Abstract
Ceftolozane-tazobactam (C/T), ceftazidime-avibactam (C/A), and meropenem/vaborbactam (M/V) are new beta-lactam/beta-lactamase combination antibiotics commonly used to treat multidrug-resistant Pseudomonas aeruginosa (MDRPA) and carbapenem-resistant Enterobacteriaceae (CRE) infections. This review reports the clinical success rates for C/T, C/A, and M/V. PubMed and EMBASE were searched from January 1, 2012, through September 2, 2020, for publications detailing the use of C/T, C/A, and M/V. A meta-analysis determined the pooled effectiveness of C/T, C/A, and M/V. The literature search returned 1950 publications; 29 publications representing 1620 patients were retained. Pneumonia was the predominant infection type (49.8%). MDRPA was the major pathogen treated (65.3%). The pooled clinical success rate was 73.3% (95% CI, 68.9%–77.5%). C/T, C/A, or M/V resistance was reported in 8.9% of the population. These antibiotics had a high clinical success rate in patients with complicated infections and limited treatment options. Larger studies comparing C/T, C/A, and M/V against other antibiotic regimens are needed.
Keywords: antimicrobial resistance, epidemiology, meta-analysis, multidrug-resistant infections
Carbapenem-resistant Enterobacteriaceae (CRE) have been identified as an urgent antibiotic-resistant threat by the Centers for Disease Control and Prevention (CDC) [1]. Infections caused by CRE are difficult to treat due to the limited number of antibiotic options available. In 2017, the CDC reported 13 100 hospital-based cases of CRE in the United States, 1100 estimated deaths (8.4% mortality rate), and costs of ~$130 million dollars annually [1]. Multidrug-resistant Pseudomonas aeruginosa (MDRPA) has been classified as a serious threat by the CDC. It infects ~32 600 people each year and contributes to 2700 deaths. Treating MDRPA costs ~$767 million annually [1]. Risk factors for CRE and MDRPA include previous antibiotic exposure, frequent contact with health care facilities, older age, and use of indwelling devices [2–7].
Ceftolozane/tazobactam (C/T) is a combination fourth-generation cephalosporin and β-lactamase inhibitor that was approved for use by the Food and Drug Administration in 2014. Ceftolozane is a potent antibiotic with activity against many gram-negative bacteria, including Pseudomonas aeruginosa. Tazobactam irreversibly binds to serine β-lactamases, thus protecting against hydrolysis by β-lactamase enzymes produced by bacteria. C/T is primarily used for the treatment of multidrug-resistant Pseudomonas aeruginosa infections (MDRPA) but can also be used to treat infections caused by extended-spectrum beta-lactamase (ESBL)–producing organisms [8, 9].
Ceftazidime/avibactam (C/A) is a combination of a third-generation cephalosporin and a novel β-lactamase inhibitor and was approved for use in 2015. Ceftazidime has increased affinity for the penicillin-binding protein (PBP)–3 that is commonly found in gram-negative organisms and inhibits bacterial cell wall synthesis. Avibactam is a non-β-lactam β-lactamase inhibitor that reduces the availability of active enzymes that can inactivate β-lactam antibiotics. C/A is primarily used for the treatment of carbapenem-resistant Enterobacteriaceae (CRE) but is also used to treat infections caused by other multidrug-resistant gram-negative organisms (MDRGNOs) [8, 10].
Meropenem/vaborbactam (M/V) is a combination carbapenem and beta-lactamase inhibitor that was approved for use in the United States in 2017 [11]. Meropenem acts by inhibiting the cell wall synthesis of gram-positive and -negative bacteria. Vaborbactam is a cyclic boronic acid–based beta-lactamase that was designed to augment the performance of carbapenem antibiotics against carbapenemase-producing organisms [12]. M/V is primarily used in the treatment of MDRGNO infections, particularly CRE.
All 3 combination antibiotics are used to treat infections from several different sources, including, but not limited to, complicated intra-abdominal and complicated urinary tract infections (cIAIs and cUTIs), and hospital- and ventilator-associated pneumonia (HAP and VAP). These drugs are often administered as salvage therapy, either when the organism is resistant to all other antibiotics or when other antibiotics have failed to treat the infection [13, 14]. Since the approval of these drugs, several medical centers have published their clinical experience with these antibiotics. However, there has not been a systematic synthesis of observational studies to determine the overall effectiveness on clinical and microbiological outcomes. The goal of this study was to determine the effectiveness of C/T, C/A, and M/V in observational studies reporting on the treatment of multidrug-resistant gram-negative infections. This analysis offers a synthesized analysis of the effectiveness of these new antibiotics against serious antibiotic-resistant organisms. This review only included studies that evaluated the antibiotics of interest independently without a comparator group.
METHODS
Article Search
This systematic review was conducted using the Meta-analyses of Observational Studies in Epidemiology (MOOSE) criteria. PubMed and EMBASE were searched from January 1, 2012, through September 2, 2020, for studies that detailed the use of C/T, C/A, or M/V for the treatment of multidrug-resistant organism (MDRO) infections. The following search terms were used to search both databases: “ceftolozane/tazobactam,” “ceftazidime/avibactam,”” meropenem/vaborbactam,” “cephalosporin/beta-lactamase inhibitor,” “Pseudomonas aeruginosa,” “multi-center study,” “observational study,” and “retrospective study.” Publications were excluded for the following reasons: in vitro studies, non-English studies, animal studies, case studies, case series with a sample size <10, studies that did not evaluate either C/T, C/A, or M/V, randomized controlled trials, studies that included <33% MDRGNO infections, and studies that did not report a clinical success rate. Studies with a sample size <10 were excluded because these studies often have a larger margin of error. As the focus of the study was the treatment of multidrug-resistant infections, studies with <33% MDRGNO infections were excluded. Authors G.W., K.W., and M.F. evaluated the studies for inclusion. Randomized controlled trials were not included in this analysis because our objective was to evaluate the real-world effectiveness, not efficacy, of C/T, C/A, and M/V.
Data Abstraction
G.W., K.W., and M.F. all independently abstracted data from the included studies. The following information was collected from each article: patient demographics and medical comorbidities, Charlson or SOFA score, infection characteristics, clinical and microbiological outcomes, development of C/T, C/A, or M/V resistance, infection recurrence, adverse events, and mortality.
Quality Assessment
Included studies were assessed for quality using the Institute of Health Economics (IHE) Case Series Quality Assessment Tool [15], which is a validated tool for assessing noncomparator observational studies. The tool contains 18 questions that can be divided into 3 categories of evaluation: study design and participant selection, outcome selection and statistical analysis, and reporting and publication bias. Each category contains 6 questions. A score of 0–2 in 1 category indicates a high risk of bias, 3–4 a moderate risk of bias, and 5–6 a low risk of bias. An overall score of 0–6 indicates that the study had a high risk of bias, 7–12 a moderate risk of bias, and 13–18 an overall low risk of bias. Study quality was assessed by G.W., K.W., and M.F. Disagreements were resolved by consensus.
Outcome Definitions
Clinical success was defined as improvement or complete resolution of signs and symptoms of infection such that no further therapy or surgical intervention was needed to address the infection. Microbiological success was defined as a negative result from a culture that was taken from the site of infection at the conclusion of the antibiotic treatment. Clinical recurrence was defined as the reappearance of signs and symptoms of infection after the end of treatment. Microbiologic recurrence was defined as a positive culture result at the end of treatment when a previous culture from the same site had been negative. Clinical failure was defined as lack of complete resolution of the signs and symptoms of infection for 1 of the following reasons: failure to respond to treatment, recurrence (clinical or microbiological) of infection following initial response to treatment, or death. The pooled recurrence rate was calculated from the patients who met the “recurrence of infection” definition for clinical failure. Salvage therapy was defined as patients who received C/T, C/A, or M/V because other available therapies had failed or the organism was resistant to other antibiotics available.
Statistical Analysis
Demographic (eg, facility region, patient age, race/ethnicity) and clinical (eg, patient comorbidities, infection type, previous treatment) variables were collected for each study. The results were summed and averaged across the included studies to determine the pooled sums and averages for the entire cohort. The 95% confidence interval for each included study was calculated using the reported clinical success rate and the standard error for each study. The standard error was determined from the effect size and sample size of each individual study. Microsoft Excel (Redmond, WA, USA) was used to calculate the pooled clinical success rates across all studies using a validated method for calculating pooled effects (fixed and random) for noncomparator studies [16]. Heterogeneity was also calculated. Subanalyses were calculated using the same method as the main pooled rate. Due to the high amount of variability in the included studies, random effects were reported for the pooled estimates.
RESULTS
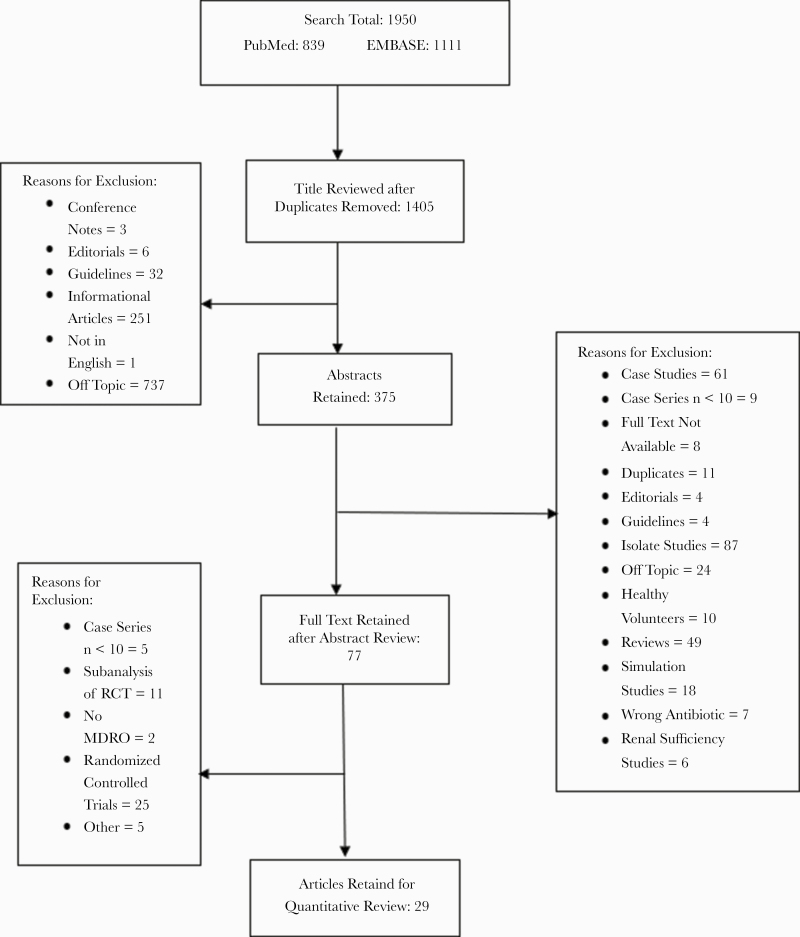
A total of 1950 publications were identified with the initial search, 839 from PubMed and 1111 from EMBASE (Figure 1). Studies were primarily excluded for the following reasons: antibiotics of interest were not evaluated, in vitro studies, case studies or case series with a sample size <10, clinical guidelines, or press releases. Because these drugs were primarily designed to treat MDROs and our aim was to review the effectiveness of these drugs in patients with infections due to MDRGNOs, we only included studies where the patient population had a >33% MDRGNO infection rate (determined by dividing the total number of patients with an MDRO by the total number of patients included in that study). After title, abstract, and full-text review, 29 studies representing 1620 patients were included in the final analysis.
Figure 1.
Publication selection flowchart. Abbreviations: MDRO, multidrug-resistant organism; RCT, randomized controlled trial.
The average study duration was 22.2 months, and the average sample size per study was 57 patients. Half of the included studies were conducted in the United States (58%) [17–27], with the remainder being from Europe (predominantly Italy and Spain) [28–37], Australia [38], and the Middle East [39] (Table 1). Most were defined as observational studies (62%), and the rest were considered extended case series with a study size of 10 or larger. The antibiotic treatment duration required for inclusion in the study was highly variable across all the studies. Most studies required patients to have at least 72 hours of treatment (31%), while almost one-fifth (17%) only required patients to receive 1 dose to be included. An additional 17% did not disclose the duration of treatment patients had in order to be included in the analysis (Table 1). There were 1620 patients represented across the 29 studies. Most patients were older and male (64.8%) (Table 1). The most frequently reported comorbidities were kidney disease (17%), solid organ transplant (15%), and cancer (14%). Other comorbidities that were not as frequently reported but represented a large percentage of the pooled population were cardiovascular disease (15%), diabetes (22%), and respiratory disease (19%). Nineteen studies reported the average Charlson score for patients, and 9 reported the average SOFA score (Table 1). There was wide variability in the infection types that were treated with C/T, C/A, and M/V. Pneumonia represented 50% of the infections treated in the pooled patient population, followed by cUTIs (13%), cIAIs (12%), skin and soft tissue infections (10%), primary bloodstream infections (9%), and bone and joint infections (5%) (Table 1). MDRPA was the most frequent organism treated (65%), while just under a quarter had CRE infections (24%).
Table 1.
Demographics of Included Studies (n = 29 Studies)
| Author, Year | Study Design | Location | Duration of Study, mo | Duration of Treatment | Sample Size | Age, y | SOT, % | Kidney Disease , % | Cancer | Pneumonia | %MDRPA | %CRE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alosaimy, 2020 | Obs. study | USA | 20 | ≥72 h MV | 40 | 58 (34–69)a | NR | 37.5 | NR | 32.5 | N/A | 84.6 |
| Bassetti, 2019 | Obs. study | Italy | 21 | ≥96 h C/T | 101 | 67 (49–74)a | 10.9 | 30.7 | 29.7 | 31.7 | 69.0 | N/A |
| Bosaeed, 2020 | Obs. study | Saudi Arabia | 24 | ≥96 h C/T | 24 | 57 (36–71)a | 8.3 | 20.8 | 20.8 | 31.6 | 100 | N/A |
| Caston, 2017 | Obs. study | Spain | 9 | NR | 12 | 67 (54–75)b | NR | 25.0 | 16.7 | 50.0 | 100 | N/A |
| De la Calle, 2019 | Obs. study | Spain | 26 | ≥72 h C/A | 23 | 58.8 (16.03)c | 21.7 | 43.5 | 21.7 | 21.7 | N/A | 100.0 |
| Diaz-Canestro, 2018 | Obs. study | Spain | 28 | C/T for ≥48 h | 58 | 60.8 (14.5)c | 1.7 | 25.9 | 32.7 | 60.3 | 97 | N/A |
| Dinh, 2017 | Obs. study | France | 9 | ≥1 dose of C/T | 15 | 48.3 (3–73)d | 33.3 | 20.0 | 26.7 | 46.7 | 100 | N/A |
| Escola-Verge, 2018 | Obs. study | Spain | 20 | ≥72 h of C/T | 38 | 59.5 (19–85)b | 28.9 | 21.1 | 28.9 | 36.8 | 100 | N/A |
| Gallagher, 2018 | Obs. study | USA | 38 | ≥24 h of C/T | 205 | 60 (48–70)a | 17.1 | 26.3 | 16.1 | 59.0 | 100 | N/A |
| Guimaraes, 2019 | Case series | Brazil | 21 | ≥48 h of C/A | 29 | 50.5e | 24.1 | 48.3 | NR | 10.3 | N/A | 100.0 |
| Haidar, 2017 | Obs. study | USA | 9 | NR | 21 | 58 (23–91)b | 38.1 | 23.8 | 9.5 | 76.2 | 100 | N/A |
| Hart, 2019 | Case series | USA | 41 | ≥24 h C/T | 70 | 57 (14)c | 67.1 | NR | NR | 55.7 | 100 | N/A |
| Jorgensen, 2019 | Obs. study | USA | 48 | ≥72 h of C/A | 203 | 62 (49–72)a | NR | 32.0 | 13.3 | 37.4 | 31 | 58 |
| Jorgensen, 2020 | Obs. study | USA | 48 | ≥72 h of C/T | 259 | 62 (52–72)a | NR | NR | 9.3 | 62.9 | 87 | N/A |
| King, 2017 | Chart review | USA | 13 | ≥24 h of C/A | 60 | 60 (51–69)a | 25.0 | 31.7 | NR | 26.7 | N/A | 83.3 |
| Molloy, 2020 | Case series | USA | Not reported | Not reported | 13 | 3 mo– 19 yf | NR | NR | 7.7 | 76.9 | 100 | N/A |
| Molnar, 2017 | Obs. study | USA | Not reported | ≥24 h C/T | 34 | 57 (42–66)a | 44.1 | NR | NR | 64.7 | 100 | N/A |
| Munita, 2017 | Obs. study | USA | Not reported | Not reported | 35 | 52.9 (16–89)d | NR | 11.4 | 25.7 | 42.9 | 77.0 | N/A |
| Nambiar, 2019 | Case series | USA | 22 | Not reported | 32 | C/A = 61 (11)c C/T = 48 (19)c | 100.0 | 12.5 | NR | 46.9 | 13.0 | 87.5 |
| Nathan, 2016 | Case series | USA | 14 | NR | 28 | 57 (18–64)d | 0.0 | NR | 39.3 | 28.6 | 50.0 | 25.0 |
| Rodriguez-Nunez, 2020 | Obs. study | USA, UK, France, Spain | 24 | ≥72 h of C/T, | 90 | 64 (16.2)c | 33 | 14.4 | 18.9 | 100 | 77.0 | N/A |
| Sacha, 2017 | Case series | USA | 10 | ≥1 dose of C/T | 49 | 65 (51–71)a | 34.7 | NR | 14.3 | 69.4 | 69 | N/A |
| Santevecchi, 2018 | Case series | USA | 12 | ≥1 dose of C/A | 10 | 53 (32–75)d | 20.0 | NR | 10.0 | 60.0 | 70 | N/A |
| Shields, 2016 | Case series | USA | 10 | ≥72 h of C/T | 37 | 64 (26–78)b | 29.7 | NR | 0.0 | 32.4 | N/A | 100.0 |
| Shields, 2020 | Obs. study | USA | 16 | MV for ≥48 h | 20 | 56 (31–83)a | NR | NR | NR | 35.0 | NR | 70.0 |
| Sousa, 2018 | Obs. study | Spain | 20 | ≥48 h of C/A | 57 | 64 (26–86)d | NR | 21.05 | 24.56 | 26.3 | N/A | 100.0 |
| Temkin, 2017 | Case series | Europe and Australia | 36 | ≥1 dose of C/A | 38 | 61 (47–67)a | 13.2 | 18.42 | NR | NR | N/A | 89.5 |
| Vena, 2020 | Obs. study | Italy | 24 | ≥72 h of C/A | 41 | 61.6 (13.0)c | NR | 26.09 | 17.39 | 48.8 | 93 | N/A |
| Xipell, 2018 | Case series | Spain | 12 | ≥72 h of C/T | 23 | 62 (41–70)a | 19.5 | 21.9 | 26.8 | 34.8 | 100 | N/A |
Abbreviations: C/A, ceftazidime/avibactam; C/T, ceftolozane/tazobactam; CRE, carbapenem-resistant Enterobacteriaceae; MDRPA, multidrug-resistant Pseudomonas aeruginosa; Obs., observational; SOT, solid organ transplant; XDRPA, extensively drug-resistant Pseudomonas aeruginosa.
aMedian (interquartile range).
bMedian (range).
cMean (SD).
dMean (range).
eMean only.
fRange only.
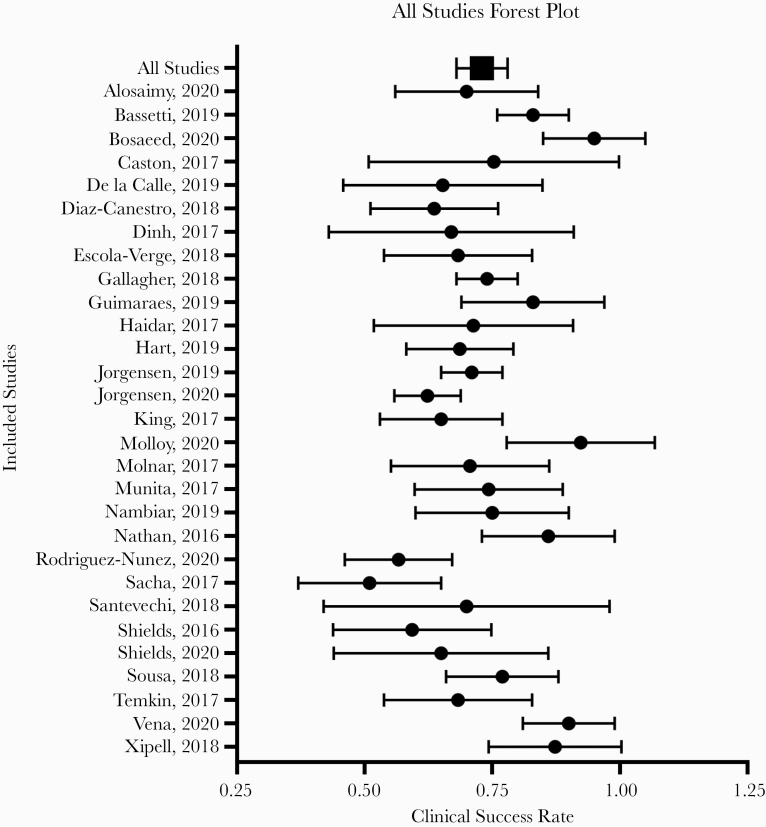
The definition for clinical success was explicitly stated in 25 of the 29 included articles. The pooled clinical success rate against MDRGNOs among all 29 studies was 73.3% (95% CI, 68.9%–77.5%) using random-effects models (Figure 2). There was a moderately high amount of heterogeneity, represented by an I2 value of 72.6%. Nineteen of the studies reported microbiological success following treatment. Microbiological success was measured in studies that reported across all culture types. The pooled microbiological success rate was 67.9% (95% CI, 58.5%–77.4%), but there was a high amount of heterogeneity (I2 = 87.9%) (Table 2). There were 4 studies included that had sample sizes of ≥100; a sensitivity analysis demonstrated that these studies did not overly influence the pooled analysis results.
Figure 2.
Forest plot of clinical success rate among studies meeting inclusion criteria (n = 29). The mean and SD of the clinical success rates for each study are represented. The pooled mean and SD are presented as the total.
Table 2.
Stratified Analyses and Subanalyses of Included Studies
| Included Studies | Pooled Rate (CI), % | I 2 Value, % | |
|---|---|---|---|
| Outcomes | |||
| Clinical success | 29 | 73.3 (68.9–77.5) | 72.6 |
| Microbiological success | 19 | 67.9 (58.8–77.4) | 87.9 |
| Recurrence rate | 14 | 33.9 (28.2–39.7) | 47.3 |
| Clinical success among subset analyses | |||
| C/T-only studies | 18 | 73.8 (67.8–79.7) | 78.5 |
| C/A-only studies | 12 | 73.0 (67.7–78.4) | 51.9 |
| Salvage therapy patients | 12 | 80.7 (78.0–83.4) | 0.0 |
| Studies with low risk of bias | 16 | 72.7 (66.8–78.6) | 80.0 |
| Studies with moderate risk of bias | 13 | 73.9 (67.7–80.1) | 55.4 |
Out of the 29 studies included, 18 exclusively evaluated the effectiveness of C/T and 12 exclusively evaluated C/A. One study combined C/T and C/A [14]; however, as the results for each drug were not reported separately, this study was not included in the subanalyses. The pooled clinical success rates were similar for C/T and C/A (73.8%; 95% CI, 67.8%–79.7%; and 73.0%; 95% CI, 67.7%–7%; respectively). Only 2 studies were identified that evaluated M/V, so a pooled subanalysis could not be completed. Of the 14 studies that reported the infection recurrence rate after treatment, the pooled rate was 33.9% (95% CI, 28.2%–39.7%). There were 12 studies that included patients receiving C/T, C/A, or M/V as salvage therapy with a pooled clinical success rate of 80.7% (95% CI, 78.0%–83.4%). There was low heterogeneity among the salvage therapy studies (Table 2).
Fourteen studies reported the adverse events (AEs) experienced by patients that were attributable to C/T or C/A treatment. The average AE rate was 10.7%, with a total of 97 events reported across all studies. The most common AE reported was acute kidney or liver injury (38%), gastrointestinal issues (35%), and rash (6%). There were no fatal AEs reported in association with any of the studied antibiotics.
Sixteen of the studies were rated as having a low risk of bias, and 13 were rated as having a moderate risk of bias based on the IHE Case Series Quality Assessment Tool (Table 3). No studies were considered to have a high risk of bias. There was no difference in the pooled clinical success rate between the studies with a low risk of bias (72.7%; 95% CI, 66.8%–78.6%) and the studies with a moderate risk of bias (73.9%; 95% CI; 67.7%–80.1%). The most common reasons for a low quality rating included incomplete or no reporting of participant inclusion and exclusion criteria and no reporting of the length of follow-up, loss to follow-up, or adverse events in the study population.
Table 3.
Quality Assessment of Included Studies
| Author, Year | Study Design/Participant Selection | Outcome Selection and Statistical Analysis | Reporting/Publication Bias | Overall Score |
|---|---|---|---|---|
| Alsoaimy, 2020 | 5/6 | 6/6 | 3/6 | 14/18 |
| Bassetti, 2019 | 6/6 | 4/6 | 4/6 | 14/18 |
| Bosaeed, 2020 | 4/6 | 6/6 | 4/6 | 14/18 |
| Caston, 2017 | 5/6 | 5/6 | 1/6 | 11/18 |
| De la Calle, 2019 | 4/6 | 4/6 | 4/6 | 12/18 |
| Diaz-Canestro, 2018 | 4/6 | 4/6 | 3/6 | 11/18 |
| Dinh, 2017 | 5/6 | 5/6 | 4/6 | 14/18 |
| Escola-Verge, 2018 | 4/6 | 5/6 | 3/6 | 12/18 |
| Gallagher, 2018 | 6/6 | 4/6 | 4/6 | 14/18 |
| Guimaraes, 2019 | 5/6 | 5/6 | 4/6 | 14/18 |
| Haidar, 2017 | 4/6 | 5/6 | 3/6 | 12/18 |
| Hart, 2019 | 3/6 | 5/6 | 2/6 | 10/18 |
| Jorgensen, 2019 | 5/6 | 6/6 | 4/6 | 15/18 |
| Jorgensen, 2020 | 5/6 | 6/6 | 5/6 | 16/18 |
| King, 2017 | 6/6 | 5/6 | 3/6 | 14/18 |
| Molloy, 2020 | 4/6 | 3/6 | 3/6 | 10/18 |
| Molnar, 2017 | 5/6 | 5/6 | 2/6 | 12/18 |
| Munita, 2017 | 5/6 | 5/6 | 3/6 | 13/18 |
| Nambiar, 2019 | 4/6 | 6/6 | 2/6 | 12/18 |
| Nathan, 2016 | 6/6 | 6/6 | 2/6 | 12/18 |
| Rodriguez-Nunez, 2020 | 6/6 | 5/6 | 4/6 | 15/18 |
| Sacha, 2017 | 4/6 | 6/6 | 3/6 | 13/18 |
| Santevecchi, 2018 | 5/6 | 6/6 | 3/6 | 14/18 |
| Shields, 2016 | 4/6 | 6/6 | 2/6 | 12/18 |
| Shields, 2020 | 4/6 | 6/6 | 3/6 | 13/18 |
| Sousa, 2018 | 5/6 | 6/6 | 4/6 | 15/18 |
| Temkin, 2017 | 4/6 | 5/6 | 2/6 | 11/18 |
| Vena, 2020 | 6/6 | 6/6 | 3/6 | 15/18 |
| Xipell, 2018 | 4/6 | 6/6 | 1/6 | 11/18 |
A score of 0–2 in 1 category indicates a high risk of bias, 3–4 a moderate risk of bias, and 5–6 a low risk of bias. An overall score of 0–6 (red) indicates a high risk of bias, 7–12 (yellow) a moderate risk of bias, and 13–18 (green) an overall low risk of bias.
DISCUSSION
The steady increase in MDRO infections globally has driven the need for new antibiotics, particularly for resistant, gram-negative bacterial infections. Over half of the antimicrobial resistance threats identified by the Centers for Disease Control and Prevention (CDC) are gram-negative bacteria, including 3 out of the 5 listed “urgent threats” [40]. Gram-negative bacteria often acquire resistance to multiple antibiotic classes, historically necessitating treatment with second- or third-line antibiotics with high toxicity and poor effectiveness. The development and approval of novel antibiotics such as C/T and C/A that have activity against MDROs causing severe infections including cIAI, cUTI, and HAP/VAP reflect a major step forward in our armamentarium against these deadly bacteria. These drugs have the potential for greater efficacy and less toxicity than established treatments. However, treatment success and AE rates in patient populations with primarily MDRO infections or in whom salvage therapy is required have not been well described. In this study, the average AE rate was 10.7% across 14 studies; this is comparable to the AE rates found for older antibiotic therapies [10, 41].
In this systematic review and meta-analysis of 29 studies that included 1620 patients primarily treated with C/T, C/A, or M/V for MDRO infections or salvage therapy, we found a high pooled clinical success rate of 73.3%. There was a moderately high amount of heterogeneity among the articles (72.6%). Clinical success rates for C/T and C/A were similar. The most common infection site reported in these studies was either HAP or VAP (44%). HAP/VAP caused by MDROs is often associated with greater clinical severity than other infection types (eg, cUTI), making infection resolution more difficult [42]. Therefore, the success rates for these drugs in treating HAP/VAP are particularly encouraging. Another finding of interest was that the studies that focused on C/T, C/A, and M/V as salvage therapy showed a higher clinical success rate than the overall analysis (79.0%). Preserving these agents primarily for salvage treatment when patients cannot have narrower-spectrum antibiotics either due to resistance or failure to cure infection may preserve the broad-spectrum activity of these drugs and decrease the development of resistance.
A recent meta-analysis primarily of randomized controlled trials found a pooled clinical success rate of 88% (95% CI, 82%–93%) for C/A and 94% (95% CI, 83%–98%) for C/T [43]. Although these results are significantly higher than what was reported herein, the authors did not restrict their inclusion criteria to the treatment of MDRO infections. Thus, including patients treated with C/T or C/A regardless of pathogen susceptibility may have contributed to the higher success rate. To our knowledge, this is the first meta-analysis of observational studies of C/T, C/A, or M/V for the treatment of MDRGNO infections. Another systematic review of treatments for MDRGNOs found that colistin, when used as either monotherapy or as part of combination therapy, had a clinical success rate ranging from 66% to 79% for the treatment of MDRPA [44]. Therefore, the pooled clinical success rate for colistin is comparable to the pooled rate for C/T for treatment of MDRPA identified in our analysis (73.8%).
Microbiological success was not evaluated in all the included studies (19/29). However, the pooled success rate for this outcome was lower than the clinical success rate (pooled success rate, 67.9%; 95% CI, 58.8%–77.4%). This result echoes the results of randomized controlled trials that found rates of microbiological clearance using these antibiotics to be lower than rates of clinical success [45, 46]. One potential reason is that it is more difficult to evaluate this outcome for certain infection types such as intra-abdominal infections. Recurrent infections accounted for 33.9% of clinical failures in our study. This is higher than the measurement recorded in other studies (8.3%–12.5%) [47, 48]. This difference is perhaps because previous studies have measured the rate of recurrence out of the total infections, whereas we felt it was more accurate to measure this outcome only out of those individuals who failed treatment.
The recent 2020 Infectious Diseases Society of America Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections recommends the use of C/A, C/T, and M/V for the treatment of MDRGNOs over polymyxins. Regardless of the culture site, these new guidelines recommend C/A and M/V for the treatment of CRE infections and C/T and C/A for MDRPA infections [49]. Our results support these recommendations by showing a high level of clinical success with the use of these antibiotics across several infection types and sources.
There are several strengths to this review. First, we offer an in-depth analysis of the pooled treatment effects of C/T, C/A, and M/V, strengthened by additional subanalyses performed to determine if the clinical success rate differed according to key variables of interest. Additionally, we expanded knowledge about the global patient population in whom these medications are primarily being targeted with these antibiotics. There were several weaknesses of this analysis. First, the analysis was limited to observational studies without a comparator or control group of patients who did not receive C/T, C/A, or M/V. Therefore, we could not evaluate the effectiveness and adverse events associated with these drugs in direct comparison with other antibiotic treatments. However, the pooled observed clinical success rate of 72.4% is comparable to clinical success rates reported with other antibiotic regimens used in the treatment of multidrug-resistant gram-negative organisms, including colistin (73%–79%) [44] and dual carbapenem therapy (72.0%) [14]. Additionally, several studies could not be included because the clinical success rate for the antibiotic was not reported. We did not include studies that had a comparator group in this analysis, so we could not evaluate the effectiveness of these new treatments compared with more traditional antibiotics. Finally, because our focus for this analysis was on C/T, C/A, and M/V use for patients with MDRO infections or as salvage therapy, we excluded several studies due to an overall low proportion of patients with MDROs. The inclusion of those studies could have resulted in a higher pooled clinical success rate than reported here. However, there were several strengths to this review. First, this was an in-depth analysis of the pooled treatment effects of C/T and C/A, strengthened by additional subanalyses performed to determine if the clinical success rate differed according to key variables of interest. Additionally, this analysis expanded the knowledge about the global patient population in whom these medications are primarily being targeted.
CONCLUSIONS
C/T, C/A, and M/V are novel antibiotics for the treatment of complicated infections and MDRO infections. The overall clinical success rate for all drugs in the treatment of patients with MDRGNOs was high (72.4%; 95% CI, 68.4%–76.4%), and the rate of adverse events was low. Interestingly, results were even higher for patients receiving C/T, C/A, and M/V for salvage therapy with limited treatment options or previously experiencing treatment failure. Our results demonstrate that C/T, C/A, and M/V can be effective in the treatment of complex and serious MDRO infections with limited treatment options. These results also support the 2020 IDSA guidelines for the use of these antibiotics for the treatment of CRE and MDRPA infections. Larger studies examining the effectiveness of these antibiotics in diverse patient populations and different infection types are needed to better evaluate their effectiveness.
Acknowledgments
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US Government.
Financial support. This work was supported by The Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, IIR 16-028 (PI: Evans, Charlesnika).
Potential conflicts of interest. All authors: no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This is a review article and does not include factors necessitating patient consent.
References
- 1.Centers for Disease Control and Prevention. Biggest Threats and Data: 2019 AR Threats Report. Atlanta: Centers for Disease Control and Prevention; 2019. [Google Scholar]
- 2. Clancy CJ, Chen L, Shields RK, et al. Epidemiology and molecular characterization of bacteremia due to carbapenem-resistant Klebsiella pneumoniae in transplant recipients. Am J Transplant 2013; 13:2619–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nadkarni AS, Schliep T, Khan L, Zeana CB. Cluster of bloodstream infections caused by KPC-2 carbapenemase-producing Klebsiella pneumoniae in Manhattan. Am J Infect Control 2009; 37:121–6. [DOI] [PubMed] [Google Scholar]
- 4. Souli M, Galani I, Antoniadou A, et al. An outbreak of infection due to beta-lactamase Klebsiella pneumoniae carbapenemase 2-producing K. pneumoniae in a Greek University Hospital: molecular characterization, epidemiology, and outcomes. Clin Infect Dis 2010; 50:364–73. [DOI] [PubMed] [Google Scholar]
- 5. van Loon K, Voor In “t Holt AF, Vos MC. A systematic review and meta-analyses of the clinical epidemiology of carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother 2018; 62:e01730-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Willems RPJ, van Dijk K, Ket JCF, Vandenbroucke-Grauls CMJE. Evaluation of the association between gastric acid suppression and risk of intestinal colonization with multidrug-resistant microorganisms: a systematic review and meta-analysis. JAMA Intern Med 2020; 180:561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaye KS, Pogue JM. Infections caused by resistant gram-negative bacteria: epidemiology and management. Pharmacotherapy 2015; 35:949–62. [DOI] [PubMed] [Google Scholar]
- 8. Lagacé-Wiens P, Walkty A, Karlowsky JA. Ceftazidime-avibactam: an evidence-based review of its pharmacology and potential use in the treatment of gram-negative bacterial infections. Core Evid 2014; 9:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Escolà-Vergé L, Pigrau C, Almirante B. Ceftolozane/tazobactam for the treatment of complicated intra-abdominal and urinary tract infections: current perspectives and place in therapy. Infect Drug Resist 2019; 12:1853–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Duin D, Bonomo RA. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis 2016; 63:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shoulders BR, Casapao AM, Venugopalan V. An update on existing and emerging data for meropenem-vaborbactam. Clin Ther 2020; 42:692–702. [DOI] [PubMed] [Google Scholar]
- 12. Petty LA, Henig O, Patel TS, et al. Overview of meropenem-vaborbactam and newer antimicrobial agents for the treatment of carbapenem-resistant Enterobacteriaceae. Infect Drug Resist 2018; 11:1461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nørgaard SM, Jensen CS, Aalestrup J, et al. Choice of therapeutic interventions and outcomes for the treatment of infections caused by multidrug-resistant gram-negative pathogens: a systematic review. Antimicrob Resist Infect Control 2019; 8:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sheu CC, Chang YT, Lin SY, et al. Infections caused by carbapenem-resistant Enterobacteriaceae: an update on therapeutic options. Front Microbiol 2019; 10:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Institute of Health Economics. Case series studies quality appraisal checklist 2019. Available at: http://www.ihe.ca/research-programs/rmd/cssqac/cssqac-about. Accessed March 2019.
- 16. Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and forest plots using a Microsoft Excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes 2012; 5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gallagher JC, Satlin MJ, Elabor A, et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: a multicenter study. Open Forum Infect Dis 2018; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haidar G, Philips NJ, Shields RK, et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin Infect Dis 2017; 65:110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hart DE, Gallagher JC, Puzniak LA, Hirsch EB. 2281. Ceftolozane–tazobactam (C/T) treatment outcomes in immunocompromised (IC) patients with multidrug-resistant (MDR) Pseudomonas aeruginosa (PA) infections. Open Forum Infect Dis 2019; XXX:XXX–XX. [Google Scholar]
- 20. King M, Heil E, Kuriakose S, et al. Multicenter study of outcomes with ceftazidime-avibactam in patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother 2017; 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Molnar E, Heil E, Claeys K, et al. Ceftolozane-tazobactam for the treatment of multi drug-resistant Pseudomonas aeruginosa (MDRPA) infections. Open Forum Infect Dis 2017; 4:S285–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Munita JM, Aitken SL, Miller WR, et al. Multicenter evaluation of ceftolozane/tazobactam for serious infections caused by carbapenem-resistant Pseudomonas aeruginosa. Clin Infect Dis 2017; 65:158–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nambiar P, Brizendine K, Athans V, Cober E. Clinical experience with novel cephalosporin/beta-lactamase inhibitor combinations in the treatment of multidrug resistant Pseudomonas aeruginosa and carbapenem-resistant Enterobacteriaceae in solid organ transplant recipients. Am J Transpl 2017;17:544–5. [Google Scholar]
- 24. Nathan RV, Alvarado FS, Prokesch RC, et al. Ceftolozane/tazobactam: outpatient treatment of gram-negative infections at physician office infusion centers (POICs). Open Forum Infect Dis 2016;. 3:2055. [Google Scholar]
- 25. Sacha GL, Neuner EA, Athans V, et al. Retrospective evaluation of the use of ceftolozane/tazobactam at a large academic medical center. Infect Dis Clin Practice 2017; 25:305–9. [Google Scholar]
- 26. Santevecchi BA, Smith TT, MacVane SH. Clinical experience with ceftazidime/avibactam for treatment of antibiotic-resistant organisms other than Klebsiella pneumoniae. Int J Antimicrob Agents 2018; 51:629–35. [DOI] [PubMed] [Google Scholar]
- 27. Shields RK, Potoski BA, Haidar G, et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 2016; 63:1615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bassetti M, Castaldo N, Cattelan A, et al. ; CEFTABUSE Study Group Ceftolozane/tazobactam for the treatment of serious Pseudomonas aeruginosa infections: a multicentre nationwide clinical experience. Int J Antimicrob Agents 2019; 53:408–15. [DOI] [PubMed] [Google Scholar]
- 29. Castón JJ, De la Torre Á, Ruiz-Camps I, et al. Salvage therapy with ceftolozane-tazobactam for multidrug-resistant Pseudomonas aeruginosa infections. Antimicrob Agents Chemother 2017; 61:e02136-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De la Calle C, Rodríguez O, Morata L, et al. Clinical characteristics and prognosis of infections caused by OXA-48 carbapenemase-producing Enterobacteriaceae in patients treated with ceftazidime-avibactam. Int J Antimicrob Agents 2019; 53:520–4. [DOI] [PubMed] [Google Scholar]
- 31. Díaz-Cañestro M, Periañez L, Mulet X, et al. Ceftolozane/tazobactam for the treatment of multidrug resistant Pseudomonas aeruginosa: experience from the Balearic Islands. Eur J Clin Microbiol Infect Dis 2018; 37:2191–200. [DOI] [PubMed] [Google Scholar]
- 32. Dinh A, Wyplosz B, Kernéis S, et al. Use of ceftolozane/tazobactam as salvage therapy for infections due to extensively drug-resistant Pseudomonas aeruginosa. Int J Antimicrob Agents 2017; 49:782–3. [DOI] [PubMed] [Google Scholar]
- 33. Escolà-Vergé L, Pigrau C, Los-Arcos I, et al. Ceftolozane/tazobactam for the treatment of XDR Pseudomonas aeruginosa infections. Infection 2018; 46:461–8. [DOI] [PubMed] [Google Scholar]
- 34. Guimarães T, Nouér SA, Martins RC, et al. Ceftazidime-avibactam as salvage therapy for infections caused by Enterobacteriales coresistant to carbapenems and polymyxins. Antimicrob Agents Chemother 2019; 63:e00528-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sousa A, Pérez-Rodríguez MT, Soto A, et al. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 2018; 73:3170–5. [DOI] [PubMed] [Google Scholar]
- 36. Temkin E, Torre-Cisneros J, Beovic B, et al. Ceftazidime-avibactam as salvage therapy for infections caused by carbapenem-resistant organisms. Antimicrob Agents Chemother 2017; 61:e01964-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xipell M, Paredes S, Fresco L, et al. Clinical experience with ceftolozane/tazobactam in patients with serious infections due to resistant Pseudomonas aeruginosa. J Glob Antimicrob Resist 2018; 13:165–70. [DOI] [PubMed] [Google Scholar]
- 38. Torres A, Zhong N, Pachl J, et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis 2018; 18:285–95. [DOI] [PubMed] [Google Scholar]
- 39. Bosaeed M, Ahmad A, Alali A, et al. Experience with ceftolozane-tazobactam for the treatment of serious Pseudomonas aeruginosa infections in Saudi tertiary care center. Infect Dis (Auckl) 2020; 13:1178633720905977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kadri SS Key takeaways from the U.S. CDC’s 2019 antibiotic resistance threats report for frontline providers. Crit Care Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Titov I, Wunderink RG, Roquilly A, et al. A randomized, double-blind, multicenter trial comparing efficacy and safety of imipenem/cilastatin/relebactam versus piperacillin/tazobactam in adults with hospital-acquired or ventilator-associated bacterial pneumonia (RESTORE-IMI 2 Study). Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barbier F, Andremont A, Wolff M, Bouadma L. Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr Opin Pulm Med 2013; 19:216–28. [DOI] [PubMed] [Google Scholar]
- 43. Nguyen CP, Dan Do TN, Bruggemann R, et al. Clinical cure rate and cost-effectiveness of carbapenem-sparing beta-lactams vs. meropenem for gram-negative infections: a systematic review, meta-analysis, and cost-effectiveness analysis. Int J Antimicrob Agents 2019; 54:790–7. [DOI] [PubMed] [Google Scholar]
- 44. Nørgaard SM, Jensen CS, Aalestrup J, et al. Choice of therapeutic interventions and outcomes for the treatment of infections caused by multidrug-resistant gram-negative pathogens: a systematic review. Antimicrob Resist Infect Control 2019; 8:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carmeli Y, Armstrong J, Laud PJ, et al. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis 2016; 16:661–73. [DOI] [PubMed] [Google Scholar]
- 46. Kaye KS, Bhowmick T, Metallidis S, et al. Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the TANGO I randomized clinical trial. JAMA 2018; 319:788–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Alosaimy S, Jorgensen SCJ, Lagnf AM, et al. Real-world multicenter analysis of clinical outcomes and safety of meropenem-vaborbactam in patients treated for serious gram-negative bacterial infections. Open Forum Infect Dis 2020; 7:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gomila A, Carratalà J, Eliakim-Raz N, et al. ; RESCUING Study Group and Study Sites Clinical outcomes of hospitalised patients with catheter-associated urinary tract infection in countries with a high rate of multidrug-resistance: the COMBACTE-MAGNET RESCUING study. Antimicrob Resist Infect Control 2019; 8:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. IDSA. Infectious Diseases Society of America guidance on the treatment of antimicrobial resistant gram-negative infections. 2020. Available at: https://www.idsociety.org/practice-guideline/amr-guidance/. Accessed November 2020.