Abstract
Background
Human babesiosis is a mild-to-severe parasitic infection that poses health concerns especially in older and other at-risk populations. The study objective was to assess babesiosis occurrence among US Medicare beneficiaries, ages 65 and older, during 2006–2017.
Methods
Our retrospective claims-based study used Medicare databases. Babesiosis cases were identified using recorded diagnosis codes. The study estimated rates (per 100 000 beneficiary-years) overall, by year, diagnosis month, demographics, and state and county of residence.
Results
Nationwide, 19 469 beneficiaries had babesiosis recorded, at a rate of 6 per 100 000 person-years, ranging from 4 in 2006 to 9 in 2017 (P < .05). The highest babesiosis rates by state were in the following: Massachusetts (62), Rhode Island (61), Connecticut (51), New York (30), and New Jersey (19). The highest rates by county were in the following: Nantucket, Massachusetts (1089); Dukes, Massachusetts (236); Barnstable, Massachusetts (213); and Dutchess, New York (205). Increasing rates, from 2006 through 2017 (P < .05), were identified in multiple states, including states previously considered nonendemic. New Hampshire, Maine, Vermont, Pennsylvania, and Delaware saw rates increase by several times.
Conclusions
Our 12-year study shows substantially increasing babesiosis diagnosis trends, with highest rates in well established endemic states. It also suggests expansion of babesiosis infections in other states and highlights the utility of real-world evidence.
Keywords: babesiosis, trends, diagnosis, elderly, Medicare
Human babesiosis is a disease caused by intraerythrocytic protozoan parasites of Babesia species. Babesia microti is the primary etiologic agent in the United States and is mainly transmitted to humans via the bite of infected tick vector Ixodes scapularis but can also be transmitted by blood transfusion [1–3]. Northeastern (Connecticut, Rhode Island, Massachusetts, New York, and New Jersey) as well as some Midwestern (Minnesota and Wisconsin) states have been considered endemic for human B. microti infections for decades. Although most cases occurred in those 7 states with well established foci of tickborne transmission, geographic range of babesiosis has been expanding into areas previously considered nonendemic, similar to the expansion pattern of Lyme disease [1, 3–6]. Human babesiosis infections are also characteristically seasonal, with peak transmission during the summer months [4–8]. In most people, babesiosis is an asymptomatic or mild disease; however, some may have prolonged subclinical carriage with possible implications for blood donation and transfusion-transmission [2, 3, 7–12]. Certain populations such as older, splenectomized, or immunocompromised persons are at higher risk for disease-related severe complications, including hemolytic anemia, acute respiratory failure, congestive heart failure, renal failure, and death [2, 3, 7, 8, 13].
Over the last couple of decades in the United States, there has been a substantial increase in reported babesiosis, both in tickborne and transfusion-transmitted cases, as well as the spread of infection outside of the well established endemic areas. This may be due to human encroachment into tick and deer habitat, growth of the deer population, climatic effects on tick populations, travel to babesiosis-endemic areas, as well as increased awareness and reporting of babesiosis by providers [1, 3–7, 14–20]. Therefore, the national surveillance of human babesiosis is critical to identification of high-risk state- and county-level areas for further development and implementation of appropriate prevention strategies (eg, enhanced public awareness, tick surveillance, deer population control). Because older persons are at an increased risk for severe complications of babesiosis, our population-based study used large Centers for Medicare & Medicaid Services (CMS) administrative databases [21, 22] to assess recorded human babesiosis infections nationally, by state and county of residence, among US Medicare beneficiaries ages 65 and older, during the 2006–2017 study period.
MATERIALS AND METHODS
Data Source
Administered by CMS, Medicare is a national health insurance program that provides healthcare coverage to persons age 65 years and older as well as to persons under age 65 who have end-stage kidney disease and/or disability. This study used the Medicare claims databases containing patients’ healthcare utilization records from professional services (ie, physician offices), skilled nursing facilities, and institutional outpatient and inpatient settings. The diagnoses and procedures are first recorded into medical records by healthcare providers (eg, physicians) and then coded and submitted to CMS for billing and reimbursement purposes and captured in the Medicare administrative databases. The study also used the Medicare Enrollment Database to assess Medicare beneficiaries’ demographic characteristics, eligibility, and enrollment status.
Study Population
To be eligible for the study in a particular year during 2006–2017, a beneficiary had to be alive at some point in the year, age 65 or older, and continuously enrolled in Medicare fee-for-service Parts A (ie, hospital care) and B (ie, outpatient care and physician services) for at least 365 consecutive days before and including their last month of enrollment in that year. Beneficiaries enrolled in Medicare Part C (ie, managed care) at any point in this 365-day period were excluded from the cohort. Incident babesiosis cases were defined based on the first recording of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) or ICD-10-CM diagnosis codes for babesiosis infection, 088.82 or B60.0, with no other instances of these codes in the prior 365 days. A beneficiary’s age was measured on babesiosis diagnosis date, or for those beneficiaries without a recorded babesiosis diagnosis, on the first day of their last enrollment month in the year.
Statistical Analysis
This retrospective claims-based study assessed recorded babesiosis diagnosis occurrence (recorded cases and corresponding unadjusted rates) overall, by state and county of residence, as well as by age, sex, race, calendar year, and diagnosis months (seasonality) among Medicare beneficiaries ages 65 years and older during 2006–2017. The overall rates estimated the number of cases per 100 000 beneficiary-years of enrollment during the study period. Annual babesiosis rates were ascertained by estimating the number of cases per 100 000 beneficiaries per year. Babesiosis occurrence by age was evaluated in 10-year categories, to facilitate the comparison with prior investigations and other public health surveillance studies [4–6, 17]. The study evaluated county- and state-level babesiosis occurrence by number of cases and rates using beneficiaries’ residence information. To help differentiate between the predominance of local or travel-associated Babesia transmission, our study investigated the state-of-service (SOS) distribution for cases residing in the states with the highest babesiosis rates, including the District of Columbia. Specifically, this analysis allows to identify the states where cases were diagnosed (ie, SOS) by state of residence (SOR), with corresponding number and percentage of cases diagnosed in the residence state and outside. Therefore, if most of the cases in a state have SOS the same as SOR, locally acquired transmission is more likely (eg, Massachusetts). If most cases in a state have SOS different from SOR, travel-associated cases are more likely (eg, Florida). The seasonality assessment was based on the number of babesiosis cases in each month among beneficiaries continuously enrolled during the 365 days before and on the first day of each month in the calendar year. Cases were included in the analysis once, at the first documentation of their babesiosis diagnosis. Cochran-Armitage test was used to evaluate babesiosis trends over time and by age groups [23, 24]. Fisher’s exact test was used to compare babesiosis rates for males versus females and for whites versus nonwhites [25]. P < .05 were used as the level of significance for all unadjusted analyses. For trend analyses by states and counties, we also calculated q-values (using R 2.4.2 qvalue package), which adjust for false-positive findings in multiple testing [26, 27]. In a separate analysis, to help account for geographical correlation in babesiosis risk between adjacent regions and improve the rate estimation in counties with small populations and low number of cases, spatially adjusted rates were ascertained using a Bayesian hierarchical model (BYM2 model) [28, 29]. This spatial autocorrelation approach was used to adjust babesiosis rates in small geographical areas by pooling observed rates in neighboring counties to predict local risk. With spatial adjustment, extreme rates, especially in small nonendemic areas, can be smoothed out. In the spatially adjusted BYM2 model, given the ease of travel across the nation, all counties in the United States are assumed to have a nonzero risk of babesiosis even in nonendemic regions with small populations. All statistical analyses in the study were performed using SAS version 9.4 and R Software R 3.6.0. Nonzero counts of less than 11 with corresponding rates are not displayed due: (1) to CMS privacy restrictions and (2) to help protect confidentiality of Medicare beneficiaries by avoiding the release of information that can be used to identify individuals. Our investigation was performed under categorical exemption by the US Food and Drug Administration’s Institutional Review Board.
RESULTS
A total of 19 469 US Medicare beneficiaries ages 65 and older had a recorded babesiosis diagnosis during 2006–2017, for an overall national rate of 6 per 100 000 beneficiary-years. Babesiosis rates (per 100 000) increased during the study period, with a significant rise in national babesiosis rates over time: from 4 in 2006 to 9 in 2017 (P < .05) (Table 1). In the 7 well established endemic states (CT, MA, RI, NJ, NY, MN, WI), babesiosis rates increased from 17 in 2006 to 42 in 2017 (P < .05) (Supplementary Table S1a); and elsewhere, in other states, rates increased from 1 in 2006 to 3 in 2017 (P < .05) (Supplementary Table S1b). Study results show significantly increasing babesiosis trends over time in females, males, whites, and nonwhites, (P < .05) (Table 1; Supplementary Table S1a and b).
Table 1.
Babesiosis Cases and Rates Among Elderly Medicare Beneficiaries, by Sex, Age, and Race, During 2006–2017
| Number of Babesiosis Cases (Babesiosis Rate per 100 000 Beneficiary-Yearsa) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex and Age | All Years | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
| All Beneficiaries | |||||||||||||
| All Ages (65+)b,c | 19 469 (6) | 995 (4) | 853 (3) | 1225 (5) | 1500 (6) | 1317 (5) | 1374 (5) | 1226 (5) | 1889 (7) | 1823 (7) | 2521 (10) | 2303 (9) | 2443 (9) |
| 65–74 | 11 162 (7) | 537 (4) | 436 (4) | 678 (6) | 833 (7) | 739 (6) | 759 (6) | 720 (6) | 1093 (9) | 1063 (8) | 1537 (11) | 1353 (10) | 1414 (10) |
| 75–84 | 6370 (6) | 353 (3) | 319 (3) | 424 (4) | 512 (6) | 447 (5) | 478 (5) | 384 (4) | 621 (7) | 591 (7) | 756 (9) | 682 (8) | 803 (9) |
| ≥85 | 1937 (4) | 105 (2) | 98 (2) | 123 (3) | 155 (3) | 131 (3) | 137 (3) | 122 (3) | 175 (4) | 169 (4) | 228 (5) | 268 (6) | 226 (5) |
| Femalesd | |||||||||||||
| All Ages (65+)b,c | 10 117 (6) | 513 (3) | 444 (3) | 649 (4) | 815 (6) | 695 (5) | 718 (5) | 655 (4) | 959 (6) | 930 (6) | 1317 (9) | 1174 (8) | 1248 (8) |
| 65–74 | 5967 (7) | 284 (4) | 242 (4) | 365 (6) | 459 (7) | 410 (6) | 428 (7) | 405 (6) | 571 (8) | 549 (8) | 819 (11) | 708 (10) | 727 (10) |
| 75–84 | 3110 (5) | 173 (3) | 154 (3) | 215 (4) | 262 (5) | 210 (4) | 230 (4) | 180 (4) | 296 (6) | 310 (6) | 365 (8) | 322 (7) | 393 (8) |
| ≥85 | 1040 (3) | 56 (2) | 48 (2) | 69 (2) | 94 (3) | 75 (2) | 60 (2) | 70 (2) | 92 (3) | 71 (2) | 133 (5) | 144 (5) | 128 (5) |
| Malesd | |||||||||||||
| All Ages (65+)b,c | 9352 (7) | 482 (4) | 409 (4) | 576 (5) | 685 (6) | 622 (6) | 656 (6) | 571 (5) | 930 (8) | 893 (8) | 1204 (10) | 1129 (10) | 1195 (10) |
| 65–74 | 5195 (7) | 253 (4) | 194 (3) | 313 (6) | 374 (7) | 329 (6) | 331 (6) | 315 (5) | 522 (9) | 514 (8) | 718 (11) | 645 (10) | 687 (10) |
| 75–84 | 3260 (7) | 180 (4) | 165 (4) | 209 (5) | 250 (7) | 237 (6) | 248 (7) | 204 (5) | 325 (9) | 281 (7) | 391 (11) | 360 (10) | 410 (11) |
| ≥85 | 897 (5) | 49 (4) | 50 (4) | 54 (4) | 61 (4) | 56 (4) | 77 (5) | 52 (3) | 83 (5) | 98 (6) | 95 (6) | 124 (8) | 98 (7) |
| Racee | |||||||||||||
| Nonwhiteb | 973 (2) | 22 (1) | 29 (1) | 47 (1) | 62 (2) | 51 (2) | 48 (1) | 43 (1) | 72 (2) | 99 (3) | 141 (4) | 165 (4) | 194 (5) |
| Whiteb | 18 496 (7) | 973 (4) | 824 (4) | 1178 (5) | 1438 (6) | 1266 (6) | 1326 (6) | 1183 (5) | 1817 (8) | 1724 (8) | 2380 (11) | 2138 (9) | 2249 (10) |
aBabesiosis rates are rounded to the nearest whole number.
bThe trend of babesiosis occurrence rates during 2006–2017 is statistically significant according to the Cochran-Armitage test for trend, using a significance level of P < .05.
cFor “All Years”, significantly declining babesiosis rates (P < .05) with advancing age, by age categories, according to the Cochran-Armitage test for trend: overall and by sex, correspondingly.
dRate comparison of males vs females, resulted in relative risk (RR) = 1.24 and 95% confidence interval (CI) = 1.21–1.28.
eRate comparison of white vs nonwhite, resulted in RR = 2.98 and 95% CI = 2.79–3.18.
Babesiosis rates differed by demographic characteristics and diagnosis months. A significant decline of babesiosis occurrence was identified with advancing age (P < .05), with the highest rate of 7 for beneficiaries ages 65–74 and the lowest rate of 4 for ages 85 and above. Approximately 90.1% of cases were ages 65–84. Babesiosis rates (per 100 000) were higher among males compared with females, 7 versus 6 (P < .05; relative risk [RR] = 1.24; 95% confidence interval [CI], 1.21–1.28), and among whites versus nonwhites, 7 versus 2 (P < .05; RR = 2.98; 95% CI, 2.79–3.18) (Table 1). Supplementary Figure S1a displays babesiosis occurrence by month of diagnosis during 2006–2017, with the highest number of cases and rates in June, July, and August, representing approximately 50.7% of all babesiosis cases. Approximately 73.8% of all cases were diagnosed in the months of May through October. Monthly trends were generally similar within and outside the 7 well established endemic states (Supplementary Figure S1b and c).
During 2006–2017, the highest babesiosis rates by state or jurisdiction of residence were identified as follows: Massachusetts (62), Rhode Island (61), Connecticut (51), New York (30), New Jersey (19), New Hampshire (12), Maine (11), Vermont (10), Maryland (7), District of Columbia (5), Pennsylvania (5), Minnesota (5), Delaware (4), Virginia (4), and Wisconsin (4), which accounted for 17 216 or 88.4% of all cases (Table 2; Supplementary Table S2). The 7 well established states (MA, RI, CT, NY, NJ, MN, WI) with prevalent local tickborne transmission accounted for 15 124 or 77.7% of all cases, and all the other states accounted for 4345 (22.3%) cases. All 7 states and many other states had significantly increasing babesiosis rates from 2006 to 2017 (P < .05). New Hampshire, Maine, Vermont, Pennsylvania, and Delaware saw the rates increase by several times during the study period, with rates in 2017 for New Hampshire, Maine, and Vermont, nearly reaching those of the top 5 well established states (Table 2; data with counts of <11 data not shown). The highest number of cases outside the 7 states were identified in the following: Florida (N = 656), Pennsylvania (N = 639), California (N = 578), Maryland (N = 444), and Virginia (N = 375), accounting for a total of 2692 cases or 62.0% of cases in other states (Table 2).
Table 2.
Babesiosis Cases and Rates by State of Residence Among Medicare Beneficiaries, Ages 65 and Older, During 2006–2017
| Number of Babesiosis Cases (Babesiosis Rate per 100 000 Beneficiary-Yearsb) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statec,d | All Years, 2006–2017 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | ||
| Cases | All Eligible Beneficiary Years | Rate | Cases (Rate) | ||||||||||||
| Totale | 19 469 | 315 245 277 | 6 | 995 (4) | 853 (3) | 1225 (5) | 1500 (6) | 1317 (5) | 1374 (5) | 1226 (5) | 1889 (7) | 1823 (7) | 2521 (10) | 2303 (9) | 2443 (9) |
| Massachusettse,f | 4625 | 7 451 471 | 62 | 76 (13) | 128 (22) | 185 (32) | 229 (39) | 231 (40) | 349 (59) | 392 (63) | 591 (92) | 633 (97) | 980 (148) | 521 (78) | 310 (46) |
| Rhode Islande,f | 554 | 909 592 | 61 | 34 (45) | 13 (18) | 30 (41) | 33 (46) | 27 (38) | 29 (39) | 21 (28) | 62 (81) | 72 (93) | 98 (121) | 69 (84) | 66 (84) |
| Connecticute,f | 2164 | 4 208 174 | 51 | 173 (44) | 166 (43) | 183 (50) | 156 (44) | 123 (35) | 153 (45) | 128 (38) | 230 (68) | 164 (49) | 196 (58) | 248 (73) | 244 (72) |
| New Yorke,f | 5361 | 17 648 490 | 30 | 399 (25) | 269 (17) | 381 (25) | 594 (40) | 537 (37) | 334 (23) | 254 (18) | 434 (30) | 344 (24) | 431 (30) | 614 (43) | 770 (54) |
| New Jerseye,f | 2039 | 10 722 779 | 19 | 80 (9) | 59 (7) | 98 (11) | 150 (17) | 113 (13) | 169 (19) | 127 (14) | 187 (21) | 226 (25) | 293 (32) | 244 (26) | 293 (33) |
| New Hampshiree,f | 239 | 1 913 692 | 12 | a | a | a | a | a | 20 (13) | 21 (13) | 12 (7) | 34 (20) | 35 (20) | 26 (14) | 59 (33) |
| Mainee,f | 211 | 2 008 861 | 11 | a | a | a | a | a | 16 (10) | a | 14 (8) | 27 (16) | 23 (14) | 36 (22) | 48 (30) |
| Vermonte,f | 96 | 990 770 | 10 | a | a | a | a | a | a | a | a | a | 16 (18) | 30 (33) | 23 (25) |
| Marylande,f | 444 | 6 714 702 | 7 | 42 (8) | 34 (7) | 60 (12) | 44 (8) | 39 (7) | 24 (4) | 28 (5) | 41 (7) | 31 (5) | 35 (6) | 17 (3) | 49 (8) |
| Pennsylvaniae,f | 639 | 12 588 796 | 5 | 28 (2) | 18 (2) | 32 (3) | 22 (2) | 35 (4) | 38 (4) | 40 (4) | 54 (5) | 58 (6) | 89 (9) | 87 (8) | 138 (13) |
| Minnesotae,f | 170 | 3 753 981 | 5 | a | a | a | 18 (5) | 17 (5) | 28 (9) | a | 16 (6) | 12 (4) | 14 (5) | 17 (7) | 21 (9) |
| Virginia | 375 | 9 159 254 | 4 | 25 (3) | 30 (4) | 58 (8) | 33 (5) | 29 (4) | 26 (4) | 22 (3) | 23 (3) | 23 (3) | 30 (4) | 33 (4) | 43 (5) |
| Wisconsine,f | 211 | 5 953 078 | 4 | a | 13 (2) | 14 (3) | a | 17 (4) | 23 (5) | a | 21 (4) | 15 (3) | 12 (3) | 36 (8) | 37 (8) |
| Floridae,f | 656 | 22 122 602 | 3 | 28 (1) | 34 (2) | 44 (2) | 71 (4) | 51 (3) | 53 (3) | 59 (3) | 55 (3) | 55 (3) | 74 (4) | 60 (3) | 72 (4) |
| Californiae,f | 578 | 25 545 752 | 2 | 15 (1) | a | 42 (2) | 52 (3) | 31 (2) | 46 (2) | 42 (2) | 52 (2) | 38 (2) | 69 (3) | 92 (4) | 89 (4) |
| Texase,f | 139 | 21 546 688 | 1 | 14 (1) | a | a | a | a | a | a | a | a | 15 (1) | 24 (1) | 25 (1) |
| North Carolinae,f | 66 | 10 893 842 | 1 | a | a | a | a | a | 0 (0) | a | a | a | a | 13 (1) | 19 (2) |
| Illinoise,f | 82 | 14 952 836 | 1 | 0 (0) | a | a | 0 (0) | a | a | a | 18 (1) | a | 18 (2) | 11 (1) | a |
aStates’ annual counts of 1–10 are masked in this table but are included in the “All Years” counts. Zero annual counts are included.
bBabesiosis rates are rounded to the nearest whole number. States are sorted by descending overall babesiosis rates (ie, from highest to lowest “all years” rates).
cStates with an overall number of babesiosis cases of 1–10 or with most of the yearly counts of 1–10 are not displayed. Cases from these states are included in the “total” row.
dAmong states not in the table, the following states/jurisdictions had a total of 11 or more cases in the last 5 years: Arizona, Arkansas, Colorado, Delaware, District of Columbia, Georgia, Indiana, Iowa, Kansas, Kentucky, Michigan, Missouri, New Mexico, Ohio, South Carolina, Tennessee, and West Virginia.
eThe trend of babesiosis occurrence rates during 2006–2017 is statistically significant according to the Cochran-Armitage test for trend, using a significance level of P < .05.
fQ-values are generated using the R 2.4.2 qvalue package to measure the proportion of false positives incurred in tests that are significant. Q-values <0.05 are marked.
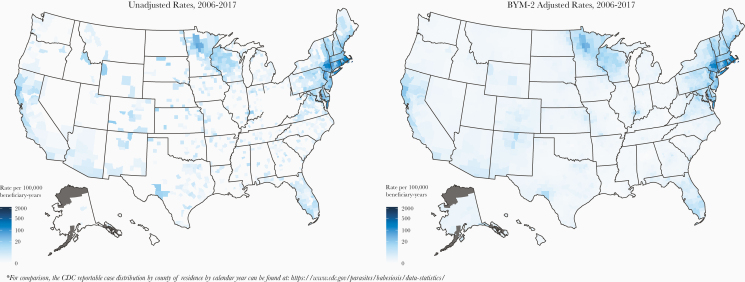
Figure 1 displays nationwide heat maps with corresponding unadjusted and adjusted babesiosis rates by county of residence during the 12-year period, which consistently show the highest risk areas in and around well established endemic Northeastern and Upper Midwestern regions. The highest babesiosis rates (per 100 000) were identified in counties of highly endemic regions: Nantucket, Massachusetts (1089); Dukes, Massachusetts (236); Barnstable, Massachusetts (213); Dutchess, New York (205); Washington, Rhode Island (187); Windham, Connecticut (177); and Plymouth, Massachusetts (177) (Table 3; Supplementary Table S3a). The highest babesiosis rates outside of well established regions were identified in the following mid-Atlantic and northeastern counties: Accomack, Virginia (113); Northampton, Virginia (68); Talbot, Maryland (51); Dorchester, Maryland (34); Pike, Pennsylvania (30); Kent, Maryland (28); Knox, Maine (26); York, Maine (25); and Chester, Pennsylvania (24) (Supplementary Table S3b). After rate adjustment, the counties with the highest rates remained largely similar to unadjusted (Supplementary Table S3a and b). Supplementary Table S4 shows the top 50 counties with the highest number of babesiosis cases, which accounted for 74.0% (N = 14 400) of all cases. Forty-four of 50 counties were in the top 5 northeastern endemic states (MA, RI, CT, NY, and NJ), representing 13 814 (71.0%) of all cases. Supplementary Table S5 shows the top 50 counties with highest number of cases in the states outside of well established endemic areas, with 39 of 50 counties in Virginia, Pennsylvania, Maryland, California, and Florida, representing a total of 1632 cases or 8.4% of all cases.
Figure 1.
Unadjusted and adjusted county-level babesiosis rates (per 100 000 medicare beneficiary-years) in the United States, 2006–2017.
Table 3.
The 50 Counties With the Highest Babesiosis Rates Among Medicare Beneficiaries, Ages 65 and Older, During 2006–2017
| Number of Babesiosis Cases (Babesiosis Rate per 100 000 Beneficiary-Yearsb) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Countyc,d | State | All Years | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
| Totale, | 12 798 (75) | 701 (49) | 568 (40) | 827 (59) | 1031 (75) | 947 (68) | 892 (64) | 794 (56) | 1321 (92) | 1241 (86) | 1728 (118) | 1383 (93) | 1365 (92) | |
| Nantucket | MA | 152 (1089) | a | a | a | a | a | 15 (1337) | 15 (1277) | 23 (1891) | 25 (2005) | 26 (2033) | a | a |
| Dukes | MA | 80 (236) | a | a | a | 12 (496) | a | a | a | 11 (366) | a | a | a | a |
| Barnstable | MA | 1217 (213) | 34 (74) | 48 (105) | 109 (239) | 109 (239) | 121 (266) | 162 (352) | 147 (309) | 206 (421) | 108 (219) | 80 (162) | 53 (105) | 40 (79) |
| Dutchesse,f | NY | 785 (205) | 40 (122) | 34 (105) | 50 (156) | 46 (146) | 56 (179) | 68 (219) | 70 (226) | 107 (343) | 67 (215) | 69 (215) | 71 (217) | 107 (323) |
| Washington | RI | 260 (187) | 23 (220) | a | 20 (191) | 25 (234) | 22 (203) | 20 (180) | a | 28 (236) | 29 (240) | 25 (196) | 23 (173) | 29 (218) |
| Windham | CT | 245 (177) | 16 (126) | 28 (228) | 20 (170) | 16 (140) | 17 (149) | 20 (180) | 15 (135) | 28 (251) | 14 (124) | 24 (212) | 23 (202) | 24 (209) |
| Plymouthe,f | MA | 1114 (177) | a | 14 (31) | 11 (24) | 46 (98) | 51 (107) | 90 (182) | 131 (248) | 166 (296) | 205 (356) | 239 (405) | 105 (174) | 54 (88) |
| Ulstere,f | NY | 367 (151) | a | a | a | a | a | a | a | 11 (55) | 12 (61) | 26 (129) | 120 (587) | 162 (783) |
| New London | CT | 506 (138) | 47 (144) | 41 (128) | 57 (183) | 45 (147) | 29 (96) | 27 (90) | 35 (117) | 59 (198) | 35 (117) | 50 (172) | 48 (158) | 33 (109) |
| Bristole,f | MA | 919 (127) | a | a | a | a | a | a | a | 42 (68) | 146 (233) | 456 (722) | 198 (314) | 43 (69) |
| Suffolke,f | NY | 2114 (121) | 265 (191) | 156 (112) | 212 (151) | 392 (279) | 347 (245) | 88 (62) | 70 (48) | 92 (62) | 94 (63) | 108 (72) | 120 (77) | 170 (104) |
| Newporte,f | RI | 133 (116) | a | 0 (0) | a | a | 0 (0) | a | a | 16 (163) | 19 (193) | 47 (471) | 26 (259) | 15 (153) |
| Accomacke,f | VA | 73 (113) | 11 (196) | a | 36 (690) | a | a | a | a | 0 (0) | 0 (0) | 0 (0) | a | a |
| Columbiae,f | NY | 92 (102) | a | a | a | a | a | a | a | 12 (164) | a | 17 (235) | 18 (249) | 18 (247) |
| Hunterdone,f | NJ | 167 (101) | a | a | a | a | a | 18 (134) | a | 21 (146) | 11 (74) | 29 (189) | 19 (123) | 27 (185) |
| Tollande,f | CT | 152 (98) | a | 16 (116) | 16 (121) | a | a | a | a | 13 (104) | 13 (103) | 23 (184) | 16 (127) | 20 (157) |
| Putname,f | NY | 96 (83) | a | a | a | a | a | 13 (134) | a | 13 (137) | a | a | a | 20 (194) |
| Middlesex | CT | 177 (80) | 19 (93) | 13 (65) | 18 (94) | 14 (76) | a | a | a | 17 (94) | 19 (106) | 18 (100) | 23 (127) | 16 (88) |
| Litchfielde,f | CT | 191 (68) | a | a | 12 (50) | 13 (55) | a | 24 (103) | a | 23 (100) | 14 (61) | 18 (79) | 16 (70) | 39 (169) |
| Sussexe,f | NJ | 115 (63) | 0 (0) | 0 (0) | a | a | a | a | a | 11 (69) | 23 (140) | a | 17 (97) | 30 (173) |
| Orangee,f | NY | 223 (55) | a | a | a | 11 (34) | a | a | a | 36 (107) | 25 (74) | 27 (78) | 40 (114) | 46 (130) |
| Fairfielde,f | CT | 455 (45) | 36 (40) | 29 (33) | 36 (42) | 31 (37) | 39 (47) | 39 (47) | 27 (33) | 46 (56) | 36 (43) | 23 (27) | 54 (63) | 59 (69) |
| Atlantic | NJ | 169 (45) | a | a | a | 19 (63) | 19 (63) | 16 (52) | a | 15 (47) | 19 (59) | 23 (67) | 16 (46) | 11 (34) |
| Warrene,f | NJ | 74 (44) | a | a | a | a | a | a | 0 (0) | a | a | 13 (88) | a | 12 (84) |
| Berkshiree,f | MA | 110 (42) | a | a | 0 (0) | a | a | 12 (56) | a | 18 (81) | 24 (107) | 31 (136) | a | a |
| Westchestere,f | NY | 430 (38) | 27 (28) | 28 (29) | 32 (34) | 40 (43) | 29 (31) | 53 (57) | 19 (20) | 52 (55) | 19 (21) | 40 (44) | 45 (49) | 46 (51) |
| New Yorke,f | NY | 528 (36) | 32 (25) | 22 (18) | 40 (33) | 52 (43) | 46 (38) | 44 (36) | 26 (21) | 46 (37) | 57 (47) | 48 (40) | 46 (38) | 69 (57) |
| Morrise,f | NJ | 216 (33) | a | 11 (22) | 11 (21) | a | 13 (25) | 14 (26) | 23 (42) | 29 (52) | 20 (36) | 25 (44) | 34 (58) | 26 (46) |
| Monmouthe,f | NJ | 278 (32) | a | a | 18 (26) | 16 (23) | a | 21 (30) | 19 (27) | 15 (21) | 37 (50) | 45 (59) | 41 (52) | 45 (59) |
| Essexe,f | MA | 296 (32) | a | 11 (15) | 12 (16) | a | 14 (19) | 19 (26) | 21 (28) | 46 (58) | 39 (48) | 43 (53) | 39 (47) | 33 (40) |
| Somersete,f | NJ | 112 (31) | a | a | a | a | a | a | a | 18 (57) | a | 22 (67) | a | 17 (53) |
| New Havene,f | CT | 285 (30) | 31 (33) | 18 (20) | 15 (18) | 25 (31) | a | 18 (23) | 18 (23) | 26 (34) | 19 (25) | 24 (32) | 42 (55) | 39 (51) |
| Norfolke,f | MA | 206 (27) | a | a | a | 11 (18) | 14 (23) | 12 (20) | 21 (32) | 24 (36) | 27 (40) | 28 (41) | 28 (40) | 26 (37) |
| Yorke,f | ME | 76 (25) | 0 (0) | a | a | a | a | a | a | a | a | a | 11 (44) | 11 (45) |
aCounties’ annual counts of 1–10 are masked in this table but are included in the “All Years” counts. Zero annual counts are included.
bBabesiosis rates are rounded to the nearest whole number. Counties are sorted in descending order by overall babesiosis rates (ie, from highest to lowest rates).
cCounties with an overall number of babesiosis cases of 1–10 or most of the yearly counts of 1–10 are not displayed in the table, including the following: Beltrami, MN; Buffalo, WI; Cass, MN; Clearwater, MN; Dorchester, MD; Greene, NY; Hubbard, MN; Kent, MD; Kent, RI; Knox, ME; Northampton, VA; Pike, PA; Renssalaer, NY; Sullivan, NY; Talbot, MD; and Washington, NY. Cases from these counties are included in the “total” row.
dAmong counties not in the table, the following counties had a total of 11 or more cases in the last 5 years: Greene, NY; Kent, RI; Knox, ME; Pike, PA; Rensselaer, NY; Sullivan, NY; and Washington, NY.
eThe trend of Babesiosis occurrence rates during 2006–2017 is statistically significant according to the Cochran-Armitage test for trend, using a significance level of P < .05.
fQ-values are generated using the R 2.4.2 qvalue package to measure the proportion of false positives incurred in tests that are significant. Q-values <0.05 are marked.
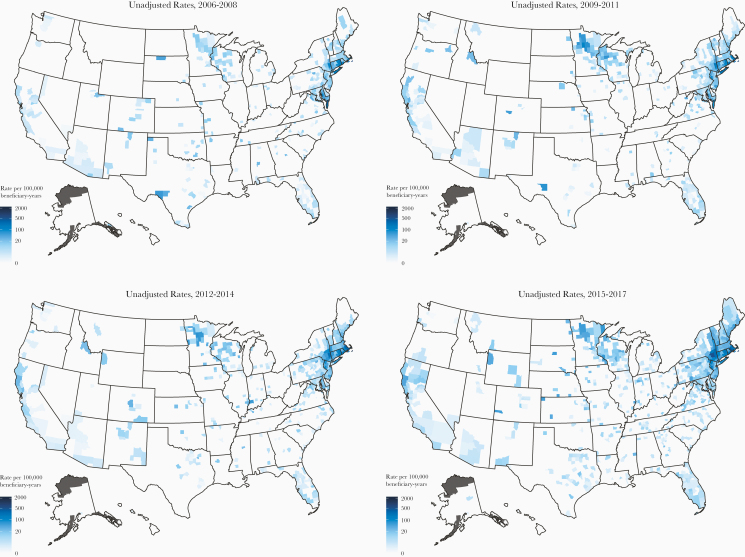
Over the 12-year period, the study observed increasing babesiosis rates in many counties of well established endemic regions and outside, most notably in counties of Maine, New Hampshire, Vermont, Pennsylvania, and other states (Figure 2). Table 3 shows significantly increasing babesiosis rates (per 100 000) during 2006–2017 (P < .05) overall, from 49 in 2006 to 92 in 2017, and in many of the top 50 counties with highest rates, including the following: Dutchess, New York; Sussex, New Jersey; Fairfield, Connecticut; Westchester, New York; New York, New York; and New Haven, Connecticut. A substantial (ie, by several times) rate increase was identified in Plymouth, Massachusetts; Ulster, New York; Newport, Rhode Island; Columbia, New York; Litchfield, Connecticut; Orange, New York; and some others. Outside the 7 states, in the top 50 counties by rate, increasing babesiosis rates were identified overall, from 16 to 32, and in many of the counties, including the following: Pike, Pennsylvania; Knox, Maine; York, Maine; Chester, Pennsylvania; Mendocino, California; Rockingham, New Hampshire; Windham, Vermont; and Merrimack, New Hampshire; all had a substantial rate increase during the study period. (Data not shown for counts less than 11.)
Figure 2.
Heat maps with unadjusted county-level babesiosis rates (per 100 000 medicare beneficiary-years) for the 3-year intervals during study period: 2006–2008, 2009–2011, 2012–2014, 2015–2017.
State-of-service distribution analysis helps to show the predominance of local or travel-associated babesiosis by SOR. During the study period, approximately 69.9% of all recorded cases had babesiosis diagnosis in the SOR, suggesting mostly local transmission (Supplementary Table S6). Figure 3 shows SOS distribution by SOR for the highest rate states, including the District of Columbia. In all of the 7 well established endemic states, except New York, most (≥70%) of the diagnosed cases had the same SOS and SOR. Massachusetts, Wisconsin, New Jersey, Minnesota, Connecticut, Rhode Island, and New York, respectively, had 96.6%, 85.3%, 84.7%, 82.9%, 81.9%, 70.4%, and 46.7% of cases diagnosed in the SOR. New York had 28.1% and 19.0% of its cases diagnosed in Massachusetts and New Jersey, respectively, suggesting potentially substantial travel-associated babesiosis (Figure 4). For the highest rate states outside of the well established endemic areas, California, Maine, Pennsylvania, Vermont, New Hampshire, Virginia, Delaware, Maryland, and Florida, respectively, had 83.0%, 73.5%, 67.4%, 64.6%, 64.4%, 58.4%, 43.3%, 35.8%, and 28.0% of cases with the same SOS and SOR (Figure 3). Florida had 34.3% of its cases with SOS in Massachusetts, the highest proportion of cases diagnosed outside of Florida (Figure 4).
Figure 3.
State-of-service (SOS) distribution for babesiosis cases with the state of residence (SOR) in the highest rate states, including the District of Columbia (DC).
Figure 4.
State-of-service (SOS) distribution for cases residing in Massachusetts (MA), Florida (FL), and New York (NY).
DISCUSSION
Our 12-year nationwide population-based study shows substantially increasing babesiosis incidence trends among the US Medicare beneficiaries ages 65 and older during 2006–2017, with rising rates not only in the well established areas but also in other areas, as supported by the literature [3–6, 17, 18, 30–37]. Consistent with published reports [1–7], our large, real-world, evidence-based investigation identified most (77.7%) of the babesiosis cases in the 7 states with well established tickborne transmission of B microti: Massachusetts, Rhode Island, Connecticut, New York, New Jersey, Minnesota, and Wisconsin. The highest babesiosis rates outside of the 7 states were identified in New Hampshire, Maine, Vermont, Maryland, Pennsylvania, Delaware, Virginia, Florida, and California, which accounted for approximately 16.9% of all cases. Overall, these 16 highest rate states accounted for approximately 94.6% of all recorded cases. The study’s SOS distribution analysis also supports substantial local transmission in the 7 endemic states and in many other higher risk states (eg, NH, ME, VT, PA, CA).
In agreement with other reports [6, 17, 33–36], our study identified substantially increasing babesiosis trends during the study period in Maine, New Hampshire, and Vermont, with latest rates comparable to the well established states and with most cases having SOS the same as the SOR, and thus suggesting mostly local (ie, nontravel-associated) Babesia transmission. Pennsylvania and Delaware, in support of the literature [6, 37], also had markedly increasing babesiosis trends, with the latest rates comparable to or higher than those for Minnesota and Wisconsin. Pennsylvania, unlike Delaware, had most of its cases diagnosed in the SOR, suggesting local transmission, as supported by the literature [37, 38]. Other states, including but not limited to California, Florida, North Carolina, and Georgia, had increasing recorded babesiosis diagnosis occurrence over time, suggesting the need for monitoring babesiosis distribution patterns in all states to help better understand local and travel-associated transmissions. The study’s investigation into SOS distribution in the highest rate states suggested possibly substantial travel-associated babesiosis among the District of Columbia, Florida, Maryland, Delaware, and New York residents, which needs further confirmation. Overall, the study results show increasing recorded babesiosis diagnosis occurrence within and outside of the 7 well established states, further supporting human Babesia infection expansion in the United States [1-6, 17, 18, 30–38].
The study findings also show variation in human babesiosis occurrence by demographic characteristics (eg, age, sex, race) and seasonality (ie, diagnosis months), likely associated with tick exposure and human activity. In support of the literature[4–6, 31–33, 39–41], among adults ages 65 and older, risk of babesiosis occurrence was identified to be higher in males versus females, in whites versus nonwhites, and in younger versus older seniors, which may be related to demographic differences in outdoor activities [42–45], especially next to the tick and deer habitat, and needs further investigations and confirmation. Most of the cases were consistently diagnosed in the months of May through October, with peaks in July and August, and corresponding to increased activity of humans during summer months as well as to the lifecycle and activity of the tick vector and mammalian hosts. [1–9, 19, 20, 31, 32, 39, 46].
Our novel population-based investigation ascertained recorded babesiosis infections by county of residence among US adults ages 65 and older. Consistent with the literature [6, 17, 18, 31, 39, 47–49], the highest recorded babesiosis occurrence was identified in areas with well established foci of tickborne transmission, suggesting the validity of CMS databases. Most of the 50 counties with highest number of cases and rates were in the highest Babesia incidence states of Massachusetts, Rhode Island, Connecticut, New York, and New Jersey, and they accounted for the majority of all babesiosis cases. During the study period, Suffolk, New York; Barnstable, Massachusetts; Plymouth, Massachusetts; and Bristol, Massachusetts had the highest number of cases, whereas Nantucket, Massachusetts; Dukes, Massachusetts; Barnstable, Massachusetts; and Dutchess, New York had the highest human babesiosis infection rates in the United States. Increasing babesiosis incidence over time was identified in the majority of top 50 counties, especially in the 5 endemic states (eg, Ulster, NY, Sussex, NJ, and Litchfield, CT). Outside of the well established states, many counties have also experienced a considerable increase in babesiosis occurrence (eg, York, ME, Chester, PA, and Rockingham, NH), suggesting expansion of recorded human babesiosis infections, as supported by the literature [3–6, 17, 33–38], and further reinforcing the importance of nationwide monitoring of babesiosis distribution in the United States. Overall, the spatially adjusted rates supported study findings and were effective in correcting high unadjusted risk in the counties with small populations and very few cases, especially in nonendemic states (eg, Colorado, Kansas, South Dakota, Texas). The study’s heat maps displaying county-level babesiosis occurrence nationally suggest a valuable way to disseminate important public health information.
The study’s limitations are related to the use of claims-based administrative databases and as such include the following: potential for misclassification of incident versus prevalent cases due to persistent parasitemia in some infected individuals; possible under- or misdiagnosis of babesiosis because some cases could have been asymptomatic and also due to unknown sensitivity and specificity of recorded diagnoses; lack of clinical details including test results for diagnosis code verification; and inability to identify Babesia species (eg, Babesia microti, Babesia duncani, Babesia divergens) in claims data. Although the SOS distribution evaluation allows for better differentiation between travel-associated or locally acquired cases, the actual location of transmission is unknown, and future investigations are needed to validate findings and ascertain precision of claims-based SOS analysis. Although the Medicare administrative databases do not provide population-based information on babesiosis risk among persons younger than 65 years, estimates of Babesia incidence may be more accurate among adults age 65 and older due to greater severity of the infections and thus better reporting in this age group, which needs further confirmation. Presence of various nucleic acid- and antibody-based laboratory diagnostic tests of unknown and different sensitivities and specificities may have added variability to the study results [1, 3, 7, 50]. In addition, babesiosis may be misdiagnosed, especially through the use of serologic testing alone, with disproportionately large misclassification possible in nonendemic areas (eg, Florida). Thus, further national population-based clinical investigations are needed to assess and validate the diagnostic testing performed and corresponding results, which were beyond the scope of this investigation. Increased babesiosis awareness by the medical community, especially outside well established areas, as well as beneficiary travel and blood component distributions throughout the country may have contributed to the babesiosis trends identified in the study.
CONCLUSIONS
In summary, our study is the largest population-based investigation on human babesiosis infection trends by state and county of residence among the US Medicare beneficiaries ages 65 and older who may be at higher risk for infection-related complications. It shows substantially increasing babesiosis occurrence during the 2006–2017 study period, with expansion of diagnosed human Babesia infections in the well established endemic areas and outside. Human encroachment into tick and deer habitat, growth of the deer population, climatic effects on tick populations, and travel to highly prevalent areas with tickborne transmission may be responsible for babesiosis spread in the United States [1–7, 9, 19, 20]. In addition, our nationwide study of recorded human babesiosis infections identified high-risk states and counties and, therefore, can support the development and implementation of appropriate prevention strategies by public health organizations at the national, state, and local levels (eg, public education, tick surveillance, deer population control). Finally, our study suggests the utility of the real-world evidence (eg, large datasets) as a tool for better understanding, monitoring, and tracking national, regional, local, seasonal, and other babesiosis transmission patterns in support of public health efforts to reduce spread of babesiosis in the United States.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Dr. Diane Gubernot and Renata Moldavskaya for contributions to the manuscript.
Disclaimer. The views expressed in this manuscript are those of the authors and do not reflect official policy of the US Food and Drug Administration (FDA) or the United States Government.
Financial support. This work was funded by the FDA, Center for Biologics Evaluation and Research.
Potential conflicts of interest. All authors: No reported conflicts of interest.
References
- 1. Krause PJ Human babesiosis. Int J Parasitol 2019; 49:165–74. [DOI] [PubMed] [Google Scholar]
- 2. Vannier E, Krause PJ. Human babesiosis. N Engl J Med 2012; 366:2397–407. [DOI] [PubMed] [Google Scholar]
- 3. Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect Dis Clin North Am 2015; 29:357–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Menis M, Forshee RA, Kumar S, et al. Babesiosis occurrence among the elderly in the United States, as recorded in large medicare databases during 2006–2013. PLoS One 2015; 10: e0140332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Menis M, Anderson SA, Izurieta HS, et al. Babesiosis among elderly Medicare beneficiaries, United States, 2006-2008. Emerg Infect Dis 2012; 18:128–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. Surveillance for Babesiosis—United States, 2017 Annual Summary Atlanta, Georgia: U.S. Department of Health and Human Services; 2019. [Google Scholar]
- 7. Gubernot DM, Nakhasi HL, Mied PA, et al. Transfusion-transmitted babesiosis in the United States: summary of a workshop. Transfusion 2009; 49:2759–71. [DOI] [PubMed] [Google Scholar]
- 8. Meldrum SC, Birkhead GS, White DJ, et al. Human babesiosis in New York State: an epidemiological description of 136 cases. Clin Infect Dis 1992; 15:1019–23. [DOI] [PubMed] [Google Scholar]
- 9. Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infect Dis Clin North Am 2008; 22:469–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krause PJ, McKay K, Gadbaw J, et al. ; Tick-Borne Infection Study Group Increasing health burden of human babesiosis in endemic sites. Am J Trop Med Hyg 2003; 68:431–6. [PubMed] [Google Scholar]
- 11. Krause PJ, Spielman A, Telford SR 3rd, et al. Persistent parasitemia after acute babesiosis. N Engl J Med 1998; 339:160–5. [DOI] [PubMed] [Google Scholar]
- 12. Krause PJ, Telford SR 3rd, Pollack RJ, et al. Babesiosis: an underdiagnosed disease of children. Pediatrics 1992; 89:1045–8. [PubMed] [Google Scholar]
- 13. Krause PJ Babesiosis. Med Clin North Am 2002; 86:361–73. [DOI] [PubMed] [Google Scholar]
- 14. Leiby DA Transfusion-transmitted Babesia spp.: bull’s-eye on Babesia microti. Clin Microbiol Rev 2011; 24:14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gubernot DM, Lucey CT, Lee KC, et al. Babesia infection through blood transfusions: reports received by the U.S. Food and Drug Administration, 1997–2007. Clin Infect Dis 2009; 48:25–30. [DOI] [PubMed] [Google Scholar]
- 16. Herwaldt BL, Linden JV, Bosserman E, et al. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011; 155:509–19. [DOI] [PubMed] [Google Scholar]
- 17. Gray EB, Herwaldt BL. Babesiosis surveillance — United States, 2011–2015. MMWR Surveill Summ 2019; 68:1–11. [DOI] [PubMed] [Google Scholar]
- 18. Linden JV, Prusinski MA, Crowder LA, et al. Transfusion-transmitted and community-acquired babesiosis in New York, 2004 to 2015. Transfusion 2018; 58:660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robinson SJ, Neitzel DF, Moen RA, et al. Disease risk in a dynamic environment: the spread of tick-borne pathogens in Minnesota, USA. Ecohealth 2015; 12:152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brownstein JS, Holford TR, Fish D. A climate-based model predicts the spatial distribution of the Lyme disease vector Ixodes scapularis in the United States. Environ Health Perspect 2003; 111:1152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Virnig BA, McBean M. Administrative data for public health surveillance and planning. Annu Rev Public Health 2001; 22:213–30. [DOI] [PubMed] [Google Scholar]
- 22. Centers for Medicare & Medicaid Services. Files for Order—General Information Baltimore: Centers for Medicare & Medicaid Services; Available at: http://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/FilesForOrderGenInfo/index.html. Accessed June 11 2019. [Google Scholar]
- 23. Cochran WG Some methods for strengthening the common χ 2 tests. Biometrics 1954; 10: 417–51. [Google Scholar]
- 24. Armitage P Tests for linear trends in proportions and frequencies. Biometrics 1955; 11: 375–86. [Google Scholar]
- 25. Fisher R A. Statistical Methods for Research Workers. In: Kotz S, Johnson NL, eds. Breakthroughs in Statistics. Springer Series in Statistics (Perspectives in Statistics). New York, NY: Springer; 1992. [Google Scholar]
- 26. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995; 57:289–300. [Google Scholar]
- 27. Storey JD, Bass AJ, Alan Dabney A, Robinson D. Q-value: Q-value estimation for false discovery rate control. R package version 2.12.0 [software]; 2015.
- 28. Rue H, Martino S, Chopin N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J R Stat Soc Series B Stat Methodol 2009; 71:319–92. [Google Scholar]
- 29. Martins TG, Simpson D, Lindgren F, Rue H. Bayesian computing with INLA: new features. Comput Stat Data Anal 2013; 67:68–83. [Google Scholar]
- 30. Klevens RM, Cumming MA, Caten E, et al. Transfusion-transmitted babesiosis: one state’s experience. Transfusion 2018; 58:2611–6. [DOI] [PubMed] [Google Scholar]
- 31. Massachusetts Department of Public Health. Tick-borne disease surveillance summaries and data: babesiosis surveillance data Available at: https://www.mass.gov/lists/tick-borne-disease-surveillance-summaries-and-data#babesiosis-surveillance-data-. Accessed 21 June 2019.
- 32. Minnesota Department of Public Health. Annual summary of communicable diseases reported to the Minnesota Department of Health, 2017. Disease Control Newsletter. Available at: https://www.health.state.mn.us/diseases/reportable/dcn/sum17/2017dcn.pdf. Accessed 21 June 2019. [Google Scholar]
- 33. Maine Center for Disease Control and Prevention. 2017 Maine reportable infectious diseases summary Available at: https://www.maine.gov/dhhs/mecdc/infectious-disease/epi/publications/#annualreports. Accessed 21 June 2019.
- 34. Smith R, Elias SP, Borelli TJ, et al. Human babesiosis, Maine, USA, 1995–2011. Emerg Infect Dis 2014; 20:1727–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vermont Department of Health. Vermont tickborne disease program: 2016 annual report Available at: http://www.healthvermont.gov/sites/default/files/documents/pdf/HS_ID_2016_TickborneDisease_Annual_Report_Digital_Version.pdf. Accessed 21 June 2019.
- 36. New Hampshire Department of Health and Human Services. Infectious disease reports: reportable communicable diseases in New Hampshire, 2014–2019 YTD Available at: https://www.dhhs.nh.gov/dphs/cdcs/documents/monthly.pdf. Accessed 21 June 2019.
- 37. Liu HH, Cushinotto L, Giger O, et al. Increasing babesiosis in Southeastern Pennsylvania, 2008–2017. Open Forum Infect Dis 2019; 6:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Edwards MJ, Russell JC, Davidson EN, et al. A 4-yr survey of the range of ticks and tick-borne pathogens in the lehigh valley region of Eastern Pennsylvania. J Med Entomol 2019; 56:1122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rhode Island Department of Health. Babesiosis surveillance 2013–2017 Available at: http://www.health.ri.gov/data/diseases/Babesiosis.pdf. Accessed 24 June 2019.
- 40. Centers for Disease Control and Prevention. National Notifiable Infectious Diseases and Conditions: United States. Table 5. Reported cases of notifiable diseases and rates per 100,000, by sex, excluding U.S. territories - - United States, 2017 Available at: https://wonder.cdc.gov/nndss/static/2017/annual/2017-table5.html. Accessed 28 June 2019.
- 41. Centers for Disease Control and Prevention. National Notifiable Infectious Diseases and Conditions: United States. Table 6. Reported cases of notifiable diseases and rates per 100,000, by race, excluding U.S. territories - - United States, 2017 Available at: https://wonder.cdc.gov/nndss/static/2017/annual/2017-table6.html. Accessed 28 June 2019.
- 42. Crespo CJ, Keteyian SJ, Heath GW, Sempos CT. Leisure-time physical activity among US adults. results from the Third National Health and Nutrition Examination Survey. Arch Intern Med 1996; 156:93–8. [PubMed] [Google Scholar]
- 43. Crespo CJ, Smit E, Andersen RE, et al. Race/ethnicity, social class and their relation to physical inactivity during leisure time: results from the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Prev Med 2000; 18:46–53. [DOI] [PubMed] [Google Scholar]
- 44. Sjögren K, Stjernberg L. A gender perspective on factors that influence outdoor recreational physical activity among the elderly. BMC Geriatr 2010; 10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. He XZ, Baker DW. Differences in leisure-time, household, and work-related physical activity by race, ethnicity, and education. J Gen Intern Med 2005; 20:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Centers for Disease Control and Prevention. National Notifiable Infectious Diseases and Conditions: United States. Table 3. Reported cases of notifiable diseases, by month, excluding U.S. territories - - United States, 2017 Available at: https://wonder.cdc.gov/nndss/static/2017/annual/2017-table3.html. Accessed July 2 2019.
- 47. New York State Department of Health. 2017 Communicable disease annual reports Available at: https://www.health.ny.gov/statistics/diseases/communicable/2017/. Accessed July 8 2019.
- 48. Connecticut State Department of Public Health. Infectious diseases statistics: number of reportable disease cases by county, by year Available at: https://portal.ct.gov/DPH/Epidemiology-and-Emerging-Infections/Infectious-Diseases-Statistics#47477. Accessed 8 July 2019.
- 49. State of New Jersey Department of Health. New Jersey reportable disease statistics and technical notes Available at: https://www.state.nj.us/health/cd/statistics/reportable-disease-stats/. Accessed 8 July 2019.
- 50. Parija SC, Kp D, Venugopal H. Diagnosis and management of human babesiosis. Trop Parasitol 2015; 5:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.