) −5.81 (3.50), LP cohort (n = 7) −4.79 (4.28) | 8 peri-operative complications in 7 patients (17.5% complication rate; 3 from the NF cohort and 5 from the PF cohort) that all resolved without clinical sequelae | 1 revision |
| Al-Khayer et al 2008150 | Retrospective, single center | 9 | IV | 24 | Hollow modular anchor, hollow screw | Lateral | autograft | Average age: 42.4 y (35–56 y). Mean duration of symptoms before surgery: 30 mo (12–84 mo) | VAS dropped from 8.1 (7–9) preoperatively to 4.6 (3–7) postoperatively Mean ODI dropped from 59 (34–70) preoperatively to 45 (28–60) postoperatively (P<0.005). | 1 complication observed; a deep wound infection, which healed with debridement and intravenous antibiotics. | 0 revisions |
| Sachs et al 2014172 | Retrospective | 144 | IV | 12 | iFuse | Lateral | No | Mean age: 58 y 71% female 62% had a history of lumbar spinal fusion | VAS pain scores from 8.6 improved by 6.1 points (5.7–6.6) to 2.7 points. Substantial clinical benefit, defined as a decrease in pain by 2.5 points or a score of 3.5 or less, was achieved in 91.9% of patients | 28 reported AEs, no intraoperative complications | 2 revisions |
| Sachs 2013118 | Retrospective | 40 | IV | 12 | iFuse | Lateral | No | Mean age: 58 y (30–81 y) 30 female, 10 male | VAS improved from 8.7 (1.5 SD) at baseline to 0.9 (1.6) at 12 mo, a 7.8-point improvement | No intraoperative complications. 2 patients presented with trochanteric bursitis, 1 incident of piriformis syndrome 1 episode of new LBP | 0 revisions |
| Sachs et al 2016171 | Retrospective, multicenter 3.7-y follow-up | 107 | IV | 44 | iFuse | Lateral | No | Mean age: 57.5 y (18–87 y) Mean BMI: 29.8 Years with SIJ pain: 5.9 y white 90.7%, black 5.6%, Hispanic 3.7% | Baseline to follow-up ODI 7.5 (1.7) to 2.6 (2.7), change score mean −4.8 (2.9) Follow-up ODI score mean 28.2 (21.3) | 3 (2.8%) procedure-related complications | 4.7% (5 of 107 patients) |
| Cummings and Capobianco 2013173 | Retrospective chart review | 18 | IV | 12 | iFuse | Lateral | No | Mean age: 64 y (39–81 y) Primarily white (83%) and female (67%). 89% of patients in cohort were obese (BMI > 30) or overweight (BMI 25–30), and 44 hypertensive. 83% had history of previous lumbar spine surgery that included fusion at 1 or more levels (73%), decompression (13%), and discectomy (13%) | VAS baseline mean 8.9 (SD 1.9), 12 mo 2.3 (SD 2.1). ODI baseline mean 52.7, 12 mo 13.2 (SD 12.6) SF-12 PCS 32.3 (SD 6.4), 12 mo 44.6 (SD 10.5). SF-12 MCS 37.6 (SD 10.2), 12 mo 53.8 (SD 9.5) | No intraoperative complications, 5% complication rate. | 0 revisions |
| Gaetani et al 2013175 | Retrospective chart review | 12 | IV | 12 | iFuse | Lateral | No | Mean age: 53.2 y (36–71 y). | Mean (±SD) ODI 31.4 (±6.3) preoperatively and 12 (±3.5) postoperatively QOL improved by 14.6 points from a baseline mean of 17.6 (±1) to 3 (±4.1) at follow-up. Mean preoperative VAS 7.7 (±1.3) and at follow-up improved by an average of 4.7 points for a mean follow-up score of 3 (±1.2) | NA | NA |
| Schroeder et al 2014174 | Retrospective review | 6 | IV | 10.25 | iFuse | Lateral | No | Average age: 50 y (25–60 y). The patients were 15.3 y on average after the original surgical procedure (range 4–25 y). | VAS score improved from 6.5 to 2.0; MCID 1.6 Back VAS score decreased from 7.83 to 2.67 mm. ODI scores dropped from 22.2 to 10.5. SRS22 scores increased from 2.93 to 3.65 | No complications | 0 revisions |
| Cleveland et al 2019181 | Retrospective review | 50 | IV | 12 | iFuse | Lateral | Mix of BMP, DBM, and autograft used | Average age: 51 y 12 males, 38 females Average BMI: 29.2 | VAS preop (n= 57) 6.2, postop 12 mo (n = 39) 3.9, 13–22 mo (n = 13) 2.8 ODI preop (n = 32) 49.2, 12 mo (n = 13) 24.6, 13–22 mo (n = 17) 20.8 DSIJQ preop (n = 20) 53.2, 12 mo (n = 10) 17.4, 13–22 mo (n=16) 20.8 | 22% complication rate | 4 revisions |
| Rudolf and Capobianco 2014178 | Prospective | 17 | IV | 60 | iFuse | Lateral | No | Mean age: 58 y (36–85 y) 77% female 47% had prior lumbar spinal fusion | VAS improved from 8.3 at baseline to 2.4 at 5 y 88% of patients reached substantial clinical benefit ODI 5 y: 21.5 | No evidence of long-term complications, implant migration or loosening | 0 revisions |
| Khurana et al 2009152 | Retrospective | 15 | IV | 17 | Hollow modular anchor hollow screw | No | Mean age: 48.7 y (37.3–62.6 y) | Mean SF-36 scores improved from 37 (23–51) to 80 (67–92) for physical function and from 53 (34–73) to 86 (70–98) for general health Mean Majeed score improved from 37 (18–54) preoperatively to 79 (63–96) postoperatively | No postoperative neurological or wound complications | 0 revisions | |
| Smith et al 2013117 | Retrospective multicenter | 263: 149 open, 114 MIS SIJF | IV | 24 | MIS only: iFuse | Lateral | No graft used for TTIs | MIS group only (n = 114): Mean age: 57.4 y 82 female 54 with prior lumbar fusion | MIS group only (n = 114): VAS baseline 8.3, VAS 12 mo 2.3, 24 mo 1.7 | No intraoperative complications occurred | Revisions (3.5%) in the MIS group were the result of suboptimal implant placement. |
| Kancherla et al 2017169 | Retrospective analysis | 45: 36 iFuse, 9 SAMBA screw | IV | 32 | iFuse | Lateral | No | Average age: 52.7 y BMI 28.6 14 female | VAS 4.0 ODI 35.2 SF-12 PCS 40.2 SF-12 MCS 45.4 | 3 complications | 1 SAMBA screw revision, 1 iFuse revision |
| Rainov et al 2019180 | Retrospective case series | 160 | IV | 12 | iFuse | Lateral | No | Mean age: 58 y 68% female | VAS pain decreased from 8.0 to 2.5 ODI decreased from 45.3 to 16.4 Follow-up was obtained in 151 patients at 3 mo, 135 patients at 6 mo, 114 at 9 mo, and 90 at 12 mo Mean (±SD) VAS of SIJ pain improved from 8.0 (±0.8) at baseline to 3.5 (±1.6) at 6 mo and 2.5 (±1.4) at 12 mo Mean (±SD) ODI improved from 45.3 (±2.3) at baseline to 22.1 (±8.8) at 6 mo and 16.4 (±6.6) at 12 mo At follow-up 12 months, 97% had a VAS improvement from baseline of at least 2 points, 97% had ODI improvement of at least 15 points, 66% had a VAS level of 2 or less, and 56% had an ODI level of 15 or less | No intraoperative complications | 0 revisions |
| Rajpal and Burneikiene 2019168 | Retrospective review | 24 | Review article | 19 | Cylindrical threaded implants | Lateral | Allograft | Average age: 62.2 y 3 males, 21 females | VAS reduction from an average baseline score of 6.6 to 3.7 postoperatively. Leg pain scores decreased from 4.8 to 1.5 | 2 patients had symptomatic subcutaneous hematomas, which resolved spontaneously, and 2 patients had superficial wound infections treated with antibiotics. | 0 revisions |
| Dorsal/Posterior | |||||||||||
| Endres and Ludwig 2013221 | Prospective | 19 | II | 13.2 | Threaded fusion cagea | Posterior | Autograft | Mean age: 60.9 y (36–76 y) | VAS: 8.5 before surgery and 6 at final follow-up Mean ODI: 64.1 before surgery and 56.97 at final follow-up | No intraoperative complications. | 0 revisions |
| Fuchs and Ruhl 2018222 | Prospective | 171 | II | 24 | Threaded fusion cagea | Posterior | Allograft | At 24 mo: MVAS improved from 63% to 36%, ODI from 51% to 33%, SF-MPQ from 50% to 31%, VAS from 74 to 37. Radiographic fusion rate 19% without BMP, 67% with BMP. | 1 complication L5 radiculitis. 33% of cases had poor implant position (implant too far into sacrum or ilium) | 1 revision | |
| McGuire et al 2012112 | Retrospective case series | 37 | IV | 40 | Fibular dowel allografta | Posterior | Allograft | Mean age: 42.5 y (23–63) 34 females, 3 males | VAS score: 3.4 (94% achieved clinical important difference at 6 mo and maintained through follow-up) Fusion achieved: 34 (89.5%); nonunion: 4 (10.5%) | Nonunion occurred at 4 sites (10.5%). Each SIJ nonunion was successfully treated by secondary autogenous bone grafting and iliosacral compression screw fixation. There were no infections. | NA |
| Wise and Dall 2008220 | Prospective | 13 | II | 12 | Threaded fusion cagea | Posterior | No | Average age: 53.1 y (45–62 y) 12 females, 1 male | VAS back pain improved an average of 4.9 VAS leg pain improved an average of 2.4 Dyspareunia improved by an average of 2.6 | No infections or neurovascular complications. | 0 revisions |
Abbreviations: AE, adverse event; BMP, bone morphogenetic protein; CM, conservative management; CTI, Cylindrical Threaded Implant; DBM, Demineralized Bone Matrix; DSIJQ; Denver Sacroiliac Joint Questionnaire, EQ-5D, Euroquol 5 dimension quality of life measure; LBP, low back pain; LP: patients with concomitant lumbar pathology treated non surgically; MCID, minimal clinically important difference; MIS indicates minimally invasive surgical; MVAS indicates Million Visual Analog Scale; SF-MPQ indicates Short Form McGill Pain Questionnaire; NF, patients with no history of spinal fusion or confounding lumbar pathology; ODI, Oswestry Disability Index; PCS, physical component summary; PF, patients with history of prior lumbar spinal fusion; PPGP, posterior pelvic girdle pain; QOL, quality of life; RCT, randomized controlled trial; SIJ, sacroiliac joint; SIJF, sacroiliac joint fusion; TDI, Triangular Dowel Implant; TTO, time trade-off index; VAS, visual analog score.
Not FDA cleared for SIJF.
Footnotes
FDA 510(k) Premarket Notification Database: K080398and K092375 (accessed 9/30/20). Companies marketing lateral SIJ devices: SI-BONE, Medtronic, Globus Medical, Xtant, Surgalign (formerly RTI), Alevio, CoreLink, Orthofix/Medical Designs, Camber Medical, Zimmer Biomet, Captiva Spine, Coorstek, Life Spine, SICage, SpineFrontier, SI-Technology, Medacta, Frontier Devices, L&K Biomed, Zavation, Huvexel.
https://www.tenonmed.com/catamaran, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K180818
http://ilionmedical.com/, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K190580
FDA 510(k) Premarket Notification Database: K080398and K092375 (accessed 9/30/20). Companies marketing lateral SI joint devices: SI-BONE, Medtronic, Globus Medical, Xtant, RTI, Alevio, CoreLink, Orthofix/Medical Designs, Camber Medical, Zimmer Biomet, Captiva Spine, Coorstek, Life Spine, SICage, SpineFrontier, SI-Technology, Medacta, Frontier Devices, L&K Biomed, Zavation, Huvexel.
Kuchazyk DW. Clinical and radiographic outcomes following minimally invasive sacroiliac joint fusion with decortication. ISASS 2020 presentation. February 27, 2020; San Juan, Puerto Rico. https://www.eventscribe.com/2015/app/presentationslides/slideshare.asp?sfp=OTU0OHwxMTcyODY0fDM5NDk4NDk1fC0x. Accessed October 20, 2020.
Rappoport LH. Minimally invasive sacroiliac joint fusion using a novel hydroxyapatite-coated screw: two-year clinical and radiographic outcomes. ISASS 2020 presentation. February 27, 2020; San Juan, Puerto Rico. https://www.eventscribe.com/2015/app/presentationslides/slideshare.asp?sfp=OTU0OHwxMTcyODY0fDM5NDk4NDk1fC0. Accessed October 20, 2020.
https://signus.com/intl/products/portfolio/diana-sacroliliac-fusion.html. Accessed October 20, 2020.
Freeman T. 107- Improvement in Sacroiliac Joint Pain 2 Years after Fusion with the Distraction Interference Arthrodesis (DIANATM) Device. Poster presented at the: ISASS 16th Annual Meeting—Oral Podium and Oral Poster Presentations; April 6; Las Vegas, Nevada. Accessed October 1, 2020. https://www.isass.org/abstracts/isass16-oral-posters/isass16-107-Improvement-in-Sacroiliac-Joint-Pain-2-Years-after-Fusion-with-the-Dis.html
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. https://www.jstatsoft.org/v036/i03.
Disclosures and COI: The authors received no funding for this update; ML – SAB Vivex Biologics; RK – no COI; AA – Consultant Surgalign.
REFERENCES
- 1.Vleeming A, Schuenke MD, Masi AT, Carreiro JE, Danneels L, Willard FH. The sacroiliac joint: an overview of its anatomy, function and potential clinical implications. J Anat. 2012;221(6):537–567. doi: 10.1111/j.1469-7580.2012.01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen SP. Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg. 2005;101(5):1440–1453. doi: 10.1213/01.ANE.0000180831.60169.EA. [DOI] [PubMed] [Google Scholar]
- 3.Dietrichs E. Anatomy of the pelvic joints—a review. Scand J Rheumatol Suppl. 1991;88:4–6. [PubMed] [Google Scholar]
- 4.Szadek KM, Hoogland PV, Zuurmond WW, de Lange JJ, Perez RS. Nociceptive nerve fibers in the sacroiliac joint in humans. Reg Anesth Pain Med. 2008;33(1):36–43. doi: 10.1016/j.rapm.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Sturesson B, Selvik G, Udén A. Movements of the sacroiliac joints. A roentgen stereophotogrammetric analysis. Spine. 1989;14(2):162–165. doi: 10.1097/00007632-198902000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Vleeming A, Albert HB, Östgaard HC, Sturesson B, Stuge B. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J. 2008;17(6):794–819. doi: 10.1007/s00586-008-0602-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikeda R. Innervation of the sacroiliac joint. Macroscopical and histological studies [in Japanese] Nihon Ika Daigaku Zasshi. 1991;58(5):587–596. doi: 10.1272/jnms1923.58.587. [DOI] [PubMed] [Google Scholar]
- 8.Szadek KM, Hoogland PVJM, Zuurmond WWA, De Lange JJ, Perez RSGM. Possible nociceptive structures in the sacroiliac joint cartilage: an immunohistochemical study. Clin Anat N Y N. 2010;23(2):192–198. doi: 10.1002/ca.20908. [DOI] [PubMed] [Google Scholar]
- 9.Forst SL, Wheeler MT, Fortin JD, Vilensky JA. The sacroiliac joint: anatomy, physiology and clinical significance. Pain Physician. 2006;9(1):61–67. [PubMed] [Google Scholar]
- 10.Murata Y, Takahashi K, Yamagata M, Takahashi Y, Shimada Y, Moriya H. Sensory innervation of the sacroiliac joint in rats. Spine. 2000;25(16):2015–2019. doi: 10.1097/00007632-200008150-00003. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa T. Study on the distribution of nerve filaments over the iliosacral joint and its adjacent region in the Japanese [in Japanese] Nihon Seikeigeka Gakkai Zasshi. 1966;40(4):419–430. [PubMed] [Google Scholar]
- 12.Solonen KA. The sacroiliac joint in the light of anatomical, roentgenological and clinical studies. Acta Orthop Scand Suppl. 1957;27:1–127. [PubMed] [Google Scholar]
- 13.Grob KR, Neuhuber WL, Kissling RO. Innervation of the sacroiliac joint of the human [in German] Z Für Rheumatol. 1995;54(2):117–122. [PubMed] [Google Scholar]
- 14.Vilensky JA, O'Connor BL, Fortin JD, et al. Histologic analysis of neural elements in the human sacroiliac joint. Spine. 2002;27(11):1202–1207. doi: 10.1097/00007632-200206010-00012. [DOI] [PubMed] [Google Scholar]
- 15.Roberts SL, Burnham RS, Ravichandiran K, Agur AM, Loh EY. Cadaveric study of sacroiliac joint innervation: implications for diagnostic blocks and radiofrequency ablation. Reg Anesth Pain Med. 2014;39(6):456–464. doi: 10.1097/AAP.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 16.Fortin JD, Washington WJ, Falco FJ. Three pathways between the sacroiliac joint and neural structures. AJNR Am J Neuroradiol. 1999;20(8):1429–1434. [PMC free article] [PubMed] [Google Scholar]
- 17.McGrath MC, Zhang M. Lateral branches of dorsal sacral nerve plexus and the long posterior sacroiliac ligament. Surg Radiol Anat. 2005;27(4):327–330. doi: 10.1007/s00276-005-0331-x. [DOI] [PubMed] [Google Scholar]
- 18.Yang AJ, McCormick ZL, Zheng PZ, Schneider BJ. Radiofrequency ablation for posterior sacroiliac joint complex pain: a narrative review. PM R. 2019;(Suppl 1):S105–S113. doi: 10.1002/pmrj.12200. [DOI] [PubMed] [Google Scholar]
- 19.Cohen SP, Chen Y, Neufeld NJ. Sacroiliac joint pain: a comprehensive review of epidemiology, diagnosis and treatment. Expert Rev Neurother. 2013;13(1):99–116. doi: 10.1586/ern.12.148. [DOI] [PubMed] [Google Scholar]
- 20.Bernard TN, Kirkaldy-Willis WH. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop. 1987;217(Apr):266–280. doi: 10.1097/00003086-198704000-00029. [DOI] [PubMed] [Google Scholar]
- 21.Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine. 1995;20(1):31–37. doi: 10.1097/00007632-199501000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Maigne JY, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine. 1996;21(16):1889–1892. doi: 10.1097/00007632-199608150-00012. [DOI] [PubMed] [Google Scholar]
- 23.Irwin RW, Watson T, Minick RP, Ambrosius WT. Age, body mass index, and gender differences in sacroiliac joint pathology. Am J Phys Med Rehabil. 2007;86(1):37–44. doi: 10.1097/PHM.0b013e31802b8554. [DOI] [PubMed] [Google Scholar]
- 24.Sembrano JN, Polly DW. How often is low back pain not coming from the back? Spine. 2009;34(1):E27–32. doi: 10.1097/BRS.0b013e31818b8882. [DOI] [PubMed] [Google Scholar]
- 25.Katz V, Schofferman J, Reynolds J. The sacroiliac joint: a potential cause of pain after lumbar fusion to the sacrum. J Spinal Disord Tech. 2003;16(1):96–99. doi: 10.1097/00024720-200302000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Maigne JY, Planchon CA. Sacroiliac joint pain after lumbar fusion. A study with anesthetic blocks. Eur Spine J. 2005;14(7):654–658. doi: 10.1007/s00586-004-0692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DePalma MJ, Ketchum JM, Saullo TR. Etiology of chronic low back pain in patients having undergone lumbar fusion. Pain Med. 2011;12(5):732–739. doi: 10.1111/j.1526-4637.2011.01098.x. [DOI] [PubMed] [Google Scholar]
- 28.Liliang P-C, Lu K, Liang C-L, Tsai Y-D, Wang K-W, Chen H-J. Sacroiliac joint pain after lumbar and lumbosacral fusion: findings using dual sacroiliac joint blocks. Pain Med. 2011;12(4):565–570. doi: 10.1111/j.1526-4637.2011.01087.x. [DOI] [PubMed] [Google Scholar]
- 29.Ivanov AA, Kiapour A, Ebraheim NA, Goel V. Lumbar fusion leads to increases in angular motion and stress across sacroiliac joint: a finite element study. Spine. 2009;34(5):E162–169. doi: 10.1097/BRS.0b013e3181978ea3. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto K, Aizawa T, Kanno H, Itoi E. Adjacent segment degeneration after fusion spinal surgery—a systematic review. Int Orthop. 2019;43(4):987–993. doi: 10.1007/s00264-018-4241-z. [DOI] [PubMed] [Google Scholar]
- 31.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J Off J North Am Spine Soc. 2004;4(6 Suppl):190S–194S. doi: 10.1016/j.spinee.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Finger T, Bayerl S, Bertog M, Czabanka M, Woitzik J, Vajkoczy P. Impact of sacropelvic fixation on the development of postoperative sacroiliac joint pain following multilevel stabilization for degenerative spine disease. Clin Neurol Neurosurg. 2016;150:18–22. doi: 10.1016/j.clineuro.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Elder BD, Ishida W, Lo S-FL, et al. Use of S2-alar-iliac screws associated with less complications than iliac screws in adult lumbosacropelvic fixation. Spine. 2017;42(3):E142–E149. doi: 10.1097/BRS.0000000000001722. [DOI] [PubMed] [Google Scholar]
- 34.Unoki E, Miyakoshi N, Abe E, Kobayashi T, Abe T, Shimada Y. Sacroiliac joint pain after multiple-segment lumbar fusion: a long-term observational study—non-fused sacrum vs. fused sacrum. Spine Surg Relat Res. 2017;1(2):90–95. doi: 10.22603/ssrr.1.2016-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unoki E, Miyakoshi N, Abe E, et al. Sacropelvic fixation With S2 alar iliac screws may prevent sacroiliac joint pain after multisegment spinal fusion. Spine. 2019;44(17):E1024–E1030. doi: 10.1097/BRS.0000000000003041. [DOI] [PubMed] [Google Scholar]
- 36.Diesing D, Franke J, Tschoeke SK, Schultheiß R, Scheufler KM. Persistent iliosacral joint syndrome following instrumentation to the sacropelvis in patients with adult spinal deformity. J Neurol Surg Part Cent Eur Neurosurg. 2019;80(1):15–25. doi: 10.1055/s-0038-1655732. [DOI] [PubMed] [Google Scholar]
- 37.Unoki E, Abe E, Murai H, Kobayashi T, Abe T. Fusion of multiple segments can increase the incidence of sacroiliac joint pain after lumbar or lumbosacral fusion. Spine. 2016;41(12):999–1005. doi: 10.1097/BRS.0000000000001409. [DOI] [PubMed] [Google Scholar]
- 38.Berven S, Wadhwa R. Sagittal alignment of the lumbar spine. Neurosurg Clin N Am. 2018;29(3):331–339. doi: 10.1016/j.nec.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Shin M-H, Ryu K-S, Hur J-W, Kim J-S, Park C-K. Comparative study of lumbopelvic sagittal alignment between patients with and without sacroiliac joint pain after lumbar interbody fusion. Spine. 2013;38(21):E1334–1341. doi: 10.1097/BRS.0b013e3182a0da47. [DOI] [PubMed] [Google Scholar]
- 40.Cho D-Y, Shin M-H, Hur J-W, Ryu K-S, Park C-K. Sagittal sacropelvic morphology and balance in patients with sacroiliac joint pain following lumbar fusion surgery. J Korean Neurosurg Soc. 2013;54(3):201–206. doi: 10.3340/jkns.2013.54.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciarpaglini R, Otten P, Sutter P, Duy VQ, Gautier E, Maestretti G. Sacroiliac joint syndrome 10 years after lumbar arthroplasty: the importance of spinopelvic alignment. Eur Spine J. 2014;23(S6):720–724. doi: 10.1007/s00586-014-3547-9. [DOI] [PubMed] [Google Scholar]
- 42.Cher D, Polly D, Berven S. Sacroiliac joint pain: burden of disease. Med Devices Evid Res. 2014;7:73–81. doi: 10.2147/MDER.S59437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cher DJ, Reckling WC. Quality of life in preoperative patients with sacroiliac joint dysfunction is at least as depressed as in other lumbar spinal conditions. Med Devices Evid Res. 2015;8:395–403. doi: 10.2147/MDER.S92070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riddle DL, Freburger JK. Evaluation of the presence of sacroiliac joint region dysfunction using a combination of tests: a multicenter intertester reliability study. Phys Ther. 2002;82(8):772–781. [PubMed] [Google Scholar]
- 45.Dreyfuss P, Michaelsen M, Pauza K, McLarty J, Bogduk N. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine. 1996;21(22):2594–2602. doi: 10.1097/00007632-199611150-00009. [DOI] [PubMed] [Google Scholar]
- 46.Robinson HS, Brox JI, Robinson R, Bjelland E, Solem S, Telje T. The reliability of selected motion- and pain provocation tests for the sacroiliac joint. Man Ther. 2007;12(1):72–79. doi: 10.1016/j.math.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Freburger JK, Riddle DL. Measurement of sacroiliac joint dysfunction: a multicenter intertester reliability study. Phys Ther. 1999;79(12):1134–1141. [PubMed] [Google Scholar]
- 48.Laslett M, Williams M. The reliability of selected pain provocation tests for sacroiliac joint pathology. Spine. 1994;19(11):1243–1249. doi: 10.1097/00007632-199405310-00009. [DOI] [PubMed] [Google Scholar]
- 49.Laslett M, Aprill CN, McDonald B. Provocation sacroiliac joint tests have validity in the diagnosis of sacroiliac joint pain. Arch Phys Med Rehabil. 2006;87(6):874. doi: 10.1016/j.apmr.2006.04.007. author reply 874–875. [DOI] [PubMed] [Google Scholar]
- 50.Laslett M. The value of the physical examination in diagnosis of painful sacroiliac joint pathologies. Spine. 1998;23(8):962–964. doi: 10.1097/00007632-199804150-00029. [DOI] [PubMed] [Google Scholar]
- 51.Szadek KM, van der Wurff P, van Tulder MW, Zuurmond WW, Perez RSGM. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10(4):354–368. doi: 10.1016/j.jpain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 52.Schneider BJ, Ehsanian R, Rosati R, Huynh L, Levin J, Kennedy DJ. Validity of physical exam maneuvers in the diagnosis of sacroiliac joint pathology. Pain Med Malden Mass. 2019 doi: 10.1093/pm/pnz183. [DOI] [PubMed]
- 53.Schneider BJ, Rosati R, Zheng P, McCormick ZL. Challenges in diagnosing sacroiliac joint pain: a narrative review. PM R. 2019 doi: 10.1002/pmrj.12175. [DOI] [PubMed]
- 54.Petersen T, Laslett M, Juhl C. Clinical classification in low back pain: best-evidence diagnostic rules based on systematic reviews. BMC Musculoskelet Disord. 2017;18(1):188. doi: 10.1186/s12891-017-1549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puhakka KB, Jurik AG, Schiøttz-Christensen B, et al. MRI abnormalities of sacroiliac joints in early spondylarthropathy: a 1-year follow-up study. Scand J Rheumatol. 2004;33(5):332–338. doi: 10.1080/03009740410005881. [DOI] [PubMed] [Google Scholar]
- 56.McKenzie-Brown AM, Shah RV, Sehgal N, Everett CR. A systematic review of sacroiliac joint interventions. Pain Physician. 2005;8(1):115–125. [PubMed] [Google Scholar]
- 57.Cohen AS, McNeill JM, Calkins E, Sharp JT, Schubart A. The “normal” sacroiliac joint. Analysis of 88 sacroiliac roentgenograms. Am J Roentgenol Radium Ther Nucl Med. 1967;100(3):559–563. [PubMed] [Google Scholar]
- 58.Elgafy H, Semaan HB, Ebraheim NA, Coombs RJ. Computed tomography findings in patients with sacroiliac pain. Clin Orthop. 2001;(382):112–118. doi: 10.1097/00003086-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 59.Puhakka KB, Boel LW, Vesterby A, Egund N, Melsen F, Jurik AG. MR imaging of the normal sacroiliac joint with correlation to histology. Skeletal Radiol. 2004;33(1):15–28. doi: 10.1007/s00256-003-0691-4. [DOI] [PubMed] [Google Scholar]
- 60.Slipman CW, Sterenfeld EB, Chou LH, Herzog R, Vresilovic E. The value of radionuclide imaging in the diagnosis of sacroiliac joint syndrome. Spine. 1996;21(19):2251–2254. doi: 10.1097/00007632-199610010-00013. [DOI] [PubMed] [Google Scholar]
- 61.Maigne JY, Boulahdour H, Chatellier G. Value of quantitative radionuclide bone scanning in the diagnosis of sacroiliac joint syndrome in 32 patients with low back pain. Eur Spine J. 1998;7(4):328–331. doi: 10.1007/s005860050083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eno J-J, Boone C, Bellino M, Bishop J. The prevalence of sacroiliac joint degeneration in asymptomatic adults. J Bone Joint Surg Am. 2015;97(11):932–936. doi: 10.2106/JBJS.N.01101. [DOI] [PubMed] [Google Scholar]
- 63.Vogler JB, Brown WH, Helms CA, Genant HK. The normal sacroiliac joint: a CT study of asymptomatic patients. Radiology. 1984;151(2):433–437. doi: 10.1148/radiology.151.2.6709915. [DOI] [PubMed] [Google Scholar]
- 64.Cihan OF, Karabulut M, Kılınçoğlu V, Yavuz N. The variations and degenerative changes of sacroiliac joints in asymptomatic adults. Folia Morphol. 2020 doi: 10.5603/FM.a2020.0032. [DOI] [PubMed]
- 65.Fortin J, Dwyer A, West S, Pier J. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: asymptomatic volunteers. Spine. 1994;19(13):1475–1482. [PubMed] [Google Scholar]
- 66.Fortin J, Aprill C, Ponthieux B, Pier J. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part II: clinical evaluation. Spine. 1994;19(13):1483–1489. [PubMed] [Google Scholar]
- 67.Kennedy DJ, Engel A, Kreiner DS, Nampiaparampil D, Duszynski B, MacVicar J. Fluoroscopically guided diagnostic and therapeutic intra-articular sacroiliac joint injections: a systematic review. Pain Med Malden Mass. 2015;16(8):1500–1518. doi: 10.1111/pme.12833. [DOI] [PubMed] [Google Scholar]
- 68.Jung MW, Schellhas K, Johnson B. Use of diagnostic injections to evaluate sacroiliac joint pain. Int J Spine Surg. 2020;14(Suppl 1):30–34. doi: 10.14444/6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murakami E, Tanaka Y, Aizawa T, Ishizuka M, Kokubun S. Effect of periarticular and intraarticular lidocaine injections for sacroiliac joint pain: prospective comparative study. J Orthop Sci Off J Jpn Orthop Assoc. 2007;12(3):274–280. doi: 10.1007/s00776-007-1126-1. [DOI] [PubMed] [Google Scholar]
- 70.Kurosawa D, Murakami E, Ozawa H, et al. A diagnostic scoring system for sacroiliac joint pain originating from the posterior ligament. Pain Med Malden Mass. 2016 doi: 10.1093/pm/pnw117. [DOI] [PubMed]
- 71.Broadhurst NA, Bond MJ. Pain provocation tests for the assessment of sacroiliac joint dysfunction. J Spinal Disord. 1998;11(4):341–345. [PubMed] [Google Scholar]
- 72.Polly D, Cher D, Whang PG, Frank C, Sembrano J INSITE Study Group. Does level of response to SI joint block predict response to SI joint fusion? Int J Spine Surg. 2016;10 doi: 10.14444/3004. article 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohen SP, Strassels SA, Kurihara C, et al. Outcome predictors for sacroiliac joint (lateral branch) radiofrequency denervation. Reg Anesth Pain Med. 2009;34(3):206–214. doi: 10.1097/AAP.0b013e3181958f4b. [DOI] [PubMed] [Google Scholar]
- 74.Cohen SP, Hurley RW, Buckenmaier CC, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279–288. doi: 10.1097/ALN.0b013e31817f4c7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel N, Gross A, Brown L, Gekht G. A randomized, placebo-controlled study to assess the efficacy of lateral branch neurotomy for chronic sacroiliac joint pain. Pain Med. 2012;13(3):383–398. doi: 10.1111/j.1526-4637.2012.01328.x. [DOI] [PubMed] [Google Scholar]
- 76.Prather H, Hunt D. Sacroiliac joint pain. Dis Mon. 2004;50(12):670–683. doi: 10.1016/j.disamonth.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 77.Prather H, Bonnette M, Hunt D. Nonoperative treatment options for patients with sacroiliac joint pain. Int J Spine Surg. 2020;14(Suppl 1):35–40. doi: 10.14444/6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neha B, Arunmozhi R, Maneesh A, Pooja A. Effectiveness of therapeutic interventions in sacroiliac joint dysfunction: a systematic review. Int J Physiother Res. 2016;4(3):1484–1488. doi: 10.16965/ijpr.2016.111. [DOI] [Google Scholar]
- 79.Stuge B, Holm I, Vøllestad N. To treat or not to treat postpartum pelvic girdle pain with stabilizing exercises? Man Ther. 2006;11(4):337–343. doi: 10.1016/j.math.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 80.Stuge B, Laerum E, Kirkesola G, Vøllestad N. The efficacy of a treatment program focusing on specific stabilizing exercises for pelvic girdle pain after pregnancy: a randomized controlled trial. Spine. 2004;29(4):351–359. doi: 10.1097/01.brs.0000090827.16926.1d. [DOI] [PubMed] [Google Scholar]
- 81.Mens JM, Snijders CJ, Stam HJ. Diagonal trunk muscle exercises in peripartum pelvic pain: a randomized clinical trial. Phys Ther. 2000;80(12):1164–1173. [PubMed] [Google Scholar]
- 82.Monticone M, Barbarino A, Testi C, Arzano S, Moschi A, Negrini S. Symptomatic efficacy of stabilizing treatment versus laser therapy for sub-acute low back pain with positive tests for sacroiliac dysfunction: a randomised clinical controlled trial with 1 year follow-up. Eur Medicophysica. 2004;40(4):263–268. [PubMed] [Google Scholar]
- 83.Visser LH, Woudenberg NP, de Bont J, et al. Treatment of the sacroiliac joint in patients with leg pain: a randomized-controlled trial. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2013;22(10):2310–2317. doi: 10.1007/s00586-013-2833-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Whang PG, Cher D, Polly D, et al. Sacroiliac joint fusion using triangular titanium implants vs. non-surgical management: six-month outcomes from a prospective randomized controlled trial. Int J Spine Surg. 2015;9 doi: 10.14444/2006. article 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sturesson B, Kools D, Pflugmacher R, Gasbarrini A, Prestamburgo D, Dengler J. Six-month outcomes from a randomized controlled trial of minimally invasive SI joint fusion with triangular titanium implants vs. conservative management. Eur Spine J. 2017;26(3):708–719. doi: 10.1007/s00586-016-4599-9. [DOI] [PubMed] [Google Scholar]
- 86.Juch JNS, Maas ET, Ostelo RWJG, et al. Effect of radiofrequency denervation on pain intensity among patients with chronic low back pain: the Mint randomized clinical trials. JAMA. 2017;318(1):68–81. doi: 10.1001/jama.2017.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cibulka MT, Delitto A. A comparison of two different methods to treat hip pain in runners. J Orthop Sports Phys Ther. 1993;17(4):172–176. doi: 10.2519/jospt.1993.17.4.172. [DOI] [PubMed] [Google Scholar]
- 88.DonTigny RL. Dysfunction of the sacroiliac joint and its treatment. J Orthop Sports Phys Ther. 1979;1(1):23–35. doi: 10.2519/jospt.1979.1.1.23. [DOI] [PubMed] [Google Scholar]
- 89.Wreje U, Nordgren B, Åberg H. Treatment of pelvic joint dysfuntion in primary care—a controlled study. Scand J Prim Health Care. 1992;10(4):310–315. doi: 10.3109/02813439209014080. [DOI] [PubMed] [Google Scholar]
- 90.Daly JM, Frame PS, Rapoza PA. Sacroiliac subluxation: a common, treatable cause of low-back pain in pregnancy. Fam Pract Res J. 1991;11(2):149–159. [PubMed] [Google Scholar]
- 91.Al-Subahi M, Alayat M, Alshehri MA, et al. The effectiveness of physiotherapy interventions for sacroiliac joint dysfunction: a systematic review. J Phys Ther Sci. 2017;29(9):1689–1694. doi: 10.1589/jpts.29.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Childs JD, Piva SR, Erhard RE. Immediate improvements in side-to-side weight bearing and iliac crest symmetry after manipulation in patients with low back pain. J Manipulative Physiol Ther. 2004;27(5):306–313. doi: 10.1016/j.jmpt.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 93.Shearar KA, Colloca CJ, White HL. A randomized clinical trial of manual versus mechanical force manipulation in the treatment of sacroiliac joint syndrome. J Manipulative Physiol Ther. 2005;28(7):493–501. doi: 10.1016/j.jmpt.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 94.Kamali F, Shokri E. The effect of two manipulative therapy techniques and their outcome in patients with sacroiliac joint syndrome. J Bodyw Mov Ther. 2012;16(1):29–35. doi: 10.1016/j.jbmt.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 95.Barbosa AC, Martins FLM, Barbosa MCSA, dos Santos RT. Manipulation and selective exercises decrease pelvic anteversion and low-back pain: a pilot study. J Back Musculoskelet Rehabil. 2013;26(1):33–36. doi: 10.3233/BMR-2012-0347. [DOI] [PubMed] [Google Scholar]
- 96.Cibulka MT, Delitto A, Koldehoff RM. Changes in innominate tilt after manipulation of the sacroiliac joint in patients with low back pain. Phys Ther. 1988;68(9):1359–1363. doi: 10.1093/ptj/68.9.1359. [DOI] [PubMed] [Google Scholar]
- 97.Tullberg T, Blomberg S, Branth B, Johnsson R. Manipulation does not alter the position of the sacroiliac joint. A roentgen stereophotogrammetric analysis. Spine. 1998;23(10):1124–1128. doi: 10.1097/00007632-199805150-00010. discussion 1129. [DOI] [PubMed] [Google Scholar]
- 98.Neufeld NJ, Jones JM. Osteopathic Medicine in Musculoskeletal Conditions. In: Gonzalez-Fernandez M, Friedman JD, editors. Physical Medicine and Rehabilitation Pocket Companion. New York, NY: Demos Medical Publishing.;; 2011. pp. 182–193. [Google Scholar]
- 99.Manchikanti L, Hansen H, Pampati V, Falco FJE. Utilization and growth patterns of sacroiliac joint injections from 2000 to 2011 in the medicare population. Pain Physician. 2013;16(4):E379–390. [PubMed] [Google Scholar]
- 100.Manchikanti L, Hirsch JA, Pampati V, Boswell MV. Utilization of facet joint and sacroiliac joint interventions in Medicare population from 2000 to 2014: explosive growth continues! Curr Pain Headache Rep. 2016;20(10):58. doi: 10.1007/s11916-016-0588-2. [DOI] [PubMed] [Google Scholar]
- 101.Salman OH, Gad SD, Mohamed AA, Rafae HH, Abdelfatah AM. Randomized, controlled blind study comparing sacroiliac intra-articular steroid injection to radiofrequency denervation for sacroiliac joint pain. Egypt J Anaesth. 2016;32:219–225. doi: 10.1016/j.egja.2015.07.005. [DOI] [Google Scholar]
- 102.Dutta K, Dey S, Bhattacharyya P, Agarwal S, Dev P. Comparison of efficacy of lateral branch pulsed radiofrequency denervation and intraarticular depot methylprednisolone injection for sacroiliac joint pain. Pain Physician. 2018;21(5):489–496. [PubMed] [Google Scholar]
- 103.Cánovas Martínez L, Orduña Valls J, Paramés Mosquera E, Lamelas Rodríguez L, Rojas Gil S, Domínguez García M. Sacroiliac joint pain: prospective, randomised, experimental and comparative study of thermal radiofrequency with sacroiliac joint block. Rev Esp Anestesiol Reanim. 2015 doi: 10.1016/j.redar.2015.08.003. [DOI] [PubMed]
- 104.Kim WM, Lee HG, Jeong CW, Kim CM, Yoon MH. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med N Y N. 2010;16(12):1285–1290. doi: 10.1089/acm.2010.0031. [DOI] [PubMed] [Google Scholar]
- 105.Wehling P, Evans C, Wehling J, Maixner W. Effectiveness of intra-articular therapies in osteoarthritis: a literature review. Ther Adv Musculoskelet Dis. 2017;9(8):183–196. doi: 10.1177/1759720X17712695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wernecke C, Braun HJ, Dragoo JL. The effect of intra-articular corticosteroids on articular cartilage: a systematic review. Orthop J Sports Med. 2015;3(5):232596711558116. doi: 10.1177/2325967115581163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patel N. Twelve-month follow-up of a randomized trial assessing cooled radiofrequency denervation as a treatment for sacroiliac region pain. Pain Pract. 2016;16(2):154–167. doi: 10.1111/papr.12269. [DOI] [PubMed] [Google Scholar]
- 108.Mehta V, Poply K, Husband M, Anwar S, Langford R. The effects of radiofrequency neurotomy using a strip-lesioning device on patients with sacroiliac joint pain: results from a single-center, randomized, sham-controlled trial. Pain Physician. 2018;21(6):607–618. [PubMed] [Google Scholar]
- 109.van Tilburg CWJ, Schuurmans FA, Stronks DL, Groeneweg JG, Huygen FJPM. Randomized sham-controlled double-blind multicenter clinical trial to ascertain the effect of percutaneous radiofrequency treatment for sacroiliac joint pain: three-month results. Clin J Pain. 2016 doi: 10.1097/AJP.0000000000000351. [DOI] [PMC free article] [PubMed]
- 110.Maas ET, Juch JNS, Ostelo RWJG, et al. Cost-effectiveness of radiofrequency denervation for patients with chronic low back pain: the MINT randomized clinical trials. Value Health J Int Soc Pharmacoeconom Outcomes Res. 2020;23(5):585–594. doi: 10.1016/j.jval.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 111.Painter CF. Excision of the os innominatum. Arthrodesis of the sacro-iliac synchrondrosis. Boston Med Surg J. 1908;159(7):205–208. [Google Scholar]
- 112.McGuire RA, Chen Z, Donahoe K. Dual fibular allograft dowel technique for sacroiliac joint arthrodesis. Evid-Based Spine-Care J. 2012;3(3):21–28. doi: 10.1055/s-0032-1327806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Buchowski JM, Kebaish KM, Sinkov V, Cohen DB, Sieber AN, Kostuik JP. Functional and radiographic outcome of sacroiliac arthrodesis for the disorders of the sacroiliac joint. Spine J Off J North Am Spine Soc. 2005;5(5):520–528. doi: 10.1016/j.spinee.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 114.Belanger TA, Dall BE. Sacroiliac arthrodesis using a posterior midline fascial splitting approach and pedicle screw instrumentation: a new technique. J Spinal Disord. 2001;14(2):118–124. doi: 10.1097/00002517-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 115.Waisbrod H, Krainick JU, Gerbershagen HU. Sacroiliac joint arthrodesis for chronic lower back pain. Arch Orthop Trauma Surg Arch Für Orthop Unf-Chir. 1987;106(4):238–240. doi: 10.1007/BF00450461. [DOI] [PubMed] [Google Scholar]
- 116.Moore MR. Surgical treatment of chronic painful sacroiliac joint dysfunction. In: Vleeming A, Mooney V, Snijders C, Dorman T, Stoeckart R, editors. Movement, Stability, and Low Back Pain: The Essential Role of the Pelvis. London, England: Churchill Livingstone; 1997. pp. 563–572. [Google Scholar]
- 117.Graham Smith A, Capobianco R, Cher D, et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res. 2013;7(1):14. doi: 10.1186/1750-1164-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sachs D. Minimally invasive versus open sacroiliac joint fusion: a comparison of process measures and description of technique. Conference Proceedings of International Society for the Advancement of Spine Surgery Annual Meeting. 2013. p. 187.
- 119.Ledonio CGT, Polly DW, Swiontkowski MF. Minimally invasive versus open sacroiliac joint fusion: are they similarly safe and effective? Clin Orthop. 2014;472(6):1831–1838. doi: 10.1007/s11999-014-3499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ledonio C, Polly D, Swiontkowski MF, Cummings J. Comparative effectiveness of open versus minimally invasive sacroiliac joint fusion. Med Devices (Auckl) 2014;7:187–193. doi: 10.2147/MDER.S60370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Avila L. Primary pyogenic infection of the sacro-iliac articulation. A new approach to the joint. J Bone Jt Surg. 1941;23(4):922–928. [Google Scholar]
- 122.Rand JA. Anterior sacro-iliac arthrodesis for post-traumatic sacro-iliac arthritis. A case report. J Bone Joint Surg Am. 1985;67(1):157–159. [PubMed] [Google Scholar]
- 123.Simpson LA, Waddell JP, Leighton RK, Kellam JF, Tile M. Anterior approach and stabilization of the disrupted sacroiliac joint. J Trauma. 1987;27(12):1332–1339. doi: 10.1097/00005373-198712000-00003. [DOI] [PubMed] [Google Scholar]
- 124.Nousiainen MT, Weil YA, Helfet DL. Sacroiliac joint arthrodesis. Conference Proceedings Orthopaedic Trauma Association Annual Meeting. 2007. p. 55.
- 125.Slinkard N, Agel J, Swiontkowski MF. Documentation of outcomes for sacroiliac joint fusion: does prior spinal fusion influence the outcome? Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2013;22(10):2318–2324. doi: 10.1007/s00586-013-2968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nystrom B, Gregebo B, Taube A, Almgren S, Schillberg B, Zhu Y. Clinical outcome following anterior arthrodesis in patients with presumed sacroiliac joint pain. Scand J Pain. 2017;17:22–29. doi: 10.1016/j.spain.2017.06005. [DOI] [PubMed] [Google Scholar]
- 127.Kibsgård TJ, Røise O, Sudmann E, Stuge B. Pelvic joint fusions in patients with chronic pelvic girdle pain: a 23-year follow-up. Eur Spine J. 2013;22(4):871–877. doi: 10.1007/s00586-012-2512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kibsgård TJ, Røise O, Stuge B. Pelvic joint fusion in patients with severe pelvic girdle pain—a prospective single-subject research design study. BMC Musculoskelet Disord. 2014;15:85. doi: 10.1186/1471-2474-15-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Murakami E, Kurosawa D, Aizawa T. Sacroiliac joint arthrodesis for chronic sacroiliac joint pain: an anterior approach and clinical outcomes with a minimum 5-year follow-up. J Neurosurg Spine. 2018;29(3):279–285. doi: 10.3171/2018.1.SPINE17115. [DOI] [PubMed] [Google Scholar]
- 130.Smith-Petersen MN. Arthrodesis of the sacroiliac joint. A new method of approach. J Bone Joint Surg. 1921;3(8):400–405. [Google Scholar]
- 131.Smith-Petersen MN, Rogers WA. Arthrodesis for tuberculosis of the sacro-iliac joint. JAMA. 1926;86(1):26–30. [Google Scholar]
- 132.Smith-Petersen MN, Rogers WA. End-result study of arthrodesis of the sacro-iliac joint for arthritis—traumatic and non-traumatic. J Bone Joint Surg. 1926;8(1):118–136. [Google Scholar]
- 133.Gaenslen FJ. Sacro-iliac arthrodesis: indications, author's technic and end-results. JAMA. 1927;89(24):2031–2035. [Google Scholar]
- 134.Bloom FA. Sacro-iliac fusion. J Bone Joint Surg. 1937;19:704–708. [Google Scholar]
- 135.Schütz U, Grob D. Poor outcome following bilateral sacroiliac joint fusion for degenerative sacroiliac joint syndrome. Acta Orthop Belg. 2006;72(3):296–308. [PubMed] [Google Scholar]
- 136.Noureldine MHA, Freeman TB, Alikhani P. Simultaneous sacroiliac joint fusion in patients with long lumbosacral constructs—case report and operative technique. World Neurosurg. 2020 doi: 10.1016/j.wneu.2020.04.188. [DOI] [PubMed]
- 137.Hasan MY, Liu G, Wong H-K, Hao TJ. Post-operative complications of S2AI versus iliac screw in spinopelvic fixation: a meta-analysis and recent trends review. Spine J Off J North Am Spine Soc. 2019 doi: 10.1016/j.spinee.2019.11.014. [DOI] [PubMed]
- 138.Guler UO, Cetin E, Yaman O, et al. Sacropelvic fixation in adult spinal deformity (ASD); a very high rate of mechanical failure. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2015;24(5):1085–1091. doi: 10.1007/s00586-014-3615-1. [DOI] [PubMed] [Google Scholar]
- 139.Colò G, Cavagnaro L, Alessio-Mazzola M, Zanirato A, Felli L, Formica M. Incidence, diagnosis and management of sacroiliitis after spinal surgery: a systematic review of the literature. Musculoskelet Surg. 2019 doi: 10.1007/s12306-019-00607-0. [DOI] [PubMed]
- 140.Smith EJ, Kyhos J, Dolitsky R, Yu W, O'Brien J. S2 Alar iliac fixation in long segment constructs, a two- to five-year follow-up. Spine Deform. 2018;6(1):72–78. doi: 10.1016/j.jspd.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 141.Mazur MD, Mahan MA, Shah LM, Dailey AT. Fate of S2-alar-iliac screws after 12-month minimum radiographic follow-up: preliminary results. Neurosurgery. 2016 doi: 10.1227/NEU.0000000000001322. [Epub]. [DOI] [PubMed]
- 142.Chang T-L, Sponseller PD, Kebaish KM, Fishman EK. Low profile pelvic fixation: anatomic parameters for sacral alar-iliac fixation versus traditional iliac fixation. Spine. 2009;34(5):436–440. doi: 10.1097/BRS.0b013e318194128c. [DOI] [PubMed] [Google Scholar]
- 143.Pham MH, Diaz-Aguilar LD, King BH, Osorio JA, Lehman RA. Quad S2-alar-iliac screw fixation via navigated spinal robotics with software planning: 2-dimensional operative video. Oper Neurosurg Hagerstown Md. 2020 doi: 10.1093/ons/opaa155. [DOI] [PubMed]
- 144.Mattei TA, Fassett DR. Combined S-1 and S-2 sacral alar-iliac screws as a salvage technique for pelvic fixation after pseudarthrosis and lumbosacropelvic instability: technical note. J Neurosurg Spine. 2013;19(3):321–330. doi: 10.3171/2013.5.SPINE121118. [DOI] [PubMed] [Google Scholar]
- 145.Park PJ, Lin JD, Makhni MC, Cerpa M, Lehman RA, Lenke LG. Dual S2 alar-iliac screw technique with a multirod construct across the lumbosacral junction: obtaining adequate stability at the lumbosacral junction in spinal deformity surgery. Neurospine. 2020;17(2):466–470. doi: 10.14245/ns.1938320.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Panico M, Chande RD, Lindsey DP, et al. The use of triangular implants to enhance sacropelvic fixation: a finite element investigation. Spine J Off J North Am Spine Soc. 2020 doi: 10.1016/j.spinee.2020.05.552. [DOI] [PubMed]
- 147.Lorio MP, Polly DW, Jr, Ninkovic I, Ledonio CGT, Hallas K, Andersson G. Utilization of minimally invasive surgical approach for sacroiliac joint fusion in surgeon population of ISASS and SMISS membership. Open Orthop J. 2014;8:1–6. doi: 10.2174/1874325001408010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Routt MLC, Meier MC, Kregor PJ, Mayo KA. Percutaneous iliosacral screws with the patient supine technique. Oper Tech Orthop. 1993;3(1):35–45. [Google Scholar]
- 149.Keating JG, Avillar MD, Price M. Sacroiliac joint arthrodesis in selected patients with low back pain. In: Vleeming A, Mooney V, Snijders C, Dorman T, Stoeckart R, editors. Movement, Stability, and Low Back Pain: The Essential Role of the Pelvis. London, England: Churchill Livingstone; 1997. pp. 573–586. [Google Scholar]
- 150.Al-Khayer A, Hegarty J, Hahn D, Grevitt MP. Percutaneous sacroiliac joint arthrodesis: a novel technique. J Spinal Disord Tech. 2008;21(5):359–363. doi: 10.1097/BSD.0b013e318145ab96. [DOI] [PubMed] [Google Scholar]
- 151.Mason LW, Chopra I, Mohanty K. The percutaneous stabilisation of the sacroiliac joint with hollow modular anchorage screws: a prospective outcome study. Eur Spine J. 2013;22(10):2325–2331. doi: 10.1007/s00586-013-2825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Khurana A, Guha AR, Mohanty K, Ahuja S. Percutaneous fusion of the sacroiliac joint with hollow modular anchorage screws: clinical and radiological outcome. J Bone Joint Surg Br. 2009;91(5):627–631. doi: 10.1302/0301-620X.91B5.21519. [DOI] [PubMed] [Google Scholar]
- 153.Frank CJ, Kondrashov D, Meyer SC, et al. Work intensity in sacroiliac joint fusion and lumbar microdiscectomy. Clin Outcomes Res. 2016;8:367–376. doi: 10.2147/CEOR.S112006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Garber T, Ledonio CGT, Polly DW. How much work effort is involved in minimally invasive sacroiliac joint fusion? Int J Spine Surg. 2015;9:58. doi: 10.14444/2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lorio M, Martinson M, Ferrara L. Paired comparison survey analyses utilizing Rasch methodology of the relative difficulty and estimated work relative value units of CPT(®) code 27279. Int J Spine Surg. 2016;10:40. doi: 10.14444/3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Polly DW, Swofford J, Whang PG, et al. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016;10 doi: 10.14444/3028. article 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dengler J, Kools D, Pflugmacher R, et al. Randomized trial of sacroiliac joint arthrodesis compared with conservative management for chronic low back pain attributed to the sacroiliac joint. J Bone Joint Surg Am. 2019;101(5):400–411. doi: 10.2106/JBJS.18.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Duhon BS, Bitan F, Lockstadt H, Kovalsky D, Cher D, Hillen T. Triangular titanium implants for minimally invasive sacroiliac joint fusion: 2-year follow-up from a prospective multicenter trial. Int J Spine Surg. 2016 doi: 10.14444/3013. Apr 20;10:article 13. [DOI] [PMC free article] [PubMed]
- 159.Whang PG, Darr E, Meyer SC, et al. Long-term prospective clinical and radiographic outcomes after minimally invasive lateral transiliac sacroiliac joint fusion using triangular titanium implants. Med Devices (Auckl) 2019 Sep 26;12:411–422. doi: 10.2147/MDER.S219862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Patel V, Kovalsky D, Meyer SC, et al. Prospective trial of sacroiliac joint fusion using 3D-printed triangular titanium implants. Med Devices (Auckl) 2020;13:173–182. doi: 10.2147/MDER.S253741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Araghi A, Woodruff R, Colle K, et al. Pain and opioid use outcomes following minimally invasive sacroiliac joint fusion with decortication and bone grafting: the evolusion clinical trial. Open Orthop J. 2017;11(1):1440–1448. doi: 10.2174/1874325001711011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rappoport LH, Luna IY, Joshua G. Minimally invasive sacroiliac joint fusion using a novel hydroxyapatite-coated screw: preliminary 1-year clinical and radiographic results of a 2-year prospective study. World Neurosurg. 2017;101:493–497. doi: 10.1016/j.wneu.2017.02.046. [DOI] [PubMed] [Google Scholar]
- 163.Dengler J, Duhon B, Whang P, et al. Predictors of outcome in conservative and minimally invasive surgical management of pain originating from the sacroiliac joint: a pooled analysis. Spine. 2017;42(21):1664–1673. doi: 10.1097/BRS.0000000000002169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Spain K, Holt T. Surgical revision after sacroiliac joint fixation or fusion. Int J Spine Surg. 2017;11:24–30. doi: 10.14444/4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vanaclocha V, Herrera JM, Sáiz-Sapena N, Rivera-Paz M, Verdú-López F. Minimally invasive sacroiliac joint fusion, radiofrequency denervation, and conservative management for sacroiliac joint pain: 6-year comparative case series. Neurosurgery. 2018;82(1):48–55. doi: 10.1093/neuros/nyx185. [DOI] [PubMed] [Google Scholar]
- 166.Claus CF, Lytle E, Kaufmann A, et al. Minimally invasive sacroiliac joint fusion using triangular titanium vs. cylindrical threaded implants: a comparison of patient-reported outcomes. World Neurosurg. 2019 doi: 10.1016/j.wneu.2019.09.150. [DOI] [PubMed]
- 167.Abbasi H, Hipp JA. The assessment of fusion following sacroiliac joint fusion surgery. Cureus. 2017;9(10):e1787. doi: 10.7759/cureus.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rajpal S, Burneikiene S. Minimally invasive sacroiliac joint fusion with cylindrical threaded implants using intraoperative stereotactic navigation. World Neurosurg. 2018 doi: 10.1016/j.wneu.2018.11.116. [DOI] [PubMed]
- 169.Kancherla VK, McGowan SM, Audley BN, Sokunbi G, Puccio ST. Patient reported outcomes from sacroiliac joint fusion. Asian Spine J. 2017;11(1):120–126. doi: 10.4184/asj.2017.11.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sachs D, Capobianco R. Minimally invasive sacroiliac joint fusion: one-year outcomes in 40 patients. Adv Orthop. 2013;2013:536128. doi: 10.1155/2013/536128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sachs D, Kovalsky D, Redmond A, et al. Durable intermediate- to long-term outcomes after minimally invasive transiliac sacroiliac joint fusion using triangular titanium implants. Med Devices (Auckl) 2016;9:213–222. doi: 10.2147/MDER.S109276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sachs D, Capobianco R, Cher D, et al. One-year outcomes after minimally invasive sacroiliac joint fusion with a series of triangular implants: a multicenter, patient-level analysis. Med Devices (Auckl) 2014;7:299–304. doi: 10.2147/MDER.S56491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cummings J, Jr, Capobianco RA. Minimally invasive sacroiliac joint fusion: one-year outcomes in 18 patients. Ann Surg Innov Res. 2013;7(1):1–7. doi: 10.1186/1750-1164-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schroeder JE, Cunningham ME, Ross T, Boachie-Adjei O. Early results of sacro–iliac joint fixation following long fusion to the sacrum in adult spine deformity. Hosp Spec Surg J. 2013;10(1):30–35. doi: 10.1007/s11420-013-9374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gaetani P, Miotti D, Risso A, et al. Percutaneous arthrodesis of sacro-iliac joint: a pilot study. J Neurosurg Sci. 2013;57(4):297–301. [PubMed] [Google Scholar]
- 176.Rudolf L. Sacroiliac joint arthrodesis-MIS technique with titanium implants: report of the first 50 patients and outcomes. Open Orthop J. 2012;6:495–502. doi: 10.2174/1874325001206010495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim J, Rudolf L, Glaser J. Outcome of percutaneous sacroiliac joint fixation with porous plasma coated titanium implants: an independent review. Open Orthop J. 2013;7:51–56. doi: 10.2174/1874325001307010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rudolf L, Capobianco R. Five-year clinical and radiographic outcomes after minimally invasive sacroiliac joint fusion using triangular implants. Open Orthop J. 2014;8:375–383. doi: 10.2174/1874325001408010375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bornemann R, Roessler PP, Strauss AC, et al. Two-year clinical results of patients with sacroiliac joint syndrome treated by arthrodesis using a triangular implant system. Technol Health Care. 2017;25(2):319–325. doi: 10.3233/THC-161272. [DOI] [PubMed] [Google Scholar]
- 180.Rainov NG, Schneiderhan R, Heidecke V. Triangular titanium implants for sacroiliac joint fusion. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2018 doi: 10.1007/s00586-018-5860-1. [DOI] [PubMed]
- 181.Cleveland A, Nhan D, Akiyama M, Kleck C, Noshchenko A, Patel V. Mini-open sacroiliac joint fusion with direct bone grafting and minimally invasive fixation using intraoperative navigation. J Spine Surg. 2019;5(1):31–37. doi: 10.21037/jss.2019.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mao G, Aldahak N, Kusyk D, et al. A consideration for the utility of the post-operative Oswestry Disability Index for measuring outcomes after sacroiliac joint fusion. Orthop Rev. 2018;10(2):7549. doi: 10.4081/or.2018.7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vanaclocha-Vanaclocha V, Verdú-López F, Sánchez-Pardo M, et al. Minimally invasive sacroiliac joint arthrodesis: experience in a prospective series with 24 patients. J Spine. 2014;3:185. doi: 10.4172/2165-7939.1000185. [DOI] [Google Scholar]
- 184.Cross WW, Delbridge A, Hales D, Fielding LC. Minimally invasive sacroiliac joint fusion: 2-year radiographic and clinical outcomes with a principles-based SIJ fusion system. Open Orthop J. 2018;12(1):7–16. doi: 10.2174/1874325001812010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kube RA, Muir JM. Sacroiliac joint fusion: one year clinical and radiographic results following minimally invasive sacroiliac joint fusion surgery. Open Orthop J. 2016;10:679–689. doi: 10.2174/1874325001610010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Heiney J, Capobianco R, Cher D. A systematic review of minimally invasive sacroiliac joint fusion utilizing a lateral transarticular technique. Int J Spine Surg. 2015 doi: 10.14444/2040. Jul 22;9:article 40. [DOI] [PMC free article] [PubMed]
- 187.Lingutla KK, Pollock R, Ahuja S. Sacroiliac joint fusion for low back pain: a systematic review and meta-analysis. Eur Spine J. 2016;25(6):1924–1931. doi: 10.1007/s00586-016-4490-8. [DOI] [PubMed] [Google Scholar]
- 188.Tran ZV, Ivashchenko A, Brooks L. Sacroiliac joint fusion methodology—minimally invasive compared to screw-type surgeries: a systematic review and meta-analysis. Pain Physician. 2019;22(1):29–40. [PubMed] [Google Scholar]
- 189.Zaidi HA, Montoure AJ, Dickman CA. Surgical and clinical efficacy of sacroiliac joint fusion: a systematic review of the literature. J Neurosurg Spine. 2015;23(1):59–66. doi: 10.3171/2014.10.SPINE14516. [DOI] [PubMed] [Google Scholar]
- 190.Whelan R, Duhon B. The evidence for sacroiliac joint surgery. Tech Orthop. 2019;34(2):87–95. doi: 10.1097/BTO.0000000000000367. [DOI] [Google Scholar]
- 191.Shamrock AG, Patel A, Alam M, Shamrock KH, Al Maaieh M. The safety profile of percutaneous minimally invasive sacroiliac joint fusion. Glob Spine J. 2019:2192568218816981. doi: 10.1177/2192568218816981. [DOI] [PMC free article] [PubMed]
- 192.Yson SC, Sembrano JN, Polly DW. Sacroiliac joint fusion: approaches and recent outcomes. PM R. 2019 doi: 10.1002/pmrj.12198. [DOI] [PubMed]
- 193.Martin CT, Haase L, Lender PA, Polly DW. Minimally invasive sacroiliac joint fusion: the current evidence. Int J Spine Surg. 2020;14(Suppl 1):20–29. doi: 10.14444/6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schoell K, Buser Z, Jakoi A, et al. Postoperative complications in patients undergoing minimally invasive sacroiliac fusion. Spine J. 2016 Nov;16(11):1324–1332. doi: 10.1016/j.spinee.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 195.Miller L, Reckling WC, Block JE. Analysis of postmarket complaints database for the iFuse SI Joint Fusion System: a minimally invasive treatment for degenerative sacroiliitis and sacroiliac joint disruption. Med Devices (Auckl) 2013 May 29;6:77–84. doi: 10.2147/MDER.S44690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cher DJ, Reckling WC, Capobianco RA. Implant survivorship analysis after minimally invasive sacroiliac joint fusion using the iFuse Implant System. Med Devices (Auckl) 2015 Nov 23;8:485–492. doi: 10.2147/MDER.S94885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cher D, Wroe K, Reckling WC, Yerby S. Postmarket surveillance of 3D-printed implants for sacroiliac joint fusion. Med Devices (Auckl) 2018 Sep 28;11:337–343. doi: 10.2147/MDER.S180958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kiapour A, Joukar A, Elgafy H, Erbulut DU, Agarwal AK, Goel VK. Biomechanics of the sacroiliac joint: anatomy, function, biomechanics, sexual dimorphism, and causes of pain. Int J Spine Surg. 2020;14(Suppl 1):3–13. doi: 10.14444/6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Casaroli G, Bassani T, Brayda-Bruno M, Luca A, Galbusera F. What do we know about the biomechanics of the sacroiliac joint and of sacropelvic fixation? A literature review. Med Eng Phys. 2019 doi: 10.1016/j.medengphy.2019.10.009. [DOI] [PubMed]
- 200.Lindsey D, Perez-Orribo L, Rodriquez-Martinez N, et al. Evaluation of a minimally invasive procedure for sacroiliac joint fusion—an in vitro biomechanical analysis of initial and cycled properties. Med Devices (Auckl) 2014 May 15;7:131–137. doi: 10.2147/MDER.S63499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lindsey DP, Kiapour A, Yerby SA, Goel VK. Sacroiliac joint stability: finite element analysis of implant number, orientation, and superior implant length. World J Orthop. 2018;9(3):14–23. doi: 10.5312/wjo.v9.i3.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Soriano-Baron H, Lindsey DP, Rodriguez-Martinez N, et al. The effect of implant placement on sacroiliac joint range of motion: posterior vs trans-articular. Spine. 2015;40(9):E525–E530. doi: 10.1097/BRS.0000000000000839. [DOI] [PubMed] [Google Scholar]
- 203.Bruna-Rosso C, Arnoux P-J, Bianco R-J, Godio-Raboutet Y, Fradet L, Aubin C-É. Finite element analysis of sacroiliac joint fixation under compression loads. Int J Spine Surg. 2016;10 doi: 10.14444/3016. article 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shih YC, Beaubien BP, Chen Q, Sembrano JN. Biomechanical evaluation of sacroiliac joint fixation with decortication. Spine J. 2018;18(7):1241–1249. doi: 10.1016/j.spinee.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 205.Cross WW, Berven SH, Slater N, Lehrman JN, Newcomb AGUS, Kelly BP. In vitro biomechanical evaluation of a novel, minimally invasive, sacroiliac joint fixation device. Int J Spine Surg. 2018 Oct 15;12(5):587–594. doi: 10.14444/5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dubé-Cyr R, Aubin C-É, Villemure I, Bianco R-J, Godio-Raboutet Y, Arnoux P-J. Biomechanical analysis of two insertion sites for the fixation of the sacroiliac joint via an oblique lateral approach. Clin Biomech (Bristol Avon) 2020 Apr;74:118–123. doi: 10.1016/j.clinbiomech.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 207.Lindsey D, Kiapour A, Gundanna M, Leasure J, Yerby SA, Kondroshov D. Biomechanics of unilateral and bilateral sacroiliac jloint stabilization: laboratory investigation. J Neurosurg Spine. 2018;28(3):326–332. doi: 10.3171/2017.7.SPINE17499. [DOI] [PubMed] [Google Scholar]
- 208.Lindsey DP, Kiapour A, Yerby SA, Goel VK. Sacroiliac joint fusion minimally affects adjacent lumbar segment motion: a finite element study. Int J Spine Surg. 2015 Nov 13;9:64. doi: 10.14444/2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Joukar A, Chande RD, Carpenter RD, et al. Effects on hip stress following sacroiliac joint fixation: a finite element study. JOR Spine. 2019;2(4):e1067. doi: 10.1002/jsp2.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cher DJ, Frasco MA, Arnold RJ, Polly DW. Cost-effectiveness of minimally invasive sacroiliac joint fusion. Clinicoecon Outcomes Res. 2015 Dec 18;8:1–14. doi: 10.2147/CEOR.S94266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ackerman SJ, Polly DW, Jr, Knight T, Holt T, Cummings J., Jr Nonoperative care to manage sacroiliac joint disruption and degenerative sacroiliitis: high costs and medical resource utilization in the United States Medicare population. J Neurosurg Spine. 2014;20(4):354–363. doi: 10.3171/2014.1.SPINE13188. [DOI] [PubMed] [Google Scholar]
- 212.Ackerman S, Polly DW, Holt T, Cummings JT, Knight T. Management of sacroiliac joint disruption and degenerative sacroiliitis with nonoperative care is medical resource-intensive and costly in a United States commercial payer population. Clin Outcomes Res. 2014;2014(6):63–74. doi: 10.2147/CEOR.S54158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ackerman S, Cummings J, Polly D, Knight T, Schneider K, Holt T. Comparison of the costs of nonoperative care to minimally invasive surgery for sacroiliac joint disruption and degenerative sacroiliitis in a United States Medicare population: potential economic implications of a new minimally-invasive technology. Clin Outcomes Res. 2013;2013(5):575–587. doi: 10.2147/CEOR.S52967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ackerman S, Knight T, Schneider K, Holt T, Cummings J, Polly D. Comparison of the costs of nonoperative care to minimally invasive surgery for sacroiliac joint disruption and degenerative sacroiliitis in a United States commercial payer population: potential economic implications of a new minimally invasive technology. Clin Outcomes Res. 2014;2014(6):283–296. doi: 10.2147/CEOR.S63757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lorio MP. ISASS Policy 2016 update—minimally invasive sacroiliac joint fusion. Int J Spine Surg. 2016;10:26. doi: 10.14444/3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lekovic GP, Han PP, Kenny KJ, Dickman CA. Bone dowels in anterior lumbar interbody fusion. J Spinal Disord Tech. 2007;20(5):374–379. doi: 10.1097/BSD.0b013e31802c1462. [DOI] [PubMed] [Google Scholar]
- 217.Kuslich SD. The Bagby and Kuslich method of lumbar interbody fusion. Spine. 1998;23(11):1267–1279. doi: 10.1097/00007632-199806010-00019. [DOI] [PubMed] [Google Scholar]
- 218.Kuslich SD, Danielson G, Dowdle JD, et al. Four-year follow-up results of lumbar spine arthrodesis using the Bagby and Kuslich lumbar fusion cage. Spine. 2000;25(20):2656–2662. doi: 10.1097/00007632-200010150-00018. [DOI] [PubMed] [Google Scholar]
- 219.Button G, Gupta M, Barrett C, Cammack P, Benson D. Three- to six-year follow-up of stand-alone BAK cages implanted by a single surgeon. Spine J. 2005;5(2):155–160. doi: 10.1016/j.spinee.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 220.Wise CL, Dall BE. Minimally invasive sacroiliac arthrodesis: outcomes of a new technique. J Spinal Disord Tech. 2008;21(8):579–584. doi: 10.1097/BSD.0b013e31815ecc4b. [DOI] [PubMed] [Google Scholar]
- 221.Endres S, Ludwig E. Outcome of distraction interference arthrodesis of the sacroiliac joint for sacroiliac arthritis. Indian J Orthop. 2013;47(5):437–442. doi: 10.4103/0019-5413.118197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Fuchs V, Ruhl B. Distraction arthrodesis of the sacroiliac joint: 2-year results of a descriptive prospective multi-center cohort study in 171 patients. Eur Spine J. 2018;27(1):194–204. doi: 10.1007/s00586-017-5313-2. [DOI] [PubMed] [Google Scholar]
- 223.Stark JG, Brown GA, Idemmili C, Meyer CL. Fusion of the sacroiliac joint: new technique and functional outcome. Conference Proceedings American Academy of Orthopaedic Surgeons Annual Meeting. 2008. In.
- 224.Morningstar. Make a case for unlisted codes. AAPC Healthc Bus Mon. 2020;42(3) https://www.aapc.com/?&utm_medium=ppc&utm_source=adwords&utm_term=%2Baapc&utm_campaign=Search_Google_Brand_AAPC-Core_BMM&hsa_cam=6492563276&hsa_tgt=aud-412910490190:kwd-312940333590&hsa_kw=%2Baapc&hsa_ver=3&hsa_ad=426824675943&hsa_acc=8669576186&hsa_grp=76662609503&hsa_src=g&hsa_net=adwords&hsa_mt=b&gclid=CjwKCAjw_NX7BRA1EiwA2dpg0pXUiVqSSj54Vvxu-FZROgzvUz5Id9srHQB7y7wwb2A0rzjOgd6WVBoCJQIQAvD_BwE&gclsrc=aw.ds Accessed on September 28, 2020. [Google Scholar]
- 225.Dengler J, Kools D, Pflugmacher R, et al. 1-year results of a randomized controlled trial of conservative management vs. minimally invasive surgical treatment for sacroiliac joint pain. Pain Physician. 2017;20(6):537–550. [PubMed] [Google Scholar]
- 226.Polly DW, Cher DJ, Wine KD, et al. Randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77(5):674–691. doi: 10.1227/NEU.0000000000000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Darr E, Cher D. 4-Year outcomes after minimally invasive transiliac sacroiliac joint fusion with triangular titanium implants. Med Devices (Auckl) 2018 Aug 29;11:287–289. doi: 10.2147/MDER.S179003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Darr E, Meyer SC, Whang PG, et al. Long-term prospective outcomes after minimally invasive trans-iliac sacroiliac joint fusion using triangular titanium implants. Med Devices (Auckl) 2018 Apr 9;11:113–121. doi: 10.2147/MDER.S160989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Patel V, Kovalsky D, Meyer SC, et al. Minimally invasive lateral transiliac sacroiliac joint fusion using 3D-printed triangular titanium implants. Med Devices Evid Res. 2019;12:203–214. doi: 10.2147/MDER.S205812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Duhon BS, Cher DJ, Wine KD, Kovalsky DA, Lockstadt H the SIFI Study Group. Triangular titanium implants for minimally invasive sacroiliac joint fusion: a prospective study. Glob Spine J. 2016;6(3):257–269. doi: 10.1055/s-0035-1562912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Duhon BS, Cher DJ, Wine KD, Lockstadt H, Kovalsky D, Soo C-L. Safety and 6-month effectiveness of minimally invasive sacroiliac joint fusion: a prospective study. Med Devices (Auckl) 2013 Dec 13;6:219–229. doi: 10.2147/MDER.S55197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Capobianco R, Cher D SIFI Study Group. Safety and effectiveness of minimally invasive sacroiliac joint fusion in women with persistent post-partum posterior pelvic girdle pain: 12-month outcomes from a prospective, multi-center trial. SpringerPlus. 2015 Oct 5;4:570. doi: 10.1186/s40064-015-1359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sachs D, Capobianco R. One year successful outcomes for novel sacroiliac joint arthrodesis system. Ann Surg Innov Res. 2012;6(1):13. doi: 10.1186/1750-1164-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bornemann R, Pflugmacher R, Webler M, et al. Clinical trial to test the iFuse implant system® in patients with sacroiliac joint syndrome: one year results [in German] Z Orthopadie Unfallchirurgie. 2016;154(6):601–605. doi: 10.1055/s-0042-110207. [DOI] [PubMed] [Google Scholar]
- 235.Rudolf L. MIS Fusion of the SI joint: does prior lumbar spinal fusion affect patient outcomes? Open Orthop J. 2013;7:163–168. doi: 10.2174/1874325001307010163. [DOI] [PMC free article] [PubMed] [Google Scholar]