Abstract
Traumatic brain injury (TBI) affects approximately 3 million Americans yearly and increases vulnerability to developing psychiatric comorbidities. Alcohol use disorder (AUD) is the most prevalent psychiatric diagnosis preceding injury and TBI may increase subsequent alcohol use. The basolateral amygdala (BLA) is a limbic structure commonly affected by TBI that is implicated in anxiety and AUD. Endocannabinoids (eCBs) regulate synaptic activity in the BLA, and BLA eCB modulation alters anxiety-like behavior and stress reactivity. Previous work from our laboratories showed that systemic eCB degradation inhibition ameliorates TBI-induced increases in anxiety-like behavior and motivation to respond for alcohol in male rats. Here, we used a lateral fluid percussion model to test moderate TBI effects on anxiety-like behavior, alcohol drinking, and eCB levels and cell signaling in BLA, as well as the effect of alcohol drinking on anxiety-like behavior and the BLA eCB system, in female rats. Our results show that TBI does not promote escalation of operant alcohol self-administration or increase anxiety-like behavior in female rats. In the BLA, TBI and alcohol drinking alter tissue amounts of 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (anandamide; AEA) 1 h post-injury, and 2-AG levels remain low 11 days post-injury. Eleven days after injury, BLA pyramidal neurons were hyperexcitable, but measures of synaptic transmission and eCB signaling were unchanged. These data show that TBI impacts BLA 2-AG tissue levels, that this effect is modified by alcohol drinking, and also that TBI increases BLA cell excitability.
Keywords: 2-arachidonoylglycerol, anandamide, anxiety, electrophysiology, fluid percussion
Introduction
Traumatic brain injury (TBI) is a common cause of morbidity and mortality in otherwise healthy individuals.1 In addition to motor, sensory, and cognitive problems, individuals with TBI are more likely to develop psychiatric comorbidities such as post-traumatic stress disorder (PTSD), anxiety disorders, and substance use disorders.2 Alcohol use disorder (AUD) is the most common psychiatric diagnosis prior to injury in individuals with TBI, and alcohol use is a major risk factor of incurring TBI, as 30–50% of TBIs can be attributed to an alcohol-related event.3–6 Past work from our group showed that alcohol intoxication at time of injury impaired resolution of TBI-induced neuroinflammation, post-TBI alcohol exposure enhanced neuroinflammation and delayed neurobehavioral recovery, and TBI produced escalation of operant alcohol self-administration in male rats.7–9
Of the nearly 3 million Americans who suffer a TBI yearly, females account for approximately 41% of TBI-related emergency department visits.10 Clinical literature suggests that female patients who have suffered a TBI display more severe post-concussive symptomatology and poorer outcomes.11–13 A study by Fann and colleagues showed that in patients without a prior history of psychiatric conditions, females are at higher risk for developing psychiatric illness in the 3 years post-TBI.14 However, most TBI studies have been performed in males because TBI is more prevalent in men, thereby limiting our understanding of sex/gender differences in physical and psychiatric complications following TBI.
The amygdala is a limbic brain structure critical for emotional learning and for orchestrating the behavioral response to stressful stimuli.15 Amygdala dysfunction has been implicated in myriad psychiatric disorders, including AUD and anxiety disorders, and in animal models of the behaviors that define those conditions.16–23 The basolateral nucleus of the amygdala (BLA) is a cortical-like structure composed mainly of glutamatergic pyramidal neurons, and it integrates sensory and affective information that arrives via inputs from the thalamus, hippocampus, and cortex.15 Glutamatergic principal neurons of BLA project to several brain regions important for addictive and anxiety-related behaviors, and inhibition of excitatory input to these neurons decreases alcohol withdrawal-induced anxiety-like behavior.24,25 The BLA is a brain structure commonly affected by TBI, and TBI can lead to BLA hyperexcitability.26–29
Within the central nervous system, the endocannabinoid (eCB) system acts to maintain synaptic homeostasis and regulate neuroinflammation. The eCB system consists of the lipid-derived eCBs, 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (anandamide; AEA); their synthetic enzymes, including diacylglycerol lipase (DAGL) and N-acyl-phosphatidyletholamine-hydrolyzing phospholipase D (NAPE-PLD); their catabolic enzymes, monoacylglycerol lipase (MAGL) and fatty acid amide hydrolase (FAAH); and their receptors, cannabinoid receptors 1 (CB1R) and 2 (CB2R). Post-synaptic activity triggers synthesis and release of eCBs that retrogradely activate pre-synaptic CB1R, a Gi/o protein coupled receptor, thereby suppressing neurotransmitter release, a phenomenon called depolarization-induced suppression of inhibition (DSI) or excitation (DSE) depending on the amino acid neurotransmitter released from the pre-synaptic terminal.30
In the BLA, regulation of synaptic input onto BLA pyramidal neurons is mediated, at least in part, by eCBs.31,32 TBI increases levels of 2-AG in the ipsilateral (whole) hemisphere acutely post-injury, and intravenous 2-AG administration is neuroprotective in a closed head, weight-drop model of TBI in male mice.33 Published data from our laboratories show that systemic administration of inhibitors of 2-AG degradation post-TBI protects blood–brain barrier integrity, blocks astrocytic and microglial activation, improves neurological impairment, and attenuates TBI-induced increases in anxiety-like behavior and motivation to consume alcohol in male rats, suggesting that global, sustained elevation of 2-AG following TBI is protective.34,35 The effects of TBI on eCB signaling in subcortical brain regions, including the amygdala, have not been studied.
Considerable data indicate that alcohol also modulates the eCB system, including within the BLA, and, conversely, changes in the eCB system contribute to alcohol-induced decreases in excitatory BLA transmission.36–39 Further, eCB signaling in the BLA modulates anxiety-like behavior: for example, increasing BLA AEA via degradation inhibition reduces anxiety-like behavior in rats.32,40 Disruption of BLA eCB signaling may therefore represent a mechanism by which TBI leads to psychiatric symptoms after injury, such as anxiety or alcohol misuse.
The effects of TBI on the alcohol-exposed brain remain inadequately understood, particularly in female animals. Therefore, the goals of this study were to examine the effects of TBI in female rats on: 1) anxiety-like behavior and alcohol self-administration; 2) the eCB system in the BLA of alcohol-naïve and alcohol-drinking rats; and 3) electrophysiological properties of BLA neurons. We hypothesized that TBI would produce escalation of alcohol self-administration and increased anxiety-like behavior in female rats, decreases in BLA eCB signaling, and increases in BLA excitability. We further hypothesized that TBI would have more pronounced effects on behavioral and molecular measures in alcohol-drinking female rats compared with alcohol-naïve animals.
Methods
Animals
A total of 180 rats were used for these experiments. Female Wistar rats (Charles River Laboratories, Raleigh, NC, USA) weighing 175–200 g on arrival were used for all alcohol-drinking and behavioral studies. Rats weighing 250–300 g on arrival were used for slice electrophysiology experiments. All rats that underwent TBI weighed 250–300 g at time of injury and were approximately 4 months old. Rats were pair-housed in temperature- and humidity-controlled rooms with a 12:12 h reverse light-dark cycle and ad libitum access to standard rat chow and water. Upon arrival, rats were allowed to acclimate to the colony room for 1 week before experiments began, during which time rats were handled daily. All experiments were conducted in the dark cycle, between 7:00AM and 5:00PM. All experiments were approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center and were conducted in accordance with the guidelines of the National Institutes of Health.
Operant alcohol self-administration
For experiments 1 and 2, rats (n = 50, Expt. 1; n = 35, Expt. 2) were trained to orally self-administer alcohol (10% w/v) or water as previously described except that self-administration sessions occurred only 3 times per week for the last 3 weeks prior to craniotomy.41 Access was permitted in a two-lever contingency (water vs. alcohol) on a fixed ratio 1 (FR1) schedule, in which one lever press resulted in delivery of 0.1 mL of water or alcohol. Upon stabilization of operant responding in 30-min sessions, rats were counterbalanced into Sham and TBI groups based on baseline alcohol intake (average intake in final four sessions before surgery). In a subset of rats, blood alcohol concentrations (BACs) were measured at baseline to ensure animals were consuming alcohol. Immediately following the penultimate operant drinking session prior to TBI, 500 μL of tail blood was collected via a small incision and analyzed using an Analox machine according to manufacturer's instructions (Analox Instruments USA, Lunenburg, MA, USA). Rats used for behavioral experiments completed five operant drinking sessions every other day for 10 days post-TBI (days 2, 4, 6, 8, and 10 post-TBI). Alcohol-naïve animals (n = 45, Expt. 1; n = 32, Expt. 2) were handled during the operant self-administration training period.
Traumatic brain injury via lateral fluid percussion
All animals received a 5-mm in diameter craniotomy above the left sensorimotor cortex (from bregma: anteroposterior [AP]: −2 mm, mediolateral [ML]: −3 mm) and were allowed 2 to 3 days to recover before undergoing TBI via lateral fluid percussion (∼2 atm; Fluid Percussion Injury (FPI), Model 01-B; Custom Design and Fabrication, Virginia Commonwealth University, Richmond, VA, USA) or sham procedures as previously described.7 Animals in the Sham group were anesthetized and received craniotomy but were not subjected to TBI (surgical controls). Immediately following the TBI or sham procedures, animals were disconnected from the FPI device and anesthesia, and apnea duration, respiratory rate, and latency to righting reflex were measured. TBI animals that did not show a physical reaction to the injury, apnea, or latency to righting reflex of at least 4 min were excluded from subsequent experimentation and analyses. A total of eight animals were excluded based on these criteria.
Elevated plus maze
Animals that were used for behavioral experiments were tested for anxiety-like behavior in the elevated plus-maze (EPM) 9 days after TBI (n = 56; Expt. 1, Table 1), 24 h after their previous alcohol-drinking session such that BACs would be zero at the time of testing. Prior to testing, animals were allowed to acclimate to the testing room for at least 30 min. Testing occurred 3–6 h into the dark cycle. For testing, rats were individually placed in the center of the EPM facing one of the open arms and allowed 5 min to explore the maze in a dimly lit (∼12 lux) room. A video camera mounted on the ceiling directly above the EPM apparatus transmitted video to a computer in the laboratory and recorded each test. Entries into and out of open and closed arms were counted once all four paws crossed the arm threshold. The percentage of total time spent in the open arms, calculated as open arm time (sec)/[open arm time (sec) + closed arm time (sec)]*100, was used as the primary index of anxiety-like behavior, where decreases in percent open arm time reflect increases in anxiety-like behavior. The number of closed arm entries was used as a measure of overall locomotor activity. Animals that fell off the maze (n = 3; 1 Sham, 2 TBI) were excluded from analysis.
Table 1.
Experimental Groups, Conditions, and Outcomes
| Groups | Treatment | Outcome measures | |
|---|---|---|---|
| Experiment 1 | Alcohol-naïve Sham | Craniotomy | Post-TBI physiological measures, alcohol SA (drinkers), EPM, BLA eCB proteins |
| Alcohol-naïve TBI | Craniotomy, TBI | ||
| Alcohol-drinking Sham | Operant conditioning, craniotomy | ||
| Alcohol-drinking TBI | Operant conditioning, craniotomy, TBI | ||
| Experiment 2 | Alcohol-naïve Sham | Craniotomy | Post-TBI physiological measures, BLA eCB levels |
| Alcohol-naïve TBI | Craniotomy, TBI | ||
| Alcohol-drinking Sham | Operant conditioning, craniotomy | ||
| Alcohol-drinking TBI | Operant conditioning, craniotomy, TBI | ||
| Experiment 3 | Alcohol-naïve Sham | Craniotomy | BLA slice electrophysiology |
| Alcohol-naïve TBI | Craniotomy, TBI |
BLA, basolateral amygdala; eCB, endocannabinoid; EPM, elevated plus maze; SA, self-administration; TBI, traumatic brain injury.
Endocannabinoid measurements
A separate cohort of animals was used to quantify amygdala eCBs after alcohol drinking and TBI (Expt. 2, Table 1). After completing alcohol self-administration training or control handling treatment, animals (n = 60) were sacrificed at 1 h or 11 days post-TBI via decapitation under deep isoflurane anesthesia, and brains were excised, flash-frozen, and stored at −80°C. Bilateral BLA punches were obtained from 0.5-mm frozen coronal brain slices. Weighed BLA samples were placed into borosilicate glass culture tubes containing 2 mL of acetonitrile and 675 pg of [2H8]AEA and 8100 pg of [2H8]2-AG. Tissue was homogenized with a glass rod and sonicated on ice for 2 h. Samples were incubated overnight at −20°C to precipitate proteins. Samples were centrifuged at 1500g, and supernatants were removed to a new glass tube and evaporated to dryness under N2 gas. The samples were resuspended in 500 μL of methanol to recapture any lipids adhering to the glass tube, and dried again. Finally, lipid extracts were suspended in 30 μL of methanol.
Following preparation, the concentrations of eCBs (AEA and 2-AG) were quantified in 5 μL of the methanol extract using stable isotope-dilution, electrospray ionization liquid chromatography/mass spectrometry of the daughter ions (LC-ESI-MS-MS). Standard curves were generated for 2-AG (10–8500 pg/μL), AEA (0.2–170 pg/μL), and internal standards [2H8]-AEA (150 pg/μL) and [2H8]-2-AG (1800 pg/μL). Concentrations of the analytes were determined from standard curves of the area ratios (standard/analyte) versus the concentration ratios (standard/analyte); [2H8]-AEA was used as the standard for AEA, whereas [2H8]-2-AG was used for 2-AG. During extraction, 2-AG partially isomerizes to 1(3)-arachidonoylglycerol and is therefore detected and analyzed as a double peak.42 The area ratios for all analytes in all samples fell within their respective standard curves.
Western blot analysis
Animals in behavioral experiments (Expt. 1) were sacrificed by decapitation under deep isoflurane anesthesia 11 days post-TBI (24 h after completing behavioral testing), and brains were removed, flash-frozen, and stored at −80°C until further processing for western analysis as previously described.43 Bilateral BLA punches were obtained from 0.5-mm frozen coronal brain slices and homogenized by sonication in lysis buffer (320 mM sucrose, 5 mM 4-(2-hydroxyethyl)-1-piperazineethane sulfonic acid [HEPES], 1 mM egtazic acid [EGTA], 1 mM ethylenediaminetetraacetic acid [EDTA], and 1% sodium dodecyl sulphate [SDS], with protease inhibitor cocktail and phosphatase inhibitor cocktails 2 and 3 diluted 1:100; Sigma, St Louis, MO, USA). Protein concentration was determined by the Lowry method (Bio-Rad, Hercules, CA, USA), and 15-μg protein samples were separated by SDS-polyacrylamide gel electrophoresis on 4–20% gradient acrylamide gels (Bio-Rad) before transfer to polyvinylidene difluoride membranes (Millipore Sigma, Burlington, MA, USA).
Membranes were blocked then incubated in primary antibody overnight against DAGLα (diluted 1:1000 in 2.5% non-fat milk; Cell Signaling, Danvers, MA, USA), DAGLβ (diluted 1:1000 in 2.5% non-fat milk; Cell Signaling), MAGL (diluted 1:1000 in 2.5% non-fat milk; Cayman Chemical, Ann Arbor, MI, USA), CB1R (diluted 1:500 in 2.5% non-fat milk; Cayman Chemical), or β-tubulin (diluted 1:1,000,000 in 2.5% non-fat milk; Santa Cruz, Dallas, TX, USA). Membranes were incubated with peroxidase-conjugated secondary antibody (1:10,000; Bio-Rad) for 1 h at room temperature and chemiluminescence was detected (Immobilon Crescendo Western HRP Substrate; Millipore Sigma). Immunoreactivity was quantified using ImageJ, and the ratio of proteins of interest to β-tubulin for each sample was calculated for normalization and statistical comparison.
Slice electrophysiology
A third cohort of alcohol-naïve animals (n = 18) were used for post-TBI electrophysiological measurements in the BLA (Expt. 3; see Table 1). Brain slice preparation, intrinsic property measurements, and assessment of action potential generation properties were performed as previously described.44 Recordings were obtained from pyramidal neurons of the BLA ipsilateral to the site of injury. Pyramidal neurons were identified based on their cell body size and pyramidal-like somatic morphology.45,46
Synaptic properties
To collect data regarding pre- and post-synaptic alterations resulting from TBI, spontaneous excitatory and inhibitory post-synaptic currents (sEPSCs, sIPSCs) were recorded. When whole cell recording configuration was established, the voltage was clamped at −70 mV (for sEPSCs) or −50 mV (for sIPSCs) and 5 min of spontaneous post-synaptic current was recorded. Spontaneous post-synaptic events were detected in these recordings by thresholding rapid excursions in current; the average event amplitude and mean frequency over the 5-min recording period were quantified.
Depolarization-induced suppression of excitation/inhibition
To measure eCB signaling post-TBI, DSE and DSI were assessed. The frequency of sEPSCs and sIPSCs were measured before and after a 10-sec depolarizing voltage step. The clamped voltage was stepped from −70 mV (for sEPSCs) or −50 mV (for sIPSCs) to 0 mV during the depolarization phase. The %DSE and %DSI were calculated by %DSE/I = (1 – post-depolarization sPSC frequency/pre-depolarization sPSC frequency) × 100.
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). Data were tested for normality using the D'Agostino and Pearson normality test and analyzed with two-way repeated measures analysis of variance (ANOVA; post-TBI alcohol self-administration), two-tailed t tests (injury pressure, electrophysiological measures), Mann-Whitney test (resting membrane potential [RMP], sEPSC amplitude; non-Gaussian distributions) or with two-way between subjects ANOVA where factors were injury (Sham vs. TBI) and alcohol history (drinking vs. naïve). Significant interactions (p < 0.05) were followed up by post hoc analysis with Tukey's multiple comparisons test. Pearson's correlation analysis was used for variables with Gaussian distributions and Spearman's correlation analysis was used for variables with non-Gaussian distributions. Outliers for dependent variables were identified with the generalized extreme Studentized deviate test and excluded from analysis. All statistical analyses were performed with GraphPad Prism 7 software (Graphpad Software Inc., La Jolla, CA, USA).
Results
Operant alcohol self-administration history does not alter physiological measures of injury severity
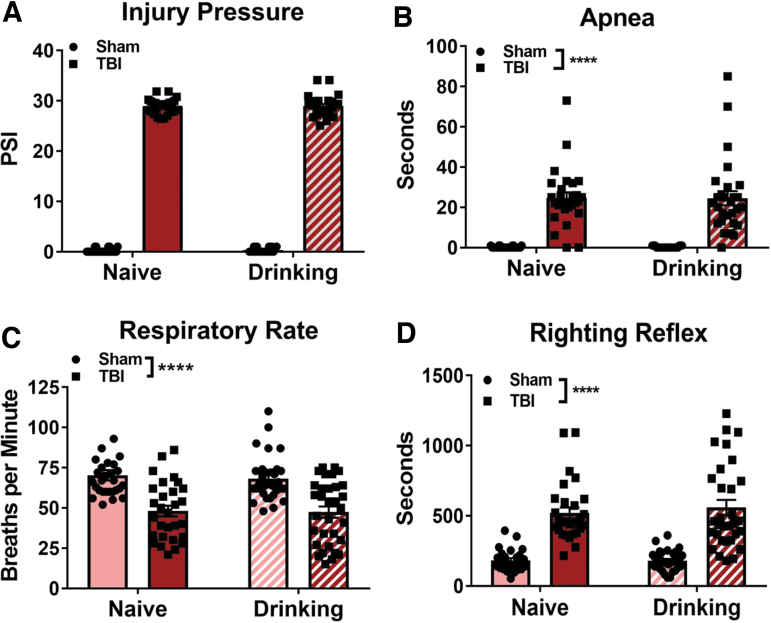
Immediately following TBI or sham injury, animals were observed and the injury pressure, and physiological parameters including length of apnea, respiratory rate, and latency to righting reflex were measured. Two-tailed unpaired t test revealed that both alcohol-naïve and alcohol-drinking TBI groups received injuries of equivalent pressures (t[53] = 0.11, p = 0.92; Fig 1A). A two-way ANOVA revealed a main effect of TBI (F[1,120] = 150.9, p < 0.0001) on apnea duration, but there was no effect of alcohol self-administration (F[1,120] = 0.0005, p = 0.98) and there was no TBI × alcohol drinking history interaction effect (F[1,120] = 0.0005, p = 0.98; Fig 1B).
FIG. 1.
Alcohol self-administration does not alter measures of TBI severity in female rats. Alcohol-naïve and alcohol-drinking TBI animals received equivalent injury pressures (A). Immediately post-TBI, alcohol-naïve and alcohol-drinking animals showed similar lengths of apnea (B), decreases in respiratory rate (C), and latency to righting reflex (D) relative to uninjured Shams. Injury pressures were compared with two-tailed unpaired t test. Other data were analyzed via two-way ANOVA, ****p < 0.0001 main effect of TBI, n = 28–35/group. Data are expressed as mean ± SEM. ANOVA, analysis of variance; SEM, standard error of the mean; TBI, traumatic brain injury.
Following the resumption of breathing, respiratory rate was measured (Fig 1C): a two-way ANOVA revealed a main effect of TBI (F[1,120] = 49, p < 0.0001), but there was no effect of alcohol self-administration (F[1,120] = 0.19, p = 0.66) and there was no TBI × alcohol drinking history interaction effect (F[1,120] = 0.06, p = 0.81). Finally, the latency for animals to regain their righting reflex was measured (Fig 1D): a two-way ANOVA revealed that TBI increases the latency to righting reflex (F[1,119] = 109.1, p < 0.0001), but there was no effect of alcohol self-administration (F[1,119] = 0.27, p = 0.60) and there was no TBI × alcohol drinking history interaction effect (F[1,119] = 0.36, p = 0.55). Mortality rates in the alcohol-naïve and alcohol-drinking TBI groups were 20% and 22.5%, respectively. Together, average pounds per square inch (psi), apnea, latency to righting reflex, and mortality rates indicate a moderate injury that was unaffected by prior alcohol self-administration.47
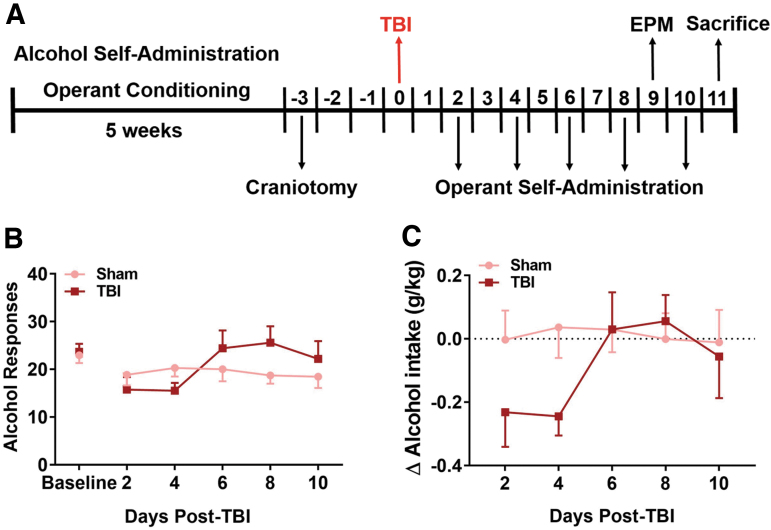
TBI does not lead to escalation of alcohol self-administration in female rats
To test the effects of TBI on operant alcohol self-administration in female rats, alcohol-drinking Sham and TBI rats from Expt. 1 underwent 30-min FR1 self-administration sessions every other day post-TBI for 10 days (i.e., five post-TBI sessions; experimental timeline shown in Fig. 2A). Average alcohol intake at baseline was 0.69 ± 0.04 g/kg, which produced average BACs of 35.9 ± 2.46 mg/dL (data not shown). Two-way repeated measures (RM) ANOVA revealed a significant main effect of time (F[5,140] = 2.54, p = 0.03) on the number of alcohol rewards post-TBI, but no effect of TBI (F[1,28] = 0.42, p = 0.52) or TBI × time interaction effect (F[5,140] = 2.13, p = 0.07; Fig 2B). When analyzing post-TBI self-administration as change in alcohol intake (g/kg) from pre-injury baseline with two-way ANOVA, there were no effects of time (F[4,112] = 0.86, p = 0.36), TBI (F[4,112] = 2.08, p = 0.09), or TBI × time interaction effects (F[4,112] = 2.31, p = 0.06; Fig 2C). There were also no statistically significant differences in post-TBI total (i.e., summated across days) alcohol intake (g/kg) between injured and uninjured animals (data not shown).
FIG. 2.
TBI does not produce escalation of alcohol self-administration in female rats in 10 days post-injury. Timeline for Expt. 1, in which behavioral data were collected and tissue was collected for western blot analysis (A). Following TBI, drinking animals underwent operant alcohol self-administration sessions every other day for 10 days (i.e., days 2, 4, 6, 8, and 10 post-TBI; A). Number of alcohol rewards (B) are not different between Sham and TBI animals or between baseline and day 10. Alcohol intake (g/kg) shown as change from baseline (dashed line; C) did not change with time or between groups. Data were analyzed via repeated measures two-way ANOVA, n = 12–18/group. Data are expressed as mean ± SEM. ANOVA, analysis of variance; EPM, elevated plus-maze; SEM, standard error of the mean; TBI, traumatic brain injury.
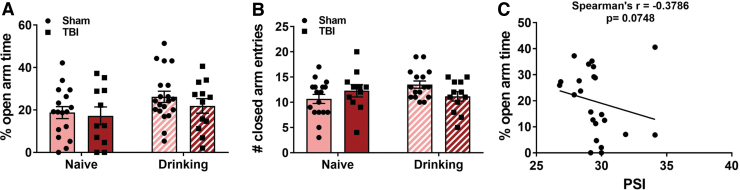
Neither TBI nor alcohol self-administration alter anxiety-like behavior in female rats
Nine days post-TBI, alcohol-naïve and alcohol-drinking Sham and TBI rats from Expt. 1 (see Fig. 2A) were tested for anxiety-like behavior on the EPM. Decreases in the time spent exploring the open arms of the maze are typically interpreted as anxiety-like behavior: a two-way ANOVA revealed no significant effect of TBI (F[1,56] = 0.81, p = 0.37), alcohol drinking history (F[1,56] = 3.51, p = 0.66), or TBI × alcohol interaction effect (F[1,56] = 0.18, p = 0.67) on % open arm time (Fig. 3A). The number of closed arm entries was used as a control measure for overall locomotor activity: a two-way ANOVA revealed no effect of TBI (F[1,56] = 0.14, p = 0.71) or operant alcohol self-administration (F[1,52] = 0.86, p = 0.36), but there was a significant TBI × alcohol interaction effect on closed arm entries (F[1,52] = 4.43, p = 0.040; Fig. 3B). Tukey's multiple comparisons test did not detect any pairwise differences between groups. Within TBI animals, there was a near significant inverse Spearman's correlation (r = −0.3786, p = 0.075) between injury pressure and % open arm time (Fig. 3C).
FIG. 3.
TBI does not increase anxiety-like behavior in female rats 9 days post-injury. Alcohol-naïve and alcohol-drinking Sham and TBI rats in Expt. 1 were tested for anxiety-like behavior via the elevated plus-maze 9 days after injury. Neither TBI nor alcohol drinking altered the percent time spent in the open arms of the elevated plus-maze (A) or the number of entries into the closed arms (B). Anxiety-like behavior may be associated with injury pressure intensity (C). Data were analyzed via two-way ANOVA, n = 11–20/group. Data are expressed as mean ± SEM. SEM, standard error of the mean; TBI, traumatic brain injury.
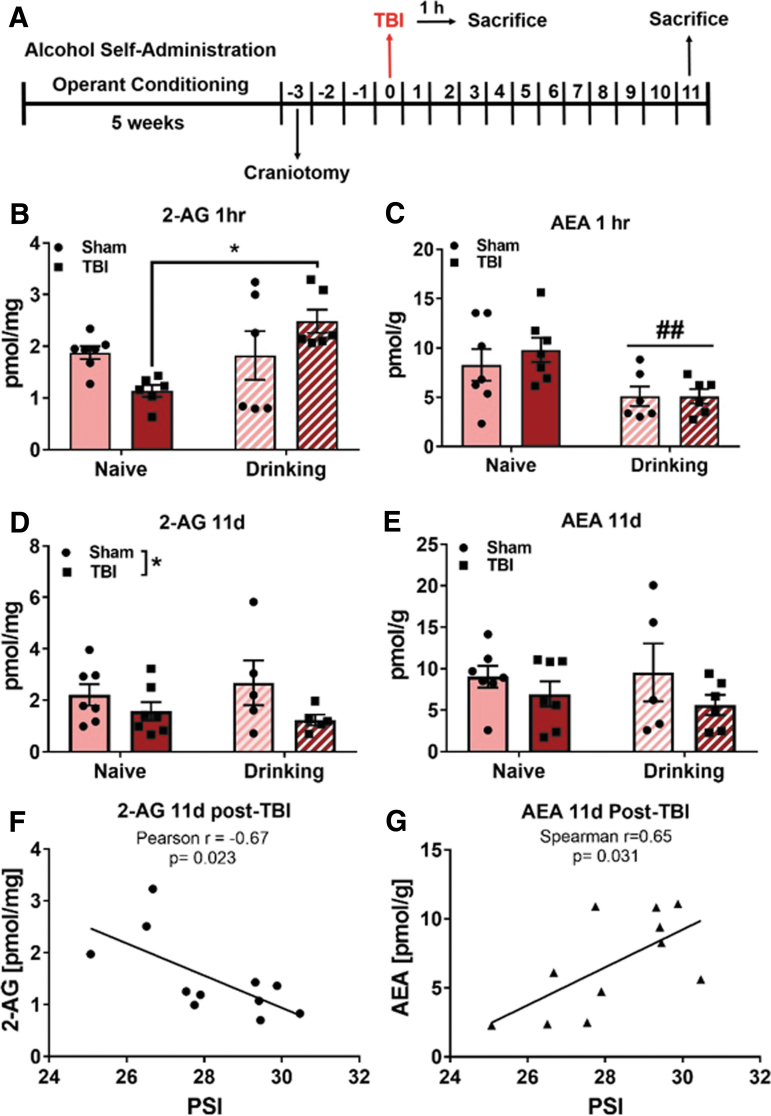
TBI and alcohol self-administration alter endocannabinoid levels in BLA
To test the effects of TBI and operant alcohol self-administration history on BLA eCB levels, alcohol-naïve and alcohol-drinking Sham and TBI rats were sacrificed either 1 h or 11 days post-TBI, and BLA eCBs were measured via liquid chromatography/mass spectroscopy in Expt. 2. In this experiment, alcohol-drinking rats underwent alcohol self-administration sessions prior to, but not after, TBI (to equate duration of alcohol access in animals sacrificed 1 h and 11 days post-injury; see timeline in Fig. 4A). Animals’ final alcohol self-administration sessions were 5 days prior to TBI. With regard to 2-AG tissue amounts 1 h after TBI, two-way ANOVA revealed a significant main effect of operant alcohol self-administration history (F[1,21] = 5.88, p = 0.024) and a significant TBI × alcohol interaction effect (F[1,21] = 6.939, p = 0.015) on 2-AG levels. Tukey's multiple comparisons tests indicated that BLA 2-AG was significantly lower in alcohol-naïve TBI animals compared with alcohol-drinking TBI animals (Fig. 4D), but Tukey's did not detect a significant difference between naïve Sham and naïve TBI 2-AG (p = 0.22).
FIG. 4.
TBI and alcohol self-administration alter eCB levels in the BLA of female rats. In Expt. 2, BLA eCBs were measured 1 h and 11 days post-TBI (A). One hour post-injury, there is a differential effect of TBI on BLA 2-AG in alcohol naïve and alcohol self-administering animals (B). Anandamide levels are decreased in BLA of alcohol-drinking animals 1 h post-TBI (C). TBI decreases BLA 2-AG (D) but not anandamide (E) 11 day after injury in alcohol naïve and alcohol-drinking animals. There was a significant inverse correlation between TBI pressure and 2-AG (F) and a positive correlation between TBI pressure and AEA (G), such that animals with high TBI pressure had lower 2-AG and higher AEA. Data were analyzed via two-way ANOVA with Tukey's post hoc analysis, *p < 0.05 naïve TBI versus drinking TBI, ##p < 0.01 main effect of alcohol self-administration, $p < 0.05 main effect of TBI, n = 5–7/group. Values are expressed as mean ± SEM. 2-AG, 2-arachidonylglyercol; AEA, anandamide; ANOVA, analysis of variance; BLA, basolateral amygdala; eCB, endocannabinoids; SEM, standard error of the mean; TBI, traumatic brain injury
With regard to AEA tissue amounts 1 h after TBI, two-way ANOVA shows a significant main effect of operant alcohol self-administration history (F[1,22] = 100.9, p = 0.004) to reduce AEA 1 h post-TBI, but no effect of TBI (F[1,22] = 0.37, p = 0.55), and no TBI × alcohol interaction effect (F[1,22] = 0.38, p = 0.55; Fig. 4B). With regard to 2-AG tissue amounts at 11 days post-TBI, two-way ANOVA revealed a main effect of TBI (F[1,20] = 4.42, p = 0.0483) to reduce BLA 2-AG levels, but no effect of alcohol self-administration (F[1,20] = 0.02, p = 0.90), and no TBI × alcohol interaction effect (F[1,20] = 0.66, p = 0.43; Fig. 4E). There were no significant effects of TBI (F[1,21] = 2.53, p = 0.13) or alcohol self-administration (F[1,21] = 0.04, p = 0.84), nor was there a TBI × alcohol interaction effect (F[1,21] = 0.24, p = 0.63) on AEA tissue amounts in BLA 11 days post-TBI (Fig. 4C). There is a significant Pearson's correlation (r = −0.67, p = 0.023) between TBI pressure and 2-AG levels (Fig. 4F) and a significant Pearson's correlation (r = 0.65, p = 0.031) between TBI pressure and AEA levels such that animals that received higher injury pressures had less 2-AG and more AEA 11 days after injury (Fig. 4G).
Alcohol self-administration, but not TBI, alters endocannabinoid protein levels in BLA
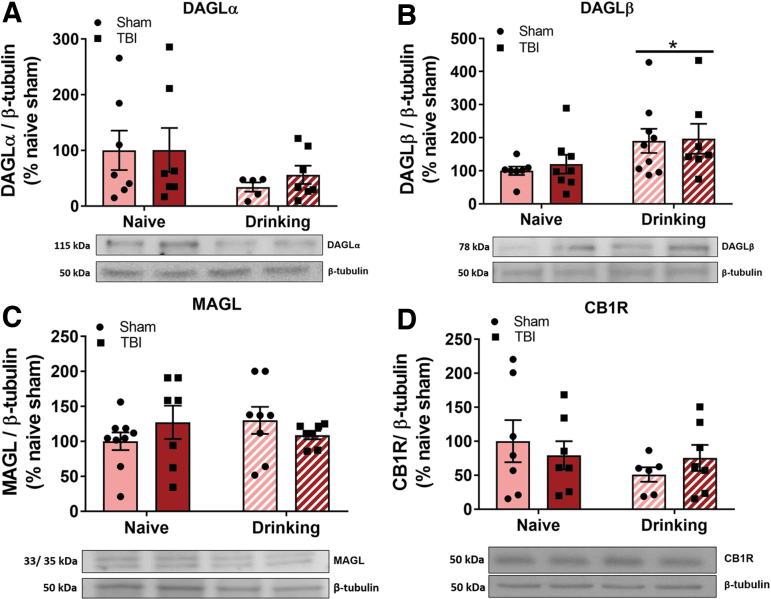
To test the hypothesis that TBI-induced decreases in BLA 2-AG are driven by decreases in 2-AG synthetic enzyme DAGL and/or increased expression of 2-AG degradative enzyme, MAGL, alcohol-naïve and alcohol-drinking Sham and TBI animals used in behavioral experiments (Expt. 1) were sacrificed 11 days post-TBI and bilateral BLA punches were used for western blot analysis. There were no effects of TBI (F[1,22] = 0.14, p = 0.71) or alcohol self-administration (F[1,22] = 3.27, p = 0.08), nor was there a TBI × alcohol interaction effect (F[1,22] = 0.12, p = 0.73) on DAGLα protein levels (Fig. 5A). A separate two-way ANOVA revealed a significant main effect of operant alcohol self-administration history (F[1,27] = 6.21, p = 0.02) on DAGLβ levels (Fig. 5B), but no effect of TBI (F[1,27] = 0.15, p = 0.70) nor a TBI × alcohol interaction effect (F[1,27] = 0.15, p = 0.85). Two-way ANOVA revealed that MAGL (Fig. 5C) and CB1R levels (Fig. 5D) in BLA were not altered by TBI (F[1,27] = 0.03, p = 0.86; F[1,23] = 0.007, p = 0.94, respectively) or alcohol self-administration (F[1,27] = 0.12, p = 0.07; F[1,23] = 1.41, p = 0.25, respectively), nor was there a TBI × alcohol interaction effect (F[1,27] = 2.06, p = 0.16; F[1,23] = 1.02, p = 0.32, respectively).
FIG. 5.
Alcohol self-administration, but not TBI, alters endocannabinoid system protein expression in the BLA of female rats 11 days post-injury. BLA collected from animals in Expt. 1 were probed for eCB system proteins via western blotting 11 days post-TBI. Alcohol-drinking animals showed an increase in DAGLβ expression (A), with a trend toward a decrease in the expression of DAGLα (B), and no change in MAGL (C), or CB1R (D). Data were analyzed via two-way ANOVA, *p < 0.05 main effect of alcohol self-administration, n = 5–9/group. Data are expressed as mean ± SEM. ANOVA, analysis of variance; BLA, basolateral amygdala; CB1R, cannabinoid receptor 1; DAGL, diacylglycerol lipase; eCB, endocannabinoids; MAGL, monoacylglycerol lipase; SEM, standard error of the mean; TBI, traumatic brain injury.
TBI increases BLA pyramidal firing rate input gain 11 days post-TBI
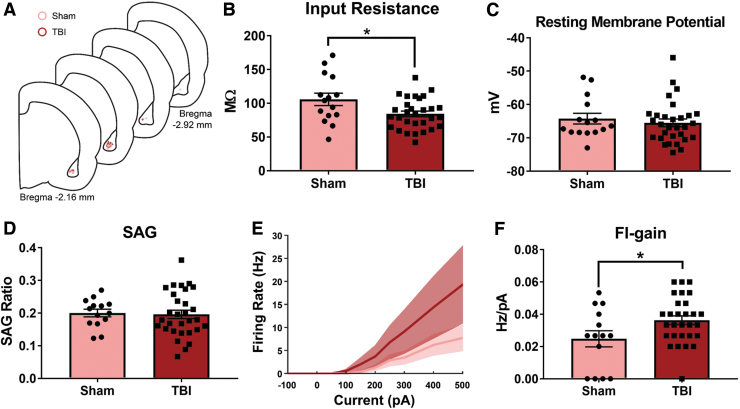
To test the hypothesis that TBI stimulates neuronal hyperexcitability in BLA pyramidal neurons, alcohol-naïve Sham and TBI animals were sacrificed 11 days post-TBI (the time-point at which TBI reduced BLA 2-AG levels regardless of alcohol drinking history) for whole-cell patch clamp slice electrophysiology. Measures of intrinsic excitability (input resistance [Rin], resting membrane potential [RMP], and rebound response to hyperpolarization-activated inward current, also known as the voltage sag [SAG]) were recorded from BLA pyramidal neurons (Fig. 6A). A two-tailed unpaired t test showed that TBI reduced input resistance in BLA pyramidal neurons (t[43] = 2.48, p = 0.02; Fig. 6B). A Mann-Whitney test showed no effect of TBI on RMP (p = 0.53, Fig. 6C). A two-tailed unpaired t test showed no TBI effect on SAG ratio (t[42] = 0.21, p = 0.84, Fig. 6D). To test the excitability of BLA pyramidal neurons, currents of increasing magnitudes were injected into neurons and the firing rate at each current step was measured (Fig. 6E), and these data were quantified as average firing rate-input (FI) gain. A two-tailed unpaired t test revealed that TBI significantly increased average FI gain of BLA pyramidal neurons (t[41] = 2.24, p = 0.03; Fig. 6F) relative to Sham controls.
FIG. 6.
TBI increases BLA pyramidal neuron excitability 11 days post-TBI in alcohol-naïve female rats. Whole-cell patch clamp slice electrophysiology was performed on BLA pyramidal neurons from alcohol naïve Sham and TBI animals 11 days post-TBI in Expt. 3 (schematic representation of recording sites shown in A). There was a decrease in the input resistance (B) of BLA pyramidal neurons from TBI animals, but there were no changes in resting membrane potential (C) or SAG ratio (D). The FI gain was significantly higher in TBI neurons relative to Sham neurons (E,F). Data were analyzed via two-tailed t test or Mann Whitney test, *p < 0.05, n = 15–30 cells/group, n = 6–9 animals/group. Data are expressed as mean ± SEM. BLA, basolateral amygdala; FI, firing rate-input; SAG, voltage sag; SEM, standard error of the mean; TBI, traumatic brain injury.
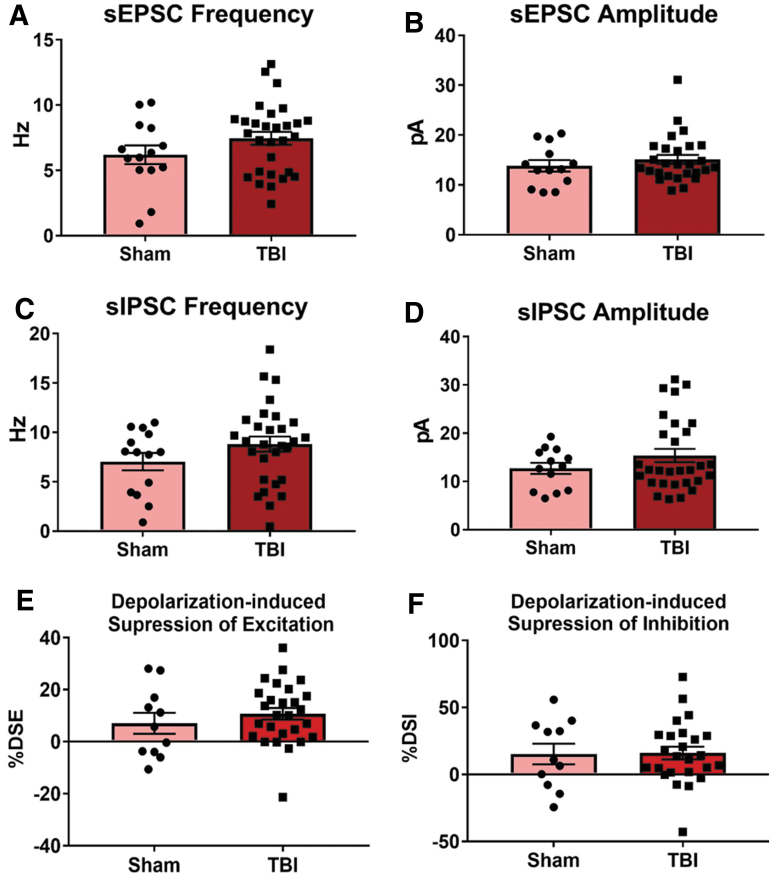
To assess whether this increase in excitability was accompanied by alterations in synaptic transmission or eCB signaling, the frequency and amplitude of sEPSCs and sIPSCs, as well as DSE and DSI were measured in BLA pyramidal neurons in Expt. 3. An unpaired two-tailed t test did not reveal any significant differences between Sham and TBI neurons in sEPSC frequency (t[41] = 1.45, p = 0.16, Fig. 7A), and a Mann-Whitney test did not show any difference in sEPSC amplitude (p = 0.51; Fig. 7B). Likewise, unpaired two-tailed t tests did not reveal any significant differences in sIPSC frequency (t[41] = 1.4, p = 0.17; Fig. 7C) or amplitude (t[40] = 1.19, p = 0.24; Fig. 7D). There were no differences in DSE (t[36] = 0.84, p = 0.41; Fig. 7E) or DSI (t[34] = 0.079, p = 0.94; Fig. 7F) between Sham and TBI neurons according to unpaired two-tailed t tests.
FIG. 7.
TBI does not alter spontaneous synaptic transmission or endocannabinoid signaling 11 days post-TBI in alcohol-naïve animals. Whole-cell patch clamp slice electrophysiology was performed on BLA pyramidal neurons from alcohol-naïve Sham and TBI animals 11 days post-TBI in Expt. 3. There were no differences in sEPSC frequency (A) or amplitude (B) nor were there changes in sIPSC frequency (C) or amplitude (D). There were also no differences in depolarization-induced suppression of excitation (DSE; E) or inhibition (DSI; F). Data were analyzed via two-tailed t test or Mann Whitney test, *p < 0.05, **p < 0.01, n = 15–30 cells/group, n = 6–9 animals/group. Data are expressed as mean ± SEM. BLA, basolateral amygdala; SEM, standard error of the mean; sEPSC, spontaneous excitatory post-synaptic current; sPSC, spontaneous inhibitory post-synaptic current; TBI, traumatic brain injury.
Discussion
We tested the effect of moderate TBI on alcohol drinking, anxiety-like behavior, electrophysiological properties of BLA cells, and expression/levels of eCB system components in the BLA of alcohol-naïve and alcohol-drinking adult female rats. Our results show that TBI does not promote escalation of alcohol drinking or anxiety-like behavior in female rats. We show that TBI interacts with alcohol to alter BLA 2-AG, and that AEA tissue contents are lower in BLA of alcohol-drinking animals 1 h post-TBI. Eleven days following injury, 2-AG BLA tissue contents were lower in TBI animals relative to Sham controls, and 2-AG amounts in BLA were negatively correlated with TBI intensity. Finally, TBI increased BLA pyramidal neuron excitability 11 days post-injury in alcohol-naïve rats, as indicated by higher FI gain in BLA cells.
Our results show that TBI does not cause escalation of alcohol self-administration in female rats, contrasting with previously published data from our laboratories demonstrating post-TBI escalation of alcohol self-administration in male rats.9 There was an apparent short-term early decrease in operant alcohol responding after TBI, which mirrors the pattern of lower alcohol use acutely after injury in humans.48 Experimental models of TBI have produced variable results regarding post-injury alcohol consumption. Following closed-skull TBI, male mice exhibit reductions in alcohol intake in the Drinking-in-the-Dark (DID) paradigm relative to sham controls, supporting the initial reduction seen here in female rats with lateral fluid percussion injury.49 One study in male Sprague-Dawley rats showed that blast exposure-induced mild TBI produced a bimodal distribution of alcohol intake in TBI animals during limited-access two-bottle choice drinking sessions, where a median split revealed higher alcohol intake in “high-drinking” injured rats compared with shams, but this effect was not found in other alcohol-drinking paradigms.50 Future studies should explore the possibility that different types of injury alter alcohol-drinking microstructure in specific ways.
Interestingly, mild TBI in juvenile female mice, but not juvenile males or adults of either sex, produced increases in alcohol drinking in adulthood.51 Limited clinical information is available on sex and gender differences in post-TBI alcohol drinking; however, one study reported that male U.S. veterans with TBI reported greater alcohol use and substance abuse compared with female U.S. veterans with TBI.52 In general, female rats consume more alcohol than males, and this difference in baseline intake may contribute to the discrepancy between these data in female rats and previously published data in male rats.53 We do not, however, attribute the lack in escalation post-TBI in female rats to a ceiling effect, because prior work has shown higher levels of operant alcohol self-administration in female Wistar rats. For example, alcohol-dependent female Wistar rats consume approximately 1.3 g/kg in a single operant session during withdrawal.53
Our results also demonstrate a lack of TBI-induced increases in anxiety-like behavior in the EPM 9 days following injury in both alcohol-naïve and alcohol-drinking female rats. However, anxiety-like behavior trended toward positively correlating with injury pressure in TBI animals, suggesting that, within injured female rats, more severe injury may be associated with higher post-injury anxiety-like behavior. We previously reported that TBI increases anxiety-like behavior in male rats in the open field 7 days post-injury.35 Studies of anxiety-like behavior in female rats have shown mixed results. A study comparing anxiety-like behavior across two different TBI models in male and female rats showed that TBI induced by weight drop on the dorsal cranium decreased time spent in the open arms of the EPM, but lateral impact injury, produced by a weight pneumatically propelled toward the left (temporal) cranium, resulted in TBI females spending more time in the open arms of the plus-maze than sham animals 3 days after injury.54 A recent study in alcohol-naïve and alcohol-drinking adult female rats did not show an effect of lateral impact TBI on anxiety-like behavior in the EPM 2 days later, but did show TBI-induced increases in anxiety-like behavior 4 days post-injury.55
Contrary to our hypothesis, post-TBI anxiety-like behavior did not significantly differ between alcohol-naïve and alcohol-drinking female rats. EPM testing occurred approximately 24 h following the most recent operant alcohol self-administration session, but mean BACs during operant sessions were far too low to be expected to elicit withdrawal-related behaviors; indeed, alcohol-drinking animals exhibited a trend toward lower anxiety-like behavior, which is the opposite of what would be expected if animals experienced withdrawal-like symptoms. It should also be noted that female rats’ behavior on the EPM may not be primarily attributable to changes in anxiety-like behavior.56 Additional studies are needed to more fully understand the impact of sex on post-TBI anxiety-like behavior. strous cycle was not tracked in these experiments, mainly to avoid potential stress effects of the swabbing procedure, but other laboratories have reported estrous effects on alcohol-drinking, anxiety-like behavior, and TBI outcomes in female rats. More work is needed to fully elucidate the contribution of estrous cycling to these post-TBI measures.57–61
We hypothesized that TBI would reduce eCB levels and signaling in the BLA. We report that BLA 2-AG levels are lower in alcohol-naïve TBI animals than alcohol-drinking TBI animals 1 h after injury, suggesting that perhaps prior alcohol use buffers against acute reductions in BLA 2-AG levels induced by TBI. Alcohol blunts other TBI effects, including pro-inflammatory cytokine expression and edema formation.62,63 Electrophysiological studies show that acute alcohol exposure results in enhanced 2-AG/CB1R signaling at glutamatergic inputs and diminished 2-AG/CB1R signaling at gamma-aminobutyric acid (GABA)ergic synapses, resulting in net increases in inhibition.36,38,64 These effects are blunted by chronic alcohol exposure, suggesting that alcohol effects on 2-AG/CB1R signaling in BLA may modify TBI effects on 2-AG.39
Conversely, although there were no effects of TBI on AEA levels 1 h post-injury, animals with an alcohol self-administration history exhibited significantly lower AEA contents regardless of injury. At this sacrifice time-point, animals’ last exposure to alcohol was 4–5 days earlier, therefore this reduction could be the result of a repeated alcohol exposure and/or an effect of alcohol abstinence. Acute alcohol reduces AEA levels in the cerebellum, hippocampus, and nucleus accumbens of male Wistar rats and in the whole amygdala of male Sprague-Dawley rats.65,66 Restraint stress reduces BLA AEA, and inhibition of BLA FAAH reduces hypothalamic-pituitary-adrenal (HPA) axis activity, and increases anxiety-like behavior.67,68 The lower BLA AEA levels we report in alcohol-drinking animals may have implications for these or similar behaviors in females, but this remains to be determined.69
Eleven days after injury, 2-AG levels were lower in BLA of TBI females relative to Sham controls, regardless of alcohol-drinking history. Further, in TBI females, BLA 2-AG levels were negatively correlated with injury pressure, suggesting that more severe injuryresults in lower 2-AG content in BLA. There was not an effect of TBI on AEA 11 days post-injury, but among TBI females, there was a significant positive correlation between injury pressure and BLA AEA levels. Although moderate TBI did not produce anxiety-like behavior in female rats in our model, prior work from other laboratories showed that 2-AG signaling in the BLA modulates fear conditioning, social behavior, and stress reactivity.67,70–71
We used western blot analysis to measure expression of the enzymes responsible for syntheizing (DAGL) and degrading (MAGL) 2-AG. Eleven days after injury, we did not observe changes in the expression of those proteins that would account for TBI-induced reductions in 2-AG levels in BLA. It is possible that: 1) proteins are differentially regulated at different synapses or in different subregions such that overall BLA amounts are not changed, 2) enzymatic activity or receptor binding is changed in the absence of a change in protein amounts, or 3) changes in 2-AG are driven by alterations in upstream processes, such as expression of post-synaptic receptors coupled to 2-AG synthesis, or upregulation of minor 2-AG catabolic pathways (e.g., COX-2, α/β-hydrolase domain enzymes).72–73 We did observe a significant increase in DAGLβ protein amounts in the BLA of alcohol-drinking Sham and TBI females relative to alcohol-naïve animals 11 days after TBI (24 h after final alcohol self-administration session).
DAGLβ is an isoform of the 2-AG synthetic enzyme predominately expressed in the central nervous system (CNS) by microglia.74 These data are in agreement with studies that used RNA-Seq analysis of cultured rat microglia to report upregulation of DAGLβ following 24-h exposure to 75 mM ethanol.75 A functional role for DAGLβ-dependent 2-AG synthesis in the BLA has not yet been established, but DAGLβ has been implicated in hippocampal neurogenesis and pro-inflammatory responses in peripheral macrophages, and systemic inhibition of DAGLβ is analgesic in mouse models of neuropathic and inflammatory pain.76–79 For these western blot data, we acknowledge the possibility that outcome measures were affected by EPM testing that occurred approximately 48 h prior to sacrifice.
We hypothesized that TBI-induced reductions in 2-AG levels in BLA 11 days after injury would confer a loss of DSE at glutamatergic inputs to BLA pyramidal neurons. Our results reveal that BLA pyramidal neurons from TBI animals are hyperexcitable as measured by increases in the average FI curve gain, but the mechanism for this enhanced excitability is currently unclear. Resting membrane potential and SAG ratio were unchanged in these neurons, and TBI decreased input resistance, which would be expected to reduce neuronal excitability. We did not observe any differences in excitatory or inhibitory synaptic transmission as measured by amplitude and frequency of spontaneous post-synaptic currents. Almeida-Suhett and colleagues found that mild controlled cortical impact TBI in Sprague-Dawley male rats decreased spontaneous and miniature IPSC amplitude and frequency in the BLA, and also enhanced α7-nAChR-mediated currents in BLA pyramidal neurons 7 days after injury.29 They also showed that TBI increases anxiety-like behavior in the open field 7 and 30 days after injury. Although we did find evidence of TBI-induced increases in BLA pyramidal neuron excitability, this effect seemingly did not alter anxiety-like behavior or alcohol self-administration in TBI female rats.
Finally, DSE and DSI were unchanged in TBI animals, and so it remains unclear how the observed decreases in 2-AG affect BLA cell signaling. The observed changes in 2-AG may be driven by changes in eCB signaling at synapses onto GABAergic interneurons (we recorded from pyramidal neurons) or by changes in specific BLA subregions that were not parsed out in this study. Further, DSE and DSI were measured using spontaneous post-synaptic currents; it's possible that stimulation of different BLA inputs (e.g., external capsule, stria terminalis) may have unveiled alterations in these measures. Importantly, 2-AG tissue contents are related to but not determined exclusively by synaptic concentrations; in fact, 2-AG concentrations are much lower in brain dialysate than in whole tissue extracts.80 Further, although DSE/DSI phenomena are attributed primarily to 2-AG, we cannot exclude the possibility that AEA influenced these outcome measures.
Our analyses revealed positive and negative correlations between TBI pressure intensity and specific outcome measures (i.e., anxiety-like behavior, brain eCB levels). Interestingly, these correlations did not extend to other indices of injury severity (i.e., apnea, respiratory rate, righting reflex). These data suggest that injury intensity is a key factor in predicting post-injury BLA dysfunction. Although studies have not explicitly evaluated graded effects of fluid percussion injury on BLA structure and function, prior behavioral and histological data do support the notion that higher pressure intensity leads to greater neurological and neurovascular impairment.81
In conclusion, results from these studies show that moderate TBI does not increase alcohol self-administration or anxiety-like behavior in adult female rats. Further, TBI and alcohol each altered eCB levels in the BLA of female rats, and TBI increases BLA pyramidal neuron excitability. Future work is necessary to clarify if differences between these data and previously published studies in male rats on alcohol self-administration and anxiety-like behavior reflect sex differences in TBI behavioral sequelae. Understanding TBI effects on psychiatric symptomatology in females is critical for developing individualized rehabilitative strategies after injury. These data add to existing evidence of post-TBI BLA dysfunction and eCB system alteration, but future studies are needed to evaluate the mechanisms and consequences of these changes in females.
Acknowledgments
We thank Garrett Sauber for performing liquid chromatography/ mass spectrometry (LC/MS) analysis for this work.
Funding Information
Support for this study was provided by National Institute on Alcohol Abuse and Alcoholism (NIAAA) training grant T32 AA007577, NIAAA fellowship award F30AA026468-01A1, NIAAA fellowship award F32 AA026779, NIAAA research grant R01AA025792-01A1, NIAAA research grant R01AA026531-01, and funds from the Louisiana State University Health Sciences Center-New Orleans Department of Physiology.
Author Disclosure Statement
N.W.G. holds shares in Glauser Life Sciences, Inc. S.E. is on the Scientific Advisory Board of Avanos. These activities have no relation to any of the work presented in this article. No competing financial interests exist for Z.F.S., E.A.F., C.J.H., J.W.M., and P.E.M.
References
- 1. Centers for Disease Control and Prevention. (2019). Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2014. Centers for Disease Control and Prevention, U.S.Department of Health and Human Services. https://www.cdc.gov/traumaticbraininjury/pdf/TBI-Surveillance-Report-508.pdf (Last accessed April19, 2020)
- 2. Koponen, S., Taiminen, T., Portin, R., Himanen, L., Isoniemi, H., Heinonen, H., Hinkka, S. and Tenovuo, O. (2002). Axis I and II psychiatric disorders after traumatic brain injury: a 30-year follow-up study. Am. J. Psychiatry 159, 1315–1321 [DOI] [PubMed] [Google Scholar]
- 3. Whelan-Goodinson, R., Ponsford, J., Johnston, L. and Grant, F. (2009). Psychiatric disorders following traumatic brain injury: their nature and frequency. J. Head Trauma Rehabil. 24, 324–332 [DOI] [PubMed] [Google Scholar]
- 4. Savola, O., Niemelä, O. and Hillbom, M. (2005). Alcohol intake and the pattern of trauma in young adults and working aged people admitted after trauma. Alcohol Alcohol. 40, 269–273 [DOI] [PubMed] [Google Scholar]
- 5. Scheenen, M.E., De Koning, M.E., Van Der Horn, H.J., Roks, G., Yilmaz, T., Van Der Naalt, J., and Spikman, J.M. (2016). Acute alcohol intoxication in patients with mild traumatic brain injury: characteristics, recovery, and outcome. J. Neurotrauma 33, 339–345 [DOI] [PubMed] [Google Scholar]
- 6. Brigode, W., Cohan, C., Beattie, G., and Victorino, G. (2019). Alcohol in traumatic brain injury: toxic or therapeutic? J. Surg. Res. 244, 196–204 [DOI] [PubMed] [Google Scholar]
- 7. Teng, S.X., and Molina, P.E. (2014). Acute alcohol intoxication prolongs neuroinflammation without exacerbating neurobehavioral dysfunction following mild traumatic brain injury. J. Neurotrauma 31, 378–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teng, S.X., Katz, P.S., Maxi, J.K., Mayeux, J.P., Gilpin, N.W., and Molina, P.E. (2015). Alcohol exposure after mild focal traumatic brain injury impairs neurological recovery and exacerbates localized neuroinflammation. Brain Behav. Immun. 45, 145–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mayeux, J.P., Teng, S.X., Katz, P.S., Gilpin, N.W., and Molina, P.E. (2015). Traumatic brain injury induces neuroinflammation and neuronal degeneration that is associated with escalated alcohol self-administration in rats. Behav. Brain Res. 279, 22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faul, M, X.L., Wald, M.M., and Coronado, V.G. (2010). Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002-2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. https://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf (Last accessed April10, 2020)
- 11. Farace, E., and Alves, W.M. (2000). Do women fare worse: a metaanalysis of gender differences in traumatic brain injury outcome. J. Neurosurg. 93, 539–545 [DOI] [PubMed] [Google Scholar]
- 12. Bazarian, J.J., Blyth, B., Mookerjee, S., He, H., and McDermott, M.P. (2010). Sex differences in outcome after mild traumatic brain injury. J. Neurotrauma 27, 527–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilde, E.A., Newsome, M., Ott, S.D., Hunter, J.V., Dash, P.K., Redell, J.B., Spruiell, M., Diaz, M., Chu, Z.D., Goodrich-Hunsaker, N.J., Petrie, J.A., Li, R., and Levin, H. (2019). Persistent disruption of brain connectivity after sports-related concussion in a female athlete. J. Neurotrauma 36, 3164–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fann, J.R., Burington, B., Leonetti, A., Jaffe, K., Katon, W.J., and Thompson, R.S. (2004). Psychiatric illness following traumatic brain injury in an adult health maintenance organization population. Arch. Gen. Psychiatry 61, 53. [DOI] [PubMed] [Google Scholar]
- 15. Janak, P.H., and Tye, K.M. (2015). From circuits to behaviour in the amygdala. Nature 517, 284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharp, B.M. (2017). Basolateral amygdala and stress-induced hyperexcitability affect motivated behaviors and addiction. Transl. Psychiatry 7, e1194–e1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wrase, J., Makris, N., Braus, D.F., Mann, K., Smolka, M.N., Kennedy, D.N., Caviness, V.S., Hodge, S.M., Tang, L., Albaugh, M., Ziegler, D.A., Davis, O.C., Kissling, C., Schumann, G., Breiter, H.C., and Heinz, A. (2008). Amygdala volume associated with alcohol abuse relapse and craving. Am. J. Psychiatry 165, 1179–1184 [DOI] [PubMed] [Google Scholar]
- 18. Gilpin, N.W., Herman, M.A., and Roberto, M. (2015). The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol. Psychiatry 77, 859–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Forster, G., Novick, A.M., Scholl, J.L., and Watt, M.J.(2012). The role of the amygdala in anxiety disorders, in: The Amygdala—A Discrete Multitasking Manager InTechOpen. https://www.intechopen.com/books/the-amygdala-a-discrete-multitasking-manager/the-role-of-the-amygdala-in-anxiety-disorders (Last accessed April16, 2020)
- 20. Tye, K.M., Prakash, R., Kim, S.-Y., Fenno, L.E., Grosenick, L., Zarabi, H., Thompson, K.R., Gradinaru, V., Ramakrishnan, C., and Deisseroth, K. (2011). Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Felix-Ortiz, A.C., Beyeler, A., Seo, C., Leppla, C.A., Wildes, C.P., and Tye, K.M. (2013). BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79, 658–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Guglielmo, G., Kallupi, M., Pomrenze, M.B., Crawford, E., Simpson, S., Schweitzer, P., Koob, G.F., Messing, R.O., and George, O. (2019). Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nature Commun. 10, 1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weera, M.M., Schreiber, A.L., Avegno, E.M., and Gilpin, N.W. (2020). The role of central amygdala corticotropin-releasing factor in predator odor stress-induced avoidance behavior and escalated alcohol drinking in rats. Neuropharmacology 166, 107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stamatakis, A.M., Sparta, D.R., Jennings, J.H., McElligott, Z.A., Decot, H., and Stuber, G.D. (2014). Amygdala and bed nucleus of the stria terminalis circuitry: implications for addiction-related behaviors. Neuropharmacology 76, Pt. B, 320–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McGinnis, M.M., Parrish, B.C., Chappell, A.M., Alexander, N.J., and McCool, B.A. (2019). Chronic ethanol differentially modulates glutamate release from dorsal and ventral prefrontal cortical inputs onto rat basolateral amygdala principal neurons. eNeuro 7, 0132–0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reger, M.L., Poulos, A.M., Buen, F., Giza, C.C., Hovda, D.A., and Fanselow, M.S. (2012). Concussive brain injury enhances fear learning and excitatory processes in the amygdala. Biol. Psychiatry 71, 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kulkarni, P., Kenkel, W., Finklestein, S.P., Barchet, T.M., Ren, J., Davenport, M., Shenton, M.E., Kikinis, Z., Nedelman, M., and Ferris, C.F. (2015). Use of anisotropy, 3D segmented atlas, and computational analysis to identify gray matter subcortical lesions common to concussive injury from different sites on the cortex. PLoS One 10, e0125748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoffman, A.N., Paode, P.R., May, H.G., Ortiz, J.B., Kemmou, S., Lifshitz, J., Conrad, C.D., and Currier Thomas, T. (2017). Early and persistent dendritic hypertrophy in the basolateral amygdala following experimental diffuse traumatic brain injury. J. Neurotrauma 34, 213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Almeida-Suhett, C.P., Prager, E.M., Pidoplichko, V., Figueiredo, T.H., Marini, A.M., Li, Z., Eiden, L.E., and Braga, M.F.M. (2014). Reduced GABAergic inhibition in the basolateral amygdala and the development of anxiety-like behaviors after mild traumatic brain injury. PLoS One 9, e102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hillard, C.J. (2015). The endocannabinoid signaling system in the CNS: a primer. Int. Rev. Neurobiol. 125, 1–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Katona, I., Rancz, E.A., Acsady, L., Ledent, C., Mackie, K., Hajos, N., and Freud, T.F. (2001). Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J. Neuroscience 21, 9506–9518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Di, S., Itoga, C.A., Fisher, M.O., Solomonow, J., Roltsch, E.A., Gilpin, N.W., and Tasker, J.G. (2016). Acute stress suppresses synaptic inhibition and increases anxiety via endocannabinoid release in the basolateral amygdala. J. Neurosci. 36, 8461–8470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Panikashvili, D., Simeonidou, C., Ben-Shabat, S., Breuer, L.H.A., Mechoulam, R., and Shohami, E. (2001). An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature 413, 527–531 [DOI] [PubMed] [Google Scholar]
- 34. Katz, P.S., Sulzer, J.K., Impastato, R.A., Teng, S.X., Rogers, E.K., and Molina, P.E. (2015). Endocannabinoid degradation inhibition improves neurobehavioral function, blood-brain barrier integrity, and neuroinflammation following mild traumatic brain injury. J. Neurotrauma 32, 297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fucich, E., Mayeux, J., McGinn, M., Gilpin, N., Edwards, S., and Molina, P. (2018). A novel role for the endocannabinoid system in ameliorating motivation for alcohol drinking and negative behavioral affect following traumatic brain injury in rats. J. Neurotrauma. 36, 1847–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perra, S., Pillolla, G., Luchicchi, A., and Pistis, M. (2008). Alcohol inhibits spontaneous activity of basolateral amygdala projection neurons in the rat: involvement of the endocannabinoid system. Alcohol. Clin. Exp. Res. 32, 443–449 [DOI] [PubMed] [Google Scholar]
- 37. Serrano, A., Rivera, P., Pavon, F.J., Decara, J., Suarez, J., Rodriguez de Fonseca, F., and Parsons, L.H. (2012). Differential effects of single versus repeated alcohol withdrawal on the expression of endocannabinoid system-related genes in the rat amygdala. Alcohol. Clin. Exp. Res. 36, 984–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Robinson, S.L., Alexander, N.J., Bluett, R.J., Patel, S., and McCool, B.A. (2016). Acute and chronic ethanol exposure differentially regulate CB1 receptor function at glutamatergic synapses in the rat basolateral amygdala. Neuropharmacology 108, 474–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Varodayan, F.P., Bajo, M., Soni, N., Luu, G., Madamba, S.G., Schweitzer, P., and Roberto, M. (2017). Chronic alcohol exposure disrupts CB1 regulation of GABAergic transmission in the rat basolateral amygdala. Addict. Biol. 22, 766–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gray, J.M., Vecchiarelli, H.A., Morena, M., Lee, T.T., Hermanson, D.J., Kim, A.B., McLaughlin, R.J., Hassan, K.I., Kuhne, C., Wotjak, C.T., Deussing, J.M., Patel, S., and Hill, M.N. (2015). Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J. Neurosci. 35, 3879–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schreiber, A.L., Lu, Y.L., Baynes, B.B., Richardson, H.N., and Gilpin, N.W. (2017). Corticotropin-releasing factor in ventromedial prefrontal cortex mediates avoidance of a traumatic stress-paired context. Neuropharmacology 113, 323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stella, N., Schweitzer, P., and Piomelli, D. (1997). A second endogenous cannabinoid that modulates long-term potentiation. Nature 388, 773–778 [DOI] [PubMed] [Google Scholar]
- 43. Mayeux, J., Katz, P., Edwards, S., Middleton, J.W., and Molina, P.E. (2017). Inhibition of endocannabinoid degradation improves outcomes from mild traumatic brain injury: a mechanistic role for synaptic hyperexcitability. J. Neurotrauma 34, 436–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fucich, E.A., Stielper, Z.F., Cancienne, H.L., Edwards, S., Gilpin, N.W., Molina, P.E., and Middleton, J.W. (2020). Endocannabinoid degradation inhibitors ameliorate neuronal and synaptic alterations following traumatic brain injury. J. Neurophysiol. 123, 707–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Washburn, M.S., and Moises, H.C. (1992). Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J. Neurosci. 12, 4066–4079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rainnie, D.G., Asprodini, E.K., and Shinnick-Gallagher, P. (1993). Intracellular recordings from morphologically identified neurons of the basolateral amygdala. J. Neurophysiol. 69, 1350–1362 [DOI] [PubMed] [Google Scholar]
- 47. Ma, C., Wu, X., Shen, X., Yang, Y., Chen, Z., Sun, X., and Wang, Z. (2019). Sex differences in traumatic brain injury: a multi-dimensional exploration in genes, hormones, cells, individuals, and society. Chin. Neurosurg. J. 5, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Beaulieu-Bonneau, S., St.-Onge, F., Blackburn, M.C., Banville, A., Paradis-Giroux, A.A., and Ouellet, M.C. (2018). Alcohol and drug use before and during the first year after traumatic brain injury. J. Head Trauma Rehabil. 33, E51–E60 [DOI] [PubMed] [Google Scholar]
- 49. Lowing, J.L., Susick, L.L., Caruso, J.P., Provenzano, A.M., Raghupathi, R., and Conti, A.C. (2014). experimental traumatic brain injury alters ethanol consumption and sensitivity. J. Neurotrauma 31, 1700–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lim, Y.W., Meyer, N.P., Shah, A.S., Budde, M.D., Stemper, B.D., and Olsen, C.M. (2015). Voluntary alcohol intake following blast exposure in a rat model of mild traumatic brain injury. PLoS One 10, e0125130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weil, Z.M., Karelina, K., Gaier, K.R., Corrigan, T.E.D., and Corrigan, J.D. (2016). Juvenile traumatic brain injury increases alcohol consumption and reward in female mice. J. Neurotrauma 33, 895–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Epstein, E.L., Martindale, S.L., VA Mid-Atlantic MIRECC Workgroup, and Miskey, H.M. (2019). Posttraumatic stress disorder and traumatic brain Injury: sex differences in veterans. Psychiatry Res. 274, 105–111 [DOI] [PubMed] [Google Scholar]
- 53. Priddy, B.M., Carmack, S.A., Thomas, L.C., Vendruscolo, J.C.M., Koob, G.F., and Vendruscolo, L.F. (2017). Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol. Biochem. Behav. 152, 61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mychasiuk, R., Hehar, H., Candy, S., Ma, I., and Esser, M.J. (2016). The direction of the acceleration and rotational forces associated with mild traumatic brain injury in rodents effect behavioural and molecular outcomes. J. Neurosci. Methods 257, 168–178 [DOI] [PubMed] [Google Scholar]
- 55. Christensen, J., Eyolfson, E., Salberg, S., Bhatt, D., Weerawardhena, H., Tabor, J., and Mychasiuk, R. (2019). When two wrongs make a right: the effect of acute and chronic binge drinking on TBI outcomes in young adult female rats. J. Neurotrauma. 37, 283–285 [DOI] [PubMed] [Google Scholar]
- 56. Fernandes, C., MI Gonzalez, M.I., Wilson, C.A., and File, S.E. (1999). Factor analysis shows that female rat behavior is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacol. Biochem. Behav. 64, 731–738 [DOI] [PubMed] [Google Scholar]
- 57. Roberts, A.J., Smith, A.D., Weiss, F., Rivier, C., and Koob, G.F. (1998) Estrous cycle effects on operant responding for ethanol in female rats. Alcohol. Clin. Exp. Res. 22, 1564–1569 [PubMed] [Google Scholar]
- 58. Ford, M.M., Eldridge, J.C., and Samson, H.H. (2002) Microanalysis of ethanol self-administration: estrous cycle phase related changes in consumption patterns. Alcohol. Clin. Exp. Res. 26, 635–643 [PubMed] [Google Scholar]
- 59. Frye, C.A., Petralia, S.M., and Rhodes, M.E. (2000). Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol. Biochem, Behav. 67, 587–596 [DOI] [PubMed] [Google Scholar]
- 60. Sayin, A., Derinoz, O., Yuksel, N., Sahin, S., and Bolay, H. (2014). The effects of the estrus cycle and citalopram on anxiety-like behaviors and c-fos expression in rats. Pharmacol. Biochem. Behav. 124, 180–187 [DOI] [PubMed] [Google Scholar]
- 61. Maghool, F., Khaksari, M., and Siahposht Khachki, A. (2013). Differences in brain edema and intracranial pressure following traumatic brain injury across the estrous cycle: involvement of female sex steroid hormones. Brain Res. 1497, 61–72 [DOI] [PubMed] [Google Scholar]
- 62. Goodman, M.D., Makley, A.T., Campion, E.M., Friend, L.A.W., Lentsch, A.B., and Pritts, T.A. (2013). Preinjury alcohol exposure attenuates the neuroinflammatory response to traumatic brain injury. J. Surg. Res. 184, 1053–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang, T., Chou, D.Y., Ding, J.Y., Fredrickson, V., Peng, C., Schafer, S., Guthikonda, M., Kreipke, C., Rafols, J.A., and Ding, Y. (2013). Reduction of brain edema and expression of aquaporins with acute ethanol treatment after traumatic brain injury. J. Neurosurg. 118, 390–396 [DOI] [PubMed] [Google Scholar]
- 64. Talani, G., and Lovinger, D.M. (2015). Interactions between ethanol and the endocannabinoid system at GABAergic synapses on basolateral amygdala principal neurons. Alcohol 49, 781–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ferrer, B., Bermúdez-Silva, F.J., Bilbao, A., Alvarez-Jaimes, L., Sanchez-Vera, I., Giuffrida, A., Serrano, A., Baixeras, E., Khaturia, S., Navarro, M., Parsons, L.H., Piomelli, D., and Rodríguez de Fonseca, F. (2007). Regulation of brain anandamide by acute administration of ethanol. Biochem. J. 404, 97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rubio, M., McHugh, D., Fernandez-Ruiz, J., Bradshaw, H., and Walker, J.M. (2007). Short-term exposure to alcohol in rats affects brain levels of anandamide, other N-acylethanolamines and 2-arachidonoyl-glycerol. Neurosci. Lett. 421, 270–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hill, M.N., McLaughlin, R.J., Morrish, A.C., Viau, V., Floresco, S.B., Hillard, C.J., and Gorzalka, B.B. (2009). Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology 34, 2733–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Morena, M., Patel, S., Bains, J.S., and Hill, M.N. (2016). Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology 41, 80–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Riebe, C.J., Hill, M.N., Lee, T.T., Hillard, C.J., and Gorzalka, B.B. (2010). Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology 35, 1265–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cavener, V.S., Gaulden, A., Pennipede, D., Jagasia, P., Uddin, J., Marnett, L.J., and Patel, S. (2018). Inhibition of diacylglycerol lipase impairs fear extinction in mice. Front. Neurosci. 12, 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Folkes, O.M., Báldi, R., Kondev, V., Marcus, D.J., Hartley, N.D., Turner, B.D., Ayers, J.K., Baechle, J.J., Misra, M.P., Altemus, M., Grueter, C.A., Grueter, B.A., and Patel, S. (2020). An endocannabinoid-regulated basolateral amygdala–nucleus accumbens circuit modulates sociability. J. Clin. Invest. 130, 1728–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kozak, K.R., Rowlinson, S.W., and Marnett, L.J. (2000). Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J. Biol. Chem. 275, 33744–33749 [DOI] [PubMed] [Google Scholar]
- 73. Blankman, J.L., Simon, G.M., and Cravatt, B.F. (2007). A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 14, 1347–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Viader, A., Ogasawara, D., Joslyn, C.M., Sanchez-Alavez, M., Mori, S., Nguyen, W., Conti, B., and Cravatt, B.F. (2016). A chemical proteomic atlas of brain serine hydrolases identifies cell type-specific pathways regulating neuroinflammation. Elife 5, e12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kalinin, S., González-Prieto, M., Scheiblich, H., Lisi, L., Kusumo, H., Heneka, M.T., Madrigal, J.L.M., Pandey, S.C., and Feinstein, D.L. (2018). Transcriptome analysis of alcohol-treated microglia reveals downregulation of beta amyloid phagocytosis. J. Neuroinflammation 15, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gao, Y., Vasilyev, D.V., Goncalves, M.B., Howell, F.V., Hobbs, C., Reisenberg, M., Shen, R., Zhang, M.Y., Strassle, B.W., Lu, P., Mark, L., Piesla, M.J., Deng, K., Kouranova, E.V., Ring, R.H., Whiteside, G.T., Bates, B., Walsh, F.S., Williams, G., Pangalos, M.N., Samad, T.A., and Doherty, P. (2010). Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J. Neurosci. 30, 2017–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hsu, K.L., Tsuboi, K., Adibekian, A., Pugh, H., Masuda, K., and Cravatt, B.F. (2012). DAGLbeta inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat. Chem. Biol. 8, 999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wilkerson, J.L., Ghosh, S., Bagdas, D., Mason, B.L., Crowe, M.S., Hsu, K.L., Wise, L.E., Kinsey, S.G., Damaj, M.I., Cravatt, B.F., and Lichtman, A.H. (2016). Diacylglycerol lipase β inhibition reverses nociceptive behaviour in mouse models of inflammatory and neuropathic pain. Brit. J. Pharmacol. 173, 1678–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wilkerson, J.L., Donvito, G., Grim, T.W., Abdullah, R.A., Ogasawara, D., Cravatt, B.F., and Lichtman, A.H. (2017). Investigation of diacylglycerol lipase alpha inhibition in the mouse lipopolysaccharide inflammatory pain model. J. Pharmacol. Exp. Ther. 363, 394–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Buczynski, M.W., and Parsons, L.H. (2010). Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls. Brit. J. Pharmacol. 160, 423–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. McIntosh, T.K., Vink, R., Noble, L., Yamakami, I., Fernyak, S., Soares, H., and Faden A.L. (1989). Traumatic brain injury in rats: characterization of the lateral fluid percussion model. Neuroscience 28, 233–244 [DOI] [PubMed] [Google Scholar]