Abstract
A single small iontophoretic injection of Phaseolus vulgaris leucoagglutinin labels projections from the area surrounding the spinal cord central canal at midthoracic (T6–T9) or lumbosacral (L6–S1) segments of the spinal cord. The projections from the midthoracic or lumbosacral level of the medial spinal cord are found: 1) ascending ipsilaterally in the dorsal column near the dorsal intermediate septum or the midline of the gracile fasciculus, respectively; 2) terminating primarily in the dorsal, lateral rim of the gracile nucleus and the medial rim of the cuneate nucleus or the dorsomedial rim of the gracile nucleus, respectively; and 3) ascending bilaterally with slight contralateral predominance in the ventrolateral quadrant of the spinal cord and terminating in the ventral and medial medullary reticular formation. Other less dense projections are to the pons, midbrain, thalamus, hypothalamus, and other forebrain structures. Projections arising from the lumbosacral level are also found in Barrington’s nucleus. The results of the present study support previous retrograde tract tracing and physiological studies from our group demonstrating that the neurons in the area adjacent to the central canal of the midthoracic or lumbosacral level of the spinal cord send long ascending projections to the dorsal column nucleus that are important in the transmission of second-order afferent information for visceral nociception. Thus, the axonal projections through both the dorsal and the ventrolateral white matter from the CC region terminate in many regions of the brain providing spinal input for sensory integration, autonomic regulation, motor and emotional responses, and limbic activation.
Indexing terms: anterograde axonal transport, dorsal column, nociception, visceral pain, sensorimotor integration, autonomic regulation
Several major ascending pathways originating from the spinal gray matter and terminating in the brain have been suggested to be involved in the transmission of visceral and somatic nociceptive information (for review see Willis and Coggeshall, 1991; Willis and Westlund, 1997). These ascending pathways include the spinothalamic tract (STT; Giesler et al., 1981; Milne et al., 1981; Foreman et al., 1984; Rucker et al., 1984; Ammons, 1987, 1989, 1990; Foreman, 1989; Hobbs et al., 1992), spinohypothalamic tract (SHT; Katter et al., 1996a,b), spinosolitary tract (SST; Menétrey and Basbaum, 1987), spinoreticular tract (SRT; Haber et al., 1982; Villanueva et al., 1988), spinoparabrachial tract (Cechetto et al., 1985; Bernard et al., 1994; Slugg and Light, 1994), and pathways projecting from the spinal cord to forebrain structures, such as the amygdala and the septal nuclei (Burstein and Giesler, 1989; Burstein and Potrebic, 1993). It is clear from these studies that there are a variety of routes by which visceral and somatic nociceptive information is relayed by the spinal cord.
Another major ascending pathway, the dorsal column (DC) pathway, has been thought of primarily in terms of transmission of tactile discriminative sensation. The DC consists of two types of ascending fibers. The first type, which can be referred to as the direct DC pathway, is composed of collaterals of primary afferent fibers whose cell bodies are located in the dorsal root ganglia. These ascending projections include both myelinated and unmyelinated primary afferent fibers that synapse in the dorsal column nuclei (DCN), including the gracile and cuneate nuclei, of the lower medulla (Kuo and De Groat, 1985; Patterson et al., 1990; Garrett et al., 1992; for review see Willis and Coggeshall, 1991). The second type, referred to as the postsynaptic dorsal column (PSDC) pathway, includes the axons of spinal neurons traveling in the dorsal funiculus to terminate synaptically in the DCN (Petit, 1972; Rustioni, 1974, 1976; Angaut-Petit, 1975a; Rustioni and Kaufman, 1977; Giesler et al., 1984; Giesler and Cliffer, 1985; Cliffer and Giesler, 1989; Cliffer and Willis, 1994). The spinal cord distribution of PSDC neurons has been studied in monkeys (Rustioni, 1976; Bennett et al., 1983; Cliffer and Willis, 1994), cats (Petit, 1972; Rustioni, 1974; Angaut-Petit, 1975a,b; Rustioni and Kaufman, 1977; Bennett et al., 1983), and rats (Giesler et al., 1984; Giesler and Cliffer, 1985; Cliffer and Giesler, 1989). A previous study in rats has shown that most of the PSDC neurons are localized in laminae III and IV of the dorsal horn, and a few cells are found in the area around the central canal (Giesler et al., 1984). The involvement of rat PSDC neurons in the transmission of nociception has remained controversial for some time (Giesler and Cliffer, 1985), although PSDC neurons are reported to respond to noxious cutaneous and heat stimuli in cats (Uddenberg, 1966, 1968; Brown and Fyffe, 1981; Brown et al., 1983).
There is growing evidence, however, showing that the DC pathway may contribute to central transmission of visceral nociceptive information (Amassian, 1951a,b, 1952; Aidar et al., 1952; Rigamonti and Hancock, 1974, 1978; Berkley and Hubscher, 1995; Al-Chaer et al., 1996a,b; Hirshberg et al., 1996; Houghton et al., 1997b; Nauta et al., 1997; Feng et al., 1998). Clinically, it has been reported that intractable pelvic cancer pain can be relieved by a limited midline myelotomy extending from the dorsal surface of the spinal cord to the level of the dorsal gray commissure (Hirshberg et al., 1996; Nauta et al., 1997). Experimentally, the recent behavioral, anatomic, and physiologic studies from our laboratories have suggested that the population of PSDC neurons in the area around the central canal that sends ascending projections to the DCN is responsible for the transmission of visceral nociceptive information. Behavioral studies demonstrated that DC lesions could reduce the nociceptive behaviors induced in rats with pancreatitis or by duodenal distention (Houghton et al., 1997a; Feng et al., 1998). Similarly, mechanical DC lesions or chemical lesions of the DCN dramatically reduced responses of thalamic neurons resulting from duodenal distention (Feng et al., 1998), from chemical stimulation of pancreatic afferent neurons in rats (Houghton et al., 1997b; Wang et al., 1998), and from colorectal distention (Al-Chaer et al., 1996a, 1997a). Anatomically, retrograde tracing studies in rats demonstrated that axons traveling in the midline of the DC to the gracile nucleus originate from the PSDC neurons in the area around the central canal (CC) of the spinal cord (Hirshberg et al., 1996; Christensen et al., 1996; Westlund et al., 1996). Electrophysiological studies in rats have shown that the PSDC neurons in the area around the CC at the L6–S1 level of the spinal cord can be excited by colorectal distention or an injection of a chemical irritant, mustard oil, into the colon (Al-Chaer et al., 1996b, 1997b). These clinical and experimental studies provide evidence in support of the hypothesis that PSDC cells around the CC whose axons ascend in the DC pathway play an important role in visceral nociceptive processing.
Neurons in the area surrounding the CC in rats have previously been shown to give rise to fibers of the STT (Giesler et al., 1979; Kevetter and Willis, 1982, 1983; Granum, 1986; Burstein et al., 1990b), SRT (Kevetter et al., 1982; Kevetter and Willis, 1982, 1983; Menétrey et al., 1983; Nahin et al., 1983, 1986; Peschanski and Besson, 1984; Nahin and Micevych, 1986; see also Ruigrock and Cella, 1995), spinomesencephalic tract (SMT; Menétrey et al., 1982; Mantyh, 1982; Liu, 1983; Wiberg et al., 1987; Yezierski and Mendez, 1991), SST (Menétrey and Basbaum, 1987; Menétrey and de Pommery, 1991), SHT (Burstein et al., 1987, 1990a; Menétrey and de Pommery, 1991), spinoparabrachial tract (Menétrey and de Pommery, 1991; Kitamura et al., 1993), and spinoamygdaloid tract (Menétrey and de Pommery, 1991; Burstein and Potrebic, 1993).
Several tract tracing studies have shown that the region around the CC receives terminations from somatic and visceral afferents (Morgan et al., 1981, 1986; Kuo et al., 1983; Nadelhaft et al., 1983; Cervero and Connell, 1984; Kuo and De Groat, 1985; Roppolo et al., 1985; Neuhuber et al., 1986). This includes some Aδ mechanical nociceptors and unmyelinated visceral afferent fibers (Light and Perl, 1979; Sugiura et al., 1989). Neurons around the CC area respond to somatic and visceral nociceptive stimulation (Cervero, 1983; Nahin et al., 1983; Honda, 1985; Honda and Perl, 1985; Tattersall et al., 1986a,b; Ness and Gebhart, 1987, 1989; Berkley et al., 1993; Al-Chaer et al., 1996b). In addition, it is well known that the region around the CC also contains clusters of preganglionic autonomic neurons (Loewy and Spyer, 1990). It should be noted here, importantly, that the cells and afferent fiber innervation in this region are localized not only within the lamina X area but also in the medial aspect of lamina VII (and the dorsal gray commissure at lumbosacral segments), based on the results of previous studies (Honda, 1985; Honda and Perl, 1985; Tattersall et al., 1986a; Ness and Gebhart, 1987; Al-Chaer et al., 1996b; Hirshberg et al., 1996; Christensen et al., 1996; Westlund et al., 1996).
Previous anterograde tracing studies with injections centered in the midline gray matter surrounding the CC of the lumbosacral cord have shown that ascending axons travel in the midline of the DC and terminate in the gracile nucleus (Hirshberg et al., 1996; Westlund et al., 1996). However, the full extent of ascending projections and terminations from the neurons in the CC area is described in detail in the present study. In addition, in the present study, distinctive differences and similarities are noted for projections from the thoracic cord, which receives a substantial projection of nociceptive information from the thoracic viscera, for example, the pancreas and duodenum.
To characterize the projections further, a single small iontophoretic microinjection of an anterograde tracer, Phaseolus vulgaris leucoagglutinin (PHA-L), was made into the CC area at either the midthoracic or the lumbosacral level of the spinal cord in a series of rats. PHA-L was chosen as the anterograde tracer in this study because previous reports have shown that PHA-L has several advantages over wheat germ agglutinin conjugated to horseradish peroxidase (WGA-HRP; Gerfen and Sawchenko, 1984; Cliffer and Giesler, 1988): 1) PHA-L is taken up mainly by cell bodies and not significantly by passing fibers, 2) it is easy to make small injections of PHA-L, 3) labeled terminal varicosities and axons are clearly identified, and 4) PHA-L is unlikely to be transported across synapses. Preliminary results have been reported in abstract form (Wang et al., 1997).
MATERIALS AND METHODS
A total of 38 male adult Sprague-Dawley rats weighing 250–340 g were used in this study. Experiments were approved by the Animal Care and Use Committee of the University of Texas Medical Branch at Galveston in accordance with NIH guidelines.
Surgery and injections of PHA-L
Rats were deeply anesthetized with sodium pentobarbital (Nembutal; 50 mg/kg, i.p.). The midthoracic or lumbosacral cord was exposed by a laminectomy, and the dura was opened. A single iontophoretic microinjection of PHA-L (2.5% in 0.05 M phosphate buffer, pH 8.0; Vector, Burlingame, CA) was made into the CC area at the midthoracic level (1.0–1.1 mm depth) or lumbosacral level (0.8 mm depth) of the spinal cord with a glass micropipette (tip diameter 15–20 μm). In some sacral animals, injections were angled into the CC area to avoid the medial dorsal spinal vessels and the DC white matter. There were no differences in terminal labeling with either method of approach to the CC. Discontinuous positive pulses of DC current (3–5 μA, 7 seconds on and off) were applied for 20 minutes. The wound was sutured, and the rats were given antibiotics (Bicillin, Wyeth Laboratories Inc.; 40,000 units i.m. total).
Immunohistochemistry
After a 4–6-week survival period, the rats were killed with an overdose of sodium pentobarbital and perfused transcardially with 150 ml of warm (37°C) heparinized saline, followed by 1,000 ml of cold (4°C) 4% paraformaldehyde and 150 ml of 30% sucrose. The brain and spinal cord were removed carefully and immersed in 30% sucrose/phosphate buffer overnight for cryoprotection. Tissue blocks were cut in 40-μm-thick coronal serial sections on a freezing microtome, and the sections were collected in ice-cold phosphate buffer containing 0.1% sodium azide (NaN3; Sigma, St. Louis, MO). After rinsing in 2% normal rabbit serum (NRS) containing 0.3% Triton X-100 at room temperature for 30 minutes, a one-in-five series of tissue sections was processed immunohistochemically with goat anti-PHA-L antibody (1:10,000; Vector)/2% NRS containing 0.3% Triton X-100 at 4°C for 36–48 hours. After rinsing in 0.1 M phosphate-buffered saline (PBS) for 20 minutes, the sections were incubated with biotinylated rabbit anti-goat IgG (1:400; Vector)/2% NRS containing 0.3% Triton X-100 at room temperature for 1 hour. Then the sections were rinsed in 0.1 M PBS for 20 minutes before incubating in avidin-biotin-horseradish peroxidase (HRP) complex solution (ABC Kit, Vector; recommended dilution ×2 in PBS) at room temperature for 1 hour. After rinsing in 0.1 M PBS for 20 minutes, the sections were reacted with 1) 0.025% DAB/phosphate buffer containing 0.0025% H2O2 or 2) 0.015% DAB/0.1 M acetate buffer (pH 6.0) containing 0.24% nickel ammonium sulfate and 0.0015% H2O2, for 5–10 minutes. Then the sections were mounted onto gelatin-coated glass slides, air dried, cleared with xylene, and coverslipped with DPX. Another one-in-five series of tissue sections was stained with cresyl violet or neutral red.
The sections were observed under conventional bright-field optics using a Nikon microphot-FXA light microscope (NCB11, HE, and ND2 filters). Photomicrographs were made with AGFAPAN APX25 professional film (batch developed in TMX RS) and printed on Kodak F4 Kodabrome II RC paper. Injection sites and labeled axons and varicosities were drawn with the aid of a camera lucida attachment. The sections were superimposed on adjacent sections stained with cresyl violet or neutral red, and the nuclei were cytoarchitectonically identified according to the stereotaxic rat brain atlas of Paxinos and Watson (1986). In addition, the nomenclature of the subnuclei of the parabrachial (PB) nucleus was adopted from the description of Fulwiler and Saper (1984). The reconstructions of injection sites were plotted on a template of the midthoracic and lumbosacral levels of the spinal cord (Fig. 1).
Fig. 1.
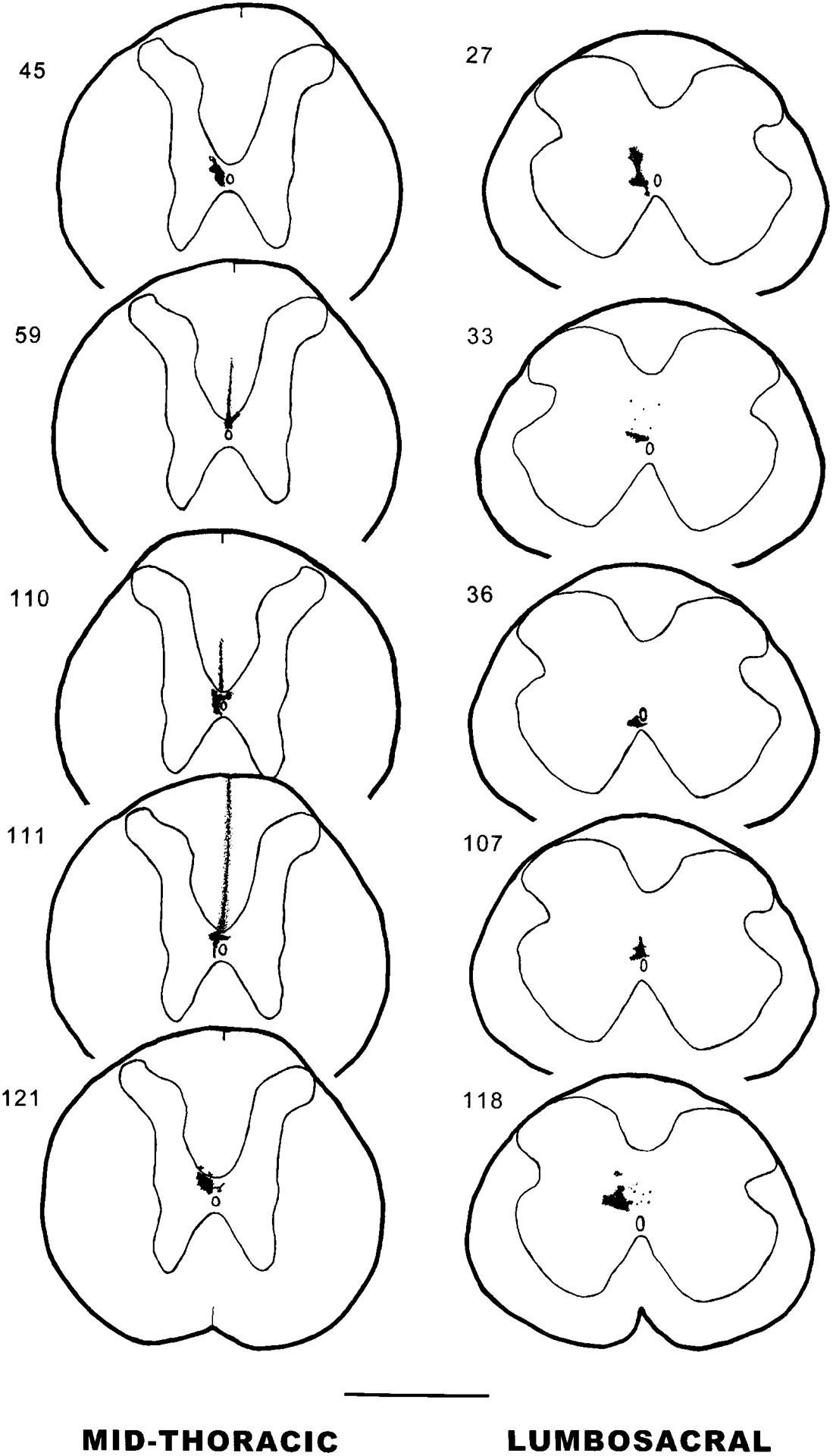
Camera lucida drawings of coronal sections of Phaseolus vulgaris-leucoagglutinin (PHA-L) injections into the area around the spinal cord central canal at midthoracic (T6–T9) or lumbosacral (L6–S1) levels of spinal cord. The numbers at left refer to the experimental animal number. Scale bar = 1 mm.
RESULTS
The present results are based on 10 selected experiments in which the single PHA-L injection site was localized in the CC area of the midthoracic or lumbosacral level of the spinal cord (n = 5 for each level; Fig. 1). These injection sites consisted of a densely stained core containing labeled cell bodies and an injection halo where diffuse extracellular staining was observed. PHA-L is thought to be preferentially taken up by the cells within the injection core but not by cells in the injection halo (Gerfen and Sawchenko, 1984). In addition, control injections were made in eight animals into the midline of the spinal cord at thoracic and lumbosacral sites that included the dorsal column (n = 3), medial zone of laminae I–V (n = 3), or lamina VII lateral to the ventral white commissure of spinal cord (n = 2; Fig. 2).
Fig. 2.
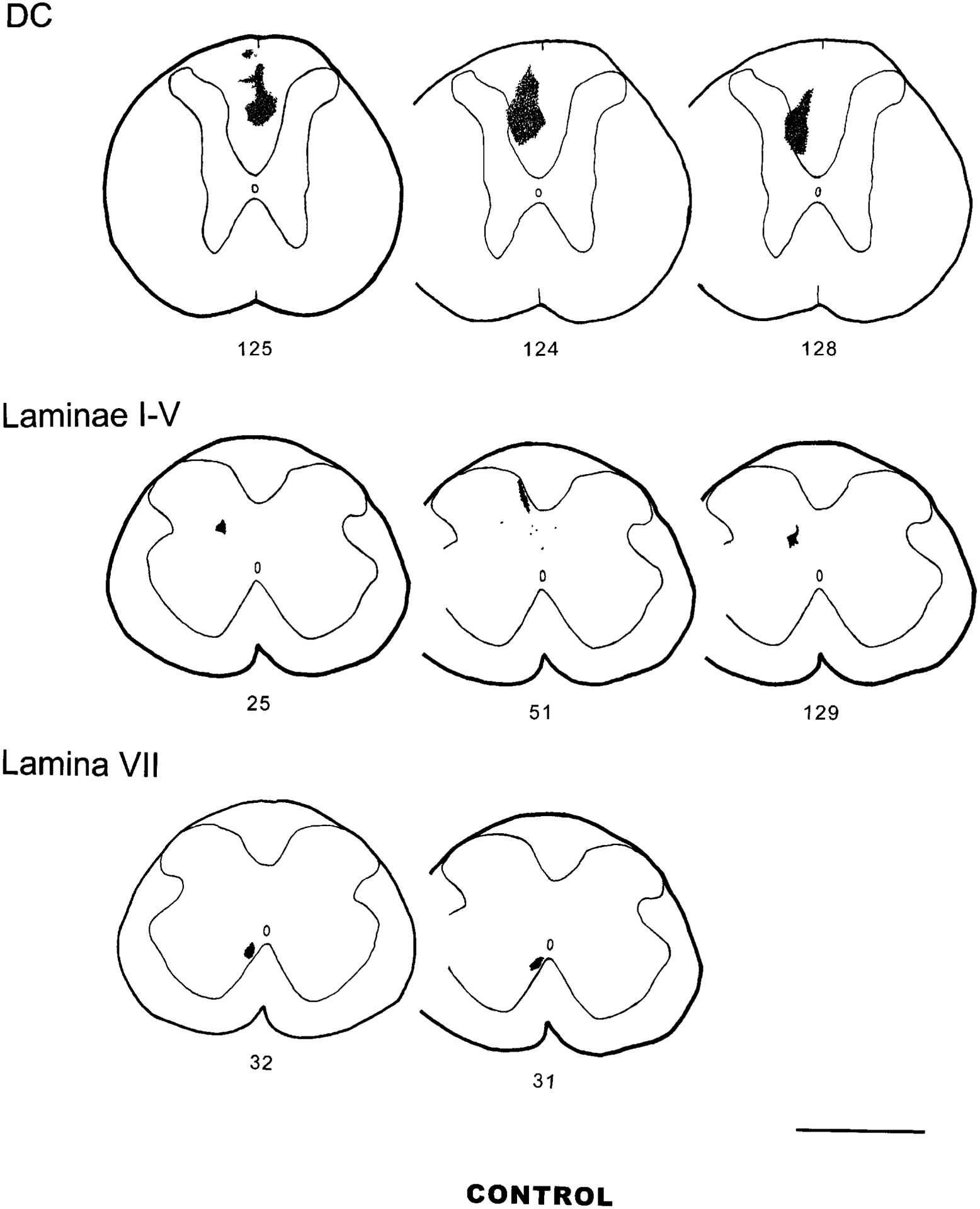
PHA-L control injection sites in the DC, laminae I–V, and VII (and VIII). Scale bar = 1 mm.
Injection sites
Midthoracic level of the spinal cord.
The midthoracic injection group description includes the results of five experiments in which the injection sites were localized in the CC area at the midthoracic level (Fig. 1, left column). In two experiments (experiments 45 and 121, injection in T7 and T9, respectively), the injection sites were restricted to the CC area. Two experiments (experiments 59 and 110, injection in T7 and T8, respectively) had injection sites in the CC area and the ventral aspect of the dorsal funiculus containing the corticospinal tract. In experiment 111, the injection site (T7) was localized not only in the CC area but also in the midline of the dorsal funiculus, including both the DC and the corticospinal tract. Experiment 110 was chosen as a representative case for camera lucida drawings and will be described in detail, because the injection site covered most of the CC area at the T8 level of the spinal cord (diameter 200–250 μm), without significant spread to the white matter.
Lumbosacral level of the spinal cord.
In five experiments, injection sites were localized in the CC area at the lumbosacral (SI) level, and the descriptions from these animals are included in the lumbosacral injection group (Fig. 1, right column). Two experiments (experiments 36 and 107) had small injection sites that targeted the CC area. Three experiments (experiments 27, 33, and 118) had larger injection sites localized in the midline of the lumbosacral level of spinal cord, including lamina X and the medial aspect of lamina VII. Experiment 118 was chosen as a representative case for camera lucida drawings and will be described in detail.
Control injections.
Control injections were made in eight animals (Fig. 2) to compare the pattern of distribution of axons from neurons in the CC area with those from adjacent regions in the medial spinal cord, e.g., the DC and the medial aspect of the laminae I–V and ventromedial aspect of lamina VII.
Pattern of ascending projections
As an overview of the results, the ascending axons from the neurons in the CC area are observed in a number of brain sites, including areas in the medulla, pons, mesencephalon, diencephalon, telencephalon, and cerebellum. Generally, a higher density of PHA-L-labeled fibers is seen at the medullary level than at other levels. The density and number of labeled axons in the diencephalon and other forebrain structures are much smaller. In comparing the pattern of ascending projections from the thoracic and lumbosacral groups of PHA-L-injected animals, a similar termination pattern was noted. However, labeled fibers in Barrington’s nucleus are found only in projections of the lumbosacral group, and the primary termination sites in the DC nuclei from thoracic and lumbosacral levels are different. Thoracic projections to the cerebellum are observed resulting from some uptake into the adjacent Clarke’s column.
The pattern of distribution of labeled terminals and axons was mapped successively starting from sections just rostral to the injection sites, at the C1 level of the spinal cord, and the brainstem through the level of the diencephalon (experiment 110, midthoracic group; experiment 118, lumbosacral group in Fig. 3). In addition, the location of terminal labeling in telencephalic regions will be described in this report. The comparative density of PHA-L-labeled fibers in selected experiments following midthoracic and lumbosacral injections is shown in Table 1.
Fig. 3.
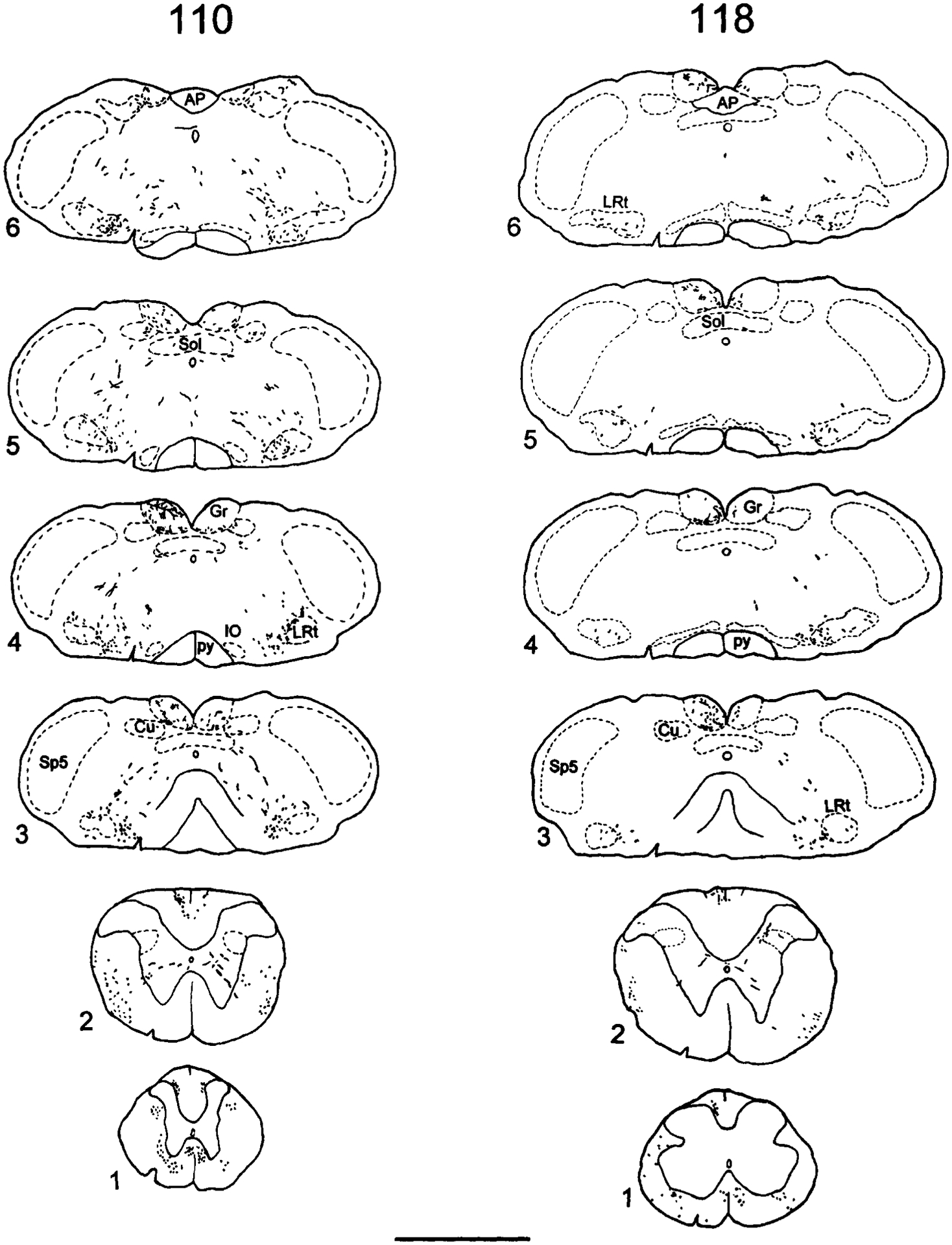
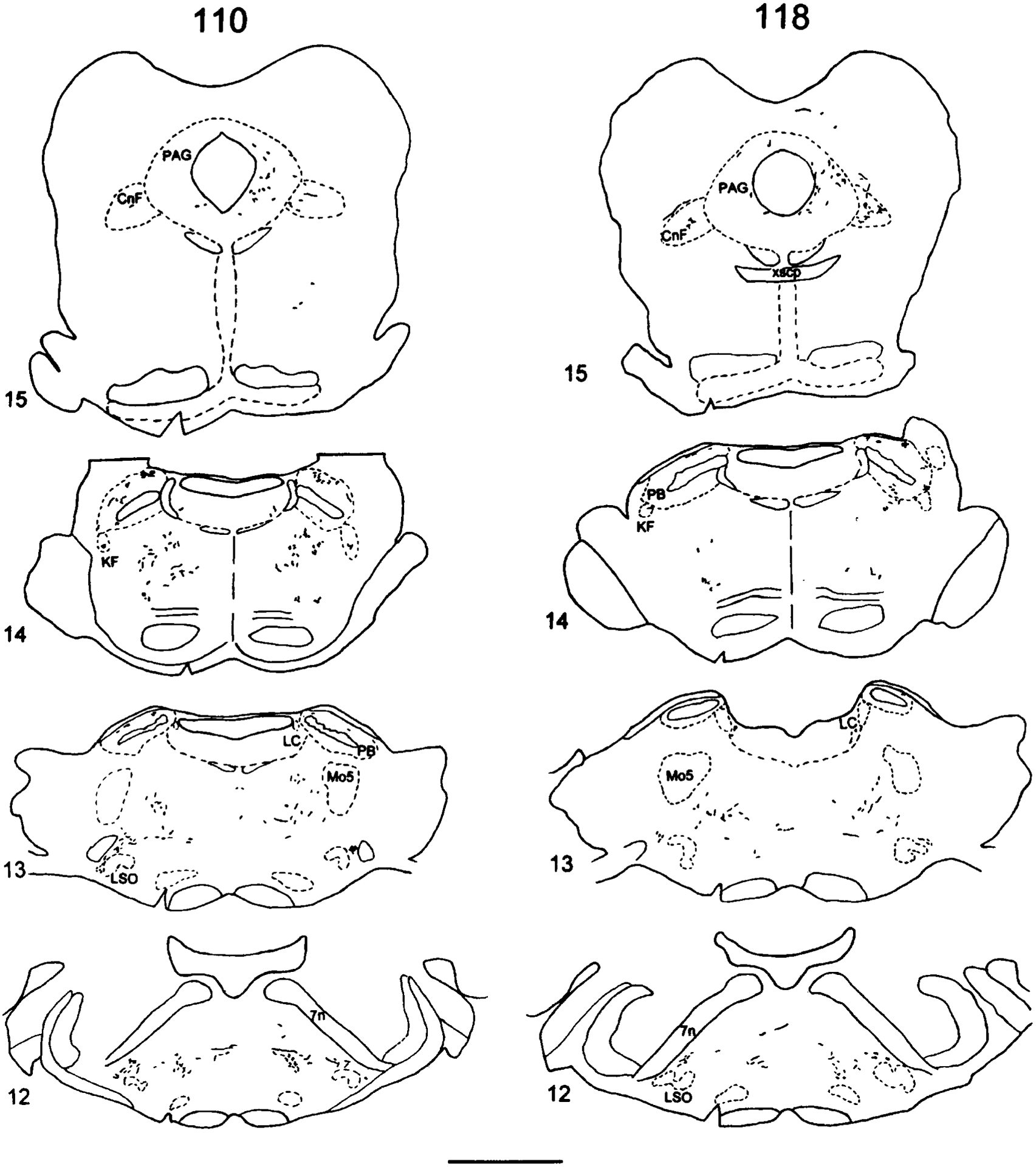
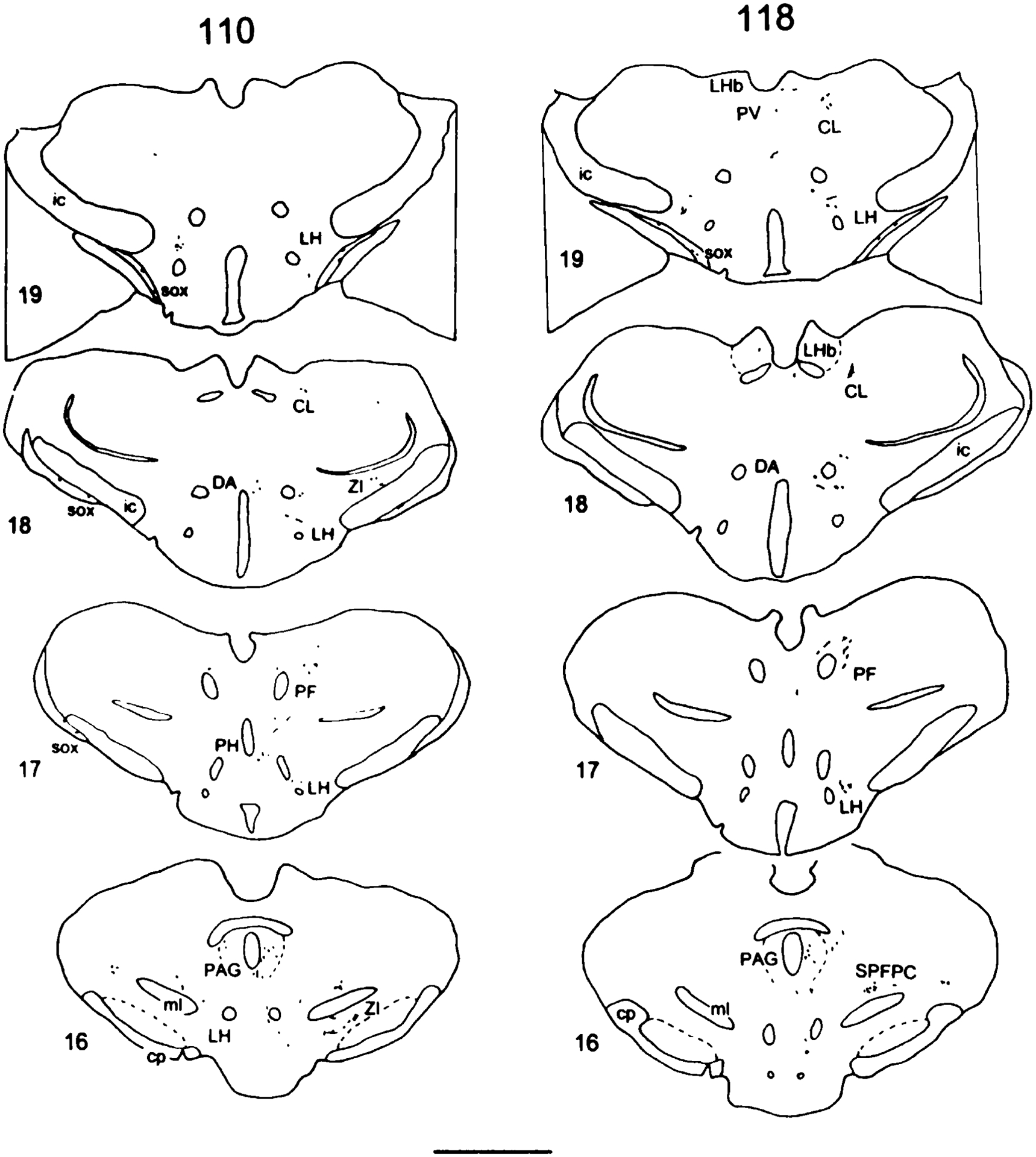
Camera lucida drawings of PHA-L-labeled fibers in a series of single transverse sections from experiment 110 in the midthoracic injection group and from experiment 118 in the lumbosacral injection group. Examples from a level several sections rostral to the injection site and sections from the C1 level of spinal cord through the level of the diencephalon. The sequence is numbered from caudal to rostral. The left side is ipsilateral to the injection site. Cytoarchitectonic delineations are based on adjacent Nissl-stained sections. The representative tissue sections from experimental animals 110 and 118 are referred to in the text by the numbers on their left. The sections are numbered sequentially from the spinal cord through the diencephalon. For abbreviations, see list. Scale bars = 2 mm.
TABLE 1.
Comparative Density of PHA-L-Labeled Fibers in Supraspinal Structures in Selected Experiments1
| Midthoracic injections (Exp. No.) | L6-S1 injections (Exp. No.) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 45 | 59 | 110 | 111 | 121 | 27 | 33 | 36 | 107 | 118 | |
| Survival time (days) | 25 | 37 | 26 | 26 | 28 | 32 | 38 | 34 | 45 | 40 |
| Medulla | ||||||||||
| Gr | ++ | ++ | +++ | +++ | +++ | ++ | + | + | ++ | +++ |
| Cu | +++ | ++ | ++ | +++ | ++ | * | 0 | + | + | 0 |
| LRt | +++ | + | +++ | +++ | +++ | ++ | +++ | ++ | ++ | ++ |
| NTS | + | + | + | + | + | + | + | + | + | + |
| SRV | ++ | + | +++ | ++ | + | + | ++ | + | ++ | + |
| SRD | + | * | + | + | + | + | + | * | + | + |
| Sp5C | * | 0 | 0 | * | * | + | 0 | 0 | 0 | 0 |
| LPGi | + | + | +++ | ++ | ++ | ++ | +++ | ++ | +++ | ++ |
| Gi | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| ROb | * | 0 | + | + | + | * | ++ | + | + | + |
| RMg | + | 0 | + | + | + | + | + | + | + | + |
| Pons | ||||||||||
| PB | + | 0 | + | + | ++ | ++ | ++ | ++ | ++ | ++ |
| KF | + | * | ++ | + | + | ++ | ++ | + | + | + |
| SubC | ++ | + | ++ | ++ | ++ | ++ | ++ | + | ++ | ++ |
| Pn | + | + | + | + | ++ | + | ++ | + | + | ++ |
| LC | 0 | 0 | + | + | 0 | + | * | + | + | * |
| Bar | 0 | 0 | 0 | 0 | 0 | + | * | + | + | + |
| Mesencephalon | ||||||||||
| PAG | +++ | + | + | + | + | ++ | +++ | ++ | +++ | ++ |
| CnF | ++ | ++ | ++ | + | + | + | + | + | + | ++ |
| DpMe | ++ | + | ++ | ++ | + | ++ | ++ | + | + | + |
| SC | + | + | + | 0 | + | + | + | + | + | * |
| InC | + | ++ | + | + | + | + | + | + | + | + |
| SNR | 0 | 0 | * | * | 0 | 0 | 0 | 0 | 0 | 0 |
| R | 0 | 0 | + | + | + | * | * | 0 | * | 0 |
| APT | 0 | + | + | * | + | + | + | + | + | + |
| Thalamus | ||||||||||
| PIL + PP | 0 | + | + | + | + | ++ | + | 0 | +++ | * |
| SPFPC | + | + | + | * | + | +++ | +++ | + | +++ | ++ |
| LHb | 0 | 0 | + | 0 | 0 | + | + | + | 0 | + |
| PV | + | + | + | * | * | * | + | + | + | + |
| PF | 0 | 0 | ++ | + | ++ | + | + | + | + | ++ |
| PrC | 0 | 0 | 0 | 0 | 0 | 0 | + | + | + | + |
| Po | * | 0 | + | 0 | + | 0 | + | + | * | + |
| VPPC | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 |
| VL | 0 | 0 | + | 0 | + | + | 0 | + | + | + |
| AVVL | 0 | 0 | 0 | 0 | + | 0 | + | + | 0 | 0 |
| VM | 0 | 0 | * | 0 | + | 0 | + | + | + | 0 |
| Re | 0 | 0 | 0 | 0 | * | 0 | + | 0 | 0 | 0 |
| CL | 0 | 0 | + | + | + | 0 | + | + | 0 | ++ |
| LD | 0 | 0 | + | 0 | + | 0 | 0 | * | 0 | 0 |
| MD | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 | + | 0 |
| CM | 0 | 0 | * | + | * | 0 | + | + | 0 | 0 |
| Hypothalamus | ||||||||||
| LH | + | + | ++ | + | + | + | + | + | ++ | + |
| ZI | + | + | ++ | + | + | +++ | + | + | + | * |
| Subl | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 | ++ | 0 |
| SUM | + | 0 | + | + | * | + | 0 | * | + | + |
| DA | + | + | 0 | 0 | + | + | + | + | + | 0 |
| PVH | 0 | 0 | 0 | 0 | + | + | 0 | 0 | ++ | 0 |
| PH | * | + | ++ | + | ++ | + | ++ | + | + | + |
| MPA | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | * | 0 |
| LPO | + | + | + | + | + | 0 | 0 | * | * | 0 |
| AH | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| sox | 0 | + | + | + | + | 0 | + | + | + | + |
| Telencephalon | ||||||||||
| Ce | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | + | 0 |
| CPU | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 |
| GP | 0 | 0 | + | + | + | 0 | + | 0 | 0 | 0 |
| SI | 0 | + | + | + | + | 0 | 0 | + | + | 0 |
| BM | + | 0 | 0 | + | 0 | 0 | + | + | 0 | 0 |
| GI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + |
++++, Very high density; +++, high density; ++, medium density; +, low density; *, sparse density; 0, no fibers found. For abbreviations, see list.
Spinal cord.
Beginning at a level several sections rostral to the injection site, the labeled axons are seen ascending at the lateral edge of the DC bilaterally as well as in the ventrolateral funiculus and the medial aspect of the ventral funiculus bilaterally. A few axons are found in the lateral funiculus of the spinal cord near the spinal gray matter (Fig. 3.1). In the lumbosacral injection group, the labeled axons then migrate to assume a position near the midline of the DC as well as in the ventrolateral funiculus at the midthoracic level of the spinal cord. A few fibers are observed in the ventral and lateral funiculus of the spinal cord. At midcervical levels the DC projection of the thoracic injection group migrates to a position adjacent to the dorsal intermediate septum.
At the C1 level of the spinal cord (Fig. 3.2), the labeled axons are seen coursing longitudinally in the DC and the ventrolateral white matter of the spinal cord. The PHA-L-labeled axons originating from the midthoracic level of the spinal cord are seen traveling primarily in the superficial part of the DC near the dorsal intermediate septum, whereas those from the lumbosacral level have migrated to ascend in the superficial part of the medial gracile fasciculus. Ascending axons in the DC arising from both the midthoracic and the lumbosacral levels of the spinal cord have bilateral projections with an ipsilateral predominance. The labeling in the ventrolateral and lateral funiculus of the spinal cord is observed bilaterally (Fig. 3.2). Propriospinal fibers are observed originating from axons traveling in the ventrolateral quadrant of the spinal cord coursing dorsomedially out of the white matter and terminating in the spinal gray matter, mainly in the area adjacent to the central canal and in the ventral horn. Propriospinal fibers leaving the DC terminate in the gray matter of the dorsal horn.
Medulla.
The fibers traveling in the DC terminate in the dorsal column nuclei (DCN) with a strong ipsilateral predominance. There is a topography of the terminations from neurons in the CC area at midthoracic and lumbosacral levels of the spinal cord in the present study. Generally, the terminal labeling is concentrated in the caudal half of the DCN. In the region of the DCN rostral to the area postrema, the density and number of fibers become less.
In three of five cases with midthoracic injections, the fibers and terminals are localized primarily in the dorsal and lateral rims of the gracile nucleus (Gr) and medial portions of the cuneate nucleus (Cu; Figs. 3.3–3.6 [left column], 4B,D). In one case, the fibers are only found in the lateral rim of the Gr, whereas, in another case, the labeling is seen exclusively in medial portion of the Cu. In the example illustrated in Figure 3 (experiment 110), however, terminations from PSDC cells in the midthoracic level of the spinal cord are also found in the dorsomedial aspect of the Gr, even though labeled fibers are seen traveling near the dorsal intermediate septum of the DC at the C1 level. Long axons with short arborizations and many terminal varicosities are seen coursing from the dorsolateral to the ventromedial aspect of the Gr (Fig. 4B). In experiment 111 with an injection site that includes the CC area and the entire midline of the DC, the labeled fibers are observed in the lateral and medial rims of the Gr and the medial aspect of the Cu. The labeling in the lateral rim of the Gr and medial portion of the Cu consists mainly of short axonal arborizations with many terminal and en passant varicosities. However, the labeling coursing along the medial rim of the Gr contains less intensely stained short axons with few varicosities.
Fig. 4.
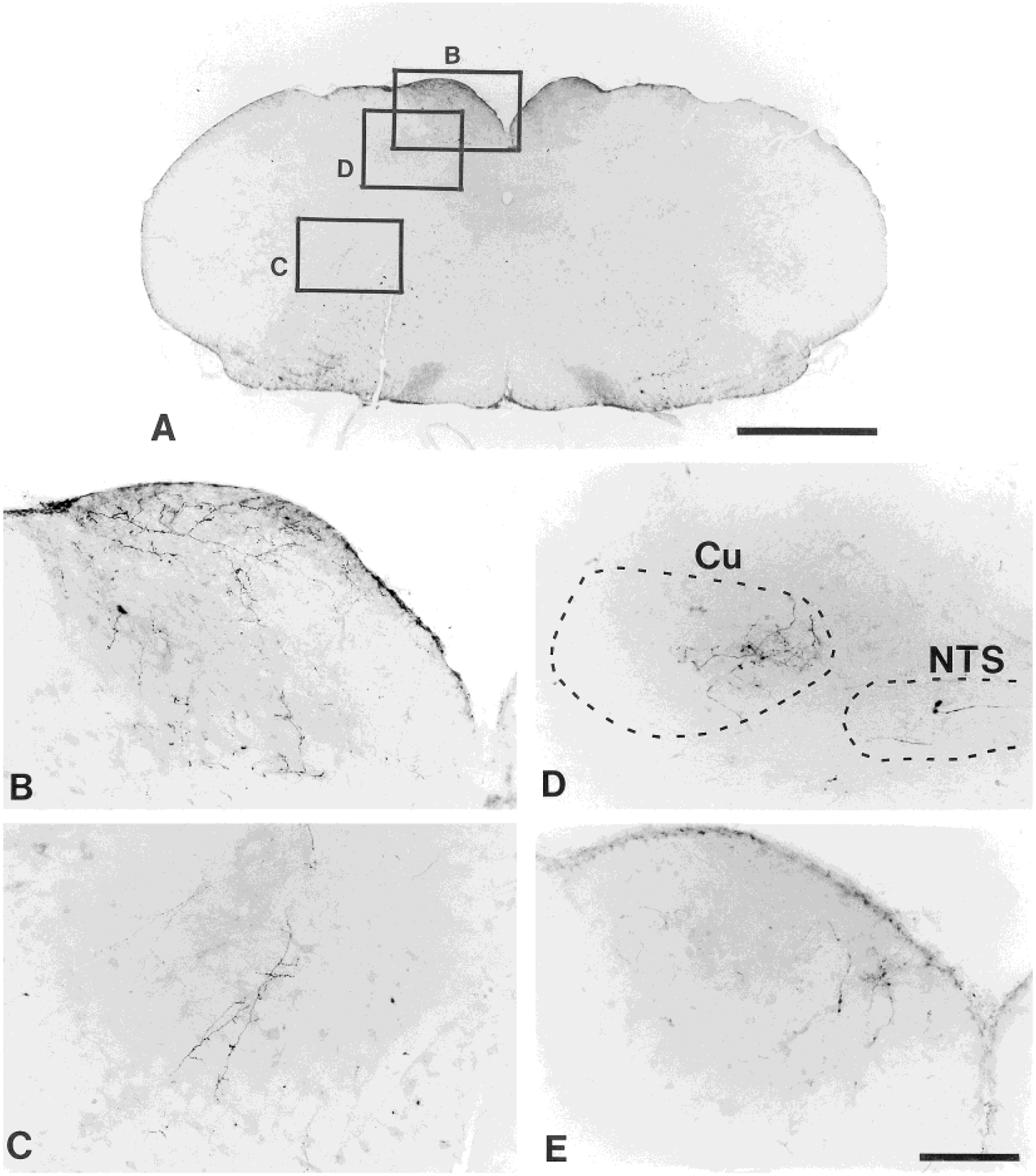
Photomicrographs illustrating PHA-L-labeled fibers and terminals in a transverse section at the caudal medulla level as shown in A from a midthoracic injection animal (experiment 110; from the section in level 4, Fig. 3). The labeling shown in the gracile nucleus (B) and the medullary reticular formation (C) is from the sites marked in A. D: The labeling in the medial aspect of the cuneate nucleus and the nucleus of solitary tract is from another midthoracic injection animal (experiment 45). The labeled fibers and terminals in the dorsomedial portion of the gracile nucleus shown in E are from experiment 27 in lumbosacral injection group. Dorsal is up, ipsilateral is left. Scale bars = 1 mm for A; 100 μm for B–E.
In all cases with injections at the lumbosacral level, terminations are observed primarily in the medial rim of the Gr (Figs. 3.3–3.6 [right column], 4E). In two cases, labeling is also seen in the ventromedial aspect of the Gr (Fig. 3.3, 3.4 [right column]). A low to medium density of terminal labeling is found in the Gr. In two cases, however, a low density of fibers is also found in the Cu.
PHA-L-labeled fibers continuing from the ventrolateral and ventral funiculi of the spinal cord are found terminating throughout the full rostrocaudal extent of the ventral medulla. The labeling is localized in the lateral reticular nucleus (LRt) and the lateral paragigantocellular reticular nucleus (LPGi); the caudal part of the nucleus of the solitary tract (NTS); and the medullary reticular formation, including the subnucleus reticularis dorsalis (SRD); the subnucleus reticularis ventralis (SRV; Villanueva et al., 1991), the gigantocellular reticular nucleus (Gi), the raphe magnus nucleus (RMg), and the raphe obscurus nucleus (ROb). In some cases, a sparse density of labeling is also seen in the trigeminal nucleus caudalis. The labeled axons and terminals in the ventral and medial medulla from midthoracic injections and those from lumbosacral injections are shown in Figure 3.3–3.9.
In general, labeled fibers are seen coursing bilaterally through the rostrocaudal extent of the ventral and medial medulla. PHA-L-labeled axons are seen extending dorsomedially from the ventral medulla across the reticular formation, including the SRD and SRV, to the region of the NTS (Figs. 3.3–3.9, 4C), or ascending longitudinally through the ventral medulla. In addition, ascending PHA-L-labeled axons localized in the ventral medulla are seen traversing the tissue section toward the midline. In the caudal medulla, a large number of labeled varicose fibers are concentrated in the area of the medial and dorsal aspect of the LRt and within the LRt (Fig. 3.3–3.8). In general, the projections to the LRt are concentrated dorsally from the midthoracic cord and ventrally from the lumbosacral cord.
At the level between the rostral end of LRt and the caudal portion of LPGi, labeled fibers are concentrated in the LRt and LPGi and ascend longitudinally (Fig. 3.7–3.9). Fibers bearing varicosities are also seen coursing dorsomedially from ventral medulla to the medial medullary reticular formation. At the level between the caudal aspect of the LPGi and the facial nucleus, labeled axons and terminals are concentrated in the LPGi bilaterally (Fig. 3.9, 3.10). PHA-L-labeled fibers are also observed coursing from the LPGi to the medial reticular formation, including the Gi and the ROb.
At the rostral medullary level, near the caudal aspect of the facial nucleus, the density of labeling becomes less intense (Fig. 3.10). In most cases, labeled fibers are seen coursing bilaterally from the ventral medulla to the medial reticular formation between the medial aspect of the facial nucleus and terminating in the RMg, ROb, and Gi. In all cases but one, a medium density of labeling in the Gi and a low density of labeled fibers in RMg and ROb are observed. The labeling in the Gi and raphe nuclei consists of axons bearing many terminal varicosities.
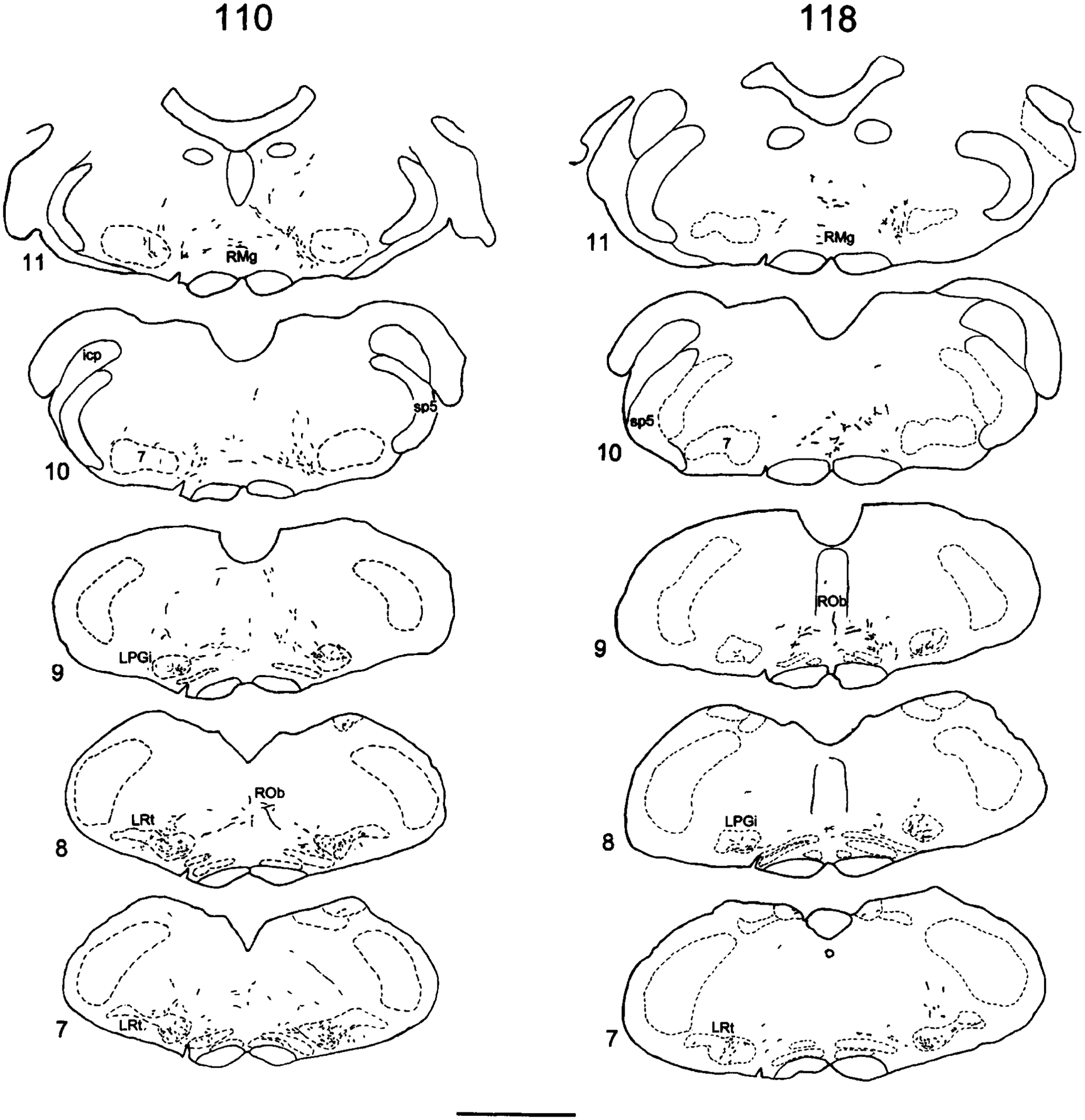
Pons.
In the pons, the PHA-L-labeled fibers from neurons in the CC area are localized in the pontine reticular formation; the ventrolateral tegmentum; the dorsolateral tegmentum, including the locus coeruleus (LC) and the subcoerulear region (SubC); and the parabrachial nucleus (PB), including the medial PB subnucleus (PBm), the internal lateral PB subnucleus (PBil), the superior lateral PB subnucleus (PBsl), the central lateral PB subnucleus (PBcl), the external lateral PB subnucleus (PBel), and the Kölliker-Fuse nucleus (KF). The labeling at this level is plotted in Figure 3.12–3.14 and Figure 6. The labeling is seen bilaterally at this level.
Fig. 6.
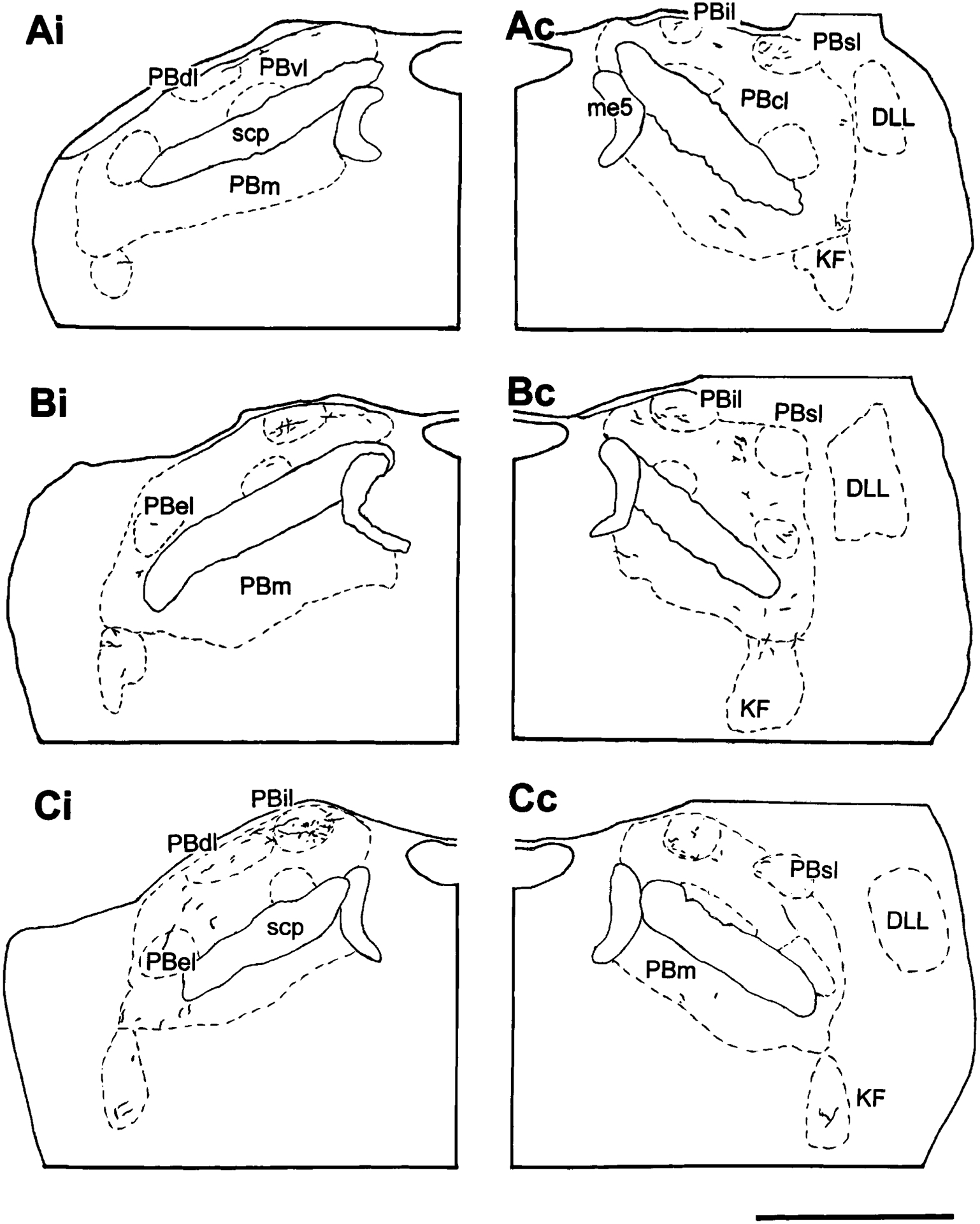
Camera lucida drawings illustrating the labeling in the ipsilateral (i) and contralateral (c) parabrachial nuclei and the Kölliker-Fuse nucleus near the pontomesencephalic junction following an injection in the area surrounding the spinal cord central canal from experiment 110 (Ai-Bi; midthoracic) and 118 (Ci; lumbosacral). The distance between Ai and Bi is 200 μm. For abbreviations, see list. Scale bar = 1 mm.
At the pontomedullary junction, a medium density of labeled fibers is observed bilaterally in the A5 region adjacent to the exit of the facial nerve, in the dorsal and lateral aspects of the lateral superior olivary nucleus (LSO; Figs. 3.12, 3.13, 5B). The labeled fibers in this region consist primarily of axons with many arborizations.
Fig. 5.
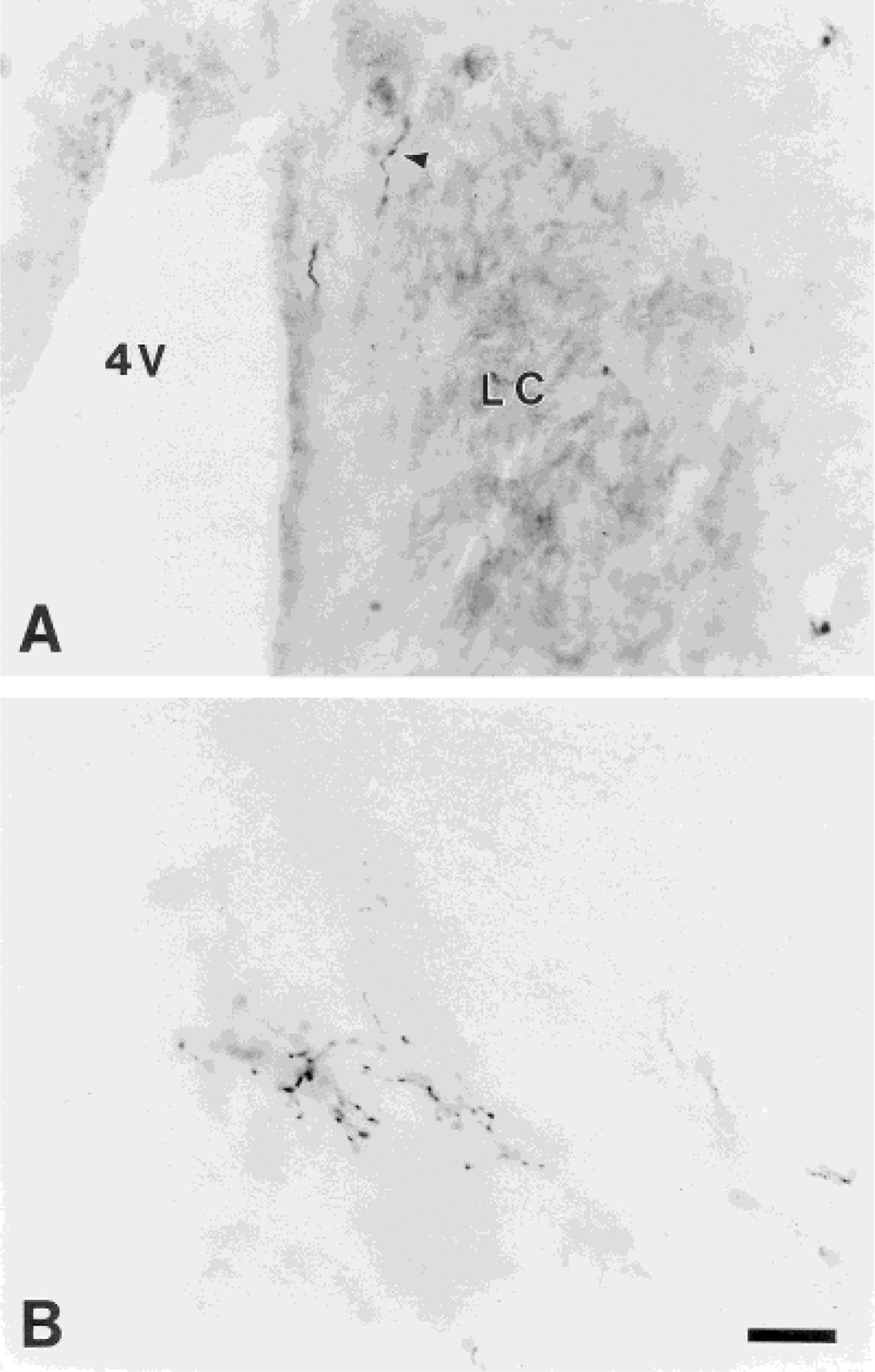
Photomicrographs of labeled fibers and terminations from neurons in the area adjacent to the spinal cord central canal in the contralateral locus coeruleus (experiment 36; A) and ventrolateral pons (A5 region; experiment 110; B; from the section in level 13 of Fig. 3). In A, the arrowhead indicates a PHA-L-labeled fiber located just medial to the locus coeruleus. Scale bar = 25 μm.
The labeled fibers in the ventrolateral tegmentum (medial to the facial nerve) continue from the rostral ventral medulla (RVM) into the caudal pons. Generally, a small number of labeled fibers are seen coursing from the dorsal and medial aspects of LSO medially and terminating in the pontine reticular formation. In addition, a medium density of labeling, in most cases, is observed coursing dorsolaterally, medial to the motor trigeminal nucleus (Mo5), and terminating diffusely in the SubC region (Fig. 3.13). Axons with several collaterals bearing varicosities are seen in the SubC region.
Fibers in the SubC region continue coursing dorsally toward the LC. In two of the five cases with midthoracic injections and all of the lumbosacral cases, a sparse to low density of the labeling is observed bilaterally in the LC. In most cases, fine and short axons bearing varicosities in the SubC are seen coursing dorsolaterally to ventral and medial aspects of the LC. In addition, labeling inside the LC is observed arising from collaterals of fibers coursing along the fourth ventricle (Fig. 5A). There is no apparent topographic organization for the innervation of the LC; fibers with boutons can be seen throughout the rostrocaudal extent of the LC for both thoracic and lumbosacral injections. At any particular level, only one or two varicose fibers can be seen within the LC (Fig. 5A). However, the labeling is focused in the caudal half of the LC. Terminal labeling is also seen in the pericoerulear region ventral and medial to the LC, including in Barrington’s nucleus. In four cases with lumbosacral injections, a low density of labeled fibers is observed in Barrington’s nucleus, whereas no fibers are found in this structure with midthoracic injections. In two cases, labeling is also seen in the mesencephalic trigeminal nucleus at this level (Me5).
In addition, ascending axons traveling in the ventrolateral tegmentum are also observed coursing dorsally with the lateral lemniscus and passing through the ventrolateral aspect of PB and KF. A medium to low density of labeled fibers and terminals is seen in the KF (Figs. 6, 7D). Most of these terminals are concentrated in the caudal half of the KF. The labeling in PB is concentrated in the middle and rostral one-third of the lateral PB subnuclei. Very few fibers are seen in the caudal aspect of PB. The labeled fibers in the lateral PB subnuclei are seen bilaterally, with a slight ipsilateral predominance. Most of the labeling is concentrated in the PBil (Figs. 6, 7B,C). Generally, the labeled fibers in the PB are long, thin axons with numerous terminal boutons. In addition, less density of fibers is seen in the PBcl, PBdl, PBsl, PBel, and KF. A sparse to low density of labeled fibers is found in the PBm.
Fig. 7.
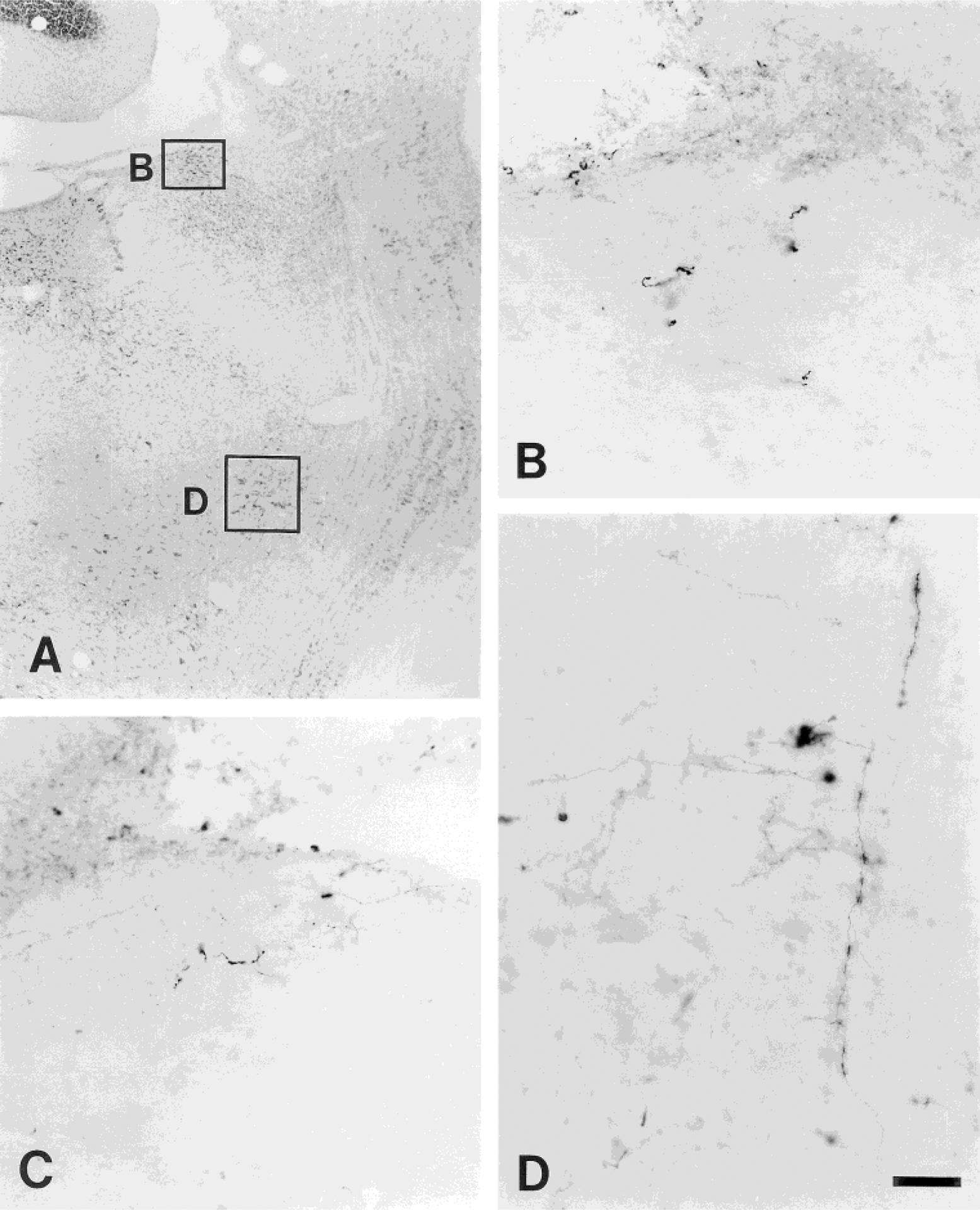
Photomicrographs showing the PHA-L labeling in the internal lateral parabrachial subnuclei ipsilateral (B) and contralateral (C) to the injection site in experiment 110 and in the Kölliker-Fuse nucleus (D) in experiment 27. The labeling shown in B and D is from the sites marked in the adjacent transverse Nissl-stain section shown in A. Scale bar = 250 μm for A; 50 μm for B–D.
Mesencephalon.
Labeled fibers are seen in the caudal lateral and ventrolateral portions of the periaqueductal gray (PAG), the cuneiform nucleus (CnF), the deep mesencephalic region (DpMe), the red nucleus, the anterior pretectal nucleus, the pars reticulata of the substantia nigra (SNR), the superior colliculus (SC), and the intercollicular region of the midbrain (InC; see Table 1; Fig. 3.15, 3.16). Basically, the labeling in the mesencephalon is bilateral with strong contralateral predominance.
The labeling in the mesencephalon (Fig. 3.15, 3.16) continues from the axons traveling in the rostral aspect of lateral PB. These axons are seen oriented from ventrolateral to dorsomedial. Low to high densities of PHA-L-labeled fibers and terminals are localized in the ventrolateral PAG. The terminals are concentrated in the caudal aspect of the ventrolateral and lateral PAG. In most cases, the labeling in the InC is of low density and scattered. In an example shown from an animal with a lumbosacral PHA-L injection (experiment 107), basket-like terminations of labeled fibers are found around cells of the Me5 adjacent to the PAG region (Fig. 8A).
Fig. 8.
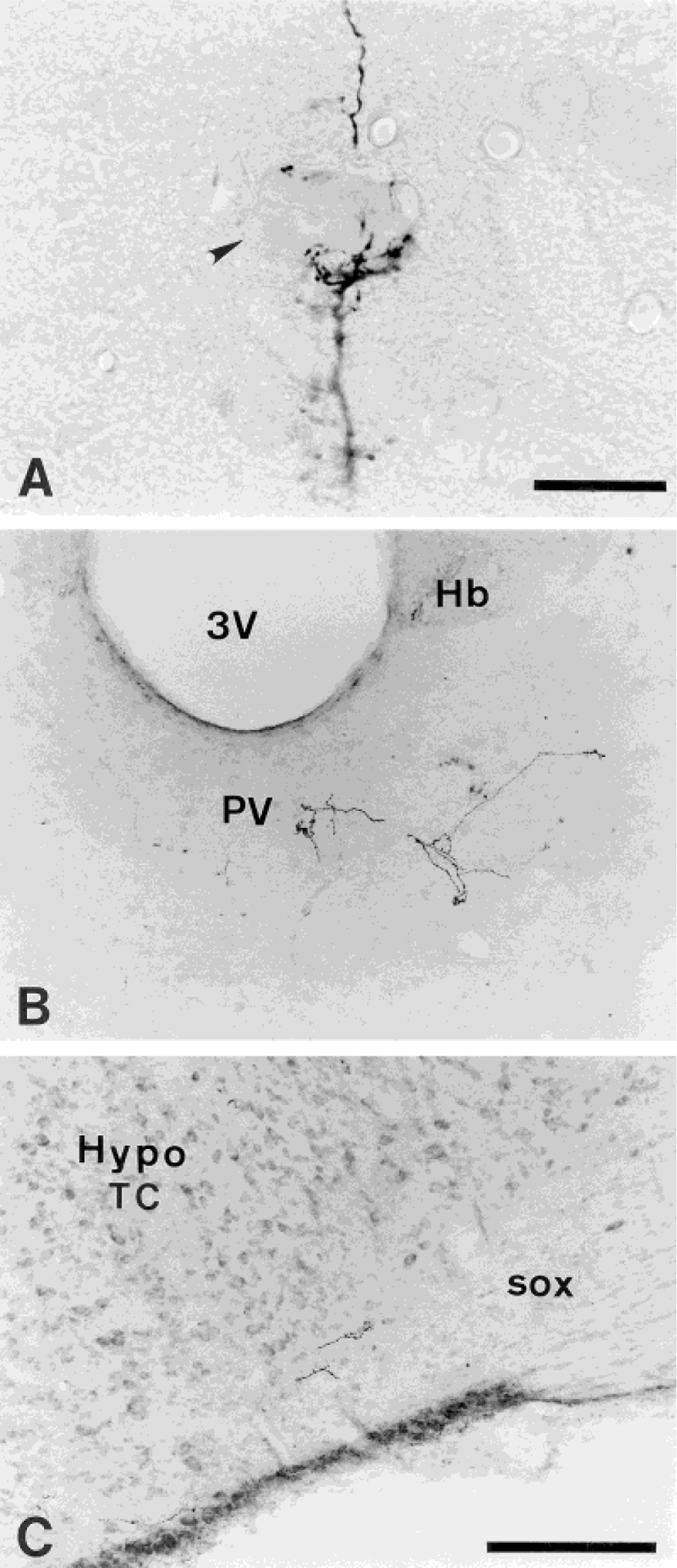
Labeled fibers and terminals in transverse sections from the neurons in the area around the central canal in experiment 107 (lumbosacral) are seen in the mesencephalic trigeminal nucleus at the periaqueductal gray level (A), the paraventricular thalamic nucleus (B), and the supraoptic decussation (C). B: Labeled terminal fibers below the habenular complex are localized in the mediodorsal thalamic nucleus. Arrowhead in A is the mesencephalic trigeminal neuron. For abbreviations, see list. Scale bars = 25 μm for A; 200 μm for B,C.
Thalamus.
The density and number of labeled axons in the area rostral to the mesencephalon is small (see Table 1). The areas in the thalamus that contain labeled fibers include the posterior thalamic nuclear group, the midline thalamic nuclei (including the paraventricular thalamic nucleus [PV; Fig. 8B], the intermediodorsal nucleus, and the reuniens nucleus), the intralaminar nuclei (including the parafascicular nucleus [Fig. 3.17], the posterior intralaminar nucleus [PIL], the central medial nucleus, and the central lateral nucleus [Fig. 3.18]), the peripeduncular nucleus (PP), the parvicellular part of subparafascicular thalamic nucleus (SPFPC; Fig. 3.16), the mediodorsal nucleus, the ventral lateral nucleus, the ventral medial nucleus, and the lateral habenular nucleus (see Table 1). In general, PHA-L labeling in the thalamus is almost entirely contralateral. In most thalamic structures, very few or a low density of fibers with a small number of boutons are observed.
A sparse to low density of labeling is observed in the SPFPC in all of the midthoracic cases and one of lumbosacral cases, and a medium to high density of labeling is seen in this structure in four cases with lumbosacral injections (Fig. 3.16). Thus, a higher density of labeling is observed in sections from animals with lumbosacral injections. In these four cases, long, varicosity-bearing, and terminal arborizations are seen throughout the entire extent of SPFPC. In the caudal aspect of SPFPC, the labeled fibers are observed coursing medially, above the region of medial lemniscus in thalamus, and to the rostral portion of SPFPC where it abuts the ventral part of the caudal pole of the ventral posterior thalamic nucleus, parvicellular part (VPPC). The labeling is focused in the caudal half of SPFPC. In most cases (four midthoracic cases and two lumbosacral cases), a sparse to low density of PHA-L labeling is observed lateral to SPFPC in the PIL and PP, but a medium to high density of labeled fibers is seen in two cases with a lumbosacral injection.
Hypothalamus.
Fibers are also localized in the hypothalamus, including the supramammillary nucleus, the posterior hypothalamic area (PH), the lateral hypothalamic area (LH; Fig. 3.16–3.19), the dorsal hypothalamic area, the zona incerta (ZI; Fig. 3.16 [left column]), the paraventricular hypothalamic nucleus, the supraoptic decussation (sox; Fig. 8C and Fig. 9A), and the medial and lateral preoptic areas (see Table 1). Generally, the labeling in the hypothalamus is bilateral with slight contralateral predominance in most cases, but two cases (one with a midthoracic and one with a lumbosacral injection) have a strong ipsilateral labeling. In addition, a higher density of labeled fibers is seen in the LH and PH than in any other areas in the hypothalamus.
Fig. 9.
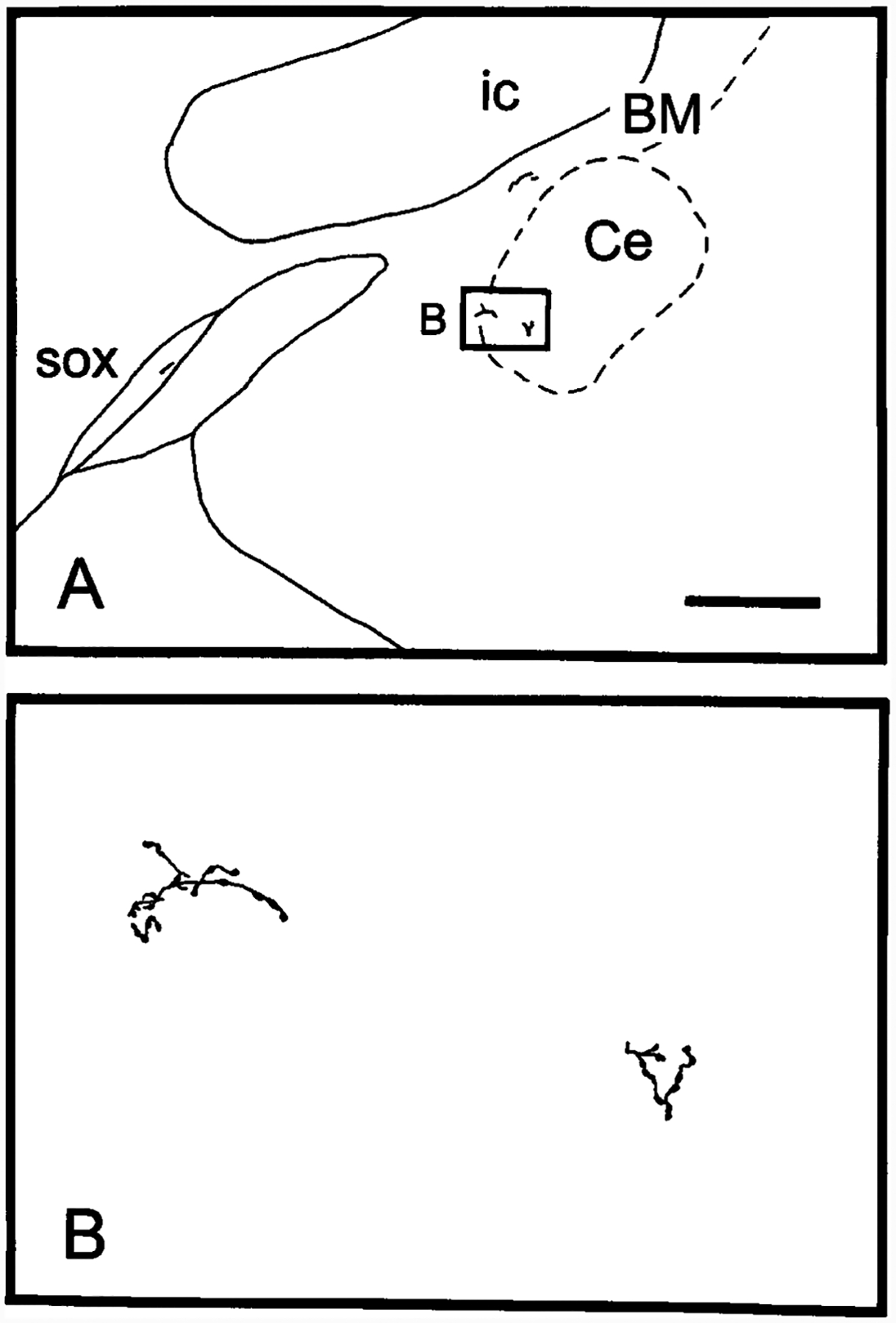
A: Camera lucida drawing illustrating PHA-L labeling in the central nucleus of the amygdala, the basal nucleus of Meynert, and the supraoptic decussation (experiment 107), contralateral to the injection site. B: Higher magnification of a camera lucida drawing illustrating PHA-L-labeled axons and varicosities in the central nucleus of amygdala. For abbreviations, see list. Scale bar = 500 μm for A; 50 μm for B.
The labeling in the ZI in most cases is sparse, except in one heavily labeled case each from a midthoracic injection (experiment 110) and from a lumbosacral injection (experiment 27). In the lumbosacral experiment 27, PHA-L-labeled fibers are primarily localized in the caudal pole of ZI. These fibers consist of many fine axons with long arborizations and terminals coursing ventromedially from lateral ZI, between the medial lemniscus and the substantia nigra, to medial ZI (Fig. 3.16).
Telencephalon.
Although the labeled fibers in the telencephalon are very scattered, PHA-L-labeled fibers are observed in the globus pallidus, the central nucleus of the amygdala (Fig. 9), the substantia innominata, and the basal nucleus of Meynert (Fig. 9A; see Table 1). The labeling in the telencephalon is generally bilateral.
Interestingly, in an experiment with a lumbosacral injection (experiment 118), labeled fibers are seen coursing in the external capsule to terminate in expanded deep layers of the caudal granular insular cortex located just outside the external capsule (2.56 mm caudal to bregma; Fig. 10). Although a few boutons are seen in layer VI of the granular insular cortex, more are found in a small, discrete region of deeper layer expansion underlying the granular insular cortex that is not labeled in any atlas available to us. Terminal boutons are observed in this discrete region scattered among multipolar neurons. The cytoarchitecture of the region matches Cajal’s description for the claustrum (DeFelipe and Jones, 1988), which is located 1.8 mm more rostrally (at 1.4 mm caudal to bregma) in the rat, so this site may represent a caudal extension of the claustrum in the rat.
Fig. 10.
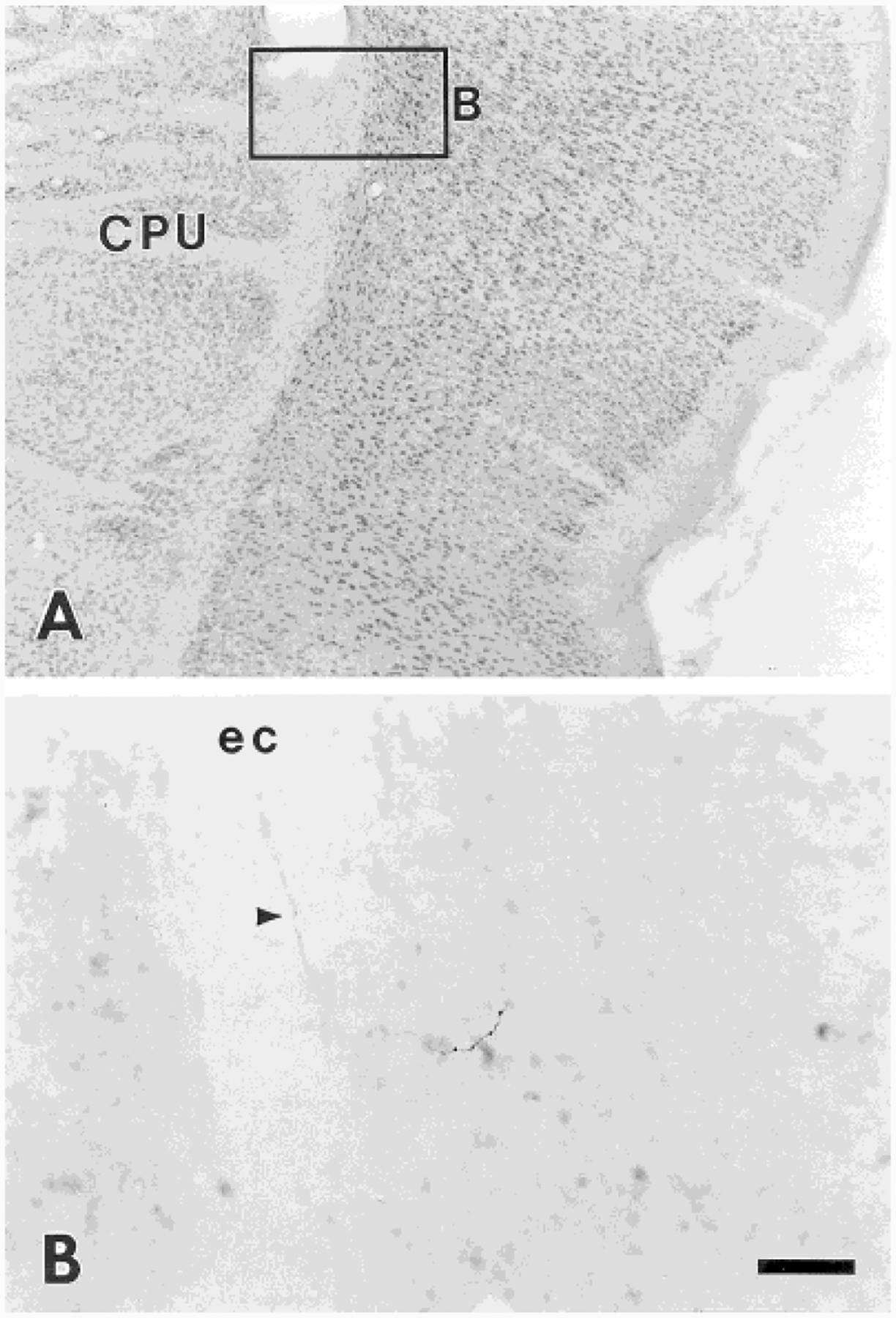
A: Nissl-stained section illustrating the location of labeled fibers from a coronal section shown in B from experiment 118. B: Labeled fiber (arrowhead) travels in the external capsule, and terminations are seen in deep layers of the granular insular cortex. For abbreviations, see list. Scale bar = 250 μm for A; 50 μm for B.
Cerebellum.
Terminal labeling in the cerebellum is present in all cases of midthoracic injections but in none of the cases with lumbosacral injections. The fibers in the cerebellum are distributed bilaterally. In the present study, the cerebellar labeling is not analyzed in detail because the labeled projections to this structure may be attributed to uptake by neurons of Clarke’s column.
Control injections.
In general, the pattern of ascending projections in control groups (Table 2) is quite different from the projections labeled by injections made in the CC area. In one control animal (experiment 125), the injection site is centered among the axons in the midline of the DC at the T8 level of the spinal cord. No cell bodies in the gray matter are labeled. Labeled fibers are seen exclusively traveling in the DC, and a low density of lightly labeled fibers with very few boutons is localized in the DCN. The quality of the stain within the fibers is different from that in the gray matter injections. In experiments 124 and 128, in which injection sites involved the medial portion of laminae III and IV, a very high density of labeled fibers is observed in the DCN, and a sparse to low density of fibers is seen in other regions of brainstem. Experiments 25 and 129, with injection sites in the medial zone of lamina V, show a low to medium density of fibers in the DCN and a sparse to low density of fibers in some structures in brainstem and diencephalon, and no fibers are found in the forebrain. A very high density of labeled fibers and terminals is observed in the DCN when the injection site is centered in laminae II–IV of the spinal cord dorsal horn (experiment 51). However, fewer fibers are seen in other brainstem structures. In experiments 31 and 32, in which the injection lies at the ventral border of laminae VII but is centered in the dorsomedial portion of lamina VIII (lateral to the ventral white commissure), no fibers are found in the DCN. Although a sparse to medium density of fibers is seen in some areas of the brain, most structures receiving a projection from the CC region have no labeled fibers.
TABLE 2.
Comparative Density of PHA-L-Labeled Fibers in Supraspinal Structures in Control Experiments1
| DC (Exp. No.) | Laminae I-V (Exp. No.) | Lamina VII (Exp. No.) | ||||||
|---|---|---|---|---|---|---|---|---|
| 125 | 124 | 128 | 25 | 51 | 129 | 32 | 31 | |
| Survival time (days) | 35 | 29 | 32 | 26 | 26 | 40 | 27 | 27 |
| Medulla | ||||||||
| Gr | + | +++ | +++ | + | ++++ | ++ | 0 | 0 |
| Cu | 0 | 0 | ++ | 0 | * | 0 | 0 | 0 |
| LRt | 0 | 0 | + | * | ++ | + | ++ | ++ |
| NTS | 0 | * | + | 0 | 0 | 0 | 0 | 0 |
| SRV | 0 | * | * | + | * | + | ++ | + |
| SRD | 0 | 0 | + | 0 | * | 0 | + | * |
| Sp5C | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 |
| LPGi | 0 | 0 | * | 0 | + | * | ++ | + |
| Gi | 0 | 0 | * | * | * | + | ++ | + |
| ROb | 0 | 0 | 0 | 0 | 0 | 0 | * | 0 |
| RMg | 0 | 0 | 0 | * | * | 0 | + | * |
| Pons | ||||||||
| PB | 0 | * | + | 0 | + | + | ++ | * |
| KF | 0 | 0 | 0 | 0 | + | + | + | 0 |
| SubC | 0 | 0 | 0 | * | + | + | ++ | * |
| Pn | 0 | 0 | * | * | 0 | * | + | + |
| LC | 0 | 0 | 0 | 0 | 0 | * | 0 | 0 |
| Bar | 0 | 0 | 0 | 0 | 0 | 0 | * | 0 |
| Mesencephalon | ||||||||
| PAG | 0 | * | * | 0 | + | + | ++ | * |
| CnF | 0 | * | * | 0 | 0 | * | + | 0 |
| DpMe | 0 | 0 | 0 | 0 | * | + | + | + |
| SC | 0 | 0 | 0 | 0 | * | + | * | 0 |
| lnC | 0 | 0 | 0 | 0 | 0 | * | * | 0 |
| SNR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| R | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 |
| APT | 0 | 0 | 0 | 0 | 0 | * | * | 0 |
| Thalamus | ||||||||
| PIL + PP | 0 | 0 | 0 | 0 | 0 | * | 0 | 0 |
| SPFPC | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 |
| LHb | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 |
| PV | 0 | 0 | 0 | 0 | + | + | + | 0 |
| PF | 0 | 0 | 0 | 0 | + | * | + | 0 |
| PrC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Po | 0 | 0 | 0 | 0 | 0 | 0 | + | * |
| VPPC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| VL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AVVL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| VM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Re | 0 | 0 | 0 | 0 | 0 | * | 0 | 0 |
| CL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| LD | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 |
| MD | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CM | 0 | 0 | 0 | * | 0 | + | 0 | 0 |
| Hypothalamus | ||||||||
| LH | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 |
| ZI | 0 | 0 | 0 | 0 | 0 | + | * | 0 |
| Subl | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SUM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DA | 0 | 0 | 0 | 0 | 0 | + | + | 0 |
| PVH | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PH | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 |
| MPA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| LPO | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AH | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| sox | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 |
| Telencephalon | ||||||||
| Ce | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CPU | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SI | 0 | 0 | 0 | 0 | 0 | 0 | * | 0 |
| BM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
++++, Very high density; +++, high density; ++, medium density; +, low density; *, sparse density; 0, no fibers found. For abbreviations, see list.
DISCUSSION
The present study provides evidence that the neurons in the CC area project to a number of specific sites in the brain, sites thought to be involved in nociceptive transmission, sensorimotor integration, autonomic regulation, motor control, emotional expression, and limbic activation. In particular, characterizations of the ascending projections and terminations of PSDC neurons in the area close to the central canal at midthoracic or lumbosacral levels are reported here.
Technical considerations
PHA-L is a reliable tract tracing method when iontophoretically applied and produces the highly restricted injection sites required for accurate mapping of projections of small neuronal populations. Previous investigators (Lee et al., 1988; Shu and Peterson, 1988) have reported that PHA-L can be transported retrogradely. However, retrograde transport of PHA-L was not evident in the present study; no PHA-L-labeled cell bodies were found anywhere in the brain. In addition, because no labeled cell bodies were found away from the injection sites, transsynaptic labeling is unlikely with PHA-L in this study. These observations are consistent with previous PHA-L studies (Cliffer and Giesler, 1989; Cliffer et al., 1991; Craig, 1991b).
Owing to the movements of the thoracic spinal cord caused by breathing, in some cases, injection sites included not only the CC area but also the midline of the DC. Previous investigators have reported that PHA-L is not effectively taken up and transported by fibers of passage (Gerfen and Sawchenko, 1984; Grove et al., 1986). However, anterograde labeling through axons of passage has been previously reported in the rat by others (Cliffer and Giesler, 1988; Rhoades et al., 1989; Schofield, 1990). The transport of PHA-L by the axons of the DC is not an important problem in this study insofar as control injections made into the DC show that 1) only lightly labeled fibers with very few boutons were found in the DCN after PHA-L injections directly into the DC (experiments 124, 125, and 128), 2) the quality of labeling from fibers of passage in the DC clearly differs from labeling arising from neurons in the CC area, 3) the DC has been shown to be somatotopically organized (Giesler et al., 1984; Cliffer and Giesler, 1989). The locations of terminations from axons in the DC, many of which arise from lower spinal levels, are quite different from those arising from neurons in the CC area in the thoracic cord. Thus, labeling through axons of passage in the DC is minor and is clearly distinguishable from that resulting from uptake by neurons in the CC area. Furthermore, to avoid the dorsal spinal vessels, the lumbosacral injections were angled into the CC area through gray matter, avoiding the DC altogether. A retrograde tracing study (Christensen et al., 1996) has confirmed the existence of a spinal projection from neurons in the CC area to the DCN. Moreover, data from the DC control injections are in agreement with previous anterograde transport studies (Cliffer and Giesler, 1988; see also discussion in Cliffer and Giesler, 1989).
Previously, an anterograde tracing study (Craig, 1995) has shown that projections and terminations from lamina I neurons are observed 1) along the entire rostrocaudal extent of the ventrolateral medulla (VLM), especially between the trigeminal nucleus caudalis and the lateral reticular nucleus (LRt) in the caudal VLM and 2) labeling in the thalamus was almost entirely contralateral. In this study, labeling in the brain could potentially have arisen from uptake by ascending fibers from STT neurons crossing in the spinal gray commissure. However, this possibility also appears unlikely insofar as very few labeled fibers and terminals are found in the VLM and the projection to the thalamus from neurons in the CC area is almost completely contralateral in the present study. Likewise, no labeled cells were seen in the dorsal horn away from the injection site. If crossing axons had been labeled, the projection to thalamus should have been bilateral. Labeling in the DCN might also have been from collateral branches of primary afferent fibers in the spinal gray matter. This is also unlikely insofar as no labeled axons or cell bodies were seen in the dorsal root ganglia.
Neurons in the spinal gray matter other than in the CC area or fibers of passage in the DC might have been labeled when electrodes were inserted into the CC area. This possibility is also unlikely; PHA-L has been shown to be transported preferentially in the anterograde direction when introduced iontophoretically (Gerfen and Sawchenko, 1984).
Anatomical considerations
The present study demonstrates that neurons in the CC area project to many specific areas in higher brain centers through the dorsal and ventrolateral funiculi of the spinal cord. The projection sites include a number of specific areas, which are believed to be involved in integration of sensory information, homeostatic regulation, motor and emotional control, and limbic activation. Many of these regions have reciprocal projections back to the spinal cord.
The area around the CC.
Anatomical studies have shown primary afferent fibers from the major splanchnic nerve (Cervero and Connell, 1984; Kuo and de Groat, 1985; Neuhuber et al., 1986), the intercostal nerve (Cervero and Connell, 1984; Neuhuber et al., 1986), the inferior cardiac nerve (Kuo et al., 1984), the left renal nerve (Kuo et al., 1983), the pelvic nerve (Morgan et al., 1981; Nadelhaft et al., 1983), and the hypogastric nerve (Neuhuber, 1982; Morgan et al., 1986) terminating near this area. Morphologically, a heterogeneous population of neurons has been found in this area (Nahin et al., 1983; Honda and Perl, 1985). For the rat, using a retrograde tract tracing method, Nahin et al. (1983) reported that fusiform, pyramidal, and stellate types of neurons were identified in the area surrounding the spinal cord central canal. However, no evident correlations between functional categories and morphological features were found (Honda and Perl, 1985). In addition, the CC area has been shown to receive inputs from neurons of the rostral medulla associated with descending modulation of nociception (Martin et al., 1981; Holstege and Kuypers, 1982; Light, 1985). A large body of immunohistochemical studies has demonstrated that several compounds, for example, serotonin (Steinbusch, 1981), substance P (Gibson et al., 1981; Sasek et al., 1984; Leah et al., 1988), enkephalin (Hökfelt et al., 1977; Gibson et al., 1981; Leah et al., 1988; Nahin, 1988), dynorphin (Sasek et al., 1984; Leah et al., 1988; Nahin, 1988), cholecystokinin (Gibson et al., 1981; Sasek et al., 1984; Leah et al., 1988), neuropeptide Y (Leah et al., 1988), bombesin (Leah et al., 1988), somatostatin (Leah et al., 1988), and vasoactive intestinal polypeptide (Gibson et al., 1981; Sasek et al., 1984; Nahin, 1988), are localized in the area around the CC. Many of these substances contribute to neural transmission or descending modulation of nociceptive information.
Although the ascending projections from the lateral portion of lamina VII were not observed in this study, according to previous anatomical studies, there is a parallel ascending projection from the lateral aspect of lamina VII. Autonomic preganglionic nuclei processing sensory information and regulating autonomic function send axons peripherally providing autonomic control, but cells in these regions have also been shown to project to supraspinal levels such as PAG and hypothalamus (Burstein et al., 1990a,b; Vanderhorst et al., 1996). Comparisons suggest that there are fewer projection neurons localized in the lateral aspect of lamina VII of the spinal cord than in the area near the CC (Kevetter et al., 1982; Menétrey et al., 1982; Burstein et al., 1990a). In addition, only a sparse density of PHA-L-labeled fibers and terminals from the lateral portions of lamina VII is observed in the PAG, PB, and KF (Bernard et al., 1995).
Projections through the dorsal funiculus.
The results of the current study demonstrate that axons originating from PSDC neurons in the CC area travel ipsilaterally in the DC and project heavily to the DCN. These findings confirm previous anatomical (Hirshberg et al., 1996; Westlund et al., 1996) and electrophysiological (Al-Chaer et al., 1996b) studies demonstrating that there are a large number of PSDC neurons in the area adjacent to the CC that relay visceral nociceptive information to the DCN. These findings contrast with those of Giesler et al. (1984), who demonstrated fewer PSDC neurons in the area around the CC in the cervical and lumbar enlargements of the spinal cord following injections of HRP into the DCN. In the present study, however, tracer was injected at levels of the spinal cord heavily innervated by visceral afferents. To label this population of PSDC neurons better, other retrograde tracers, such as Fluoro-Gold, have been reported to be more sensitive than HRP (Burstein et al., 1990b). Results obtained in our laboratories (Christensen et al., 1996) have confirmed using Fluoro-Gold that large numbers of PSDC neurons are localized in the area around the CC at lumbosacral levels of the spinal cord.
As was mentioned above, fibers originating from neurons in the CC area at the midthoracic and lumbosacral levels of the spinal cord travel in the DC near the dorsal intermediate septum and midline, respectively. These findings indicate that ascending projections from PSDC neurons near the CC are somatoviscerotopically organized at the spinal cord level. The results are similar to those previously described for PSDC neurons in the spinal cord dorsal horn (Giesler et al., 1984; Cliffer and Giesler, 1989).
With regard to the terminals in the DCN, in some cases the results from this study show that projections from midthoracic segments of the spinal cord terminate ipsilaterally not only in the ventral aspect of Gr and the medial aspect of Cu but also in the dorsal aspect of Gr, whereas those from the lumbosacral level are localized in the dorsomedial and ventral aspects of Gr. Thus, together, projections from the CC area of the spinal cord form a shell around the Gr. These findings are consistent with those of an anterograde tracing study (Cliffer and Giesler, 1989) in which labeling was also seen in the ventral part of the Gr following injection of PHA-L into the dorsal horn of the cervical, thoracic, or lumbar cord.
Projections through the ventrolateral funiculi.
The present study demonstrates that neurons in the CC area project to many specific areas in the brainstem and forebrain through the ventrolateral funiculi of the spinal cord. Particularly, structures in the brainstem receive most of the projections and terminations from neurons in the CC area. The projections and terminations are observed primarily in the caudal portion of the NTS, the ventral and medial pontine and medullary reticular formation (including the RMg, Rob, LRt, LPGi, and Gi), the noradrenergic cell regions (including the LC, SubC, A5, and KF), the PB, the caudal ventrolateral and lateral portions of the PAG, and the Cnf. These projections and terminations are generally bilateral in the medulla; however, the PAG receives predominantly contralateral projections. Forebrain structures also receive minor projections from neurons in the CC area. The regions innervated include the thalamus, zona incerta (ZI), hypothalamus, granular insular cortex, basal nucleus of Meynert, central nucleus of the amygdala (Ce), globus pallidus, and substantia innominata.
Many of the findings of the present study are consistent with results of previous anterograde and retrograde studies. Earlier studies of silver-stained degeneration (Lund and Webster, 1967; Mehler, 1969; Zemlan et al., 1978) or anterograde tract tracing studies with the use of WGA-HRP (Wiberg and Blomqvist, 1984; Wiberg et al., 1987; Yezierski, 1988) or PHA-L (Cliffer et al., 1991; Slugg and Light, 1994; Bernard et al., 1995; Feil and Herbert, 1995; Newman et al., 1996) reported that these areas received spinal inputs. Neurons in the area surrounding the spinal cord CC have been retrogradely labeled from the NTS, medullary and pontine reticular formation, PB, LC, PAG, thalamus, hypothalamus, and other forebrain structures (Giesler et al., 1979; Kevetter et al., 1982; Kevetter and Willis, 1982, 1983; Chaouch et al., 1983; Liu, 1983; Menétrey et al., 1982, 1983, 1992; Nahin et al., 1983, 1986; Peschanski and Besson, 1984; Nahin and Miceyvich, 1986; Menétrey and Basbaum, 1987; Leah et al., 1988; Hylden et al., 1989; Burstein et al., 1990a,b; Aston-Jones et al., 1991; Menétrey and de Pommery, 1991; Yezierski and Mendez, 1991; Burstein and Potrebic, 1993; Kitamura et al., 1993). In terms of efferent projections, the CC region contains not only neurons with projections to higher brain centers but also preganglionic neurons (Petras and Cummings, 1972; Hancock and Peveto, 1979). Previous literature reporting spinal projections to SPFPC, ZI, or Me5 could not be found.
Comparisons to the projections from lamina I neurons.
The pattern of projections from neurons in the CC area is different from the findings of previous anterograde transport studies of lamina I neurons of the spinal cord (Craig, 1991a,b, 1995), particularly at spinal and medullary levels. For example, the present study demonstrated that axons originating from neurons in the CC area travel in the dorsal column and in the ventrolateral funiculus of spinal cord bilaterally. Fiber terminations are concentrated in the ventral and medial reticular formation, including RMg, ROb, LRt, LPGi, and Gi. By contrast, projections from lamina I neurons are concentrated in the middle of the contralateral lateral funiculus of the spinal cord and terminate heavily in the ventral lateral portion of medullary reticular formation between the trigeminal nucleus caudalis and the LRt. In addition, the labeling from lamina I neurons decreases in the ventrolateral medulla (VLM) at the level between the LRt and facial nucleus and then intensifies in the rostral VLM, near the caudal aspect of the facial nucleus (Craig, 1995). However, this tendency is not observed in this study.
It is noteworthy that the present study demonstrates innervation of the raphe nuclei in the rostral medulla. Raphe nuclei, including both the raphe magnus nucleus and raphe obscurus nucleus, receive direct afferent inputs from neurons in the CC area. Compared to the previous study describing lamina I projections (Craig, 1995), the present study demonstrates that a more prominent projection to the raphe nuclei and ventromedial reticular formation arises from neurons in the CC area relative to the sparse projection from lamina I neurons. The lamina I projection terminates most heavily in the ventrolateral reticular formation. Although the CC region is a key nociceptive and autonomic processing region, a significant direct spinal input to raphe nuclei has not been described previously (cf. Abols and Basbaum, 1981). Previously, it has been shown that the raphe nuclei receive projections primarily from supraspinal structures, such as the PAG, hypothalamus, and reticular formation (Holstege, 1987; Hermann et al., 1997).
The present study shows that the fiber projections to catecholamine cell regions from neurons in the CC area are generally bilateral. The labeling is concentrated in the caudal half of the LC and KF. These findings are similar to the projections from lamina I neurons (Craig, 1992, 1995; Westlund and Craig, 1996). However, the pattern of projections from lamina I neurons to the LC, SubC, and KF appears different from that from neurons in the CC area. 1) Axons originating from spinal lamina I neurons terminate in the LC and SubC bilaterally with a strong contralateral predominance. However, in the present study, a strong contralateral predominance is not observed. 2) Axons of lamina I neurons enter the LC from the ventrolateral or dorsolateral pontine tegmentum and the continuation of fibers from the LC terminate in the SubC. However, in the present study, the projections from neurons in the CC area continue rostrally from the RVM and terminate in the SubC regions. Labeled fibers in the SubC regions continue dorsally into the LC and the pericoerulear region from the medial side.
The labeled fibers in the PAG are primarily localized in the caudal portions of the ventral and ventrolateral PAG in the current study. The pattern of distribution of terminations originating from the neurons in the CC area is similar to that from lamina I neurons in the cat and monkey (Craig, 1995). Unlike the case with the observations by Craig (1995), in which a clear topographic organization existed in the lateral column of PAG of cats and monkeys, no topographic organization is observed in this area in the present study for the rat. However, the possibility of the presence of such a topographic organization in the projections from the neurons in the CC area could not be ruled out, because restricted injections were made in a relatively small population of experimental animals.
For the thalamus, the present study revealed labeled fibers in the parvicellular part of subparafascicular nucleus (SPFPC), the peripeduncular thalamic nucleus (PP), and the posterior intralaminar nucleus (PIL). Unlike the case with the termination from lamina I neurons (Craig, 1991b), no projections from neurons in the CC area to the thalamus were found in the ventrobasal complex and submedius nucleus of the thalamus.
Functional considerations
The CC ascending projections appear to provide afferent information from the spinal cord to many areas in the brain involved in processing and transmission of sensory information, autonomic and homeostatic regulation, and emotional control. In the following sections, the functional characteristics of neurons near the CC area and CC projections will be described.
Neurons in the area near the CC.
Neurons in the gray matter surrounding the CC receive inputs from cutaneous Aδ mechanical nociceptors and unmyelinated visceral afferent fibers that are activated by noxious stimuli (Light and Perl, 1979; Sugiura et al., 1989). Neurons in the area adjacent to the CC have been shown to respond to both visceral and somatic input (Honda, 1985; Honda and Perl, 1985; Ness and Gebhart, 1987, 1989; Berkley et al., 1993; Al-Chaer et al., 1996b). Neurons in this region may respond to innocuous stimuli, to noxious stimuli, and to both in rats and cats. In contrast, Nahin et al. (1983) reported that all of the neurons identified in this area responded exclusively to noxious stimuli. Al-Chaer et al. (1996b) demonstrated that a population of PSDC neurons located in and around the CC area projects to the DCN and that these PSDC neurons respond to innocuous and noxious cutaneous mechanical stimuli and to mechanical and chemical noxious visceral stimuli.
Integration of sensory information.
The results of the present study show that neurons in the CC area project to higher brain centers involved in processing and transmission of sensory information (including nociception). Importantly, this study provides conclusive anatomical evidence in support of previous electrophysiological studies by (Al-Chaer et al., 1996a,b), which demonstrated that a population of PSDC neurons localized near the central canal is excited by noxious colonic visceral stimulation. The visceral nociceptive information is conveyed by way of PSDC neurons in the medial area of the lumbosacral spinal cord level near the CC to the Gr, which in turn activates neurons in the VPL nucleus of thalamus. Transmission of pelvic visceral nociceptive information can be nearly eliminated by a midline lesion of the DC at the T10 level in rats (Al-Chaer et al., 1996a,b) or at the T8 or T10 level in humans (Hirshberg et al., 1996; Nauta et al., 1997). The conclusion of previous studies from our laboratories is that the DC pathway involving PSDC neurons is more important for pelvic visceral nociceptive transmission than are pathways in the ventrolateral funiculus of the spinal cord, such as the spinothalamic tract. In contrast, pathways in the ventrolateral funiculus are more important for transmitting somatic nociceptive information. In addition, DC lesions in the upper cervical spinal cord extending beyond the dorsal intermediate septum can reverse the home-cage behavior following the induction of pancreatitis in rats (Houghton et al., 1997a) and can block the behavioral responses and nociceptive transmission from duodenal distention (Feng et al., 1998) and chemical stimulation of pancreatic afferent fibers (Houghton et al., 1997b; Wang et al., 1998). Unlike the study by Al-Chaer et al. (1996b), which demonstrated neurons in the dorsomedial part of Gr that could be excited by colonic nociceptive stimuli, the present study shows that fibers from lumbosacral spinal cord project not only to the dorsomedial aspect but also to the ventral portion of the Gr. Therefore, these findings suggest that the visceral input to the DCN, from projection neurons in the CC area, may be involved in a variety of functions.
In previous electrophysiological studies, nociceptive responses have been recorded in the posterior thalamic group (for review see Albe-Fessard et al., 1985; Poggio and Mountcastle, 1960; Carstens and Yokota, 1980; Guilbaud et al., 1980; Peschanski et al., 1981), the intralaminar thalamic nuclei (Berkley et al., 1995), the medial thalamic nuclei (Dostrovsky and Guilbaud, 1990), the SPFPC (Dong et al., 1978), and the lateral habenular nucleus (Benabid and Jeaugey, 1989). Most of the neurons in the intralaminar nuclei respond exclusively to noxious somatic and visceral stimuli (Peschanski et al., 1981; Berkley et al., 1995). In addition, neurons in the intralaminar thalamic nuclei respond exclusively to noxious, but not to weak, mechanical stimuli (Perl, 1984). For conscious human subjects, it has been reported that electrical stimulation of the intralaminar thalamic nuclei causes feelings of burning pain (Perl, 1984; Lenz and Dougherty, 1997).
Previous investigators (for references see Burstein et al., 1990a) have shown that neurons in the hypothalamus respond to a variety of somatosensory (including mechanical, thermal, and visceral) stimuli. Neurons in the dorsal horn of the lumbosacral spinal cord have been shown to respond to both noxious somatic and visceral stimuli (Burstein et al., 1991; Katter et al., 1996b). However, the SHT neurons near the CC have not been well characterized.
Projections from the CC area to the LRt, rostral ventral and medial reticular formation (including the RMg, Rob, and Gi), A5 region, LC, KF, and PAG are also observed in the present study. Previous studies have shown that electrical or chemical stimulation of these areas can produce antinociceptive effects (Willis et al., 1977; Basbaum and Fields, 1978; Haber et al., 1980; Tattersall et al., 1986b; Hodge et al., 1986; Yaksh, 1986; Janss et al., 1987; Jones and Gebhart, 1988; Zhao and Duggan 1988; Burnett and Gebhart, 1991; Miller and Proudfit, 1991; Proudfit, 1992; Yeomans et al., 1992; Yeomans and Proudfit, 1992) through known descending projections (Westlund and Coulter, 1980; Kuypers and Martin, 1982).
Autonomic regulation.
This study provides evidence that projections from neurons in the CC area are localized in areas such as NTS, LRt, A5 region, LC, SubC, KF, PB, PAG, ZI, hypothalamus, Ce, and GI, which are responsible for autonomic (cardiovascular, respiratory, micturition, and sexual) regulation. These areas involving autonomic regulation have been discussed in other comprehensive articles (Fulwiler and Saper, 1984; Cechetto and Saper, 1990; Loewy and Spyer, 1990; Depaulis and Bandler, 1991; Proudfit, 1992; Craig, 1995). Neurons in these areas have been shown to respond to somatosensory (including noxious) stimuli (Kruger and Albe-Fessard, 1960; Eickhoff et al., 1978; Rose, 1979; Gelsema et al., 1989; Bernard and Besson, 1990; Bernard et al., 1994; Hubscher and Berkley, 1994; Bester et al., 1995; Willis et al., 1997). Fos protein can be induced in the NTS, LC, SubC, KF, lateral PB, PAG, and hypothalamus following noxious cutaneous (Bullitt, 1990; Keay et al., 1994; Buritova et al., 1998) or visceral chemical (Hammond et al., 1992; Bon et al., 1996, 1997) or mechanical stimuli (Lantéri-Minet et al., 1993; Traub et al., 1996a,b). In addition, electrical or chemical stimulation in the medullary reticular formation, NTS, A5 region, LC, PB, PAG, ZI, Ce, and hypothalamus produces changes in cardiovascular reflexes (Loewy et al., 1979a, 1986; Cox et al., 1987; Iwata et al., 1987; Lovick, 1985; Sved and Felsten, 1987; Randich et al., 1988; Spencer et al., 1988, 1989; Bonham and Jeske, 1989; Chamberlin and Saper, 1992) and respiration (Feldman and Ellenberger, 1988; Motekaitis et al., 1994). Furthermore, Strack et al. (1989) demonstrated that neurons in the A5 region, ventromedial medulla, caudal raphe nuclei, and PVH send descending projections to innervate the sympathetic preganglionic neurons in the intermediolateral cell column (IML) involved in cardiovascular control. Therefore, cardiovascular and respiratory response patterns might be influenced or elicited by somatic and visceral input transmitted through ascending projections from neurons in the CC area of the thoracic spinal cord.
The brainstem projections from neurons near the CC area at lumbosacral levels of the spinal cord are likely related to autonomic regulation of pelvic organs. Brainstem control of eliminative and sexual functions has been reviewed (Morrison, 1987; de Groat and Steers, 1990). In the present study, it is noteworthy that Barrington’s nucleus, an area involved in micturition control, receives ascending projections only from neurons in the CC area at lumbosacral levels of the spinal cord. Anterograde tracing studies in the rat (Loewy et al., 1979b), cat (Holstege et al., 1979, 1986), and monkey (Westlund and Coulter, 1980) have shown that neurons in Barrington’s nucleus project directly to the sacral intermediolateral cell group, which contains the parasympathetic preganglionic neurons innervating the bladder. In addition, neurons in the CC area at lumbosacral levels project to the RMg, parapyramidal reticular formation, A5 and A7 region, LC, SubC, PAG, and hypothalamus. These findings are consistent with those from other retrograde transneuronal transport studies showing direct brainstem projections to parasympathetic preganglionic neurons in the sacral spinal cord. These transneuronal tracing studies with injections of pseudorabies virus into the rat urinary bladder and urethra and into the rat penis and clitoris have shown that these areas are involved in micturition reflexes and sexual functions (Nadelhaft et al., 1992; Marson et al., 1993; Marson, 1995; Vizzard et al., 1995).
Motor control and emotional expression.
The results of the present study also demonstrate projections from neurons in the CC area to areas involved in motor control, such as the LRt, R, and basal ganglia. The somatotopic relationships detailed previously in the literature for the LRt were evident in these studies (for review see Ruigrok and Cella, 1995). The LRt, R, and basal ganglia have been suggested to play a role in processing of sensory information related to motor functions (for reviews see Lidsky et al., 1985; Ruigrok and Cella, 1995). Therefore, the direct spinal projections to the basal ganglia, demonstrated in the present study, may provide a route for sensory input, including nociceptive information, to this region for modulation of motor functions.
Surprisingly, labeled fibers are seen forming baskets of terminations encasing cells in the mesencephalic trigeminal nucleus (Me5), the cell bodies of proprioceptors involved in reflexive jaw movements (Linden, 1978). The data presented here showing projections from neurons around the central canal area to the Me5 suggest that this direct projection may provide input from the spinal cord for jaw tensing, teeth baring, or exclamatory jaw opening.
Limbic activation.
The present study determined that the area around the CC sends direct projections to limbic structures, such as Ce, SI, and BM. The amygdala, particularly the Ce, is involved in the affective-emotional and behavioral responses to noxious events (Burstein and Potrebic, 1993). Previous studies have shown involvement of Ce in fear and emotional memory and behavior (for references see Bernard et al., 1996). Activity of neurons in the Ce can be recorded after noxious stimuli are applied (Bernard et al., 1990, 1992). Colorectal distention results in an increase in expression of Fos protein in this structure (Traub et al., 1996b). Decreased regional cerebral blood flow is observed in the amygdala region after noxious stimuli are applied in healthy humans (Derbyshire et al., 1997). Thus, in combining the results of the current study with those of previous studies, it appears that the Ce plays an important role in affective-emotional and behavioral responses to noxious events.
CONCLUSIONS
The results of the present study clearly show that the ascending pathways originating from the neurons around the central canal project to a variety of specific structures involved in integration of sensory information, homeostatic regulation, motor control, emotional expression, and limbic activation. These findings are consistent with the hypothesis that projections of neurons around the central canal play a role in relaying sensory information, particularly visceral nociception, from the internal and external environment to the central nervous system. Regions innervated include sensory, emotional, motor, and autonomic integration sites as well as limbic regions. In particular, the new ascending pathway transmitting visceral nociceptive information via PSDC neurons in the CC area through the DC is clearly evident in this study. Thus, the present findings are strongly in support of recent clinical studies (Hirshberg et al., 1996; Nauta et al., 1997) and experimental studies (Al-Chaer et al., 1996b; Houghton et al., 1997; Feng et al., 1998) that have postulated the existence of such a pathway.
ACKNOWLEDGMENTS
The authors thank G. Gonzales for assistance with the prints and K. Sawyer for assistance with the manuscript. This work was supported by NIH grants NS11255 to W.D.W. and NS32778 to K.N.W.
Abbreviations
- 3V
third ventricle
- 4V
fourth ventricle
- 7
facial nucleus
- 7n
facial nerve
- AH
anterior hypothalamic area
- AP
area postrema
- APT
anterior pretectal nucleus
- Aq
cerebral aqueduct
- AVVL
anterolateral thalamic nucleus, ventrolateral part
- BM
basal nucleus of Meynert
- Bar
Barrington’s nucleus
- CC
area around the spinal cord central canal
- Ce
central nucleus of amygdala
- CL
centrolateral thalamic nucleus
- CM
central medial thalamic nucleus
- CnF
cuneiform nucleus
- cp
cerebral peduncle
- CPU
caudate putamen
- Cu
cuneate nucleus
- DA
dorsal hypothalamic area
- DC
dorsal column
- DCN
dorsal column nucleus
- DpMe
deep mesencephalic nucleus
- ec
external capsule
- fr
fasciculus retroflexus
- GI
granular insular cortex
- Gi
gigantocullular reticular nucleus
- GP
globus pallidus
- Gr
gracile nucleus
- Hb
habenular complex
- Hypo
hypothalamus
- icp
inferior cerebellar peduncle
- InC
intercollicular nucleus
- IO
inferior olivary nucleus
- KF
Kölliker-Fuse nucleus
- LC
locus coeruleus
- LD
laterodorsal thalamic nucleus
- LH
lateral hypothalamic area
- LHb
lateral habenular nucleus
- LPGi
lateral paragigantocellular nucleus
- LPO
lateral preoptic area
- LRt
lateral reticular nucleus
- LSO
lateral superior olivary nucleus
- MD
mediodorsal thalamic nucleus
- ml
medial lemniscus
- Mo5
motor trigeminal nucleus
- MPA
medial preoptic area
- NTS
nucleus of solitary tract
- PAG
periaqueductal gray
- PB
parabrachial nucleus
- PBcl
central lateral parabrachial subnucleus
- PBdl
dorsal lateral parabrachial subnucleus
- PBel
external lateral parabrachial subnucleus
- PBil
internal lateral parabrachial subnucleus
- PBm
medial parabrachial subnucleus
- PBsl
superior lateral parabrachial subnucleus
- PBvl
ventral lateral parabrachial subnucleus
- PF
parafascicular thalamic nucleus
- PH
posterior hypothalamic area
- PHA-L
Phaseolus vulgaris leucoagglutinin
- PIL
posterior intralaminar thalamic nucleus
- Pn
pontine reticular nucleus
- Po
posterior thalamic nuclear group
- PP
peripeduncular nucleus
- PR
prerubral field
- PrC
precommissural nucleus
- PSDC
postsynaptic dorsal column
- PV
paraventricular nucleus of thalamus
- PVH
paraventricular hypothalamic nucleus
- py
pyramidal tract
- R
red nucleus
- Re
reuniens thalamic nucleus
- RI
rostral interstitial nucleus of medial longitudinal fasciculus
- RMg
raphe magnus nucleus
- Rob
raphe obscurus nucleus
- RVM
rostral ventral medulla
- SC
superior colliculus
- scp
superior cerebellar peduncle
- SHT
spinohypothalamic tract
- SI
substantia innominata
- SMT
spinomesencephalic tract
- SNR
substantia nigra pars reticulata
- sox
supraoptic decussation
- Sp5
spinal trigeminal nucleus
- sp5
spinal trigeminal tract
- Sp5C
trigeminal nucleus caudalis
- SPFPC
subparafascicular thalamic nucleus, parvicellular part
- SRD
subnucleus reticularis dorsalis
- SRT
spinoreticular tract
- SRV
subnucleus reticularis ventralis
- SST
spinosolitary tract
- STT
spinothalamic tract
- SubC
subcoerulear region
- SUM
supramammillary nucleus
- TC
tuber cinereum
- VB
ventrobasal complex of thalamus
- VL
ventrolateral thalamic nucleus
- VLM
ventrolateral medulla
- VM
ventromedial thalamic nucleus
- VMH
ventromedial hypothalamic nucleus
- VPL
ventral posterolateral thalamic nucleus
- VPM
ventral posteromedial thalamic nucleus
- VPPC
ventral posterior thalamic nucleus, parvicellular part
- xscp
decussation of the superior cerebellar peduncle
- ZI
zona incerta
LITERATURE CITED
- Abols IA, Basbaum AI. 1981. Afferent connections of the rostral medulla of the cat: A neural substrate for midbrain-medullary interactions in the modulation of pain. J Comp Neurol 201:285–297. [DOI] [PubMed] [Google Scholar]
- Aidar O, Geohegan WA, Ungewitter LH. 1952. Splanchnic afferent pathways in the central nervous system. J Neurophysiol 15:131–138. [DOI] [PubMed] [Google Scholar]
- Albe-Fessard D, Berkley KJ, Kruger L, Ralston HJ III, Willis WD. 1985. Diencephalic mechanisms of pain sensation. Brain Res Rev 9:217–296. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. 1996a. Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J Neurophysiol 76:2661–2674. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. 1996b. Pelvic visceral input the nucleus gracilis is largely mediated by post-synaptic dorsal column pathway. J Neurophysiol 76:2675–2690. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Westlund KN, Willis WD. 1997a. Nucleus gracilis: an integrator for visceral and somatic information. J Neurophysiol 78:521–527. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Westlund KN, Willis WD. 1997b. Sensitization of postsynaptic dorsal column neuronal responses by colon inflammation. Neuroreport 8:3267–3273. [DOI] [PubMed] [Google Scholar]
- Amassian VE. 1951a. Cortical representation of visceral afferents. J Neurophysiol 14:433–444. [DOI] [PubMed] [Google Scholar]
- Amassian VE. 1951b. Fiber groups and spinal pathways of cortically represented visceral afferents. J Neurophysiol 14:445–460. [DOI] [PubMed] [Google Scholar]
- Amassian VE. 1952. Interaction in the somato-visceral projection system. Res Publ Assoc Res Nerv Ment Dis 30:371–402. [PubMed] [Google Scholar]
- Ammons WS. 1987. Characteristics of spinoreticular and spinothalamic neurons with renal inputs. J Neurophysiol 58:480–495. [DOI] [PubMed] [Google Scholar]
- Ammons WS. 1989. Primate spinothalamic cell responses to ureteral occlusion. Brain Res 496:124–130. [DOI] [PubMed] [Google Scholar]
- Ammons WS. 1990. Cardiopulmonary sympathetic afferent excitation of lower thoracic spinoreticular and spinothalamic neurons. J Neurophysiol 64:1907–1916. [DOI] [PubMed] [Google Scholar]
- Angaut-Petit D 1975a. The dorsal column system. I. Existence of long ascending postsynaptic fibres in the cat’s fasciculus gracilis. Exp Brain Res 22:457–470. [DOI] [PubMed] [Google Scholar]
- Angaut-Petit D 1975b. The dorsal column system. II. Functional properties and bulbar relay of the postsynaptic fibres of the cat’s fasciculus gracilis. Exp Brain Res 22:471–493. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B, Charléty P, Valentino RJ, Williams JT. 1991. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Progr Brain Res 88:47–75. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. 1978. Endogenous pain control mechanisms: Review and hypothesis. Ann Neurol 4:451–462. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Jeaugey L. 1989. Cells of the rat lateral habenula respond to high-threshold somatosensory inputs. Neurosci Lett 96:289–294. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Seltzer Z, Lu GW, Nishikawa N, Dubner R. 1983. The cells of origin of the dorsal column postsynaptic projection in the lumbosacral enlargements of cats and monkeys. Somatosens Res 1:131–149. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Hubscher CH. 1995. Are there separate central nervous system pathways for touch and pain? Nature Med 1:766–773. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Hubscher CH, Wall PD. 1993. Neuronal responses to stimulation of cervix, uterus, colon, and skin in the rat spinal cord. J Neurophysiol 69:545–556. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Benoist J-M, Gautron M, Guilbaud G. 1995. Responses of neurons in the caudal intralaminar thalamic complex of the rat to stimulation of the uterus, vagina, cervix, colon and skin. Brain Res 695:92–95. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Besson JM. 1990. The spino(trigemino)-pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol 63:473–490. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM. 1990. Effect of noxious somesthetic stimulation on the activity of neurons of the nucleus centralis of the amygdala. Brain Res 523:347–350. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM. 1992. The nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol 68:551–569. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM. 1994. The parabrachial area: electrophysiological evidence for an involvement in visceral nociceptive processes. J Neurophysiol 71:1646–1660. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Dallel R, Raboisson P, Villanueva L, Le Bars D. 1995. Organization of the efferent projections from the spinal cervical enlargement to the parabrachial area and periaqueductal gray: a PHA-L study in the rat. J Comp Neurol 353:480–505. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Bester H, Besson JM. 1996. Involvement of the spinoparabrachio-amygdaloid and -hypothalamic pathways in the autonomic and affective emotional aspects of pain. Progr Brain Res 107:243–255. [DOI] [PubMed] [Google Scholar]
- Bester H, Menendez L, Besson JM, Bernard JF. 1995. The spino(trigemino)-parabrachiohypothalamic pathway: Electrophysiological evidence for an involvement in pain processes. J Neurophysiol 73:568–585. [DOI] [PubMed] [Google Scholar]
- Bon K, Lantéri-Minet M, de Pommery J, Michiels JF, Menétrey D. 1996. Cyclophosphamide cystitis as a model of visceral pain in rats. A survey of hindbrain structures involved in visceroception and nociception using the expression of c-Fos and Krox-24 proteins. Exp Brain Res 108:404–416. [DOI] [PubMed] [Google Scholar]
- Bon K, Lantéri-Minet M, de Pommery J, Michiels JF, Menétrey D. 1997. Cyclophosphamide cystitis as a model of visceral pain in rats: minor effects at mesodiencephalic levels as revealed by the expression of c-Fos, with a note on Krox-24. Exp Brain Res 113:249–264. [DOI] [PubMed] [Google Scholar]
- Bonham AC, Jeske I. 1989. Cardiorespiratory effects of DL-homocysteic acid in caudal ventrolateral medulla. Am J Physiol 256:H688–H696. [DOI] [PubMed] [Google Scholar]
- Brown AG, Fyffe REW. 1981. Form and function of dorsal horn neurones with axons ascending the dorsal columns in cat. J Physiol (London) 321:31–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG, Brown PB, Fyffe REW, Pubols LM. 1983. Receptive field organization and response properties of spinal neurons with axons ascending the dorsal columns in the cat. J Physiol (London) 377:575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullitt E 1990. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol 296:517–530. [DOI] [PubMed] [Google Scholar]
- Buritova J, Besson JM, Bernard JF. 1998. Involvement of the spinoparabrachial pathway in inflammatory nociceptive processes: a c-Fos protein study in the awake rat. J Comp Neurol 397:10–28. [DOI] [PubMed] [Google Scholar]
- Burnett A, Gebhart GF. 1991. Characterization of descending modulation of nociception from the A5 cell group. Brain Res 546:271–281. [DOI] [PubMed] [Google Scholar]
- Burstein R, Giesler GJ. 1989. Retrograde labeling of neurons in spinal cord that project directly to nucleus accumbens or the septal nuclei in the rat. Brain Res 497:149–154. [DOI] [PubMed] [Google Scholar]
- Burstein R, Potrebic S. 1993. Retrograde labeling of neurons in the spinal cord that project directly to the amygdala or the orbital cortex in the rat. J Comp Neurol 335:469–485. [DOI] [PubMed] [Google Scholar]
- Burstein R, Cliffer KD, Giesler GJ. 1987. Direct somatosensory projections from the spinal cord to the hypothalamus and telencephalon. J Neurosci 7:4159–4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Cliffer KD, Giesler GJ Jr. 1990a. Cells of origin of the spinohypothalamic tract in the rat. J Comp Neurol 291:329–344. [DOI] [PubMed] [Google Scholar]
- Burstein R, Dado RJ, Giesler GJ. 1990b. The cells of origin of the spinothalamic tract of the rat: a quantitative reexamination. Brain Res 511:329–337. [DOI] [PubMed] [Google Scholar]
- Burstein R, Dado RJ, Cliffer KD, Giesler GJ. 1991. Physiological characterization of spinohypothalamic tract neurons in the lumbar enlargement of rats. J Neurophysiol 66:261–284. [DOI] [PubMed] [Google Scholar]
- Carstens E, Yokota T. 1980. Viscerosomatic convergence and responses to intestinal distension of neurons at the junction of midbrain and posterior thalamus in the cat. Exp Neurol 70:392–402. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. 1990. Role of the cerebral cortex in autonomic function In: Loewy AD, Spyer KM, editors. Central regulation of autonomic functions. New York: Oxford University Press; p 208–223. [Google Scholar]
- Cechetto DF, Standaert DG, Saper CB. 1985. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol 240:153–160. [DOI] [PubMed] [Google Scholar]
- Cervero F 1983. Somatic and visceral inputs to the thoracic spinal cord of the cat: effects of noxious stimulation of the biliary system. J Physiol 337:51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F, Connell LA. 1984. Distribution of somatic and visceral primary afferent fibers within the thoracic spinal cord of the cat. J Comp Neurol 230:88–98. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. 1992. Topographic organization of cardiovascular responses to electrical and glutamate microstimulation of the parabrachial nucleus in the rat. J Comp Neurol 326:245–262. [DOI] [PubMed] [Google Scholar]
- Chaouch A, Menétrey D, Binder D, Besson JM. 1983. Neurons at the origin of the medial component of the bulbopontine spinoreticular tract in the rat: an anatomical study using horseradish peroxidase retrograde transport. J Comp Neurol 214:309–320. [DOI] [PubMed] [Google Scholar]
- Christensen MD, Willis WD, Westlund KN. 1996. Anatomical evidence for cells of origin of a postsynaptic dorsal column visceral pathway: sacral spinal cord cells innervating the medial nucleus gracilis. Soc Neurosci Abstr 22:109. [Google Scholar]
- Cliffer KD, Burstein R, Giesler GJ Jr. 1991. Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J Neurosci 11:852–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffer KD, Giesler GJ Jr. 1988. PHA-L can be transported anterogradely through fibers of passage. Brain Res 458:185–191. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Giesler GJ Jr. 1989. Postsynaptic dorsal column pathway of the rat. III. Distribution of ascending afferent fibers. J Neurosci 9:3146–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffer KD, Willis WD. 1994. Distribution of the postsynaptic dorsal column projection in the cuneate nucleus of monkeys. J Comp Neurol 345: 84–93. [DOI] [PubMed] [Google Scholar]
- Cox GE, Jordan D, Paton JFR, Spyer KM, Wood LM. 1987. Cardiovascular and phrenic nerve responses to stimulation of the amygdala central nucleus in the anaesthetized rabbit. J Physiol (London) 389:541–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. 1991a. Spinal distribution of ascending lamina I axons anterogradely labeled with Phaseolus vulgaris leucoagglutinin (PHA-L) in the cat. J Comp Neurol 313:377–393. [DOI] [PubMed] [Google Scholar]
- Craig AD. 1991b. Supraspinal pathways and mechanisms relevant to central pain In: Casey KL, editor. Pain and central nervous system disease: the central pain syndromes. New York: Raven Press; p 157–170. [Google Scholar]
- Craig AD. 1992. Spinal and trigeminal lamina I input to the locus coeruleus anterogradely labeled with Phaseolus vulgaris leucoagglutinin (PHA-L) in the cat and the monkey. Brain Res 584:325–328. [DOI] [PubMed] [Google Scholar]
- Craig AD. 1995. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol 361:225–248. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Jones EG. 1988. Cajal on the cerebral cortex: an annotated translation of the complete writings In: Corsi P, Jones EG, Shepherd GM, editors. History of neuroscience. No. 1 New York: Oxford University Press. [Google Scholar]
- De Groat WC, Steers WD. 1990. Autonomic regulation of the urinary bladder and sexual organs In: Loewy AD, Spyer KM, editors. Central regulation of autonomic functions. New York: Oxford University Press; p 310–333. [Google Scholar]
- Depaulis A, Bandler R. 1991. The midbrain periaqueductal gray matter. New York: Plenum Press. [Google Scholar]
- Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL. 1997. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain 73:431–445. [DOI] [PubMed] [Google Scholar]
- Dong WK, Ryu H, Wagman IH. 1978. Nociceptive responses of neurons in medial thalamus and their relationship to spinothalamic pathways. J Neurophysiol 41:1592–1613. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Guilbaud G. 1990. Nociceptive responses in medial thalamus of the normal and arthritic rat. Pain 40:93–104. [DOI] [PubMed] [Google Scholar]
- Eickhoff R, Handwerker HO, McQueen DS, Schick E. 1978. Noxious and tactile input to medial structures of midbrain and pons in the rat. Pain 5:99–113. [DOI] [PubMed] [Google Scholar]
- Feil K, Herbert H. 1995. Topographic organization of spinal and trigeminal somatosensory pathways to the rat parabrachial to Kölliker-Fuse nuclei. J Comp Neurol 353:506–528. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Ellenberger HH. 1988. Central coordination of respiratory and cardiovascular control in mammals. Annu Rev Physiol 50:593–606. [DOI] [PubMed] [Google Scholar]
- Feng Y, Cui M, Al-Chaer ED, Willis WD. 1998. Epigastric antinociception by cervical dorsal column lesion in rats. J Anesthesiol 89:411–420. [DOI] [PubMed] [Google Scholar]
- Foreman RD. 1989. Organization of the spinothalamic tract as a relay for cardiopulmonary sympathetic afferent fiber activity In: Progress in sensory physiology. Vol. 9 Heidelberg: Springer-Verlag; p 1–51. [Google Scholar]
- Foreman RD, Blair RW, Weber RN. 1984. Viscerosomatic convergence onto T2–T4 spinoreticular, spinoreticular-spinothalamic, and spinothalamic neurons in the cat. Exp Neurol 85:597–619. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. 1984. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res Rev 7:229–259. [DOI] [PubMed] [Google Scholar]
- Garrett L, Coggeshall RE, Patterson JT, Chung K. 1992. Numbers and proportions of unmyelinated axons at cervical levels in the fasciculus gracilis of monkey and cat. Anat Rec 232:301–304. [DOI] [PubMed] [Google Scholar]
- Gelsema AJ, Roe MJ, Calaresu FR. 1989. Neurally mediated cardiovascular responses to stimulation of cell bodies in the hypothalamus of the rat. Brain Res 482:67–77. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Sawchenko P. 1984. An anterograde neuroanatomical tracing method that shows the detail morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L). Brain Res 290:219–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SJ, Polak JM, Bloom SR, Wall PD. 1981. The distribution of nine peptides in rat spinal cord with special emphasis on the substantia gelatinosa and on the area around the central canal (lamina X). J Comp Neurol 201:65–79. [DOI] [PubMed] [Google Scholar]
- Giesler GJ Jr, Cliffer KD. 1985. Postsynaptic dorsal column pathway of the rat. II. Evidence against an important role in nociception. Brain Res 326:347–356. [DOI] [PubMed] [Google Scholar]
- Giesler GJ Jr, Menétrey D, Basbaum AI. 1979. Differential origins of spinothalamic tract projections to medial and lateral thalamus in the rat. J Comp Neurol 184:107–126. [DOI] [PubMed] [Google Scholar]
- Giesler GJ Jr, Spiel HR, Willis WD. 1981. Organization of spinothalamic tract axons within the rat spinal cord. J Comp Neurol 195:243–252. [DOI] [PubMed] [Google Scholar]
- Giesler GJ Jr, Nahin RL, Madsen AM. 1984. Postsynaptic dorsal column pathway of the rat. I. Anatomical studies. J Neurophysiol 51:260–275. [DOI] [PubMed] [Google Scholar]
- Granum SL. 1986. The spinothalamic system of the rat. I. Locations of cells of origin. J Comp Neurol 247:159–180. [DOI] [PubMed] [Google Scholar]
- Grove EA, Domesick VB, Nauta WHH. 1986. Light microscopic evidence of striatal input to intrapallidal neurons of cholinergic cell group Ch4 in the rat: A study employing the anterograde tracer Phaseolus vulgaris leucoagglutinin (PHA-L). Brain Res 367:379–384. [DOI] [PubMed] [Google Scholar]
- Guilbaud G, Peschanski M, Gautron M, Binder D. 1980. Neurones responding to noxious stimulation in VB complex and caudal adjacent regions in the thalamus of the rat. Pain 8:303–318. [DOI] [PubMed] [Google Scholar]
- Haber LH, Martin RF, Chung JM, Willis WD. 1980. Inhibition and excitation of primate spinothalamic tract neurons by stimulation in region of nucleus reticularis gigantocellularis. J Neurophysiol 43:1578–1593. [DOI] [PubMed] [Google Scholar]
- Haber LH, Moore BD, Willis WD. 1982. Electrophysiological response properties of spinoreticular neurons in the monkey. J Comp Neurol 207:75–84. [DOI] [PubMed] [Google Scholar]
- Hammond DL, Presley R, Gogas KR, Basbaum AI. 1992. Morphine or U-50,488 suppresses Fos protein-like immunoreactivity in the spinal cord and nucleus tractus solitarii evoked by a noxious visceral stimulus in the rat. J Comp Neurol 315:244–253. [DOI] [PubMed] [Google Scholar]
- Hancock MB, Peveto CA. 1979. A preganglionic autonomic nucleus in the dorsal gray commissure of the lumbar spinal cord of the rat. J Comp Neurol 183:75–84. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. 1997. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars? Demonstrated by iontophoretic application of choleratoxin (subunit b). J Chem Neuroanat 13:1–21. [DOI] [PubMed] [Google Scholar]
- Hirshberg RM, Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. 1996. Is there a pathway in the posterior funiculus that signals visceral pain? Pain 67:291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs SF, Chandler MJ, Bolser DC, Foreman RD. 1992. Segmental organization of visceral and somatic input onto C3–T6 spinothalamic tract cells of the monkey. J Neurophysiol 68:1575–1588. [DOI] [PubMed] [Google Scholar]
- Hodge CJ Jr, Apkarian AV, Stevens RT. 1986. Inhibition of dorsal-horn cell responses by stimulation of the Kölliker-Fuse nucleus. J Neurosurg 65:825–833. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Elde R, Johansson O, Terenius L, Stein L. 1977. The distribution of enkephalin-immunoreactive cell bodies in the rat central nervous system. Neurosci Lett 5:25–31. [DOI] [PubMed] [Google Scholar]
- Holstege G 1987. Some anatomical observations on the projections from the hypothalamus to brainstem and spinal cord: an HRP and autoradio-graphic tracing study in the cat. J Comp Neurol 260:98–126. [DOI] [PubMed] [Google Scholar]
- Holstege G, Kuypers HGJM. 1982. The anatomy of brain stem pathways to the spinal cord in the cat: a labeled amino-acid tracing study. Progr Brain Res 57:145–175. [DOI] [PubMed] [Google Scholar]
- Holstege G, Kuypers HGJM, Boer RC. 1979. Anatomical evidence for direct brain stem projections to the somatic motoneuronal cell groups and autonomic preganglionic cell groups in cat spinal cord. Brain Res 171:329–333. [DOI] [PubMed] [Google Scholar]
- Holstege G, Griffiths D, de Wall H, Dalm E. 1986. Anatomical and physiological observations on supraspinal control of bladder and urethral sphincter muscles in the cat. J Comp Neurol 250:449–461. [DOI] [PubMed] [Google Scholar]
- Honda CN. 1985. Visceral and somatic afferent convergence onto neurons near the central canal in the sacral spinal cord of the cat. J Neurophysiol 53:1059–1078. [DOI] [PubMed] [Google Scholar]
- Honda CN, Perl ER. 1985. Functional and morphological features of neurons in the midline region of the caudal spinal cord of the cat. Brain Res 340:285–295. [DOI] [PubMed] [Google Scholar]
- Houghton AK, Kadura S, Westlund KN. 1997a. Dorsal column lesions reverse the reduction of homecage activity in rats with pancreatitis. Neuroreport 8:3795–3800. [DOI] [PubMed] [Google Scholar]
- Houghton AK, Wang C-C, Kadura S, Willis WD, Westlund KN. 1997b. The effect of dorsal column lesion upon the central actions of pancreatic afferent fibers in the rat. Soc Neurosci Abstr 23:1000. [Google Scholar]
- Hubscher CH, Berkley KJ. 1994. Responses of neurons in caudal solitary nucleus of female rats to simulation of vagina, cervix, uterine horn and colon. Brain Res 664:1–8. [DOI] [PubMed] [Google Scholar]
- Hylden JLK, Anton F, Nahin RL. 1989. Spinal lamina I projection neurons in the rat: collateral innervation of parabrachial area and thalamus. Neuroscience 28:27–37. [DOI] [PubMed] [Google Scholar]
- Iwata J, Chida K, LeDoux JE. 1987. Cardiovascular responses elicited by stimulation of neurons in the central amygdaloid nucleus in awake but not anesthetized rats resemble conditioned emotional responses. Brain Res 418:183–188. [DOI] [PubMed] [Google Scholar]
- Janss AJ, Cox BF, Brody MJ, Gebhart GF. 1987. Dissociation of antinociceptive from cardiovascular effects of stimulation in the lateral reticular nucleus in the rat. Brain Res 405:140–149. [DOI] [PubMed] [Google Scholar]
- Jones SL, Gebhart GF. 1988. Inhibition of spinal nociceptive transmission from the midbrain, pons and medulla in the rat: activation of descending inhibition by morphine, glutamate and electrical stimulation. Brain Res 460:281–296. [DOI] [PubMed] [Google Scholar]
- Katter JT, Dado RJ, Kostarczyk E, Giesler GJ Jr. 1996a. Spinothalamic and spinohypothalamic tract neurons in the sacral spinal cord of rats. I. Locations of antidromically identified axons in the cervical cord and diencephalon. J Neurophysiol 75:2581–2605. [DOI] [PubMed] [Google Scholar]
- Katter JT, Dado RJ, Kostarczyk E, Giesler GJ Jr. 1996b. Spinothalamic and spinohypothalamic tract neurons in the sacral spinal cord of rats. II. Responses to cutaneous and visceral stimuli. J Neurophysiol 75:2606–2628. [DOI] [PubMed] [Google Scholar]
- Keay KA, Clement CI, Owler B, Depaulis A, Bandler R. 1994. Convergence of deep somatic and visceral nociceptive information onto a discrete ventrolateral midbrain periaqueductal gray region. Neuroscience 61: 727–732. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Willis WD. 1982. Spinothalamic cells in the rat lumbar cord with collaterals to the medullary reticular formation. Brain Res 238:181–185. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Willis WD. 1983. Collaterals of spinothalamic cells in the rat. J Comp Neurol 215:453–464. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Haber LH, Yezierski RP, Chung JM, Martin RF, Willis WD. 1982. Cells of origin of the spinoreticular tract in the monkey. J Comp Neurol 207:61–74. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Yamada J, Sato H, Yamashita K. 1993. Cells of origin of the spinoparabrachial fibers in the rat: a study with fast blue and WGA-HRP. J Comp Neurol 328:449–461. [DOI] [PubMed] [Google Scholar]
- Kruger L, Albe-Fessard D. 1960. Distribution of responses to somatic afferent stimuli in the diencephalon of the cat under chloralose anesthesia. Exp Neurol 2:442–467. [DOI] [PubMed] [Google Scholar]
- Kuo DC, De Groat WC. 1985. Primary afferent projections of the major splanchnic nerve to the spinal cord and gracile nucleus of the cat. J Comp Neurol 231:421–434. [DOI] [PubMed] [Google Scholar]
- Kuo DC, Nadelhaft I, Hisamitsu T, De Groat WC. 1983. Segmental distribution and central projections of renal afferent fibers in the cat studies by transganglionic transport of horseradish peroxidase. J Comp Neurol 216:162–174. [DOI] [PubMed] [Google Scholar]
- Kuo DC, Oravitz JJ, De Groat WC. 1984. Tracing of afferent and efferent pathways in the left inferior cardiac nerve of the cat using retrograde and transganglionic transport of horseradish peroxidase. Brain Res 321:111–118. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM, Martin GF. 1982. Descending pathways to the spinal cord Progr Brain Res Vol. 57 Amsterdam: Elsevier Biomedical Press. [Google Scholar]
- Lantéri-Minet M, Isnardon P, De Pommery J, Menétrey D. 1993. Spinal and hindbrain structures involved in visceroception and visceronociception as revealed by the expression of Fos, Jun and Knox-24 proteins. Neuroscience 55:737–753. [DOI] [PubMed] [Google Scholar]
- Leah J, Menétrey D, De Pommery J. 1988. Neuropeptides in long ascending spinal tract cells in the rat: evidence for parallel processing of ascending information. Neuroscience 24:195–207. [DOI] [PubMed] [Google Scholar]
- Lee CL, McFarland DJ, Wolpaw JR. 1988. Retrograde transport of the lectin Phaseolus vulgaris leucoagglutinin (PHA-L) by rat spinal motoneurons. Neurosci Lett 86:133–138. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Dougherty PM. 1997. Pain processing in the human thalamus In: Steriade M, Jones EG, McCormick DA, editors: Thalamus. Vol. 2. Experimental and clinical aspects Amsterdam: Elsevier; p 617–651. [Google Scholar]
- Lidsky TI, Manetto C, Schneider JS. 1985. A consideration of sensory factors involved in motor functions of the basal ganglia. Brain Res Rev 9:33–146. [DOI] [PubMed] [Google Scholar]
- Light AR. 1985. The spinal terminations of single, physiologically characterized axons originating in the pontomedullary raphe of the cat. J Comp Neurol 234:536–548. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. 1979. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibres. J Comp Neurol 186:133–150. [DOI] [PubMed] [Google Scholar]
- Linden RWA. 1978. Properties of intraoral mechanoreceptors represented in the mesencephalic nucleus of the fifth nerve in the cat. J Physiol 279:395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RP. 1983. Laminar origins of spinal projection neurons to the periaqueductal gray of the rat. Brain Res 264:118–122. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Spyer KM. 1990. Central regulation of autonomic functions. New York: Oxford University Press. [Google Scholar]
- Loewy AD, McKellar S, Saper CB. 1979a. Direct projections from the A5 catecholamine cell group to the intermediolateral cell column. Brain Res 174:309–314. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Saper CB, Baker RP. 1979b. Descending projections from the pontine micturition center. Brain Res 172:533–539. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Marson L, Parkinson D, Perry MA, Sawyer WB. 1986. Descending noradrenergic pathways involved in the A5 depressor response. Brain Res 386:313–324. [DOI] [PubMed] [Google Scholar]
- Lovick TA. 1985. Ventrolateral medullary lesions block the antinociceptive and cardiovascular responses elicited by stimulating the dorsal periaqueductal grey matter in rats. Pain 21:241–252. [DOI] [PubMed] [Google Scholar]
- Lund RD, Webster KE. 1967. Thalamic afferents from the spinal cord and trigeminal nuclei. J Comp Neurol 130:313–328. [DOI] [PubMed] [Google Scholar]
- Mantyh PW. 1982. The ascending input to the midbrain periaqueductal gray of the primate. J Comp Neurol 211:50–64. [DOI] [PubMed] [Google Scholar]
- Marson L 1995. Central nervous system neurons identified after injection of pseudorabies virus into the rat clitoris. Neurosci Lett 190:41–44. [DOI] [PubMed] [Google Scholar]
- Marson L, Platt KB, McKenna KE. 1993. Central nervous system innervation of the penis as revealed by the transneuronal transport of pseudorabies virus. Neuroscience 55:263–280. [DOI] [PubMed] [Google Scholar]
- Martin GF, Cabana T, Humbertson AO, Laxson LC, Panneton WM. 1981. Spinal projections from the medullary reticular formation of the North American opossum: Evidence for connectional heterogeneity. J Comp Neurol 196:663–682. [DOI] [PubMed] [Google Scholar]
- Mehler WR. 1969. Some neurological species differences: a posteriori. Ann NY Acad Sci 167:424–468. [Google Scholar]
- Menétrey D, Basbaum AI. 1987. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. J Comp Neurol 255:439–450. [DOI] [PubMed] [Google Scholar]
- Menétrey D, de Pommery J. 1991. Origins of spinal ascending pathways that reach central areas involved in visceroception and visceronociception in the rat. Eur J Neurosci 3:249–259. [DOI] [PubMed] [Google Scholar]
- Menétrey D, Chaouch A, Binder D, Besson JM. 1982. The origin of the spinomesencephalic tract in the rat: an anatomical study using the retrograde transport of horseradish peroxidase. J Comp Neurol 206:193–207. [DOI] [PubMed] [Google Scholar]
- Menétrey D, Roudier F, Besson JM. 1983. Spinal neurons reaching the lateral reticular nucleus as studied in the rat by retrograde transport of horseradish peroxidase. J Comp Neurol 220:439–452. [DOI] [PubMed] [Google Scholar]
- Menétrey D, de Pommery J, Thomasset M, Baimbridge KG. 1992. Calbindin-D28K (CaBP28k)-like immunoreactivity in ascending projections. II. Spinal projections to brain stem and mesencephalic areas. Eur J Neurosci 4:70–76. [DOI] [PubMed] [Google Scholar]
- Miller JF, Proudfit HK. 1990. Antagonism of stimulation-produced antinociception from ventrolateral pontine sites by intrathecal administration of α-adrenergic antagonists and naloxone. Brain Res 530:20–34. [DOI] [PubMed] [Google Scholar]
- Milne RJ, Foreman RD, Giesler GJ, Willis WD. 1981. Convergence of cutaneous and pelvic visceral nociceptive inputs onto primate spinothalamic neurons. Pain 11:163–183. [DOI] [PubMed] [Google Scholar]
- Morgan C, Nadelhaft I, De Groat WC. 1981. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol 201:415–440. [DOI] [PubMed] [Google Scholar]
- Morgan C, de Groat WC, Nadelhaft I. 1986. The spinal distribution of sympathetic preganglionic and visceral primary afferent neurons that send axons into the hypogastric nerves of the cat. J Comp Neurol 243:23–40. [DOI] [PubMed] [Google Scholar]
- Morrison JFB. 1987. Bladder control: role of higher levels of the central nervous system In: Torrens M, Morrison JFB, editors. Physiology of the lower urinary tract. New York: Springer. [Google Scholar]
- Motekaitis AM, Solomon IC, Kaufman MP. 1994. Stimulation of parabrachial nuclei dilates airways in cats. J Appl Physiol 76:1712–1718. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Roppolo J, Morgan C, De Groat WC. 1983. Parasympathetic preganglionic neurons and visceral primary afferents in monkey sacral spinal cord revealed following application of horseradish peroxidase to pelvic nerve. J Comp Neurol 216:36–52. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL, Card JP, Miselis RR. 1992. Central nervous system neurons labeled following the injection of pseudorabies virus into the rat urinary bladder. Neurosci Lett 143:271–274. [DOI] [PubMed] [Google Scholar]
- Nahin RL. 1988. Immunocytochemical identification of long ascending, peptidergic lumbar spinal neurons terminating in either the medial or lateral thalamus in the rat. Brain Res 443:345–349. [DOI] [PubMed] [Google Scholar]
- Nahin RL, Micevych PE. 1986. A long ascending pathway of enkephalin-like immunoreactive spinoreticular neurons in the rat. Neurosci Lett 65:271–276. [DOI] [PubMed] [Google Scholar]
- Nahin RL, Madsen AM, Giesler GJ. 1983. Anatomical and physiological studies of the gray matter surrounding the spinal cord central canal. J Comp Neurol 220:321–335. [DOI] [PubMed] [Google Scholar]
- Nahin RL, Madsen AM, Giesler GJ. 1986. Funicular location of the ascending axons of neurons adjacent to the spinal cord central canal in the rat. Brain Res 384:367–372. [DOI] [PubMed] [Google Scholar]
- Nauta HJW, Hewitt E, Westlund KN, Willis WD. 1997. Surgical interruption of a midline dorsal column visceral pain pathway. J Neurosurg 86:538–542. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. 1987. Characterization of neuronal responses to noxious visceral and somatic stimuli in the medial lumbosacral spinal cord of the rat. J Neurophysiol 57:1867–1892. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. 1989. Characterization of superficial T13–L2 dorsal horn neurons encoding for colorectal distension in the rat: comparison with neurons in deep laminae. Brain Res 486:301–309. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL. 1982. The central projections of visceral primary afferent neurons of the inferior mesenteric plexus and hypogastric nerve and the location of the related sensory and preganglionic sympathetic cell bodies in the rat. Anat Embryol 164:413–425 [DOI] [PubMed] [Google Scholar]
- Neuhuber WL, Sandoz PA, Fryscak T. 1986. The central projections of primary afferent neurons of greater splanchnic and intercostal nerves in the rat. a horseradish peroxidase study. Anat Embryol 174:123–44. [DOI] [PubMed] [Google Scholar]
- Newman HM, Stevens RT, Apkarian AV. 1996. Direct spinal projections to limbic and striatal areas: anterograde transport studies from the upper cervical spinal cord and the cervical enlargement in squirrel monkey and rat. J Comp Neurol 365:640–658. [DOI] [PubMed] [Google Scholar]
- Patterson JT, Coggeshall RE, Lee WT, Chung K. 1990. Long ascending unmyelinated primary afferent axons in the rat dorsal column: immunohistochemical localizations. Neurosci Lett 108:6–10. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 1986. The rat brain in stereotaxic coordinates, 2nd ed. New York: Academic Press. [Google Scholar]
- Perl ER. 1984. Pain and nociception In: Darian-Smith I, editor. Handbook of physiology. Section 1. The nervous system, Vol. III. Sensory Processes. Part 2 Bethesda, MD: American Physiological Society; p 915–975. [Google Scholar]
- Peschanski M, Besson JM. 1984. A spino-reticulo-thalamic pathway in the rat: an anatomical study with reference to pain transmission. Neuroscience 12:165–178. [DOI] [PubMed] [Google Scholar]
- Peschanski M, Guilbaud M, Gautron M. 1981. Posterior intralaminar region in the rat: neuronal responses to noxious and non-noxious cutaneous stimuli. Exp Neurol 72:226–238. [DOI] [PubMed] [Google Scholar]
- Petit D. 1972. Postsynaptic fibres in the dorsal columns and their relay in the nucleus gracilis. Brain Res 48:380–384. [DOI] [PubMed] [Google Scholar]
- Petras JM, Cummings JF. 1972. Autonomic neurons in the spinal cord of the rhesus monkey: a correlation of the findings of cytoarchitectonics and sympathectomy with fibre degeneration following dorsal rhizotomy. J Comp Neurol 146:189–218. [DOI] [PubMed] [Google Scholar]
- Poggio GF, Mountcastle VB. 1960. A study of the functional contributions of the lemniscal and spinothalamic systems to somatic sensibility. Bull Johns Hopkins Hosp 106:266–316. [PubMed] [Google Scholar]
- Proudfit HK. 1992. The behavioral pharmacology of the noradrenergic descending system In: Besson J-M, Guilbaud G, editors. Towards the use of noradrenergic agonists for the treatment of pain. New York: Elsevier; p 119–136. [Google Scholar]
- Randich A, Roose MG, Gebhart GF. 1988. Characterization of antinociception produced by glutamate microinjection in the nucleus tractus solitarius and the nucleus reticularis ventralis. J Neurosci 6:4675–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades RW, Fish SE, Chiaia NL, Bennett-Clarke C, Mooney RD. 1989. Organization of the projections from the trigeminal brainstem complex to the superior colliculus in the rat and hamster: anterograde tracing with Phaseolus vulgaris leucoagglutinin and intraaxonal injection. J Comp Neurol 289:641–656. [DOI] [PubMed] [Google Scholar]
- Rigamonti DD, Hancock MB. 1974. Analysis of field potentials elicited in the dorsal column nuclei by splanchnic nerve A-beta afferents. Brain Res 77:326–329. [DOI] [PubMed] [Google Scholar]
- Rigamonti DD, Hancock MB. 1978. Viscerosomatic convergence in the dorsal column nuclei. Exp Neurol 61:337–348. [DOI] [PubMed] [Google Scholar]
- Roppolo JR, Nadelhaft I, De Groat WC. 1985. The organization of pudendal motoneurons and primary afferent projections in the spinal cord of the rhesus monkey revealed by horseradish peroxidase. J Comp Neurol 234:475–488. [DOI] [PubMed] [Google Scholar]
- Rose JD. 1979. Anatomical distribution and sensory properties of brain stem and posterior diencephalic neurons responding to genital, somatosensory, and nociceptive stimulation in the squirrel monkey. Exp Neurol 66:169–185. [DOI] [PubMed] [Google Scholar]
- Rucker HK, Holloway JA, Keyser GF. 1984. Response characteristics of cat spinothalamic tract neurons to splanchnic nerve stimulation. Brain Res 291:383–387. [DOI] [PubMed] [Google Scholar]
- Ruigrok TJ, Cella F. 1995. Precerebellar nuclei and red nucleus In: Paxinos G, editor. The rat nervous system. San Diego: Academic Press; p 277–308. [Google Scholar]
- Rustioni A 1974. Non-primary afferents to the cuneate nucleus in the brachial dorsal funiculus of the cat. Brain Res 75:247–259. [DOI] [PubMed] [Google Scholar]
- Rustioni A 1976. Spinal neurons project to the dorsal column nuclei of rhesus monkeys. Science 196:656–658. [DOI] [PubMed] [Google Scholar]
- Rustioni A, Kaufman AB. 1977. Identification of cells or origin of non-primary afferents to the dorsal column nuclei of the cat. Exp Brain Res 27:1–14. [DOI] [PubMed] [Google Scholar]
- Sasek CA, Seybold VS, Elde RP. 1984. The immunohistochemical localization of nine peptides in the sacral parasympathetic nucleus and the dorsal gray commissure in rat spinal cord. Neuroscience 12:855–873. [DOI] [PubMed] [Google Scholar]
- Schofield BR. 1990. Uptake of Phaseolus vulgaris leucoagglutinin (PHA-L) by axons of passage. J Neurosci Methods 35:47–56. [DOI] [PubMed] [Google Scholar]
- Shu SY, Peterson GM. 1988. Anterograde and retrograde axonal transport of Phaseolus vulgaris leucoagglutinin (PHA-L) from the globus pallidus to the striatum of the rat. J Neurosci Methods 25:175–180. [DOI] [PubMed] [Google Scholar]
- Slugg RM, Light AR. 1994. Spinal cord and trigeminal projections to the pontine parabrachial region in the rat as demonstrated with Phaseolus vulgaris leucoagglutinin. J Comp Neurol 339:49–61. [DOI] [PubMed] [Google Scholar]
- Spencer SE, Sawyer WB, Loewy AD. 1988. L-Glutamate stimulation of the zona incerta in the rat decreases heart rate and blood pressure. Brain Res 458:72–81. [DOI] [PubMed] [Google Scholar]
- Spencer SE, Sawyer WB, Loewy AD. 1989. Cardiovascular effects produced by L-glutamate stimulation of the lateral hypothalamus area. Am J Physiol 257:H540–H552. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. 1981. Distribution of serotonin-immunoreactivity in the central nervous system of the rat. Cell bodies and terminals. Neuroscience 6:557–618. [DOI] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. 1989. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res 491:156–162. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Terui N, Hosoya Y. 1989. Difference in distribution of central terminals between visceral and somatic unmyelinated primary afferent fibers. J Neurophysiol 62:834–840. [DOI] [PubMed] [Google Scholar]
- Sved A, Felsten G. 1987. Stimulation of the locus coeruleus decreases arterial pressure. Brain Res 414:119–132. [DOI] [PubMed] [Google Scholar]
- Tattersall JEH, Cervero F, Lumb BM. 1986a. Effects of reversible spinalization on the visceral input to viscerosomatic neurons in the lower thoracic spinal cord of the cat. J Neurophysiol 56:785–796. [DOI] [PubMed] [Google Scholar]
- Tattersall JEH, Cervero F, Lumb BM. 1986b. Viscerosomatic neurons in the lower thoracic spinal cord of the cat: excitations and inhibitions evoked by splanchnic and somatic nerve volleys and by stimulation of brain stem nuclei. J Neurophysiol 56:1411–1423. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Sengupta JN, Gebhart GF. 1996a. Differential c-Fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distention in the rat. Neuroscience 74:873–884. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Silva E, Gebhart GF, Solodkin A. 1996b. Noxious colorectal distention induced-c-Fos protein in limbic brain structures in the rat. Neurosci Lett 215:165–168. [DOI] [PubMed] [Google Scholar]
- Uddenberg N 1966. Studies on modality segregation and second-order neurones in the dorsal funiculus. Experientia 22:441–442. [DOI] [PubMed] [Google Scholar]
- Uddenberg N 1968. Functional organization of long, second-order afferents in the dorsal funiculus. Exp Brain Res 4:377–382. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VGJM, Mouton LJ, Blok BFM, Holstege G 1996. Distinct cell groups in the lumbosacral cord of the cat project to different areas in the periaqueductal gray. J Comp Neurol 376:361–385. [DOI] [PubMed] [Google Scholar]
- Villanueva L, Bouhassira D, Bing D, Le Bars D. 1988. Convergence of heterotopic nociceptive information onto subnucleus reticularis dorsalis neurons in the rat medulla. J Neurophysiol 60:980–1009. [DOI] [PubMed] [Google Scholar]
- Villanueva L, de Pommery J, Menétrey D, Le Bars D. 1991. Spinal afferent projections to subnucleus reticularis dorsalis in the rat. Neurosci Lett 134:98–102. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Erickson VL, Card JP, Roppolo JR, de Groat WC. 1995. Transneuronal labeling of neurons in the adult rat brainstem and spinal cord after injection of pseudorabies virus into the urethra. J Comp Neurol 355:629–640. [DOI] [PubMed] [Google Scholar]
- Wang C-C, Lu Y, Willis WD, Westlund KN. 1997. A new visceral nociceptive pathway in the dorsal column: a PHA-L study of ascending projections from the area around the central canal of T7 or S1 spinal cord in rats. Soc Neurosci Abstr 23:2349. [Google Scholar]
- Wang C-C, Houghton AK, Westlund KN. 1998. Pancreatic nociceptive information is transmitted by the post-synaptic dorsal column pathway. Soc Neurosci Abstr 24:394. [Google Scholar]
- Westlund KN, Coulter JD. 1980. Descending projections of the locus coeruleus and subcoeruleus/medial parabrachial nuclei in monkey: axonal transport studies and dopamine β-hydroxylase immunocytochemistry. Brain Res 2:235–264. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Craig AD. 1996. Association of spinal lamina I projections with brainstem catecholamine neurons in the monkey. Exp Brain Res 110:151–162. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Hirshberg RM, Lawand NB, Al-Chaer ED, Willis WD. 1996. Anatomical evidence for a visceral pain pathway in the dorsal column (abstract). Int Assoc Stud Pain, 8 p 447. [Google Scholar]
- Wiberg M, Blomqvist A. 1984. The spinomesencephalic tract in the cat: its cells of origin and termination pattern as demonstrated by the intraaxonal transport of horseradish peroxidase. Brain Res 291:1–18. [DOI] [PubMed] [Google Scholar]
- Wiberg M, Westman J, Blomqvist A. 1987. Somatosensory projection to the mesencephalon: an anatomical study in the monkey. J Comp Neurol 264:92–117. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. 1991. Sensory mechanisms of the spinal cord. New York: Plenum Press. [Google Scholar]
- Willis WD, Westlund KN. 1997. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol 14:2–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD, Haber LH, Martin RF. 1977. Inhibition of spinothalamic tract cells and interneurons by brain stem stimulation in the monkey. J Neurophysiol 40:968–981. [DOI] [PubMed] [Google Scholar]
- Willis WD, Westlund KN, Al-Chaer ED. 1997. Spinal pathways for colorectal input into the solitary nucleus. Soc Neurosci Abstr 23:2350. [Google Scholar]
- Yaksh TL. 1986. The effects of intrathecally administered opioid and adrenergic agents on spinal function In: Yaksh TL, editor. Spinal afferent processing. New York: Plenum Press; p 505–540. [Google Scholar]
- Yeomans DC, Proudfit HK. 1992. Antinociception induced by microinjection of substance P into the A7 catecholamine cell group in the rat. Neuroscience 49:681–691. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Clark FM, Paice JA, Proudfit HK. 1992. Antinociception induced by electrical stimulation of spinally projecting noradrenergic neurons in the A7 catecholamine cell group of the rat. Pain 48:499–461. [DOI] [PubMed] [Google Scholar]
- Yezierski RP. 1988. Spinomesencephalic tract: projections from the lumbosacral spinal cord of the rat, cat, and monkey. J Comp Neurol 267:131–146. [DOI] [PubMed] [Google Scholar]
- Yezierski RP, Mendez CM. 1991. Spinal distribution and collateral projections of rat spinomesencephalic tract cells. Neuroscience 44:113–130. [DOI] [PubMed] [Google Scholar]
- Zemlan FP, Leonard CM, Kow L-M, Pfaff DW. 1978. Ascending tracts of the lateral columns of the rat spinal cord: a study using the silver impregnation and horseradish peroxidase techniques. Exp Neurol 62:298–334. [DOI] [PubMed] [Google Scholar]
- Zhao Z-Q, Duggan AW. 1988. Idazoxan blocks the action of noradrenaline but not spinal inhibition from electrical stimulation of the locus coeruleus and nucleus Kölliker-Fuse of the cat. Neuroscience 25:997–1005. [DOI] [PubMed] [Google Scholar]


