Abstract
Objective.
Recent advances in neural engineering have restored mobility to people with paralysis, relieved symptoms of movement disorders, reduced chronic pain, restored the sense of hearing, and provided sensory perception to individuals with sensory deficits.
Approach.
This progress was enabled by the team-based, interdisciplinary approaches used by neural engineers. Neural engineers have advanced clinical frontiers by leveraging tools and discoveries in quantitative and biological sciences and through collaborations between engineering, science, and medicine. The movement toward bioelectronic medicines, where neuromodulation aims to supplement or replace pharmaceuticals to treat chronic medical conditions such as high blood pressure, diabetes and psychiatric disorders is a prime example of a new frontier made possible by neural engineering. Although one of the major goals in neural engineering is to develop technology for clinical applications, this technology may also offer unique opportunities to gain insight into how biological systems operate.
Main results.
Despite significant technological progress, a number of ethical and strategic questions remain unexplored. Addressing these questions will accelerate technology development to address unmet needs. The future of these devices extends far beyond treatment of neurological impairments, including potential human augmentation applications. Our task, as neural engineers, is to push technology forward at the intersection of disciplines, while responsibly considering the readiness to transition this technology outside of the laboratory to consumer products.
Significance.
This article aims to highlight the current state of the neural engineering field, its links with other engineering and science disciplines, and the challenges and opportunities ahead. The goal of this article is to foster new ideas for innovative applications in neurotechnology.
Keywords: neural engineering, neurotechnology, innovation, applications, knowledge gaps, collaboration/teams, future
Introduction
A recent surge in technologies that interface with the nervous system has led to devices that greatly improve quality of life for people with neurological disorders. Neural engineers are instrumental players in the continued advancement and clinical translation of these emerging neuro-technologies. Toward this goal, research-and-development minded individuals from fields within basic and applied sciences and medicine come together to understand and modulate neural systems [1]. Neural engineering as a discipline can be broadly defined as the application of neuroscientific and engineering approaches to understand, repair, replace, enhance, or exploit the properties of neural systems, as well as to design solutions to problems associated with neurological limitations and dysfunction [2, 3]. Often accompanied by scientific research directed at the interface between living neural systems and non-living components [2], this field brings together teams of engineers, neuroscientists, biologists, chemists, therapists, and physicians. Neural engineers value the heterogeneity of their colleagues and seek out multiple perspectives to inform the development of their technology. The sub-specialties illustrated in figure 1 demonstrate the vast range of professionals involved in neural engineering advancements. With this article, we invite multi-disciplinary participation in the process of developing neural technology. We provide a brief overview of the current state of the field, illustrate the need for involvement of individuals with different training backgrounds, and describe some key future considerations.
Figure 1.
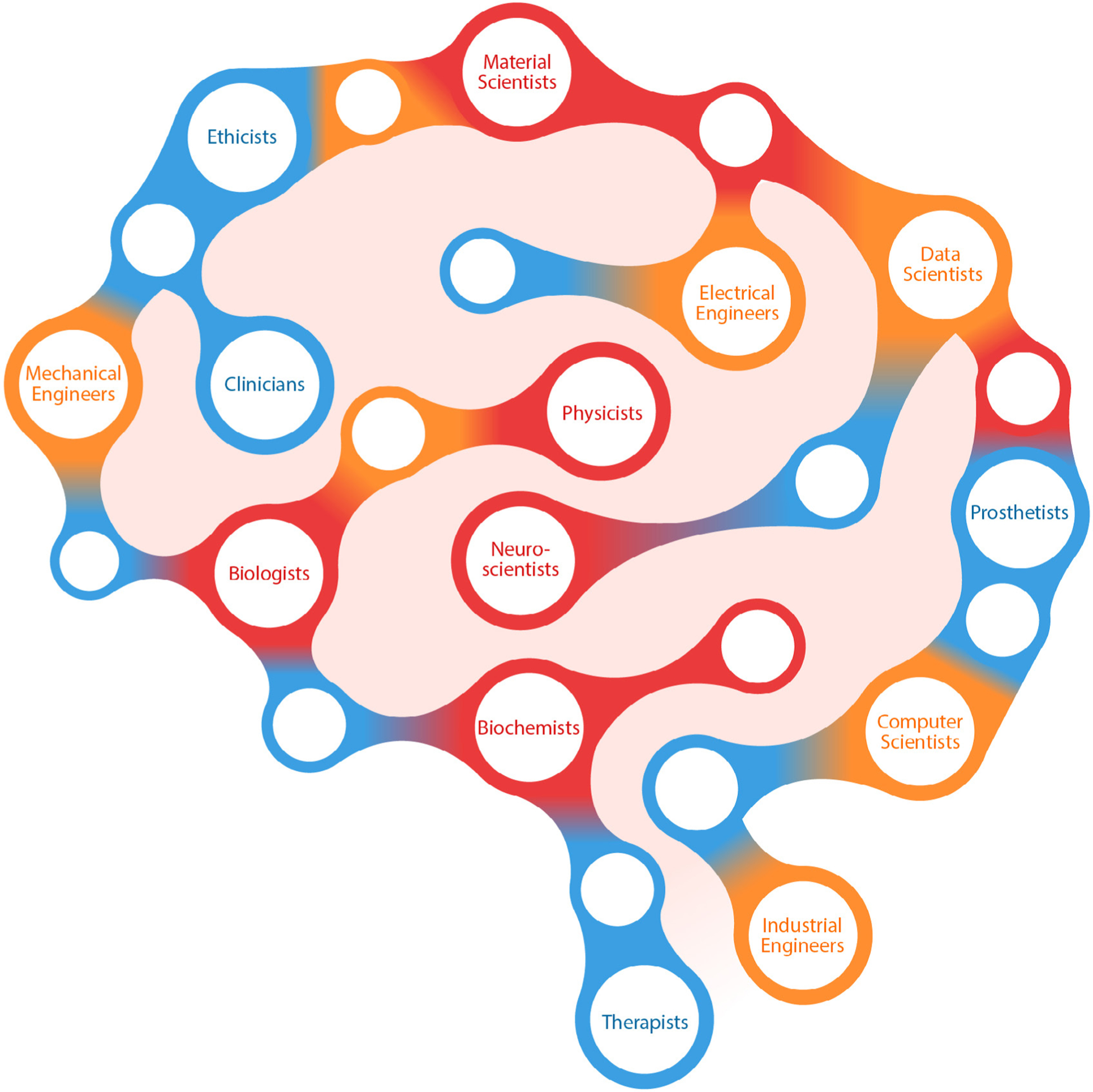
Who is a neural engineer? This illustration highlights the professionals actively involved in developing and translating neural technology. These professionals include sub-specialties ranging from scientists, technical experts, clinicians, and others involved in the clinical setting.
Applications of neural engineering
Neural engineering devices interface with the nervous system to measure or modulate neural activity and are most commonly applied to understand or address challenges associated with neurological dysfunction. These devices cross a spectrum of invasiveness, from externally worn to those that are implanted in the body. Advances in neural engineering have led to important innovations in medical science with the potential to restore function or alleviate symptoms. This has garnered attention in both therapeutic and consumer applications. Numerous neural engineering technologies have transitioned from their research (proof-of-concept) origins to becoming viable treatment options in medical and clinical applications.
In the example of deep brain stimulation (DBS), the implantation and targeted stimulation of specific brain areas have been studied to treat symptoms associated with various motor impairments, such as Parkinson’s disease, essential tremor, dystonia, and psychiatric disorders such as obsessive-compulsive disorder and depression [4–12]. After extensive research and clinical trials, commercially available DBS devices have become increasingly prevalent [13]. This technology also epitomizes how neural engineering has benefited from previous biomedical engineering innovations. DBS technology, particularly the first clinical devices, has leveraged approaches developed for cardiac pacemakers [14]. Although implanted electrodes and associated leads have been designed specifically for targeted neuroanatomy, multiple device techniques and components, such as stimulator electronics, battery, and packaging are borrowed from cardiac applications.
Similarly, spinal cord stimulation has become an established method for treating chronic pain. Clinical trials for various stimulation strategies, including tonic, high frequency, and bursting paradigms, have shown significant long-term reductions in pain experienced by patients. Further, ongoing research into new stimulation targets, advanced neurostimulator technologies, and improved stimulation patterns are rapidly expanding the neuromodulation market for pain therapy [15–18].
Existing neural devices also mitigate seizures and partially restore hearing, sight, and movement. Cochlear implants are a remarkably successful neural engineering technology that improve the quality of life for individuals with hearing impairment. They are widely prescribed for deaf individuals to partially restore hearing by electrically stimulating the auditory nerve as a means of bypassing the damaged inner ear [19]. Retinal implants can help partially restore sight to those affected by retinal diseases by bypassing damaged photoreceptors and electrically stimulating remaining retinal neurons [20]; however, this technology remains in the early stages of development. Sacral neuromodulation therapy is used to help alleviate symptoms of pelvic floor disorders such as overactive bladder resulting in urinary incontinence [21]. Vagus nerve stimulation may reduce the frequency of seizures in people with epilepsy who do not fully respond to standard medication [22]. Responsive or closed loop brain stimulation has also been shown to reduce seizure frequency, while dramatically reducing the total amount of energy delivered to a patient’s body [23, 24]. Finally, there are numerous neuromuscular electrical stimulation devices and therapies to partly restore impaired movement [25, 26].
In addition to these established neural engineering technologies, there are many emerging interventions currently transitioning from research into clinical care that is producing a rapid growth of career and clinical opportunities for neurotechnology engineers. The global market for neuromodulation devices is expected to grow to around US $5 billion by 2022, doubling its estimated size from 2017 [27]. In fact, several technologies have recently received regulatory approval and are now available for commercial use, including the NeuroPace RNS® system for refractory epilepsy, Allergan TrueTear® nasal stimulation to increase tear production, and the Inspire Upper Airway Stimulator for obstructive sleep apnea. See table 1 for a more comprehensive list.
Table 1.
Selection of neural engineering devices recently approved by the US Food and Drug Administration. PMA: premarket approval; HDE: humanitarian device exemption.
| Device | Manufacturer or Sponsor | Indication | Year approved | Regulatory path | Innovations |
|---|---|---|---|---|---|
| Inspire | Inspire Medical Systems, Inc. | Treat obstructive sleep apnea | 2014 | PMA | Stimulation of the hypoglossal nerve during inspiration |
| TrueTear® | Allergan plc | Increase in real tear production | 2018 | de novo | Electrical stimulation to sensory neurons of the nasal cavities |
| Maestro® | EnteroMedics, Inc. | Weight reduction in people with obesity | 2015 | PMA | Electrical stimulation to block abdominal nerve trunks of the vagus nerve |
| gammaCore™ | electroCore, Inc. | Acute treatment of migraine headache | 2018 | 510(k) | Noninvasive vagus nerve stimulation (nVNS) on the side of the neck |
| Drug Relief® | DyAnsys, Inc. | Reduce opiod withdrawal symptoms | 2018 | 510(k) | Electrical stimulation at branches of cranial nerve |
| RNS® | NeuroPace, Inc. | Reducing the frequency of seizures for people with epilepsy | 2013 | PMA | Cranially implantable neurostimulator that senses brain’s electrical activity and delivers stimulation to epileptogenic foci |
| Argus® II Retinal Prosthesis System | Second Sight Medical Products, Inc. | Inducing visual perception in blind patients due to retinitis pigmentosa | 2013 | HDE | Electrical stimulation of retina |
| Medtronic DBS System for Epilepsy | Medtronic, Inc. | Expand use of DBS system for treatment of epilepsy | 2018 | PMA | Bilateral stimulation of the anterior nucleus of the thalamus |
Many nascent neurotechnologies are on the cusp of transitioning from research and pre-clinical environments to clinical implementation. For example, advanced artificial limbs can interface with nerves that remain following amputation. This enables intuitive closed-loop prosthetic control by both recording and stimulating residual nerves, and can be facilitated by a variety of techniques, including peripheral nerve interfaces and reinnervation surgeries. These techniques allow users to both move their devices and receive relevant sensory feedback from their missing limbs [28–33]. These technologies are beginning to blur the line between human and machine, and hold the potential to integrate these assistive devices as a part of one’s body [34, 35]. Additionally, for people with spinal cord injury, emerging neural technologies may include stimulating neural circuitry above or below the injury to help restore and modulate the control of both upper and lower limbs [36–41], respiration [42–44], and urinary or bowel control [25, 45]. Finally, existing technologies have opened avenues to potential treatment options in new and unexpected ways. Beyond their conventional applications, DBS and vagus nerve stimulation are currently being tested in clinical trials for the treatment of a number of neurological and psychiatric conditions such as depression, Tourette’s syndrome, Alzheimer’s, dementia, and addiction [46–48].
Neural engineering technologies have achieved significant milestones and future potential of emerging technologies is clear. There remains, however, a need to develop improved devices that seamlessly integrate into the lives of users to the point that these individuals are indistinguishable from able-bodied individuals. Future progress will be enabled by three key principles: (1) optimizing clinical benefit through the close collaboration of applied clinical, quantitative, and basic sciences; (2) uncovering a mechanistic understanding of neural systems and interventions to help inform treatment strategies; and (3) understanding user needs and the context of their disease management to improve end-user acceptance.
Engineering innovation
Training is required to successfully develop technology across disciplines. Neural engineers must develop and incorporate skills in systems-level project management, and user needs assessment, and gain sufficient interdisciplinary experience to speak the technical language of multiple disciplines and backgrounds. The ability for neural engineers to effectively communicate with multiple disciplines becomes more crucial with a diverse team that includes scientists that are guided by discovery, engineers that are design driven, and end-users/clinicians that are generally more concerned with outcome benefits and accessibility of the technology. The need for expertise from multiple disciplines requires neural engineers to build strong communication and interpersonal skills to foster effective collaboration in teams.
Neural engineers have long been performing convergent engineering research, which was recently identified as a priority by the National Academies of Sciences, Engineering, and Medicine [49, 50]. Convergent research integrates several disciplines to address a specific challenge, resulting in the combination of diverse disciplines’ knowledge and methods. The combination of these disciplines also develops efficient ways of communicating across disciplines and possibly even a new scientific language. As basic science discoveries related to neurological diseases and the integration and function of neurotechnologies are achieved, they must be translated to the clinical environment. This process relies on communication between scientists, medical professionals, and neural engineers, as they concurrently feed information to one another and enable the progression of the field (figure 2).
Figure 2.
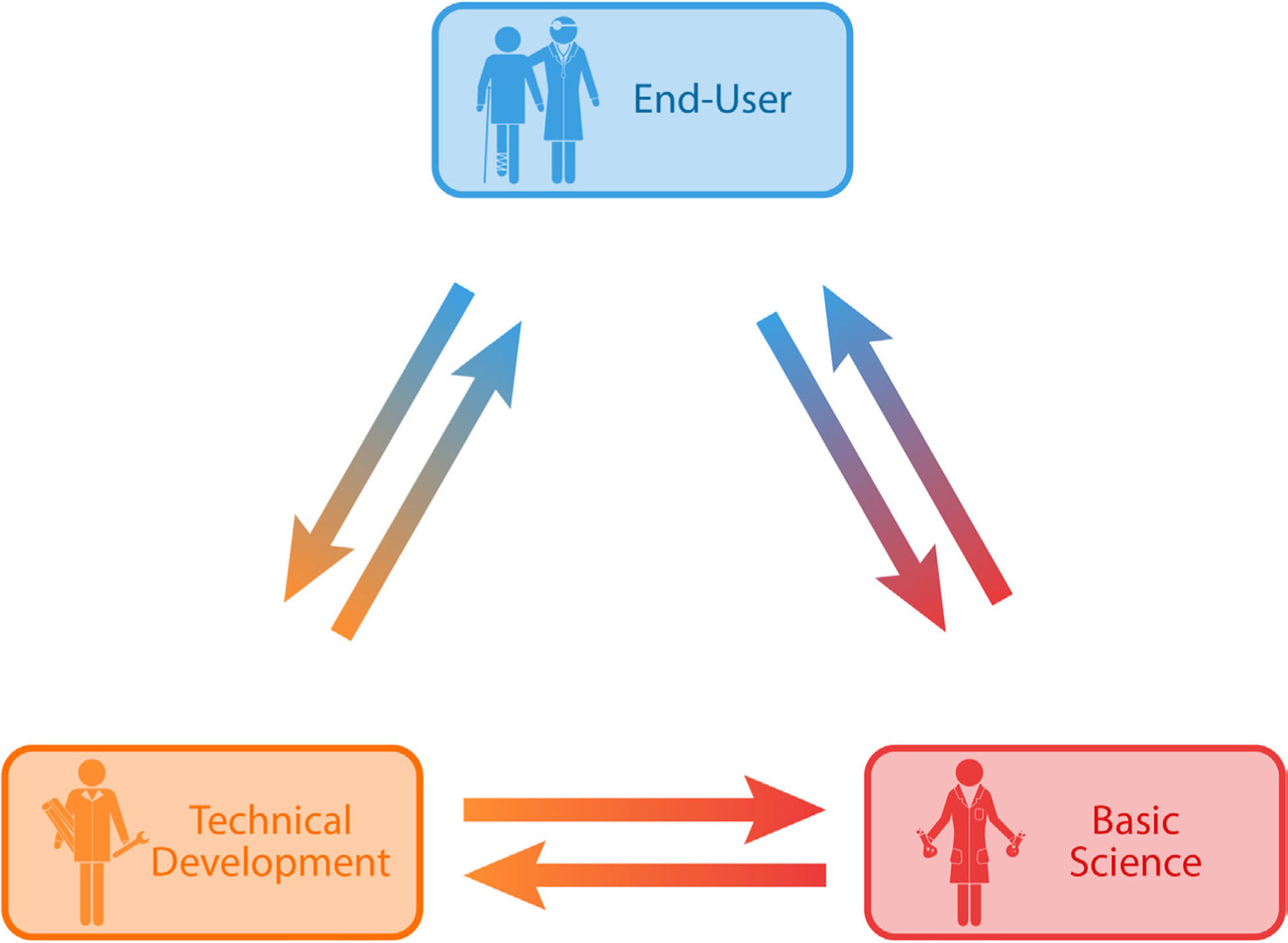
Developmental cycle of neurotechnology process. Information flow in the process of developing and translating neurotechnology.
Neural engineering technology depends on an effective cycle of scientific discovery, innovative development of next-generation technology, and evaluation of feasibility and efficacy in the clinic. There is a need for scientists to continue learning about the nervous system on a physiological, cellular, and molecular level. In parallel, engineers need to incorporate information from scientists and end-users (both clinicians and patients) to guide the development of next-generation tools and identify unmet needs that are necessary for advancing knowledge and treatment of the nervous system. The knowledge gained provides insight and approaches needed for clinicians to understand, diagnose, and treat their patients. As an example of this process, clinicians identified the incompatibility of DBS electrodes with magnetic resonance imaging (MRI) as a limitation to providing standard patient care [51]. Innovations in materials science provided specialized MRI-compatible substrates, which engineers incorporated into implantable electrodes [52]. These improved electrodes allow clinicians to use standard imaging procedures to monitor disease progression while also enabling new scientific studies of brain activity during stimulation [52].
As the field of neural engineering continues to grow, there will be an emerging need for trained and experienced neural engineers. Unlike other disciplines, there is no formal pathway to practicing as a neural engineer. Instead, it is typically comprised of a combination of related education paired with experience in the field. A neural engineer may be formally trained in a constituent field such as neuroscience, engineering, or medicine, and accumulate experience through immersion in multifaceted teams. Through this experience, they learn to leverage the teams’ expertise and address challenges across a spectrum of biological and engineering disciplines. Therefore, it is important that training environments foster opportunities to actively interact with teams of scientists, engineers, and clinicians. Furthermore, trainees will benefit from formal course work in complementary fields, which will help them more effectively communicate and bridge fields. It is crucial that trainees themselves seek to understand challenges from multiple perspectives and learn to frame problems and apply techniques in contexts that extend beyond their formal area of expertise.
Addressing knowledge gaps to advance neural engineering
Neural engineering technologies have provided substantial capabilities for understanding and communicating with the nervous system. These advances in basic neuroscience, in turn, have provided essential insight that enables the development of neuromodulatory interventions. Despite these advances, essential gaps in knowledge remain. One gap is our limited understanding of how recording and stimulating electrodes interact and interface with nervous tissue, as well as strategies to seamlessly integrate electrode technologies with nervous tissue and optimize their functionality during interactions with the physiology of the implanted organ system. Specifically, a discontinuity exists between brain tissue and interfacing neurotechnology that may contribute to the inflammatory response following implantation of intracortical microelectrodes [53–57]. Inflammation can lead to declines in chronic recording quality observed within months and a decreased lifespan for the implanted device [58–61].
A second gap is present in understanding neurological pathways related to pain and autonomic, sensory, cognitive/emotional, and motor systems. Specifically, questions remain about device mechanisms of action, neural encoding, and neural changes at the cellular and molecular level. For example, newly gained knowledge in modulating the autonomic nervous system has found applications in atrial arrhythmia, pain, and hypertension [62–65]. An improved understanding of how the autonomic nervous system responds to electrical stimulation could further improve outcomes and expand applications. Similarly, greater understanding of neural encoding would allow devices that interact with the sensorimotor system to better engage with existing neural circuitry. Furthermore, with the growth of novel techniques and advanced materials to interface with the nervous system, there is an increasing demand for new standards to ascertain their performance, efficacy, reliability, and safety. There has been an effort to improve this aspect of neurotechnology development in recent years. For example, in vitro protocols have been developed to rapidly test the durability and cytotoxicity of neural implants in environments that simulates in vivo conditions [66–70]. However, common methodologies have yet to be adopted across research groups to consistently characterize tissue response, neural recording quality, and chronic performance. This limits the ability to compare across devices and applications and hampers device translation to the clinic. It is important to note that interventions with positive outcomes in animal models do not always translate to human clinical trials. These limitations reflect a need for comprehensive study of the underlying neurobiology and mechanisms, which is a fertile opportunity for collaboration between neural engineers and basic neuroscientists.
Before innovative neurotechnology can reach its users, most devices will need regulatory approval prior to clinical investigation and commercialization. Fundamentally, regulation is a balance between promoting innovative solutions and ensuring patient safety. Within the last several years, there has been a remarkable number of novel neural engineering devices approved by the US Food and Drug Administration (FDA). Certain key realms of innovation have seen huge advances, such as closed-loop technology [71], high-frequency neuromodulation, non-invasive device technology, and DBS for novel indications (see table 1). The FDA’s Center for Devices and Radiological Health (CDRH) established several recent programs that are designed to promote innovation in device technology. For example, the Breakthrough Devices Program was initiated to help patients gain timely access to breakthrough technology and accelerate device development [72]. A neural engineering device that uses cortical stimulation system for sight restoration, the Orion Visual Cortical Prosthesis from Second Sight Medical Products, Inc., is among the 54 devices granted breakthrough status [73]. CDRH has also implemented the Early Feasibility Study (EFS) program for clinical studies [74]. The FDA is encouraging early feasibility studies to emphasize performing discovery science and physiological research in clinical trials, bridging basic science data and clinical findings. This program has doubled in size over the last five years, with neurological devices claiming the second-highest number of submissions, behind cardiac devices. One of the forerunners in the EFS program was the Networked Neuroprosthesis, from the Institute for Functional Restoration. Based on their initial success from EFS, a pivotal trial for this device is in the near future. Beyond the number of EFS submissions, the review process has become more streamlined, with the time to approval for EFS clinical studies reduced to an average of 68 d.
The data collected from clinical studies substantially contribute to understanding the impact of these neurotechnologies and how neural engineers can optimize them to improve functionality. Clinical trials of neurotechnologies should collect rich patient feedback in multiple forms, including user experience surveys and in-person interviews of perspectives [75, 76]. Moreover, the insight provided by end-users will continue to be critical to the advancement of these neurotechnologies. The device design process should include user feedback at every stage of the development process, from initial conceptualization to final testing. Factors such as optimization of current technologies, the need for additional features, and the design and usability of these devices will improve considerably with this important input.
Although the FDA’s CDRH has released guidance documents specific to innovative medical technology, certain product types face a more difficult regulatory path. Additional testing and/or regulatory review time may be required for devices that involve novel materials, are combined products with biologics (such as growth factors, stem cells or gene therapy), or are modular systems with multiple product types integrated into a single device (such as brain-controlled neuroprosthetic systems) [77]. Other challenges arise from the design of clinical trials for small patient populations, including appropriate controls for neurostimulation or outcome metrics for devices designed to allow novel capabilities [78]. Continued dialogue between the FDA and the neural engineering community can begin to address these issues. For instance, the FDA’s public workshop on brain-computer interface devices for patients with paralysis or amputation solicited community input on challenges for BCI devices [79]. This meeting resulted in a draft guidance document that lays out the FDA’s thoughts on a diversity of pre-clinical, clinical, and device development issues [80]. Among many valuable recommendations, this draft document lays out a role for Patient Reported Outcome Measures in clinical trial design, which can be a key element for devices that improve user quality of life.
The need to continue exploring available pathways is stoking collaborations across institutions and conversations about the future of neurotechnology. For example, the National Institutes of Health hosted a meeting, SCI 2020: Launching a Decade for Disruption in Spinal Cord Injury Research, to discuss recent progress and current gaps in the understanding and treatment of spinal cord injury, as well as foster collaboration between scientists, clinicians, and patient advocates. The Cleveland Neural Engineering Workshop has been fostering communication between scientists, engineers, medical professionals, industry leaders, government funding agencies, regulatory experts, and end-users since 2011. Their expressed goal has been to ‘bring together the neural engineering stakeholders with the specific purpose of developing a strategic plan, an infrastructure plan and best practices for the community’ to advance neural technology for the next century [81, 82]. Conferences that have previously focused on case studies and reports from clinicians, such as the North American Neuromodulation Society Conference, as well as conferences that have traditionally been organized as collaborative environments, such as the Neural Interfaces Conference and the Gordon Research Conferences (e.g. recent specific topics relevant to neural engineering include the Bioelectronics and Neuroelectronic Interfaces Gordon Conferences), are increasing their focus on scientific evidence, raising the bar for discussions and presentation of data from technology, scientific, and clinical perspectives.
Neural engineering faces an exciting time for growth in which we can leverage breakthroughs in other technologies to expand existing applications. For example, advancements in electronics and fabrication techniques facilitating hardware miniaturization allowed multi-channel and multi-unit recordings from nervous tissue. This technology both enabled large-scale recording and accelerated the associated signal processing [83, 84]. Innovations in recording neurotechnology, such as optical tools [85] and high-density silicon probes [86, 87], have allowed monitoring of single-cell activity in large populations of neurons. Advancements in the capabilities of neurotechnologies have facilitated further insight into the function of neural circuitry as well as the role of individual cells during activity and associated behavior [88, 89]. As the amount, variety, and complexity of data sources grows, the neural engineering field stands to gain from the ongoing development of big data approaches [90]. Data collected from these emerging breakthroughs can be utilized to alleviate the burden of many diseases and their symptoms.
Neural engineers may also employ their tools to continue building our understanding of the central and peripheral nervous system, which is one of the great challenges in biology and science [91]. To advance the science of neural engineering, it is necessary to invest in the development of technologies that are designed to expand scientific knowledge and therapeutic applications. Therefore, it is crucial to continue research support for the science of neural engineering, which is often too risky for the commercial sector at the early stages of innovation.
Future potential of neural engineering
Exploration of neural engineering traces back to the late 18th century when Volta showed that electrical stimulation in the ears produced sensations of sound [92]. In recent decades, the field of neural engineering has rapidly accelerated. A search of scientific literature indexed on PubMed returned 2242 journal articles describing neural engineering-related research published in the last 30 years, 91% of which were published in the last 10 years (figure 3). There are numerous success stories of neural technology helping people with disabling medical conditions. It has been estimated that the global market for neurotechnology products will grow to $13.3 billion by 2022, a 12% growth from 2018 [93]. The leading segments in this market belong to neuromodulation systems for spinal cord and deep brain stimulation [93]. Notably, neuroprosthetics systems are predicted to have a 57% growth from $2.1 billion to $3.3 billion from 2018 to 2022 [93].
Figure 3.
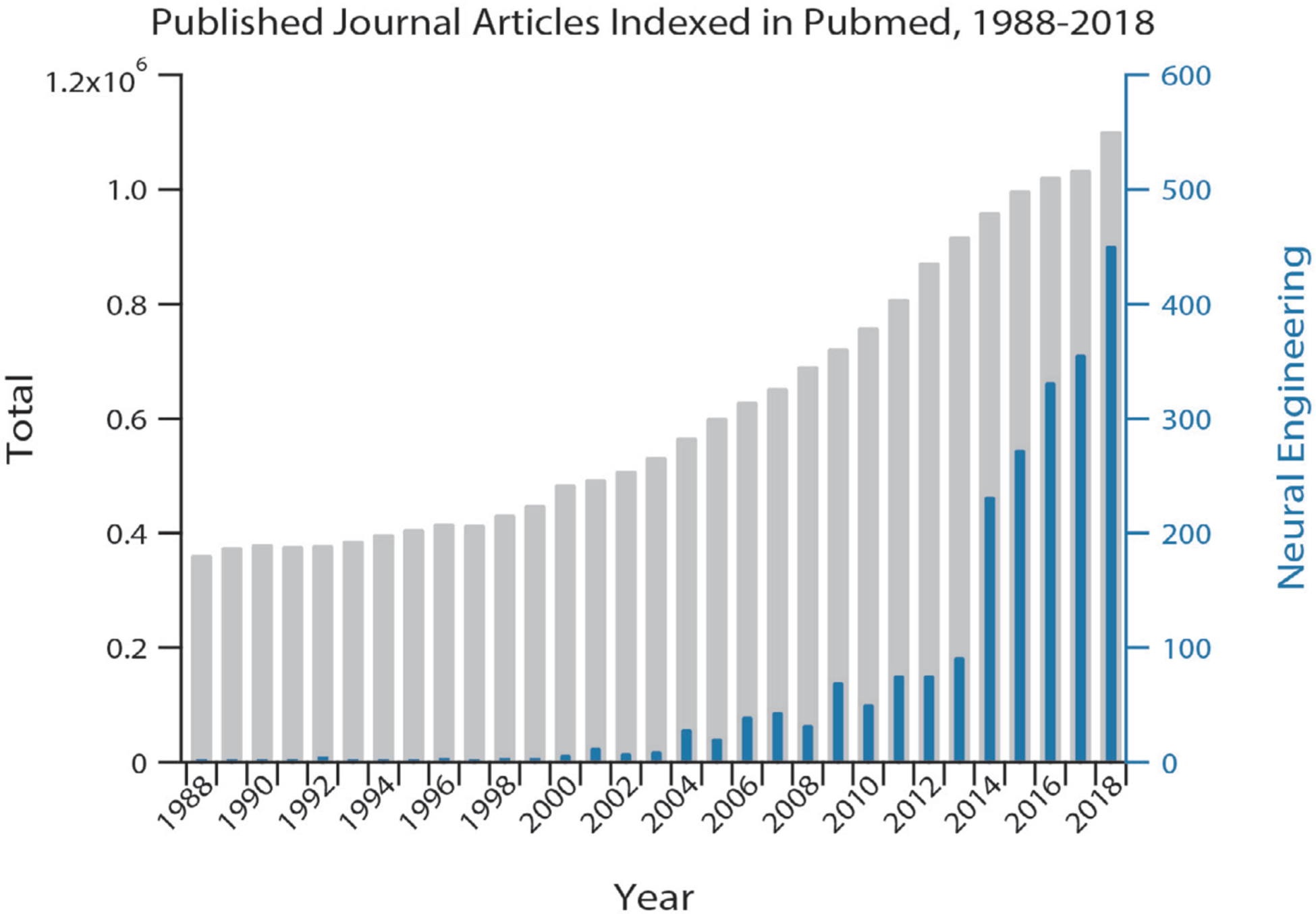
Growth of neural engineering over the past 30 years. Total (left axis, grey) and neural-engineering-related (right axis, blue) journal articles published and indexed in PubMed over the past 30 years. A search of PubMed with the publication type restricted to ‘journal article’ returned the total number of journal articles and adding AND (‘neural engineering’ OR ‘neurotechnology’ OR ‘neuro technology’ OR ‘neural interface’) returned the number of journal articles about neural engineering.
Many opportunities exist to further improve the functionality, efficacy, and efficiency of neural devices. Some of the immediate next steps may include leveraging recent progress in battery technology, microfabrication techniques, and biocompatible materials. Furthermore, limitations in brain-to-machine communication may be overcome by leveraging increases in computing power to offload portions of device control into learning algorithms that draw on contextual or environmental cues.
Several neural engineering technologies may benefit from developing closed-loop systems, in which biological feedback is used to maintain effective treatment in the presence of the dynamic central and peripheral nervous systems. Such closed-loop systems often require reliable and accessible biomarkers to be utilized as feedback to the system. Despite proof-of-concept studies supporting closed-loop neural engineering systems [94], additional research initiatives are required to identify biomarkers for a variety of disorders and ascertain their suitability. Emerging neurotechnology provides researchers with new tools and perspectives to understand, treat, and perhaps even cure or guide cures for brain injuries and neurodegenerative diseases.
Neurotechnology has matured to the point where there is unprecedented commercial interest. Pharmaceutical and technological companies have invested into neurotechnology development as an alternative to conventional medicine. Initiatives by GlaxoSmithKline and investments by Alphabet’s Verily Life Sciences in recent years are among a few examples of expanding support for ‘bioelectronic medicine’. In this emerging field, novel therapeutics intervene by electrically modulating the body’s neural signals. Such solutions offer the potential of user-specific treatments that could be selectively tuned based on individual user needs, provide real-time symptom relief, and reduce off-target side effects.
There is also notable enthusiasm for augmentation of people without medical conditions. Companies hope to take advantage of recent advances in artificial intelligence to develop brain-computer interfaces that could enhance the human brain’s processing power. The Defense Advanced Research Projects Agency (DARPA) has shown particular interest in human augmentation using neural engineering techniques by announcing programs such as Targeted Neuroplasticity Training (TNT) [95], which aims to augment brain function to achieve rapid learning. In fact, commercial interests have grown in this area and companies such as Kernel, Neuralink, and Facebook have already invested in developing products that could expand human cognition or improve the communication bandwidth between human and computer. Similar to efforts to restore function in people with disability, there is much work to be done before these technologies can meet or exceed the functionality of the intact and healthy nervous system. At the same time, ethical concerns around such new technologies should be carefully considered and addressed [96–98].
Further investment into neural engineering is critical to push the field forward. Visionary early investments by the National Institutes of Health (e.g. Brain Research through Advancing Innovative Neurotechnologies [BRAIN] Initiative and Stimulating Peripheral Activity to Relieve Conditions [SPARC] program), National Science Foundation, and DARPA (e.g. TNT and Electrical Prescriptions [ElectRx] programs) have supported high-risk, high-reward projects that have enabled the field to achieve the current level of success. Despite promising investment interest from the private sector, it is crucial that government agencies continue to support foundational technology development and early-stage investigational approaches. Nonetheless, the burgeoning commercial interest in neural engineering is an excellent indicator of the maturity of this exciting field, and its tremendous potential to enhance quality of life in the future.
Conclusion
Neural engineering is a rapidly expanding and exciting field. There are already numerous clinical neurotechnologies improving the lives of persons with neural deficits or disorders. However, opportunities abound for involvement of experts with diverse backgrounds. The active engagement of such experts is imperative to tackle complex problems integrating the nervous system with non-biological tools. More effort should be dedicated to foster growth of the next generation of neural engineers by encouraging and facilitating training outside of trainees’ formal expertise. In addition, further investment is needed to develop and refine next-generation technologies that will substantially expand quality of life. However, investment should not be limited to those technologies with a clear path to market. Research initiatives should promote development of new tools and novel techniques, as well as work that investigates underlying mechanisms of neurological conditions and interactions with neurotechnology. Neural engineering both benefits from and enables enhanced understanding of basic science, which is crucial to enable progress in the field and innovation of neural devices. Although any landmark progress in the field requires technical innovation, several key non-technical groups are essential to a successful development process, including funding agencies, regulatory agents, end-users, neuroethicists, and the private sector. Therefore, it is essential to develop pathways for early engagement between regulatory bodies and those who develop neurotechnology, as well as between academia and industry. By working closely together, we can translate research discoveries into neurotechnologies that impact society.
Acknowledgments
The authors would like to acknowledge the Cleveland Neural Engineering Workshop (Cleveland NEW) for bringing everyone together to discuss these ideas and visions for the future. This work was supported by the US Department of Veterans Affairs awards: Cleveland FES Center (Dustin J Tyler, PhD) and Cleveland APT Center (Dustin J Tyler, PhD). The authors also thank Madeline Newcomb for her assistance rendering figures and Dr Erik J Peterson for his contributions to this paper.
References
- [1].Bassett DS, Khambhati AN and Grafton ST 2017. Emerging frontiers of neuroengineering: a network science of brain connectivity Ann. Rev. Biomed. Eng 19 327–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hetling J 2008. Comment on ‘What is neural engineering?’ J. Neural Eng 5 360. [DOI] [PubMed] [Google Scholar]
- [3].Durand DM. What is neural engineering? J. Neural Eng. 2006;4 [Google Scholar]
- [4].Budman E et al. 2018. Potential indications for deep brain stimulation in neurological disorders: an evolving field Eur. J. Neurol 25 434–e30 [DOI] [PubMed] [Google Scholar]
- [5].Deuschl G et al. 2006. A randomized trial of deep-brain stimulation for Parkinson’s disease New Engl. J. Med 355 896–908 [DOI] [PubMed] [Google Scholar]
- [6].Lipsman N, Neimat JS and Lozano AM 2007. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: the search for a valid target Neurosurgery 61 1–13 [DOI] [PubMed] [Google Scholar]
- [7].Goodman WK and Insel TR 2009. Deep brain stimulation in psychiatry: concentrating on the road ahead Biol. Psychiatry 65 263–6 [DOI] [PubMed] [Google Scholar]
- [8].Kupsch A et al. 2006. Pallidal deep-brain stimulation in primary generalized or segmental dystonia New Engl. J. Med 355 1978–90 [DOI] [PubMed] [Google Scholar]
- [9].Cavanna AE et al. 2011. An approach to deep brain stimulation for severe treatment-refractory Tourette syndrome: the UK perspective Br. J. Neurosurg 25 38–44 [DOI] [PubMed] [Google Scholar]
- [10].Weaver FM et al. (CSP 468 Study Group) 2009. Bilateral deep brain stimulation versus best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial JAMA 301 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Widge AS, Malone DA Jr and Dougherty DD 2018. Closing the loop on deep brain stimulation for treatment-resistant depression Focus 16 305–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Graat I, Figee M and Denys D 2017. The application of deep brain stimulation in the treatment of psychiatric disorders Int. Rev. Psychiatry 29 178–90 [DOI] [PubMed] [Google Scholar]
- [13].Market Research Report 2018. Deep Brain Stimulation Devices Market Size, Share & Trends Analysis Report By Application (Pain Management, Epilepsy, Essential Tremor, OCD, Depression, Dystonia, Parkinson’s), By Region, And Segment Forecasts, 2018–2025 (978-1-68038-335-5)
- [14].Sarem-Aslani A and Mullett K 2011. Industrial perspective on deep brain stimulation: history, current state, and future developments Frontiers Integr. Neurosci 5 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dones I and Levi V 2018. Spinal cord stimulation for neuropathic pain: current trends and future applications Brain Sci. 8 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Slavin KV 2014. Spinal stimulation for pain: future applications Neurotherapeutics 11 535–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vannemreddy P and Slavin KV 2011. Spinal cord stimulation: current applications for treatment of chronic pain Anesthesia Essays Res. 5 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Verrills P, Sinclair C and Barnard A 2016. A review of spinal cord stimulation systems for chronic pain J. Pain Res 9 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Crowson MG et al. 2017. Quality of life and cost-effectiveness of cochlear implants: a narrative review Audiol. Neurotol 22 236–58 [DOI] [PubMed] [Google Scholar]
- [20].Damle S, Lo Y-H and Freeman WR 2017. High visual acuity retinal prosthesis: understanding limitations and advancements toward functional prosthetic vision LWW Retina. 37 1423–7 [DOI] [PubMed] [Google Scholar]
- [21].Tahseen S 2018. Role of sacral neuromodulation in modern urogynaecology practice: a review of recent literature Int. Urogynecol. J 29 1081–91 [DOI] [PubMed] [Google Scholar]
- [22].Howland R 2014. Vagus nerve stimulation Curr. Behav. Neurosci. Rep 1 64–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Geller EB et al. 2017. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy Epilepsia 58 994–1004 [DOI] [PubMed] [Google Scholar]
- [24].Bergey GK et al. 2015. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures Neurology 84 810–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ho CH et al. 2014. Functional electrical stimulation and spinal cord injury Phys. Med. Rehabil. Clin 25 631–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Takeda K, Tanino G and Miyasaka H 2017. Review of devices used in neuromuscular electrical stimulation for stroke rehabilitation Med. Devices 10 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Prochazka A 2017. Neurophysiology and neural engineering: a review J. Neurophysiol 118 1292–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dhillon GS and Horch KW 2005. Direct neural sensory feedback and control of a prosthetic arm IEEE Trans. Neural Syst. Rehabil. Eng 13 468–72 [DOI] [PubMed] [Google Scholar]
- [29].Raspopovic S. et al. Restoring natural sensory feedback in real-time bidirectional hand prostheses. Sci. Transl. Med. 2014;6:222ra19. doi: 10.1126/scitranslmed.3006820. [DOI] [PubMed] [Google Scholar]
- [30].Tan DW. et al. A neural interface provides long-term stable natural touch perception. Sci. Transl. Med. 2014;6:257ra138. doi: 10.1126/scitranslmed.3008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Davis T. et al. Restoring motor control and sensory feedback in people with upper extremity amputations using arrays of 96 microelectrodes implanted in the median and ulnar nerves. J. Neural Eng. 2016;13:036001. doi: 10.1088/1741-2560/13/3/036001. [DOI] [PubMed] [Google Scholar]
- [32].Charkhkar H. et al. High-density peripheral nerve cuffs restore natural sensation to individuals with lower-limb amputations. J. Neural Eng. 2018;15:056002. doi: 10.1088/1741-2552/aac964. [DOI] [PubMed] [Google Scholar]
- [33].Marasco PD. et al. Illusory movement perception improves motor control for prosthetic hands. Science Transl. Med. 2018;10:eaao6990. doi: 10.1126/scitranslmed.aao6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Marasco PD et al. 2011. Robotic touch shifts perceptionof embodiment to a prosthesis in targeted reinnervation amputees Brain 134 747–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Graczyk EL. et al. Home use of a neural-connected sensory prosthesis provides the functional and psychosocial experience of having a hand again. Sci. Rep. 2018;8:9866. doi: 10.1038/s41598-018-26952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rejc E. et al. Motor recovery after activity-based training with spinal cord epidural stimulation in a chronic motor complete paraplegic. Sci. Rep. 2017;7:13476. doi: 10.1038/s41598-017-14003-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gad P et al. 2018. Noninvasive activation of cervical spinal networks after severe paralysis J. Neurotrauma 35 2145–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gill ML. et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 2018;24:1677. doi: 10.1038/s41591-018-0175-7. [DOI] [PubMed] [Google Scholar]
- [39].Wagner FB. et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563:65. doi: 10.1038/s41586-018-0649-2. [DOI] [PubMed] [Google Scholar]
- [40].Angeli CA et al. 2018. Recovery of over-ground walking after chronic motor complete spinal cord injury New Engl. J. Med 379 1244–50 [DOI] [PubMed] [Google Scholar]
- [41].Inanici F et al. 2018. Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia IEEE Trans. Neural Syst. Rehabil. Eng 26 1272–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Brown R et al. 2006. Respiratory dysfunction and management in spinal cord injury Respiratory Care 51 853–70 [PMC free article] [PubMed] [Google Scholar]
- [43].DiMarco AF et al. 2019. Complete restoration of respiratory muscle function in three subjects with spinal cord injury: pilot interventional clinical trial Ame. J. Phys. Med. Rehabil 98 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].DiMarco AF et al. 2006. Inspiratory muscle pacing in spinal cord injury: case report and clinical commentary J. Spinal Cord Med 29 95–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bourbeau DJ et al. 2018. Genital nerve stimulation increases bladder capacity after SCI: A meta-analysis J. Spinal Cord Med 41 426–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Larson PS 2008. Deep brain stimulation for psychiatric disorders Neurotherapeutics 5 50–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cimpianu C-L et al. 2017. Vagus nerve stimulation in psychiatry: a systematic review of the available evidence J. Neural Transm 124 145–58 [DOI] [PubMed] [Google Scholar]
- [48].Guiraud D. et al. Vagus nerve stimulation: state of the art of stimulation and recording strategies to address autonomic function neuromodulation. J. Neural Eng. 2016;13:041002. doi: 10.1088/1741-2560/13/4/041002. [DOI] [PubMed] [Google Scholar]
- [49].National Academies of Sciences, Engineering, and Medicine 2015. The Integration of Immigrants Into American Society (Washington, DC: The National Academies Press; ) pp 458 Bocskor A, Hunyadi M and Vince D 2017 Intersections 3 157–61 [Google Scholar]
- [50].National Academies of Sciences, Engineering, and Medicine 2017. A New Vision for Center-Based Engineering Research (Washington, DC: The National Academies Press; ) pp 102 [Google Scholar]
- [51].Zrinzo L et al. 2011. Clinical safety of brain magnetic resonance imaging with implanted deep brain stimulation hardware: large case series and review of the literature World Neurosurg. 76 164–72 [DOI] [PubMed] [Google Scholar]
- [52].Zhao S et al. 2016. Graphene encapsulated copper microwires as highly MRI compatible neural electrodes Nano Lett. 16 7731–8 [DOI] [PubMed] [Google Scholar]
- [53].Michelson NJ. et al. Multi-scale, multi-modal analysis uncovers complex relationship at the brain tissue-implant neural interface: new emphasis on the biological interface. J. Neural Eng. 2018;15:033001. doi: 10.1088/1741-2552/aa9dae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ereifej ES. et al. Implantation of intracortical microelectrodes elicits oxidative stress. Frontiers Bioeng. Biotechnol. 2018;6:9. doi: 10.3389/fbioe.2018.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jorfi M. et al. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J. Neural Eng. 2015;12:011001. doi: 10.1088/1741-2560/12/1/011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Salatino JW. et al. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 2017;1:862. doi: 10.1038/s41551-017-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bennett C et al. 2019. Neuroinflammation, oxidative stress, and blood-brain barrier (BBB) disruption in acute Utah electrode array implants and the effect of deferoxamineas an iron chelator on acute foreign body response Biomaterials 188 144–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Barrese JC. et al. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J. Neural Eng. 2013;10:066014. doi: 10.1088/1741-2560/10/6/066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Karumbaiah L et al. 2013. Relationship between intracortical electrode design and chronic recording function Biomaterials 34 8061–74 [DOI] [PubMed] [Google Scholar]
- [60].Kozai TDY et al. 2015. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies ACS Chem. Neurosci 6 48–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chestek CA. et al. Long-term stability of neural prosthetic control signals from silicon cortical arrays in rhesus macaque motor cortex. J. Neural Eng. 2011;8:045005. doi: 10.1088/1741-2560/8/4/045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Linz D et al. 2013. Application in hypertension of renal sympathetic denervation–a review. Int Cardiol. Rev 8 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lanza GA, Barone L and Di Monaco A 2012. Effect of spinal cord stimulation in patients with refractory angina: evidence from observational studies Neuromodulation 15 542–9 [DOI] [PubMed] [Google Scholar]
- [64].Shen MJ et al. 2011. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines Circulation 123 2204–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hucker WJ. et al. Device-based approaches tomodulate the autonomic nervous system and cardiac electrophysiology. Arrhythmia Electrophysiol. Rev. 2014;3:30. doi: 10.15420/aer.2011.3.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Takmakov P. et al. Rapid evaluation of the durabilityof cortical neural implants using accelerated aging with reactive oxygen species. J. Neural Eng. 2015;12:026003. doi: 10.1088/1741-2560/12/2/026003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Charkhkar H et al. 2014. Use of cortical neuronal networksfor in vitro material biocompatibility testing Biosens. Bioelectron 53 316–23 [DOI] [PubMed] [Google Scholar]
- [68].Polikov VS et al. 2006. In vitro model of glial scarring around neuroelectrodes chronically implanted in the CNS Biomaterials 27 5368–76 [DOI] [PubMed] [Google Scholar]
- [69].Ereifej ES et al. 2013. Comparative assessment of iridium oxide and platinum alloy wires using an in vitro glial scar assay Biomed. Microdevices 15 917–24 [DOI] [PubMed] [Google Scholar]
- [70].Ereifej ES et al. 2013. Examining the inflammatory response to nanopatterned polydimethylsiloxane using organotypic brain slice methods J. Neurosci. Methods 217 17–25 [DOI] [PubMed] [Google Scholar]
- [71].Young D and Cong P 2019. Wireless implantable sensors: from lab to technology breakthrough ambitions Sensors Actuators A 294 81–90 [Google Scholar]
- [72].US Food and Drug Administration 2018. Breakthrough Devices Program (www.fda.gov/media/108135/download)
- [73].Second Sight 2017. Second Sight Receives FDA Expedited Access Pathway Designation for the Orion Cortical Visual Prosthesis System (http://investors.secondsight.com/node/8311/pdf)
- [74].US Food and Drug Administration 2013. Investigational Device Exemptions (IDEs) for Early Feasibility Medical Device Clinical Studies, Including Certain First in Human (FIH) Studies (www.fda.gov/media/81784/download)
- [75].Goering S and Klein E 2019. Neurotechnologies and justice by, with, and for disabled people The Oxford Handbook of Philosophy and Disability ed Cureton A abd Wasserman D (Oxford: Oxford University Press; ) ( 10.1093/oxfordhb/9780190622879.013.33) [DOI] [Google Scholar]
- [76].Gilbert F, O’brien T and Cook M 2018. The effects of closed-loop brain implants on autonomy and deliberation: what are the risks of being kept in the loop? Camb. Q. Healthcare Ethics 27 316–25 [DOI] [PubMed] [Google Scholar]
- [77].National Institute of Neurological Disorders and Stroke 2016. NIH Workshop on Standards and Modularity of Brain-Computer Interfaces and Neuroprostheses (https://meetings.ninds.nih.gov/Home/Index/14639)
- [78].Bowsher K. et al. Brain-computer interface devices for patients with paralysis and amputation: a meeting report. J. Neural Eng. 2016;13:023001. doi: 10.1088/1741-2560/13/2/023001. [DOI] [PubMed] [Google Scholar]
- [79].US Food and Drug Administration. FDA Public Workshop: Brain-Computer Interface Devices for Patients With Paralysis and Amputation. 2014.
- [80].US Food and Drug Administration 2014. Implanted Brain-Computer Interface (BCI) Devices for Patients with Paralysis or Amputation–Non-clinical Testing and Clinical Considerations (www.fda.gov/media/120362/download)
- [81].Tyler DJ et al. 2019. Cleveland neural engineering workshop 2017: strategic evaluation of neural engineering Bioelectronic Med. 5 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Anderson K et al. 2018. Proceedings of the second biennial Cleveland neural engineering workshop 2013 Bioelectronic Med. 4 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Berényi A et al. 2013. Large-scale, high-density (up to 512 channels) recording of local circuits in behaving animals J. Neurophysiol 111 1132–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Viventi J. et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci. 2011;14:1599. doi: 10.1038/nn.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].MacLean JN and Yuste R 2009. Imaging action potentials with calcium indicators Cold Spring Harb. Protoc 11 [DOI] [PubMed] [Google Scholar]
- [86].Du J, Roukes ML and Masmanidis SC 2009. Dual-side and three-dimensional microelectrode arrays fabricated from ultrathin silicon substrates J. Micromech. Microeng 19 075008 [Google Scholar]
- [87].Jun JJ. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature. 2017;551:232. doi: 10.1038/nature24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Alivisatos AP et al. 2012. The brain activity map project and the challenge of functional connectomics Neuron 74 970–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ramirez-Zamora A et al. 2018. Evolving applications, technological challenges and future opportunities in neuromodulation: proceedings of the fifth annual deep brain stimulation think tank Frontiers Neurosci. 11 734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sejnowski TJ, Churchland PS and Movshon JA 2014. Putting big data to good use in neuroscience Nat. Neurosci 17 1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bargmann CI and Newsome WT 2014. The brain research through advancing innovative neurotechnologies (BRAIN) initiative and neurology JAMA Neurol. 71 675–6 [DOI] [PubMed] [Google Scholar]
- [92].Miller G 2008. Engineering a fix for broken nervous systems Science 322 847–7 [DOI] [PubMed] [Google Scholar]
- [93].Cavuoto J 2018. The market for neurotechnology: 2018–2022 Neurotech Report 1 (http://www.neurotechreports.com/pages/reportsumm.html) [Google Scholar]
- [94].McIntyre CC et al. 2015. Engineering the next generation of clinical deep brain stimulation technology Brain Stimul. 8 21–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].McClure-Begley T 2016. Targeted Neuroplasticity Training (TNT) (www.darpa.mil/program/targeted-neuroplasticity-trainingg)
- [96].Gilbert F and Tubig P 2018. Cognitive enhancement with brain implants: the burden of abnormality J. Cogn. Enhanc 2 364–8 [Google Scholar]
- [97].Yuste R. et al. Four ethical priorities for neurotechnologies and AI. Nat. News. 2017;551:159. doi: 10.1038/551159a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Pham M et al. 2018. Asilomar survey: researcher perspectives on ethical principles and guidelines for BCI research Brain–Comput. Interfaces 5 97–111 [Google Scholar]


