The role of the implantable cardioverter-defibrillator (ICD) is well established as a lifesaving intervention in patients with both severe ischemic (ICM) and non-ischemic (NICM) cardiomyopathy, primarily by aborting ventricular arrhythmias (VA) including monomorphic ventricular tachycardia (MVT), polymorphic ventricular tachycardia (PMVT) and ventricular fibrillation (VF). Since the use of anti-tachycardia pacing (ATP) was first described in 1987 to successfully terminate MVT in 22 patients predominantly with ischemic heart disease by Lindsay and colleagues,1 the use of ATP has become a mainstay of transvenous ICD therapy without requiring shocks. Subsequent larger studies have demonstrated the efficacy of ATP to terminate MVT, such as the PainFree RX study, which found that ATP was successful even in fast MVT episodes up to 250 bpm in patients with coronary artery disease.2 The early ATP studies assessed the efficacy of ATP primarily in patients with ischemic coronary disease. There was a perception that scar-based re-entrant VT is more susceptible to ATP and more common in ICM. In contrast, the substrate in NICM was previously not as well defined, and it was unknown whether scar-based MVT may be less common in NICM and whether VT in NICM may be as easily terminated with ATP. Hence, one could argue that implanting ICDs without ATP capabilities such as the SICD may be preferable in patients with NICM.
In this month’s issue of the Journal of Cardiovascular Electrophysiology, Dr. Cheng and colleagues3 sought to answer the specific questions of 1) whether the prevalence of MVT is similar in NICM compared to ICM patients and 2) whether ATP is just as effective for VA in NICM compared to ICM. In this meta-analysis of the Shock-Less,4 PainFree SST,5 and PREPARE6 studies of a total of 6,127 patients, the investigators found that patients with NICM experienced MVT and PMVT/VF at similar rates and proportions compared to patients with ICM. Additionally, ATP was equally effective at terminating MVT in patients with NICM vs ICM (76% vs 75%).
These results support the hypothesis that ATP is just as effective in patients with NICM compared to ICM, and that the prevalence of MVT is similar in the two populations. Although the study does not specifically investigate the mechanisms underlying MVT in patients with NICM, these results do suggest that there may be some similarities in the behavior of MVT in NICM compared to ICM. In fact, an attempt to explain these results may shed some light on the substrate and potential mechanisms underlying VA in patients with NICM.
Although the mechanisms of ventricular tachycardia include autonomic, triggered and re-entry, scar-based re-entrant VT is the most common and is due to underlying structural abnormalities that create a slowly conducting critical isthmus. ATP terminates re-entrant VT because stimulation of the excitable gap renders that tissue refractory and causes collision of the antidromic pacing and orthodromic tachycardia wavefronts, extinguishing the reentry. In the case of triggered and autonomic VT, the ability of overdrive pacing to terminate these mechanisms is not as well known.
One possible explanation why ATP works in patients with NICM is that these patients also frequently have scar substrate. As advanced cardiac imaging and invasive electroanatomic mapping technologies have enabled visualization of underlying VT substrate at higher resolution, we have learned that myocardial fibrosis is present in many types of dilated, familial and idiopathic non-ischemic cardiomypathies. In contrast to ischemic cardiomyopathy where fibrosis is located endocardially, fibrosis is often located in the mid-myocardial or epicardial layers in NICM.7 Furthermore, in many non-ischemic cardiomyopathies the fibrosis can be seen located in characteristic regions, such as the peri-mitral or aortic annuli.8 In a meta-analysis of 2850 NICM patients, late gadolinium enhancement (LGE) on MRI was a predictor of VA,9 and the size of the LGE region also correlated with inducibility of VT in a recently published study by Ghannam and colleagues.10
We present a recent example of a young patient with a history of Lamin A/C mutation cardiomyopathy who recently was referred to our center with VT storm. He had multiple VT episodes and most of them were successfully terminated with ATP therapy (Figure 1). Coronary angiography showed no obstructive coronary disease. He underwent VT mapping and ablation. Bipolar and unipolar mapping showed peri-aortic and mitral low voltage as a surrogate for scar (Figure 2 A). Constant and progressive fusion confirmed a re-entrant VT mechanism (Figure 2 B), and entrainment and activation mapping localized the VT to the basal anteroseptal LV at the borderzone of the scar region. This arrhythmia was easily pace-terminated with burst pacing at 75% tachycardia cycle length from the RV apex (Figure 2 C). This case illustrates a scar-based monomorphic VT that is commonly seen with NICM and demonstrates the ability of these arrhythmias to be pace-terminated with ATP.
Figure 1.
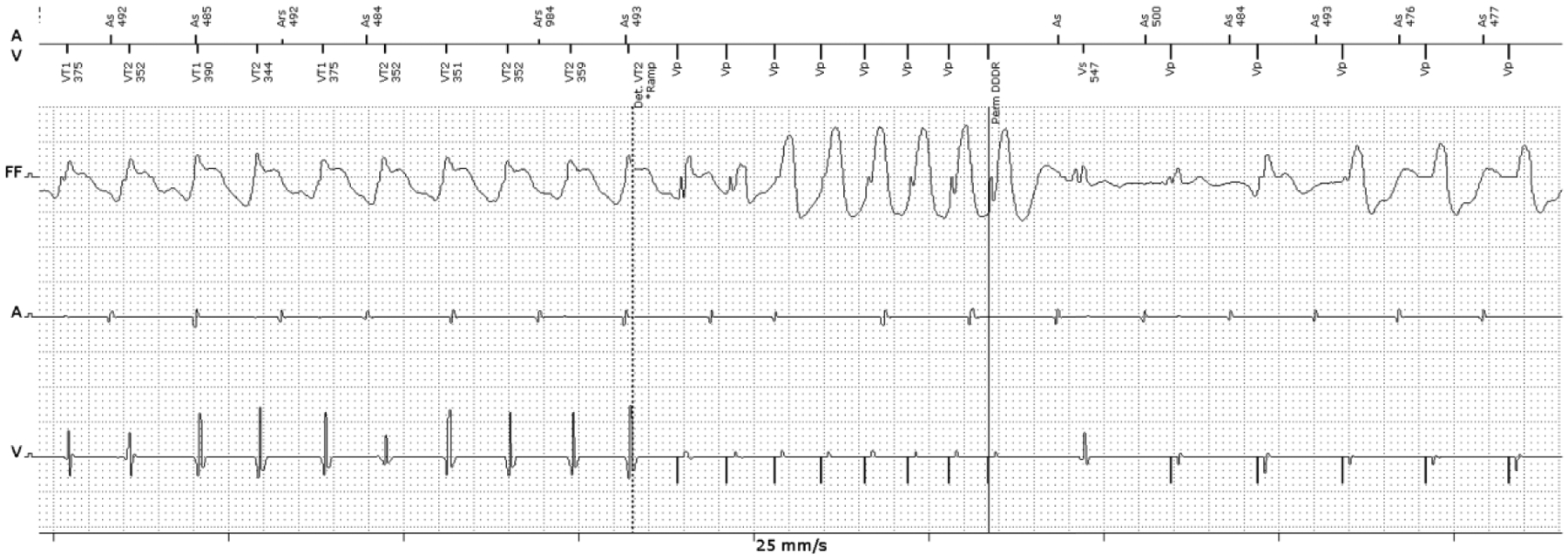
Example of a monomorphic VT terminated by ATP in a patient with non-ischemic cardiomyopathy. This patient has a history of non-ischemic, Lamin A/C mutation cardiomyopathy. Intracardiac ICD electrogram of the episode shows monomorphic VT (162 bpm) terminated with 1 round of burst ATP.
(VT: ventricular tachycardia, ICD: implantable cardioverter-defibrillator, ATP: anti-tachycardia pacing)
Figure 2.

A) Limited unipolar voltage map of the example patient with non-ischemic Lamin A/C mutation cardiomyopathy showing low voltage in the basal anteroseptal and peri-aortic region. The VT was localized to the borderzone of the scar. B) Manifest fusion is demonstrated by comparing pure RV pacing during sinus on the left and entrainment pacing on the right showing manifest fusion, confirming re-entrant VT. C) Successful termination of VT with burst pacing at 75% TCL.
(VT: ventricular tachycardia, TCL: tachycardia cycle length)
The recent advances in characterizing the fibrotic substrate of NICM provide a rationale to explain why ATP is just as effective in NICM compared to ICM. This is the largest study to specifically investigate this question and takes advantage of a robust data set of interrogations of ICD therapies from three well designed prospective studies. Although this study was overall well conducted, there are a few limitations. The number of patients with MVT were low in both groups at ~10%, and thus analysis of the MVT events treated with ATP came from a small number of patients with multiple episodes, which limits generalizability of the data.
Nevertheless, the success rates of ATP for the entire population were comparable to prior studies.1,2 Additionally, it is too often the case we know much less about the female patient population. Of the 165 NICM patients who experienced MVT, only 14 and 28 patients were female with a CRT-D and ICD device respectively.
This study does not address whether the efficacy of ATP translates into hard outcomes such as mortality benefit, prevention of inappropriate shocks, and risks of pro-arrhythmia. The Boston Scientific S-ICD PRAETORIAN and UNTOUCHED11 studies showed that implantation of the S-ICD resulted in no difference in mortality or inappropriate shocks as compared to transvenous ICDs, suggesting that the ability to provide ATP may not be critical. On the other hand, ATP provides a potential for shock reduction, improved quality of life and indirect mortality benefit.12 The results of this study do support the fact that for the purposes of reducing ICD shocks, ATP may be just as useful in patients with NICM.
Ultimately the choice between ATP-capable ICDs compared to SICD should not become a polarized debate between device manufacturers but be a decision based on patient characteristics. The decision to implant an ATP-capable ICD for a patient with cardiomyopathy should not solely be based on whether the patient has NICM versus ICM, in light of the findings from this study. Rather, the decision to select an ICD with ATP capability should take into consideration the potential mechanisms of VA in each patient and other patient factors such as susceptibility to bloodstream infections. For example, ATP may not be useful in a young Brugada patient who may be prone to VF. Finally, characterization of the extent of scar may also be useful in determining a patient’s risk of scar-based VT to help tailor ATP programming.
Funding Sources
This work was supported by the American Heart Association (AHA 19CDA34760021) and the National Institutes of Health (NIH 1KL2TR001444).
Footnotes
Disclosures
Dr. Ho has received grant support from the American Heart Association (AHA 19CDA34760021), National Institutes of Health (NIH 1KL2TR001444) and Abbott Laboratories, fellowship support from Abbott, Boston Scientific, Biotronik, and Medtronic and owns equity in Vektor Inc.
Dr Birgersdotter-Green received grant support from Abbott Laboratories, honoraria from Medtronic, Biotronik, Abbott and Boston Scientific.
References
- 1.Lindsay BD, Saksena S, Rothbart ST, Wasty N & Pantopoulos D Prospective evaluation of a sequential pacing and high-energy bidirectional shock algorithm for transvenous cardioversion in patients with ventricular tachycardia. Circulation 76, 601–609 (1987). [DOI] [PubMed] [Google Scholar]
- 2.Wathen MS et al. Shock reduction using antitachycardia pacing for spontaneous rapid ventricular tachycardia in patients with coronary artery disease. Circulation 104, 796–801 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Cheng A et al. Characteristics of Ventricular Tachyarrhythmias and their Susceptibility to ATP Termination in Patients with Ischemic and Non-Ischemic Cardiomyopathy: A Patient-level Meta- Analysis of 3 Large Clinical Trials. J. Cardiovasc. Electrophysiol (2020). [DOI] [PubMed] [Google Scholar]
- 4.Silver MT et al. Feedback to providers improves evidence-based implantable cardioverter-defibrillator programming and reduces shocks. Hear. Rhythm 12, 545–553 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Auricchio A et al. Low inappropriate shock rates in patients with single- and dual/triple-chamber implantable cardioverter-defibrillators using a novel suite of detection algorithms: PainFree SST trial primary results. Hear. Rhythm 12, 926–936 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Wilkoff BL et al. Strategic Programming of Detection and Therapy Parameters in Implantable Cardioverter-Defibrillators Reduces Shocks in Primary Prevention Patients. Results From the PREPARE (Primary Prevention Parameters Evaluation) Study. J. Am. Coll. Cardiol 52, 541–550 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Soejima K et al. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: The importance of low-voltage scars. J. Am. Coll. Cardiol 43, 1834–1842 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Zeppenfeld K Ventricular Tachycardia Ablation in Nonischemic Cardiomyopathy. JACC Clin. Electrophysiol 4, 1123–1140 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Disertori M et al. Myocardial fibrosis assessment by LGE is a powerful predictor of ventricular tachyarrhythmias in ischemic and nonischemic LV dysfunction: a meta-analysis. JACC Cardiovasc. Imaging 9, 1046–1055 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Ghannam M et al. Risk stratification in patients with nonischemic cardiomyopathy and ventricular arrhythmias based on quantification of intramural delayed enhancement on cardiac magnetic resonance imaging. J. Cardiovasc. Electrophysiol 0–2 (2020) doi: 10.1111/jce.14514. [DOI] [PubMed] [Google Scholar]
- 11.Boersma LV et al. Understanding Outcomes with the EMBLEM S-ICD in Primary Prevention Patients with Low EF Study (UNTOUCHED): Clinical characteristics and perioperative results. Hear. Rhythm 16, 1636–1644 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Poole JE et al. Prognostic importance of defibrillator shocks in patients with heart failure. N. Engl. J. Med 359, 1009–1017 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]


