Abstract
Background
Atopic dermatitis (AD) is characterized by chronic, relapsing skin inflammation (eczema) with itchy sensation. Keratinocytes, which are located at the outermost part of our body, are supposed to play important roles at the early phase of type 2 inflammation including AD pathogenesis.
Objective
The purpose of this study was to evaluate whether keratinocytes-derived reactive oxygen species (ROS) could be produced by the allergens or non-allergens, and the keratinocytes-derived ROS could modulate a set of biomarkers for type 2 inflammation of the skin.
Methods
Normal human epidermal keratinocytes (NHEKs) were treated with an allergen of house dust mites (HDM) or a non-allergen of compound 48/80 (C48/80). Then, biomarkers for type 2 inflammation of the skin including those for neurogenic inflammation were checked by reverse transcriptase-polymerase chain reaction and western immunoblot experiments.
Results
HDM or C48/80 was found to upregulate expression levels of our tested biomarkers, including type 2 T helper-driving pathway (KLK5, PAR2, and NFκB), epithelial-cell-derived cytokines (thymic stromal lymphopoietin, interleukin [IL]-25, IL-33), and neurogenic inflammation (NGF, CGRP). The HDM- or C-48/80-induced expression levels of the biomarkers could be blocked by an antioxidant treatment with 5 mM N-acetyl-cysteine. In contrast, pro-oxidant treatment with 1 mM H2O2 could upregulate expression levels of the tested biomarkers in NHEKs.
Conclusion
Our results reveal that keratinocytes-derived ROS, irrespective to their origins from allergens or non-allergens, have a potential to induce type 2 inflammation of AD skin.
Keywords: Atopic dermatitis, House dust mite, Keratinocytes, Reactive oxygen species, Type 2 inflammation of the skin
INTRODUCTION
Atopic dermatitis (AD) is caused by type 2 inflammation encompassing type 2 T helper (Th2) response in the skin, manifesting with recurrent attacks of skin inflammation with itching sensation (pruritus). AD can be induced or aggravated by a wide variety of factors, including allergenic (such as house dust mites [HDM]) and non-allergenic factors, such as pathogens, chemicals, and environmental pollutants. Innate and adaptive immune cells (mast cells, basophils, eosinophils, Langerhans cells, macrophage, and lymphocytes) have been reported as major cells that induce the type 2 immune response of AD skin1,2,3,4,5. A triad of epithelial-cell-derived cytokines, i.e., thymic stromal lymphopoietin (TSLP), interleukin (IL)-25, and IL-33, which are produced from keratinocytes, are regarded as crucial mediators that induce type 2 inflammation in the AD skin6. They can activate not only adaptive immune cells, including mast cells, basophils, and eosinophils, but also innate lymphoid cells (ILCs), including invariant natural killer T (iNKT) cells and innate type-2 lymphoid cells (ILC2)7. Taken together, keratinocytes are actively implicated in the immune response, as they are located at the outermost layer of the skin to respond to various environmental factors in the AD skin7,8,9.
TSLP and IL-33 are critical cytokines that are expressed at the interface between the environment and our body with local and systemic immune responses9. They are potent activators of mast cells, leading to the production of type-2 cytokines and chemokines in the skin1. HDM induces type 2 inflammation by upregulating IL-25 and IL-33, while they activate toll-like receptors (TLRs) 1 and 6 signaling in keratinocytes8,10. IL-25-producing dendritic cells (DCs) induce type-2 cell response to inhibit filagrin synthesis, suggesting that IL-25 plays a role in bridging the gap between inflammation and skin barrier dysfunction in AD11. Collectively, keratinocytes are supposed to play an active role in the early stage of type 2 inflammation of the skin by producing epithelial-cell-derived cytokines of TSLP, IL-25, and IL-33, which play a key role to activate and/or attract innate immune cells, including cutaneous DC12. Kallikrein is expressed in the outermost layer of the epidermis to execute a biological role in maintaining the epidermal barrier13,14. Elevated expression levels of kallikrein 5 (KLK5), proteinase-activated receptor (PAR2), and TSLP were detected in an AD mouse model12. In humans, dysregulated KLK5 directly activates PAR2, which triggers TSLP expression in the AD-like skin lesions of Netherton syndrome15.
In relation with itchy sensation of AD patients, type 2 inflammation of the AD skin was reported to be mediated by a set of neurotransmitters, including cortisol, substance P (SP), and nerve growth factor (NGF)16. In the AD skin, the local release of such inflammatory mediators from afferent neurons induces ‘neurogenic inflammation’. The released neuropeptides stimulate adjacent mast cells to release histamine, which in turn evokes the release of SP and CGRP from nerve cells, forming a bidirectional interaction of nerves with mast cells17,18. Therefore, afferent C-fibers- or keratinocytes-releasing neuropeptides play an important role in producing itchy sensation as well as type 2 inflammation in the AD skin19,20,21. As an allergen to induce AD, HDM was reported to mediate neurogenic inflammation as well as Th2-type responses in the skin and spleen22. Clinically, AD is frequently aggravated by emotional stress, and expression levels of biomarkers for neurogenic inflammation were closely related to AD severity23,24,25,26. For this study, we selected NGF and CGRP as biomarkers for neurogenic inflammation as well as those for type 2 inflammation of the skin, which can be regarded as crucial mediators to induce itchy sensation in the AD skin.
Oxidative stress was reported to be one of factor to induce inflammatory conditions of AD including skin barrier dysfunction by modulating genes that code proinflammatory cytokines and structural proteins in the skin. For examples, reactive oxygen species (ROS) is implicated in the pathogenesis of type 2 inflammation in the airways of asthma and allergic rhinitis27 as well as in the skin with AD28,29,30,31. The increase of ROS could be detected in the circulating system of AD patients30,31. HDM has been reported to be a source of ROS from immune-related or inflammatory cells, inducing Th2 response in AD or asthma. Der-f1-producing ROS activated ERK and p38 MAPK phosphorylation in human basophilic cells32. Der-f or Der-f1 could induce the production of ROS from neutrophils of asthmatic patients33. Unfortunately, there is still controversial on the role of oxidative stress in the pathogenesis of type 2 inflammation in the skin including AD, partly due to the low number of studies. In this study, we evaluated whether HDM (allergen) or compound 48/80 (non-allergen) could be sources for keratinocytes-derived ROS, and further evaluated the role of ROS in the pathogenesis of type 2 inflammation in the skin. For the purpose, we checked the expression level of the following biomarkers for type 2 inflammation in the skin in normal human epidermal keratinocytes (NHEKs), which were treated with HDM- or C48/80: Th2-driving pathway (KLK5, PAR2, and NFκB); epithelial-cell-derived cytokines (TSLP, IL-25, and IL-33); and neurogenic inflammation (NGF and CGRP). To verify the role of ROS in type 2 inflammation in the skin, the effect of N-acetyl cysteine (NAC), an antioxidant and hydrogen peroxide (H2O2), a pro-oxidant, were evaluated in NHEKs.
MATERIALS AND METHODS
Cell and culture
NHEKs (C0015C) were purchased from EpiLife (Cascade Biologics, Portland, OR, USA). The cells were cultured in basal keratinocyte growth media (EpiLife) supplemented with human keratinocyte growth supplement and antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin) in a 5% CO2 incubator. Passages 2~9 were used for all experiments.
MTT asay
To test cell viability, NHEKs were seeded into 96-well plates and treated with different concentrations of HDM and C48/80 for 24 hours. Cell viability was assayed with the colorimetric 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide (MTT) kit (Chemicon International, Inc., Billerica, MA, USA), according to the manufacturer's instructions. Briefly, 10 mL of MTT solution was added to each well (0.5 mg/ml), and plates were incubated at 37℃ for 2 hours. The resulting formazan crystals were dissolved with 100 ml of dimethylsulfoxide, and absorbance was read at 570 nm in an ELISA reader (Molecular Devices, Sunnyvale, CA, USA).
Measurement of ROS
Fluorogenic 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA; Invitrogen, Carlsbad, CA, USA) reacts with oxidant species and can be detected by confocal microscopy. Briefly, NHEKs were seeded in each well of a 60-mm culture dish at a density of 5×105 cells per well and were cultured up to approximately 70% confluence. Cells were treated with HDM (10 mg/ml) or C48/80 (10 µg/ml) for 6 hours in the absence and presence of NAC, and the intracellular ROS produced was detected with a confocal microscope using DCF-DA dye. Counter staining was performed with 4′,6-diamidino-2-phenylindole (DAPI), and fluorescence signals were detected with wavelengths of 492 to 495 nm for excitation and 517 to 527 nm for emission. Images were visualized using a confocal microscope with a 409 objective lens on the laser-scanning microscope (LSM 510; Carl Zeiss, Jena, Germany), and analyzed by the LSM 5 browser imaging software (ZEN 2.6 blue edition; Carl Zeiss).
Reverse transcriptase-polymerase chain reaction
Total RNA was isolated using the RNeasy mini kit (Qiagen, Fremont, CA, USA), according to the manufacturer's instructions. cDNA was generated from 1 µg of total RNA using the Omniscript RT kit (Qiagen). A polymerase chain reaction (PCR) pre-mixture kit (ELPIS, Daejeon, Korea) was used for reverse transcriptase-PCR (RT-PCR). PCR reactions were performed with the primers for each enzyme shown in Supplementary Table 1. PCR products were analyzed using 1.5% agarose gel electrophoresis, stained with Sybr Safe DNA gel stain buffer (Invitrogen), and visualized by luminescence (LAS 3000; Fujifilm, Tokyo, Japan). Expression levels were normalized to endogenous GAPDH as a control.
Western blot analysis
Nuclear and cytosolic extracts were collected with the NE-PER nuclear and cytoplasmic extraction reagents (Pierce, Rockford, IL, USA). For Western blotting, protein bands were probed with the antibodies to anti-Phospho NFκB p65 (Ser536, 1:1,000; Cell Signaling Technology, Danvers, MA, USA), anti-IkBα (sc-1643, 1:500; Santa Cruz Biotechnology, Dallas, TX, USA) or anti-lamin B (C-20, 1:200; Santa Cruz Biotechnology) overnight at 4℃. HRP-conjugated rabbit anti-mouse immunoglobulin G (IgG) (1:5,000; Santa Cruz Biotechnology) were used for secondary antibodies. The protein bands were visualized by luminescence (LAS 3000, Fujifilm). Densitometric analyses were performed by using the Multi Gauge V3.0 software (Fujifilm). Expression levels were normalized to an endogenous control β-actin. All data represent three experiments.
Immunocytochemistry for NFκB
Immunocytochemistry for confocal microscopy was performed on NFκB. NHEKs were seeded in each well of the slide chamber at a density of 1×104 cells per well. The cells were treated with HDM (10 mg/ml) or C48/80 (10 µg/ml) for 6 hours. After being washed with phosphate buffered saline (PBS), cells were fixed in 4% paraformaldehyde in PBS for 10 minutes, and then permeabilized with PBS containing 0.5% Triton X-100 (PBS-T) for 15 minutes. The cells were incubated with PBS-T containing 1% bull serum albumin for 1 hour and stained with the anti-NFκB (1:150; Abcam, Cambridge, MA, USA) overnight at 4℃, followed by incubation with Alexa Fluor 488 goat anti-rabbit IgG (1:500; Invitrogen) for 1 hour. Images were visualized using confocal microscopy with a 20X objective on an LSM 510 laser scanning microscope (Carl Zeiss) and analyzed using the LSM 5 browser imaging software.
Statistics
All experiments were carried out in triplicate, and the results are expressed as the mean standard deviation. Comparisons between samples were carried out using Student's t-test or one-way analysis of variance (ANOVA) test with a post hoc Tukey's test using SPSS program ver. 13.0 (SPSS Inc., Chicago, IL, USA). p-value<0.05 was considered a statistically significant difference.
RESULTS
Setting-up the optimal experimental conditions to test biomarkers for type 2 inflammation of the skin in NHEKs
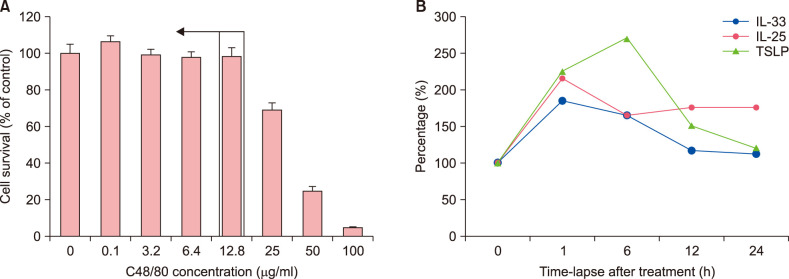
In our preliminary experiments using the MTT cell-viability assay, 0.1~12.8 µg/ml of C48/80 were the optimum without cytotoxicity to NHEKs (Fig. 1A). From the result, for the next experiments, NHEKs were treated with 10 µg/ml of C48/80. To decide on the optimal time-point for our experiments, we checked time-course changes in mRNA expression levels of the triad of epithelial cell-derived cytokines, i.e., TSLP, IL-25, and IL-33. When NHEKs were treated with 10 µg/ml of C48/80, the optimal time-point to evaluate their expression levels was found to be 1~6 hours after treatment, showing a change in mRNA expression levels of tested biomarkers in a time-dependent manner (Fig. 1B). From experiments repeated at least three times, the optimal time-point for each biomarker was found for the following experiments: 10 minutes for NFκB; 1 hour for KLK5, PAR2, IL-25, IL-33, and CGRP; 6 hours for TSLP and CGRP; and 12 hours for NGF (data not shown).
Fig. 1. Establishing the optimal dose and time-point for evaluating candidate biomarkers for type 2 inflammation of the skin. (A) To find out the non-toxic experimental dose of compound 48/80 (C48/80) for cells, MTT assay was performed in normal human epidermal keratinocytes (NHEKs), which were treated with different concentrations of C48/80 between 0.1 µg/ml and 100 µg/ml for 24 hours. (B) When 10 µg/ml of C48/80 was set as the optimal experimental dose for our experiments, expression levels of the triad of epithelial-cell-derived cytokines (TSLP, IL-25, IL-33) were measured by reverse transcriptase-polymerase chain reaction experiments in C48/80-treated NHEKs. From densitometric analysis, time-dependent changes in relative mRNA expression levels for the three cytokines are depicted. TSLP: thymic stromal lymphopoietin, IL: interleukin.
House dust mites or C48/80 induces the production of ROS from keratinocytes
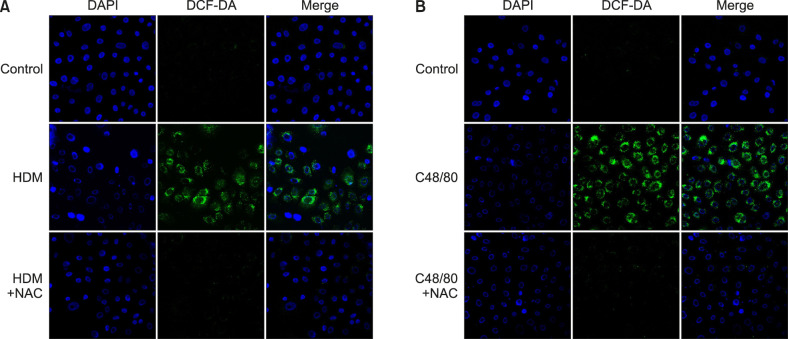
Besides HDM as an allergenic factor, several studies demonstrated that non-allergenic factors, such as diesel exhaust particles (DEP), cigarette smoke, and ultraviolet B (UVB) rays, could induce type 2 inflammation of AD32,34. From our hypothesis that ROS might be a common mediator to induce type 2 inflammation in the skin, we tested whether HDM (allergen) or C48/80 (non-allergen) could produce ROS from keratinocytes. In immunocytochemical staining, DCF-DA-positive signals were strongly detected in the cytoplasm of HDM-treated (Fig. 2A) or C48/80-treated (Fig. 2B) NHEKs. The signals were markedly suppressed by NAC treatment (5 mM), indicating that HDM or C48/80 is a source of ROS in keratinocytes (Fig. 2).
Fig. 2. HDM or C48/80 induces reactive oxygen species (ROS) production from keratinocytes. To find out whether HDM (allergen) or C48/80 (non-allergen) could produce ROS from normal human epidermal keratinocytes (NHEKs), cells were treated with HDM (10 mg/ml) or C48/80 (10 µg/ml), and DCF-DA-positive signals were detected with a confocal microscope. A DCF-DA (+) signal was detected from the (A) HDM-treated NHEKs or (B) C48/80-treated NHEKs in the absence or presence of NAC (+NAC). HDM: house dust mites, NAC: N-acetyl cysteine.
House dust mites or C48/80 upregulates KLK5 and PAR2, as they activate NFκB via ROS
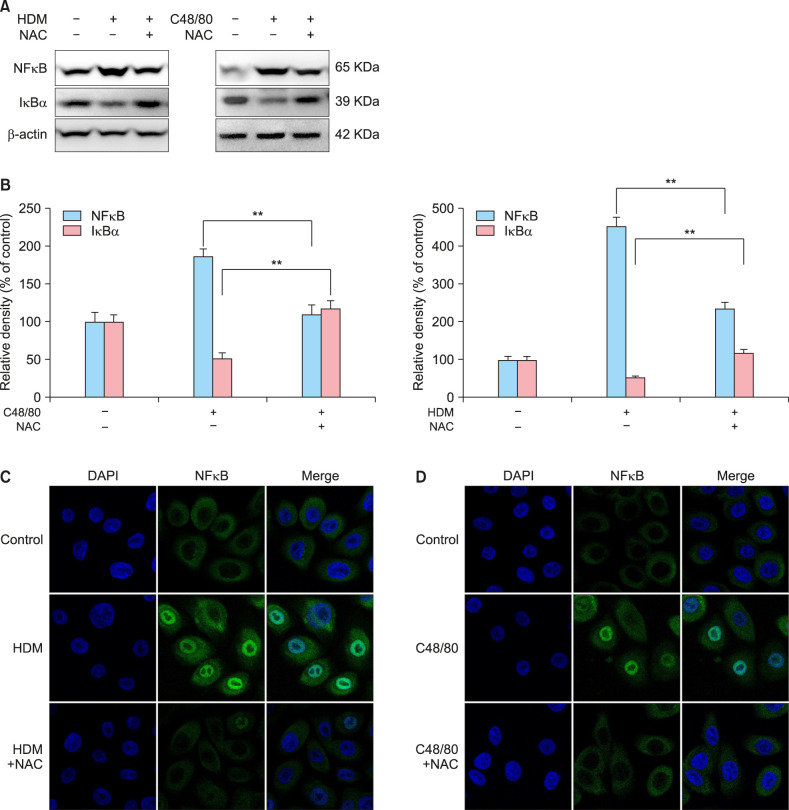
Next, we tested whether HDM or C48/80 could modulate the expression levels of KLK5, PAR2, and NFκB, which are known to drive type 2 inflammation in the skin. In RT-PCR experiments, HDM or C48/80 induced the upregulation of mRNA levels of KLK5 and PAR2, which could be inhibited by 5 mM NAC treatment (Fig. 3A), showing a statistically significant difference by densitometric analysis (p<0.05, n=3; Fig. 3B). In Western immunblots with a nuclear extract of NHEKs, NFκB protein expression was upregulated, but IκB, a NFκB inhibitor, protein expression was downregulated, by HDM or C48/80 treatment (Fig. 4A). The HDM- or C48/80-modulated expression levels of NFκB and IκB could be reversed by NAC treatment (Fig. 4A), showing a statistically significant change by densitometric analysis (p<0.01) (Fig. 4B). In immunocytochemical staining, NFκB was upregulated and translocated to the nuclei by HDM or C48/80 treatment, which could be blocked by NAC treatment (Fig. 4C, D), indicating that HDM or C48/80 activates NFκB via ROS in keratinocytes.
Fig. 3. HDM or C48/80 induces the upregulation of KLK5 and PAR2 as well as NFκB activation via reactive oxygen species. (A) Normal human epidermal keratinocytes (NHEKs) were treated with HDM (10 mg/ml; left panel) or C48/80 (10 µg/ml; right panel) in the absence (−) or presence (+) of NAC for 1 hour, and cells were harvested for reverse transcriptase-polymerase chain reaction (RT-PCR) experiments of KLK5 and PAR2 as described in the Materials and Methods. The pictures demonstrate the typical results of three repetitive RT-PCR experiments for KLK5 and PAR2 in the HDM-treated NHEKs (left panel) and C48/80-treated NHEKs (right panel). (B) Relative mRNA expression levels were semi-quantified by densitometric analysis. HDM: house dust mites, NAC: N-acetyl cysteine, KLK5: kallikrein 5, PAR2: proteinase-activated receptor 2. *p<0.05.
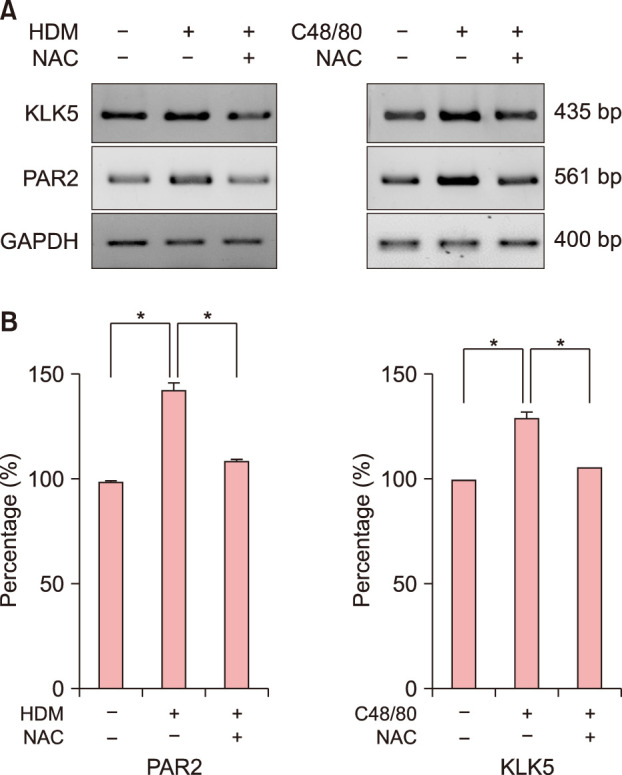
Fig. 4. HDM or C48/80 induces NFκB activation via reactive oxygen species. (A) For evaluating the activation of NFκB, nuclear extracts of cells were harvested to perform western immunoblots of NFκB and IκB. Normal human epidermal keratinocytes (NHEKs) were treated with HDM (10 mg/ml; left panel) or C48/80 (10 µg/ml; right panel) in the absence (−) or presence (+) of NAC. (B) Relative mRNA expression levels were semi-quantified by densitometric analysis. Left: HDM-treated NHEKs. Right: C48/80-treated NHEKs. Immunocytochemical staining for NFκB expression was performed in (C) HDM-treated NHEKs and (D) C48/80-treated NHEKs. HDM: house dust mites, NAC: N-acetyl cysteine, KLK5: kallikrein 5, PAR2: proteinase-activated receptor 2. **p<0.01.
House dust mites or C48/80 upregulates biomarkers for type 2 inflammation in the skin, including epithelial cell-derived cytokines and neurogenic inflammation, via keratinocytes-producing ROS
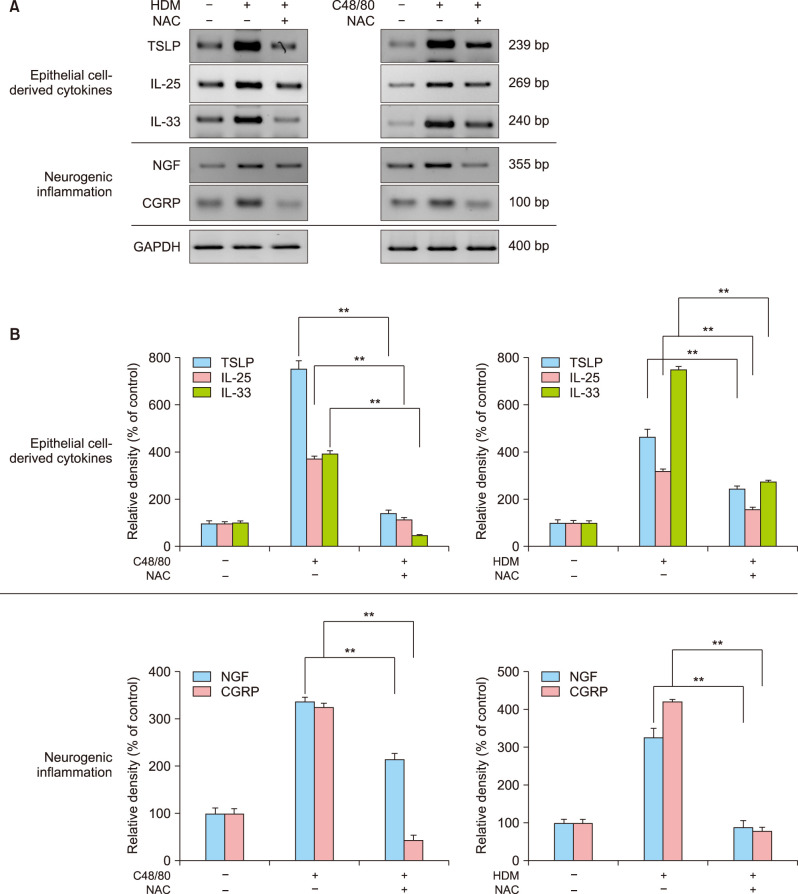
Next, we tested whether HDM or C48/80 could modulate biomarkers for type 2 inflammation of the skin. In RT-PCR experiments, HDM or C48/80 upregulated mRNA expression levels of epithelial-cell-derived cytokines, including TSLP, IL-25, and IL-33, which could be inhibited by NAC treatment (Fig. 5A). As biomarkers for neurogenic inflammation as well as for type 2 inflammation in the skin, HDM or C48/80 upregulated mRNA expression levels of NGF and CGRP, which could be inhibited by NAC treatment (Fig. 5A). In densitometric analysis, there was a statistically significant difference in mRNA expression levels between the two compared groups (p<0.01; Fig. 5B).
Fig. 5. HDM or C48/80 upregulates the biomarkers for epithelial cell-derived type 2 cytokines and neurogenic inflammation via reactive oxygen species. (A) Normal human epidermal keratinocytes (NHEKs) were treated with HDM (10 mg/ml, left panel) or C48/80 (10 µg/ml, right panel) in the absence (−) or presence (+) of NAC, and cell extracts were harvested to perform reverse transcriptase-polymerase chain reaction (RT-PCR) experiments for epithelial cell-derived type 2 cytokines (TSLP, IL-25, IL-33) and for neurogenic inflammation (NGF, CGRP) as described in the Materials and Methods. The pictures show the typical results of three repetitive RT-PCR experiments for type 2 cytokines in HDM-treated NHEKs and C48/80-treated NHEKs. (B) Relative mRNA expression levels were semi-quantified by densitometric analysis. Left: HDM-treated NHEKs. Right: C48/80-treated NHEKs. HDM: house dust mites, NAC: N-acetyl cysteine, TSLP: thymic stromal lymphopoietin, IL: interleukin, NGF: nerve growth factor. **p<0.01.
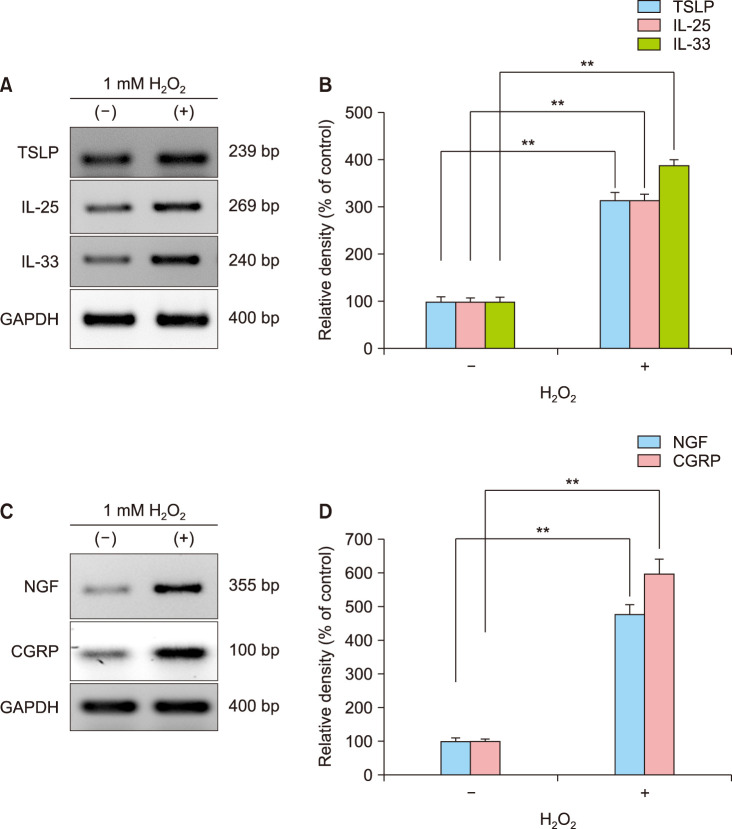
H2O2 induces the upregulation of epithelial-cell-derived cytokines and biomarkers for neurogenic inflammation in keratinocytes
To verify the active role of ROS, NHEKs were treated with 1 mM H2O2, instead of HDM or C48/80, as a pro-oxidant agent to drive ROS production from NHEKs. In RT-PCR, H2O2 could upregulate mRNA expression levels of the triad of epithelial-cell-derived cytokines including biomarkers for neurogenic inflammation, i.e., TSLP, IL-25, IL-33, NGF, and CGRP (Fig. 6A, C). In densitometric analysis, there was a statistically significant difference in mRNA expression levels between the H2O2-treated and H2O2-non-treated control groups (p<0.01) (Fig. 6B, D), indicating that ROS plays an active role in modulating the expression of those cytokines in keratinocytes.
Fig. 6. H2O2, a pro-oxidant, induces the expression of a set of biomarkers for type 2 inflammation of the skin from keratinocytes. To verify the role of keratinocytes-derived reactive oxygen species to induce type 2 inflammation of the skin, NHEKs were treated with H2O2 (1 mM), and then cells were harvested at 6h post-treatment to check mRNA expression levels of epithelial-derived type 2 cytokines (TSLP, IL-25, IL-33) and biomarkers for neurogenic inflammation (NGF, CGRP). (A) Reverse transcriptase-polymerase chain reaction (RT-PCR) experiment of the triad of epithelial-derived type 2 cytokines. (B) Relative mRNA expression levels of TSLP, IL-25, and IL-33. (C) RT-PCR experiment of biomarkers for neurogenic inflammation. (D) Relative mRNA expression levels of NGF and CGRP. TSLP: thymic stromal lymphopoietin, IL: interleukin, NGF: nerve growth factor. **p<0.01.
DISCUSSION
From this study, allergen- or non-allergen-producing ROS are revealed to induce a set of biomarkers for type 2 inflammation in the skin, including KLK5, PAR2, TSLP, IL-25, IL-33, NGF, and CGRP, from keratinocytes. The results could be further confirmed by antioxidant (NAC) and pro-oxidant (H2O2) experiments. Therefore, keratinocytes-producing ROS are found to play an important role to induce type 2 inflammation in the skin by modulating a triad of epithelial-cell-derived cytokines as well as biomarkers for neurogenic inflammation. Previously, glutathionedepletion-induced oxidative stress resulted in a Th2-polarized immune response in APCs5. Consistently, H2O2-induced IL-12 overproduction promotes the Th2-predominant response in mouse peritoneal macrophages4.
Many studies have demonstrated that DCs and innate ILCs play crucial roles in polarizing the type 2 inflammation in AD skin12,35,36. From this study, we found that keratinocytes are also active cells that respond to various environmental factors (allergens or non-allergens) by producing ROS, which are closely implicated in the early stage of type 2 inflammation in the skin. Consistently, TSLP and IL-33 were released from keratinocytes (or epithelial cells of airways) by danger signals, including those from pathogens, allergens, and chemicals10. TSLP is known to be expressed in the epithelial cells at barrier surfaces, such as the skin, gut, and lung37. TSLP could activate resident LCs in the epidermis to migrate into draining lymph nodes, which promoted differentiation of Naïve T cells into Th2 cells37,38,39. IL-25 was reported to increase Th2-type response by promoting the expression and functions of TSLP-DCs-activated Th2 memory cells40. ILC2 was also activated by IL-25 and IL-33, which led to the production of Th2-type cytokines (IL-5 and IL-13) in the lesional skin of AD41. IL-33, a member of the IL-1 family, can be regarded as alarmin-bridging tissue damage and immune response42. IL-33 plays an important role in bridging innate and adaptive immunity for tissue homeostasis42,43,44. From this study, ROS, irrespective of their origins from allergens or non-allergens, are found to play important mediators to activate type 2 inflammation of the skin, including the AD skin.
ROS derived from environmental pollutants and UV light was reported to induce skin barrier dysfunction and aggravation of AD45. Also, keratinocytes are major epithelial cells to be exposed to high levels of ROS produced by various environmental stimuli. Consistently, our study demonstrates that a non-allergic chemical agent (C48/80) can induce type 2 inflammation of the skin by producing ROS from keratinocytes. This result supports our notion that AD can be induced or aggravated by non-allergic environmental factors, such as DEP, cigarette smoke, and chemicals (volatile organic compounds, formaldehyde, toluene, or nitrogen dioxide), and UVB rays28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46: DEP could induce TSLP expression as well as Th2/Th17-type cytokines47,48; cigarette smoke aggravated type 2 inflammation by increasing IL-4 and IL-5 production49; and UVB irradiation induced the production of cytokines for type 2 inflammation including TSLP from keratinocytes46. Clinically, most AD patients developed their symptoms in infancy or childhood; approximately 80% of AD develops before age 5 years. As a plausible reason for infants or children to have the higher incidence of AD, we speculate that the young persons have underdeveloped antioxidant systems, which might be related with the higher oxidative stress level due to the impaired ROS-scavenging system in the skin.
TSLP is regarded as a master cytokine to control type 2 inflammation of the skin. We found that an allergen (HDM) or non-allergen (C48/80) can activate the KLK5-PAR2-NF κB pathway by the mediation of ROS, which might induce TSLP expression in keratinocytes. In an AD mouse model, KLK5-PAR2-TSLP cascade was stimulated by skin alkalization, implying that non-allergens including skin pH can modulate type 2 inflammation of the skin13. Consistently, ROS-dependent type 2 inflammation was also reported in the respiratory system of asthma and allergic rhinitis27.
Neurogenic inflammation in the skin is mediated by orchestrated expression of cytokines, chemokines, neurotrophins, and neuropeptides50. The complex network between the nervous system and target cells in the skin is mediated by immune-related cells (mast cells, T lymphocytes, DCs, macrophages, and eosinophils) as well as by skin-resident cells (keratinocytes and dermal fibroblasts)50,51. A set of neurotrophins and neuropeptides, which could be released from the nervous system and keratinocytes, were found to play a key role in inducing both type 2 inflammation and itching in AD16,19,20,21,52. From this study, oxidative stress is revealed to be implicated in the neurognic inflammation of the skin, which might elicit itching sensation in the AD skin. We found that allergens (HDM) or non-allergens (C48/80) could upregulate biomarkers for neurogenic inflammation via keratinocytes-producing ROS, which might be one of pathways to activate type 2 inflammation in the AD skin. To support our results, hydrogen peroxide or tertbutyl hydroperoxide was reported to provoke itching in mice53, and antixodiant desferrioxamine treatment was reported to suppress IL-4 expression in mercuric chrolide-induced Th2-polarized immune response in rat54.
In summary, allergens or non-allergens can induce type 2 inflammation of the skin by modulating keratinocytes-producing ROS, which induces expression of epithelial-cell-derived cytokines and mediators for neurogenic inflammation (Supplementary Fig. 1). The scenario to be observed in keratinocytes are strongly mimic to the early stage of type 2 inflammation in the AD skin. Our results further strengthens the evidence that oxidative stress or redox imbalance is one of important pathogenic mechanisms to induce AD by modulating a set of genes that regulate type 2 inflammation in the skin. Also, our results suggest us that efficient delivery of antioxidants to scavenge ROS in the skin is a promising therapeutic modality for proper management of AD.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: This project was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2015R1D1A1A01060023).
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPLEMENTARY MATERIALS
Supplementary data can be found via http://anndermatol.org/src/sm/ad-33-026-s001.pdf.
Primers for RT-PCR
Diagram for the role of allergens- or non-allergens-producing ROS in the early stage of type 2 inflammation in the skin. ROS is proposed as one of key molecules to drive type 2 inflammation of the skin in keratinocytes. Allergens (HDMs) or non-allergens (C48/80) are one of factors to stimulate keratinocytes to produce ROS. The keratinocytes-producing ROS upregulates a set of epithelial cell-derived type 2 cytokines (TSLP, IL-25, IL-33) as well as biomarkers for neurogenic inflammation (NGF, CGRP), which might play an important role to induce type 2 inflammation of the skin at the early phase. Antioxidants to scavenge ROS can be used as efficient drugs to inhibit type 2 inflammation of the skin. HDM: house dust mites, ROS: reactive oxygen species, KLK5: kallikrein 5, PAR2: proteinase-activated receptor 2, TSLP: thymic stromal lymphopoietin, IL: interleukin, NGF: nerve growth factor.
References
- 1.Cornelissen C, Brans R, Czaja K, Skazik C, Marquardt Y, Zwadlo-Klarwasser G, et al. Ultraviolet B radiation and reactive oxygen species modulate interleukin-31 expression in T lymphocytes, monocytes and dendritic cells. Br J Dermatol. 2011;165:966–975. doi: 10.1111/j.1365-2133.2011.10487.x. [DOI] [PubMed] [Google Scholar]
- 2.Dobashi K, Aihara M, Araki T, Shimizu Y, Utsugi M, Iizuka K, et al. Regulation of LPS induced IL-12 production by IFN-gamma and IL-4 through intracellular glutathione status in human alveolar macrophages. Clin Exp Immunol. 2001;124:290–296. doi: 10.1046/j.1365-2249.2001.01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murata Y, Shimamura T, Hamuro J. The polarization of T(h)1/T(h)2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. Int Immunol. 2002;14:201–212. doi: 10.1093/intimm/14.2.201. [DOI] [PubMed] [Google Scholar]
- 4.Obata F, Hoshino A, Toyama A. Hydrogen peroxide increases interleukin-12 p40/p70 molecular ratio and induces Th2-predominant responses in mice. Scand J Immunol. 2006;63:125–130. doi: 10.1111/j.1365-3083.2005.01718.x. [DOI] [PubMed] [Google Scholar]
- 5.Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci U S A. 1998;95:3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Divekar R, Kita H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr Opin Allergy Clin Immunol. 2015;15:98–103. doi: 10.1097/ACI.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 8.Jang YH, Choi JK, Jin M, Choi YA, Ryoo ZY, Lee HS, et al. House dust mite increases pro-Th2 cytokines IL-25 and IL-33 via the activation of TLR1/6 signaling. J Invest Dermatol. 2017;137:2354–2361. doi: 10.1016/j.jid.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 9.Ryu WI, Lee H, Kim JH, Bae HC, Ryu HJ, Son SW. IL-33 induces Egr-1-dependent TSLP expression via the MAPK pathways in human keratinocytes. Exp Dermatol. 2015;24:857–863. doi: 10.1111/exd.12788. [DOI] [PubMed] [Google Scholar]
- 10.Saluja R, Zoltowska A, Ketelaar ME, Nilsson G. IL-33 and Thymic Stromal Lymphopoietin in mast cell functions. Eur J Pharmacol. 2016;778:68–76. doi: 10.1016/j.ejphar.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 11.Deleuran M, Hvid M, Kemp K, Christensen GB, Deleuran B, Vestergaard C. IL-25 induces both inflammation and skin barrier dysfunction in atopic dermatitis. Chem Immunol Allergy. 2012;96:45–49. doi: 10.1159/000331871. [DOI] [PubMed] [Google Scholar]
- 12.Carmi-Levy I, Homey B, Soumelis V. A modular view of cytokine networks in atopic dermatitis. Clin Rev Allergy Immunol. 2011;41:245–253. doi: 10.1007/s12016-010-8239-6. [DOI] [PubMed] [Google Scholar]
- 13.Jang H, Matsuda A, Jung K, Karasawa K, Matsuda K, Oida K, et al. Skin pH is the master switch of kallikrein 5-mediated skin barrier destruction in a murine atopic dermatitis model. J Invest Dermatol. 2016;136:127–135. doi: 10.1038/JID.2015.363. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka RJ, Ono M, Harrington HA. Skin barrier homeostasis in atopic dermatitis: feedback regulation of kallikrein activity. PLoS One. 2011;6:e19895. doi: 10.1371/journal.pone.0019895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135–1147. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liezmann C, Klapp B, Peters EM. Stress, atopy and allergy: a re-evaluation from a psychoneuroimmunologic persepective. Dermatoendocrinol. 2011;3:37–40. doi: 10.4161/derm.3.1.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foreman JC. Substance P and calcitonin gene-related peptide: effects on mast cells and in human skin. Int Arch Allergy Appl Immunol. 1987;82:366–371. doi: 10.1159/000234229. [DOI] [PubMed] [Google Scholar]
- 18.Rosa AC, Fantozzi R. The role of histamine in neurogenic inflammation. Br J Pharmacol. 2013;170:38–45. doi: 10.1111/bph.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botchkarev VA, Yaar M, Peters EM, Raychaudhuri SP, Botchkareva NV, Marconi A, et al. Neurotrophins in skin biology and pathology. J Invest Dermatol. 2006;126:1719–1727. doi: 10.1038/sj.jid.5700270. [DOI] [PubMed] [Google Scholar]
- 20.Salomon J, Baran E. The role of selected neuropeptides in pathogenesis of atopic dermatitis. J Eur Acad Dermatol Venereol. 2008;22:223–228. doi: 10.1111/j.1468-3083.2007.02399.x. [DOI] [PubMed] [Google Scholar]
- 21.Ständer S, Steinhoff M. Pathophysiology of pruritus in atopic dermatitis: an overview. Exp Dermatol. 2002;11:12–24. doi: 10.1034/j.1600-0625.2002.110102.x. [DOI] [PubMed] [Google Scholar]
- 22.Huang CH, Kuo IC, Xu H, Lee YS, Chua KY. Mite allergen induces allergic dermatitis with concomitant neurogenic inflammation in mouse. J Invest Dermatol. 2003;121:289–293. doi: 10.1046/j.1523-1747.2003.12356.x. [DOI] [PubMed] [Google Scholar]
- 23.Ostlere LS, Cowen T, Rustin MH. Neuropeptides in the skin of patients with atopic dermatitis. Clin Exp Dermatol. 1995;20:462–467. doi: 10.1111/j.1365-2230.1995.tb01378.x. [DOI] [PubMed] [Google Scholar]
- 24.Teresiak-Mikołajczak E, Czarnecka-Operacz M, Jenerowicz D, Silny W. Neurogenic markers of the inflammatory process in atopic dermatitis: relation to the severity and pruritus. Postepy Dermatol Alergol. 2013;30:286–292. doi: 10.5114/pdia.2013.38357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toyoda M, Nakamura M, Makino T, Hino T, Kagoura M, Morohashi M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br J Dermatol. 2002;147:71–79. doi: 10.1046/j.1365-2133.2002.04803.x. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi J, Aihara M, Kobayashi Y, Kambara T, Ikezawa Z. Quantitative analysis of nerve growth factor (NGF) in the atopic dermatitis and psoriasis horny layer and effect of treatment on NGF in atopic dermatitis. J Dermatol Sci. 2009;53:48–54. doi: 10.1016/j.jdermsci.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Huerta-Yepez S, Baay-Guzman GJ, Bebenek IG, Hernandez-Pando R, Vega MI, Chi L, et al. Hypoxia inducible factor promotes murine allergic airway inflammation and is increased in asthma and rhinitis. Allergy. 2011;66:909–918. doi: 10.1111/j.1398-9995.2011.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji H, Li XK. Oxidative stress in atopic dermatitis. Oxid Med Cell Longev. 2016;2016:2721469. doi: 10.1155/2016/2721469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HR, Kim JH, Choi EJ, Lee YK, Kie JH, Jang MH, et al. Hyperoxygenation attenuated a murine model of atopic dermatitis through raising skin level of ROS. PLoS One. 2014;9:e109297. doi: 10.1371/journal.pone.0109297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Omata N, Tsukahara H, Ito S, Ohshima Y, Yasutomi M, Yamada A, et al. Increased oxidative stress in childhood atopic dermatitis. Life Sci. 2001;69:223–228. doi: 10.1016/s0024-3205(01)01124-9. [DOI] [PubMed] [Google Scholar]
- 31.Sivaranjani N, Rao SV, Rajeev G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J Clin Diagn Res. 2013;7:2683–2685. doi: 10.7860/JCDR/2013/6635.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi MH, Kim HP, Jeong KY, Kim CR, Kim TY, Yong TS. House dust mite allergen Der f 1 induces IL-8 in human basophilic cells via ROS-ERK and p38 signal pathways. Cytokine. 2015;75:356–364. doi: 10.1016/j.cyto.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Fukunaga M, Gon Y, Nunomura S, Inoue T, Yoshioka M, Hashimoto S, et al. Protease-mediated house dust mite allergen-induced reactive oxygen species production by neutrophils. Int Arch Allergy Immunol. 2011;155 Suppl 1:104–109. doi: 10.1159/000327492. [DOI] [PubMed] [Google Scholar]
- 34.Wu D, Zhang F, Lou W, Li D, Chen J. Chemical characterization and toxicity assessment of fine particulate matters emitted from the combustion of petrol and diesel fuels. Sci Total Environ. 2017;605-606:172–179. doi: 10.1016/j.scitotenv.2017.06.058. [DOI] [PubMed] [Google Scholar]
- 35.Haniffa M, Gunawan M, Jardine L. Human skin dendritic cells in health and disease. J Dermatol Sci. 2015;77:85–92. doi: 10.1016/j.jdermsci.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim BS, Wojno ED, Artis D. Innate lymphoid cells and allergic inflammation. Curr Opin Immunol. 2013;25:738–744. doi: 10.1016/j.coi.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cianferoni A, Spergel J. The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert Rev Clin Immunol. 2014;10:1463–1474. doi: 10.1586/1744666X.2014.967684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 39.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milovanovic M, Volarevic V, Radosavljevic G, Jovanovic I, Pejnovic N, Arsenijevic N, et al. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res. 2012;52:89–99. doi: 10.1007/s12026-012-8283-9. [DOI] [PubMed] [Google Scholar]
- 43.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz C, O'Grady K, Lavelle EC, Fallon PG. Interleukin 33: an innate alarm for adaptive responses beyond Th2 immunity-emerging roles in obesity, intestinal inflammation, and cancer. Eur J Immunol. 2016;46:1091–1100. doi: 10.1002/eji.201545780. [DOI] [PubMed] [Google Scholar]
- 45.Niwa Y, Sumi H, Kawahira K, Terashima T, Nakamura T, Akamatsu H. Protein oxidative damage in the stratum corneum: evidence for a link between environmental oxidants and the changing prevalence and nature of atopic dermatitis in Japan. Br J Dermatol. 2003;149:248–254. doi: 10.1046/j.1365-2133.2003.05417.x. [DOI] [PubMed] [Google Scholar]
- 46.Jang Y, Jeong SH, Park YH, Bae HC, Lee H, Ryu WI, et al. UVB induces HIF-1α-dependent TSLP expression via the JNK and ERK pathways. J Invest Dermatol. 2013;133:2601–2608. doi: 10.1038/jid.2013.203. [DOI] [PubMed] [Google Scholar]
- 47.Manners S, Alam R, Schwartz DA, Gorska MM. A mouse model links asthma susceptibility to prenatal exposure to diesel exhaust. J Allergy Clin Immunol. 2014;134:63–72. doi: 10.1016/j.jaci.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takai T. TSLP expression: cellular sources, triggers, and regulatory mechanisms. Allergol Int. 2012;61:3–17. doi: 10.2332/allergolint.11-RAI-0395. [DOI] [PubMed] [Google Scholar]
- 49.Singh SP, Mishra NC, Rir-Sima-Ah J, Campen M, Kurup V, Razani-Boroujerdi S, et al. Maternal exposure to secondhand cigarette smoke primes the lung for induction of phosphodiesterase-4D5 isozyme and exacerbated Th2 responses: rolipram attenuates the airway hyperreactivity and muscarinic receptor expression but not lung inflammation and atopy. J Immunol. 2009;183:2115–2121. doi: 10.4049/jimmunol.0900826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scholzen T, Armstrong CA, Bunnett NW, Luger TA, Olerud JE, Ansel JC. Neuropeptides in the skin: interactions between the neuroendocrine and the skin immune systems. Exp Dermatol. 1998;7:81–96. doi: 10.1111/j.1600-0625.1998.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 51.Peters EM, Ericson ME, Hosoi J, Seiffert K, Hordinsky MK, Ansel JC, et al. Neuropeptide control mechanisms in cutaneous biology: physiological and clinical significance. J Invest Dermatol. 2006;126:1937–1947. doi: 10.1038/sj.jid.5700429. [DOI] [PubMed] [Google Scholar]
- 52.Antúnez C, Torres MJ, López S, Rodriguez-Pena R, Blanca M, Mayorga C, et al. Calcitonin gene-related peptide modulates interleukin-13 in circulating cutaneous lymphocyte-associated antigen-positive T cells in patients with atopic dermatitis. Br J Dermatol. 2009;161:547–553. doi: 10.1111/j.1365-2133.2009.09318.x. [DOI] [PubMed] [Google Scholar]
- 53.Liu T, Ji RR. Oxidative stress induces itch via activation of transient receptor potential subtype ankyrin 1 in mice. Neurosci Bull. 2012;28:145–154. doi: 10.1007/s12264-012-1207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Z, Holwill SD, Oliveira DB. Desferrioxamine modulates chemically induced T helper 2-mediated autoimmunity in the rat. Clin Exp Immunol. 2004;135:194–199. doi: 10.1111/j.1365-2249.2003.02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers for RT-PCR
Diagram for the role of allergens- or non-allergens-producing ROS in the early stage of type 2 inflammation in the skin. ROS is proposed as one of key molecules to drive type 2 inflammation of the skin in keratinocytes. Allergens (HDMs) or non-allergens (C48/80) are one of factors to stimulate keratinocytes to produce ROS. The keratinocytes-producing ROS upregulates a set of epithelial cell-derived type 2 cytokines (TSLP, IL-25, IL-33) as well as biomarkers for neurogenic inflammation (NGF, CGRP), which might play an important role to induce type 2 inflammation of the skin at the early phase. Antioxidants to scavenge ROS can be used as efficient drugs to inhibit type 2 inflammation of the skin. HDM: house dust mites, ROS: reactive oxygen species, KLK5: kallikrein 5, PAR2: proteinase-activated receptor 2, TSLP: thymic stromal lymphopoietin, IL: interleukin, NGF: nerve growth factor.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.