Abstract
Objectives:
The serotonergic system is involved in the regulation of socio-emotional behavior and heavily innervates the amygdala, a key structure of social brain circuitry. We quantified serotonergic axon density of the four major nuclei of the amygdala in humans, and examined our results in light of previously published data sets in chimpanzees and bonobos.
Materials and methods:
Formalin-fixed postmortem tissue sections of the amygdala from six humans were stained for serotonin transporter (SERT) utilizing immunohistochemistry. SERT-immunoreactive (ir) axon fiber density in the lateral, basal, accessory basal, and central nuclei of the amygdala was quantified using unbiased stereology. Nonparametric statistical analyses were employed to examine differences in SERT-ir axon density between amygdaloid nuclei within humans, as well as differences between humans and previously published data in chimpanzees and bonobos.
Results:
Humans displayed a unique pattern of serotonergic innervation of the amygdala, and SERT-ir axon density was significantly greater in the central nucleus compared to the lateral nucleus. SERT-ir axon density was significantly greater in humans compared to chimpanzees in the basal, accessory basal, and central nuclei. SERT-ir axon density was greater in humans compared to bonobos in the accessory basal and central nuclei.
Conclusions:
The human pattern of SERT-ir axon distribution in the amygdala complements the redistribution of neurons in the amygdala in human evolution. The present findings suggest that differential serotonergic modulation of cognitive and autonomic pathways in the amygdala in humans, bonobos, and chimpanzees may contribute to species-level differences in social behavior.
Keywords: amygdala, brain evolution, serotonin, social behavior
1 |. INTRODUCTION
While social species are found across the animal kingdom, primates have particularly complex social lives (Smuts, Cheney, Seyfarth, Wrangham, & Struhsaker, 1987; Strum, 2001). Such social complexity is mediated by neural substrates that simultaneously process and evaluate external social stimuli and integrate this information with one’s own emotional state in order to guide behavior and successfully navigate the social environment (Brothers & Ring, 1992). The amygdala, a subcortical structure in the medial temporal lobe, has significant bidirectional connectivity to temporal and orbitofrontal association cortices involved in higher order cognitive processing, as well as direct projections to autonomic centers in the brain, and is therefore well-situated to mediate the internal and external environments (Barbas, Zikopoulos, & Timbie, 2011; Gallagher & Holland, 1994; Stefanacci & Amaral, 2000, 2002). Functionally, the amygdala is thought to play a primary role in the detection and categorization of emotionally salient environmental stimuli, as well as the behavioral and physiological modulation of emotion, and is therefore considered a central structure of social brain circuitry (Kling & Dunne, 1976).
Four distinct subdivisions of the amygdala—the lateral, basal, accessory basal, and central nuclei—proportionally constitute the majority of the structure and can be distinguished from each other based on cellular distribution, as well as connectivity to other brain areas, which can be sorted into two somewhat distinct, but overlapping loops of the social brain network. The lateral, basal, and accessory basal nuclei, collectively referred to as the “deep” or “basolateral” nuclei, demonstrate significant bidirectional connectivity to orbitofrontal and temporal cortical association areas, and are considered to be components of the “cognitive” loop of the amygdala. In contrast, the central nucleus has little cortical connectivity, and instead receives heavy projections from the basolateral nuclei and serves as the primary output of the amygdala to regulatory regions in the brainstem and hypothalamus (Stefanacci & Amaral, 2000, 2002), representing the autonomic/physiological loop of the amygdala. Comparative postmortem studies in humans and nonhuman apes have demonstrated differential changes in neuron number and volume of amygdala nuclei that suggest a reorganization of the amygdala in humans that emphasized the cognitive loop (Barger et al., 2012; Barger, Stefanacci, & Semendeferi, 2007), in line with predictions with the social brain hypothesis of human brain evolution (Dunbar, 1998; Frith & Frith, 2010).
While several comparative neuroanatomical studies in primates, including humans, have targeted cellular distribution and volume of the major amygdaloid nuclei in order to elucidate possible relationships between evolutionarily derived changes in neural substrates and social behavior, (Barger et al., 2007, 2012; Barton & Aggleton, 2002; Stephan & Andy, 1977) little is known about the neuromodulatory systems which regulate neuronal activity of the amygdala within social brain circuits. The serotonergic system is involved in the suppression of emotional arousal through neuromodulation of limbic circuits (Soubrié, 1986), and the amygdala is a major serotonergic target (Azmitia & Gannon, 1986; Freedman & Shi, 2001; O’Rourke & Fudge, 2006). Serotonin decays rapidly after death, and is therefore difficult to examine in formalin-fixed human and ape postmortem tissue. However, serotonin transporter (SERT), a protein involved in serotonin reuptake, remains relatively stable in postmortem tissue (Asan, Steinke, & Lesch, 2013; Verney, Lebrand, & Gaspar, 2002). SERT has virtually identical labeling patterns as serotonin, and given its stability is most commonly used to examine the chemoarchitecture and anatomy of the serotonin system in postmortem tissue (Asan et al., 2013; Austin, Whitehead, Edgar, Janosky, & Lewis, 2002; Azmitia & Gannon, 1986; Azmitia & Nixon, 2008; Azmitia, Singh, & Whitaker-Azmitia, 2011; Bauman & Amaral, 2005; Raghanti et al., 2008). A comparative histological study by Stimpson et al. (2015) utilized an anti-SERT antibody to examine serotonergic axon density of the four major subdivisions of the amygdala in the postmortem brains of bonobos and chimpanzees, two closely related species that display profound differences in social behavior (de Waal, 1995; Palagi, 2006; Parish, 1996; Parish & de Waal, 2000). Specifically, chimpanzees frequently respond to conflict with aggression (Parish & de Waal, 2000), while bonobos more often engage in prosocial strategies such as sexual interaction and play to mediate conflict (de Waal, 1995; Palagi, 2006; Parish, 1996). Stimpson et al. (2015) observed significantly more serotonergic axons in the amygdala in bonobos compared to chimpanzees as well as species differences in the regional distribution of SERT-ir axon density across the amygdala, and hypothesized that this may contribute to differential processing of social stimuli by the amygdala and could underlie their distinct sociobehavioral differences.
The present study is the first quantitative stereological study to examine serotonergic innervation of the major subdivisions of the amygdala in humans. Utilizing immunohistochemistry and stereological counting methods, we quantified the density of SERT-immunoreactive (SERT-ir) axons in the four major subdivisions of the human amygdala—the lateral, basal, accessory basal, and central nuclei—in order to quantify regional distribution of serotonergic axons within the amygdala in humans. Given the significant reorganization of the amygdala in human evolution, which includes an expansion of the lateral nucleus and reduction of the central nucleus (Barger et al., 2007, 2012); we predicted that relative regional differences in serotonergic axon density across the human amygdala would be the greatest between the lateral and central nuclei. We additionally compared our human data set with the published data sets in chimpanzees and bonobos, which utilized the same regions of interest and the same anti-SERT antibody and stereological software as the present study (Stimpson et al., 2015). Given that the findings of increased serotonergic axon density in bonobos compared to chimpanzees may be attributable to differences in social behavior between the two species, we hypothesized that humans, as “ultra-social” primates (Tomasello, 2014), would demonstrate greater serotonergic innervation of the amygdala compared to chimpanzees and bonobos.
2 |. MATERIALS AND METHODS
Postmortem amygdala tissue was obtained from six neurotypical adult male and female human subjects (Table 1). The amygdala from one hemisphere per subject was examined, and determined to be sufficient for analysis, as no asymmetry in the human amygdala has been observed in either postmortem histological or neuroimaging studies (Barger et al., 2007; Brierley, Shaw, & David, 2002). For three subjects (Subjects 1, 3, and 6 in Table 1), formalin-fixed whole tissue blocks containing the rostro-caudal extent of the amygdala were obtained through the NIH NeuroBioBank. Tissue sections for the remaining three subjects (Subjects 2, 4, and 5 in Table 1) were obtained from the laboratory of Cynthia Schumann (MIND Institute, UC Davis School of Medicine) and received as a 1-in-20 series of 50 μm-thick free-floating formalin-fixed frozen tissue sections spanning the rostro-caudal extent of the amygdala.
TABLE 1.
Human subject background
| Subject # | Subject ID | Age at death (years) | Sex | Hemisphere | Cause of death | Postmortem interval (hours) |
|---|---|---|---|---|---|---|
| 1 | 4916 | 19 | Male | Right | Drowning | 5 |
| 2 | H-11-02 | 27 | Male | Left | Cardiovascular disease | 16 |
| 3 | 5758 | 43 | Female | Right | Sepsis | 22 |
| 4 | H-19-01 | 44 | Male | Left | Unknown | 26 |
| 5 | H-11-98 | 67 | Male | Left | Unknown | <30 |
| 6 | 5493 | 69 | Male | Right | Acute coronary thrombosis | 23 |
2.1 |. Tissue processing
Formalin-fixed tissue blocks were cryoprotected until saturated in a gradient of sucrose and 0.1 M phosphate buffer solutions (10, 20, 30%) in preparation for cutting. Tissue was frozen with dry ice and cut along the coronal plane on a Leica SM sliding microtome. Tissue was cut in either alternating 80 and 40 μm sections (Subjects 1, 3, and 6), or alternating 100 and 50 μm sections (subjects 2, 4, and 5). A 1-in-10 series of either 80 or 100 μm sections per individual was immediately mounted and stained for Nissl substance, and the remaining series were placed in cryoprotectant solution and stored in a −20° C freezer until use.
A 1-in-20 series of either 40 μm (Subjects 1, 3, and 6) or 50 μm (Subjects 2, 4, and 5) sections was selected from the frozen tissue to be stained with mouse monoclonal antibody against SERT (MAB5618, EMD Millipore, Billerica, MA) for each subject. While serotonin decays rapidly after death, serotonin transporter (SERT) remains relatively stable in postmortem tissue (Verney et al., 2002). SERT removes serotonin from the synaptic cleft and extracellular space, and is therefore responsible for the recycling of serotonin in the presynaptic neuron, as well as the termination of serotonergic effects (Asan et al., 2013). Although involved in re-uptake, SERT has virtually identical labeling patterns as serotonin, and given its stability is most commonly used to examine the chemoarchitecture and anatomy of the serotonin system in postmortem tissue (Asan et al., 2013; Austin et al., 2002; Azmitia et al., 2011; Azmitia & Gannon, 1986; Azmitia & Nixon, 2008; Bauman & Amaral, 2005; Raghanti et al., 2008). Antibody specificity for mouse monoclonal antibody against SERT has been demonstrated at 60–70 kDa (Ramsey & DeFelice, 2017; Serafeim et al., 2002), and two previous studies to date (Raghanti et al., 2008; Stimpson et al., 2015) have demonstrated the selectivity of the antibody used in this study in formalin-fixed human and nonhuman primate neural tissue using a heat-based antigen retrieval immunohistochemical staining protocol. An avidin-biotin peroxidase method for heavily fixed tissue (Raghanti et al., 2008), was used, with slight modifications based on results of several pilot studies in our laboratory. Free-floating sections were removed from cryoprotectant and rinsed in PBS, then pretreated for antigen retrieval by incubation in a 0.05% citraconic acid solution and submerged in a water bath set at 95°C for 1 hr. Sections were again rinsed with PBS and incubated for 20 min in a 75% methanol, 2.5% hydrogen peroxide solution to quench endogenous peroxidases. Sections were rinsed and incubated for 1 hr in a PBS dilution buffer of 4% normal horse serum, 5% bovine serum albumin, and 0.6% Triton-X detergent, rinsed again, then incubated in the primary antibody (1:10,000 dilution with PBS) for 3 days on an orbital shaker (24 hr at room temperature, 48 hr at 4°C). After removal from primary antibody, sections were rinsed and then incubated in biotinylated anti-mouse secondary antibody (1:200 dilution, BA-2000, Vector Laboratories, Burlingame, CA) with 4% normal horse serum and PBS for 1 hr. Sections were again rinsed and treated with an Avidin-Biotin Peroxidase Complex kit (PK-4000, Vector Laboratories). A DAB Chromogen kit (SK-4100, Vector Laboratories) was used to visualize immunoreactive SERT-positive fibers, and then rinsed in PBS to halt the reaction. Before mounting, free-floating stained sections were chilled in the refrigerator for 24–48 hr to reduce the likelihood of tissue tears during handling.
2.2 |. Data collection
All data were collected by CHL after establishing intra-rater reliability by replicating quantitative results in a single subject three times with >95% concordance. Stereologic sampling to determine length density of SERT-immunoreactive axons was conducted using the Space Balls probe in the Stereoinvestigator software suite (MBF BioScience, Williston, VT) on a Dell workstation receiving live video feed from a Lumenera color video camera (Ottawa, Ontario) attached to an Eclipse 80i microscope equipped with a Ludl MAC5000 stage (Hawthorn, NY) and a Heiden z-axis encoder (Plymouth, MN). Serotonergic axon density was quantified separately in the lateral, basal, accessory basal, and central nuclei, which are distinguished from each other by their connections with other brain areas, and have distinct boundaries that are consistently identified across individuals (Schumann & Amaral, 2005). For each SERT-ir stained section examined, boundaries of the amygdaloid nuclei were first traced in Stereoinvestigator at 1x magnification, utilizing an adjacent Nissl-stained section to ensure precision of boundaries as described in Lew et al. (2018) (Figure 1a). After boundaries were identified, the Space Balls probe was employed at ×100 magnification (1.4 numerical aperture, oil lens). Using systematic random sampling determined by a predefined grid size, Space Balls employs a virtual isotropic hemisphere (probe parameters in the current study utilized a radius of 7 and a 1 μm guard zone on the top and bottom of the section) that the investigator toggles through the section on the z-axis, marking the hemisphere each time an axon crosses the probe’s circumference. Different grid sizes were used for each nucleus given the difference in nucleus size (1,000 × 1000 μm2 lateral nucleus, 900 × 900 μm2 basal nucleus; 600 × 600 μm2 accessory basal nucleus; 400 × 400 μm2 central nucleus) so that an average of 150 sampling sites per nucleus was achieved. Tissue thickness was measured at every third sampling site. Coefficient of error (m = 1) for axon length estimates were between 0.03 and 0.08 for each region of interest in each human subject, indicating that the parameters used were sufficient for accurate stereological estimation. Total SERT-ir axon length density (from here on referred to as SERT-ir axon density) was calculated by dividing the total axon length by the planimetric reference volume calculated by the Stereoinvestigator software.
FIGURE 1.

(a) Boundaries of the lateral (L), basal (B), accessory basal (AB) and central (C) nuclei of the amygdala; (b) SERT-ir axons of each nucleus at ×60 magnification
2.3 |. Statistical analyses
Descriptive statistics were employed to obtain the mean, SD, and coefficient of variation for each nucleus examined, and SERT-ir axon density data was additionally run through a Grubbs’ test (p < .05) to detect possible outliers. Due to the small number of subjects, nonparametric statistical tests were employed for all analyses. Spearman rank order correlation tests were used to identify any age, sex, hemisphere, or postmortem interval (PMI) effects on SERT-ir axon density. We utilized the Friedman test with Dunn’s test for multiple comparisons to identify possible differences between the lateral, basal, accessory basal, and central nuclei. We additionally compared the human data with the published data sets in seven adult chimpanzees and six adult bonobos (Stimpson et al., 2015 Table S2) utilizing the Kruskal-Wallis test with Dunn’s test for multiple comparisons. All data analysis were performed using Prism v.8 statistical software (GraphPad Software, La Jolla, CA).
3 |. RESULTS
Mean SERT-ir axon density in humans was the highest in the central nucleus (0.00656 ± 0.00309 μm/μm3), followed by the accessory basal nucleus (0.00572 ± 0.00270 μm/μm3), then the basal nucleus (0.00458 ± 0.00182 μm/μm3) and the lowest in the lateral nucleus (0.00368 ± 0.00101 μm/μm3). Significant differences in SERT-ir axon density were found between the lateral and central nuclei only (p = .0048, Z = 3.35; Figure 2; Table 2). The mean group pattern was present in four of the six individuals, while the remaining two individuals demonstrated very little difference in SERT-ir axon density across the four nuclei examined (Figure 3). The coefficient of variation for mean SERT-ir axon density was high across all four nuclei (lateral = 27.54%; basal = 39.71%; accessory basal = 47.14%; central = 47.16%). No correlations were found between age, sex, hemisphere, or PMI and SERT-ir axon density, and no outliers were identified. However, the single female subject (Subject #3) demonstrated the highest SERT-ir axon density of all subjects in all four nuclei (Figure 2).
FIGURE 2.

Mean SERT-ir axon density and standard deviation (error bars; μm/μm3) in the lateral, basal, accessory basal, and central nuclei of the human amygdala. Asterisks indicate significant differences between nuclei. **p < .005
TABLE 2.
Statistical results (p-values and Z-scores) of the Friedman test with Dunn’s test for multiple comparisons of each nucleus compared to each nucleus in the humans
| Comparison | p-value | Z-score |
|---|---|---|
| Lateral vs. basal | >0.9999 | 1.342 |
| Lateral vs. accessory basal | 0.0834 | 2.460 |
| Lateral vs. central | 0.0048a | 3.354 |
| Basal vs. accessory basal | >0.9999 | 1.118 |
| Basal vs. central | 0.2650 | 2.012 |
| Accessory basal vs. central | >0.9999 | 0.8944 |
Statistically significant value.
FIGURE 3.
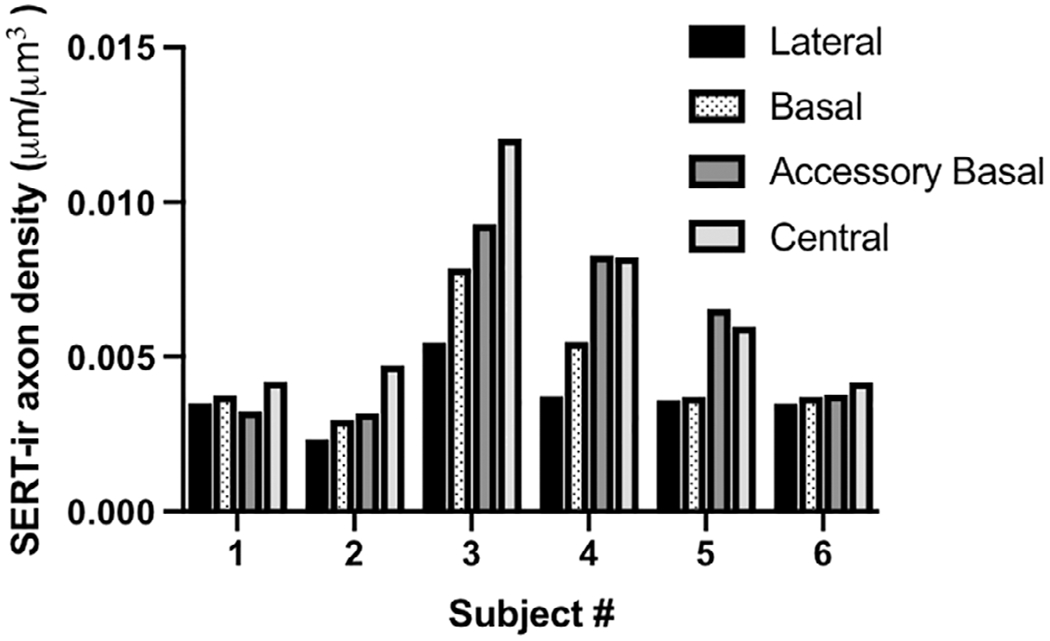
SERT-ir axon density (μm/μm3) in the lateral, basal, accessory basal, and central nuclei of each human subject
Compared to the Stimpson et al. (2015) ape data sets, mean SERT-ir axon density in humans was greater than chimpanzees in all four amygdala nuclei examined (Figures 4 and 5), reaching significance in the basal (p = .0421; Z = 2.46), accessory basal (p = .0023; Z = 3.36), and central (p = .0035; Z = 3.25). Mean SERT-ir axon density in humans was greater than bonobos in the accessory basal and central nuclei, and slightly lower in the lateral and basal nuclei, but no significant differences were observed (Table 3; Figures 4 and 5).
FIGURE 4.

Mean SERT-ir axon density and standard deviation (error bars; μm/μm3) in humans alongside previously published data in chimpanzees and bonobos (Stimpson et al., 2015). Asterisks indicate significant differences between species. *p < .05; **p < .005. aData set obtained from Stimpson et al., 2015
FIGURE 5.
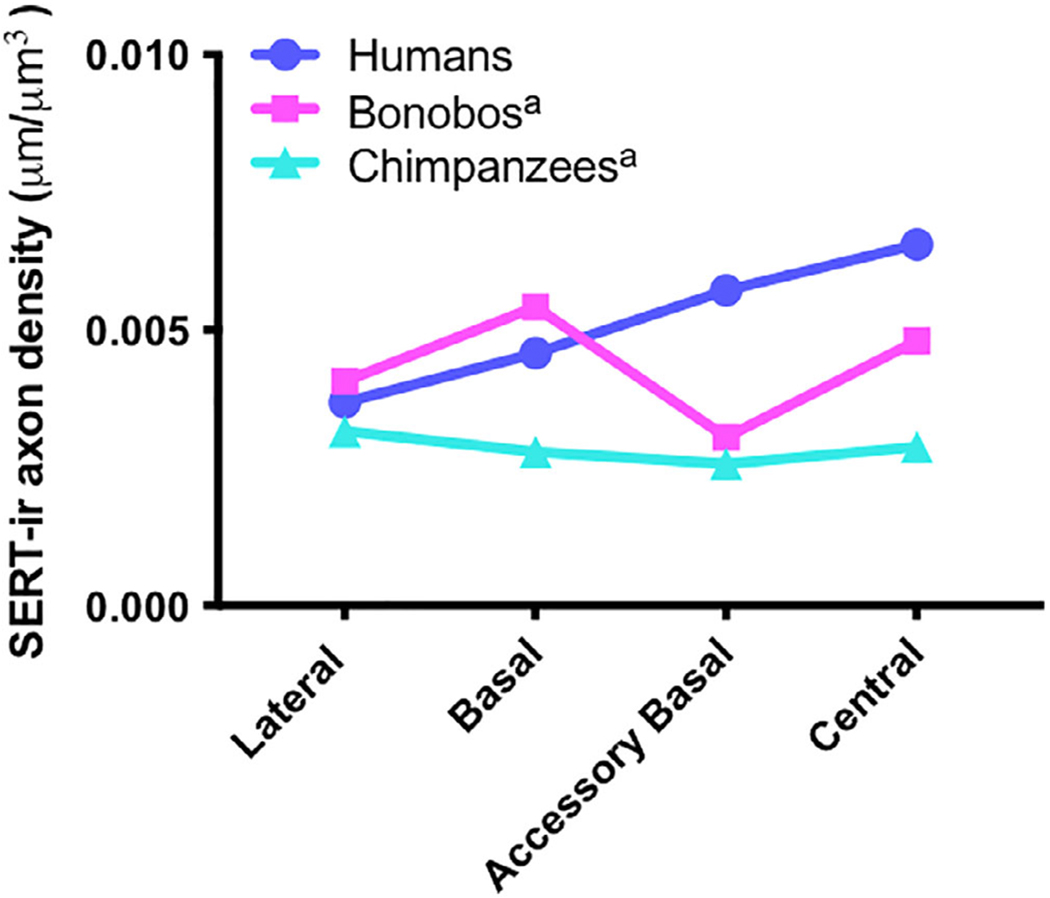
Pattern of mean SERT-ir axon density in micrometers (μm/μm3) across the four nuclei of the amygdala in each species.
aData set obtained from Stimpson et al., 2015
TABLE 3.
Statistical results (p-values and Z-scores) of the Kruskal-Wallis test with Dunn’s test for multiple comparisons between humans and bonobos and chimpanzees in each nucleus
| Comparison | p-value | Z-score | |
|---|---|---|---|
| Humans vs. chimpanzees | |||
| Lateral | >0.9999 | 0.8670 | |
| Basal | 0.0421a | 2.456 | |
| Accessory basal | 0.0023b | 3.361 | |
| Central | 0.0035b | 3.247 | |
| Humans vs. bonobos | |||
| Lateral | 0.9892 | 0.9747 | |
| Basal | 0.9892 | 0.9747 | |
| Accessory basal | 0.2434 | 1.744 | |
| Central | >0.9999 | 0.4104 |
Statistically significant value.
p-value of less than 0.005.
4 |. DISCUSSION
The present study quantitatively examined SERT-ir axon density in the major subdivisions of the human amygdala. Our major finding was the regional distribution of mean SERT-ir axon density across the four major subdivisions of the amygdala in humans (central>accessory basal>basal>lateral; Figure 2). In line with our first hypothesis, regional differences in the humans were the greatest between the central and lateral nuclei, reaching statistical significance. This pattern of serotonergic innervation appears unique to humans (Figure 5)—in bonobos, the highest and lowest SERT-ir densities were in the basal nucleus and accessory basal nucleus, respectively, and in chimpanzees, SERT-ir axon density was relatively homogenous across the four nuclei (Stimpson et al., 2015). As previously discussed, human/nonhuman ape comparative studies in the amygdala have found the basolateral and central nuclei to be differentially affected in humans compared to nonhuman apes, with humans displaying a significant increase in volume and the number of neurons in the lateral nucleus, the primary site of neocortical input into the amygdala, and a significant decrease in volume and the number of neurons in the central nucleus, the primary site of amygdala projections to subcortical autonomic centers (Barger et al., 2007, 2012). These findings suggest an increased emphasis in excitatory input in the cognitive loop of the amygdala and a decreased emphasis in excitatory input in the central nucleus of the amygdala in humans. While the effects of serotonin on neuronal activity are complex, it appears to have a primarily inhibitory effect on projection neurons in the amygdala (Rainnie, 1999, 2003; Stutzmann & LeDoux, 1999). The present findings of a human-specific pattern of serotonergic innervation in the amygdala, which includes significantly greater SERT-ir axon density in the central nucleus compared to the lateral nucleus, may reflect a shift in inhibitory modulation that complements the redistribution of excitatory neurons in the amygdala in human evolution.
In addition to examining relative patterns of serotonergic distribution across the human amygdala, we used nonparametric statistical analyses to quantitatively compare our findings in the human amygdala with previously published data sets in chimpanzees and bonobos. Our major finding was that mean SERT-ir axon density in the basal, accessory basal, and central nuclei was significantly greater in humans compared to chimpanzees. Of all four amygdala subdivisions examined, the accessory basal nucleus stood out as demonstrating the greatest difference in humans compared to the two nonhuman ape species. The accessory basal was the only nucleus in which we found a statistically significant difference between humans and chimpanzees, but not bonobos and chimpanzees, and although the difference between humans and bonobos in this nucleus was not statistically significant, mean SERT-ir axon density was ~2x greater in humans compared to bonobos (Figures 4 and 5). The accessory basal nucleus is a major site of integration in the amygdala between information from external sensory stimuli (via cortical projections from the lateral nucleus) on the one hand, and information about the internal environment (via projections from the hypothalamus) on the other hand (Allen, Saper, Hurley, & Cechetto, 1991; Pitkanen et al., 1995). Additionally, the accessory basal nucleus projects heavily to the central nucleus, providing input from the basolateral division of the amygdala (primarily implicated in amygdala-cortical connections underlying cognitive processing) to autonomic circuitry between the central nucleus of the amygdala and brainstem (Aggleton, Burton, & Passingham, 1980; Carmichael & Price, 1995; Leichnetz & Astruc, 1976, 1977; Leichnetz, Povlishock, & Astruc, 1976; Stefanacci & Amaral, 2000, 2002). The present findings of greater serotonergic innervation in the accessory basal nucleus in humans suggest that an increased emphasis on the modulation of amygdaloid circuitry between cognitive and autonomic pathways by the serotonergic system may be an important human-specific specialization.
In contrast to the accessory basal nucleus, SERT-ir axon density in the lateral nucleus was similar in all three species, and was the only subdivision examined that did not demonstrate a significant increase in SERT-ir axon density in humans compared to the chimpanzees (Figures 4 and 5; Table 3). These findings are intriguing, as previous comparative studies examining volume and neuron number of the same four nuclei of the amygdala assessed here found that the human lateral nucleus was significantly larger and contained significantly more neurons compared to the nonhuman apes, suggesting an expansion of the lateral nucleus in human evolution (Barger et al., 2007, 2012). The lateral nucleus projects heavily to the accessory basal nucleus (Pitkanen et al., 1995), so it is possible that increased serotonergic neuromodulation in the accessory basal nucleus in humans represents a compensatory mechanism for increased excitatory input from the lateral nucleus.
It is of interest that the relative distribution of mean SERT-ir axon density across the amygdaloid nuclei is heterogeneous in humans and bonobos, but relatively homogenous in chimpanzees (Figures 4 and 5), and that furthermore in quantitative terms, serotonergic innervation of the amygdala is more similar in humans and bonobos than in bonobos and chimpanzees. These findings lead to three major questions that will be further addressed in the sections below: (a) How might serotonergic innervation of the amygdala contribute to differences in social behavior in these species?; (b) what was the pattern of serotonergic innervation of the amygdala in last common ancestor of humans, chimpanzees, and bonobos?; and (c) what is a potential genetic mechanism for differences in serotonergic innervation of the amygdala between species?
4.1 |. Specializations of the serotonergic system may underlie differences in social behavior
Studies in captive apes have demonstrated that bonobos are more cooperative and perform better at social cognition tasks compared to chimpanzees (Hare, Melis, Woods, Hastings, & Wrangham, 2007; Herrmann, Hare, Call, & Tomasello, 2010; Tan, Ariely, & Hare, 2017). Along a similar vein, a study by Hermann and colleagues (2007) found that human children out-perform captive chimpanzees in tasks utilizing social cognition, but not physical cognition, leading some to suggest that social cognition represents a particularly important human adaptation (Boyd & Richerson, 2009; Tomasello, 2014). A pertinent possible explanation of these species differences in social behavior in relation to the amygdala and the serotonergic system is the emotional reactivity hypothesis, as proposed by Hare and Tomasello (2005)) and Hare et al. (2007)). These authors suggest that selective evolutionary specialization occurred in the limbic system of prosocial species, leading to greater social tolerance that reduced the constraints of rigid responses of fear and aggression in social interactions, enabling more cooperative interaction for successful social problem solving. The present findings of greater SERT-ir axon density and differential patterns of serotonergic innervation within distinct subdivisions of the amygdala in humans and bonobos as compared to chimpanzees may support greater behavioral flexibility in response to social stimuli in these species. Specifically within humans, two findings suggest that the serotonergic system may be specialized for reduced emotional reactivity and greater behavioral control, which would facilitate highly complex social interactions. First is the relative distribution of significantly more SERT-ir axons in the central nucleus compared to the lateral nucleus within humans, which suggests increased serotonergic neuromodulation of projections out of the amygdala, critical to physiological mechanisms contributing to emotion and behavioral response. Second is the increase in SERT-ir axon density in the accessory basal nucleus in humans compared to chimpanzees and bonobos, which, as a site of integration of information from the neocortex and regulatory centers, may imply greater integration of cognitive and autonomic circuitry.
4.2 |. Serotonergic innervation of the amygdala in the last common ancestor
Given the close phylogenetic relationship of humans, chimpanzees, and bonobos, the comparisons of these three species alone cannot support a hypothesis about the SERT-ir expression in the amygdala of the last common ancestor (LCA), or when changes in SERT-ir expression occurred. Very few studies have examined the serotonergic system in the primate brain, and no study to date has quantified SERT-ir axon density in the postmortem monkey brain. Only two studies in the literature are of some relevance. The first study examined the relative distribution of serotonin (5-HT) axons across the amygdaloid nuclei in three perfused macaque brains (Bauman & Amaral, 2005). While 5-HT and SERT are different biomarkers, studies in perfused animal brains have found both to display virtually identical labeling patterns (Asan et al., 2013), so these findings of relative distribution across the amygdala may be comparable to the present study. However, Bauman and Amaral (2005) found 5-HT axon density to be the highest in the central nucleus, intermediate in the lateral and basal nuclei, and the lowest in the accessory basal nucleus in macaques, a distribution pattern dissimilar to humans, chimpanzees, and bonobos. The second possibly relevant publication is a comparative study of SERT-ir axon density in the cortex in humans, chimpanzees, and macaques, which found that the ratio of SERT-ir axon density to neuron density was significantly increased in the infragranular layers of the prefrontal cortex in humans and chimpanzees compared to macaques (Raghanti et al., 2008). However, unlike the present study, no significant differences were found between humans and chimpanzees (Raghanti et al., 2008), suggesting that changes to the serotonergic system in the amygdala and the prefrontal cortex may have occurred at different time points in primate evolution. A future study, which includes a more extensive comparative examination of SERT-ir axon density in the postmortem amygdaloid nuclei of macaques as well as other apes is needed, as the current available evidence is insufficient to make predictions about when changes in serotonergic innervation of the amygdala occurred in primate evolution.
4.3 |. Differences in SERT-ir expression may be linked to allelic variation of SERT precursor gene
While it is unclear when changes in serotonergic innervation of the amygdala occurred, the genetic mechanism underlying the observed species differences in serotonergic innervation of the amygdala may be associated with species-specific differences in transcriptional variation of the gene SLC6A4, which codes for SERT (Heils et al., 1996). A repeat-length polymorphism in the promoter region of the SLC6A4 gives rise to either a long (5-HTTLPR-l) or short (5-HTTLPR-s) variant of the allele that results in differential expression and function of SERT (Heils et al., 1996). This allelic variation has been linked to individual variation in amygdala reactivity to socially aversive stimuli in humans (Bertolino et al., 2005; Caspi, Ahmad, Holmes, Rudolf, & Terrie, 2010; Hariri, Mattay, Tessitore, & Kolachana, 2002; Pezawas et al., 2005), and species-specific differences have been observed in number of repeat elements in this allele in humans and nonhuman primates (Inoue-Murayama et al., 2000; Lesch et al., 1997). A future study examining SERT-ir axon density as well as 5-HTTLPR genotype of each subject would offer a powerful approach for understanding the relationship between these common polymorphisms and variability of SERT expression, both between and within species.
Postmortem studies are limited by sample size and opportunistic sampling based on tissue availability, as brain tissue, human and ape alike, is a scarce resource. While the mean pattern of SERT-ir axon density was present in most individuals, individual variation in SERT-ir axon density values in the human subjects was substantial (Figure 3), which may have had an effect on results. The high coefficient of variation is at least partially driven by the single female subject included in the study (Subject #3), which had the highest SERT-ir axon density values of all subjects in all four nuclei (Figure 3). No previous studies have examined sex differences in serotonergic innervation, so it is unclear whether the high serotonergic density of this subject is associated with sex differences, or whether another variable such as sepsis as a cause of death, which is associated with neuropathological changes including neurotransmitter imbalance (Stubbs, Yamamoto, & Menon, 2013), may be implicated.
Small sample size and opportunistic sampling may also have had an effect on comparisons between species. Both the human data set and the ape data sets in Stimpson et al. (2015) were similar in size (six humans, seven chimpanzees, six bonobos), and ape and human subjects, although all adults, were not matched for age, sex, or hemisphere, so the possible effects of these variables on the results cannot be ruled out. Another limitation of this study, particularly regarding the direct comparisons of SERT-ir axon density values in the humans and apes, is the possibility of differential shrinkage of human and ape tissue during processing and immunohistochemical staining. This variable cannot be corrected in quantitative measures of length, including measures used to calculate SERT-ir axon density (MBF Bioscience Stereo Investigator User Guide V9; West, 2018). Additionally, the present human study and the Stimpson et al. (2015) study were conducted by different research groups, so inter-rater reliability was not established between our human data set and the published ape data sets. However, both studies utilized the same anatomical boundaries and anti-SERT antibody, similar antigen retrieval and immunohistochemical staining methods, and the same stereological probe (Spaceballs, MBF BioScience) to quantify serotonergic axon density, which significantly enhances the accuracy of quantification and limits subjective aspects of data collection (West, 2018). Furthermore, the possibility of differential shrinkage and inter-rater differences in quantitative measures between the human and ape data sets does not affect the differences observed in relative distribution of SERT-ir axons within the amygdala in humans compared to the apes.
The present study is the first to quantitatively examine serotonergic innervation in the human postmortem amygdala. We found regional differences within humans that are the greatest between the central and lateral nuclei of the amygdala, which have undergone significant volumetric and neuronal redistribution in human evolution, suggesting a complementary redistribution of the serotonergic system. Additionally, differences in serotonergic axon density in humans, chimpanzees, and bonobos may be evidence of evolutionary shifts in serotonergic neuromodulation of cognitive and autonomic amygdala circuits that may underlie differences in emotional reactivity, cognitive control of behavior, and social tolerance. Future studies examining species-level differences in other neuromodulators such as glutamate, which may interact with the serotonergic system to regulate amygdala activity (Tran, Lasher, Young, & Keele, 2013), or the distribution of specific neuromodulatory cell subtypes, can deepen our understanding of the ways in which the chemoarchitecture of the amygdala may underlie variation in social responses, and additionally, the ways in which these systems may have been altered in great ape and human evolution.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health P01 NICHD033113, 5R03MH103697, and R56MH109587. A subset of the human’s tissue used in this project was obtained from the University of Maryland Brain and Tissue Bank, which is a Brain and Tissue Repository of NIH NeuroBioBank. We wish to thank the tissue donors and their families whose gift to science made this study possible. We additionally thank Erin Carlson at the MIND Institute for her assistance with procuring human tissue from the Schumann brain collection. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding information
National Institute of Child Health and Human Development, Grant/Award Number: P01NICHD033113; National Institute of Mental Health, Grant/Award Numbers: 5R03MH103697, R56MH109587
REFERENCES
- Aggleton JP, Burton MJ, & Passingham RE (1980). Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta). Brain Research, 190(2), 347–368. 10.1016/0006-8993(80)90279-6 [DOI] [PubMed] [Google Scholar]
- Allen GV, Saper CB, Hurley KM, & Cechetto DF (1991). Organization of viseral and limbic connections of the insular cortex of the rat. Journal of Comparative Neurology, 311, 1–16. [DOI] [PubMed] [Google Scholar]
- Asan E, Steinke M, & Lesch K-P (2013). Serotonergic innervation of the amygdala: Targets, receptors, and implications for stress and anxiety. Histochemistry and Cell Biology, 139(6), 785–813. 10.1007/s00418-013-1081-1 [DOI] [PubMed] [Google Scholar]
- Austin MC, Whitehead RE, Edgar CL, Janosky JE, & Lewis D.a. (2002). Localized decrease in serotonin transporter-immunoreactive axons in the prefrontal cortex of depressed subjects committing suicide. Neuroscience, 114(3), 807–815. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, & Gannon PJ (1986). The primate serotonergic system: A review of human and animal studies and a report on Macaca fascicularis. Advances in Neurology, 43, 407–468. [PubMed] [Google Scholar]
- Azmitia EC, & Nixon R (2008). Dystrophic serotonergic axons in neurodegenerative diseases. Brain Research, 1217, 185–194. 10.1016/j.brainres.2008.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC, Singh JS, & Whitaker-Azmitia PM (2011). Increased serotonin axons (immunoreactive to 5-HT transporter) in postmortem brains from young autism donors. Neuropharmacology, 60(7–8), 1347–1354. 10.1016/j.neuropharm.2011.02.002 [DOI] [PubMed] [Google Scholar]
- Barbas H, Zikopoulos B, & Timbie C (2011). Sensory pathways and emotional context for action in primate prefrontal cortex. Biological Psychiatry, 69(12), 1133–1139. 10.1016/j.biopsych.2010.08.008 [DOI] [PubMed] [Google Scholar]
- Barger N, Stefanacci L, Schumann CM, Sherwood CC, Annese J, Allman JM, … Semendeferi K (2012). Neuronal populations in the basolateral nuclei of the amygdala are differentially increased in humans compared with apes: A stereological study. The Journal of Comparative Neurology, 520(13), 3035–3054. 10.1002/cne.23118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger N, Stefanacci L, & Semendeferi K (2007). A comparative volumetric analysis of the Amygdaloid complex and Basolateral division in the human and ape brain. American Journal of Physical Anthropology, 403(134), 392–403. 10.1002/ajpa [DOI] [PubMed] [Google Scholar]
- Barton RA, & Aggleton JP (2002). Primate evolution and the amygdala In Aggleton JP (Ed.), The amygdala: A functional analysis (pp. 479–508). Oxford: Oxford University Press. [Google Scholar]
- Bauman MD, & Amaral DG (2005). The distribution of serotonergic fibers in the macaque monkey amygdala: An immunohistochemical study using antisera to 5-hydroxytryptamine. Neuroscience, 136(1), 193–203. 10.1016/j.neuroscience.2005.07.040 [DOI] [PubMed] [Google Scholar]
- Bertolino A, Arciero G, Rubino V, Latorre V, De Candia M, Mazzola V, … Scarabino T (2005). Variation of human amygdala response during threatening stimuli as a function of 50HTTLPR genotype and personality style. Biological Psychiatry, 57(12), 1517–1525. 10.1016/j.biopsych.2005.02.031 [DOI] [PubMed] [Google Scholar]
- Boyd R, & Richerson PJ (2009). Culture and the evolution of human cooperation. Philosophical Transactions of the Royal Society B: Biological Sciences, 364, 3281–3288. 10.1098/rstb.2009.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley B, Shaw P, & David a. S. (2002). The human amygdala: A systematic review and meta-analysis of volumetric magnetic resonance imaging. Brain Research. Brain Research Reviews, 39(1), 84–105. [DOI] [PubMed] [Google Scholar]
- Brothers L, & Ring B (1992). A neuroethological framework for the representation of minds. Journal of Cognitive Neuroscience, 4(2), 107–118. 10.1162/jocn.1992.4.2.107 [DOI] [PubMed] [Google Scholar]
- Carmichael ST, & Price JL (1995). Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. The Journal of Comparative Neurology, 363515, 641. [DOI] [PubMed] [Google Scholar]
- Caspi A, Ahmad R,H, Holmes A, Rudolf U, & Terrie EM. (2010). Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry, 167(5), 509–527. 10.1176/appi.ajp.2010.09101452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal FB (1995). Sex as an alternative to aggression in the bonobo In Abramson P & Pinkerton S (Eds.), Sexual Nature, Sexual Culture (pp. 37–56). Chicago: University of Chicago Press. [Google Scholar]
- Dunbar R (1998). The social brain hypothesis. Evolutionary Anthropology, 6(5), 178–190. [Google Scholar]
- Freedman LJ, & Shi C (2001). Monoaminergic innervation of the macaque extended amygdala. Neuroscience, 104(4), 1067–1084. 10.1016/S0306-4522(01)00157-9 [DOI] [PubMed] [Google Scholar]
- Frith U, & Frith C (2010). The social brain: Allowing humans to boldly go where no other species has been. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 365(1537), 165–176. 10.1098/rstb.2009.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, & Holland PC (1994). The amygdala complex: Multiple roles in associative learning and attention. Proceedings of the National Academy of Sciences of the United States of America, 91(25), 11771–11776. 10.1073/pnas.91.25.11771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare B, Melis A, Woods V, Hastings S, & Wrangham RW (2007). Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Current Biology, 17, 619–623. 10.1016/j.cub.2007.02.040 [DOI] [PubMed] [Google Scholar]
- Hare B, & Tomasello M (2005). Human-like social skills in dogs? Trends in Cognitive Sciences, 9, 439–444. 10.1016/j.tics.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Hariri A, Mattay V, Tessitore A, & Kolachana B (2002). Serotonin transporter genetic variation and the response of the human amygdala. Science, 297(July), 400–404. 10.1126/science.1071829 [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel B, & Lesch KP (1996). Allelic variation of human serotonin transporter gene expression. Journal of Neurochemistry, 6, 2621–2624. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Hare B, Call J, & Tomasello M (2010). Differences in the cognitive skills of bonobos and chimpanzees. PLoS One, 5(8), 2–5. 10.1371/journal.pone.0012438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue-Murayama M, Niimi Y, Takenaka O, Okada K, Matsuzaki I, Ito S, et al. (2000). Allelic variation of the serotonin transporter gene polymorphic region in apes. Primates, 41, 267–273. [DOI] [PubMed] [Google Scholar]
- Kling A, & Dunne K (1976). Social-environmental factors affecting behavior and plasma testosterone in normal and amygdala lesioned M. speciosa. Primates, 17(1), 23–42. 10.1007/BF02381564 [DOI] [Google Scholar]
- Leichnetz GR, & Astruc J (1976). The efferent projections of the medial prefrontal cortex in the squirrel monkey (Saimiri sciureus). Brain Research, 109, 455–472. 10.1016/0006-8993(76)90027-5 [DOI] [PubMed] [Google Scholar]
- Leichnetz GR, & Astruc J (1977). The course of some prefrontal corticofugals to the pallidum, substantia innominata, and amygdaloid complex in monkeys. Experimental Neurology, 54(1), 104–109. 10.1016/0014-4886(77)90238-2 [DOI] [PubMed] [Google Scholar]
- Leichnetz GR, Povlishock JT, & Astruc J (1976). A prefrontoamygdaloid projection in the monkey: Light and electron microscopic evidence. Neuroscience Letters, 2(5), 261–265. 10.1016/0304-3940(76)90157-9 [DOI] [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, Flügge G, Hinney A, Hebebrand J, …Heils A. (1997). The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: Alternative biallelic variation in rhesus monkeys. Journal of Neural Transmission, 104(11–12), 1259–1266. 10.1007/BF01294726 [DOI] [PubMed] [Google Scholar]
- Lew CH, Groeniger K, Bellugi U, & Semendeferi K (2018). A postmortem stereological study of the amygdala in Williams Syndrome. Brain Structure and Function, 223(4), 1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke H, & Fudge JL (2006). Distribution of serotonin transporter labeled fibers in amygdaloid subregions: Implications for mood disorders. Biological Psychiatry, 60(5), 479–490. 10.1016/j.biopsych.2005.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palagi E (2006). Social play in bonobos (Pan paniscus) and chimpanzees (Pan troglodytes): Implications for natural social systems and interindividual relationships. American Journal of Physical Anthropology, 129, 418–426. [DOI] [PubMed] [Google Scholar]
- Parish AR (1996). Female relationships in bonobos (Pan paniscus). Human Nature, 7, 61–96. [DOI] [PubMed] [Google Scholar]
- Parish AR, & de Waal FB (2000). The other “closest living relative”. How bonobos (Pan paniscus) challenge traditional assumptions about females, dominance, intra- and intersexual interactions, and hominid evolution. Annual New York Academy of Sciences, 907, 97–113. [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, & Weinberger DR (2005). 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience, 8(6), 828–834. 10.1038/nn1463 [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Stefanacci L, Farb CR, Go GG, LeDoux JE, & Amaral DG (1995). Intrinsic connections of the rat amygdaloid complex: Projections originating in the lateral nucleus. Journal of Comparative Neurology, 361(2), 345–368. 10.1002/cne.903610211 [DOI] [PubMed] [Google Scholar]
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, & Sherwood CC (2008). Differences in cortical serotonergic innervation among humans, chimpanzees, and macaque monkeys: A comparative study. Cerebral Cortex (New York, N.Y. : 1991), 18(3), 584–597. 10.1093/cercor/bhm089 [DOI] [PubMed] [Google Scholar]
- Rainnie DG (1999). Serotonergic modulation of neurotransmission in the rat Basolateral amygdala. Journal of Neurophysiology, 82(1), 69–85. 10.1152/jn.1999.82.1.69 [DOI] [PubMed] [Google Scholar]
- Rainnie DG (2003). Inhibitory and excitatory circuitries in amygdala nuclei. Annual NY Academy of Sciences, 22(4), 17–20. 10.1016/j.appet.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Ramsey IS, & DeFelice LJ (2017). Serotonin transporter function and pharmacology are sensitive to expression level. Journal of Biological Chemistry, 277(17), 14475–14482. 10.1074/jbc.m110783200 [DOI] [PubMed] [Google Scholar]
- Schumann CM, & Amaral DG (2005). Stereological estimation of the number of neurons in the human amygdaloid complex. The Journal of Comparative Neurology, 491(4), 320–329. 10.1002/cne.20704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafeim A, Grafton G, Chamba A, Gregory CD, Blakely RD, Bowery NG, … Gordon J (2002). 5-Hydroxytrptamine drives apoptosis in Burkitt lymphoma cells: Reversal by selective serotonin reuptake inhibitors. Blood, 99(7), 2545–2553. [DOI] [PubMed] [Google Scholar]
- Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, & Struhsaker TT (1987). Primate Societies. Chicago: University of Chicago Press. [Google Scholar]
- Soubrié P (1986). Reconciling the role of central serotonin neurons in human and animal behavior. Behavioral and Brain Sciences, 9(02), 319–335. 10.1017/S0140525X00022871 [DOI] [Google Scholar]
- Stefanacci L, & Amaral DG (2000). Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: A retrograde tracing study. Journal of Comparative Neurology, 421(January), 52–79. 10.1002/(SICI)1096-9861(20000522)421 [DOI] [PubMed] [Google Scholar]
- Stefanacci L, & Amaral DG (2002). Some observations on cortical inputs to the macaque monkey amygdala: An anterograde tracing study. Journal of Comparative Neurology, 451(May), 301–323. 10.1002/cne.10339 [DOI] [PubMed] [Google Scholar]
- Stephan H, & Andy OJ (1977). Quantitative comparison of the amygdala in insectivores and primates. Cells, Tissues, Organs, 98(2), 130–153. 10.1159/000144789 [DOI] [PubMed] [Google Scholar]
- Stimpson CD, Barger N, Taglialatela JP, Gendron-Fitzpatrick A, Hof PR, Hopkins WD, & Sherwood CC (2015). Differential serotonergic innervation of the amygdala in bonobos and chimpanzees. Social Cognitive and Affective Neuroscience, 11(3), 1–10. 10.1002/elan [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strum S (2001). Almost human: A journey into the world of baboons. Chicago, IL: University of Chicago Press. [Google Scholar]
- Stubbs DJ, Yamamoto AK, & Menon DK (2013). Imaging in sepsis-associated encephalopathy - insights and opportunities. Nature Reviews Neurology, 9(10), 551–561. 10.1038/nrneurol.2013.177 [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, & LeDoux JE (1999). GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: A mechanism for modulation of sensory inputs related to fear conditioning. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 19(11), RC8 10.1523/JNEUROSCI.19-11-j0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Ariely D, & Hare B (2017). Bonobos respond prosocially toward members of other groups. Scientific Reports, 7, 14733 10.1038/s41598-017-15320-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M (2014). The ultra-social animal. European Journal of Social Psychology, 44(3), 187–194. 10.1002/ejsp.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, Lasher BK, Young KA, & Keele NB (2013). Depletion of serotonin in the basolateral amygdala elevates glutamate receptors and facilitates fear-potentiated startle. Translational Psychiatry, 3(9),e298–e298. 10.1038/tp.2013.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney C, Lebrand C, & Gaspar P (2002). Changing distribution of monoaminergic markers in the developing human cerebral cortex with special emphasis on the serotonin transporter. Anatomical Record, 267, 87–93. [DOI] [PubMed] [Google Scholar]
- West MJ (2018). Space balls revisited: Stereological estimates of length with virtual isotropic surface probes. Frontiers in Neuroanatomy, 12 (June), 1–6. 10.3389/fnana.2018.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]


