Abstract
Prior studies have reported white matter abnormalities associated with a history of cumulative concussion and/or repetitive head impacts (RHI) in contact sport athletes. Growing evidence suggests these abnormalities may begin as more subtle changes earlier in life in active younger athletes. We investigated the relationship between prior concussion and contact sport exposure with multi-modal white matter microstructure and macrostructure using magnetic resonance imaging. High school and collegiate athletes (n = 121) completed up to four evaluations involving neuroimaging. Linear mixed-effects models examined associations of years of contact sport exposure (i.e., RHI proxy) and prior concussion across multiple metrics of white matter, including total white matter volume, diffusion tensor imaging (DTI) metrics, diffusion kurtosis imaging (DKI) metrics, and quantitative susceptibility mapping (QSM). A significant inverse association between cumulative years of contact sport exposure and QSM was observed, F(1, 237.77) = 4.67, p = 0.032. Cumulative contact sport exposure was also associated with decreased radial diffusivity, F(1, 114.56) = 5.81, p = 0.018, as well as elevated fractional anisotropy, F(1, 115.32) = 5.40, p = 0.022, and radial kurtosis, F(1, 113.45) = 4.03, p = 0.047. In contrast, macroscopic white matter volume was not significantly associated with cumulative contact sport exposure (p > 0.05). Concussion history was not significantly associated with QSM, DTI, DKI, or white matter volume (all, p > 0.05). Cumulative contact sport exposure is associated with subtle differences in white matter microstructure, but not gross white matter macrostructure, in young active athletes. Longitudinal follow-up is required to assess the progression of these findings to determine their contribution to potential adverse effects later in life.
Keywords: concussion, diffusion kurtosis imaging, diffusion tensor imaging, quantitative susceptibility mapping, repetitive head impacts
Introduction
Multiple prior studies have documented various macroscopic and microstructural chronic brain abnormalities in white matter among former contact sport athletes, including those with and without a history of concussion.1–5 While alterations in white matter microstructure have routinely been documented following acute concussion6-8 and have been observed more chronically following a single concussion,9-11 growing evidence suggests that cumulative contact sport exposure may have differential effects on white matter structure in former contact sport athletes. For example, in retired older athletes, increased white matter signal abnormalities (i.e., hypointensities on T1-weighted images) have been observed as being positively associated with repetitive head impacts (RHI) based on an advanced quantification metric of RHI.5,12 Further, years of contact sport exposure, a common proxy measure for RHI, has been observed as significantly predicting white matter rarefaction (i.e., decreased macroscopic white matter integrity) on autopsy.13
Findings based on active younger athletes increasingly support the notion that adverse long-term outcomes associated with cumulative concussion and/or contact sport exposure may begin at earlier periods in life. For example, Churchill and colleagues demonstrated white matter differences (i.e., elevated fractional anisotropy [FA] and decreased mean diffusivity [MD]) among 37 active collegiate sport athletes with a history of remote concussion, as compared to matched controls with no prior history of concussion.14 The study also reported greater density and coherence of orientation of neurites within white matter among the same cohort of athletes. Additionally, preliminary evidence for the cumulative effects of RHI among younger active athletes has been observed, as a single season of American football participation has been associated with changes in white matter microstructure.15,16
Most studies to this point have investigated white matter integrity abnormalities in former and current athletes through various means, such as gross macroscopic volume or microstructural changes as measured via water diffusion properties (i.e., diffusion magnetic resonance imaging [MRI]). As a quantitative extension of routinely applied susceptibility-weighted MRI (SWI) sequences, quantitative susceptibility mapping (QSM) utilizes raw MRI signal extracted from SWI to estimate a scalar magnetic susceptibility value for each imaged tissue voxel.17 Our group recently reported on the efficacy of QSM in detecting magnetism changes within the white matter acutely following sport-related concussion.18 Given these previous findings and the inherent sensitivity of QSM to changes in tissue microstructure and composition, QSM may also be useful in elucidating the processes underlying white matter tissue abnormalities associated with remote prior concussion and cumulative RHI. The association between QSM with cumulative contact sport exposure and remote concussion history, particularly as they relate to other macroscopic and microstructural measures of white matter, have not been investigated.
Examining structural brain abnormalities using multi-modal metrics of white matter, particularly within younger active athletes, contains the potential to provide insight into the process underlying the effects of cumulative RHI and/or concussion on long-term outcomes later in life. Specifically, patterns of findings across modalities (i.e., DTI and QSM) have been shown to provide complimentary information regarding underlying white matter pathology following experimental traumatic brain injury.19 Further, examining these changes in younger athletes can provide a greater understanding of the temporal nature of changes across the lifespan (e.g., possible select microstructural changes earlier in life in the absence of macrostructural volume atrophy observed later in life). The aim of the current study is to investigate the association between cumulative contact sport exposure and remote concussion history in young, active athletes with measures of white matter macrostructure (i.e., volume) and microstructure (i.e., diffusion tensor and kurtosis metrics and QSM).
Methods
Participants
This study was approved by the Medical College of Wisconsin Institutional Review Board and the U.S. Department of Defense Human Research Protection Office. As part of a prospective study on the acute effects of concussion, high school and collegiate athletes were enrolled between July 2015 and June 2020. Adult participants and parents of minor participants provided written informed consent. Written assent was provided by minor participants.
As part of the parent study (described in greater detail elsewhere),20 a cohort of football players and various non-contact athletes without a recent concussion (at least 6 months) were enrolled and completed a pre-season visit that included a detailed evaluation and comprehensive structured interview (demographic, health history, academic history). Self-reported years of exposure to contact sport (e.g., football, soccer) throughout the lifespan was recorded and serves as a proxy of RHI. Athletes also reported the number of prior concussions throughout their lifespan after being provided with a standard definition of concussion based on the U.S. Department of Defense definition, which included “a blow to the head followed by a variety of symptoms that may include any of the following: headache, dizziness, loss of balance, blurred vision, seeing stars, feeling in a fog or slowed down, memory problems, poor concentration, nausea or throwing up. Getting knocked out or being unconscious does not always occur with a concussion.”21 In addition to baseline visits, participants completed multiple in-season neuroimaging visits that corresponded with the schedule of acute injury visits for the parent study (i.e., <48 h, 8 days, 15 days, and 45 days).
Exclusion criteria for this study included the following: history or suspicion of conditions known to influence cognition (e.g., epilepsy, moderate to severe TBI, or other neurological disease); injury during the study period that would preclude participation in the study protocol (e.g., concussion); current psychotic disorder or narcotic use, or any contraindication to study procedures (e.g., unable to undergo MRI); or poor scan quality or processing errors (e.g., due to motion artifacts; see below).
Imaging protocol and processing
Images were acquired on a 3 Tesla General Electric MR750 whole-body magnetic resonance scanner using a 32-channel receive coil array. High resolution T1-weighted images (1 mm × 1 mm × 1 mm) were acquired via a magnetization-prepared rapid gradient-echo sequence with the following parameters: field of view = 256 mm; acquisition matrix = 256; 160 slices; slice thickness = 1 mm; repetition time/echo time/inversion time = 7.592/3.008/900 msec; flip angle = 8°. Cortical surfaces were reconstructed and tissue segmentation was performed on the T1-weighted images using the following steps implemented in FreeSurfer v5.3: removal of non-brain tissue, automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter structures, intensity normalization, tessellation of the gray/white matter boundary, automated topology correction, and surface deformation following the intensity gradients of the image in order to accurately place the boundaries between gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). The longitudinal stream was used to extract reliable volume estimates.22 Visual inspection of each step of the processing stream was conducted. For the purposes of this study, the primary macroscopic structural outcome of interest included total segmented cerebral white matter volume.
QSM processing
QSM images were acquired at 0.75 mm × 0.96 mm in-plane resolution across 2-mm axial slices. Phase-sensitive MRI acquired at four echo times (10.4, 17.4, 24.4, and 31.4) msec and a repetition time of 58.6 msec were used to construct magnetic field perturbation maps. The longitudinal component the static magnetic fields, which were utilized for QSM estimation, were derived from the phase-sensitive MRI data. for each reconstructed echo-time image set. Following background field removal from the field maps, susceptibility inversion was performed using an adapted localized processing formulation of the Morphology-Enabled Dipole Inversion (MEDI) algorithm.23 Brain regions of sufficient QSM stability within the anatomic template space were identified by computing coefficients of variation (CV) across a control cohort (n = 68 examinations) from an independent study that utilized identical acquisition parameters.
A stability mask was then constructed from this CV map at a threshold of CV <0.8, which is a QSM stability threshold that has been utilized in previous published studies.18 Individual and group analyses were enabled via subject image registrations to 2 mm isotropic MNI neurological template space.24 Registrations were performed using software contained within the FSL suite. Regions of interest (ROIs) were obtained from the Johns Hopkins University (JHU) atlas.25,26 Mean QSM was calculated in a primary global white matter ROI derived from the JHU, as well as individual JHU ROIs for secondary, post hoc analyses (Table 1). The utilized compartments were refined using a physics-based masking operation that restricted white matter as χ < 0.03 susceptibility thresholds.27 Reported QSM values are referenced to a CSF baseline, which was computed as the mean CSF compartment susceptibility value averaged across the subject cohort.
Table 1.
Individual ROIs Comprising Global White Matter
| White matter ROIs |
|---|
| Contact sport exposure |
| L. Anterior thalamic radiation |
| R. Anterior thalamic radiation |
| L. Corticospinal tract |
| R. Corticospinal tract |
| L. Cingulum (cingulate gyrus) |
| R. Cingulum (cingulate gyrus) |
| L. Cingulum (hippocampus) |
| R. Cingulum (hippocampus) |
| Forceps major |
| Forceps minor |
| L. Inferior fronto-occipital fasciculus |
| R. Inferior fronto-occipital fasciculus |
| L. Inferior longitudinal fasciculus |
| R. Inferior longitudinal fasciculus |
| L. Superior longitudinal fasciculus |
| R. Superior longitudinal fasciculus |
| L. Uncinate fasciculus |
| R. Uncinate fasciculus |
| L. Superior longitudinal fasciculus (temporal) |
| R. Superior longitudinal fasciculus (temporal) |
ROI, region of interest; L, left; R, right.
DKI/DTI processing
Diffusion-weighted images for DKI were obtained using a single-shot Spin-Echo EPI sequence with 3-mm isotropic voxels and b-values of 1000 sec/mm2 and 2000 sec/mm2, with 30 diffusion directions for each b-value. An additional non–diffusion-weighted Spin-Echo EPI scan was acquired with the phase encode polarity reversed to correct for distortions due to magnetic susceptibility variations using post-processing software tools. Each diffusion-weighted image set was visually inspected for artifacts and those that cleared were processed using an established preprocessing pipeline.33,34 First, spatial distortions caused by magnetic susceptibility variations were corrected using the TOPUP function of the FSL software (version 6.0.0, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki).35 Motion and eddy current related distortions were corrected using the EDDY function of FSL.36 After the preprocessing, images were inspected again to ensure that the processing did not introduce artifacts or distortions. DKE software was utilized to estimate kurtosis and diffusion tensors (www.nitrc.org/projects/dke)28,29 and derive maps of kurtosis fractional anisotropy (KFA), mean kurtosis (MK), radial kurtosis (RK), axial kurtosis (AK), fractional anisotropy (FA), radial diffusivity (RD), axial diffusivity (AD), and mean diffusivity (MD). Images were normalized to standard space using whole-brain white matter skeleton was extracted using the FSL toolbox, Tract-Based Spatial Statistics (TBSS).30 Mean diffusion metrics were obtained from ROIs, as above. Analyses were limited to identical ROI as used in the QSM to maintain consistency with QSM analyses.25,26
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics version 24 (Armonk, NY). Generalized linear mixed-effects models (GLMM) were fit to determine the association of prior concussion and years of contact sport exposure with white matter volume, global white matter diffusion, and global white matter QSM. Age, and body mass index were used as a priori covariates in the analyses. Intracranial volume was included as an additional covariate for the model analyzing white matter volume as the outcome of interest. Concussion history was binned into three groups based on the distribution of the sample, consisting of groups with 0, 1, or 2+ prior concussions. Participant was modeled as a random effect to account for repeat scanning over time. Successive scans were performed over a 45-day period for each participant and sensitivity analyses revealed no current sport group (contact vs. non-contact) by time interaction (i.e., no within-season changes in metrics due to current sport participation). Significant effects of concussion or contact sport history on global diffusion or QSM white matter ROI were followed up with exploratory, post hoc GLMMs to assess subcompartments within that global metric (i.e., individual white matter ROIs that comprise the global white matter metric highlighted in Table 1). Given our a priori hypotheses, statistical significance was evaluated at the 0.05 uncorrected alpha level. For exploratory post hoc ROI analyses, statistical significance was evaluated at the 0.10 level for descriptive purposes.
Results
Participant characteristics
In total, 121 participants (75 football players and 46 non-contact sport athletes) met the criteria for inclusion. The sample mean age was 18.52 years (standard deviation [SD] = 1.74) with an average of 5.01 years (SD = 4.49) of prior contact sport participation and approximately 24.8% of the sample had one or more prior concussions. Demographic history is provided in Table 2.
Table 2.
Demographics and Sample Characteristics
| Demographic | M(SD) or n |
|---|---|
| N | 121 |
| Age | 18.52 (1.74) |
| Race (n) | |
| White | 98 (81.0%) |
| Person of Color | 22 (18.2%) |
| Other | 1 (0.8%) |
| Ethnicity (n) | |
| Non-Hispanic/Latino | 108 (89.3%) |
| Hispanic/Latino | 7 (5.8%) |
| Not reported | 5 (4.2%) |
| Education level | |
| College | 95 (78.4%) |
| High school | 26 (21.6%) |
| WTAR standard score | 101.46 (11.41) |
| BMI | 26.94 (4.68) |
| Years of contact sport exposure | 5.01 (4.49) |
| Number of concussions (n) | |
| 0 | 91 (75.2%) |
| 1 | 15 (12.4%) |
| 2+ | 15 (12.4%) |
M, mean: SD, standard deviation; WTAR, Wechsler Test of Adult Reading, BMI, body mass index.
Association of prior concussion and contact sport exposure with white matter
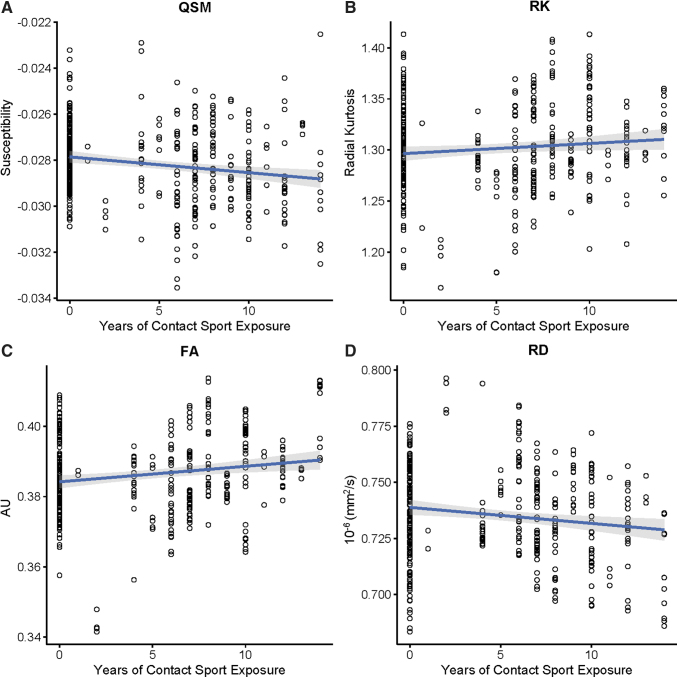
GLMM demonstrated a significant inverse association between cumulative years of contact sport exposure and QSM, [F(1, 237.77) = 4.67, B = -0.0001, 95% confidence interval [CI] = -0.0001, -0.00007, p = 0.032; Fig. 1; Table 3]. Cumulative contact sport exposure was also inversely associated with RD [F(1, 114.56) = 5.81, B = -0.001, 95% CI = -0.002, -0.0002, p = 0.018]. Conversely, cumulative contact sport exposure was positively predictive of FA [F(1, 115.32) = 5.40, B = 0.0006, 95% CI = 0.0009, .001, p = 0.022, and RK, F(1, 113.45) = 4.03, B = 0.002, 95% CI = 0.0003, 0.004, p = 0.047]. White matter volume was not significantly associated with prior cumulative contact sport exposure (p > 0.05). Similarly, concussion history was not significantly associated with QSM, DTI, DKI, or white matter volume (all, p > 0.05).
FIG. 1.
Scatterplots of significant quantitative susceptibility mapping (QSM), diffusion tensor imaging (DTI), and diffusion kurtosis imaging (DKI) metrics in which global white matter changes were associated increased contact sport exposure. FA, fractional anisotropy; RD, radial diffusivity. Color image is available online.
Table 3.
Association of Contact Sport Exposure and Prior Concussion on Global White Matter
| Contact sport exposure | F | B | 95% CI | p Value |
|---|---|---|---|---|
| Quantitative Susceptibility mapping | 4.67 | -.0001 | -.0001, -.00007 |
.032 |
| Fractional anisotropy | 5.4 | .00062 | .0009, 0.001 |
.022 |
| Axial diffusivity | 1.34 | .00053 | -.000401, .00152 | .25 |
| Radial diffusivity | 5.81 | -.00119 | -.002164, -.000212 | .018 |
| Mean diffusivity | 2.85 | -.00064 | -.001400, .000111 | .094 |
| Kurtosis fractional anisotropy | 2.29 | .000456 | -.000141, 0.001053 | .133 |
| Axial kurtosis | .21 | -.000151 | -.000801, .000498 | .645 |
| Radial kurtosis | 4.03 | .001901 | .000025, .003778 | .047 |
| Mean kurtosis | 1.83 | .000691 | -.000321, .001702 | .179 |
| White matter volume | .26 | 296.56 | -859.60, 1452.71 | .612 |
| Concussion history | F | B | 95% CI | p Value |
| Quantitative Susceptibility mapping | .14 | -.00008 | -0.0005, 0.0003 | .71 |
| Fractional anisotropy | .06 | -.00039 | -0.004, 0.003 |
.81 |
| Axial diffusivity | 1.38 | .00342 | -0.002351, 0.00920 | .24 |
| Radial diffusivity | .54 | .00216 | -0.003680, 0.007994 | .47 |
| Mean diffusivity | .56 | .00284 | -0.001681, 0.00737 | .22 |
| Kurtosis fractional anisotropy | .87 | .001694 | -.001906, .005294 | .353 |
| Axial kurtosis | .02 | .000308 | -.003587, .004202 | .876 |
| Radial kurtosis | .62 | -.004468 | -.015753, .006817 | .435 |
| Mean kurtosis | .95 | -.002984 | -.009053, .003085 | .332 |
| White matter volume | 0.0 | -45.39 | -7033.05, 6942.27 | .99 |
CI, confidence interval.
Post hoc ROIs
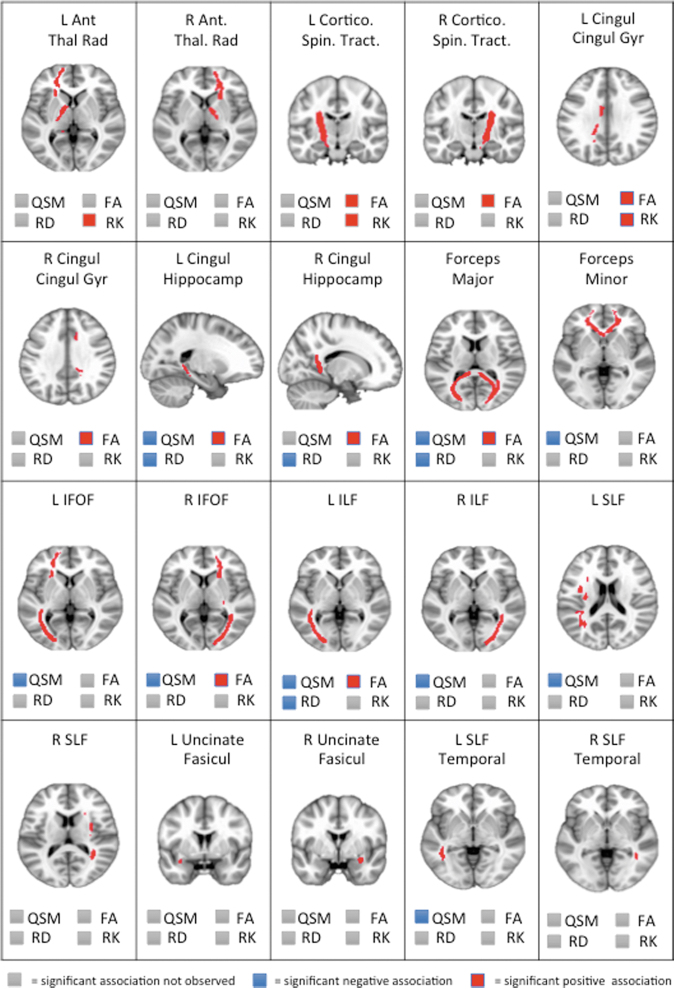
Given significant associations of cumulative contact sport participation and global white matter compartment for QSM and select DTI (FA and RD) and DKI (RK) metrics, similar, post hoc GLMM models were fit as above for individual white matter ROIs. Various significant associations between cumulative contact sport participation with QSM, FA, and RD were observed (Fig. 2). RD showed significant effects in four ROIS, FA in eight ROIs, and QSM in nine ROIs. Ultimately, there was minimal overlap between QSM and DTI metrics. Of the possible 20 ROIs analyzed, QSM effects overlapped minimally with FA (n = 4; 20.0%) and RD effects (n = 3; 15.0%). Specifically, within the left cingulum (hippocampus) forceps major, and left inferior longitudinal fasciculus, cumulative contact sport exposure was significantly associated with lower QSM, elevated FA, and lower RD (all, p < 0.10). QSM (25.0%), DTI (10.0%), and DKI (5.0%) metrics were also differentially associated with independent ROIs, as displayed in Figure 2.
FIG. 2.
Individual white matter regions of interest and their associations with quantitative susceptibility mapping (QSM), diffusion tensor imaging (DTI), and diffusion kurtosis imaging (DKI) metrics in which significant associations were observed for global white matter. Significance was evaluated at the 0.10 level for descriptive purposes; red = significant positive association; blue = significant negative association; gray = significant association not observed. L Ant Thal Rad, left anterior thalamic radiation; R Ant Thal Rad, right anterior thalamic radiation; L Cortico Spin Trac, left corticospinal tract; R Cortico Spin Track, right corticospinal tract ; L Cingul Gyr, left cingulum (cingulate gyrus); R Cingul Gyr, right cingulum (cingulate gyrus); L Cingul Hippocamp, left cingulum (hippocampus); R Cingul Hippocamp, right cingulum (hippocampus); L IFOF, left inferior fronto-occipital fasciculus; R IFOF, right inferior fronto-occipital fasciculus; L ILF, Left inferior longitudinal fasciculus; R ILF, right inferior longitudinal fasciculus; L SLF, left superior longitudinal fasciculus; R SLF, right superior longitudinal fasciculus; L Uncinate Fasicul, left uncinate fasciculus; R Uncinate Fasicul, right uncinate fasciculus; L SLF Temporal, left superior longitudinal fasciculus (temporal); R SLF Temporal, right superior longitudinal fasciculus (temporal). Color image is available online.
Discussion
The current study observed significant associations between cumulative contact sport exposure and multiple metrics of white matter microstructure (QSM, DTI, and DKI) in young, active athletes. Conversely, gross white matter macrostructure was not significantly associated with cumulative contact sport exposure. Independent of contact sport exposure, history of concussion was not associated with either microstructural or macroscopic white matter metrics. These findings are consistent with the growing body of literature suggesting that subtle differences in brain structure related to cumulative head impacts can be observed earlier in life, prior to the development of adverse outcomes that have been reported in subsets of older athletes.1–5 Longitudinal follow-up and monitoring of these subtle changes are required to determine the long-term significance of these findings and their relationship, if any, with the adverse outcomes later in life.
White matter microstructure and head impacts in active athletes
Growing evidence suggests that white matter changes over a single season of contact sport exposure without a concussive injury in multiple contact sports (football, hockey, soccer) can be detect with diffusion MRI, though the direction (i.e., increase vs. decrease) of these changes have been variable across studies.15,31–35 Further, these changes in white matter microstructure have been associated with direct measures of head impact exposure (i.e., telemetry or head impact measurements technology).36–39 Interestingly, multiple studies have demonstrated that changes associated with head impact exposure reverse back toward pre-season baseline levels at follow-up visits at least 6 months after the contact season ends.32,40 While reversing in trend, the diffusion metrics still significantly differ from non-contact controls and fall short of returning completely to baseline.
In the context of the current findings showing white matter microstructural changes associated with cumulative years of contact sport exposure, it is possible that abnormalities in white matter incrementally accumulate with each year of cumulative contact sport participation. This raises the need to further investigate related factors, such as length of contact sport season and the potentially compounding effect of simultaneous or successive contact sport seasons (e.g., participation of soccer in the fall and hockey in the winter). Attempts to consider these factors, such as “time between hits” and “time to post-season assessment,” have been included within novel methods of weighting the effects of measured repetitive head impacts, which have significantly increased the amount of variance explained in the association between telemetry system recorded head impacts and pre- to post-season DTI differences among collegiate football players.38 Longitudinal, multi-year follow-up of contact sport athletes is required to prospectively investigate the etiology of these annual changes.
Relatedly, while statistically significant, the associations between years of contact sport exposure and white matter abnormalities observed in the current study are relatively subtle and the clinical significance, if any, is unclear. Within the same cohort of young athletes, our group has failed to observed statistically significant associations between years of contact sport exposure and several clinical measures (i.e., symptom report inventories and neurocognitive assessments).41,42
Prior to the current study, QSM had not yet been utilized to investigate the cumulative effects of contact sport exposure, though one study failed to observed a significant association between a single season of high school football participation and alterations in cortical and deep gray matter using QSM.43 Two studies to date have examined the association between QSM of deep white matter and acute concussion.18,44 Findings from these studies were conflicting regarding the sensitivity of QSM to detect the acute and subacute effects of concussion, though these were likely due to study design and sample differences (i.e., baseline to post-injury vs. well-matched group, large vs. limited sample size, and ROI vs. voxel-wise approach). Of the larger more diverse sample, QSM was sensitive to the acute effects of concussion (within 24 h) and persisted through subacute periods (8 days).
Interestingly, the QSM changes following acute concussion (i.e., increased susceptibility in white matter) was in the opposite direction of the association between contact sport exposure and QSM (i.e., decreased susceptibility) in the current study, suggesting a potential discrepancy in acute effects of concussion versus the chronic effects of contact sport exposure on QSM. Potential mechanistic explanations for this are discussed below. Within the above study, the acute effects of concussion on QSM were observed as resolving over time, as statistically significant differences between the concussed and control group at 6-month follow-up were not observed.18 This would be consistent with the findings of the current study, in which remote history of concussion (at least 6 months prior) was not associated QSM.
Outcome differences in head impacts and concussion
Within the current study, years of contact sport exposure, not concussion, was associated with microstructural changes in young active athletes. Growing evidence suggests that these different mechanisms of insult may have differential effects on outcomes later in life.5,12,45-47 A number of previously published studies also have observed head impact exposure, independent of concussion history, as being significantly associated with neuropathology (i.e., CTE),47-49 neurobehavioral functioning,12,50 markers of inflammatory microglia,51 and neurochemistry (i.e., creatine).52 Prior studies investigating the association of white matter signal alterations and history of head injury among active, younger athletes have primarily focused on history of remote (greater than 6 months duration) concussion, rather than cumulative years of contact sport exposure. Of these studies, differences in white matter microstructure associated with concussion history were observed across two independent samples of active hockey and diverse collegiate athletes.14,53
One potential explanation for the discrepancy in the prior findings and results from the current study could be found in the differences of samples and study design, as one of the studies was comprised of 50% of athletes who sustained a concussion during that season.53 Additionally, within the study of 68 athletes from diverse collegiate sports, the effects of prior concussion on white matter were examined by analyzing group differences across 37 athletes with a history of concussion and 31 athletes without a history of concussion. The sample of the study was recruited from both contact and non-contact sports (volleyball, hockey, soccer, football, rugby, basketball, and lacrosse).14 Given that contact sport athletes are at greater risk for sustaining a concussion, the concussion history group may have been disproportionately comprised of contact sport athletes, and the effects of the concussion history group may more accurately reflect history of contact sport exposure, rather prior concussion.
White matter abnormalities and adverse outcomes in older populations
The current study observed associations between cumulative contact sport exposure and microstructural white matter within younger, asymptomatic athletes, in the absence of white matter volume differences. A number of studies have reported on similar diffusion-based white matter abnormalities among older, retired former contact sport athletes1–5,10 and that years of participation was associated with white matter rarefaction (i.e., degradation), independent of other cardiovascular risk factors.13 The extent to which subtle microscopic white matter abnormalities in the absence of gross macroscopic differences early in life translate to adverse long-term neurobehavioral and neurobiological outcomes is not entirely clear at this point.54-56 Findings from a well-designed study of former athletes (mean age of approximately 59 years) suggest that changes in white matter microstructure may reflect an initial stage of change that precedes functional decline, macroscopic brain changes (i.e., gross atrophy) and frank impairments.10 Further, white matter abnormalities (i.e., white matter hyperintensities on MRI T2 FLAIR and diffusion differences) among older non-athlete populations have been observed as independently predictive of neuropathology (t-tau and beta-amyloid), cerebral atrophy, and various aspects of functional decline (cognitive, neuropsychiatric, daily function) at follow-up within longitudinal studies.57–60 Further work is required to investigate whether the microscopic white matter abnormalities observed in early life among younger, active healthy athletes in the current study are associated with greater risk for any of the adverse outcomes listed above later in life.
Pattern of white matter abnormalities and potential underlying mechanisms
The pattern of global white matter differences recorded in the current study included elevated FA and RK, as well as decreased RD and susceptibility (QSM). Decreases in diffusion measures perpendicular to the main fiber direction (i.e., radial diffusivity) were associated with greater cumulative head impact histories, with increases in radial kurtosis and FA also being evident as expected from their mathematical relationships to one another. While there was some degree of overlap between QSM and diffusion metrics in individual ROIs showing significant effects of cumulative contact sport exposure, there was clear heterogeneity observed for ROIs across and within methods. Specifically, QSM and DTI exhibited consistent effects in 15%–20% of anatomical ROIs, but were unique in 25% and 10%, respectively. This potentially suggests that microscopic markers based on diffusion and magnetization could be providing unique information related to the underlying brain changes.
Although we are unable to directly identify the underlying mechanism driving the observed white matter abnormalities, there are a number of potential explanations that would be consistent with the pattern of white matter findings observed. One consideration for the mechanistic explanation of the current findings involves gliosis in response to cumulative head impacts, as previous studies have also demonstrated significant associations between increased anisotropy and kurtosis metrics (particularly RK) with gliosis (astrocyte and oligodendrocyte) reactivity following TBI.61–64 Given the previously reported relationship between myelin changes with RD65,66 and QSM,19,27 the pattern of white matter abnormalities in the current study could potentially be indicative of remyelination in response to head impacts, as some have previously suggested similar findings in other clinical samples (e.g., stroke).14,67
Within the acute concussion literature, the role of cytotoxic edema secondary to altered gated ion channel function and increased intracellular water has been proposed as one underlying source for elevated anisotropy following head injury.68,69 Although no obvious DWI lesions reflective of cytotoxic edema are evident within concussion or cumulative head impacts as they are prominently in stroke, it is possible that subvoxel changes below the level of detection only emerge across sufficiently powered group studies. While investigations into the relationship between QSM and cytotoxic edema are limited, one study of transient cerebral ischemia in mice demonstrated significantly decreased susceptibility (similar to the current study) following reperfusion after 24 and 48 h, potentially representing inflammation and oxidative stress.70 Cytotoxic or vasogenic edema as a plausible underlying explanation for the pattern of findings is also indirectly supported by the fact that DTI-related effects (significant differences across several ROIs in FA and RD), but minimal DKI-related effects were observed in the current study. Several studies have shown that lower diffusion weighting (i.e., DTI compared to DKI) is significantly affected by intravoxel incoherent motion (IVIM)71 via interstitial flow (ISF) through the glymphatic system,72-74 and ISF has been hypothesized to be a potential source of water instigating ionic edema (originating in from the CSF).75,76 Recent studies of blood–brain barrier disruption associated with concussion and RHI would be consistent with this possibility.77
Limitations
Given that studies have shown changes in diffusion over the course of a single season, one potential limitation of the study is that athletes completed scans during their competitive season. Sensitivity analyses found no differences in metrics over the ∼45-day scanning period as a function of group (i.e., current sport as football versus non-contact). Nevertheless, sport participation difference within the window of study may have subtle influences on the associations (or lack thereof) observed. Secondly, the current study quantified contact sport exposure by aggregating each year of contact sport participation across all possible contact sports, based on the theoretical notion that more seasons of contact sport, regardless of the sport, will result in greater exposure to RHI. While this approach is advantageous in capturing cumulative head impacts and is likely the most generalizable, information can be lost and associations with outcomes may contain some degree of error, as the frequency and magnitude of head impacts can differ across sports, position, playing style, etc.
Finally, primary analyses focused on global white matter using an ROI approach. Although secondary analyses investigated individual ROI, it is possible that this approach is insensitive to smaller, focal abnormalities that might be captured by voxel-based approaches. Moreover, future studies investigating subject-specific abnormalities that do not assume that concussion and contact sport participation are associated with abnormalities in the same anatomical regions across all participants are warranted. Finally, we conducted multiple comparisons. Given the high degree of correlation between our outcome measures, as well as their non-independence, Bonferroni correction would be overly conservative. Nevertheless, the observed effects were subtle and we cannot rule out type I error.
Conclusion
Within a sample of young, active athletes, cumulative contact sport exposure was significantly associated with white matter abnormalities across multiple metrics of white matter microstructure, but not gross white matter macrostructure. These findings are consistent with the growing body of literature suggesting that subtle differences in brain structure related to cumulative head impacts can be observed earlier in life. Longitudinal follow-up of these changes is required assess their progression and true contribution to risk of later life decline.
Acknowledgments
The authors would like to thank Ashley LaRoche and Alexa Wild from the Department of Neurosurgery at the Medical College of Wisconsin for study coordination. The authors would also like to thank Brad Swearingen and Lezlie España for their efforts on image management and processing. We are grateful for the participation of the student athletes without whom this research would not be possible.
Funding Information
This research was supported by the Department of Defense Broad Agency Announcement for Extramural Medical Research through award number W81XWH-14-1-0561. BLB acknowledges support from the National Institute of Neurological Disorders and Stroke under the National Institutes of Health under the award NO L30 NS113158. TBM acknowledges support from the National Institute of Neurological Disorders and Stroke of the NIH under award number R01NS102225. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The REDCap electronic database used for this project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR001436. This research was completed in part with computational resources and technical support provided by the Research Computing Center at the Medical College of Wisconsin.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Clark, M.D., Varangis, E.M.L., Champagne, A.A., Giovanello, K.S., Shi, F., Kerr, Z.Y., Smith, J.K., and Guskiewicz, K.M. (2018). Effects of career duration, concussion history, and playing position on white matter microstructure and functional neural recruitment in former college and professional football athletes. Radiology 286, 967–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Multani, N., Goswami, R., Khodadadi, M., Ebraheem, A., Davis, K.D., Tator, C.H., Wennberg, R., Mikulis, D.J., Ezerins, L., and Tartaglia, M.C. (2016). The association between white-matter tract abnormalities, and neuropsychiatric and cognitive symptoms in retired professional football players with multiple concussions. J. Neurol. 263, 1332–1341 [DOI] [PubMed] [Google Scholar]
- 3. Koerte, I.K., Ertl-Wagner, B., Reiser, M., Zafonte, R., and Shenton, M.E. (2012). White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA 308, 1859–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hart, J.Jr., Kraut, M.A., Womack, K.B., Strain, J., Didehbani, N., Bartz, E., Conover, H., Mansinghani, S., Lu, H., and Cullum, C.M. (2013). Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: a cross-sectional study. JAMA Neurol. 70, 326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alosco, M.L., Koerte, I.K., Tripodis, Y., Mariani, M., Chua, A.S., Jarnagin, J., Rahimpour, Y., Puzo, C., Healy, R.C., Martin, B., Chaisson, C.E., Cantu, R.C., Au, R., McClean, M., McKee, A.C., Lin, A.P., Shenton, M.E., Killiany, R.J., and Stern, R.A. (2018). White matter signal abnormalities in former National Football League players. Alzheimers Dement. (Amst.) 10, 56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Churchill, N.W., Hutchison, M.G., Richards, D., Leung, G., Graham, S.J., and Schweizer, T.A. (2017). Neuroimaging of sport concussion: persistent alterations in brain structure and function at medical clearance. Sci. Rep. 7, 8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lancaster, M.A., Olson, D.V., McCrea, M.A., Nelson, L.D., LaRoche, A.A., and Muftuler, L.T. (2016). Acute white matter changes following sport-related concussion: a serial diffusion tensor and diffusion kurtosis tensor imaging study. Hum. Brain Mapp. 37, 3821–3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mustafi, S.M., Harezlak, J., Koch, K.M., Nencka, A.S., Meier, T.B., West, J.D., Giza, C.C., DiFiori, J.P., Guskiewicz, K.M., Mihalik, J.P., LaConte, S.M., Duma, S.M., Broglio, S.P., Saykin, A.J., McCrea, M., McAllister, T.W., and Wu, Y.C. (2018). Acute white-matter abnormalities in sports-related concussion: a diffusion tensor imaging study from the NCAA-DoD CARE Consortium. J. Neurotrauma 35, 2653–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henry, L.C., Tremblay, J., Tremblay, S., Lee, A., Brun, C., Lepore, N., Theoret, H., Ellemberg, D., and Lassonde, M. (2011). Acute and chronic changes in diffusivity measures after sports concussion. J. Neurotrauma 28, 2049–2059 [DOI] [PubMed] [Google Scholar]
- 10. Tremblay, S., Henry, L.C., Bedetti, C., Larson-Dupuis, C., Gagnon, J.F., Evans, A.C., Theoret, H., Lassonde, M., and De Beaumont, L. (2014). Diffuse white matter tract abnormalities in clinically normal ageing retired athletes with a history of sports-related concussions. Brain 137, 2997–3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lancaster, M.A., Meier, T.B., Olson, D.V., McCrea, M.A., Nelson, L.D., and Muftuler, L.T. (2018). Chronic differences in white matter integrity following sport-related concussion as measured by diffusion MRI: 6-nonth follow-up. Hum. Brain Mapp. 39, 4276-4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montenigro, P.H., Alosco, M.L., Martin, B.M., Daneshvar, D.H., Mez, J., Chaisson, C.E., Nowinski, C.J., Au, R., McKee, A.C., Cantu, R.C., McClean, M.D., Stern, R.A. and Tripodis, Y. (2017). Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J. Neurotrauma 34, 328–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alosco, M.L., Stein, T.D., Tripodis, Y., Chua, A.S., Kowall, N.W., Huber, B.R., Goldstein, L.E., Cantu, R.C., Katz, D.I., Palmisano, J.N., Martin, B., Cherry, J.D., Mahar, I., Killiany, R.J., McClean, M.D., Au, R., Alvarez, V., Stern, R.A., Mez, J., and McKee, A.C. (2019). Association of white matter rarefaction, arteriolosclerosis, and tau with dementia in chronic traumatic encephalopathy. JAMA Neurol. 76, 1298–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Churchill, N.W., Caverzasi, E., Graham, S.J., Hutchison, M.G., and Schweizer, T.A. (2017). White matter microstructure in athletes with a history of concussion: Comparing diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI). Hum. Brain Mapp. 38, 4201–4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bahrami, N., Sharma, D., Rosenthal, S., Davenport, E.M., Urban, J.E., Wagner, B., Jung, Y., Vaughan, C.G., Gioia, G.A., Stitzel, J.D., Whitlow, C.T., and Maldjian, J.A. (2016). Subconcussive head impact exposure and white matter tract changes over a single season of youth football. Radiology 281, 919–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuzminski, S.J., Clark, M.D., Fraser, M.A., Haswell, C.C., Morey, R.A., Liu, C., Choudhury, K.R., Guskiewicz, K.M., and Petrella, J.R. (2018). White matter changes related to subconcussive impact frequency during a single season of high school football. AJNR Am. J. Neuroradiol. 39, 245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang, Y. and Liu, T. (2015). Quantitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker. Magn. Reson. Med. 73, 82–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koch, K.M., Meier, T.B., Karr, R., Nencka, A.S., Muftuler, L.T., and McCrea, M. (2018). Quantitative Susceptibility Mapping after Sports-Related Concussion. AJNR Am. J. Neuroradiol. 39, 1215–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soni, N., Vegh, V., To, X.V., Mohamed, A.Z., Borges, K., and Nasrallah, F.A. (2020). Combined diffusion tensor imaging and quantitative susceptibility mapping discern discrete facets of white matter pathology post-injury in the rodent brain. Front. Neurol. 11, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chin, E.Y., Nelson, L.D., Barr, W.B., McCrory, P., and McCrea, M.A. (2016). Reliability and validity of the Sport Concussion Assessment Tool-3 (SCAT3) in high school and collegiate Athletes. Am. J. Sports Med. 44, 2276–2285 [DOI] [PubMed] [Google Scholar]
- 21. Carney, N., Ghajar, J., Jagoda, A., Bedrick, S., Davis-O'Reilly, C., du Coudray, H., Hack, D., Helfand, N., Huddleston, A., Nettleton, T., and Riggio, S. (2014). Concussion guidelines step 1: systematic review of prevalent indicators. Neurosurgery 75 Suppl 1, S3–S15 [DOI] [PubMed] [Google Scholar]
- 22. Reuter, M., Schmansky, N.J., Rosas, H.D., and Fischl, B. (2012). Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61, 1402–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu, J., Liu, T., de Rochefort, L., Ledoux, J., Khalidov, I., Chen, W., Tsiouris, A.J., Wisnieff, C., Spincemaille, P., Prince, M.R., and Wang, Y. (2012). Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage 59, 2560–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mazziotta, J., Toga, A., Evans, A., Fox, P., Lancaster, J., Zilles, K., Woods, R., Paus, T., Simpson, G., Pike, B., Holmes, C., Collins, L., Thompson, P., MacDonald, D., Iacoboni, M., Schormann, T., Amunts, K., Palomero-Gallagher, N., Geyer, S., Parsons, L., Narr, K., Kabani, N., Le Goualher, G., Feidler, J., Smith, K., Boomsma, D., Hulshoff Pol, H., Cannon, T., Kawashima, R., and Mazoyer, B. (2001). A four-dimensional probabilistic atlas of the human brain. J. Am. Med. Inform. Assoc. 8, 401–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Desikan, R.S., Segonne, F., Fischl, B., Quinn, B.T., Dickerson, B.C., Blacker, D., Buckner, R.L., Dale, A.M., Maguire, R.P., Hyman, B.T., Albert, M.S., and Killiany, R.J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 [DOI] [PubMed] [Google Scholar]
- 26. Mori, S., Wakana, S., Nagae-Poetscher, L.M., and van Zijl, P. (2005). MRI Atlas of Human White Matter, 1st ed. Elsevier: New York, NY [Google Scholar]
- 27. Li, W., Wu, B., Batrachenko, A., Bancroft-Wu, V., Morey, R.A., Shashi, V., Langkammer, C., De Bellis, M.D., Ropele, S., Song, A.W., and Liu, C. (2014). Differential developmental trajectories of magnetic susceptibility in human brain gray and white matter over the lifespan. Hum. Brain Mapp. 35, 2698–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jensen, J.H., Helpern, J.A., Ramani, A., Lu, H., and Kaczynski, K. (2005). Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 53, 1432–1440 [DOI] [PubMed] [Google Scholar]
- 29. Jensen, J.H. and Helpern, J.A. (2010). MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 23, 698–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith, S.M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T.E., Mackay, C.E., Watkins, K.E., Ciccarelli, O., Cader, M.Z., Matthews, P.M., and Behrens, T.E. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505 [DOI] [PubMed] [Google Scholar]
- 31. Sollmann, N., Echlin, P.S., Schultz, V., Viher, P.V., Lyall, A.E., Tripodis, Y., Kaufmann, D., Hartl, E., Kinzel, P., Forwell, L.A., Johnson, A.M., Skopelja, E.N., Lepage, C., Bouix, S., Pasternak, O., Lin, A.P., Shenton, M.E., and Koerte, I.K. (2018). Sex differences in white matter alterations following repetitive subconcussive head impacts in collegiate ice hockey players. Neuroimage Clin. 17, 642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yuan, W., Barber Foss, K.D., Thomas, S., DiCesare, C.A., Dudley, J.A., Kitchen, K., Gadd, B., Leach, J.L., Smith, D., Altaye, M., Gubanich, P., Galloway, R.T., McCrory, P., Bailes, J.E., Mannix, R., Meehan, W.P. 3rd, and Myer, G.D. (2018). White matter alterations over the course of two consecutive high-school football seasons and the effect of a jugular compression collar: A preliminary longitudinal diffusion tensor imaging study. Hum. Brain Mapp. 39, 491–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andersson, J.L., Skare, S., and Ashburner, J. (2003). How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage 20, 870–888 [DOI] [PubMed] [Google Scholar]
- 34. Andersson, J.L., and Skare, S. (2002). A model-based method for retrospective correction of geometric distortions in diffusion-weighted EPI. Neuroimage 16, 177–199 [DOI] [PubMed] [Google Scholar]
- 35. Barber Foss, K.D., Yuan, W., Diekfuss, J.A., Leach, J., Meehan, W., DiCesare, C.A., Solomon, G., Schneider, D.K., MacDonald, J., Dudley, J., Cortes, N., Galloway, R., Halstead, M., Walker, G. and Myer, G.D. (2019). Relative head impact exposure and brain white matter alterations after a single season of competitive football: a pilot comparison of youth versus high school football. Clin. J. Sport Med. 29, 442-450 [DOI] [PubMed] [Google Scholar]
- 36. Myer, G.D., Barber Foss, K., Thomas, S., Galloway, R., DiCesare, C.A., Dudley, J., Gadd, B., Leach, J., Smith, D., Gubanich, P., Meehan, W.P., 3rd, Altaye, M., Lavin, P., and Yuan, W. (2018). Altered brain microstructure in association with repetitive subconcussive head impacts and the potential protective effect of jugular vein compression: a longitudinal study of female soccer athletes. Br. J. Sports Med. 53, 1539–1551 [DOI] [PubMed] [Google Scholar]
- 37. Merchant-Borna, K., Asselin, P., Narayan, D., Abar, B., Jones, C.M., and Bazarian, J.J. (2016). Novel method of weighting cumulative helmet impacts improves correlation with brain white matter changes after one football season of sub-concussive head blows. Ann. Biomed. Eng. 44, 3679–3692 [DOI] [PubMed] [Google Scholar]
- 38. Jang, I., Chun, I.Y., Brosch, J.R., Bari, S., Zou, Y., Cummiskey, B.R., Lee, T.A., Lycke, R.J., Poole, V.N., Shenk, T.E., Svaldi, D.O., Tamer, G.G.Jr., Dydak, U., Leverenz, L.J., Nauman, E.A., and Talavage, T.M. (2019). Every hit matters: white matter diffusivity changes in high school football athletes are correlated with repetitive head acceleration event exposure. Neuroimage Clin. 24, 101930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Davenport, E.M., Whitlow, C.T., Urban, J.E., Espeland, M.A., Jung, Y., Rosenbaum, D.A., Gioia, G.A., Powers, A.K., Stitzel, J.D., and Maldjian, J.A. (2014). Abnormal white matter integrity related to head impact exposure in a season of high school varsity football. J. Neurotrauma 31, 1617–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Davenport, E.M., Apkarian, K., Whitlow, C.T., Urban, J.E., Jensen, J.H., Szuch, E., Espeland, M.A., Jung, Y., Rosenbaum, D.A., Gioia, G.A., Powers, A.K., Stitzel, J.D. and Maldjian, J.A. (2016). Abnormalities in diffusional kurtosis metrics related to head impact exposure in a season of high school varsity football. J. Neurotrauma 33, 2133–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nilsson, K.J., Flint, H.G., Gao, Y., Kendrick, L., Cutchin, S., Pentecost, R., and Pardue, K. (2019). Repetitive head impacts in youth football: description and relationship to white matter structure. Sports Health 11, 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mayinger, M.C., Merchant-Borna, K., Hufschmidt, J., Muehlmann, M., Weir, I.R., Rauchmann, B.S., Shenton, M.E., Koerte, I.K., and Bazarian, J.J. (2018). White matter alterations in college football players: a longitudinal diffusion tensor imaging study. Brain Imaging Behav. 12, 44–53 [DOI] [PubMed] [Google Scholar]
- 43. Brett, B.L., Savitz, J., Nitta, M., Espana, L., Kent Teague, T., Nelson, L.D., McCrea, M.A., and Meier, T.B. (2020). Systemic inflammation moderates the association of prior concussion with hippocampal volume and episodic memory in high school and collegiate athletes. Brain Behav. Immun. 89, 380–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brett, B.L., Huber, D.L., Wild, A., Nelson, L.D. and McCrea, M.A. (2019). Age of first exposure to american football and behavioral, cognitive, psychological, and physical outcomes in high school and collegiate football players. Sports Health 1941738119849076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gong, N.J., Kuzminski, S., Clark, M., Fraser, M., Sundman, M., Guskiewicz, K., Petrella, J.R., and Liu, C. (2018). Microstructural alterations of cortical and deep gray matter over a season of high school football revealed by diffusion kurtosis imaging. Neurobiol. Dis. 119, 79–87 [DOI] [PubMed] [Google Scholar]
- 46. Weber, A.M., Pukropski, A., Kames, C., Jarrett, M., Dadachanji, S., Taunton, J., Li, D.K.B., and Rauscher, A. (2018). Pathological insights from quantitative susceptibility mapping and diffusion tensor imaging in ice hockey players pre and post-concussion. Front. Neurol. 9, 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tagge, C.A., Fisher, A.M., Minaeva, O.V., Gaudreau-Balderrama, A., Moncaster, J.A., Zhang, X.L., Wojnarowicz, M.W., Casey, N., Lu, H., Kokiko-Cochran, O.N., Saman, S., Ericsson, M., Onos, K.D., Veksler, R., Senatorov, V.V.Jr., Kondo, A., Zhou, X.Z., Miry, O., Vose, L.R., Gopaul, K.R., Upreti, C., Nowinski, C.J., Cantu, R.C., Alvarez, V.E., Hildebrandt, A.M., Franz, E.S., Konrad, J., Hamilton, J.A., Hua, N., Tripodis, Y., Anderson, A.T., Howell, G.R., Kaufer, D., Hall, G.F., Lu, K.P., Ransohoff, R.M., Cleveland, R.O., Kowall, N.W., Stein, T.D., Lamb, B.T., Huber, B.R., Moss, W.C., Friedman, A., Stanton, P.K., McKee, A.C., and Goldstein, L.E. (2018). Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain 141, 422–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McKee, A.C., Alosco, M.L., and Huber, B.R. (2016). Repetitive head impacts and chronic traumatic encephalopathy. Neurosurg. Clin. N. Am. 27, 529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stein, T.D., Alvarez, V.E., and McKee, A.C. (2015). Concussion in chronic traumatic encephalopathy. Curr. Pain Headache Rep. 19, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McKee, A.C., Stern, R.A., Nowinski, C.J., Stein, T.D., Alvarez, V.E., Daneshvar, D.H., Lee, H.S., Wojtowicz, S.M., Hall, G., Baugh, C.M., Riley, D.O., Kubilus, C.A., Cormier, K.A., Jacobs, M.A., Martin, B.R., Abraham, C.R., Ikezu, T., Reichard, R.R., Wolozin, B.L., Budson, A.E., Goldstein, L.E., Kowall, N.W., and Cantu, R.C. (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mez, J., Daneshvar, D.H., Abdolmohammadi, B., Chua, A.S., Alosco, M.L., Kiernan, P.T., Evers, L., Marshall, L., Martin, B.M., Palmisano, J.N., Nowinski, C.J., Mahar, I., Cherry, J.D., Alvarez, V.E., Dwyer, B., Huber, B.R., Stein, T.D., Goldstein, L.E., Katz, D.I., Cantu, R.C., Au, R., Kowall, N.W., Stern, R.A., McClean, M.D., Weuve, J., Tripodis, Y. and McKee, A.C. (2019). Duration of American football play and chronic traumatic encephalopathy. Ann. Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alosco, M.L., Tripodis, Y., Baucom, Z.H., Mez, J., Stein, T.D., Martin, B., Haller, O., Conneely, S., McClean, M., Nosheny, R., Mackin, S., McKee, A.C., Weiner, M.W., and Stern, R.A. (2020). The late contributions of repetitive head impacts and TBI to depression symptoms and cognition. Neurology 95, e793–e804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cherry, J.D., Tripodis, Y., Alvarez, V.E., Huber, B., Kiernan, P.T., Daneshvar, D.H., Mez, J., Montenigro, P.H., Solomon, T.M., Alosco, M.L., Stern, R.A., McKee, A.C., and Stein, T.D. (2016). Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol. Commun. 4, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alosco, M.L., Tripodis, Y., Rowland, B., Chua, A.S., Liao, H., Martin, B., Jarnagin, J., Chaisson, C.E., Pasternak, O., Karmacharya, S., Koerte, I.K., Cantu, R.C., Kowall, N.W., McKee, A.C., Shenton, M.E., Greenwald, R., McClean, M., Stern, R.A., and Lin, A. (2019). A magnetic resonance spectroscopy investigation in symptomatic former NFL players. Brain Imaging Behav. 14, 1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sasaki, T., Pasternak, O., Mayinger, M., Muehlmann, M., Savadjiev, P., Bouix, S., Kubicki, M., Fredman, E., Dahlben, B., Helmer, K.G., Johnson, A.M., Holmes, J.D., Forwell, L.A., Skopelja, E.N., Shenton, M.E., Echlin, P.S., and Koerte, I.K. (2014). Hockey Concussion Education Project, Part 3. White matter microstructure in ice hockey players with a history of concussion: a diffusion tensor imaging study. J Neurosurg 120, 882–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McKee, A.C., Cantu, R.C., Nowinski, C.J., Hedley-Whyte, E.T., Gavett, B.E., Budson, A.E., Santini, V.E., Lee, H.S., Kubilus, C.A. and Stern, R.A. (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 68, 709–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mez, J., Daneshvar, D.H., Kiernan, P.T., Abdolmohammadi, B., Alvarez, V.E., Huber, B.R., Alosco, M.L., Solomon, T.M., Nowinski, C.J., McHale, L., Cormier, K.A., Kubilus, C.A., Martin, B.M., Murphy, L., Baugh, C.M., Montenigro, P.H., Chaisson, C.E., Tripodis, Y., Kowall, N.W., Weuve, J., McClean, M.D., Cantu, R.C., Goldstein, L.E., Katz, D.I., Stern, R.A., Stein, T.D., and McKee, A.C. (2017). Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 318, 360–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lepage, C., Muehlmann, M., Tripodis, Y., Hufschmidt, J., Stamm, J., Green, K., Wrobel, P., Schultz, V., Weir, I., Alosco, M.L., Baugh, C.M., Fritts, N.G., Martin, B.M., Chaisson, C., Coleman, M.J., Lin, A.P., Pasternak, O., Makris, N., Stern, R.A., Shenton, M.E., and Koerte, I.K. (2018). Limbic system structure volumes and associated neurocognitive functioning in former NFL players. Brain Imaging Behav. 13, 725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Puzo, C., Labriola, C., Sugarman, M.A., Tripodis, Y., Martin, B., Palmisano, J.N., Steinberg, E.G., Stein, T.D., Kowall, N.W., McKee, A.C., Mez, J., Killiany, R.J., Stern, R.A., and Alosco, M.L. (2019). Independent effects of white matter hyperintensities on cognitive, neuropsychiatric, and functional decline: a longitudinal investigation using the National Alzheimer's Coordinating Center Uniform Data Set. Alzheimers Res. Ther. 11, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Araque Caballero, M.A., Suarez-Calvet, M., Duering, M., Franzmeier, N., Benzinger, T., Fagan, A.M., Bateman, R.J., Jack, C.R., Levin, J., Dichgans, M., Jucker, M., Karch, C., Masters, C.L., Morris, J.C., Weiner, M., Rossor, M., Fox, N.C., Lee, J.H., Salloway, S., Danek, A., Goate, A., Yakushev, I., Hassenstab, J., Schofield, P.R., Haass, C.m and Ewers, M. (2018). White matter diffusion alterations precede symptom onset in autosomal dominant Alzheimer's disease. Brain 141, 3065–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mayo, C.D., Mazerolle, E.L., Ritchie, L., Fisk, J.D., and Gawryluk, J.R.; Alzheimer's Disease Neuroimaging Initiative. (2017). Longitudinal changes in microstructural white matter metrics in Alzheimer's disease. Neuroimage Clin 13, 330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ly, M., Canu, E., Xu, G., Oh, J., McLaren, D.G., Dowling, N.M., Alexander, A.L., Sager, M.A., Johnson, S.C., and Bendlin, B.B. (2014). Midlife measurements of white matter microstructure predict subsequent regional white matter atrophy in healthy adults. Hum. Brain Mapp. 35, 2044–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Braeckman, K., Descamps, B., Pieters, L., Vral, A., Caeyenberghs, K., and Vanhove, C. (2019). Dynamic changes in hippocampal diffusion and kurtosis metrics following experimental mTBI correlate with glial reactivity. Neuroimage Clin. 21, 101669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Budde, M.D., Janes, L., Gold, E., Turtzo, L.C., and Frank, J.A. (2011). The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain 134, 2248–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lee, J.B., Affeldt, B.M., Gamboa, Y., Hamer, M., Dunn, J.F., Pardo, A.C. and Obenaus, A. (2018). Repeated pediatric concussions evoke long-term oligodendrocyte and white matter microstructural eysregulation eistant from the injury. Dev. Neurosci. 40, 358–375 [DOI] [PubMed] [Google Scholar]
- 66. Guglielmetti, C., Veraart, J., Roelant, E., Mai, Z., Daans, J., Van Audekerke, J., Naeyaert, M., Vanhoutte, G., Delgado, Y.P.R., Praet, J., Fieremans, E., Ponsaerts, P., Sijbers, J., Van der Linden, A., and Verhoye, M. (2016). Diffusion kurtosis imaging probes cortical alterations and white matter pathology following cuprizone induced demyelination and spontaneous remyelination. Neuroimage 125, 363–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Song, S.K., Sun, S.W., Ramsbottom, M.J., Chang, C., Russell, J., and Cross, A.H. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17, 1429–1436 [DOI] [PubMed] [Google Scholar]
- 68. Song, S.K., Yoshino, J., Le, T.Q., Lin, S.J., Sun, S.W., Cross, A.H., and Armstrong, R.C. (2005). Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26, 132–140 [DOI] [PubMed] [Google Scholar]
- 69. Jiang, Q., Zhang, Z.G., Ding, G.L., Silver, B., Zhang, L., Meng, H., Lu, M., Pourabdillah-Nejed, D.S., Wang, L., Savant-Bhonsale, S., Li, L., Bagher-Ebadian, H., Hu, J., Arbab, A.S., Vanguri, P., Ewing, J.R., Ledbetter, K.A., and Chopp, M. (2006). MRI detects white matter reorganization after neural progenitor cell treatment of stroke. Neuroimage 32, 1080–1089 [DOI] [PubMed] [Google Scholar]
- 70. Mayer, A.R., Ling, J., Mannell, M.V., Gasparovic, C., Phillips, J.P., Doezema, D., Reichard, R., and Yeo, R.A. (2010). A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 74, 643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Simard, J.M., Kent, T.A., Chen, M., Tarasov, K.V., and Gerzanich, V. (2007). Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 6, 258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vaas, M., Deistung, A., Reichenbach, J.R., Keller, A., Kipar, A. and Klohs, J. (2018). Vascular and tissue changes of magnetic susceptibility in the mouse brain after transient cerebral ischemia. Transl. Stroke Res 9, 426–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vieni, C., Ades-Aron, B., Conti, B., Sigmund, E.E., Riviello, P., Shepherd, T.M., Lui, Y.W., Novikov, D.S., and Fieremans, E. (2020). Effect of intravoxel incoherent motion on diffusion parameters in normal brain. Neuroimage 204, 116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ahlgren, A., Knutsson, L., Wirestam, R., Nilsson, M., Stahlberg, F., Topgaard, D., and Lasic, S. (2016). Quantification of microcirculatory parameters by joint analysis of flow-compensated and non-flow-compensated intravoxel incoherent motion (IVIM) data. NMR Biomed. 29, 640–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sepehrband, F., Cabeen, R.P., Choupan, J., Barisano, G., Law, M., and Toga, A.W.; Alzheimer's Disease Neuroimaging Initiative. (2019). Perivascular space fluid contributes to diffusion tensor imaging changes in white matter. Neuroimage 197, 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Peckham, M.E., Anderson, J.S., Rassner, U.A., Shah, L.M., Hinckley, P.J., de Havenon, A., Kim, S.E., and McNally, J.S. (2018). Low b-value diffusion weighted imaging is promising in the diagnosis of brain death and hypoxic-ischemic injury secondary to cardiopulmonary arrest. Crit. Care 22, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Thrane, A.S., Rangroo Thrane, V., and Nedergaard, M. (2014). Drowning stars: reassessing the role of astrocytes in brain edema. Trends Neurosci. 37, 620–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Stokum, J.A., Gerzanich, V., and Simard, J.M. (2016). Molecular pathophysiology of cerebral edema. J. Cereb. Blood Flow Metab. 36, 513–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. O'Keeffe, E., Kelly, E., Liu, Y., Giordano, C., Wallace, E., Hynes, M., Tiernan, S., Meagher, A., Greene, C., Hughes, S., Burke, T., Kealy, J., Doyle, N., Hay, A., Farrell, M., Grant, G.A., Friedman, A., Veksler, R., Molloy, M.G., Meaney, J.F., Pender, N., Camarillo, D., Doherty, C.P., and Campbell, M. (2020). Dynamic blood-brain barrier regulation in mild traumatic brain injury. J. Neurotrauma 37, 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]