Abstract
Background:
Both obesity and the presence of collagenolytic bacterial strains (Enterococcus faecalis) can increase the risk of anastomotic leak. The aim of this study was to determine whether mice chronically fed a high-fat Western-type diet (WD) develop anastomotic leak in association with altered microbiota, and whether this can be mitigated by a short course of standard chow diet (SD; low fat/high fibre) before surgery.
Methods:
Male C57BL/6 mice were assigned to either SD or an obesogenic WD for 6 weeks followed by preoperative antibiotics and colonic anastomosis. Microbiota were analysed longitudinally after operation and correlated with healing using an established anastomotic healing score. In reiterative experiments, mice fed a WD for 6 weeks were exposed to a SD for 2, 4 and 6 days before colonic surgery, and anastomotic healing and colonic microbiota analysed.
Results:
Compared with SD-fed mice, WD-fed mice demonstrated an increased risk of anastomotic leak, with a bloom in the abundance of Enterococcus in lumen and expelled stool (65–90 per cent for WD versus 4–15 per cent for SD; P = 0·010 for lumen, P = 0·013 for stool). Microbiota of SD-fed mice, but not those fed WD, were restored to their preoperative composition after surgery. Anastomotic healing was significantly improved when WD-fed mice were exposed to a SD diet for 2 days before antibiotics and surgery (P < 0·001).
Conclusion.
The adverse effects of chronic feeding of a WD on the microbiota and anastomotic healing can be prevented by a short course of SD in mice.
Introduction
The biological variables that drive secure and safe anastomotic healing are complex, and involve multiple aspects including surgical technique, blood flow, tension and inflammatory factors. Because the gut is constantly exposed to a high burden of microbial organisms, healing may be dependent on a delicate balance between the health-promoting microbiota and the virulence of pathobiota. Studies1–6 in animals as early as 1955 demonstrated a key role for the colonic microbiota in the pathogenesis of anastomotic healing. A more recent study7 examining anastomotic tissues in rodents showed a significant loss of health-promoting taxa such as Ruminococcus, Bacteroidales family S24–7 and Prevotellaceae, with a significant bloom in Escherichia and Enterococcus. Functional profiling of predominate strains colonizing anastomotic tissues in these studies predicted the production of multiple virulence factors, including those involved in collagen degradation7. Given that Enterococcus is currently considered to be a causative agent in anastomotic disruption8–10, it is possible that the combination of loss of the normal microbiota and domination of pathobiota in anastomotic tissues contributes to anastomotic disruption. As intestinal microbiota have co-evolved with host tissues, it is not surprising that they have a positive effect on the host immune system and healing when injury occurs11,12. Yet, in modern intestinal surgery the intestinal microbiota are routinely disrupted by the use of antibiotics and cleansing purgatives, by diets that consist mainly of liquid foods, and by the exposure of intestinal tissues to ambient oxygen leading to elimination of key health-promoting Bacteroides species7.
Among the many variables that may affect both gut microbiota composition and anastomotic healing is diet. A high-fat obesogenic Western-type diet (WD) is known to alter gut microbial composition13,14. In addition, obesity is a known risk factor for anastomotic leak15. However, it is unknown whether an obesogenic diet affects the microbiota at the site of intestinal anastomosis and whether such changes are associated with leak. Therefore, the aims of this study were to determine the effect of intake of WD versus standard chow diet (SD) on the anastomotic microbiome and on anastomotic healing following colorectal surgery in mice. A longitudinal study was performed tracking the microbiome in expelled stool, in luminal content at the anastomotic site and in anastomotic tissues, to encompass both the regional and spatial context of the microbiome and determine its association with anastomotic healing. Finally, whether mice chronically fed a WD could be prehabilitated with SD to mitigate the effects of WD on anastomotic healing was determined.
Methods
All experiments were undertaken using mice, which were maintained in accordance with the guidelines prepared by the institutional animal care and use committee at the University of Chicago. Experimental steps and ethics were approved by the Institutional Animal Care and Use Committees Protocol 72417.
Mouse model
Male C57BL/6 mice aged 6–7 weeks (Charles River Laboratories, Raleigh, North Carolina, USA) were used in all experiments and maintained in accordance with the guidelines at the University of Chicago. Mice were housed under standard laboratory conditions in a temperature-controlled room (22–24°C) with a 12-h light–dark diurnal cycle. Mice were allowed free access to food and water.
Sixty-two mice were assigned to either a high-fat/low-fibre WD or SD of rodent chow (Table S1, supporting information). Mice were maintained for 6 weeks on their respective diets and weighed weekly. After 6 weeks, six WD mice and four SD mice were killed, and samples of intestinal luminal content and tissues were obtained to determine the preoperative microbiota status. The remaining mice underwent a low colorectal anastomosis as described previously9,16. Thirty minutes before surgery, mice received a subcutaneous injection of cefoxitin (40 mg/kg), and were anaesthetized with intraperitoneal ketamine (100 mg/kg) and xylazine (10 mg/kg). A midline abdominal incision was made, followed by colonic transection and anastomosis using interrupted sutures. Anastomotic integrity was confirmed by distending the distal colon with normal saline via an enema. All animals were resuscitated with 1 ml 0.9 per cent normal saline administered subcutaneously. Mice were maintained for 4 weeks on their respective diets after operation.
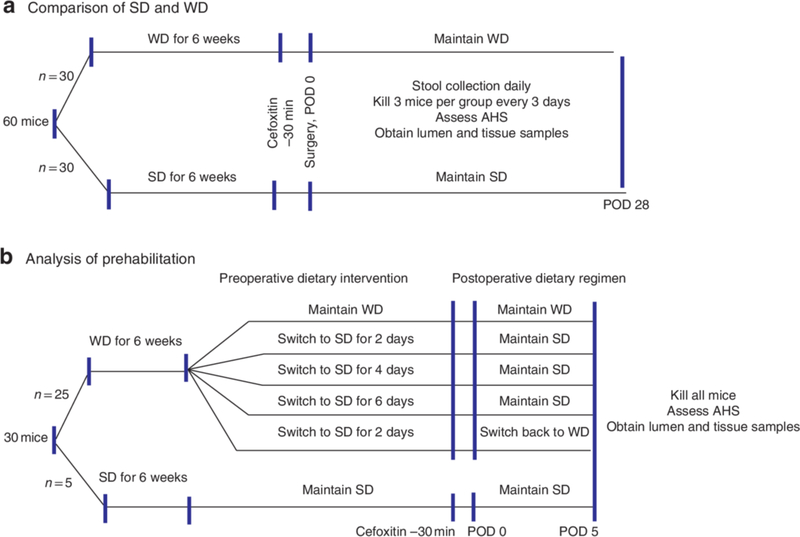
For longitudinal analysis of the microbiome during the postoperative phase, expelled stool samples were collected daily. Luminal and tissue-associated microbiota were assessed by killing three mice from each group on postoperative day (POD) 1, 3, 7, 13, 16, 24 and 28. Anastomotic healing was assessed using the anastomotic healing score (AHS), as described previously9, 16: AHS 0, normal healing; AHS 1, flimsy adhesions; AHS 2, dense adhesions without abscess; AHS 3, dense adhesions with gross abscess at the anastomotic site; and AHS 4, gross leak with peritonitis. At the time of death, luminal contents and anastomotic tissues were collected and, with expelled stool, analysed for microbiota composition by 16S ribosomal RNA (rRNA) measurement. The experimental design is outlined in Fig. 1a. Reiterative experiments were performed to directly compare anastomotic healing on POD 5 between mice fed a WD or SD (5 per group).
Fig.1. Experimental design.
a Comparison of Western diet (WD) and standard chow diet (SD); b testing effect of prehabilitation with SD. POD, postoperative day; AHS, anastomotic healing score.
To test the ability of SD to reverse the effects of WD feeding, a crossover experiment was performed (Fig. 1b). Mice consumed a WD for 6 weeks, and were then assigned to groups consuming a SD for 2, 4 or 6 days before surgery (5 per group), and continued on either a SD or WD after operation. Reiterative experiments were undertaken to determine the effect of 2 days of chow feeding (10 per group). All mice were killed on POD 5, and anastomotic healing was evaluated using the AHS. In addition, anastomotic tissues were collected and assayed by culture for the presence of collagenolytic Enterococcus.
Microbiota analysis of expelled stool, luminal contents and anastomotic tissues.
Expelled stool samples from each mouse were weighed daily. Luminal and tissue samples were obtained from groups of mice at the time of death. Luminal contents were collected by flushing the anastomotic site with 100 μl sterile water. A 0.5-cm section of anastomotic tissue was collected proximal and distal to the anastomosis. Tissue samples containing mucosa and overlying mucus were homogenized, and divided into two for DNA extraction and culture. Some tissue samples were analysed for collagenolytic Enterococcus.
Isolation of DNA and 16S ribosomal RNA sequencing
DNA was extracted using the DNeasy PowerSoil HTP 96 Kit (Qiagen, Hilden, Germany). One faecal pellet was used for each stool sample, and 0.25 g material for each tissue sample. The DNA extraction protocol followed that of the Earth Microbiome Project (EMP)17, with heating performed in a Lab Armor Bead Bath (Fisher Scientific, Cornelius, Oregon, USA) to minimize contamination.
For library preparation, DNA was amplified using the barcoded 12-bp Golay primer set designed for the EMP17. A mitochondrial peptide nucleic acid blocker (PNA Bio, Thousand Oaks, California, USA) was used to prevent the amplification of the murine 16S mitochondrial region. For each sample, PCR was performed according to the protocol using the EMP primers, mPNA, AccuStart™ II PCR ToughMix (Quantabio, Beverly, Massachusetts, USA), and the extracted DNA. The results of the quantification were used to normalize the amount of DNA for sequencing, and to ensure that each amplicon was represented evenly during sequencing. The volumes were consolidated sequentially into a single tube using a liquid handler (OpenTrons, Brooklyn, New York, USA) running a custom Python script. Finally, an aliquot of the final pool was taken, and the DNA was purified using an Agencourt® AMPure XP PCR* purification system (Beckman-Coulter, Indianapolis, Indiana, USA). The samples were run on an MiSeq® system (Illumina, San Diego, California, USA) at University of Illinois at Urbana-Champaign (150 base pairs × 2). The sequence data are deposited in the EBI Database (accession number ERP113631).
16S rRNA sequence data analysis
For 16S rRNA analysis, the paired-end reads were joined using the join_paired_ends.py script followed by quality filtering and de-multiplexing using the split_libraries_fastq.py script in QIIME18. The final set of de-multiplexed sequences were then selected for exact sequence variant (ESV) picking using the DeBlur pipeline19. To improve downstream network analysis, ESVs present in ten or fewer samples were removed. The data were also rarefied to a depth of 10000 reads per sample. The α and β diversity were analyzed using the Phyloseq and MicrobiomeSeq package in the statistical software R (R Foundation for Statistical Computing, Vienna, Austria). The Shannon index was used for evaluation of α diversity, and β diversity was analysed using non-metric multidimensional scaling plots that were generated based on a weighted UniFrac dissimilarity matrix. Assessment of the statistical significance of α and β diversity trends was performed using permutational ANOVA. To determine significantly different ESVs between groups of interest, the analyses of composition of microbiome pipeline was used, with a P value (false discovery rate, FDR) cut-off of less than 0.050.
Enterococcus culture analysis for collagenolytic activity
Anastomotic tissues were plated on selective media for Enterococcus (Enterococcosel™ agar medium; BD Difco, Franklin Lakes, New Jersey, USA) with the addition of 15 per cent skimmed milk, prepared as described previously9. Enterococcus collagenolytic species producing black colonies surrounded by a clearing halo were counted, and colony-forming unit counts were normalized to the tissue weight.
Statistical analysis
Data are reported as mean(s.d.) unless indicated otherwise, with statistical significance determined by ANOVA or t test, as appropriate. P < 0.050 was considered statistically significant, unless stated otherwise. Statistical analysis for the AHS was performed on the mean score by group and POD using ANOVA.
Results
WD alters gut function and delays anastomotic healing
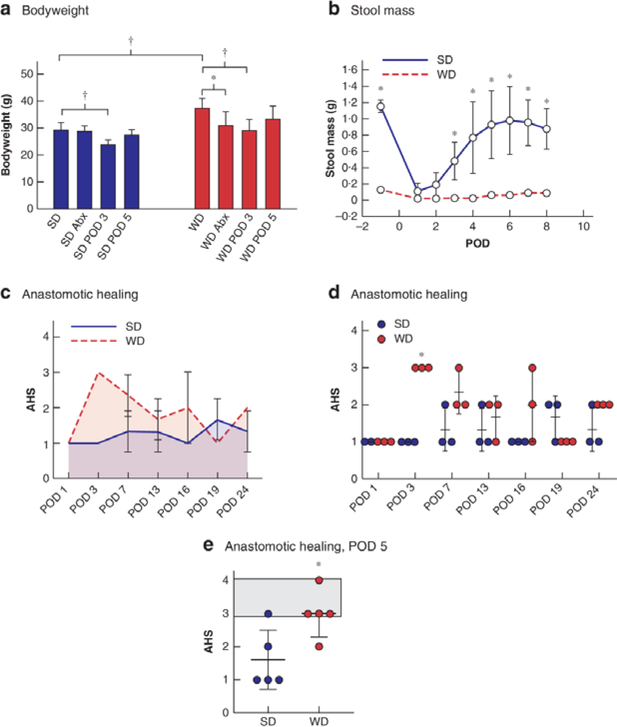
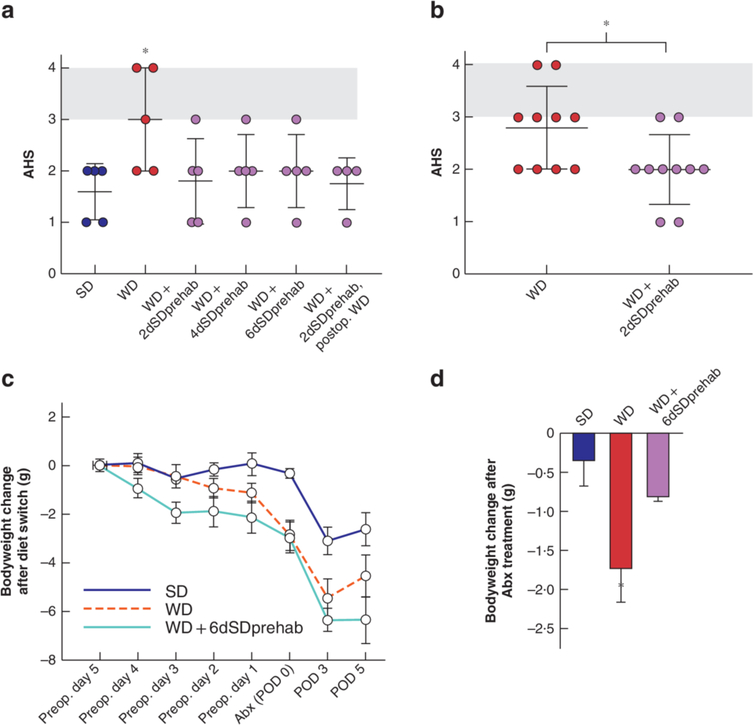
Consuming a WD resulted in a significant increase in bodyweight (Fig. 2a). WD mice showed increased weight loss after antibiotics compared with SD mice. By POD 3, both groups had experienced similar weight loss. By POD 5, weights in both groups had normalized to preoperative values. The WD resulted in a significant decrease in preoperative and postoperative stool mass (Fig. 2b). The stool mass in SD mice decreased significantly after surgery, but recovered to preoperative levels by POD 7. The AHS differed significantly between SD and WD groups across the entire study (P = 0.003) (Fig. 2c), with the most pronounced difference evident on POD 3 (P < 0.001) (Fig. 2d). Given the differences in AHS on POD 3, a further comparison was made of WD and SD groups on POD 5 (5 mice per group), which revealed a significant difference in AHS between the groups (Fig. 2e).
Fig.2. WD mice developed altered gut function and anastomotic healing.
a Mean(s.d.) bodyweight of mice after 6 weeks on a standard (SD) or Western (WD) diet. Abx, after antibiotics; POD, postoperative day. *P < 0.050, †P < 0.010 (unpaired 2-tailed t test, 5 mice per group). b Longitudinal analysis of stool mass. F (1,8) = 33.87, P < 0.001 between groups (2-way ANOVA; 30 per group at the start of the experiment, decreasing to 3 per group at the end as mice were killed every 3 days). *P < 0.050 versus WD (Student’s t test). c Longitudinal analysis of anastomotic healing score (AHS), showing area under the curve for AHS. F (1,28) = 18.78, P < 0.001 between groups (2-way ANOVA). d Mean(s.d.) AHS and individual values. *P < 0.001 versus SD (t test). e Mean(s.d.) AHS on POD 5 in separate groups of WD and SD mice exposed to antibiotics and colorectal surgery. Shaded area indicates anastomotic leak. *P = 0.021 versus SD (t test). Four of five WD-fed mice had an AHS of 3 (abscess) or 4 (peritonitis), whereas only one of five of SD-fed mice had an AHS of 3 (abscess).
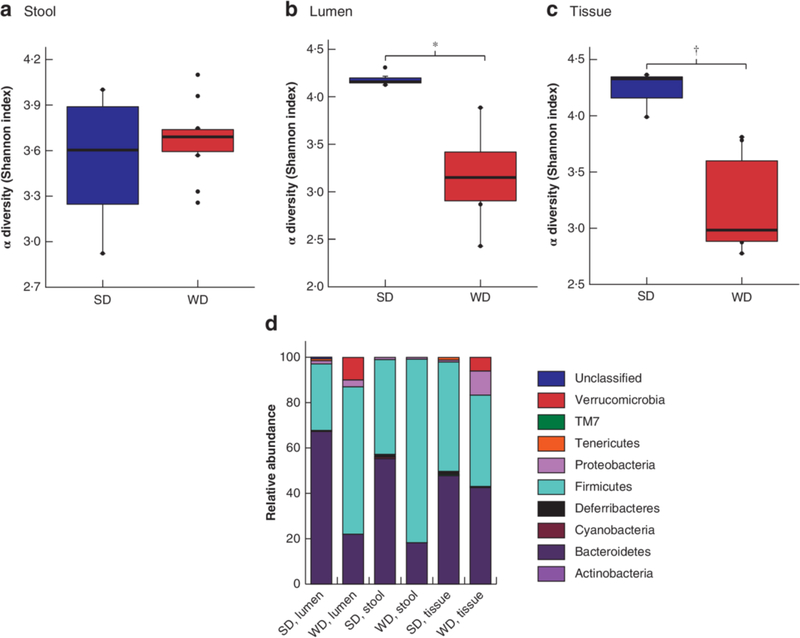
WD shifts the colonic microbiota before surgery
Loss of diversity in both luminal content and colonic tissue was observed in WD mice (Fig. 3a–c). WD mice had a significant shift in microbiota composition compared with SD mice, characterized by a significant decrease in Bacteroidetes in luminal content and expelled stool, and emergence of Proteobacteria in colonic tissue (Fig. 3d). Enterococcus abundance was low in both groups: 0.5 per cent in the tissue of WD mice and 0.008 per cent in that of SD mice (P = 0.001).
Fig.3. 16S ribosomal RNA analysis of colonic microbiota in preoperative samples.
a–c Comparison of α diversity, based on Shannon index, in standard chow (SD)- and Western (WD)-fed mice before surgery and antibiotic exposure in expelled stool (a), colonic lumen (b) and colonic tissue (c). Mean values (bold lines), i.q.r. (boxes), and range (error bars) excluding outliers (dots) are shown. *P = 0.034, †P = 0.005 (permutational ANOVA). d Relative abundance plots at the level of phyla.
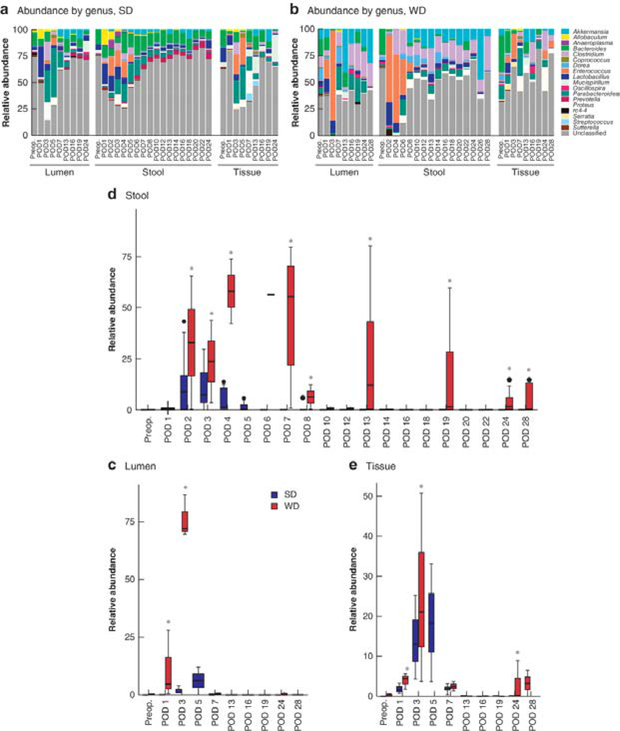
Longitudinal tracking of microbiota in expelled stool identified domination by Enterococcus in WD mice
Three compartments were analysed using 16S rRNA: expelled stool, colonic luminal content and anastomotic tissue. To determine whether expelled stool could serve as a trackable biomarker in this model, all three compartments were compared and analysed. In the expelled stool and luminal content of WD mice, there was a dominance of Enterococcus which comprised 65–90 per cent of the microbiota within the first week after surgery (Fig. 4b). In comparison, in SD mice, Enterococcus accounted for 4–15 per cent of the microbiota in expelled stool and luminal content (P = 0.013 for stool, P = 0.010 for lumen) (Fig. 4a,c,d). At the tissue level, differences in the relative abundance of Enterococcus were not as pronounced, but Enterococcus was still significantly more abundant in WD mice (Fig. 4e). Enterococcus could be detected up to 1 month in expelled stool and anastomotic tissues in WD mice, but was completely absent from SD mice after POD 5 (Fig. 4d,e). In summary, WD mice had a spike in Enterococcus within the first week after surgery and the increase persisted for up to 1 month after surgery. These changes were consistent between tissue and expelled stool, thus validating expelled stool as a potential marker of microbiota status following colonic surgery. Finally there was a notable loss of Bacteroides coupled with an increase in Akkermansia and Clostridium in the stool of WD mice (P < 0.050, FDR) (Fig. 4b).
Fig.4. Longitudinal 16S ribosomal RNA analysis of colonic microbiota in postoperative samples.
a,b Relative abundance (rarefied to 10 000 reads/sample) of bacterial taxa at the genus level in lumen, expelled stool and tissue microbiota of mice fed standard chow (SD) (a) and Western diet (WD) (b). c–e Relative abundance of Enterococcus in lumen (c), expelled stool (d) and tissue (e) microbiota. Mean values (bold lines), i.q.r. (boxes), and range (error bars) excluding outliers (dots) are shown. *P < 0.050 versus SD (analysis of composition of microbiome (ANCOM), with Benjamini–Hochberg false discovery rate correction). There was a significant difference in relative abundance of Enterococcus over time for all sample types in each group (P < 0.050, ANCOM), demonstrating a significant flux of Enterococcus at certain times in both SD and WD groups. Preop., preoperative.
Composition of the colonic microbiota is restored in SD- but not WD- fed mice
Using weighted Unifrac as a measure of β diversity, microbiota composition was noted to be significantly altered immediately after surgery in both SD and WD fed mice (Fig.5). However, β diversity appeared to progress toward preoperative levels within the first two weeks after surgery in SD mice (Fig. 5A). This was in contrast to the persistent disturbance of β diversity for up to 4 weeks after operation in WD mice (Fig.5B).
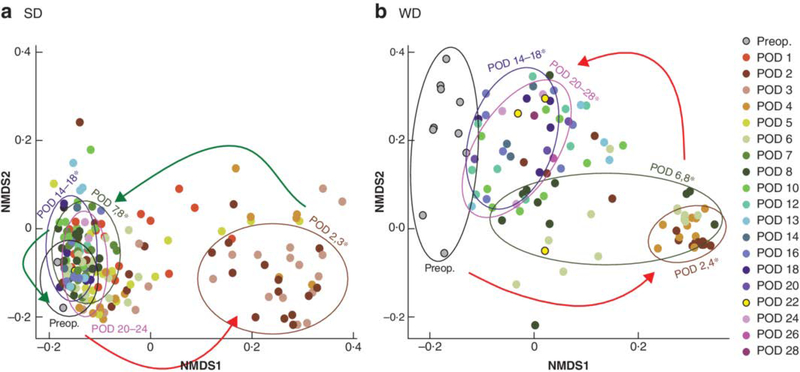
Fig.5. 16S ribosomal RNA analysis for restoration of microbiota after disruption caused by surgery.
Weighted UniFrac-based non-metric multidimensional scaling (NMDS) plots of expelled stool microbial community composition by time in mice fed a standard chow (SD) and b Western (WD) diet. Red arrows indicate disruption of microbiota in the early postoperative phase (postoperative day (POD) 2,3). Green arrows depict reversal in the microbiota toward that of the preoperative (preop.) phase. *P < 0.050, false discovery rate, for postoperative versus preoperative composition permutational ANOVA). There was a significant difference in surgery-related influences on microbial composition in SD (P = 0.010) and WD (P = 0.020) groups (permutational ANOVA).
Collagenolytic Enterococcus proliferates to a high degree in WD mice
Taken that previous studies demonstrated a role for collagenolytic Enterococcus in the pathogenesis of anastomotic leak, culture analysis for collagenolytic Enterococcus was undertaken. Anastomotic tissues were cultured on Enterococcosel™ agar medium with the addition of skimmed milk9. Both WD and SD mice were equally colonized by collagenolytic populations of Enterococcus on POD 3. However, by POD 5, the density of collagenolytic Enterococcus had decreased in SD mice, but had increased further in WD mice (Fig. 6).
Fig.6. Collagenolytic potential of Enterococcus population in anastomotic tissue.
Fold increase in collagenolytic Enterococcus relative to preoperative level in mice fed a standard chow (SD) and b Western (WD) diet. *P = 0.022 versus SD (unpaired 2-tailed t test, 5 per group).
Short-term prehabilitation on SD reverses the adverse effect of chronic WD feeding on anastomotic healing
To determine whether short-term preoperative switching from a WD to a SD affects anastomotic healing, mice fed a WD for 6 weeks were switched to SD for 2, 4 or 6 days before antibiotic treatment and colonic surgery (Fig. 1b); these groups were termed WD + 2dSDprehab, WD + 4dSDprehab and WD + 6dSDprehab respectively. Independent of the duration of SD feeding, there was a significant improvement in anastomotic healing on POD 5 (Fig. 7a). To account for the effect of the chow-based postoperative diet, a separate group of mice received the WD after surgery, resulting in an equally protective effect on anastomotic healing. Taken together, these results indicate that short-term consumption of a SD in the preoperative phase had a significant and durable effect on anastomotic healing independent of the postoperative diet.
Fig.7. Preoperative short-term switch to standard diet improves anastomotic healing in mice chronically fed a Western diet.
a Mean(s.d.) anastomotic healing score (AHS) on postoperative day (POD) 5 in standard chow (SD) and Western (WD) diet groups, and in mice fed a WD for 6 weeks followed by SD for 2 days (WD + 2dSDprehab), 4 days (WD + 4dSDprehab) or 6 days (WD + 6dSDprehab) before antibiotic treatment, and those that received a WD for 6 weeks followed by SD for 2 days before antibiotic treatment and switching back to WD after colonic surgery (WD + 2dSDprehab, postop. WD). *P = 0.025 versus WD + 2dSDprehab (unpaired t test, 5 per group). b Mean(s.d.) AHS on POD 5. *P = 0.025 (unpaired t test, 10 per group). c,d Effects of prehabilitation on changes in bodyweight, demonstrating reduction in weight (c) and reduced drop in weight after antibiotic (Abx) treatment (difference between preoperative (preop.) day 1 and POD 0) (d) in WD + 6dSDprehab group. *P < 0.010 versus WD + 6dSDprehab (t test, 5 per group).
Given the improvement in AHS in the WD + 2dSDprehab group, additional studies were conducted to confirm the reversal effect with ten mice per group. Six of ten WD-fed mice developed an AHS score of 3 or higher (indicating clinically significant leakage) compared with two of ten in the WD +2dSDprehab group (P = 0.025) (Fig. 7b). Prehabilitation with SD had two main effects on mouse weight: weight loss (Fig. 7c), and significant attenuation of antibiotic-induced weight loss (Fig. 7d).
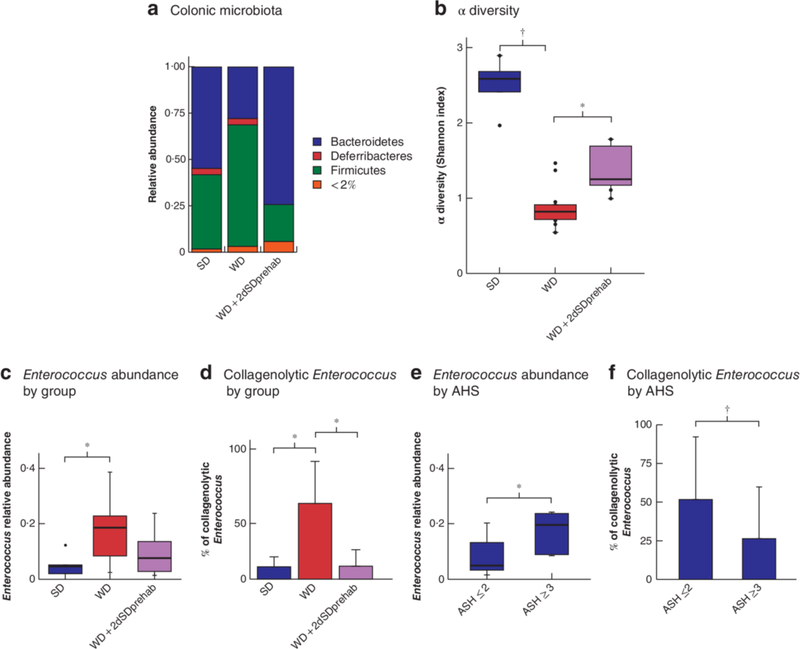
Short-term SD preoperative feeding restores Bacteroidetes in chronic WD- fed mice, improves overall diversity and decreases postoperative collagenolytic Enterococcus
Short-term preoperative SD feeding attenuated WD-induced distortion of the colonic microbiota composition, evident by maintaining the abundance of Bacteroidetes in the colonic lumen of preoperative samples (P = 0.029 for SD versus WD; P < 0.001 for WD versus WD + 2dSDprehab group) (Fig. 8a). Short-term dietary prehabilitation was also able to minimize the loss of diversity in the colonic microbiota seen in WD mice (Fig. 8b). The total relative abundance of Enterococcus in tissues in the WD + 2dSDprehab group was decreased compared with that in mice maintained on a WD (Fig. 8c), although the difference was not statistically significant. However, a significant decrease in the collagenolytic population of Enterococcus was observed in anastomotic tissues from WD + 2dSDprehab mice (P < 0.001 for SD versus WD; P < 0.001 for WD versus WD + 2dSDprehab) (Fig. 8d). Comparisons between leaking and non-leaking anastomoses across all groups showed a statistically significant difference in both the overall relative abundance of Enterococcus (P = 0.001) (Fig. 8e) and percentage of collagenolytic Enterococcus (P = 0.038) (Fig. 8f).
Fig.8. Preoperative short-term switch to standard diet prevents Western diet-mediated distortion of colonic microbiota.
a–d Comparison of relative abundance of bacteria in standard chow (SD) and Western (WD) diet groups, and in mice fed a WD for 6 weeks followed by SD for 2 days (WD + 2dSDprehab). a Relative abundance plots at the level of phyla in preoperative colonic lumen microbiota. b α diversity based on Shannon index comparing colonic lumen microbiota on postoperative day (POD) 5. *P = 0.016, †P < 0·001 (permutational ANOVA). c Relative abundance of Enterococcus in anastomotic tissue on POD 5 by 16S rRNA analysis. *P = 0.037 (ANOVA, Tukey honestly significant difference (HSD) test, 5 in SD group, 15 in WD group, 15 in WD + 2SDprehab group). d Mean(s.d) percentage of collagenolytic colonies among total Enterococcus populations in colonic lumen and anastomotic tissue microbiota on POD 5. *P < 0.001 (ANOVA, Tukey HSD test). e,f Relative abundance of Enterococcus (e) and mean(s.d.) percentage of collagenolytic Enterococcus (f) on POD 5 according to anastomotic healing: clinically relevant leaking (anastomotic healing score (AHS) 3 or 4) versus non-leaking (AHS 0–2) anastomoses. *P = 0.001, †P = 0.038 (Student’s t test, 12 in leaking group, 23 in non-leaking group). Box plots in b,c and e show mean values (bold line), i.q.r. (boxes), and range (error bars) excluding outliers (dots).
Discussion
Historically, preoperative dietary modulation to reduce postoperative complications has been a major consideration among surgeons, but mostly involves the use of chemically defined, liquid sterile supplements. However, their effect size on postoperative complications seems to be diminishing, given the advances in anaesthesia and pain control, and use of minimally invasive techniques and enhanced recovery programmes. It may be important to recognize that the current recommendation of enhanced recovery programmes specifically suggests ‘solid’ food to be provided to patients after surgery20. Therefore, it may be time to consider the consumption of customary unprocessed foodstuffs beyond their effect on BMI, serum protein markers and other standard nutritional parameters15,21.
Although obesity increases the probability of postoperative healing complications, especially infections, it is important to recognize that the majority of obese patients do not develop complications and non-obese patients can experience serious complications including infections22. In this regard, independent of their ability to increase BMI, high-fat/low-fibre diets may increase the risk of postoperative complications, which in the present report has been demonstrated for anastomotic leakage. That this effect may be mediated, in part, by alterations to the microbiome bears further consideration.
The authors’ laboratory has provided compelling evidence that bacteria with the capacity to produce collagenase can play a key and causal role in anastomotic leak. First, Enterococcus faecalis and Pseudomonas aeruginosa were identified as two highly collagenolytic bacteria capable of causing leaks in both rats and mice8,23. The discovery of E. faecalis as a leak pathogen is particularly important as it is the most common pathogen to be cultured from a leaking anastomosis in patients3,24–26 and the most difficult to eradicate from the intestinal tract27,28. Although E. faecalis is classified as a low-abundance commensal species among the normal microbiota, when properly provoked, it can bloom and behave as a virulent pathogen29–31.
The present study has demonstrated an important link between diet, antibiotic exposure and anastomotic surgery in terms of their aggregate effect on the microbiome and their potential role in anastomotic healing. Data from this study suggest that a high-fibre/low-fat diet like standard rodent chow can preserve the health-promoting Bacteroidetes, which may competitively exclude and suppress pathogens such as E. faecalis and P. aeruginosa from impairing anastomotic healing32. Although obesity, defined by BMI, has been identified as a risk factor for postoperative infection-related complications33, diet quality may be a more relevant parameter to consider given that a high-fat/low-fibre diet can change the microbiome independent of its effect on weight34. The ability to rapidly shift the observed microbiota imbalance in WD-fed mice with only 2 days of dietary prehabilitation with a low-fat/high-fibre chow-based diet is not unexpected, given the wealth of evidence demonstrating the near-immediate effect of a dietary change on the animal and human microbiome35.
An important finding of the present study was the fidelity in the 16S rRNA analysis (community structure and membership) between expelled stool, luminal content and anastomotic tissues. This potentially validates the use of expelled stool to determine a patient’s microbiome status before surgery and modulate the diet accordingly.
This study has some limitations, including that the WD used was completely lacking fibre, in contrast to a standard low-fibre WD. This was done to amplify the effects of fibre in the model. It is important to note that Western dietary fibre typically consists of non-fermentable sources of little benefit to the microbiota36. As with any murine study, translation to humans requires validation as there are major variations between mouse and human microbiomes; however, the global changes of the microbiota to diet and antibiotics in human and mice tend to be similar37.
Supplementary Material
Acknowledgements
S.K.H., C.A. and K.W. are joint first authors; H.v.G., O.Z. and J.C.A. are senior authors. The work was supported by Honours student grants, Radboud University Medical Centre Health Academy, and in part by National Institutes for Health grant R01-GM062344-18 (J.C.A.). All data generated or analysed during this study are included in this published article and in the supporting information. The data sets generated are available in the European Bioinformatics Institute database (project accession number PRJEB31116, accession number ERP113631).
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Cohn I, Rives JD. Antibiotic protection of colon anastomoses. Ann Surg 1955; 141: 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bothin C, Okada M, Midtvedt T, Perbeck L. The intestinal flora influences adhesion formation around surgical anastomoses. Br J Surg 2001; 88: 143–145. [DOI] [PubMed] [Google Scholar]
- 3.Gaines S, Shao C, Hyman N, Alverdy JC. Gut microbiome influences on anastomotic leak and recurrence rates following colorectal cancer surgery. Br J Surg 2018; 105: e131–e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mik M, Berut M, Trzcinski R, Dziki L, Buczynski J, Dziki A. Preoperative oral antibiotics reduce infections after colorectal cancer surgery. Langenbecks Arch Surg 2016; 401: 1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schardey HM, Joosten U, Finke U, Staubach KH, Schauer R, Heiss A et al. The prevention of anastomotic leakage after total gastrectomy with local decontamination. A prospective, randomized, double-blind, placebo-controlled multicenter trial. Ann Surg 1997; 225: 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeVeen HH, Wapnick S, Falk G, Olivas O, Bhat D, Gaurdre M et al. Effects of prophylactic antibiotics on colonic healing. Am J Surg 1976; 131: 47–53. [DOI] [PubMed] [Google Scholar]
- 7.Shogan BD, Smith DP, Christley S, Gilbert JA, Zaborina O, Alverdy JC. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome 2014; 2: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shogan BD, Belogortseva N, Luong PM, Zaborin A, Lax S, Bethel C et al. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med 2015; 7: 286ra268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiegerinck M, Hyoju SK, Mao J, Zaborin A, Adriaansens C, Salzman E et al. Novel de novo synthesized phosphate carrier compound ABA-PEG20k-Pi20 suppresses collagenase production in Enterococcus faecalis and prevents colonic anastomotic leak in an experimental model. Br J Surg 2018; 105: 1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shakhsheer BA, Versten LA, Luo JN, Defazio JR, Klabbers R, Christley S et al. Morphine promotes colonization of anastomotic tissues with collagenase-producing Enterococcus faecalis and causes leak. J Gastrointest Surg 2016; 20: 1744–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okada M, Bothin C, Kanazawa K, Midtvedt T. Experimental study of the influence of intestinal flora on the healing of intestinal anastomoses. Br J Surg 1999; 86: 961–965. [DOI] [PubMed] [Google Scholar]
- 12.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science 2012; 336: 1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiao Y, Sun J, Xie Z, Shi Y, Le G. Propensity to high-fat diet-induced obesity in mice is associated with the indigenous opportunistic bacteria on the interior of Peyer’s patches. J Clin Biochem Nutr 2014; 55: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol 2010; 299: G440–G448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frasson M, Flor-Lorente B, Rodríguez JL, Granero-Castro P, Hervás D, Alvarez Rico MA et al. ; ANACO Study Group. Risk factors for anastomotic leak after colon resection for cancer: multivariate analysis and nomogram from a multicentric, prospective, national study with 3193 patients. Ann Surg 2015; 262: 321–330. [DOI] [PubMed] [Google Scholar]
- 16.Hyoju SK, Klabbers RE, Aaron M, Krezalek MA, Zaborin A, Wiegerinck M et al. Oral polyphosphate suppresses bacterial collagenase production and prevents anastomotic leak due to Serratia marcescens and Pseudomonas aeruginosa. Ann Surg 2018; 267: 1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 2012; 6: 1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010; 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amir A, McDonald D, Navas-Molina JA, Kopylova E, Morton JT, Zech Xu Z et al. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems 2017; 2: e00191–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr 2017; 36: 623–650. [DOI] [PubMed] [Google Scholar]
- 21.Aiolfi A, Asti E, Rausa E, Bonavina G, Bonitta G, Bonavina L. Use of C-reactive protein for the early prediction of anastomotic leak after esophagectomy: systematic review and Bayesian meta-analysis. PLoS One 2018; 13: e0209272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tjeertes EK, Hoeks SE, Beks SB, Valentijn TM, Hoofwijk AG, Stolker RJ. Obesity – a risk factor for postoperative complications in general surgery? BMC Anesthesiol 2015; 15: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivas AD, Shogan BD, Valuckaite V, Zaborin A, Belogortseva N, Musch M et al. Intestinal tissues induce an SNP mutation in Pseudomonas aeruginosa that enhances its virulence: possible role in anastomotic leak. PLoS One 2012; 7: e44326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belmouhand M, Krohn PS, Svendsen LB, Henriksen A, Hansen CP, Achiam MP. The occurrence of Enterococcus faecium and faecalis is significantly associated with anastomotic leakage after pancreaticoduodenectomy. Scand J Surg 2018; 107: 107–113. [DOI] [PubMed] [Google Scholar]
- 25.Ohigashi S, Sudo K, Kobayashi D, Takahashi T, Nomoto K, Onodera H. Significant changes in the intestinal environment after surgery in patients with colorectal cancer. J Gastrointest Surg 2013; 17: 1657–1664. [DOI] [PubMed] [Google Scholar]
- 26.Hirst NA, Tiernan JP, Millner PA, Jayne DG. Systematic review of methods to predict and detect anastomotic leakage in colorectal surgery. Colorectal Dis 2014; 16: 95–109. [DOI] [PubMed] [Google Scholar]
- 27.Jahansepas A, Aghazadeh M, Rezaee MA, Hasani A, Sharifi Y, Aghazadeh T et al. Occurrence of Enterococcus faecalis and Enterococcus faecium in various clinical infections: detection of their drug resistance and virulence determinants. Microb Drug Resist 2018; 24: 76–82. [DOI] [PubMed] [Google Scholar]
- 28.Goh HMS, Yong MHA, Chong KKL, Kline KA. Model systems for the study of enterococcal colonization and infection. Virulence 2017; 8: 1525–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christoffersen TE, Jensen H, Kleiveland CR, Dørum G, Jacobsen M, Lea T. In vitro comparison of commensal, probiotic and pathogenic strains of Enterococcus faecalis. Br J Nutr 2012; 108: 2043–2053. [DOI] [PubMed] [Google Scholar]
- 30.Foulquié Moreno MR, Sarantinopoulos P, Tsakalidou E, De Vuyst L. The role and application of enterococci in food and health. Int J Food Microbiol 2006; 106: 1–24. [DOI] [PubMed] [Google Scholar]
- 31.de Almeida CV, Taddei A, Amedei A. The controversial role of Enterococcus faecalis in colorectal cancer. Therap Adv Gastroenterol 2018; 11: 1756284818783606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wexler AG, Goodman AL. An insider’s perspective: Bacteroides as a window into the microbiome. Nat Microbiol 2017; 2: 17026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierpont YN, Dinh TP, Salas RE, Johnson EL, Wright TG, Robson MC et al. Obesity and surgical wound healing: a current review. ISRN Obes 2014; 2014: 638936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009; 137: 1716.e2–1724.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505: 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab 2014; 20: 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen TL, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis Model Mech 2015; 8: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.