Abstract
Olfactory dysfunction (OD) and gustatory dysfunction (GD) has been reported as one of the presenting symptoms amongst COVID-19 patients. However the literature available is disjunct on this aspect. This study is conducted to identify the prevalence of olfactory and/or gustatory dysfunction in patients with coronavirus disease in Northern part of India. It’s a cross-sectional observation study, conducted over 387 COVID-19 positive patients, at ENT dept of tertiary care hospital. A retrospective survey was conducted using a pre designed questionnaire and details of Olfactory and Gustatory dysfunction was collected. The patient’s demographic details, disease course and recovery time for olfactory (OD) and/or gustatory dysfunctions (GD) were collected. A total of 387 patients with COVID-19 completed the study. 228 (58.9%) patients suffered from influenza like illness (ILI) (fever, sore throat, dry cough, malaise, and myalgia). There was significant positive association seen between with ILI and OD and / or GD. 167/387 (43.15%) patients reported OD, and 153/387 (39.53%) reported GD. 43.71% and 50.3% patients had mild OD & GD respectively. Recovery rates for both OD and GD are high and almost similar, with 161 (96.4%) and 148 (96.73%) patients had complete recovery of smell and taste. Maximum recovery was noticed between 4 and 6 weeks. COVID-19 patients with habits have significantly high probability of developing OD &/or GD. There is a significant correlation between OD and GD and there is high probability that patients who reported to have OD will also have GD or vice versa. Prevalence of OD and GD in Indian population may not be as high as mentioned in western literature, however, both are frequent and early symptoms of COVID-19. Recent onset of these should be considered as red flag symptoms for COVID-19.
Keywords: COVID-19, Olfactory dysfunction, Gustatory dysfunction, Chemosensory disorders
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), emerged from Wuhan city in China in Dec 2019 and since then had spread across the world causing pandemic. Entire world and specially the health care system is thrown out of gears rendering them grappling and struggling to find methods to contain and cure the spread of disease. As per WHO corona virus dashboard there were 4,29,66,344 cumulative cases worldwide with 79,09,959 cases in India with more than 1,152,604 deaths worldwide and more than a million in India, with daily new cases numbers hovering around 70 thousand [1]. Most recent data for India, suggests despite infection rate of 516 cases per 100,000 population, case fatality is 1.5% and death per 100,000 population is 8.80 [2].
Individuals of all ages are at risk for infection. SARS-CoV-2 virus (COVID-19) causes a wide gamut of symptoms and signs ranging from mild symptoms like fever, cough, and sore throat, myalgia to severe or fatal pneumonia with acute respiratory distress syndrome (ARDS). Amongst these varied symptoms, reports started coming that COVID-19 patients are presenting with sudden onset olfactory dysfunction [3, 4] and gustatory dysfunction as preliminary symptoms, without associated rhinorrhea or nasal obstruction [5, 6]. Various studies have quoted olfactory dysfunction (OD) and gustatory dysfunction (GD) ranging from 5 to 88% in COVID-19 patients, depending upon geographic location of study conducted [4–11].
Studies have revealed that in both symptomatic and asymptomatic COVID-19 patients, the nasal cavity have higher viral loads than in the pharynx, hinting that the nasal cavity is the first gateway for the initial infection [12].
The mechanism by which SARS-CoV-2 virus causes OD and/or GD in COVID-19 patient is still a matter of debate. The pathogenesis is discussed in detail later.
Studies have further concluded that OD and GD could be preliminary symptoms in otherwise asymptomatic carriers. Such asymptomatic carriers can silently spread infections to others which could be life threatening for persons with comorbidities or at risk population [13]. Presently very limited data on COVID-19 induced OD and GD is available for Indian population. With our study we have attempted to ascertain the prevalence and association of OD and GD laboratory confirmed COVID-19 patients from Northern India.
Materials and Methods
It is a cross-sectional observation study, conducted at an ENT center of a tertiary care hospital of Northern India, actively involved in management of COVID-19 patients. The study was carried out in accordance with the ethical standards of the Helsinki Declaration and was approved by the by Institutional ethics committee. Consent has been taken from all participating individuals.
The study is conducted on 387 patients who suffered from COVID-19 from 01 May 2020 to 15 Aug 2020. All these patients were laboratory confirmed COVID-19 by RT-PCR done over nasopharyngeal and oropharyngeal swabs. The details of the patient was extracted from hospital database. These patients either had ILI symptoms or when patient was admitted for any other illness in triage ward and underwent mandatory test as per institutional protocol. Patients were then interviewed either telephonically using a structured questionnaire or by online survey.
The primary objective was to analyse prevalence of OD and GD in COVID-19 patients.
Secondary objectives were to identify association of OD and GD with influenza like illness (ILI), subjectively grade the degree of OD and GD, time taken to recover from these chemosensory deficits and to look for association between OD and GD amongst COVID-19 patients.
Sample size was calculated using data from a large meta-analysis, with prevalence of 41% for OD and 38.2% for GD [14]. A required sample size of 372 cases of COVID-19 was calculated keeping level of confidence interval at 95%, precision of 5%. Since OD and GD are overlapping symptoms therefore we finally recruited 387 patients.
The inclusion criteria were: RT-PCR proven COVID-19 patients. Patients of more than 18 years of age, patient willing to participate in the study. Patients with mild to moderate severity of illness, defined as lack of ICU care and assisted ventilation requirement.
Exclusion criteria were multiple responses/ incomplete responses by patients, patients who claimed to have hyposmia/anosmia or dysgeusia prior to onset of pandemic, patients with previous history of undergoing any rhinosinusal surgery, patients with neuropsychiatric disorders, patients with severe symptoms who were admitted in ICU for assisted ventilation and patients who responded albeit being outside the study duration.
To help patients in assessing severity of OD and GD, a simple grading was proposed to patients. The odorants used for assessing grading were common household odorants to which Indian population are familiar with; for example baby talcum powder, floral/fruity smell, smell of spices, tea/coffee, garlic, petroleum products, peppermint etc. Similarly for gustatory assessment, patients were asked to answer whether they can taste sweet, salty, sour, bitter and pungent food articles. Patients were then asked to rate dysfunctions appropriately on a scale of 1–10 from mild to severe dysfunction.
Statistical analysis was performed using SPSS software, version 22.0 (IBM Corp, Armonk, NY, USA). Frequencies and percentages were calculated for the demographic and clinical data. The association between the factors and outcome variables (Olfactory and Gustatory dysfunction) was analysed using chi square test. p value < 0.05 with 95% confidence interval is considered as statistically significant.
Results
A total of 1500 COVID-19 positive patients were contacted through telephone/short message service and were interviewed telephonically or by online survey. However, out of 1500 COVID-19 patients only 451 (30%) consented to participate. After excluding patients as per laid down criteria, 387 patients were selected for final analysis based on the completeness of the data.
The epidemiological characteristics data pertaining to OD and GD is presented in Table 1. Out of 387 patients, 255 (65.9%) were males and 132 (34.1%) were females. The p value is 0.47. However, this skewed difference between male and female patients appears to be due reasons like social inhibition or lack of initiative in participating in survey, also access to smart phones/internet could be a possible reason for poor female patients participation, rather than actual difference in infectivity rates.
Table 1.
Epidemiological and clinical characteristics of patients with COVID 19 disease
| Variables | Total n = 387 |
with OD n = 167 |
without OD n = 220 |
p value | with GD n = 153 |
without GD n = 234 |
p value | with OD & GD n = 105 |
without OD & GD n = 282 |
p value |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||||
| Male | 255 | 113 | 142 | 0.52 | 100 | 155 | 0.42 | 70 | 185 | 0.47 |
| Female | 132 | 54 | 78 | 53 | 79 | 35 | 97 | |||
| Age group (years) | ||||||||||
| 18–35 | 180 | 87 | 93 | 0.25 | 70 | 110 | 0.63 | 51 | 129 | 0.92 |
| 36–50 | 121 | 48 | 73 | 52 | 69 | 32 | 89 | |||
| 51–65 | 82 | 28 | 45 | 25 | 48 | 18 | 55 | |||
| > 65 | 17 | 4 | 9 | 6 | 7 | 4 | 9 | |||
| ILI Symptoms | 228 | 123 | 105 | < 0.001 | 132 | 96 | < 0.001 | 92 | 136 | < 0.001 |
| Specific risk factor | ||||||||||
| HCW | 81 | 35 | 46 | 0.49 | 35 | 46 | 0.22 | 26 | 55 | 0.12 |
| Non HCW | 306 | 132 | 174 | 118 | 188 | 79 | 227 | |||
| Habits (Consume Alcohol, Smoking, tobacco chewing) | ||||||||||
| Present | 66 | 32 | 34 | 0.16 | 30 | 36 | 0.14 | 24 | 42 | 0.03 |
| Absent | 321 | 135 | 186 | 123 | 198 | 81 | 240 | |||
| Co Morbidities | ||||||||||
| Cadio-vascular diseases | 59 | 24 | 35 | 0.81 | 29 | 30 | 0.36 | 18 | 41 | 0.52 |
| Diabetes mellitus | 32 | 13 | 19 | 0.76 | 14 | 18 | 0.61 | 11 | 21 | 0.33 |
| CLD | 11 | 5 | 6 | 0.87 | 6 | 5 | 0.3 | 5 | 6 | 0.16 |
| CKD | 2 | 2 | 0 | – | 2 | 0 | – | 2 | 0 | – |
| Malignancy | 2 | 2 | 0 | – | 2 | 0 | – | 2 | 0 | – |
Bold numbers signifies that p value is less than 0.05, which means all those factors in bold had significant chances of developing Olfactory and gustatory dysfunction
COVID 19 corona virus disease 2019, OD olfactory dysfunction, GD gustatory dysfunction, ILI influenza like illness, HCW health care worker, CLD chronic lung disease, CKD chronic kidney disease, CVD cardiovascular disease.
Among the 387 patients, 180/387 (46.5%) were from 18 to 35 years age group. 228 (58.9%) patients suffered from ILI (fever, sore throat, dry cough, malaise, and myalgia). There was significant positive association seen between with ILI and OD and/or GD with p value < 0.001 at the level of significance 0.05.
81/387 patients (20.9%) were health care workers (HCW) and 306 (79.1%) were general population (non HCW). 35/81 had OD, 35/81 had GD, out of these 26/81 had both OD and GD. p value for HCW with or without OD is 0.49, with or without GD is 0.22 and with olfactory-gustatory dysfunction (OGD) or without OGD is 0.12. p value signifies that there is no statistical difference between HCW or general population to develop either OD, GD or both. This also means that there was no observational bias created by including HCW.
66 patients reported having habits like alcohol consumption, smoking and tobacco chewing. 24 out of 66 patients had both OD and GD. On chi square test, p value is 0.03, which is < 0.05 at level significance 0.05 meaning patients with habits have significant chance of developing OGD symptoms.
In our study, the most common co morbidities found amongst patients with COVID-19 were cardiovascular disease (CVD), diabetes mellitus, chronic lung disease, chronic kidney diseases and malignancy. However, no significant association could be deciphered between comorbidities and chances of having OD and GD.
Out of 387 COVID-19 patients, 167 (43.15%) patients had OD, 153 (39.53%) patients had GD and since OD and GD are overlapping symptoms, 105 (27.1%) patients had both as depicted in Fig. 1.
Fig. 1.
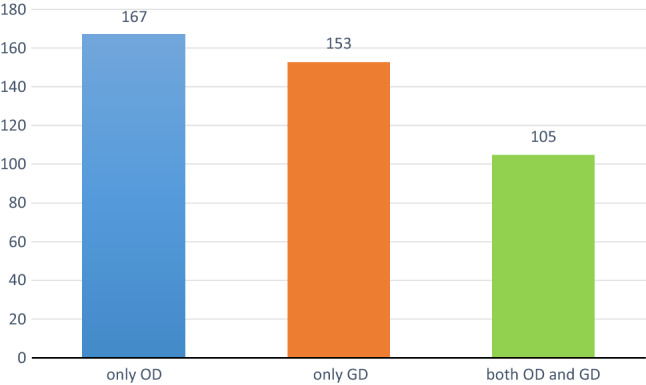
Proportion of OD, GD and both OD and GD in COVID 19 patients
As far as grading of severity of OD and GD is concerned, most patients had mild OD (43.71%) and GD (50.3%), further detail are mentioned in Table 2. Recovery rates for both OD and GD were almost similar, with 161 (96.4%) and 148 (96.73%) patients had complete recovery of smell and taste. Maximum recovery was noticed between 4 and 6 weeks. Only 6 (3.59%) and 5 (3.27%) patients continued to have OD and GD beyond 8 weeks. (Table 2 and Fig. 2).
Table 2.
Severity and recovery characteristics of OD and GD in COVID-19 patients
| Patients with OD n = 167 |
Percentage (%) | Patients with GD n = 153 |
Percentage (%) | |
|---|---|---|---|---|
| Severity | ||||
| Mild | 73 | 43.71 | 77 | 50.3 |
| Moderate | 53 | 31.74 | 52 | 34 |
| Severe | 41 | 24.33 | 24 | 15.7 |
| Recovery | ||||
| Complete recovery | ||||
| Between 0–2 weeks | 6 | 3.59 | 15 | 9.8 |
| Between 2–4 weeks | 9 | 5.39 | 11 | 7.19 |
| Between 4–6 weeks | 144 | 86.23 | 119 | 77.78 |
| Between 6–8 weeks | 2 | 1.20 | 3 | 1.96 |
| Incomplete recovery | 6 | 3.59 | 5 | 3.27 |
Fig. 2.
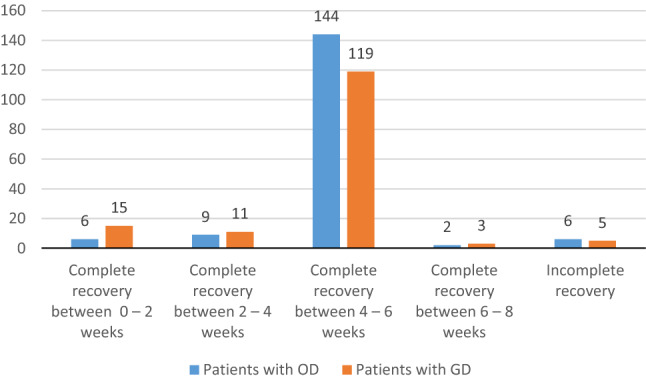
Recovery rate of COVID 19 patients with OD and GD
On correlating OD with GD [Table 3], p value is < 0.00001 at the level of 0.05. With Yates correction p value is 0.00001 at level of confidence of 0.05, meaning, there is high probability of patients who reported to have OD will also have GD or vice versa.
Table 3.
Correlation between olfactory and gustatory dysfunction in COVID 19 patients
| Gustatory dysfunction | p value* | |||
|---|---|---|---|---|
| Yes | No | |||
| Olfactory dysfunction | Yes | 105 | 62 | < 0.00001 |
| No | 48 | 172 | ||
Discussion
Since the onset of pandemic, several studies have suggested OD and/or GD as presenting symptoms amongst COVID-19 patients [4, 8, 15]. A large meta-analysis showed a loss of smell in 55% (95% confidence interval 38% to 70%) of patients with COVID-19. A large online questionnaire based survey found that, in COVID-19, loss of smell is usually severe and sudden in onset, but transient in most patients, although 10.6% of patients showed no improvement at one month [16, 17].
Despite extensive research, the pathophysiological mechanisms by which SARS-CoV-2 infection causes OD or GD are still imprecise [4, 6, 14, 18, 19]. SARS-CoV-2 is a single-stranded RNA, enveloped virus, similar to severe acute respiratory syndrome coronavirus (SARS-CoV). Angiotensin converting enzyme 2 (ACE2) and Trans membrane Serine Protease 2 (TMPRSS2) are functional receptor for SARS-CoV-2 and are highly expressed in nasal epithelial cells. It has been found that these receptors are required for host cell entry and facilitate accumulation, replication and binding of SARS-CoV-2 and thereby invades the central nervous system through the olfactory neuroepithelium, including the olfactory bulb and spread to CNS [20–23].
ACE2 receptors are also detected on glial cells and neurons in the brain, alveolar epithelial cells, and mucosa of oral cavity, intestine, kidney and heart. High expression of ACE2 over tongue than other oral cavity subsites and can induce dysguesia via a complex mechanism [19, 24, 25].
Thus SARS Cov-2 virus are known to be neurotropic and neuroinvasive and these underlying pathogenic mechanisms by which it disrupts the biochemical and electrophysiological homeostasis could explain chemosensory disorders in COVID-19 patients [5, 26].
It is also evident that most patients with a reduced sense of smell or taste did not report nasal congestion [27]. In 2007, Suzuki et al., demonstrated that coronavirus may be detected in the nasal discharge of patients with olfactory dysfunction. They observed that some patients with normal acoustic rhinometry did not recover their olfaction, suggesting that nasal inflammation and related obstruction were not the only etiological factors underlying the olfactory dysfunction in viral infection. This could explain the presence of olfactory dysfunction without nasal congestion [28]. In a small study, magnetic resonance imaging of brain of patients with COVID-19 with anosmia, also noted abnormalities of the olfactory bulb and olfactory nerve with development of a microvascular phenomenon and injury such as micro bleeding and/or a blood–brain barrier break [29].
In our study, to accrue the data we relied on a questionnaire which was filled by the patient online or by telephonic interview from proven COVID-19 patients. Various other studies have also relied on such methodology [6, 30–32]. While others have relied on objective testing, performed by the health worker or self-administered after explaining the procedure [9, 33–35].
Our rationale for opting for contactless questionnaire based study was to avoid risk of potential cross infection to health care workers. We performed assessment of degree of OD and GD by asking patient to score on the scale of 0 to 10, with 0 being “no symptoms” and 10 being “worst/severe symptoms”.
Evidence available till now shows that there is noticeable differences in prevalence of OD and GD amongst COVID-19 patients, which is high, as quoted by European and American studies as compared to studies conducted in Asian region. As per Lechien et al., they reported up to 85.6% COVID-19 patients with olfactory dysfunction and 88% patients had gustatory dysfunctions [6]. Similar results of 68% and 71% regarding prevalence of smell and taste disorder has been found in COVID-19 patients in a study from USA, [36] whereas, low prevalence rates (5.1 to 24.8% for OD; 5.6 to 20.6% for GD) are mentioned in studies from Asian region [5, 31, 32, 37].
The data available on Indian population is scant and varied. One study on Indian population, found prevalence of 14.8% of new onset anosmia, which is significantly less as compared to European population [38]. Whereas, another study on Indian population showed that 81.6% patient had OD and 84.2% had GD in COVID-19 positive cases [39].
In our study the overall prevalence of OD is 43.15%, whereas GD is 39.53%. 27.1% patients had both OD and GD. The reasons for this variation in OD and/or GD amongst different geographical regions is unclear, however, few studies have studied the mechanisms for these variations. As per Forester et al., 03 major variants, named type A, B, and C have been identified in a phylogenetic network analysis of 160 SARS-CoV-2 genomes. They found that type A followed by type C was predominant in Europe and the United States, while type B was most common in East Asia. Phenotypic characteristics might differ between these variants, including those related to the prevalence of OD and GD. In addition, the affinity of the virus for certain tissues and individuals might partially explain the clinical differences between patients in different parts of the world. The expression level of ACE2, the receptor for SARS-CoV-2, in different tissues might be critical for the susceptibility, symptoms, and outcomes of COVID-19 infection [40]. Another study, by comparing 15 expression quantitative trait locus (eQTLs) variants of the ACE2 gene, found a large number of ACE2 polymorphisms and differences in expression levels between the European and Asian populations [41]. Lechien et al., also mentioned about possibility of variable affinity of the COVID-19 virus to different tissue sites in different ethnic groups [6]. The other reason for higher prevalence of OD and GD in European studies could be due to use of objective tests for evaluation, where patient were able to differentiate between covert hyposmia/anosmia and taste dysfunctions [34].
In light of results from our study, we also concur with the findings of Moein et al., which suggest that self-reported OD or GD underestimates the frequency of chemosensitive disorders [42]. Varia et al., noticed that, 10.3 per cent of patients who self-reported normal function, had mild or moderate chemosensory disorder on objective testing [34].
We found that most patients with OD and/or GD in age group of 18–35 years, with male gender predominance. Cultural, socio-economic status, level of education/awareness and willingness to participate in the study are possible reasons for these results. Further case control studies would be warranted to overcome this bias. We also found that 81 (20.9%) of HCW responded to the survey. We assumed the HCW would be more prone to develop symptoms, being more exposed to COVID-19 patients, they may have more viral load and maybe more aware of symptomatology. However the p values for OD and/or GD in HCW population versus non HCW was not significant, meaning that HCW were equally prone to develop symptoms as non HCW.
With the data available from our study we also infer that there is strong association between OD and GD amongst COVID-19 patients. Similar results have been quoted by various studies [7, 8, 36].
Recovery from OD and GD were a norm in our study and most patients recovered from the symptoms within 4 to 6 weeks, with complete recovery noticed in almost 96.4% patients. Only 3.59% and 3.27% patients had partial recovery even beyond 8 weeks of follow-up. Similar results have been quoted by Klopfenstein et al., they reported a mean duration of anosmia of 9 days, with a complete recovery occurring in almost all patients within 4 weeks [43].
Lechien et al., also reported that 72.6% of patients recovered their olfactory and gustatory functions completely within the first 8 days following resolution of the disease [6].
The spontaneous recovery may be a result of regeneration or rapid turnover of the damaged olfactory epithelium and taste buds [44, 45].
Regarding treatment initiation, the evidence is again divided, however, it is important to consider both the likelihood of spontaneous recovery and the potential risks of treatments. As on date, The European Rhinologic Society (ERS) and the ENT UK association advises against giving systemic corticosteroids to patients with sudden olfactory dysfunction since recovery can occur in the first weeks after onset. Administration of intranasal corticosteroids (INCS) in patients with anosmia is also controversial and ERS recommends against its use. Some studies have mentioned beneficial effects of Zinc, intranasal vitamin A and omega 3 as supplements for early recovery [46]. As per Damm et al., olfactory training can also play a role in recovery of OD in patients who start it within 12 months after onset [47]. As more evidence evolves, further studies addressing therapeutic approaches to OD and/or GD among infected patients will be needed.
Our study is limited by the fact that it is a retrospective analysis based on online/telephonic questionnaire which is bound to have recall bias; also cultural, educational and demographic biases are present, as most replies are given by young patients—who have access to smartphones, well versed with the software, more educated, understands the importance of this survey, aware about the symptoms post media reporting of such symptoms and thus more inclined to participate in the study.
Prevailing wearying conditions due to COVID-19 with cases still surging in India and compulsion to avoid cross infection while performing objective tests, handicapped us from conducting objective tests for grading of OD and GD and had to rely on subjective evaluation by the patients. Although not aimed but another potential limitation which we could not assess is, whether any intervention in form of use of antibiotics, antivirals, steroids, multivitamins or any home based remedy lead to early recovery from olfactory and gustatory dysfunction. We are not sure whether use of intranasal corticosteroids (INCS) can assist in early recovery as no patient was prescribed INCS. All of these limitations should be considered in future studies to investigate and characterize the olfactory and gustatory dysfunctions in COVID-19 patients with a possibility of developing interventions which can result in early recovery of symptoms.
Conclusion
To best of our knowledge and literature review, it is presently the study with largest sample size describing the prevalence of OD and GD in RT-PCR confirmed COVID-19 positive Indian population. Prevalence of OD and GD in Indian population is lower than that quoted in western literature. However, our study has shown that new onset OD and GD are early and frequent presenting symptom in COVID-19 patients. There is a strong association of OD and GD in COVID-19 patients.
It is recommended that Otorhinolaryngologists and even general practitioners should focus on recent onset OD and/or GD as significant symptom. These may precede full blown clinical case and might be valuable in early diagnosis, thereby forewarn patients and medical authorities to carry out appropriate steps to manage and contain the spread of disease.
Further studies aimed at assessing olfactory and gustatory dysfunction objectively would be required to understand the symptomatology, progression and long term after effects and also to study possible interventions to avoid long term morbidities.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Taken.
Human and Animal Rights
Research involves human Participants.
Informed Consent
Written informed consent was taken from individual patients included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.https://covid19.who.int/table. Accessed on 27 Oct 2020
- 2.https://coronavirus.jhu.edu/data/mortality. Accessed on 27 Oct 2020
- 3.Russell B, Moss C, Rigg A, Hopkins C, Papa S, Van Hemelrijck M. Anosmia and ageusia are emerging as symptoms in patients with COVID-19: What does the current evidence say? Ecancermedicalscience. 2020 doi: 10.3332/ecancer.2020.ed98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome. Rhinology. 2020;58(3):299–301. doi: 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- 5.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, Chekkoury-Idrissi Y. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spinato G, Fabbris C, Polesel J, Cazzador D, Borsetto D, Hopkins C, Boscolo-Rizzo P. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323(20):2089–2090. doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, Rusconi S, Gervasoni C, Ridolfo AL, Rizzardini G, Antinori S. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71(15):889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaira LA, Deiana G, Fois AG, Pirina P, Madeddu G, De Vito A, Babudieri S, Petrocelli M, Serra A, Bussu F, Ligas E. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck. 2020;42(6):1252–1258. doi: 10.1002/hed.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beltrán-Corbellini Á, Chico-García JL, Martínez-Poles J, Rodríguez-Jorge F, Natera-Villalba E, Gómez-Corral J, Gómez-López A, Monreal E, Parra-Díaz P, Cortés-Cuevas JL, Galán JC. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case–control study. Eur J Neurol. 2020;27(9):1738–1741. doi: 10.1111/ene.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heidari F, Karimi E, Firouzifar M, Khamushian P, Ansari R, Ardehali MM, Heidari F. Anosmia as a prominent symptom of COVID-19 infection. Rhinology. 2020;58(3):302–303. doi: 10.4193/Rhin20.140. [DOI] [PubMed] [Google Scholar]
- 12.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia J, Guo Q. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kronbichler A, Kresse D, Yoon S, Lee KH, Effenberger M, Shin JI. Asymptomatic patients as a source of COVID-19 infections: a systematic review and meta-analysis. Int J Infect Dis. 2020;98:180–186. doi: 10.1016/j.ijid.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R (2020) Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis In: Mayo clinic proceedings, Elsevier [DOI] [PMC free article] [PubMed]
- 15.Eliezer M, Hautefort C, Hamel AL, Verillaud B, Herman P, Houdart E, Eloit C. Sudden and complete olfactory loss of function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg. 2020;146(7):674–675. doi: 10.1001/jamaoto.2020.0832. [DOI] [PubMed] [Google Scholar]
- 16.Borsetto D, Hopkins C, Philips V, Obholzer R, Tirelli G, Polesel J, Boscolo-Rizzo P. Self-reported alteration of sense of smell or taste in patients with COVID-19: a systematic review and meta-analysis on 3563 patients. Rhinology. 2020;58(5):430–436. doi: 10.4193/Rhin20.185. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology. 2020;58(3):295–298. doi: 10.4193/Rhin20.116. [DOI] [PubMed] [Google Scholar]
- 18.Ralli M, Di Stadio A, Greco A, de Vincentiis M, Polimeni A. Defining the burden of olfactory dysfunction in COVID-19 patients. Eur Rev Med PharmacolSci. 2020;24(7):3440–3441. doi: 10.26355/eurrev_202004_20797. [DOI] [PubMed] [Google Scholar]
- 19.Lovato A, de Filippis C, Marioni G (2020) Upper airway symptoms in coronavirus disease 2019 (COVID-19). Am J Otolaryngol 15 [DOI] [PMC free article] [PubMed]
- 20.Bilinska K, Jakubowska P, Von Bartheld CS, Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem Neurosci. 2020;11(11):1555–1562. doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baig AM. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS NeurosciTher. 2020;26(5):499. doi: 10.1111/cns.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conde G, Pájaro LD, Marzola ID, Villegas YR, Salazar LR. Neurotropism of SARS-CoV 2: mechanisms and manifestations. J Neurol Sci. 2020;412:116824. doi: 10.1016/j.jns.2020.116824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):1–5. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng X, Deng Y, Dai Z, Meng Z. COVID-19 and anosmia: a review based on up-to-date knowledge. Am J Otolaryngol. 2020;41(5):102581. doi: 10.1016/j.amjoto.2020.102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mercante G, Ferreli F, De Virgilio A, Gaino F, Di Bari M, Colombo G, Russo E, Costantino A, Pirola F, Cugini G, Malvezzi L. Prevalence of taste and smell dysfunction in coronavirus disease 2019. JAMA Otolaryngol Head Neck Surg. 2020;146(8):723–728. doi: 10.1001/jamaoto.2020.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, Murakami S. Identification of viruses in patients with postviral olfactory dysfunction. The Laryngoscope. 2007;117(2):272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aragão MD, Leal MC, CartaxoFilho OQ, Fonseca TM, Valença MM. Anosmia in COVID-19 associated with injury to the olfactory bulbs evident on MRI. Am J Neuroradiol. 2020;41(9):1703–1706. doi: 10.3174/ajnr.A6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dell’Era V, Farri F, Garzaro G, Gatto M, Aluffi Valletti P, Garzaro M. Smell and taste disorders during COVID-19 outbreak: a cross-sectional study on 355 patients. Head Neck. 2020;42(7):1591–1596. doi: 10.1002/hed.26288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Ani RM, Acharya D. Prevalence of anosmia and ageusia in patients with COVID-19 at a primary health center, Doha, Qatar. Indian J Otolaryngol Head Neck Surg. 2020;19:1–7. doi: 10.1007/s12070-020-02064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song J, Deng YK, Wang H, Wang ZC, Liao B, Ma J, He C, Pan L, Liu Y, Alobid I, Wang DY (2020) Self-reported taste and smell disorders in patients with COVID-19: distinct features in China. medRxiv [DOI] [PMC free article] [PubMed]
- 33.Vaira LA, Hopkins C, Salzano G, Petrocelli M, Melis A, Cucurullo M, Ferrari M, Gagliardini L, Pipolo C, Deiana G, Fiore V. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck. 2020;42(7):1560–1569. doi: 10.1002/hed.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaira LA, Hopkins C, Petrocelli M, Lechien JR, Chiesa-Estomba CM, Salzano G, Cucurullo M, Salzano FA, Saussez S, Boscolo-Rizzo P, Biglioli F. Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J LaryngolOtol. 2020;1:1–7. doi: 10.1017/S0022215120001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaira LA, Hopkins C, Petrocelli M, Lechien JR, Soma D, Giovanditto F, Rizzo D, Salzano G, Piombino P, Saussez S, De Riu G. Do olfactory and gustatory psychophysical scores have prognostic value in COVID-19 patients? A prospective study of 106 patients. J Otolaryngol Head Neck Surg. 2020;49(1):1. doi: 10.1186/s40463-020-00449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10(7):806–813. doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee Y, Min P, Lee S, Kim SW. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. 2020;35(18):e174. doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishra P, Gowda V, Dixit S, Kaushik M. Prevalence of new onset anosmia in COVID-19 patients: Is the trend different between European and Indian population? Indian J Otolaryngol Head Neck Surg. 2020;21:1–4. doi: 10.1007/s12070-020-01986-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bidkar V, Mishra M, Selvaraj K, Joshi P, Shrikrishna BH, Dabhekar S, Prathipati KK, Rathod BS, Shendre P, Gondode P. Testing olfactory and gustatory dysfunctions among quarantine COVID-19 suspects. Indian J Otolaryngol Head Neck Surg. 2020;14:1–6. doi: 10.1007/s12070-020-02210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forster P, Forster L, Renfrew C, Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. ProcNatlAcadSci. 2020;117(17):9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao Y, Li L, Feng Z, Wan S, Huang P, Sun X, Wen F, Huang X, Ning G, Wang W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6(1):1–4. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020;10(8):944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klopfenstein T, Kadiane-Oussou NJ, Toko L, Royer PY, Lepiller Q, Gendrin V, Zayet S. Features of anosmia in COVID-19. Médecine et Maladies Infect. 2020;50(5):436–439. doi: 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soler ZM, Patel ZM, Turner JH, Holbrook EH, Hopkins C, Surda P, Kumar N, Lechien JR, Gane SB, Kelly C, Hopkins C. A primer on viral-associated olfactory loss in the era of COVID-19. Int Forum Allergy Rhinol. 2020;10:814–820. doi: 10.1002/alr.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oakley B, Riddle DR. Receptor cell regeneration and connectivity in olfaction and taste. ExpNeurol. 1992;115(1):50–54. doi: 10.1016/0014-4886(92)90220-k. [DOI] [PubMed] [Google Scholar]
- 46.Vroegop AV, Eeckels AS, Van Rompaey V, Abeele DV, Schiappoli M, Alobid I, Hummel T, De Dorlodot C, Levie P, Huart C, Eloy P. COVID-19 and olfactory dysfunction-an ENT perspective to the current COVID-19 pandemic. B-ENT. 2020;16(1):81–85. [Google Scholar]
- 47.Damm M, Pikart LK, Reimann H, Burkert S, Göktas Ö, Haxel B, Frey S, Charalampakis I, Beule A, Renner B, Hummel T. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. The Laryngoscope. 2014;124(4):826–831. doi: 10.1002/lary.24340. [DOI] [PubMed] [Google Scholar]


