Abstract
Argonaute 2 (Ago2) is the main component of the RNA-induced silencing complex. We recently showed that liver-specific Ago2-deficiency in mice (L-Ago2 knockout [KO] mice) enhances mitochondrial oxidation and alleviates obesity-associated pathophysiology. However, the precise mechanisms behind the role of hepatic Ago2 in regulating the mitochondrial oxidation associated with glucose metabolism are still unclear. Here, we show that hepatic Ago2 regulates the function of peroxisome proliferator–activated receptor α (PPARα) for oxidative metabolism. In both genetically and diet-induced severe obese conditions, L-Ago2 KO mice developed obesity and hepatic steatosis but exhibited improved glucose metabolism accompanied by lowered expression levels of pathologic microRNAs (miRNAs), including miR-802, miR-103/107, and miR-152, and enhanced expression of PPARα and its target genes regulating oxidative metabolism in the liver. We then investigated the role of hepatic Ago2 in the outcomes of vertical sleeve gastrectomy (VSG) in which PPARα plays a crucial role in a drastic transcription reprogram associated with improved glycemia post VSG. Whereas VSG reduced body weight and improved fatty liver in wild-type mice, these effects were not observed in hepatic Ago2-deficient mice. Conversely, glucose metabolism was improved in a hepatic Ago2-dependent manner post VSG. Treating Ago2-deficient primary hepatocytes with WY-14643, a PPARα agonist, showed that Ago2-deficiency enhances sensitivity to WY-14643 and increases expression of PPARα target genes and mitochondrial oxidation. Our findings suggest that hepatic Ago2 function is intrinsically associated with PPARα that links Ago2-mediated RNA silencing with mitochondrial functions for oxidation and obesity-associated pathophysiology.
Keywords: obesity, bariatric surgery, RNA silencing, Argonaute 2, PPARα
The worldwide prevalence of obesity has reached pandemic proportions. These individuals are often at high risk of insulin resistance and type 2 diabetes (T2D), but treatment options are limited (1-3). Bariatric surgery is an effective therapeutic option for obesity, most commonly using vertical sleeve gastrectomy (VSG). In VSG, approximately 80% of the stomach is removed and the gastric remnant forms a functional tube of relatively fixed caliber (4-7). While it is conventionally accepted that the metabolic benefits post VSG are due mainly to weight loss, it is becoming increasingly clear there are other unexplained mechanisms that contribute to the remission of insulin resistance and T2D (8-10). For example, a recent study in rodents revealed that VSG improves glycemia in a manner independent of weight loss by restoring hepatic insulin sensitivity, suggesting that the liver is one of the critical organs that facilitates the remission of glycemic conditions prior to weight loss post VSG (11). Intriguingly, significant transcriptional reprogramming is associated with the improved glycemia in the liver post VSG, and 2 hepatic transcription factors (peroxisome proliferator–activated receptor α [PPARα] and hepatocyte nuclear factor 4 α [HNF4α]), appear to play key roles in these changes (11). However, the molecular mechanism that translates to these metabolic benefits remains poorly understood. Identification of the molecules and the molecular mechanism in the liver associated with the improvement of glucose metabolism post VSG may provide a possible nonsurgical treatment for T2D and obesity.
PPARα is a nuclear receptor highly expressed in the liver; it has a robust oxidative activity that plays a critical role in metabolism, especially during fasting (12-14). Accumulating knowledge indicates that PPARα regulates the expression of genes involved in mitochondrial functions such as mitochondrial biogenesis and β-oxidation. It also plays a crucial role in the hepatic responses to fasting, including gluconeogenesis and elevated fatty acid (FA) oxidation and ketogenesis, which are impaired in PPARα-deficient mouse models (15-17). Therefore, PPARα is an attractive therapeutic target for improving lipid metabolism in obesity and fatty liver diseases. Indeed, several PPARα-agonists, such as fenofibrate and bezafibrate, have been developed and used for these metabolic conditions (18). Additionally, recent evidence indicates that PPARα agonists significantly improve glucose metabolism in patients with diabetes and impaired glucose tolerance (19, 20). The function of PPARα in glucose metabolism is also evidenced by studies of single-nucleotide variations (formerly single-nucleotide polymorphisms), indicating that gene variants of PPARα influence the progression of T2D in humans (21-23). These findings suggest that PPARα also plays a role in glycemic control, likely by enhancing mitochondrial oxidation, and that activation of PPARα in the liver can improve systemic glucose metabolism. This PPARα-mediated event appears to be associated with improved glycemia post VSG (11). However, the molecular mechanism of action of PPARα and how PPARα function is regulated in obesity and in response to VSG remain unclear.
There is emerging interest in microRNA (miRNA)-mediated posttranscriptional regulation of glycemic control as a method of identifying promising targets for treating obesity-associated diabetes and related conditions (24-26). We recently reported that hepatic Argonaute 2 (Ago2), which is a central component of RNA-induced silencing complex, regulates the biogenesis of the pathologic miRNAs (such as miR-802, miR-103/107, and miR-148a/148b/152) that silence the genes critical for metabolism; this silencing disrupts glucose metabolism (27-31) and subsequently deteriorates metabolic controls, including mitochondrial oxidation, in obesity (32). Consequently, hepatic Ago2-deficiency in mice (L-Ago2 knockout [KO] mice) enhances mitochondrial oxidation with enhanced expression and activation of adenosine 5′-monophosphate–activated protein kinase α (AMPK α) and protects from diet-induced glucose intolerance and insulin resistance (32). These findings suggest that Ago2-mediated RNA silencing in the liver is intrinsically linked to AMPK activation and mitochondrial oxidation during glucose metabolism. However, L-Ago2 KO mice improve glucose metabolism even in an AMPK α1-deleted condition, indicating that there is an additional factor or factors that regulate mitochondrial oxidation and glucose metabolism in an Ago2-dependent manner. Gene expression profile analyses of the obese liver showed that the expression of genes regulating mitochondrial functions, including PPARα and PPAR γ coactivator (Pgc1α), was significantly increased in the absence of Ago2 (32). These findings suggest that hepatic Ago2-dependent RNA-silencing impairs mitochondrial functions via regulating the gene expression in the pathogenesis of obesity. In this study, we investigated the role of hepatic Ago2 in models of severe obesity and in the metabolic improvements observed post VSG. We also assessed whether hepatic Ago2 regulates mitochondrial oxidation by modulating the functions of PPARα.
Materials and Methods
Animals
Animal care and experiments were performed according to procedures approved by the animal care committees of Cincinnati Children’s Hospital Medical Center. Ago2fl/fl, Albumincre/cre, and Lepob/J mice were obtained from the Jackson Laboratory (Stock Nos.: 016520, 003574, and 000632, respectively) to generate liver-specific Ago2-deficient mice (L-Ago2 KO) and liver-specific Ago2-deficient ob/ob mice (L-Ago2 KO-ob/ob); all mice were on a C57BL/6 background. Male mice in the diet-induced obesity model were fed a high-fat diet (HFD: 60% fat, 20% protein, and 20% carbohydrate kcal; Research Diets No. D12492) and others received normal chow (13% fat, 29% protein, and 58% carbohydrate kcal; LAB Diet No. 5010) ad libitum, beginning at age 4 weeks, with free access to water. Glucose tolerance tests (GTTs) were performed by intraperitoneal glucose injection (0.5 g/kg) following an overnight fast for 14 hours. Insulin tolerance tests (ITTs) were performed by intraperitoneal insulin injection (2 IU/kg) following daytime food withdrawal for 6 hours. Mouse plasma insulin levels were measured using the Mouse Ultrasensitive Insulin ELISA (enzyme-linked immunosorbent assay kit; ALPCO). The body composition of the mice was analyzed by using magnetic resonance spectroscopy (EchoMRI). Lipid profiling was performed by the University of Cincinnati’s Mouse Metabolic Phenotyping Core. Serum and hepatic triglyceride (TG) levels were measured using a Lab Assay Triglyceride kit (Wako).
Vertical Sleeve Gastrectomy and Postoperative Care
Male mice placed on an HFD for 34 weeks underwent VSG, as described in our previous studies (6, 7). Briefly, after incision of the abdominal wall in anesthetized mice, the stomach was exposed, and 80% of the stomach was excised to leave a tubular gastric remnant in continuity with the esophagus superiorly and the pylorus and duodenum inferiorly using an Endopath Linear Cutter (Ethicon Endo-Surgery). The sham procedure involved analogous isolation of the stomach, followed by manually applying pressure with blunt forceps along a vertical line between the esophageal and pyloric sphincters. Mice were on a liquid diet (Osmolite) from 1 day before to 3 days post surgery. A single dose of buprenorphine (analgesics) (1.0 mg/kg body weight) the day of surgery and a single dose per day of meloxicam (an anti-inflammatory drug) (5.0 mg/kg bodyweight) starting the day of surgery to 3 days post surgery were given subcutaneously. Mice were fed an HFD from day 4 post surgery. Tissues and plasma were harvested from mice 4 weeks post surgery and processed as required for the experiments.
Plasma alanine aminotransferase (ALT) analysis
Plasma collected at 4 weeks post-surgery was used to measure alanine aminotransferase (ALT) using the DiscretPak ALT Reagent Kit (Catachem). ALT enzyme kinetics were determined over a 5-minute interval by measuring the photometric absorbance at 340 nm.
Liver Histopathology Analysis
Liver tissue was harvested from mice 4 weeks post surgery, fixed in 10% formalin, and sectioned in a microtome to generate 5-μm sections for histologic analyses. The sections were stained with eosin and hematoxylin and analyzed to determine the nonalcoholic fatty liver disease (NAFLD) activity score (NAS) by a single independent pathologist who was blinded to the experimental design and treatment groups. In NAS, the liver histology is graded on steatosis (score 0-3), lobular inflammation (score 0-3), and ballooning (score 0-2) (33).
Primary Hepatocyte Isolation and Treatment With Peroxisome Proliferator–Activated Receptor Agonists
Primary hepatocytes were isolated from L-Ago2 WT and KO mice using the perfusion method reported previously (34). Briefly, the mouse was anesthetized by isoflurane, and then an incision was made to expose the abdominal organs. A catheter (size 24-gauge) was inserted in the inferior vein cava to perfuse the liver with warm Hank’s balanced salt solution (HBSS) and again with warm HBSS containing collagenase X (Wako). The liver capsule was removed from the perfused liver, and the parenchymal cells were dispersed carefully into cold, low-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) in a cell culture dish. Then, the primary hepatocytes were purified using 40% density gradient buffer. After purification, the cells were cultured in William’s medium E (Invitrogen) for 2 to 3 hours and changed to 10% fetal bovine serum in low-glucose DMEM overnight. After the cells adhered to the plate, they were treated with 10-µM WY14643 (PPARα agonist, Sigma), 100-µM rosiglitazone (PPARγ agonist, Tocris), and 100-µM GW0742 (PPARδ agonist, Tocris) for 24 hours. Then, the cells were collected in Trizol (Invitrogen) for total RNA isolation.
Quantitative Real-Time Polymerase Chain Reaction Analysis
For miRNA quantification, total RNA was extracted using an miRNeasy Micro Kit (Qiagen) according to the manufacturer’s instructions. TaqMan miRNA assays (Life Technologies) were used and real-time polymerase chain reaction (PCR) analyses were carried out for mature miRNA quantification on a real-time PCR machine (QuantStudio 6 Flex and QuantStudio 3 Real-Time PCR system; Thermo Fisher Scientific). Primary miRNAs were quantified using TaqMan Pri-miRNA assays using Sno202 as an internal control. For mRNA quantification, total RNA was extracted using Trizol reagent and converted to the first-strand complementary DNA (cDNA) using a SuperScript VILO cDNA Synthesis Kit (Invitrogen). Quantitative real-time PCR analysis was performed using a SYBR Select Master Mix (Applied Biosystems) on a real-time PCR machine (QuantStudio 6 Flex and QuantStudio 3 Real-Time PCR system). The primers used are listed in Supplementary Table 1 (35).
Oxygen Consumption Rate
Oxygen consumption rate (OCR) was measured on an XF96 Seahorse extracellular flux analyzer (Agilent) as reported previously (32). Primary mouse hepatocytes from L-Ago2 WT and KO mice were plated at a density of 8 × 103 cells per well of an XF 96-well cell culture microplate (Agilent, 101085-004), with at least 10 samples per condition. The next day, cells were washed once in Williams E medium and incubated with or without the indicated concentration of PPARα agonist for 48 hours. After the incubation period, the assay was initiated after washing cells once in unbuffered Seahorse assay medium (Agilent, 102353-100, pH7.4) and preequilibrating for 60 minutes at 37 °C without CO2 while the assay cartridge was prepared and calibrated. After determination of OCRs at the basal level, primary hepatocytes were sequentially treated with oligomycin A (2 μM), FCCP (carbonilcyanide p-trifluoromethoxyphenylhydrazone; 2 μM), and antimycin A (1 μM) along with rotenone (1 μM) to examine the effects of PPARα agonist on mitochondrial oxidative activity.
Immunoblotting and Antibodies
Liver tissues were homogenized in cold mammalian cell lysis buffer with protease and phosphatase inhibitor cocktails as described previously (32). Tissue lysates were then centrifuged and the resulting supernatants were used for immunoblot analyses. Ago2 primary antibody (36) and α-Tubulin primary antibody (37) were purchased from Cell Signaling Technology. PPARα primary antibody (38) was purchased from Santa Cruz. Antimouse immunoglobulin G (IgG) secondary antibody (39) was purchased from GE Life Sciences. Horseradish peroxidase–conjugated goat antirabbit IgG (H+L) secondary antibody (40) was purchased from Invitrogen.
Statistical Analysis
Data are presented as mean ± SEM. Unpaired 2-tailed t test, multiple sample t tests, and one-way analysis of variance (ANOVA) were performed to identify statistically significant differences between experimental groups. Immunoblots were quantified using ImageJ (National Institutes of Health). Comparisons between multiple time points were analyzed using repeated-measures ANOVA with 2-way ANOVA, followed by Tukey test for post hoc analysis using the GraphPad Prism software. A P value of less than .05 was considered statistically significant.
Results
Role of Hepatic Argonaute 2 in a Genetically Severe Obese Model
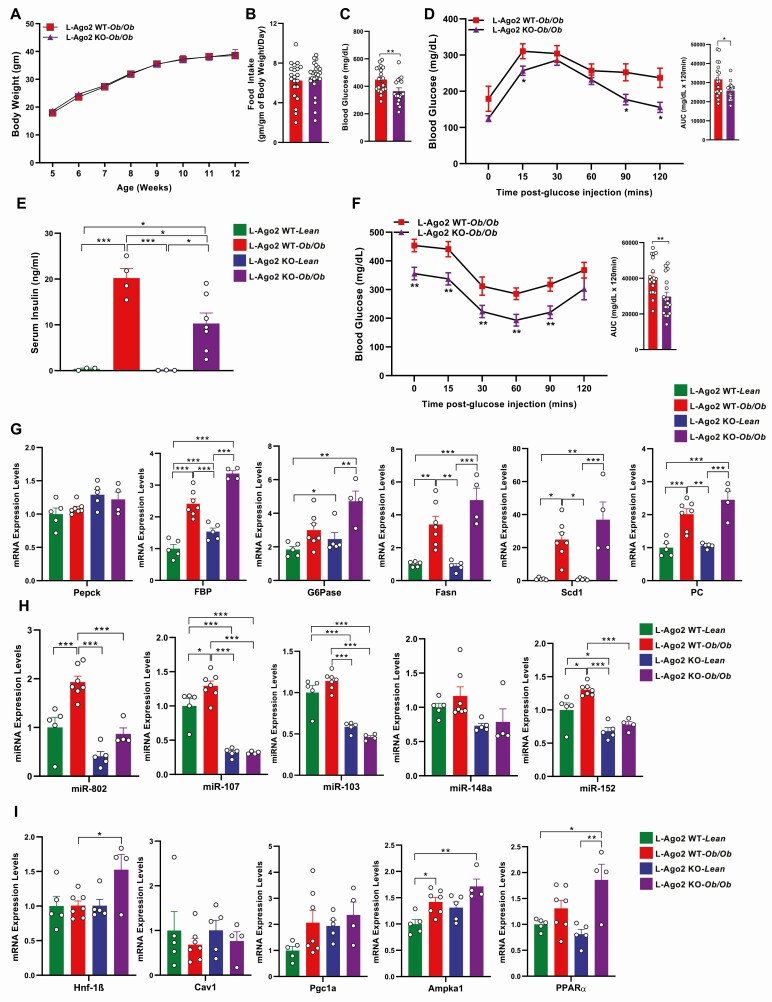
We recently reported that L-Ago2 KO mice exhibited higher energy expenditure with improved glucose metabolism compared to L-Ago2 WT mice on an HFD (32). To further investigate the importance of hepatic Ago2 in regulating glucose metabolism in severe obesity and diabetic conditions, we generated L-Ago2 KO-ob/ob mice and their WT littermates (L-Ago2 WT-ob/ob). In the C57BL/6J-ob/ob genetic background, L-Ago2 WT and L-Ago2 KO mice exhibited similar gain weight and developed severe obesity with comparable daily food intake (Fig. 1A and 1B). Analysis of body composition using EchoMRI showed no significant difference in absolute body fat or lean mass between genotypes in the ob/ob background (Supplementary Fig. 1A [35]). However, compared to L-Ago2 WT-ob/ob mice, L-Ago2 KO-ob/ob mice exhibited lower blood glucose levels (Fig. 1C) and significantly improved glucose clearance after a glucose challenge in an overnight food withdrawal condition in GTTs (Fig. 1D). Consistent with these striking observations, serum insulin levels of L-Ago2 KO-ob/ob mice were lower than those of L-Ago2 WT-ob/ob mice (Fig. 1E). In addition, blood glucose levels post insulin administration in L-Ago2 KO-ob/ob mice became lower than those in L-Ago2 WT-ob/ob mice under a daytime 6-hour food withdrawal condition compared to their controls (Fig. 1F). These results suggest that hepatic Ago2 deficiency in the ob/ob background improves systemic glucose metabolism even though expression levels of key genes regulating gluconeogenesis are comparable between the genotypes (Fig. 1G). These metabolic phenotypes of hepatic Ago2-deficiency in the ob/ob genetic background were consistently observed by others (41). Despite the improvement in glucose metabolism, L-Ago2 KO-ob/ob mice still developed hepatic TG accumulation (Supplementary Fig. 1B [35]), hepatic steatosis, and NAS (Supplementary Fig. 1C and 1D [35]). Consistent with these phenotypes, there was a trend that the levels of genes involved in lipid biosynthesis, stearoyl-CoA desaturase-1 (Scd1) and FA synthase (Fasn), in the liver of L-Ago2 KO-ob/ob mice were higher than those of L-Ago2 WT-ob/ob mice (Fig. 1G).
Figure 1.
Effect of hepatic Argonaute 2 (Ago2) deficiency on glucose metabolism of diabetic obese mice. A, Body weight of liver-specific Ago2-deficiency (L-Ago2) wild-type (WT)-ob/ob (n = 16), and L-Ago2 knockout (KO)-ob/ob (n = 13) mice on normal chow (CD) from age 5 to 12 weeks. B, Food intake normalized by body weight per day of L-Ago2 WT-ob/ob (n = 21), and L-Ago2 KO-ob/ob (n = 24) mice at age 11 weeks. C, Blood glucose levels after 6 hours of fasting of L-Ago2 WT-ob/ob (n = 20) and L-Ago2 KO-ob/ob (n = 16) at age 13 weeks. D, Glucose tolerance test (GTT) and its area under the curve (AUC) analysis in L-Ago2 WT-ob/ob (n = 18) and L-Ago2 KO-ob/ob (n = 18) mice at age 7 weeks. E, Serum insulin levels after 6 hours of fasting of L-Ago2 WT-Lean (n = 3), L-Ago2 WT-ob/ob (n = 4), L-Ago2 KO-Lean (n = 3), and L-Ago2 KO-ob/ob (n = 7) mice at age 13 weeks. F, Insulin tolerance test (ITT) and its AUC analysis in L-Ago2 WT-ob/ob (n = 18) and L-Ago2 KO-ob/ob (n = 18) mice at age 9 weeks. G, Messenger RNA (mRNA) expression levels of de novo lipogenesis and gluconeogenesis in livers of L-Ago2 WT-Lean (n = 5), L-Ago2 WT-ob/ob (n = 10), L-Ago2 KO-Lean (n = 5), and L-Ago2 KO-ob/ob (n = 4) mice after overnight fasting at age 13 weeks. H, Metabolic disease–associated microRNAs (MD-miRNAs) and I, their target mRNA expression levels in livers of L-Ago2 WT-Lean (n = 5), L-Ago2 WT-ob/ob (n = 10), L-Ago2 KO-Lean (n = 5), and L-Ago2 KO-ob/ob (n = 4) mice after overnight fasting at age 13 weeks. Data are shown as mean ± SEM of group size (n). Statistical analyses were performed by unpaired 2-tailed t test for C, D, and F or by 2-way analysis of variance followed by Tukey post hoc test for E, G, H, and I. *P less than or equal to .05, **P less than or equal to .01, ***P less than or equal to .001.
We previously demonstrated that hepatic Ago2 regulates the expression of a subset of metabolic disease–associated miRNAs (MD-miRNAs, such as miR-802, miR-103/107, and miR-148a/148b/152) that are critical for glucose metabolism (32). We investigated the effect of hepatic Ago2 on the expression of these MD-miRNAs in the liver of ob/ob mice. The levels of MD-miRNAs, miR-802, miR-103/miR-107, and miR-148b/152 were decreased in the livers of L-Ago2 KO-ob/ob mice compared with controls (Fig. 1H). These results indicate that although hepatic Ago2 is dispensable for the regulation of body weight in the ob/ob background, hepatic Ago2 deficiency results in a reduction of the subset of MD-miRNAs accompanied by improved glucose metabolism even in severe obesity. We and others previously showed that these MD-miRNAs target mRNAs involved in glycemic control, including hepatocyte nuclear factor 1 homeobox B (Hnf1β), Pgc1α, and Ampkα1 (27-32). Although the reduction of the subset of MD-miRNAs expression was not always linked to increased expression of their known target mRNAs in the liver of ob/ob mice (Fig. 1I), there was a trend that the levels of target miRNAs were higher in the Ago2-deficient condition.
Glucose Metabolism in Hepatic Argonaute 2–Deficient Mice in a Long-Term High-Fat Diet Condition
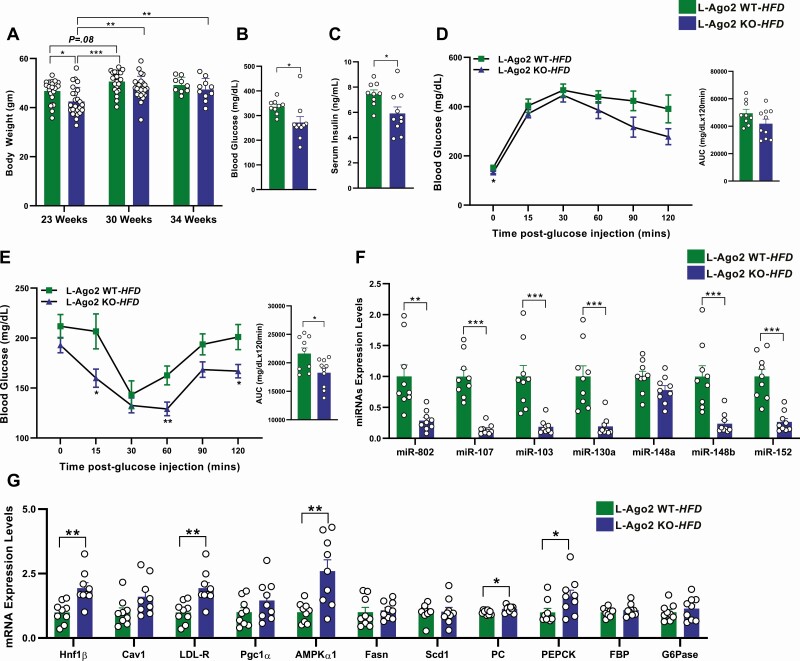
We previously showed that the bodyweight gain in L-Ago2 KO mice in a diet-induced obese mouse model (L-Ago2 KO-HFD) was less than that of controls (L-Ago2 WT-HFD), with the difference reaching statistical significance at age approximately 23 weeks (32). Given that body weight gain is comparable between the genotypes in the ob/ob background, we further examine the role of hepatic Ago2 in the regulation of body weight and glucose metabolism in an extended HFD condition. The bodyweight of L-Ago2 WT-HFD mice appeared to reach a plateau at age around 30 weeks and did not further increase by age 34 weeks on the HFD. Intriguingly, the body weight of L-Ago2 KO-HFD mice was lower than that of L-Ago2 WT-HFD mice at age 23 weeks as reported; however, a subpopulation of L-Ago2 KO mice gained weight at a similar level to L-Ago2 WT mice at age 30 weeks, and an average of body weight became comparable between the genotypes on HFD at age 30 and 34 weeks (Fig. 2A). These results suggest that while hepatic Ago2 deficiency in mice enables them to alleviate the gained weight during the course of diet-induced obesity, the effect is limited and the body weight of L-Ago2 KO mice eventually hits the plateau of body weight to levels similar to those observed in L-Ago2 WT mice on the HFD. To examine whether L-Ago2 KO mice show better glucose metabolism independent of body weight at the extended HFD feeding condition as observed in the ob/ob background, we measured blood glucose and serum insulin levels in L-Ago2 WT and KO mice of which body weight was comparable. Despite the similar body weights, L-Ago2 KO HFD mice at age 34 weeks exhibited lower blood glucose and serum insulin levels (Fig. 2B and 2C). Consistent with this, there were improvements in glucose clearance after a glucose challenge and significant reduction of blood glucose levels post insulin administration in L-Ago2 KO-HFD mice, although the results of the glucose clearance did not reach statistical significance (Fig. 2D and 2E). Of note, after long-term HFD feeding, the levels of serum TG, hepatic steatosis, NAS, and lipogenic genes, Scd1 and Fasn, were comparable between genotypes (Supplementary Fig. 2A-2C [35] and Fig. 2G). These results suggest that hepatic Ago2 deficiency improves systemic glucose metabolism during long-term HFD feeding, and that this improvement appears to be uncoupled from body weight and hepatic steatosis, which are consistent with what was observed in the ob/ob background.
Figure 2.
Effect of hepatic Argonaute 2 (Ago2) deficiency on glucose metabolism in a long-term high-fat diet (HFD) condition. A, Body weight of liver-specific Ago2-deficiency (L-Ago2) wild-type (WT) and L-Ago2 knockout (KO) mice on an HFD at age 23 (n = 22 and 25, respectively), 30 (n = 22 and 25, respectively), and 34 (n = 9 and 10, respectively) weeks. B, Blood glucose levels after 6 hours of fasting of L-Ago2 WT (n = 9) and L-Ago2 KO (n = 10) mice at age 34 weeks. C, Serum insulin levels of L-Ago2 WT (n = 9) and L-Ago2 KO (n = 10) mice at age 34 weeks. D, Glucose tolerance test (GTT) and its area under the curve (AUC) analysis in L-Ago2 WT (n = 9) and L-Ago2 KO (n = 10) mice at age 30 weeks. E, Insulin tolerance test (ITT) and its AUC analysis in L-Ago2 WT (n = 9), and L-Ago2 KO (n = 10) mice at age 31 weeks. F, Metabolic disease–associated microRNAs (MD-miRNAs) and their G, target messenger RNA (mRNA) expression levels in livers of L-Ago2 WT (n = 9) and L-Ago2 KO (n = 9) mice at age 34 weeks. Data are shown as mean ± SEM of group size (n). Statistical analyses were performed by ordinary one-way analysis of variance, followed by Tukey post hoc test for A or unpaired 2-tailed test for B to G. *P less than or equal to .05, **P less than or equal to .01, ***P less than or equal to .001.
As L-Ago2 KO mice showed better glucose metabolism despite similar body weight to that of L-Ago2 WT mice under the long-term HFD condition, we analyzed the expression levels of the subset of MD-miRNAs that are critical for glucose metabolism. Consistent with our observation in the ob/ob background, the expression of miR-802, miR-103/miR-107, and miR-148b/152 was also decreased in the liver of L-Ago2 KO-HFD mice compared with controls even in the similar body weight condition under long-term HFD feeding (Fig. 2F), indicating the universal role of Ago2 in the regulation of the subset of MD-miRNA expression. While expression levels of key gluconeogenic genes were not reduced in the liver of L-Ago2 KO-HFD mice, known target genes of the MD-miRNAs, such as Hnf1β and Ampkα1, were enhanced (Fig. 2G), which likely contributes to the improved glucose metabolism in hepatic Ago2 deficiency. Analyses of L-Ago2 KO mice in the ob/ob background and in the extended HFD condition indicate that changes in expression of MD-miRNAs and their target miRNAs are associated with systemic glucose metabolism but are uncoupled from bodyweight.
Role of Hepatic Argonaute 2 in Glycemic Control and Metabolic Disease–Associated MicroRNA Expression Post Vertical Sleeve Gastrectomy
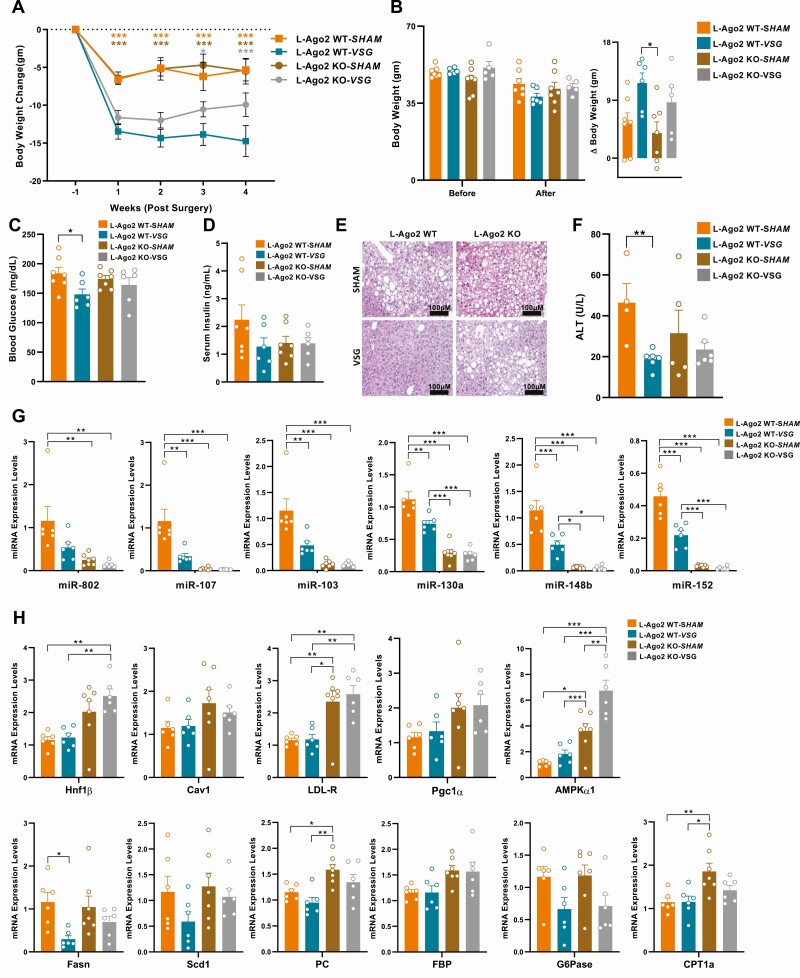
A recent study in rodents demonstrated that VSG improves glycemia in a manner independent of weight loss by restoring hepatic insulin sensitivity, and the liver is one of the critical organs that facilitates the remission of glycemic conditions prior to weight loss post VSG (11). As L-Ago2 KO mice improved systemic glucose metabolism uncoupled from body weight in obesity, we investigated the effect of hepatic Ago2 in the VSG-mediated metabolic regulation. We fed HFD to both L-Ago2 WT and L-Ago2 KO mice for 34 weeks. The L-Ago2 WT and L-Ago2 KO mice were randomly distributed between the VSG and sham surgery groups. We observed a drastic reduction of body weight in the L-Ago2 WT mice undergoing VSG compared with those undergoing sham surgery (Fig. 3A). In contrast, VSG was significantly less effective at reducing the body weight of mice in the L-Ago2 KO group post VSG (Fig. 3A and 3B). These results suggest that hepatic Ago2 plays an important role in sustaining weight loss post VSG. We then examined the glycemic control post VSG and observed that fasting blood glucose levels in the L-Ago2-WT-VSG group became lower than those in the L-Ago2-WT-SHAM group (Fig. 3C). Conversely, while there was a trend that fasting blood glucose levels of L-Ago2-KO-SHAM mice became lower compared to those of L-Ago2-WT-SHAM mice, the effect of VSG on the blood glucose levels was blunted in L-Ago2 KO mice and the levels were kept lower with or without VSG (see Fig. 3C). A similar trend was observed in serum insulin levels, although the reduction in insulin in the L-Ago2-WT-VSG group compared with the L-Ago2-WT-SHAM group showed no statistical significance (Fig. 3D). We examined the histology of the liver and observed an improvement in hepatosteatosis in the livers of L-Ago2-WT-VSG mice compared with L-Ago2-WT-SHAM; however, no significant difference was observed in hepatosteatosis between the VSG and sham groups in the L-Ago2 KO group (Fig. 3E). Plasma ALT concentrations were decreased significantly in the L-Ago2-WT-VSG group compared to L-Ago2-WT-SHAM. Although L-Ago2-KO-VSG mice showed a reduction of plasma ALT levels compared to L-Ago2-KO-SHAM, these did not reach statistical significance (Fig. 3F). These results raise the possibility that VSG modifies Ago2’s function, for example, Ago2’s slicer activity and Ago2-mediated RNA silencing, in the liver, and the functional changes are associated with weight loss and improved hepatosteatosis post VSG. As glycemia in L-Ago2 KO mice was maintained with or without VSG whereas VSG improves that in L-Ago2 WT mice, hepatic Ago2 also appears to be involved in glycemic control post VSG; however, it is still possible that VSG improves glycemia independent of hepatic Ago2 function.
Figure 3.
Effects of vertical sleeve gastrectomy (VSG) surgery on glycemic control and metabolic disease–associated microRNA (MD-miRNA) expression in liver-specific Ago2-deficiency (L-Ago2) knockout (KO) mice. A, Body weight gain of L-Ago2 wild-type (WT)-SHAM (n = 7) and L-Ago2 WT-VSG (n = 6), L-Ago2 KO-SHAM (n = 7), and L-Ago2 KO-VSG (n = 6) mice post surgery. B, Body weight loss of L-Ago2 WT-SHAM (n = 7) and L-Ago2 WT-VSG (n = 6), L-Ago2 KO-SHAM (n = 7), and L-Ago2 KO-VSG (n = 5) mice pre surgery and post surgery. C, Blood glucose levels after 6 hours of fasting of L-Ago2 WT-SHAM (n = 7), L-Ago2 WT-VSG (n = 6), L-Ago2 KO-SHAM (n = 7), and L-Ago2 KO-VSG (n = 6) mice 4 weeks post surgery. D, Serum insulin levels after 6 hours of fasting of L-Ago2 WT-SHAM (n = 7), L-Ago2 WT-VSG (n = 6), L-Ago2 KO-SHAM (n = 7), and L-Ago2 KO-VSG (n = 6) mice 4 weeks post surgery. E, hematoxylin-eosin–stained liver section of L-Ago2 WT-SHAM, L-Ago2 WT-VSG, L-Ago2 KO-SHAM, and L-Ago2 KO-VSG mice 4 weeks post surgery. F, Plasma alanine aminotransferase (ALT) levels of L-Ago2 WT-SHAM (n = 4), L-Ago2 WT-VSG (n = 6), L-Ago2 KO-SHAM (n = 6), and L-Ago2 KO-VSG (n = 6) mice 4 weeks post surgery. G, MD-miRNAs and their target messenger RNA (mRNA), de novo lipogenesis, and H, gluconeogenesis gene expression levels in livers of L-Ago2 WT-SHAM (n = 6), L-Ago2 WT-VSG (n = 6), L-Ago2 KO-SHAM (n = 7), and L-Ago2 KO-VSG (n = 6) mice 4 weeks post surgery. Data are shown as mean ± SEM of group size (n). Statistical analyses were performed by multiple t test for A or by unpaired 2-tailed test for C and F, or by 2-way analysis of variance followed by Tukey post hoc test for B, G, and H. *P less than or equal to .05, * P less than or equal to .01, *** P less than or equal to .001.
As our study indicates that hepatic Ago2–mediated glycemic control is associated with expression levels of a subset of MD-miRNAs in obesity, we next investigated the expression patterns of MD-miRNAs in the livers of these mice post VSG. Importantly, expression levels of MD-miRNAs, including miR-802, miR-107/103, miR-130a, were robustly downregulated in the liver of L-Ago2-WT-VSG mice compared to those of L-Ago2-WT-SHAM (Fig. 3G). Conversely, this effect of VSG was blunted in the L-Ago2 KO group, and the expression levels of MD-miRNAs were kept lower in the liver of L-Ago2 KO mice with or without VSG (see Fig. 3G). These trends of MD-miRNA expression appeared to be associated with glycemic control post VSG, and known target genes of these MD-miRNAs remained higher in the L-Ago2 KO group compared to the WT groups (Fig. 3H). However, VSG had a minimal or no effect on the expression of these known target genes. In addition, although VSG appeared to suppress the expression of lipogenic genes, Scd-1 and Fasn, and a gluconeogenic gene, glucose 6-phosphatase (G6Pase), there were no obvious differences in the expression levels between genotypes (see Fig. 3H). Therefore, there may be different sets of genes regulated by MD-miRNAs for metabolic improvements associated with Ago2’s function post VSG.
Role of Hepatic Argonaute 2 in Regulating the Peroxisome Proliferator–Activated Receptor α Pathway
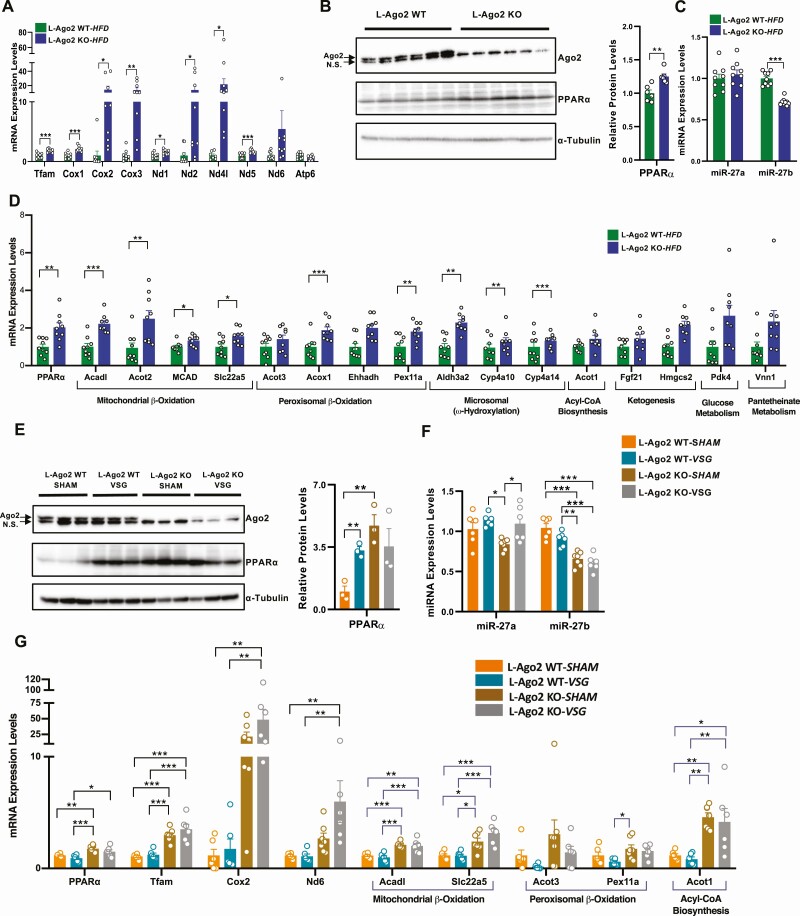
We previously demonstrated that Ago2 deficiency leads to enhanced mitochondrial oxidation with increased activation of AMPKα and expression genes related to the Tfam-mitochondrial pathway in hepatocytes (32). While Ago2-mediated energy metabolism is linked to AMPKα activation that enhances mitochondrial biogenesis and function (42), as L-Ago2 KO mice improve glucose metabolism even in the hepatic AMPKα1-deleted condition (32), there appear to be additional factors that are responsible for Ago2-mediated regulation of mitochondrial oxidation and glycemia. We hypothesized that one such factor is hepatic PPARα being involved in various metabolic pathways, including mitochondrial and peroxisomal oxidation, and in transcriptional reprogramming post VSG (11). Increased expression of genes related to the Tfam-mitochondrial pathway in the livers of HFD L-Ago2 KO mice was caused by enhanced mitochondrial quality (18), which was still observed under the long-term HFD condition (Fig. 4A). As expected, we saw increased levels of hepatic PPARα protein in the livers of long-term HFD L-Ago2 KO mice compared with controls (Fig. 4B). Importantly, by searching significant miRNAs whose expression levels are downregulated in hepatic Ago2 deficiency in our miRNA-sequencing profiles published previously (32), we found that miR-27b, which negatively regulates PPARα (43, 44), was reduced in the liver of L-Ago2 KO-HFD mice. Consistent with these observations, the mRNA expression of PPARα and its target genes involved in mitochondrial β-oxidation (Acadl, Acot2, MCAD, Slc22a5), peroxisomal β-oxidation (Acot3, Acox1, Ehhadh, Pex11a), and microsomal ω-hydroxylation (Aldh3a2, Cyp4a10, Cyp4a14) was increased in the livers of L-Ago2 KO mice compared with controls under the long-term HFD condition (Fig. 4D).
Figure 4.
Hepatic Argonaute 2 (Ago2) deficiency enhances the expression of the peroxisome proliferator–activated receptor α (PPARα) pathway. A, Messenger RNA (mRNA) expression of Tfam-mitochondrial genes in livers of liver-specific Ago2-deficiency (L-Ago2) wild-type (WT) (n = 9) and L-Ago2 knockout (KO) (n = 9) mice at age 34 weeks. B, Western blot analysis of PPARα protein expression in the liver of L-Ago2 WT (n = 6) and L-Ago2 KO (n = 6) mice at age 34 weeks. C, miR-27a and miR-27b expression in livers of L-Ago2 WT (n = 9) and L-Ago2 KO (n = 9) mice at age 34 weeks. D, PPARα and its target mRNA expression in livers of L-Ago2 WT (n = 9) and L-Ago2 KO (n = 9) mice at age 34 weeks. E, Western blot analysis of PPARα protein expression in the liver of L-Ago2 WT-SHAM (n = 3), L-Ago2 WT-VSG (n = 3), L-Ago2 KO-SHAM (n = 3), and L-Ago2 KO-VSG (n = 3) mice 4 weeks post surgery. F, miR-27a and miR-27b expression in livers of L-Ago2 WT-SHAM (n = 6), L-Ago2 WT-VSG (n = 6), L-Ago2 KO-SHAM (n = 7), and L-Ago2 KO-VSG (n = 6) mice 4 weeks post surgery. G, PPARα and its target mRNA expression in livers of L-Ago2 WT-SHAM (n = 6), L-Ago2 WT-VSG (n = 6), L-Ago2 KO-SHAM (n = 7), and L-Ago2 KO-VSG (n = 6) mice 4 weeks post surgery. NS, nonspecific band. Data are shown as mean ± SEM of group size (n). Statistical analyses were performed by unpaired 2-tailed test for A to D, by multiple t test for E, or by 2-way analysis of variance followed by Tukey post hoc test for F and G. *P less than or equal to .05, * P less than or equal to .01, *** P less than or equal to .001.
A recent study showed that hepatic PPARα mRNA and protein levels were increased in the liver of ob/ob mice post VSG compared with sham controls (11). PPARα protein levels were significantly elevated in the livers of the L-Ago2-WT-VSG group compared with the L-Ago2-WT-SHAM group (Fig. 4E). Importantly, PPARα protein levels were higher in the livers of L-Ago2 KO-SHAM mice compared with L-Ago2 WT-SHAM, but the expression was not increased post VSG in L-Ago2 KO mice. Expression levels of miR-27b were lower in the liver of L-Ago2 KO mice (Fig. 4F), although there was no effect of VSG on its expression in L-Ago2 WT mice. Similarly, we observed that the expression of mRNA levels of PPARα, its target genes, and mitochondrial genes became higher in hepatic Ago2 deficiency both in the sham and VSG groups, whereas there was no obvious effect of VSG on the expression of those genes in the L-Ago2 WT mice (Fig. 4G). These data suggest that Ago2-mediated RNA-silencing regulates the expression of PPARα expression in obesity and post VSG, although the significance of PPARα in the transcriptional regulation of its target genes post VSG remains to be elucidated.
Effects of Peroxisome Proliferator–Activated Receptor α Agonist in Argonaute 2–Deficient Primary Hepatocytes
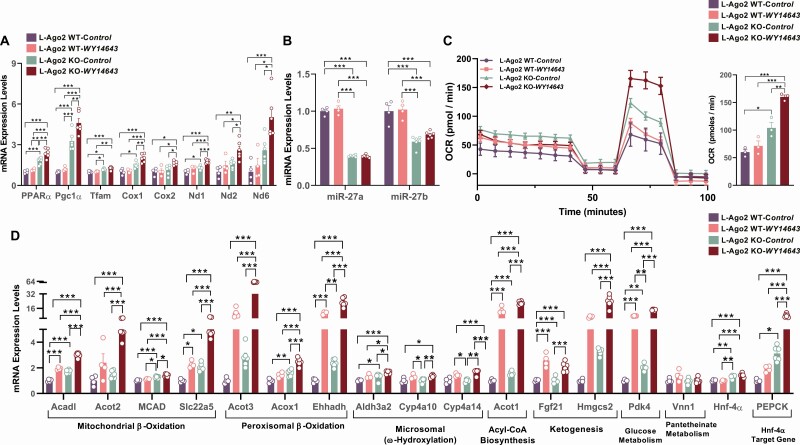
Because Ago2 regulates the expression of PPARα and its target genes and genes relevant to mitochondrial functions are increased in Ago2-deficient obese liver, we further investigated whether hepatic Ago2 is involved in the regulation of the PPARα pathway and PPARα-mediated cellular events in primary hepatocytes. Consistent with the gene expression profile observed in the liver of mice fed a long-term HFD and post VSG (see Fig. 4B and 4E), mRNA levels of PPARα and mitochondrial genes were higher in Ago2-deficient hepatocytes compared with WT controls (Fig. 5A). Treatment with the PPARα agonist WY-16463 significantly increased these mRNA levels, and the effect was much more robust in Ago2-deficient hepatocytes (see Fig. 5A). Consistent with the increased expression of PPARα, miR-27b, and also miR-27a, both of which negatively regulate PPARα expression, were downregulated in Ago2-deficient primary hepatocytes (Fig. 5B). To further confirm the role of Ago2 in regulating PPARα functions, we examined whether Ago2-deficiency affects PPARα agonist–induced oxidative metabolism. Analysis of mitochondrial OCR in WT and Ago2-deficient hepatocytes demonstrated that Ago2-deficiency leads to an increased OCR compared with WT controls (Fig. 5C). Importantly, the mitochondrial OCR of Ago2-deficient hepatocytes was significantly higher after treatment with a PPARα agonist compared with WT hepatocytes (see Fig. 5C). Increased OCR of Ago2-deficient hepatocytes also appeared to be associated with the induction of PPARα target genes in the presence or absence of the PPARα agonist (Fig. 5D). Further, we have investigated the effect of Ago2 deficiency on other PPAR family transcription factors, PPARγ and PPARδ, in primary hepatocytes. Treatment of hepatocytes with either PPARγ agonist (rosiglitazone) or PPARδ agonist (GW0742) induced the expression of their target genes both in WT and Ago2-deficient hepatocytes at a similar level (Supplementary Fig. 3 [35]). These data indicate that Ago2 regulates the activity of PPARα but not PPARγ or PPARδ.
Figure 5.
Effects of peroxisome proliferator-activated receptor (PPAR) agonist in Argonaute 2 (Ago2)-deficient primary hepatocytes. A, messenger RNA (mRNA) expression of Tfam-mitochondrial genes in liver-specific Ago2-deficiency (L-Ago2) wild-type (WT) (n = 6), and L-Ago2 knockout (KO) (n = 6) primary hepatocytes treated with or without 10-µM WY14643 (a PPARα agonist) for 24 hours. B, miR-27a and miR-27b expression in L-Ago2 WT (n = 6) and L-Ago2 KO (n = 6) primary hepatocytes treated with or without 10-µM WY14643 (PPARα agonist) for 24 hours. C, Oxygen consumption rate (OCR) of L-Ago2 WT (n = 12) and KO (n = 10–12) primary hepatocytes were measured in the presence or absence of a PPARα agonist (WY14643: 10 μM) pretreatment for 48 hours. D, PPARα target mRNA expression in L-Ago2 WT (n = 6) and L-Ago2 KO (n = 6) primary hepatocytes treated with or without 10-µM WY14643 (PPARα agonist) for 24 hours. Data are shown as mean± SEM. Statistical analyses were performed by 2-way analysis of variance for A, B, and D; and ordinary 1-way analysis of variance followed by Tukey post hoc test for C. *P less than or equal to .05, * P less than or equal to .01, *** P less than or equal to .001.
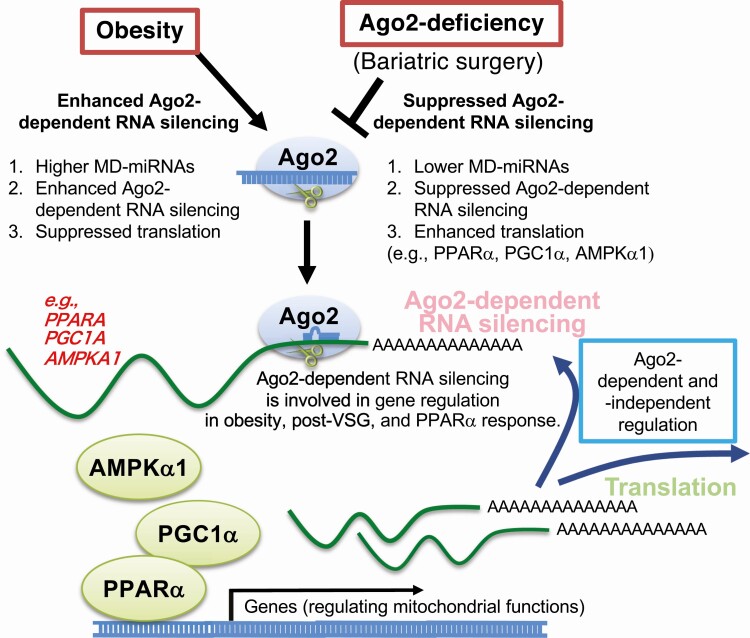
Taken together, this study shows that hepatic Ago2 is a critical regulator of PPARα expression and function, and that its deficiency increases PPARα-mediated oxidative metabolism. Ago2-dependent RNA silencing is involved in gene regulation in obesity, post-VSG, and the PPARα response (Fig. 6), which are critical for glycemic control.
Figure 6.
A proposed molecular mechanism of the role of hepatic Argonaute 2 (Ago2) in obesity, post bariatric surgery, and in peroxisome proliferator–activated receptor α (PPARα) response. Hepatic Ago2 is required for the expression of metabolic disease–associated microRNA (MD-miRNA), which is enhanced in obesity but suppressed post bariatric surgery. Hepatic Ago2-dependent RNA silencing plays a critical role in gene regulation relevant to mitochondrial functions, including PPARα, adenosine 5′-monophosphate–activated protein kinase α (AMPKα1), and PPAR γ coactivator (PGC1α), and PPARα-mediated oxidative metabolism in obesity, post–vertical sleeve gastrectomy (VSG), and PPARα response.
Discussion
Understanding the molecular links between obesity and bariatric surgery outcomes may help identify novel therapeutic targets and strategies for the treatment of obesity-associated sequelae. Bariatric surgery has significant metabolic benefits, including resolution of T2D, and some of these benefits cannot be explained simply by weight loss alone (4, 8, 9). However, the mechanisms responsible for these weight-independent metabolic benefits are poorly understood. Animal studies, including a recent finding in rodents with deficient leptin signaling, also indicate that weight loss post VSG is not responsible for improved glycemic control (11). Instead, this improvement could be attributed to increased peripheral insulin sensitivity, particularly in the liver, but not to insulin secretion from β cells. These observations suggest that functional changes in the molecule or molecules regulating insulin sensitivity play a central role in glycemic improvement post surgery. The present study indicates that hepatic Ago2–mediated regulation of glucose tolerance and insulin sensitivity is strikingly uncoupled from bodyweight in obesity. Also, glycemic improvements were not observed in L-Ago2 KO mice post VSG, suggesting that hepatic Ago2 is dispensable for modulating systemic glucose metabolism post surgery. Therefore, hepatic Ago2 is likely involved in the metabolic benefits of bariatric surgery, a feature consistent with the role of hepatic Ago2 in regulating glucose tolerance and insulin sensitivity in a weight-independent manner. Nevertheless, further studies are needed to delineate the precise nature of this potentially intricate biology in obesity and response to surgery.
Our group and others recently reported that Ago2 in the liver is critical for the development of obesity-associated pathophysiology (32, 41). In the present study, we demonstrated that 2 independent mouse models of hepatic Ago2 deficiency were protected against severe obesity-induced insulin resistance and diabetes, and that the glucose-related phenotypes were uncoupled from body weight and hepatosteatosis. We next examined whether hepatic Ago2-dependent molecular mechanisms were involved in the improved metabolic outcomes after bariatric surgery. Our results revealed that hepatic Ago2 plays a crucial role in the regulation of glucose homeostasis in severe obesity, both before and after surgery. Importantly, bariatric surgery markedly reduced the expression of a subset of MD-miRNAs (including miR-802 and miR-107/103) that are known to detrimentally affect glucose metabolism. Of note, Ago2 possesses an endoribonuclease (“slicer”) activity (45), and we recently showed that this slicer activity is required for the expression of mature MD-miRNAs (32). These findings raise the possibility that hepatic Ago2 slicer activity is increased in obesity (32) and that this activity is suppressed by bariatric surgery. This leads to the reduced biogenesis of MD-miRNAs, which is accompanied by improved glycemic control. A previous report suggested that phosphorylation of Ago2 at serine 388 inhibits Ago2 slicer activity (46). Together, these findings raise the possibility that bariatric surgery improves glycemia at least in part by inhibiting hepatic Ago2 slicer activity, which may be executed by altering the cell signaling pathways that phosphorylate hepatic Ago2 post surgery. Thus, our findings suggest that inhibiting hepatic Ago2–mediated RNA silencing might be a critical step in improving glycemia post bariatric surgery and a viable interventional strategy for the treatment of established metabolic disorders.
Importantly, our study might resolve a gap in current models describing how VSG increases PPARα protein levels (11). Specifically, while the activity of Ago2-mediated RNA silencing is higher under conditions of obesity, which results in enhanced expression of MD-miRNAs and reduction of their target mRNA translation, VSG-mediated suppression of Ago2 slicer activity appears to contribute to the increased PPARα protein levels (see Fig. 6). Consequently, PPARα protein levels are higher in the livers of L-Ago2 KO mice compared with controls, which may be associated with the increased oxidation and expression of mitochondrial genes in obesity and in response to VSG. Consistent with these observations, Ago2-deficient hepatocytes were more sensitive to a PPARα-agonist and exhibited enhanced mitochondrial oxidation, suggesting that Ago2 is a negative regulator of PPARα function. Importantly, PPARα is known to enhance FA oxidation; however, no obvious improvement in lipid metabolism was observed in the liver of L-Ago2 KO mice under the extended HFD condition even though levels of PPARα and mitochondrial genes increased. As Ago2 deficiency in the liver prevents hepatosteatosis with enhanced energy expenditure during the pathogenesis of mild obesity (32), there appear to be additional unknown mechanisms of how hepatic Ago2 deficiency affects lipid metabolism during the development of severe obesity. Reduction in hepatic mitochondrial oxidation may play an important role during the course of the lipid accumulation in the liver by suppressing lipid oxidation. Conversely, increased hepatic mitochondrial oxidation might lead to increased formation of reactive oxygen species and damaged mitochondrial respiratory chain. This negative effect of increased hepatic mitochondrial oxidation may be one of the reasons that L-Ago2 KO mice temporarily show less hepatic steatosis (32) and then develop severe steatosis during the extended HFD feeding. Intriguingly, glycemic improvements were observed consistently in L-Ago2 KO mice, where PPARα levels in the liver were higher compared to L-Ago2 WT, in mild and severe obesity and in insulin antagonist–induced insulin-resistant conditions (32). PPARα is a key player in lipid metabolism, and there is genetic evidence in humans that PPARα single-nucleotide variations and variants are associated with the progression of T2D; these observations are supported by animal studies (23). In addition, PPARα agonist treatment improves glucose metabolism in patients with diabetes and impaired glucose tolerance (19, 20). These findings raise the possibility that the increased expression and function of PPARα and resulting changes in associated mitochondrial functions may contribute to the glycemic improvements observed under hepatic Ago2–deficient conditions in obesity. Additional studies will address whether PPARα is required for the improved glycemic control observed in L-Ago2 KO mice.
In conclusion, this work supports the hypothesis that hepatic Ago2 plays an important role in regulating MD-miRNA expression and the progression and persistence of glucose intolerance during obesity. Because Ago2 slicer activity is required for MD-miRNA expression (32), inhibition of Ago2-dependent RNA silencing appears to be a critical step for achieving positive metabolic outcomes after bariatric surgery. Also, by investigating the role of Ago2 in the expression of PPARα, which was recently identified as a key factor involved in VSG-mediated glycemic improvement (11), this work reveals the significant role of hepatic Ago2 in the regulation of PPARα expression and PPARα-mediated mitochondrial oxidation. Future studies to identify the molecular mechanisms that suppress hepatic Ago2–dependent RNA silencing post VSG might lead to a novel non-surgical therapeutic intervention to achieve glycemic control in T2D.
Acknowledgments
We thank Drs Vivian Hwa, Andrew Vonberg, and Tomoki Yagai for the scientific input and discussion.
Financial Support: This work was supported by the National Institute of Health (NIH; R01 grant Nos. R01DK100314 to R.K., R01DK107530 to T.N., and P30DK078392 for the Digestive Disease Research Core Center in Cincinnati.).
Author Contributions: R.K. and T.N. conceived the research. J.B., V.J.B., E.S.B.S., R.K., and T.N. designed the methodology and experiments. J.B., V.J.B., E.S.B.S., C.Z., K.M., R.K.G., A.K., J.K.K., R.S., and M.W. conducted experiments. J.B., R.K. and T.N. wrote the manuscript with input from all authors.
Glossary
Abbreviations
- Ago2
Argonaute 2
- ALT
alanine aminotransferase
- AMPK
adenosine 5′-monophosphate–activated protein kinase
- ANOVA
analysis of variance
- FA
fatty acid
- GTT
glucose tolerance test
- HFD
high-fat diet
- HNF4α
hepatocyte nuclear factor 4 α
- ITT
insulin tolerance test
- KO
knockout
- L-Ago2 KO
liver-specific Ago2-deficiency
- MD-miRNA
metabolic disease–associated microRNA
- mRNA
messenger RNA
- miRNA
microRNA
- NAS
nonalcoholic fatty liver disease (NAFLD) activity score
- OCR
oxygen consumption rate
- PCR
polymerase chain reaction
- PPARα
peroxisome proliferator–activated receptor α
- T2D
type 2 diabetes
- TG
triglyceride
- VSG
vertical sleeve gastrectomy
- WT
wild-type
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Afshin A, Forouzanfar MH, Reitsma MB, et al. ; GBD 2015 Obesity Collaborators . Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177-185. [DOI] [PubMed] [Google Scholar]
- 3. Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510(7503):84-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brito JP, Montori VM, Davis AM. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. JAMA. 2017;317(6):635-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Myronovych A, Salazar-Gonzalez RM, Ryan KK, et al. The role of small heterodimer partner in nonalcoholic fatty liver disease improvement after sleeve gastrectomy in mice. Obesity (Silver Spring). 2014;22(11):2301-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Myronovych A, Kirby M, Ryan KK, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring). 2014;22(2):390-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inge TH, Courcoulas AP, Jenkins TM, et al. ; Teen–LABS Consortium . Five-year outcomes of gastric bypass in adolescents as compared with adults. N Engl J Med. 2019;380(22):2136-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shah AS, D’Alessio D, Ford-Adams ME, Desai AP, Inge TH. Bariatric surgery: a potential treatment for type 2 diabetes in youth. Diabetes Care. 2016;39(6):934-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abu-Gazala S, Horwitz E, Ben-Haroush Schyr R, et al. Sleeve gastrectomy improves glycemia independent of weight loss by restoring hepatic insulin sensitivity. Diabetes. 2018;67(6):1079-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dubois V, Eeckhoute J, Lefebvre P, Staels B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J Clin Invest. 2017;127(4):1202-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bougarne N, Weyers B, Desmet SJ, et al. Molecular actions of PPARα in lipid metabolism and inflammation. Endocr Rev. 2018;39(5):760-802. [DOI] [PubMed] [Google Scholar]
- 14. Montagner A, Polizzi A, Fouché E, et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 2016;65(7):1202-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fan W, Evans R. PPARs and ERRs: molecular mediators of mitochondrial metabolism. Curr Opin Cell Biol. 2015;33:49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aoyama T, Peters JM, Iritani N, et al. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα). J Biol Chem. 1998;273(10):5678-5684. [DOI] [PubMed] [Google Scholar]
- 17. Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor α (PPARα) in the cellular fasting response: the PPARα-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A. 1999;96(13):7473-7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. 2017;13(1):36-49. [DOI] [PubMed] [Google Scholar]
- 19. Feng X, Gao X, Jia Y, Xu Y. PPAR-α agonist fenofibrate reduces insulin resistance in impaired glucose tolerance patients with hypertriglyceridemia: a cross-sectional study. Diabetes Ther. 2017;8(2):433-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Teramoto T, Shirai K, Daida H, Yamada N. Effects of bezafibrate on lipid and glucose metabolism in dyslipidemic patients with diabetes: the J-BENEFIT study. Cardiovasc Diabetol. 2012;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flavell DM, Ireland H, Stephens JW, et al. Peroxisome proliferator-activated receptor α gene variation influences age of onset and progression of type 2 diabetes. Diabetes. 2005;54(2):582-586. [DOI] [PubMed] [Google Scholar]
- 22. Andrulionyte L, Kuulasmaa T, Chiasson JL, Laakso M; STOP-NIDDM Study Group . Single nucleotide polymorphisms of the peroxisome proliferator-activated receptor-α gene (PPARA) influence the conversion from impaired glucose tolerance to type 2 diabetes: the STOP-NIDDM trial. Diabetes. 2007;56(4):1181-1186. [DOI] [PubMed] [Google Scholar]
- 23. Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62(3):720-733. [DOI] [PubMed] [Google Scholar]
- 24. Arner P, Kulyté A. MicroRNA regulatory networks in human adipose tissue and obesity. Nat Rev Endocrinol. 2015;11(5):276-288. [DOI] [PubMed] [Google Scholar]
- 25. Ross SA, Davis CD. The emerging role of microRNAs and nutrition in modulating health and disease. Annu Rev Nutr. 2014;34:305-336. [DOI] [PubMed] [Google Scholar]
- 26. Salem ESB, Vonberg AD, Borra VJ, Gill RK, Nakamura T. RNAs and RNA-binding proteins in immuno-metabolic homeostasis and diseases. Front Cardiovasc Med. 2019;6:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trajkovski M, Hausser J, Soutschek J, et al. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474(7353):649-653. [DOI] [PubMed] [Google Scholar]
- 28. Kornfeld JW, Baitzel C, Könner AC, et al. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature. 2013;494(7435):111-115. [DOI] [PubMed] [Google Scholar]
- 29. Wagschal A, Najafi-Shoushtari SH, Wang L, et al. Genome-wide identification of microRNAs regulating cholesterol and triglyceride homeostasis. Nat Med. 2015;21(11):1290-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goedeke L, Rotllan N, Canfrán-Duque A, et al. MicroRNA-148a regulates LDL receptor and ABCA1 expression to control circulating lipoprotein levels. Nat Med. 2015;21(11):1280-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soronen J, Yki-Järvinen H, Zhou Y, et al. Novel hepatic microRNAs upregulated in human nonalcoholic fatty liver disease. Physiol Rep. 2016;4(1):e12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang C, Seo J, Murakami K, et al. Hepatic Ago2-mediated RNA silencing controls energy metabolism linked to AMPK activation and obesity-associated pathophysiology. Nat Commun. 2018;9(1):3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kleiner DE, Brunt EM, Van Natta M, et al. ; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6): 1313-1321. [DOI] [PubMed] [Google Scholar]
- 34. Salem ESB, Murakami K, Takahashi T, et al. Isolation of primary mouse hepatocytes for nascent protein synthesis analysis by non-radioactive L-azidohomoalanine labeling method. J Vis Exp. 2018;(140):e58323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bhattacharjee J, Borra VJ, Salem ESB, et al. Data from: Hepatic Ago2 regulates PPARα for oxidative metabolism linked to glycemic control in obesity and post bariatric surgery. figshare. Deposited December 30, 2020. doi: 10.6084/m9.figshare.13506804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. RRID:AB_2096291, https://scicrunch.org/resolver/RRID:AB_2096291 [Google Scholar]
- 37. RRID:AB_2619646, https://scicrunch.org/resolver/AB_2619646 [Google Scholar]
- 38. RRID:AB_2885073, https://scicrunch.org/resolver/AB_2885073 [Google Scholar]
- 39. RRID:AB_772210, https://scicrunch.org/resolver/RRID:AB_772210 [Google Scholar]
- 40. RRID:AB_228341, https://scicrunch.org/resolver/RRID:AB_228341 [Google Scholar]
- 41. Yan X, Wang Z, Bishop CA, et al. Control of hepatic gluconeogenesis by Argonaute2. Mol Metab. 2018;18:15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sun L, Trajkovski M. MiR-27 orchestrates the transcriptional regulation of brown adipogenesis. Metabolism. 2014;63(2):272-282. [DOI] [PubMed] [Google Scholar]
- 44. Kida K, Nakajima M, Mohri T, et al. PPARα is regulated by miR-21 and miR-27b in human liver. Pharm Res. 2011;28(10):2467-2476. [DOI] [PubMed] [Google Scholar]
- 45. Meister G. Argonaute proteins: functional insights and emerging roles. Nat Rev Genet. 2013;14(7):447-459. [DOI] [PubMed] [Google Scholar]
- 46. Horman SR, Janas MM, Litterst C, et al. Akt-mediated phosphorylation of Argonaute 2 downregulates cleavage and upregulates translational repression of microRNA targets. Mol Cell. 2013;50(3):356-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”