Abstract
Goodpasture’s syndrome is a rare vasculitis associated with anti-glomerular basement membrane (anti-GBM) autoantibodies that target type IV collagen found in the basement membranes of glomeruli and alveoli. We present a case of a 79-year-old man with seronegative Goodpasture’s syndrome with predominant respiratory symptoms and mild acute kidney injury that initially improved. Final diagnosis was made by immunofluorescent staining on open lung biopsy which also revealed concomitant organising pneumonia. The patient underwent treatment with corticosteroids, cyclophosphamide, haemodialysis and plasmapheresis. This was an atypical presentation wherein the patient only exhibited pulmonary symptoms early in the course of illness in the setting of negative anti-GBM antibody serum testing, which made diagnosis challenging. With this case, we emphasise that clinicians should have a high suspicion for Goodpasture’s syndrome in the setting of unexplained severe pulmonary or renal disease despite negative anti-GBM antibody testing.
Keywords: cardiothoracic surgery, vasculitis, acute renal failure, interstitial lung disease, pathology
Background
Goodpasture’s syndrome, also known as anti-glomerular basement membrane (anti-GBM) disease, is a rare autoimmune condition characterised by physician Earnest Goodpasture in 1919 during the influenza pandemic.1 Its aetiology was discovered in the 1960s when researchers found anti-GBM autoantibodies, which target the alpha-3 chain of type IV collagen in the alveolar membrane and GBM. Incidence of Goodpasture’s syndrome is reported to be 0.5–1.8 cases/million/year with European and Asian populations at greater risk. Men are more frequently affected than women in a 2:1 ratio with a bimodal distribution, typically being found in ages 20–30 or ages 60–80.2 The syndrome encompasses a triad of circulating antibodies, pulmonary haemorrhage and rapidly progressing glomerulonephritis.1 A majority of cases (60%–80%) present as both pulmonary and renal disease, but it also can present as an isolated renal disease in 20%–40% of cases or an isolated alveolar disease in 10% of cases.3 We submit a case of seronegative anti-GBM disease with an atypical clinical presentation of pulmonary predominant disease with concomitant organising pneumonia, mild acute kidney injury without haematuria, and an absence of haemoptysis.
Case presentation
A 79-year-old Dutch American man presented to a suburban community hospital emergency department with a 2-week history of dyspnoea associated with non-productive cough and disorientation. He denied chest pain, diarrhoea, haemoptysis, abdominal pain, recent travel or sick contacts. His medical history was notable for prior 70 pack-year smoking history, mild dementia, hypertension, hyperlipidaemia and atrial fibrillation on apixaban. Vital signs were temperature 100.4°F, blood pressure 166/77 mm Hg and oxygen saturation 88% on room air. Physical examination found irregularly irregular heart rhythm, crackles involving two-third of the bilateral lung fields and pitting oedema of the bilateral lower extremities extending to the level of the knees.
Investigations
Initial workup was notable for white blood cell (WBC) count of 11.1×109/L, haemoglobin 10.4 g/dL, creatinine 2.05 mg/dL with prior baseline of 0.95 mg/dL (3 years prior to admission), procalcitonin 0.07 ng/mL, troponin I of 0.028 ng/mL, lactic acid 0.9 mmol/L, pro-b-type natriuretic peptide 3807 pg/mL and C reactive protein (CRP) 13.4 mg/L. Respiratory virus PCR panel was negative. Microscopic urinalysis was notable for 2 red blood cells (RBCs)/high power field (hpf) with negative sediments and 0 WBCs. ECG revealed known atrial fibrillation with rate of 71 beats/min. Chest X-ray (CXR) demonstrated right upper lobe pneumonia with consolidation in the left perihilar region (figure 1). The patient was started on ceftriaxone, doxycycline and furosemide. Transthoracic echocardiogram found left ventricular ejection fraction of 71%–75%; dilated left atrium, right ventricle and right atrium; mild mitral regurgitation; moderate/severe tricuspid regurgitation and mild pulmonary hypertension.
Figure 1.
Chest X-ray on admission.
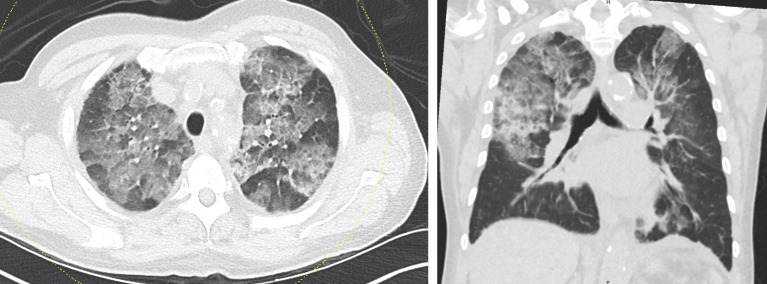
By the third day of hospitalisation, the patient’s creatinine had steadily improved to 1.44 mg/dL, but his oxygen requirement had worsened with interval progression of bilateral perihilar pneumonia, left worse than right, seen on CXR. Repeat procalcitonin was negative. Blood cultures from admission had no growth. Sputum culture could not be performed due to lack of sputum production. However, CRP continued to rise to 16.5 mg/L, and erythrocyte sedimentation rate was elevated at 85 mm/hour. CT chest without intravenous contrast (figure 2) revealed severe symmetric bilateral ground glass opacities with small areas of intervening normal lung with upper zone predominance. It also showed areas of confluent heterogenous airspace opacity in the right midlung and in the superior segment of the right lower lobe that were concerning for superimposed bacterial pneumonia. Ceftriaxone was discontinued after three doses, but the patient was continued on doxycycline to complete a 10-day course.
Figure 2.
Initial CT chest without contrast.
Due to the patient’s lack of clinical improvement, further infectious and rheumatologic diagnostic work-up was pursued as summarised in table 1. On the seventh day of hospitalisation, diagnostic bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial lung biopsy revealed on sequential lavage increasing numbers of RBCs (4×1012/L to 11×1012/L to 14×1012/L) with preliminary pathology consistent with diffuse alveolar haemorrhage (DAH). Primarily lymphocytes and monocytes were seen with no eosinophils. The patient was started on methylprednisolone for possible inflammatory lung disease, and the patient’s apixaban was discontinued. Due to finding of DAH and anaemia with haemoglobin in the 8.0–8.5 g/dL range, the patient’s anticoagulation was held for several days and then later limited to subcutaneous heparin at deep vein thrombosis prophylaxis dosing in addition to continued sequential compression device use on his lower extremities. On the ninth day of hospitalisation, the patient’s renal function acutely worsened with a creatinine of 2.5 mg/dL. Repeat microscopic urinalysis on hospital day 20 found 4 RBCs/hpf and 4 WBCs/hpf, with second microscopic urinalysis on hospital day 23 finding no RBCs or WBCs. Final bronchoscopy pathology report revealed organising pneumonia pattern with high flow cytometry CD4: CD8 ratio. Fungal and acid-fast bacteria cultures of BAL were negative. After the patient had been on corticosteroids for 5 days, CT chest showed unchanged diffuse parenchymal abnormality with upper lobe predominance, particularly increased density over the right upper lobe. The patient was intubated for video-assisted thoracoscopic surgery (VATS) lung biopsy that was complicated by small right apical pneumothorax requiring chest tube placement, but the patient was successfully extubated 4 days postprocedure. Preliminary pathology was consistent with organising pneumonia with negative antineutrophil cytoplasmic antibody (ANCA). Final pathology with immunohistochemistry confirmed anti-GBM disease of the right lower lobe (figure 3). Right upper lobe specimen showed organising pneumonia (figure 4) and lung damage without positive staining for anti-GBM disease. Serum anti-GBM IgG was negative (<0.2).
Table 1.
Summary of laboratory data
| Lab | Reference range, adults | Result |
| Fungitell (1–3)-β-D-Glucan assay | <60 pg/mL | <31 |
| GBM-Ab IgG | 0.0–0.9 AI | <0.2 |
| Mycoplasma pneumonia by PCR | Not detected | |
| HIV-1/HIV-2 Ab/antigen screen | Negative | |
| Cold agglutinins titre | <1:32 | <1:32 |
| Respiratory syncytial virus antigen | Negative | |
| Respiratory virus PCR panel | Not detected | |
| SS-A/B Sjogren’s Ab | 0.0–0.9 AI | <0.2 |
| SM Ab | 0.0–0.9 AI | <0.2 |
| RNP Ab | 0.0–0.9 AI | <0.2 |
| Anti-SM/RNP | 0.0–0.9 AI | <0.2 |
| Anti-ribosomal P | 0.0–0.9 AI | <0.2 |
| Scleroderma (Scl-70) Ab | 0.0–0.9 AI | <0.2 |
| Jo-IgG Ab | 0.0–0.9 AI | <0.2 |
| Chromatin (histone) Ab | 0.0–0.9 AI | <0.2 |
| Centromere Ab screen | 0.0–0.9 AI | <0.2 |
| DsDNA Ab | 0.0–4.0 IU/mL | 1.0 |
| ANA titre | <1:160 | 1:160 |
| ANCA vasculitis IgG Ab | 0.0–0.9 AI | <0.2 |
| ANA | Positive | |
| C3 complement | 90–180 mg/dL | 133 |
| Angiotensin converting enzyme | 8–52 U/L | 10 |
| QuantiFERON-TB | Indeterminate |
Ab, antibody; ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibody; DsDNA, double-stranded DNA; GBM, glomerular basement membrane; RNP, ribonucleoprotein; SM, Smith; SS, Sjogren's syndrome.
Figure 3.
Right lower lobe pathology from video-assisted thoracoscopic surgery lung biopsy. Immunohistochemistry demonstration of areas with positive staining for IgG, IgA, C3, lambda and fibrinogen. Findings indicate anti-glomerular basement membrane disease.
Figure 4.

Right upper lobe pathology from video-assisted thoracoscopic surgery lung biopsy. Demonstration of areas of organising pneumonia containing typical plugs of recent fibroblast proliferation that fill airspaces.
Differential diagnosis
Initially, the patient was treated for community-acquired pneumonia and heart failure exacerbation. After minimal clinical response, CT chest revealed symmetric ground-glass opacities. Tests for opportunistic infections, such as Pneumocystis pneumonia, cytomegalovirus, herpes simplex virus and respiratory syncytial virus were all negative. The patient’s inflammatory markers were up-trending, so autoimmune diseases were investigated with testing for anti-GBM, Smith antibody, Sjogren's syndrome A/B antibody, scleroderma antibody, ANCA IgG antibody, double-stranded DNA antibody (table 1), all of which were negative. Diagnostic bronchoscopy with transbronchial lung biopsy revealed DAH, which increased diagnostic suspicion for an underlying inflammatory disease. Other causes of DAH, such as bacterial, fungal and viral infections were ruled out. Final bronchoscopy pathology report showed organising pneumonia, which was thought to be the primary diagnosis. However, the patient continued to show minimal improvement despite immunosuppressive therapy and later developed renal injury that required haemodialysis. Thus, we proceeded with VATS lung biopsy with immunofluorescence staining, and pathology showed organising pneumonia with immunofluorescent studies indicative of anti-GBM disease in the right lower lobe. The pathologist noted that while the immunofluorescent studies were most suggestive of Goodpasture’s syndrome, diffuse alveolar damage and organising pneumonia are not the typical presentations of Goodpasture’s syndrome. Goodpasture’s typically presents as DAH with prominent haemosiderin deposition both within alveolar macrophages and within the interstitium, which were not seen in these biopsies. While the histologic presentation was not classic, pathology found the positive immunofluorescent studies in the setting of worsening renal function consistent with Goodpasture’s syndrome.
Treatment
Once organising pneumonia was confirmed on bronchoscopy pathology, the patient was continued on high-dose steroids and was started on cyclophosphamide. On the twentieth day of hospitalisation, the patient was started on haemodialysis and received a total of two sessions prior to final open lung biopsy pathology results confirming diagnosis of Goodpasture’s syndrome. Afterwards, the patient was continued on corticosteroids and cyclophosphamide and was also started on plasmapheresis. The night following first plasmapheresis therapy, the patient became hypoxemic requiring re-intubation, and the patient was started on vancomycin and piperacillin–tazobactam.
Outcome and follow-up
The patient received a total of three plasmapheresis treatments, but his respiratory status worsened with his hospital course further complicated by thrombocytopenia and atrial fibrillation with rapid ventricular response. After discussion of goals of care, the patient’s family pursued comfort measures only with terminal extubation.
Discussion
Traditionally, a diagnosis of Goodpasture’s syndrome is confirmed either by the presence of anti-GBM antibody in the serum, which has high sensitivity (>95%) and specificity (>97%) or by renal biopsy demonstrating IgG in linear pattern of deposition along the GBM by immunofluorescence consistent with crescentic glomerulonephritis.4 In patients with isolated pulmonary disease or delayed/minor renal presentations, lung biopsy may be indicated.5 An estimated 2%–3% of cases of Goodpasture’s syndrome are seronegative, and these patients without anti-GBM antibodies present with more indolent symptoms and thus more difficult to diagnose.6 We found two other case reports of seronegative anti-GBM by Fernandes et al and Salama et al that describe the diagnosis being confirmed by renal biopsy.6 7
Multiple hypotheses to explain the absence of anti-GBM antibodies in Goodpasture’s syndrome exist in the literature. One proposed rationale is that the presence of low circulating levels of pathogenic antibodies with an additional trigger is sufficient to cause enough damage to tissue to manifest the clinical signs and symptoms of Goodpasture’s syndrome, especially in early stage of disease.8 It has also been theorised that the responsible antibody may be of different immunoglobulin subtypes, such as IgA or IgG4 which are not picked up by standard ELISA.8 Another theory is that the responsible antibodies may disappear prior to the resolution of symptoms, leading to negative test results even while patients are symptomatic.8
Regardless of the clinical presentation or the seropositivity, Goodpasture’s syndrome is typically managed by triple therapy, including plasmapheresis, to remove the circulating autoantibodies along with immunosuppressants such as cyclophosphamide and corticosteroids to halt production of the responsible antibodies. There are no standard protocols for treatment, but the guideline published by the American Society for Aphresis in 2016 recommends treatment with plasmapheresis daily or every other day for a course of 10–20 days.9 In patients who cannot tolerate cyclophosphamide due to side effects or allergies, rituximab (anti-CD20 monoclonal antibody) has shown to be beneficial, especially in those with glomerular disease. One-year survival rate was reported to be between 75% and 90% in the most recent case series of anti-GBM disease.10
Our case offers multiple unique characteristics to highlight. First, our patient had an atypical presentation with non-specific pulmonary symptoms, including shortness of breath and non-productive cough, in the absence of haemoptysis. While the patient initially presented with acute kidney injury, his renal function steadily improved early in his hospitalisation, and he had no evidence of haematuria. Later in his hospital course, he developed acute kidney injury requiring haemodialysis, but a subsequent urinalysis found only 4 RBCs/hpf, and a final urinalysis performed on hospital day 23 found 0 RBCs/hpf. Second, the absence of anti-GBM antibodies in setting of delayed renal disease made the diagnosis more challenging as the work up was driven by the need to investigate his worsening pulmonary status with bronchoscopy and later surgical lung biopsy. Other cases of seronegative Goodpasture’s syndrome have been diagnosed with renal biopsy, but in our case, the pathology results from a surgical lung biopsy served as the mechanism of diagnosis.
Another atypical feature was the presence of organising pneumonia on lung pathology. After extensive search of the medical literature, we found seven reported cases of pre-existing chronic interstitial pneumonia in the setting of newly diagnosed Goodpasture’s syndrome, and this was reported as a poor prognostic factor.11 However, we did not find any cases of concurrent Goodpasture’s syndrome and organising pneumonia, so we cannot rely on the experiences of others in formulating conclusions regarding the prognosis of that combination of disease processes. Intuitively, we would expect those patients to have lower lung tissue reserve and worse outcomes, which is what we saw in our patient. Finally, the pathogenesis of anti-GBM disease is thought to be influenced by viral infections, but our patient’s influenza and respiratory virus panel testing were negative. Of note, SARS-CoV-2 testing was not available at the time, as this patient’s hospitalisation occurred roughly 2 months before the WHO’s global pandemic declaration and the spike of COVID-19 cases in nearby New York City in March 2020. Our patient had no known travel history, sick contacts or other risk factors to contract SARS-CoV-2 at a time when there was no evidence of widespread community transmission in the USA, but infection with novel coronavirus was not ruled out.
Learning points.
We advise clinicians to have high index of suspicion for Goodpasture’s syndrome in the setting of pulmorenal presentation, particularly if environmental risk factors, such as tobacco smoke, cocaine and hydrocarbon chemical exposure are present.
Clinicians should be aware that atypical presentations with only renal manifestations or only pulmonary manifestations may represent up to 40% of cases, and these cases are at risk of being diagnosed later in the disease course.
In the setting of unexplained severe pulmonary or renal disease, Goodpasture’s syndrome should remain on the differential even in the setting of negative anti-glomerular basement membrane antibody testing.
Goodpasture’s syndrome is typically managed by triple therapy, including plasmapheresis along with cyclophosphamide and corticosteroids, but there are no standard protocols for treatment, and the use of immunosuppressants heightens the risk of harm if the patient has active infection.
Acknowledgments
We’d like to thank Donna Belcinski, Greenwich Hospital Medical Librarian, for her assistance in acquiring articles to be used as references.
Footnotes
Contributors: JYB was responsible for conception of this work, data collection from patient chart and wrote initial drafts of each section. KIH performed the literature search, data collection from patient chart and authored critical revisions of the article. EL contributed critical revisions of the article and data collection in the form of image selection. HMA contributed critical revisions of the article and gave final approval of the version to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer-reviewed.
References
- 1.Lettieri C, Pina J. Goodpasture's syndrome: a case of delayed appearance of autoantibodies and renal disease. Mil Med 2001;166:827–30. 10.1093/milmed/166.9.827 [DOI] [PubMed] [Google Scholar]
- 2.Benoit FL, Rulon DB, Theil GB, et al. Goodpasture's syndrome: a clinicopathologic entity. Am J Med 1964;37:424–44. 10.1016/0002-9343(64)90199-8 [DOI] [PubMed] [Google Scholar]
- 3.Edward JA, Lee JS, Moore PK, et al. A man in his 50S with hemoptysis, dyspnea, and bilateral patchy Ground-Glass opacities. Chest 2019;156:e41–5. 10.1016/j.chest.2019.03.021 [DOI] [PubMed] [Google Scholar]
- 4.Sinico RA, Radice A, Corace C, et al. Anti-glomerular basement membrane antibodies in the diagnosis of Goodpasture syndrome: a comparison of different assays. Nephrol Dial Transplant 2006;21:397–401. 10.1093/ndt/gfi230 [DOI] [PubMed] [Google Scholar]
- 5.Abboud RT, Chase WH, Ballon HS, et al. Goodpasture's syndrome: diagnosis by transbronchial lung biopsy. Ann Intern Med 1978;89:635–8. 10.7326/0003-4819-89-5-635 [DOI] [PubMed] [Google Scholar]
- 6.Fernandes R, Freitas S, Cunha P, et al. Goodpasture's syndrome with absence of circulating anti-glomerular basement membrane antibodies: a case report. J Med Case Rep 2016;10:205. 10.1186/s13256-016-0984-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salama AD, Dougan T, Levy JB, et al. Goodpasture's disease in the absence of circulating anti-glomerular basement membrane antibodies as detected by standard techniques. Am J Kidney Dis 2002;39:1162–7. 10.1053/ajkd.2002.33385 [DOI] [PubMed] [Google Scholar]
- 8.Glassock RJ Atypical anti-glomerular basement membrane disease: lessons learned. Clin Kidney J 2016;9:653–6. 10.1093/ckj/sfw068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz J, Padmanabhan A, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical Practice-Evidence-Based approach from the writing Committee of the American Society for apheresis: the seventh special issue. J Clin Apher 2016;31:149–338. 10.1002/jca.21470 [DOI] [PubMed] [Google Scholar]
- 10.Pusey CD Anti-glomerular basement membrane disease. Kidney Int 2003;64:1535–50. 10.1046/j.1523-1755.2003.00241.x [DOI] [PubMed] [Google Scholar]
- 11.Tashiro H, Takahashi K, Ikeda Y, et al. Pre-existing chronic interstitial pneumonia is a poor prognostic factor of Goodpasture's syndrome: a case report and review of the literature. J Med Case Rep 2017;11:102. 10.1186/s13256-017-1273-8 [DOI] [PMC free article] [PubMed] [Google Scholar]