Abstract
A 56-year-old woman with a 12-year history of recurrent triple-negative invasive carcinoma of the breast presented with progressive enlargement of lymph nodes in the setting of established rupture of the ipsilateral silicone breast implant. Although this was proven to be benign on cytology, its progressive nature led to repeated core biopsies for histology, which were necessary given the high-risk nature of triple-negative breast cancer and the multiple proven previous recurrences. The histology demonstrated features of silicone deposits without evidence of malignancy. This case demonstrates the dilemma in surveillance of high-risk patients with breast cancer who have had previous silicone lymphadenopathy.
Keywords: breast cancer, radiology, oncology
Background
Breast cancer is a disease of immense psychological burden,1 2 and the prospect of potential recurrence continues to cause distress for affected women.3 Recurrence is unfortunately the reality for some.4 5 Furthermore, so-called triple-negative breast cancers (TNBC), used to describe those that are negative for oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2,6 7 tend to be associated with a higher rate of recurrence in visceral and soft tissues8 and a lower overall survival rate compared with non-TNBC.8 9
Surveillance post breast cancer treatment is important for detection of early recurrence, as well as providing psychological reassurance for the patient. Regional lymphadenopathy may be the first sign of recurrence; however, other differential diagnoses need to be considered. These include reactive lymph nodes secondary to infection such as HIV and infective mononucleosis; haematological malignancies; and, for patients who have reconstruction, reaction secondary to both intact and ruptured implants. Silicone-induced lymphadenopathy arises from silicone in breast implants leaking into adjacent lymph nodes. This is usually in the context of implant rupture and is a reasonably common finding; however, it is uncommon for these lymph nodes to enlarge over time.
Case presentation
We present a 56-year-old woman who was diagnosed with a TNBC of her left breast 13 years prior (2007) at age 43. She is a smoker with no other risk factors for breast cancer, in particular no family history of breast or ovarian cancer. She was tested for BRCA1 and BRCA2 gene mutations during her initial treatment, and no mutations were identified. She underwent a left breast wide local excision and axillary lymph node clearance, which demonstrated a completely excised 37 mm grade 3 TNBC with associated high-grade ductal carcinoma in situ (DCIS) and macrometastases in 2 out of 14 lymph nodes. She received adjuvant chemotherapy with four cycles of anthracycline and cyclophosphamide and then four cycles of docetaxel, followed by 50 Gy of whole-breast radiotherapy in 25 fractions, with 10 Gy boost to the surgical site.
Five years later (2012), she was found to have an invasive recurrence in her left breast as well as intermediate-to-high-grade DCIS in the contralateral right breast. She underwent completion of left simple mastectomy and right hookwire-localised wide local excision with right sentinel lymph node biopsy. The specimen showed a 20 mm grade 3 TNBC in her left breast and in the right breast 10 mm of high-grade DCIS. Surgical margins were clear, and all four of her right sentinel lymph nodes were negative for cancer. The patient subsequently received four cycles of docetaxel and cyclophosphamide.
In 2013, joint decision between the treating team and the patient was to proceed with a completion right prophylactic mastectomy with immediate bilateral reconstruction using latissimus dorsi myocutaneous flaps and two 350cc Mentor SILTEX Round Moderate Profile Gel Breast Implant Cohesive II implants. There was no further evidence of hyperplasia or DCIS found in the mastectomy specimen.
Unfortunately, 6 years after her reconstruction (2019), she developed a second left breast cancer recurrence, located in the axillary tail. Core biopsy confirmed presence of invasive high-grade TNBC with associated chondroid metaplasia. The patient was staged with a chest, abdomen and pelvis CT scan, which revealed prominent lymph nodes in the right axilla and subpectoral region and in the right internal mammary chain nodes (figure 1A) with right breast combined intracapsular and extracapsular implant rupture (figure 1B). This was further investigated with a silicone-specific MRI that demonstrated silicone uptake in right axillary lymph nodes, right internal mammary chain (figure 2) and epicardial lymph nodes.
Figure 1.

(A) Staging chest, abdomen and pelvis CT scan (2019) showing prominent lymph nodes in the right axilla and subpectoral region and in the right internal mammary chain nodes with right breast implant rupturefigure 1. (B) Staging chest, abdomen and pelvis CT scan (2019) showing prominent lymph nodes in the right axilla and subpectoral region and in the right internal mammary chain nodes with right breast implant rupture.
Figure 2.

Silicone-specific MRI (2019) demonstrating silicone uptake in right internal mammary lymph nodes.
With the additional knowledge of no proven distant metastasis, the patient’s recurrent cancer was removed with wide local excision. There was grade 3 TNBC (two foci with 6 mm and 7 mm in size each) with Ki67 index of 60%–70%. The cancer was present at the inferomedial surgical margin, which was abutting the breast implant. She then received four cycles of docetaxel and cyclophosphamide as adjuvant chemotherapy, as well as a further 50 Gy dose of radiotherapy over her left chest wall in 25 fractions.
On clinical follow-up later in 2019, the surgeon noted a palpable right axillary lymph node. Subsequent ultrasound (US) of the right axilla demonstrated a 1.7 cm lobulated node with thickened cortex measuring up to 5 mm (figure 3). Furthermore, the lymph node displayed a snowstorm appearance, characteristic of silicone lymphadenopathy as described in the literature.10 A fine-needle aspirate (FNA) with cytology was performed to confirm reactive lymphoid cells without malignancy.
Figure 3.

Ultrasound of the right axilla (2019) demonstrated a 1.7 cm lobulated node with thickened cortex measuring up to 5 mm.
The patient was managed with sequential clinical assessment and annual breast surveillance mammogram and US as per our hospital protocol. The patient presented 1 year later (2020) with left shoulder and chest wall pain. A repeat staging CT scan showed no evidence of recurrence but an interval increase in the size of her right internal mammary and right axillary lymph nodes (figure 4). Although the cytology from the previous year suggested silicone lymphadenopathy, previous recurrences and the high Ki67 index necessitated further diagnostic certainty. The case was again referred to the breast cancer multidisciplinary meeting, and decision was made to core biopsy to rule out a synchronous malignancy.
Figure 4.

Repeat CT scan (2020) showing interval increase in size of patient’s right internal mammary and right axillary lymph nodes.
An internal mammary lymph node biopsy was subsequently performed without complication. The specimen showed no malignancy but only foreign body giant cell reaction and clear spaces containing refractile material (figure 5), which was consistent with descriptions of silicone lymphadenopathy in the literature.11–17
Figure 5.
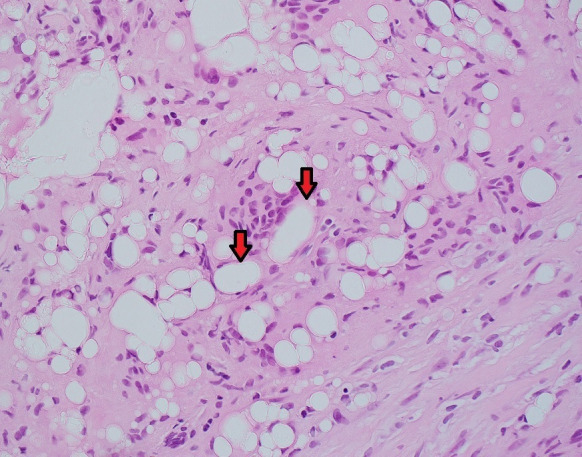
H&E stain, original magnification ×40—histiocytes and multinucleated giant cells forming granulomatous inflammatory reaction around refractile clear spaces containing foreign material, likely silicone (red arrows).
Treatment
Aside from the investigative procedures such as the FNA in 2019 and the core biopsy in 2020, she did not require any further surgical management for the lymphadenopathy. However, the stress and anxiety surrounding the uncertainty of the diagnosis and the fear of recurrence required extensive psychological support.
Outcome and follow-up
At last follow-up in May 2020, the patient continues to have pain and discomfort in relation to her left shoulder and chest wall but has no evidence of recurrent disease. The right axillary and internal mammary lymphadenopathy remain asymptomatic. She underwent bilateral implant exchange and capsulotomy in May 2020 without complication.
She will continue to be reviewed every 6 months.
Discussion
Silicone breast implants are made in the form of a silicone elastomer shell, which is then filled with silicone gel of varying cohesiveness.18 Although silicone does offer cosmetic advantages over the saline alternative,18 19 its previously perceived chemical and biological inertness may not be true.20
Silicone lymphadenopathy is a physiological response to release or migration of silicone from the breast implant into surrounding tissue and the subsequent migration to lymph nodes21 via physical migration or by transport from macrophages.20 Although associated with rupture of the implant, this is not always necessary,22 as low-molecular-weight compounds of silicon can migrate through the shell.20
There have been rare significant complications associated with silicone gel implants, which include formation of anaplastic large-cell lymphoma20 23 at a rate of 1:12 000–30 000,20 as well as autoimmune conditions such as autoimmune/inflammatory syndrome induced by adjuvants24 or silicone incompatibility syndrome.25 However, we were unable to identify in the literature if the presence of silicone in lymph nodes exacerbated or modified the risk of these systemic conditions. Aside from these, there do not appear to be other significant adverse effects from this phenomenon. Therefore, conservative management is appropriate.21 26
However, there is a potential for a lymph node recurrence or metastasis to be misdiagnosed as silicone lymphadenopathy. Without a method of clearly differentiating between the two pathologies, there is a risk of overinvestigating a benign lymph node found on a surveillance scan or conversely missing a recurrence leading to delayed treatment. Thus, this phenomenon offers a diagnostic dilemma for the treating clinician and provides a challenge to accurate breast cancer surveillance.21
Presence of lymphadenopathy is common following breast implant surgery. Enlarged internal mammary lymph nodes can be seen in up to 37.6% of patients 1 year after breast reconstruction with silicone implants.27 Enlarged lymph nodes are due to silicone lymphadenopathy rather than recurrent disease in up to 99.5% of cases.27
In our literature search, we have explored various methods of stratifying patients who are more likely to have malignancy rather than silicone-infiltrated lymph nodes. Although the only definitive method of diagnosis is via a biopsy, it is possible to initially investigate via less invasive methods, which can then be used to assist discussion regarding the merit of a biopsy. However, further research would be beneficial.
Risk stratification
Especially in cases where there is doubt, it may be helpful to divide patients into high-risk and low-risk categories. For example, recurrence risk of postmastectomy patients is seen with increasing tumour size and nodal burden.28 Furthermore, histological-type, malignancy-grade, chest wall invasion, extracapsular nodal extension and extensive lymphovascular invasion can also be considered in assessing risk.28 Finally, immunohistochemical markers and molecular subtypes can also identify patients with worse prognosis.29 Timing of presentation can also play a crucial role as up to three-quarters of eventual local recurrences occur during the first 5 years following treatment.30
Imaging
Silicone lymphadenopathy may present as palpable lymph nodes, as part of surveillance scans, or as an incidental radiological finding. A US is usually ordered to investigate the enlarged lymph nodes. As well as providing detailed morphological information,13 31 this can also demonstrate subtle, ill-defined posterior acoustic shadow arising from the lymph node mediastinum.13 The finding is named a ‘snowstorm sign’ and has a sensitivity as high as 87.5% when correlated to silicon infiltration.31
On the other hand, silicone-selective MRI is a useful technique to evaluate implant rupture32 but has a low sensitivity for detecting silicone in lymph nodes, which may be as low as 20%.31 While both MRI and US have 100% specificity, the higher sensitivity of US may better aid in clinical decision making.
Silicone-infiltrated lymph nodes can be avid in fluorodeoxyglucose positron emission tomography (PET) scans.33 Furthermore, the sensitivity of PET scan for detecting breast cancer varies from 64.4% to 87.8%,34 35 and a study by Pritchard et al found that the sensitivity was only 23.7% in detection of axillary lymph node metastasis,36 thus making non-avidity not as diagnostically helpful.
Timing
In our literature search, we were able to identify case reports that have reported on silicone lymphadenopathy mimicking metastasis,11–17 37 but none discussed the differentiating behavioural characteristics between the two pathologies over time. In our case, the lymph node continued to grow, thus imitating the growth pattern that is expected of a malignant lymph node. As the biopsy showed that our patient did not have a recurrence, we highlight that this characteristic may not be relied on as an exclusive sign of malignancy. Hence, there may be benefit in further research to study the temporal behaviour of silicone lymphadenopathy to compare against metastatic lymph nodes.
Learning points.
Silicone lymphadenopathy is common, and in fact, up to 99.5% of lymphadenopathies post silicone implant insertion are due to silicone deposition.
Silicone-containing lymph nodes may continue to grow at a pace mimicking that of metastasis, although this is uncommon.
In high-risk patients, such growth is difficult to be ignored as there is chance of synchronous malignancy.
Silicone-containing lymph nodes may be positron emission tomography avid.
There is currently no imaging technique that can accurately differentiate silicone lymphadenopathy from metastasis. Therefore, fine-needle aspirate or core biopsy if technically feasible is recommended in high-risk patients.
Acknowledgments
We would like to thank Dr James Gullifer and the department of anatomical pathology for providing us with the histology slides used in the case report.
Footnotes
Contributors: RPC: as the main author, I was responsible for collecting case notes, conducting literature review and writing the case report. SC-HT helped with editing the case report and verifying facts in literature review. He also provided guidance in writing the Discussion section. CBB helped with editing the case report, verifying facts in literature review and providing guidance in writing Discussion section. JL helped with editing the case report and verifying facts in literature review. She is also the main treating doctor for the patient. She made sure that the chronology of the patient’s case presentation was accurate.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Tsaras K, Papathanasiou IV, Mitsi D, et al. Assessment of depression and anxiety in breast cancer patients: prevalence and associated factors. Asian Pac J Cancer Prev 2018;19:1661–9. 10.22034/APJCP.2018.19.6.1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansen S, Cvancarova M, Ruland C. The effect of cancer patients' and their family caregivers' physical and emotional symptoms on caregiver burden. Cancer Nurs 2018;41:91–9. 10.1097/NCC.0000000000000493 [DOI] [PubMed] [Google Scholar]
- 3.Hawley ST, Janz NK, Griffith KA, et al. Recurrence risk perception and quality of life following treatment of breast cancer. Breast Cancer Res Treat 2017;161:557–65. 10.1007/s10549-016-4082-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher B, Jeong J-H, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 2002;347:567–75. 10.1056/NEJMoa020128 [DOI] [PubMed] [Google Scholar]
- 5.Geurts YM, Witteveen A, Bretveld R, et al. Patterns and predictors of first and subsequent recurrence in women with early breast cancer. Breast Cancer Res Treat 2017;165:709–20. 10.1007/s10549-017-4340-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gadi VK, Davidson NE. Practical approach to triple-negative breast cancer. J Oncol Pract 2017;13:293–300. 10.1200/JOP.2017.022632 [DOI] [PubMed] [Google Scholar]
- 7.Leon-Ferre RA, Polley M-Y, Liu H, et al. Impact of histopathology, tumor-infiltrating lymphocytes, and adjuvant chemotherapy on prognosis of triple-negative breast cancer. Breast Cancer Res Treat 2018;167:89–99. 10.1007/s10549-017-4499-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008;26:1275–81. 10.1200/JCO.2007.14.4147 [DOI] [PubMed] [Google Scholar]
- 9.Li X, Yang J, Peng L, et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat 2017;161:279–87. 10.1007/s10549-016-4059-6 [DOI] [PubMed] [Google Scholar]
- 10.Kim JR, Chang M-C, Kim YM. Usefulness of sonography for diagnosis of siliconomas mimicking metastatic lymphadenopathy on computed tomography. J Ultrasound Med 2015;34:167–9. 10.7863/ultra.34.1.167 [DOI] [PubMed] [Google Scholar]
- 11.D'hulst L, Nicolaij D, Beels L, et al. False-Positive Axillary Lymph Nodes Due to Silicone Adenitis on (18)F-FDG PET/CT in an Oncological Setting. J Thorac Oncol 2016;11:e73–5. 10.1016/j.jtho.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 12.Rivero MA, Schwartz DS, Mies C. Silicone lymphadenopathy involving intramammary lymph nodes: a new complication of silicone mammaplasty. AJR Am J Roentgenol 1994;162:1089–90. 10.2214/ajr.162.5.8165987 [DOI] [PubMed] [Google Scholar]
- 13.Collado-Mesa F, Yepes M, Doshi P, et al. Contralateral intramammary silicone lymphadenitis in a patient with an intact standard dual-lumen breast implant in the opposite reconstructed breast. J Radiol Case Rep 2013;7:24–31. 10.3941/jrcr.v7i11.1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dragoumis DM, Assimaki AS, Vrizas TI, et al. Axillary silicone lymphadenopathy secondary to augmentation mammaplasty. Indian J Plast Surg 2010;43:206–9. 10.4103/0970-0358.73453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinke K, Brook P, Ramuz O. Radiological pitfall: Siliconoma in internal mammary lymph node mimics breast cancer recurrence. Radiol Case Rep 2011;6:601. 10.2484/rcr.v6i4.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee Y, Song SE, Yoon E-S, et al. Extensive silicone lymphadenopathy after breast implant insertion mimicking malignant lymphadenopathy. Ann Surg Treat Res 2017;93:331–5. 10.4174/astr.2017.93.6.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams ST, Cox J, Rao GS. Axillary silicone lymphadenopathy presenting with a lump and altered sensation in the breast: a case report. J Med Case Rep 2009;3:6442. 10.1186/1752-1947-3-6442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown MH, Shenker R, Silver SA. Cohesive silicone gel breast implants in aesthetic and reconstructive breast surgery. Plast Reconstr Surg 2005;116:768–79. discussion 80-1. 10.1097/01.prs.0000176259.66948.e7 [DOI] [PubMed] [Google Scholar]
- 19.Rocco N, Rispoli C, Moja L, et al. Different types of implants for reconstructive breast surgery. Cochrane Database Syst Rev 2016;5:CD010895. 10.1002/14651858.CD010895.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caravantes-Cortes M-I, Roldan-Valadez E, Zwojewski-Martinez R-D, et al. Breast prosthesis syndrome: pathophysiology and management algorithm. Aesthetic Plast Surg 2020;44:1423–37. 10.1007/s00266-020-01663-9 [DOI] [PubMed] [Google Scholar]
- 21.Zambacos GJ, Molnar C, Mandrekas AD. Silicone lymphadenopathy after breast augmentation: case reports, review of the literature, and current thoughts. Aesthetic Plast Surg 2013;37:278–89. 10.1007/s00266-012-0025-9 [DOI] [PubMed] [Google Scholar]
- 22.Klang E, Amitai MM, Raskin S, et al. Association between enlarged axillary lymph nodes and silicone breast implant ruptures seen on magnetic resonance imaging. Isr Med Assoc J 2016;18:719–24. [PubMed] [Google Scholar]
- 23.Berlin E, Singh K, Mills C, et al. Breast implant-associated anaplastic large cell lymphoma: case report and review of the literature. Case Rep Hematol 2018;2018:2414278. 10.1155/2018/2414278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watad A, Quaresma M, Brown S, et al. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld's syndrome) - An update. Lupus 2017;26:675–81. 10.1177/0961203316686406 [DOI] [PubMed] [Google Scholar]
- 25.Fuzzard SK, Teixeira R, Zinn R. A review of the literature on the management of silicone implant incompatibility syndrome. Aesthetic Plast Surg 2019;43:1145–9. 10.1007/s00266-019-01407-4 [DOI] [PubMed] [Google Scholar]
- 26.Wazir U, Kasem A, Mokbel K. The clinical implications of poly implant prothèse breast implants: an overview. Arch Plast Surg 2015;42:4–10. 10.5999/aps.2015.42.1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutton EJ, Watson EJ, Gibbons G, et al. Incidence of internal mammary lymph nodes with silicone breast implants at MR imaging after oncoplastic surgery. Radiology 2015;277:381–7. 10.1148/radiol.2015142717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Leij F, Elkhuizen PHM, Bartelink H, et al. Predictive factors for local recurrence in breast cancer. Semin Radiat Oncol 2012;22:100–7. 10.1016/j.semradonc.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 29.Kyndi M, Sørensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish breast cancer Cooperative group. J Clin Oncol 2008;26:1419–26. 10.1200/JCO.2007.14.5565 [DOI] [PubMed] [Google Scholar]
- 30.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087–106. 10.1016/S0140-6736(05)67887-7 [DOI] [PubMed] [Google Scholar]
- 31.Klang E, Yosepovich A, Krosser A, et al. Detection of pathologically proven silicone lymphadenopathy: ultrasonography versus magnetic resonance imaging. J Ultrasound Med 2018;37:969–75. 10.1002/jum.14434 [DOI] [PubMed] [Google Scholar]
- 32.Schneider E, Chan TW. Selective MR imaging of silicone with the three-point Dixon technique. Radiology 1993;187:89–93. 10.1148/radiology.187.1.8451442 [DOI] [PubMed] [Google Scholar]
- 33.Soudack M, Yelin A, Simansky D, et al. Fluorodeoxyglucose--positive internal mammary lymph node in breast cancer patients with silicone implants: is it always metastatic cancer? Eur J Cardiothorac Surg 2013;44:79–82. 10.1093/ejcts/ezs625 [DOI] [PubMed] [Google Scholar]
- 34.Groheux D, Cochet A, Humbert O, et al. ¹⁸F-FDG PET/CT for staging and Restaging of breast cancer. J Nucl Med 2016;57(Suppl 1):17S–26. 10.2967/jnumed.115.157859 [DOI] [PubMed] [Google Scholar]
- 35.Avril N, Rosé CA, Schelling M, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol 2000;18:3495–502. 10.1200/JCO.2000.18.20.3495 [DOI] [PubMed] [Google Scholar]
- 36.Pritchard KI, Julian JA, Holloway CMB, et al. Prospective study of 2-[¹⁸F]fluorodeoxyglucose positron emission tomography in the assessment of regional nodal spread of disease in patients with breast cancer: an Ontario clinical oncology group study. J Clin Oncol 2012;30:1274–9. 10.1200/JCO.2011.38.1103 [DOI] [PubMed] [Google Scholar]
- 37.Borghol K, Gallagher G, Skelly BL. Silicone granuloma from ruptured breast implants as a cause of cervical lymphadenopathy. Ann R Coll Surg Engl 2016;98:e118–20. 10.1308/rcsann.2016.0166 [DOI] [PMC free article] [PubMed] [Google Scholar]


