Abstract
Measles viruses (MV) are rapidly inactivated by anti-measles neutralizing antibodies which has limited their clinical performance as oncolytic agents. Here, by substituting the H and F surface glycoproteins of MV with those from the homologous canine distemper virus (CDV) and engineering the CDV H attachment protein to target EGF receptor or CD38, we generated a fully retargeted MV capable of resisting neutralization by measles-immune human serum. The resultant recombinant MVs encoding retargeted CDV envelope glycoproteins had similar growth kinetics as the control MV, showed the expected engineered receptor specificities for cell entry, intercellular fusion and target cell killing, and were blind to native CDV receptors. In contrast to the control MV, recombinant MVs incorporating CDV F and H glycoproteins retained full infectivity when exposed to high concentrations of pooled measles-immune human serum. Comparing viruses bearing MV or CDV glycoproteins in the SKOV3ip.1 model, only the virus bearing an EGFR retargeted CDV envelope glycoprotein complex was capable of limiting tumor growth and extending the survival in measles immune mice. MV, “stealthed” and retargeted using engineered CDV surface glycoproteins, may be a promising platform to advance for systemic cancer therapy in measles-immune patients.
Keywords: Ovarian cancer, oncolytic virus, measles, virus engineering, EGFR, CD38, antibody targeting, canine distemper virus
Introduction:
Oncolytic virotherapy using engineered measles viruses (MV) derived from the Edmonston vaccine lineage is a promising experimental approach to the treatment of cancer1. MV can be engineered to selectively infect, replicate in, and destroy tumor cells with minimal toxicity to normal cells1, 2. Clinical trials using engineered MVs to treat a variety of human cancers3 including nervous system4, 5 hematological6–8 gynecologic9, 10 and urothelial malignancies11, have shown promising results.
A major barrier to the systemic deployment of MV as an anticancer agent is the high prevalence of anti-measles antibodies in the general population6, 8, 12. Also, while dose limiting toxicities have not yet been encountered in MV clinical trials12, even with systemic MV doses as high as 1011TCID50, the concern remains that the systemic deployment of genetically armed MVs could be toxic to noncancerous cells expressing natural MV receptors, SLAMF1 and NECTIN4. We have therefore been working to engineer oncolytic MV whose receptor attachment and entry specificity is targeted to antigens overexpressed on the tumor cell surface, and which have been further “stealthed” to evade neutralization by pre-existing anti-measles antibodies.
MV has two surface glycoproteins, the receptor-binding attachment protein, H, and its fusogenic partner, F, that together mediate virus entry and intercellular fusion between infected and uninfected cells expressing the native virus receptors. By a poorly understood mechanism, H receptor binding triggers the depolymerization of the F trimer leading to membrane fusion. We previously developed a versatile system to redirect the specificity of MV attachment and entry by fusing specificity determining polypeptide ligands to the carboxy terminus of H and mutating key residues to disrupt its interactions with the native receptors13–15.
MVs retargeted in this way were shown to escape neutralization by monoclonal antibodies targeting the receptor binding sites on H16, 17 but remained highly susceptible to neutralization by polyclonal anti-measles neutralizing antibodies in measles-immune serum16. Since all MV neutralizing antibodies target H or F 18, 19, we chose to substitute these surface glycoproteins with the corresponding proteins from a homologous morbillivirus.
Canine Distemper Virus (CDV) is a morbillivirus endemic in domestic dogs. CDV and MV surface glycoproteins are structurally similar but serologically distinct. A proof of concept study was previously reported for the substitution of MV F and H proteins with homologous CDV glycoproteins to circumvent antibody mediated neutralization of MV20–22. However, the previously reported viruses were not specific for clinically relevant tumor-selective receptors21 and were not blinded for native receptor use, so could still infect nontargeted human cells via the human NECTIN4 receptor23.
To generate fully retargeted chimeric MVs capable of escaping neutralization by measles-immune human serum, we here substituted the MV F and H genes with homologous CDV F and CDV H genes mutated to destroy native receptor interactions and retargeted via C-terminally fused single chain antibody fragments (scFv), specific for clinically relevant receptors EGFR or CD38 that have been previously well characterized for specificity in the context of retargeted MV13. These retargeted viruses show the expected reprogramming of their receptor tropisms and are selectively oncolytic for receptor-positive tumors, even in mice passively immunized with measles-immune antiserum. Fully retargeted chimeric MVs that evade measles neutralizing antibodies may be suitable for clinical translation in measles-immune cancer patients.
Materials and methods:
Cell culture.
The African green monkey kidney cells (Vero), baby hamster kidney cells (BHK), human glioblastoma cell line (U87-MG), human embryonic kidney cells (HEK293), human Burkitt’s lymphoma Raji and Ramos cell lines, and Chinese hamster ovary (CHO) cells were purchased from American Type Tissue Culture collection (ATCC) and grown in the ATCC recommended media. ATCC authenticates cell lines using short tandem repeat DNA profiling. The modified cell lines: SKOV3ip.1-FLuc 24 and CHO-NECTIN4 25 CHO-αHIS13, CHO-EGFR14, CHO-CD3826, CHO-dogSLAMtag27 and Vero-αHIS13 were cultured as previously described. The SKOV3ip.1-FLuc was authenticated using short tandem repeat DNA profiling, all other modified cell lines were not further authenticated. All cells were grown at 37 °C in a 5% CO2 humidified incubator. All cell lines were tested 48 hours post thawing for mycoplasma contamination using a Universal Mycoplasma Detection Kit (ATCC; 30–1012K) and all cell lines routinely tested negative. Cells were not maintained in tissue culture for more than 20 passages.
Generation of expression plasmids and recombinant viruses.
CDV H from the 5804P wild type strain 25 or the previously generated SLAM Blind construct 26, were inserted between the PacI/SpeI restriction sites in the pCG vector or the PacI/SfiI restriction sites pTN vector bearing the respective scFvs13 by ligation cloning to generate pCG CDV H, pTN CDV H -CD38, pTN CDV HRB-CD38 and pTN CDV HRB-EGFR expression plasmids(Fig.1A) The pCG CDV F plasmid was generated by excising out the MV F gene in pCG-MV F with HpaI and PacI and ligating an in-frame CDV F DNA coding sequence PCR amplified from a CDV field isolate. Recombinant viruses were generated by replacing MV F and H genes in the MV Moraten vaccine antigenome 22 (with an additional transcription unit after the P gene encoding for enhanced Green Fluorescence protein (eGFP) to facilitate visualization of virus during rescue) with the NarI/PacI CDV F gene fragment (from pCG-CDVF) and the PacI/SpeI CDVHRB EGFR or CDVHRB-CD38 fragments (from pTN CDVHRB EGFR and pTN CDVHRB-CD38, respectively), in compliance with the rule that recombinant MV genomes are hexametric. The sequences of all generated plasmids were verified by sanger sequencing. Viruses were rescued as described previously22 and grown on Vero-αHis cells at 32 °C.
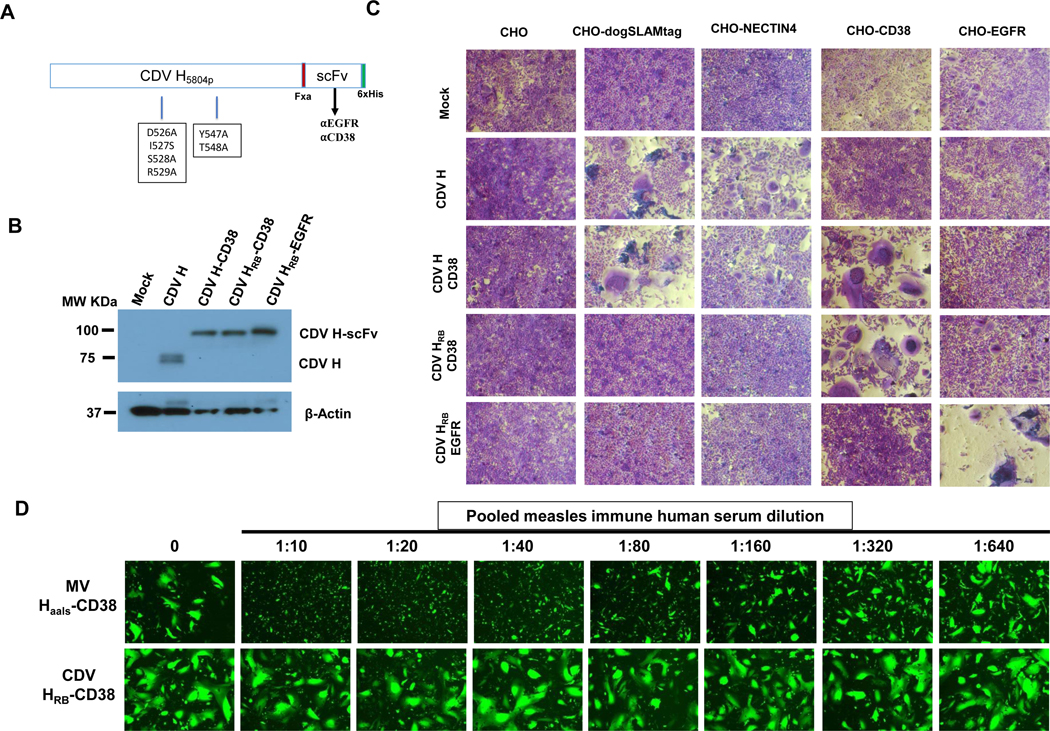
Figure 1: Retargeting the wild type CDV Hemagglutinin (H) protein to EGFR and CD38.
A) Schematic representation of retargeted CDV H protein constructs (not drawn to scale). Standard one-letter amino acid abbreviations are used to denote the mutations that ablate the use of the native receptors (dogSLAM and NECTIN4) thereby generating receptor blind CDV H (CDV HRB). The single-chain antibody fragment (scFv) and a six-histidine tag (6xHis) is displayed as a C-terminal extension of H with a flexible linker containing the Factor Xa, Fxa, (IEGR) cleavage site. (B) Immunoblot analysis of HEK293 cell lysates after transient transfection of engineered CDV H expression plasmids (CDV H, CDV H-CD38, CDV HRB-CD38, CDV HRBEGFR) using an antibody directed against the cytoplasmic tail of CDV H. C) Co-transfection experiments evaluating receptor-specific intercellular fusion mediated by engineered CDV H and CDV F glycoproteins. CHO Cells expressing the desired receptors were co-transfected with a CDV F expression plasmid and the respective engineered CDV H expression plasmids, stained with crystal violet 48 hours post transfection and imaged (100X magnification). (D) Impact of pooled measles immune human serum on Intercellular fusion mediated by retargeted CDV envelope. CHO-CD38 cells were either co-transfected with the CDVHRB-CD38 and CDV F or the homologous MV Haals-CD38 and MV F expression plasmids, and a GFP expression plasmid for visualization of fusion, and incubated with the indicated dilutions (made with culture medium) of pooled human serum (PRN = 256; anti-MV IgG titer = 300 EU/mL). Photographs were taken at 48 hours post transfection under a fluorescence microscope. 100X magnification
Approval to generate MV that evade neutralization was obtained from the Mayo Clinic Institutional Biosafety Committee (IBC) before initiating this project.
Measles immune human serum:
The pooled human immune-measles AB serum used for all experiments was obtained from Valley Biomedical Inc. (Lot #C80553). This serum has anti-MV IgG of 300 EU/mL and titer determined to be 256 by plaque reduction neutralization titer (PRN)24. Serum dilutions were made using Opti-MEM or cell culture medium.
Intercellular fusion assay:
1 × 104 cells seeded in each well of a 96 well tissue culture plate (Corning) were transfected the next day with 50ng each of pCG CDV F and the indicated engineered CDV H plasmid using FugeneHD (Promega) according to the manufacturer’s instructions. 48 hours post transfection, cells were washed once with PBS, stained with 1% (weight/vol.) crystal violet solution and imaged with a light microscope at 100X magnification.
To assess sensitivity of intercellular fusion to measles immune human serum, 1 × 104 CHO-CD38 cells in each well of a 96 well plate were co-transfected the next day with 50ng each of either the pCG CDV F, pTN CDV HRB-CD38 and pCDNA3.1-eGFP, or the pCG-MV F, pTN MV HaalsCD38 and pCDNA3.1 GFP using SuperFect (Qiagen) according to the manufacturer’s instructions. Two hours after transfection, the transfection medium was replaced with culture medium containing two-fold serial dilutions of heat inactivated pooled measles immune human AB serum. Photographs were taken 48 hours post transfection under a fluorescence microscope at 100X magnification.
Western Blot:
To determine expression of engineered CDV H proteins, HEK293 cells (2×106/well in 6 well plate) were transfected with 2μg of the corresponding CDV H plasmids. 48 hours later, cells were washed with PBS, lysed with RIPA buffer for 30 mins at 4ºC, and centrifuged at 13000 RPM for 20 mins at 4ºC to clarify the lysate. 10 μL of lysate was mixed with an equal volume of 2X Laemmli buffer (Biorad), boiled at 95ºC for 5 mins, fractionated on a 7.5% Tris-HCl gel and transferred to a PVDF membrane. Immunoblotting to detect the CDV H was performed as previously described22 using the following antibody dilutions: rabbit anti-CDV H cyt antibody (MC712, 1:5000)21 kindly provided by Dr. Roberto Cattaneo, horse radish peroxidase (HRP)-conjugated goat anti-rabbit (Thermo Fisher Scientific; 1:10000), and HRP- conjugated mouse anti-B-actin (Sigma-Aldrich, A3854; 1:25000).
For immunoblot analysis of chimeric viruses, 2 × 105 TCID50 of each virus stock was directly mixed with an equal volume of denaturing loading buffer, boiled at 95ºC for 5 mins and fractionated on a 7.5% gel. Proteins were transferred to a PVDF membrane and immunoblotted in the standard fashion using a rabbit antibody against MV N (N12), rabbit antibodies directed against the cytoplasmic tails of CDV H (MC712; 1:2000)21 or MV H (1:2000)21, (all kind gifts of Dr. Roberto Cattaneo) primary antibodies; and HRP-conjugated goat anti-rabbit (1:10000) secondary antibody as previously described22
Virus growth kinetics:
2 × 105 Vero-αHis cells in each well of a 6 well plate were infected with the respective viruses at an MOI of 0.03 for 2 hours in 1 mL optiMEM at 37ºC. After which, the infection media was replaced with 2 ml / well of fresh growth media and the cells incubated at 37°C. Cells were harvested at the indicated times by scraping into 1mL of growth media, subjected to 3 freeze-thaw cycles, centrifuged at 1200RPM for 10 mins at 4ºC and viral titers in the supernatant determined on Vero-αHis. The experiment was repeated 3 times.
Flow cytometry:
The surface expression of EGFR and CD38 on human tumor cell lines was performed as previously described13 using an Alexa Fluor647-conjugated anti-human EGFR (matuzumab) Mab (R&D systems; FAB10023R) and FITC-conjugated Mouse anti-Human CD38 (BD Biosciences; Cat: 555459), respectively.
In vitro infection, virus neutralization, and cell killing:
104 adherent cells or 105 suspension cells were infected in one well of a 96 well plate at an MOI of 0.5 (titers determined on Vero-αHis cells) in 50 μL Opti-MEM for 3 hours at 37°C before the addition of complete growth medium. 48 hours after infection, cells were examined and photographed under a fluorescence microscope at 100X magnification.
For the neutralization assay, 30 infectious virus units of each virus was incubated with serial dilutions of heat inactivated pooled AB human serum (Valley Biomedical, Inc. Lot # C80553) in Opti-MEM at 37°C in quadruplicates for hour. The mix was then used to infect a 90% confluent monolayer of Vero-αHis cells in a 96 well plate for 2 hours at 37°C. After the infection, fresh culture medium was added, and the cells cultured at 37°C. 72 hours later, wells were observed for the appearance of viral cytopathic effect (CPE). The reciprocal of the dilution at which there was no viral CPE in all 4 wells for each virus is reported as the neutralization titer. The experiment was repeated 2 times.
For in vitro cytotoxicity, 1×104 SKOV3ip.1-Fluc cells in each well of 96-well plate were infected with the respective viruses in quadruplicates in Opti-MEM media for 3 hours at an MOI of 10 and cultured at 37°C in culture medium. 72 hours later, cytotoxicity was determined by MTT assay (Promega) according to the manufacturer’s instructions.
In vivo experiments:
All animal experiments were approved by the Mayo Clinic Institutional Animal Care and Use Committee (IACUC) and performed in accordance with IACUC established guidelines. For all experiments, we used female 6–8 week old athymic nude mice (Taconic).
To assess specificity of infection in vivo 5 × 106 cells in 200μL PBS were implanted i.p. followed 10 days later by 6 i.p. virus treatments of 2 ×106 TCID50 (200 μL) MVCDVenv-EGFR or MVCDVenv-CD38 (n=4/group) or control treatment (200 μL of Vero-αHis cells lysate; n=3) every other day. To compare efficacy of MVEGFR to MVCDVenvEGFR 5 × 106 cells in 200μL PBS were implanted i.p. followed 10 days later by a single treatment of 200 μL control (Vero-αHis cells lysate) or 2 × 106 TCID50 of MVEGFR or MVCDVenvEGFR (n=5/group). For the immune evasion studies, 2.5 × 106 SKOV3ip.1-Fluc cells were implanted into the peritoneal cavity of athymic nude mice (n=10/group) followed 6 days later by passive immunization of animals in the serum group as previously described24. For these experiments we used 200 μL of pooled human measles immune serum (Valley Biomedical, Inc; Lot # C80553). Three hours following passive immunization, animals were treated with a single intraperitoneal injection of 200 μL control treatment (Vero-αHis cells lysate) or 2 × 106 TCID50 of MVEGFR or MVCDVenvEGFR. Tumor burden was monitored by weekly luminescence imaging (IVIS Xenogen; Perkins) and total body luminescence was quantified from these images using the Live Imaging Software (IVIS Xenogen; Perkins) according to the manufacturer’s protocol. Animals were euthanized when they developed ascites. For the subcutaneous model, 5 × 106 SKOV3ip.1-Fluc cells in 100 μL PBS were implanted subcutaneously in the right flank and treated with 6 intratumoral injections of 100 μL control treatment (Vero-αHis cells lysate; n=3) or 1 × 106 TCID50 of MVEGFR or MVCDVenvEGFR (n=4/group) every other day. Tumor volume was monitored by caliper measurements and animals sacrificed if they met euthanasia criteria. Tumor volume was calculated by using the formula 0.5 × length × width × width.
Statistical analysis
Statistical analyses and calculations were performed with the GraphPad Prism software (Graphpad Software, San Diego, CA). We used the Kaplan-Maier method to graph the survival of treated animals, and the Log-Rank test to compare the differences in the survival curves. Differences in subcutaneous tumor volumes and total body luminescence were assessed by two-way ANOVA with Turkey’s multiple comparison test to correct for multiple comparisons. Unpaired Student’s t test was used to compare differences between two groups. A p < 0.05 was considered to be statistically significant for all analyses.
Results:
1. Retargeting CDV H to EGFR and CD38
Since the vaccine strains of CDV have evolved to use other uncharacterized receptor(s) in addition to the known CDV receptors30 we focused our engineering efforts on a wild type CDV H. The CDV H coding sequence of the wild type strain (5804P)28 or the previously engineered receptor blind variant (CDV H RB)29 were cloned as shown in Fig. 1A into an expression vector with or without C-terminally fused scFvs targeting EGFR or CD38 to generate expression plasmids for CDV H, CDV H-CD38, CDV HRB-CD38 and CDV HRB-EGFR. These plasmids were transiently transfected into human embryonic kidney (HEK293) cells and the lysates assessed for protein expression by western blot using an antibody directed against the cytoplasmic tail of CDV H protein21. The immunoblot in Fig. 1B shows that all proteins were equally expressed and the scFv-tagged proteins (CDV H-CD38, CDV HRB-CD38 and CDV HRB-EGFR) had the expected ~25kDa increase in molecular weight compared to the unmodified CDV H protein.
Next, to assess receptor specificity of the engineered CDV H proteins, we co-transfected a panel of CHO cells expressing the relevant receptors with each of the CDV H expression plasmids in combination with a CDV F expression plasmid. The readout for these experiments was syncytia formation resulting from intercellular fusion. As expected, we observed syncytia formation with the wild type CDV H protein only in CHO cells expressing native CDV receptors canine SLAM or NECTIN4, and not in the parental CHO, CHO-EGFR, or CHO-CD38 cell lines (Fig. 1C). By contrast, the six mutations introduced into the CDV H receptor binding sites selectively eliminated native receptor-dependent fusion without impairing retargeted fusion via displayed ligands (compare the CDV H-CD38 row to the CDV HRB-CD38 or CDV HRB-EGFR rows). After confirming full retargeting of CDV H, we next evaluated the impact of measles neutralizing antibodies on the intercellular fusion mediated by the engineered CDV envelope. As shown in Fig. 1E intercellular fusion mediated by the retargeted CDV H-CD38 was resistant to neutralization by pooled measles-immune human serum even with serum dilutions as low as 1:10. In contrast intercellular fusion mediated by MV H-CD38 co-transfected with the MV F protein in CHO-CD38 cells was strongly inhibited even at high serum dilutions.
Taken together, these data demonstrate that wild type CDV H can be fully retargeted and that intercellular fusion mediated by retargeted CDV H in concert with CDV F is resistant to neutralization by antibodies present in measles-immune human serum.
2. Generation and in vitro characterization of chimeric MV incorporating retargeted CDV envelope glycoproteins (MVCDVenv)
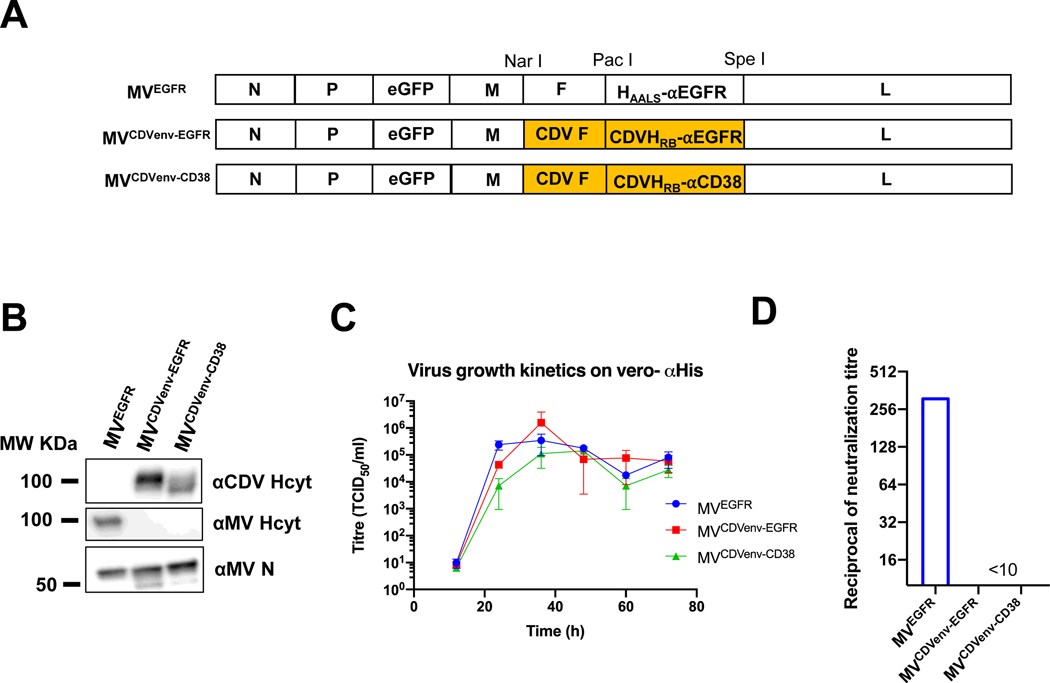
To generate fully retargeted chimeric MV bearing retargeted CDV envelopes, we substituted the MV F and H genes into the MV Moraten vaccine (vac2) genome (with an eGFP cistron inserted upstream of the P gene)22 with the respective CDV F and CDV HRB-EGFR or CDV HRB-CD38 protein-encoding genes. The resulting viruses were named MVCDVenv-EGFR and MVCDVenv-CD38 respectively. As control, we also generated MVEGFR, in which the MV H gene was replaced by a previously engineered sequence encoding a native receptor blind MV H protein displaying a C-terminal scFv against EGFR (Haals-EGFR) (Fig.2A). All three viruses were rescued and propagated using the six-histidine tag rescue (STAR) system13 and the incorporation of chimeric envelopes confirmed by immunoblot analysis (Fig. 2B). The retargeted viruses all exhibited slower growth kinetics compared to unmodified MV-Moraten, but compared to one another had similar growth kinetics on the Vero-αHis cell line (Fig. 2C) and yielded stocks with similar maximum titers of 3 × 108TCID50/mL for MVEGFR, 2 × 108TCID50/mL, MVCDVenv-EGFR, and 6 × 107TCID50/mL for MVCDVenv-CD38. In vitro neutralization assays showed that the viruses bearing the retargeted CDV envelopes maintained their infectivity even in the presence of a high concentration (<1:10 dilution) of pooled measles immune human serum. In contrast the virus bearing the retargeted MV envelope was efficiently neutralized at serum dilutions as high as 1:320 (Fig. 2D). Collectively, these data demonstrate that chimeric measles viruses with the retargeted CDV envelope can be generated and propagated with the STAR pseudo-receptor system and that these viruses are resistant to neutralization in vitro by measles immune serum.
Figure 2: Generation and in vitro characterization of chimeric MeV bearing the CDV F and CDV H retargeted to EGFR and CD38 glycoproteins.
A) Schematic representation of recombinant MV genome constructs. The MV F and MV H retargeted to EGFR genes in the MV vac2 anti-genome were substituted with the homologous CDV F and either CDVHRB-EGFR or CDVHRB-CD38. There is an additional gene encoding for the enhanced green fluorescent protein (eGFP) inserted after the P gene to facilitate visualization of viruses during rescue, propagation, and in in vitro experiments. B) Western blot analysis of viral stocks (1×105TCID50) using antibodies specific for MV N (αMV N), the cytoplasmic tail of CDV H (αCDV Hcyt) or the cytoplasmic tail of MV H (αMV Hcyt) demonstrated that engineered retargeted CDV H glycoproteins were incorporated into the chimeric virions. C) Multi-step growth curves on Vero-⍺His cells showed that the newly generated chimeric viruses had similar growth kinetics as the control MVEGFR virus. Vero-⍺His cells were infected at an MOI of 0.03 and cell pellets were collected at the indicated time points and titrated on Vero-⍺His cells after 3 three freeze thaw cycles. Error bars indicated SEM between 3 experiments. D) Neutralization assays using pooled measles-immune human serum, showed that the retargeted chimeric viruses with the CDV envelope were less susceptible to neutralization. Viruses were exposed to two-fold serial dilutions of pooled measles immune serum prior to infecting Vero-⍺His cells. The reciprocal of the lowest antibody dilution at which there was full viral CPE in two independent experiments is reported as the neutralization titer.
3. Receptor specificity of chimeric MV bearing retargeted CDV envelopes
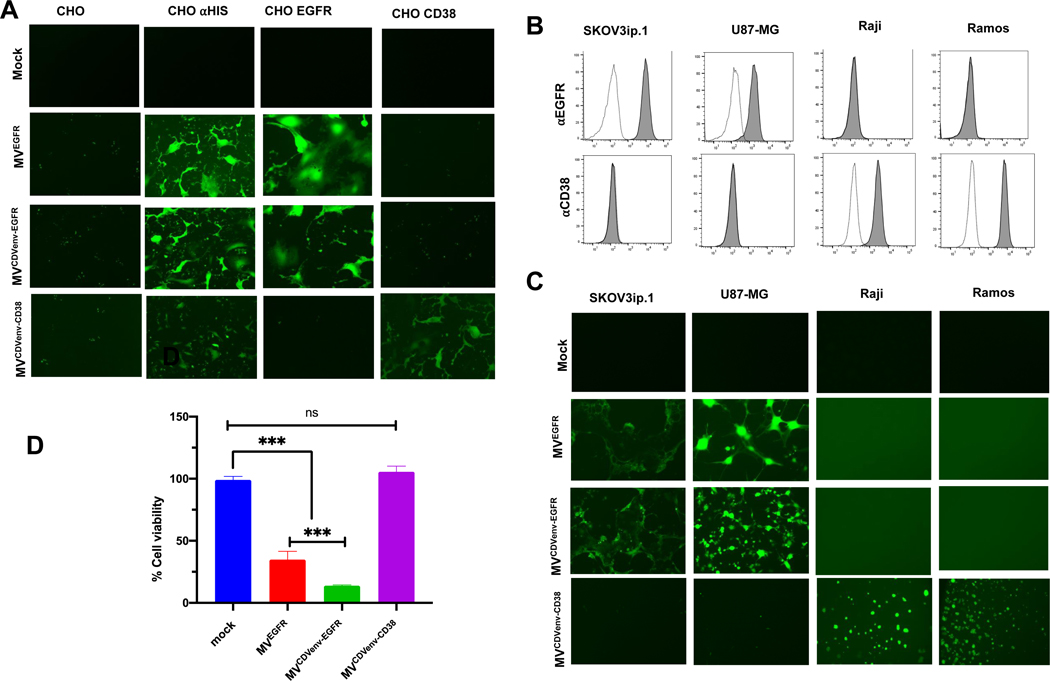
To assess the receptor specificity of the chimeric retargeted viruses we infected the panel of CHO cells expressing the relevant receptors and monitored for GFP expressing syncytia 48 hours post virus exposure as readout for infection. As shown in Fig 3A, all chimeric viruses exhibited the expected receptor specificities. None infected the parental CHO cells whereas all viruses infected the CHO-αHis cell line via the interaction between their displayed His tag and the αHis pseudoreceptor. MVEGFR and MVCDVenv-EGFR specifically infected CHO-EGFR cells with no discernable infection in CHO-CD38 cells, while MVCDVenv-CD38 specifically infected the CHO-CD38 cells without infecting the CHO-EGFR cells. None of the viruses infected CHO cells expressing native CDV receptors, SLAM or NECTIN4.
Figure 3. Receptor specificity of chimeric MV bearing the retargeted CDV envelope in vitro.
(A) Chimeric MV bearing CDV F and CDV H retargeted to EGFR or CD38 maintain receptor-specific infectivity on a panel of CHO cells with the desired receptors. Cells were infected with the respective viruses at an MOI of 0.5 and photographed 48 hours later using a fluorescence microscope; 100X magnification. (B) Flow cytometry analysis of selected human tumor cell lines that express either EGFR or CD38 stained (filled histograms) with an antibody specific for EGFR (⍺EGFR) or CD38 (⍺CD38). Unstained controls are shown as unfilled histograms. (C) Chimeric MV bearing CDV F and CDV H retargeted to EGFR or CD38 maintain receptor-specific infectivity selected human tumor cell lines that express either EGFR or CD38. Cells were infected with the respective viruses at an MOI of 0.5 and photographed 48 hours later using a fluorescence microscope; 100X magnification. (D) In vitro cytotoxicity of retargeted chimeric viruses on the EGFR over-expressing SKoV3ip-Fluc tumor cell line showing that the specific infection by the viruses retargeted to EGFR leads to in vitro cytotoxicity. Cells were infected with respective viruses at an MOI of 10 and cytotoxicity determined 72 hours later by an MTT assay. Error bars indicate SEM between two experiments; ns = not significant, ***p<0.001
Entry specificity was further tested in a panel of four human tumor cell lines known to express either EGFR or CD38: the SKOV3ip.1 ovarian cancer and U87-MG brain tumor cell lines are positive for EGFR and negative for CD38, whereas the Raji and Ramos Burkitt’s lymphoma lines are positive for CD38 and negative for EGFR as confirmed by flow cytometric staining (Fig. 3B). As expected, MVEGFR and MVCDVenv-EGFR infected only the EGFR positive tumor cell lines while MVCDVenv-CD38 infected only the CD38 positive tumor cell lines (Fig. 3C). Receptor-dependent killing of SKOV3ip.1 tumor cells by the retargeted viruses was evaluated using an MTT assay as readout for cell viability 72 hours post infection. Consistent with the cell infectivity data, only the EGFR-retargeted viruses significantly diminished cell viability, (Fig. 3D) indicating that EGFR-dependent entry and intercellular fusion translates to receptor-dependent cell killing.
4. In vivo specificity and oncolytic activity of chimeric viruses in an EGFR positive ovarian cancer tumor model
To evaluate the in vivo specificity and oncolytic activity of the retargeted chimeric viruses we utilized the EGFR-overexpressing SKOV3ip.1-FLuc orthotopic ovarian cancer xenograft tumor model 24, 31, 32. SKOV3ip.1-Fluc cells express luciferase which allows for non-invasive monitoring of tumor burden by bioluminescence (IVIS Xenogen) imaging (BLI). To assess for specificity of infection and oncolytic efficacy in vivo, SKOV3ip.1Fluc cells were implanted into the peritoneal cavity in athymic mice to develop a model of disseminated carcinomatosis peritonei, followed 10 days later by 6 intraperitoneal treatments of 2 × 106 TCID50 of MVCDVenv-EGFR, MVCDVenv-CD38 or an equivalent volume of control cell lysate (from the producer Vero-αHis cell line), given every other day. Figure 4A shows that only the MVCDVenv-EGFR virus was able to control tumor growth, as demonstrated by a marked reduction in the tumor luminescence signal in this treatment group compared to the control treatment groups. Control of tumor growth was associated with significant prolongation in survival of animals in the MVCDVenv-EGFR treatment group compared to the MVCDVenv-CD38 or control groups (median survival of 54 vs 30 days; p = 0.01) (Fig. 4B), indicating that the specificity of infection observed in vitro for the MVCDVenv-EGFR translates to specific oncolytic efficacy in vivo.
Figure 4: In vivo specificity of chimeric MV in an EGFR positive human ovarian tumor xenograft model.
MVCDVenv-EGFR is specifically oncolytic in i.p. EGFR positive SKOV3i.p.1-Fluc tumors treated 10 days after tumor implantation with 6 doses of 2 X 106 TCID50 of indicated virus treatment or control (n=3 control and n=4 for treatment group). (A) Serial bioluminescent imaging on the indicated days. (B) Overall survival of treated animals. ns = not significant; * p<0.05
5. Comparing the oncolytic activity of MVEGFR versus MVCDVenv-EGFR
To directly compare the oncolytic activities of the MVCDVenv-EGFR and MVEGFR viruses, we used both subcutaneous and intraperitoneal SKOV3ip.1-Fluc tumor models. In the subcutaneous model we assessed the effect of 6 intratumoral treatments of 1 × 106 TCID50 of virus versus control cell lysate administered every other day starting 10 days after tumor implantation. The tumor volumes of subcutaneous tumors assessed by caliper measurements shown in Fig. 5A, demonstrate that MVCDVenv-EGFR and MVEGFR were equally effective in this model, significantly impacting tumor volumes compared to control (p=0.0019 for MVEGFR and p<0.0001 compared to control treatment). Tumor volumes (mean ±SD) were 27.5 ± 9.1 mm3, 23.4 ± 11. 8 mm3 and 20.9 ± 2.5 mm3 before virus injection and 1346.7 ± 527.6 mm3, 67.3 ± 76.6 mm3 and 9.8 ± 19.3 mm3, 30 days after the first virus treatment for control, MVEGFR, and MVCDVenv-EGFR, respectively. There was no statistical significance in tumor growth curves between the two virus treatments (P=0.2234). Next, in the orthotopic SKO3ip.1-Fluc model we compared the oncolytic activity of a single intraperitoneal virus treatment (2 × 106 TCID50) versus control therapy 10 days after tumor cell implantation. Tumor burden was monitored weekly by luciferase imaging. As shown in Fig. 5B, the luciferase signal (i.e. tumor burden) increased more rapidly in the animals in the control group versus those in either virus treatment group. There was a statistically significant decrease in the whole body luminescence of animals in the treatment groups compared to control treated animals on day 14 (p = 0.0473 for MVEGFR and p = 0.0324 for MVCDVenv-EGFR compared to control), with no statistically significant difference in signal intensities between the two treatment groups on day 14 (p>0.9999) (Fig. 5C). This decrease in luminescence correlated with a statistically significant increase in median survival from 18 days for the control treatment to 45 days for the MVEGFR and 53 days for MVCDVenv-EGFR treatment groups (p=0.0063 and p =0.0015, respectively), with no difference in survival between the two treatment groups (p=0.6217, (Fig. 5D). Overall, these data show that MVCDVenv-EGFR is equipotent compared to the previously reported MVEGFR in an EGFR-overexpressing tumor model.
Figure 5: Comparison of the oncolytic efficacy of the EGFR retargeted viruses in the EGFR positive ovarian cancer tumor model.
(A) MVEGFR and MVCDVenvEGFR have similar oncolytic efficacy in a subcutaneous tumor model. Tumor volumes of subcutaneously implanted SKOV3i.p.1-Fluc cells treated with 6 intratumoral doses of 2 X 106 TCID50 of the respective viruses or control (producer Vero-⍺His lysate). (B-D) MVEGFR and MVCDVenvEGFR have similar oncolytic efficacy in an orthotopic, intra peritoneal model. (B) Serial bioluminescent imaging on the indicated days to monitor tumor burden of mice with i.p. SKOV3i.p.1-Fluc tumors treated with 2 X 106 TCID50 of indicated virus treatment or control (n=5 per group) 10 days post tumor implantation. (C) Quantification of total body luminescence (tumor burden) on day 14 post treatment; error bars indicate SEM. (D) Overall survival of treated animals. ns= not significant; *p<0.05
6. In vivo oncolytic activity of chimeric viruses in measles immune mice bearing EGFR positive SKOV3ip.1 tumors
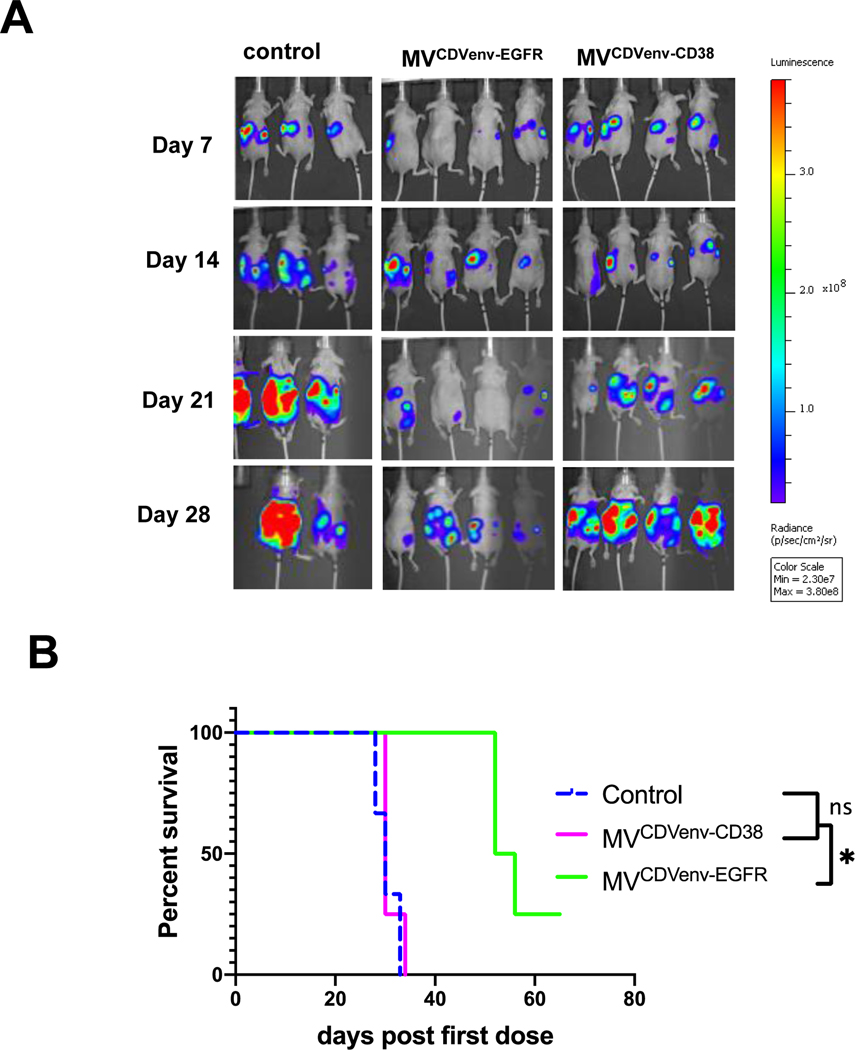
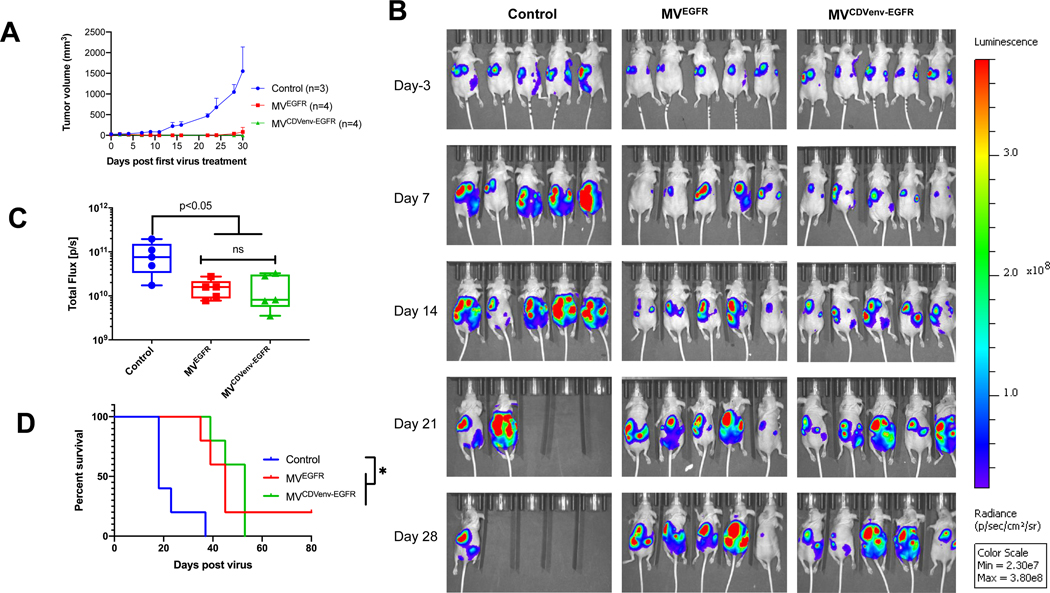
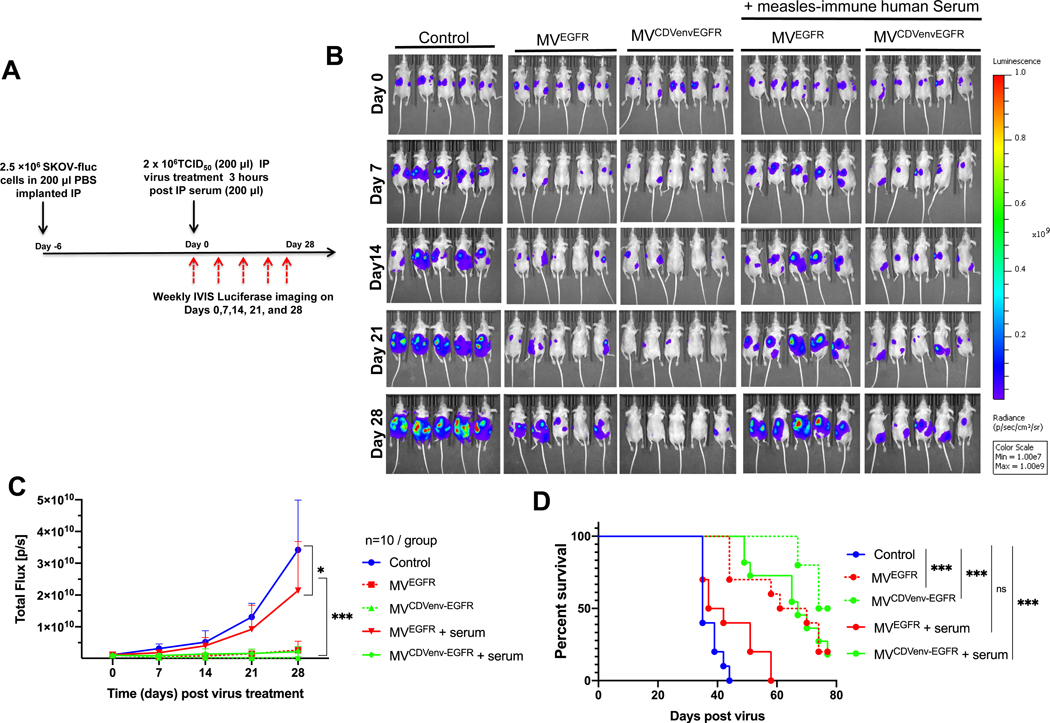
To assess the impact of measles neutralizing antibodies on the oncolytic activity of MVEGFR and MVCDVenv-EGFR, athymic mice with established intraperitoneal SKOV3ip.1-Fluc tumor xenografts were passively immunized with measles immune human AB serum (60 EU per mouse) and treated 3 hours later with a single intraperitoneal virus dose of 2 × 106 TCID50 (Fig. 6A). Tumor growth was monitored by serial bioluminescence imaging as shown in Fig. 6B with the corresponding quantification of total body luminescence graphed in Fig. 6C. As expected, both MVEGFR and MVCDVenv-EGFR reduced tumor burden(P<0.0001) and prolonged the survival (P<0.0001) of treated animals compared to control treatment in the absence of measles neutralizing antibodies. However, as anticipated, the efficacy of the MVEGFR was severely diminished in the presence of measles neutralizing antibodies, resulting in a statistically significant increase in the luminescence signal (P<0.0001) and corresponding decrease in survival (P<0.0018) for passively immunized mice compared to naïve mice treated with MVEGFR. In contrast, the oncolytic activity of MVCDVenv-EGFR was maintained in the presence of neutralizing measles antibodies, with no statistically significant difference in tumor luminescence (P=0.7083) or overall survival (P=0.3381) for passively immunized mice compared to nonimmunized control mice treated with MVCDVenv-EGFR. Together, these results demonstrate that MVCDVenv-EGFR retains full antitumor activity in vivo even in the presence of a high body fluid concentration of MV neutralizing antibodies.
Figure 6: Impact of pre-existing measles neutralizing antibodies on the oncolytic activity of EGFR-retargeted viruses.
MVCDVenvEGFR maintained oncolytic efficacy in the presence of pre-existing anti-measles neutralizing antibodies. A) schematic of experimental design. Athymic mice with i.p. SKOV3ip.1Fluc tumors were treated with a single i.p. injection of 2 × 106 TCID50 of MVEGFR, MVCDVenvEGFR or 200 μL control (producer vero-⍺His lysate), with or without 200 μL (60 EU) of pooled human measles-immune neutralizing serum added 3 hours before virus treatment (n=10 mice/group). Representative (5 animals/ group) images (B) quantification (C) of serial bioluminescent imaging and (D) overall survival of treated animals *p<0.05; *** P<0.0001.
Discussion:
Here, we substituted the MV F and H genes with CDV F and CDV H retargeted to EGFR or CD38, and thereby generated fully retargeted oncolytic MVs capable of escaping neutralization by preexisting measles antibodies. MVCDVenv-EGFR was potently oncolytic in athymic mice bearing aggressive, intraperitoneally disseminated, EGFR-overexpressing, SKOV3ip.1 human ovarian cancer tumors, even after the mice had been passively immunized against measles virus using pooled human serum.
Ovarian cancer, the second most common cancer of the female genitourinary tract, is a lethal disease accounting for an estimated 13,980 number of deaths in the US in 201933. Intraperitoneal EGFR-targeted MV virotherapy is an interesting possibility for the treatment of this disease. Ovarian cancer often remains localized to the peritoneal cavity even in patients with relapsed and treatment refractory disease and we have therefore been developing MV as a potential intraperitoneal therapy for this malignancy9, 10, 24, 31. A recently completed phase I clinical trial of intraperitoneally administered MV doubled the median overall survival of heavily pretreated, platinum-resistant ovarian cancer patients compared to historical controls10, despite the fact that the enrolled patients had high titers of measles neutralizing antibodies in their blood and peritoneal fluid10. Our preclinical studies have underscored the importance of circumventing antibody neutralization of MV to maximize the antitumor potency of the approach and, with this goal in mind, we are clinically evaluating the intraperitoneal administration of ex-vivo infected autologous mesenchymal stem cell carriers as an antibody resistant virus delivery system 24.
In the current study we have pursued an alternative approach to circumvent antibody neutralization, using envelope glycoprotein exchange in conjunction with tropism engineering via surface display of single chain antibodies (scFv). The current study builds on previous reports of chimeric MV bearing CDV envelope glycoproteins 20–22. However, in contrast to previous studies, our current approach has clinical translational relevance because we have targeted the virus to EGFR, a clinically relevant ovarian cancer marker34 also expressed at high levels in several other cancers35, 36, and to CD38, a clinically relevant target in multiple myeloma37. The scFvs we displayed in this study are derived from monoclonal antibodies—EGFR (Matuzumab) and CD38 (Daratumumab)—that have already been well characterized for specificity and toxicity in humans, as well as in the context of retargeted MV13. We elected here to substitute measles H with a wild type CDV H (5804) because of its narrower range of receptor use compared to the H proteins from CDV vaccine strains30. While the mutations (D526A, I527S, S528A, R529A, Y547A, T548A) we introduced into the CDV H protein were initially reported to ablate only SLAM-dependent fusion29 we have confirmed here (Fig.1C) as others have shown38 that these mutations also ablate NECTIN4-dependent fusion making our vector completely “blind” to native pathogenicity-determining CDV receptors. Given the similarity of the CDV and MV envelopes, we anticipate that there will be little limitation to the diversity of tumor associated surface antigens that can be targeted using these chimeric viruses15.
MV does not replicate efficiently in murine cells39, due to as yet undefined intracellular barriers to the viral replication cycle, independent of receptor expression. Thus, we did not test our viruses in syngeneic immunocompetent tumor models. Instead, we used the extensively characterized intraperitoneal SKOV3ip.1-Fluc tumor model in athymic mice in which we could adoptively transfer measles-immune human serum. Therefore, we did not assess the potential contributions of the other compartments of the immune system to the antitumor efficacy of this new vector. However, data previously generated in the SKOV3ip.1 model was deemed sufficiently informative to justify initial clinical testing of MV in ovarian cancer, and the measles antibody titers achieved by passive immunization in our efficacy studies are comparable to the ascites concentrations present in ovarian cancer patients24. Clinical testing of the viruses described in this manuscript may therefore be warranted.
It is worth noting that despite the high doses of virus used in our ovarian cancer studies, neither MVCDVenv-EGFR or MVEGFR therapy was curative. Treated animals eventually succumbed to progressive intraperitoneal disease and were typically euthanized due to ascites formation. Examination of explanted peritoneal tumors from virus treated animals under fluorescent light showed strong expression of GFP indicating that these tumors were persistently infected with the virus. The presence of an intact T-cell immune response might facilitate clearance of these virus infected cells, and this will be tested in future adoptive T cell transfer experiments which may also facilitate the evaluation of immunotherapy combination approaches such as MV virotherapy plus checkpoint inhibition to achieve more durable cures40. While the current study focused exclusively on the SKOV3ip.1 ovarian cancer model, we do plan to investigate the systemic administration of MVCDVenv-EGFR in other EGFR-overexpressing tumors such as lung cancers35, and glioblastoma36. Furthermore, MVCDVenv-CD38 will be evaluated in CD38 overexpressing models of multiple myeloma26
A critical parameter requiring further optimization for the effective clinical translation of the stealthed and retargeted MVs described in this manuscript is to improve the virus manufacturing yield. While we were eventually able to generate viral stocks with titers as high as 108 TCID50/mL of the fully retargeted chimeric viruses for our in vivo animal experiments, retargeted MVs generally grow to much lower titers compared to MVs with native envelope glycoproteins, and are more challenging to propagate. Following their initial rescue, the viruses propagate very slowly until they have been through several tissue culture passages. This could be related to the use of an artificial receptor (the αHis pseudoreceptor) on the cells that are used for virus amplification, or to altered kinetics of virus maturation in infected cells. Significant further optimization of virus yield may be achievable using alternative cell substrates41, alternative receptors for virus propagation, or by virus engineering, perhaps through cytoplasmic tail truncations of the surface glycoproteins42 or substitution of the M gene20, 43. An alternative approach to bypass the MV manufacturing difficulty might be to incorporate the targeted CDV envelope glycoprotein complexes into other viral platforms such as adenovirus14 or VSV44, 45 that are easier to manufacture at high titer.
By design, we used the Edmonston lineage measles vaccine backbone with a remarkable safety record both as a vaccine and oncolytic agent in clinical trials to construct our viruses. The genome of this backbone has remained stable, with no reversions to pathogenicity reported to date, in the past 50+ years since routine childhood measles vaccination was introduced. Since there are over 40 different attenuating mutations dispersed all over the genome, of which mutations in the innate immune antagonizing P/V/C gene are the most important46, we retained all of the core MV proteins, to ensure that our viruses would be safe and not cause disease in humans. Moreover, these core proteins harbor T-cell epitopes that could enhance the anti-tumor effect of MV by eliminating residual virus infected tumor cells 47. While wild type CDV can cause severe disease in dogs48 and has been reported capable of pathogenicity in other species including non-human primates49, the viruses described in this manuscript do not use any of the natural virus receptors responsible for CDV pathogenicity48 and are fully retargeted to receptors that are not compatible with normal virus biology50. Moreover, most pet dogs are vaccinated against CDV. Of course, additional extensive formal toxicology studies in consultation with the FDA will be needed before any human clinical trials can be planned.
In summary, envelope glycoprotein exchange between MV and CDV allowed us to generate chimeric MVs retargeted to EGFR or CD38 that were resistant to neutralization by anti-measles antibodies in serum from measles immune human subjects. As such, these viruses may warrant clinical advancement for the treatment of measles immune patients with disseminated malignancies overexpressing EGFR or CD38. Furthermore, the platform might be suitable for generating additional “stealthed” and retargeted MV tailored for the treatment of a variety of human cancers.
Acknowledgements
We are grateful to Dr. Roberto Cattaneo (Mayo Clinic) for the anti-CDVH cyt, anti-MV H cyt and Anti MV-N antibodies used for western blot. We also thank the Mayo Clinic Microscopy and Cell Analysis Core for experimental and technical support. This work was funded by the Mayo Foundation. E.S.B is supported by the Mayo Clinic Medical Scientist Training Program (MSTP) grant (T32GM065841) and Initiative for maximizing student diversity (IMSD) grant (R25GM055252) from the NIH.
Financial information:
This work was funded by the Mayo Foundation. E.S.B is supported by the Mayo Clinic Medical Scientist Training Program (MSTP) grant (T32GM065841) and Initiative for maximizing student diversity (IMSD) grant (R25GM055252) from the NIH.
Footnotes
Conflicts of Interest
S.J.R and K.W.P are co-founders and officers of VYRIAD and Mayo Clinic holds equity in VYRIAD. All the other authors declare no potential conflicts of interest.
Reference:
- 1.Nakamura T. and Russell SJ, Oncolytic measles viruses for cancer therapy. Expert Opin Biol Ther, 2004. 4(10): p. 1685–92. [DOI] [PubMed] [Google Scholar]
- 2.Russell SJ and Peng KW, Measles virus for cancer therapy. Curr Top Microbiol Immunol, 2009. 330: p. 213–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson S. and Galanis E, Potential and clinical translation of oncolytic measles viruses. Expert Opin Biol Ther, 2017. 17(3): p. 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurokawa C, et al. , Constitutive Interferon Pathway Activation in Tumors as an Efficacy Determinant Following Oncolytic Virotherapy. J Natl Cancer Inst, 2018. 110(10): p. 1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaccine Therapy in Treating Patients With Malignant Peripheral Nerve Sheath Tumor That is Recurrent or Cannot Be Removed by Surgery. https://ClinicalTrials.gov/show/NCT02700230.
- 6.Dispenzieri A, et al. , Phase I trial of systemic administration of Edmonston strain of measles virus genetically engineered to express the sodium iodide symporter in patients with recurrent or refractory multiple myeloma. Leukemia, 2017. 31(12): p. 2791–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinzerling L, et al. , Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood, 2005. 106(7): p. 2287–94. [DOI] [PubMed] [Google Scholar]
- 8.Russell SJ, et al. , Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin Proc, 2014. 89(7): p. 926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galanis E, et al. , Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res, 2010. 70(3): p. 875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galanis E, et al. , Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res, 2015. 75(1): p. 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trial of Intravesical Measles Virotherapy in Patients With Bladder Cancer Who Are Undergoing Radical Cystectomy. https://ClinicalTrials.gov/show/NCT03171493.
- 12.Msaouel P, et al. , Clinical Trials with Oncolytic Measles Virus: Current Status and Future Prospects. Curr Cancer Drug Targets, 2018. 18(2): p. 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura T, et al. , Rescue and propagation of fully retargeted oncolytic measles viruses. Nat Biotechnol, 2005. 23(2): p. 209–14. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T, et al. , Antibody-targeted cell fusion. Nat Biotechnol, 2004. 22(3): p. 331–6. [DOI] [PubMed] [Google Scholar]
- 15.Msaouel P, et al. , Oncolytic measles virus retargeting by ligand display. Methods Mol Biol, 2012. 797: p. 141–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lech PJ, et al. , Antibody neutralization of retargeted measles viruses. Virology, 2014. 454–455: p. 237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kneissl S, et al. , Measles virus glycoprotein-based lentiviral targeting vectors that avoid neutralizing antibodies. PLoS One, 2012. 7(10): p. e46667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Swart RL, Yuksel S, and Osterhaus AD, Relative contributions of measles virus hemagglutinin- and fusion protein-specific serum antibodies to virus neutralization. J Virol, 2005. 79(17): p. 11547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouche FB, Ertl OT, and Muller CP, Neutralizing B cell response in measles. Viral Immunol, 2002. 15(3): p. 451–71. [DOI] [PubMed] [Google Scholar]
- 20.Rouxel RN, Svitek N, and von Messling V, A chimeric measles virus with canine distemper envelope protects ferrets from lethal distemper challenge. Vaccine, 2009. 27(36): p. 4961–6. [DOI] [PubMed] [Google Scholar]
- 21.Miest TS, et al. , Envelope-chimeric entry-targeted measles virus escapes neutralization and achieves oncolysis. Mol Ther, 2011. 19(10): p. 1813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munoz-Alia MA and Russell SJ, Probing Morbillivirus Antisera Neutralization Using Functional Chimerism between Measles Virus and Canine Distemper Virus Envelope Glycoproteins. Viruses, 2019. 11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noyce RS, Delpeut S, and Richardson CD, Dog nectin-4 is an epithelial cell receptor for canine distemper virus that facilitates virus entry and syncytia formation. Virology, 2013. 436(1): p. 210–20. [DOI] [PubMed] [Google Scholar]
- 24.Mader EK, et al. , Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin Cancer Res, 2009. 15(23): p. 7246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu YP, et al. , Ablation of nectin4 binding compromises CD46 usage by a hybrid vesicular stomatitis virus/measles virus. J Virol, 2014. 88(4): p. 2195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng KW, et al. , Oncolytic measles viruses displaying a single-chain antibody against CD38, a myeloma cell marker. Blood, 2003. 101(7): p. 2557–62. [DOI] [PubMed] [Google Scholar]
- 27.Tatsuo H, Ono N, and Yanagi Y, Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J Virol, 2001. 75(13): p. 5842–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Messling V, et al. , A ferret model of canine distemper virus virulence and immunosuppression. J Virol, 2003. 77(23): p. 12579–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Messling V, et al. , Nearby clusters of hemagglutinin residues sustain SLAM-dependent canine distemper virus entry in peripheral blood mononuclear cells. J Virol, 2005. 79(9): p. 5857–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujita K, et al. , Host range and receptor utilization of canine distemper virus analyzed by recombinant viruses: Involvement of heparin-like molecule in CDV infection. Virology, 2007. 359(2): p. 324–35. [DOI] [PubMed] [Google Scholar]
- 31.Peng KW, et al. , Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res, 2002. 62(16): p. 4656–62. [PubMed] [Google Scholar]
- 32.Peng KW, et al. , Pharmacokinetics of oncolytic measles virotherapy: eventual equilibrium between virus and tumor in an ovarian cancer xenograft model. Cancer Gene Ther, 2006. 13(8): p. 732–8. [DOI] [PubMed] [Google Scholar]
- 33.Siegel RL, Miller KD, and Jemal A., Cancer statistics, 2019. CA Cancer J Clin, 2019. 69(1): p. 7–34. [DOI] [PubMed] [Google Scholar]
- 34.Morrison J, et al. , Epidermal growth factor receptor blockers for the treatment of ovarian cancer. Cochrane Database Syst Rev, 2018. 10: p. CD007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selvaggi G, et al. , Epidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non-small-cell lung cancer. Ann Oncol, 2004. 15(1): p. 28–32. [DOI] [PubMed] [Google Scholar]
- 36.Brennan CW, et al. , The somatic genomic landscape of glioblastoma. Cell, 2013. 155(2): p. 462–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lokhorst HM, et al. , Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med, 2015. 373(13): p. 1207–19. [DOI] [PubMed] [Google Scholar]
- 38.Langedijk JP, et al. , Canine distemper virus infects canine keratinocytes and immune cells by using overlapping and distinct regions located on one side of the attachment protein. J Virol, 2011. 85(21): p. 11242–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanagi Y, et al. , Measles virus infects mouse fibroblast cell lines, but its multiplication is severely restricted in the absence of CD46. Arch Virol, 1994. 138(1–2): p. 39–53. [DOI] [PubMed] [Google Scholar]
- 40.Hardcastle J, et al. , Immunovirotherapy with measles virus strains in combination with anti-PD-1 antibody blockade enhances antitumor activity in glioblastoma treatment. Neuro Oncol, 2017. 19(4): p. 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott JV and Choppin PW, Enhanced yields of measles virus from cultured cells. J Virol Methods, 1982. 5(3–4): p. 173–9. [DOI] [PubMed] [Google Scholar]
- 42.Moll M, Klenk HD, and Maisner A, Importance of the cytoplasmic tails of the measles virus glycoproteins for fusogenic activity and the generation of recombinant measles viruses. J Virol, 2002. 76(14): p. 7174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dietzel E, et al. , Canine distemper virus matrix protein influences particle infectivity, particle composition, and envelope distribution in polarized epithelial cells and modulates virulence. J Virol, 2011. 85(14): p. 7162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayala-Breton C, et al. , Amalgamating oncolytic viruses to enhance their safety, consolidate their killing mechanisms, and accelerate their spread. Mol Ther, 2013. 21(10): p. 1930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanauer JDS, et al. , CD30-targeted oncolytic viruses as novel therapeutic approach against classical Hodgkin lymphoma. Oncotarget, 2018. 9(16): p. 12971–12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bankamp B, et al. , Genetic characterization of measles vaccine strains. J Infect Dis, 2011. 204 Suppl 1: p. S533–48. [DOI] [PubMed] [Google Scholar]
- 47.Jaye A, et al. , Ex vivo analysis of cytotoxic T lymphocytes to measles antigens during infection and after vaccination in Gambian children. J Clin Invest, 1998. 102(11): p. 1969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rendon-Marin S, et al. , Tropism and molecular pathogenesis of canine distemper virus. Virol J, 2019. 16(1): p. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakai K, et al. , Lethal canine distemper virus outbreak in cynomolgus monkeys in Japan in 2008. J Virol, 2013. 87(2): p. 1105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfeffermann K, et al. , Morbillivirus Pathogenesis and Virus-Host Interactions. Adv Virus Res, 2018. 100: p. 75–98. [DOI] [PubMed] [Google Scholar]