Abstract
Background:
In children with prenatal alcohol exposure, spatial working memory is affected and brain regions important for spatial working memory performance exhibit atypical neurodevelopment. We therefore hypothesized that children with prenatal alcohol exposure may also have atypical development of spatial working memory ability.
Method:
We examined the relation between spatial working memory and age using a cross-sectional developmental trajectory approach in youth with and without histories of heavy prenatal alcohol exposure. The CANTAB Spatial Working Memory subtest was administered to children 5.0–16.9 years old.
Results:
While the controls and children with prenatal alcohol exposure showed similar performance at younger ages, larger group differences were observed in older children. This effect was replicated in a separate sample.
Conclusions:
The atypical brain development that has previously been reported in children with heavy prenatal alcohol exposure may have clinically relevant implications for cognitive development; however, longitudinal cognitive analyses are needed.
Keywords: FASD, prenatal alcohol, development, cognition, spatial working memory
Introduction
Alcohol can disrupt prenatal development, yielding a variety of protracted effects and impairment across the lifespan. Such exposure can be particularly damaging to the nascent brain (Riley et al. 2011) and disrupt its progressive development. This early insult to brain development can result in neurobehavioral deficits (Mattson et al., 2011; 2019) and other difficulties in mental health, social, and adaptive function that persist into adulthood (Streissguth et al. 2004). A better understanding of how neurodevelopment in children with histories of prenatal alcohol exposure differs from typical development may help to inform more effective interventions to prevent poor outcomes later in life, including difficulty living independently, low educational attainment, trouble with the law, increased risk of substance use, and other psychiatric disorders (Moore & Riley, 2015).
Working memory, the ability to manipulate temporarily stored information, is critical for a number of cognitive processes and frequently affected in children with prenatal alcohol exposure (Kingdon et al. 2016). While prenatal alcohol exposure has been associated with a general working memory deficit, it also appears to have a particularly detrimental impact on spatial working memory. When tested on executive function tasks, children with prenatal alcohol exposure generally scored lower than non-exposed controls; however, the effect size was largest for a spatial working memory task (Green et al. 2009). Children with prenatal alcohol exposure who were tested with the Cambridge Neuropsychological Test Automated Battery (CANTAB) showed clinical levels of impairment on the Spatial Span and Spatial Working Memory subtests, which involve assessment of spatial working memory (Rasmussen et al., 2011). Additionally, the CANTAB Spatial Working Memory subtest appears to be useful for distinguishing children with prenatal alcohol exposure who meet criteria for a diagnosis from those who do not (Astley, 2004) as well as from children with minimal or no exposure who meet criteria for attention-deficit/hyperactivity disorder (Mattson et al. 2013).
While prenatal alcohol exposure can result in deficits in spatial working memory, the impact of age on this neurocognitive skill is not known. Magnetic resonance imaging (MRI) studies have not only documented that prenatal alcohol exposure has a detrimental impact on brain structure and function, but also that it results in atypical trajectories of brain development (for review see Moore et al., 2014). For example, a longitudinal structural MRI study showed that several posterior cortical regions, including the parietal areas, showed significantly different developmental trajectories between control children and children with prenatal alcohol exposure (Lebel et al. 2012). Developmental effects have also been noted in longitudinal functional MRI studies. On a visuo-spatial attention task, children with heavy prenatal alcohol exposure and controls demonstrated differential blood oxygen level dependent (BOLD) response patterns as they got older (Gautam et al., 2015). While the control group showed increased BOLD response in frontal, parietal, and temporal areas over time, the group with AE showed decreases, suggesting differential functional development of the brain regions underlying visuospatial attention, a critical skill that would influence spatial working memory performance. The brain regions shown to have atypical developmental patterns in children and adolescents with heavy prenatal alcohol exposure are important for spatial working memory performance. Indeed, spatial working memory performance is related to connectivity strength among dorsal attention and fronto-parietal networks (Liu et al. 2017).
Given these developmental differences in brain regions implicated in spatial working memory, children with prenatal alcohol exposure may also have an atypical developmental trajectory of this cognitive skill. We examined spatial working memory performance as a function of age to determine if heavy prenatal alcohol exposure impacts its developmental trajectory. We hypothesized that the relation between age and spatial working memory ability in children with heavy prenatal alcohol exposure would be atypical such that larger group differences would be noted at older ages, in comparison to controls.
Materials and Methods
General Method
Participants were recruited as part of ongoing Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) studies at the Center for Behavioral Teratology at San Diego State University (San Diego, CA) between 2003–2017 (CIFASD phases I-III, N = 270), and Emory University (Atlanta, GA) between 2007–2017 (CIFASD phases II-III, N = 226). Data from the San Diego site served as the primary sample, whereas data from the Atlanta site were used as a replication sample. At each site, recruitment occurred through clinical referral, community outreach, and word of mouth. In accordance with the Institutional Review Boards at each site, the parents or legal guardians underwent informed consent procedures and assent was obtained from all child participants. A financial incentive was provided for participation.
Subjects
Individuals aged 5.0–16.9 years were included in this study. Comparisons were made between children with histories of heavy prenatal alcohol exposure (AE, San Diego n=128, Atlanta n =114) and typically developing children with no or minimal prenatal alcohol exposure (CON, San Diego n=141, Atlanta n=109) using criteria as defined by the CIFASD (Mattson et al. 2010). Children were recruited through advertisements, word of mouth, use of national registers, through in-school studies and, additionally, children in the AE group were recruited through clinician and dysmorphologist referrals. Documented prenatal alcohol exposure histories demonstrating either heavy exposure (AE group) or no/minimal exposure (CON group) were necessary for inclusion in this study. For both AE and CON groups, alcohol exposure histories were determined retrospectively through maternal self-report using questionnaires and/or in person interviews, or via a record review. When direct maternal report was available, heavy prenatal alcohol exposure was defined as consumption of greater than four drinks per occasion at least once per week or greater than thirteen drinks per week for the majority of the pregnancy. Minimal exposure to alcohol was defined as never more than one drink per week on average and never more than two drinks on any one occasion during pregnancy. When direct maternal report was not available, reports from family members who had knowledge of the biological mother’s alcohol use during pregnancy and/or review of medical history, birth records, adoption tellings, and social services records were used to determine exposure histories. Corroborative evidence supporting alcohol abuse or dependence during pregnancy was sufficient for inclusion in the AE group. Clinical judgment was used to assess this corroborative evidence, which varied on a case-by-case basis but may include records indicating that the mother experienced trouble with the law due to alcohol intoxication during pregnancy (e.g., receipt of DUIs or violations for public intoxication, etc.). Clear records indicating that no alcohol use occurred during pregnancy was required for inclusion in the CON group if maternal report was unavailable. While children in the AE group were not required to meet diagnostic criteria for fetal alcohol syndrome (FAS), if a CIFASD dysmorphologist rendered such a diagnosis for a child with suspected alcohol exposure it was considered de facto confirmation that the child was heavily exposed. During recruitment, CON group participants are matched, by site, to the AE group on the basis of age (+/− 6 months), race/ethnicity, sex, socioeconomic status, and geographic region. Exclusion criteria for both groups were significant head trauma, loss of consciousness >30 minutes, or a significant physical (e.g., uncorrected visual impairment, hemiparesis) or psychiatric (e.g., psychosis) disability that hindered the ability to participate in study tasks. Exclusionary criteria were assessed at the time of recruitment via parent report during a phone screening. Additional methodological details for CIFASD studies are described in Mattson et al. (2010).
Procedure and Measures
All subjects completed the Cambridge Neuropsychological Test Automated Battery (CANTAB) Spatial Working Memory (SWM) task as part of a larger neuropsychological battery. This task was common across all phases of CIFASD at both data collection sites. The specific neuropsychological battery differed across the three phases of CIFASD. Details on the specific tests administered during each phase of the CIFASD are available at cifasd.org. Length of testing varied by phase, ranging from 3–6 hours and the administration time for the SWM task was approximately four minutes. Standardized procedures were followed and the SWM task was administered at the same point within the CIFASD phase-specific battery for all children. While in most cases separate groups of children were tested in each phase, there were some cases where the same child was tested in multiple phases. In these instances, the first testing session was selected for analysis.
The SWM task was administered on a touch screen tablet. A number of colored boxes were presented on the screen. These boxes were either empty or contained a token. The aim was to use a process of elimination to find the tokens hidden within the boxes. The number of boxes gradually increased until the subject was required to search 8 boxes to find the token. The variables of interest for our analysis were the raw scores for Total Errors (number of times that boxes already found to be empty or have a token were revisited) and Strategy (number of inefficient searches, determined from the number of searches started using a different box). The Total Errors and Strategy z-scores were used in analyses.
Additionally, each subject completed a standardized test of general cognitive ability. While SWM task data was consistently collected for every child tested between the years 2000–2017, the overall neuropsychological battery changed over these years. Three different tests were used to measure general cognitive ability over the years, and test scores for either the Leiter International Performance Scale Revised (Leiter-R) Brief IQ (Roid & Miller, 1997), Wechsler Intelligence Scale for Children, Fourth Edition Full Scale IQ (WISC-IV FSIQ; Wechsler, 2004), or the Differential Abilities Scale, Second Edition General Conceptual Ability (DAS-II GCA; Elliott, 2007) scores were collected.
Statistical Analyses
Analyses were conducted using SPSS v.26 (SPSS, 2019). Results were considered significant at p < .05 unless otherwise stated. Differences between the groups at each site in terms of race, ethnicity, sex, and handedness were analyzed using Pearson’s chi-square statistics. Analysis of variance was used to examine age and indices of general cognitive ability. Separate analyses were conducted for each of the three general cognitive indices: Leiter-R Brief IQ, WISC-IV FSIQ, or the DAS-II GCA.
Group differences in developmental trajectory of SWM task performance were evaluated by examining the z-scores for Total Errors and Strategy. These variables were first assessed for univariate and multivariate outliers within each exposure group and site. Univariate outliers were assessed with box plots and defined as scores with an absolute value greater than three times the interquartile mean. Two subjects from the AE group and one subject from the CON group at the Atlanta site were identified as univariate outliers and removed. Multivariate outliers were identified by obtaining the Mahalanobis Distance for the scores and calculating its chi square probability distribution; scores that were significantly outside this distribution (<.001) were considered outliers. One subject from the San Diego site and three subjects from the Atlanta site, all in the control group, were removed based on this criterion.
Demographic variables were evaluated as potential covariates. To meet criteria for inclusion as a covariate, there must be a significant relationship between the covariate and the dependent variables (Total Errors and Strategy), and the covariate must not interact with the predictor variables (Exposure, Age, and Sex). Neither race, ethnicity, nor handedness met these criteria due to a lack of relationship between these variables and either Total Errors or Strategy. General cognition scores were not evaluated as a potential covariate as different tests were used to determine the general cognition scores. Furthermore, lower scores on general cognitive measures is a feature of FASD, and thus statistically controlling for this would remove variance of interest (see Dennis et al. 2009).
SWM task performance was evaluated with a multivariate general linear model. The predictors of interest were Exposure, Age, Sex, and their interaction. Age was statistically centered around 5 years in the main multivariate analysis. Significant effects were followed up with separate univariate analysis to isolate group differences. Additional univariate tests were planned to examine the quadratic effects of age. To rule-out the possibility that any Age-by-Group interactions observed in the SWM task analyses were general effects rather than specific to this test, we also conducted separate general linear models evaluating relations between Exposure, Age, and their interaction on each of the indices of general cognitive ability: Leiter-R Brief IQ, WISC-IV FSIQ, or the DAS-II GCA.
Results
Demographic Information
Subject characteristics are reported in Table 1. Groups were not statistically different on age, sex, ethnicity, or handedness. At both sites, groups significantly differed on race, behavioral problems, and cognitive ability. For the San Diego site, there was a larger proportion of caregivers who identified their children as White in the CON group, and a larger proportion of caregivers identified their children as Black/African American in the AE group. For the Atlanta site, a larger proportion of caregivers identified their children as Black/African American in the CON group, whereas a larger proportion of caregivers identified their children as White in the AE group. At both sites, as compared to the CON group, the AE group had more caregiver-reported behavioral problems, and performed worse on measures of cognitive ability, regardless of which test was used.
Table 1.
Demographic characteristics of the prenatal alcohol exposed (AE) and control (CON) groups.
| San Diego | Atlanta | |||
|---|---|---|---|---|
| AE n = 128 |
CON n = 141 |
AE n = 114 |
CON n = 109 |
|
| Age [M (SD)] | 11.3 (3.11) | 11.0 (3.16) | 10.3 (3.42) | 10.2 (3.88) |
| 5–7y [n (%)] | 28 (21.9) | 45 (31.9) | 44 (38.6) | 45 (41.3) |
| 8–10y [n (%)] | 29 (22.7) | 29 (20.6) | 19 (16.7) | 11 (10.1) |
| 11–13y [n (%)] | 41 (32.0) | 40 (28.4) | 33 (28.9) | 26 (23.9) |
| 14–16y [n (%)] | 30 (23.4) | 31 (22.0) | 20 (17.5) | 28 (25.7) |
| Sex [n (%)] | ||||
| Male | 69 (53.9) | 69 (48.9) | 62 (54.4) | 53 (48.6) |
| Female | 59 (46.1) | 72 (51.1) | 52 (45.6) | 56 (51.4) |
| Race [n (%)] | ||||
| American Indian/Alaskan Native | 3 (2.3) | 1 (0.7) | 0 (0.0) | 0 (0.0) |
| Asian | 3 (2.3) | 3 (2.1) | 0 (0.0) | 1 (0.9) |
| Black/African American | 15 (11.7)* | 4 (2.8) | 69 (60.5)* | 88 (80.7) |
| White | 81 (63.3)* | 103 (73.0) | 36 (31.6)* | 13 (11.9) |
| More than one race | 20 (15.6) | 24 (17.0) | 8 (7.0) | 6 (5.5) |
| Other | 1 (0.8) | 0 (0.0) | 0 (0.0) | 1 (0.9) |
| Unknown/Not Reported | 5 (3.9) | 6 (4.3) | 1 (0.9) | 0 (0.0) |
| Ethnicity [n (%)] | ||||
| Hispanic or Latino | 37 (28.9) | 42 (29.8) | 10 (8.8) | 3 (2.8) |
| Not Hispanic or Latino | 88 (68.8) | 97 (68.8) | 104 (91.2) | 104 (95.4) |
| Unknown/Not Reported | 3 (2.3) | 2 (1.4) | 0 (0.0) | 2 (1.8) |
| Handedness [n (%)] | ||||
| Right dominant | 109 (85.2) | 126 (89.4) | 102 (89.5) | 103 (94.5) |
| Left dominant | 16 (12.5) | 13 (9.2) | 9 (7.9) | 5 (4.6) |
| Mixed handedness | 3 (2.3) | 2 (1.4) | 3 (2.6) | 1 (0.9) |
| CBCL Total Problems [M (SD)] | 65.6 (10.08)* | 42.3 (9.60) | 61.1 (11.14)* | 47.1 (9.53) |
| General Cognition [M (SD)] | ||||
| Leiter-R | 95.7 (15.47)* | 108.2 (14.70) | - | - |
| WISC-IV | 86.4 (17.66)* | 107.9 (11.70) | 80.0 (15.24)* | 108.6 (14.47) |
| DAS-II | 87.0 (12.38)* | 106.8 (12.33) | 85.7 (12.66)* | 91.5 (12.57) |
Note: Data were collected across 15 years of research studies (2003–2017), and the tests measuring general cognition differed across the years. The Leiter-R was administered between 2003–2007, the WISC-IV between 2007–2012, and the DAS-II with 2012–2017. In San Diego, the sample size for each cognitive test are as follows: Leiter-R AE n=37, CON n=32; WISC-IV AE n=36, CON n=48; DAS-II AE n=55, CON n=61. For Atlanta, WISC-IV AE n=30, CON n=19; DAS-II AE n=83, CON n=89. The Atlanta site did not participate in CIFASD phase I; therefore, no children were tested with the Leiter-R. Asterisk indicates significant difference between AE and CON groups within each site.
Linear Trajectories for SWM Task
Developmental trajectories are depicted in Figure 1. The initial MANOVA examined main effects of Age, Exposure, Sex, and their interactions in the San Diego data. In this test, we identified significant effects of Exposure (p = .005), Age (p < .001), and an Exposure*Age interaction (p = .01); the main effect of Sex (p = .34), and the interactions of Sex*Age (p = .13), Exposure*Sex (p = .48), and Exposure*Sex*Age (p = .47) were not significant. For parsimony, we removed sex from our model. The subsequent MANOVA omnibus test conducted on the San Diego site data revealed a significant main effect of Exposure [F(2, 264) = 5.2, p = .006, η2 = .04], a significant main effect of Age [F(2, 264) = 73.0, p < .001, η2 = .36], and a significant Exposure*Age interaction [F(2, 264) = 5.1, p = .007, η2 = .04].
Figure 1. Developmental Trajectory of Spatial Working Memory.
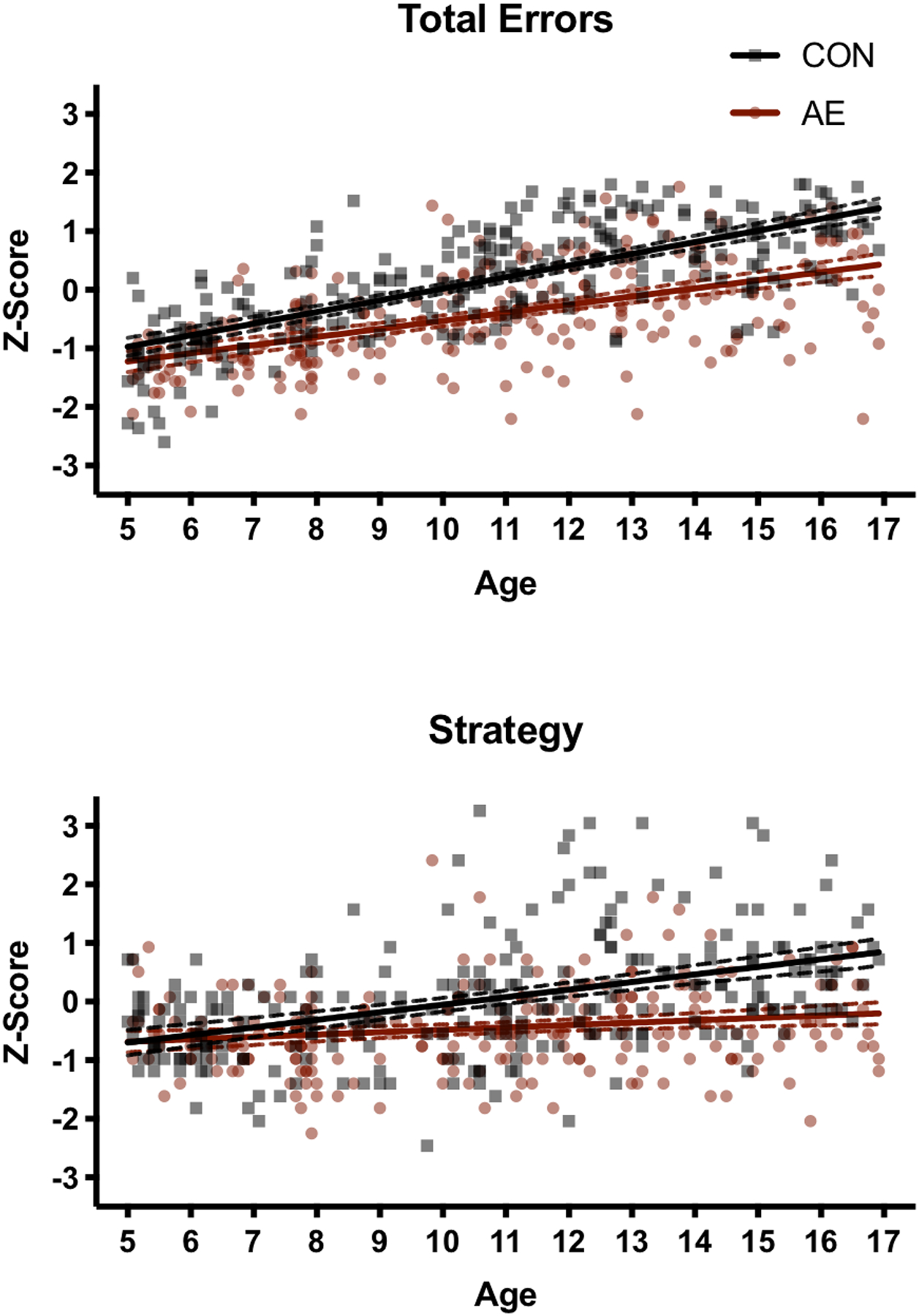
Linear developmental trajectories for Total Errors (top) and Strategy (bottom); dotted lines represent the 95% confidence intervals. As the effects seen at the San Diego site were replicated at the Atlanta site, data were combined. For the CON group, developmental improvements occurred at a faster rate as compared to the AE group.
The univariate follow-up tests on the San Diego data detected a significant main effect of Exposure for Total Errors [F(1, 265) = 5.2, p = .02, η2 = .02], but not for Strategy (p = .72). Significant main effects of Age were found for both Total Errors [F(1, 265) = 140.3, p < .001, η2 = .35] and Strategy [F(1, 265) = 30.7, p < .001, η2 = .10]. A significant Age*Exposure interaction was detected for both Total Errors [F(1, 265) = 4.8, p = .03, η2 = .02] and Strategy [F(1, 265) = 10.2, p = .002, η2 = .04]. The AE group improved at a slower rate than the CON group for both Total Errors (B = .06, p = .03, η2 = .02) and Strategy (B = .11, p = .002, η2 = .04). To determine at what age group differences emerged, follow-up tests were conducted by centering on each whole year of age. The significance threshold for these tests was adjusted to correct for multiple comparisons (.05/12 = .004). Group differences in total errors emerged by age 6 (p < .004), but the group differences in strategy did not emerge until age 10 (p < .001).
Similar results were found when data from the Atlanta site were analyzed, replicating the major findings from the analysis of the San Diego site data. When sex was included in the model, the MANOVA omnibus test revealed a significant main effect of Age (p < .001) and an interaction between Exposure*Age (p < .05), but the main effect of Exposure (p = .85), Sex (p = .56), and interactions between Sex*Age (p = .49), Exposure*Sex (p = .83), and Exposure*Sex*Age (p = .48) were not significant. Therefore, once again we removed sex from the model. In the subsequent analysis, a significant effect of Age [F(2, 218) = 98.8, p < .001, η2 = .48] and a significant Exposure*Age interaction [F(2, 218) = 4.0, p = .02, η2 = .04] were observed but there was no main effect of Exposure (p = .81).
In the univariate follow-up tests, significant main effects of Age were identified in both Total Errors [F(1, 219) = 198.4, p < .001, η2 = .48] and Strategy [F(1, 219) = 26.3, p < .001, η2 = .11]. Significant Age*Exposure interactions were detected for Total Errors [F(1, 219) = 4.9, p = .03, η2 = .02] and Strategy [F(1, 219) = 6.0, p = .02, η2 = .03]. Again, the AE group improved at a slower rate than the CON group for both Total Errors (B = .06, p = .03, η2 = .02) and Strategy (B = .07, p = .02, η2 = .03). We probed at what age group differences emerged by adjusting the age intercept in our univariate analyses and considered results significant at p < .004. We found that group differences in total errors emerged by age 9 (p = .001), later than observed in the San Diego cohort. However, similar to San Diego, group differences in strategy were apparent at age 10 (p < .001).
Quadratic Trajectories for SWM Task
Given that developmental trajectories are often nonlinear, we chose to conduct an additional analysis evaluating linear and quadratic effects of age and their interaction with group. As the linear trajectories were similar between the two sites, we combined the data to examine potential quadratic relationships between age and spatial working memory. Because we did not observe any main effects of sex or interactions between sex and age in our prior analyses, we excluded sex from the models. Stepwise multiple regression models were used to determine if the quadratic effect of age resulted in improved model fit over the linear effect of age. Results are shown in Table 2. For Total Errors, adding the quadratic term resulted in a significant R2 change (p < .001) but only explained an additional 3% of the variance. While this change is statistically significant, its practical significance is limited; therefore, we chose to retain the linear model. For strategy, the linear model was significant and explained 20% of the variance but adding the quadratic term did not significantly improve model fit (p = .98).
Table 2.
Linear and quadratic effects of age on CANTAB Spatial Working Memory Total Error and Strategy z-scores.
| Total Error | Strategy | |||
|---|---|---|---|---|
| Linear Model B (SE) |
Quadratic Model B (SE) |
Linear Model B (SE) |
Quadratic Model B (SE) |
|
| Exposure | .247 (.120)a | .153 (.169) | −.015 (.146) | −.036 (.211) |
| Age | .138 (.013)c | .265 (.049)c | .040 (.016)a | .028 (.062) |
| Exposure*Age | .060 (.018)c | .134 (.066)a | .089 (.022)c | .098 (.083) |
| Age2 | - | −.011 (.004)b | - | .001 (.005) |
| Exposure*Age2 | - | −.007 (.006) | - | −.001 (.007) |
| Constant | −1.220 (.088)c | −1.476 (.129)c | −.683 (.107)c | −.658 (.161)c |
| R2 | .486d | .515e | .204d | .204 |
Note: Data were combined across San Diego and Atlanta sites to test model fit for linear and quadratic trajectories.
p<.05;
p<.01;
p<.001;
indicates significant R2;
indicates significant R2 change.
General Cognitive Ability
As it is possible that as the children with AE age into adolescence they fall behind in a number of cognitive areas, we sought to determine if the differential age relations may also generalize to general cognitive ability. Because the measures of cognitive ability differed across the years of CIFASD data collection, we conducted three separate analyses examining the effects of Group, Age and their interaction on the Leiter-R Brief IQ, WISC-IV FSIQ, or the DAS-II GCA. As Atlanta did not participate in CIFASD I, the Leiter-R Brief IQ analyses include only the San Diego site; however, for the WISC-IV FSIQ and the DAS-II GCA analyses the data from San Diego and Atlanta were combined.
Leiter-R Brief IQ: A significant main effect of age [F(1, 65) = 7.0, p = .01, η2 = .10] was identified but no significant main effect of group (p = .37, η2 = .01) or age-by-group interaction (p = .59, η2 = .005) were noted.
WISC-IV FSIQ: A significant main effect of group [F(1, 129) = 11.8, p = .001, η2 = .08] was observed but neither the main effect of age (p = .11, η2 = .02) nor the interaction term (p = .74, η2 = .001) were significant.
DAS-II GCA: Significant main effects of group [F(1, 284) = 11.5, p = .001, η2 = .04] and age [F(1, 284) = 6.6, p = .01, η2 = .02] were identified but the interaction term was not significant (p = .32, η2 = .003).
Discussion
As expected, older children performed better on the SWM task than younger children, regardless of exposure history; however, based on this cross-sectional data, the performance of typically developing children appears to improve at a faster rate than that of children with heavy prenatal alcohol exposure. At age 5, the two groups performed similarly in terms of Total Errors and Strategy, but by age 16 there is nearly a whole standard deviation difference between the groups on these two performance measures. Indeed, the heavily exposed children at age 16 performed more similarly to control children at age 10–11. The differential relation between age and task performance between the groups did not extend to general cognitive ability as measured by either the Leiter-R Brief IQ, WISC-IV FSIQ, or DAS-II GCS, indicating that this altered trajectory is not uniform across cognitive domains.
As typically developing children age into adolescence, they become more efficient at cognitively processing, storing, and manipulating information, and developmental performance gains are observed across a number of cognitive domains (Akshoomoff et al. 2014). Our findings suggest that heavy prenatal alcohol-exposure may alter the trajectory of cognitive development, at least in regard to spatial working memory during childhood through adolescence. It is not yet known how this atypical development may manifest during later developmental stages. That the young children with AE show similar spatial working memory ability as compared to the young typically developing children while adolescents with AE demonstrate worse performance suggests that a slower rate of development occurs. However, this leaves open the possibility that individuals with AE may “catch up” to their typically developing peers later in life. Alternatively, those with AE may experience a developmental arrest at some point in the future where performance stops improving, and their abilities remain below that of typically developing individuals. A prior study examining spatial working memory ability in a small sample of children age 7–12 years and adults age 18–33 years did not observe differential performance based on age in the AE group, suggesting that catch up does not occur in spatial working memory by young adulthood (Malisza et al. 2005). Additional work in older ages will be necessary to understand the nature of the development of spatial working memory ability in those with AE.
Performance on spatial working memory tasks can be improved with effective strategy use. An efficient strategy on the CANTAB SWM task is to consistently use a specific search sequence to identify tokens. Lower Strategy scores on the SWM task indicate that the subject was not consistently starting with the same box when conducting their searches, with the lowest scores assigned when a seemingly random selection of boxes is utilized during the search. Young children in both groups tended to lack efficient strategies on the task. In comparison, the older typically developing children were more likely to use a more efficient strategy than the older children with AE. Previous studies that compared younger (5–6 years) and older (8–10 years) children with FASD to controls reported that similar strategies to complete working memory tasks were used in both groups, but that children with FASD perhaps used the strategies less consistently or effectively as compared to controls (Rasmussen et al. 2009). Our study suggests that as children with FASD age into adolescence, they do not develop an effective strategy use as quickly as controls. However, early interventions aimed at teaching children how to effectively use strategies may help improve memory and prevent children with FASD from falling substantially behind their peers. Indeed, strategy training has been shown to improve working memory span in children with FASD ages 4–11 years (Loomes et al. 2008).
That differential relations between general cognitive ability and age in the AE and control groups were not observed indicates that there is some specificity for these age-related differences. However, the fact that there was a different relation between our groups in terms of the strategy employed and age suggests that our finding may not be specific to spatial working memory per se. Prior studies have evaluated the relation between age and other neuropsychological measures in children with prenatal alcohol exposure (Taylor & Enns, 2018; Davies et al. 2017; Panczakiewicz et al. 2016; Tamana et al. 2014; Williams et al. 2014; Rasmussen & Bisanz, 2009; Kully-Martens et al. 2013; Stevens et al. 2015; Crocker et al. 2009; Fagerlund et al. 2012). Many of these prior studies were limited by small sample sizes or were only able to examine age-effects within an AE group; however, there is evidence that children with AE may have atypical developmental trajectories on other measures of executive function and learning/memory. Within an AE group, age negatively correlated with standard scores on letter fluency and inhibition/switching tasks, indicating that the children with AE were developing at a slower rate than the normative sample (measured with DKEFS; Rasmussen & Bisanz, 2009). On an Iowa Gambling Task, age was highly correlated with performance in a control group but not in an AE group, suggesting that the children with AE were not developing their decision-making abilities at the same rate as control children (Kully-Martens et al. 2013). Additionally, on a task that required sequencing while alternating between letters and numbers (Trails B) and on learning trials for an auditory verbal learning test, older participants with AE had a larger discrepancy relative to norms than did younger participants (Tamana et al. 2014). Crocker et al. (2009) assessed adaptive functioning in children and adolescents with prenatal alcohol exposure as compared to controls and children with ADHD. They found a negative relation between age and both communication and socialization standard scores in the AE group that was not observed in controls or children with ADHD. This suggests that as children with AE age they may also fall further behind their peers in adaptive behavior, which is consistent with early reports that adolescents and adults with FASD have the adaptive functioning skills that are more similar to those observed in children (Streissguth et al. 1991). Given that executive function skills have been shown to predict adaptive behavior in children with FASD (Ware et al. 2012), it is possible that differences in developmental trajectories in executive function may also contribute to poor adaptive behavior in adolescents and adults with FASD. In contrast, in a large CIFASD sample that compared performances in young (5–7 years) and older (10–16 years) age children with and without heavy prenatal alcohol exposure on a number of neuropsychological variables, Panczakiewicz et al. (2016) did not find any age-by-group interactions that met their significance threshold. However, they did report a trend (p=0.01) for an age-by-group interaction on a visuospatial processing task (NEPSY-II Arrows subtest; Panczakiewicz et al. 2016). Importantly, this study examined developmental stage as a categorical variable, which precluded an examination of rate of change with age. Thus, further study is warranted not only to replicate our findings but also to examine additional cognitive and behavioral domains, such as executive function and adaptive behavior, ideally using longitudinal methods.
Postnatal environmental factors can also influence cognitive and behavioral development. Many studies have shown the positive effects of enriched environments and the detrimental effects of stressors on development (Miguel et al. 2019; Boersma et al. 2014; Malarbi et al. 2017; Goltermann et al. 2020). It is not uncommon for individuals with prenatal alcohol exposure to be involved with child welfare services or have several placements within the foster care system (Fuchs et al. 2010; Koponen et al. 2009; Lange et al. 2013). Streissguth and colleagues (2004) showed that for those with FASD, having a stable home environment was associated with more positive outcomes while harmful experiences such as child abuse or exposure to domestic violence were associated with adverse outcomes. Thus, it should also be considered that prenatal alcohol exposure in combination with the range of adverse experiences children with such exposure may have, may have a compounding effect on cognition over time.
That children with AE have divergent development of spatial working memory ability is supported by MRI studies that demonstrate that children have atypical structural development of brain regions important for spatial relations and working memory, including the frontal and parietal cortices (Lebel et al. 2012; Hendrickson et al. 2018; Treit et al. 2014; Infante et al. 2015). Additionally, a functional MRI study of visuospatial attention development found that while controls had significant increases in activation intensities with age, the children with AE did not. As the children age, differences in activation patterns were seen within frontal, temporal, and parieto-occipital regions in children with AE as compared to controls (Gautam et al. 2015). This suggests that visuospatial attention develops differently in children with AE. While visuospatial attention and spatial working memory are distinct cognitive skills, they recruit overlapping brain regions and share processing resources (Feng et al. 2012). Thus, atypical visuospatial attention development may have contributed to the slowed rate of improvement on the spatial working memory task in the children with AE in this study.
Alcohol exposure can disrupt the earliest stages of prenatal development, which can have cascading effects throughout life. Most neuronal proliferation and migration transpire during the prenatal period, while glial proliferation and migration continues throughout childhood and adulthood (Houston et al. 2014). After migration, synaptogenesis occurs, and this process continues beyond the prenatal period. Starting prenatally but continuing throughout childhood, more cells and connections are generated than are needed, and refining these connections with myelination to increase signal speed and elimination of unnecessary connections to improve efficiency largely occurs across childhood and adolescence (Stiles and Jernigan, 2010). These developmental processes occur in a heterochronous fashion and, generally speaking, sensory areas mature sooner while areas associated with complex cognitive functions, such as the frontal lobe, mature later (Gogtay et al. 2004). These processes are critical for the typical trajectory of brain and cognitive development. The particular impact of alcohol on these processes are contingent on the timing of the exposure, and severity of the effects can be affected by genetics, nutrition, as well as a number of other factors that confer risk or protection for an individual (Riley et al. 2011). However, in animal models, such developmental alcohol exposure has been shown to result in alterations at the cellular level in neurodevelopmental processes later in life (Klintsova et al. 2007; Helfer et al. 2009).
Limitations, Strengths, and Future Directions
We used a between-subjects comparison to probe the relation between age and task performance across groups. Because our study was not longitudinal, we cannot make inferences about developmental trajectories. Thus, our study is limited by its cross-sectional design, which is subject to potential bias (Kraemer et al. 2000; Pfefferbaum & Sullivan, 2015). Furthermore, our findings are limited to the CANTAB SWM task and it is possible that these results may simply be a by-product of characteristics of this test. Thus, it will be important to replicate this effect using another measure of the same construct. It should also be noted that there are other factors that may contribute to our results, for example, potential differences in socioeconomic status, adverse childhood experiences, comorbid conditions and/or psychoactive medications in the AE group. However, the strengths of our study include the large sample of children ages 5–16 who were recruited and tested over 15 years of CIFASD projects, in two separate U.S. samples of children that allowed for replication. Our analyses suggest that children with heavy prenatal alcohol exposure may experience cognitive gains slower than typically developing children, at least in regard to spatial working memory. However, in order to understand the nature of potential differences in developmental trajectories, longitudinal studies are essential. Furthermore, longitudinal studies should examine additional cognitive domains, including other learning/memory tasks, processing speed, and executive function measures, as the atypical relations between age and performance in children with AE may not be selective to spatial working memory.
Acknowledgements
The authors thank the families who graciously participate in our studies. The authors have no financial or other conflicts of interest. All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Additional information about CIFASD can be found at www.cifasd.org. Research described in this paper was supported by NIAAA grant number U01 AA014834. Additional support was provided by U24 AA014811, U24 AA014815, and K99/R00 AA022661.
References
- Akshoomoff N, Newman E, Thompson WK, McCabe C, Bloss CS, Chang L, Amaral DG, Casey BJ, Ernst TM, Frazier JA, Gruen JR, Kaufmann WE, Kenet T, Kennedy DN, Libiger O, Mostofsky S, Murray SS, Sowell ER, Schork N, Dale AM, Jernigan TL (2014). The NIH Toolbox Cognition Battery: results from a large normative developmental sample (PING). Neuropsychology, 28(1), 1–10. doi: 10.1037/neu0000001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley SJ (2004). Validation of the fetal alcohol spectrum disorder (FASD) 4-digit diagnostic code. J Popul Ther Clin Pharmacol, 20(3), e416–467. [PubMed] [Google Scholar]
- Boersma GJ, Bale TL, Casanello P, Lara HE, Lucion AB, Suchecki D, & Tamashiro KL (2014). Long‐term impact of early life events on physiology and behaviour. Journal of neuroendocrinology, 26(9), 587–602. [DOI] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, & Mattson SN (2009). Comparison of adaptive behavior in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcohol Clin Exp Res, 33(11), 2015–2023. doi: 10.1111/j.1530-0277.2009.01040.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies LA, Cockcroft K, Olinger L, Chersich M, Urban M, Chetty Makkan CM, Turnbull OH, Olivier L, Viljoen D (2017). Alcohol exposure during pregnancy altered childhood developmental trajectories in a rural South African community. Acta Paediatr, 106(11), 1802–1810. doi: 10.1111/apa.13978 [DOI] [PubMed] [Google Scholar]
- Elliott CD (2007). Differential ability scales—second edition (DAS-II). San Antonio: Harcourt Assessment. [Google Scholar]
- Fagerlund A, Autti-Ramo I, Kalland M, Santtila P, Hoyme HE, Mattson SN, & Korkman M (2012). Adaptive behaviour in children and adolescents with foetal alcohol spectrum disorders: a comparison with specific learning disability and typical development. Eur Child Adolesc Psychiatry, 21(4), 221–231. doi: 10.1007/s00787-012-0256-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Pratt J, & Spence I (2012). Attention and visuospatial working memory share the same processing resources. Frontiers in psychology, 3, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs D, Burnside L, Marchenski S, & Mudry A (2010). Children with FASD-related disabilities receiving services from child welfare agencies in Manitoba. International journal of mental health and addiction, 8(2), 232–244. [Google Scholar]
- Gautam P, Nunez SC, Narr KL, Mattson SN, May PA, Adnams CM, Riley EP, Jones KL, Kan EC, Sowell ER (2015). Developmental Trajectories for Visuo-Spatial Attention are Altered by Prenatal Alcohol Exposure: A Longitudinal FMRI Study. Cereb Cortex, 25(12), 4761–4771. doi: 10.1093/cercor/bhu162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC … & Thompson PM (2004). Dynamic mapping of human cortical development during childhood through early adulthood. PNAS, 101(21):8174–8179. 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goltermann J, Redlich R, Grotegerd D, Dohm K, Leehr EJ, Böhnlein J, … & Repple J (2020). Childhood maltreatment and cognitive functioning: the role of depression, parental education, and polygenic predisposition. Neuropsychopharmacology, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CR, Mihic AM, Nikkel SM, Stade BC, Rasmussen C, Munoz DP, & Reynolds JN (2009). Executive function deficits in children with fetal alcohol spectrum disorders (FASD) measured using the Cambridge Neuropsychological Tests Automated Battery (CANTAB). J Child Psychol Psychiatry, 50(6), 688–697. doi: 10.1111/j.1469-7610.2008.01990.x [DOI] [PubMed] [Google Scholar]
- Helfer JL, Goodlett CR, Greenough WT, & Klintsova AY (2009). The effects of exercise on adolescent hippocampal neurogenesis in a rat model of binge alcohol exposure during the brain growth spurt. Brain research, 1294, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson TJ, Mueller BA, Sowell ER, Mattson SN, Coles CD, Kable JA, Jones KL, Boys CJ, Lee S, Lim KO, Riley EP, Wozniak JR (2018). Two-year cortical trajectories are abnormal in children and adolescents with prenatal alcohol exposure. Dev Cogn Neurosci, 30, 123–133. doi: 10.1016/j.dcn.2018.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston SM, Herting MM, & Sowell ER (2014). The neurobiology of childhood structural brain development: Conception through adulthood. Curr Top Behav Neurosci, 16:3–17. doi: 10.1007/7854_2013_265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante MA, Moore EM, Bischoff-Grethe A, Migliorini R, Mattson SN, & Riley EP (2015). Atypical cortical gyrification in adolescents with histories of heavy prenatal alcohol exposure. Brain Res, 1624, 446–454. doi: 10.1016/j.brainres.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon D, Cardoso C, & McGrath JJ (2016). Research Review: Executive function deficits in fetal alcohol spectrum disorders and attention-deficit/hyperactivity disorder - a meta-analysis. J Child Psychol Psychiatry, 57(2), 116–131. doi: 10.1111/jcpp.12451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, & Greenough WT (2007). Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcoholism: Clinical and experimental research, 31(12), 2073–2082. [DOI] [PubMed] [Google Scholar]
- Koponen AM, Kalland M, & Autti-Rämö I (2009). Caregiving environment and socio-emotional development of foster-placed FASD-children. Children and Youth Services Review, 31(9), 1049–1056. [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, & Kupfer D (2000). How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry, 157, 163–171. doi: 10.1176/appi.ajp.157.2.163 [DOI] [PubMed] [Google Scholar]
- Kully-Martens K, Treit S, Pei J, & Rasmussen C (2013). Affective decision-making on the Iowa gambling task in children and adolescents with fetal alcohol spectrum disorders. J Int Neuropsychol Soc, 19(2), 137–144. doi: 10.1017/S1355617712001026 [DOI] [PubMed] [Google Scholar]
- Lange S, Shield K, Rehm J, & Popova S (2013). Prevalence of fetal alcohol spectrum disorders in child care settings: a meta-analysis. Pediatrics, 132(4), e980–e995. [DOI] [PubMed] [Google Scholar]
- Lebel C, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, Bookheimer SY, O’Connor MJ, Narr KL, Kan E, Abaryan Z, Sowell ER (2012). A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. J Neurosci, 32(44), 15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Xia M, Dai Z, Wang X, Liao X, Bi Y, & He Y (2017). Intrinsic brain hub connectivity underlies individual differences in spatial working memory. Cerebral cortex, 27(12), 5496–5508. [DOI] [PubMed] [Google Scholar]
- Loomes C, Rasmussen C, Pei J, Manji S, & Andrew G (2008). The effect of rehearsal training on working memory span of children with fetal alcohol spectrum disorder. Research in Developmental Disabilities, 29(2), 113–124. [DOI] [PubMed] [Google Scholar]
- Malarbi S, Abu-Rayya HM, Muscara F, & Stargatt R (2017). Neuropsychological functioning of childhood trauma and post-traumatic stress disorder: A meta-analysis. Neuroscience & Biobehavioral Reviews, 72, 68–86. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Allman AA, Shiloff D, Jakobson L, Longstaffe S, & Chudley AE (2005). Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: a functional magnetic resonance imaging study. Pediatric research, 58(6), 1150–1157. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Bernes GA, & Doyle LR (2019). Fetal alcohol spectrum disorders: a review of the neurobehavioral deficits associated with prenatal alcohol exposure. Alcohol Clin Exp Res, 43(6), 1046–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, & Nguyen TT (2011). Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev, 21(2), 81–101. doi: 10.1007/s11065-011-9167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund A, Autti-Rämö I, May PA, Andams CM, Konovalova V, Wetherill L, Arenson AD, Barnett WK, Riley EP, CIFASD. (2010). Collaborative initiative on fetal alcohol spectrum disorders: methodology of clinical projects. Alcohol, 44(7–8), 635–641. doi: 10.1016/j.alcohol.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Andams CM, Jones KL, Riley EP, CIFASD. (2013). Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res, 37(3), 517–528. doi: 10.1111/j.1530-0277.2012.01952.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel PM, Pereira LO, Silveira PP, & Meaney MJ (2019). Early environmental influences on the development of children’s brain structure and function. Developmental Medicine & Child Neurology, 61(10), 1127–1133. [DOI] [PubMed] [Google Scholar]
- Moore EM, Migliorini R, Infante MA, & Riley EP (2014). Fetal Alcohol Spectrum Disorders: Recent Neuroimaging Findings. Curr Dev Disord Rep, 1(3), 161–172. doi: 10.1007/s40474-014-0020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, & Riley EP (2015). What Happens When Children with Fetal Alcohol Spectrum Disorders Become Adults? Current Developmental Disorders Reports, 2(3), 219–227. doi: 10.1007/s40474-015-0053-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panczakiewicz AL, Glass L, Coles CD, Kable JA, Sowell ER, Wozniak JR, Jones KL, Riley EP, Mattson SN, CIFASD. (2016). Neurobehavioral Deficits Consistent Across Age and Sex in Youth with Prenatal Alcohol Exposure. Alcohol Clin Exp Res, 40(9), 1971–1981. doi: 10.1111/acer.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, & Sullivan EV (2015). Cross-sectional versus longitudinal estimates of age-related changes in the adult brain: overlaps and discrepancies. Neurobiol Aging, 36(9), 2563–2567. doi: 10.1016/j.neurobiolaging.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C, & Bisanz J (2009). Executive functioning in children with Fetal Alcohol Spectrum Disorders: profiles and age-related differences. Child Neuropsychol, 15(3), 201–215. doi: 10.1080/09297040802385400 [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Soleimani M, & Pei J (2011). Executive functioning and working memory deficits on the CANTAB among children with prenatal alcohol exposure. J Popul Ther Clin Pharmacol, 18(1), e44–53. [PubMed] [Google Scholar]
- Riley EP, Infante MA, & Warren KR (2011). Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev, 21(2), 73–80. doi: 10.1007/s11065-011-9166-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid GH, & Miller LJ (1997). Leiter international performance scale-revised (Leiter-R). Wood Dale, IL: Stoelting. [Google Scholar]
- Stevens SA, Dudek J, Nash K, Koren G, & Rovet J (2015). Social Perspective Taking and Empathy in Children with Fetal Alcohol Spectrum Disorders. J Int Neuropsychol Soc, 21(1), 74–84. doi: 10.1017/S1355617714001088 [DOI] [PubMed] [Google Scholar]
- Stiles J & Jernigan TL (2010). The Basics of Brain Development. Neuropsychol Rev, 20(4):327–348. doi: 10.1007/s11065-010-9148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, & Smith DF (1991). Fetal alcohol syndrome in adolescents and adults. JAMA, 265(15), 1961–1967. [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, & Young JK (2004). Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr, 25(4), 228–238. [DOI] [PubMed] [Google Scholar]
- Tamana S, Pei J, Massey D, Massey V, & Rasmussen C (2014). Neuropsychological Impairments and Age-Related Differences in Children and Adolescents with Fetal Alcohol Spectrum Disorders. J Popul Ther Clin Pharmacol, 21(2), e167–180. [PubMed] [Google Scholar]
- Taylor NM, & Enns LN (2018). Age-related differences in neuropsychological assessment of fetal alcohol spectrum disorder: a cross-sectional study. Biochem Cell Biol, 96(2), 252–259. doi: 10.1139/bcb-2017-0081 [DOI] [PubMed] [Google Scholar]
- Treit S, Zhou D, Lebel C, Rasmussen C, Andrew G, & Beaulieu C (2014). Longitudinal MRI reveals impaired cortical thinning in children and adolescents prenatally exposed to alcohol. Hum Brain Mapp, 35(9), 4892–4903. doi: 10.1002/hbm.22520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware AL, Crocker N, O’Brien JW, Deweese BN, Roesch SC, Coles CD, … & Mattson SN (2012). Executive function predicts adaptive behavior in children with histories of heavy prenatal alcohol exposure and attention-deficit/hyperactivity disorder. Alcoholism: Clinical and Experimental Research, 36(8), 1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2004). Manual for the Wechsler Intelligence Scale for Children–Fourth Edition Integrated. PsychCorp, San Antonio, TX. [Google Scholar]
- Williams L, Jackson CP, Choe N, Pelland L, Scott SH, & Reynolds JN (2014). Sensory-motor deficits in children with fetal alcohol spectrum disorder assessed using a robotic virtual reality platform. Alcohol Clin Exp Res, 38(1), 116–125. doi: 10.1111/acer.12225 [DOI] [PubMed] [Google Scholar]


