Abstract
Background
Recent epidemiological studies have demonstrated that air pollution is associated with the inflammatory response and may aggravate inflammatory skin diseases such as atopic dermatitis (AD). However, it is unclear whether particulate matter (PM) aggravates AD symptoms.
Objective
The aim of this study was to investigate whether PM exposure affects the skin barrier dysfunction and aggravates AD symptoms using human keratinocytes (HaCaT) cells and a mouse model of oxazolone-induced AD-like skin.
Methods
Standard reference material (SRM) 1649b, which mainly comprises polycyclic aromatic hydrocarbons, was used as the reference PM. HaCaT cells and mouse model of oxazolone-induced AD-like skin were treated with PM. The mRNA or protein expression levels of stratum corneum (SC) and tight junction (TJ) proteins, inflammatory cytokines, as well as clinical and histological changes of the AD-like skin of mouse model were evaluated. The expression of genes and proteins was analyzed by real-time polymerase chain reaction and Western blotting. Levels of inflammatory cytokines were measured by enzyme-linked immunosorbent assay.
Results
The results revealed that PM downregulates the expression levels of several SC and TJ-related proteins in the mouse model with AD-like skin. Clinically, epidermal and dermal thickness was significantly increased and dermal inflammation was prominent in PM treated AD-like skin.
Conclusion
In conclusion, we found that PM aggravates skin barrier dysfunction, clinically augmenting epidermal and dermal thickening with dermal inflammation in AD-like skin. These results suggest that PM may trigger the exacerbation of AD symptoms via skin barrier dysfunction-related mechanisms.
Keywords: Air pollution, Atopic dermatitis, Epidermis, Particulate matter
INTRODUCTION
Particulate air pollutants (particulate matter, PM) are a complex mixture containing various substances such as metals, minerals, organic toxins, and various contaminants (smog, tobacco smoke, pollen, and house dust mite allergens)1,2,3. Ambient PM is known to have harmful effects on human health4,5,6. The World Health Organization estimates that PM contributes to approximately 800,000 premature deaths each year, ranking it the 13th leading cause of mortality worldwide5. In particular, ambient PM is highly associated with the morbidity of respiratory and cardiovascular diseases7,8. Furthermore, recent epidemiological investigations have demonstrated that PM is also correlated with inflammatory skin diseases such as atopic dermatitis (AD), acne, psoriasis, and allergic reactions9.
The skin is the largest organ in the human body and acts as an essential barrier to protect against harmful external factors such as ultraviolet radiation and ambient PM10. The barrier function of the skin is primarily dependent on two major barrier structures: the stratum corneum (SC), the outermost layer of the epidermis, and the tight junction (TJ) proteins, intercellular junctions that seal adjacent keratinocytes in the stratum granulosum1,11,12,13. However, various environmental pollutants can downregulate the expression of the structural proteins of the SC and TJ proteins3,14,15,16, leading to skin barrier dysfunction and skin inflammation3,16,17. Furthermore, PM can penetrate the skin through hair follicles and cause epidermal thickening and dermal inflammation in disrupted skin17,18.
AD is a chronic relapsing inflammatory skin disease characterized by complex interactions among genetic predisposition, environmental factors, immune dysregulation, and skin barrier dysfunction19,20,21,22. Recently, several epidemiologic studies have investigated the effects of PM in patients with AD1,22,23,24,25,26. However, the results of these studies are controversial: several studies demonstrated that ambient PM is related to the exacerbation of AD symptoms22,23,24,27; in contrast, others report that AD is not related to urbanization and industrialization25,26. Moreover, most previous studies primarily focused on the epidemiological perspective; thus, studies on the mechanism by which PM causes or worsens AD symptoms have not been well performed. Therefore, in this study, we aimed to investigate whether PM exposure affects the exacerbation of AD-like symptoms and elucidate the mechanisms by which PM affects AD-like skin.
MATERIALS AND METHODS
Chemicals and preparation
The SRMs 1648a and 1649b were purchased from the National Institute of Standards and Technology (Gaithersburg, MD, USA) and dispersed in distilled water. SRM 1648a mainly contains heavy metals, whereas SRM 1649b includes polycyclic aromatic hydrocarbons, polychlorinated biphenyl congeners, and pesticides3. Recombinant human interleukin (IL)-4 and IL-13 were purchased from PeproTech (Rocky Hill, NJ, USA). 4-Ethoxymethylene-2-phenyl-2-oxazolin-5-one (OXA) was purchased from Sigma-Aldrich (St Louis, MO, USA). OXA was dissolved in vehicle (acetone: olive oil=4:1 mixture). For western blot analysis, anti-occludin, anti-claudin-1, anti-involucrin, and anti-β-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-loricrin and anti-filaggrin were purchased from LifeSpan Biosciences (Seattle, WA, USA). Anti-E-cadherin and anti-GAPDH antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-ZO-1 was purchased from Thermo Fisher Scientific (Waltham, MA, USA). A mouse immunoglobulin E (IgE) ELISA kit was purchased from Thermo Fisher Scientific.
Cell culture
Human keratinocyte (HaCaT) cells (American Tissue Cell Collection [ATCC] obtained in 2002, USA) were cultured in Dulbecco's modified Eagle's medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Cells were propagated at 37℃ in a humidified atmosphere of 5% CO2. When the cultures reached confluence, cells were treated with 0.05% trypsin/0.53 mM EDTA for 5 minutes at 37℃. HaCaT cells were used in the experiments between passages 20 to 24.
HaCaT cells were treated with IL-4, IL-13, and particulate matters
Since type 2 helper T cell (Th2) cytokines IL-4 and IL-13 have been implicated in the pathogenesis of AD, HaCaT cells were treated with 50 ng/ml concentrations of recombinant human IL-4 and IL-13 to establish an in vitro model of AD. After incubation for 72 hours, the cells were treated with PMs (SRM 1648a and 1649b) at concentrations of 50 µg/cm2 and 25 µg/cm2, respectively. The plates were then incubated at 37℃ for 24 hours. After incubation, all of the HaCaT cells, including the control, were evaluated at the same time point. PMs were used at the optimal concentrations mentioned in prior studies28,29.
Animals
Six-week-old female BALB/c mice (n=5 in each experimental condition; n=20 in total) were housed under specific pathogen-free conditions and allowed to acclimatize for 1 week before starting the experiment. All animal experiments were performed in accordance with the regulations and upon the approval of the Institutional Animal Care and Use Committee (IACUC) of the Chung-Ang University (IRB Approval No. 2018-00097).
Induction of atopic dermatitis-like skin lesions and exposure to particulate matter
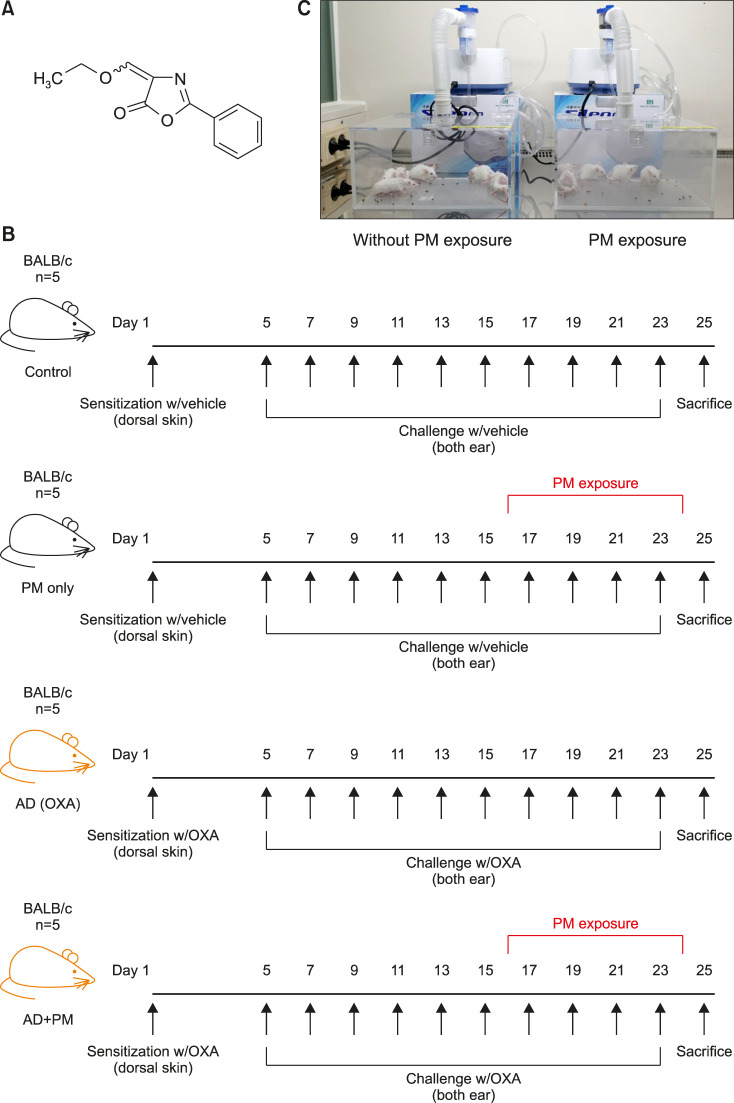
The mouse model with OXA-induced AD-like skin is a well-known animal model for AD-like symptoms. It is induced by multiple epicutaneous administrations of OXA (Fig. 1A), a potent hapten that induces a chronic Th2 hypersensitivity reaction resembling the features of initial human AD30.
Fig. 1. Experimental protocol of this study. (A) Chemical structure of oxazolone. (B) Experimental protocol for the induction of AD-like skin and PM exposure. (C) Closed-system chamber attached to a nebulizer for PM exposure. AD: atopic dermatitis, PM: particulate matter, OXA: oxazolone.
The dorsal hair of the mice was shaved 2 days before initiating the process of sensitization. Then, 10 mice each were sensitized with 20 µl of 1% OXA or vehicle applied to the shaved dorsal skin. Four days after sensitization, the ears of 10 mice sensitized with vehicle were repeatedly challenged with vehicle, and the ears of 10 mice sensitized with OXA were repeatedly challenged with 20 µl of 0.1% OXA once every other day for 18 days (Fig. 1B). After performing the OXA or vehicle challenge six times, 5 mice from each group were exposed to 100 µg/cm3 SRM 1649b for 1 h/day in a closed-system chamber attached to a nebulizer (Macjin Medical, Seoul, Korea), once every other day for 10 days (Fig. 1C). The other 5 mice from each group were left in the same closed-system chamber attached to a nebulizer but without exposing to PM (Fig. 1C).
Ear thickness was measured using a digital caliper (Mitutoyo, Tokyo, Japan) before every challenge and immediately before sacrifice. Ear skin and blood samples were collected after the mice were anesthetized using 2,2,2-tribromoethanol (Avertin; Sigma-Aldrich). OXA treatment was performed and SRM 1649b exposure conditions were in accordance with previously described protocols with modifications21,30,31,32,33.
RNA extraction and quantitative real-time PCR analysis
Total RNA was extracted from the cells and ear tissues using TRIzol® reagent (Welgene, Seoul, Korea) according to the manufacturer's protocol. cDNA templates were synthesized from mRNA by reverse transcription using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) and incubated for 1 hours at 42℃. The resultant cDNA was then amplified by quantitative real-time PCR (qPCR) with primers specific for IL-1α, IL-1β, IL-4, IL-6, IL-13, CXCL1, CXCL2, CXCL5, MMP13, TNF-α, TSLP, TARC, TLR2, TLR4, involucrin, loricrin, filaggrin, claudin-1, ZO-1, and GAPDH. qPCR assays were performed on a real-time thermal cycler (Applied Biosystems; Thermo Fischer Scientific) using PowerUp SYBR Green Master mix (Applied Biosystems; Thermo Fisher Scientific). All results were normalized to the level of GAPDH. Relative quantitation was analyzed using the comparative ΔΔCt method according to the manufacturer's instructions.
Western blot analysis
Protein samples were prepared from the skin tissues, and the HaCaT cells were cultured with 1% Triton-X radioimmunoprecipitation assay buffer containing a protease inhibitor cocktail. The protein concentrations of the lysed cells and homogenized skin tissue samples were determined using the bicinchoninic acid assay. Equal amounts of protein were loaded onto 8%~10% sodium dodecyl sulfate-polyacrylamide gels. After electrophoresis, the proteins were transferred onto nitrocellulose membranes. The membranes were blocked with 5% skim milk for 1 hour. Subsequently, the membranes were incubated overnight with primary antibodies at 4℃; subsequently, they were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. Protein expression was detected using the EzWestLumi Plus system (ATTO, Tokyo, Japan), and images were captured by exposing the membranes to a ChemiDoc™ XRS image analyzer (Bio-Rad, Hercules, CA, USA).
Enzyme-linked immunosorbent assay
Total IgE levels were analyzed using enzyme-linked immunosorbent assay (ELISA). Blood samples were collected from the intraorbital vein of the mice on sacrifice. The collected whole blood was left undisturbed at room temperature to allow the blood to clot. Serum samples were separated from the whole blood and stored at −80℃ until analysis. Total IgE levels were quantified using ELISA kits (Thermo Fisher Scientific) according to the manufacturer's protocol.
Histological examination
The ear skin of each mouse was fixed in 4% paraformaldehyde solution. Then, tissues were embedded in paraffin and sliced into 5-µm-thick sections; the tissue sections were stained with hematoxylin and eosin (H&E). Histological changes were examined via light microscopy (Leica D750; Leica Microsystems, Wetzlar, Germany). Means of SC, epidermal, and dermal thickness was quantified by measuring the thickness of 10 randomly selected area of the tissue section with the aid of ImageJ Software (NIH, Bethesda, MD, USA; http://imagej.nih.gov/ij).
Statistical analysis
Each in vivo assay was performed independently at least twice. Data from each assay are presented as the mean± standard deviation (SD). Data were analyzed using oneway or two-way analysis of variance (ANOVA) with the multiple correction test algorithm of Bonferroni. We considered p-values<0.05 significant. Statistical analysis was performed using SPSS (PASW statistics ver. 18; IBM Corp., Armonk, NY, USA).
RESULTS
Effects of SRM 1648a and 1649b on HaCaT cells pretreated with IL-4 and IL-13
First, we investigated the effects of SRM 1648a and 1649b on the SC-related proteins and TJ proteins in HaCaT cells pretreated with IL-4 and IL-13.
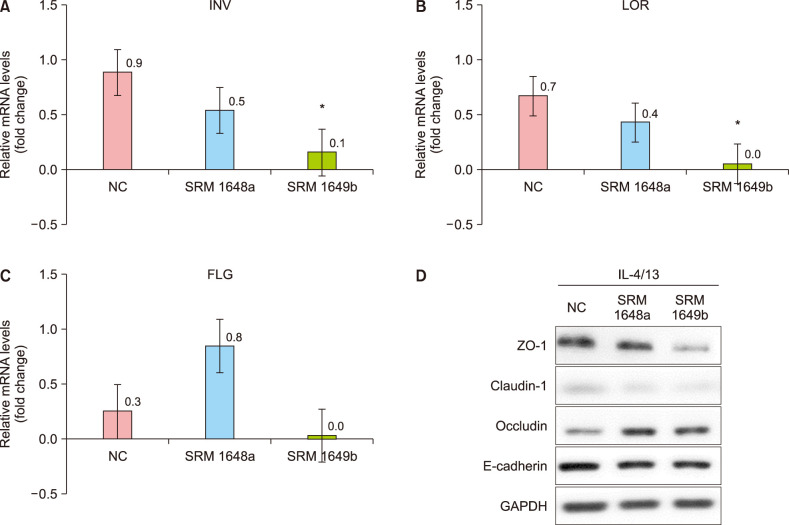
As shown in Fig. 2, exposure to SRM 1649b significantly decreased the mRNA expression of involucrin and loricrin and the protein expression of claudin-1 and ZO-1 in HaCaT cells pretreated with IL-4 and IL-13. Exposure to SRM 1649b also decreased the mRNA expression of filaggrin; however, the changes were not significant. In contrast, the decreased mRNA expression of involucrin and loricrin with exposure to SRM 1648a was not significant, and the mRNA expression of filaggrin was rather increased. However, these changes were not statistically significant. In general, there was a greater decrease in the mRNA and protein expression levels in HaCaT cells exposed to SRM 1649b than in those exposed to 1648a. These results indicate that SRM 1649b comprising polycyclic aromatic hydrocarbons, polychlorinated biphenyl congeners, and pesticides is more toxic to human keratinocytes than 1648a comprising heavy metals. These results are also consistent with the results of previous studies, which indicated that 1649b, but not 1648a, significantly disrupted the SC and TJ proteins3. Therefore, in the following animal experiments, only SRM 1649b was used.
Fig. 2. Standard reference material (SRM) 1649b significantly decreased the mRNA expression levels of INV and LOR, and the protein expression of claudin-1 and ZO-1 in HaCaT cells pretreated with interleukin (IL)-4 and IL-13. On the other hand, although SRM 1648a decreased the mRNA expression levels of INV and LOR, the changes were not statistically significant. In general, there was a greater decrease in the mRNA and protein expression levels in HaCaT cells exposed to SRM 1649b than in those exposed to SRM 1648a. The mRNA expression levels of (A) INV, (B) LOR, and (C) FLG in HaCaT cells were determined by real-time quantitative PCR. (D) The protein levels of ZO-1, claudin-1, occludin, and E-cadherin were determined by western blotting. Cells were treated with SRM 1648a (50 µg/cm2) and SRM 1649b (25 µg/cm2) for 24 hours following IL-4 and IL-13 (50 ng/ml) pre-treatment for 72 hours, respectively. Values are presented as mean±standard deviation of two independent experiments (n=2). NC: normal control, INV: involucrin, LOR: loricrin, FLG: filaggrin. *p<0.05 vs. NC.
Effects of SRM 1649b on ear thickness in the mouse model with oxazolone-induced atopic dermatitis-like skin
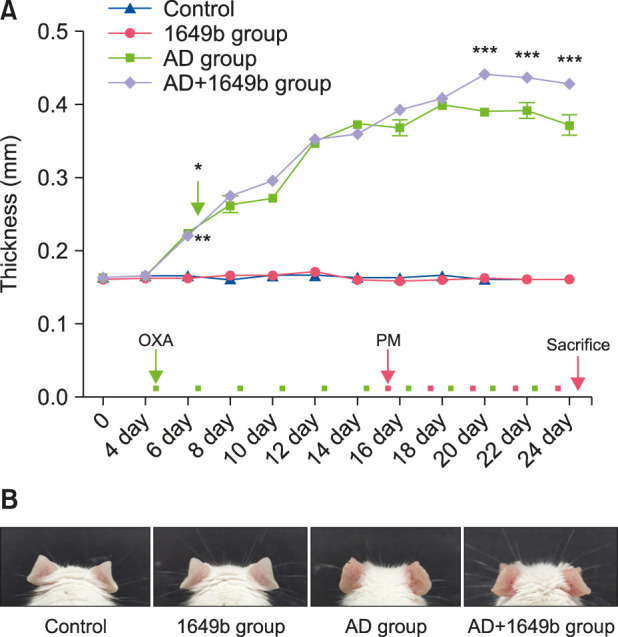
To examine the effects of repeated SRM 1649b exposure on skin thickness in the mouse model with OXA-induced AD-like skin lesions, we measured ear thickness using a digital caliper before each OXA challenge (which was performed once every other day) and immediately before sacrifice. There was no significant difference between the normal control and the SRM 1649b-exposed control group (1649b group); however, there was a significant increase in ear thickness in the OXA-induced AD group (AD group) and the SRM 1649b-exposed OXA-induced AD group (AD+1649b group). In addition, ear thickness was significantly higher in the AD+1649b group than in the AD group after exposure (three times) to SRM 1649b (Fig. 3).
Fig. 3. (A) Repeated exposure to standard reference material (SRM) 1649b increased the thickness of the AD-like skin in the oxazolone (OXA)-induced AD-like mouse model. Ear thickness significantly increased in the AD groups. Furthermore, ear thickness was further increased in the AD+1649b group compared to the AD group. Ear thickness was measured using a digital caliper before each OXA challenge and immediately before sacrifice. (B) Exposure to SRM 1649b induced changes in morphology in the OXA-induced AD-like mouse model. Clinical features were observed in the images taken during the repeated application of OXA and SRM 1649b in the mouse model. Values are presented as mean±standard deviation of two independent experiments (n=2). AD: atopic dermatitis, PM: particulate matter, 1649b group: SRM 1649b-exposed control group, AD+1649b group: SRM 1649b-exposed OXA-induced AD group, AD group: OXA-induced AD group. *p<0.01 1649b group vs. AD+1649b group. *p<0.01 Control group vs. AD group. ***p<0.001 AD group vs. AD+1649b group.
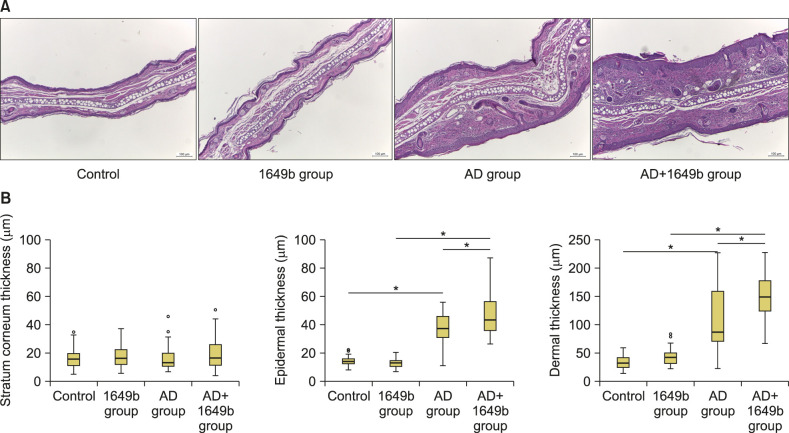
Histological evaluation further confirmed that the hyperkeratosis and epidermal acanthosis were more pronounced and dermal inflammation was increased in the AD+1649b group (Fig. 4A). When we measured the thickness of each compartment (SC, epidermis, and dermis), the epidermal and dermal thickness was significantly increased in the AD+1649b group compared to AD group (Fig. 4B).
Fig. 4. (A) Histological examination confirmed that epidermal and dermal thickness were increased with dermal inflammatory cell infiltration in the AD+1649b group compared to the AD group (H&E, Scale bar=100 µm). Hematoxylin and eosin stain. (B) The epidermal and dermal thickness was significantly increased in the AD+1649b group compared to AD group. The thicknesses of each compartment (stratum corneum, epidermis, and dermis) were quantified by measuring the thickness of 10 randomly selected area of the tissue section with the aid of ImageJ Software (NIH, Bethesda, MD, USA; http://imagej.nih.gov/ij). AD: atopic dermatitis, 1649b group: standard reference material (SRM) 1649b-exposed control group, AD+1649b group: SRM 1649b-exposed oxazolone (OXA)-induced AD group, AD group: OXA-induced AD group. *p<0.05 using ANOVA with the multiple correction test algorithm of Bonferroni.
Effects of SRM 1649b on inflammatory cytokine, chemokine, and toll-like receptor production in the mouse model with oxazolone-induced atopic dermatitis-like skin
To investigate the effects of SRM 1649b on inflammatory responses in OXA-induced AD-like skin lesions, we determined the levels of inflammatory cytokines, chemokines, and toll-like receptor using qPCR. Although mice do not have a human IL-8 homolog, CXCL1 (KC), CXCL2 (MIP-2), and CXCL5 (LIX) are recognized as functional homologs17,34. Therefore, the mRNA levels of IL-1α, IL-1β, IL-4, IL-6, IL-13, CXCL1 (KC), CXCL2 (MIP-2), CXCL5 (LIX), TNF-α, TSLP, TARC, TLR2, TLR4, and MMP13 were analyzed.
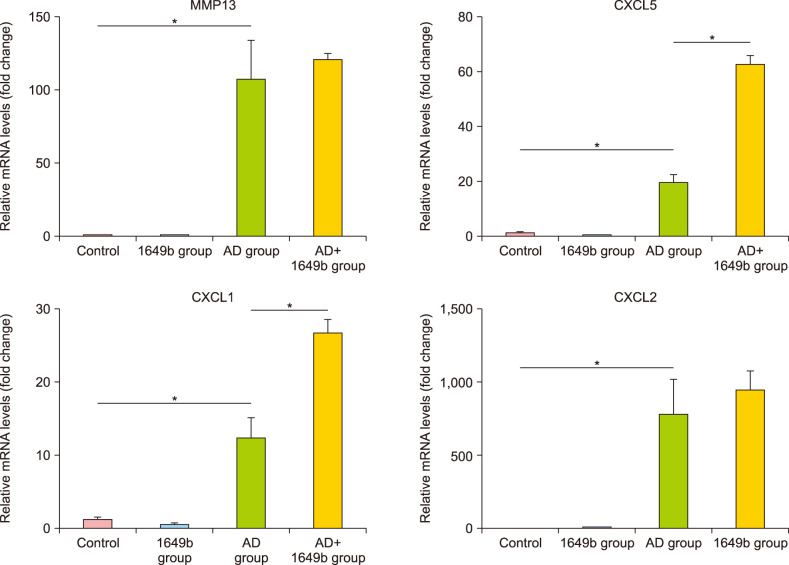
The mRNA levels of MMP13, CXCL1, CXCL2, and CXCL5 significantly increased in the AD group compared to the control and 1649b group. Furthermore, subsequent PM exposure increased all four mRNA levels, and in particular the mRNA levels of CXCL1 and CXCL5 significantly increased in AD+1649b compared to AD group (Fig. 5). There was no statistically significant difference in the mRNA expression levels of other inflammatory cytokines, chemokines, and toll-like receptor between the groups.
Fig. 5. Effects of standard reference material (SRM) 1649b on the inflammatory response in oxazolone (OXA) induced dermatitis-like mice model. The mRNA levels of MMP13, CXCL1, CXCL2, and CXCL5 significantly increased in the atopic dermatitis (AD) group compared to the control and 1649b group. The mRNA levels of CXCL1 and CXCL5 significantly increased in AD+1649b compared to AD group. The mRNA expression of were measured by real-time quantitative PCR. The relative mRNA and protein expression levels in AD-like mice model are shown. These levels were normalized to those of β-actin. Values are presented as mean±standard deviation of two independent experiments (n=2). 1649b group: SRM 1649b-exposed control group, AD+1649b group: SRM 1649b-exposed OXA-induced AD group, AD group: OXA-induced AD group. *p<0.05.
Effects of SRM 1649b on stratum corneum-related protein and tight junction protein expression in the mouse model with oxazolone-induced atopic dermatitis-like skin
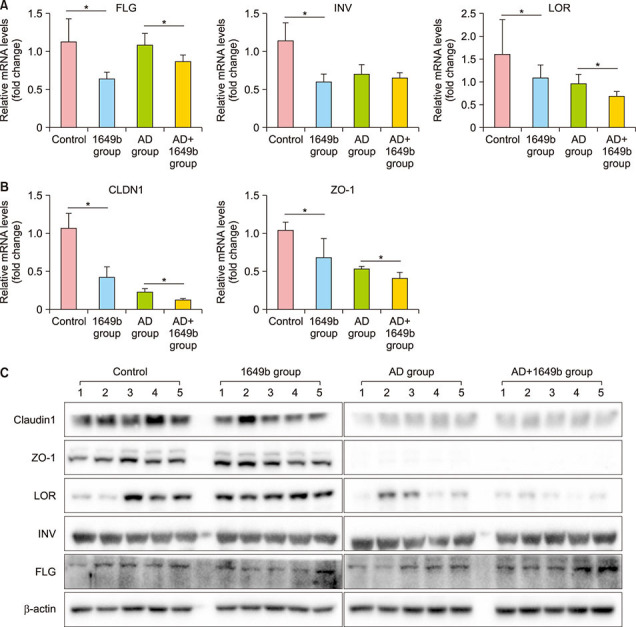
To investigate the effects of SRM 1649b on the expression of SC-related proteins and TJ proteins in the mouse model with OXA-induced AD-like skin lesions, we analyzed the expression levels of involucrin, loricrin, filaggrin, claudin-1, and ZO-1 using qPCR and western blot analysis. As presented in Fig. 6, SRM 1649b exposure reduced the mRNA and protein expression levels of filaggrin, involucrin, loricrin, claudin-1, and ZO-1 in both the control and OXA-induced AD groups. However, the decrease in the expression of the SC-related proteins and TJ, proteins after SRM 1649b exposure was comparable between the two groups. These results suggest that PM downregulates the expression of SC-related proteins and TJ proteins in both healthy and AD skin.
Fig. 6. Standard reference material (SRM) 1649b exposure reduced the mRNA and protein expression levels of FLG, INV, LOR, CLDN-1, and ZO-1 in both control and oxazolone (OXA)-induced atopic dermatitis (AD) groups. However, the decrease in the expression of the stratum corneum-related proteins and tight junction proteins after SRM exposure was comparable between the two groups. The mRNA and protein levels of skin barrier-related proteins were analyzed using (A, B) real-time quantitative PCR and (C) western blotting, respectively. The relative mRNA and protein expression levels in the AD-like mouse model are shown. These levels were normalized to the mRNA expression levels of β-actin. Values are presented as mean±standard deviation of two independent experiments (n=2). FLG: filaggrin, INV: involucrin, LOR: loricrin, CLDN-1: claudin-1, 1649b group: SRM 1649b-exposed control group, AD+1649b group: SRM 1649b-exposed OXA-induced AD group, AD group: OXA-induced AD group. *p<0.05.
Effects of SRM 1649b on immunoglobulin E production in the mouse model with oxazolone-induced atopic dermatitis-like skin
To investigate whether SRM 1649b induces IgE production in mice, we determined the serum IgE levels using ELISA analysis. For ELISA analysis, serum was isolated from the collected whole blood of the mice. The serum IgE levels were higher in the OXA-induced AD groups (AD group and AD+1649b group) than in the control group. However, there was no significant difference in the serum IgE levels between the AD group and the AD+1649b group (data not shown).
DISCUSSION
In this study, we investigated whether PM aggravates ADlike symptoms by evaluating the epidermal thickness and histological changes in a mouse model with OXA-induced AD-like skin. We also investigated whether PM affects the expression levels of SC-related proteins and TJ proteins because the decreased expression of these proteins is related to various skin disorders and can cause skin barrier dysfunction.
As shown in Fig. 3, we found that the ear thickness of the mouse model with AD-like skin was significantly increased after exposure to PM. Histological evaluation further confirmed significant increase in the epidermal and dermal thickness with heavy dermal cell infiltration after PM exposure in AD-like skin. These results show that PM exposure clinically exacerbates AD symptoms characterized by epidermal acanthosis and dermal inflammation.
Recently, the focus on the pathogenesis of AD has evolved from concentration on immune system dysfunction as the primary abnormality in disease development to the incorporation of the concept of epidermal barrier dysfunction35. Numerous alterations and deficiencies in the SC-related proteins and TJ proteins in the skin of atopic individuals have been implicated in the disturbed skin barrier, highlighting the importance of the skin barrier in disease development. For instance, in both the lesional and nonlesional skin of patients with AD, filaggrin mutation has been shown to contribute to AD pathogenesis. Loricrin and involucrin, two other important proteins that facilitate the terminal differentiation of the epidermis and the formation of the skin barrier, are known to be downregulated in AD skin36. Furthermore, humans with filaggrin mutations show decreased expression of TJ proteins such as occludin and ZO-137. In addition, reduced expression of the TJ protein claudin-1 has also been demonstrated in the nonlesional skin of individuals with AD38.
The results of our study showed that PM downregulates the expression of SC-related proteins and TJ proteins in both control and AD skin. These results are also consistent with those of previous studies. Pan et al.3 have shown that SRM 1649b disrupted the SC and TJs in pig skin. Some proteins related to the barrier function, such as cytokeratin, filaggrin, and E-cadherin, were also diminished after 1649b treatment. Lehmann et al.39 have also shown that PMs damage the TJs of the lung epithelial barrier. Lee et al.16 found that PM induced the downregulation of filaggrin protein and mRNA expression in a time- and dose-dependent manner in human keratinocytes via cyclooxygenase 2 expression and prostaglandin E2 production. However, all these previous studies have only evaluated the effects of PM on the skin barrier function in non-AD, normal, intact skin. None of the previous studies have evaluated the effects of PM on the skin barrier function in AD skin.
Our results suggest that PM exposure disrupts normal keratinocyte differentiation in both normal and AD skin. These effects were neither more specific nor greater in AD skin than in normal intact skin. However, in AD skin, where the skin barrier is already impaired, the disruption in normal keratinocyte differentiation would halt barrier repair and further aggravate skin barrier dysfunction. For instance, deregulation of keratinocyte differentiation and activation markers have been implicated in the non-healing edges of patients with venous ulcer40. Similarly, PM exposure, which leads to the deregulation of keratinocyte differentiation, would disturb the normal healing process of the impaired skin barrier in patients with AD.
The impaired skin barrier would allow further allergen exposure, which subsequently drives inflammation and the release of cytokines, which, in turn, reduce the skin barrier function, leading to a vicious cycle41,42. In our previous study, we showed that PM exposure can induce the expression of pro-inflammatory cytokines in HaCaT cells when the cells are directly treated with PM29. In this study, we found that the mRNA levels of functional homologues of human IL-8 cytokine (CXCL1, CXCL2, and CXCL5) and MMP13 increased following PM exposure in AD skin, although statistically significant changes were observed only in CXCL1 and CXCL5. These results are in concordance with previous reports by Jin et al.17 where they found that the mRNA levels of CXCL1, CXCL2, CXCL5, and MMP13 were significantly increased when PM was directly applied with transparent film dressing on the tape-stripped dorsal skin of mice for 6 consecutive days. Although similar results were observed in our study, statistical significances were only achieved for CXCL1 and CXCL5. This may be due to the limited exposure to PM in our study (mice were exposed to 100 µg/cm3 PM for 1 h/day, once every other day for 10 days), which may not have been enough to experimentally prove the changes in the inflammatory cytokines and chemokines in the in vivo mouse model.
There are several limitations of this study. First, regarding the skin barrier function, we did not evaluate the effect of PM on intercellular lipids of SC including free fatty acids, ceramides, and cholesterol. It is well known that the composition of lipids in SC is important on the integrity of the SC and previous studies have shown that in patients with AD, ceramide levels were lowered and cholesterol was increased in SC43. Furthermore, a few epidemiological studies have shown that people living in heavily polluted areas had higher sebum levels and a lower ratio of squalene/cholesterol44,45. More importantly, a recent study using transcriptomic analysis has shown that PM2.5 could disturb cholesterol homeostasis, and 3-dimensional in vitro skin model treated with PM2.5 showed that the cholesterol levels were much higher than that of the control group46. Thus, PM can impair skin barrier function by interfering with intercellular lipids. However, it should also be noted that barrier disruption itself induces lipid synthesis to promote repair of the skin barrier. Since cholesterol is an important component of cell membranes, damaged skin accelerates the rate of cell proliferation and a large amount of cholesterol is synthesized to supplement keratinocyte, intercellular lipids and repair the damage SC47. Therefore, it is still unclear whether PM directly impairs homeostasis of intercellular lipids of SC. The impaired cholesterol homeostasis may be the compensatory response of the damaged skin barrier to repair itself. Thus, future studies should be performed to elucidate this. Second, although we observed increase in dermal inflammation in the tissue section of AD+1649b skin, the changes in inflammatory cytokines and chemokines were inconsistent following PM exposure. This may be due to the effect of PM directly penetrating into the dermis of barrier-damaged skin as shown by Jin et al17. To elucidate this, further evaluation for gene expressions of NF-κB and quantifying dermal inflammation by immunohistochemical staining for myeloperoxidase are required in the future studies.
In conclusion, we investigated the effects of PM on AD skin using HaCaT cells and a mouse model with AD-like skin. Our results showed that PM exposure decreases the expression levels of SC-related proteins and TJ proteins. Clinically, we found that PM exposure significantly increased the epidermal and dermal thickness of the ears of the model mice with AD-like skin (Fig. 3). Based on these findings, we suggest that PM can aggravate AD symptoms, and that this may be related to the negative effects of PM on the skin barrier function. Therefore, further research should be performed to elucidate the detailed mechanism and biological crosstalk between the epidermis and dermis, when AD skin is exposed to PM.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP; Ministry of Science, ICT &Future Planning) (No. NRF-2017R1C1B5017825).
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Kim KE, Cho D, Park HJ. Air pollution and skin diseases: adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016;152:126–134. doi: 10.1016/j.lfs.2016.03.039. [DOI] [PubMed] [Google Scholar]
- 2.Lee CW, Lin ZC, Hsu LF, Fang JY, Chiang YC, Tsai MH, et al. Eupafolin ameliorates COX-2 expression and PGE2 production in particulate pollutants-exposed human keratinocytes through ROS/MAPKs pathways. J Ethnopharmacol. 2016;189:300–309. doi: 10.1016/j.jep.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Pan TL, Wang PW, Aljuffali IA, Huang CT, Lee CW, Fang JY. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J Dermatol Sci. 2015;78:51–60. doi: 10.1016/j.jdermsci.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Kappos AD, Bruckmann P, Eikmann T, Englert N, Heinrich U, Höppe P, et al. Health effects of particles in ambient air. Int J Hyg Environ Health. 2004;207:399–407. doi: 10.1078/1438-4639-00306. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: a review of the effects of particulate matter air pollution on human health. J Med Toxicol. 2012;8:166–175. doi: 10.1007/s13181-011-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancebo SE, Wang SQ. Recognizing the impact of ambient air pollution on skin health. J Eur Acad Dermatol Venereol. 2015;29:2326–2332. doi: 10.1111/jdv.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinelli N, Olivieri O, Girelli D. Air particulate matter and cardiovascular disease: a narrative review. Eur J Intern Med. 2013;24:295–302. doi: 10.1016/j.ejim.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 9.Krutmann J, Liu W, Li L, Pan X, Crawford M, Sore G, et al. Pollution and skin: from epidemiological and mechanistic studies to clinical implications. J Dermatol Sci. 2014;76:163–168. doi: 10.1016/j.jdermsci.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Hänel KH, Cornelissen C, Lüscher B, Baron JM. Cytokines and the skin barrier. Int J Mol Sci. 2013;14:6720–6745. doi: 10.3390/ijms14046720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004;17 Suppl 1:43–48. doi: 10.1111/j.1396-0296.2004.04s1005.x. [DOI] [PubMed] [Google Scholar]
- 12.Wickett RR, Visscher MO. Structure and function of the epidermal barrier. Am J Infect Control. 2006;34(Suppl 2):S98–S110. [Google Scholar]
- 13.Egawa G, Kabashima K. Barrier dysfunction in the skin allergy. Allergol Int. 2018;67:3–11. doi: 10.1016/j.alit.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Kang Z, Jiang S, Zhao J, Yan S, Xu F, et al. Effects of ambient fine particles PM2.5 on human HaCaT cells. Int J Environ Res Public Health. 2017;14:72. doi: 10.3390/ijerph14010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao R, Guo Z, Zhang R, Deng C, Xu J, Dong W, et al. Nasal epithelial barrier disruption by particulate matter ≤ 2.5 μm via tight junction protein degradation. J Appl Toxicol. 2018;38:678–687. doi: 10.1002/jat.3573. [DOI] [PubMed] [Google Scholar]
- 16.Lee CW, Lin ZC, Hu SC, Chiang YC, Hsu LF, Lin YC, et al. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci Rep. 2016;6:27995. doi: 10.1038/srep27995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin SP, Li Z, Choi EK, Lee S, Kim YK, Seo EY, et al. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J Dermatol Sci. 2018;91:175–183. doi: 10.1016/j.jdermsci.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Lademann J, Schaefer H, Otberg N, Teichmann A, Blume-Peytavi U, Sterry W. [Penetration of microparticles into human skin] Hautarzt. 2004;55:1117–1119. doi: 10.1007/s00105-004-0841-1. [DOI] [PubMed] [Google Scholar]
- 19.Ahn K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol. 2014;134:993–999. doi: 10.1016/j.jaci.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M, et al. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129:1892–1908. doi: 10.1038/jid.2009.133. [DOI] [PubMed] [Google Scholar]
- 21.Kim JY, Jeong MS, Park MK, Lee MK, Seo SJ. Time-dependent progression from the acute to chronic phases in atopic dermatitis induced by epicutaneous allergen stimulation in NC/Nga mice. Exp Dermatol. 2014;23:53–57. doi: 10.1111/exd.12297. [DOI] [PubMed] [Google Scholar]
- 22.Jung Y, Kim JC, Park NJ, Bong SK, Lee S, Jegal H, et al. Eupatilin, an activator of PPARα, inhibits the development of oxazolone-induced atopic dermatitis symptoms in Balb/c mice. Biochem Biophys Res Commun. 2018;496:508–514. doi: 10.1016/j.bbrc.2018.01.098. [DOI] [PubMed] [Google Scholar]
- 23.Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Krämer U, et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med. 2008;177:1331–1337. doi: 10.1164/rccm.200701-036OC. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Kim EH, Oh I, Jung K, Han Y, Cheong HK, et al. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J Allergy Clin Immunol. 2013;132:495–498. doi: 10.1016/j.jaci.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Huss-Marp J, Eberlein-König B, Breuer K, Mair S, Ansel A, Darsow U, et al. Influence of short-term exposure to airborne Der p 1 and volatile organic compounds on skin barrier function and dermal blood flow in patients with atopic eczema and healthy individuals. Clin Exp Allergy. 2006;36:338–345. doi: 10.1111/j.1365-2222.2006.02448.x. [DOI] [PubMed] [Google Scholar]
- 26.Pénard-Morand C, Raherison C, Charpin D, Kopferschmitt C, Lavaud F, Caillaud D, et al. Long-term exposure to close-proximity air pollution and asthma and allergies in urban children. Eur Respir J. 2010;36:33–40. doi: 10.1183/09031936.00116109. [DOI] [PubMed] [Google Scholar]
- 27.Suárez-Varela MM, Gallardo-Juan A, García-Marcos L, Gimeno-Clemente N, Silvarrey-Varela AL, Miner-Canflanca I, et al. The impact of atmospheric pollutants on the prevalence of atopic eczema in 6-7-year-old schoolchildren in Spain; ISAAC Phase III. Iran J Allergy Asthma Immunol. 2013;12:220–227. [PubMed] [Google Scholar]
- 28.Kim HO, Kim JH, Cho SI, Chung BY, Ahn IS, Lee CH, et al. Improvement of atopic dermatitis severity after reducing indoor air pollutants. Ann Dermatol. 2013;25:292–297. doi: 10.5021/ad.2013.25.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim M, Kim JH, Jeong GJ, Park KY, Lee MK, Seo SJ. Particulate matter induces pro-inflammatory cytokines via phosphorylation of p38 MAPK possibly leading to dermal inflammaging. Exp Dermatol. 2019;28:809–815. doi: 10.1111/exd.13943. [DOI] [PubMed] [Google Scholar]
- 30.Kim JH, Kim M, Kim JM, Lee MK, Seo SJ, Park KY. Afzelin suppresses proinflammatory responses in particulate matter-exposed human keratinocytes. Int J Mol Med. 2019;43:2516–2522. doi: 10.3892/ijmm.2019.4162. [DOI] [PubMed] [Google Scholar]
- 31.Jin H, He R, Oyoshi M, Geha RS. Animal models of atopic dermatitis. J Invest Dermatol. 2009;129:31–40. doi: 10.1038/jid.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Man MQ, Hatano Y, Lee SH, Man M, Chang S, Feingold KR, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008;128:79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SY, Kim JK, Park SH, Kim BG, Jang AS, Oh SH, et al. Effects of inhaled particulate matter on the central nervous system in mice. Neurotoxicology. 2018;67:169–177. doi: 10.1016/j.neuro.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Kim BG, Lee PH, Lee SH, Kim YE, Shin MY, Kang Y, et al. Long-term effects of diesel exhaust particles on airway inflammation and remodeling in a mouse model. Allergy Asthma Immunol Res. 2016;8:246–256. doi: 10.4168/aair.2016.8.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320–322. doi: 10.1038/jid.2008.252. [DOI] [PubMed] [Google Scholar]
- 36.Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126:332–337. doi: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gruber R, Elias PM, Crumrine D, Lin TK, Brandner JM, Hachem JP, et al. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am J Pathol. 2011;178:2252–2263. doi: 10.1016/j.ajpath.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127:773–786.e1-7. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehmann AD, Blank F, Baum O, Gehr P, Rothen-Rutishauser BM. Diesel exhaust particles modulate the tight junction protein occludin in lung cells in vitro. Part Fibre Toxicol. 2009;6:26. doi: 10.1186/1743-8977-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stojadinovic O, Pastar I, Vukelic S, Mahoney MG, Brennan D, Krzyzanowska A, et al. Deregulation of keratinocyte differentiation and activation: a hallmark of venous ulcers. J Cell Mol Med. 2008;12:2675–2690. doi: 10.1111/j.1582-4934.2008.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, et al. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006;177:3677–3685. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 42.Traidl-Hoffmann C, Mariani V, Hochrein H, Karg K, Wagner H, Ring J, et al. Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J Exp Med. 2005;201:627–636. doi: 10.1084/jem.20041065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998;78:27–30. doi: 10.1080/00015559850135788. [DOI] [PubMed] [Google Scholar]
- 44.Lefebvre MA, Pham DM, Boussouira B, Bernard D, Camus C, Nguyen QL. Evaluation of the impact of urban pollution on the quality of skin: a multicentre study in Mexico. Int J Cosmet Sci. 2015;37:329–338. doi: 10.1111/ics.12203. [DOI] [PubMed] [Google Scholar]
- 45.Lefebvre MA, Pham DM, Boussouira B, Qiu H, Ye C, Long X, et al. Consequences of urban pollution upon skin status. A controlled study in Shanghai area. Int J Cosmet Sci. 2016;38:217–223. doi: 10.1111/ics.12270. [DOI] [PubMed] [Google Scholar]
- 46.Liao Z, Nie J, Sun P. The impact of particulate matter (PM2.5) on skin barrier revealed by transcriptome analysis: focusing on cholesterol metabolism. Toxicol Rep. 2019;7:1–9. doi: 10.1016/j.toxrep.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Proksch E, Fölster-Holst R, Jensen JM. Skin barrier function, epidermal proliferation and differentiation in eczema. J Dermatol Sci. 2006;43:159–169. doi: 10.1016/j.jdermsci.2006.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.