INTRODUCTION
The myelodysplastic syndromes (MDS) are a heterogeneous group of clonal hematopoietic stem cell disorders that typically affect older adults (median age, 76 years1) but do occur less commonly in children and young adults. There is increasing recognition of an inherited predisposition to MDS as well as acute myeloid leukemia (AML) in both children and older individuals. Specific syndromes and gene mutations are infrequent but, collectively, inherited predisposition to myeloid malignancy represents a significant proportion of these diagnoses, with at least 5% of cases having a germline cause2,3 and with a prevalence up to 10% to 15% in certain patient cohorts.4–8 Germline predisposition to MDS can occur as a part of a syndrome or multisystem disorder or as a seemingly sporadic disease. The timely diagnosis of an underlying genetic predisposition is critical because it has broad implications for treatment, transplant considerations, long-term surveillance, and family counseling. It is more common for pediatric providers to consider these phenotypes, and thus increasing awareness for adult providers is becoming more important as clinicians realize that these disorders can present in older patients too. This article highlights the current state of knowledge for germline genetic causes of MDS (in children and adults); in addition, it provides a framework for the diagnosis and management of genetic predisposition to MDS/AML in the clinic for patients of all ages.
GERMLINE MYELODYSPLASTIC SYNDROME PREDISPOSITION SYNDROMES
Because of the increasing recognition of germline predisposition to MDS/AML and the impact on clinical care, germline predisposition to myeloid neoplasm was incorporated into the World Health Organization classification, and diagnostic recommendations were added to the most recent National Comprehensive Cancer Network (NCCN) practice guidelines.9,10 NCCN guidelines provide relevant guidance on how to test patients but are lacking in explanations to clinicians for identification of appropriate candidates for testing. As such, some institutions have developed their own approach to the diagnosis and management of hereditary myeloid malignancies,6,11–13 and consensus guidelines for surveillance and management exist for several specific MDS predisposition syndromes.14–16 Common to all of these approaches is the appreciation that patients can have a germline predisposition to MDS with the absence of other syndromic features on history and physical and without a family history. Atypical or cryptic cases of the classic pediatric bone marrow failure (BMF) syndromes can become apparent only in adulthood, and MDS or AML can be the first presenting feature of these syndromes.
The major hereditary MDS/AML syndromes to date are summarized in Table 1 with references to large case series detailing comprehensive features of each syndrome that have been published since the genetic diagnosis was established. The syndromes can be divided into the following categories:
Table 1.
Genes involved in predisposition to myelodysplastic syndrome/acute myeloid leukemia and important clinical features
| Syndrome | Gene(s) | Inheritance Mutation Types | Age of MDS/AML Onset (range, y) | Hematologic Features | Extrahematopoietic Features | Other Cancers | Implications for Management | References |
|---|---|---|---|---|---|---|---|---|
| Myeloid Neoplasms with Germline Predisposition Without Preexisting Disorder or Organ Dysfunction | ||||||||
| Familial AML with CEBPA mutations | CEBPA | AD Missense, FS |
Adult>Ped (range 1–62) | AML | — | — | Chemosensitive Risk of second primary AML |
47,48,81–86 |
| Familial MDS/AML with mutated DDX41 | DDX41 | AD Missense, FS, NS, CNV |
Older adult (range 40–89) | MDS, AML, CML Lymphoma |
Granulomatous and autoimmune disorders in a few families | — | — | 70,87–91 |
| Myeloid neoplasms with germline predisposition and preexisting platelet disorders | ||||||||
| ANKRD26-related thrombocytopenia | ANKRD26 | AD UTR variants; coding NS, missensea |
Adult (range 26–70) | MDS, AML, CML CMML, CLL Thrombocytopenia |
— | — | Mild bleeding tendency | 92–98 |
| ETV6-related thrombocytopenia | ETV6 | AD Missense, FS, NS |
Ped-Adult (range 8–82) | B-ALL, MDS, AML, CMML, DLBCL Variable thrombocytopenia |
Not shared across pedigrees (developmental delay, dysmorphisms, autoimmunities) | Colon, breast, meningioma | Mild to moderate bleeding tendency | 99–106 |
| Familial platelet disorder with propensity to AML | RUNX1 | AD Missense, FS, NS, CNV, rearrangements |
Adult>Ped (range 5–72) | MDS, AML, T-ALL, hairy cell leukemia, CMML Mild to moderate thrombocytopenia |
Case report of co-occurring eczema | — | — | 50,112–120 |
| Myeloid neoplasms with germline predisposition and other organ dysfunction | ||||||||
| Germline SAMD9/SAMD9L |
SAMD9 SAMD9L |
AD Missense |
Ped, rare adult (range 1–56) | AA, MDS, AML, CMMLa Increased prevalence of monosomy 7 |
MIRAGE syndrome (SAMD9) Ataxia-Pancytopenia (SAMD9L) |
— | — | 7,37,38,121–124 |
| Familial MDS/AML with GATA2 mutation | GATA2 | AD Missense, NS, FS, splicing, regulatory, CNV |
AYA (range 3–78) | AA, MDS, AML, CMML Increased prevalence of monosomy 7 |
Infection Lymphedema Pulmonary alveolar proteinosis Hearing loss |
— | — | 8,19,44,65,107–111 |
| Diamond-Blackfan anemia |
GATA1 RPL5 RPL11 RPL15 RPL23 RPL26 RPL27 RPL31 RPL35a RPL36 RPS7 RPS10 RPS15 RPS17 RPS19 RPS24 RPS26 RPS27 RPS27A RPS28 RPS29 TSR2 |
AD, X linked (GATA1, TSR2) Missense, FS, NS, splicing, CNV, 3′ UTR |
Adult>Ped (range 2–57) | Red cell aplasia, MDS, AML | Growth retardation, congenital malformations | Osteosarcoma, colon, possibly others | — | 69,125–128 |
| Fanconi anemia |
FANCA FANCB FANCC FANCD1/BRCA2 FANCD2 FANCE FANCF FANCG FANCI FANCJ/BRIP1 FANCL FANCM FANCN/PALB2 FANCO/RAD51C FANCP/SLX4 FANCQ/ERCC4 FANCR/RAD51 FANCS/BRCA1 FANCT/UBE2T FANCU/XRCC2 FANCV |
AR AD (FANCR) X linked (FANCB) Missense, FS, NS, splicing, CNV |
AYA (range 1–57) | AA, MDS, AML ALL with FANCD1 |
Short stature, developmental delay, skeletal and renal abnormalities | SCC of head, neck and anogenital region | Require attenuated therapy, radiosensitive | 61,69,129–132 |
| Short telomere syndromes |
ACD/TPP1 CTC1 DKC1 NAF1 NHP2 NOP10 PARN POT1 RTEL1 TR TERT TINF2 WRAP53/TCAB1 ZCCHC8 |
AD, AR, X linked Missense, FS, NS, splicing, CNV TR: SNV, INDELs |
Adult>Ped (range 2–77) | AA, MDS, AML | Mucocutaneous features, pulmonary fibrosis, liver disease, immunodeficiency, enteropathy, severe congenital anomalies in some | SCC of head, neck and anogenital region | Attenuated regimen, radiosensitive | 69,79,80,133–144 |
| Shwachman-Diamond |
SBDS DNAJC21 EFL1 |
AR Missense, FS, NS, splicing, CNV |
AYA (range 2–53) | Neutropenia, MDS, AML | Exocrine pancreatic insufficiency, neurodevelopmental and skeletal abnormalities | — | — | 2,38,69,145–147 |
| Traditional Hereditary Cancer Predisposition Syndromes | ||||||||
| Li-Fraumeni |
TP53 CHEK2 |
AD Missense, FS, NS, splicing, intronic, CNV |
Ped + adult (range 4–50) | ALL, MDS, AML, CML, lymphoma | — | Breast, sarcoma, CNS, adreno cortical carcinoma | — | 6,148–152 |
Abbreviations: AA, aplastic anemia; AD, autosomal dominant; ALL, acute lymphoblastic leukemia; AR, autosomal recessive; AYA, adolescent and young adult population; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; CNV, copy number variant; DLBCL, diffuse large B-cell lymphoma; FS, frameshift; MIRAGE, myelodysplasia, infection, restriction of growth, adrenal hypoplasia, genital phenotypes and enteropathy; NS, nonsense; Ped, pediatric-onset disease; SCC, squamous cell carcinoma.
Finding reported in a single family or case report.
Myeloid neoplasms with germline predisposition without a preexisting disorder or organ dysfunction (CEBPA, DDX41)
Myeloid neoplasms with germline predisposition and preexisting platelet disorders (RUNX1, ANKRD26, ETV6)
Myeloid neoplasms with germline predisposition and other organ dysfunction (GATA2, short telomere syndromes, other inherited BMF syndromes)
Traditional hereditary cancer predisposition syndromes (now understood to include hematologic malignancies in addition to solid tumors)
INDICATIONS FOR GENETIC TESTING
Certain clinical and laboratory features enrich for populations with inherited predisposition, and those populations warrant comprehensive screening for germline mutations as outlined later. However, limiting testing to only these high-risk patients could overlook a diagnosis in those older patients, nonsyndromic patients, or patients without family history who present with what seems to be de novo MDS but who carry a genetic predisposition.
Germline genetic testing is recommended in:
Young-onset MDS, before 50 years of age; a proportion have negative family history and no other suggestive features
MDS with any clinical or pathologic feature of a BMF syndrome (including lifelong history of cytopenias, even if MDS diagnosis made at a later age)
Familial cases with MDS, acute leukemia, aplastic anemia, unexplained cytopenias, or bleeding history in 2 or more relatives (first or second degree)
Individuals or families with MDS/AML clustering with extrahematopoietic manifestations characteristic of the BMF syndromes (see Table 1)
Personal history of MDS and multiple primary malignancies and/or strong family history of cancers at early ages
Individuals with mutations in genes known to be associated with hereditary MDS/AML found on somatic tumor testing (discussed later)
Consider germline genetic testing in:
Hypoplastic MDS at any age (without paroxysmal nocturnal hemoglobinuria [PNH] clone)
Personal history of thrombocytopenia (diagnosed as autoimmune) not responsive to standard therapies
Patients with chemotherapy toxicity more severe than experienced by most patients
Related donor with unexplained cytopenias or poor peripheral blood stem cell mobilization; donor-derived malignancy after related donor hematopoietic stem cell transplant (HSCT)
Certain patients with therapy-related myeloid malignancies may be more likely to harbor germline variants in predisposition genes
EVALUATION FOR GERMLINE PREDISPOSITION TO MYELODYSPLASTIC SYNDROME
Standard diagnostic criteria for MDS apply to patients with germline predisposition and all the conventional diagnostic evaluations should be done. The underlying germline predisposition may not be evident on usual evaluations, so a high index of suspicion and dedicated work-up are needed. Mutations in some of the genes known to cause hereditary myeloid malignancy, such as CEBPA, RUNX1, or TP53, can also arise somatically in the clonal MDS/AML population. Clinically theses scenarios can lack clarity without further evaluation. Published guidelines now include consideration of additional molecular and genetic testing specifically for hereditary hematologic malignancies.10 Patients should receive both pretest and posttest counseling according to standard genetic testing clinical practice guidelines.17,18
Bone Marrow Studies
MDS arising from an underlying marrow failure state can have distinguishing syndrome-specific features19–21 or can appear to be sporadic MDS. A marrow evaluation is imperative in all cases. In the case of a hypocellular or patchy marrow in which MDS is suspected, increased cluster of differentiation (CD) 34 count, which can be quantified by flow cytometry of bone marrow aspirates or immunohistochemistry on the core biopsy, favors hypoplastic MDS rather than aplastic anemia.22,23 Cytogenetics may also not grow or be nondiagnostic, and in that case fluorescence in situ hybridization (FISH) studies can be added to evaluate for the common aberrations.13,24 In patients with noninformative or nondiagnostic cytogenetics, in cases in which this information may change management, single nucleotide polymorphism (SNP) microarrays could be considered as an alternative karyotyping tool to detect most cytogenetic aberrations.25–27 In patients with cytopenias and suspicion of an inherited syndrome, repeat marrow examinations may be necessary to establish the diagnosis of MDS because of hypocellular or patchy marrows and background dysplasia. This testing can be done at the time of clinical changes with the interval between marrows informed by the severity of cytopenias.
Tiered Approached to Genetic Testing
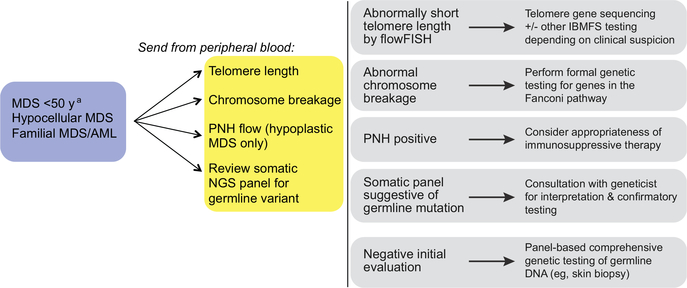
The authors’ practice is to use a stepwise approach to the genetic evaluation of patients with MDS (Fig. 1). The tiered methodology allows for attention to detail, managing appropriate patient-centered issues, and parallel testing (of potential donor) if appropriate or necessary; there must also be acknowledgment of both the financial and emotional cost of these pathways at the bedside. In patients in whom there is a presumed higher risk for a genetic predisposition to MDS or features suspicious for these diagnoses, initial screening is done from the peripheral blood if applicable. Notably these tests include flow cytometry for a PNH clone,28 especially if hypoplastic; telomere length measurement by CLIA (Clinical Laboratory Improvement Amendments)-certified flow cytometry and FISH (flowFISH)29; and chromosome breakage with diepoxybutane (DEB).30 Polymerase chain reaction–based quantification of telomere length is not a reliable measure and should not be used in clinical settings.29,31 The results of these initial tests help to further guide appropriate germline genetic testing.
Fig. 1.
Screening and evaluation for genetic predisposition to MDS. flowFISH, flow cytometry and FISH; IBMFS, inherited bone marrow failure syndrome; NGS, next-generation sequencing.
a Limiting screening to patients less than 50 years old misses cases of inherited MDS/AML.
The benefit of this approach is that these tests can be readily done from peripheral blood, are less expensive, and have a shorter turnaround time than most next-generation sequencing (NGS) platforms. This process quickly identifies patients at risk for short telomere syndromes and Fanconi anemia who are at high risk for increased toxicity from certain therapies, and the finding of a PNH clone, suggesting an acquired disorder, precludes the need for further genetic testing.32 A tiered approach also allows for expectation management and reassurance to patients and families when possible. However, it can be less efficient, even if it is resource conscious. Further, it is important to facilitate appropriate work-up for both the patients and any related potential donors. Related donors should be screened in a targeted fashion if a predisposition syndrome is identified in the recipient. This screening is relevant for fully matched siblings or haploidentical siblings, children, or parental donors. If the choice has been made a priori to use an unrelated donor, then the added stress of a familial work-up can be deferred or delayed until the patient has been treated.
Germline Source of DNA
It is critical at the bedside to evaluate the proper genetic material to document a germline disorder. Mutations in some of the genes known to cause hereditary myeloid malignancy can occur somatically. Thus, a germline source of DNA is imperative to distinguish acquired from inherited mutations. In addition, chromosomal aberrations and, in rare cases, revertant somatic mosaicism can obscure allele frequencies and may cause the genetic diagnosis to be missed if sequencing is only done from the peripheral blood.33–38
The preferred source of germline material for sequencing is DNA derived from skin fibroblasts cultured in a CLIA-certified laboratory.39 Some sequencing laboratories require up to 5 μg of DNA for complete testing, which is difficult to obtain from other tissue sources (ie, hair roots and nail clippings40). Fibroblasts are usually obtained from a 3-mm skin punch biopsy, which can be done at the bedside even in very young patients or thrombocytopenic patients. Because of the time required to culture fibroblasts (3–6 weeks), a skin biopsy should be obtained as early as clinically feasible in the diagnostic evaluation. Saliva, buccal swab, and DNA from a skin biopsy directly yield sufficient DNA but can be contaminated with circulating cells.41 In clinical situations in which a genetic diagnosis is needed urgently, these sources can be used initially with confirmatory testing done on cultured fibroblasts.11
Germline Next-Generation Sequencing Approaches
In the work-up of MDS, especially in adult hematology/oncology clinics, the use of NGS testing most often refers to targeted panels of somatic mutations known to be associated with myeloid malignancies.42 These panels are distinct from alternative panels specific to germline mutations. Because of the phenotypic overlap of syndromes and nonclassic presentations, a comprehensive panel-based approach inclusive of the many genes implicated in genetic predisposition to MDS/AML is imperative.
Attention to the details of the testing ordered is vital because, without specific knowledge of the results obtained, false reassurance could come from a negative test that does not cover the relevant genetic markers for the inherited syndrome; this may require specific consultation with the genetic counselors as well as molecular pathologists before testing or at the time of interpretation of the results. The use of NGS methodology to detect point mutations in addition to copy number changes including large deletions/duplications is key.4,43 It is also important that the NGS panel is specifically designed to capture certain noncoding regions that are known to be involved in disorders that predispose to MDS: the promoter in the 5′ untranslated region (UTR) of ANKRD26 (most families, reviewed by Godley43) and the enhancer region deep in intron 4 of GATA2 (NM_032638, accounting for at least 10% of families8,44). Capture of the UTR of ANRKD26 is variable on standard clinical whole-exome sequencing, and variants deep in the middle of the large GATA2 intron 4 are likely to be missed unless specifically targeted. Furthermore, somatic prognostic panels for MDS/AML, many of which include GATA2, do not capture these intronic variants nor report known pathogenic synonymous variants. There are several clinically available genetic testing panels for hereditary MDS/AML, and testing methodology, genes included and interpretation expertise, in addition to turnaround time and cost, should be assessed prior to test selection.
Variants in Known Predisposition Genes Identified on Somatic Panels
As discussed earlier, it is recommended as standard of care to consider molecular testing for somatic mutations associated with MDS.10 Increasingly these panels guide discussions of biology, prognosis, treatment pathways, and in rare instances targeted therapy on clinical trials. NGS of tumor samples using somatic panels may inadvertently identify patients at risk for germline predisposition to MDS/AML that were not otherwise appreciated to be high risk. Acquired pathogenic/likely pathogenic mutations in the same genes associated with genetic predisposition to MDS (ANKRD26, CEBPA, DDX41, ETV6, GATA2, RUNX1, or TP53) may be detected in more than 20% of patients tested with somatic myeloid malignancy panels.45 Because of gross chromosomal rearrangements and more subtle gains and losses, the variant allele frequency of mutations in peripheral blood–derived DNA from patients with MDS is commonly unreliable, and deleterious variants identified on prognostic panels, especially in DDX41 and GATA2,45 should be investigated for germline origin regardless of allele frequency.46 Ten percent of patients with biallelic CEBPA variants possess 1 of the 2 mutations in their germlines.47,48 In genes such as RUNX1 and TP53, the same variants can occur either in the germline or somatically in the MDS/AML clone. In these cases, the presence of a variant in the Catalogue Of Somatic Mutations In Cancer (COSMIC) database does not preclude it from being carried in the germline.49,50 Variants in other pathways, such as telomerase and telomere maintenance genes, are rarely found in sequenced MDS/AML samples.51 Germline confirmatory testing should be done in these cases to assess whether the somatically detected variant is really in the germline. In contrast, it is noteworthy that the absence of pathogenic variants on somatic panels does not exclude a germline predisposition and should not be used as a substitute for dedicated germline testing.
Interpretation of Somatic Gene Variants with Germline Allele Frequencies
In general, variants with allele frequencies between 40% and 60% could be germline, as opposed to acquired, in the malignant population. When these occur in genes known to be associated with an MDS predisposition syndrome, as described earlier and in Table 1, further consideration is warranted. When the variant occurs in genes known only to have a somatic role in MDS/AML, the clinician should be cautious before ascribing too much significance to it. It is possible that the variant could still be somatic and the allele frequency explained by the disease burden at the time of testing, or it could be a germline benign polymorphism. These potentially germline and inheritable variants should be approached carefully so as to ensure proper counseling but also avoidance of undue testing burden.
Interpretation of Germline Variants of Unknown Significance
When reviewing the results of somatic panel testing, it is possible for mutations that have a germline association to receive annotation. How these are codified and interpreted may vary by report. Interpretation of dedicated germline sequencing should be done according to guidelines for variant classification from the American College of Medical Genetics and Genomics, which recommends identified variants be assigned 1 of 5 categories: pathogenic, likely pathogenic, uncertain significance, likely benign, and benign.52 In patients referred to the laboratory in an academic center for panel-based testing of hereditary MDS/AML, a pathogenic or likely pathogenic variant established the diagnosis in 15–20% of patients, but more than one-third of the patients carried variants of uncertain significance (VUSs) in known genes.4 VUSs pose a challenge to clinicians and patients; thus, consultation with a geneticist may be indicated. Extreme caution must be taken when basing treatment decisions on the presence of a VUS so as to avoid ascribing a disorder to a nonpathogenic mutation. All individuals carry numerous heterozygous nonsynonymous coding variants in their germlines, many of which are common in the general population and likely benign polymorphisms.53 Determining the frequency of germline VUSs in the general population is useful in assessing their potential pathogenicity.54 In a rare mendelian disorder such as these myeloid diseases and syndromes, an allele frequency in the general population that is greater than expected for the disorder is less likely to be driving the disease. However, this assumption is less reliable in diseases with later onset in life.52,55,56 Functional studies, such as telomere length measurement or chromosome breakage, if not previously done, can aid in assessing the pathogenicity of VUSs where applicable. Use of genomic tumor boards or multidisciplinary groups with germline expertise, in practice at some institutions to assess VUSs, can aid in variant interpretation before clinical decisions are made.57 Variant pathogenicity should also be reevaluated as new cohorts are sequenced and new evidence is accrued. For example, TERT variants A202T, H412Y, and A1046T were at one time thought to be pathogenic, but evidence now suggests these are common polymorphisms.6,58,59 Additional assessments of variants, such as segregation of VUSs in asymptomatic relatives and in vitro functional studies, should be done on a research basis.
CLONAL HEMATOPOIESIS IN GERMLINE MYELODYSPLASTIC SYNDROMES
The mechanisms by which these germline predisposition syndromes are leukemogenic are not fully understood. There are a few recurrent chromosomal aberrations and somatic mutations that are important to highlight. These mutations can be seen recurrently within MDS/AML arising from a single syndrome, such as somatic TP53 mutations in Shwachman-Diamond, or shared across MDS arising from different syndromes, such as monosomy 7.
Recurrent Chromosomal Aberrations
The selective pressure of the failing marrow in several of the inherited BMF syndromes drives recurrent, nonrandom chromosomal aberrations,13,24 which are not necessarily leukemogenic but can affect prognosis. There is an increased prevalence of monosomy 7 in genetically mediated MDS compared with de novo, most commonly reported to date in SAMD9/9L in younger children and GATA2 in adolescents, but this can also be seen in the other syndromes. The loss of chromosome 7 or del(7q) in patients with SAMD9/9L mutations (located on chromosome 7q) deletes the mutant allele, alleviating the growth repression caused by the gain-of-function germline mutation. The outcome of this acquired monosomy 7 ranges from normalization of the karyotype and bone marrow (through duplication of the nonmutated allele) or progression to advanced MDS, thought to occur through acquisition of additional somatic driver mutations. The functional role of monosomy 7 in GATA2-deficient BMF is less clear. Isochromosome 7q and del20q occur in Shwachman-Diamond syndrome,60 and 1q+ and 3q26q29 amplifications are common in Fanconi anemia.61–63 Recurrent changes may be found in other inherited BMF syndromes as more cases are systematically studied. Review of the literature, even for case reports at the time of identification of these changes in a single patient, will be important as additional knowledge emerges.
Clonal Evolution Through Acquired Mutations
Driver and cooperating mutations are important for the pathogenesis of both de novo MDS/AML and MDS arising from a germline predisposition. The understanding of these co-mutational patterns is rapidly evolving; these acquired mutations may explain some of the variable penetrance and expressivity seen within families. For this reason, it is vital in older patients with previously undiagnosed predisposition syndromes to have both somatic and germline testing if applicable. In addition, patients with therapy-related myeloid malignancies may be more likely to harbor germline variants in predisposition genes and should be considered for testing as well.64
In unaffected RUNX1 carriers <50 years, 6 of 9 (67%) harbored detectable somatic mutations, and all of the patients with RUNX1-mediated MDS/AML (5 of 5) had somatic mutations, suggesting clonal hematopoiesis occurs before the development of overt MDS/AML in RUNX1 carriers.65 However, most of these mutations detected by exome sequencing occurred in genes different than those seen recurrently mutated in MDS. In patients with germline GATA2 mutations who developed MDS/AML, three-quarters (22 of 29) harbored MDS-associated somatic mutations; recurrent mutations in ASXL1 (40%, loss of function) and STAG2 (28%) were most common.66,67 In relatives also carrying GATA2 mutations but without MDS, it was less common for those with essentially normal marrows to have somatic driver mutations (1 of 5 studied), whereas, in those with abnormal marrows, 5 of 7 had somatic mutations, including 3 with ASXL1, suggesting this may be an intermediate phenotype in transition to an MDS state.67
Acquired TP53 mutations were seen in 7 of 7 young adults with biallelic SBDS mutations before HSCT for MDS.2 Further, ultradeep sequencing of patients with Shwachman-Diamond without MDS/AML has also identified acquired TP53 mutations in half (13 of 27 patients), although at exceptionally low allele frequencies (median 0.36%, range 0.05%–3.1%) of unclear clinical significance.68 The role of these recurrent events in the transformation to MDS/AML is an active area of research. Application of these somatic findings to monitoring and prevention is not yet established. Furthermore, the lack of prospective observational data limits the clinical ability to incorporate the knowledge of somatic variants’ presence for prognostication in predisposed patients before the development of MDS.
SURVEILLANCE AND MANAGEMENT
There are many bedside benefits of real-time diagnosis of a germline MDS predisposition disorder. Some germline MDS predisposition disorders are characterized by extrahematopoietic manifestations, which can contribute to significant morbidity and for which screening can change management (see Table 1). Another important clinical advantage is insight into the natural history of the disorder. Most importantly, the added knowledge of a germline syndrome can alter or facilitate more appropriate therapy in affected individuals. Presumed lack of responsiveness to noncurative therapies for a germline disease likely prompts HSCT evaluation sooner from an unrelated donor. Recognition of the possibility of these diagnostic and treatment interactions is only the first step and then appropriate referrals with the use of resources can follow.71
Selection of Related Donors
Recognition of an inherited disorder is important before assessment and selection of a related donor. Unexplained cytopenias, recurrent/severe infections, or failure to mobilize stem cells in a donor may be caused by an underlying marrow failure syndrome and warrant additional investigation. However, completely asymptomatic related donors may still be carriers of the familial mutation because there can be significant heterogeneity for hematologic and extrahematopoietic manifestations within and across families carrying the same mutation. If a mutation has been identified in the recipient, potential related donors should be counseled and then offered targeted testing to screen for this mutation. Results of this testing are important both for decision on use as a donor as well as to identify the person’s own potential predisposition and disease risk. These aspects should be explained clearly to the patient and related donor as the process is ongoing. Using a related donor carrying the familial mutation puts both the donor and recipient at risk for complication.72,73
If the personal or family history is suspicious for a germline MDS predisposition syndrome but no causative mutation can be identified, an attempt should be made to find a matched unrelated donor in hopes of avoidance of the conceivable risk of transplanting the causative mutation. If there is no HLA-matched unrelated donor or there is an urgent indication for HSCT, the potential risks and benefits should be discussed with both the recipient and the donor.73 It is also possible to use second-degree relatives as haploidentical donors, so there may be less traditional options to find a suitable donor for potentially curative HSCT in these families as well.74 Regardless, bone marrow studies and thorough hematologic evaluation in relatives under consideration for allograft donation is strongly encouraged.
Recipient Specific Implications
Diagnosis of a germline MDS predisposition disorder also has implications for timing of transplant, preparative regimens, and posttransplant care. Understanding the natural history of individual disorders is critical to making decisions at the bedside. Patients with Fanconi anemia and short telomere syndromes can experience increased toxicity from standard chemotherapy and radiation and need attenuated regimens; however, there is more published experience with HSCT for BMF than MDS.75–77 Patients with short telomere syndromes post-HSCT continue to be at risk for additional short telomere manifestations as they age, which can occur at an earlier age in this setting. Patients with Fanconi anemia have an increased cancer risk post-HSCT.69,78 Patients with germline CEBPA mutations are at risk of second primary AMLs, but prospective trials have not been done to assess the best timing for HSCT in this situation.
Familial Implications
Identification of a genetic predisposition to MDS has implications for both the immediate and extended family. This possibility should be discussed with the patients by genetic counselors or clinicians experienced in these issues before genetic testing is pursued. In children, parental testing to determine whether a variant is inherited or de novo should be obtained before screening asymptomatic siblings. Genetic testing of asymptomatic individuals, especially children, should only be undertaken after consultation of risks and benefits with a genetic counselor. This consultation can become more complicated with adult children of older patients with MDS, given that it is less expected in these ages to diagnose a germline condition. Dialogues surrounding the repercussions must be had transparently with all those involved.
EMERGING IDEAS
Discovery
The genetic cause for more than half of familial MDS/AML cases remains unsolved.6,38,65,80 Further study of these families, especially of those who present as adults, has the potential to identify new mechanisms of disease in known genes (regulatory mutations, synonymous mutations) and identify new genes and pathways. This knowledge will aid in diagnosis, surveillance, and management, and also may provide insight into leukemogenic mechanisms for prevention and targeted therapy in both germline and somatic conditions.
Prevention and Treatment
Increasing use of targeted treatments in MDS/AML may apply to familial cases with sporadic mutations in these same pathways. Understanding of the pathogenesis in specific syndromes may also lead to targeted therapies that can be used for prevention of MDS/AML. It is hoped that additional investigation of genetic and epigenetic alterations in these patients will elucidate the mechanisms of reduced penetrance in some syndromes and also lead to prevention strategies. Acquisition of multiple somatic mutations in genes recurrently mutated in myeloid malignancy is high risk for transformation. No prospective trials exist to guide treatment decisions, but consideration of intervention should be discussed with the patient.
SUMMARY
Germline predisposition to MDS, even in adults, is more common than was previously recognized and important to diagnose in real time before treatment, and especially before HSCT. Individual syndromes may be rare but, collectively, represent a significant risk in both pediatric and adult patients. Awareness of the risk as well as methods of identification are increasingly important steps in providing high-quality care to all patients with MDS. Growing knowledge about this has the potential to lead to personalized treatment paradigms for these patients that also have broader implications for the more numerous patients with somatic mutations in similar pathways.
KEY POINTS.
Inherited predisposition to myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) occurs in children as well as older adults.
Analysis of the genetics of the disease is now standard of care in the evaluation of patients with MDS.
Patients without syndromic features and negative family histories can still have a germline predisposition to MDS.
Somatic tumor panels cannot replace dedicated genetic evaluations for germline mutations in the many genes implicated in genetic predisposition to MDS/AML.
Diagnosis of an inherited predisposition has important implications for counseling and management.
ACKNOWLEDGEMENTS
The work of K.E.S. is supported by NIH T32HL007525.
Footnotes
DISCLOSURE
The authors declare no relevant conflicts of interest.
REFERENCES
- 1.Ma X Epidemiology of myelodysplastic syndromes. Am J Med 2012;125(7 Suppl):S2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindsley RC, Saber W, Mar BG, et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med 2017;376(6):536–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Nichols KE, Downing JR. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med 2016;374(14):1391. [DOI] [PubMed] [Google Scholar]
- 4.Guidugli L, Johnson AK, Alkorta-Aranburu G, et al. Clinical utility of gene panel-based testing for hereditary myelodysplastic syndrome/acute leukemia predisposition syndromes. Leukemia 2017;31(5):1226–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch CM, Przychodzen BP, Radivoyevitch T, et al. Molecular features of early onset adult myelodysplastic syndrome. Haematologica 2017;102(6):1028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keel SB, Scott A, Sanchez-Bonilla M, et al. Genetic features of myelodysplastic syndrome and aplastic anemia in pediatric and young adult patients. Haematologica 2016;101(11):1343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz JR, Ma J, Lamprecht T, et al. The genomic landscape of pediatric myelodysplastic syndromes. Nat Commun 2017;8(1):1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wlodarski MW, Hirabayashi S, Pastor V, et al. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood 2016;127(11):1387–97 [quiz: 1518]. [DOI] [PubMed] [Google Scholar]
- 9.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127(20):2391–405. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg PL, Stone RM, Al-Kali A, et al. Myelodysplastic syndromes, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017;15(1):60–87. [DOI] [PubMed] [Google Scholar]
- 11.University of Chicago Hematopoietic Malignancies Cancer Risk Team. How I diagnose and manage individuals at risk for inherited myeloid malignancies. Blood 2016;128(14):1800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk 2016;16(7):417–28.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godley LA, Shimamura A. Genetic predisposition to hematologic malignancies: management and surveillance. Blood 2017;130(4):424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frohnmayer D, Frohnmayer L, Guinan E, et al. Fanconi Anemia: Guidelines for Diagnosis and Management. 4th edition. Eugene, (OR): Fanconi Anemia Research Fund, Inc; 2014. [Google Scholar]
- 15.Vlachos A, Ball S, Dahl N, et al. Diagnosing and treating Diamond Blackfan anaemia: results of an international clinical consensus conference. Br J Haematol 2008;142(6):859–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savage SA, Cook EF. Dyskeratosis Congenita and Telomere Biology Disorders: Diagnosis and Management Guidelines. 1st edition. New York: Dyskeratosis Congenita Outreach, Inc; 2015. [Google Scholar]
- 17.Lu KH, Wood ME, Daniels M, et al. American Society of Clinical Oncology Expert Statement: collection and use of a cancer family history for oncology providers. J Clin Oncol 2014;32(8):833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanislaw C, Xue Y, Wilcox WR. Genetic evaluation and testing for hereditary forms of cancer in the era of next-generation sequencing. Cancer Biol Med 2016;13(1):55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvo KR, Vinh DC, Maric I, et al. Myelodysplasia in autosomal dominant and sporadic monocytopenia immunodeficiency syndrome: diagnostic features and clinical implications. Haematologica 2011;96(8):1221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proytcheva MA. Diagnostic pediatric hematopathology. Cambridge (United Kingdom): Cambridge University Press; 2011. [Google Scholar]
- 21.Kallen ME, Dulau-Florea A, Wang W, et al. Acquired and germline predisposition to bone marrow failure: Diagnostic features and clinical implications. Semin Hematol 2019;56(1):69–82. [DOI] [PubMed] [Google Scholar]
- 22.Matsui WH, Brodsky RA, Smith BD, et al. Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia 2006;20(3):458–62. [DOI] [PubMed] [Google Scholar]
- 23.DeZern AE, Sekeres MA. The challenging world of cytopenias: distinguishing myelodysplastic syndromes from other disorders of marrow failure. Oncologist 2014;19(7):735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy AL, Shimamura A. Genetic predisposition to MDS: clinical features and clonal evolution. Blood 2019;133(10):1071–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medeiros BC, Othus M, Estey EH, et al. Unsuccessful diagnostic cytogenetic analysis is a poor prognostic feature in acute myeloid leukaemia. Br J Haematol 2014;164(2):245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiu RV, Gondek LP, O’Keefe CL, et al. New lesions detected by single nucleotide polymorphism array-based chromosomal analysis have important clinical impact in acute myeloid leukemia. J Clin Oncol 2009;27(31):5219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dougherty MJ, Wilmoth DM, Tooke LS, et al. Implementation of high resolution single nucleotide polymorphism array analysis as a clinical test for patients with hematologic malignancies. Cancer Genet 2011;204(1):26–38. [DOI] [PubMed] [Google Scholar]
- 28.Dezern AE, Borowitz MJ. ICCS/ESCCA consensus guidelines to detect GPI-deficient cells in paroxysmal nocturnal hemoglobinuria (PNH) and related disorders part 1 - clinical utility. Cytometry B Clin Cytom 2018;94(1):16–22. [DOI] [PubMed] [Google Scholar]
- 29.Alder JK, Hanumanthu VS, Strong MA, et al. Diagnostic utility of telomere length testing in a hospital-based setting. Proc Natl Acad Sci U S A 2018;115(10): E2358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet 2015;85:8.7.1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin-Ruiz CM, Baird D, Roger L, et al. Reproducibility of telomere length assessment–an international collaborative study. Int J Epidemiol 2015;44(5): 1749–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeZern AE, Symons HJ, Resar LS, et al. Detection of paroxysmal nocturnal hemoglobinuria clones to exclude inherited bone marrow failure syndromes. Eur J Haematol 2014;92(6):467–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alder JK, Stanley SE, Wagner CL, et al. Exome sequencing identifies mutant TINF2 in a family with pulmonary fibrosis. Chest 2015;147(5):1361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gross M, Hanenberg H, Lobitz S, et al. Reverse mosaicism in Fanconi anemia: natural gene therapy via molecular self-correction. Cytogenet Genome Res 2002;98(2–3):126–35. [DOI] [PubMed] [Google Scholar]
- 35.Jongmans MC, Verwiel ET, Heijdra Y, et al. Revertant somatic mosaicism by mitotic recombination in dyskeratosis congenita. Am J Hum Genet 2012;90(3): 426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pastor VB, Sahoo SS, Boklan J, et al. Constitutional SAMD9L mutations cause familial myelodysplastic syndrome and transient monosomy 7. Haematologica 2018;103(3):427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tesi B, Davidsson J, Voss M, et al. Gain-of-function SAMD9L mutations cause a syndrome of cytopenia, immunodeficiency, MDS, and neurological symptoms. Blood 2017;129(16):2266–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bluteau O, Sebert M, Leblanc T, et al. A landscape of germ line mutations in a cohort of inherited bone marrow failure patients. Blood 2018;131(7):717–32. [DOI] [PubMed] [Google Scholar]
- 39.Teer JK, Zhang Y, Chen L, et al. Evaluating somatic tumor mutation detection without matched normal samples. Hum Genomics 2017;11(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padron E, Ball MC, Teer JK, et al. Germ line tissues for optimal detection of somatic variants in myelodysplastic syndromes. Blood 2018;131(21):2402–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Looi ML, Zakaria H, Osman J, et al. Quantity and quality assessment of DNA extracted from saliva and blood. Clin Lab 2012;58(3–4):307–12. [PubMed] [Google Scholar]
- 42.Steensma DP. How I use molecular genetic tests to evaluate patients who have or may have myelodysplastic syndromes. Blood 2018;132(16):1657–63. [DOI] [PubMed] [Google Scholar]
- 43.Godley LA. Inherited predisposition to acute myeloid leukemia. Semin Hematol 2014;51(4):306–21. [DOI] [PubMed] [Google Scholar]
- 44.Hsu AP, Johnson KD, Falcone EL, et al. GATA2 haploinsufficiency caused by mutations in a conserved intronic element leads to MonoMAC syndrome. Blood 2013;121(19):3830–7. S3831–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drazer MW, Kadri S, Sukhanova M, et al. Prognostic tumor sequencing panels frequently identify germ line variants associated with hereditary hematopoietic malignancies. Blood Adv 2018;2(2):146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Churpek JE. Familial myelodysplastic syndrome/acute myeloid leukemia. Best Pract Res Clin Haematol 2017;30(4):287–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pabst T, Eyholzer M, Haefliger S, et al. Somatic CEBPA mutations are a frequent second event in families with germline CEBPA mutations and familial acute myeloid leukemia. J Clin Oncol 2008;26(31):5088–93. [DOI] [PubMed] [Google Scholar]
- 48.Taskesen E, Bullinger L, Corbacioglu A, et al. Prognostic impact, concurrent genetic mutations, and gene expression features of AML with CEBPA mutations in a cohort of 1182 cytogenetically normal AML patients: further evidence for CEBPA double mutant AML as a distinctive disease entity. Blood 2011;117(8): 2469–75. [DOI] [PubMed] [Google Scholar]
- 49.Osato M Point mutations in the RUNX1/AML1 gene: another actor in RUNX leukemia. Oncogene 2004;23(24):4284–96. [DOI] [PubMed] [Google Scholar]
- 50.Preudhomme C, Renneville A, Bourdon V, et al. High frequency of RUNX1 biallelic alteration in acute myeloid leukemia secondary to familial platelet disorder. Blood 2009;113(22):5583–7. [DOI] [PubMed] [Google Scholar]
- 51.Walter MJ, Shen D, Shao J, et al. Clonal diversity of recurrently mutated genes in myelodysplastic syndromes. Leukemia 2013;27(6):1275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17(5):405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.1000 Genomes Project Consortium, Abecasis GR, Auton A, Brooks LD, et al. An integrated map of genetic variation from 1,092 human genomes. Nature 2012; 491(7422):56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karczewski K, Francioli LC, Tiao G, et al. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. bioRxiv 2019. [Google Scholar]
- 55.de Andrade KC, Frone MN, Wegman-Ostrosky T, et al. Variable population prevalence estimates of germline TP53 variants: a gnomAD-based analysis. Hum Mutat 2019;40(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soussi T, Leroy B, Devir M, et al. High prevalence of cancer-associated TP53 variants in the gnomAD database: a word of caution concerning the use of variant filtering. Hum Mutat 2019;40(5):516–24. [DOI] [PubMed] [Google Scholar]
- 57.van der Velden DL, van Herpen CML, van Laarhoven HWM, et al. Molecular Tumor Boards: current practice and future needs. Ann Oncol 2017;28(12):3070–5. [DOI] [PubMed] [Google Scholar]
- 58.Alder JK, Chen JJ, Lancaster L, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A 2008;105(35):13051–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du HY, Pumbo E, Ivanovich J, et al. TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood 2009;113(2):309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maserati E, Minelli A, Pressato B, et al. Shwachman syndrome as mutator phenotype responsible for myeloid dysplasia/neoplasia through karyotype instability and chromosomes 7 and 20 anomalies. Genes Chromosomes Cancer 2006;45(4):375–82. [DOI] [PubMed] [Google Scholar]
- 61.Cioc AM, Wagner JE, MacMillan ML, et al. Diagnosis of myelodysplastic syndrome among a cohort of 119 patients with fanconi anemia: morphologic and cytogenetic characteristics. Am J Clin Pathol 2010;133(1):92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quentin S, Cuccuini W, Ceccaldi R, et al. Myelodysplasia and leukemia of Fanconi anemia are associated with a specific pattern of genomic abnormalities that includes cryptic RUNX1/AML1 lesions. Blood 2011;117(15):e161–70. [DOI] [PubMed] [Google Scholar]
- 63.Tonnies H, Huber S, Kuhl JS, et al. Clonal chromosomal aberrations in bone marrow cells of Fanconi anemia patients: gains of the chromosomal segment 3q26q29 as an adverse risk factor. Blood 2003;101(10):3872–4. [DOI] [PubMed] [Google Scholar]
- 64.Churpek JE, Larson RA. The evolving challenge of therapy-related myeloid neoplasms. Best Pract Res Clin Haematol 2013;26(4):309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Churpek JE, Pyrtel K, Kanchi KL, et al. Genomic analysis of germ line and somatic variants in familial myelodysplasia/acute myeloid leukemia. Blood 2015; 126(22):2484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ding LW, Ikezoe T, Tan KT, et al. Mutational profiling of a MonoMAC syndrome family with GATA2 deficiency. Leukemia 2017;31(1):244–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McReynolds LJ, Yang Y, Yuen Wong H, et al. MDS-associated mutations in germline GATA2 mutated patients with hematologic manifestations. Leuk Res 2019;76:70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xia J, Miller CA, Baty J, et al. Somatic mutations and clonal hematopoiesis in congenital neutropenia. Blood 2018;131(4):408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alter BP, Giri N, Savage SA, et al. Cancer in the National Cancer Institute inherited bone marrow failure syndrome cohort after fifteen years of follow-up. Haematologica 2018;103(1):30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quesada AE, Routbort MJ, DiNardo CD, et al. DDX41 mutations in myeloid neoplasms are associated with male gender, TP53 mutations and high-risk disease. Am J Hematol 2019;94(7):757–66. [DOI] [PubMed] [Google Scholar]
- 71.Clifford M, Bannon S, Bednar EM, et al. Clinical applicability of proposed algorithm for identifying individuals at risk for hereditary hematologic malignancies. Leuk Lymphoma 2019;60(12):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Churpek JE, Artz A, Bishop M, et al. Correspondence regarding the consensus statement from the worldwide network for blood and marrow transplantation standing committee on donor issues. Biol Blood Marrow Transplant 2016; 22(1):183–4. [DOI] [PubMed] [Google Scholar]
- 73.Gadalla SM, Wang T, Haagenson M, et al. Association between donor leukocyte telomere length and survival after unrelated allogeneic hematopoietic cell transplantation for severe aplastic anemia. JAMA 2015;313(6):594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elmariah H, Kasamon YL, Zahurak M, et al. Haploidentical bone marrow transplantation with post-transplant cyclophosphamide using non-first-degree related donors. Biol Blood Marrow Transplant 2018;24(5):1099–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gadalla SM, Sales-Bonfim C, Carreras J, et al. Outcomes of allogeneic hematopoietic cell transplantation in patients with dyskeratosis congenita. Biol Blood Marrow Transplant 2013;19(8):1238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barbaro P, Vedi A. Survival after hematopoietic stem cell transplant in patients with dyskeratosis congenita: systematic review of the literature. Biol Blood Marrow Transplant 2016;22(7):1152–8. [DOI] [PubMed] [Google Scholar]
- 77.Dietz AC, Orchard PJ, Baker KS, et al. Disease-specific hematopoietic cell transplantation: nonmyeloablative conditioning regimen for dyskeratosis congenita. Bone Marrow Transpl 2011;46(1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anur P, Friedman DN, Sklar C, et al. Late effects in patients with Fanconi anemia following allogeneic hematopoietic stem cell transplantation from alternative donors. Bone Marrow Transplant 2016;51(7):938–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gable DL, Gaysinskaya V, Atik CC, et al. ZCCHC8, the nuclear exosome targeting component, is mutated in familial pulmonary fibrosis and is required for telomerase RNA maturation. Genes Dev 2019;33(19–20):1381–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holme H, Hossain U, Kirwan M, et al. Marked genetic heterogeneity in familial myelodysplasia/acute myeloid leukaemia. Br J Haematol 2012;158(2):242–8. [DOI] [PubMed] [Google Scholar]
- 81.Smith ML, Cavenagh JD, Lister TA, et al. Mutation of CEBPA in familial acute myeloid leukemia. N Engl J Med 2004;351(23):2403–7. [DOI] [PubMed] [Google Scholar]
- 82.Sellick GS, Spendlove HE, Catovsky D, et al. Further evidence that germline CEBPA mutations cause dominant inheritance of acute myeloid leukaemia. Leukemia 2005;19(7):1276–8. [DOI] [PubMed] [Google Scholar]
- 83.Stelljes M, Corbacioglu A, Schlenk RF, et al. Allogeneic stem cell transplant to eliminate germline mutations in the gene for CCAAT-enhancer-binding protein alpha from hematopoietic cells in a family with AML. Leukemia 2011;25(7): 1209–10. [DOI] [PubMed] [Google Scholar]
- 84.Tawana K, Wang J, Renneville A, et al. Disease evolution and outcomes in familial AML with germline CEBPA mutations. Blood 2015;126(10):1214–23. [DOI] [PubMed] [Google Scholar]
- 85.Pathak A, Seipel K, Pemov A, et al. Whole exome sequencing reveals a C-terminal germline variant in CEBPA-associated acute myeloid leukemia: 45-year follow up of a large family. Haematologica 2016;101(7):846–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yan B, Ng C, Moshi G, et al. Myelodysplastic features in a patient with germline CEBPA-mutant acute myeloid leukaemia. J Clin Pathol 2016;69(7):652–4. [DOI] [PubMed] [Google Scholar]
- 87.Polprasert C, Schulze I, Sekeres MA, et al. Inherited and somatic defects in DDX41 in myeloid neoplasms. Cancer cell 2015;27(5):658–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cardoso SR, Ryan G, Walne AJ, et al. Germline heterozygous DDX41 variants in a subset of familial myelodysplasia and acute myeloid leukemia. Leukemia 2016;30(10):2083–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lewinsohn M, Brown AL, Weinel LM, et al. Novel germ line DDX41 mutations define families with a lower age of MDS/AML onset and lymphoid malignancies. Blood 2016;127(8):1017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li R, Sobreira N, Witmer PD, et al. Two novel germline DDX41 mutations in a family with inherited myelodysplasia/acute myeloid leukemia. Haematologica 2016;101(6):e228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kobayashi S, Kobayashi A, Osawa Y, et al. Donor cell leukemia arising from preleukemic clones with a novel germline DDX41 mutation after allogenic hematopoietic stem cell transplantation. Leukemia 2017;31(4):1020–2. [DOI] [PubMed] [Google Scholar]
- 92.Noris P, Perrotta S, Seri M, et al. Mutations in ANKRD26 are responsible for a frequent form of inherited thrombocytopenia: analysis of 78 patients from 21 families. Blood 2011;117(24):6673–80. [DOI] [PubMed] [Google Scholar]
- 93.Pippucci T, Savoia A, Perrotta S, et al. Mutations in the 5’ UTR of ANKRD26, the ankirin repeat domain 26 gene, cause an autosomal-dominant form of inherited thrombocytopenia, THC2. Am J Hum Genet 2011;88(1):115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Al Daama SA, Housawi YH, Dridi W, et al. A missense mutation in ANKRD26 segregates with thrombocytopenia. Blood 2013;122(3):461–2. [DOI] [PubMed] [Google Scholar]
- 95.Noris P, Favier R, Alessi MC, et al. ANKRD26-related thrombocytopenia and myeloid malignancies. Blood 2013;122(11):1987–9. [DOI] [PubMed] [Google Scholar]
- 96.Marquez R, Hantel A, Lorenz R, et al. A new family with a germline ANKRD26 mutation and predisposition to myeloid malignancies. Leuk Lymphoma 2014; 55(12):2945–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perez Botero J, Oliveira JL, Chen D, et al. ASXL1 mutated chronic myelomonocytic leukemia in a patient with familial thrombocytopenia secondary to germline mutation in ANKRD26. Blood Cancer J 2015;5:e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marconi C, Canobbio I, Bozzi V, et al. 5’UTR point substitutions and N-terminal truncating mutations of ANKRD26 in acute myeloid leukemia. J Hematol Oncol 2017;10(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moriyama T, Metzger ML, Wu G, et al. Germline genetic variation in ETV6 and risk of childhood acute lymphoblastic leukaemia: a systematic genetic study. Lancet Oncol 2015;16(16):1659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Noetzli L, Lo RW, Lee-Sherick AB, et al. Germline mutations in ETV6 are associated with thrombocytopenia, red cell macrocytosis and predisposition to lymphoblastic leukemia. Nat Genet 2015;47(5):535–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Topka S, Vijai J, Walsh MF, et al. Germline ETV6 mutations confer susceptibility to acute lymphoblastic leukemia and thrombocytopenia. Plos Genet 2015;11(6): e1005262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang MY, Churpek JE, Keel SB, et al. Germline ETV6 mutations in familial thrombocytopenia and hematologic malignancy. Nat Genet 2015;47(2):180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Melazzini F, Palombo F, Balduini A, et al. Clinical and pathogenic features of ETV6-related thrombocytopenia with predisposition to acute lymphoblastic leukemia. Haematologica 2016;101(11):1333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Poggi M, Canault M, Favier M, et al. Germline variants in ETV6 underlie reduced platelet formation, platelet dysfunction and increased levels of circulating CD341 progenitors. Haematologica 2017;102(2):282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Di Paola J, Porter CC. ETV6-related thrombocytopenia and leukemia predisposition. Blood 2019;134(8):663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rampersaud E, Ziegler DS, Iacobucci I, et al. Germline deletion of ETV6 in familial acute lymphoblastic leukemia. Blood Adv 2019;3(7):1039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hahn CN, Chong CE, Carmichael CL, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet 2011;43(10):1012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hsu AP, Sampaio EP, Khan J, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood 2011;118(10):2653–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kazenwadel J, Secker GA, Liu YJ, et al. Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood 2012; 119(5):1283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Spinner MA, Sanchez LA, Hsu AP, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood 2014;123(6):809–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fisher KE, Hsu AP, Williams CL, et al. Somatic mutations in children with GATA2-associated myelodysplastic syndrome who lack other features of GATA2 deficiency. Blood Adv 2017;1(7):443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Song WJ, Sullivan MG, Legare RD, et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat Genet 1999;23(2):166–75. [DOI] [PubMed] [Google Scholar]
- 113.Buijs A, Poddighe P, van Wijk R, et al. A novel CBFA2 single-nucleotide mutation in familial platelet disorder with propensity to develop myeloid malignancies. Blood 2001;98(9):2856–8. [DOI] [PubMed] [Google Scholar]
- 114.Beri-Dexheimer M, Latger-Cannard V, Philippe C, et al. Clinical phenotype of germline RUNX1 haploinsufficiency: from point mutations to large genomic deletions. Eur J Hum Genet 2008;16(8):1014–8. [DOI] [PubMed] [Google Scholar]
- 115.Owen CJ, Toze CL, Koochin A, et al. Five new pedigrees with inherited RUNX1 mutations causing familial platelet disorder with propensity to myeloid malignancy. Blood 2008;112(12):4639–45. [DOI] [PubMed] [Google Scholar]
- 116.Sorrell A, Espenschied C, Wang W, et al. Hereditary leukemia due to rare RUNX1c splice variant (L472X) presents with eczematous phenotype. Int J Clin Med 2012;3(7):607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schmit JM, Turner DJ, Hromas RA, et al. Two novel RUNX1 mutations in a patient with congenital thrombocytopenia that evolved into a high grade myelodysplastic syndrome. Leuk Res Rep 2015;4(1):24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Latger-Cannard V, Philippe C, Bouquet A, et al. Haematological spectrum and genotype-phenotype correlations in nine unrelated families with RUNX1 mutations from the French network on inherited platelet disorders. Orphanet J Rare Dis 2016;11:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kanagal-Shamanna R, Loghavi S, DiNardo CD, et al. Bone marrow pathologic abnormalities in familial platelet disorder with propensity for myeloid malignancy and germline RUNX1 mutation. Haematologica 2017;102(10):1661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chisholm KM, Denton C, Keel S, et al. Bone marrow morphology associated with germline RUNX1 mutations in patients with familial platelet disorder with associated myeloid malignancy. Pediatr Dev Pathol 2019;22(4):315–28. [DOI] [PubMed] [Google Scholar]
- 121.Chen DH, Below JE, Shimamura A, et al. Ataxia-pancytopenia syndrome is caused by missense mutations in SAMD9L. Am J Hum Genet 2016;98(6): 1146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Narumi S, Amano N, Ishii T, et al. SAMD9 mutations cause a novel multisystem disorder, MIRAGE syndrome, and are associated with loss of chromosome 7. Nat Genet 2016;48(7):792–7. [DOI] [PubMed] [Google Scholar]
- 123.Buonocore F, Kuhnen P, Suntharalingham JP, et al. Somatic mutations and progressive monosomy modify SAMD9-related phenotypes in humans. J Clin Invest 2017;127(5):1700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sarthy J, Zha J, Babushok D, et al. Poor outcome with hematopoietic stem cell transplantation for bone marrow failure and MDS with severe MIRAGE syndrome phenotype. Blood Adv 2018;2(2):120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vlachos A, Rosenberg PS, Atsidaftos E, et al. Incidence of neoplasia in Diamond Blackfan anemia: a report from the Diamond Blackfan Anemia Registry. Blood 2012;119(16):3815–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arbiv OA, Cuvelier G, Klaassen RJ, et al. Molecular analysis and genotype-phenotype correlation of Diamond-Blackfan anemia. Clin Genet 2018;93(2): 320–8. [DOI] [PubMed] [Google Scholar]
- 127.Simkins A, Bannon SA, Khoury JD, et al. Diamond-Blackfan anemia predisposing to myelodysplastic syndrome in early adulthood. JCO Precis Oncol 2017;(1):1–5. [DOI] [PubMed] [Google Scholar]
- 128.Vlachos A, Rosenberg PS, Atsidaftos E, et al. Increased risk of colon cancer and osteogenic sarcoma in Diamond-Blackfan anemia. Blood 2018;132(20):2205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alter BP. Fanconi anemia and the development of leukemia. Best Pract Res Clin Haematol 2014;27(3–4):214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Savage SA, Walsh MF. Myelodysplastic syndrome, acute myeloid leukemia, and cancer surveillance in Fanconi anemia. Hematol Oncol Clin North Am 2018; 32(4):657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. J Clin Oncol 2013;31(13): 1669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kutler DI, Singh B, Satagopan J, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood 2003;101(4):1249–56. [DOI] [PubMed] [Google Scholar]
- 133.Dokal I, Bungey J, Williamson P, et al. Dyskeratosis congenita fibroblasts are abnormal and have unbalanced chromosomal rearrangements. Blood 1992; 80(12):3090–6. [PubMed] [Google Scholar]
- 134.Yamaguchi H, Baerlocher GM, Lansdorp PM, et al. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood 2003;102(3):916–8. [DOI] [PubMed] [Google Scholar]
- 135.Ortmann CA, Niemeyer CM, Wawer A, et al. TERC mutations in children with refractory cytopenia. Haematologica 2006;91(5):707–8. [PubMed] [Google Scholar]
- 136.Armanios MY, Chen JJ, Cogan JD, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 2007;356(13):1317–26. [DOI] [PubMed] [Google Scholar]
- 137.Kirwan M, Vulliamy T, Marrone A, et al. Defining the pathogenic role of telomerase mutations in myelodysplastic syndrome and acute myeloid leukemia. Hum Mutat 2009;30(11):1567–73. [DOI] [PubMed] [Google Scholar]
- 138.Jyonouchi S, Forbes L, Ruchelli E, et al. Dyskeratosis congenita: a combined immunodeficiency with broad clinical spectrum–a single-center pediatric experience. Pediatr Allergy Immunol 2011;22(3):313–9. [DOI] [PubMed] [Google Scholar]
- 139.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet 2012; 13(10):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gorgy AI, Jonassaint NL, Stanley SE, et al. Hepatopulmonary syndrome is a frequent cause of dyspnea in the short telomere disorders. Chest 2015; 148(4):1019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stanley SE, Rao AD, Gable DL, et al. Radiation sensitivity and radiation necrosis in the short telomere syndromes. Int J Radiat Oncol Biol Phys 2015;93(5): 1115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Burris AM, Ballew BJ, Kentosh JB, et al. Hoyeraal-Hreidarsson syndrome due to PARN mutations: fourteen years of follow-up. Pediatr Neurol 2016;56:62–68 e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cardoso SR, Ellison ACM, Walne AJ, et al. Myelodysplasia and liver disease extend the spectrum of RTEL1 related telomeropathies. Haematologica 2017; 102(8):e293–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wagner CL, Hanumanthu VS, Talbot CC Jr, et al. Short telomere syndromes cause a primary T cell immunodeficiency. The J Clin Invest 2018;128(12): 5222–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Donadieu J, Fenneteau O, Beaupain B, et al. Classification of and risk factors for hematologic complications in a French national cohort of 102 patients with Shwachman-Diamond syndrome. Haematologica 2012;97(9):1312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Myers KC, Bolyard AA, Otto B, et al. Variable clinical presentation of Shwachman-Diamond syndrome: update from the North American Shwachman-Diamond Syndrome Registry. J Pediatr 2014;164(4):866–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Boocock GR, Morrison JA, Popovic M, et al. Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nat Genet 2003;33(1):97–101. [DOI] [PubMed] [Google Scholar]
- 148.Schlegelberger B, Kreipe H, Lehmann U, et al. A child with Li-Fraumeni syndrome: modes to inactivate the second allele of TP53 in three different malignancies. Pediatr Blood Cancer 2015;62(8):1481–4. [DOI] [PubMed] [Google Scholar]
- 149.Swaminathan M, Bannon SA, Routbort M, et al. Hematologic malignancies and Li-Fraumeni syndrome. Cold Spring Harb Mol Case Stud 2019;5(1) [pii: a003210]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Talwalkar SS, Yin CC, Naeem RC, et al. Myelodysplastic syndromes arising in patients with germline TP53 mutation and Li-Fraumeni syndrome. Arch Pathol Lab Med 2010;134(7):1010–5. [DOI] [PubMed] [Google Scholar]
- 151.Lynch HT, Weisenburger DD, Quinn-Laquer B, et al. Family with acute myelocytic leukemia, breast, ovarian, and gastrointestinal cancer. Cancer Genet Cytogenet 2002;137(1):8–14. [DOI] [PubMed] [Google Scholar]
- 152.Janiszewska H, Bak A, Skonieczka K, et al. Constitutional mutations of the CHEK2 gene are a risk factor for MDS, but not for de novo AML. Leuk Res 2018;70:74–8. [DOI] [PubMed] [Google Scholar]