Abstract
Background:
Thyroid cancer is diagnosed at relatively young ages compared to most other malignancies, and the incidence is higher in females than males beginning in early adolescence. However, few in utero and early-life risk factors have been identified.
Methods:
Using population-based registry data from four Nordic countries, we conducted a nested case-control study of thyroid cancer risk in offspring in relation to maternal medical history, pregnancy complications, and birth characteristics. Each thyroid cancer case was matched with up to ten controls on birth year, sex, country, and county of birth. Conditional logistic regression models were used to calculate odds ratios (ORs) with 95% confidence intervals (CIs).
Results:
Of the 2,437 cases, 81% were papillary carcinomas, 77% were female, and 57% were diagnosed before age 30 (range: 0-48 years). Higher birth weight (OR per kg=1·14, 95% CI 1·05-1·23), congenital hypothyroidism (OR=4·55, 95% CI 1·58-13·1), and maternal diabetes before pregnancy (OR=1·69, 95% CI 0·98-2·93), postpartum hemorrhage (OR=1·28, 95% CI 1·06-1·55), and (from registry data in Denmark) maternal benign thyroid disease before/during pregnancy (hypothyroidism, OR=18·1, 95% CI 10·5-31·2; hyperthyroidism, OR=11·9, 95% CI 6·80-20·9; goiter, OR=67·4, 95% CI 39·9-114; and benign neoplasms, OR=22·5, 95% CI 6·93-73·1) were each positively associated with risk of thyroid cancer in offspring.
Interpretation:
In utero exposures, particularly those related to maternal thyroid disorders, may have a long-term influence on thyroid cancer risk in offspring.
Funding:
Intramural Research Program of the National Cancer Institute, National Institutes of Health.
INTRODUCTION
Over the past few decades, the incidence of thyroid cancer has increased substantially in all regions of the world, including the Nordic countries.1,2 While much of the increase has been attributed to more widespread use of diagnostic ultrasonography and fine-needle aspiration biopsy, environmental and hormonal exposures also may have contributed.2 Thyroid cancer is now the fifth leading cancer diagnosis among women worldwide and the second most common cancer among women under age 40.3 The higher female-to-male sex ratio for thyroid cancer, with an age-standardized incidence of 7.3/100,000 women versus 2.6/100,000 men in the Nordic countries (2012-2016), is apparent beginning in adolescence and peaks during the reproductive years (ages 15-44).1-4 The unusual age at diagnosis and sex patterns for thyroid cancer incidence remain largely unexplained, with epidemiologic studies providing conflicting evidence on the relationship between hormonal and reproductive factors (e.g. parity, age at menarche and menopause, exogenous hormone use) and risk of thyroid cancer in women.2,4 However, these surrogate indicators may not capture the specific hormonal exposures or critical exposure time window for thyroid cancer development and progression.2,4
To date, the only established modifiable risk factors for thyroid cancer are obesity and childhood exposure to ionizing radiation, with evidence suggesting stronger associations for younger versus older ages at exposure.5,6 Some studies have suggested a possible association between increased risk of thyroid cancer and in utero exposure to ionizing radiation, maternal diagnosis of hyperemesis gravidarum, and birth weight.7-10 Studying the role of the intrauterine environment in thyroid cancer development is challenging because it requires large study populations and complete and long-term follow-up of individuals from young ages until thyroid cancer onset. Accurate exposure information is desirable, but it is rarely feasible to collect direct measurements of exposure to the developing fetus, and self-reported exposure information is subject to recall and misclassification bias. Population-based medical registries offer a unique opportunity to study the relationship between in utero exposures and long-term risks of cancer, while overcoming many of the challenges described above.4
To gain additional insight about the role of intrauterine exposures in thyroid cancer etiology, we conducted a pooled nested case-control analysis of linked population-based registry data from four Nordic countries. In this study, we explored the associations for a wide range of perinatal characteristics, pregnancy complications, and birth outcomes, with thyroid cancer risk in offspring.
METHODS
Study population
We combined information on thyroid cancer from the national cancer registries in Denmark, Finland, Norway, and Sweden with information on maternal and offspring characteristics retrieved from the national medical birth registries (MBRs). The Nordic countries provide mainly publicly funded and organized health care.11 The cancer registries cover the entire population in Denmark, Finland, Norway and Sweden starting in 1943, 1953, 1953 and 1958, respectively, while the MBRs contain information on all births since 1973, 1987, 1967 and 1973, respectively. The Nordic cancer registries and MBRs offer high-quality, and virtually complete, nationwide coverage of cancer diagnoses and births.11-13 The Nordic MBRs include electronically recorded data obtained from standardized forms that are highly similar across the four countries and completed shortly after birth by the attending healthcare provider, documenting maternal health prior to pregnancy, during pregnancy, and at birth, in addition to selected offspring and birth outcomes. Additional linkages with the National Patient Registries (NPRs) in Sweden (since 1964) and Denmark (since 1977) were used to provide additional data on maternal (Denmark only) and offspring (Sweden and Denmark) diagnoses. Linkages of individual-level data across the registries were conducted using unique identification numbers provided to all citizens of the four countries.
This study was approved by the ethical committees in Norway and Sweden. In Denmark, the study was approved by the Data Protection Agency. In Finland, we obtained permission to use health registry and population data from the Finnish Institute for Health and Welfare and the Digital and Population Data Services Agency after approval by the data protection authority.
Case and control selection
Included were all thyroid cancer cases identified among individuals with recorded births in the four MBRs. Thyroid cancer cases were individuals diagnosed with first primary thyroid cancer (International Statistical Classification of Diseases and Related Health Problems [ICD]-10 C73 or the equivalent) in Denmark (1973-2015), Finland (1987-2015), Norway (1967-2015), or Sweden (1973-2015) (Supplementary Table 1). ICD-O-3 morphology codes were used to classify cases as papillary (8050, 8260, 8340-8344, 8350, 8450-8460), follicular (8290, 8330-8335), medullary (8345, 8510-8513), and anaplastic (8020-8035) carcinoma. In general, ten controls identified from the MBRs were sampled for each case, matched on country of residence, birth year, sex, and county of birth. Excluded were cases and controls with a previous diagnosis of cancer other than non-melanoma skin cancer, at the time of thyroid cancer diagnosis of the case (T0). Both cases and controls had to be alive and residing in the country of birth as of T0.
Exposures
MBR-derived variables included calendar year of birth, maternal age at birth, maternal marital status, birth order, multiple versus singleton birth, birth weight and length, and small/large for gestational age (SGA/LGA; weight by mean gestational age ±2 standard deviations14), preterm birth, delivery type (Cesarean, vaginal), and maternal pregnancy complications (hyperemesis, pregnancy anemia, placental abruption, placenta previa, pre-eclampsia/eclampsia, postpartum hemorrhage). Information on maternal smoking early and/or late in pregnancy was available in Denmark, Finland, Norway, and Sweden since 1991, 1987/1990, 1999, and 1982/1999, respectively, but largely incomplete. Pre-pregnancy body mass index (BMI) was incomplete and available mainly in Sweden. Offspring diagnoses of congenital hypothyroidism and neonatal jaundice were captured in the first year after birth. Data on maternal diagnoses of benign thyroid diseases were complete only in Denmark (see Supplementary Table 1 for codes).
Statistical analysis
We used conditional logistic regression models to calculate odds ratios (ORs) with 95% confidence intervals (CIs) for associations of maternal diagnosis of thyroid disease (prior to and during pregnancy), pregnancy complications, and birth outcomes with thyroid cancer risk in offspring, conditioned on matching variables. Additional adjustments were made for birth order. Analyses also were conducted separately by sex, urban (defined by counties that included a capital city) versus rural areas, birth year (<1986, ≥1986), calendar year of diagnosis (<2005, ≥2005), histologic type of thyroid cancer, and stage at thyroid cancer diagnosis (localized versus regional/distant). Models for papillary and follicular carcinomas were further stratified by age at diagnosis (<30, ≥30 years).
Role of the funding source
The funder had no role in the study design, data collection, data analysis, or data interpretation. DSD and TB had access to the raw data. The corresponding author had final responsibility for the decision to submit for publication.
RESULTS
In total, 2,437 first primary thyroid cancer cases and 24,362 matched controls were identified from the registries (Table 1). The breakdown of cases by histologic type (81% papillary, 12% follicular, 6% medullary, <1% anaplastic) was similar by country, although Denmark included a slightly lower proportion of papillary (77%) and slightly higher proportion of follicular (16%) carcinomas. Most cases were diagnosed in females (77%) and before age 30 (57%). The mean age at diagnosis was 27·5±8·7 years; cases were generally younger in Finland due to more recent establishment of the MBR.
Table 1.
Characteristics of 2,437 thyroid cancer casesa included in the study population
| Denmark | Finland | Norway | Swedenb | Total | |
|---|---|---|---|---|---|
| Total thyroid cancer cases (n) | 400 | 140 | 986 | 911 | 2,437 |
| Histologic typea | |||||
| Papillary carcinoma | 306 (77%) | 114 (81%) | 817 (83%) | 730 (82%) | 1,967 (81%) |
| Follicular carcinoma | 65 (16%) | 19 (14%) | 111 (11%) | 86 (10%) | 281 (12%) |
| Medullary carcinoma | 24 (6%) | 6 (4%) | 46 (5%) | 61 (7%) | 137 (6%) |
| Anaplastic carcinoma | <5c | <5c | <5c | <5c | 6 (<1%) |
| Other histology | 5 (1%) | <5c | 9 (<1%) | 13 (1%) | 28 (1%) |
| Sex | |||||
| Female | 301 (75%) | 110 (79%) | 760 (77%) | 709 (78%) | 1,880 (77%) |
| Male | 99 (25%) | 30 (21%) | 226 (23%) | 202 (22%) | 557 (23%) |
| Year of birth | |||||
| 1967-1969 | N/A | N/A | 243 (25%) | N/A | 243 (10%) |
| 1970-1979 | 222 (56%) | N/A | 502 (51%) | 451 (50%) | 1,175 (48%) |
| 1980-1989 | 144 (36%) | 56 (40%) | 184 (19%) | 352 (39%) | 736 (30%) |
| 1990-1999 | 32 (8%) | 79 (56%) | 52 (5%) | 94 (10%) | 257 (11%) |
| 2000-2013 | <5b | 5 (4%) | 5 (<1%) | 14 (2%) | 26 (1%) |
| Age at diagnosis (years) | |||||
| 0-9 | 8 (2%) | 9 (6%) | 7 (<1%) | 24 (3%) | 48 (2%) |
| 10-19 | 73 (18%) | 61 (44%) | 110 (11%) | 185 (20%) | 429 (18%) |
| 20-29 | 163 (41%) | 70 (50%) | 299 (30%) | 375 (41%) | 907 (37%) |
| 30-39 | 155 (39%) | <5c | 393 (40%) | 312 (34%) | 860 (35%) |
| 40-48 | <5c | <5c | 177 (18%) | 15 (2%) | 193 (8%) |
| Mean age (years) at diagnosis (SD) | 26·2 (7·8) | 18·9 (4·8) | 30·9 (8·7) | 25·7 (7·9) | 27·5 (8·7) |
| Year of cancer diagnosis | |||||
| 1985-1989 | 6 (2%) | 0 (0%) | 32 (3%) | 10 (1%) | 48 (2%) |
| 1990-1999 | 45 (11%) | 2 (1%) | 133 (13%) | 111 (12%) | 291 (12%) |
| 2000-2009 | 166 (42%) | 39 (28%) | 346 (35%) | 369 (41%) | 920 (38%) |
| 2010-2015 | 183 (46%) | 99 (71%) | 475 (48%) | 421 (46%) | 1,178 (48%) |
N/A = not applicable
The relatively high number of cases in Norway and Sweden was mainly a function of the year of establishment of the birth registry (with Norway having the oldest, allowing for more time between birth and thyroid cancer diagnosis) and the size of the underlying population (with Sweden having the largest).
18 unspecified cases from Sweden were excluded
Numbers suppressed due to <5 cases and/or controls
Risk of thyroid cancer in offspring was associated with birth weight (OR per kg=1·14, 95% CI 1·05-1·23), maternal pre-pregnancy diabetes (OR=1·69, 95% CI 0·98-2·93), postpartum hemorrhage (OR=1·28, 95% CI 1·06-1·55) (Table 2). These results did not materially change with additional adjustment for birth order, and mutual adjustment of birth weight and maternal history of diabetes had almost no influence on those results (data not shown). In Denmark, maternal diagnosis of benign thyroid diseases prior to or during pregnancy was associated with increased risk of thyroid cancer in offspring, with ORs of 18·1 (95% CI 10·5-31·2 for hypothyroidism, 11·9 (95% CI 6·80-20·9) for hyperthyroidism, 67·4 (95% CI 39·9-114) for goiter, and 22·5 (95% CI 6·93-73·1) for benign thyroid neoplasms (Figure). Of the neonatal conditions evaluated, congenital hypothyroidism was positively associated with thyroid cancer (OR=4·55, 95% CI 1·58-13·1), based on five exposed cases. In a sensitivity analysis unrestricted on age at congenital hypothyroidism diagnosis, the association strengthened slightly (OR=5·33, 95% CI 2·26-12·6), based on 8 exposed cases and 15 exposed controls.
Table 2.
Odds ratios (ORs)a for thyroid cancer risk in offspring in relation to maternal, paternal, and birth characteristics and maternal and neonatal medical conditions
| Cases (n=2,437) | Controls (n=24,362) | OR | 95% CI | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age at birth (years) | ||||
| <25 | 891 (37%) | 8,694 (36%) | 1·00 | Reference |
| 25-29 | 886 (36%) | 8,676 (36%) | 0·99 | 0·90-1·10 |
| 30-39 | 620 (25%) | 6,611 (27%) | 0·91 | 0·82-1·02 |
| 40+ | 40 (2%) | 380 (2%) | 1·02 | 0·73-1·43 |
| Missing | 0 (0%) | 1 (0%) | ||
| Age at birth (per year) | 2,437 (100%) | 24,361 (100%) | 0·99 | 0·99-1·00 |
| Smoking during pregnancy | ||||
| No | 367 (15%) | 3,826 (16%) | 1·00 | Reference |
| Yes | 123 (5%) | 1,150 (5%) | 1·12 | 0·90-1·40 |
| Missing | 1,947 (80%) | 19,386 (80%) | ||
| Marital status | ||||
| Married/co-inhabitant | 1,909 (78%) | 19,196 (79%) | 1·00 | Reference |
| Single/widowed/divorced | 444 (18%) | 4,386 (18%) | 1·02 | 0·90-1·14 |
| Missing | 84 (3%) | 780 (3%) | ||
| Paternal characteristics | ||||
| Age at birth (per year) | 978 (40%) | 9,791 (40%) | 1·00 | 0·99-1·01 |
| Missing | 1,459 (60%) | 14,571 (60%) | ||
| Birth characteristics | ||||
| Preterm birth | ||||
| No | 2,316 (95%) | 23,200 (95%) | 1·00 | Reference |
| Yes | 116 (5%) | 1,148 (5%) | 1·01 | 0·83-1·23 |
| Missing | 5 (0·2%) | 14 (0·1%) | ||
| Multiple birth | ||||
| No | 2,383 (98%) | 23,919 (98%) | 1·00 | Reference |
| Yes | 54 (2%) | 443 (2%) | 1·22 | 0·92-1·63 |
| Cesarean delivery | ||||
| No | 2,254 (92%) | 22,482 (92%) | 1·00 | Reference |
| Yes | 181 (7%) | 1,875 (8%) | 0·96 | 0·82-1·13 |
| Missing | 2 (0·1%) | 5 (0%) | ||
| Small-for-gestational age | ||||
| No | 2,262 (93%) | 22,778 (94%) | 1·00 | Reference |
| Yes | 70 (3%) | 676 (3%) | 1·04 | 0·81-1·34 |
| Missing | 105 (4%) | 908 (4%) | ||
| Large-for-gestational age | ||||
| No | 2,275 (93%) | 22,932 (94%) | 1·00 | Reference |
| Yes | 57 (2%) | 522 (2%) | 1·10 | 0·83-1·45 |
| Missing | 105 (4%) | 908 (4%) | ||
| Birth weight (g) | ||||
| <1,500 | 7 (0·3%) | 88 (0·4%) | 0·85 | 0·39-1·84 |
| 1,500-2,499 | 89 (4%) | 932 (4%) | 1·02 | 0·81-1·27 |
| 2,500-3,499 | 1,096 (45%) | 11,708 (48%) | 1·00 | Reference |
| 3,500-4,499 | 1,171 (48%) | 11,016 (45%) | 1·14 | 1·04-1·24 |
| 4,500+ | 53 (3%) | 514 (2%) | 1·21 | 0·93-1·58 |
| Missing | 9 (0·4%) | 45 (0·2%) | ||
| Birth weight (per kg) | 2,428 (100%) | 24,319 (100%) | 1·14 | 1·05-1·23 |
| Birth order | ||||
| 1 | 1,009 (41%) | 9,756 (40%) | 1·00 | Reference |
| 2 | 897 (37%) | 8,569 (35%) | 1·01 | 0·92-1·11 |
| 3 | 354 (15%) | 3,973 (16%) | 0·86 | 0·76-0·98 |
| 4 | 104 (4%) | 1,330 (5%) | 0·75 | 0·61-0·93 |
| 5+ | 72 (3%) | 716 (3%) | 0·97 | 0·75-1·24 |
| Missing | 1 (0%) | 18 (0·1%) | ||
| Maternal conditions diagnosed before pregnancy | ||||
| Diabetes | 15 (0·6%) | 89 (0·4%) | 1·69 | 0·98-2·93 |
| Hypertension | 2 (0·1%) | 22 (0·1%) | 0·90 | 0·21-3·85 |
| Maternal conditions diagnosed before/during pregnancy (Denmark only) | ||||
| Hypothyroidism | 38 (10%) | 24 (0·6%) | 18·1 | 10·5-31·2 |
| Hyperthyroidism | 27 (7%) | 24 (0·6%) | 11·9 | 6·8-20·9 |
| Goiter | 115 (29%) | 30 (0·8%) | 67·4 | 39·9-114 |
| Thyroiditis | <5b | <5b | 3·33 | 0·90-12·3 |
| Benign thyroid neoplasms | <5b | <5b | 22·5 | 6·93-73·1 |
| Maternal conditions diagnosed during pregnancy | ||||
| Gestational hypertension (yes vs. no) | 31 (1%) | 402 (2%) | 0·77 | 0·53-1·11 |
| Hyperemesis (yes vs. no) | 23 (1%) | 185 (1%) | 1·24 | 0·81-1·92 |
| Pregnancy anemia (yes vs. no) | 20 (1%) | 217 (1%) | 0·92 | 0·58-1·46 |
| Gestational diabetes (yes vs. no) | 9 (0·4%) | 72 (0·3%) | 1·25 | 0·62-2·52 |
| Placental abruption (yes vs. no) | 7 (0·3%) | 86 (0·4%) | 0·81 | 0·37-1·76 |
| Placenta previa (yes vs. no) | 6 (0·2%) | 69 (0·3%) | 0·87 | 0·38-2·01 |
| Preeclampsia/eclampsia (yes vs. no) | 32 (0·1%) | 337 (1%) | 1·03 | 0·78-1·35 |
| Postpartum hemorrhage (yes vs. no) | 132 (5%) | 1,047 (4%) | 1·28 | 1·06-1·55 |
| Neonatal conditions diagnosed in the first year after birth | ||||
| Congenital hypothyroidism (yes vs. no) | 5 (0·2%) | 11 (0·04%) | 4·55 | 1·58-13·1 |
| Neonatal jaundice (yes vs. no) | 27 (1%) | 226 (1%) | 1·20 | 0·80-1·81 |
NE= not estimable
From conditional logistic regression models, conditioned on birth year (of the case), sex, country, and county of birth
Numbers suppressed due to <5 cases and/or controls
Figure 1. Odds ratios (ORs) and 95% confidence intervals (CIs) for maternal thyroid conditions and risk of thyroid cancer in offspring (Denmark).
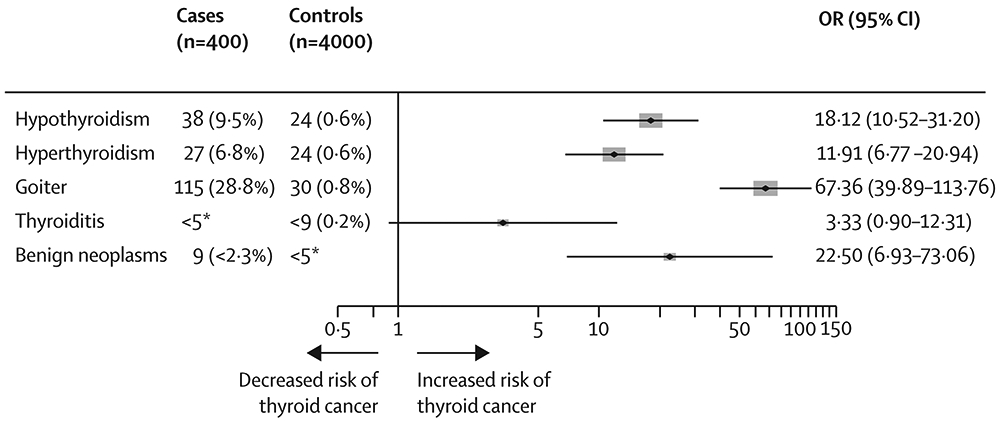
Results were based on conditional logistic regression models, conditioned on birth year, sex, and county of birth. Numbers of cases and controls under five were suppressed, in line with the regulations of the Danish Health Data Agency.
In models stratified by sex (Table 3), birth weight (per kg) was positively associated with risk of thyroid cancer in both male (1·16, 95% CI 0·99-1·36) and female (1·13, 95% CI 1·03-1·24) offspring. Being born LGA was associated with thyroid cancer risk in males (OR=1·65, 95% CI 1·00-2·71), but not in females (OR=0·95, 95% CI 0·68-1·32). Maternal pre-pregnancy diabetes was associated with increased thyroid cancer risk in females (OR=1·95, 95% CI 1·07-3·55), but not males (OR=0·91, 95% CI 0·21-3·88). Associations for maternal benign thyroid diseases were driven by females; we could not estimate ORs for males as all cases were unexposed. Congenital hypothyroidism was positively associated with thyroid cancer risk in females (OR=3·64, 95% CI 1·16-11·4); case numbers in male offspring were small.
Table 3.
Odds ratios (ORs)a for thyroid cancer risk in offspring in relation to maternal, paternal, and birth characteristics and maternal and neonatal medical conditions, by sex, urban/rural, year of birth, and year of diagnosis
| Sex | Urban vs. rural | Year of birth | Year of diagnosis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males (n=557 cases) |
Females (n=1,880 cases) |
Urban (n=417) | Rural (n=2,020) | Up to 1985 (n=1,873) |
1986+ (n=564) | Up to 2004 (n=672) |
2005+ (n=1,765) | |||||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Maternal characteristics | ||||||||||||||||
| Age at birth (years) | ||||||||||||||||
| <25 | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference |
| 25-29 | 0·89 | 0·72-1·09 | 1·03 | 0·92-1·15 | 1·13 | 0·88-1·45 | 0·97 | 0·87-1·08 | 1·00 | 0·89-1·11 | 0·98 | 0·78-1·22 | 0·98 | 0·82-1·18 | 1·00 | 0·89-1·13 |
| 30-39 | 0·86 | 0·68-1·07 | 0·93 | 0·82-1·05 | 1·04 | 0·79-1·36 | 0·89 | 0·78-1·00 | 0·92 | 0·81-1·04 | 0·89 | 0·71-1·12 | 0·83 | 0·67-1·03 | 0·94 | 0·83-1·07 |
| 40+ | 1·00 | 0·49-2·02 | 1·03 | 0·70-1·51 | 1·19 | 0·53-2·69 | 1·01 | 0·70-1·45 | 1·01 | 0·68-1·50 | 1·06 | 0·57-1·98 | 0·96 | 0·49-1·86 | 1·05 | 0·71-1·55 |
| Age at birth (per year) | 0·99 | 0·97-1·01 | 1·00 | 0·99-1·01 | 1·00 | 0·98-1·02 | 0·99 | 0·98-1·00 | 1·00 | 0·99-1·100 | 0·99 | 0·97-1·01 | 0·99 | 0·97-1·01 | 1·00 | 0·99-1·01 |
| Smoking during pregnancy (yes vs. no) | 1·33 | 0·82-2·16 | 1·08 | 0·84-1·38 | 1·25 | 0·79-1·96 | 1·09 | 0·85-1·40 | 1·33 | 0·88-2·00 | 1·05 | 0·81-1·37 | 0·68 | 0·36-1·29 | 1·22 | 0·96-1·54 |
| Marital status | ||||||||||||||||
| Married/co-inhabitant | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference |
| Single/widowed/divorced | 1·04 | 0·82-1·33 | 1·01 | 0·88-1·15 | 0·96 | 0·73-1·26 | 1·04 | 0·91-1·18 | 1·02 | 0·89-1·16 | 1·01 | 0·78-1·31 | 1·07 | 0·86-1·33 | 1·00 | 0·87-1·14 |
| Paternal characteristics | ||||||||||||||||
| Age at birth (per year) | 0·99 | 0·97-1·01 | 1·00 | 0·99-1·01 | 1·04 | 1·00-1·07 | 0·99 | 0·98-1·00 | 0·99 | 0·98-1·01 | 1·02 | 0·99-1·05 | 0·99 | 0·97-1·01 | 1·00 | 0·99-1·01 |
| Birth characteristics | ||||||||||||||||
| Preterm birth (yes vs. no) | 1·07 | 0·73-1·56 | 0·99 | 0·79-1·25 | 1·37 | 0·89-2·09 | 0·94 | 0·75-1·18 | 0·88 | 0·70-1·12 | 1·38 | 0·99-1·95 | 1·05 | 0·74-1·50 | 0·99 | 0·79-1·26 |
| Multiple birth (yes vs. no) | 1·32 | 0·72-2·43 | 1·20 | 0·87-1·65 | 1·43 | 0·77-2·64 | 1·22 | 0·88-1·68 | 1·17 | 0·83-1·65 | 1·37 | 0·82-2·28 | 1·05 | 0·58-1·92 | 1·28 | 0·93-1·78 |
| Cesarean delivery (yes vs. no) | 0·90 | 0·64-1·27 | 0·98 | 0·82-1·17 | 1·12 | 0·79-1·58 | 0·94 | 0·78-1·12 | 0·95 | 0·78-1·15 | 0·99 | 0·75-1·31 | 0·98 | 0·71-1·35 | 0·96 | 0·79-1·15 |
| Small-for-gestational age (yes vs. no) | 1·21 | 0·72-2·01 | 0·99 | 0·75-1·33 | 1·45 | 0·87-2·41 | 0·95 | 0·71-1·27 | 0·98 | 0·74-1·30 | 1·31 | 0·76-2·26 | 1·05 | 0·66-1·68 | 1·04 | 0·77-1·39 |
| Large-for-gestational age (yes vs. no) | 1·65 | 1·00-2·71 | 0·95 | 0·68-1·32 | 1·06 | 0·51-2·22 | 1·08 | 0·79-1·46 | 1·00 | 0·71-1·41 | 1·34 | 0·84-2·14 | 1·33 | 0·81-2·19 | 1·02 | 0·73-1·42 |
| Birth weight (g) | ||||||||||||||||
| <1,500 | 0·70 | 0·09-5·26 | 0·88 | 0·38-2·04 | 1·16 | 0·27-5·02 | 0·77 | 0·31-1·94 | 0·72 | 0·26-1·98 | 1·14 | 0·34-3·75 | 0·87 | 0·20-3·67 | 0·85 | 0·34-2·12 |
| 1,500-2,499 | 0·93 | 0·55-1·58 | 1·04 | 0·81-1·33 | 1·70 | 1·10-2·64 | 0·86 | 0·66-1·13 | 0·95 | 0·73-1·24 | 1·26 | 0·80-1·98 | 1·03 | 0·67-1·59 | 1·01 | 0·78-1·32 |
| 2,500-3,499 | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference |
| 3,500-4,499 | 1·20 | 1·00-1·45 | 1·12 | 1·02-1·24 | 1·12 | 0·90-1·39 | 1·14 | 1·04-1·26 | 1·13 | 1·02-1·25 | 1·17 | 0·98-1·41 | 1·33 | 1·12-1·57 | 1·07 | 0·97-1·19 |
| 4,500+ | 1·34 | 0·87-2·07 | 1·15 | 0·82-1·62 | 1·50 | 0·80-2·81 | 1·15 | 0·86-1·55 | 1·21 | 0·89-1·66 | 1·22 | 0·74-2·00 | 1·96 | 1·23-3·11 | 1·00 | 0·72-1·38 |
| Birth weight (per kg) | 1·16 | 0·99-1·36 | 1·13 | 1·03-1·24 | 0·96 | 0·80-1·15 | 1·18 | 1·08-1·29 | 1·16 | 1·06-1·27 | 1·07 | 0·92-1·25 | 1·27 | 1·09-1·47 | 1·09 | 0·99-1·19 |
| Birth order | ||||||||||||||||
| 1 | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference |
| 2 | 0·96 | 0·79-1·17 | 1·03 | 0·92-1·14 | 1·19 | 0·95-1·49 | 0·97 | 0·88-1·08 | 0·97 | 0·87-1·08 | 1·16 | 0·98-1·42 | 0·84 | 0·70-1·01 | 1·08 | 0·97-1·21 |
| 3 | 0·72 | 0·55-0·94 | 0·91 | 0·78-1·04 | 0·87 | 0·63-1·21 | 0·85 | 0·74-0·98 | 0·86 | 0·75-0·99 | 0·85 | 0·65-1·12 | 0·79 | 0·62-1·00 | 0·89 | 0·77-1·03 |
| 4 | 0·58 | 0·36-0·95 | 0·80 | 0·64-1·02 | 1·10 | 0·63-1·93 | 0·70 | 0·56-0·89 | 0·70 | 0·55-0·90 | 0·93 | 0·61-1·42 | 0·65 | 0·44-0·98 | 0·79 | 0·62-1·02 |
| 5+ | 0·88 | 0·51-1·52 | 0·99 | 0·75-1·32 | 1·35 | 0·70-2·59 | 0·92 | 0·70-1·21 | 0·84 | 0·63-1·14 | 1·51 | 0·92-2·47 | 0·90 | 0·56-1·43 | 0·99 | 0·74-1·34 |
| Maternal conditions diagnosed before pregnancy | ||||||||||||||||
| Diabetes (yes vs. no) | 0·91 | 0·21-3·88 | 1·95 | 1·07-3·55 | 1·66 | 0·48-5·68 | 1·85 | 0·99-3·45 | 1·50 | 0·77-2·93 | 2·26 | 0·86-5·98 | 0·95 | 0·22-4·06 | 1·93 | 1·06-3·50 |
| Hypertension (yes vs. no) | NE | NE | 1·24 | 0·29-5·40 | 1·27 | 0·16-10·3 | 0·66 | 0·09-5·01 | 1·67 | 0·37-7·45 | NE | NE | 1·67 | 0·20-13·8 | 0·62 | 0·98-4·68 |
| Maternal conditions diagnosed before/during pregnancy (Denmark only)b | ||||||||||||||||
| Hypothyroidism (yes vs. no) | NE | NE | 19·1 | 11·0-33·1 | 38·4 | 10·9-136 | 11·1 | 6·35-19·5 | 17·6 | 10·2-30·4 | 3·33 | 0·67-16·5 | 28·0 | 10·1-77·7 | 11·2 | 6·23-20·0 |
| Hyperthyroidism (yes vs. no) | NE | NE | 11·9 | 6·8-20·9 | 5·60 | 1·64-19·2 | 8·91 | 5·05-15·7 | 8·32 | 4·81-14·4 | 6·00 | 1·43-25·1 | 4·72 | 1·60-13·9 | 9·40 | 5·24-16·9 |
| Goiter (yes vs. no) | NE | NE | 82·8 | 46·6-147 | 51·0 | 19·6-133 | 34·6 | 22·4-53·5 | 50·2 | 31·5-80·1 | 10·0 | 4·16-24·0 | 33·6 | 14·5-78·0 | 38·4 | 24·5-60·0 |
| Thyroiditis (yes vs. no) | NE | NE | 3·33 | 0·90-12·3 | 10·0 | 0·63-160 | 2·22 | 0·48-10·3 | 3·33 | 0·90-12·3 | NE | NE | 3·33 | 0·35-32·1 | 2·86 | 0·59-13·8 |
| Benign thyroid neoplasms (yes vs. no) | NE | NE | 22·5 | 6·93-73·1 | NE | NE | 22·5 | 6·93-73·1 | 17·5 | 5·12-59·8 | 20·0 | 1·81-221 | NE | NE | 14·0 | 4·44-44·1 |
| Maternal conditions diagnosed during pregnancy | ||||||||||||||||
| Gestational hypertension (yes vs. no) | 0·80 | 0·40-1·59 | 0·75 | 0·49-1·17 | 1·26 | 0·60-2·65 | 0·67 | 0·44-1·03 | 0·68 | 0·43-1·09 | 0·96 | 0·52-1·75 | 0·70 | 0·32-1·51 | 0·79 | 0·52-1·20 |
| Hyperemesis (yes vs. no) | 0·66 | 0·16-2·78 | 1·36 | 0·68-2·15 | 1·27 | 0·49-3·27 | 1·21 | 0·74-1·98 | 1·10 | 0·65-1·89 | 1·64 | 0·77-3·48 | 1·09 | 0·43-2·74 | 1·30 | 0·79-2·13 |
| Pregnancy anemia (yes vs. no) | 1·11 | 0·39-3·14 | 0·88 | 0·52-1·48 | 0·45 | 0·11-1·88 | 1·03 | 0·63-1·69 | 0·78 | 0·43-1·41 | 1·26 | 0·59-2·66 | 1·38 | 0·64-2·96 | 0·76 | 0·42-1·36 |
| Gestational diabetes (yes vs. no) | 1·00 | 0·13-7·96 | 1·29 | 0·62-2·72 | 1·35 | 0·40-4·53 | 1·20 | 0·51-2·83 | 1·07 | 0·33-3·52 | 1·37 | 0·58-3·26 | 2·00 | 0·58-6·91 | 1·05 | 0·45-2·46 |
| Placental abruption (yes vs. no) | 1·51 | 0·44-5·11 | 0·60 | 0·22-1·66 | 1·25 | 0·28-5·59 | 0·70 | 0·28-1·74 | 0·74 | 0·30-1·85 | 1·05 | 0·26-4·52 | 0·80 | 0·18-3·42 | 0·82 | 0·33-2·04 |
| Placenta previa (yes vs. no) | 1·43 | 0·32-6·29 | 0·73 | 0·26-2·01 | 0·70 | 0·09-5·39 | 0·91 | 0·36-2·27 | 1·13 | 0·48-2·65 | NE | NE | 2·71 | 0·89-8·27 | 0·37 | 0·09-1·51 |
| Preeclampsia/eclampsia (yes vs. no) | 1·14 | 0·64-2·04 | 1·00 | 0·74-1·36 | 0·52 | 0·21-1·30 | 1·16 | 0·87-1·54 | 1·11 | 0·82-1·52 | 0·81 | 0·46-1·43 | 1·34 | 0·83-2·14 | 0·92 | 0·66-1·28 |
| Postpartum hemorrhage (yes vs. no) | 1·23 | 0·79-1·90 | 1·29 | 1·05-1·59 | 1·33 | 0·86-2·04 | 1·27 | 1·03-1·57 | 1·19 | 0·96-1·47 | 1·74 | 1·16-2·61 | 1·06 | 0·73-1·55 | 1·37 | 1·10-1·70 |
| Neonatal conditions diagnosed in the first year after birth | ||||||||||||||||
| Congenital hypothyroidism (yes vs. no) | NE | NE | 3·64 | 1·16-11·4 | NE | NE | 5·56 | 1·86-16·6 | 3·33 | 0·67-16·5 | 6·00 | 1·43-25·1 | 10·0 | 0·63-160 | 4·00 | 1·25-12·8 |
| Neonatal jaundice (yes vs. no) | 0·64 | 0·23-1·80 | 1·41 | 0·90-2·21 | 0·61 | 0·19-1·97 | 1·41 | 0·91-2·19 | 1·92 | 0·94-3·93 | 1·00 | 0·61-1·65 | 1·15 | 0·40-3·34 | 1·21 | 0·78-1·89 |
From conditional logistic regression models, conditioned on birth year (of the case), sex, country, and county of birth
Results were generally similar by urban versus rural residence, year of birth of the offspring, and calendar year of thyroid cancer diagnosis (Table 3). The association for birth weight was stronger among offspring born up to 1985 and those diagnosed with thyroid cancer up to 2004. The association for maternal diabetes was restricted to thyroid cancers diagnosed after 2004 (OR=1·93, 95% CI 1·06-3·50). Associations of maternal hypothyroidism and goiter were stronger in urban versus rural residents and offspring born up to 1985. Associations of maternal benign thyroid neoplasms were only observed in rural residents and thyroid cancer diagnosed after 2004. The association of congenital hypothyroidism was restricted to rural residents (OR=5·56, 95% CI 1·86-16·6).
In models stratified by histologic type (Table 4), birth weight was positively associated with risk of both papillary (OR=1·11, 95% CI 1·02-1·21) and follicular (OR=1·38, 95% CI 1·10-1·75) thyroid carcinoma. In Denmark, maternal diagnosis of hypothyroidism (OR=25·1, 95% CI 13·5-46·5), thyroiditis (OR=3·75, 95% CI 0·99-14·1), and benign thyroid neoplasm (OR=20·0, 95% CI 5·00-80·0) were more clearly positively associated with risk of papillary than follicular thyroid carcinoma due partly to smaller number of follicular cases. Maternal hyperthyroidism and goiter were associated with both histologic types, though ORs were slightly stronger for follicular thyroid carcinoma (17·8, 95% CI 3·22-98·5; 86·4, 95% CI 26·2-285, respectively). The association for postpartum hemorrhage was also slightly stronger for follicular (OR=1·70, 1·02-2·82) versus papillary (OR=1·20, 95% CI 0·97-1·49) carcinoma. Neonatal diagnosis of congenital hypothyroidism was associated with follicular (OR=20·0, 95% CI 1·81-221), but not papillary, carcinoma. Of all the factors evaluated, risk of medullary carcinoma (n=137) was only associated with preterm birth (OR=1·94, 95% CI 1·02-3·72; other results not shown). We did not separately evaluate associations for other thyroid cancer types due to small case numbers.
Table 4.
Odds ratios (ORs)a for thyroid cancer risk in offspring in relation to maternal, paternal, and birth characteristics and maternal and neonatal medical conditions, by histologic type and stage at diagnosis
| Papillary (n=1,967) | Follicular (n=281) | Localized (n=869) | Regional/distant (n=842) | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Maternal characteristics | ||||||||
| Age at birth (per year) | 1·00 | 0·99-1·00 | 0·98 | 0·95-1·00 | 1·00 | 0·99-1·02 | 1·00 | 0·98-1·01 |
| Smoking during pregnancy (yes vs. no) | 1·12 | 0·87-1·43 | 1·06 | 0·57-1·98 | 1·25 | 0·81-1·94 | 0·83 | 0·54-1·30 |
| Marital status | ||||||||
| Married/co-inhabitant | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference |
| Single/widowed/divorced | 1·04 | 0·91-1·18 | 0·90 | 0·63-1·26 | 1·29 | 1·06-1·57 | 0·92 | 0·75-1·13 |
| Paternal characteristics | ||||||||
| Age at birth (per year) | 1·00 | 0·99-1·01 | 0·98 | 0·95-1·01 | 1·00 | 0·99-1·02 | 0·99 | 0·98-1·01 |
| Birth characteristics | ||||||||
| Preterm birth (yes vs. no) | 0·90 | 0·71-1·13 | 1·19 | 0·70-2·02 | 0·81 | 0·56-1·16 | 0·90 | 0·64-1·28 |
| Multiple birth (yes vs. no) | 1·24 | 0·90-1·70 | 0·60 | 0·18-1·92 | 1·25 | 0·76-2·04 | 1·02 | 0·62-1·70 |
| Cesarean delivery (yes vs. no) | 0·91 | 0·76-1·10 | 1·25 | 0·80-1·93 | 1·03 | 0·77-1·36 | 0·91 | 0·68-1·21 |
| Small-for-gestational age (yes vs. no) | 1·09 | 0·83-1·43 | 0·54 | 0·20-1·50 | 1·09 | 0·72-1·65 | 1·15 | 0·77-1·73 |
| Large-for-gestational age (yes vs. no) | 1·24 | 0·92-1·68 | 0·56 | 0·20-1·56 | 1·08 | 0·69-1·70 | 1·35 | 0·85-2·14 |
| Birth weight (g) | ||||||||
| <1,500 | 0·74 | 0·30-1·85 | NE | NE | NE | NE | 1·27 | 0·38-4·21 |
| 1,500-2,499 | 1·00 | 0·78-1·29 | 0·88 | 0·42-1·85 | 1·10 | 0·77-1·59 | 0·96 | 0·64-1·44 |
| 2,500-3,499 | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference |
| 3,500-4,499 | 1·08 | 0·98-1·19 | 1·51 | 1·17-1·95 | 1·14 | 0·99-1·32 | 1·19 | 1·02-1·37 |
| 4,500+ | 1·30 | 0·98-1·73 | 0·69 | 0·25-1·95 | 1·20 | 0·77-1·87 | 1·53 | 1·00-2·35 |
| Birth weight (per kg) | 1·11 | 1·02-1·21 | 1·38 | 1·10-1·75 | 1·20 | 1·05-1·37 | 1·17 | 1·03-1·34 |
| Birth order | ||||||||
| 1 | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference |
| 2 | 1·01 | 0·91-1·12 | 1·02 | 0·77-1·34 | 0·97 | 0·83-1·14 | 0·97 | 0·83-1·14 |
| 3 | 0·83 | 0·72-0·96 | 0·82 | 0·57-1·19 | 0·99 | 0·81-1·21 | 0·88 | 0·71-1·08 |
| 4 | 0·75 | 0·59-0·94 | 0·71 | 0·38-1·31 | 0·82 | 0·59-1·15 | 0·69 | 0·48-0·98 |
| 5+ | 1·02 | 0·77-1·34 | 0·53 | 0·21-1·32 | 0·98 | 0·66-1·47 | 0·75 | 0·48-1·18 |
| Maternal conditions diagnosed before pregnancy | ||||||||
| Diabetes (yes vs. no) | 1·65 | 0·89-3·05 | 3·00 | 0·83-10·9 | 2·34 | 0·95-5·76 | 1·14 | 0·41-3·23 |
| Hypertension (yes vs. no) | 1·05 | 0·24-4·50 | NE | NE | 2·86 | 0·59-13·8 | NE | NE |
| Maternal conditions diagnosed before/during pregnancy (Denmark only) | ||||||||
| Hypothyroidism (yes vs. no) | 25·1 | 13·5-46·5 | 3·33 | 0·35-32·1 | 10·0 | 3·97-25·2 | 30·1 | 14·2-63·8 |
| Hyperthyroidism (yes vs. no) | 11·9 | 6·5-21·9 | 17·8 | 3·22-98·5 | 11·4 | 4·14-31·5 | 5·71 | 2·81-11·6 |
| Goiter (yes vs. no) | 59·9 | 33·4-108 | 86·4 | 26·2-285 | 36·0 | 17·9-72·5 | 35·4 | 20·3-61·5 |
| Thyroiditis (yes vs. no) | 3·75 | 0·99-14·1 | NE | NE | NE | NE | 6·00 | 1·43-25·1 |
| Benign neoplasms (yes vs. no) | 20·0 | 5·00-80·0 | 10·0 | 0·63-160 | NE | NE | 7·50 | 1·68-33·5 |
| Maternal conditions diagnosed during pregnancy | ||||||||
| Gestational hypertension (yes vs. no) | 0·69 | 0·45-1·06 | 0·91 | 0·32-2·55 | 0·86 | 0·46-1·59 | 0·59 | 0·29-1·21 |
| Hyperemesis (yes vs. no) | 1·02 | 0·60-1·74 | 1·68 | 0·58-4·90 | 1·27 | 0·63-2·55 | 1·48 | 0·76-2·89 |
| Pregnancy anemia (yes vs. no) | 0·99 | 0·61-1·62 | 1·05 | 0·24-4·59 | 0·83 | 0·33-2·08 | 1·30 | 0·59-2·88 |
| Gestational diabetes (yes vs. no) | 1·20 | 0·51-2·83 | 1·44 | 0·32-6·40 | 0·80 | 0·19-3·39 | 1·68 | 0·65-4·36 |
| Placental abruption (yes vs. no) | 0·67 | 0·27-1·67 | 1·26 | 0·15-10·7 | 0·63 | 0·15-2·61 | 0·52 | 0·09-3·93 |
| Placenta previa (yes vs. no) | 0·91 | 0·36-2·28 | 1·00 | 0·13-7·81 | 0·74 | 0·18-3·12 | 0·71 | 0·09-5·43 |
| Preeclampsia/eclampsia (yes vs. no) | 1·00 | 0·74-1·35 | 0·86 | 0·34-2·16 | 1·07 | 0·68-1·69 | 0·68 | 0·41-1·14 |
| Postpartum hemorrhage (yes vs. no) | 1·20 | 0·97-1·49 | 1·70 | 1·02-2·82 | 1·47 | 1·09-1·99 | 1·58 | 1·16-2·16 |
| Neonatal conditions diagnosed in the first year after birth | ||||||||
| Congenital hypothyroidism (yes vs. no) | 2·22 | 0·48-10·3 | 20·0 | 1·81-221 | 13·3 | 2·98-59·6 | 2·00 | 0·23-17·1 |
| Neonatal jaundice (yes vs. no) | 1·33 | 0·85-2·07 | 0·37 | 0·05-2·79 | 1·21 | 0·57-2·58 | 1·64 | 0·88-3·05 |
NE= not estimable
From conditional logistic regression models, conditioned on birth year (of the case), sex, country, and county of birth
Few differences were observed by stage at diagnosis (Table 4). Birth weight and postpartum hemorrhage were positively associated with risk of thyroid cancer irrespective of stage, and risk of regional/distant stage thyroid cancer was positively associated with all maternal thyroid diseases examined. Only localized thyroid cancer was associated with single/widowed/divorced maternal marital status. Localized thyroid cancer also was more strongly associated with maternal pre-pregnancy diabetes, maternal hyperthyroidism, and congenital hypothyroidism. Risk of regional/distant thyroid cancer was higher among cohort members with maternal hypothyroidism.
Findings were largely similar by offspring age at thyroid cancer diagnosis (<30 vs. ≥30 years) (Table 5). However, the association for maternal diabetes was restricted to papillary carcinoma cases diagnosed before age 30 (OR=2·25, 95% CI 1·09-4·63). The association of maternal hypothyroidism was stronger for papillary carcinoma diagnosed before age 30, whereas the association of maternal hyperthyroidism was stronger for both papillary and follicular thyroid cancer diagnosed after age 30. Maternal goiter appeared to be more strongly associated with papillary and follicular thyroid carcinoma diagnosed after age 30. There was limited power to evaluate these factors in relation to thyroid cancer diagnosed before age 20.
Table 5.
Odds ratios (ORs)a for thyroid cancer risk in offspring (papillary, n=1,968 and follicular, n=280) in relation to maternal and neonatal characteristics, stratified on age at diagnosis.
| Papillary | Follicular | |||||||
|---|---|---|---|---|---|---|---|---|
| <30 years | 30+ years | <30 years | 30+ years | |||||
| OR1 | 95% | OR1 | 95% | OR1 | 95% | OR1 | 95% | |
| Maternal characteristics | ||||||||
| Age at birth (per birth) | 0.99 | 0.98-1·00 | 1·00 | 0.99-1·01 | 0.97 | 0.94-1·01 | 0.98 | 0.95-1·02 |
| Smoking during pregnancy (yes vs. no) | 1·14 | 0.89-1·47 | 0.29 | 0.03-2·66 | 0.98 | 0.52-1·86 | 7·94 | 0.49-128 |
| Marital status | ||||||||
| Married/ cohabitant | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference |
| Single/widow/divorced/other | 1·08 | 0.91-1·28 | 1·00 | 0.82-1·21 | 0.83 | 0.52-1·33 | 0.98 | 0.59-1·63 |
| Paternal characteristics | ||||||||
| Age at birth (per year) | 1·00 | 0.98-1·02 | 1·00 | 0.98-1·01 | 0.99 | 0.94-1·04 | 0.97 | 0.93-1·01 |
| Birth characteristics | ||||||||
| Preterm birth (yes vs. no) | 1·04 | 0.78-1·37 | 0.69 | 0.46-1·04 | 1·06 | 0.50-2·22 | 1·36 | 0.63-2·93 |
| Multiple birth (yes vs. no) | 1·18 | 0.77-1·81 | 1·32 | 0.83-2·12 | 0.33 | 0.04-2·43 | 1·00 | 0.23-4·31 |
| Cesarean delivery (yes vs. no) | 0.90 | 0.72-1·13 | 0.94 | 0.69-1·27 | 1·09 | 0.63-1·88 | 1·63 | 0.78-3·38 |
| Small-for-gestational age (yes vs. no) | 1·22 | 0.86-1·75 | 0.94 | 0.62-1·43 | 0.28 | 0.04-2·06 | 0.80 | 0.24-2·64 |
| Large-for-gestational age (yes vs. no) | 1·26 | 0.84-1·88 | 1·23 | 0.78-1·92 | 0.59 | 0.18-1·95 | 0.48 | 0.06-3·59 |
| Birth weight (g) | ||||||||
| <1,500 | 1·15 | 0.46-2·91 | NE | NE | NE | NE | NE | NE |
| 1,500-2,499 | 1·09 | 0.78-1·51 | 0.90 | 0.61-1·33 | 1·22 | 0.47-3·15 | 0.59 | 0.18-1·96 |
| 2,500-3,499 | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference |
| 3,500-4,499 | 1·15 | 1·01-1·31 | 1·01 | 0.87-1·16 | 1·61 | 1·13-2·28 | 1·41 | 0.97-2·06 |
| 4,500+ | 1·30 | 0.87-1·93 | 1·31 | 0.87-1·97 | 1·00 | 0.35-2·90 | NE | NE |
| Birth weight (per kg) | 1·11 | 0.99-1·25 | 1·11 | 0.97-1·26 | 1·44 | 1·06-1·97 | 1·31 | 0.92-1·88 |
| Birth order | ||||||||
| 1 | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference | 1·00 | Reference |
| 2 | 0.99 | 0.86-1·14 | 1·03 | 0.88-1·20 | 1·21 | 0.83-1·75 | 0.84 | 0.55-1·27 |
| 3 | 0.78 | 0.65-0.95 | 0.89 | 0.72-1·09 | 0.89 | 0.53-1·49 | 0.78 | 0.46-1·33 |
| 4 | 0.78 | 0.57-1·07 | 0.70 | 0.49-1·00 | 0.68 | 0.29-1·64 | 0.72 | 0.30-1·75 |
| 5+ | 1·33 | 0.93-1·91 | 0.71 | 0.46-1·11 | 0.46 | 0.11-1·95 | 0.57 | 0.17-1·92 |
| Maternal conditions diagnosed before pregnancy | ||||||||
| Diabetes (yes vs. no) | 2·25 | 1·09-4·63 | 0.91 | 0.27-2·99 | 1·11 | 0.14-8·77 | 20.0 | 1·81-221 |
| Hypertension (yes vs. no) | 0.58 | 0.08-4·39 | 5·00 | 0.45-55·1 | NE | NE | NE | NE |
| Maternal conditions diagnosed before/during pregnancy (Denmark only) | ||||||||
| Hypothyroidism (yes vs. no) | 42·5 | 17·5-103 | 12·2 | 4·88-30.7 | 5·00 | 0.45-55·1 | NE | NE |
| Hyperthyroidism (yes vs. no) | 9·63 | 3·99-23·2 | 14·4 | 6·17-33·8 | NE | NE | 8·22 | 1·13-60.1 |
| Goiter (yes vs. no) | 48·7 | 22·8-104 | 77·8 | 30.7-197 | 28·4 | 7·66-105 | NEb | NEb |
| Thyroiditis (yes vs. no) | 2·50 | 0.28-22·5 | 5·00 | 0.92-27·3 | NE | NE | NE | NE |
| Benign neoplasms (yes vs. no) | 20.0 | 3·66-109 | 20.0 | 1·81-221 | NE | NE | 10.0 | 0.63-160 |
| Maternal conditions diagnosed during pregnancy | ||||||||
| Gestational hypertension (yes vs. no) | 0.74 | 0.43-1·28 | 0.62 | 0.30-1·26 | NE | NE | 2·53 | 0.84-7·67 |
| Hyperemesis (yes vs. no) | 1·02 | 0.51-2·03 | 1·02 | 0.44-2·36 | 2·20 | 0.61-7·87 | 1·00 | 0.13-7·81 |
| Pregnancy anemia (yes vs. no) | 1·39 | 0.77-2·51 | 0.58 | 0.23-1·43 | 0.83 | 0.10-6·55 | 1·43 | 0.18-11·6 |
| Gestational diabetes (yes vs. no) | 1·19 | 0.47-3·05 | 1·25 | 0.16-9·99 | 2·27 | 0.48-10.8 | NE | NE |
| Placental abruption (yes vs. no) | 0.69 | 0.21-2·25 | 0.65 | 0.15-2·70 | 1·46 | 0.17-12·8 | NE | NE |
| Placenta previa (yes vs. no) | 1·07 | 0.32-3·54 | 0.74 | 0.17-3·13 | NE | NE | 1·67 | 0.20-13·8 |
| Preeclampsia/eclampsia (yes vs. no) | 1·00 | 0.68-1·47 | 1·01 | 0.63-1·63 | 0.33 | 0.04-2·42 | 1·44 | 0.50-4·19 |
| Postpartum hemorrhage (yes vs. no) | 1·15 | 0.86-1·54 | 1·27 | 0.93-1·74 | 1·63 | 0.78-3·37 | 1·78 | 0.88-3·59 |
| Neonatal conditions diagnosed in the first year after birth | ||||||||
| Congenital hypothyroidism (yes vs. no) | 2·86 | 0.59-13·8 | NE | NE | 20.0 | 1·81-221 | NE | NE |
| Neonatal jaundice (yes vs. no) | 1·36 | 0.85-2·18 | 1·05 | 0.25-4·52 | 0.37 | 0.05-2·79 | NE | NE |
From conditional logistic regression models, conditioned on birth year (of the case), sex, country, and county of birth
In unconditional logistic regression models (adjusting for the matching factors), the OR is 216 (95% CI 87-541), based on 19 exposed cases (59%) and 3 exposed controls (1%)
In sensitivity analyses restricted to cases and controls from Sweden for whom data were available, adjustment for smoking information during pregnancy (324 cases; 3,290 controls) and pre-pregnancy BMI (246 cases; 2,456 controls) had almost no influence on the magnitude of the associations (data not shown).
DISCUSSION
We pooled nationwide population-based registry data from four Nordic countries to evaluate a maternal medical history, pregnancy complications, and birth characteristics in relation to risk of thyroid cancer in offspring. Maternal benign thyroid conditions diagnosed before or during pregnancy were strongly associated with thyroid cancer risk in offspring, with ORs ranging from >3 (for maternal thyroiditis and risk of papillary carcinoma) to >80 (for goiter and risk of follicular carcinoma). These findings were only evident in female offspring due to the lack of exposed male cases and were similar for thyroid cancers diagnosed before and after age 30. Modest positive associations were observed for birth weight, maternal history of diabetes, and maternal postpartum hemorrhage, while an inverse association with risk was found for birth order, apart from the highest category (five or more). Congenital hypothyroidism was strongly associated with follicular thyroid carcinoma (OR=20). Together, these findings generate new hypotheses regarding two unique features of thyroid cancer compared to other malignancies—the relatively young age at onset and the higher incidence of the disease in women compared to men—and provide additional insights into the role of early-life exposures in thyroid cancer etiology.
Our study is the first, to our knowledge, to evaluate maternal thyroid diseases in relation to thyroid cancer risk in offspring. The associations were strong in magnitude and consistent with previously observed associations of maternal thyroid disease in pregnancy with thyroid function in adolescent offspring,15 and associations of benign thyroid conditions with increased risk of subsequent thyroid cancer.16 Thyroid diseases are more common in women than men and often manifest for the first time during pregnancy due to the substantial physiological, hormonal, and immunological changes that occur over the course of gestation, resulting in additional stress on the thyroid and contributing to changes in thyroid hormone levels.17 In iodine sufficient regions, undiagnosed hypothyroidism is prevalent in about 2-3% of pregnancies, most commonly due to Hashimoto’s thyroiditis (an autoimmune condition), while the prevalence of undiagnosed hyperthyroidism in pregnancy ranges from about 0·1-1·0%.17 As iodine is essential for thyroid hormone synthesis, severe iodine deficiency contributes to the development of maternal (and fetal) hypothyroidism, as well as other benign thyroid conditions, such as simple and nodular goiter, due to increased production of thyroid stimulating hormone (TSH).17 Maternal dietary iodine requirements are increased during pregnancy due to increased maternal thyroid hormone synthesis, increased renal iodine losses, and some transfer of iodine to the fetus.18 Residing in a region characterized by mild-to-moderate iodine deficiency (e.g. Denmark, prior to mandatory iodine supplementation of salt and bread in the early 2000s) greatly increases the risk of developing severe iodine deficiency in pregnancy.17,18,19 Evidence from animal and epidemiologic studies generally supports a link between iodine deficiency, as well as simple and nodular goiter, and increased incidence of follicular thyroid carcinoma,16,19,20 while the relationship between iodine status and papillary thyroid carcinoma has been less clear.20 The strong associations for maternal hyperthyroidism, goiter, and congenital hypothyroidism with risk of follicular thyroid carcinoma support a role of iodine deficiency in thyroid cancer development. The slightly stronger association of congenital hypothyroidism with thyroid cancer for offspring born after 1985 may reflect more accurate or complete early diagnostic information. Including all cases of congenital hypothyroidism, regardless of age at diagnosis, slightly strengthened the association for congenital hypothyroidism and thyroid cancer risk in offspring. The association for congenital hypothyroidism was stronger among rural residents, which may reflect the poorer iodine status of rural regions. Our results for maternal hypothyroidism, hyperthyroidism, and thyroiditis potentially also suggest a role of inherited thyroid autoimmunity in thyroid cancer development. Recent studies have similarly shown positive associations between diagnosis of autoimmune thyroid conditions (Hashimoto’s thyroiditis, Graves’ disease) and increased risk of thyroid cancer within the same individuals; this increased risk persisted for years following benign thyroid disease diagnosis and was observed for localized and regional stage thyroid cancer.16
Some of the other variables examined, such as birth weight, may be surrogates for fetal exposure to sex steroid hormones and growth factors. Similar to our study, birth weight was associated with risk of childhood and adulthood thyroid cancer in registry linkage studies in Denmark and California, USA.9,10 Umbilical cord levels of insulin-like growth factor (IGF)-I, IGF binding protein (IGFBP)-3, and leptin have been shown to be elevated in larger babies, and there is some evidence that these hormones have a direct influence on thyroid cancer development.21 Birth weight is associated with higher maternal sex steroid hormone concentrations;22,23 however, maternal and fetal concentrations of these hormones appear to be modestly correlated.24 While confounding by excess weight or weight gain during pregnancy could explain the positive associations of maternal diabetes and postpartum hemorrhage,25 results did not change after adjusting for pre-pregnancy BMI. Other variables associated with thyroid and sex steroid hormone levels,26 such as preterm birth, preeclampsia/gestational hypertension, gestational diabetes, and maternal smoking, were not associated with thyroid cancer risk. Studies that directly assess hormonal concentrations in pregnancy in relation to thyroid cancer risk among offspring may provide greater insight on underlying biological mechanisms.
The large source population obtained from combining nationwide registry data from four Nordic countries, virtually complete cancer incidence follow-up over several decades (important considering the potentially long latency period between exposure and thyroid cancer diagnosis), and use of routinely collected and recorded birth and medical information helped to overcome some common challenges in studying the relation between in utero exposures and thyroid cancer risk. Due to mandatory reporting of birth information to the MBRs, the level of missingness was low for most variables. Some thyroid cancer risk factors (e.g. exposure to ionizing radiation in childhood, genetic factors) were not captured by the registries, but these factors were unlikely to have been strong confounders. For uncontrolled confounding to account for the observed associations, the confounder would have needed to be even more strongly associated with both the exposure and the outcome.27 Smoking and pre-pregnancy BMI, available for only a small proportion of cases, were not found to be important confounders. We lacked information on breastfeeding in this study. This is an important limitation considering the high rates of breastfeeding in the Nordic countries.28 In the presence of suboptimal iodine intake, breastfeeding mothers are more susceptible to iodine deficiency, as likely would be their child;29 thus, breastfeeding potentially could have a direct influence on thyroid cancer risk in offspring or modify the associations of other factors examined in the current study.
Detection bias is an important concern in epidemiologic studies of thyroid cancer. Exposures being evaluated could serve as surrogates of healthcare access and utilization, and thus increased likelihood of incidental detection of thyroid cancer. However, detection bias is a less plausible explanation in the Nordic countries, which are characterized by high-quality and universal healthcare. Indeed, the proportion of cases due to overdiagnosis has been estimated to be much lower in the Nordic countries (~20-40% in 1988-2007 to ~40-65% in 2008-12) compared to other high-income, higher-resource countries, like South Korea, Italy, and the United States.30 Also, thyroid cancer screening has not been available or encouraged to the same extent in the Nordic countries compared to countries like South Korea.30 Individual matching of cases and controls at the county level should have helped to further minimize biases related to healthcare access, while controlling for other sources of confounding, including regional differences in iodine intake and radioactive fallout. Some of the exposures examined may be risk factors for thyroid disease rather than thyroid cancer, per se. Individuals diagnosed with thyroid disorders are more closely monitored for thyroid abnormalities and, thus, more likely to have incidentally-detected thyroid cancer.16 Similarly, individuals whose mothers were diagnosed with thyroid disorders may be under greater surveillance for thyroid abnormalities. However, our study restricted maternal thyroid disorders to those diagnosed before or during pregnancy, as we hypothesized that pregnancy was a susceptible period in offspring thyroid cancer development. Also, we expected that any influence of maternal thyroid conditions on diagnostic scrutiny among offspring to decrease over time, but similar elevated risks of thyroid cancer were observed for thyroid cancer diagnosed before and after age 30. All the positive associations remained after restricting the outcome to regional/distant stage thyroid cancers, which represent clinically meaningful cases that have progressed and require treatment. Finally, the association for congenital hypothyroidism was restricted to rural residents with potentially lower access to care. This suggests that our results cannot be entirely explained by detection bias.
From the Danish data, we could not determine whether the maternal thyroid diseases were diagnosed before or during pregnancy and, thus, could not disentangle effects due to the diseases themselves, their underlying causes, or exposure to treatments received by mothers during pregnancy. Future studies would ideally incorporate this level of detail.
Our study, using population-based registry data, supports a link between in utero exposures, particularly those related to maternal thyroid disorders, and risk of thyroid cancer later in life. Future studies should explore whether these findings are attributable to treatments received during pregnancy, fetal iodine deficiency, shared genetic susceptibility to thyroid disorders including autoimmune disease, enhanced medical surveillance, or other factors.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
We searched PubMed for studies published between January 1, 1990 and October 9, 2020, that addressed the associations of maternal birth characteristics, perinatal exposures, and birth outcomes with risk of thyroid cancer in offspring, and more broadly, the association between early-life exposures as risk factors for thyroid cancer, using the search terms “early-life,” “in utero,” “pregnancy,” “prenatal,” “infant,” “birth characteristics,” “birth outcomes,” “maternal,” and “thyroid cancer,” and “risk.” Few previous studies evaluated the role of the intrauterine environment in thyroid cancer development. Previous studies suggested a possible association between in utero exposure to ionizing radiation, maternal diagnosis of hyperemesis gravidarum, and birth weight with increased risk of thyroid cancer in offspring.
Added value of this study
Our study provides evidence in support of a link between in utero exposures, particularly those related to maternal thyroid disorders, and risk of thyroid cancer later in life. The strong associations observed for maternal hyperthyroidism, goiter, and congenital hypothyroidism with risk of follicular thyroid carcinoma support a potential etiologic role of iodine deficiency.
Implications of all the available evidence
These findings may motivate additional research into early-life exposures involved in the etiology of thyroid cancer, which may ultimately help to identify modifiable risk factors and targets for primary prevention of the disease.
Footnotes
Declaration of interests: All authors declare no competing interests.
Data sharing: The datasets analysed during the current study are not freely available due to national regulations, but similar data can be obtained from the register authorities.
REFERENCES
- 1.Danckert B, Ferlay J, Engholm G, et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 8.2 (26March2019). [Google Scholar]; Association of the Nordic Cancer Registries. Danish Cancer Society. Available from http://www.ancr.nu. Last accessed on October 2, 2020. [Google Scholar]
- 2.Kitahara CM, Schneider AB, Brenner AV Thyroid Cancer In: Michael Thun MSL, Cerhan James R., Haiman Christopher A., and Schottenfeld David, (ed). Schottenfeld and Fraumeni Cancer Epidemiology and Prevention, 4th Edition. New York, NY: Oxford University Press; 2018. [Google Scholar]
- 3.Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: IARC, 2018. Available from: https://gco.iarc.fr/today. Last accessed on May 1, 2020. [Google Scholar]
- 4.Troisi R, Bjorge T, Gissler M, et al. The role of pregnancy, perinatal factors and hormones in maternal cancer risk: a review of the evidence. J Intern Med 2018;283:430–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res 1995;141:259–277. [PubMed] [Google Scholar]
- 6.Kitahara CM, Gamborg M, Berrington de Gonzalez A, et al. Childhood height and body mass index were associated with risk of adult thyroid cancer in a large cohort study. Cancer Res 2014;74:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatch M, Brenner AV, Cahoon EK, et al. Thyroid Cancer and Benign Nodules After Exposure In Utero to Fallout From Chernobyl. J Clin Endocrinol Metab 2019;104:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandraas KF, Vikanes AV, Stoer NC, et al. Hyperemesis gravidarum and risk of cancer in offspring, a Scandinavian registry-based nested case-control study. BMC Cancer 2015;15:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aarestrup J, Kitahara CM, Baker JL Birthweight and risk of thyroid cancer and its histological types: A large cohort study. Cancer Epidemiol 2019;62:101564. [DOI] [PubMed] [Google Scholar]
- 10.Deziel N, Zhang Y, Wang R, et al. Birth characteristics and risk of pediatric thyroid cancer: a population-based record-linkage study in California. Thyroid 2020. (Online ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 2019;11:563–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langhoff-Roos J, Krebs L, Klungsøyr K, et al. The Nordic medical birth registers—a potential goldmine for clinical research. Acta Obstet Gynecol Scand 2014;93:132–137. [DOI] [PubMed] [Google Scholar]
- 13.Pukkala E, Engholm G, Højsgaard Schmidt LK, et al. Nordic Cancer Registries - an overview of their procedures and data comparability. Acta Oncol 2018; 57:440–455. [DOI] [PubMed] [Google Scholar]
- 14.Skjærven R, Gjessing HK, Bakketeig LS Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand 2000;79:440–9. [PubMed] [Google Scholar]
- 15.Pakkila F, Mannisto T, Surcel HM, et al. Maternal thyroid dysfunction during pregnancy and thyroid function of her child in adolescence. J Clin Endocrinol Metab 2013;98:965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitahara CM, Körmendiné Farkas D, Jørgensen JOL, et al. Benign Thyroid Diseases and Risk of Thyroid Cancer: A Nationwide Cohort Study. J Clin Endocrinol Metab 2018;103:2216–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stagnaro-Green A, Pearce E Thyroid disorders in pregnancy. Nat Rev Endocrinol 2012;8:650–658. [DOI] [PubMed] [Google Scholar]
- 18.Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017;27:315–389. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann MB, Boelaert K Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol 2015;3:286–295. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann MB, Galetti V Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res 2015;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt JA, Allen NE, Almquist M, et al. Insulin-like growth factor-I and risk of differentiated thyroid carcinoma in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 2014;23:976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peck JD, Hulka BS, Savitz DA, et al. Accuracy of fetal growth indicators as surrogate measures of steroid hormone levels during pregnancy. Am J Epidemiol 2003;157:258–266. [DOI] [PubMed] [Google Scholar]
- 23.Mucci LA, Lagiou P, Tamimi RM, et al. Pregnancy estriol, estradiol, progesterone and prolactin in relation to birth weight and other birth size variables (United States). Cancer Causes Control 2003;14:311–318. [DOI] [PubMed] [Google Scholar]
- 24.van de Beek C, Thijssen JH, Cohen-Kettenis PT, et al. Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: what is the best source of information to investigate the effects of fetal hormonal exposure? Horm Behav 2004;46:663–669. [DOI] [PubMed] [Google Scholar]
- 25.Blomberg M Maternal obesity and risk of postpartum hemorrhage. Obstet Gynecol 2011;118:561–568. [DOI] [PubMed] [Google Scholar]
- 26.Shields B, Hill A, Bilous M, et al. Cigarette smoking during pregnancy is associated with alterations in maternal and fetal thyroid function. J Clin Endocrinol Metab 2009;94:570–574. [DOI] [PubMed] [Google Scholar]
- 27.Steenland K, Schubauer-Berigan MK, Vermeulen R, et al. Risk of bias assessments and evidence syntheses for observational epidemiologic studies of environmental and occupational exposures: strengths and limitations. Environ Health Perspect 2020;128:95002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hörnell A, Langström H, Lande B, Thorsdottir I Breastfeeding, introduction of other foods and effects on health: a systematic literature review for the 5th Nordic Nutrition Recommendations. Food Nutr Res 2013;57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aakre I, Morseth MS, Dahl L, et al. Iodine status during pregnancy and at 6 weeks, 6, 12 and 18 months post-partum. Matern Child Nutr 2020;e13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M, Dal Maso L, Vaccarella S Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol 2020;8:468–470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


