Abstract
Aim: Lifetime risk (LTR) indicates the absolute risk of disease during the remainder of an individual's lifetime. We aimed to assess the LTRs for coronary heart disease (CHD) mortality associated with blood pressure (BP) and total cholesterol levels in an Asian population using a meta-analysis of individual participant data because no previous studies have assessed this risk.
Methods: We analyzed data from 105,432 Japanese participants in 13 cohorts. Apart from grade 1 and 2–3 hypertension groups, we defined “normal BP” as systolic/diastolic BP < 130/< 80 mmHg and “high BP” as 130–139/80–89 mmHg. The sex-specific LTR was estimated while considering the competing risk of death.
Results: During the mean follow-up period of 15 years (1,553,735 person-years), 889 CHD deaths were recorded. The 10-year risk of CHD mortality at index age 35 years was ≤ 0.11%, but the corresponding LTR was ≥ 1.84%. The LTR of CHD at index age 35 years steeply increased with an increase in BP of participants with high total cholesterol levels [≥ 5.7 mmol/L (220 mg/dL)]. This risk was 7.73%/5.77% (95% confidence interval: 3.53%–10.28%/3.83%–7.25%) in men/women with grade 2–3 hypertension and high total cholesterol levels. In normal and high BP groups, the absolute differences in LTRs between the low and high total cholesterol groups were ≤ 0.25% in men and ≤ 0.40% in women.
Conclusions: High total cholesterol levels contributed to an elevated LTR of CHD mortality in hypertensive individuals. These findings could help guide high-risk young individuals toward initiating lifestyle changes or treatments.
Keywords: Blood pressure, Cholesterol, Coronary heart disease, Cohort study, Lifetime risk
See editorial vol. 28: 1–2
Introduction
Hypertension and hyperlipidemia are strongly and independently associated with coronary heart disease (CHD). Individuals with both elevated blood pressure (BP) and elevated total cholesterol (TC) levels have an increased risk of CHD when compared with those with either of the two conditions1–3). Using relative risk estimation, we have previously demonstrated the synergic effect of elevated BP and TC levels on the risk of CHD death in an Asian population4). However, information regarding the long-term absolute risk of CHD according to BP and TC in Asians is limited.
This synergistic effect is not limited to elderly individuals4). As a result, lifestyle interventions or drug initiation early in life may be required5). However, most of the guidelines for the management of hypertension or hyperlipidemia in young individuals struggle to identify or explain the absolute cardiovascular risks in this population, as their short-term risks are extremely low3, 5, 6). Thus, these guidelines used relative risk as an alternative3, 5, 6).
The American Heart Association (AHA)/American College of Cardiology (ACC) Guideline on the Management of Blood Cholesterol recommends estimating lifetime risk (LTR) to determine the intensity of primary preventative measures in young adults7). LTR is a cumulative assessment of the incidence of disease risk factors from a given age (index age) until the end of life; therefore, it provides an estimation of the absolute risk of CHD during the remainder of an individual's lifetime8). As short-term risk (e.g., < 10 years) is considerably affected by age, LTR is a suitable index for informing young populations about their current and future risk of CHD. Relative risk indicates the risk ratio relative to status without exposure, whereas LTR shows the concrete risk difference among groups.
The LTR of CHD mortality based on BP values has been examined previously9–11). However, prior studies did not evaluate cholesterol levels, which interact with BP to increase CHD risk4, 12). Furthermore, irrespective of other factors, the LTR assessment of CHD according to serum cholesterol levels alone is also scarce in Asian populations13).
Aim
We assessed the LTR of CHD mortality according to the cross-classification by BP and TC in Asian populations through a meta-analysis of individual participant data (IPD) from the Evidence for Cardiovascular Prevention from Observational Cohorts in Japan (EPOCH–JAPAN).
Methods
Study Design and Populations
The EPOCH–JAPAN study is a meta-analysis of individual data from Japanese cohorts4, 14). It is based on databases that include an overview of individual participants' data from longitudinal observational studies in Japan. All studies contributing to the EPOCH–JAPAN project received ethical approval from their respective institutional review boards. Moreover, the EPOCH–JAPAN study received ethical approval from the institutional review board of Shiga University of Medical Science (23-125-1) and the Ethics Committee of the Keio University School of Medicine (20110192). The former is a data management center, and the latter is the university in which the primary investigator of EPOCH–JAPAN works.
In 2017, the EPOCH–JAPAN cardiovascular database included 14 Japanese cohorts. Of the 121,003 participants with codes for causes of death, 2,489 participants in the Tanno-Sobetsu cohort15) were excluded because there was no information on the past history of cardiovascular disease. For the current analyses, 13,082 individuals were excluded because of age [< 35 years old throughout the follow-up period (n = 685)], lack of BP (n = 1,713) or TC data (n = 2,620), and lack of data on the history of cardiovascular disease (n = 869) or a previous history of cardiovascular disease (n = 7,195). Finally, 105,432 participants without a history of cardiovascular disease from 13 cohorts were analyzed16–28).
BP Measurement
BP was measured with a mercury sphygmomanometer, with each participant in a seated position, except in the Ohasama study16), where an automated device was used. Participants rested before any measurement in all the studies, except those in the Ohsaki study17). One reading at the examination center was obtained and used in the analysis, except in the cases of the Ohasama16) and Suita18) studies (two readings) and the Hisayama study19) (three readings). In one study (JACC20)), BP was self-recorded after it had been measured at a health check-up. Methods for obtaining other clinical data have been presented previously4, 29).
On the basis of hypertension guidelines3, 30) and our previous reports29, 31), we first set grade (G) 1 hypertension as a systolic/diastolic BP range within 140–159/90–99 mmHg and G2–3 hypertension as a BP of ≥ 160/≥ 100 mmHg3, 30, 32, 33). We additionally defined a BP of < 130/< 80 mmHg as the “normal group” and a BP range within 130–139/80–89 mmHg as the “high BP group,” which is defined as stage 1 hypertension by the ACC/AHA Guideline6). If systolic and diastolic BP fell within different groups (e.g., systolic high, diastolic normal), participants were assigned to the higher BP category.
Data Collection and Measurement of Serum TC
In all cohorts, a questionnaire was used to obtain detailed information on each participant's drinking and smoking habits. Smoking and drinking (alcohol consumption) habits were categorized into current smoker or nonsmoker and current habitual drinker or nondrinker, respectively, in which the number of cigarettes and the amount of alcohol were not considered. Body mass index was determined by body weight in kilograms divided by height in meters squared. Serum TC was determined by automated enzymatic methods on venous blood samples in all cohorts, with the exception of the NIPPON DATA80 cohort24), in which TC levels were measured by the Liebermann–Burchard direct method. We divided the participants into groups according to a TC cut-off value of 5.7 mmol/L (220 mg/dL); this TC level is equivalent to an LDL value of 3.6 mmol/L (140 mg/dL), which is the cut-off point for dyslipidemia screening in Japan34). For the sensitivity analysis, we also used a TC cut-off point of 6.2 mmol/L (240 mg/dL), which is equivalent to an LDL value of 4.1 mmol/L (160 mg/dL)34).
Outcomes
In accordance with the Family Registration Law in Japan, all death certificates are forwarded to the Ministry of Health, Labor, and Welfare via the public health center in the area of residence35). Registration of death is required by law. Other sources used in some studies included autopsy reports18, 19), medical records16, 18, 19), health examinations16, 17), and questionnaires. The underlying causes of death were coded according to the 9th International Classification of Disease (ICD-9) generated at the end of 1994, and the 10th International Classification of Disease (ICD-10) generated at the beginning of 1995. The cause of death was defined as follows: CHD (410 to 414; I20 to I25).
Statistical Analysis
LTR was calculated using the practical incidence estimator (PIE) macro8). Values were adjusted for cause of death other than CHD with a double-decrement approach, taking into consideration the occurrence of the outcome of interest and all-cause mortality8, 10, 36–38). We have indicated the schema of methods used to calculate intermediate risk and LTR (Supplementary Fig. 1). Age categories began at the age of 35 years, and the highest age category was set at age ≥ 85 years. However, age ≥ 85 years was later changed to 85 years, as the small number of observations in each age group ≥ 85 years caused unstable LTRs. Accordingly, sex-specific LTRs of CHD death at 35, 45, 55, 65, and 75 years were each estimated. Similarly, mortality-adjusted cumulative rates for 10-, 20-, 30-, 40-, and 50-year risks of CHD death were estimated as intermediate-term risks. For sensitivity analysis, we estimated LTRs after excluding two studies22, 28) based on young workers. We also performed stratified analysis according to the use of antihypertensive drugs using available data from 63,960 individuals. The LTRs and their 95% confidence intervals at the age of 45 years were calculated instead of those at 35 years if LTR was not estimated because of the small sample size. SAS software, version 9.4 (SAS Institute, Cary, NC) was used in our study. Values were expressed as mean ± standard deviation (SD), unless otherwise noted.
Supplementary Fig. 1.
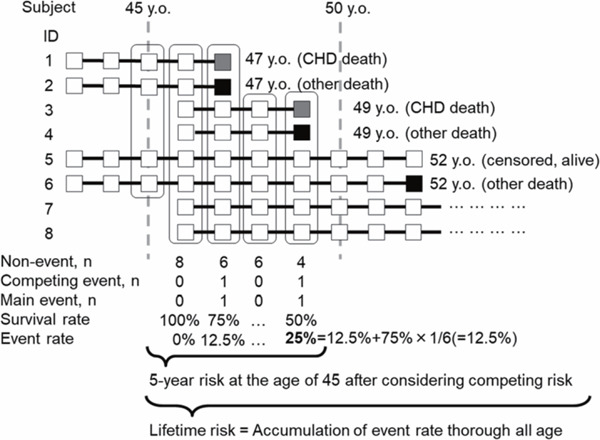
Schema of intermediate- and lifetime risk calculation CHD, coronary heart disease.
Results
Baseline Characteristics
Of the 105,432 participants (42.5% men; mean age, 55.0 ± 12.5 years), 40,901 (38.8%), 29,793 (28.3%), 23,575 (22.4%), and 11,163 (10.6%) showed normal BP, high BP, G1 hypertension, and G2–3 hypertension, respectively, and 28,919 (27.4%) had TC ≥ 5.7 mmol/L. Baseline characteristics according to the BP and TC categories are shown in Table 1, and characteristics stratified by cohort are shown in Supplementary Table 1.
Table 1. Baseline Characteristics.
| Category: SBP/DBP, mmHg |
|||||
|---|---|---|---|---|---|
| TC | Variables | Normal: < 130/< 80 (n = 40,901) |
High BP: 130–139/80–89 (n = 29,793) |
Grade 1 HT: 140–159/90–99 (n = 23,575) |
Grade 2–3 HT: ≥ 160/≥ 100 (n = 11,163) |
| < 5.7 mmol/L | |||||
| (< 220 mg/dL) | N | 31,707 | 21,152 | 16,152 | 7,502 |
| Age, years | 50.1 ± 12.5 | 54.2 ± 12.1 | 58.8 ± 11.4 | 61.5 ± 11.2 | |
| Men, % | 38.8 | 49.8 | 51.5 | 53.7 | |
| Body mass index, kg/m2 | 22.1 ± 2.8 | 23.0 ± 3.0 | 23.4 ± 3.2 | 23.7 ± 3.4 | |
| Current smoking, % | 26.8 | 29.5 | 28.9 | 31.0 | |
| Alcohol consumption, % | 42.6 | 49.9 | 49.8 | 51.4 | |
| Systolic BP, mmHg | 113.0 ± 9.5 | 129.2 ± 7.3 | 144.6 ± 7.9 | 167.5 ± 14.8 | |
| Diastolic BP, mmHg | 68.0 ± 6.9 | 79.8 ± 6.1 | 85.6 ± 7.9 | 95.1 ± 11.3 | |
| Total cholesterol, mmol/L | 4.6 ± 0.6 | 4.7 ± 0.6 | 4.8 ± 0.6 | 4.7 ± 0.6 | |
| ≥ 5.7 mmol/L | |||||
| (≥ 220 mg/dL) | N | 9,194 | 8,641 | 7,423 | 3,661 |
| Age, years | 54.6 ± 10.9 | 56.7 ± 10.5 | 59.6 ± 10.2 | 61.4 ± 10.4 | |
| Men, % | 30.2 | 36.2 | 32.8 | 33.5 | |
| Body mass index, kg/m2 | 22.9 ± 2.9 | 23.8 ± 3.1 | 24.3 ± 3.2 | 24.8 ± 3.4 | |
| Current smoking, % | 20.9 | 20.3 | 18.3 | 18.2 | |
| Alcohol consumption, % | 36.5 | 40.1 | 37.1 | 37.5 | |
| Systolic BP, mmHg | 114.1 ± 9.1 | 129.5 ± 7.2 | 145 ± 7.8 | 167.4 ± 15.0 | |
| Diastolic BP, mmHg | 69.0 ± 6.6 | 80.2 ± 6.1 | 85.9 ± 8.0 | 95.7 ± 11.4 | |
| Total cholesterol, mmol/L | 6.3 ± 0.6 | 6.3 ± 0.6 | 6.4 ± 0.6 | 6.4 ± 0.7 | |
A total of 105,432 Japanese individuals from 13 cohorts are included. Data on body mass index, smoking status, alcohol consumption status, and total cholesterol are unavailable in 395, 4,148, and 4,193 individuals, respectively. Data are shown as mean ± SD, unless otherwise stated. BP, blood pressure; HT, hypertension.
Supplementary Table 1. Characteristics of Study Participants in Each Cohort: EPOCH–JAPAN.
| Cohorts | Baseline Year | N | Men, % | Age, years | Systolic BP, mmHg | Diastolic BP, mmHg | Total Cholesterol, mmol/L | CHD Death, n |
|---|---|---|---|---|---|---|---|---|
| Ohsaki1) | 1994 | 15,551 | 42.0 | 62.2 ± 9.4 | 131.2 ± 17.7 | 78.6 ± 11.0 | 5.3 ± 0.9 | 107 |
| Ohasama2) | 1987 | 2,617 | 39.1 | 55.8 ± 12.1 | 129.4 ± 16.7 | 73.3 ± 11.1 | 5.1 ± 0.9 | 25 |
| Oyabe3) | 1988 | 5,124 | 30.8 | 56.8 ± 11.3 | 126.5 ± 19.9 | 75.5 ± 11.2 | 5.0 ± 0.9 | 28 |
| YKK workers4) | 1990 | 6,507 | 63.8 | 38.7 ± 9.7 | 118.7 ± 14.7 | 71.4 ± 11.8 | 4.9 ± 0.9 | 5 |
| Suita5) | 1989 | 6,241 | 47.2 | 55.0 ± 13.3 | 127.1 ± 22.1 | 77.4 ± 12.3 | 5.4 ± 1.0 | 79 |
| RERF cohort6) | 1986 | 4,243 | 31.9 | 61.6 ± 12.2 | 134.2 ± 22.5 | 82.2 ± 11.9 | 5.4 ± 1.0 | 68 |
| Hisayama7) | 1988 | 2,636 | 42.1 | 59.1 ± 11.9 | 133.8 ± 21.4 | 77.8 ± 11.3 | 5.3 ± 1.1 | 22 |
| JACC study8) | 1988 | 26,454 | 35.9 | 57.1 ± 9.6 | 133.0 ± 19.3 | 79.1 ± 11.3 | 5.1 ± 0.9 | 260 |
| NIPPON DATA 80 9) | 1980 | 9,173 | 43.8 | 50.7 ± 13.2 | 135.9 ± 21.3 | 81.3 ± 12.2 | 4.9 ± 0.9 | 177 |
| NIPPON DATA 90 10) | 1990 | 7,125 | 41.6 | 52.3 ± 13.7 | 135.1 ± 20.6 | 81.2 ± 11.9 | 5.2 ± 1.0 | 61 |
| Osaka11) | 1985 | 3,613 | 32.9 | 51.2 ± 12.0 | 125.6 ± 16.6 | 78.2 ± 10.3 | 5.4 ± 1.0 | 9 |
| JMS12) | 1992 | 10,744 | 38.6 | 55.5 ± 11.0 | 129.3 ± 20.9 | 77.4 ± 12.2 | 5.0 ± 0.9 | 45 |
| Aichi workers13) | 2002 | 5,404 | 78.5 | 48.0 ± 7.1 | 126.2 ± 15.5 | 77.7 ± 11.5 | 5.4 ± 0.9 | 3 |
A total of 105,432 Japanese individuals from 13 cohorts were included. BP, blood pressure; CHD, coronary heart disease; JACC, Japan Collaborative Cohort; JMS, Jichi Medical School; NIPPON DATA, National Integrated Project for Prospective Observation of Non*communicable Disease And its Trends in the Aged; RERF, Radiation Effects Research Foundation; YKK, Yoshida Kogyo Kabushikigaisya.
LTRs and Intermediate-Term Risks of CHD Mortality
During the mean follow-up period of 15 years (1,553,735 person-years), 889 deaths from CHD were recorded. The follow-up person-years according to age group are shown in Table 2. In men, the 10-year risk of CHD death at the index age of 35 years was at most 0.11% (Table 3). The LTR of CHD death was at least 2.65% (Table 3). The LTRs increased with incremental increases in BP, and the association of BP with the LTRs was steeper in participants with TC ≥ 5.7 mmol/L than in those with TC < 5.7 mmol/L. In hypertensive men, the differences in LTRs between the TC < 5.7 mmol/L and ≥ 5.7 mmol/L groups were ≈2.6%. In men with high BP, the LTR in the TC < 5.7 mmol/L group did not differ from that in the TC ≥ 5.7 mmol/L group (3.93% vs 3.70%) (Table 3). In women with high BP, although similar results were observed, the difference in the LTR between the TC < 5.7 mmol/L and ≥ 5.7 mmol/L groups was only significant in the G2–3 hypertension group (Table 4).
Table 2. Number of CHD deaths and the follow-up person-years.
| Strata | CHD Mortality Rates |
||
|---|---|---|---|
| Number of Deaths | Person-Years | Mortality per 1,000 Person-Years | |
| Men | |||
| 35–44 | 3 | 64,686 | 0.05 |
| 45–54 | 19 | 137,563 | 0.14 |
| 55–64 | 63 | 167,670 | 0.38 |
| 65–74 | 126 | 157,433 | 0.80 |
| 75–84 | 197 | 77,557 | 2.54 |
| 85 ≤ | 84 | 31,580 | 2.66 |
| Women | |||
| 35–44 | 0 | 70,139 | 0.00 |
| 45–54 | 3 | 166,302 | 0.02 |
| 55–64 | 14 | 247,515 | 0.06 |
| 65–74 | 80 | 253,615 | 0.32 |
| 75–84 | 175 | 125,198 | 1.40 |
| 85 ≤ | 125 | 54,477 | 2.29 |
Data shows the number of CHD death and the follow-up person-years in each age group according to sex. CHD, coronary heart disease. The total number of CHD deaths in men and women are 492 and 397, respectively.
Table 3. Risk Estimates (95% Confidence Intervals) for CHD Mortality in Men at the Index Age of 35.
| TC, mmol/L | Category: SBP/DBP, mmHg | Intermediate-Term Risk, % |
|||||
|---|---|---|---|---|---|---|---|
| 10-Year | 20-Year | 30-Year | 40-Year | 50-Year | LTR, % | ||
| < 5.7 | |||||||
| (< 220 mg/dL) | Normal: | 0.06 | 0.14 | 0.27 | 0.51 | 1.81 | 2.90 |
| < 130/< 80 | (0.00–0.14) | (0.02–0.25) | (0.11–0.42) | (0.28–0.73) | (0.97–2.37) | (1.55–3.71) | |
| High BP: | 0.00 | 0.12 | 0.5 | 1.27 | 2.66 | 3.93 | |
| 130–139/80–89 | (0.00–0.00) | (0.00–0.24) | (0.28–0.72) | (0.89–1.61) | (1.80–3.26) | (2.58–4.77) | |
| G1 hypertension: | 0.11 | 0.26 | 0.51 | 1.15 | 2.75 | 4.03 | |
| 140–159/90–99 | (0.00–0.33) | (0.00–0.55) | (0.18–0.83) | (0.72–1.54) | (1.93–3.34) | (2.76–4.80) | |
| G2–3 hypertension: | 0.00 | 0.79 | 1.22 | 2.01 | 4.20 | 5.11 | |
| ≥ 160/≥ 100 | (0.00–0.00) | (0.00–1.60) | (0.32–2.08) | (1.02–2.93) | (2.73–5.31) | (3.24–6.32) | |
| ≥ 5.7 | |||||||
| (≥ 220 mg/dL) | Normal: | 0.00 | 0.00 | 0.42 | 0.85 | 2.15 | 2.65 |
| < 130/< 80 | (0.00–0.00) | (0.00–0.00) | (0.04–0.79) | (0.18–1.45) | (0.23–3.51) | (0.12–4.32) | |
| High BP: | 0.00 | 0.10 | 0.33 | 1.07 | 2.65 | 3.70 | |
| 130–139/80–89 | (0.00–0.00) | (0.00–0.30) | (0.00–0.66) | (0.40–1.68) | (1.01–3.87) | (1.18–5.38) | |
| G1 hypertension: | 0.00 | 0.26 | 0.92 | 2.19 | 5.06 | 6.63 | |
| 140–159/90–99 | (0.00–0.00) | (0.00–0.63) | (0.29–1.52) | (1.19–3.08) | (2.73–6.66) | (3.39–8.63) | |
| G2–3 hypertension: | 0.00 | 0.35 | 1.77 | 3.49 | 5.76 | 7.73 | |
| ≥ 160/≥ 100 | (0.00–0.00) | (0.00–1.03) | (0.51–2.95) | (1.79–5.03) | (3.04–7.82) | (3.53–10.28) | |
A total of 44,761 Japanese men from 13 cohorts were included and the total number of observations was 636,489 person-years. Intermediate-term risks were defined as mortality-adjusted cumulative rates for 10-, 20-, 30-, 40-, and 50-year risk of CHD death. CHD, coronary heart disease; DBP, diastolic blood pressure; SBP, systolic blood pressure; LTR, lifetime risk; TC, total cholesterol.
Table 4. Risk Estimates (95% Confidence Intervals) for CHD Mortality in Women at the Index Age of 35.
| TC | Category: SBP/DBP, mmHg | Intermediate-Term Risk, % |
|||||
|---|---|---|---|---|---|---|---|
| 10-Year | 20-Year | 30-Year | 40-Year | 50-Year | LTR, % | ||
| < 5.7 | |||||||
| (< 220 mg/dL) | Normal: | 0.00 | 0.00 | 0.05 | 0.14 | 0.96 | 1.83 |
| < 130/< 80 | (0.00–0.00) | (0.00–0.00) | (0.00–0.09) | (0.04–0.23) | (0.45–1.37) | (0.94–2.50) | |
| High BP: | 0.00 | 0.00 | 0.04 | 0.42 | 1.40 | 3.02 | |
| 130–139/80–89 | (0.00–0.00) | (0.00–0.00) | (0.00–0.09) | (0.21–0.60) | (0.85–1.86) | (1.91–3.88) | |
| G1 hypertension: | 0.00 | 0.06 | 0.06 | 0.34 | 1.63 | 3.55 | |
| 140–159/90–99 | (0.00–0.00) | (0.00–0.18) | (0.00–0.18) | (0.13–0.54) | (1.03–2.12) | (2.48–4.36) | |
| G2–3 hypertension: | 0.00 | 0.20 | 0.26 | 0.68 | 1.96 | 3.78 | |
| ≥ 160/≥ 100 | (0.00–0.00) | (0.00–0.59) | (0.00–0.67) | (0.15–1.17) | (1.06–2.71) | (2.34–4.82) | |
| ≥ 5.7 | |||||||
| (≥ 220 mg/dL) | Normal: | 0.00 | 0.05 | 0.08 | 0.18 | 1.30 | 1.84 |
| < 130/< 80 | (0.00–0.00) | (0.00–0.16) | (0.00–0.20) | (0.01–0.34) | (0.43–2.01) | (0.67–2.78) | |
| High BP: | 0.00 | 0.00 | 0.08 | 0.36 | 1.60 | 2.62 | |
| 130–139/80–89 | (0.00–0.00) | (0.00–0.00) | (0.00–0.18) | (0.14–0.57) | (0.87–2.24) | (1.42–3.60) | |
| G1 hypertension: | 0.00 | 0.00 | 0.00 | 0.46 | 1.51 | 2.79 | |
| 140–159/90–99 | (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | (0.20–0.70) | (0.88–2.04) | (1.70–3.64) | |
| G2–3 hypertension: | 0.00 | 0.00 | 0.58 | 1.34 | 3.73 | 5.77 | |
| ≥ 160/≥ 100 | (0.00–0.00) | (0.00–0.00) | (0.00–1.16) | (0.57–2.07) | (2.4–4.85) | (3.83–7.25) | |
A total of 60,671 Japanese individuals from 13 cohorts were included and the total number of observations was 917,246 person-years, considering the mean follow-up period of 15 years. Intermediate-term risks were defined as mortality-adjusted cumulative rates for 10-, 20-, 30-, 40-, and 50-year risk of CHD death. CHD, coronary heart disease; DBP, diastolic blood pressure; SBP, systolic blood pressure; LTR, lifetime risk; TC, total cholesterol.
Fig. 1 shows summarized data for the LTRs at each index age. The corresponding intermediate-risk and LTR values at the index age of 45 years, 55 years, 65 years, and 75 years are shown in Supplementary Table 2, Supplementary Table 3, Supplementary Table 4, and Supplementary Table 5, respectively. Although LTR decreased with age, the association between both BP and TC, and LTR was similar irrespective of the age index (Fig. 1). The LTR of CHD mortality at the index age of 75 years in the G2–3 hypertension group with TC ≥ 5.7 mmol/L was 5.71 (1.66–8.49)% in men and 5.19 (3.37–6.71)% in women.
Fig. 1.
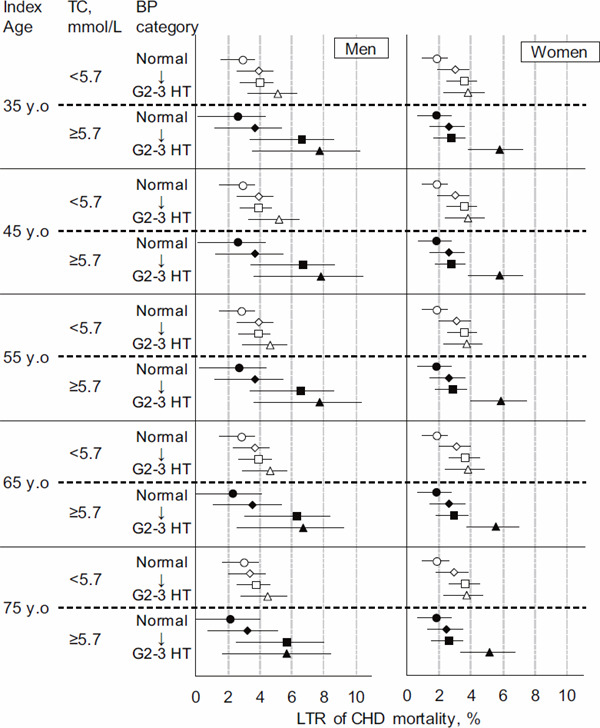
Sex- and blood pressure-specific lifetime risk of CHD mortality after stratification of the total cholesterol level across five age groups
A total of 105,432 Japanese individuals from 13 cohorts were included, and the mean follow-up period was 15 years (1,553,735 person-years). Open and closed symbols indicate the TC < 5.7 mmol/L and ≥ 5.7 mmol/L groups, respectively. CHD, coronary heart disease; DBP, diastolic blood pressure; SBP, systolic blood pressure; LTR, lifetime risk.
Supplementary Table 2. Sex- and Blood Pressure-Specific Risk Estimates (95% Confidence Intervals) for Coronary Heart Disease Mortality at the Index Age of 45 After Stratification of Total Cholesterol Level.
| Intermediate-Term Risk, % |
|||||
|---|---|---|---|---|---|
| Strata | 10-Year Risk | 20-Year Risk | 30-Year Risk | 40-Year Risk | LTR, % |
| Men | |||||
| TC < 5.7 mmol/L | |||||
| Normal | 0.08 (0.00–0.16) | 0.21 (0.08–0.34) | 0.46 (0.24–0.66) | 1.76 (0.93–2.32) | 2.86 (1.51–3.67) |
| High BP | 0.12 (0.00–0.24) | 0.51 (0.28–0.72) | 1.27 (0.89–1.62) | 2.67 (1.81–3.28) | 3.95 (2.59–4.80) |
| Grade 1 HT | 0.15 (0.00–0.34) | 0.40 (0.15–0.65) | 1.05 (0.68–1.38) | 2.66 (1.87–3.22) | 3.96 (2.70–4.71) |
| Grade 2–3 HT | 0.80 (0.00–1.63) | 1.24 (0.34–2.12) | 2.04 (1.05–2.99) | 4.27 (2.80–5.41) | 5.20 (3.32–6.44) |
| TC ≥ 5.7 mmol/L | |||||
| Optimal | 0.00 (0.00–0.00) | 0.42 (0.04–0.80) | 0.85 (0.18–1.46) | 2.16 (0.24–3.54) | 2.67 (0.14–4.35) |
| High BP | 0.10(0.00–0.30) | 0.34 (0.00–0.67) | 1.08 (0.41–1.69) | 2.67 (1.02–3.89) | 3.72 (1.20–5.41) |
| Grade 1 HT | 0.27 (0.00–0.64) | 0.93 (0.30–1.54) | 2.21 (1.22–3.11) | 5.11 (2.79–6.73) | 6.70 (3.46–8.72) |
| Grade 2–3 HT | 0.35 (0.00–1.04) | 1.79 (0.54–2.99) | 3.53 (1.84–5.10) | 5.83 (3.11–7.92) | 7.83 (3.64–10.41) |
| Women | |||||
| TC < 5.7 mmol/L | |||||
| Normal | 0.00 (0.00–0.00) | 0.05 (0.00–0.09) | 0.14 (0.04–0.23) | 0.96 (0.46–1.37) | 1.83 (0.95–2.51) |
| High BP | 0.00 (0.00–0.00) | 0.04 (0.00–0.09) | 0.42 (0.22–0.61) | 1.41 (0.85–1.87) | 3.04 (1.93–3.90) |
| Grade 1 HT | 0.06 (0.00–0.18) | 0.06 (0.00–0.18) | 0.34 (0.13–0.54) | 1.64 (1.04–2.13) | 3.57 (2.49–4.38) |
| Grade 2–3 HT | 0.20 (0.00–0.59) | 0.26 (0.00–0.68) | 0.68 (0.15–1.18) | 1.97 (1.07–2.72) | 3.80 (2.36–4.84) |
| TC ≥ 5.7 mmol/L | |||||
| Normal | 0.05 (0.00–0.16) | 0.08 (0.00–0.20) | 0.18 (0.01–0.34) | 1.30 (0.44–2.03) | 1.85 (0.69.2–80) |
| High BP | 0.00 (0.00–0.00) | 0.08 (0.00–0.18) | 0.36 (0.14–0.57) | 1.60 (0.87–2.24) | 2.62 (1.42–3.60) |
| Grade 1 HT | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.46 (0.21–0.70) | 1.52 (0.89–2.05) | 2.81 (1.72–3.66) |
| Grade 2–3 HT | 0.00 (0.00–0.00) | 0.58 (0.00–1.16) | 1.34 (0.57–2.07) | 3.73 (2.40–4.85) | 5.77 (3.83–7.25) |
Normal, high BP, grade (G) 1 hypertension and G2–3 hypertension was defined as systolic/diastolic BPs < 130/< 80 mmHg, 130–139/80–89, 140–159/90–99, ≥ 160/≥ 100 mmHg, respectively. BP, blood pressure; CHD, coronary heart disease; LTR, lifetime risk; TC, total cholesterol.
Supplementary Table 3. Sex- and Blood Pressure-Specific Risk Estimates (95% Confidence Intervals) for Coronary Heart Disease Mortality at the Index Age of 55 After Stratification of Total Cholesterol Level.
| Intermediate-Term Risk, % |
||||
|---|---|---|---|---|
| Strata | 10-Year Risk | 20-Year Risk | 30-Year Risk | LTR, % |
| Men | ||||
| TC < 5.7 mmol/L | ||||
| Normal | 0.13 (0.02–0.23) | 0.38 (0.18–0.57) | 1.71 (0.88–2.27) | 2.83 (1.48–3.65) |
| High BP | 0.39 (0.20–0.58) | 1.18 (0.82–1.51) | 2.61 (1.76–3.22) | 3.92 (2.57–4.78) |
| Grade 1 HT | 0.26 (0.08–0.42) | 0.91 (0.59–1.21) | 2.57 (1.80–3.12) | 3.91 (2.66–4.65) |
| Grade 2–3 HT | 0.47 (0.12–0.79) | 1.32 (0.76–1.81) | 3.68 (2.42–4.52) | 4.66 (2.94–5.65) |
| TC ≥ 5.7 mmol/L | ||||
| Optimal | 0.43 (0.04–0.81) | 0.86 (0.20–1.48) | 2.19 (0.27–3.59) | 2.70 (0.18–4.41) |
| High BP | 0.24 (0.00–0.51) | 1.00 (0.36–1.59) | 2.62 (0.99–3.85) | 3.69 (1.18–5.41) |
| Grade 1 HT | 0.68 (0.17–1.18) | 1.99 (1.07–2.84) | 4.96 (2.66–6.58) | 6.59 (3.36–8.63) |
| Grade 2–3 HT | 1.49 (0.44–2.52) | 3.30 (1.74–4.77) | 5.69 (3.04–7.75) | 7.76 (3.60–10.35) |
| Women | ||||
| TC < 5.7 mmol/L | ||||
| Normal | 0.05 (0.00–0.09) | 0.14 (0.04–0.23) | 0.97 (0.47–1.39) | 1.85 (0.97–2.54) |
| High BP | 0.04 (0.00–0.09) | 0.42 (0.22–0.61) | 1.42 (0.87–1.89) | 3.07 (1.96–3.94) |
| Grade 1 HT | 0.00 (0.00–0.00) | 0.29 (0.11–0.45) | 1.61 (1.02–2.09) | 3.57 (2.50–4.39) |
| Grade 2–3 HT | 0.07 (0.00–0.19) | 0.49 (0.14–0.81) | 1.82 (1.00–2.48) | 3.70 (2.30–4.69) |
| TC ≥ 5.7 mmol/L | ||||
| Normal | 0.03 (0.00–0.09) | 0.12 (0.00–0.25) | 1.26 (0.41–1.98) | 1.82 (0.65–2.77) |
| High BP | 0.08 (0.00–0.18) | 0.36 (0.14–0.57) | 1.62 (0.88–2.26) | 2.64 (1.45–3.63) |
| Grade 1 HT | 0.00 (0.00–0.00) | 0.47 (0.21–0.71) | 1.54 (0.91–2.08) | 2.84 (1.75–3.70) |
| Grade 2–3 HT | 0.59 (0.00–1.19) | 1.38 (0.61–2.12) | 3.82 (2.49–4.97) | 5.91 (3.97–7.42) |
Normal, high BP, grade (G) 1 hypertension and G2–3 hypertension were defined as systolic/diastolic BPs < 130/< 80 mmHg, 130–139/80–89, 140–159/90–99, ≥ 160/≥ 100 mmHg, respectively. BP, blood pressure; CHD, coronary heart disease; LTR, lifetime risk; TC, total cholesterol.
Supplementary Table 4. Sex- and Blood Pressure-Specific Risk Estimates (95% Confidence Intervals) for Coronary Heart Disease Mortality at the Index Age of 65 After Stratification of Total Cholesterol Level.
| Intermediate-Term Risk, % |
|||
|---|---|---|---|
| Strata | 10-Year Risk | 20-Year Risk | LTR, % |
| Men | |||
| TC < 5.7 mmol/L | |||
| Normal | 0.27 (0.09–0.43) | 1.67 (0.85–2.25) | 2.85 (1.50–3.71) |
| High BP | 0.83 (0.52–1.11) | 2.33 (1.50–2.95) | 3.71 (2.36–4.59) |
| Grade 1 HT | 0.71 (0.43–0.97) | 2.48 (1.73–3.04) | 3.91 (2.67–4.69) |
| Grade 2–3 HT | 0.94 (0.50–1.35) | 3.55 (2.33–4.40) | 4.64 (2.93–5.66) |
| TC ≥ 5.7 mmol/L | |||
| Optimal | 0.45 (0.00–0.96) | 1.83 (0.00–3.23) | 2.37 (0.00–4.10) |
| High BP | 0.79 (0.21–1.33) | 2.46 (0.85–3.71) | 3.57 (1.07–5.33) |
| Grade 1 HT | 1.40 (0.61–2.13) | 4.55 (2.28–6.19) | 6.28 (3.06–8.38) |
| Grade 2–3 HT | 1.94 (0.74–3.07) | 4.48 (1.99–6.41) | 6.70 (2.58–9.27) |
| Women | |||
| TC < 5.7 mmol/L | |||
| Normal | 0.09 (0.01–0.17) | 0.94 (0.44–1.37) | 1.84 (0.96–2.54) |
| High BP | 0.39 (0.20–0.58) | 1.42 (0.87–1.90) | 3.11 (2.00–4.00) |
| Grade 1 HT | 0.30 (0.12–0.46) | 1.66 (1.07–2.16) | 3.69 (2.62–4.53) |
| Grade 2–3 HT | 0.45 (0.12–0.75) | 1.84 (1.02–2.51) | 3.79 (2.40–4.82) |
| TC ≥ 5.7 mmol/L | |||
| Normal | 0.10 (0.00–0.21) | 1.26 (0.41–1.99) | 1.82 (0.66–2.79) |
| High BP | 0.29 (0.10–0.48) | 1.58 (0.85–2.23) | 2.63 (1.44–3.64) |
| Grade 1 HT | 0.48 (0.22–0.73) | 1.58 (0.94–2.13) | 2.91 (1.82–3.79) |
| Grade 2–.3 HT | 0.82 (0.34–1.29) | 3.37 (2.17–4.41) | 5.56 (3.69–7.02) |
Normal, high BP, grade (G) 1 hypertension and G2–3 hypertension were defined as systolic/diastolic BPs < 130/< 80 mmHg, 130–139/80–89, 140–159/90–99, ≥ 160/≥ 100 mmHg, respectively. BP, blood pressure; CHD, coronary heart disease; LTR, lifetime risk; TC, total cholesterol
Supplementary Table 5. Sex- and Blood Pressure-Specific Risk Estimates (95% Confidence Intervals) for Coronary Heart Disease Mortality at the Index Age of 75 after Stratification of Total Cholesterol Level.
| Intermediate-Term Risk, % |
||
|---|---|---|
| Strata | 10-Year Risk | LTR, % |
| Men | ||
| TC < 5.7 mmol/L | ||
| Normal | 1.60 (0.79–2.23) | 2.94 (1.60–3.90) |
| High BP | 1.75 (0.96–2.38) | 3.34 (2.02–4.31) |
| Grade 1 HT | 2.10 (1.39–2.69) | 3.79 (2.57–4.66) |
| Grade 2–3 HT | 3.19 (2.03–4.11) | 4.51 (2.84–5.67) |
| TC ≥ 5.7 mmol/L | ||
| Optimal | 1.55 (0.00–3.01) | 2.15 (0.00–4.01) |
| High BP | 1.92 (0.40–3.21) | 3.20 (0.73–5.11) |
| Grade 1 HT | 3.65 (1.48–5.37) | 5.65 (2.47–7.95) |
| Grade 2–3 HT | 3.05 (0.79–4.94) | 5.71 (1.66–8.49) |
| Women | ||
| TC < 5.7 mmol/L | ||
| Normal | 0.90 (0.41–1.35) | 1.86 (0.98–2.60) |
| High BP | 1.09 (0.58–1.57) | 2.90 (1.80–3.83) |
| Grade 1 HT | 1.47 (0.91–1.97) | 3.65 (2.59–4.54) |
| Grade 2–3 HT | 1.53 (0.78–2.19) | 3.69 (2.33–4.78) |
| TC ≥ 5.7 mmol/L | ||
| Normal | 1.22 (0.37–1.97) | 1.81 (0.65–2.82) |
| High BP | 1.35 (0.64–2.00) | 2.45 (1.27–3.48) |
| Grade 1 HT | 1.17 (0.59–1.70) | 2.60 (1.53–3.50) |
| Grade 2–3 HT | 2.80 (1.68–3.81) | 5.19 (3.37–6.71) |
Normal, high BP, grade (G) 1 hypertension and G2–3 hypertension were defined as systolic/diastolic BPs < 130/< 80 mmHg, 130–139/80–89, 140–159/90–99, ≥ 160/≥ 100 mmHg, respectively. BP, blood pressure; CHD, coronary heart disease; LTR, lifetime risk; TC, total cholesterol.
Sensitivity Analyses
Despite excluding two studies on the basis of participant demographics, which could have caused imbalances in participants' characteristics (young workers22, 28)), the results remained unchanged (Supplementary Table 6). We also performed the same analysis after excluding three studies using multiple BP readings, and the highest LTR of CHD mortality was observed in participants with TC ≥ 5.7 mmol/L combined with G2–3 hypertension (Supplementary Table 7).
Supplementary Table 6. Sex- and Blood Pressure-Specific Lifetime Risk (95% Confidence Intervals) for Coronary Heart Disease Mortality at the Index Age of 35 After Excluding 2 Studies Based Young Workers.
| Sex | Strata | LTR at the Index Age of 35 Years, % |
|---|---|---|
| Men | ||
| TC< 5.7 mmol/L | ||
| Normal | 3.02 (1.65–3.84) | |
| High BP | 3.98 (2.62–4.83) | |
| Grade 1 HT | 3.92 (2.66–4.67) | |
| Grade 2–3 HT | 5.29 (3.35–6.59) | |
| TC ≥ 5.7 mmol/L | ||
| Optimal | 2.59 (0.04–4.24) | |
| High BP | 3.81 (1.26–5.49) | |
| Grade 1 HT | 6.71 (3.45–8.74) | |
| Grade 2–3 HT | 8.13 (3.87–10.8) | |
| Women | ||
| TC < 5.7 mmol/L | ||
| Normal | 1.83 (0.95–2.50) | |
| High BP | 3.02 (1.91–3.87) | |
| Grade 1 HT | 3.56 (2.48–4.36) | |
| Grade 2–3 HT | 3.78 (2.33–4.82) | |
| TC ≥ 5.7 mmol/L | ||
| Optimal | 1.86 (0.69–2.80) | |
| High BP | 2.62 (1.43–3.60) | |
| Grade 1 HT | 2.80 (1.70–3.65) | |
| Grade 2–3 HT | 5.78 (3.83–7.26) | |
Supplementary Table 7. Sex- and Blood Pressure-Specific Lifetime Risk (95% Confidence Intervals) for Coronary Heart Disease Mortality at the Index Age of 35 After Excluding 3 Studies using multiple BP readings.
| Sex | Strata | LTR at the Index Age of 35 Years, % |
|---|---|---|
| Men | ||
| TC < 5.7 mmol/L | ||
| Normal | 2.99 (1.06–4.18) | |
| High BP | 4.07 (2.07–5.28) | |
| Grade 1 HT | 4.38 (2.53–5.49) | |
| Grade 2–3 HT | 4.98 (2.61–6.49) | |
| TC ≥ 5.7 mmol/L | ||
| Optimal | 1.81 (0.00–3.87) | |
| High BP | 3.50 (0.03–5.81) | |
| Grade 1 HT | 6.26 (1.62–8.93) | |
| Grade 2–3 HT | 8.25 (2.48–11.68) | |
| Women | ||
| TC< 5.7 mmol/L | ||
| Normal | 2.49 (1.06–3.57) | |
| High BP | 3.94 (2.22–5.26) | |
| Grade 1 HT | 3.51 (2.00–4.60) | |
| Grade 2–3 HT | 4.30 (2.23–5.74) | |
| TC ≥ 5.7 mmol/L | ||
| Optimal | 1.52 (0.05–2.71) | |
| High BP | 1.82 (0.57–2.86) | |
| Grade 1 HT | 2.85 (1.32–4.03) | |
| Grade 2–3 HT | 5.52 (3.01–7.40) | |
Next, we used a TC of 6.2 mmol/L instead of 5.7 mmol/L as the cut-off value of high TC. In the G2–3 hypertension group, the LTRs at the index age of 45 years were 5.19% (men)/4.00% (women) for the TC < 6.2 mmol/L group and 11.08% (men)/6.76% (women) for the TC ≥ 6.2 mmol/L group (Table 5). In both men and women of the high BP group aged 45 years, little difference in LTRs between the TC groups was observed (Table 5).
Table 5. Sex- and Blood Pressure-Specific Lifetime Risk (95% Confidence Intervals) for CHD Mortality After Stratification of Total Cholesterol Level of 6.2 mmol/L (240 mg/dL).
| LTR at each Index Age, % |
|||||
|---|---|---|---|---|---|
| Strata | 35 Years Old | 45 Years Old | 55 Years Old | 65 Years Old | 75 Years Old |
| Men | |||||
| TC < 6.2 mmol/L | |||||
| Normal | 2.95 (1.69–3.72) | 2.92 (1.66–3.69) | 2.90 (1.64–3.68) | 2.83 (1.57–3.64) | 2.89 (1.64–3.79) |
| High BP | 3.81 (2.57–4.58) | 3.82 (2.59–4.60) | 3.78 (2.55–4.57) | 3.57 (2.35–4.38) | 3.22 (2.02–4.11) |
| Grade 1 HT | 4.25 (3.04–4.98) | 4.20 (3.01–4.92) | 4.17 (2.99–4.90) | 4.16 (2.98–4.91) | 3.92 (2.76–4.76) |
| Grade 2–3 HT | 5.11 (3.41–6.20) | 5.19 (3.49–6.31) | 4.77 (3.17–5.71) | 4.63 (3.06–5.60) | 4.28 (2.74–5.35) |
| TC ≥ 6.2 mmol/L | |||||
| Normal | 1.92 (0.00–4.32) | 1.93 (0.00–4.33) | 1.96 (0.00–4.41) | 2.05 (0.00–4.61) | 1.84 (0.00–4.56) |
| High BP | 4.99 (0.00–8.23) | 5.01 (0.03–8.27) | 5.11 (0.12–8.43) | 5.09 (0.11–8.50) | 4.53 (0.003–8.26) |
| Grade 1 HT | 7.70 (1.80–11.18) | 7.70 (1.80–11.18) | 7.36 (1.48–10.87) | 6.86 (0.97–10.53) | 6.82 (0.93–10.90) |
| Grade 2.3 HT | 10.95 (3.11–15.47) | 11.08 (3.24–15.65) | 11.08 (3.28–15.75) | 9.79 (1.97–14.53) | 10.00 (2.18–15.48) |
| Women | |||||
| TC< 6.2 mmol/L | |||||
| Normal | 1.75 (0.99–2.34) | 1.76 (1.00–2.35) | 1.78 (1.02–2.38) | 1.78 (1.01–2.38) | 1.79 (1.03–2.43) |
| High BP | 2.66 (1.77–3.36) | 2.67 (1.78–3.37) | 2.70 (1.81–3.41) | 2.72 (1.83–3.44) | 2.49 (1.62–3.24) |
| Grade 1 HT | 3.35 (2.44–4.03) | 3.37 (2.46–4.05) | 3.38 (2.47–4.07) | 3.48 (2.58–4.19) | 3.38 (2.48–4.12) |
| Grade 2.3 HT | 3.98 (2.72–4.92) | 4.00 (2.74–4.94) | 3.93 (2.71–4.83) | 3.89 (2.68–4.80) | 3.78 (2.60–4.74) |
| TC ≥ 6.2 mmol/L | |||||
| Normal | 2.21 (0.41–3.67) | 2.24 (0.44–3.70) | 2.13 (0.34–3.59) | 2.11 (0.33–3.60) | 2.07 (0.29–3.61) |
| High BP | 3.71 (1.64–5.37) | 3.71 (1.64–5.37) | 3.75 (1.68–5.42) | 3.81 (1.73–5.53) | 3.68 (1.62–5.47) |
| Grade 1 HT | 2.91 (1.43–4.08) | 2.91 (1.43–4.08) | 2.93 (1.46–4.11) | 3.01 (1.53–4.22) | 2.67 (1.24.3–91) |
| Grade 2–3 HT | No data* | 6.76 (3.94–8.88) | 6.89 (4.07–9.04) | 6.76 (3.99–8.92) | 6.19 (3.49–8.45) |
LTR was not calculated as there were no participants within the index age.
BP, blood pressure; CHD, coronary heart disease; HT, hypertension; LTR, lifetime risk. TC, total cholesterol.
We confirmed that 8,712 subjects were treated with antihypertensive therapy, whereas 55,248 participants were not. The results in untreated participants were similar to the main results (Table 6). However, this was not true in treated participants. The LTRs at the index age of 45 years in treated men/women with G2–3 hypertension were 7.87%/7.29% for the TC < 5.7 mmol/L group and 8.43%/6.15% for the TC ≥ 5.7 mmol/L group, respectively, resulting in little difference in the LTRs of CHD mortality between the low and high TC groups (Table 6).
Table 6. Sex- and Blood Pressure-Specific Lifetime Risk (95% Confidence Intervals) for CHD Mortality at the Index Age of 45 After Stratification of the Usage of Antihypertensive Treatment.
| LTR at the Index Age of 45 Years*, % |
|||
|---|---|---|---|
| Sex | Strata | Untreated with Antihypertensive Medication (CHD death/ n = 353/ 55,248) |
Treated with Antihypertensive Medication (CHD death/ n = 160/ 8,712) |
| Men | |||
| TC< 5.7 mmol/L | |||
| Normal | 2.39 (0.45–3.55) | 6.97 (0.00–11.67) | |
| High BP | 4.30 (2.07–5.67) | 2.19 (0.00–4.19) | |
| Grade 1 HT | 4.71 (2.48–6.02) | 3.63 (0.44–5.49) | |
| Grade 2–3 HT | 3.67 (1.31–5.33) | 7.87 (2.97–11.44) | |
| TC ≥ 5.7 mmol/L | |||
| Optimal | 2.25 (0.00–4.80) | 2.96 (0.00–8.69) | |
| High BP | 4.61 (0.00–7.86) | 2.24 (0.00–5.31) | |
| Grade 1 HT | 5.05 (0.66–7.71) | 9.43 (0.00–15.54) | |
| Grade 2–3 HT | 7.88 (0.28–12.19) | 8.43 (0.14–13.79) | |
| Women | |||
| TC< 5.7 mmol/L | |||
| Normal | 2.57 (0.99–3.75) | 3.59 (0.00–7.20) | |
| High BP | 3.86 (1.85–5.42) | 4.51 (0.94–7.17) | |
| Grade 1 HT | 3.39 (1.57–3.30 | (0.74–5.13) | |
| Grade 2–3 HT | 2.51 (0.40–3.86) | 7.29 (2.83–11.09) | |
| TC ≥ 5.7 mmol/L | |||
| Normal | 1.82 (0.00–3.38) | 0.88 (0.00–2.61) | |
| High BP | 2.23 (0.43–3.69) | 2.81 (0.00–5.46) | |
| Grade 1 HT | 3.19 (1.22–4.72) | 2.46 (0.00–4.37) | |
| Grade 2.3 HT | 6.12 (2.48–8.70) | 6.15 (2.02–9.42) | |
Because of the small sample size, the LTRs at the age of 45 years were calculated instead of those at 35 years. BP, blood pressure; LTR, lifetime risk; HT, hypertension; TC, total cholesterol.
Discussion
The present study assessed the LTR of CHD mortality according to BP levels after stratification of TC levels, by considering the threshold for hypertension determined by the ACC/AHA Guideline. There was a greater LTR of CHD mortality with the presence of hypertension in the TC ≥ 5.7 mmol/L and TC ≥ 6.2 mmol/L groups than at lower TC values. LTR was highest in men and women with G2–3 hypertension and high TC. In the high BP group, the absolute differences in LTR between low and high TC were 0.23% (low TC, 3.93%; high TC, 3.70%) in men and 0.40% (low TC, 3.02%; high TC, 2.62%) in women.
There are few studies assessing the LTR of CHD in Asian populations11, 36). This is primarily due to the large number of subjects and extensive data collection necessary to detect LTRs, as the number of CHD events is limited in Asian populations11, 36). The Suita study is the only study reporting the LTR of CHD events according to hypertension status10) or hypercholesterolemia13) in an Asian population. According to this study, the LTR of CHD was 26.95% for Japanese hypertensive men and 14.85% for Japanese hypertensive women10). Similarly, a raised cholesterol level was associated with an elevated LTR of CHD13). The LTRs of CHD in the Suita study were higher than those in our study, which is likely because LTR was estimated based on incidence and not mortality10). However, as their Suita study included only 5,834 participants with 204 CHD events, a detailed stratification analysis according to both hypertension and hypercholesterolemia could not be performed10, 13). The present study estimated the BP- and TC- specific LTRs of CHD death based on 105,432 individuals. The participants were followed up for 15 years, resulting in 1,553,735 person-years. The 10-year risk of CHD mortality at the index age of 35 years was ≤ 0.11% regardless of sex, BP, or TC. The LTRs were higher than the 10-year risks and clearly increased with an increase in BP levels, as we have reported previously9). The LTRs of CHD mortality in men aged 35 years with G1 and G2–3 hypertension increased from 4.03% and 5.11% in the TC < 5.7 mmol/L group to 6.63% and 7.73% in the TC ≥ 5.7 mmol/L group, respectively (Table 3). A similar increase in LTR on stratification based on high TC was also observed in women, although only in the G2–3 hypertension group (Table 4). The LTR of CHD mortality in hypertensive individuals with TC ≥ 6.2 mmol/L (240 mg/dL) appears to be higher than in those with TC ≥ 5.7 mmol/L (Table 5). In our previous study, we noted that the LTRs of CHD mortality at the age of 35 years in men and women with G2–3 hypertension were 5.3%–7.2% and 4.0%–6.9%, respectively9). These results suggest that high TC contributes to an elevation in LTR of CHD death in hypertensive individuals. These results are supported by the response-to-injury hypothesis39) and the inflammation hypothesis, in which the combination of hypertension and hypercholesterolemia contributes to long-term mortality rates40).
The ACC/AHA Guideline for the Prevention, Detection, Evaluation and Management of High BP in Adults lowered the diagnostic threshold for hypertension from ≥ 140/≥ 90 mmHg to ≥ 130/≥ 80 mmHg (systolic/diastolic)6). The use of BP-lowering medications is indicated when patients are presented with a BP of 130–139/80–89 mmHg, in the presence of a ≥ 10% atherosclerotic cardiovascular disease risk. TC has been noted as one of the significant risk factors for atherosclerotic cardiovascular disease6). However, whether this recommendation should be adopted in Asian populations is still controversial. In the present study, the LTR of CHD mortality did not differ based on TC levels in the high BP group, which is defined as stage 1 hypertension by the ACC/AHA Guideline6). Although high TC is likely to increase the adjusted risk ratio of CHD mortality4), our findings suggest that the impact of high TC on the LTR of CHD mortality is low in Asian populations.
The LTR of CHD mortality in those with G2–3 hypertension in the presence of high TC levels decreased with age in this study. We estimated the LTR to be 7.73% for men (Table 3) and 5.77% for women in the 35-year-old group (Table 4) and only 5.71% for men and 5.19% for women in the 75-year-old group (Supplementary Table 5). Thus, our data suggest that in individuals with hypertension and hyperlipidemia, it is important to intervene early in life, especially for men. As the short-term absolute CHD risk in younger populations is quite low9, 10), it is often difficult to implement and maintain healthy lifestyles in young high-risk individuals. LTR is far easier to comprehend for patients than a relative risk estimation41), and patients prefer health risks to be indicated in absolute terms42). Thus, the estimated LTR in the present study could help encourage young, high-risk individuals to make lifestyle changes or begin treatments, which in turn could lead to lower rates of CHD mortality.
The LTRs in untreated participants in this study were almost identical to the overall main findings (Table 6). In treated G2–3 hypertensive men and women, the LTR of CHD mortality at the age of 45 years was 7.87% and 7.29% in the TC < 5.7 mmol/L group, respectively, which was similar to the results observed in the TC ≥ 5.7 mmol/L group in the same BP level group (Table 6). Patients undergoing treatment who still presented with G2–3 hypertension could have pharmacologically resistant hypertension, which has previously been shown to be related to cardiovascular complications3, 43). Therefore, the LTRs of CHD mortality in treated patients with G2–3 hypertension may have already increased irrespective of current TC levels. In normotensive men undergoing treatment, the LTR of CHD mortality at the age of 45 years was 6.97%, even though the corresponding 95% confidence interval ranged from 0.00% to 11.67%. Although a limited number of observations may have caused the high LTR observed in this group, it may also partly reflect a J-curve relationship between BP, cardiovascular risk, and LTR in these patients44). In treated women with G2–3 hypertension, the TC < 5.7 mmol/L group had a slightly higher LTR of CHD mortality than the TC ≥ 5.7 mmol/L group (7.29% vs 6.15% in Table 6). We previously reported that only TC ≥ 6.72 mmol/L was significantly associated with the higher relative risk of CHD mortality in women aged 40–69 years45). The cut-off point of a higher TC level may change this inverse association of TC with LTR of CHD mortality in women with uncontrolled BP, because the present study could not perform further stratified analysis according to a high TC cut-off point such as TC ≥ 6.7 mmol/L due to the limited number of CHD deaths.
The present findings must be interpreted within the context of their potential limitations. First, although our findings demonstrated the impact of BP and TC on long-term absolute CHD mortality risk, no direct inference can be made regarding the benefit of antihypertensive treatment because of the observational study design. Second, our findings cannot be generalized to Western populations where the rate of CHD and total cardiovascular disease is substantially higher. The Cooper Center Longitudinal Study in the United States reported that the LTRs of CHD mortality were 13.7% in men with a family history of premature CHD and 8.9% in men without a family history46). Because the highest LTR of CHD mortality in 45-year-old men was 7.8% in the present study (Supplementary Table 2) even in the hypertension groups, the Japanese population can have a lower LTR of CHD than the United States population. Third, the model estimating LTR assumed that the exposure related to the risks remained stable. For example, patients with G3 hypertension at baseline are assumed to maintain the same BP levels even if they are treated with antihypertensive medication during follow-up. However, the intensity or presence of hypertension could have changed due to lifestyle modifications and/or treatment initiation over the follow-up period. As such, our findings could underestimate the LTR according to hypertension or high TC levels, although this limitation exists in other studies10, 37, 47). Furthermore, the model for the estimation of LTR was not adjusted for confounding factors in this study. There is a possibility that some other confounding factors affected the association of TC and BP with the LTR of CHD. Participants in the cohort study were generally volunteers, who may have different characteristics compared with nonparticipants. This could also cause an underestimation of LTR in the present study. Fourth, a cholesterol-lowering therapy was not considered because this information was only available for a small portion of the participants (28,576 participants, 27.1%). However, statin use in Japan began only in the late 1980s, and because baseline surveys in EPOCH–JAPAN were primarily performed around the year 1989, it is unlikely that the use of statins at baseline affected the present findings. Furthermore, the Framingham48) and Japanese49) risk score for cardiovascular disease and the atherosclerotic cardiovascular disease risk estimator50) do not consider statin therapy because statin therapy appears not to alter the impact of TC on cardiovascular risk. Finally, the classification system for death certificate diagnosis was changed from ICD-9 to ICD-10 in 1995 in Japan, which caused a decrement of heart failure death and an increment of CHD death51). This may lead to the underestimation of CHD mortality that occurred before 1995. We cannot assess the effect of changing the ICD code on our findings because the present database does not include baseline year in each participant's data. However, this effect could be limited because the mean follow-up duration was 15 years and most CHD deaths occurred after 1995.
Perspectives
BP was found to be strongly associated with the LTR of CHD mortality in those with high TC. This suggests that even in nonelderly hypertensive individuals, high TC contributes to an elevated LTR of CHD mortality. Further, the LTR of CHD mortality in participants with elevated BP and TC was higher in non-elderly individuals than in elderly individuals. The LTR estimated in the present study could be a useful tool to inform the general public about the dangers of hypertension and hyperlipidemia. These findings on LTR could help guide high-risk young individuals toward initiating lifestyle changes or treatments.
Notice of Grant Support
This research was supported by a grant-in-aid from the Ministry of Health, Labour and Welfare, Health and Labor Sciences research grants, Japan (Research on Health Services: H17-Kenkou-007; Comprehensive Research on Cardiovascular Disease and Life-Related Disease: H18-Junkankitou [Seishuu]-Ippan-012; Comprehensive Research on Cardiovascular Disease and Life-Related Disease: H19-Junkankitou [Seishuu]-Ippan-012; Comprehensive Research on Cardiovascular and Life-Style Related Diseases: H20-Junkankitou [Seishuu]-Ippan-013; Comprehensive Research on Cardiovascular and Life-Style Related Diseases: H23-Junkankitou [Seishuu]-Ippan-005), and an Intramural Research Fund (22-4-5) for Cardiovascular Diseases of National Cerebral and Cardiovascular Center; Comprehensive Research on Cardiovascular and Life-Style Related Diseases: H26-Junkankitou [Seisaku]-Ippan-001; and H29-Junkankitou-Ippan-003.
Acknowledgements
We are grateful to all the participants in each cohort study. The following investigators are part of the Evidence for Cardiovascular Prevention from Observational Cohorts in Japan (EPOCH–JAPAN) Research Group. Chairperson: Hirotsugu Ueshima (Shiga University of Medical Science); Co–Chairperson: Tomonori Okamura (Keio University); Executive committee: Hirotsugu Ueshima (Shiga University of Medical Science), Yutaka Imai (Tohoku University Graduate School of Pharmaceutical Sciences), Takayoshi Ohkubo (Teikyo University School of Medicine), Fujiko Irie (Ibaraki Prefecture), Hiroyasu Iso, Akihiko Kitamura (Osaka University Graduate School of Medicine), Yutaka Kiyohara (Kyushu University Graduate School of Medicine), Katsuyuki Miura (Shiga University of Medical Science), Yoshitaka Murakami (Toho University), Hideaki Nakagawa (Kanazawa Medical University), Takeo Nakayama (Kyoto University School of Public Health), Tomonori Okamura (Keio University), Akira Okayama (Japan Anti–Tuberculosis Association), Toshimi Sairenchi (Dokkyo Medical University), Shigeyuki Saitoh (Sapporo Medical University), Kiyomi Sakata (Iwate Medical University), Akiko Tamakoshi (Hokkaido University Graduate School of Medicine), Ichiro Tsuji (Tohoku University Graduate School of Medicine), Michiko Yamada (Radiation Effects Research Foundation), Masahiko Kiyama (Osaka Center for Cancer and Cardiovascular Disease Prevention), Yoshihiro Miyamoto (National Cerebral and Cardiovascular Center), Shizukiyo Ishikawa (Jichi Medical University) and Hiroshi Yatsuya (Fujita Health University).
Disclosures/ Conflicts of Interest
The authors have declared that no competing interests exist.
References
- 1). Lowe LP, Greenland P, Ruth KJ, Dyer AR, Stamler R, Stamler J. Impact of major cardiovascular disease risk factors, particularly in combination, on 22-year mortality in women and men. Arch Intern Med, 1998; 158: 2007-2014 [DOI] [PubMed] [Google Scholar]
- 2). Asia Pacific Cohort Studies Collaboration. Joint effects of systolic blood pressure and serum cholesterol on cardiovascular disease in the Asia Pacific region. Circulation, 2005; 112: 3384-3390 [DOI] [PubMed] [Google Scholar]
- 3). Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, Horio T, Hoshide S, Ikeda S, Ishimitsu T, Ito M, Ito S, Iwashima Y, Kai H, Kamide K, Kanno Y, Kashihara N, Kawano Y, Kikuchi T, Kitamura K, Kitazono T, Kohara K, Kudo M, Kumagai H, Matsumura K, Matsuura H, Miura K, Mukoyama M, Nakamura S, Ohkubo T, Ohya Y, Okura T, Rakugi H, Saitoh S, Shibata H, Shimosawa T, Suzuki H, Takahashi S, Tamura K, Tomiyama H, Tsuchihashi T, Ueda S, Uehara Y, Urata H, Hirawa N. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res, 2019; 42: 1235-1481 [DOI] [PubMed] [Google Scholar]
- 4). Satoh M, Ohkubo T, Asayama K, Murakami Y, Sakurai M, Nakagawa H, Iso H, Okayama A, Miura K, Imai Y, Ueshima H, Okamura T, Evidence for Cardiovascular Prevention From Observational Cohorts in Japan Research Group. Combined effect of blood pressure and total cholesterol levels on long-term risks of subtypes of cardiovascular death: evidence for cardiovascular prevention from observational cohorts in Japan. Hypertension, 2015; 65: 517-524 [DOI] [PubMed] [Google Scholar]
- 5). Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, Authors/Task Force Members. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens, 2018; 36: 1953-2041 [DOI] [PubMed] [Google Scholar]
- 6). Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT, Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension, 2018; 71: 1269-1324 [DOI] [PubMed] [Google Scholar]
- 7). Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr., Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol, 2019; 73: e285-e350 [Google Scholar]
- 8). Beiser A, D'Agostino RB Sr., Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer's disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med, 2000; 19: 1495-1522 [DOI] [PubMed] [Google Scholar]
- 9). Satoh M, Ohkubo T, Asayama K, Murakami Y, Sugiyama D, Yamada M, Saitoh S, Sakata K, Irie F, Sairenchi T, Ishikawa S, Kiyama M, Ohnishi H, Miura K, Imai Y, Ueshima H, Okamura T, EPOCH-JAPAN Research Group. Lifetime Risk of Stroke and Coronary Heart Disease Deaths According to Blood Pressure Level: EPOCH-JAPAN (Evidence for Cardiovascular Prevention From Observational Cohorts in Japan). Hypertension, 2019; 73: 52-59 [DOI] [PubMed] [Google Scholar]
- 10). Turin TC, Okamura T, Raheen Afzal A, Rumana N, Watanabe M, Higashiyama A, Nakao YM, Nakai M, Takegami M, Nishimura K, Kokubo Y, Okayama A, Miyamoto Y. Impact of hypertension on the lifetime risk of coronary heart disease. Hypertens Res, 2016; 39: 548-551 [DOI] [PubMed] [Google Scholar]
- 11). Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet, 2014; 383: 1899-1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Tsukinoki R, Okamura T, Watanabe M, Kokubo Y, Higashiyama A, Nishimura K, Takegami M, Murakami Y, Okayama A, Miyamoto Y. Blood pressure, low-density lipoprotein cholesterol, and incidences of coronary artery disease and ischemic stroke in Japanese: the Suita study. Am J Hypertens, 2014; 27: 1362-1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Sugiyama D, Turin TC, Yeasmin F, Rumana N, Watanabe M, Higashiyama A, Takegami M, Kokubo Y, Okamura T, Miyamoto Y. Hypercholesterolemia and Lifetime Risk of Coronary Heart Disease in the General Japanese Population: Results from the Suita Cohort Study. J Atheroscler Thromb, 2020; 27: 60-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Murakami Y, Hozawa A, Okamura T, Ueshima H, Evidence for Cardiovascular Prevention From Observational Cohorts in Japan Research Group. Relation of blood pressure and all-cause mortality in 180,000 Japanese participants: pooled analysis of 13 cohort studies. Hypertension, 2008; 51: 1483-1491 [DOI] [PubMed] [Google Scholar]
- 15). Ohnishi H, Saitoh S, Takagi S, Katoh N, Chiba Y, Akasaka H, Nakamura Y, Shimamoto K. Incidence of type 2 diabetes in individuals with central obesity in a rural Japanese population: The Tanno and Sobetsu study. Diabetes Care, 2006; 29: 1128-1129 [DOI] [PubMed] [Google Scholar]
- 16). Ohkubo T, Kikuya M, Metoki H, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol, 2005; 46: 508-515 [DOI] [PubMed] [Google Scholar]
- 17). Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA, 2006; 296: 1255-1265 [DOI] [PubMed] [Google Scholar]
- 18). Mannami T, Konishi M, Baba S, Nishi N, Terao A. Prevalence of asymptomatic carotid atherosclerotic lesions detected by high-resolution ultrasonography and its relation to cardiovascular risk factors in the general population of a Japanese city: the Suita study. Stroke, 1997; 28: 518-525 [DOI] [PubMed] [Google Scholar]
- 19). Arima H, Tanizaki Y, Kiyohara Y, Tsuchihashi T, Kato I, Kubo M, Tanaka K, Ohkubo K, Nakamura H, Abe I, Fujishima M, Iida M. Validity of the JNC VI recommendations for the management of hypertension in a general population of Japanese elderly: the Hisayama study. Arch Intern Med, 2003; 163: 361-366 [DOI] [PubMed] [Google Scholar]
- 20). Iso H, Date C, Yamamoto A, Toyoshima H, Tanabe N, Kikuchi S, Kondo T, Watanabe Y, Wada Y, Ishibashi T, Suzuki H, Koizumi A, Inaba Y, Tamakoshi A, Ohno Y, JACC Study Group. Perceived mental stress and mortality from cardiovascular disease among Japanese men and women: the Japan Collaborative Cohort Study for Evaluation of Cancer Risk Sponsored by Monbusho (JACC Study). Circulation, 2002; 106: 1229-1236 [DOI] [PubMed] [Google Scholar]
- 21). Soyama Y, Miura K, Morikawa Y, Nishijo M, Nakanishi Y, Naruse Y, Kagamimori S, Nakagawa H, Oyabe S. High-density lipoprotein cholesterol and risk of stroke in Japanese men and women: the Oyabe Study. Stroke, 2003; 34: 863-868 [DOI] [PubMed] [Google Scholar]
- 22). Yoshita K, Miura K, Morikawa Y, Ishizaki M, Kido T, Naruse Y, Soyama Y, Suwazono Y, Nogawa K, Nakagawa H. Relationship of alcohol consumption to 7-year blood pressure change in Japanese men. J Hypertens, 2005; 23: 1485-1490 [DOI] [PubMed] [Google Scholar]
- 23). Nakanishi S, Yamada M, Hattori N, Suzuki G. Relationship between HbA(1)c and mortality in a Japanese population. Diabetologia, 2005; 48: 230-234 [DOI] [PubMed] [Google Scholar]
- 24). Ueshima H, Choudhury SR, Okayama A, Hayakawa T, Kita Y, Kadowaki T, Okamura T, Minowa M, Iimura O. Cigarette smoking as a risk factor for stroke death in Japan: NIPPON DATA80. Stroke, 2004; 35: 1836-1841 [DOI] [PubMed] [Google Scholar]
- 25). Okamura T, Hayakawa T, Kadowaki T, Kita Y, Okayama A, Ueshima H, NIPPON DATA Research Group. The inverse relationship between serum high-density lipoprotein cholesterol level and all-cause mortality in a 9.6-year follow-up study in the Japanese general population. Atherosclerosis, 2006; 184: 143-150 [DOI] [PubMed] [Google Scholar]
- 26). Imano H, Kitamura A, Sato S, Kiyama M, Ohira T, Yamagishi K, Noda H, Tanigawa T, Iso H, Shimamoto T. Trends for blood pressure and its contribution to stroke incidence in the middle-aged Japanese population: the Circulatory Risk in Communities Study (CIRCS). Stroke, 2009; 40: 1571-1577 [DOI] [PubMed] [Google Scholar]
- 27). Ishikawa S, Gotoh T, Nago N, Kayaba K, Jichi Medical School Cohort Study Group. The Jichi Medical School (JMS) Cohort Study: design, baseline data and standardized mortality ratios. J Epidemiol, 2002; 12: 408-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Uemura M, Yatsuya H, Hilawe EH, Li Y, Wang C, Chiang C, Otsuka R, Toyoshima H, Tamakoshi K, Aoyama A. Breakfast Skipping is Positively Associated With Incidence of Type 2 Diabetes Mellitus: Evidence From the Aichi Workers' Cohort Study. J Epidemiol, 2015; 25: 351-358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Asayama K, Satoh M, Murakami Y, Ohkubo T, Nagasawa SY, Tsuji I, Nakayama T, Okayama A, Miura K, Imai Y, Ueshima H, Okamura T, Evidence for Cardiovascular Prevention From Observational Cohorts in Japan Research Group. Cardiovascular risk with and without antihypertensive drug treatment in the Japanese general population: participant-level meta-analysis. Hypertension, 2014; 63: 1189-1197 [DOI] [PubMed] [Google Scholar]
- 30). Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J, 2013; 34: 2159-2219 [Google Scholar]
- 31). Fujiyoshi A, Ohkubo T, Miura K, Murakami Y, Nagasawa S, Okamura T, Ueshima H, Observational Cohorts in Japan (EPOCH-JAPAN) Research Group. Blood pressure categories and long-term risk of cardiovascular disease according to age group in Japanese men and women. Hypertens Res, 2012; 35: 947-953 [DOI] [PubMed] [Google Scholar]
- 32). National Institute for Health and Clinical Excellence (NICE). Hypertension (CG127): clinical management of primary hypertension in adults. Available at: https://www.nice.org.uk/guidance/CG127. Accessed 22 Nov, 2019 [Google Scholar]
- 33). Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., Jones DW, Materson BJ, Oparil S, Wright JT Jr., Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension, 2003; 42: 1206-1252 [DOI] [PubMed] [Google Scholar]
- 34). Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S, Committee for Epidemiology Clinical Management of Atherosclerosis. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). The Ministry of Health Labour and Welfare. Mannual to fill in a death certificate (in Japanese). In The Ministry of Health, Labour, and Welfare, ed. 2014. Available at: http://www.mhlw.go.jp/toukei/manual/. Accessed 22 Nov, 2019 [Google Scholar]
- 36). Allen N, Berry JD, Ning H, Van Horn L, Dyer A, Lloyd-Jones DM. Impact of blood pressure and blood pressure change during middle age on the remaining lifetime risk for cardiovascular disease: the cardiovascular lifetime risk pooling project. Circulation, 2012; 125: 37-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Turin TC, Okamura T, Afzal AR, Rumana N, Watanabe M, Higashiyama A, Nakao Y, Nakai M, Takegami M, Nishimura K, Kokubo Y, Okayama A, Miyamoto Y. Hypertension and lifetime risk of stroke. J Hypertens, 2016; 34: 116-122 [DOI] [PubMed] [Google Scholar]
- 38). Takahashi I, Geyer SM, Nishi N, Ohshita T, Takahashi T, Akahoshi M, Fujiwara S, Kodama K, Matsumoto M. Lifetime risk of stroke and impact of hypertension: estimates from the adult health study in Hiroshima and Nagasaki. Hypertens Res, 2011; 34: 649-654 [DOI] [PubMed] [Google Scholar]
- 39). Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med, 1999; 340: 115-126 [DOI] [PubMed] [Google Scholar]
- 40). Libby P. Inflammation in atherosclerosis. Nature, 2002; 420: 868-874 [DOI] [PubMed] [Google Scholar]
- 41). Karmali KN, Lloyd-Jones DM. Adding a life-course perspective to cardiovascular-risk communication. Nat Rev Cardiol, 2013; 10: 111-115 [DOI] [PubMed] [Google Scholar]
- 42). Fortin JM, Hirota LK, Bond BE, O'Connor AM, Col NF. Identifying patient preferences for communicating risk estimates: a descriptive pilot study. BMC Med Inform Decis Mak, 2001; 1: 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Oikawa T, Obara T, Ohkubo T, Kikuya M, Asayama K, Metoki H, Komai R, Murai K, Hashimoto J, Totsune K, Imai Y, J-HOME Study Group. Characteristics of resistant hypertension determined by self-measured blood pressure at home and office blood pressure measurements: the J-HOME study. J Hypertens, 2006; 24: 1737-1743 [DOI] [PubMed] [Google Scholar]
- 44). Bohm M, Schumacher H, Teo KK, Lonn EM, Mahfoud F, Mann JFE, Mancia G, Redon J, Schmieder RE, Sliwa K, Weber MA, Williams B, Yusuf S. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Lancet, 2017; 389: 2226-2237 [DOI] [PubMed] [Google Scholar]
- 45). Nagasawa SY, Okamura T, Iso H, Tamakoshi A, Yamada M, Watanabe M, Murakami Y, Miura K, Ueshima H, Evidence for Cardiovascular Prevention from Observational Cohorts in Japan Research Group. Relation between serum total cholesterol level and cardiovascular disease stratified by sex and age group: a pooled analysis of 65 594 individuals from 10 cohort studies in Japan. J Am Heart Assoc, 2012; 1: e001974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46). Bachmann JM, Willis BL, Ayers CR, Khera A, Berry JD. Association between family history and coronary heart disease death across long-term follow-up in men: the Cooper Center Longitudinal Study. Circulation, 2012; 125: 3092-3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Turin TC, Kokubo Y, Murakami Y, Higashiyama A, Rumana N, Watanabe M, Okamura T. Lifetime risk of acute myocardial infarction in Japan. Circ Cardiovasc Qual Outcomes, 2010; 3: 701-703 [DOI] [PubMed] [Google Scholar]
- 48). Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation, 1998; 97: 1837-1847 [DOI] [PubMed] [Google Scholar]
- 49). Nishimura K, Okamura T, Watanabe M, Nakai M, Takegami M, Higashiyama A, Kokubo Y, Okayama A, Miyamoto Y. Predicting coronary heart disease using risk factor categories for a Japanese urban population, and comparison with the framingham risk score: the suita study. J Atheroscler Thromb, 2014; 21: 784-798 [DOI] [PubMed] [Google Scholar]
- 50). American College of Cardiology. ASCVD Risk Estimator Plus. Available at: http://tools.acc.org/ASCVD-Risk-Estimator-Plus/#!/calculate/estimate/ Accessed 22 Nov, 2019 [Google Scholar]
- 51). Saito I. Review of death certificate diagnosis of coronary heart disease and heart failure in Japan. Nihon Koshu Eisei Zasshi, 2004; 51: 909-916 [PubMed] [Google Scholar]
References for Supplement Data
- 1). Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA, 2006; 296: 1255-1265 [DOI] [PubMed] [Google Scholar]
- 2). Ohkubo T, Kikuya M, Metoki H, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol, 2005; 46: 508-515 [DOI] [PubMed] [Google Scholar]
- 3). Soyama Y, Miura K, Morikawa Y, Nishijo M, Nakanishi Y, Naruse Y, Kagamimori S, Nakagawa H, Oyabe S. High-density lipoprotein cholesterol and risk of stroke in Japanese men and women: the Oyabe Study. Stroke, 2003; 34: 863-868 [DOI] [PubMed] [Google Scholar]
- 4). Yoshita K, Miura K, Morikawa Y, Ishizaki M, Kido T, Naruse Y, Soyama Y, Suwazono Y, Nogawa K, Nakagawa H. Relationship of alcohol consumption to 7-year blood pressure change in Japanese men. J Hypertens, 2005; 23: 1485-1490 [DOI] [PubMed] [Google Scholar]
- 5). Mannami T, Konishi M, Baba S, Nishi N, Terao A. Prevalence of asymptomatic carotid atherosclerotic lesions detected by high-resolution ultrasonography and its relation to cardiovascular risk factors in the general population of a Japanese city: the Suita study. Stroke, 1997; 28: 518-525 [DOI] [PubMed] [Google Scholar]
- 6). Nakanishi S, Yamada M, Hattori N, Suzuki G. Relationship between HbA(1)c and mortality in a Japanese population. Diabetologia, 2005; 48: 230-234 [DOI] [PubMed] [Google Scholar]
- 7). Arima H, Tanizaki Y, Kiyohara Y, Tsuchihashi T, Kato I, Kubo M, Tanaka K, Ohkubo K, Nakamura H, Abe I, Fujishima M, Iida M. Validity of the JNC VI recommendations for the management of hypertension in a general population of Japanese elderly: the Hisayama study. Arch Intern Med, 2003; 163: 361-366 [DOI] [PubMed] [Google Scholar]
- 8). Iso H, Date C, Yamamoto A, Toyoshima H, Tanabe N, Kikuchi S, Kondo T, Watanabe Y, Wada Y, Ishibashi T, Suzuki H, Koizumi A, Inaba Y, Tamakoshi A, Ohno Y, JACC Study Group. Perceived mental stress and mortality from cardiovascular disease among Japanese men and women: the Japan Collaborative Cohort Study for Evaluation of Cancer Risk Sponsored by Monbusho (JACC Study). Circulation, 2002; 106: 1229-1236 [DOI] [PubMed] [Google Scholar]
- 9). Ueshima H, Choudhury SR, Okayama A, Hayakawa T, Kita Y, Kadowaki T, Okamura T, Minowa M, Iimura O. Cigarette smoking as a risk factor for stroke death in Japan: NIPPON DATA80. Stroke, 2004; 35: 1836-1841 [DOI] [PubMed] [Google Scholar]
- 10). Okamura T, Hayakawa T, Kadowaki T, Kita Y, Okayama A, Ueshima H, NIPPON DATA Research Group. The inverse relationship between serum high-density lipoprotein cholesterol level and all-cause mortality in a 9.6-year follow-up study in the Japanese general population. Atherosclerosis, 2006; 184: 143-150 [DOI] [PubMed] [Google Scholar]
- 11). Imano H, Kitamura A, Sato S, Kiyama M, Ohira T, Yamagishi K, Noda H, Tanigawa T, Iso H, Shimamoto T. Trends for blood pressure and its contribution to stroke incidence in the middle-aged Japanese population: the Circulatory Risk in Communities Study (CIRCS). Stroke, 2009; 40: 1571-1577 [DOI] [PubMed] [Google Scholar]
- 12). Ishikawa S, Gotoh T, Nago N, Kayaba K, Jichi Medical School Cohort Study Group. The Jichi Medical School (JMS) Cohort Study: design, baseline data and standardized mortality ratios. J Epidemiol, 2002; 12: 408-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Uemura M, Yatsuya H, Hilawe EH, Li Y, Wang C, Chiang C, Otsuka R, Toyoshima H, Tamakoshi K, Aoyama A. Breakfast Skipping is Positively Associated With Incidence of Type 2 Diabetes Mellitus: Evidence From the Aichi Workers' Cohort Study. J Epidemiol, 2015; 25: 351-358 [DOI] [PMC free article] [PubMed] [Google Scholar]


