We used a novel approach to estimate the spatial contraction of sawfish populations and guide recovery efforts.
Abstract
Extinctions on land are often inferred from sparse sightings over time, but this technique is ill-suited for wide-ranging species. We develop a space-for-time approach to track the spatial contraction and drivers of decline of sawfishes. These iconic and endangered shark-like rays were once found in warm, coastal waters of 90 nations and are now presumed extinct in more than half (n = 46). Using dynamic geography theory, we predict that sawfishes are gone from at least nine additional nations. Overfishing and habitat loss have reduced spatial occupancy, leading to local extinctions in 55 of the 90 nations, which equates to 58.7% of their historical distribution. Retention bans and habitat protections are urgently necessary to secure a future for sawfishes and similar species.
INTRODUCTION
The ocean comprises 99.8% of the habitable volume of our planet, yet its resources are not inexhaustible (1). Around the world, the intensity, spatial reach, and technical capacity of fisheries have expanded enormously over the past half-century (2, 3). As a consequence, overfishing is unquestionably the primary threat to ocean biodiversity (4, 5). Even as the stock health of some managed and monitored fisheries improves (6), many exploited species go unmonitored, making it difficult to track the extinction of marine fish species (7–9). The statistical approaches used to infer extinctions are typically based on time series of sightings data, which are difficult to obtain for wide-ranging species, particularly marine fishes (10, 11). As a consequence, marine extinctions have been overlooked, as many marine populations have been exploited to the point of collapse long before monitoring began (9, 12, 13).
There is a rich body of theory describing how population abundance drives spatial patterns of site occupancy, whereby habitat occupancy and geographic range contract as numerical abundance declines (14–19). This dynamic geography theory posits that high-quality habitat is preferentially occupied first by individuals until, as density increases, they are forced to occupy poorer-quality habitats (20). Thus, a key insight of dynamic geography is that habitat quality can be effectively defined by the population growth rate r and can be derived from the abundance-occupancy relationship [Fig. 1A; (17, 18, 21)]. Specifically, the shape of the relationship may directly reflect the habitat quality through population-specific parameters [e.g., death rates (17)]. Consequently, increasing death rates by overfishing may drive local population growth rate below zero more quickly when habitat is lost; specifically, effective habitat quality is reduced per unit of available habitat [Fig. 1, A and B; (18)]. Thus, habitat loss can encompass the reduction in combined habitat availability and quality. This theory yields the prediction that the probability that populations are extinct is greater in locations with higher fishing pressure and lower ecological carrying capacity. Increasing fishing pressure has the ability to reduce the carrying capacity of a given population, leading to decreased levels of maximum population size [Fig. 1A; (22)]. Even in the absence of large-scale, long-term population and abundance time series, we can draw on dynamic geography theory to track and attribute causality of local marine extinctions using occurrence records, complemented by indices of ecological carrying capacity and fishing pressure.
Fig. 1. Linking dynamic geography to abundance-occupancy.
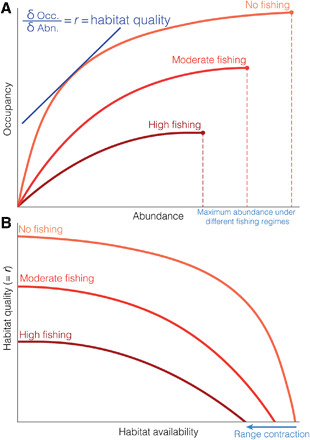
(A) Changes in abundance-occupancy with varying levels of fishing shown in different shades of red. Slope tangent to the line represents r, the population growth rate, which is synonymous with, and indeed a definition of, habitat quality. Increasing fishing pressure causes the occupancy curve to approach its asymptotic limit for a smaller given abundance compared to no fishing pressure. The maximum abundance of a given population shrinks under stronger fishing regimes, as shown with the point and the dashed line. (B) Curves derived from (A) showing changes in habitat quality (= r) with habitat availability with varying levels of fishing. When fishing pressure is high, the abundance-occupancy curve approaches its asymptotic limit (r = 0) at a lower given occupancy (A), resulting in a steeper decline in population growth rate and habitat quality for a given available habitat, resulting in a geographic range contraction.
The sawfishes (family Pristidae) are among the world’s most threatened families of marine fishes (23). The imperiled status of many populations was only recently recognized long after major declines and local extinctions had occurred (24, 25). Three of the five species are Critically Endangered according to the International Union for Conservation of Nature (IUCN) Red List of Threatened Species: Largetooth Sawfish (Pristis pristis), Smalltooth Sawfish (Pristis pectinata), and Green Sawfish (Pristis zijsron). The other two species—Dwarf Sawfish (Pristis clavata) and Narrow Sawfish (Anoxypristis cuspidata)—are Endangered (26). Sawfishes are highly vulnerable to population depletion: their tooth-studded rostra are easily entangled in nets, they live primarily nearshore in heavily exploited tropical and subtropical regions, and they have low reproductive outputs, yielding some of the largest ova in the animal kingdom (23, 27). Sawfish fins are among the most valuable in the global shark fin trade as a celebratory dish in some Asian cultures (23). Sawfish rostra are sold for curios and medicine, while rostral teeth are prized as spurs for cockfighting (28). In the absence of adequate fishing restrictions, intensely exploited populations collapsed rapidly in the early 20th century. Today, sawfishes remain among the world’s most valuable internationally traded wildlife, although most commercial international trade has been prohibited since 2007 under the Convention on International Trade in Endangered Species of Wild Fauna and Flora (4, 27). In places where they persist without enforced protections, sawfishes that are caught are often retained for consumption or sale, while others are killed in an effort to recover fishing gear or protect fishers (23, 25). Sawfishes are an exemplar of one of the fundamental challenges of tracking biodiversity change: discerning severe population declines from local extinction for a group that is scarce, relatively infrequently encountered, and for which there is little systematic monitoring [e.g., (29)].
We combine national-level sawfish occurrence surveys with ecological, socioeconomic, and political drivers in a space-for-time substitution framework (i.e., using spatial occupancy as a substitute for time series of abundances) to attribute causality of local extinctions. Here, we (i) report on the current status of sawfish occurrence, (ii) attribute causality with underlying mechanisms of local extinction using the listed threats from the IUCN Red List (fig. S1), and (iii) predict the probability of extinction in data-deficient nations (i.e., Presence Uncertain status) using 13 indices of ecological carrying capacity, fishing pressure, and management capacity (Table 1 and fig. S2). Note that the use of the term “extinct” or “near extinct” refers to the local extinction, or the increasing risk of extinction, of a population and does not reflect the IUCN Red List category of Extinct (26).
Table 1. Variables considered in the BRT model.
Only variables included in the model are shown.
| Variable | Description | Support |
| Indirect fishing pressures | ||
| ln coastal population | Number of people living in urban and rural areas within 100 km of the nation’s coastline as of 2011 |
Large coastal populations may drive unsustainable fishing practices (30) |
| ln marine protein consumption | Marine fish food supply, g capita−1 day−1 | The reliance on marine fish products for dietary protein and economic stability (42) |
| Direct fishing pressures | ||
| ln chondrichthyan landings | Total metric tons summed for all Aquatic Sciences and Fisheries Information System species of sharks, rays, and chimaeras. Total of 146 species-specific and 23 aggregate not elsewhere indicated categories |
Shark, ray, and chimaera products are of high economic value (59); thus, sawfishes that are caught opportunistically would have high economic return (28) |
| ln gear-specific marine fisheries landings | Total metric tons summed for catches with bottom trawls, gillnets, otter trawls, shrimp trawls, small-scale gillnets, small-scale longlines, small-scale trammel nets, and trammel nets |
Sawfishes have high catchability in specific fishing gears (53) |
| ln fishing effort | Fishing effort (kW) for artisanal and subsistence sectors from the EEZ of each nation |
Sawfishes have high catchability in small-scale, in-shore fisheries (53) |
| Management capacity | ||
| World Governance Index | On the basis of the control of corruption government effectiveness, political stability, rule of law, regulatory quality, and voice and accountability |
Governance is required for effective and sustainable fisheries management (60) |
| ln Gross Domestic Product | Measured in million USD | Nations with high income and high development status have better fisheries management than low-income countries (60, 61) |
| Human Development Index | Measures life expectancy, education, and Gross National Income |
Nations with high income and high development status have better fisheries management than low-income countries (60, 61) |
| Ecological carrying capacity | ||
| ln continental shelf area | Measured as the area found within the distribution maps for each species clipped to the maximum depth of each reported species (km2) |
Larger habitats can potentially support larger sawfish populations (62) |
| ln marine primary productivity | Chlorophyll a per nation, mg m−3 | Marine primary productivity linked to diversifying food webs in marine habitats by supporting higher trophic guild individuals (63) |
| ln estuarine discharge rate | Mean freshwater input, m3 s−1 | Sawfishes are highly associated with river and estuarine systems (25, 64) |
| ln mangrove area | Mean mangrove area (km2) | Sawfishes are highly associated with mangroves (53) |
| Sea surface temperature | Monthly means from 1981 to 2018 in °C | Sawfishes are associated with tropical waters with a lower lethal temperature between 8° and 12°C (64) |
RESULTS AND DISCUSSION
The 2014 publication of the IUCN Species Survival Commission Shark Specialist Group’s (SSC SSG) Global Sawfish Conservation Strategy helped reveal the plight of sawfishes to the world (23, 28). We reviewed sawfish research activity from personal correspondences, published, and gray literature and documented 251 activities from 64 nations from 2014 to 2019. These activities were the combination of ongoing research efforts and targeted sawfish searches to determine the current status of sawfish occurrence (Fig. 2A).
Fig. 2. The historical presence, extinction, and uncertainty of the presence of sawfishes.
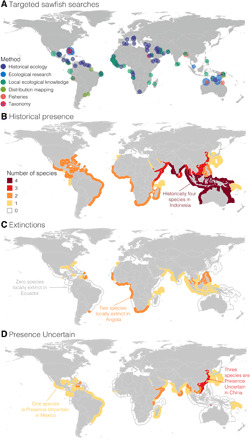
(A) Global sawfish search effort with each color representing the different activities and the size of the point representing the number of activities in each nation, where the smallest point represents one activity and the largest point represents 14 activities. (B) The historical distribution of sawfish species richness across 90 nations. (C) The number of sawfish species extinct in each nation. (D) The number of sawfish species with Presence Uncertain status; no color means the presence status is known. For (B) to (D), statuses are colored in the exclusive economic zone (EEZ) of each nation’s coastal waters and where greater species richness is denoted by the warmer colors.
All five sawfish species were historically found in the coastal waters of 90 nations, with the greatest species richness occurring in the Indo-West Pacific nations of India, Indonesia, Malaysia, Papua New Guinea, and Australia (Fig. 2B). Now, sawfishes are presumed extinct from more than half (n = 46) of these nations: 18 nations are missing at least one species, and 28 nations are missing two species (Fig. 2C). At least one species of sawfish still persists in 38 nations (fig. S3). Nevertheless, the uncertainty that remains due to the challenge of detecting rare marine species (30) means that the presence of sawfishes remains uncertain (Presence Uncertain) in 42 nations because either (i) the current presence of all sawfishes is unknown or (ii) although the presence of some species can be confirmed, the presence of others is unknown (Fig. 2D). We used the presence-absence data generated from these geographic distribution maps to predict the probability of extinction of sawfishes in the remaining Presence Uncertain nations (see Materials and Methods).
We find that most of the variation in sawfish occurrence in each nation is explained by ecological carrying capacity, fishing pressure, and management capacity indices, which accounted for 46.5, 35.8, and 16.5% of the summed variable importance from 1000 bootstrapped Boosted Regression Trees (BRTs), respectively (cross-validated pseudo r2 = 0.57 and bootstrapped range = 0.44 to 0.66; Fig. 3 and table S1). We found that species was not a substantial contributor to the model (sum of variable importance of all five species <2%; Fig. 3, N to R, and table S1). This suggests that, although the ecologies of all five species are different, the pathways of extinction are similar when assessed at the national level. The probability of local extinction of sawfish populations was higher in nations with low habitat availability and quality (Fig. 3, A to E), high fishing pressure (Fig. 3, F to J), and low management capacity (Fig. 3, K to M). Although all 13 indices best explain spatial patterns of extinction in sawfishes, the three indices with the highest variable importance values were shelf area (a measure of habitat availability; Fig. 3A; median variable importance = 25.0%, range = 22.8 to 28.6%), mangrove area (a proxy for habitat quality; Fig. 3B; 14.8%, 13.0 to 17.0%), and gear-specific landings, measured as the total tonnage of all fishes caught using gears that have high sawfish catchability (a direct measure of fishing pressure; see Table 1 and Fig. 3F; 14.5%, 13.0 to 17.3%).
Fig. 3. Marginal effects of ecological carrying capacity, fishing pressure, and management capacity on extinction risk in sawfishes.
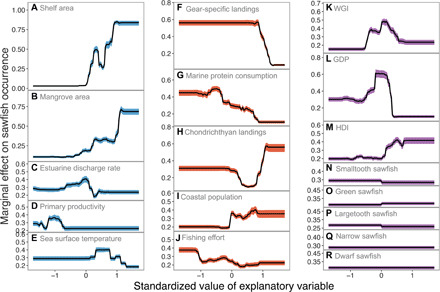
Partial dependence plots calculated for each predictor when all other indices are averaged. The solid black curve is the median of 1000 bootstrapped samples and the shaded ribbon shows the minimum and maximum fitted response for (A to E) ecological carrying capacity [(A) shelf area (km2; 25.0%), (B) mangrove area (km2; 14.8%), (C) estuarine discharge rate (m3 s−1; 3.06%), (D) marine primary productivity (mg m−3; 2.10%), and (E) sea surface temperature (°C; 1.52%)], (F to J) indices of fishing pressure [(F) gear-specific landings (metric tons; 14.5%), (G) marine protein consumption (metric tons; 9.38%), (H) total chondrichthyan landings (4.86%), (I) coastal human population (4.82%), and (J) fishing effort (2.22%)], (K to M) management capacity [(K) World Governance Index (WGI; 7.18%), (L) Gross Domestic Product (GDP; 5.66%), and (M) Human Development Index (HDI; 3.67%)], and (N to R) species identity [(N) Smalltooth Sawfish, P. pectinata (0.54%); (O) Green Sawfish, P. zijsron (0.42%); (P) Largetooth Sawfish, P. pristis (0.21%); (Q) Narrow Sawfish A. cuspidata (0%); and (R) Dwarf Sawfish, P. clavata (0%)]. The response is shown with standardized values of predictor variables (calculated by subtracting the mean and dividing by the SD) for presentation purposes, whereas the analysis was run with unscaled values.
The prediction that localized extinction occurs through range contractions and changes in the dynamic geography relationships among a species’ population holds true (18). The probability of occupancy by sawfishes increased with habitat availability (shelf area); however, this relationship was strongly mediated by fishing pressure (gear-specific landings; Fig. 4, A and B) and weakly mediated by habitat quality (mangrove area; Fig. 4B and fig. S4). As fishing pressure increased, the probability of occupancy for a given habitat size decreased. This is particularly apparent when comparing the median habitat required for 5% occupancy across levels of fishing pressure (Fig. 4C). When fishing pressure (i.e., gear-specific landings) is set to zero, the median habitat required to ensure 5% occupancy of sawfish populations is 110 km2; however, the median shelf area required increases to 493 km2 when fishing pressure is low, to 1410 km2 when fishing pressure is moderate, to 7332 km2 when fishing pressure is high, and to 26,903 km2 when fishing pressure is maximum (Fig. 5C). This latter amount is roughly equivalent to the shelf area of Venezuela or Oman. Although a similar pattern exists for mangrove area, where decreasing the total area of mangroves increases the habitat required to achieve 5% occupancy, this pattern is much weaker than that of fishing pressure (fig. S4). Dynamic geography theory predicts that species extinctions are most likely to occur at range edges, as immigration is likely to be lower, which has been borne out by the well-documented disappearance of sawfishes from the range edges of the United States, South America, South Africa, eastern Australia, and the Mediterranean Sea (24, 31). The listed threats from the IUCN Red List Assessments highlight that the major drivers endangering sawfishes are direct biological resource use [i.e., fishing (26)] and habitat loss (e.g., due to residential and commercial development, natural system modifications, etc.; fig. S1). Consequently, although local extinction of sawfishes can be attributed to the combination of ecological carrying capacity, fishing pressure, and management capacity, extinction is ultimately driven by overfishing and habitat loss.
Fig. 4. Dynamic geography of sawfish populations.
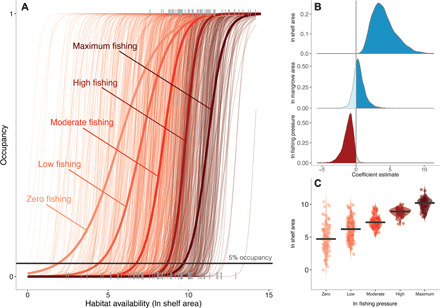
The effects of increasing fishing pressure (gear-specific landings), habitat quality (mangrove area), and habitat availability (shelf area) on occupancy in sawfishes. (A) Logistic regression where the thin curves show draws from the posterior distribution and the thick colored curves are the mean posterior estimates. Curves are colored and predicted by levels of fishing pressure (where mangrove area is held at its mean): zero fishing shown in the lightest orange, low fishing in orange, moderate fishing in red, high fishing in dark red, and maximum fishing in darkest red. The thick gray line shows the intersection where 5% occupancy occurs. Light gray rugs show the data. (B) Posterior distributions of the coefficient estimates from the logistic regression for shelf area (blue), mangrove area (blue), and fishing pressure (i.e., gear-specific landings; red), where the majority of the posterior is darker. Shelf area had a strong positive effect on the occupancy of sawfishes [mean estimate = 4.08, 95% credible interval (CI) = 1.52 to 8.05; 100% of the posterior > 0], whereas mangrove area had a small positive effect (0.48, 95% CI = −0.99 to 2.54; 72.1% of the posterior distribution > 0), and fishing pressure had a strong negative effect on the occupancy of sawfishes (−1.17, 95% CI = −3.05 to 0.03; 97.2% of the posterior distribution < 0). (C) Estimated habitat required to have 5% occupancy drawn from the posterior distribution through different levels of fishing. Violin plots and points show spread of the predicted draws and thick lines show the median value. Points have been jittered for ease of interpretation.
Fig. 5. Sawfish extinction risk and national conservation potential.
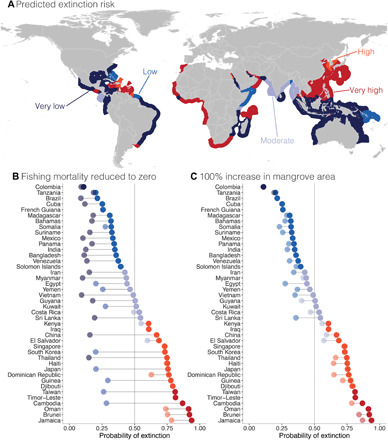
(A) Predicted probability of extinction from 1000 bootstrapped BRTs combined with current nations of occurrence represented in the EEZ. (B and C) Changes in predicted probability of extinction (current risk in dark colored points, predicted risk in transparent points) for Presence Uncertain nations where (B) fishing mortality (except coastal human population) is zero and (C) mangrove area is doubled. Dark blue, nations where sawfishes are present or have the lowest probability of extinction (<0.2); light blue, low probability of extinction (0.2 to 0.4); lightest blue, moderate probability of extinction (0.4 to 0.6); red, high probability of extinction (0.6 to 0.8); dark red, extremely high probability of extinction (0.8 to 1.0) or are already recorded as locally extinct.
In addition to the 46 nations where sawfishes are essentially confirmed to be locally extinct (Fig. 2C), we predict that sawfishes have disappeared from the coasts of at least nine additional former range states (median extinction probability >80.0%). In other words, sawfishes are likely locally extinct in a total of 55 nations and 58.7% of their historical distribution. Ordered from low-to-high extinction probability, we predict that sawfishes are locally extinct in China (median extinction probability = 80.8%), Iraq (81.8%), Haiti (81.8%), Japan (81.9%), Timor-Leste (83.4%), El Salvador (84.4%), Taiwan (85.4%), Djibouti (85.9%), and Brunei (88.2%) (Fig. 5A and table S2). In a number of nations, although we confirm that some species are locally extinct, other species are still listed as Presence Uncertain. In four of these nations, we predict that the last remaining sawfishes are locally extinct: Jamaica (94.8%), Singapore (86.9%), Guinea (85.7%), and Cambodia (85.2%). We also predict that one species has disappeared from Oman (84.8%; Fig. 5A and table S2).
To illustrate the conservation potential of reducing fishing mortality and increasing habitat quality, we tested two hypothetical scenarios on the probability of extinction in sawfishes: eliminating all forms of sawfish fishing pressure (i.e., setting gear-specific landings, marine protein consumption, chondrichthyan landings, and fishing effort to zero) or increasing the habitat quality by 100% (i.e., doubling the mangrove area). In nations where extinction probability is high, eliminating sawfish fishing mortality yields the greatest reduction in extinction risk. For example, eliminating all fishing pressures in Venezuela is predicted to result in a fourfold reduction in the probability of extinction in sawfishes, from 37.5 to 13.4% (Fig. 5B). Similarly, doubling the mangrove forest area in certain nations, especially those where restoration measures are already in place [e.g., Vietnam (32)], is predicted to also reduce extinction risk in sawfishes (Fig. 5C). Globally, reducing all fishing pressure to zero would decrease the probability of extinction of sawfishes by 20.7%, whereas doubling the mangrove area would decrease global extinction risk by 10.1% (Fig. 5, B and C). Although minimizing sawfish fishing mortality or increasing habitat quality in most nations can yield a large decline in extinction risk, the magnitude of this effect is not uniform across the globe. In many nations, even eliminating sawfish fishing pressure completely at this point may still be insufficient, leaving a relatively high probability of extinction compared to other nations (e.g., the probability of extinction in the Dominican Republic is only reduced from 76.3 to 62.8%; Fig. 5B). As such, targeted conservation efforts tailored to each nation’s unique combination of fishing pressure, management capacity, and ecological carrying capacity are required to minimize the probability of extinction of sawfishes.
Despite several international treaty mandates, most of the nations where sawfish presence is uncertain and extinction probability is very low have yet to establish legal protections for sawfishes (e.g., Madagascar, Colombia, and Panama). It is important to stress that sawfish protections are still urgently warranted in nations with low predicted probabilities of extinction; abundance in these nations is still relatively low and likely declining as a myriad of threats remain. The actual status of sawfishes in all nations lacking adequate protection may be much worse than predicted. Conservation action should be informed by the long-term knowledge of experts with insight into relevant local conditions that we have not accounted for (e.g., cultural importance of sawfishes).
Our analysis can guide sawfish conservation efforts around the world, including targeted assistance, particularly in nations with high likelihoods of sawfish presence and relatively high management capacity. We recommend the following eight nations that have very low predicted extinction probability but currently Presence Uncertain status(es) (median extinction probability <20.0%) as priorities for initiating or continuing specialized surveys to determine sawfish status and enacting protections: Cuba (median extinction probability = 9.4%), Tanzania (12.2%), Colombia (12.6%), Madagascar (13.3%), Panama (15.5%), Brazil (18.0%), Mexico (18.6%), and Sri Lanka (18.6%) (Fig. 5A and table S2). Achieving prescribed sawfish conservation policies in these eight nations, when added to the 38 nations where the presence of sawfishes has been confirmed, would amount to protection in 71.5% of their historical global distribution (Fig. 5).
Currently, Australia and the United States can be considered “lifeboat” nations: sawfishes are relatively well protected and are still present in these nations. Largetooth Sawfish are locally extinct in the United States, but the Smalltooth Sawfish population is considered to be increasing, due to strict prohibitions, public outreach, habitat protection, and coastal gillnet bans (33, 34). Removal or relaxation of any key safeguards would pose an immediate threat to this lifeboat population. Australia’s protections have ensured that the four Indo-West Pacific species persist, although further mitigation is required to reduce ongoing bycatch in commercial trawl and gillnet fisheries. Adjacent to both lifeboat nations are “beacon of hope” nations where sawfishes are present but remain largely unprotected (i.e., the Bahamas and Papua New Guinea). In these two nations, scientists and conservationists are working to understand the viability of the species and to secure protections.
There are other beacon-of-hope nations with varying geopolitical and macroeconomic barriers to conservation. Although there are a number of nations that have implemented legal protections for sawfishes (35), there is a mismatch between conservation implementation and probability of extinction. For example, despite the adoption of national sawfish protections in South Africa in 1997, it cannot currently act as a beacon-of-hope nation because sawfishes are considered locally extinct there (35). Conversely, the combination of fishing pressure, management capacity, and ecological carrying capacity in Cuba results in a very low predicted probability of extinction of sawfishes (9.4%). Given the relatively strong capacity to implement and enforce marine conservation actions (36), Cuba has the potential to transform from a beacon-of-hope to a lifeboat nation. To do so, sawfish-specific legal protections would need to be implemented, strictly enforced, and complemented by educational programs. Other beacon-of-hope nations include Brazil and several nations bordering the Red Sea and the Arabian/Persian Gulf. Sawfish recovery warrants ideally strict, species-specific prohibitions (on killing, retention, and trade), complementary educational and enforcement programs, bycatch mitigation measures, and habitat conservation, supported by strategic research. Ongoing public, political, and financial support as well as, in many cases, capacity building, bolster the effectiveness of these measures (23, 28). Such programs can also benefit similar species of threatened elasmobranchs, particularly wedgefishes and giant guitarfishes (37).
Here, we have presented the first well-documented near-extinction of an iconic family of marine fishes due to overfishing and habitat limitations. Our approach offers an opportunity to improve the detection of the disappearance of wide-ranging species, attribute causal factors, and identify the relative benefits of different conservation solutions. There are key, yet fleeting, opportunities to prevent further nation-scale extinctions and reverse population declines through immediate and stringent conservation action in remaining range states. Without such action, the repeated losses of populations of these extraordinary species are likely to serve as stepping stones toward the first global extinction of a marine fish species. Our space-for-time approach offers the capability to track spatial declines and probabilities of extinctions of widespread, rare species as well as identify threatening processes and priority nations for action.
MATERIALS AND METHODS
Geographic distribution maps
The IUCN SSC SSG convened 26 experts to reassess the geographic status of sawfishes, building upon a review of the recently published, gray, and online literature and email communication with 174 members of the IUCN SSC SSG network (38). We reviewed all sawfish-related activities, projects, and literature between 2014 and 2019, and these activities were classified as (i) local ecological knowledge surveys (interviews) of primarily subsistence fishing communities, (ii) ecological research (field-based ecology, habitat use, movements, life history, and molecular ecology), (iii) fisheries-related research (including examination of bycatch and analyses of bather protection program data), (iv) distribution mapping (synthesizing field, encounter, and/or museum records; environmental DNA surveys aiming to map occurrence), (v) historical ecology, and (vi) taxonomy.
We modeled our spatial analyses of geographic distribution following the methods of a previous status report on sawfishes [i.e., 100 m maximum bathymetry and exclusion of distant offshore islands; see (23) for further details]. For each species and historical range state (see Fig. 2B), nations were scored as either Extant (1), Possibly Extinct (0), or Presence Uncertain using the IUCN spatial presence codes (39). Note that the use of Possibly Extant was ignored, as this dataset is an update from a previous status report on sawfishes, where Possibly Extant was only used for P. clavata for uncertain point estimates within the Australian Coral Sea (23). The use of expert opinion, extensive search protocols, and bathymetry considerations has vastly improved the resolution of sawfish occurrence compared to other model-based distribution maps [e.g., AquaMaps are created using distribution models with relative probabilities of occurrence based on the species’ environmental envelopes (40)]. As such, this new analysis yields a comprehensive assessment of sawfish populations through local status surveys throughout much of their historic range.
Data collation
We focused our analysis at the national level (including countries and their territories where data were available) owing to the resolution of our data, but also because species protection typically occurs at the national or subnational scale. Note that we could not use traditional species distribution models because of the absence of point records for sawfishes outside the United States and Australia. Furthermore, because of the scale of our analysis, we could not reliably use indicators of successful management protocols that achieve fisheries management objectives, as they are limited in the target fisheries and the spatial scale considered [e.g., Melnychuk et al. (41) only considered 10 directed fisheries for 28 countries (42)]. To both reconcile these spatial gaps and build upon previous climate and conservation vulnerability work [e.g., (43)], we focused on general indicators of fishing pressures specific to sawfishes [table S1; (42, 44–46)]. Ideally, we would include sawfish-specific data (e.g., stock assessments, total sawfish landings, etc.); however, owing to the scarcity and high economic value of sawfishes, accurate data do not exist at a global scale. Attempts at establishing and enforcing strict national protection are lagging or otherwise inadequate for sawfishes (35) and only apply to a subset of nations; thus, we omitted direct measurements of conservation action from our analyses and focused on general indicators of management capacity (Table 1). We excluded nations that either (i) never harbored sawfish due to unfavorable physical conditions [i.e., Namibia was excluded from all sawfish distribution maps because it is predominantly an upwelling ecosystem and has a steep bathymetric shelf; (23)] or (ii) were otherwise lacking adequate governmental or fisheries data (e.g., Western Sahara and a number of European territories in the Caribbean).
We modeled the occurrence of sawfishes using (i) five indices of indirect and direct fishing pressures, (ii) three indicators of the capacity at which a nation can implement effective fisheries management processes, and (iii) five indicators of the ecological carrying capacity of the available habitat (Table 1). First, we separated fishing pressures into indirect and direct fishing pressures, where we used coastal human population size (https://sedac.ciesin.columbia.edu/data/set/nagdc-population-landscape-climate-estimates-v3) and marine protein consumption (47) as indirect measures of fishing pressure (42). Because sawfishes have a high catchability in specific fishing gears, we used total landings with specific gear types (Food and Agriculture Organization gear types named: bottom trawls, otter trawls, shrimp trawls, gillnets, small-scale gillnets, small-scale longlines, small-scale trammel nets, and trammel nets), the total chondrichthyan landings, and the fishing effort from subsistence and artisanal sectors as direct fishing pressures (47, 48). Second, we used three measures of governance and literacy to reflect the capacity of management to undertake conservation: World Governance Index (WGI; https://databank.worldbank.org/source/worldwide-governance-indicators), Human Development Index (HDI; http://hdr.undp.org/en/content/human-development-index-hdi), and Gross Domestic Product (GDP in USD; https://data.worldbank.org/indicator/NY.GDP.MKTP.CD). Last, to characterize ecological carrying capacity, we used the continental shelf area (km2) as a measurement of the total habitat available (see Supplementary text). We only measured shelf area as the area found within the geographic distribution maps for each species clipped to the maximum depth bathymetry for each species [see Supplementary text; (23, 26)]. We also used marine primary productivity [mg m−3; (49)], mangrove cover [km2; (50)], total freshwater estuarine discharge rate [m3 s−1; (51)], and sea surface temperature [°C; (52)] to characterize ecological carrying capacity. We selected these indicators because of the habitat preferences of sawfishes for shallow, inshore waters in tropical regions [Table 1; (53)].
Analysis
We used BRTs to model the occurrence of sawfishes and to predict the probability of extinction in the Presence Uncertain nations (n = 42; table S2). We also predicted the probability of extinction under two hypothetical scenarios: (i) if all fishing pressure was zero [i.e., marine protein consumption, chondrichthyan landings, fishing effort, and gear-specific landings (not coastal population) values were all set to zero] or (ii) if mangrove area increased by 100% in Presence Uncertain nations (Fig. 5, B and C). Using the geographic distribution maps for each species (excluding the Presence Uncertain nations), we were able to use a Bernoulli loss function to predict the probability of extinction as the difference between one and the probability of occurrence [P(extinct) = 1 − P(occurrence)]. BRTs are a powerful statistical method with high predictive accuracy because they combine many decision trees and a boosting algorithm and are not restricted by nonlinear relationships, complex interactions, or missing data (54). To improve model performance, we ln-transformed chondrichthyan landings, gear-specific landings, coastal human population, marine protein consumption, GDP, fishing effort, continental shelf area, primary productivity, mangrove cover, and total estuarine discharge rate; species was coded as a dummy variable. Although BRTs can handle collinear variables, removing highly collinear variables may sometimes improve model fit (54). We initially considered the total landings of marine fisheries production and the catches of illegal, unreported, and unregulated fishing as indicators of fishing pressure, but removed them from further analyses due to high collinearity with multiple variables, and they were both classified as indicators of fishing pressure (|r| > 0.8; fig. S2). Although coastal human population size and GDP were also highly correlated (r = 0.84), we chose to keep both variables in the model because they are not within the same indices (classified under fishing pressure and management capacity, respectively; Table 1 and fig. S2).
We randomly separated our data into a training set (80%; n = 102 species-nation combinations) and a test set (20%; n = 25). We selected hyperparameters by varying the learning rate and tree complexity and assessed model fit based on minimizing the root mean squared error. Our final model used a learning rate = 0.005, a tree complexity = 10, and a bag fraction = 0.5 and was optimized using 10-fold cross-validation. Because of the stochastic building process of BRTs, we used the median of the variable importance and partial dependence values of each variable from 1000 bootstrapped samples. Our final BRT model explained 67.6% (range = 49.0 to 81.5%) of the deviance in the training dataset and 27.1% (range = 15.6 to 31.0%) of the deviance in the test set across 1000 bootstrapped samples. Despite the low percentage of deviance explained in the test set, which could be due to the variability due to a small sample size (n = 25), this model had high predictive accuracy where our bootstrapped cross-validated AUC (area under the curve) of the receiver operating characteristics curve was 0.83 (range = 0.73 to 0.88) and our evaluation AUC of the test set was 0.84 (range = 0.81 to 0.88). We performed all BRT analyses using the gbm v.2.1.4 (55) and dismo v.1.1-4 (56), packages in R v.3.5.2 (57).
To test the dynamic geography of sawfishes (14–19), we used a generalized linear mixed model in a Bayesian framework with a logit link and the default non/weakly informative priors in the brms package (58). We modeled occupancy as a function of habitat availability (ln shelf area), habitat quality (ln mangrove area), and fishing pressure (ln gear-specific landings) and used nation as a grouping factor by specifying random intercepts. For this model, we used four Markov chain Monte Carlo chains simultaneously, each with 2000 iterations and 1000 warm-up iterations. We achieved convergence on all four chains (rhat = 1.00 for all coefficient estimates). We ran all dynamic geography analyses in R v.3.5.2 (57), using Stan through the brms v.2.10.0 package (58).
Acknowledgments
We thank all researchers, members of the sawfish network, and members of the IUCN SSC Shark Specialist Group who contributed distribution data and knowledge; the Georgia Aquarium and A. Dove for hosting a workshop on sawfish priorities; R. Pollom and M. Clark for workshop organization; and all workshop attendees, including J. Carlson, P. Carlson, P. Charvet, D. Grubbs, K. Herman, O. Koubrak, K. Mileham, and T. Wiley-Lescher. We thank H. V. Watkins, B. R. Howard, D. A. Greenberg, C. J. Brown, and Stats Beerz at Simon Fraser University for helpful discussions on the analysis; the Dulvy Lab for helpful feedback on the manuscript; and two anonymous reviewers for insightful and constructive comments. The majority of baseline data on the current sawfish distribution were provided by small-scale fishers throughout the tropics; we are grateful for their contribution. Funding: This work was funded by the Barrett Foundation, the Georgia Aquarium, and the Shark Conservation Fund. In addition, P.M.K. was supported by the Marine Biodiversity Hub, a collaborative partnership supported through funding from the Australian Government’s National Environmental Science Program (NESP). R.H.L. was supported by funding from the National Oceanic and Atmospheric Administration, the National Marine Fisheries Service (grant no. NA15NMF4690193), and the Save Our Seas Foundation. L.N.K.D. was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Canada Graduate Scholarship (CGS) and by a postdoctoral fellowship from the Biodiversity Research Centre at the University of British Columbia. S.V.F.’s sawfish work was funded by the Henry Foundation, the Curtis and Edith Munson Foundation, and the Disney Conservation Fund. N.K.D. was funded by NSERC and the Canada Research Chairs Program. Author contributions: H.F.Y., N.K.D., L.N.K.D., P.M.K., B.F., R.W.J., R.H.L., and S.V.F. conceived this study. P.M.K., B.F., R.W.J., and R.H.L. compiled sawfish distribution data. H.F.Y., D.H.D., and L.N.K.D. compiled spatial variables. H.F.Y. and L.N.K.D. compiled socioeconomic variables. D.H.D. and L.N.K.D. completed spatial analyses and range contraction analyses. H.F.Y., R.P.F., and N.K.D. conducted abundance-occupancy analyses. H.F.Y. and N.K.D. conducted the BRT analysis and drafted the manuscript with input from all authors. Competing interests: P.K., R.W.J., R.H.L., B.F., S.V.F., and N.K.D. were working in the IUCN Species Survival Commission Shark Specialist Group during this work. All other authors declare that they have no competing interests. Data and materials availability: All data and annotated R code can be found at https://github.com/helenfyan/Sawfish_Prioritization. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/7/eabb6026/DC1
REFERENCES AND NOTES
- 1.Dawson M. N., Species richness, habitable volume, and species densities in freshwater, the sea, and on land. Front. Biogeogr. 4, 105–116 (2012). [Google Scholar]
- 2.Amoroso R. O., Pitcher C. R., Rijnsdorp A. D., McConnaughey R. A., Parma A. M., Suuronen P., Eigaard O. R., Bastardie F., Hintzen N. T., Althaus F., Baird S. J., Black J., Buhl-Mortensen L., Campbell A. B., Catarino R., Collie J., Cowan J. H. Jr., Durholtz D., Engstrom N., Fairweather T. P., Fock H. O., Ford R., Gálvez P. A., Gerritsen H., Góngora M. E., González J. A., Hiddink J. G., Hughes K. M., Intelmann S. S., Jenkins C., Jonsson P., Kainge P., Kangas M., Kathena J. N., Kavadas S., Leslie R. W., Lewis S. G., Lundy M., Makin D., Martin J., Mazor T., Gonzalez-Mirelis G., Newman S. J., Papadopoulou N., Posen P. E., Rochester W., Russo T., Sala A., Semmens J. M., Silva C., Tsolos A., Vanelslander B., Wakefield C. B., Wood B. A., Hilborn R., Kaiser M. J., Jennings S., Bottom trawl fishing footprints on the world’s continental shelves. Proc. Natl. Acad. Sci. 115, E10275–E10282 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroodsma D. A., Mayorga J., Hochberg T., Miller N. A., Boerder K., Ferretti F., Wilson A., Bergman B., White T. D., Block B. A., Woods P., Sullivan B., Costello C., Worm B., Tracking the global footprint of fisheries. Science 359, 904–908 (2018). [DOI] [PubMed] [Google Scholar]
- 4.McClenachan L., Cooper A. B., Dulvy N. K., Rethinking trade-driven extinction risk in marine and terrestrial megafauna. Curr. Biol. 26, 1640–1646 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Ripple W. J., Wolf C., Newsome T. M., Betts M. G., Ceballos G., Courchamp F., Hayward M. W., Van Valkenburgh B., Wallach A. D., Worm B., Are we eating the world’s megafauna to extinction? Conserv. Lett. , e12627 (2019). [Google Scholar]
- 6.Hilborn R., Amoroso R. O., Anderson C. M., Baum J. K., Branch T. A., Costello C., de Moor C. L., Faraj A., Hively D., Jensen O. P., Kurota H., Little L. R., Mace P., McClanahan T., Melnychuk M. C., Minto C., Osio G. C., Parma A. M., Pons M., Segurado S., Szuwalski C. S., Wilson J. R., Ye Y., Effective fisheries management instrumental in improving fish stock status. Proc. Natl. Acad. Sci. 117, 2218–2224 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cashion M. S., Bailly N., Pauly D., Official catch data underrepresent shark and ray taxa caught in Mediterranean and Black Sea fisheries. Mar. Policy 105, 1–9 (2019). [Google Scholar]
- 8.Ricard D., Minto C., Jensen O. P., Baum J. K., Examining the knowledge base and status of commercially exploited marine species with the RAM Legacy Stock Assessment Database. Fish Fish. 13, 380–398 (2012). [Google Scholar]
- 9.Dulvy N. K., Fowler S. L., Musick J. A., Cavanagh R. D., Kyne P. M., Harrison L. R., Carlson J. K., Davidson L. N., Fordham S. V., Francis M. P., Pollock C. M., Simpfendorfer C. A., Burgess G. H., Carpenter K. E., Compagno L. J., Ebert D. A., Gibson C., Heupel M. R., Livingstone S. R., Sanciangco J. C., Stevens J. D., Valenti S., White W. T., Extinction risk and conservation of the world’s sharks and rays. eLife 3, e00590 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boakes E. H., Rout T. M., Collen B., Inferring species extinction: The use of sighting records. Methods Ecol. Evol. 6, 678–687 (2015). [Google Scholar]
- 11.MacNeil M. A., Chapman D. D., Heupel M., Simpfendorfer C. A., Heithaus M., Meekan M., Harvey E., Goetze J., Kiszka J., Bond M. E., Currey-Randall L. M., Speed C. W., Sherman C. S., Rees M. J., Udyawer V., Flowers K. I., Clementi G., Valentin-Albanese J., Gorham T., Adam M. S., Ali K., Pina-Amargós F., Angulo-Valdés J. A., Asher J., Barcia L. G., Beaufort O., Benjamin C., Bernard A. T. F., Berumen M. L., Bierwagen S., Bonnema E., Bown R. M. K., Bradley D., Brooks E., Brown J. J., Buddo D., Burke P., Cáceres C., Cardeñosa D., Carrier J. C., Caselle J. E., Charloo V., Claverie T., Clua E., Cochran J. E. M., Cook N., Cramp J., D’Alberto B., de Graaf M., Dornhege M., Estep A., Fanovich L., Farabough N. F., Fernando D., Flam A. L., Floros C., Fourqurean V., Garla R., Gastrich K., George L., Graham R., Guttridge T., Hardenstine R. S., Heck S., Henderson A. C., Hertler H., Hueter R., Johnson M., Jupiter S., Kasana D., Kessel S. T., Kiilu B., Kirata T., Kuguru B., Kyne F., Langlois T., Lédée E. J. I., Lindfield S., Luna-Acosta A., Maggs J., Manjaji-Matsumoto B. M., Marshall A., Matich P., McCombs E., McLean D., Meggs L., Moore S., Mukherji S., Murray R., Kaimuddin M., Newman S. J., Nogués J., Obota C., O’Shea O., Osuka K., Papastamatiou Y. P., Perera N., Peterson B., Ponzo A., Prasetyo A., Quamar L. M. S., Quinlan J., Ruiz-Abierno A., Sala E., Samoilys M., Schärer-Umpierre M., Schlaff A., Simpson N., Smith A. N. H., Sparks L., Tanna A., Torres R., Travers M. J., van Zinnicq Bergmann M., Vigliola L., Ward J., Watts A. M., Wen C., Whitman E., Wirsing A. J., Wothke A., Zarza-Gonzâlez E., Cinner J. E., Global status and conservation potential of reef sharks. Nature 583, 801–806 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Sadovy Y., Cheung W. L., Near extinction of a highly fecund fish: The one that nearly got away. Fish Fish. 4, 86–99 (2003). [Google Scholar]
- 13.Webb T. J., Mindel B. L., Global patterns of extinction risk in marine and non-marine systems. Curr. Biol. 25, 506–511 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Burgess M. G., Costello C., Fredston-Hermann A., Pinsky M. L., Gaines S. D., Tilman D., Polasky S., Range contraction enables harvesting to extinction. Proc. Natl. Acad. Sci. 114, 3945–3950 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher J., Frank K., Abundance-distribution relationships and conservation of exploited marine fishes. Mar. Ecol. Prog. Ser. 279, 201–213 (2004). [Google Scholar]
- 16.Freckleton R. P., Gill J. A., Noble D., Watkinson A. R., Large-scale population dynamics, abundance-occupancy relationships and the scaling from local to regional population size. J. Anim. Ecol. 74, 353–364 (2005). [Google Scholar]
- 17.Freckleton R. P., Noble D., Webb T. J., Distributions of habitat suitability and the abundance-occupancy relationship. Am. Nat. 167, 260–275 (2006). [DOI] [PubMed] [Google Scholar]
- 18.A. D. MacCall, Dynamic Geography of Marine Fish Populations (Washington Sea Grant Program, 1990). [Google Scholar]
- 19.Webb T. J., Dulvy N. K., Jennings S., Polunin N. V. C., The birds and the seas: Body size reconciles differences in the abundance–occupancy relationship across marine and terrestrial vertebrates. Oikos 120, 537–549 (2011). [Google Scholar]
- 20.Jensen O. P., Branch T. A., Hilborn R., Marine fisheries as ecological experiments. Theor. Ecol. 5, 3–22 (2012). [Google Scholar]
- 21.Holt R. D., Lawton J. H., Gaston K. J., Blackburn T. M., On the relationship between range size and local abundance: Back to basics. Oikos 78, 183–190 (1997). [Google Scholar]
- 22.Kindsvater H. K., Mangel M., Reynolds J. D., Dulvy N. K., Ten principles from evolutionary ecology essential for effective marine conservation. Ecol. Evol. 6, 2125–2138 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dulvy N. K., Davidson L. N. K., Kyne P. M., Simpfendorfer C. A., Harrison L. R., Carlson J. K., Fordham S. V., Ghosts of the coast: Global extinction risk and conservation of sawfishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 26, 134–153 (2016). [Google Scholar]
- 24.Everett B. I., Cliff G., Dudley S. F. J., Wintner S. P., van der Elst R. P., Do sawfish Pristis spp. represent South Africa’s first local extirpation of marine elasmobranchs in the modern era? Afr. J. Mar. Sci. 37, 275–284 (2015). [Google Scholar]
- 25.Fernandez-Carvalho J., Imhoff J. L., Faria V. V., Carlson J. K., Burgess G. H., Status and the potential for extinction of the largetooth sawfish Pristis pristis in the Atlantic Ocean. Aquat. Conserv. Mar. Freshw. Ecosyst. 24, 478–497 (2014). [Google Scholar]
- 26.IUCN, The IUCN Red List of Threatened Species, version 2020-2 (2020); www.iucnredlist.org/.
- 27.Thorson T. B., The impact of commercial exploitation on sawfish and shark populations in Lake Nicaragua. Fisheries 7, 2–10 (1982). [Google Scholar]
- 28.L. R. Harrison, N. K. Dulvy, Sawfish: A Global Strategy for Conservation (IUCN Species Survival Commission’s Shark Specialist Group, 2014). [Google Scholar]
- 29.Gómez-Rodríguez S., Caldas J. P., Arturo A.-P., Martínez-Silva M. A., Sáenz-Okuyama P., Lasso C. A., Lasso-Alcalá O. M., Geographic distribution and conservation status of sawfish Pristis spp (Pristiformes: Pristidae) in the southern Caribbean Sea. Biota Colomb. 15, 109–117 (2014). [Google Scholar]
- 30.Dulvy N. K., Ellis J. R., Goodwin N. B., Grant A., Reynolds J. D., Jennings S., Methods of assessing extinction risk in marine fishes. Fish Fish. 5, 255–276 (2004). [Google Scholar]
- 31.Ferretti F., Morey Verd G., Seret B., Sulić Šprem J., Micheli F., Falling through the cracks: The fading history of a large iconic predator. Fish Fish. 17, 875–889 (2016). [Google Scholar]
- 32.Nam V. N., Sasmito S. D., Murdiyarso D., Purbopuspito J., MacKenzie R. A., Carbon stocks in artificially and naturally regenerated mangrove ecosystems in the Mekong Delta. Wetl. Ecol. Manag. 24, 231–244 (2016). [Google Scholar]
- 33.Carlson J. K., Osborne J., Schmidt T. W., Monitoring the recovery of smalltooth sawfish, Pristis pectinata, using standardized relative indices of abundance. Biol. Conserv. 136, 195–202 (2007). [Google Scholar]
- 34.Norton S. L., Wiley T. R., Carlson J. K., Frick A. L., Poulakis G. R., Simpfendorfer C. A., Designating critical habitat for juvenile endangered Smalltooth Sawfish in the United States. Mar. Coast. Fish. 4, 473–480 (2012). [Google Scholar]
- 35.J. M. Lawson, S. V. Fordham, Sharks Ahead: Realizing the Potential of the Convention on Migratory Species to Conserve Elasmobranchs (Shark Advocates International, The Ocean Foundation, 2018), p. 76.
- 36.Perera Valderrama S., Hernández Ávila A., González Méndez J., Moreno Martínez O., Cobián Rojas D., Ferro Azcona H., Milián Hernández E., Caballero Aragón H., Alcolado P. M., Pina Amargós F., Hernández González Z., Espinosa Pantoja L., Rodríguez Farrat L. F., Marine protected areas in Cuba. Bull. Mar. Sci. (2018), in press. [Google Scholar]
- 37.Kyne P. M., Jabado R. W., Rigby C. L., Dharmadi, Gore M. A., Pollock C. M., Herman K. B., Cheok J., Ebert D. A., Simpfendorfer C. A., Dulvy N. K., The thin edge of the wedge: Extremely high extinction risk in wedgefishes and giant guitarfishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 30, 1337–1361 (2020). [Google Scholar]
- 38.S. V. Fordham, R. W. Jabado, P. M. Kyne, P. Charvet, N. K. Dulvy, Saving Sawfish: Progress and Priorities (IUCN Shark Specialist Group, 2018), 6 pp.
- 39.IUCN, Mapping standards and data quality for the IUCN Red List categories and criteria version 1.16 (2018); https://nc.iucnredlist.org/redlist/resources/files/1539098236-Mapping_Standards_Version_1.16_2018.pdf.
- 40.K. Kaschner, K. Kesner-Reyes, C. Garilao, J. Segschneider, J. Rius-Barile, T. Rees, R. Froese, AquaMaps: Predicted range maps for aquatic species (2019); www.aquamaps.org.
- 41.Melnychuk M. C., Peterson E., Elliott M., Hilborn R., Fisheries management impacts on target species status. Proc. Natl. Acad. Sci. 114, 178–183 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidson L. N. K., Krawchuk M. A., Dulvy N. K., Why have global shark and ray landings declined: Improved management or overfishing? Fish Fish. 17, 438–458 (2016). [Google Scholar]
- 43.Smit B., Wandel J., Adaptation, adaptive capacity and vulnerability. Glob. Environ. Change. 16, 282–292 (2006). [Google Scholar]
- 44.McClanahan T. R., Cinner J. E., Maina J., Graham N. A. J., Daw T. M., Stead S. M., Wamukota A., Brown K., Ateweberhan M., Venus V., Polunin N. V. C., Conservation action in a changing climate. Conserv. Lett. 1, 53–59 (2008). [Google Scholar]
- 45.Dickman A. J., Hinks A. E., Macdonald E. A., Burnham D., Macdonald D. W., Priorities for global felid conservation. Conserv. Biol. 29, 854–864 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Davidson L. N. K., Dulvy N. K., Global marine protected areas to prevent extinctions. Nat. Ecol. Evol. 1, 40 (2017). [DOI] [PubMed] [Google Scholar]
- 47.FAO, FISHStatJ: Software for fishery and aquaculture statistical time series (FAO Fisheries and Aquaculture Department, 2013); www.fao.org/fishery/statistics/software/fishstat/en.
- 48.D. Pauly, D. Zeller, Sea Around Us concepts, design and data (2015); http://seaaroundus.org.
- 49.NASA Ocean Biology (OB.DAAC), Mean annual sea surface chlorophyll-a concentration for the period 2009-2013 (composite dataset created by UNEP-WCMC), Moderate Resolution Imaging Spectroradiometer (MODIS) Aqua Ocean Colour website (NASA OB.DAAC, 2014), http://oceancolor.gsfc.nasa.gov/cgi/l3 [accessed 28 November 2014]; Cambridge (UK): UNEP World Conservation Monitoring Centre. URL: https://data.unep-wcmc.org/datasets/37.
- 50.Bunting P., Rosenqvist A., Lucas R. M., Rebelo L.-M., Hilarides L., Thomas N., Hardy A., Itoh T., Shimada M., Finlayson C. M., The global mangrove watch—A new 2010 global baseline of mangrove extent. Remote Sens. (Basel) 10, 1669 (2018). [Google Scholar]
- 51.Alder J., Putting the coast in the “Sea Around Us”. Sea Us Newsl. 15, 1–2 (2003). [Google Scholar]
- 52.Reynolds R. W., Rayner N. A., Smith T. M., Stokes D. C., Wang W., An improved in situ and satellite SST analysis for climate. J. Climate 15, 1609–1625 (2002). [Google Scholar]
- 53.Simpfendorfer C. A., Predicting population recovery rates for endangered western Atlantic sawfishes using demographic analysis. Environ. Biol. Fishes 58, 371–377 (2000). [Google Scholar]
- 54.Elith J., Leathwick J. R., Hastie T., A working guide to boosted regression trees. J. Anim. Ecol. 77, 802–813 (2008). [DOI] [PubMed] [Google Scholar]
- 55.G. Ridgeway, gbm: Generalized boosted regression models (R package version 2.1.4, 2015); https://cran.r-project.org/web/packages/gbm/gbm.pdf.
- 56.R. J. Hijmans, S. Phillips, J. Leathwick, J. Elith, dismo: Species distribution modeling (R Package Version 1.1–4, 2011); https://cran.r-project.org/web/packages/dismo/dismo.pdf.
- 57.R Core Team, R: A language and environment for statistical computing (R Foundation for Statistical Computing, 2017); www.R-project.org/.
- 58.Bürkner P.-C., brms: An R package for Bayesian multilevel models using Stan. J. Stat. Softw. 80, 1–28 (2017). [Google Scholar]
- 59.Clarke S. C., McAllister M. K., Milner-Gulland E. J., Kirkwood G. P., Michielsens C. G. J., Agnew D. J., Pikitch E. K., Nakano H., Shivji M. S., Global estimates of shark catches using trade records from commercial markets. Ecol. Lett. 9, 1115–1126 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Pitcher T., Kalikoski D., Pramod G., Short K., Not honouring the code. Nature 457, 658–659 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Mora C., Myers R. A., Coll M., Libralato S., Pitcher T. J., Sumaila R. U., Zeller D., Watson R., Gaston K. J., Worm B., Management effectiveness of the world’s marine fisheries. PLOS Biol. 7, e1000131 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Myers R. A., MacKenzie B. R., Bowen K. G., Barrowman N. J., What is the carrying capacity for fish in the ocean? A meta-analysis of population dynamics of North Atlantic cod. Can. J. Fish. Aquat. Sci. 58, 1464–1476 (2001). [Google Scholar]
- 63.Chassot E., Bonhommeau S., Dulvy N. K., Mélin F., Watson R., Gascuel D., Pape O. L., Global marine primary production constrains fisheries catches. Ecol. Lett. 13, 495–505 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Poulakis G. R., Stevens P. W., Timmers A. A., Stafford C. J., Simpfendorfer C. A., Movements of juvenile endangered Smalltooth Sawfish, Pristis pectinata, in an estuarine river system: Use of non-main-stem river habitats and lagged responses to freshwater inflow-related changes. Environ. Biol. Fishes 96, 763–778 (2013). [Google Scholar]
- 65.P. Last, W. White, M. de Carvalho, B. Séret, M. Stehmann, G. Naylor, Rays of the World (CSIRO publishing, 2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/7/eabb6026/DC1


