Summary
Body composition estimates are widely used in clinical research and field studies as measures of energy-nutrient balance, functionality and health. Despite their broad relevance and multiple applications, important gaps remain in techniques available for accurately and precisely quantifying body composition in infants and children from birth through 5 years. Identifying these gaps and highlighting research needs in this age group were the topics of a National Institutes of Health workshop held in Bethesda, MD, USA, 30–31 May 2019. Experts reviewed available methods (multicompartment models, air-displacement plethysmography, dual-energy X-ray absorptiometry, weight-length and height indices, bioimpedance analysis, anthropometry-skinfold techniques, quantitative magnetic resonance, optical imaging, omics and D3-creatine dilution), their limitations in this age range and high priority research needs. A summary of their individual and collective workshop deliberations is provided in this report.
Keywords: early childhood phenotyping, growth, nutritional assessment
1 ∣. INTRODUCTION
Measurement of body composition during infancy and early childhood has potential importance in primary and specialty health care settings, clinical research and national surveys and surveillance. Health care providers can use body composition information to screen for current and future health risks, provide anticipatory guidance, monitor therapeutic progress and tailor treatment as in precision medicine. Knowledge of body tissue compartment sizes can allow researchers to better understand the longitudinal associations between body composition, physiologic and metabolic processes and various health and disease outcomes throughout the lifespan, beginning at birth and the impact of various obesity prevention and weight management interventions. National reference data for body composition by age, sex and race/ethnicity from survey measurements (e.g., National Health and Nutrition Examination Survey, NHANES) can be used as a benchmark for monitoring the health of populations and for evaluation of programs for young children (e.g., Special Supplemental Nutrition Program for Women, Infants, and Children [WIC], Head Start).
Different approaches have been used to estimate or directly measure various aspects of body composition (fat mass [FM], fat-free mass [FFM], bone mineral content [BMC] and total body water [TBW]) at different ages. These include anthropometry (recumbent length, stature, weight, circumferences and skinfold thicknesses), bioelectrical impedance analysis (BIA), magnetic resonance imaging (MRI), multicompartment models including TBW by isotope dilution, body density by air-displacement plethysmography (ADP; previously hydrostatic weighing) and BMC by dual X-ray absorptiometry (DXA). However, limitations have been identified across the various measurement procedures that can produce inaccurate estimates of body composition outcomes. Accurate body composition assessment is especially challenging among younger children, for example, because of prediction equations that tend to be specific to the population from which they were derived (anthropometry and BIA), inaccuracies due to crying/movement during testing (ADP or MRI), exposure to radiation (DXA) and high-cost or significant technical examiner expertise (MRI). These limitations and challenges are significant barriers to obtaining accurate and reliable body composition measurements in children from birth through 5 years. There are presently no ‘off the shelf’ approaches that can be used across all ages from birth to adolescence or adulthood for tracking the natural history of obesity or the longitudinal outcomes of interventions on body composition.
2 ∣. WORKSHOP OBJECTIVE
A workshop ‘Body Composition Measurements from Birth through 5 Years: Challenges, Gaps, and Existing & Emerging Technologies’ was held on the campus of the National Institutes of Health, Bethesda, MD, 30–31 May 2019. The overarching objective was to identify specific needs for research that will address existing knowledge gaps and identify improvements that can be made for assessing body composition components in children from birth through age 5 years. A special focus was on measures that can be used longitudinally and for evaluation of intervention studies (Box 1). The discussed studies were all previously approved by respective institutional review boards (IRBs) as indicated in the provided bibliography.
Box 1. Workshop goals.
Review current state of science for available techniques to assess body composition from birth through 5 years.
Identify limitations and gaps in current assessment methodologies.
Identify research needs for refinement of existing and development of new approaches.
Introduce novel approaches, for example, use of ‘omics’ for biomarkers to quantify body compartments.
Generate expert opinion to encourage development of new approaches and technologies and instruments for assessing body composition in children.
Consider applications across various settings such as research laboratory/clinics, specialty care clinics and surveys to establish national reference data.
Discuss appropriate criterion methods for validation—advantages and limitations.
3 ∣. BACKGROUND
Accurately tracking changes in body composition during the first 5 years of life poses multiple challenges to research investigators. Young children often are unable to hold still for prolonged periods of time or to understand or follow detailed verbal protocol instructions, which complicates obtaining accurate measurements. Rapid changes in body composition, notably early changes in fluid balance, influence the validity of prediction models and formulas used to quantify FM and FFM. Interindividual variability can be large, further degrading the accuracy of models designed around group averages. Nevertheless, investigators have pieced together a general picture of how body weight and composition change during the first 5 years of life. The following provides a brief overview of these earlier findings.
In addition to genetic predisposition, composition of the newborn body is shaped by multiple in utero factors that may occur during fetal development to influence tissue accretion rates and tissue proportions at the time of birth (Figure 1). The implication of these observations is that body composition across newborns can differ in terms of fat and FFM, independent of body weight. Since birth is the beginning of the life course based on when physical measurements can be directly obtained ex utero, body composition at birth should be viewed in the context of these complex in utero factors.
FIGURE 1.
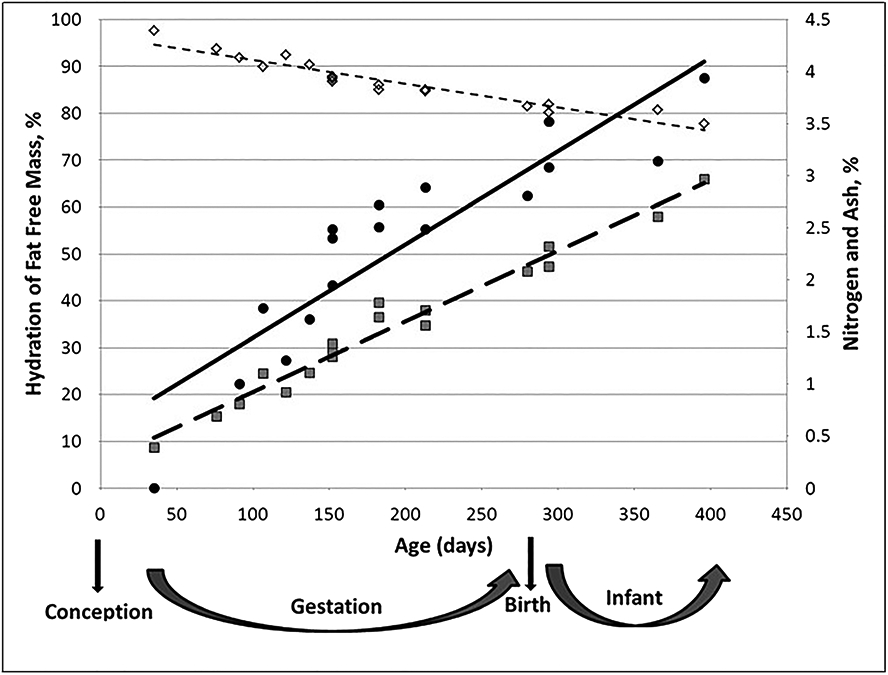
Effect of age on fat-free mass composition. Adapted from Moulton1 as published in Toro-Ramos et al,2 with permission. ◊ (–––), hydration of fat-free mass (%) = −0.05 × age (days) + 96.31, R2 = 0.91 ( standard error estimate (SEE) = 1.63%); (–), ash (%) = 0.009 × age (days) + 0.55, R2 = 0.81 (SEE = 0.443%); (–—), nitrogen (%) = 0.007 × age (days) + 0.25, R2 = 0.97 (SEE = 1.123%)
Body composition changes progressively from infancy as children grow and mature, reflecting dynamic differences in FM and FFM. Rapid changes in body weight and composition occur soon after birth.3 Hull et al4 reported that mean weights at 25–48 and at 48–72 h after birth did not differ but were significantly less than mean weight at less than 24 h. A weight loss of 5% to 10% in the neonate has been reported during the first week after birth.4
The immediate postbirth weight loss is largely due to loss in body water, an adaptation to the extra-uterine environment. The mechanisms of water equilibrium in the body were reviewed by Barbosa Baker and Lopes Moreira in 2012.5 The newborn has higher hydration of FFM (i.e., TBW/FFM, expressed in percent) than a child at 1 year, approximately 83% versus 78–79%.6,7 A summary of published studies on body composition for girls and boys from birth to 1 year was reported by Toro-Ramos et al.2
Total percent body fat averages 11–15% at age 2 weeks in healthy full-term newborns.2,6,8,9 Body fat is primarily located in the subcutaneous layer, and there are negligible amounts of intra-abdominal or visceral adipose tissue at birth.10 Total percent body fat increased to about 30% by 6 months of age,7 and at 2 years, body fat decreased to 19.5% for boys and 20.4% for girls, whereas at 5 years, body fat was 14.6% in boys and 16.7% in girls. Compared with birth, body fat declines to about 19% in girls and 14% in boys at 10 years.7 The timing and distribution of changes in body fat have important implications for future health including the risk of developing metabolic complications associated with adiposity.
Boys older than 1 year and up to 5 years were reported to have 9.1 kg of FFM (protein as percentage of FFM of 13.5%, calculated as nitrogen × 6.25) at 18 months, 10.1 kg (14% protein) at 2 years and 16.0 kg (15.8% protein) at 5 years; girls had 8.4 kg (13.5% protein) at 18 months, 9.5 kg (13.9% protein) at 2 years and 14.7 kg (15.0% protein) at 5 years.7 In another study, boys had 8.55 kg of FFM (12.9% protein) at 18 months and 9.13 kg (13.5% protein) at 2 years; girls had 7.99 kg (12.7% protein) at 18 months and 8.99 kg (13.1% protein) at 2 years.6 Based on these findings, protein accretion as an indicator of lean mass occurs at a steady pace during early ages with modest variability between sexes and across studies.
Chemical analysis of body tissues is the only direct measure of body composition; all other assessment procedures currently available are considered indirect estimates. Chemical analysis studies of cadavers for body composition have not been performed for children under 5 years of age. Available data are based on indirect methods (e.g., skinfold thicknesses and BIA) which use algorithms or prediction equations to provide estimates of body composition. Ideally, future studies aimed at establishing the chemical composition of children younger than 5 years will be conducted that define reference values for this age group (Table 1). Another approach is to reduce the number of inherent model assumptions when evaluating young children by combining methods. Limited data are available based on these multicompartment models that account for variation in hydration during early life. Butte et al provided longitudinal data to 2 years,6 and Fomon et al7 published data through 10 years of age. Ellis et al9 reported reference values in children, age 3–5 years, and of different ethnic backgrounds using BIA, DXA and total body potassium. Short-term changes in body composition over days or weeks with interventions can be tracked using a combination of body composition methods, including metabolic balance studies,12,13 that account for hydration effects.
TABLE 1.
Summary of workshop identified gaps, challenges and opportunities
| Research gap or challenge | Recommendations | Targeted advance |
|---|---|---|
| Obtaining normative chemical maturation values and normative anatomical organ and tissues weights for children ages birth to 5 years | Perform chemical and anatomical analyses of cadavers in ages 0 to 5 years. | Robust reference data for normative chemical maturation values and anatomical weights in infants and children at different ages would serve as a standard for the validation of indirect methods. |
| Obtaining total body density between 6 to 24 months | Working collaboratively with ADP equipment manufacturer attempt to validate the Pediatric Option™ in ages 6–24 months using 4C as criterion method. *Probably software improvements with the testing frequency and increasing the testing cycle (Hz)* also, attempt to fill the “dead air” space | Further refinement of Pediatric Option™ |
| Understand the impact of movement and clothing (diaper/blanket) on test results | Use known infant/adolescent phantoms and movement during the test. Replicate early work with movement and blankets/diapers Koo et al11 | Improved DXA protocol |
| Genetic architecture of fat and fat-free mass in childhood | Longitudinal; newborn and childhood body composition together with collection of intrauterine and environment factors | Discovery-based genetic studies (GWAS, NGS, etc.) |
| Mechanisms underlying association of the intrauterine environment with childhood body composition | Longitudinal; newborn and childhood body composition together with collection of intrauterine and environmental factors as well as biosamples for omics studies | Enhance understanding of epigenetic, metabolomic and microbiome factors |
| Genetic architecture of fat and fat-free mass in childhood | Longitudinal; newborn and childhood body composition together with collection of intrauterine and environment factors | Discovery-based genetic influences (GWAS, NGS, etc.) |
| Identifying which behavioural factors are likely to have strongest causal associations with childhood body composition or metabolic health | Analysis of well-designed cohort using modern causal inference techniques | Targets for future research |
| Measurement approaches that do not require the child to hold still | Laser scanning or optical approaches that can measure children while freely moving in a free-living environment, validated against validated standard body composition measures | Low-cost, portable, optical scanning device that can be used in the field to provide fat mass and fat-free mass measures |
| Measurement approaches that could estimate body composition from 2D images | Optical analysis of two-dimensional photographs of children, validated against gold standard body composition measures | Machine learning algorithms to generate measures of body fat and fat-free mass from digital two-dimensional media |
These collective observations describe the first 5 years as a dynamic phase of life for rapid growth associated with changes in body composition that are challenging to quantify with a high level of accuracy and precision. Improvements in quantification methods, using newer methodologies, promise to refine our understanding of these growth processes and through these pathways inform link-ages with health and disease later in life. The sections that follow review these developments and identify areas of potential future research.
4 ∣. SESSION I: OVERVIEW OF PRINCIPLES AND ASSUMPTIONS UNDERLYING BODY COMPOSITION MEASUREMENT APPROACHES
4.1 ∣. Overview of multi-compartment models: The foundation for existing measurement methods. Steven B. Heymsfield, MD
Among the many challenges of studying body composition in this age group, one of the most important is the ‘chemical maturation’ that occurs during this early life phase (Figure 1).1 As cells proliferate and grow during development, corresponding changes in the extracellular space follow. The net effect of these growth processes is that TBW declines as a fraction of nonadipose tissues.7,14 These and other related effects can have an impact on body composition models including the two-compartment TBW method for quantifying body fat and FFM, DXA, two-compartment body density model and methods such as BIA.6
These chemical maturation issues impact measurement methods and are an important topic for future review and research. ‘Constants’ used in evaluating body composition in adults may not be accurate when applied in models for children. Another issue is that the level of participant tolerance and compliance with measurement protocols that can be anticipated in very young children is limited. Several methods (e.g., ADP) require participant cooperation. Normative chemical maturation values for this age group created from large and diverse samples are lacking or are in an early phase of development and would benefit from further research. Lastly, there is a need for novel/new approaches for quantifying body composition in this age group. Cross-disciplinary teams including biomedical, engineering and other expertise are needed to develop new and innovative approaches.
4.2 ∣. Limitations of currently available methods: sensitivity and specificity in ages birth through 5 years. Dympna Gallagher, EdD
A primary limitation or barrier to body composition assessment is a shortage of validated measurement methods in the birth through 5-year age range. Testing creative ideas to advance research for development of more effective methods that assess body composition in children must first be grounded in validation. Body composition assessment during infancy and childhood has unique challenges. Based on currently available measurement approaches, these challenges include the need for age-specific accurate prediction equations, measurement procedures that require minimal movement of infant or child, equipment designed for small bodies and appropriate validation studies at various ages across the birth through 5-year age range, to describe changes that occur over time. The timing and accretion rates for fat and lean tissues during fetal development and the rates of growth of these and other tissues in the first weeks and months of postnatal life are dynamic. Rapid changes in body composition occur immediately after birth with weight loss of 5% to 10% in the neonate during the first week, attributed in part to a loss in TBW.2,3,6 Newborns have higher TBW relative to body weight, which equates to a higher hydration of FFM compared with an older infant/child at any age, and the hydration of FFM rapidly decreases after birth. The mineral content of FFM increases during postnatal life. An implication from these ongoing changes is the difficulty in generating reference data for specific age groups or populations. Accordingly, in the absence of robust reference data, the development of in vivo body composition assessment methods is hindered.
Each in vivo body composition measurement method or approach incorporates a set of theoretical assumptions, which if violated in the individual or population being tested will produce inaccurate results. For example, a method developed using data collected on infants ages 2–4 months will lack validity if applied to infants ages 6–8 months. Age- and sex-specific density of FFM constants developed in healthy children may not apply in disease states. Due to heterogeneity across methodologies, body composition estimates for fat, FFM or percentage body fat collected using different methods cannot be merged. Training technicians to maintain high precision (low intraobserver variability) is important for some methodologies.
Studies involving chemical analysis of cadavers for body composition are needed for children through 5 years of age. The analysis of cadavers for body composition involves the dissection of whole-body tissues or samples of tissues and organs. Tissues and organs undergo chemical analysis for quantification of fat, water, nitrogen and protein, ash, calcium, phosphorus, sodium, potassium, magnesium, iron, copper, zinc and iodine. Anatomical analyses provide data on gross weights of the individual dissected components of the body including skin, muscle, adipose tissue, bone and organs. The results of chemical and anatomical analyses performed on infants and children at different ages would serve as a standard for the validation of indirect methods. Currently, this is a fundamental gap.
4.3 ∣. Air-displacement plethysmography (ADP) and dual-energy X-ray absorptiometry (DXA)—bringing in a practical perspective, hands-on experience, and limitations. David A. Fields, PhD
The evaluation and tracking of infant body composition, including during postnatal growth, is important for understanding the quality of weight gain. However, body composition assessment in infancy and in paediatric populations is challenging, even for the most experienced of clinicians and laboratory personnel. Currently, the two most widely used methods for body composition in early life are ADP and DXA. To date, both methods lack robust and in-depth validation studies with few studies reporting on sources of error that are both known and unknown, controllable and random.
Procedural limitations of DXA include violation of compliance in some way (movement usually occurs in some fashion), radiation exposure may be a concern (infant dose 0.8956 mR; more than 6 months 0.216 mR), impact of how much child movement during scan acquisition is too much such that error is unacceptable, impact of blanket used to swaddle infant while on the scanning bed on fat and FFM results, and impact of the constraints used to restrain child from moving during scan on fat and FFM results.
Longitudinal challenges when using DXA include IRBs that will not allow more than two yearly scans; some IRBs may not approve DXA scans in infants younger than 3 months; compliance with requirement for child to remain motionless is particularly a challenge for children ages 1–3 years. In countries outside of the United States, the use of DXA for non-clinical purposes in paediatric age groups may be restricted or not allowed.
A strength of DXA is that it provides the only assessment of BMC and regional estimates for arms, legs and trunk, for bone, fat tissue and bone-free lean tissue.
There are two ADP systems, the small volume PEA POD body composition system (COSMED USA, Inc.) and the larger volume BOD POD (COSMED USA, Inc.). A procedural limitation of ADP-PEA POD includes the approximately 20–30% of infants 4 months and older who are larger than can be accommodated by the instrument, to fit in the tray. Note also that ADP has not been validated in children between 6 and 24 months, and sources of error are either unknown or unaccounted for (e.g., movement during the test and influence of unavoidable body fluids such as urine in measurement tray during the measurement procedure).
Longitudinal challenges using ADP include loss to follow-up, which will occur in the 2- to 6-year age group due to instrument size limitations. Only two studies to date15,16 have validated the paediatric adapter option for ADP in the 2–6 year age range, and with limited success. The Pediatric Option™ includes a customized seat insert that fits within the BOD POD, a modified Windows®-based software program and calibration standard.
Strengths of ADP are that multiple assessments can be made with no restrictions or concern for risk to the child. Starting at 24 months, ADP-BOD POD could in theory be used throughout the life span given that it can accommodate individuals as large as 150 kg. For continuity of measures to become a reality, there is a need to resolve issues associated with child movement and behaviour that negates the use of ADP for children ages 6–24 months and produces invalid results in many children ages 2–5 years.
There is also a need to further understand the differences and similarities between DXA system approaches for measurement of soft tissues (fat and lean mass) and bone. Understanding variation across DXA systems is important beginning at birth and across the years involving growth and development. For example, is the calibrated density unit the same across all DXA systems, both within and across manufacturers? An important advance would be the development of imaging methods and techniques using existing or new platforms for the assessment of visceral fat. Lastly, there is a need for imaging technologies that are radiation-free and that are equivalent in cost to current methods.
Future areas of research include the need for large validation studies of DXA with multiple ethnicities and multiple centres using standard reference criterion-based methods and innovative study designs to better understand sources of error. A first step could include the development and validation of paediatric phantoms for equipment calibration and standardization.
5 ∣. SESSION II: CHALLENGES ASSOCIATED WITH MEASUREMENT APPROACHES FOR LONGITUDINAL MONITORING FROM BIRTH THROUGH 5 YEARS
5.1 ∣. Transitioning from weight-for-length to weight-for-height—challenges. Cynthia Ogden, PhD
The most significant findings related to surveillance of weight and height in the United States and the 2000 Centers for Disease Control and Prevention (CDC) growth charts are from the work by Ogden and colleagues.17-20 Results show that between 1976–1980 and 2011–2014, there was no change in the prevalence of low or high weight-for-age and weight-for-length among infants and toddlers; however, the prevalence of high length-for-age decreased. A significant trend in relative weight gain between birth and time of survey participation was observed among non-Hispanic black children.17 Among children 2–5 years, the pattern has been more complicated over the past decade, with a quadratic trend in obesity prevalence.20 According to NHANES, 9% of infants and 14% of toddlers have excess weight based on different measures (weight-for-recumbent length for infants and BMI-for-age for toddlers) and reference populations (World Health Organization [WHO] versus CDC). The most pressing questions relate to the challenge of how to use growth charts to assess growth across the age range when there is a change in measurement method or reference chart. Using growth charts to monitor and track weight is challenging for several reasons. CDC recommends that the WHO growth charts be used until 2 years of age after which the CDC growth charts are recommended. Specific challenges include (1) physical measurement changes from recumbent length to stature at 2 years of age and (2) using WHO weight-for-length charts until age 2 years, then CDC BMI-for-age from 2 through 5 years. BMI-for-age and weight-for-stature are not interchangeable. Moreover, using the WHO versus the CDC growth charts to assess underweight or obesity will provide inconsistent results19; and (3) using the CDC growth charts to track children with extreme values of BMI is problematic. Extrapolation of z scores beyond the 97th percentile is not recommended as a wide range of high BMIs compress to a very narrow range of high z scores.
Measurements, indicators, reference populations, and cut-off points are all important. Measured weight and stature are recommended over reliance on self- or proxy-reported values. Among children 2–5 years, proxies tend to underreport stature (and to a lesser extent weight) which results in an overestimate of BMI and consequently an overestimate of obesity prevalence in young children.
Recumbent length is measured up to age 2 years, while stature is usually measured after age 2. In the CDC growth chart data, there is an average of 0.8-cm difference between recumbent length and stature. Typically, in the United States, weight-for-length is used to monitor growth and define excess weight in children under age 2 years, and BMI-for-age is used for children over age 2 years.
Excess weight in children is defined in relation to a reference population. Various reference populations have been used including those for the WHO growth standards, the International Obesity Task Force (IOTF) growth reference and the 2000 CDC growth charts. In some countries, different charts are used for epidemiological studies compared with those used for clinical care.
WHO: A growth standard for birth to 5 years based on data from sites in select countries. Children were included based on optimal nutrition/feeding, environment and care, and children with excess weight were excluded. Charts for both weight-for-length and BMI-for-age exist. The CDC recommends the use of WHO weight-for-length charts up to 24 months.
CDC: A growth reference, not a standard, based on the overall US population in the 1960s through the early 1990s. Weight-for-length can be used for birth up to 36 months (if the child cannot stand unassisted) and BMI-for-age starting at age 2 years.
IOTF: A growth reference based on data from select countries, with BMI-for-age starting at age 2 years. The data used to create the CDC growth charts are included in the reference population.
The cut-off point for obesity depends on the chart used. The WHO growth standard uses +2 z scores, which is equivalent to the 98th percentile; the CDC growth chart uses the 95th percentile; and the IOTF reference uses the percentile that corresponds to a BMI of 30 at age 18 years.
Small changes in weight or small errors in weight measurement can change BMI categorization. For example, a 1.36 kg (3 lb) weight change in a 2-year-old girl who is 0.84 m (33 in.) tall can result in a change from the 75th to the 96th percentile. Different cut-off points and reference populations can change prevalence estimates. Agreement between children ages 2 years in NHANES categorized using the CDC BMI 95th percentile and +2 z score of WHO weight-for-length is 97%. Obesity or high weight-for-length reflects underlying reference distribution parameters (i.e., weight, height, age and gender). Race/ethnic disparities in the United States become much more apparent in children ages 2–5 years based on BMI-for-age compared with children ages birth to 24 months based on weight-for-length. BMI (weight/length2) is not used universally for younger than 24 months, in part because length can be difficult to measure accurately. Because BMI uses a squared term for the linear measure, the error is squared, which magnifies the impact of the error term.
5.2 ∣. Anthropometry and bioimpedance analysis (BIA)—limitations and challenges. Shumei Sun, PhD
The need to track body FM and FFM in individual subjects and the need to assess changes in the prevalence of obesity in populations over time underscore the importance of documenting changes in body composition with age. A critical barrier to progress has been the rarity of long-term prospective birth cohorts with serial data for body composition and frequently inventoried status of health and disease.
US national infant growth charts are available for weight and weight-for-length during the first 36 months of life. These parameters reflect infants' growth and nutritional status. Infants with abnormally low values for weight and weight-for-length may be receiving inadequate nutrition or experiencing a health-related condition. At the other end of the scale, body composition assessments for infants and young children are needed to better understand the secular increase in the prevalence of childhood obesity that has occurred over the past three decades. This secular change presages an increase in the prevalence of type 2 diabetes as early as the second decade of life.
In large-scale field or epidemiological studies, it is difficult to determine levels and distributions of body fatness accurately and reliably. Anthropometric indices coupled with BIA are often used because they are safe, portable, easy to apply and inexpensive to obtain. A practical solution is to use easy-to-measure variables to predict fat and FFM in prediction equations.
A variety of skinfold thicknesses has been used to predict body fat. However, these anthropometric measurements have inherent errors. The measurement errors may be compounded in infants and young children owing to excessive movement during the examination. In children from birth to 4 years of age, the reliability of skinfold thickness measurements ranges from 60% to 70%. Weight, recumbent length and stature are easier to measure and have better reliability than skinfolds.
BIA is easy and reliable to measure and is an appropriate method to consider for use in field studies. BIA measures are inversely proportional to the volume of TBW after adjustment for conductor length of the subject. BIA, expressed as length2/resistance, in combination with body weight has been used increasingly as independent variables to predict FFM. Most epidemiological studies use single frequency bioelectrical impedance at 50 kHz to estimate body fatness using prediction equations. These equations tend to predict well in samples like those from which they are developed, but performance decreases as body fatness increases.
Statistical methods are available to develop prediction equations for body composition from anthropometry and BIA. Key assumptions are normality, linearity and homogeneity, meaning that the response variable is normally distributed, the relationships between response and predictor variables are linear, and the variance of the response variable is a constant. Other relevant statistical considerations involve the quality of the equation, notably goodness of fit, accuracy of prediction and parsimony, meaning how well the equation describes the relationships between dependent and independent variables, and the accuracy of performance of the equation when applied to an independent sample, as well as parsimonious use of the data.
For large epidemiologic and field studies, a subsample is built to develop the parsimonious equations. The subsample consists of simple reliable measures for the dependent and independent variables. The developed equation is applied to the epidemiological study, so the sample characteristics of the data used to develop the equation are like the data to be applied. A five-centre BIA project designed to ascertain a national distribution of body composition illustrates how to obtain age-, sex- and race-specific national body composition distributions.18,21
Several gaps and issues need to be considered in children. The first of these gaps reflects a series of issues related to prediction formulas. There is a need for standardized data collection processes for each method that can be made available to researchers, including practical tips on how to measure across age ranges. Large epidemiological and field-based studies are needed for the development of simple body composition equations. When applying the developed equations to other study samples, investigators need to ensure that the new study sample characteristics are similar to the sample in which the equations were developed. There is a need to build equations based on individual populations and to be aware that it may not be possible to generalize equations to broader populations in the absence of cross-validation studies.
Method-specific standardized protocols need to be developed, and method-specific measurement errors need to be quantified. It is important to consider how errors in measurement methods affect cross-sectional outcomes. The model regression coefficient can result in bias in the outcome measure; therefore, it is necessary to adjust for this form of measurement error. Longitudinal measurements can be made, but they are problematic because error is multiplied depending on number of assessments. Serial data are misleading because of regression to the mean, and random effects models should be used, but this does not address measurement error, just regression for serial measures. Analyses should adjust for measurement error across time.
To predict body fat from skinfold thicknesses, there should be correction for error in regression. When using skinfold thicknesses longitudinally, the absolute skinfold thickness values should be used rather than calculating %BF from skinfolds. There is a need to develop age-, sex- and race-specific body composition reference distributions. For longitudinal studies, there is a need to develop portable methods that can be used at home and other non-clinical settings over time.
Prevailing challenges and barriers include the following: The four-component model has assumptions, each component has an error, and the errors are additive. Use of the four-component model is labour intensive, time consuming and expensive, making this approach impractical for field studies. A need exists to establish prediction equations to predict FFM from BIA and body weight. The predicted FFM can then be converted into total body fat and %BF. Several important fundamental issues emerge from this review that could be addressed through targeted research: For monitoring child health, we need to reconsider the advantages and disadvantages of body composition reference data beyond weight and weight-for-length reference data. A consensus is needed on the use of a four-component model in children from birth to 5 years of age. For large epidemiologic field studies, BIA needs to be developed such that, with body weight it can be used to predict FFM, from which %BF can be derived. An agreement is also needed on strategies to develop and validate prediction equations.
6 ∣. SESSION III: EMERGING MEASUREMENT TECHNOLOGIES: BIRTH TO 5 YEARS
6.1 ∣. Quantitative magnetic resonance (QMR). Aline Andres, PhD, RD
The QMR technology has been widely used in experimental research (rodents and other species) for decades. In the past 10 years, the technology has been applied to humans, and specifically to children, with systems that can evaluate body composition in infants (EchoMRI-Infant, Echo Medical Systems, Houston, TX) or in children weighing 3 to 50 kg (EchoMRI-AH). This technology does not require stillness or exposure to radiation and can estimate body composition of infants and children weighing up to 12 kg (EchoMRI Infants)22 and children weighing 3 to 50 kg (EchoMRI-AH).23 A larger system is available for use in adults thereby allowing this approach to cover the lifespan. Accuracy and reproducibility are high. This technology provides estimates of FM, lean mass, TBW and total body free water.
The Arkansas Children's Nutrition Center (ACNC) has validated the first EchoMRI-AH released using carcass analyses in piglets24 and using deuterium dilution (D2O), DXA and ADP in children weighing 3.3 to 49.9 kg.23 In the later study, the percentages of compliance were 98% for QMR, 75% for DXA, 94% for BOD POD and 95% for PEA POD. Although QMR precision was high (coefficient of variation = 1.42%), it overestimated FM approximately 10% compared with the four-component model in children 6 years or older and was less concordant with four-component or D2O models for infants less than or equal to 8 kg.25 Using a mathematical model to adjust FM in infants, results suggested that the paediatric QMR provided a fast and precise method for assessing FM longitudinally in infants and in children weighing up to 50 kg.26
Since the validation study, the ACNC has used QMR to collect multiple longitudinal measures in infants and children for the determination of FM, lean mass and TBW trajectories under different paradigms of infant feeding or in utero exposures.25,26
There are several challenges and barriers to the use of QMR technology in the birth through 5-year age group. The system does not assess the distribution of FM or lean mass; it requires infants and children to be free of metal (no dental metals); the device may require calibration and validation; very few instruments are available in the United States for paediatric evaluations; and the cost is substantial (approximately $450,000) and may be prohibitive for most research centres.
Several important gaps and issues also need to be considered for QMR. There is a need for standardized data collection processes and validation techniques for quantifying between-instrument variations. Errors in measurement across the age range, as demonstrated in a previous study,23 need to be considered. Some prevailing questions remain: How can the QMR instruments be validated to ensure continuity of measures from infants to adolescents to adult instruments? Can the technology be developed for large-scale distribution to lower the cost?
6.2 ∣. Optical imaging. John Shepherd, PhD
Surrogate measures of body composition, including visceral and subcutaneous adipose tissue, percentage body fat and appendicular lean mass, have been estimated in children from 5 to 17 years using 3D optical scanning.27 3D optical scanners create surface renderings from tens of thousands of point estimates on the body in 3D space. From these scans, automated anthropometry measures including circumferences, lengths, volumes and surface areas can be generated. By combining these automated measurements, body composition equations have been derived and validated for children27 and adults.28 Optical scans take less than 1 min, and commercial systems generate over 300 anthropometric measurements that would otherwise be time prohibitive to acquire using manual techniques. Evaluations are low cost and free of ionizing radiation.
Using 3D optical scanning in children younger than 5 years has not been demonstrated to date due to several technical challenges. First, it is difficult for children this age to hold still for the required 1 min. Second, the current systems are not optimized for small children. Third, there are few criterion body composition measures available that cover the entire age range of 5 years and below to calibrate body shape. Should technical limitations that currently preclude its use in younger children be overcome, this technique has much promise. An ‘ideal’ measurement approach should be fast (short measurement time), insensitive to child movement and to body pose, and be validated against a criterion measure that is either diagnostic or predictive of future childhood and adult health risks. Lastly, optical scans are ideal for advanced statistical and machine learning approaches. Body shape as described by principal component analysis is one example and has been applied to adult scans to describe total body and regional body composition with higher accuracy and precision than 3D automated anthropometry.29
Important questions for future consideration include the following: Children in this age range are unable to conform to requirements that involve following instructions and remaining motionless during the scan. What approaches can be used to quantify body composition accurately when the young children cannot be restrained to limit motion? Available body composition measurement methods for use in the under 5-year age range have limitations. What should the body composition criterion measure be, against which new technologies can be compared? Do models of body composition apply to children in this age range associated with differences from adult hydration levels, body size and tissue compartmental composition?
7 ∣. ARE THERE BIOMARKERS OF BODY COMPOSITION AND OTHER APPROACHES TO UNDERSTANDING EARLY LIFE ADIPOSITY?
7.1 ∣. Omics. William Lowe, PhD
Genome-wide association studies in mothers and newborns from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study identified a locus on chromosome 3 (3q25) associated with newborn sum of skinfolds, a measure of adiposity.29 This locus was associated with ponderal index in other studies.30,31 A recent study from the Early Growth Genetics (EGG) consortium identified more than 140 fetal loci associated with birth weight.32 A genetic risk score generated from 60 of these loci was associated with newborn sum of skinfolds,33 although which of these 60 genetic variants are driving the association has not been determined.
Use of metabolomics to identify metabolic signatures associated with maternal hyperglycaemia and obesity during pregnancy and the relationship of the maternal metabolome to newborn size and body composition are important and emerging areas. A recent study demonstrated that at 1-h post glucose load, maternal levels of amino acids, fatty acids and lipid metabolites were positively associated with newborn sum of skinfolds.34 In the fasting state, a more limited group of maternal metabolites were positively (pyruvate and triglycerides) or negatively (arachidoyl carnitine) associated with newborn sum of skinfolds. Maternal phenotypes during pregnancy, including obesity and glycaemia, were associated with cord blood metabolites, reflecting a potential influence of maternal phenotype on the fetal metabolome.35
FFM and adiposity are determined, in part, by genetics. This has been clearly demonstrated in adults, and evidence for genetic determinants for size at birth, including newborn adiposity, has also been reported.30-32,36,37 While genetic variants important for obesity and body composition in adults appear to play some role in newborns and children,38 discovery-based analyses to define the genetics of body composition in early childhood have not been undertaken. Such studies would allow for better definition of the genetic architecture of body composition during childhood and the development of polygenic risk scores or other approaches at birth to predict subsequent body composition and identify children at risk for greater adiposity. This risk is modulated by many environmental factors; thus, well-phenotyped cohorts with longitudinal measures from birth across childhood would be required for these discovery-based studies. Body composition, like other anthropometric and metabolic traits,39 appears to be determined by many genetic variants with small effect size. Thus, large cohorts are likely to be required to define the genetic architecture of body composition in childhood. To date, large cohorts of newborns and children have typically been recruited using relatively crude phenotypes obtained through personal report or medical records. New approaches need to be considered to achieve the requisite cohorts for discovery-based studies with careful phenotyping to examine the desired traits.
Beyond genetics, epigenetics is another important area that may reflect the impact of the intrauterine environment and influence subsequent development. Again, similarly large, well-phenotyped cohorts are needed for these studies with phenotyping that also includes characterization of the intrauterine environment (e.g., maternal BMI, weight gain and glycaemia). Finally, biosamples needed for additional omics technologies (e.g., metabolomics and the microbiome) could easily be collected in these studies to undertake a more comprehensive omics-based approach to define determinants of body composition in childhood.
Important questions for future consideration include the following: How can ongoing efforts be further enhanced to develop biomarkers for body composition in early childhood, especially biomarkers measurable at birth that enable better prediction of future adiposity and FFM? What is the genetic architecture of childhood adiposity and lean body mass and what are the genetic variants associated with these measures? What are the mechanisms underlying the association of the intrauterine environment with childhood adiposity and FFM and how does the intrauterine environment interact with offspring genetics to influence childhood body composition? Are there maternal and newborn metabolic signatures associated with childhood body composition?
7.2 ∣. Creatine dilution for estimating muscle mass in infants and children. William Evans, PhD
D3-creatine dilution provides a novel method for measurement of the total body creatine pool size.40,41 Using the assumption that approximately 98% of the creatine pool is found in muscle, creatine can serve as an index of muscle mass. Creatine is found in the contractile component of muscle and the creatine pool size may be a measure of ‘functional muscle mass.’ The developed method is non-invasive, although validation research studies are needed for infants and children.41 For infants, urine is collected from a diaper at least 48 h after dosing and a minimum of three diapers should be collected in infants to obtain uncontaminated (by stool) samples. The simultaneous administration of 2H2O for measurement of TBW (saliva sample between 1 and 3 h post dose) allows for an estimate of FFM. The combined measures of D3Cr and 2H2O (administered together) provides an assessment of muscle mass, TBW (FFM) and (by subtraction from body mass) FM using a single dose.
Important questions for future consideration include the following: How does nutritional status effect the accretion of muscle mass in infants and young children? How do early life changes in body composition and muscle mass influence the risk of obesity? How variable is muscle mass accretion in young children?
8 ∣. SESSION IV: PRACTICAL APPLICATION FOR PREDICTING OBESITY AND OTHER HEALTH OUTCOMES
8.1 ∣. Prediction of metabolic co-morbidities from early life adiposity measures. Emily Oken, MD
Leveraging longitudinal clinical and research cohorts has helped identify numerous early life exposures that predict size and body composition in early life as well as cardiometabolic health later in life.42,43 It is important to characterize prenatal and postnatal environmental, behavioural and social exposures that influence growth, including adiposity as well as later health and disease risk, to understand how to translate observational findings into clinical or public health interventions. Oken and colleagues have characterized the roles of several strong predictors of child outcomes including parental weight (maternal and paternal obesity and gestational weight gain especially in the first trimester), environmental exposures (smoking, air pollution and chemicals), infant diet and feeding behaviours.42-48 Applying advanced statistical methods to longitudinal child cohort data will be essential to moving beyond describing simple exposure-outcome relationships. Examples include modelling exposure mixtures (e.g., accounting both chemicals and nutrients), modelling trajectories of exposures and outcomes, rather than single time points of each, and incorporating advanced causal methods that can account for time-varying confounders and mediators.49-51
Abundant evidence indicates that overall size, adiposity, and patterns of growth in early life predict later life body composition and cardiometabolic risk.52,53 However, it is important to note that although in epidemiologic or clinical analyses early childhood size or body composition may be used as an ‘exposure,’ or predictor of later health outcomes, they are likely not exposures in the true causal sense. Rather, growth and body composition are the outcomes or products of multiple exposures (genetic, behavioural, environmental and nutritional), and it is likely these exposures are causing the later health status, perhaps mediated by body composition. This distinction is important when translating observational results to planning interventions. Caution should be exercised with use of language and extrapolation of findings for studies examining both predictors and outcomes of body composition. Furthermore, since exposures never travel in isolation in the real world, identifying important causal factors is not straightforward. For example, diet is perhaps one of the most logical and well-studied exposures related to growth and body composition. However, multiple challenges exist: diet is difficult to measure accurately as it typically relies on recall; the critical time windows of exposure are not always clear; diet encompasses a great deal of complexity, as nutrients, foods, food groups, and dietary patterns are inter-correlated; and diet is perhaps irretrievably entangled with multiple environmental, behavioural and sociodemographic confounders. We should use novel statistical approaches already being applied in the causal inference and environmental health communities to identify the most influential dietary and other behavioural factors.
8.2 ∣. What do clinical researchers need to measure body composition in young children when designing short-term or longer-term studies through adolescence? Julie Lumeng, MD
Researchers working with young children and their parents must consider the well-being of the child and the comfort of the parent with the study procedures. Ensuring the child's and parent's comfort is important for ethical reasons and to improve the likelihood the study will achieve its intended goals regarding data completeness, participant retention and recruitment. Children and their parents who find the experience too distressing may withdraw assent (in the case of the child) and consent (in the case of the parent) or decline to return for future study visits. These issues are especially pertinent to studies with longitudinal follow-up goals as well as those seeking to enroll healthy, typically developing children who will experience no direct benefit from participating in the research study.
Ensuring that a large majority of age-eligible children can participate in the measurement approach is also important for generalizability of the study findings. Children who have temperaments characterized by greater negativity, emotionality, negative mood, fear, surgency and distress to limitations, as well as weaker self-control, emotion regulation, ability to delay gratification, attention span, soothability and inhibitory control, are more likely to have both excessive weight gain and to be underweight.54 These same temperamental characteristics likely make it more difficult for children to participate in body composition measures that require the child to be separated from the parent, hold still, inhibit an impulse and stay calm in a new environment. Thus, the children most at risk for unhealthy body composition are also those least able to participate in measures that place high behavioural demands on the child.
Measurement approaches that require the child to be separated from the parent, even briefly, may lead to substantial distress for children with less secure attachment patterns, which occurs in about one in three children. Less secure attachment patterns have likewise been linked with both poor growth and excessive weight gain.55,56 Therefore, again, children with less healthy growth patterns may also be those who are least likely to be able to successfully participate in measurement approaches requiring them to remain calm when separated from the parent.
Children's cognitive and language development will also influence their ability to participate in some measurement approaches. Typically, developing children ages 1–2 years can generally only follow a one- to two-step verbal instruction (e.g., ‘Stop’ and ‘Wait here’). By 3–4 years, typically developing children can generally follow a three-step verbal instruction (e.g., ‘Sit, hold still, and look at me.’). Importantly, executive function, which is defined by planning, organization and the ability to follow multistep instructions, has been reported to be weaker among children with obesity.57 These cognitive and language milestones also apply to typically developing children only. About 15% of children in the United States have a degree of developmental delay or learning difference that qualifies them for special education services. Children with developmental delays and in particular autism are especially at risk for differences in growth, weight gain and body composition and would therefore be a subgroup likely to benefit the most from approaches to measurement that put little behavioural demand on the child.58
Although it is essential to note that most parents of children with overweight or underweight are competent parents faced with parenting in a challenging food environment, children who are overweight have been reported to have parents who are more likely to be especially strict, demonstrating less sensitivity and more expectations for self-control.59 Thinner children who are pickier eaters have also been reported to have parents who are more anxious.60 Parents of children with either underweight or overweight may also be less able to tolerate measurement procedures that ‘press’ for child ‘disobedience’ or distress, and yet these populations of children are those who may benefit the most from studies of body composition.
Some consideration should be given to child and parent sensitivities around measuring the child's body shape, even at this young age. Parents may be sensitive about obesity-related stigma even in very young children. Many parents are also attentive to ensure children understand principles of ‘inappropriate touching’ by strangers and may be reluctant to appear to observe and endorse a stranger touching the child's body outside the physician's office in a manner that the child is not accustomed to and that may be confusing.
Finally, parents receive substantial messaging from public health initiatives about appropriate child caregiving practices, and when these guidelines are not adhered to by researchers in an academic research centre, it can send confusing messages or lead parents to question the credibility of the researchers in working with children. Practice guidelines indicate, for example, that infants should sleep in a supine position, on a firm surface, and only placed prone while observed and awake. Swaddling should not be used after an infant exhibits signs of attempting to roll.61 Children should avoid all screen media younger than 18 months and limit screen media to less than an hour per day in the preschool age range, with high-quality programming only.62 Food should also never be used as a reward. In summary, researchers should be sensitive to and knowledgeable about paediatric practice guidelines when interacting with young children to obtain body composition measures.
In summary, clinical researchers working with children need body composition measurement methods that are rapid, require the child to hold still as little as possible, do not require the child to be separated from the parent, do not require the child to remove their clothes and do not require behavioural compliance. This is a substantial challenge, but achieving these goals will ultimately enable the greatest generalizability of study findings to the populations of children at greatest risk for unhealthy or atypical body composition.
Important projects for future consideration include the following: Develop measurement approaches that can be used ‘in the field’ that are easily portable (i.e., for measuring via visits in the community to homes or schools) while also reliable and valid. Develop measurement approaches that can be completed lightly clothed in the child's own clothing. Develop measurement approaches that can be used longitudinally from birth to 5 years (allowing comparison across age). Develop measurement approaches that are very rapid (to allow for developmentally typical child behaviours) and allow the parent to remain with the child.
9 ∣. PANEL DISCUSSION
Based on speaker presentations, key comments and questions related to the evaluation of body composition from birth to 5 years were as follows:
- Several assumptions are involved with the DXA approach that include the amount of bone marrow fat, calcium content of bone and head size. These assumptions represent information that is largely proprietary to the manufacturers. The underlying validity of these assumptions and the extent to which assumption errors impact body composition results are unknown.
- Models must be adapted according to age. In young children, they exclude the head for DXA bone density. Hands and feet are also excluded in the DXA analysis, especially in children.
- DXA does not estimate brown fat. No validated methods, apart from fluro-deoxyglucose positron emission scanning, are available.
- The criteria to exclude a DXA scan due to movement are not defined.
- Infants and children under 6 months of age can be swaddled and strapped to the DXA table to prevent movement during the scan. Swaddling children older than 6 months is ineffective at keeping them motionless. Positioning a child on their stomach may help reduce movement during a DXA scan with young children. At one centre, the technician holds the child's feet while the child is consoled by a parent.
For the BOD POD, children may be allowed to have ‘play time’ in the device prior to measurement to familiarize them with the procedure.
New smart phone-based applications to measure body density through optical imaging techniques should be explored. Similarly, a critical analysis of research-grade 3D optical scanning methods is needed in children below the age of 5 years. Are specially designed devices for this age group needed? How precise are these methods? What reference methods should they be standardized against for estimating body composition?
- Two-compartment models have limitations—the density of fat (adipose tissue) and TBW are not fixed or constant (variable and changing) in early life, especially in newborns.
- We do not know what body density values to use in infants and children, an important concern with respect to the two-compartment body density model.
- Chemical analysis data are based on very old data, and very little information is available in children below the age of 5 years. We need modern cohorts of fetuses/children to obtain updated direct measurements of chemical composition. These values are important in body composition models.
- The four-component model, a frequently used reference method, has multiple assumption and measurement errors, so it is not clear why it is the accepted reference standard. One explanation is that the errors are independent of each other; therefore, they may stabilize the model and mitigate error. A critical analysis is needed to establish the best body composition “reference” standard for young children. Other available methods can then be calibrated against and compared with this standard.
The following topics (also summarized in Table 1) were suggested by workshop participants to help bridge the gaps and move the field forward. Two areas emerged: (1) development and (2) conduct of research studies.
- Develop:
- Collaborative partnerships between academic researchers and industry to share innovative ideas and advance technology development to meet research and applied needs using equipment designed for children less than 5 years of age.
- Approaches that are quick, simple to administer, affordable, not sensitive to movement or body position and validated against a criterion method that is diagnostic or predictive of future body composition, risk biomarkers, and morbidity.
- Portable technology and measurements that are practical for field use.
- Measurement approaches that can be used longitudinally across the lifespan.
- Conduct research:
- To inform agreement on best strategies for developing and validating prediction equations.
- On validation studies of prediction equations and technology-based algorithms using best available criterion methods.
- To compare body composition data obtained across different technologies and within a technology across different vendors (e.g., to address use of different proprietary algorithms and prediction equations that yield varying results).
- To develop biomarkers for body composition that are measurable at birth and that predict future adiposity and lean mass.
- On the genetic architecture of body composition and genetic variants associated with body composition measures.
- To explore maternal and newborn metabolic signatures associated with childhood body composition.
- To develop reference data for the main body compartments (e.g., FM, FFM and TBW) against which to compare data from individual research studies.
- Using large longitudinal multisite, multiethnic, criterion-based cohort studies with well-defined protocols and body composition methods.
10 ∣. SUMMARY
This workshop was designed to bring together authorities in the field of body composition assessment methodologies as well as clinical paediatric and epidemiologic research to share experiences and perspectives that will help inform future directions through research. Speakers were invited to present an update on state-of-the-art body composition assessment methodologies currently in use and in development. This report summarizes highlights of concepts presented during the workshop with a focus on infants and young children through age 5 years. Expert opinion on limitations, future needs, remaining questions and emerging opportunities for future research and development are delineated.
ACKNOWLEDGEMENTS
We thank Cynthia Ogden, PhD, National Center for Health Statistics, Centers for Disease Control and Prevention, Hyattsville, MD, for reviewing and providing feedback on the summary of her talk included here.
Footnotes
CONFLICT OF INTEREST
The following authors declare no conflict of interest: DG, AA, DAF, JCL, EO, RK, SS and WLL. JS received research grants from Hologic and GE Healthcare and received ‘in kind’ research support from Western Digital, Fit3D, Hologic, iCAD, Styku and Sizestream; SBH is a member of the Tanita Corp. Medical Advisory Board. WJE receives grant support from the Duchenne UK, Solid Biosciences, Hologic, and the Bill and Melinda Gates Foundation.
REFERENCES
- 1.Moulton CR. Age and chemical development in mammals. J Biol Chem. 1923;57(1):79–97. [Google Scholar]
- 2.Toro-Ramos T, Paley C, Pi-Sunyer FX, Gallagher D. Body composition during fetal development and infancy through the age of 5 years. Eur J Clin Nutr. 2015;69(12):1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demerath EW, Fields DA. Body composition assessment in the infant. Am J Hum Biol. 2014;26(3):291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hull HR, Thornton JC, Ji Y, et al. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol. 2011;205(3):e211–e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbosa Baker Méio M, Lopes Moreira M. Total body water in newborns In: Preedy VR, ed. Handbook of Anthropometry: Physical Measures of Human Form in Health and Disease. Vol.2 New York: Springer; 2012:1121. [Google Scholar]
- 6.Butte NF, Hopkinson JM, Wong WW, Smith EO, Ellis KJ. Body composition during the first 2 years of life: an updated reference. Pediatr Res. 2000;47(5):578–585. [DOI] [PubMed] [Google Scholar]
- 7.Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982;35(5 Suppl):1169–1175. [DOI] [PubMed] [Google Scholar]
- 8.Andres A, Shankar K, Badger TM. Body fat mass of exclusively breastfed infants born to overweight mothers. J Acad Nutr Diet. 2012;112(7):991–995. [DOI] [PubMed] [Google Scholar]
- 9.Ellis KJ, Abrams SA, Wong WW. Body composition reference data for a young multiethnic female population. Appl Radiat Isot. 1998;49(5–6):587–588. [DOI] [PubMed] [Google Scholar]
- 10.Harrington TA, Thomas EL, Frost G, Modi N, Bell JD. Distribution of adipose tissue in the newborn. Pediatr Res. 2004;55(3):437–441. [DOI] [PubMed] [Google Scholar]
- 11.Koo WW, Hockman EM, Hammami M. Dual energy X-ray absorptiometry measurements in small subjects: conditions affecting clinical measurements. J Am Coll Nutr. 2004;23(3):212–219. [DOI] [PubMed] [Google Scholar]
- 12.Muller MJ, Bosy-Westphal A. Effect of over- and underfeeding on body composition and related metabolic functions in humans. Curr Diab Rep. 2019;19(11):1–11, 108. https://link.springer.com/content/pdf/10.1007/s11892-019-1221-7.pdf [DOI] [PubMed] [Google Scholar]
- 13.Rudman D, Chyatte SB, Patterson JH, et al. Metabolic effects of human growth hormone and of estrogens in boys with Duchenne muscular dystrophy. J Clin Invest. 1972;51(5):1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lohman TG. Applicability of body composition techniques and constants for children and youths. Exerc Sport Sci Rev. 1986;14:325–357. [PubMed] [Google Scholar]
- 15.Crook TA, Armbya N, Cleves MA, Badger TM, Andres A. Air displacement plethysmography, dual-energy X-ray absorptiometry, and total body water to evaluate body composition in preschool-age children. J Acad Nutr Diet. 2012;112(12):1993–1998. [DOI] [PubMed] [Google Scholar]
- 16.Fields DA, Allison DB. Air-displacement plethysmography pediatric option in 2-6 years old using the four-compartment model as a criterion method. Obesity (Silver Spring). 2012;20(8):1732–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akinbami LJ, Kit BK, Carroll MD, Fakhouri THI, Ogden CL. Trends in anthropometric measures among US children 6 to 23 months, 1976-2014. Pediatrics. 2017;139(3):1–10, e20163374. https://pediatrics.aappublications.org/content/139/3/e20163374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chumlea WC, Guo SS, Kuczmarski RJ, et al. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26(12):1596–1609. [DOI] [PubMed] [Google Scholar]
- 19.Grummer-Strawn LM, Reinold C, Krebs NF, Centers for Disease C, Prevention. Use of World Health Organization and CDC growth charts for children aged 0-59 months in the United States. MMWR Recomm Rep. 2010;59(RR-9):1–15. [PubMed] [Google Scholar]
- 20.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018;319(16):1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun SS, Chumlea WC, Heymsfield SB, et al. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am J Clin Nutr. 2003;77(2):331–340. [DOI] [PubMed] [Google Scholar]
- 22.Toro-Ramos T, Paley C, Wong WW, et al. Reliability of the EchoMRI infants system for water and fat measurements in newborns. Obesity (Silver Spring). 2017;25(9):1577–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andres A, Gomez-Acevedo H, Badger TM. Quantitative nuclear magnetic resonance to measure fat mass in infants and children. Obesity (Silver Spring). 2011;19(10):2089–2095. [DOI] [PubMed] [Google Scholar]
- 24.Andres A, Mitchell AD, Badger TM. QMR: validation of an infant and children body composition instrument using piglets against chemical analysis. Int J Obes (Lond). 2010;34(4):775–780. [DOI] [PubMed] [Google Scholar]
- 25.Heard-Lipsmeyer M, Diaz E, Sims C, et al. Maternal adiposity is associated with fat mass accretion in female but not male offspring during the first two years of life. Obesity (Silver Spring). 2020;28(3):624–630. 10.1002/oby.22735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diaz EC, Cleves MA, DiCarlo M, et al. Parental adiposity differentially associates with newborn body composition. Pediatr Obes. 2019;15(4):1–11, e12596. 10.1111/ijpo.12596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong MC, Ng BK, Kennedy SF, et al. Children and adolescents' anthropometrics body composition from 3-D optical surface scans. Obesity (Silver Spring). 2019;27(11):1738–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng BK, Hinton BJ, Fan B, Kanaya AM, Shepherd JA. Clinical anthropometrics and body composition from 3D whole-body surface scans. Eur J Clin Nutr. 2016;70(11):1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng BK, Sommer MJ, Wong MC, et al. Detailed 3-dimensional body shape features predict body composition, blood metabolites, and functional strength: the Shape Up! studies. Am J Clin Nutr. 2019;110(6):1316–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horikoshi M, Yaghootkar H, Mook-Kanamori DO, et al. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nat Genet. 2013;45(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urbanek M, Hayes MG, Armstrong LL, et al. The chromosome 3q25 genomic region is associated with measures of adiposity in newborns in a multi-ethnic genome-wide association study. Hum Mol Genet. 2013;22(17):3583–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warrington NM, Beaumont RN, Horikoshi M, et al. Maternal and fetal genetic effects on birth weight and their relevance to cardiometabolic risk factors. Nat Genet. 2019;51(5):804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes AE, Nodzenski M, Beaumont RN, et al. Fetal genotype and maternal glucose have independent and additive effects on birth weight. Diabetes. 2018;67(5):1024–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadakia R, Nodzenski M, Talbot O, et al. Maternal metabolites during pregnancy are associated with newborn outcomes and hyperinsulinaemia across ancestries. Diabetologia. 2019;62(3):473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowe WL Jr, Bain JR, Nodzenski M, et al. Maternal BMI and glycemia impact the fetal metabolome. Diabetes Care. 2017;40(7):902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karasik D, Zillikens MC, Hsu YH, et al. Disentangling the genetics of lean mass. Am J Clin Nutr. 2019;109(2):276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Y, Day FR, Gustafsson S, et al. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat Commun. 2016;7:1–15, 10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khera AV, Chaffin M, Wade KH, et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell. 2019;177(3):587–596. e589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barroso I, McCarthy MI. The genetic basis of metabolic disease. Cell. 2019;177(1):146–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stimpson SA, Leonard MS, Clifton LG, et al. Longitudinal changes in total body creatine pool size and skeletal muscle mass using the D-creatine dilution method. J Cachexia Sarcopenia Muscle. 2013;4(3):217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stimpson SA, Turner SM, Clifton LG, et al. Total-body creatine pool size and skeletal muscle mass determination by creatine-(methyl-D3) dilution in rats. J Appl Physiol (1985). 2012;112(11):1940–1948. [DOI] [PubMed] [Google Scholar]
- 42.Gingras V, Aris IM, Rifas-Shiman SL, Switkowski KM, Oken E, Hivert MF. Timing of complementary feeding introduction and adiposity throughout childhood. Pediatrics. 2019;144(6):1–9, e20191320. https://pediatrics.aappublications.org/content/pediatrics/144/6/e20191320.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gingras V, Rifas-Shiman SL, Taveras EM, Oken E, Hivert MF. Dietary behaviors throughout childhood are associated with adiposity and estimated insulin resistance in early adolescence: a longitudinal study. Int J Behav Nutr Phys Act. 2018;15(1):1–12, 129. https://ijbnpa.biomedcentral.com/track/pdf/10.1186/s12966-018-0759-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleisch AF, Luttmann-Gibson H, Perng W, et al. Prenatal and early life exposure to traffic pollution and cardiometabolic health in childhood. Pediatr Obes. 2017;12(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleisch AF, Rifas-Shiman SL, Koutrakis P, et al. Prenatal exposure to traffic pollution: associations with reduced fetal 7growth and rapid infant weight gain. Epidemiology. 2015;26(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fleisch AF, Rifas-Shiman SL, Mora AM, et al. Early-life exposure to perfluoroalkyl substances and childhood metabolic function. Environ Health Perspect. 2017;125(3):481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mora AM, Oken E, Rifas-Shiman SL, et al. Prenatal exposure to perfluoroalkyl substances and adiposity in early and mid-childhood. Environ Health Perspect. 2017;125(3):467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond). 2008;32(2):201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aris IM, Oken E. Childhood adiposity trajectories: discerning order amongst the chaos. Am J Clin Nutr. 2019;110(5):1049–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oken E, Aris IM, Young JG. Pre-pregnancy weight and preterm birth: a causal relation? Lancet Diabetes Endocrinol. 2019;7(9):663–665. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X, Tilling K, Martin RM, et al. Analysis of ‘sensitive’ periods of fetal and child growth. Int J Epidemiol. 2019;48(1):116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aris IM, Rifas-Shiman SL, Li LJ, et al. Association of weight for length vs body mass index during the first 2 years of life with cardiometabolic risk in early adolescence. JAMA Netw Open. 2018;1(5):1–16, e182460. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2703136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kramer MS, Zhang X, Martin RM, Oken E, Aris IM, Yang S. Growth during infancy and early childhood and its association with metabolic risk biomarkers at 11.5 years. Am J Epidemiol. 2019pii: kwz234 10.1093/aje/kwz234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anzman-Frasca S, Stifter CA, Birch LL. Temperament and childhood obesity risk: a review of the literature. J Dev Behav Pediatr. 2012;33(9):732–745. [DOI] [PubMed] [Google Scholar]
- 55.Diener MJ, Geenen R, Koelen JA, et al. The significance of attachment quality for obesity: a meta-analytic review. Can J Behav Sci. 2016;48(4):255–265. [Google Scholar]
- 56.Ward MJ, Kessler DB, Altman SC. Infant-mother attachment in children with failure-to-thrive. Inf Mental Hlth J. 1993;14(3):208–220. [Google Scholar]
- 57.Reinert KR, Po'e EK, Barkin SL. The relationship between executive function and obesity in children and adolescents: a systematic literature review. J Obes. 2013;2013:1–10, 820956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Curtin C, Jojic M, Bandini LG. Obesity in children with autism spectrum disorder. Harv Rev Psychiatry. 2014;22(2):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rhee KE, Lumeng JC, Appugliese DP, Kaciroti N, Bradley RH. Parenting styles and overweight status in first grade. Pediatrics. 2006;117(6):2047–2054. [DOI] [PubMed] [Google Scholar]
- 60.Pliner P, Loewen ER. Temperament and food neophobia in children and their mothers. Appetite. 1997;28(3):239–254. [DOI] [PubMed] [Google Scholar]
- 61.Task Force On Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: updated 2016 recommendations for a safe infant sleeping environment. Pediatrics. 2016;138(5):1–12, e20162938. [DOI] [PubMed] [Google Scholar]
- 62.Chassiakos YR, Radesky J, Christakis D, Moreno MA, Cross C, Media CC. Children and adolescents and digital media. Pediatrics. 2016;138(5):e1–e18, e20162593. [DOI] [PubMed] [Google Scholar]


