Abstract
Background
Acral melanoma occurs on glabrous skin or the nail apparatus and is distinct from ultraviolet-related melanoma due to differing genetic alteration patterns. Although the pathogenesis of acral melanoma is not well understood, mechanical stress is thought to induce acral melanoma. The incidence of gene mutation and promoter methylation has been reported in tumors from acral melanoma; however, an association between genetic/epigenetic alterations and mechanical stress in acral melanoma remains unclear.
Objective
To investigate the relationship between clinical/genetic factors and mechanical stress in acral melanoma.
Methods
A retrospective review of 52 patients diagnosed with acral melanoma was performed. We reviewed the clinical characteristics of patients, tumor status, and tumor location. Mutations in BRAF, NRAS, and the TERT promoter, along with KIT amplification and PTEN promoter methylation were analyzed in the tumors.
Results
The heel (34/52, 65.4%) was the most common anatomical tumor site. Mutations in BRAF (6/48, 12.5%), NRAS (6/49, 12.2%), and the TERT promoter (4/33, 12.1%), along with KIT amplification (3/37, 8.1%) and PTEN promoter hypermethylation (12/48, 25.0%) were observed in the tumors. On the forefoot, heel, and hallux, PTEN promoter hypermethylation was significantly associated with Breslow thickness (p=0.001) and ulceration rate (p=0.042). On the midfoot and lesser toes, there was no significant difference in Breslow thickness or ulceration rate regardless of PTEN promoter hypermethylation (p>0.05).
Conclusion
PTEN promoter hypermethylation is associated with Breslow thickness and tumor ulceration on the forefoot, heel, and hallux in acral melanoma in Korean patients.
Keywords: Acral melanoma, Malignant melanoma, Mechanical stress, Methylation, Phosphatase and tensin homolog
INTRODUCTION
Cutaneous melanoma, which is a heterogeneous group of tumors, is subdivided into four types: chronic sun damage-induced melanoma (CSD), non-CSD, mucosal melanoma, and acral melanoma1. Among them, acral melanoma occurs on glabrous skin or the nail apparatus and is distinct from ultraviolet (UV)-related melanoma due to differing genetic alteration patterns2,3. Acral melanoma mostly displays histologic features of acral lentiginous melanoma (>80%)4. Acral melanoma accounts for 2% to 8% of melanomas in Caucasians, whereas it accounts for more than 40% of melanomas in Asians5. Acral melanoma is the most common type of melanoma in the Korean population4.
Melanoma is one of cancer that harbors the highest somatic mutational burdens among all solid malignancies6. Acral melanoma is genetically distinct from other cutaneous melanomas. Molecular genetics research has demonstrated several mutational differences between UV-related melanoma and non-UV related melanoma. Acral melanomas have a low mutational burden, differing mutated genes, and a differing type of genetic alteration3. UV-related mutational signatures, C>T or CC>TT, are typically not detected in acral melanomas2. BRAF mutations (14.8%) and NRAS mutations (13.3%) are less frequently observed in acral melanomas than in other cutaneous melanomas (45%~60% and 15%~25%)7. TERT promoter mutations are reported in 33%~65% of cutaneous melanomas, but in less than 10% of acral melanomas8. Acral melanomas show recurrent genomic copy number alterations, which include gains of TERT (5p15), KIT (4q12), CCND1 (11q13), and AURKA (20q13)2. These results suggest that acral melanomas may have different genetic causal pathways than UV-related melanomas9.
Besides genetic alterations, a recent study had reported that epigenetic alterations, such as RARB and PTEN promoter hypermethylation, are prognostic markers in cutaneous melanoma10. Although promoter hypermethylation frequency is lower in tumors from Asian individuals than in tumors from Caucasian individuals, PTEN promoter hypermethylation has been shown to be an independent prognostic factor for survival for melanoma in Asian populations11. However, the clinical significance of promoter methylation and somatic mutation in acral melanoma is unclear.
Besides genetic factors, other factors, such as mechanical stress, are considered factors for non-UV related melanoma12,13,14. Several reports have shown a high incidence of acral melanomas on the weight-bearing portion of the sole15,16,17, and more oral mucosal melanomas have occurred on the hard palatal mucosa and maxillary gingiva than on other areas of the oral mucosal surface14. From studies on sole pressure while standing and walking18,19, the heel and forefoot showed the highest peak pressures. The little toes and midfoot are relatively low-pressure areas. Also, recent studies have shown that more acral melanomas occur on the heel than on any other areas of the plantar surface12,13. Based on the relationship between acral melanoma and pressure, we investigated whether there are genetic or clinical factors associated with pressure. We evaluated mutations in BRAF, NRAS, and in the TERT promoter, along with KIT amplification and PTEN promoter hypermethylation in primary tumors on the plantar surface in Korean acral melanoma patients. We assessed the association of these genetic/epigenetic alterations with tumor status, mechanical stress, and clinical characteristics.
MATERIALS AND METHODS
This study included 52 acral melanoma patients. A retrospective review of 52 patients diagnosed with acral melanoma was carried out. Medical records and mutation analysis results were reviewed from acral melanoma patients who were treated at Severance Hospital and Yonsei Cancer Hospital from 2002 to 2012. We selected 52 patients with genetic and epigenetic alteration results for analysis. Clinical data that included age, sex, body mass index, tumour-node-metastasis stage, Breslow thickness, and ulceration (both macroscopic and microscopic) were collected. From the genetic and epigenetic alteration results, mutations in BRAF, NRAS, and the TERT promoter, along with KIT amplification and PTEN promoter hypermethylation were reviewed. This retrospective study was approved by the Institutional Review Board of Gangnam Severance Hospital (IRB no. 2019-0487-001). The requirement of informed consent was exempted.
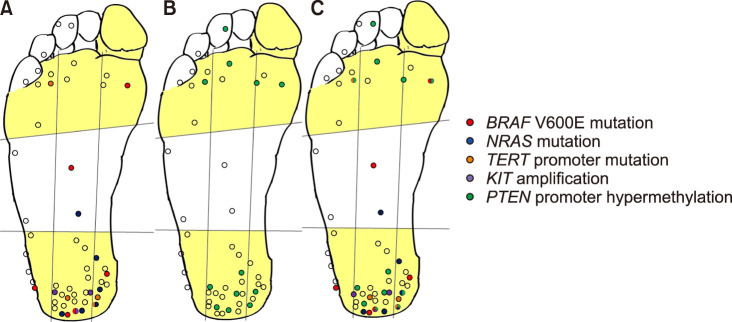
All lesions with clinical pictures were standardized as a composite image and plotted by location according to pressure (Fig. 1). We divided the sole into 4 regions (heel, midfoot, forefoot, and toes), and each region was subdivided into various areas (center, medial, lateral, lesser toes, hallux) based on previous pedobarographic pressure studies18,19,20. The forefoot included the metatarsal lesion and transverse arch. The midfoot included the cuboid bone and the medial and lateral arches. The heel included the calcaneus and talus bones. From previously published literature15,16,17 and in consideration of pedobarographic pressure20 and the 3 arches (2 longitudinal and 1 transverse), we divided plantar foot into two areas (heel, forefoot, hallux vs. midfoot, lesser toes) (Fig. 1). The staging was determined according to the American Joint Committee on Cancer (AJCC) 8th guidelines for melanoma.
Fig. 1. Anatomical mapping of genetic and epigenetic alterations in acral melanoma on the glabrous foot. Heel, forefoot, and hallux are yellow-colored and the centers of the lesions are plotted (n=52). Each dot is color-coded according to the mutation type (A) or PTEN promoter hypermethylation (B). (C) Both mutations and PTEN promoter hypermethylation are shown in each dot.
DNA preparation and mutation analysis were performed as previously described21,22. Formalin-fixed, paraffin-embedded tissue blocks diagnosed as acral melanoma were retrieved. Exon 15 (codon 600) of the BRAF gene and exons 1 and 2 (codon 12, 13, and 61) of the NRAS gene were amplified by polymerase chain reaction (PCR) in order to detect hotspot mutations. PCR amplification of the TERT promoter region was also performed. The primer sequences are listed in Table 1. Pyrosequencing using a PyroMark Q24 (Qiagen, Germantown, MD, USA) was performed at room temperature. Gold Q24 Reagents (Qiagen) were used according to the manufacturer's instructions. Sequencing analysis was performed using PyroMark Q24 software ver. 1.0.10 (Qiagen) in allele quantification analysis mode.
Table 1. Primers used in referred studies.
| Gene | Exon | Sequence |
|---|---|---|
| BRAF | 15 | F: 5′-biotin-GCTTGCTCTGATAGGAAAATGA-3′ |
| R: 5′-GACAACTGTTCAAACTGATGGG-3′ | ||
| S: 5′-CCACTCCATCGAGATTT-3 | ||
| NRAS | 1 | F: 5′-GGTGTGAAATGACTGAGTACAAACTGG-3′ |
| R: 5′-biotin-CATATTCATCTACAAAGTGGTT CTGGA-3′ | ||
| S: 5′-CAAACTGGTGGTGGTTGGAG-3′ | ||
| 2 | F: 5′-GATTCTTACAGAAAACAAGTGGTTAT AGAT-3′ | |
| R: 5′-biotin-GCAAATACACAGAGGAAGCCT TCG-3′ | ||
| S: S: 5′-GACATACTGGATACAGCTGG-3′ | ||
| TERT promoter | F: 5′-CCCACGTGCGCAGCAGGAC-3′ | |
| R: 5′-Biotin-CTCCCAGTGGATTCGCGGGC-3′ | ||
| S: 5′-AGGGGCTGGGAGGGC | ||
| KIT | 17 | F: 5′-AAAGATTTGTGATTTTGGTCTAGC-3′ |
| R: 5′-GAAACTAAAAATCCTTTGCA-3′ | ||
| GAPDH | 2 | F: 5′-CACTAGGCGCTCACTGTTCT-3′ |
| R: 5′-GCGAACTCACCCGTTG-3′ | ||
| PTE promoter | F: 5′-GGATGTGGGTGTTTGTGTAATTA-3′ | |
| R: 5′-biotin-AATTCCCACTCCCCAATAATAAC-3′ | ||
| S: 5′-TTTGTGTAATTAGTTTTTTA-3′ |
F: forward primer, R: reverse primer, S: sequencing primer, GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
KIT amplification was analyzed as previously described21. KIT copy number was assessed by quantitative real-time PCR using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the control gene (KIT exon 17 and GAPDH primers are listed in Table 1). PCR reactions were performed in the Rotor-Gene 2000 Real-Time Cycler (Corbett Research, Mortlake, Australia) using the QuantiTect SYBR Green PCR KIT (Qiagen) with a 20 µl total volume and 100 ng of genomic DNA. Relative copy numbers were calculated by the ΔΔCt method, where Ct is the threshold cycle for amplification. For each sample, ΔCt for KIT vs. GAPDH was calculated as ΔCt=Ct (KIT)−Ct (GAPDH). The ΔCt value for each experimental test sample was calibrated to a reference pool of human genomic DNA (Promega, Madison, WI, USA) using the formula ΔΔCt=ΔCt (test sample)−ΔCt (reference pool). Relative DNA copy number was calculated using the formula 2−ΔΔCt.
PTEN promoter methylation was analyzed as previously described11. Five potential promoter regions, spanning 1,333 base pairs upstream and 1,297 base pairs downstream of the PTEN gene transcription start site, were analyzed. CpG islands were identified within the core promoter region. For primer design, DNA sequences were converted in silico to the methylated form of CpG as follows: CG motifs were converted to YG with Y equalling either C/T or G/A, and then C was converted to T. Using this converted sequence, methylation-specific primers for quantitative sequencing (pyrosequencing) of PTEN CpGs were designed using the Biotage Assay Design software (PyroMark Assay Design 2.0) and the pyrosequencer PyroMark Q24 version 1.0.10 software (Qiagen). The primer sequences are listed in Table 1. Fifty nanogram of bisulfite-treated DNA was used in the PCR reaction with 200 nmol/L forward and reverse primers. PCR conditions for PTEN were 95℃ for 15 minutes; 50 cycles of 95℃ for 40 seconds, 55℃ for 40 seconds, and 72℃ for 40 seconds; and 72℃ for 10 minutes. PCR reactions included 0.5 U of Amplitaq Gold (Applied Biosystems, Austin, TX, USA). The percentage methylated fraction (C/T ratio) was automatically calculated. Each site was analyzed as a C/T polymorphism where a 100% C-reading denotes a fully methylated C in the original genomic DNA sample, and a 100% T-reading denotes that the C was unmethylated in the genomic DNA sample. Intermediate C/T percentages denote partial methylation in the sample. The methylation values were calculated as the peak height methylated/(peak height methylated+peak height unmethylated)×100.
All statistical analyses were performed using SPSS ver. 23.0 software (IBM Corp., Armonk, NY, USA). Categorical data were described using frequencies and percentages. Continuous data, such as age, were described using mean±standard deviation or median (range) for normally distributed data. A chi-square test or Fisher exact test was used to differentiate the rates of different groups. Differences in measurement data between two groups were evaluated by unpaired t-test or Mann–Whitney test. Hierarchical clustering was performed to identify PTEN promoter “hypermethylated” and “hypomethylated” samples. All statistical analyses were two-sided, and significance was assigned at p<0.05.
RESULTS
Baseline clinical characteristics
Fifty-two acral melanoma patients were analyzed (Table 2). There were 26 males and 26 females with a sex ratio of 1:1. The median age was 65 years (range, 37~89 years). Ulceration was present in 17 patients (39.5%), with a mean Breslow thickness of 2.99 mm. There was no significant relationship between high body mass index (BMI) and acral melanoma on the heel, forefoot, and hallux (p>0.05; Table 2). The mutation rate was 12.5% for BRAF (6/48), 12.2% for NRAS (6/49), and 12.1% for the TERT promoter (4/33). The gene amplification rate for KIT was 8.1% (3/37). PTEN promoter hypermethylation (12/48) was also observed in 25.0% of patients. The center of each acral melanoma lesion was plotted on the composite image. Each dot is color-coded according to the gene alteration type (Fig. 1A) or PTEN promoter hypermethylation (Fig. 1B). All BRAF mutations (n=6) were BRAF V600E. In the 6 patients with NRAS mutations, a mutation in codon 61 was identified in 2 patients. In the other four patients, mutations resulting in G12R in one patient and G13R in 3 patients were also detected. A G>A transition in the TERT promoter was found at position −124 bp (relative to the ATG start site) in 4 patients.
Table 2. Baseline clinical characteristics of acral melanoma patients.
| Variable | Total | Heel, forefoot and hallux | Midfoot and lesser toes | p-value |
|---|---|---|---|---|
| Patient | 52 (100.0) | 43 (82.7) | 9 (17.2) | |
| Median age, yr (range) | 65 (37~89) | 63 (37~89) | 67 (37~80) | 0.341 |
| Female sex | 26 (50.0) | 22 (51.2) | 4 (44.4) | 0.725 |
| BMI, kg/m2 (n=51) | 24.04±3.14 | 24.36±2.85 | 22.37±3.90 | 0.675 |
| ≤23 | 20 | 15 (35.7) | 5 (55.6) | |
| 23<BMI≤25 | 10 | 9 (21.4) | 1 (11.1) | |
| >25 | 21 | 18 (42.9) | 3 (33.3) | |
| Ulceration (n=43) | 0.071 | |||
| Yes | 17 | 16 (47.1) | 1 (11.1) | |
| No | 26 | 18 (52.9) | 8 (88.9) | |
| Thickness, mm (n=48) | 2.99±2.93 | 3.34±3.15 | 1.37±0.93 | 0.088 |
| Gene alteration | ||||
| BRAF (n=48) | 6 (12.5) | 5 | 1 | |
| NRAS (n=49) | 6 (12.2) | 5 | 1 | |
| TERT promoter (n=33) | 4 (12.1) | 4 | 0 | |
| KIT amplification (n=37) | 3 (8.1) | 3 | 0 | |
| PTEN promoter hypermethylation (n=48) | 12 (25.0) | 11 | 1 |
Values are presented as number (%), mean (range), mean±standard deviation, or number only.
Anatomical mapping of acral melanoma
Among the 52 patients, the vertical distribution of lesions were as follows: 34 lesions (65.4%) on the heel, 4 lesions (7.7%) on the midfoot, 9 lesions (17.3%) on the forefoot, and 5 lesions (9.6%) on the toes. The heel (n=34, 65.4%) was the most common site. The parallel distribution of lesions was as follows: 12 lesions (23.1%) on the medial side, 14 lesions (26.9%) on the lateral side, and 26 lesions (50.0%) in the central region. The anatomic mapping of site distribution is shown in Fig. 1C.
Analysis of the relationship between clinical/genetic factors and pressure
To analyze the relationship with pressure, the foot was divided into a pressure prone area and a lesser pressure prone area. Heel, forefoot, and hallux are yellow-colored on Fig. 1. We also analyzed the relationship between clinical/genetic factors and pressure. Mutations in BRAF, NRAS, and the TERT promoter and KIT amplification profiles showed no association with Breslow thickness or ulceration rate on both groups (p>0.05; Table 3). However, PTEN promoter hypermethylation showed an association with Breslow thickness (p=0.001; Table 4) and ulceration (p=0.042; Table 4) on the heel, forefoot, and hallux. In the presence of PTEN promoter hypermethylation, Breslow thickness was increased only on the heel, forefoot, and hallux. In the midfoot and lesser toes, there were no significant differences in Breslow thickness or ulceration rate associated with PTEN promoter hypermethylation (p>0.05; Table 4).
Table 3. Genetic alteration profiles associated with Breslow thickness and ulceration in acral melanoma.
| Category | BRAF | NRAS | KIT amplification | TERT promoter | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Heel, forefoot, hallux | ||||||||
| Breslow thickness | ||||||||
| ≤1 | 1 (20.0) | 9 (27.3) | 0 | 10 (29.4) | 0 | 9 (33.3) | 1 (33.3) | 7 (28.0) |
| >1.0~2.0 | 1 (20.0) | 10 (30.3) | 1 (25.0) | 10 (29.4) | 2 (50.0) | 6 (22.2) | 1 (33.3) | 2 (8.0) |
| >2.0~4.0 | 0 | 6 (18.2) | 2 (50.0) | 4 (11.8) | 0 | 2 (7.4) | 0 | 6 (24.0) |
| >4 | 3 (60.0) | 8 (24.2) | 1 (25.0) | 10 (29.4) | 1 (66.6) | 10 (37.0) | 1 (33.3) | 10 (40.0) |
| p-value | 0.239 | 0.295 | 0.325 | 0.543 | ||||
| Ulceration | ||||||||
| Yes | 2 (66.7) | 13 (43.3) | 2 (50.0) | 13 (44.8) | 2 (66.6) | 10 (43.5) | 0 | 11 (52.4) |
| No | 1 (33.3) | 17 (56.7) | 2 (50.0) | 16 (55.2) | 1 (25.0) | 13 (56.5) | 2 (100.0) | 10 (47.6) |
| p-value | 0.579 | >0.999 | 0.425 | 0.478 | ||||
| Midfoot, lesser toes | ||||||||
| Breslow thickness | ||||||||
| ≤1 | 0 | 4 (50.0) | 0 | 4 (50.0) | 0 | 3 (50.0) | 0 | 0 |
| >1.0~2.0 | 0 | 2 (25.0) | 1 (100) | 1 (12.5) | 0 | 2 (33.3) | 0 | 3 (75.0) |
| >2.0~4.0 | 1 (100) | 2 (25.0) | 0 | 3 (37.5) | 0 | 1 (16.7) | 0 | 1 (25.0) |
| >4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| p-value | 0.222 | 0.556 | NA | NA | ||||
| Ulceration | ||||||||
| Yes | 0 | 1 (12.5) | 0 | 1 (12.5) | 0 | 0 | 0 | 0 |
| No | 1 (100) | 7 (87.5) | 1 (100) | 7 (87.5) | 1 (100) | 6 (100) | 1 (100) | 4 (100) |
| p-value | >0.999 | >0.999 | NA | NA | ||||
Values are presented as number (%).
Table 4. PTEN promoter hypermethylation associated with Breslow thickness and ulceration in acral melanoma.
| Category | PTEN promoter hypermethylation | ||
|---|---|---|---|
| Yes | No | p-value | |
| Heel, forefoot, hallux | |||
| Breslow thickness | 0.001* | ||
| ≤1 | 0 | 9 (33.3) | |
| >1.0~2.0 | 1 (11.1) | 8 (29.6) | |
| >2.0~4.0 | 0 | 6 (22.2) | |
| >4 | 8 (88.9) | 4 (14.8) | |
| Ulceration | 0.042* | ||
| Yes | 6 (75.0) | 7 (30.4) | |
| No | 2 (25.0) | 16 (69.6) | |
| Midfoot, lesser toes | |||
| Breslow thickness | 0.556 | ||
| ≤1 | 0 | 2 (25.0) | |
| >1.0~2.0 | 0 | 4 (50.0) | |
| >2.0~4.0 | 1 (100) | 2 (25.0) | |
| >4 | 0 | 0 | |
| Ulceration | 0.125 | ||
| Yes | 1 (100) | 0 | |
| No | 0 | 7 (100) | |
Values are presented as number (%). *Statistically significant (p<0.05).
DISCUSSION
In the present study, we investigated whether there are genetic or clinical factors associated with pressure in Korean acral melanoma patients. We discovered that PTEN promoter hypermethylation was associated with foot melanoma thickness and ulceration rate only on the heel, forefoot, and hallux. We found that 25.0% of acral melanoma patients had PTEN promoter hypermethylation. The hypermethylation rate (12/48, 25.0%) of Korean acral melanoma patients is consistent with the rate found in a previous Korean study (31/158, 19.6%)11. We checked the association between PTEN promoter hypermethylation and Breslow thickness, and there was no significant correlation (p=0.238), which is also consistent with the previous study11. Somatic mutation rates of acral melanoma patients were also similar to other studies23. No other somatic alterations (mutations in BRAF, NRAS, and the TERT promoter, KIT amplification) were associated with Breslow thickness or ulceration rate in acral melanoma. These results are consistent with an analysis of 48 cases of acral melanoma in Brazil23.
Recently, several studies have suggested that mechanical stress is a possible factor in promoting acral melanoma, particularly on the sole24. Several reports have shown a high incidence of acral melanoma on the weight-bearing portion of the soles15,16,17,25. Sheen et al.25 recently showed that acral melanomas tended to develop on weight-bearing areas, and the distribution pattern was not associated with clinical and prognostic factors. Our study show that heel (34/52, 65.4%) was the most common anatomical site of tumors and there was no significant relationship between high pressure area and other clinical factors (sex, age, BMI, Breslow thickness, ulceration), which is consistent results with previous study25.
We aimed to evaluate the role of long-term mechanical stress in acral melanoma by analyzing clinical and genetic factors on the heel, forefoot, and hallux. There was no significant difference in Breslow thickness or ulceration rate on the heel, forefoot and hallux compared to the midfoot and lesser toes. Our data showed that tumor thickness and ulceration rate were only related to PTEN promoter hypermethylation on the heel, forefoot, and hallux.
There are various types of mechanical stresses, which include pressure, friction, shearing forces, and stretching24. The relationship between mechanical stress and disease has been studied in Hidradenitis suppurativa, callus, and diabetic foot ulcers, but little has been reported in acral melanoma. Friction primarily applies to the epidermis, which causes intertrigo and hidradenitis suppurativa26, whereas shear injury affects deeper skin layers and causes pressure ulcers. Shear stress is defined as force per unit area exerted parallel to the sole plane while walking27. When standing, the pressure acts perpendicular to the plane and extends across the entire skin layer (epidermis, dermis, subcutaneous tissue)24. In acral melanoma patients, standing pressure and shear stress mainly affect the foot.
Physiologically, shear stress elicits an increase in cutaneous microvascular reactivity and endothelial function27. In the tumor microenvironment, shear stress activates transforming growth factor-β (TGF-β) signalling, inducing the epithelial to mesenchymal transition28. TGF-β signalling, which is associated with tissue fibrosis and the tumor microenvironment have predominant roles by stimulating the non-canonical hedgehog pathway in the epithelial-mesenchymal transition. This stimulation is important in melanoma invasion and metastasis. However, how macroscopic mechanical forces regulate cell fate through genetic/epigenetic alterations remains unclear in acral melanoma. Further prospective investigation of mechanical stress and genetic/epigenetic alterations will be needed.
PTEN, a tumor-suppressor gene, is implicated in cellular differentiation, reproduction, and apoptosis, as well as in cellular adhesion and mobility. Multiple studies have shown reduced, but not absent, PTEN in melanoma29,30. Recent studies have shown that complete or partial loss of PTEN in melanoma is associated with poor overall survival31,32. However, PTEN loss cannot be fully explained by genetic alteration. In the New York University and the Cancer Genome Atlas (TCGA) melanoma cohorts, PTEN mutations and deletions were relatively uncommon in melanoma32. PTEN promoter hypermethylation has also been associated with loss of PTEN in melanoma. In the TCGA and Korean melanoma cohorts, PTEN promoter methylation was a significant negative prognostic marker of survival for melanoma patients7,11. Our study showed that PTEN promoter methylation is associated with increased Breslow thickness and higher ulceration rates in acral melanomas. This result was limited to the heel, forefoot, and hallux. Based on these clinical results, additional studies will be needed to investigate whether epigenetic alterations of PTEN, such as hyper- or hypomethylation and histone modifications, are associated with PTEN loss in acral melanoma. In selected acral melanoma patients, induction of tumor PTEN expression above a threshold level might suppress Akt activity and tumor growth and promote anti-tumor immunity to improve patient survival33.
There are a number of limitations of this study. First, as a retrospective single-center study, the sample size is small. Further large-scale and population-based analysis containing more samples, especially on midfoot and lesser toes, is needed to clearly show the relationship between mechanical stress and genetic/epigenetic alterations. Second, the methodology used to analyze weight bearing area was different from other studies. However, there is no study clarifying where is weight bearing area of sole. In this study, sole was divided into two parts considering pedobarography results of non-melanoma patients while walking and standing. In future study, measuring pedobarography of acral melanoma patients may help to consider not only weight bearing portion of sole, but also the external pressure details such as personal walking habits, pressure caused by shoes, and pressure when lying down during sleep.
In conclusion, we have characterized genetic/epigenetic alterations and the relationship with pressure in 52 acral melanoma patients. We have shown that about 25% of acral melanoma patients harbor PTEN promoter hypermethylation. Moreover, epigenetic alterations, like PTEN promoter hypermethylation, are related to increased Breslow thickness and higher ulceration rates on the heel, forefoot, and hallux in acral melanoma patients. These results could help identify the possible role of mechanical stress in promoting acral melanoma. Further prospective investigation of molecular alterations will be needed to understand the relationship between mechanical stress and genetic/epigenetic alterations.
ACKNOWLEDGMENT
We wish to thank Sinae Kim, a biostatistician at the Yonsei Medical Research Center, for advice on statistical analysis.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2017R1C1B2005574).
DATA SHARING STATEMENT
Research data are not shared.
References
- 1.Viros A, Fridlyand J, Bauer J, Lasithiotakis K, Garbe C, Pinkel D, et al. Improving melanoma classification by integrating genetic and morphologic features. PLoS Med. 2008;5:e120. doi: 10.1371/journal.pmed.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–180. doi: 10.1038/nature22071. [DOI] [PubMed] [Google Scholar]
- 3.Merkel EA, Gerami P. Malignant melanoma of sun-protected sites: a review of clinical, histological, and molecular features. Lab Invest. 2017;97:630–635. doi: 10.1038/labinvest.2016.147. [DOI] [PubMed] [Google Scholar]
- 4.Jang HS, Kim JH, Park KH, Lee JS, Bae JM, Oh BH, et al. Comparison of melanoma subtypes among Korean patients by morphologic features and ultraviolet exposure. Ann Dermatol. 2014;26:485–490. doi: 10.5021/ad.2014.26.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Zhao Y, Ma S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer. 2016;16:691. doi: 10.1186/s12885-016-2747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zebary A, Omholt K, Vassilaki I, Höiom V, Lindén D, Viberg L, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72:284–289. doi: 10.1016/j.jdermsci.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Liau JY, Tsai JH, Jeng YM, Chu CY, Kuo KT, Liang CW. TERT promoter mutation is uncommon in acral lentiginous melanoma. J Cutan Pathol. 2014;41:504–508. doi: 10.1111/cup.12323. [DOI] [PubMed] [Google Scholar]
- 9.Carrera C, Puig-Butille JA. Clinical, epidemiological, and molecular heterogeneity in acral melanoma. J Invest Dermatol. 2018;138:254–255. doi: 10.1016/j.jid.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 10.de Unamuno Bustos B, Murria Estal R, Pérez Simó G, Simarro Farinos J, Pujol Marco C, Navarro Mira M, et al. Aberrant DNA methylation is associated with aggressive clinicopathological features and poor survival in cutaneous melanoma. Br J Dermatol. 2018;179:394–404. doi: 10.1111/bjd.16254. [DOI] [PubMed] [Google Scholar]
- 11.Roh MR, Gupta S, Park KH, Chung KY, Lauss M, Flaherty KT, et al. Promoter methylation of PTEN is a significant prognostic factor in melanoma survival. J Invest Dermatol. 2016;136:1002–1011. doi: 10.1016/j.jid.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Minagawa A, Omodaka T, Okuyama R. Melanomas and mechanical stress points on the plantar surface of the foot. N Engl J Med. 2016;374:2404–2406. doi: 10.1056/NEJMc1512354. [DOI] [PubMed] [Google Scholar]
- 13.Costello CM, Pittelkow MR, Mangold AR. Acral melanoma and mechanical stress on the plantar surface of the foot. N Engl J Med. 2017;377:395–396. doi: 10.1056/NEJMc1706162. [DOI] [PubMed] [Google Scholar]
- 14.Rambhia PH, Stojanov IJ, Arbesman J. Predominance of oral mucosal melanoma in areas of high mechanical stress. J Am Acad Dermatol. 2019;80:1133–1135. doi: 10.1016/j.jaad.2018.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosokawa M, Kato T, Seiji M, Abe R. Plantar malignant melanoma. Statistical and clinicopathological studies. J Dermatol. 1980;7:137–142. doi: 10.1111/j.1346-8138.1980.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 16.Dwyer PK, Mackie RM, Watt DC, Aitchison TC. Plantar malignant melanoma in a white Caucasian population. Br J Dermatol. 1993;128:115–120. doi: 10.1111/j.1365-2133.1993.tb15138.x. [DOI] [PubMed] [Google Scholar]
- 17.Jung HJ, Kweon SS, Lee JB, Lee SC, Yun SJ. A clinicopathologic analysis of 177 acral melanomas in Koreans: relevance of spreading pattern and physical stress. JAMA Dermatol. 2013;149:1281–1288. doi: 10.1001/jamadermatol.2013.5853. [DOI] [PubMed] [Google Scholar]
- 18.Stucke S, McFarland D, Goss L, Fonov S, McMillan GR, Tucker A, et al. Spatial relationships between shearing stresses and pressure on the plantar skin surface during gait. J Biomech. 2012;45:619–622. doi: 10.1016/j.jbiomech.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galica AM, Hagedorn TJ, Dufour AB, Riskowski JL, Hillstrom HJ, Casey VA, et al. Hallux valgus and plantar pressure loading: the Framingham foot study. J Foot Ankle Res. 2013;6:42. doi: 10.1186/1757-1146-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skopljak A, Muftic M, Sukalo A, Masic I, Zunic L. Pedobarography in diagnosis and clinical application. Acta Inform Med. 2014;22:374–378. doi: 10.5455/aim.2014.22.374-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, Kim JE, Jang HS, Park KH, Oh BH, Shin SJ, et al. Genetic alterations among Korean melanoma patients showing tumor heterogeneity: a comparison between primary tumors and corresponding metastatic lesions. Cancer Res Treat. 2018;50:1378–1387. doi: 10.4143/crt.2017.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roh MR, Park KH, Chung KY, Shin SJ, Rha SY, Tsao H. Telomerase reverse transcriptase (TERT) promoter mutations in Korean melanoma patients. Am J Cancer Res. 2017;7:134–138. [PMC free article] [PubMed] [Google Scholar]
- 23.Vazquez Vde L, Vicente AL, Carloni A, Berardinelli G, Soares P, Scapulatempo C, et al. Molecular profiling, including TERT promoter mutations, of acral lentiginous melanomas. Melanoma Res. 2016;26:93–99. doi: 10.1097/CMR.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 24.Boer J, Nazary M, Riis PT. The role of mechanical stress in hidradenitis suppurativa. Dermatol Clin. 2016;34:37–43. doi: 10.1016/j.det.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Sheen YS, Liao YH, Lin MH, Chen JS, Liau JY, Tseng YJ, et al. A clinicopathological analysis of 153 acral melanomas and the relevance of mechanical stress. Sci Rep. 2017;7:5564. doi: 10.1038/s41598-017-05809-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pressure ulcers in America: prevalence, incidence, and implications for the future. An executive summary of the National Pressure Ulcer Advisory Panel monograph. Adv Skin Wound Care. 2001;14:208–215. doi: 10.1097/00129334-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Hodges GJ, Stewart DG, Davison PJ, Cheung SS. The role of shear stress on cutaneous microvascular endothelial function in humans. Eur J Appl Physiol. 2017;117:2457–2468. doi: 10.1007/s00421-017-3732-8. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Zhou F, Shen Y, Zhang Y, Yin H, Zeng Y, et al. Fluid shear stress induces epithelial-mesenchymal transition (EMT) in Hep-2 cells. Oncotarget. 2016;7:32876–32892. doi: 10.18632/oncotarget.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikhail M, Velazquez E, Shapiro R, Berman R, Pavlick A, Sorhaindo L, et al. PTEN expression in melanoma: relationship with patient survival, Bcl-2 expression, and proliferation. Clin Cancer Res. 2005;11:5153–5157. doi: 10.1158/1078-0432.CCR-05-0397. [DOI] [PubMed] [Google Scholar]
- 30.Zhou XP, Gimm O, Hampel H, Niemann T, Walker MJ, Eng C. Epigenetic PTEN silencing in malignant melanomas without PTEN mutation. Am J Pathol. 2000;157:1123–1128. doi: 10.1016/S0002-9440(10)64627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bucheit AD, Chen G, Siroy A, Tetzlaff M, Broaddus R, Milton D, et al. Complete loss of PTEN protein expression correlates with shorter time to brain metastasis and survival in stage IIIB/C melanoma patients with BRAFV600 mutations. Clin Cancer Res. 2014;20:5527–5536. doi: 10.1158/1078-0432.CCR-14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giles KM, Rosenbaum BE, Berger M, Izsak A, Li Y, Illa Bochaca I, et al. Revisiting the clinical and biologic relevance of partial PTEN loss in melanoma. J Invest Dermatol. 2019;139:430–438. doi: 10.1016/j.jid.2018.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hopkins BD, Parsons RE. Molecular pathways: intercellular PTEN and the potential of PTEN restoration therapy. Clin Cancer Res. 2014;20:5379–5383. doi: 10.1158/1078-0432.CCR-13-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.