Abstract
Breast cancer is a hormonally-driven cancer, and various dietary factors are associated with estrogen metabolism, including dietary fiber. Several studies report associations between dietary fiber and breast cancer; however, research on whether fiber influences circulating estrogens through the gut microbiota is rare. The objective of this cross-sectional study among 29 newly-diagnosed (stage 0-II), post-menopausal breast cancer patients is to examine associations between dietary fiber and the gut microbiota that are linked with β-glucuronidase activity, and purportedly increase circulating estrogens. Spearman’s and partial correlations controlling for body mass index and age were performed using dietary recall data, Illumina MiSeq generated microbiota relative abundance, and HPLC-mass spectrometry-derived estradiol and estrone levels.
Major findings are: (1) total dietary fiber is inversely associated with Clostridium hathewayi (r = −0.419; p = 0.024); (2) soluble fiber is inversely associated with Clostridium (r=−0.11; p = 0.02); (3) insoluble fiber is positively associated with Bacteroides uniformis sp. (r = 0.382; p = 0.041); and (4) serum estradiol and estrone levels are not correlated with species/genera or dietary fiber, though there is a trend toward an inverse association between soluble fiber and estradiol levels (r= −0.30; p = 0.12). More studies are needed to understand the complex interaction between dietary fiber, intestinal microbiota, and hormonal levels in older females.
Introduction
Breast cancer affects many people worldwide. It is estimated that in 2018, approximately 266,120 US women will be diagnosed with invasive and 63,960 with noninvasive breast cancer (1). Roughly 40,920 American women will die of this disease, making it the second leading cause of cancer-related death among U.S. females (1). While breast cancer claims the lives of many women, many more are survivors. Currently, it is estimated that more than 3.1 million women are alive in the U.S. with a history of breast cancer. These women are either cancer-free or continue to live with active disease (2). Considering the threat breast cancer poses to so many women, the concern for the prevention of this disease has become forefront in today’s society.
Both weight status and dietary factors appear to be associated with breast cancer risk among post-menopausal women. The mechanisms regulating estrogen production in postmenopausal women might play a role in this association (see Figure 1). Numerous studies have shown that post-menopausal obese women have a 20% to 40% increased risk of developing breast cancer compared to women of normal weight (3, 4). Each 5-unit increase in BMI is associated with a 12% increase in the risk of breast cancer in postmenopausal women (3–5). It appears that a high-fat diet, and increased circulating levels of total cholesterol and triglycerides play a role in increasing breast cancer risk (6, 7). Furthermore, high protein intake, particularly increased red meat consumption, is associated with a 13% increase in the risk of breast cancer (6, 8). On the other hand, consumption of a plant-based dietary pattern is associated with a decreased risk of breast cancer and provides protection against this prevalent disease (9).
Figure 1.
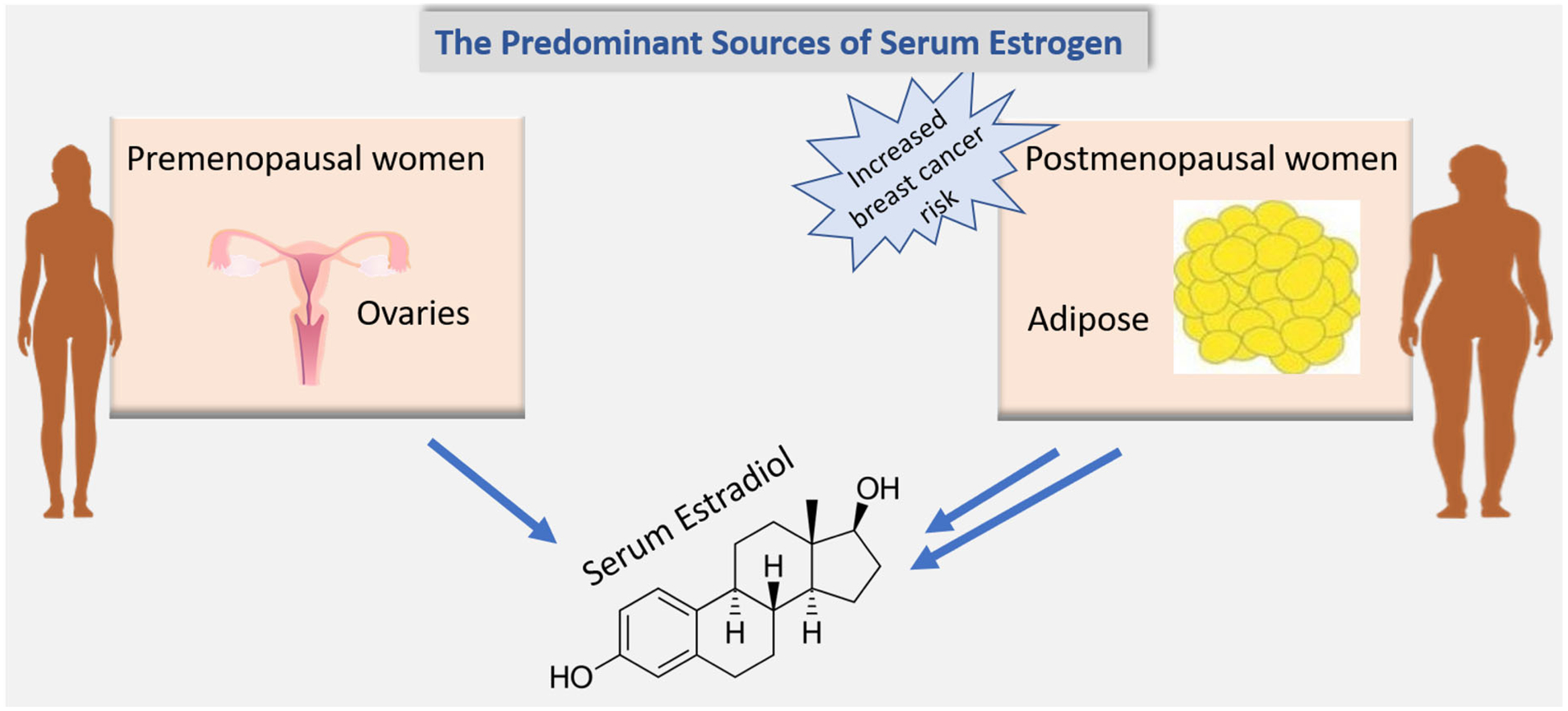
A framework indicating sites of conversion of androgen to estrogen. In premenopausal women, the ovaries are the predominant source of serum estradiol; however, in postmenopausal women, estradiol mainly comes from aromatization of adrenal and ovarian androgens in fat tissues.
In plant-based diets, fiber intake is prominent and associated with significantly lower breast cancer risk (10, 11). A high-fiber diet provides many health benefits. It may enhance weight loss and lower high cholesterol levels, as well as decrease insulin sensitivity (12). Considering that increased estrogen levels are associated with breast cancer development (13), the relationship between fiber and estrogen metabolism may play an important role in breast cancer prevention. It is postulated that fiber reduces circulating estrogen levels by altering the gut microbiota and decreasing deconjugation and reabsorption of estrogen. By accelerating intestinal transit and binding to estrogen in the intestine, fiber decreases serum estrogen concentrations and prevents free hormones, such as 17β-estradiol, to be reabsorbed (see Figure 2) (10, 14).
Figure 2.
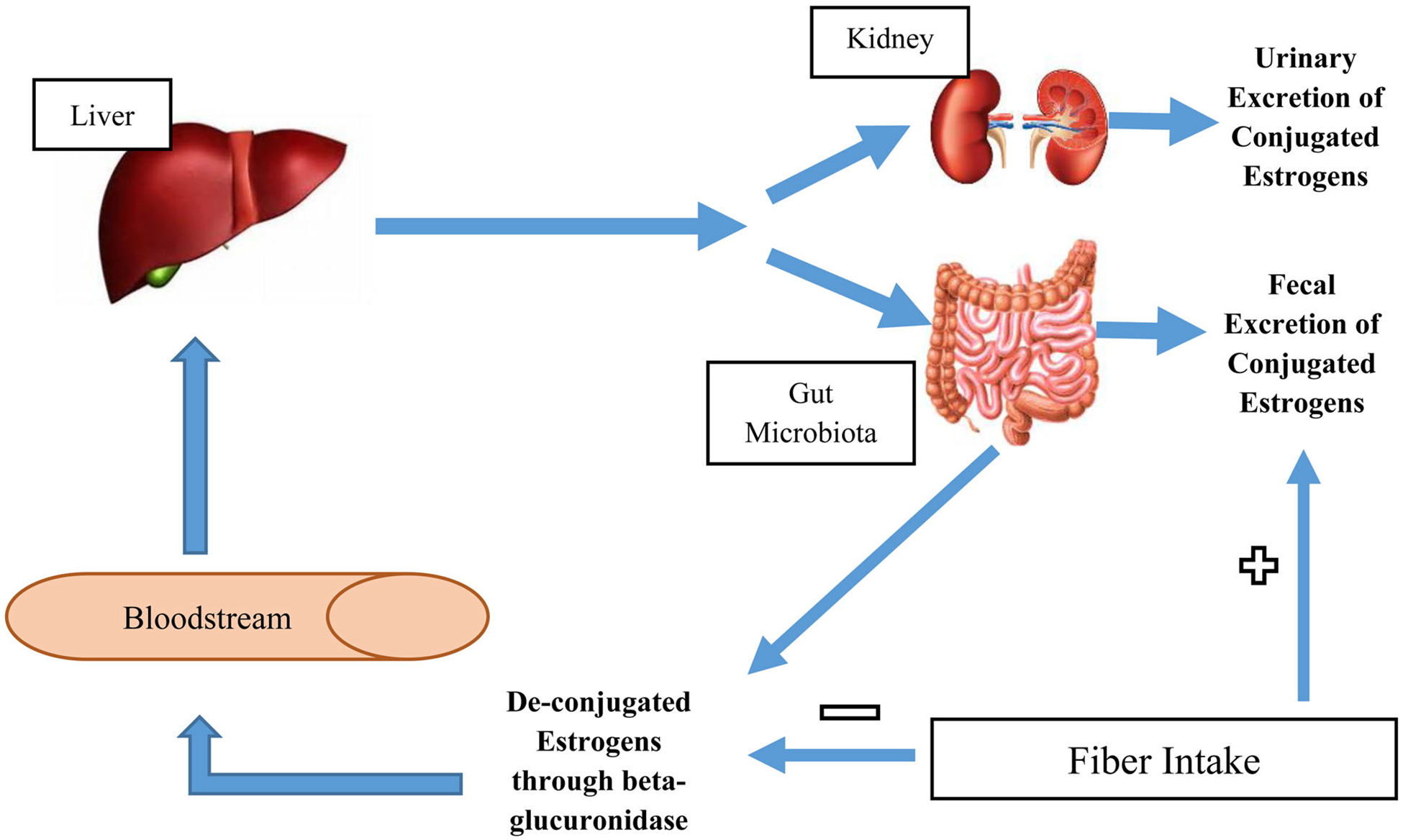
Conceptual Framework Exhibiting the Relationship between Fiber Intake, Gut Microbiota and Estrogen Metabolism (Adopted from Kwa, et al. 2016) (18).
Further, dietary fiber may alter the gut microbiota and influence estradiol metabolism through specific enzyme activities, such as β-glucuronidase (14). Normally, estrogens circulate throughout the body until they reach the liver where they are inactivated through conjugation. Inactivated estrogens are then transported to the intestine for excretion into the stool. However, specific bacterial genera encode β-glucuronidase, which re-activates conjugated estrogens in the gut (see Figure 3). Deconjugated estrogens are reabsorbed and influence estrogen metabolism which is associated with hormone-dependent cancers, such as breast cancer (15, 16). To better understand the association between fiber intake and breast cancer, this study investigates associations between dietary fiber and the gut microbiota that promote β-glucuronidase activity and explores associations with estrogen levels in the blood. We hypothesize that higher levels of dietary fiber will be associated with lower abundance of intestinal microbiota that promote β-glucuronidase activity while lower abundance of intestinal microbiota will be significantly associated with lower levels of circulating 17β-estradiol and estrone.
Figure 3.
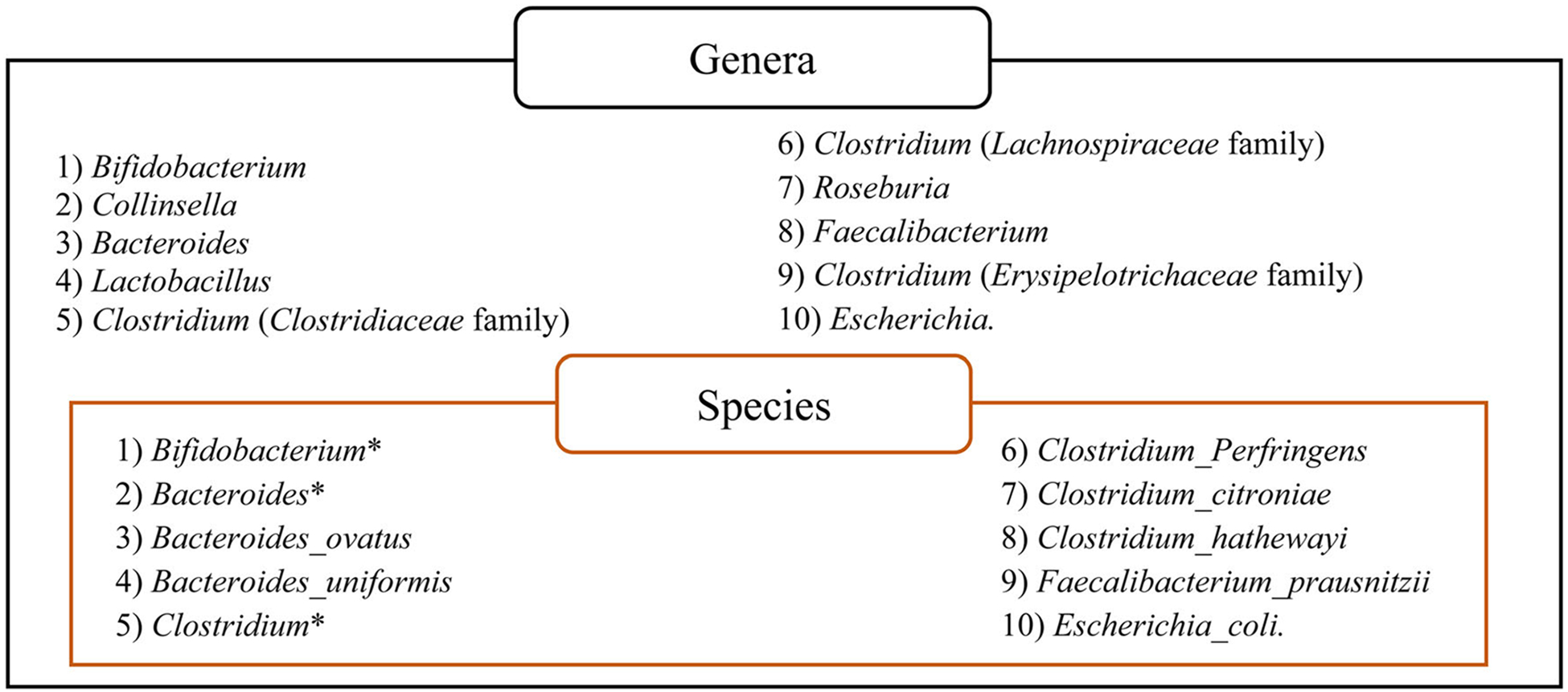
ß-glucuronidase encoding bacteria (Adapted from Kwa, et al. 2016) (18).
*Represents unnamed but previously identified bacterial species/OTUs within the genera
Materials and Methods
Experimental Plan
Study Design
This cross-sectional study includes baseline data from a randomized controlled trial of weight loss conducted among 29 post-menopausal women who were newly-diagnosed with stage 0–II breast cancer and treatment naïve. The detailed methods of this trial have been published previously and are summarized below (17).
Recruitment/Consent
Study subjects were recruited from the University of Alabama at Birmingham (UAB) Kirklin Interdisciplinary Breast Health Clinic (Birmingham, Alabama, USA). The trial was registered with the National Clinical Trials database (NCT02224807) and approved by the UAB Institutional Review Board (IRB-130325009).
Eligibility criteria for the study included being overweight or obese (BMI of 25–60 kg/m2) with histopathologically-confirmed stage 0–II breast cancer and scheduled for surgery as primary treatment. Patients were screened to ensure there were no preexisting medical conditions that would prevent adherence to unsupervised exercise. Also, physician clearance for any conditions, such as resting blood pressures >99 diastolic or >159 systolic, or cardiac anomaly was obtained. Participants had no current medical conditions that would affect weight status, such as Cushing’s syndrome or untreated hypothyroidism, nor additional active malignancy. The participants were newly diagnosed with breast cancer, and hence had yet to begin hormonal therapy, and none of our subjects reported nephritis, enteritis, antibiotic/antimicrobial use, or the use of mitochondrial uncouplers (laxative use was not assessed). Those who met these criteria were informed about the study, provided written informed consent, and were enrolled.
Baseline Assessment
Study staff collected and recorded data for demographics, height/weight, medical history, and medication use (including recent use of antibiotics) (17). A multiple-pass method was used to collect two 24-hour dietary recalls that represented normal eating habits for one weekday and one weekend day. The data were entered and analyzed using the Nutrition Data System for Research (NDSR 2014, Minneapolis, Minnesota, USA). Phlebotomy was performed after a 12-hour fast and serum was obtained. Stool samples were collected using a sterile wipe after a bowel movement prior to the baseline assessment. All samples were stored at −80 °C.
LC-MS Analysis of 17β-Estradiol and Estrone
Assays for 17β-estradiol and estrone in serum were performed in the UAB Targeted Metabolomics and Proteomics Laboratory. Estrogen analyses were determined by isotope dilution HPLC-electrospray ionization-multiple reaction ion mass spectrometry adapted from the method of Tai and Welch (18). 17β-Estradiol and estrone standards were prepared in 0.05% BSA. Sera (500 μl) were diluted 1:1 with MilliQ H2O. Samples and standards were spiked with 0.5 ng/ml 13C6-estradiol (CIL, Tewksbury, MA) internal standard. Diluted samples (1 ml) and standards were loaded onto individual 30 mg Polymeric Strata-X Solid Phase Extraction cartridge columns (Phenomenex, Torrance, CA). The cartridges were washed with MilliQ H2O (1 ml) and 40% methanol (1 ml) followed by elution of 17β-estradiol with 1 ml of methanol. Sample eluents were dried under a gentle stream of N2. Sodium bicarbonate (50 ml, 100 mM, pH 10.5) and dansyl-chloride (50 μl, 1 mg/ml) in acetone were added to samples which were incubated at 60 °C for 10 min. Samples were dried once more under a gentle stream of N2 followed by reconstitution in 100 ml of 40% methanol/0.1% formic acid (FA). Chromatography was carried out using an Ace Excel C18-Aromatic 1.7 mm 50 × 3.0 mm IS column at 50 °C using a 20AD Prominence HPLC (Shimadzu, Kyoto, Japan) in tandem with 6500 Qtrap mass spectrometer (SCIEX, Framingham, MA). LC-MS operation and data collection were under the control of Analyst 1.6.2 software (SCIEX). The mobile phases were composed of (A) 0.1% FA and (B) acetonitrile 0.1% FA; the flow rate was 300 μl/min. Gradient starting conditions were 50% B which was held for 1 min, a linear increase of B to 100% B at 4 min, held at 100% B until 4.75 min, and returned to 50% B at 5 mins, to equilibrate to starting conditions until 7 min. LC flow was diverted to waste for the first 1.8 min to prevent salt contaminating the MS front end. The MS was operated in positive electrospray ionization mode with the following parameters: curtain gas 30, collision gas medium, temperature 500, ion spray voltage 5000, collision energy 25, GS1 60 and GS2 60. Mass transitions for multiple-reaction-monitoring mode were m/z 506/171 for dansyl-17β-estradiol, 504/171 for dansyl-estrone and m/z 512/171 for 13C6-dansyl-17β-estradiol. The standard curve ranged from 5 – 5000 pg/ml over seven points. All data were processed, and concentration factors were corrected using Multiquant 3.0.1 (SCIEX).
Fecal Microbe Analysis
Stool samples were collected by participants after defecation using a sterile wipe, placed in a plastic bag, and kept in their home freezer until baseline assessment, at which time the samples were collected and stored at −80 °C until analyzed.
Fecal DNA extraction was carried-out using Zymo Fecal DNA Miniprep kit. Microbiome analysis targeting the V4 region of the 16S rRNA gene was performed using an Illumina MiSeq (19). The post DNA sequence analysis used Quantitative Insight into Microbial Ecology (QIIME) suite, V.1.7 with modifications as described in Kumar et al. (19) and Fruge et al. (20, 21).
Variables and Statistical Analyses
This study was a secondary analysis that utilized data on 29 post-menopausal women. The outcome variables of interest were dietary intake of fiber, serum 17β-estradiol, BMI, and the bacterial genera and species that colonize the human intestinal tract that encode for ß-glucuronidase based on the findings of Kwa et al. (15).
Normality tests for all variables of interest were assessed. Due to non-normal distribution, the Spearman rank test was utilized to reveal any potential associations between gut microbiota, 17β-estradiol, estrone, and dietary fiber (total, soluble and insoluble). In addition to the individual correlations between gut microbiota at the genera and species levels, the microbiota linked at phylum, family and class levels were combined, and the correlations between these, 17β-estradiol, estrone and the dietary fiber types were explored. Further analyses, including partial correlation tests between the dietary fiber types and specific microbiota, were performed by controlling for BMI and age. Control for the presence of diabetes was considered, but because no significant differences were found in the microbiota among participants with this comorbidity vs. those without, it was not used as a covariate. All analyses were performed using IBM SPSS (version 24.0).
Results
A total of 32 women enrolled in the study and provided fasting blood and fecal samples. We excluded premenopausal (n = 2) and perimenopausal (n = 1) women because fluctuating hormone levels affect circulating estrogen concentrations. Characteristics of participants are provided in Table 1. The study participants had a mean age of 62.4 years and 82.1% were obese. Most were Non-Hispanic White or African-American, the remainder were of mixed race. Most were recently diagnosed with invasive cancers that were both estrogen- and progesterone-positive, though none had begun hormonal therapy. Other medications found to affect the microflora, eg., antibiotics/antimicrobials and mitochondrial uncouplers were not reported by any participants.
Table 1.
Characteristics of post-menopausal treatment-naïve women with stage 0-II breast cancer (n = 29).
| Variable | Mean (SD) | Range |
|---|---|---|
| Age (years) | 62.3 (8.5) | 51–85 |
| BMI (kg/m2) | 34.6 (5.8) | 25.9–47.8 |
| Weight (kg) | 90.1 (16.6) | 58.4–124.7 |
| N | % | |
| Clinical Stage | ||
| In situ | 7 | 24.1 |
| Invasive | 22 | 75.9 |
| Biopsy Grade | ||
| Low | 2 | 6.9 |
| Low-Intermediate | 1 | 3.5 |
| Intermediate | 8 | 27.6 |
| Intermediate-High | 9 | 31 |
| High | 9 | 31 |
| Hormone receptor status | ||
| Estrogen Receptor Positive | 26 | 89.7 |
| Progesterone Receptor Positive | 20 | 69 |
| Comorbidities | ||
| 0 | 1 | 3.4 |
| 1–2 | 10 | 34.5 |
| 3+ | 18 | 62 |
| Race | ||
| African-American | 12 | 41.4 |
| Non-Hispanic White | 15 | 51.7 |
| More than one race | 2 | 6.9 |
| Cardiovascular disease | 4 | 13.8 |
| Diabetes mellitus | 9 | 31 |
| Smoker | 2 | 6.9 |
Table 2 presents data on dietary fiber, microbiota, estradiol concentrations, and the relative abundance of investigated species and genera. Total dietary fiber intake was roughly 14 g/day of which insoluble fiber comprised the major proportion. The relative abundance values of each phylum for each subject are presented in Figure 4. The most common phyla were Firmicutes and Bacteroidetes. At the genus and species levels, a higher abundance of Faecalibacterium and a lower abundance of Bifidobacterium, Clostridium-perfringens, and Clostridium hathewayi were detected in the samples (Table 2).
Table 2.
Dietary fiber intake, fecal microbiota, and serum estradiol concentrations (N = 29).
| Mean | SD | Range | |
|---|---|---|---|
| Total Dietary Fiber (g/day) | 14.22 | 6.94 | 5.23 – 39.67 |
| Soluble Dietary Fiber (g/day) | 4.54 | 1.79 | 2.01 – 8.88 |
| Insoluble Dietary Fiber (g/day) | 9.67 | 5.96 | 2.32 – 33.81 |
| Serum estradiol (pg/ml)* | 17.68 | 15.12 | 4.3 – 79.5 |
| Serum estrone (pg/ml)* | 198.30 | 114.43 | 21.8 – 535.1 |
| Bacteroides;s__ | 2.63% | 4.77% | 0.002 – 22.301% |
| Bacteroides;s__ovatus | 0.02% | 0.03% | 0.000 – 0.157% |
| Bacteroides;s__uniformis | 0.69% | 1.11% | 0.000 – 4.352% |
| Clostridium;s__ | 0.47% | 0.51% | 0.002 – 1.959% |
| Clostridium;s__perfringens | 0.01% | 0.04% | 0.000 – 0.198% |
| Clostridium;s__citroniae | 0.92% | 3.95% | 0.000 – 21.321% |
| Clostridium;s__hathewayi | 0.01% | 0.03% | 0.000 – 0.106% |
| Faecalibacterium;s__prausnitzii | 9.24% | 8.56% | 0.011 – 29.509% |
| Escherichia;s__coli | 1.63% | 4.46% | 0.002 – 21.331% |
| g__Bifidobacterium | 1.26% | 2.00% | 0.004 – 7.285% |
| g__Collinsella | 1.48% | 1.91% | 0.000 – 6.333% |
| g__Bacteroides | 5.79% | 8.63% | 0.005 – 31.364% |
| g__Lactobacillus | 0.69% | 1.20% | 0.031 – 6.360% |
| g__Clostridium (Clostridiaceae family) | 0.56% | 0.56% | 0.002 – 1.959% |
| g__Clostridium (Lachnospiraceae family) | 0.94% | 3.95% | 0.002 – 21.353% |
| g__Roseburia | 3.61% | 4.03% | 0.020 – 16.531% |
| g__Faecalibacterium | 9.32% | 8.64% | 0.010 – 29.688% |
| g__Clostridium (Erysipelotrichaceae family) | 0.17% | 0.44% | 0.000 – 1.830% |
| g__Escherichia | 1.63% | 4.46% | 0.002 – 21.331% |
Sample size reduced from 29 to 28 from lack of blood sample for one participant.
Figure 4.
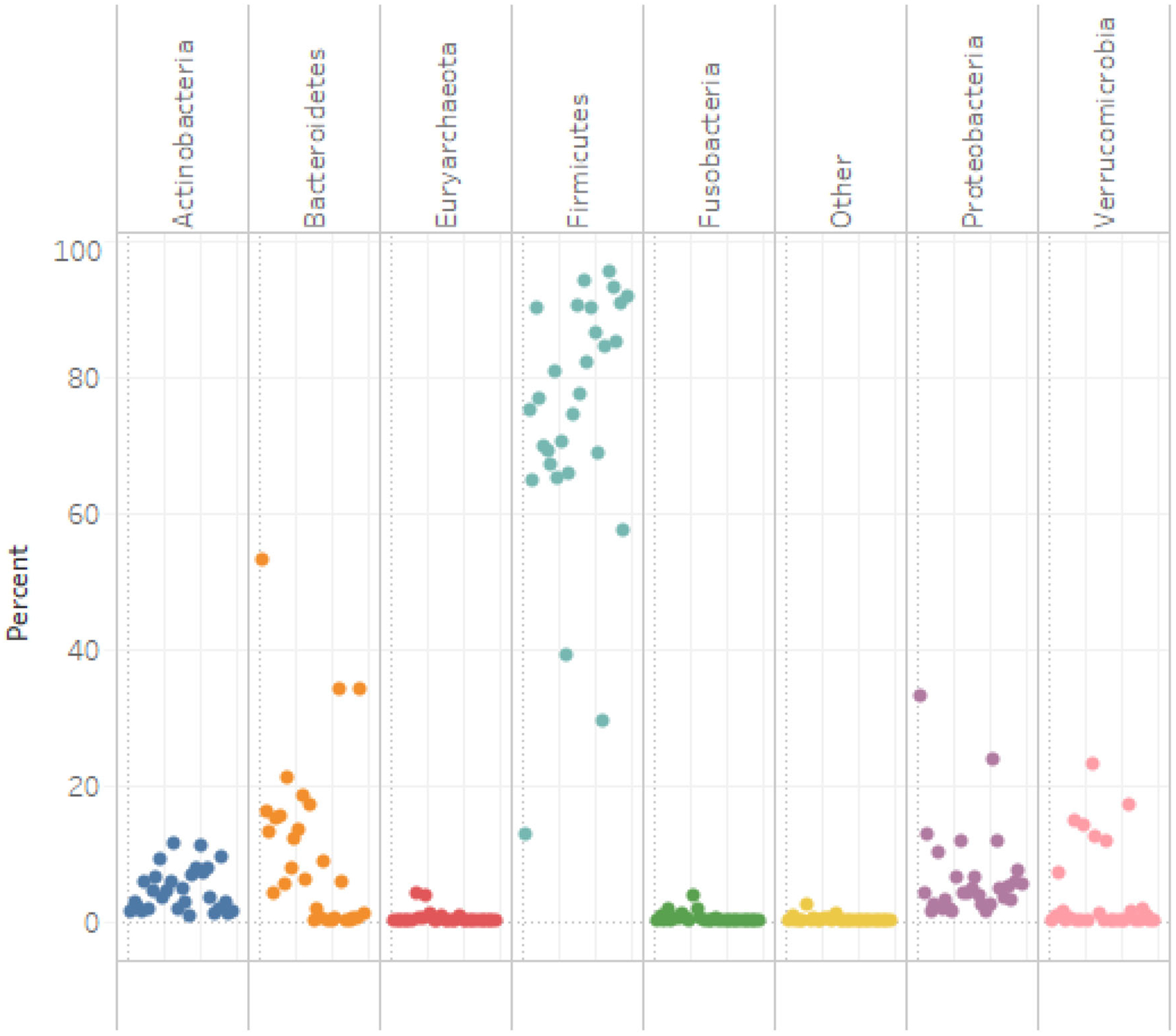
Phylum Level Relative Abundance of fecal bacteria from 29 treatment-naïve post-menopausal women with stage 0-II breast cancer.
Correlations between dietary fiber types, gut microbiota, 17β-estradiol and estrone concentrations are displayed in Table 3. The results indicate that total dietary fiber intake is significantly and inversely associated with Clostridium hathewayi sp. (r= −0.419; p = 0.024). While the strength of association was somewhat weaker, soluble dietary fiber was significantly and inversely associated with Clostridium (r=−0.11; p = 0.02). Both of these inverse relationships continued to be observed after controlling for age and BMI. Also, insoluble dietary fiber was significantly and positively associated with Bacteroides uniformis sp. (r = 0.382; p= 0.041), again an association that remained after controlling for age and BMI. There were marginally significant correlations between certain gut microbiota and dietary fiber types (Table 3). For example, the association between insoluble dietary fiber and Clostridium hathewayi sp. was marginally significant (r= −0.31 p = 0.066). There also were mar-significant positive associations between Escherichia coli sp., Escherichia and total dietary fiber (r = 0.35; p = 0.059).
Table 3.
Associations between dietary fiber intake, gut microbiota, and serum estradiol concentrations among stage 0-II breast cancer patients (n = 29).
| Total Dietary Fiber (g/day) | Soluble Dietary Fiber (g/day) | Insoluble Dietary Fiber (g/day) | |
|---|---|---|---|
| Serum Estradiol (pg/ml)** | −0.21 (p = 0.28) | −0.30 (p = 0.12) | −0.08 (p = 0.70) |
| Serum Estrone (pg/ml)** | −0.08 (p = 0.70) | −0.17 (p = 0.40) | 0.07 (p = 0.74) |
| Species | |||
| Bifidobacterium;s__ | 0.07 (p = 0.71) | 0.11 (p = 0.56) | 0.02 (p = 0.91) |
| Bacteroides;s__ | 0.18 (p = 0.35) | 0.00 (p = 1.00) | 0.28 (p = 0.14) |
| Bacteroides;s__ovatus | 0.14 (p = 0.46) | −0.01 (p = 0.96) | 0.22 (p = 0.26) |
| Bacteroides;s__uniformis | 0.30 (p = 0.11) | 0.12 (p = 0.54) | .382* (p = 0.04) |
| Clostridium;s__ | 0.17 (p = 0.39) | 0.10 (p = 0.62) | 0.22 (p = 0.26) |
| Clostridium;s__perfringens | 0.08 (p = 0.69) | 0.06 (p = 0.77) | 0.04 (p = 0.83) |
| Clostridium;s__citroniae | −0.12 (p = 0.53) | −0.24 (p = 0.22) | −.0.08 (p = 0.68) |
| Clostridium;s__hathewayi | − .419* (p = 0.02) | −0.31 (p = 0.10) | −0.35 (p = 0.07) |
| Faecalibacterium;s_prausnitzii | 0.09 (p = 0.63) | 0.00 (p = 0.98) | 0.12 (p = 0.54) |
| Escherichia;s__coli | 0.35 (p = 0.06) | 0.14 (p = 0.48) | 0.30 (p = 0.12) |
| Genera | |||
| g__Bifidobacterium | 0.27 (p = 0.16) | 0.29 (p = 0.13) | 0.19 (p = 0.31) |
| g__Collinsella | −0.08 (p = 0.67) | −0.16 (p = 0.41) | −0.07 (p = 0.70) |
| g__Bacteroides | 0.25 (p = 0.20) | 0.06 (p = 0.77) | 0.34 (p = 0.07) |
| g__Lactobacillus | −0.07 (p = 0.71) | −0.12 (p = 0.54) | −0.03 (p = 0.89) |
| g__Clostridium (Clostridiaceae family) | 0.20 (p = 0.29) | 0.15 (p = 0.43) | 0.23 (p = 0.23) |
| g__Clostridium (Lachnospiraceae family) | −0.21 (p = 0.28) | −0.22 (p = 0.25) | −0.17 (p = 0.36) |
| g__Roseburia | −0.08 (p = 0.66) | −0.17 (p = 0.37) | −0.10 (p = 0.62) |
| g__Faecalibacterium | 0.09 (p = 0.63) | 0.00 (p = 0.98) | 0.12 (p = 0.54) |
| g__Clostridium (Erysipelotrichaceae family) | −0.16 (p = 0.40) | −0.11* (p = 0.02) | −0.15 (p = 0.44) |
| g__Escherichia | 0.35 (p = 0.06) | 0.14 (p = 0.48) | 0.30 (p = 0.12) |
| SUM_Species | 0.29 (p = 0.12) | 0.22 (p = 0.26) | 0.29 (p = 0.13) |
| SUM_Genera | 0.33 (p = 0.08) | 0.22 (p = 0.26) | 0.31 (p = 0.10) |
significant at the p<.05 level; these associations were stable after controlling for BMI and age.
n = 28 for this analysis since serum was unavailable for one participant.
Relatedly, there was a trend toward an inverse association between soluble dietary fiber and 17β-estradiol levels (r= −0.30; p = 0.12), albeit statistically significant. Serumin 17β-estradiol and estrone levels were not correlated either with species/genera nor with dietary fiber types. Moreover, combined genera and species at the phylum, family and class levels and the summative proportion of the microbes at the genus and species levels did not reveal any significant associations.
Discussion
Our study is one of the few to examine the relationship between the gut microbiota that promote ß-glucuronidase activity, dietary fiber and circulating 17β-estradiol and estrone. Contrary to our hypothesis, the results of this study indicate that dietary fiber intake had no relationship to estrogen levels in the blood. However, we found that higher levels of total and soluble dietary fibers correlate with lower levels of Clostridium hathewayi sp. and Clostridium (Erysipelotrichaceae family), respectively. These bacteria promote ß-glucuronidase activity and the inverse relationship between them and dietary fiber continued to be observed after controlling for age and BMI. Contrary to these findings, we also found a positive and significant relationship between insoluble fiber and Bacteroides uniformis which also promotes ß-glucuronidase activity. Given the important role of ß-glucuronidase enzyme in luminal hormone metabolism, this exploratory analysis provides valuable insights for future studies.
To date, no studies have found a link between Clostridium hathewayi and dietary fiber. However, our results demonstrated the significant inverse relationship between total dietary fiber intake and Clostridium hathewayi, a newly discovered Clostridium species (22), which has been implicated in clinical diseases, such as sepsis and infection (23–27). Thus, the association between total dietary fiber and Clostridium hathewayi may have important implications regarding the prevention of both infectious diseases, as well as those that are hormonally driven.
Another inverse relationship observed was between soluble dietary fiber and Clostridium. However, this result is not in accordance with findings reported by Chinda et al. (28) and Bang et al. (29) who reported that pectin, which is a soluble fiber, was associated with higher levels of Clostridium. These studies were conducted exclusively in healthy males, unlike our study which included female postmenopausal breast cancer patients, and included far fewer study participants, ie., n = 14 and n = 3, respectively. Also, our outcome variables included total soluble dietary fiber, rather than pectin alone. Because Clostridium produces the short chain fatty acids acetate and butyrate it might be important for enhancing intestinal peristalsis and in preventing/treating constipation, diarrhea, and colitis (29). Therefore, further research is warranted to clarify the relationship between dietary fiber and Clostridium and elucidate specific mechanistic relationships.
Among the three dietary fiber types, the only significant positive relationship we found was between insoluble fiber and Bacteroides uniformis. Higher levels of insoluble fiber were associated with higher levels of Bacteroides uniformis. This finding may relate to the influence of Bacteroides uniformis on glycolysis pathways. According to a study that was conducted by Benítez-Páez on 75 full-term newborns, “B. uniformis strains exhibit an expanded glycolytic capability when compared with other Bacteroides species” (30). Enhanced glycolysis is related to higher glucose uptake, and it is currently used as an indicator of malignancy since it represents an evident characteristic of many cancers (31, 32). Considering the relationship between Bacteroides uniformis and glycolysis, our results, thereby, might shed light on understanding the influence of Bacteroides uniformis on glycolysis and its possible physiological effects in breast cancer.
Although we found some significant associations, we did not find support for our hypothesis regarding the relationship between dietary fiber and levels of circulating estrogens. We found only a weak inverse association between soluble fiber and 17β-estradiol. This result is consistent with the findings of Gaskins et al. (2009) who observed a significant and inverse relationship between dietary fiber consumption and 17β-estradiol concentrations among 250 premenopausal healthy women (aged 18–44 y) (13). In this much larger prospective cohort study, both soluble and insoluble fiber had an inverse relationship with 17β-estradiol concentrations (β = −0.222, p = 0.01; β = −0.057, p = 0.02, respectively) (13). Despite highly fluctuating 17β-estradiol levels among premenopausal women, the significant associations they found suggest that if this relationship was explored among larger samples of postmenopausal women, significant results might be attained.
Ours is the first study to correlate dietary constituents and 17β-estradiol/estrone levels, and to explore their relationship with gut microbiota involved with β-glucuronidase activity among post-menopausal women with breast cancer. However, it is important to recognize study limitations, which include a small sample size and cross-sectional design that resulted in a limited ability to detect associations and reduced the statistical power. Although we adjusted for BMI and age, our results could still have been affected by residual or unmeasured confounding; for example, we did not collect data on laxative use which may have affected the results. The range of 17β-estradiol and estrone levels in post-menopausal women, for example, is narrow and levels are low - thus the distribution may not have been sufficient to detect correlations. Moreover, because our sample was fairly circumscribed, ie., post-menopausal breast cancer patients, our results may not generalize to other populations, such as females without breast cancer and males. Finally, multiple testing and the chance of uncovering associations that are spurious is a limitation of this study. Despite these limitations, this study makes a unique contribution, first because very few studies have investigated microbiota among populations with breast cancer, and second, this study relies on dietary data that includes soluble, insoluble, and total fiber.
Conclusion
The role of bacterial ß-glucuronidase activity in breast cancer risk is still obscure. The influence of dietary fiber on this activity has an important bearing on understanding the link between estrogen metabolism and breast cancer. By being one of the few studies investigating the triad of these overlapping relationships among dietary fiber, ß-glucuronidase activity and serum estrogens, our study provides insight and direction for future studies.
Funding
This study was supported by The National Cancer Institute R21 (CA178359), P30 (CA013148), and R25 (CA047888), UAB Cancer Research Experiences for Students (CaRES) Program, 5R25CA076023, UAB Center for Clinical Translational Science (UL1TR00065, UL1TR001417), the Heflin Center for Genomic Sciences, and the UAB Health Services Foundation General Endowment Fund (funding for the mass spectrometer used in this study). The following are acknowledged for their support of the Microbiome Resource at the University of Alabama at Birmingham: Comprehensive Cancer Center (P30AR050948), Center for Clinical Translational Science (UL1TR001417), University Wide Institutional Core, Heflin Center for Genomic Sciences and Microbiome Center.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Breast Cancer Facts & Figures 2018. Atlanta: American Cancer Society, 2018. [Google Scholar]
- 3.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36(1):114–36. doi: 10.1093/epirev/mxt010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X [DOI] [PubMed] [Google Scholar]
- 5.Rossi RE, Pericleous M, Mandair D, Whyand T, Caplin ME. The role of dietary factors in prevention and progression of breast cancer. Anticancer Res. 2014;34(12):6861–75. [PubMed] [Google Scholar]
- 6.Kapil U, Bhadoria AS, Sareen N, Singh P, Dwivedi SN. Total cholesterol and triglyceride levels in patients with breast cancer. J Breast Cancer. 2013;16(1): 129–30. doi: 10.4048/jbc.2013.16.1.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Dietary protein sources in early adulthood and breast cancer incidence: prospective cohort study. BMJ. 2014;348:g3437doi: 10.1136/bmj.g3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catsburg C, Kim RS, Kirsh VA, Soskolne CL, Kreiger N, Rohan TE. Dietary patterns and breast cancer risk: a study in 2 cohorts. Am J Clin Nutr. 2015;101(4): 817–23. doi: 10.3945/ajcn.114.097659 [DOI] [PubMed] [Google Scholar]
- 9.Link LB, Canchola AJ, Bernstein L, Clarke CA, Stram DO, Ursin G, Horn-Ross PL. Dietary patterns and breast cancer risk in the California Teachers Study cohort. Am J Clin Nutr. 2013;98(6):1524–32. doi: 10.3945/ajcn.113.061184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aune D, Chan DSM, Greenwood DC, Vieira AR, Rosenblatt DAN, Vieira R, Norat T. Dietary fiber and breast cancer risk: a systematic review and meta-analysis of prospective studies. Ann Oncol. 2012;23(6): 1394–402. doi: 10.1093/annonc/mdr589 [DOI] [PubMed] [Google Scholar]
- 11.Anderson JW, Baird P, Davis RH, Ferreri S, Knudtson M, Koraym A, Waters V, Williams CL. Health benefits of dietary fiber. Nutr Rev. 2009;67(4): 188–205. doi: 10.1111/j.1753-4887.2009.00189.x [DOI] [PubMed] [Google Scholar]
- 12.Fujii H, Iwase M, Ohkuma T, Ogata-Kaizu S, Ide H, Kikuchi Y, Idewaki Y, Joudai T, Hirakawa Y, Uchida K, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12: 159. doi: 10.1186/1475-2891-12-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaskins AJ, Mumford SL, Zhang C, Wactawski-Wende J, Hovey KM, Whitcomb BW, Howards PP, Perkins NJ, Yeung E, Schisterman EF, BioCycle Study Group, et al. Effect of daily fiber intake on reproductive function: the BioCycle study. Am J Clin Nutr. 2009;90(4):1061–9.: doi: 10.3945/ajcn.2009.27990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki R, Rylander-Rudqvist T, Ye W, Saji S, Adlercreutz H, Wolk A. Dietary fiber intake and risk of postmenopausal breast cancer defined by estrogen and progesterone receptor status-a prospective cohort study among Swedish women. Int J Cancer. 2008; 122(2):403–12. doi: 10.1002/ijc.23060 [DOI] [PubMed] [Google Scholar]
- 15.Kwa M, Plottel CS, Blaser MJ, Adams S. The intestinal microbiome and estrogen receptor–positive female breast cancer. J National Cancer Inst. 2016; 108:djw029. doi: 10.1093/jnci/djw029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raftogianis R, Creveling C, Weinshilboum R, Weisz J. Estrogen metabolism by conjugation. JNCI Monogr. 2000;2000(27):113–24. doi: 10.1093/oxfordjournals.jnci-monographs.a024234 [DOI] [PubMed] [Google Scholar]
- 17.Tsuruta Y, Rogers LQ, Krontiras H, Grizzle WE, Frugé AD, Oster RA, Umphrey HR, Jones LW, Azrad M, Demark-Wahnefried W, et al. Exploring effects of presurgical weight loss among women with stage 0-II breast cancer: protocol for a randomised controlled feasibility trial. BMJ Open. 2016;6(9):e012320. doi: 10.1136/bmjopen-2016-012320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tai SS, Welch MJ. Development and evaluation of a reference measurement procedure for the determination of estradiol-17beta in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2005;77(19):6359–63. doi: 10.1021/ac050837i [DOI] [PubMed] [Google Scholar]
- 19.Kumar R, Eipers P, Little RB, Crowley M, Crossman DK, Lefkowitz EJ, Morrow CD. Getting started with microbiome analysis: sample acquisition to bioinformatics. Curr Protoc Hum Genet. 2014;82:18.8.1–29. doi: 10.1002/0471142905.hg1808s82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frugé AD, Ptacek T, Tsuruta Y, Morrow CD, Azrad M, Desmond RA, Hunter GR, Rais-Bahrami S, Demark-Wahnefried W. Dietary changes impact the gut microbe composition in overweight and obese men with prostate cancer undergoing radical prostatectomy. J Acad Nutr Diet. 2018;118(4): 714–23.e1. doi: 10.1016/j.jand.2016.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fruge AD, Van der Pol W, Rogers LQ, Morrow CD, Tsuruta Y, et al. Fecal akkermansia muciniphila is associated with body composition and microbiota diversity in overweight and obese women with breast cancer participating in a presurgical weight loss trial. J Acad Nutr Diet. 2020;120(4):650–9. doi: 10.1016/j.jand.2018.08.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steer T, Collins MD, Gibson GR, Hippe H, Lawson PA. Clostridium hathewayi sp. nov., from human faeces. Syst Appl Microbiol. 2001;24(3):353–7. doi: 10.1078/0723-2020-00044 [DOI] [PubMed] [Google Scholar]
- 23.Linscott AJ, Flamholtz RB, Shukla D, Song Y, Liu C, Finegold SM. Fatal septicemia due to Clostridium hathewayi and Campylobacter hominis. Anaerobe. 2005;11(1–2):97–8. doi: 10.1016/j.anaerobe.2004.10.002 [DOI] [PubMed] [Google Scholar]
- 24.Woo PCY, Lau SKP, Woo GKS, Fung AMY, Yiu VPY, Yuen K-Y. Bacteremia due to Clostridium hathewayi in a patient with acute appendicitis. J Clin Microbiol. 2004;42(12):5947–9. doi: 10.1128/jcm.42.12.5947-5949.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tena D, Losa C, Medina-Pascual MJ, Sáez-Nieto JA. Fournier’s gangrene caused by Actinomyces funkei, Fusobacterium gonidiaformans and Clostridium hathewayi. Anaerobe. 2014;27:14–6. doi: 10.1016/j.anaerobe.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 26.Dababneh AS, Nagpal A, Palraj BR, Sohail MR. Clostridium hathewayi bacteraemia and surgical site infection after uterine myomectomy. BMJ Case Rep. 2014;2014. doi: 10.1136/bcr-2013-009322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sameer E, Kunyan Z. Human infection caused by Clostridium hathewayi. Emerg Infect Dis J. 2004;10: 1950. doi: 10.3201/eid1011.040006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinda D, Nakaji S, Fukuda S, Sakamoto J, Shimoyama T, Nakamura T, Fujisawa T, Terada A, Sugawara K. The fermentation of different dietary fibers is associated with fecal clostridia levels in men. J Nutr. 2004;134(8):1881–6. doi: 10.1093/jn/134.8.1881 [DOI] [PubMed] [Google Scholar]
- 29.Bang S-J, Kim G, Lim MY, Song E-J, Jung D-H, Kum J-S, Nam Y-D, Park C-S, Seo D-H. The influence of in vitro pectin fermentation on the human fecal microbiome. AMB Express. 2018;8(1):98. doi: 10.1186/s13568-018-0629-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benítez-Páez A, Gómez del Pulgar EM, Sanz Y. The glycolytic versatility of bacteroides uniformis CECT 7771 and its genome response to oligo and polysaccharides. Front Cell Infect Microbiol. 2017;7:383. doi: 10.3389/fcimb.2017.00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivenzon-Segal D, Margalit R, Degani H. Glycolysis as a metabolic marker in orthotopic breast cancer, monitored by in vivo (13)C MRS. Am J Physiol Endocrinol Metab. 2002;283(4):E623–E630. doi: 10.1152/ajpendo.00050.2002 [DOI] [PubMed] [Google Scholar]
- 32.van der Hiel B, Pauwels EK, Stokkel MP. Positron emission tomography with 2-[18F]-fluoro-2-deoxy-D-glucose in oncology. Part IIIa: therapy response monitoring in breast cancer, lymphoma and gliomas. J Cancer Res Clin Oncol. 2001;127(5):269–77. doi: 10.1007/s004320000191 [DOI] [PubMed] [Google Scholar]


