ABSTRACT
Expression of synphilin-1 in neurons induces hyperphagia and obesity in a Drosophila model. However, the molecular pathways underlying synphilin-1-linked obesity remain unclear. Here, Drosophila models and genetic tools were used to study the synphilin-1-linked pathways in energy balance by combining molecular biology and pharmacological approaches. We found that expression of human synphilin-1 in flies increased AMP-activated kinase (AMPK) phosphorylation at Thr172 compared with that in non-transgenic flies. Knockdown of AMPK reduced AMPK phosphorylation and food intake in non-transgenic flies, and further suppressed synphilin-1-induced AMPK phosphorylation, hyperphagia, fat storage and body weight gain in transgenic flies. Expression of constitutively activated AMPK significantly increased food intake and body weight gain in non-transgenic flies, but it did not alter food intake in the synphilin-1 transgenic flies. In contrast, expression of dominant-negative AMPK reduced food intake in both non-transgenic and synphilin-1 transgenic flies. Treatment with STO-609 also suppressed synphilin-1-induced AMPK phosphorylation, hyperphagia and body weight gain. These results demonstrate that the AMPK signaling pathway plays a critical role in synphilin-1-induced hyperphagia and obesity. These findings provide new insights into the mechanisms of synphilin-1-controlled energy homeostasis.
KEY WORDS: Synphilin-1, Obesity, AMPK, Energy homeostasis, Hyperphagia
Summary: Genetic, behavioral and pharmacological approaches revealed that the synphilin-1-regulated AMPK signaling pathway mediates positive energy homeostasis, resulting in hyperphagia and obesity in Drosophila.
INTRODUCTION
Obesity has become a worldwide epidemic and a major health problem. However, the etiology of obesity is still not fully understood. Recent studies suggest that synphilin-1 is involved in regulating food intake and body weight (Li et al., 2012; Liu et al., 2012). Synphilin-1 is a 919-amino acid protein and is expressed in various tissues (Engelender et al., 1999; Nagano et al., 2003; Ribeiro et al., 2002), such as in neurons in many regions of the brain, including the hypothalamus, a central nervous system site critical for energy homeostasis (Engelender et al., 1999; Li et al., 2010, 2012; Nagano et al., 2003; Ribeiro et al., 2002; Wakabayashi et al., 2000). The biological functions of synphilin-1 are not clear. Previous studies have shown that synphilin-1 can interact with multiple proteins (e.g. alpha-synuclein), and has implications in the pathogenesis of Parkinson's disease related to protein aggregation (Alvarez-Castelao and Castaño, 2011; Avraham et al., 2005; Chung et al., 2001; Engelender et al., 1999; Lee et al., 2004; Marx et al., 2007; O'Farrell et al., 2001; Ribeiro et al., 2002; Ryo et al., 2006; Smith et al., 2010, 2005; Szargel et al., 2008; Wakabayashi et al., 2000). We and others have demonstrated that synphilin-1 has neurotrophic and neuroprotective effects (Giaime et al., 2006; Hernández-Vargas et al., 2011; Li et al., 2010; Smith et al., 2010). Our previous studies demonstrated that synphilin-1 fosters a positive energy balance, resulting in hyperphagia and obesity in transgenic mice and flies (Li et al., 2012; Liu et al., 2012). However, it is not clear how synphilin-1 contributes to this energy balance.
Recently, using in vitro cell culture models, we found that synphilin-1 interacts with AMP-activated protein kinase (AMPK, a serine/threonine kinase), and regulates cellular energy currency, ATP (Li et al., 2014, 2020). AMPK is an integrator of regulatory signals monitoring cellular and systemic energy status (Hue and Rider, 2007; Kola et al., 2008; Misra, 2008; Ruderman et al., 2013; Winder and Thomson, 2007) in response to nutrition changes. AMPK is also a master regulator of food intake by integrating nutrient and hormonal signals (Ruderman et al., 2013; Xue and Kahn, 2006). A decrease in hypothalamic AMPK activity is associated with decreased feeding, whereas activation of AMPK leads to increased food intake. Insulin and leptin suppress hypothalamic AMPK activity (Minokoshi et al., 2004). Orexigenic peptides, such as the gut-derived hormone ghrelin and the neuropeptide agouti-related peptide (Minokoshi et al., 2004), stimulate hypothalamic AMPK activity to increase food intake (Andersson et al., 2004; Hardie, 2007, 2008; Kim et al., 2004; Moran and Gao, 2006; Sinnett and Brenman, 2016). However, it is unclear whether synphilin-1 interacting with AMPK plays a role in synphilin-1-induced food intake and body weight gain.
In this study, we used our synphilin-1 transgenic Drosophila model to investigate whether synphilin-1 alters AMPK signaling, thereby resulting in hyperphagia and obesity. By combining extensive genetic, behavioral and pharmacological approaches, we found that expression of human synphilin-1 in flies activates the AMPK signaling event, and leads to increased food intake, fat storage and body weight. Our results demonstrate that synphilin-1-linked AMPK signaling regulates feeding behavior and metabolism in vivo.
RESULTS
Synphilin-1 increased AMPK phosphorylation in Drosophila
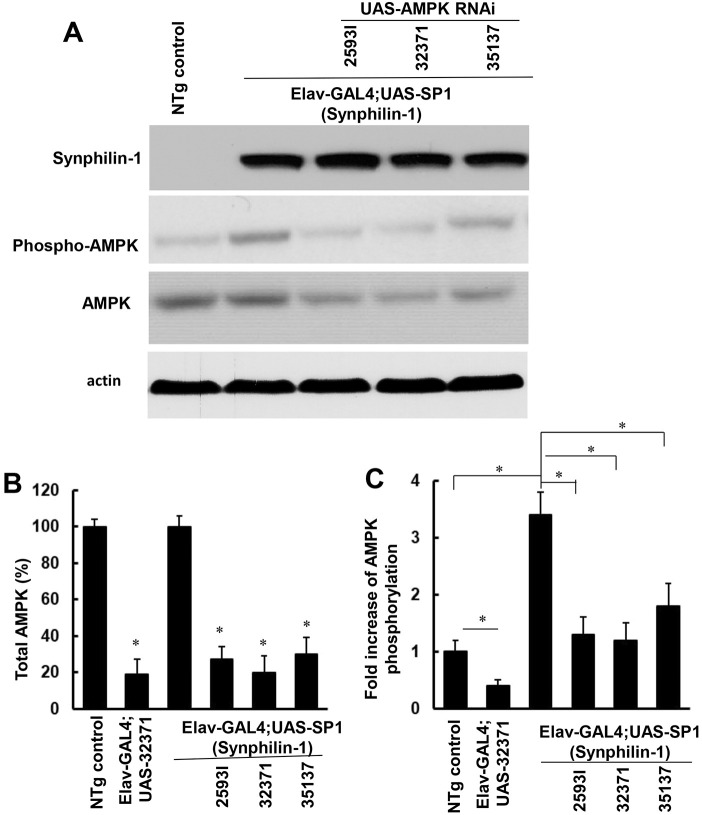
Our previous studies found that that synphilin-1 interacts with, and activates, AMPK in cell model systems (Li et al., 2020). Expression of synphilin-1 in Drosophila increases food intake and body weight (Liu et al., 2012). Given that there is not a homologous gene of human synphilin-1 in Drosophila, the human synphilin-1 transgenic Drosophila model provides a unique system to study the functions of human synphilin-1 protein in regulating energy balance. To further assess whether synphilin-1 alters AMPK in vivo, head homogenates of Elav-GAL4;UAS-SP1 and non-transgenic flies were subjected to western blot analysis using anti-phosphorylated AMPK antibodies. The Elav-Gal4 driver can lead to expression of synphilin-1 in pan neurons in flies. We found that there was significantly increased AMPK phosphorylation at Thr172, up to 3.4-fold, in flies expressing human synphilin-1 compared with non-transgenic control flies (Fig. 1). These results further support the notion that synphilin-1 upregulates AMPK activation in vivo.
Fig. 1.
Synphilin-1 increases AMPK phosphorylation in flies. (A-C) The homogenates from adult non-transgenic and Elav-GAL4;UAS-SP1 transgenic 10-day-old flies with or without UAS-AMPK RNAi (25931, 32371 and 35137) were subjected to western blot analysis (A, representative blots) using anti-phospho-AMPK, anti-AMPK, anti-synphilin-1 and anti-actin antibodies. In each experiment, three fly heads were pooled together for one sample. There were six samples in each experimental group for western blot analysis. The quantification of total (B, *P<0.05 versus NTg group) and phosphorylated (C, *P<0.05 represents significance between groups as indicated) AMPK levels was normalized to actin. Data are mean±s.e.m. and were analyzed by ANOVA followed by Tukey's post-hoc test.
Knockdown of AMPK suppressed synphilin-1-induced hyperphagia and obesity-like phenotypes
AMPK is highly conserved from Drosophila to mammal. In Drosophila melanogaster, mono-allelic expression of AMPK-α, -β and -γ produces a single heterotrimeric energy sensor that regulates energetic homeostasis (Sinnett and Brenman, 2016). Unlike mammalian AMPK, which has multiple subunit isoforms (α1–2, β1–2 and γ1–3) resulting in 12 unique heterotrimers, Drosophila has a single gene for each AMPK subunit and only form one AMPK heterotrimer (Sinnett and Brenman, 2016). To further assess whether AMPK signaling alters synphilin-1-linked obesity-like phenotypes, three UAS-AMPKα siRNA lines (25931, 32371 and 35137) targeting the Drosophila AMPKα gene (also known as SNF1A) were used to knockdown AMPKα in Elav-GAL4;UAS-SP1 transgenic flies. Total AMPK (Fig. 1A,B) and phosphorylated AMPK (Fig. 1A,C) were significantly decreased in both non-transgenic and synphilin-1 transgenic flies when they co-expressed AMPKα siRNA. The UAS-AMPKα siRNA (32371) line displayed the highest knockdown efficiency on AMPK levels in synphilin-1 transgenic flies.
To assess whether AMPK activation alters synphilin-1-induced feeding behavior, a capillary feeding (CAFE) assay was used to measure food intake. Knockdown of AMPK significantly reduced the daily food intake by ∼40% in non-transgenic flies (Fig. 2). Importantly, knockdown of AMPK by the 25931 or 32371 AMPKα-RNAi siRNA lines almost reversed the synphilin-1-induced hyperphagia in transgenic flies (Fig. 2). Consistent with the levels of AMPK knockdown, the UAS-AMPK siRNA (32371) line had the greatest effect on reducing food intake. The knockdown of AMPK also reduced the body weight gain of non-transgenic and synphilin-1 transgenic flies (Fig. 3A). Triglyceride (TAG) is one of the major intracellular forms of stored energy in flies, representing fat storage. Knockdown of AMPK also reduced the TAG levels in non-transgenic flies and attenuated the synphilin-1-induced fat storage in transgenic flies (Fig. 3B). These results suggest that AMPK signaling mediates synphilin-1-induced hyperphagia and fat deposition.
Fig. 2.
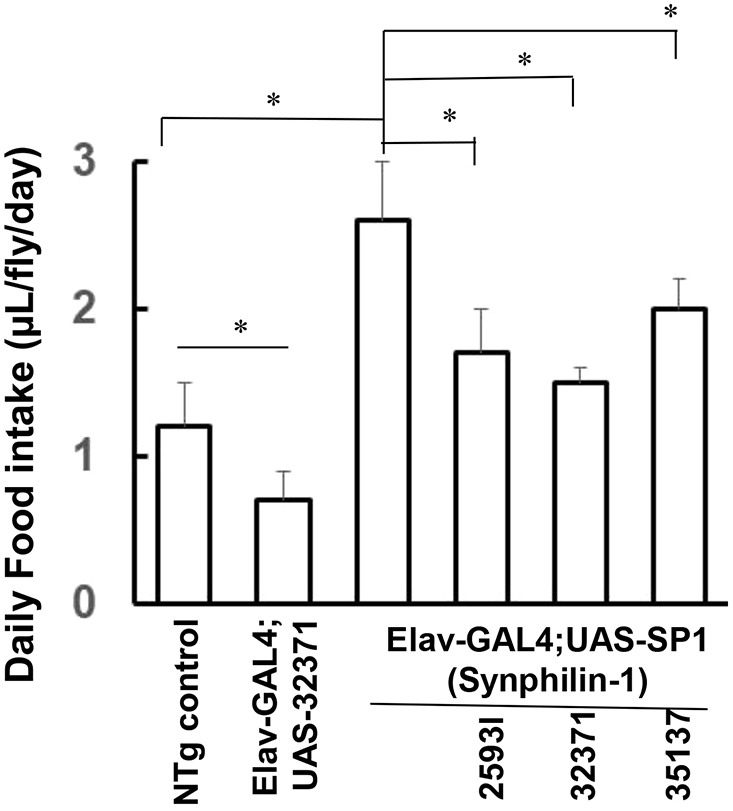
Knockdown of AMPK suppressed synphilin-1-induced hyperphagia in flies. Bar graph showing food intake of various flies as indicated (observed at 1 to 5 days post-eclosion). Knockdown of AMPK significantly reduced the daily food intake by ∼40% in non-transgenic flies. Knockdown of AMPK by the 25931 or 32371 AMPKα-RNAi siRNA lines almost reversed the synphilin-1-induced hyperphagia in transgenic flies. There were 60 flies in each group. Data are mean±s.e.m. *P<0.05 (ANOVA followed by Tukey's post-hoc test; significance between groups as indicated).
Fig. 3.
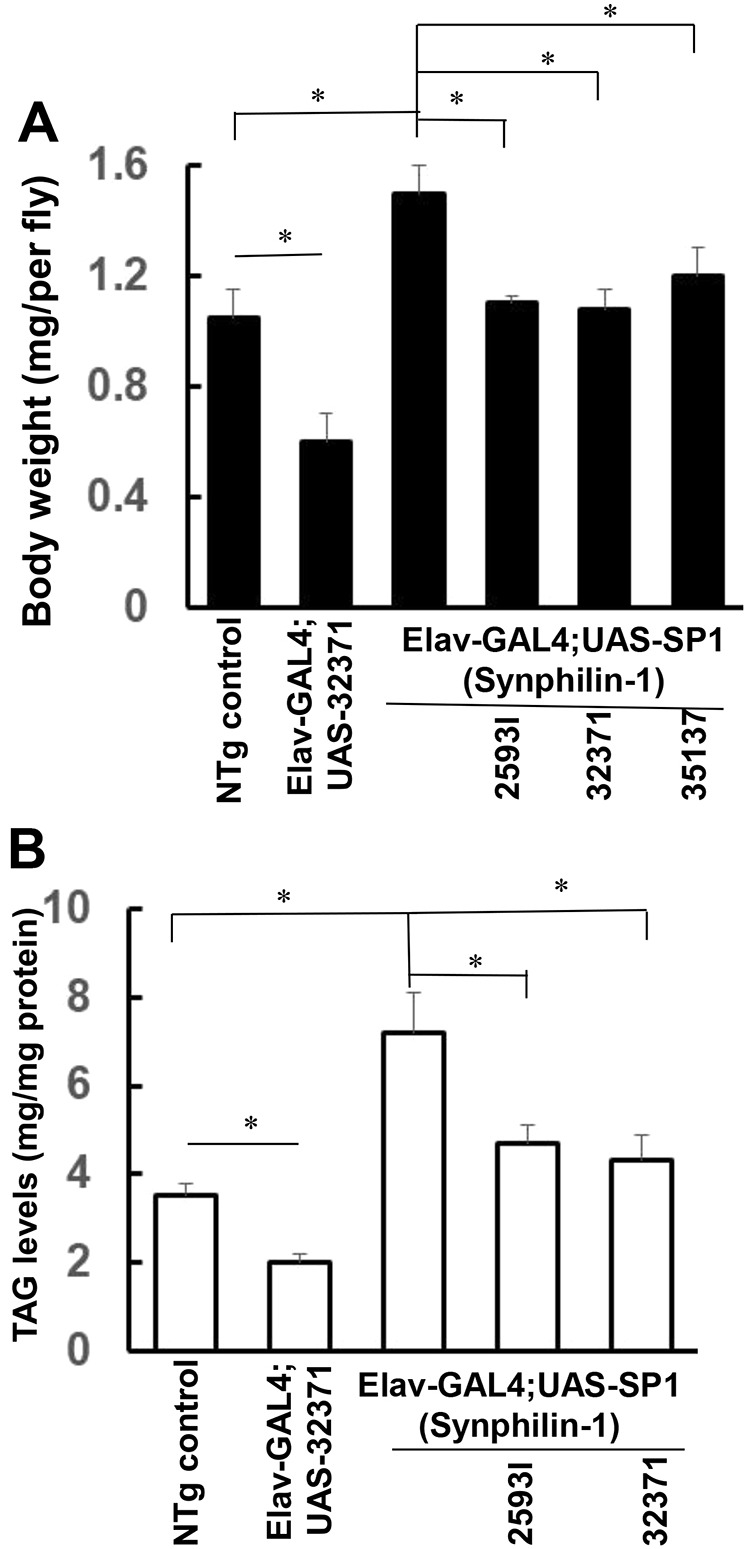
Knockdown of AMPK suppressed synphilin-1-induced body weight gain in flies. (A) Bar graph showing the body weight of various flies as indicated (measured at 10 days of age). There were 60 flies in each group. (B) Bar graph showing that knockdown of AMPK reduced the TAG levels in both non-transgenic and synphilin-1 transgenic flies. TAG levels of various flies as indicated. Data are mean±s.e.m. * P<0.05 (ANOVA followed by Tukey's post-hoc test; significance between groups as indicated).
Expression of DN-AMPK reduced food intake and body weight gain in synphilin-1 flies
To further study the roles of AMPK in synphilin-1-induced obesity-like phenotypes, Elav-Gal4;UAS-SP1 flies were crossed with UAS-AMPKαK57A [a dominant-negative (DN) form of AMPK] or UAS-AMPKαT184D (a constitutively active form of AMPK). Expression of AMPKαK57A reduced daily food intake in normal flies by ∼35% (Fig. 4), and suppressed synphilin-1-induced hyperphagia (Fig. 4A) and body weight gain (Fig. 4B). In contrast, expression of AMPKαT184D increased food intake and body weight in normal flies (Fig. 4), but it did not alter the food intake and body weight in synphilin-1 transgenic flies (Fig. 4). This may be a result of synphilin-1-induced hyperphagia that could not further increase food intake by AMPKαT184D expression.
Fig. 4.
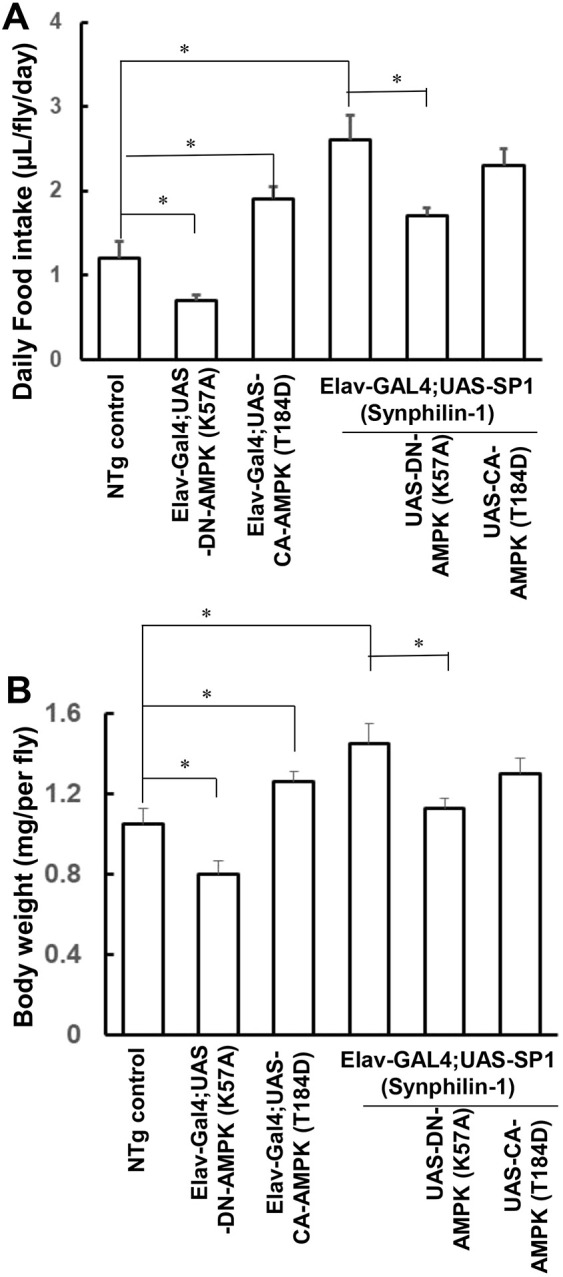
Expression of DN-AMPK K57A suppressed synphilin-1-induced food intake and body weight gain in flies. Elav-Gal4;SP1 flies were crossed with UAS-AMPK-DN. (A) Food intake of various flies as indicated was observed at 1 to 5 days post-eclosion. (B) The body weight of various flies as indicated was measured at 10 days of age. There were 60 flies in each group. Data are mean±s.e.m. *P<0.05 (ANOVA followed by Tukey’s post-hoc test; significance between groups as indicated.
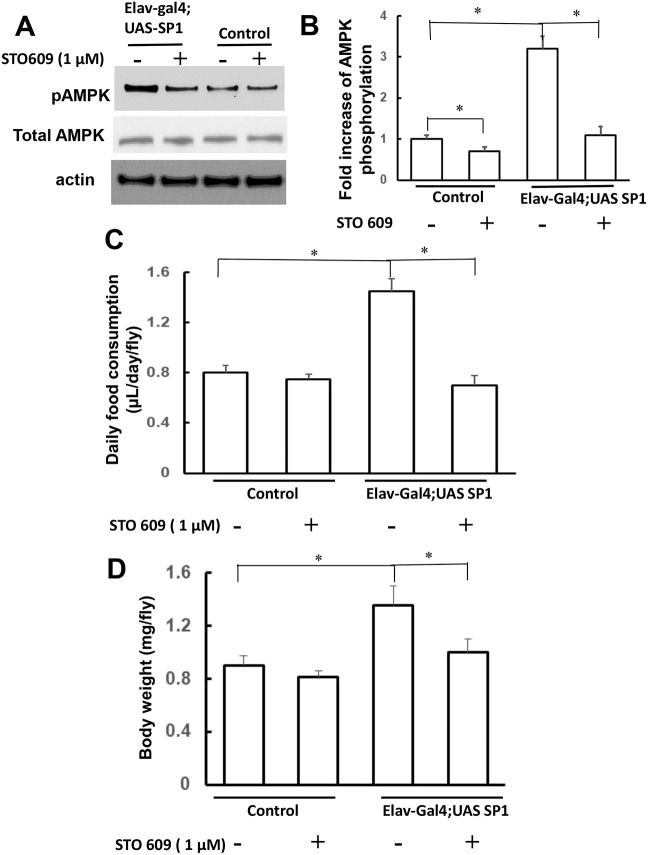
Inhibition of AMPK phosphorylation reduced food intake in synphilin-1 flies
Previous studies report that STO-609 can reduce AMPK phosphorylation, as it is a relative specific inhibitor of Ca2+/calmodulin-dependent protein kinase kinase isoform 2 (CaM-KK2), an upstream kinase of AMPK (Tokumitsu et al., 2003). STO-609 (1 μM) was added to the food of the Elav-Gal4;SP1 flies beginning on the first day of eclosion for 10 days. STO-609 significantly attenuated synphilin-1-induced AMPK phosphorylation (Fig. 5A,B). Moreover, STO-609 significantly suppressed synphilin-1-induced food intake (Fig. 5C) and body weight gain (Fig. 5D) in transgenic flies compared with untreated synphilin-1 flies. However, treatment with STO-609 at 1 μM in fly food only slightly (with no statistical significance) altered food intake and body weight in normal flies. This could be a result of STO-609 more effectively suppressing food intake and body weight gain in synphilin-1 transgenic flies with elevated AMPK phosphorylation than in normal flies with basal levels of AMPK phosphorylation. Our results suggest that synphilin-1 regulates the AMPK signaling pathway, thereby inducing hyperphagia and body weight gain.
Fig. 5.
STO-609 attenuated synphilin-1-induced food consumption in flies. STO-609 (1 µM) was added to the fly food, beginning on day 1 of eclosion and continuing for 10 days. (A,B) The fly homogenates were subjected to western blot analysis (A, representative blots) using anti-phospho-AMPK, anti-AMPK and anti-actin antibodies. The quantification of phosphorylated AMPK levels (B) was normalized to actin. (B) Bar graph showing that STO-609 significantly attenuated synphilin-1-induced AMPK phosphorylation. (C,D) Bar graphs showing that STO-609 significantly suppressed synphilin-1-induced food intake (C) and body weight gain (D) in transgenic flies (Elav-Gal4;UAS-SP1) compared with untreated synphilin-1 flies (control). There were 60 flies in each group. Data are mean± s.e.m. *P<0.05 (paired t-test; significance between groups as indicated).
DISCUSSION
The main finding of this study was that synphilin-1 regulated AMPK signalling, thereby leading to hyperphagia and obesity-like phenotypes in Drosophila. Our data revealed that the expression of synphilin-1 in neurons in flies increased AMPK phosphorylation (activation). Knockdown of AMPK or expression of DN-AMPK reduced food intake and body weight gain in normal flies and further attenuated synphilin-1-induced hyperphagia, fat deposition and body weight gain. Treatment with STO-609 also reduced synphilin-1-induced hyperphagia and obesity-like phenotypes in flies. These results indicate that the synphilin-1-linked AMPK pathway plays a critical role in controlling food intake and body weight.
AMPK is an energy sensor that monitors cellular energy status in response to nutritional changes (Hue and Rider, 2007; Kola et al., 2008; Misra, 2008; Ruderman et al., 2013; Winder and Thomson, 2007) via energy-producing catabolic pathways and energy-consuming anabolic pathways (Ruderman et al., 2013). Disruption of the AMPK pathway leads to metabolic dysregulation and causes various metabolic disorders. AMPK modulates a wide range of metabolic pathways and has become a therapeutic target for the treatment of obesity, type II diabetes mellitus and other related disorders. Our previous cell culture studies report that synphilin-1 binds with and activates AMPK (Li et al., 2020). In this study, we found that knockdown of AMPK or expression of DN-AMPK reduced food intake and body weight gain in normal flies. Moreover, we further validated that overexpression of synphilin-1 increases AMPK phosphorylation (activation) in flies in vivo. Knockdown of AMPK with siRNA, or expression of DN-AMPK, reduced both total and phosphorylated AMPK levels in human synphilin-1 transgenic flies, and further reduced synphilin-1-induced hyperphagia and body weight gain. Furthermore, overexpression of AMPK-DN-K57A, or treatment with STO-609, also suppressed synphilin-1-induced hyperphagia and body weight gain. Our studies are consistent with previous studies in mice, in which knockdown of hypothalamic AMPK reduces food intake and prevents diet-induced body weight gain (Oh et al., 2016). In line with this notion, an increase of hypothalamic AMPK activity by orexigenic peptides (e.g. ghrelin) leads to increased food intake (Andersson et al., 2004; Minokoshi et al., 2004; Moran and Gao, 2006). Fasting increases AMPK activity in the hypothalamus resulting in compensatory increases in food intake, whereas refeeding suppresses it (Minokoshi et al., 2004; Xue and Kahn, 2006). Together, these findings provide the first in vivo evidence that AMPK signaling mediates synphilin-1-induced positive energy balance.
Previous studies report that AMPK can be directly phosphorylated on Thr172 and activated by CaM-KK2 in response to metabolic hormones (Carling et al., 2011; Hardie, 2011; Hardie et al., 2012; Zhang et al., 2009). Our results also demonstrated that STO-609, a relative specific CaM-KK2 inhibitor, attenuated synphilin-1-induced AMPK phosphorylation, food consumption and body weight gain in transgenic flies. These findings suggest that CaM-KK2 may be an upstream kinase that phosphorylates AMPK, together playing a critical role in synphilin-1 action in regulating the energy balance. Whether synphilin-1 directly alters CaM-KK2 requires further investigation. Nevertheless, our findings demonstrated that a synphilin-1 regulated AMPK signaling event mediated positive energy balance, thereby resulting in the hyperphagia and obese phenotypes in flies.
Given that AMPK is a central regulator of energy metabolism (Carling et al., 2011; Hardie, 2011; Hardie et al., 2012; Zhang et al., 2009), targeting of the synphilin-1-linked AMPK pathway could be a potential therapeutic strategy for obesity intervention. In vitro studies in cell models have shown that synphilin-1 interacts with, and activates, AMPK, resulting in elevated cellular ATP levels (Li et al., 2014, 2020). In Drosophila models, synphilin-1 activates AMPK signaling pathways and induces hyperphagia and obesity (Liu et al., 2012). The inhibition of the AMPK signaling pathway by STO-609 reduced synphilin-1-induced food consumption, providing the proof of principle for further drug development. Our synphilin-1 obesity fly model provides a useful model for future pathogenesis and therapeutic studies of obesity and other related disorders.
In summary, this study provides the first in vivo evidence that the synphilin-1-regulated AMPK signaling pathway mediates positive energy homeostasis, resulting in hyperphagia and obesity. These findings not only provide a novel insight into synphilin-1 actions in energy homeostasis but also provide a potential therapeutic target for obesity intervention.
MATERIALS AND METHODS
Materials
Commercial antibodies were used in this study, including anti-phosphorylated-AMPKα (Thr172) antibody (1:1000; catalog number 2535S, Cell Signaling Technology, USA), anti-total-AMPKα 1/2 antibody (1:1000; catalog number B1297, Creative Diagnostics, USA) and anti-β-actin antibody (1:2000; catalog number A2228, Sigma-Aldrich, USA). The homemade anti-human synphilin-1 polyclonal antibody was generated as described previously (Engelender et al., 1999). STO-609 (a CaM-KK2 inhibitor) (Tokumitsu et al., 2003) was obtained from Sigma-Aldrich.
Fly stocks and maintenance
The UAS-human synphilin-1 (UAS-SP1) fly line was generated and maintained in our laboratory as described previously (Liu et al., 2012). The Elav-Gal4 driver flies were obtained from Dr Craig Montel (Johns Hopkins University, Baltimore, MD, USA). The three UAS-AMPK RNAi lines targeting the AMPKα gene, including AMPKαTRiP.JF01951 (25931), AMPKαTRiP.HMS00362 (32371), AMPKαTRiP.GL00004 (35137), UAS AMPKαK57A (32112) and UAS AMPKαT184D (32110), were obtained from the Bloomington Drosophila Stock Center. All flies were cultured in 25 ml fly culture vials with standard cornmeal medium, except during the feeding experiments. All flies were kept in a Microprocessor Controlled Low Temperature Illuminated Incubator (Thermo Fisher Scientific) at 25°C, with humidity at 55%, and with a 12 h light-12 h dark cycle.
Western blot analysis
Fly heads were harvested in RIPA buffer (Cell Signaling Technology) with protease inhibitors (10 µg/ml aprotinin, 5 mM phenylmethylsulfonyl fluoride, 10 µg/ml pepstatin and 10 µg/ml leupeptin) as described previously (Li et al., 2010). Three fly heads pooled together for one sample. There were six samples in each experimental group for western blot analysis. Proteins from each sample were separated on 4-12% NuPAGE Bis-Tris gels and transferred onto PVDF membranes (Invitrogen). The PVDF membranes were blocked with 5% non-fat milk and incubated with various primary and secondary detection antibodies. Enhanced chemiluminescence reagents (PerkinElmer) were used to detect the proteins in PVDF membranes. The quantification of total or phosphorylated AMPK levels was normalized to actin levels. The fold increase of protein levels was relative to non-transgenic control files (without any genetic alteration or treatment).
Body weight and food intake measurement
Body weight of adult flies was measured using a Sartorius Microbalance ME5 (Thermo Fisher Scientific) with the threshold reading up to 0.0001 mg as described previously. Ten flies were measured together and each individual weight value was the average of ten flies. Fly food intake was measured using CAFE assays at the age of 1 to 10 days post-eclosion, as described previously (Ja et al., 2007; Liu et al., 2012). Briefly, the CAFE assay was performed using a fly vial with 1% agar (15 ml) covered with a foam plug. A truncated 200 µl pipette tip was inserted through the foam plug and a glass capillary tube was placed inside this tip with 5% sugar solution. Each experiment was performed under a 12-h-light/12-h-dark cycle for 3 days followed by 4 h of starvation. The food intake value was the average of 3 days. A CAFE chamber without flies was employed as a control for evaporative losses. The readings for evaporative loss were subtracted from the fly food intake readings (Ja et al., 2007; Liu et al., 2012).
TAG measurement
Flies were homogenized in PBS buffer containing 1% Triton X-100, and incubated at 70°C for 10 min. Heat-treated homogenates were added with free glycerol reagent (Sigma-Aldrich) for 5 min at 37°C. Samples were subjected to a BioTek Synergy HT microplate spectrophotometer at 540 nm. TAG levels were normalized to protein levels.
STO-609 treatment
STO-609 was added to the fly food from day 1 of eclosion and continued for 10 days. Fresh food with drugs was provided every 3 to 4 days. Food intake and body weight were recorded during the treatment.
Data analysis
Quantitative data were represented as mean±s.e.m. All data from each experiment were subjected to a normal distribution test using Kolmogorov–Smirnov and Shapiro–Wilk methods. Once we confirmed normal distribution of the data, comparisons of protein levels, food intake, body weight and protein levels were analyzed using parametric tests including t-test or one-way or two-way ANOVA followed by Tukey's post-hoc test using SigmaStat 3.1 statistical software (Aspire Software International). Comparisons of the effects of STO-609 were analyzed using paired t-tests. P<0.05 was considered significant.
Acknowledgements
We thank Dr Craig Montel for providing the Elav-Gal4 driver flies.
Footnotes
Competing interests
The human synphilin-1 transgenic Drosophila line is a tangible property of the University of Maryland.
Author contributions
Conceptualization: J.L., T.H.M., W.W.S.; Methodology: J.L., X.W., R.M., T.L., G.G.; Validation: J.L., X.W., R.M., T.L., B.N., W.W.S.; Formal analysis: J.L.; Investigation: J.L., X.W., R.M., T.L., G.G., B.N., W.W.S.; Data curation: W.W.S.; Writing - original draft: J.L., W.W.S.; Writing - review & editing: T.H.M., W.W.S.; Supervision: W.W.S.; Project administration: W.W.S.; Funding acquisition: W.W.S.
Funding
This work was supported by National Institutes of Health (DK083410 to W.W.S.). Deposited in PMC for release after 12 months.
References
- Alvarez-Castelao, B. and Castaño, J. G. (2011). Synphilin-1 inhibits alpha-synuclein degradation by the proteasome. Cell. Mol. Life Sci. 68, 2643-2654. 10.1007/s00018-010-0592-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, U., Filipsson, K., Abbott, C. R., Woods, A., Smith, K., Bloom, S. R., Carling, D. and Small, C. J. (2004). AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 279, 12005-12008. 10.1074/jbc.C300557200 [DOI] [PubMed] [Google Scholar]
- Avraham, E., Szargel, R., Eyal, A., Rott, R. and Engelender, S. (2005). Glycogen synthase kinase 3β modulates synphilin-1 ubiquitylation and cellular inclusion formation by SIAH: implications for proteasomal function and Lewy body formation. J. Biol. Chem. 280, 42877-42886. 10.1074/jbc.M505608200 [DOI] [PubMed] [Google Scholar]
- Carling, D., Mayer, F. V., Sanders, M. J. and Gamblin, S. J. (2011). AMP-activated protein kinase: nature's energy sensor. Nat. Chem. Biol. 7, 512-518. 10.1038/nchembio.610 [DOI] [PubMed] [Google Scholar]
- Chung, K. K. K., Zhang, Y., Lim, K. L., Tanaka, Y., Huang, H., Gao, J., Ross, C. A., Dawson, V. L. and Dawson, T. M. (2001). Parkin ubiquitinates the α-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat. Med. 7, 1144-1150. 10.1038/nm1001-1144 [DOI] [PubMed] [Google Scholar]
- Engelender, S., Kaminsky, Z., Guo, X., Sharp, A. H., Amaravi, R. K., Kleiderlein, J. J., Margolis, R. L., Troncoso, J. C., Lanahan, A. A., Worley, P. F.et al. (1999). Synphilin-1 associates with α-synuclein and promotes the formation of cytosolic inclusions. Nat. Genet. 22, 110-114. 10.1038/8820 [DOI] [PubMed] [Google Scholar]
- Giaime, E., Sunyach, C., Herrant, M., Grosso, S., Auberger, P., McLean, P. J., Checler, F. and da Costa, C. A. (2006). Caspase-3-derived C-terminal product of synphilin-1 displays antiapoptotic function via modulation of the p53-dependent cell death pathway. J. Biol. Chem. 281, 11515-11522. 10.1074/jbc.M508619200 [DOI] [PubMed] [Google Scholar]
- Hardie, D. G. (2007). AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 8, 774-785. 10.1038/nrm2249 [DOI] [PubMed] [Google Scholar]
- Hardie, D. G. (2008). AMPK and Raptor: matching cell growth to energy supply. Mol. Cell 30, 263-265. 10.1016/j.molcel.2008.04.012 [DOI] [PubMed] [Google Scholar]
- Hardie, D. G. (2011). AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 25, 1895-1908. 10.1101/gad.17420111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie, D. G., Ross, F. A. and Hawley, S. A. (2012). AMP-activated protein kinase: a target for drugs both ancient and modern. Chem. Biol. 19, 1222-1236. 10.1016/j.chembiol.2012.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Vargas, R., Fonseca-Ornelas, L., López-González, I., Riesgo-Escovar, J., Zurita, M. and Reynaud, E. (2011). Synphilin suppresses alpha-synuclein neurotoxicity in a Parkinson's disease Drosophila model. Genesis 49, 392-402. 10.1002/dvg.20740 [DOI] [PubMed] [Google Scholar]
- Hue, L. and Rider, M. H. (2007). The AMP-activated protein kinase: more than an energy sensor. Essays Biochem. 43, 121-138. 10.1042/bse0430121 [DOI] [PubMed] [Google Scholar]
- Ja, W. W., Carvalho, G. B., Mak, E. M., de la Rosa, N. N., Fang, A. Y., Liong, J. C., Brummel, T. and Benzer, S. (2007). Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. USA 104, 8253-8256. 10.1073/pnas.0702726104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E.-K., Miller, I., Aja, S., Landree, L. E., Pinn, M., McFadden, J., Kuhajda, F. P., Moran, T. H. and Ronnett, G. V. (2004). C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J. Biol. Chem. 279, 19970-19976. 10.1074/jbc.M402165200 [DOI] [PubMed] [Google Scholar]
- Kola, B., Grossman, A. B. and Korbonits, M. (2008). The role of AMP-activated protein kinase in obesity. Front. Horm. Res. 36, 198-211. 10.1159/000115366 [DOI] [PubMed] [Google Scholar]
- Lee, G., Tanaka, M., Park, K., Lee, S. S., Kim, Y. M., Junn, E., Lee, S.-H. and Mouradian, M. M. (2004). Casein kinase II-mediated phosphorylation regulates α-synuclein/synphilin-1 interaction and inclusion body formation. J. Biol. Chem. 279, 6834-6839. 10.1074/jbc.M312760200 [DOI] [PubMed] [Google Scholar]
- Li, X., Liu, Z., Tamashiro, K., Shi, B., Rudnicki, D. D., Ross, C. A., Moran, T. H. and Smith, W. W. (2010). Synphilin-1 exhibits trophic and protective effects against Rotenone toxicity. Neuroscience 165, 455-462. 10.1016/j.neuroscience.2009.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Tamashiro, K. L. K., Liu, Z., Bello, N. T., Wang, X., Aja, S., Bi, S., Ladenheim, E. E., Ross, C. A., Moran, T. H.et al. (2012). A novel obesity model: synphilin-1-induced hyperphagia and obesity in mice. Int. J. Obes. 36, 1215-1221. 10.1038/ijo.2011.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T., Liu, J. and Smith, W. W. (2014). Synphilin-1 binds ATP and regulates intracellular energy status. PLoS ONE 9, e115233 10.1371/journal.pone.0115233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T., Liu, J., Guo, G., Ning, B., Li, X., Zhu, G., Yang, D., Moran, T. H. and Smith, W. W. (2020). Synphilin-1 interacts with AMPK, and increases AMPK phosphorylation. Cells 21, 4352 10.3390/ijms21124352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Li, T., Yang, D., Ma, R., Moran, T. H. and Smith, W. W. (2012). Synphilin-1 alters metabolic homeostasis in a novel Drosophila obesity model. Int. J. Obes. 36, 1529-1536. 10.1038/ijo.2012.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx, F. P., Soehn, A. S., Berg, D., Melle, C., Schiesling, C., Lang, M., Kautzmann, S., Strauss, K. M., Franck, T., Engelender, S.et al. (2007). The proteasomal subunit S6 ATPase is a novel synphilin-1 interacting protein--implications for Parkinson's disease. FASEB J. 21, 1759-1767. 10.1096/fj.06-6734com [DOI] [PubMed] [Google Scholar]
- Minokoshi, Y., Alquier, T., Furukawa, N., Kim, Y.-B., Lee, A., Xue, B., Mu, J., Foufelle, F., Ferré, P., Birnbaum, M. J.et al. (2004). AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428, 569-574. 10.1038/nature02440 [DOI] [PubMed] [Google Scholar]
- Misra, P. (2008). AMP activated protein kinase: a next generation target for total metabolic control. Expert Opin Ther. Targets 12, 91-100. 10.1517/14728222.12.1.91 [DOI] [PubMed] [Google Scholar]
- Moran, T. H. and Gao, S. (2006). Looking for food in all the right places? Cell Metab. 3, 233-234. 10.1016/j.cmet.2006.03.004 [DOI] [PubMed] [Google Scholar]
- Nagano, Y., Yamashita, H., Takahashi, T., Kishida, S., Nakamura, T., Iseki, E., Hattori, N., Mizuno, Y., Kikuchi, A. and Matsumoto, M. (2003). Siah-1 facilitates ubiquitination and degradation of synphilin-1. J. Biol. Chem. 278, 51504-51514. 10.1074/jbc.M306347200 [DOI] [PubMed] [Google Scholar]
- O'Farrell, C., Murphy, D. D., Petrucelli, L., Singleton, A. B., Hussey, J., Farrer, M., Hardy, J., Dickson, D. W. and Cookson, M. R. (2001). Transfected synphilin-1 forms cytoplasmic inclusions in HEK293 cells. Brain Res. Mol. Brain Res. 97, 94-102. 10.1016/S0169-328X(01)00292-3 [DOI] [PubMed] [Google Scholar]
- Oh, T. S., Cho, H., Cho, J. H., Yu, S.-W. and Kim, E.-K. (2016). Hypothalamic AMPK-induced autophagy increases food intake by regulating NPY and POMC expression. Autophagy 12, 2009-2025. 10.1080/15548627.2016.1215382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, C. S., Carneiro, K., Ross, C. A., Menezes, J. R. L. and Engelender, S. (2002). Synphilin-1 is developmentally localized to synaptic terminals, and its association with synaptic vesicles is modulated by α-synuclein. J. Biol. Chem. 277, 23927-23933. 10.1074/jbc.M201115200 [DOI] [PubMed] [Google Scholar]
- Ruderman, N. B., Carling, D., Prentki, M. and Cacicedo, J. M. (2013). AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Invest. 123, 2764-2772. 10.1172/JCI67227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryo, A., Togo, T., Nakai, T., Hirai, A., Nishi, M., Yamaguchi, A., Suzuki, K., Hirayasu, Y., Kobayashi, H., Perrem, K.et al. (2006). Prolyl-isomerase Pin1 accumulates in lewy bodies of parkinson disease and facilitates formation of α-synuclein inclusions. J. Biol. Chem. 281, 4117-4125. 10.1074/jbc.M507026200 [DOI] [PubMed] [Google Scholar]
- Sinnett, S. E. and Brenman, J. E. (2016). The Role of AMPK in Drosophila melanogaster. Exp. Suppl. 107, 389-401. 10.1007/978-3-319-43589-3_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, W. W., Margolis, R. L., Li, X., Troncoso, J. C., Lee, M. K., Dawson, V. L., Dawson, T. M., Iwatsubo, T. and Ross, C. A. (2005). α-synuclein phosphorylation enhances eosinophilic cytoplasmic inclusion formation in SH-SY5Y cells. J. Neurosci. 25, 5544-5552. 10.1523/JNEUROSCI.0482-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, W. W., Liu, Z., Liang, Y., Masuda, N., Swing, D. A., Jenkins, N. A., Copeland, N. G., Troncoso, J. C., Pletnikov, M., Dawson, T. M.et al. (2010). Synphilin-1 attenuates neuronal degeneration in the A53T alpha-synuclein transgenic mouse model. Hum. Mol. Genet. 19, 2087-2098. 10.1093/hmg/ddq086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szargel, R., Rott, R. and Engelender, S. (2008). Synphilin-1 isoforms in Parkinson's disease: regulation by phosphorylation and ubiquitylation. Cell. Mol. Life Sci. 65, 80-88. 10.1007/s00018-007-7343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumitsu, H., Inuzuka, H., Ishikawa, Y. and Kobayashi, R. (2003). A single amino acid difference between α and β Ca2+/calmodulin-dependent protein kinase kinase dictates sensitivity to the specific inhibitor, STO-609. J. Biol. Chem. 278, 10908-10913. 10.1074/jbc.M213183200 [DOI] [PubMed] [Google Scholar]
- Wakabayashi, K., Engelender, S., Yoshimoto, M., Tsuji, S., Ross, C. A. and Takahashi, H. (2000). Synphilin-1 is present in Lewy bodies in Parkinson's disease. Ann. Neurol. 47, 521-523. [DOI] [PubMed] [Google Scholar]
- Winder, W. W. and Thomson, D. M. (2007). Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochem. Biophys. 47, 332-347. 10.1007/s12013-007-0008-7 [DOI] [PubMed] [Google Scholar]
- Xue, B. and Kahn, B. B. (2006). AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J. Physiol. 574, 73-83. 10.1113/jphysiol.2006.113217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B. B., Zhou, G. and Li, C. (2009). AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 9, 407-416. 10.1016/j.cmet.2009.03.012 [DOI] [PubMed] [Google Scholar]