Abstract
Background:
Prenatal development is a time when the brain is acutely vulnerable to insult and alteration by environmental factors (e.g., toxins, maternal health). One important risk factor is maternal obesity (Body Mass Index > 30). Recent research indicates that high maternal BMI during pregnancy is associated with increased risk for numerous physical health, cognitive, and mental health problems in offspring across the lifespan. It is possible that heightened maternal prenatal BMI influences the developing brain even before birth.
Methods:
The present study examines this possibility at the level of macrocircuitry in the human fetal brain. Using a data-driven strategy for parcellating the brain into subnetworks, we test whether MRI functional connectivity within or between fetal neural subnetworks varies with maternal prenatal BMI in 109 fetuses between the ages of 26 and 39weeks.
Results:
We discovered that strength of connectivity between two subnetworks, left anterior insula/inferior frontal gyrus (aIN/ IFG) and bilateral prefrontal cortex (PFC), varied with maternal BMI. At the level of individual aIN/IFG-PFC connections, we observed both increased and decreased between-network connectivity with a tendency for increased within-hemisphere connectivity and reduced cross-hemisphere connectivity in higher BMI pregnancies. Maternal BMI was not associated with global differences in network topography based on network-based statistical analyses.
Conclusions:
Overall effects were localized in regions that will later support behavioral regulation and integrative processes, regions commonly associated with obesity-related deficits. By establishing onset in neural differences prior to birth, this study supports a model in which maternal BMI-related risk is associated with fetal connectome-level brain organization with implications for offspring long-term cognitive development and mental health.
Keywords: Functional connectivity, obesity, fMRI, prenatal, resting-state
Introduction
Obesity is on the rise in the United States and worldwide (Bentham et al., 2017) and is associated with numerous negative health outcomes. Among these, obesity is increasingly linked to mental health and cognitive problems, including depression (Milaneschi, Simmons, van Rossum, & Penninx, 2019), anxiety (Rajan & Menon, 2017), and neurodegenerative and neurodevelopmental disorders (Cortese et al., 2016; Whitmer et al., 2008; Zheng et al., 2017), as well as reduced executive functioning (Yang, Shields, Guo, & Liu, 2018), memory and learning problems (Gunstad, Lhotsky, Wendell, Ferrucci, & Zonderman, 2010), differences in reward response and motivation (Kenny, 2011; Volkow, Wang, & Baler, 2011), and mild cognitive impairment (Rochette et al., 2016).
Obesity-related risk can also be transferred from mother to child during the prenatal period. That is, children born to mothers with body mass index (BMI) greater than 30 show increased risk for many of the same problems seen in individuals who are obese (Contu & Hawkes, 2017; Edlow, 2017). Specifically, elevated prenatal BMI has been associated with differences in offspring cognitive performance at age 5 (Basatemur et al., 2013) and affective and social functioning at ages 5 and 6, respectively (Jo et al., 2015; Robinson et al., 2013; Rodriguez, 2010). High prenatal BMI has also been associated with developmental disorders, including attention-deficit/hyperactivity disorder (ADHD) and autism in early and middle childhood (Getz, Anderka, Werler, & Jick, 2016; Sanchez et al., 2018). It is not yet certain precisely when differences emerge, but early neurodevelopmental processes appear sensitive to heightened BMI during pregnancy. Given rising rates of obesity, especially in women of childbearing age (Fisher, Kim, Sharma, Rochat, & Morrow, 2013), and given new knowledge that high prenatal BMI may negatively influence child brain development, an important open question relates to the fetal brain targets of elevated maternal BMI.
Studies in animals with diet-induced obesity during pregnancy provide foundational evidence that maternal obesity affects offspring intrauterine brain development. These studies report differences in neuron proliferation, differentiation, and maturation (Chang, Gaysinskaya, Karatayev, & Leibowitz, 2008; Niculescu & Lupu, 2009; Stachowiak et al., 2012), as well as altered gene expression and DNA methylation patterns (Grissom et al., 2014). Data show that these differences persist in postnatal life (Glendining, Fisher, & Jasoni, 2018; Naef et al., 2011; Schmitz et al., 2018; Tozuka et al., 2010; Vucetic, Kimmel, Totoki, Hollenbeck, & Reyes, 2010) and extend to other domains, including functional and neurochemical processing (Coleman & Parkington, 2016; Sullivan et al., 2010). Brain areas most frequently implicated by prenatal obesity are important for reward processing, higher order cognitive functioning, and mental health, including the prefrontal cortex (Glendining, Fisher, & Jasoni, 2018; Grissom et al., 2014), nucleus accumbens (Naef et al., 2011; Vucetic et al., 2010), and hippocampus (Niculescu & Lupu, 2009; Tozuka et al., 2010). Many of these neurological findings have been linked to alterations in cognitive (e.g., spatial learning), reward, and social behavior, as well as increased anxious and ADHD-like traits (Menting et al., 2019; Sullivan, Nousen, & Chamlou, 2014). Taken together, these studies demonstrate that prenatal obesity influences the developing brain before birth, and they provide insight into the specific neurological pathways by which obesity-related transfer of risk may occur.
In contrast to what has been discovered in animal studies, our understanding of the impact of maternal BMI on human intrauterine brain development is limited. Elevated prenatal BMI is a risk factor for neural tube defects (Rasmussen, Chu, Kim, Schmid, & Lau, 2008) and congenital anomalies (Vasudevan, Renfrew, & McGuire, 2011), but normative prenatal neurodevelopmental processes sensitive to maternal BMI have yet to be examined.
Studies of human neonates and infants inform hypotheses about prenatal neural variation associated with elevated maternal BMI. A primary example comes from Salzwedel and colleagues (Salzwedel et al., 2019), who observed associations between BMI and functional connectivity (FC) in two-week-old neonates. They report positive associations between BMI and FC in regions critical for cognitive, sensory cue, and motor control processing, and mixed effects in reward processing regions. In a related line of work, Li et al. (2016) report decreased FC between dorsal anterior cingulate and prefrontal cortices when mothers had higher body fat percentage early in pregnancy. Salzwedel et al. (2019) also performed graph analysis of neonatal functional networks, observing alterations in global degree and efficiency in reward and cognitive control regions in neonates with high-BMI mothers. Additionally, studies have examined white matter (WM) changes related to maternal BMI, demonstrating that high BMI during pregnancy was related to decreased WM integrity in two-week-old neonates in multiple brain regions (Ou, Thakali, Shankar, Andres, & Badger, 2015), and that prenatal BMI-related WM differences can persist into adulthood (Verdejo-Román et al., 2018). These studies provide initial evidence that maternal prenatal BMI may influence intrauterine brain development in humans and implicate sensory, reward, and cognitive control systems as the potential bases of cognitive and behavioral functioning differences in children born to mothers with elevated BMI.
The goal of the present study is to assess associations between maternal BMI and human intrauterine brain development. Leveraging recent advances in resting-state functional MRI (rs-fMRI) methodology, we examine functional connectivity across large-scale networks in the human fetal brain. Recent fetal rs-fMRI studies confirm that there are individual differences in prenatal brain network development (Jakab et al., 2014; Wheelock et al., 2019), and that such differences relate to exposures (Thomason et al., 2019), to future preterm delivery (Thomason et al., 2017), and to future infant behavior (Thomason et al., 2018). We obtained maternal demographic, health, and fetal rs-fMRI data in 124 mothers and fetuses in order to evaluate BMI-brain associations and potential confounding variables. Based on areas of behavioral impairment associated with high maternal prenatal BMI, we hypothesized that maternal BMI would be associated with variation in fetal FC in the prefrontal cortex, insula, and striatum. We utilized a data-driven strategy for defining subnetworks of the fetal brain followed by enrichment and permutation to determine whether the quantity of nodal connectivity differences within and between subnetworks surpassed the number expected by chance.
Methods
Participants
A community sample of 124 pregnant women with singleton pregnancies (age 18–38 years) was recruited during routine obstetrical appointments. Exclusions for participation included presence of suspected fetal central nervous system abnormality as determined by 20-week ultrasound and/or contraindication for MRI (e.g., pacemaker, ferromagnetic material in mother’s body, claustrophobia). Fetuses ranged in age from 20 to 39 weeks gestational age (GA), with GA determined by physician ultrasound examination within one week of MRI scanning. Maternal BMI ranged from 18.6 to 47.8 at MRI scan. Pre-pregnancy BMI data in participants’ medical records were limited. Thus, pre-pregnancy BMI was estimated following prior approaches (cf. Dietz et al., 2006), wherein a constant of 1.25 kg was subtracted from maternal weight at the time of MRI then multiplied for weeks >12 by a weight gain rate of 0.4375 kg/week and divided by height (in meters) squared. All participants provided informed written consent, and all study procedures were approved by the Wayne State University Institutional Review Board. Fifteen fetal participants were excluded prior to group-level analyses due to (a) estimated low pre-pregnancy BMI (<18.5; n = 6), (b) low birthweight or preterm birth (n = 6), or (c) GA <24 weeks at scan (n = 3), leaving a total of 109 participants with fetal ages ranging from 26.4 to 39.6 weeks. fMRI data from 87 of these participants were recently published in a study of sex differences in prenatal brain development (Wheelock et al., 2019).
Measures
Maternal BMI was obtained at the time of MRI assessment and calculated in metric units: weight (kg)/ height (m)2. In order to account for weight gain over the course of gestation, BMI values used in study analyses were adjusted for GA at scan by computing residual values using the regression BMI ~ GA + error. BMI was treated as a continuous variable, given lack of standardized cut point for defining obesity during pregnancy. Additionally, we collected demographic information (age, race, level of education), physical and mental health measures (physical health habits, depression, anxiety), and birth outcomes (GA at birth, birthweight). Maternal physical health was assessed using an adapted version of the Health Practices Scale (HPS; Jackson, 2006) that measured five domains of health: diet, exercise, medical adherence, substance abuse, and sleep. Maternal mental health was assessed using the State-Trait Anxiety Inventory (STAI; Spielberger, 1983), a two-subscale measure of current symptoms and longstanding traits of anxiety (scores range from 40 to 160, delineating no, low, medium, and high anxiety), and the Center for Epidemiological Studies-Depression scale (CES-D; Radloff, 1977), a measure used to screen for depression in the general population in clinical and research settings (scores > 16 indicate clinically significant depression symptoms). We calculated Spearman correlations between maternal BMI and health measures and computed descriptive statistics to characterize the demographic make-up and birth outcomes in our sample. We conducted post hoc analyses to determine whether the average overall effect of maternal BMI on fetal FC would be related to maternal education.
Image acquisition
MRI scans were conducted on a Siemens Verio 70 cm open-bore 3T system using a lightweight (500 g) abdominal four-channel Siemens Flex Coil centered approximately at the fetal head. Resting-state fMRI data were acquired using a gradient echo planar imaging (EPI) sequence, TR/TE 2,000/30 ms, flip angle 80°, 360 frames, slice thickness 4 mm (axial), voxel size 3.4 ×3.4 × 4 mm3, 12 min. When possible, rs-fMRI scans were repeated to attain up to 24 min of functional resting-state data per participant. The average specific absorption rate (SAR), an estimated measure of radio-frequency energy, was 0.20 W/kg (SD = 0.07).
Functional MRI data preprocessing
Data were preprocessed following methods described previously (Thomason et al., 2014, 2017; Thomason et al., 2013). In brief, preprocessing included visual inspection of data to identify segments (time periods) of quiescence, or periods of low fetal movement. All participants included in group-level analyses had at least 100 timeframes and lower than 0.53mm average translational motion. 3D fetal brain masks were created using BrainSuite (Shattuck & Leahy, 2002) on a functional reference frame for each low-motion segment. Masks were binarized and applied to all frames in the segment. Segments were reoriented manually, and SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) was used to realign segments to the mean BOLD volume, resample to 2 mm3 isotropic voxels, and normalize to a 32-week fetal template (Serag et al., 2012). All realigned, normalized segments were concatenated into one run, realigned to the mean BOLD volume to correct for intrasegment error, and smoothed with a 4 mm fixed-width half-maximum (FWHM) Gaussian kernel. CONN functional connectivity toolbox (v14n; Whitfield-Gabrieli & Nieto-Castanon, 2012) was used to conduct linear detrending, nuisance regression of six head motion parameters and five principal components extracted from the 32-week fetal atlas white matter and CSF mask using aCompCor (Behzadi, Restom, Liau, & Liu, 2007), and band-pass filtering at 0.008–0.09 Hz.
Network analysis
Global network organization was examined to assess a potential relationship between maternal BMI and global topological organization of the reconstructed fetal brain networks (Marqués-Iturria et al., 2015). Total network strength (S) was computed as the (non-thresholded) total sum of the brain networks. Next, of the proportional thresholded network, the weighed global clustering coefficient (C) was computed to assess the level of local connectedness of the node of the network. Global efficiency (GE) was computed as a measure of how efficiently the network can exchange information given its layout of connections (Latora & Marchiori, 2001), here computed as the inverse of the harmonic mean of the communication path length in the network. S, C, and GE were computed for all fetal brain networks and normalized to S, C, and GE values of population of random networks (1,000 random networks). The relationship between-network organization and BMI was examined using correlation analysis.
Next, we computed a network-based statistic (NBS) (Verstraete et al., 2014; Zalesky, Fornito, & Bullmore, 2010) to asses a potential relationship between a specific subnetwork of network connections and BMI. This NBS analysis included the following steps. First, for each network connection the relationship with BMI was computed by means of correlation analysis. Connections showing an effect with a statistical p < .05 were kept, and across the network, the largest connected component of BMI-related connections was computed. To evaluate the statistical significance of this subnetwork, the same procedure was repeated for 1,000 random networks, in which BMI values were permuted and subnetwork size was similarly stored. The original effect was assigned an NBS subnetwork p-level as the number of random networks that exceeded the original size of the subnetwork of BMI-related connections.
Derivation of fetal brain networks
Fetal brain subnetwork distribution and extent were defined using a data-driven Infomap community detection algorithm (Rosvall & Bergstrom, 2008). In brief, 197 similarly sized, spatially contiguous regions of interest (ROIs) were clustered according to similar patterns of functional connectivity. That is, the average activation time series was computed for each ROI, and the Pearson correlation between the time courses in each ROI pair was computed for the 19,306 possible ROI-ROI pairs. The set of 197 × 197 ROI correlation matrices was thresholded at multiple thresholds (with degrees of sparseness ranging from 1% to 10%, in steps of 0.01%). The Infomap community detection algorithm (Rosvall & Bergstrom, 2008) was applied at each threshold, and the solutions for each threshold were combined using an algorithmic consensus procedure that produced a final, optimal model of fetal brain network structure consisting of 16 functional connectivity networks. The benefit of this widely used parcellation method is that the resulting networks are derived from and preserve the heterogeneity of spatiotemporal patterns in the brain.
Enrichment analysis
Following methods described by Eggebrecht et al. (2017), adapted from large-scale genome-wide association studies and similar to prior fMRI approaches (Sripada et al., 2014), enrichment analysis was used to identify significant differences in connectivity within and between fetal brain subnetworks related to maternal BMI residual values, described above. The enrichment approach has been used in recent fetal and infant resting-state studies (Eggebrecht et al., 2017; Marrus et al., 2018; McKinnon et al., 2019; Thomason et al., 2019; Wheelock et al., 2018, 2019). Enrichment uses Spearman rank correlation for each ROI pair then uses Chi-square test (χ2, df = 1) to determine whether the number of significant connections, thresholded at p < .05, within each network pair is greater than would be expected by chance. This approach identifies network pairs with significantly more BMI-related ROI-ROI connections than would be expected if the overall number of significant BMI-related connections was uniformly distributed across all within-and between-network comparisons. Empirical significance values were determined by random permutation of 10,000 pairings of FC matrices with randomly swapped BMI values (Eggebrecht et al., 2017). Thus, the family-wise error rate was controlled using permutation, and the reported permutation-based p-values represented the probability of observing the actual enrichment values in our data at the 5% false-positive level. A schematic of the group-level statistical approach is provided in Figure 1. Networks that were significantly enriched for BMI-FC associations were further examined at the level of ROI-ROI pairs to describe positive and negative BMI-FC correlations representing increased and decreased FC associated with higher BMI. All analyses and visualizations were carried out in MATLAB (Release 2016a, Mathworks).
Figure 1.
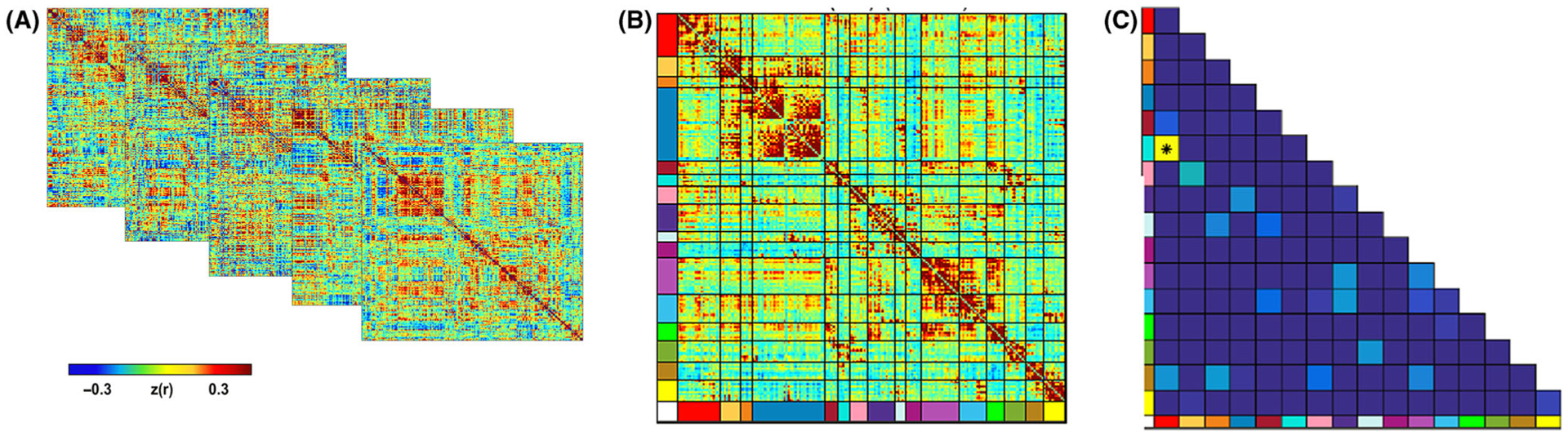
Overview of fetal fMRI statistical approach. Pearson correlation matrices (panel A) reflecting 197 ROIs for each participant were analyzed to create a subnetwork model of the fetal brain. The complete set of unique n = 19,306 ROI pair functional connectivity (Fisher-z) values from all participants were then averaged, producing a 197 × 197 connectivity matrix (panel B). In order to test the effects of maternal BMI at the network-pair level, enrichment analysis was then performed to identify individual ROI pairs with functional connectivity related to maternal prenatal BMI. Subsequently Chi-squared statistic was used to determine whether the number of significant ROI pairs (p < .05) within a network pair was greater than expected by chance (panel C). This approach was developed by Eggebrecht et al. (2017).
Results
Participant characteristics
The 109 fetuses included in this study were between 26.4 and 39.6 weeks GA at the time of MRI (mean = 33.5, SD = 3.7), born between 34.1 and 42.1 weeks (mean = 39.0, SD = 1.5), and weighed on average 3208.0 g (SD = 517.6) at birth. Average maternal age at MRI was 25.2 years (SD = 4.4). Mothers were 86% African American, 10% Caucasian, and 4% multiracial. Mothers whose highest level of education is high school diploma or GED constituted 39% of participants, mothers with some college were 36%, those with a 2-year, 4-year, or graduate degree were 6%, and mothers with no diploma or GED made up 17% of the sample. Participant characteristics are summarized in Table 1. BMI at the time of MRI scan (without adjusting for pregnancy) ranged from 22.5 to 47.8 (mean = 33.2, SD = 6.3; Figure 2) and was not significantly correlated with physical health (total composite score, five subscales) or mental health (STAI, CES-D); Spearman correlations ranged from −0.04 to 0.13 (p = .20 to .72, uncorrected). BMI was also unrelated to motion parameters; Spearman correlations 0.05 to 0.08 (p = .39–.60, uncorrected). Neither BMI nor BMI adjusted for pregnancy was correlated with fetal GA; Spearman correlations were −0.13 and −0.08 (p = .18 and .42), respectively (Table 1).
Table 1.
Data summary: Participant characteristics and BMI Spearman correlations
| Prenatal variables | Mean | SD |
|---|---|---|
| Fetal gestational age at MRI (weeks) | 33.5 | 3.7 |
| Maternal age at MRI (years) | 25.2 | 4.4 |
| Maternal BMI | 33.2 | 6.3 |
| Maternal physical health | ||
| Total (Composite) | 191.2 | 27.1 |
| Diet | 63.2 | 13.3 |
| Exercise | 28.8 | 7.1 |
| Medical adherence | 28.1 | 5.2 |
| Substance abuse | 49.2 | 5.8 |
| Sleep | 22.6 | 5.6 |
| Maternal mental health | ||
| STAI total | 35.8 | 8.3 |
| CES-D total | 13.5 | 8.6 |
| Maternal ethnicity, n (%) | ||
| African American | 92 | 8% |
| Caucasian | 9 | 84% |
| Multiracial | 4 | 4% |
| Not reported | 4 | 4% |
| Maternal education, n (%) | ||
| No GED/Diploma | 18 | 17% |
| GED/Diploma | 42 | 39% |
| Some college | 39 | 36% |
| 2-year degree | 1 | 1% |
| 4-year degree | 3 | 3% |
| Graduate degree | 2 | 2% |
| Not reported | 4 | 4% |
| fMRI data characteristics | ||
| # fMRI frames analyzed | 158.2 | 42.9 |
| Translational movement (mm) | 0.2 | 0.1 |
| Rotational movement (degrees) | 0.4 | 0.2 |
| Birth outcomes | ||
| Fetal gestational age at birth (weeks) | 39.0 | 1.5 |
| Birth weight (g) | 3208.0 | 517.6 |
| Correlations with BMI (Spearman) | r | pa |
| Fetal gestational age at MRI (weeks) | −.13 | .18 |
| Health total (Composite) | .10 | .30 |
| Diet | .13 | .20 |
| Exercise | .04 | .68 |
| Medical adherence | .10 | .32 |
| Substance abuse | .09 | .38 |
| Sleep | .07 | .48 |
| STAI total | .08 | .43 |
| CES-D total | −.04 | .72 |
| Translational movement (mm) | .05 | .60 |
| Rotational movement (degrees) | .08 | .39 |
| Correlations with BMI adjusted for pregnancy (Spearman) | ||
| Fetal gestational age at MRI (weeks) | −.08 | .42 |
Summary of maternal and fetal participant characteristics, correlations between BMI and potential confound variables. Maternal physical and mental health were measured using self-report rating scales. The adapted Health Practices Scale consists of 53 items on which participants rank from 1 = never to 6 = always the frequency of engaging in health behaviors (e.g., Have blood pressure checked regularly). The State-Trait Anxiety Inventory (STAI) consists of two 20-item subscales measuring current and longstanding anxiety. Per subscale, scores indicate ‘no or low anxiety’ (20–37), ‘moderate anxiety’ (37–44), and ‘high anxiety’ (45–80). The Center for Epidemiological Studies-Depression scale (CES-D) consists of 20 items on which participants rank their experience of depression symptoms during the past week. Scores above 16 indicate clinically significant depression symptoms.
Uncorrected.
Figure 2.
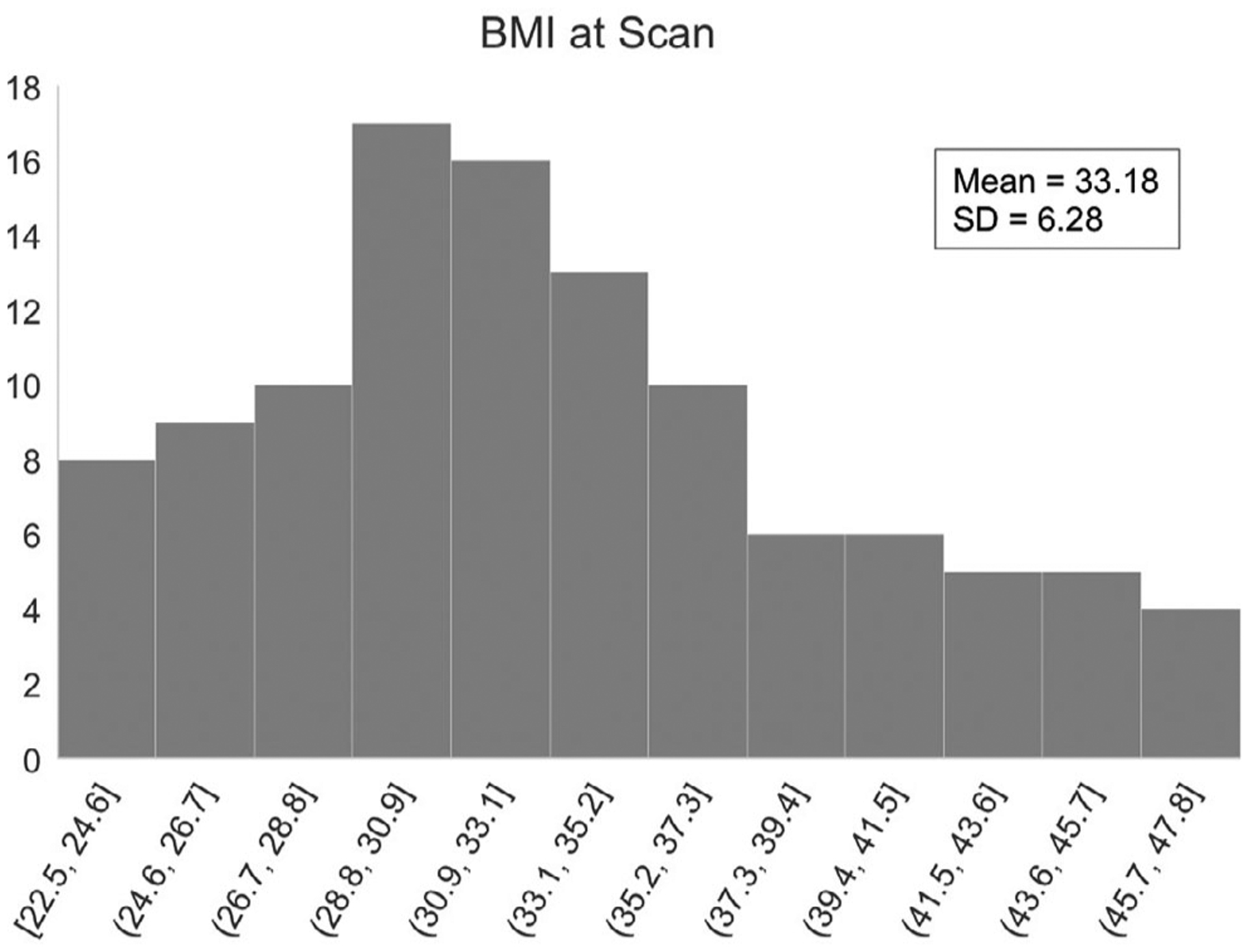
Distribution of maternal BMI at the time of MRI assessment. Participants (N = 109) varied in length of pregnancy from 26 to 39 weeks.
Network analysis
No specific effects were found for the association between BMI and global network organization. S, C, and GE as well as their normalized counterparts did not reveal a significant association with maternal BMI (all p > .05). NBS analysis also did not reveal one specific subcomponent of maternal BMI-related connections (p > .05). These findings suggest that global fetal functional neural systems are organized as expected, with BMI effects not specifically tuned to a single aspect of topographical brain network organization.
Variation in fetal functional connectivity linked to maternal BMI
Community detection analysis generated a 16 functional network consensus model (Figure 3) that became the basis for significant relationships between maternal prenatal BMI and fetal brain subnetwork connectivity.
Figure 3.
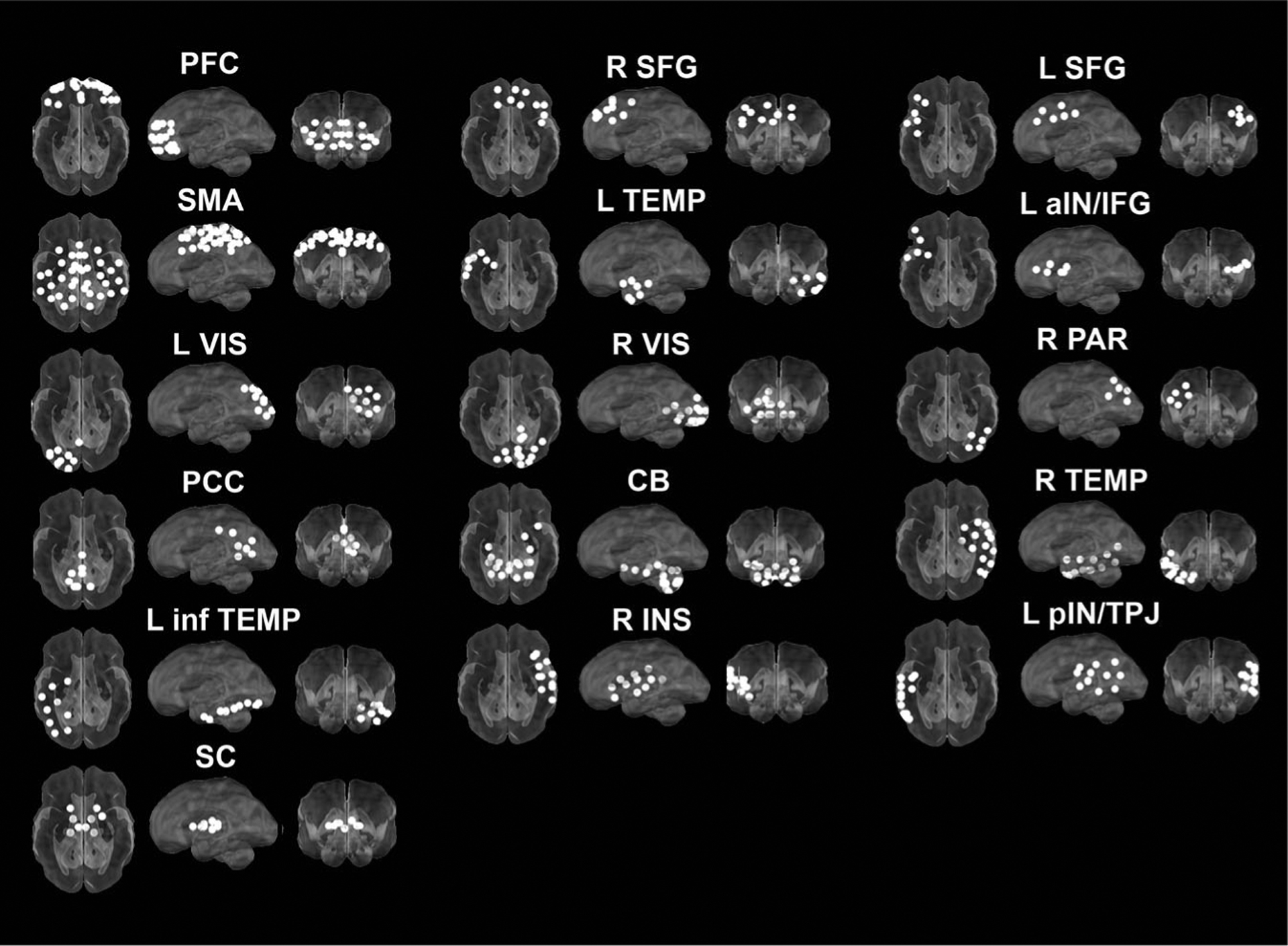
Automated consensus procedure for identifying fetal brain subnetworks. Infomap community detection algorithm was used to assign ROIs to neural subnetworks based on maximization of within-module random walks applied to adjacency matrices at each threshold. Solutions for each threshold were combined using an automated consensus procedure to provide a single model of the community structure by maximizing the normalized mutual information of groups of neighboring solutions and then maximizing modularity. This network solution resulted in an optimal solution of 16 fetal brain subnetworks encompassing fetal cortex, subcortical structures, and the cerebellum
We observed that maternal BMI was associated with variation in strength of functional connectivity between a bilateral prefrontal cortical network (PFC) and a network encompassing the left anterior insula and inferior frontal gyrus (aIN/IFG) in the fetal brain (Figure 4). Multiple individual connections between pairs of ROIs gave rise to this network-level effect, and in these ROI-ROI pairs, the directionality of the association was mixed. That is, both positive and negative associations gave rise to the observed significant cross-network effect of maternal BMI, with the following proportions: 47% and 53%, respectively (Figure 4B). We further observed the spatial organization of the significant connections, noting that positive BMI-FC associations occurred predominantly unilaterally in the left hemisphere (of nine within-hemisphere connections, 67% were positive), whereas negative BMI-FC associations tended to cross the midline (of the six cross-hemisphere connections, 83% were negative) (Figure 4). The latter finding suggests that maternal BMI-related FC effects are associated with increased within-hemispheric connectivity and decreased cross-hemispheric connectivity in prefrontal and left insular cortical regions.
Figure 4.
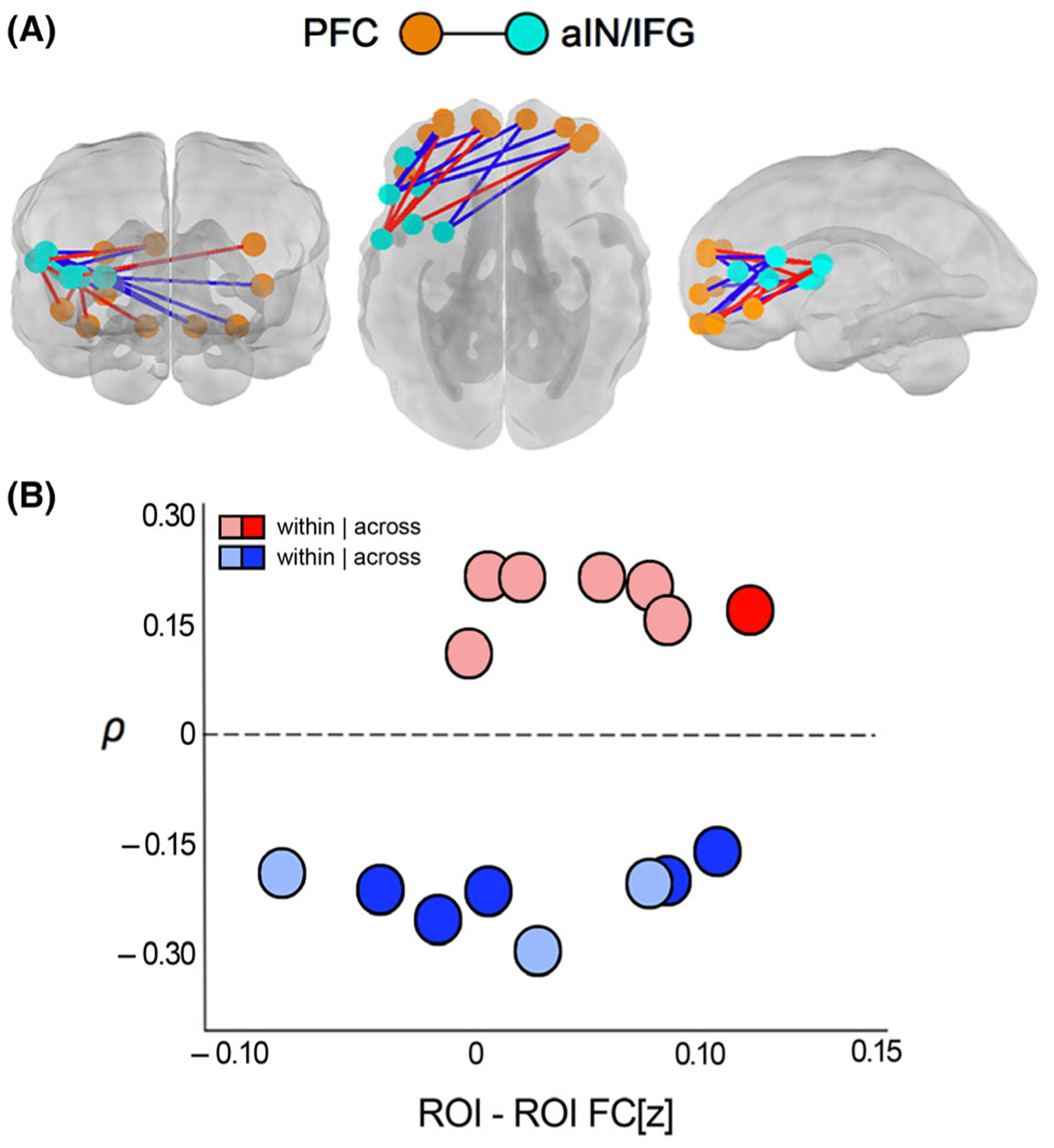
Maternal BMI was associated with variation in fetal brain functional connectivity (FC) across subnetworks that encompass bilateral anterior prefrontal cortical regions (orange spheres) and left anterior insula/inferior frontal gyrus (teal spheres; panel A). Both positive (red lines) and negative (blue lines) BMI-FC correlations were observed, indicating that increasing BMI related to both increases and decreases in FC (panel A). Strength of significant BMI-FC correlations (FC[z]) ranged from −0.80 to 1.06 (panel B). Increased FC associated with increasing BMI tended to connect regions unilaterally within the left hemisphere, whereas decreased FC was more often observed in cross-hemispheric connections (panel B)
Post hoc analyses assessing the relation between the average overall BMI-FC effect (including both positive and negative associations) and maternal level of education (as a proxy for socioeconomic status) did not reveal a significant association (p = .22). Further, we did not observe significant interaction between maternal BMI and education level in relation to fetal FC effects (p > .05).
Discussion
This study demonstrates an association between maternal BMI and systems-level organization of the developing fetal brain in humans. Using a whole-brain, data-driven approach, we discovered maternal BMI-related variation in fetal frontal (prefrontal, IFG) and insular brain regions. These findings support hypotheses that (a) variation in maternal prenatal BMI relates to development of neural systems in utero in humans, and that (b) fetal brain regions that show BMI-related connectivity differences are similar to those that will later support processes that are frequently impaired in individuals with high BMI and those who were exposed to high maternal BMI during prenatal development.
The observed association between fetal prefrontal and insular connectivity and maternal BMI is perhaps not surprising, as these regions have long been of interest in research into behavioral health and obesogenic cognitive effects. The prefrontal cortex plays a central role in cognitive control, including control of eating behavior (Han, Boachie, Garcia-Garcia, Michaud, & Dagher, 2018), and the insula plays a central role in processing food-and appetite-related information (Frank, Kullmann, & Veit, 2013; Rolls, 2006). Alterations in prefrontal structure and function are robustly documented in humans with high BMI (Devoto et al., 2018; García-García et al., 2019; Reinert et al., 2013). Moreover, altered prefrontal brain development is a key finding in studies of prenatal obesity exposure in animals (Rivera et al., 2015; Vucetic et al., 2010) and high BMI exposure in human neonates (Li et al., 2016; Salzwedel et al., 2019). The effects we observed in the insular cortices were predominately in the anterior portion, which is regarded as primary gustatory cortex (Rolls, 2006), and is well-known for integrating cognitive, affective, sensory, and reward-related information (Gogolla, 2017).The anterior insula has been implicated in studies of obesity-related differences in sensory and reward processing (Brooks, Cedernaes, & Schöth, 2013; Devoto et al., 2018). Given consistent evidence that prefrontal and insular circuits are key neural targets of obesogenic processes, it is notable that in this study, we have discovered that differences in these circuits may already be present prior to birth.
We observed significant enrichment effects along with null NBS results. This difference may be attributed to the level of analyses in these complimentary approaches. Having observed significance for enrichment suggests that local, focal effects of BMI are not accompanied by differences in global organization of the full brain network. This has been seen in prior studies by our group and others (van den Heuvel, Mandl, Stam, Kahn, & Hulshoff Pol, 2010). Findings such as this have led some to suggest there may be compensatory processes in other parts of the brain that may in part contribute to lack of effects at the level of global topographic organization (de Lange et al., 2019).
There are a number of mechanisms actively being studied in animal models to explore how maternal diet and obesity may influence the developing CNS of the fetus. The present study identifies regions that seem to be relevant for later behavioral development, and animal research provides crucial insight into the pathways by which maternal obesity may influence fetal neural development. Proposed mechanisms include intrauterine exposures to excess nutrients, metabolic hormones, and inflammatory cytokines, which impact neuroendocrine, brain functional and structural, and microbiome development in the fetus (Menting et al., 2019; Sullivan et al., 2014). For example, recent work by Sanguinetti et al. (2019) showed that maternal high-fat diet in a murine model led to changes in the offspring microbiome before weaning, and those changes were later associated with cognitive impairment in the adult animal. Epigenetic mechanisms have also been considered as possible bases of prenatal obesity-related transfer of risk. In particular, obesity-exposed animals have shown epigenetic and neurochemical alterations in prefrontal and medial temporal brain regions (Glendining et al., 2018; Grissom et al., 2014; Vucetic et al., 2010). For example, a study by Vucetic and colleagues showed that obesity-related epigenetic changes (DNA hypomethylation) in offspring led to alterations in gene expression resulting in up-regulation (increases) of the dopamine reuptake transporter and μ-opioid receptor in the prefrontal cortex, both of which are neurochemical systems critically involved in regulating the intake of palatable foods (Grissom et al., 2014; Vucetic et al., 2010). Another study by Glendining and colleagues revealed altered epigenetic markers in several areas, including the prefrontal cortex in which they observed downregulation of a gene (GADD45B) associated with cell growth, synaptic plasticity, and response to environmental stresses (Glendining et al., 2018). Importantly, these studies in animals have linked maternal obesity-related epigenetic and neurochemical differences to behavioral variation in offspring, including preference for sugar and fat (Vucetic et al., 2010) and increased impulsivity (Grissom et al., 2014). Overall, studies in animals provide a basis for future consideration of the mechanistic pathways that might underpin the association we have observed in humans between maternal prenatal BMI and prefrontal/insular brain development in utero.
Maternal BMI-related risk was associated with both positive and negative differences in fetal brain functional connectivity. Notably, positive BMI-FC associations were predominately within-hemisphere connections, whereas negative BMI-FC associations were predominately cross-hemisphere connections (Figure 4). This is preliminary evidence that matenal BMI-related FC effects are associated with increased within-hemisphere connectivity and decreased cross-hemisphere connectivity in prefrontal and left insular cortical regions. Prior research has shown that cross-hemisphere and longer-range connectivity in the fetal brain increases across the gestational stages tested in the present study (second/third trimester) (Jakab et al., 2014; Thomason et al., 2013). Thus, having observed less robust cross-hemisphere connectivity in fetuses of mothers with higher BMI may reflect less mature functional organization. Future research will be needed to test this possibility in a longitudinal framework and to link this potential mechanism to future neurobehavioral health and development.
The left laterality of our insula/IFG connectivity effects is congruent with precedent in the adult literature (Brooks et al., 2013; Devoto et al., 2018; Wijngaarden et al., 2015). For example, Devoto and colleagues showed that the left insula responds to food-related visual stimuli and is differentially engaged during hunger states in individuals with high compared to healthy BMI (Devoto et al., 2018; Wijngaarden et al., 2015). In healthy weight adults, Jakab and colleagues found that structural connections of the left anterior insula are more extensive than the right, particularly with prefrontal and frontal brain regions (Jakab, Molnár, Bogner, Béres, & Berényi, 2012). The authors note that this asymmetry corresponds to putative biomarkers of overeating behavior in adults with obese BMI. Laterality has also been reported in brain development prior to birth. Asymmetries in fetal brain morpho-metric, microstructural, and functional development are well documented (Clouchoux & Limperopoulos, 2012; Galaburda, LeMay, Kemper, & Geschwind, 1978; Toga & Thompson, 2003). Thus, data from this study of prenatal BMI-related risk raise questions as to the origin of asymmetry differences and whether these could arise from interactions between intrauterine developmental processes and heightened maternal BMI.
In this study, the measure of BMI was derived from assessment at the time of the MRI, correcting for duration of the pregnancy. A limitation of this approach is that measurement of BMI at different stages of pregnancy has potential to yield different effects (Luzzo et al., 2012). In addition, BMI is a relatively coarse measure and has shown variable accuracy across sex and ethnicity (Gallagher et al., 1996; Sumner, Ricks, Sen, & Frempong, 2007). We also expect that maternal body composition, weight and weight gain, and nutritional resources all have potential to impact the developing fetal brain in unique ways. Useful future directions will be to examine weight change over the course of pregnancy, to perform more in-depth assessment of maternal diet, and to examine maternal body composition using techniques such as anthropometry, densitometry, hydrometry, and MRI-based methods, such as Dixon and liver fat content MR imaging (Dixon, 1984; Most, Marlatt, Altazan, & Redman, 2018). With these data, it will be possible to begin to map specific physiological or nutritive pathways associated with early human brain development.
There are additional limitations of the present study that warrant consideration. The cross-sectional nature of our study does not permit us to assess how the observed effects map onto postnatal brain and behavioral development. Future longitudinal research will have potential to elucidate developmental processes sensitive to maternal prenatal diet and body composition and identify associated differences in brain and behavioral outcomes. Another methodological challenge is motion, particularly in fetal data. Although our motion threshold is consistent with the fetal literature (e.g., Jakab et al., 2014; Schöpf, Kasprian, Brugger, & Prayer, 2012; Thomason et al., 2013), it is liberal relative to that used in non-fetal resting-state studies. This is an ongoing challenge for the relatively nascent field of fetal imaging (Di Martino et al., 2014). Lastly, environmental factors such as maternal socioeconomic status, food insecurity, or health behavior have potential to interact with maternal body composition and/or diet to influence fetal brain development. Here, we did not observe an association between fetal FC and maternal level of education; however, this does not eliminate possibility that alternative factors may have contributed to effects observed here. A major goal for future studies will be to examine these associations at a much larger scale to begin to disentangle complex relationships between prenatal contextual programming factors and specific alterations in human fetal neural circuitry.
Important questions also remain regarding the contribution of genetic and postnatal environmental factors to the link between prenatal maternal BMI and child developmental outcomes. Overall, existing data indicate that genetic and environmental factors play a prominent role but do not fully account for variation observed in developmental outcomes. Indeed, population studies indicate that developmental differences related to maternal prenatal diet and body weight persist even when controlling for genetic and postnatal environmental factors (Lumey, Stein, & Susser, 2011). For example, the offspring of women that conceived and carried during the Dutch Hunger Winter, a famine that affected the western Netherlands during World War II, later demonstrated higher rates of depression, schizophrenia spectrum disorders, and antisocial behavior (Hoek, Brown, & Susser, 1998; Neugebauer, Hoek, & Susser, 1999; Stein, Pierik, Verrips, Susser, & Lumey, 2009), greater age-related decline in cognitive ability (de Rooij, Wouters, Yonker, Painter, & Roseboom, 2010), and differences in brain morphometry (Hulshoff Pol et al., 2000). This work confirms that atypical maternal body weight and nutrition before birth can increase risk for offspring mental health and cognitive problems over and above genetic and environmental factors. Additionally, it is clear that both low and high maternal health extremes have potential to influence fetal neurodevelopment, and investigation of differential neural effects, mechanisms, or child outcomes remains an important area for ongoing and future research.
In conclusion, the present study provides evidence that maternal prenatal BMI is associated with variation in functional connectivity in large-scale networks in the fetal brain, specifically in frontal and insular neural circuitry. Given the role of these brain regions in cognitive control and food regulation behavior, as well as in integration of neural connectivity, the present data support a model of risk transfer wherein maternal prenatal BMI relates to fetal brain development with implications for both future body health and neurobehavioral outcomes. Replication and longitudinal research are needed to confirm our findings, to elucidate the significance of lateralized and hemispheric organizational effects, and to determine the ways in which BMI-related changes in fetal brain development relate to later neurodevelopmental and behavioral outcomes. Armed with this information, it may be possible to develop interventions that address prenatal maternal physical health to improve offspring health and cognitive outcomes.
Key points and relevance.
What’s known:
Obesity is an increasingly prevalent problem.
Elevated maternal BMI during pregnancy is linked to cognitive and mental health problems in children. Animal studies indicate that differences may originate during prenatal brain development.
What’s new:
Recent advances in neuroimaging methodology allow for non-invasive examination of human fetal brain development in utero.
What’s relevant:
Our results provide initial evidence that the brain regions that underlie behavioral impairments seen in children exposed to high prenatal BMI show differences in functional connectivity before birth.
Obesity in women of childbearing age may be an important target for intervention to promote long-term child health and well-being.
Acknowledgements
This project was supported by awards to M.E.T. from the National Institutes of Health, R01 MH110793, R34 DA050287, R01 MH122447, R21 ES026022, and P30 ES020957, and by a NARSAD Young Investigator Award. The authors thank Pavan Jella, Toni Lewis, Sydney Rooks, Sophia Neuenfeldt, and Janessa Manning for their assistance in data acquisition. The authors also thank participant families who generously shared their time.
M.E.T. designed the studies, provided experimental and data analysis oversight and interpretation, and authored the manuscript. M.E.N. contributed to the experimental design, data analysis and interpretation, and authored the manuscript. J.L.H. contributed to data acquisition, data analysis and interpretation, and provided computational support. C.J.L. and M.vdH. contributed to data analysis and authoring the manuscript. The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- Basatemur E, Gardiner J, Williams C, Melhuish E, Barnes J, & Sutcliffe A (2013). Maternal prepregnancy BMI and child cognition: A longitudinal cohort study. Pediatrics, 131, 56–63. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentham J, Di Cesare M, Bilano V, Bixby H, Zhou B, Stevens GA, … & Paciore CJ (2017). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. The Lancet, 390, 2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, Cedernaes J, & Schiöth HB (2013). Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: A meta-analysis of fMRI studies. PLoS One, 8, e60393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, & Leibowitz SF (2008). Maternal high-fat diet and fetal programming: Increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. Journal of Neuroscience, 28, 12107–12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouchoux C, & Limperopoulos C (2012). Novel applications of quantitative MRI for the fetal brain. Pediatric Radiology, 42, 24–32. [DOI] [PubMed] [Google Scholar]
- Coleman HA, & Parkington HC (2016). Maternal obesity in pregnancy: Consequences for brain function in the offspring. Neuromethods, 109, 203–219. [Google Scholar]
- Contu L, & Hawkes CA (2017). A review of the impact of maternal obesity on the cognitive function and mental health of the offspring. International Journal of Molecular Sciences, 18, 1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, Moreira-Maia CR, St Fleur D, Morcillo-Peñalver C, Rohde LA, & Faraone SV (2016). Association between ADHD and obesity: A systematic review and metaanalysis. American Journal of Psychiatry, 173, 34–43. [DOI] [PubMed] [Google Scholar]
- de Lange SC, Scholtens LH, van den Berg LH, Boks MP, Bozzali M, Cahn W, … & van den Heuvel MP (2019). Shared vulnerability for connectome alterations across psychiatric and neurological brain disorders. Nature Human Behaviour, 3, 988–998. [DOI] [PubMed] [Google Scholar]
- de Rooij SR, Wouters H, Yonker JE, Painter RC, & Roseboom TJ (2010). Prenatal undernutrition and cognitive function in late adulthood. Proceedings of the National Academy of Sciences USA, 107, 16881–16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto F, Zapparoli L, Bonandrini R, Berlingeri M, Ferrulli A, Luzi L, … & Paulesu E. (2018). Hungry brains: A meta-analytical review of brain activation imaging studies on food perception and appetite in obese individuals. Neuroscience and Biobehavioral Reviews, 94, 271–285. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Fair DA, Kelly C, Satterthwaite TD, Castellanos FX, Thomason ME, … & Milham MP(2014) Unraveling the miswired connectome: A developmental perspective. Neuron, 83, 1335–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz PM, Callaghan WM, Cogswell ME, Morrow B, Ferre C, & Schieve LA (2006). Combined effects of prepregnancy body mass index and weight gain during pregnancy on the risk of preterm delivery. Epidemiology, 17, 170–177. [DOI] [PubMed] [Google Scholar]
- Dixon WT (1984). Simple proton spectroscopic imaging. Radiology, 153, 189–94. [DOI] [PubMed] [Google Scholar]
- Edlow AG (2017). Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenatal Diagnosis, 37, 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggebrecht AT, Elison JT, Feczko E, Todorov A, Wolff JJ, Kandala S, … & Pruett JR (2017). Joint attention and brain functional connectivity in infants and toddlers. Cerebral Cortex, 27, 1709–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SC, Kim SY, Sharma AJ, Rochat R, & Morrow B(2013). Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Preventive Medicine, 56, 372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Kullmann S, & Veit R (2013). Food related processes in the insular cortex. Frontiers in Human Neuroscience, 7, 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda A, LeMay M, Kemper T, & Geschwind N (1978). Right-left asymmetrics in the brain. Science, 199, 852–856. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, & Heymsfield SB (1996). How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? American Journal of Epidemiology, 143, 228–239. [DOI] [PubMed] [Google Scholar]
- García-García I, Michaud A, Dadar M, Zeighami Y, Neseliler S, Collins DL, … & Dagher A (2019). Neuroanatomical differences in obesity: Meta-analytic findings and their validation in an independent dataset. International Journal of Obesity, 43, 943–951. [DOI] [PubMed] [Google Scholar]
- Getz KD, Anderka MT, Werler MM, & Jick SS (2016). Maternal pre-pregnancy body mass index and autism spectrum disorder among offspring: A population-based case-control study. Paediatric and Perinatal Epidemiology, 30, 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendining KA, Fisher LC, & Jasoni CL (2018). Maternal high fat diet alters offspring epigenetic regulators, amygdala glutamatergic profile and anxiety. Psychoneuroendocrinology, 96, 132–141. [DOI] [PubMed] [Google Scholar]
- Gogolla N (2017). The insular cortex. Current Biology, 27, R580–R586. [DOI] [PubMed] [Google Scholar]
- Grissom NM, Lyde R, Christ L, Sasson IE, Carlin J, Vitins AP, … & Reyes TM (2014). Obesity at conception programs the opioid system in the offspring brain. Neuropsychopharmacology, 39, 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad J, Lhotsky A, Wendell CR, Ferrucci L, & Zonderman AB (2010). Longitudinal examination of obesity and cognitive function: Results from the baltimore longitudinal study ofaging. Neuroepidemiology, 34, 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JE, Boachie N, Garcia-Garcia I, Michaud A, & Dagher A (2018). Neural correlates of dietary self-control in healthy adults: A meta-analysis of functional brain imaging studies. Physiology and Behavior, 192, 98–108. [DOI] [PubMed] [Google Scholar]
- Hoek HW, Brown AS, & Susser E (1998). The Dutch Famine and schizophrenia spectrum disorders. Social Psychiatry and Psychiatric Epidemiology, 33, 373–379. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Hoek HW, Susser E, Brown AS, Dinge-mans A, Schnack HG, … & Kahn RS (2000). Prenatal exposure to famine and brain morphology in schizophrenia. American Journal of Psychiatry, 157, 1170–1172. [DOI] [PubMed] [Google Scholar]
- Jackson T (2006). Relationships between perceived close social support and health practices within community samples of american women and men. The Journal of Psychology, 140, 229–246. [DOI] [PubMed] [Google Scholar]
- Jakab A, Molñar PP, Bogner P, Béres M, & Berényi EL (2012). Connectivity-based parcellation reveals interhemispheric differences in the insula. Brain Topography, 25(3), 264–271. [DOI] [PubMed] [Google Scholar]
- Jakab A, Schwartz E, Kasprian G, Gruber GM, Prayer D, Schöpf V, & Langs G (2014). Fetal functional imaging portrays heterogeneous development of emerging human brain networks. Frontiers in Human Neuroscience, 8, 852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo H, Schieve LA, Sharma AJ, Hinkle SN, Li R, & Lind JN (2015). Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics, 135, e1198–e1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ (2011). Reward mechanisms in obesity: New insights and future directions. Neuron, 69, 664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latora V, & Marchiori M (2001). Efficient behavior of small-world networks. Physical Review Letters, 87, 198701. [DOI] [PubMed] [Google Scholar]
- Li X, Andres A, Shankar K, Pivik RT, Glasier CM, Ramakrishnaiah RH, … & Ou X. (2016). Differences in brain functional connectivity at resting state in neonates born to healthy obese or normal-weight mothers. International Journal of Obesity, 40, 1931–1934. [DOI] [PubMed] [Google Scholar]
- Lumey LH, Stein AD, & Susser E (2011). Prenatal famine and adult health. Annual Review of Public Health, 32, 237–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, … & Moley KH (2012). High fat diet induced developmental defects in the mouse: Oocyte meiotic aneu-ploidy and fetal growth retardation/brain defects. PLoS One, 7, e49217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqués-Iturria I, Scholtens LH, Garolera M, Pueyo R, García-García I, González-Tartiere P, … & van den Heuvel MP (2015). Affected connectivity organization of the reward system structure in obesity. NeuroImage, 111, 100–106. [DOI] [PubMed] [Google Scholar]
- Marrus N, Eggebrecht AT, Todorov A, Elison JT, Wolff JJ, Cole L, … & Pruett JR (2018). Walking, gross motor development, and brain functional connectivity in infants and toddlers. Cerebral Cortex, 28, 750–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon CJ, Eggebrecht AT, Todorov A, Wolff JJ, Elison JT, Adams CM, … & McKinstry RC (2019). Restricted and repetitive behavior and brain functional connectivity in infants at risk for developing autism spectrum disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menting MD, van de Beek C, Mintjens S, Wever KE, Korosi A, Ozanne SE, … & Painter RC (2019). The link between maternal obesity and offspring neurobehavior: A systematic review of animal experiments. Neuroscience and Biobehavioral Reviews, 98, 107–121. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Simmons WK, vanRossum EFC, &Penninx BW (2019). Depression and obesity: evidence of shared biological mechanisms. Molecular Psychiatry, 24, 18–33. [DOI] [PubMed] [Google Scholar]
- Most J, Marlatt KL, Altazan AD, & Redman LM (2018). Advances in assessing body composition during pregnancy. European Journal of Clinical Nutrition, 72, 645–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naef L, Moquin L, Dal Bo G, Giros B, Gratton A, & Walker CD (2011). Maternal high-fat intake alters presy-naptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience, 176, 225–236. [DOI] [PubMed] [Google Scholar]
- Neugebauer R, Hoek HW, & Susser E (1999). Prenatal exposure to wartime famine and development of antisocial personality disorder in early adulthood. JAMA, 282, 455. [DOI] [PubMed] [Google Scholar]
- Niculescu MD, & Lupu DS (2009). High fat diet-induced maternal obesity alters fetal hippocampal development. International Journal of Developmental Neuroscience, 27, 627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X, Thakali KM, Shankar K, Andres A, & Badger TM(2015). Maternal adiposity negatively influences infant brain white matter development. Obesity, 23, 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale. Applied Psychological Measurement, 1, 385–401. [Google Scholar]
- Rajan T, & Menon V (2017). Psychiatric disorders and obesity: A review of association studies. Journal of Postgraduate Medicine, 63, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SA, Chu SY, Kim SY, Schmid CH, & Lau J (2008). Maternal obesity and risk of neural tube defects: A metaanalysis. American Journal of Obstetrics and Gynecology, 198, 611–619. [DOI] [PubMed] [Google Scholar]
- Reinert KRS, Po’e EK, & Barkin SL (2013). The relationship between executive function and obesity in children and adolescents: A systematic literature review. Journal of Obesity, 2013, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera HM, Kievit P, Kirigiti MA, Bauman LA, Baquero K, Blundell P, … & Sullivan EL (2015). Maternal high-fat diet and obesity impact palatable food intake and dopamine signaling in nonhuman primate offspring. Obesity, 23, 2157–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M, Zubrick SR, Pennell CE, Van Lieshout RJ, Jacoby P, Beilin LJ, … & Oddy WH (2013). Prepregnancy maternal overweight and obesity increase the risk for affective disorders in offspring. Journal of Developmental Origins of Health and Disease, 4, 42–48. [DOI] [PubMed] [Google Scholar]
- Rochette AD, Spitznagel MB, Strain G, Devlin M, Crosby RD, Mitchell JE, … & Gunstad J (2016). Mild cognitive impairment is prevalent in persons with severe obesity. Obesity, 24, 1427–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A (2010). Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 51, 134–143. [DOI] [PubMed] [Google Scholar]
- Rolls ET (2006). Brain mechanisms underlying flavour and appetite. Philosophical Transactions of the Royal Society B: Biological Sciences, 361, 1123–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall M, & Bergstrom CT (2008). Maps of random walks on complex networks reveal community structure. Proceedings of the National Academy of Sciences of the United States of America, 105, 1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzwedel AP, Gao W, Andres A, Badger TM, Glasier CM, Ramakrishnaiah RH, … & Ou X. (2019). Maternal adiposity influences neonatal brain functional connectivity. Frontiers in Human Neuroscience, 12, 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez CE, Barry C, Sabhlok A, Russell K, Majors A, Kollins SH, & Fuemmeler BF (2018). Maternal prepregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obesity Reviews, 19, 464–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti E, Guzzardi MA, Tripodi M, Panetta D, Selma-Royo M, Zega A, … & Iozzo P. (2019). Microbiota signatures relating to reduced memory and exploratory behaviour in the offspring of overweight mothers in a murine model. Scientific Reports, 9, 12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz L, Kuglin R, Bae-Gartz I, Janoschek R, Appel S, Mesaros A, … & Hucklenbruch-Rother E. (2018). Hippocampal insulin resistance links maternal obesity with impaired neuronal plasticity in adult offspring. Psychoneuroendocrinology, 89, 46–52. [DOI] [PubMed] [Google Scholar]
- Schöpf V, Kasprian G, Brugger PC, & Prayer D (2012). Watching the fetal brain at “rest”. International Journal of Developmental Neuroscience, 30, 11–17. [DOI] [PubMed] [Google Scholar]
- Serag A, Aljabar P, Ball G, Counsell SJ, Boardman JP, Rutherford MA, … & Rueckert D (2012). Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. NeuroImage, 59, 2255–2265. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, & Leahy RM (2002). Brainsuite: An automated cortical surface identification tool. Medical Image Analysis, 6, 129–142. [DOI] [PubMed] [Google Scholar]
- Spielberger CD (1983). Manual for the state-trait anxiety inventory STAI (Form Y), Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Sripada C, Kessler D, Fang Y, Welsh RC, Prem Kumar K, & Angstadt M (2014). Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Human Brain Mapping, 35, 4693–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak EK, Oommen S, Vasu VT, Srinivasan M, Stachowiak M, Gohil K, & Patel MS (2012). Maternal obesity affects gene expression and cellular development in fetal brains. Nutritional Neuroscience, 16, 96–103. [DOI] [PubMed] [Google Scholar]
- Stein AD, Pierik FH, Verrips GHW, Susser ES, & Lumey LH (2009). Maternal exposure to the Dutch famine before conception and during pregnancy: Quality of life and depressive symptoms in adult offspring. Epidemiology, 20, 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, … & Grove KL (2010). Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. The Journal of Neuroscience, 30, 3826–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Nousen EK, & Chamlou KA (2014). Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiology and Behavior, 123, 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner AE, Ricks M, Sen S, & Frempong BA (2007). How current guidelines for obesity underestimate risk in certain ethnicities and overestimate risk in others. Current Cardiovascular Risk Reports, 1, 97–101. [Google Scholar]
- Thomason ME, Brown JA, Dassanayake MT, Shastri R, Marusak HA, Hernandez-Andrade E, … & Romero R.(2014). Intrinsic functional brain architecture derived from graph theoretical analysis in the human fetus. PLoS One, 9, e94423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Dassanayake MT, Shen S, Katkuri Y, Alexis M, Anderson AL, … & Romero R. (2013). Cross-hemispheric functional connectivity in the human fetal brain. Science translational medicine, 5, 173ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Hect JL, Rauh VA, Trentacosta C, Wheelock MD, Eggebrecht AT, … & Burt SA (2019). Prenatal lead exposure impacts cross-hemispheric and long-range connectivity in the human fetal brain. NeuroImage, 191, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Hect J, Waller R, Manning JH, Stacks AM, Beeghly M, … & Romero R (2018). Prenatal neural origins of infant motor development: Associations between fetal brain and infant motor development. Development and Psychopathology, 30, 763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Scheinost D, Manning JH, Grove LE, Hect J, Marshall N, … & Romero R (2017). Weak functional connectivity in the human fetal brain prior to preterm birth. Scientific Reports, 7, 39286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, & Thompson PM (2003). Mapping brain asymmetry. Nature Reviews Neuroscience, 4, 37–48. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Kumon M, Wada E, Onodera M, Mochizuki H, & Wada K (2010). Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochemistry International, 57, 235–247. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RCW, Stam CJ, Kahn RS, & Hulshoff Pol HE (2010). Aberrant frontal and temporal complex network structure in schizophrenia: A graph theoretical analysis. Journal of Neuroscience, 30, 15915–15926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan C, Renfrew M, & McGuire W (2011). Fetal and perinatal consequences of maternal obesity. Archives of Disease in Childhood - Fetal and Neonatal Edition, 96, F378–F382. [DOI] [PubMed] [Google Scholar]
- Verstraete E, Veldink JH, van den Berg LH, & van den Heuvel MP (2014). Structural brain network imaging shows expanding disconnection of the motor system in amyotrophic lateral sclerosis. Human Brain Mapping, 35, 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, & Baler RD (2011). Reward, dopamine and the control of food intake: Implications for obesity. Trends in Cognitive Sciences, 15, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, & Reyes TM (2010). Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology, 151, 4756–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MD, Austin NC, Bora S, Eggebrecht AT, Melzer TR, Woodward LJ, & Smyser CD (2018). Altered functional network connectivity relates to motor development in children born very preterm. NeuroImage, 183, 574–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MD, Hect JL, Hernandez-Andrade E, Hassan SS, Romero R, Eggebrecht AT, & Thomason ME (2019). Sex differences in functional connectivity during fetal brain development. Developmental Cognitive Neuroscience, 36, 100632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn : A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2, 125–141. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, & Yaffe K (2008). Central obesity and increased risk of dementia more than three decades later. Neurology, 71, 1057–1064. [DOI] [PubMed] [Google Scholar]
- Wijngaarden MA, Veer IM, Rombouts SARB, van Buchem MA, Willems van Dijk K, Pijl H, & van der Grond J (2015). Obesity is marked by distinct functional connectivity in brain networks involved in food reward and salience. Behavioural Brain Research, 287, 127–134. [DOI] [PubMed] [Google Scholar]
- Yang Y, Shields GS, Guo C, & Liu Y (2018). Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neuroscience and Biobehavioral Reviews, 84, 225–244. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, & Bullmore ET (2010). Network-based statistic: Identifying differences in brain networks. NeuroImage, 53, 1197–1207. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Zhang L, Li S, Zhao F, Wang Y, Huang L, … & Mu D (2017). Association among obesity, overweight and autism spectrum disorder: A systematic review and metaanalysis. Scientific Reports, 7, 11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Román J, Björnholm L, Muetzel RL, Torres-Espínola FJ, Lieslehto J, Jaddoe V, … El Marroun H (2019). Maternal prepregnancy body mass index and offspring white matter microstructure: results from three birth cohorts. International Journal of Obesity, 43, 1995–2006. 10.1038/s41366-018-0268-x [DOI] [PubMed] [Google Scholar]


