Abstract
Background
Phytochemical medicines containing tanshinol and ligustrazine are commonly used in the treatment of stable angina in China, but their clinical effectiveness and risk have not been adequately assessed. In this paper, we conducted a systematic review and meta-analysis to evaluate the clinical efficacy.
Methods
Relevant randomized controlled trials (RCTs) of phytochemical medicines containing tanshinol and ligustrazine in the treatment of stable angina were searched in electronic databases. The search date was up to March 31, 2020, and the languages of the RCTs were limited to English and Chinese.
Results
A total of 28 studies, including 2518 patients, were included in the meta-analysis. It was shown that the adjunctive therapy of phytochemical medicines containing tanshinol and ligustrazine was better than the conventional therapies in the improvement of stable angina according to the clinical efficacy in symptoms (n = 2518, RR = 1.24, 95% CI: 1.20 to 1.29, P < 0.01) and clinical efficacy in electrocardiography (n = 1766, RR = 1.29, 95% CI: 1.19 to 1.40, P < 0.01).
Conclusion
The meta-analysis supported the use of phytochemical medicines containing tanshinol and ligustrazine in the treatment of stable angina. However, quality of the evidence for this finding was low due to a high risk of bias in the included studies. Therefore, well-designed RCTs are still needed to further evaluate the efficacy.
1. Introduction
Stable angina is caused by fixed blockages in coronary arteries [1]. It typically occurs during activities, and the main symptoms are chest tightness and shortness of breath, which can be alleviated after a rest or administration of sublingual nitroglycerin [2–4]. Stable angina is a chronic coronary disease compared with unstable angina; however, it seriously affects patients' lives, such as restricting daily activities [5]. Then, the treatment aims to reduce morbidity and improve symptoms.
Currently, the main treatment of stable angina is medicine, such as nitroglycerin, beta-blockers, or calcium channel blockers, which focus on decreasing heart's workload and prevent episodes [6–9]. In China, phytochemical medicines are also used by many physicians. For example, Shao et al. [10] conducted a meta-analysis to assess the efficacy of danshen injection (main component: salvianic aid A) in the treatment of angina pectoris and concluded that it is more effective than antianginal agents alone. Yu et al. [11] and Wang et al. [12] conducted randomized controlled trials in the treatment of stable angina, respectively, and found that xinxuekang capsule (main component: steroidal saponins) had a better efficacy compared with danshen tablets. In addition, for the treatment of unstable angina, many researchers have supported different phytochemical medicines, such as puerarin injection [13], safflower yellow injection [14], and danshen chuanxiongqin injection [15].
In these phytochemical medicines, tanshinol and ligustrazine are the commonly used components. Tanshinol is also named salvianic aid A, with a molecular formula C9H10O5 [16]. Ligustrazine's molecular formula is C8H12N2 [17]. Tanshinol has antioxidant capacity [18]; it can attenuate oxidative stress by decreasing the expressions of FoxO3a signaling [19] and improve cardiovascular injury by scavenging reactive oxygen species [20]. In addition, tanshinol can attenuate endothelial cell apoptosis, which helps reduce the aortic atherosclerotic lesion area [21]. Ligustrazine has effects on calcium channels; the research of Ren et al. [22] showed that ligustrazine could significantly suppress calcium transient and contraction in rabbits. It was also reported that the ligustrazine exhibits an anti-inflammatory effect; as Guo et al. described, the salvia ligustrazine injection could decrease high-sensitivity C-reactive protein and interleukin-6 levels [17, 23]. Ligustrazine was also found to suppress acid-sensing ion channels and reduce ischemia-induced infarct size in rats with angina [24]. The combination of tanshinol and ligustrazine has efficacy in dilating coronary arteries, reducing blood viscosity, promoting blood circulation, and removing blood stasis through synergistic action [25–27]. Ye et al. [28] investigated the anti-inflammatory effect of danshen, chuanxiong, and their combination and found that their combination has a dual anti-inflammatory effect on macrophages and endothelial cells. All these findings provide a biological basis of tanshinol and ligustrazine in the treatment of angina.
Tanshinol and ligustrazine are the main compounds of danshen and chuanxiong. There are several phytochemical medicines whose main components are tanshinol and ligustrazine, such as danshen chuanxiongqin injection, guanxinning injection, and shenxiong glucose injection. Several systematic reviews have been conducted to evaluate the efficacy of these medicines in the treatment of angina pectoris. Jia et al. [29] analyzed eligible RCTs using guanxinning injection, Zhang et al. [15] assessed danshen chuanxiongqin injection in treating unstable angina pectoris, and Liu and Ding [30] assessed shenxiong glucose injection in the treatment of unstable angina pectoris. In addition, many randomized controlled trials have been published to support the use of danshen and chuanxiongqin in the treatment of stable angina. However, in the treatment of stable angina, no relevant meta-analysis has been conducted to assess the clinical efficacy or the risk of phytochemical medicines containing tanshinol and ligustrazine. Therefore, in this study, a meta-analysis was conducted to evaluate the efficacy of phytochemical medicines in the treatment of stable angina.
2. Methods
The protocol of this study was registered in PROSPERO with the registration number CRD42018105921.
2.1. Database and Search Strategies
The following electronic databases were searched by two independent reviewers (Gao L. and Wang J.): Web of Science, Cochrane Library, PubMed, Chinese Biomedical Literature Database, Chinese National Knowledge Infrastructure, Chinese Scientific Journal Database, and Wanfang Database. The search date was up to March 31, 2020, and the languages of the publications were limited to English and Chinese. The following search terms were used: (tanshinol OR salvianic acid A OR β-(3,4-dihydroxyphenyl) lactic acid OR danshensu OR danshen OR radix salvia OR salvia miltiorrhiza) AND (ligustrazine OR chuanxiong OR chuanxiongzine OR tetramethylpyrazine) AND (stable angina OR angina OR angina pectoris OR stenocardia OR angor pectoris) AND (randomized controlled trial).
2.2. Inclusion Criteria
The included studies must be RCTs.
Participants: patients who were diagnosed with stable angina were included. The stable angina was diagnosed according to the criteria [31, 32], with tests such as electrocardiography (ECG), exercise ECG, and symptoms of the patients.
Interventions: interventions using phytochemical medicines containing tanshinol and ligustrazine as a main treatment were chosen. The dosages of tanshinol and ligustrazine should be described specifically.
Comparators: the control groups received conventional treatments, such as taking medicines to treat and prevent angina attacks. Placeboes were also included.
Outcomes: the primary outcome was the clinical efficacy in symptoms and ECG; the secondary outcome is adverse event.
2.3. Exclusion Criteria
The exclusion criteria in the meta-analysis included (a) non-RCTs, case studies, experience summaries, animal experiments, and unpublished or repeated studies; (b) studies that used herbal medicines as the main intervention in addition to tanshinol and ligustrazine; (c) studies that used acupuncture or cupping as combined therapies; (d) patients who were identified as unstable angina; and (e) patients who have complications of heart failure, diabetes, stroke, or some other serious organic diseases.
2.4. Data Extraction and Quality Assessment
Four reviewers (Gao L, Wu T, Jia C, and Xiao Z) independently performed the data extraction and quality assessments. Meta-analysis was conducted using RevMan 5.3 software, and the risk of bias was assessed according to the Cochrane handbook [33]. Any disagreement was resolved by discussions among all reviewers.
3. Results
3.1. Description of the Included Studies
In this meta-analysis, 1613 studies were identified through database searching. But 500 repeated studies were excluded and 882 irrelevant studies were excluded through title and abstract reviewing. The full texts of 231 studies were assessed and 203 studies were excluded, including 75 studies that used some other herbal medicines in addition to tanshinol and ligustrazine in the intervention group, 96 studies included patients with unstable angina, 2 study lacked data on the dosages of tanshinol and ligustrazine, 1 study lacked data to judge the efficacy, and 29 studies had patients with complications. At last, a total of 28 studies [34–61] were included in the meta-analysis. The screening process is summarized in a PRISMA flow diagram (Figure 1).
Figure 1.
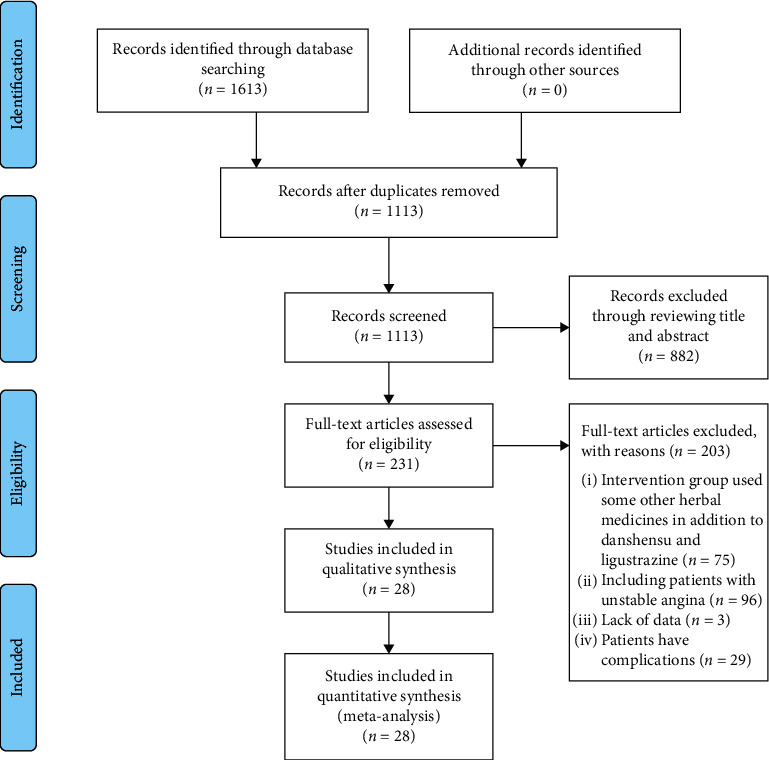
PRISMA flow diagram of the screening process.
Details of the 28 studies are summarized in Table 1. There were 2518 patients in total, including 1276 patients in the intervention group and 1242 patients in the control group. Sample sizes of the studies were small, and only 8 studies had sample sizes greater than 100 patients. The youngest patient in these studies was 46 years old, while most of the studies reported patients older than 60 years old. Many patients had a long course of the disease, and the longest course was 25 years. In the control group, conventional treatments were used, such as nitroglycerin, beta-blockers, and calcium channel blockers. No study used a placebo. In the intervention group, phytochemical medicines containing tanshinol and ligustrazine were used based on the control group, except that one study that used a phytochemical medicine containing tanshinol and ligustrazine alone. The uses of tanshinol and ligustrazine were in different forms, 21 studies used danshen chuanxiongqin injection (DCI), 5 studies used shenxiong glucose injection (SGI), and 2 studies used danshen injection (DI) combined with ligustrazine injection (LI). The nature of constituents is botanical. 1 ml DCI contains 0.4 mg tanshinol and 20 mg ligustrazine, 1 ml SGI contains 0.2 mg tanshinol and 1 mg ligustrazine, and 1 ml DI contains 0.2 mg tanshinol. Details of the constituents of the 28 included studies are shown in Appendix table (available here). The treatment duration lasted from 7 days to 30 days. All the studies used clinical efficacy in symptoms as the main outcome, and 18 studies used clinical efficacy in ECG.
Table 1.
Details of the 28 included studies on phytochemical medicines containing tanshinol and ligustrazine in the treatment of stable angina.
| Study | Sample size | Age (years) | Course of disease (years) | Intervention group | Control group | Treatment duration (days) | Main outcomes |
|---|---|---|---|---|---|---|---|
| Cao and Wang [34] | 118 (59/59) | 63.0 ± 14.0 61.5 ± 14.5 |
NR | DCI (10 ml) + TCR | Nifedipine 30–60 mg/d; metoprolol 100–200 mg/d; aspirin 100–300 mg/d; nitroglycerin when necessary | 14 | CES + ECG |
| Chen [35] | 100 (50/50) | 57.24 ± 9.64 | NR | DCI (10 ml) | Aspirin 100 mg/d; atorvastatin 20 mg/d; trimetazidine 60 mg/d; nitroglycerin 10 mg/d | 7 | CES |
| Ding [36] | 60 (30/30) | 56–72 | NR | DCI (10 ml) + TCR | Nitroglycerin | 10 | CES |
| Han [37] | 60 (30/30) | 64.9 ± 5.89 65.7 ± 7.93 |
NR | DCI (10 ml) + TCR | Nitrates; aspirin; calcium channel blockers | 14 | CES |
| He and Li [38] | 70 (35/35) | 48.2 ± 2.1 | NR | DCI (10 ml) + TCR | Aspirin; nitrates | 14 | CES |
| Hu [39] | 58 (30/28) | 60 ± 8 59 ± 9 |
NR | DCI (10 ml) + TCR | Nitrates; beta-blockers; calcium channel blockers; aspirin | 14 | CES + ECG |
| Hua et al. [40] | 70 (35/35) | 64.7 ± 8.4 63.3 ± 8.3 |
0.5–10 | DCI (10 ml) + TCR | Isosorbide mononitrate 25 mg/d; quinapril 10 mg/d; metoprolol 50 mg/d; aspirin 100 mg/d | 14 | CES + ECG |
| Jia [41] | 76 (38/38) | 62.04 ± 2.15 61.92 ± 2.13 |
NR | DCI (10 ml) + TCR | Aspirin 100 mg/d; atorvastatin 20 mg/d; trimetazidine 60 mg/d; isosorbide mononitrate 40 mg/d | 7 | CES |
| Lan [42] | 216 (116/100) | 57.6 ± 4.6 58.1 ± 5.2 |
7.62 ± 3.87 8.91 ± 4.28 |
DCI (10 ml) + TCR | Nitrates; beta-blockers; calcium channel blockers; aspirin | 14 | CES + ECG |
| Li et al. [43] | 80 (40/40) | 67.3 ± 6.20 | 3.5 ± 1.6 | DCI (10 ml) + TCR | Isosorbide mononitrate 40 mg/d; metoprolol 47.5 mg/d; aspirin 100 mg/d; trimetazidine 60 mg/d | 14 | CES + ECG |
| Li and Li [44] | 80 (40/40) | 58.93 ± 2.07 58.42 ± 2.31 |
4.45 ± 1.43 4.37 ± 1.52 |
DCI (10 ml) + TCR | Aspirin 100 mg/d; atorvastatin 20 mg/d; trimetazidine 60 mg/d; isosorbide mononitrate 40 mg/d | 7 | CES |
| Li [45] | 216 (108/108) | 64.7 ± 4.8 | 10.6 ± 1.2 | DCI (10 ml) + TCR | Aspirin; calcium channel blockers; beta-blockers; nitrates | 14 | CES + ECG |
| Liu and Li [46] | 60 (30/30) | 63.7 ± 7.7 64.9 ± 9.4 |
NR | SGI (100 ml) + TCR | Nitrates; aspirin; beta-blockers; calcium channel blockers; ACE inhibitors; ARBs | 14 | CES |
| Ma et al. [47] | 80 (40/40) | NR | NR | DCI (10 ml) + TCR | Nitrates; beta-blockers; calcium channel blockers; aspirin | 14 | CES + ECG |
| Ma et al. [48] | 104 (52/52) | 63.74 ± 11.83 62.34 ± 10.63 |
NR | SGI (100 ml) + TCR | Aspirin 100 mg/d; isosorbide mononitrate 40 mg/d; metoprolol 50 mg/d; atorvastatin 20 mg/d | 14 | CES |
| Ou [49] | 60 (30/30) | 60.33 ± 10.04 61.21 ± 9.36 |
0.08–12 0.16–13 |
SGI (100 ml) + TCR | Nitrates; beta-blockers; calcium channel blockers; antiplatelet drug | 14 | CES + ECG |
| Pang and Liu [50] | 64 (32/32) | 77.2 ± 5.6 | 6–20 | SGI (200 ml) + TCR | Antiplatelet drug; beta-blockers; statins; ACE inhibitors; ARBs; nitrates; lipid-lowering drug; hypotensor; nitroglycerin; isosorbide mononitrate 20 mg/d | 14 | CES + ECG |
| Sun [51] | 80 (40/40) | 72.3 ± 0.2 71.9 ± 0.4 |
5.2 ± 0.6 5.1 ± 0.4 |
DCI (5 ml) + TCR | Antiplatelet drug; thrombolytic drug; lipid-lowering drug; hypotensor; digoxin when necessary | 30 | CES + ECG |
| Tian [52] | 62 (32/30) | 61.68 ± 10.98 60.39 ± 9.76 |
NR | DCI (20 ml) + TCR | Nitrates; calcium channel blockers | 14 | CES |
| Wang and Wang [54] | 62 (31/31) | 46–58 | 3–18 | DI (20 ml) + LI (80 mg) + TCR | Nitrates; beta-blockers; calcium channel blockers | 14 | CES + ECG |
| Wang and Lian [53] | 105 (56/49) | 51–75 | 1.6–25 | DCI (10 ml) + TCR | Nitrates; beta-blockers; calcium channel blockers; aspirin | 14 | CES + ECG |
| Xi [55] | 80 (40/40) | 59.3 ± 6.4 62.4 ± 5.3 |
5.9 ± 0.6 4.8 ± 0.8 |
DCI (10 ml) + TCR | Aspirin; atorvastatin; nitroglycerin | 14 | CES |
| Xie and Zhu [56] | 104 (52/52) | 51–79 | 1.6–25 | DI (20–30 ml) + LI (40–80 mg) + TCR | Nitrates; beta-blockers; calcium channel blockers; aspirin | 14 | CES + ECG |
| Xing [57] | 100 (50/50) | 66.2 ± 5.60 | 2.8 ± 1.8 | DCI (10 ml) + TCR | Isosorbide mononitrate 40 mg/d; aspirin 100 mg/d | 14 | CES + ECG |
| Xiong and Wang [58] | 85 (46/39) | 52–71 | 1.6–25 | DCI (10 ml) + TCR | Nitrates; beta-blockers; calcium channel blockers; aspirin | 14 | CES + ECG |
| Xu and Qin [59] | 84 (42/42) | 56.14 ± 7.40 51.20 ± 7.30 |
1–13 2–12 |
DCI (10 ml) + TCR | Nitrates; aspirin; beta-blockers; nitroglycerin when necessary | 14 | CES + ECG |
| Yu and Fang [60] | 94 (47/47) | NR | NR | DCI (5–10 ml) + TCR | Antiplatelet drug; thrombolytic drug; lipid-lowering drug; hypotensor; digoxin when necessary | 20 | CES + ECG |
| Zhang [61] | 90 (45/45) | 68.21 ± 9.26 68.52 ± 9.39 |
NR | SGI (200 ml) + TCR | Antiplatelet drug; anticoagulant; ACE inhibitors; beta-blockers; calcium channel blockers; statins; nitrates | 10–14 | CES + ECG |
NR: not reported; DCI: danshen chuanxiongqin injection; SGI: shenxiong glucose injection; DI: danshen injection; LI: ligustrazine injection; GI: guanxinning injection; TCR: treatments in the control group; ACE: angiotensin-converting enzyme; ARBs: angiotensin receptor blockers; CES: clinical efficacy in symptoms; ECG: electrocardiography.
3.2. Risk of Bias
The risk of bias was high in the included studies (Figure 2). All the studies were described using randomization, but only five of these studies [35, 38, 44, 47, 55] reported using an appropriate method of random sequence generation. None of the studies described the method for allocation concealment, blinding of participants and personnel, and blinding of the outcome assessment.
Figure 2.
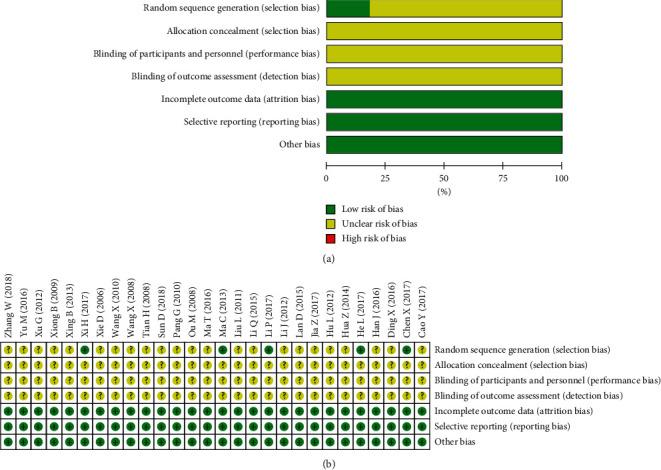
Risk of bias graph: (a) risk of bias in all included studies; (b) risk of bias summary.
3.3. Outcome Measurements
The outcome measurements of the included studies include clinical efficacy in symptoms, clinical efficacy in ECG, and adverse events.
3.3.1. Clinical Efficacy in Symptoms
The criteria for clinical efficacy in symptoms are defined as follows [62]: effective (the frequency of angina or the amount of nitroglycerine used is reduced by more than 50%) and no effect (the frequency of angina or the amount of nitroglycerine used is reduced by less than 50%).
All the studies showed that phytochemical medicines containing tanshinol and ligustrazine have better clinical efficacy in symptoms. Since low heterogeneity was observed in the meta-analysis (I2 = 43%, which is lower than 50%), a model of fixed effects was used to calculate the pooled estimation with an analysis of the dichotomous data using relative risk (RR), including 95% confidence intervals (CIs). The total meta-analysis showed favorable effects of phytochemical medicines on clinical efficacy (n = 2518, RR = 1.24, 95% CI: 1.20 to 1.29, P < 0.01) compared with the control group (Figure 3).
Figure 3.
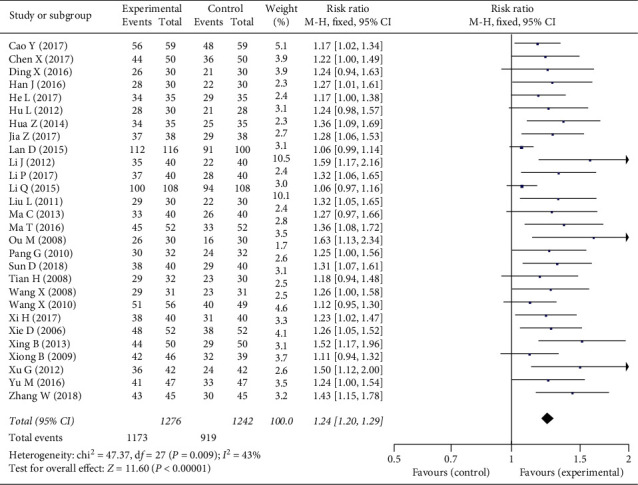
Forest plot of the clinical efficacy in symptoms of phytochemical medicines containing tanshinol and ligustrazine in the treatment of stable angina.
3.3.2. Clinical Efficacy in ECG
The criteria for clinical efficacy in ECG are defined as follows [62]: effective (recovery of ST-segment depression is more than 0.05 mV, or amplitude of the inverted T wave reduces more than 50%, or the shape of T wave changes from flat to upright) and no effect (no improvements in ECG compared with before).
Since high heterogeneity was observed in the meta-analysis (I2 = 64%, which is higher than 50%), a model of random effects was used. The total meta-analysis showed favorable effects of phytochemical medicines on ECG (n = 1766, RR = 1.29, 95% CI: 1.19 to 1.40, P < 0.01) compared with the control group (Figure 4).
Figure 4.
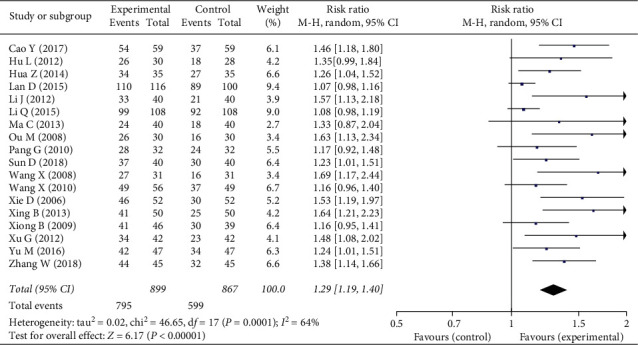
Forest plot of the clinical efficacy in ECG of phytochemical medicines containing tanshinol and ligustrazine in the treatment of stable angina.
3.3.3. Adverse Events (AEs)
Only 10 studies reported AEs, of which 7 studies [35, 39, 40, 49, 54, 56, 59] reported that there were no AEs. In the other three studies [38, 42, 55], two studies reported AEs in the intervention group, including 2 cases of skin rash, 1 case of epigastric discomfort, 1 case of insomnia, and 1 case of tiredness, and three studies reported AEs in the control group, including 2 cases of nausea, 1 case of stomachache, 1 case of dizziness, 2 cases of skin rash, 4 cases of epigastric discomfort, 3 cases of insomnia, and 3 cases of tiredness. Other studies did not report AEs.
4. Discussion
Currently, phytochemical medicines containing tanshinol and ligustrazine have been widely utilized by physicians to treat stable angina in China. However, it is controversial since there was no systematic review to assess the therapy's clinical efficacy. Therefore, this meta-analysis aimed to evaluate the efficacy or risk of phytochemical medicines in the treatment of stable angina.
In this meta-analysis, DCI and SGI were used by most studies. Both DCI and SGI consist of tanshinol and ligustrazine. As reported, DCI and SGI have been studied in the treatment of acute myocardial infarction [63], myocardial ischemia/reperfusion injury [64], and focal cerebral ischemia [65]. In the theory of traditional Chinese medicine, angina pectoris should be treated by supplementing qi and activating blood circulation. Tanshinol and ligustrazine are extracted from danshen and chuanxiong, which are two commonly used herbs in the treatment of cardiac diseases in China.
Tanshinol is the drug used for promoting blood circulation and removing blood stasis, which can improve cardiac function by increasing coronary blood flow and slowing the heart rate down [66]. Clinical practice has proved that salvia has a curative effect on myocardial hypoxia caused by myocardial infarction, and it plays a role in anticoagulation by dilating peripheral vessels to reduce blood pressure and improving cAMP (cyclic adenosine monophosphate) in cells [67, 68]. Ligustrazine is a kind of active alkaloid, which is effective to dilate coronary arteries and reduce coronary resistance. Ligustrazine is efficient in increasing coronary blood flow and improving myocardial oxygen supply, making it commonly to be used to inhibit platelet aggregation and depolymerize the aggregated platelets [69, 70]. In the treatment of cardiac diseases, tanshinol and ligustrazine can promote blood circulation, dilate coronary arteries, and inhibit platelet aggregation [29, 71], which provides a rationale in the treatment of stable angina.
Heterogeneity in this meta-analysis was moderate, with clinical efficacy in symptoms of I2 = 43%, and clinical efficacy in ECG of I2 = 64%. The reasons for this may be that different conventional treatments were used in different control groups. Therapies in the intervention group were based on the control group; different therapies make the efficacy hard to be assessed.
The high risk of bias of the included studies makes the methodological quality for this finding low. There are several limitations to this systematic review. First, for most of the included studies, the methods for randomization, allocation concealment, and blinding were not reported clearly. Second, in the 28 included studies, only 8 studies had sample sizes greater than 100 patients, and the small sample sizes in most studies made meaningful conclusions difficult to be drawn. Third, clinical efficacy was the main outcome measurement for most studies, but a bias from the physicians may decrease the reliability and validity of the studies. Fourth, all the studies were published in China, which may limit the generalization of the findings.
5. Conclusion
In conclusion, this meta-analysis included 28 studies that used phytochemical medicines containing tanshinol and ligustrazine in the treatment of stable angina, and the results supported their clinical application. However, the studies analyzed to date are of relatively low quality. More rigorous RCTs with large sample sizes are needed to further evaluate the clinical efficacy and the adverse effects.
Acknowledgments
This study was supported by China Postdoctoral Science Foundation (Grant no. 2019M650598), Fundamental Research Funds for the Central Universities (Grant no. 2019-JYB-JS-005), National Natural Science Foundation of China (Grant no. 81874514), and National Key Research and Development Program of China (Grant no. 2017YFC1700102).
Abbreviations
- RCTs:
Randomized controlled trials
- ECG:
Electrocardiography
- DCI:
Danshen chuanxiongqin injection
- SGI:
Shenxiong glucose injection
- DI:
Danshen injection
- LI:
Ligustrazine injection
- CIs:
Confidence intervals
- AEs:
Adverse events
- TCR:
Treatments in the control group
- ACE:
Angiotensin-converting enzyme
- ARBs:
Angiotensin receptor blockers
- CES:
Clinical efficacy in symptoms.
Contributor Information
Chunhua Jia, Email: chjia11@163.com.
Wei Wang, Email: wangwei@bucm.edu.cn.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Additional Points
Highlights. (i) It was the first meta-analysis assessing the efficacy of phytochemical medicines containing tanshinol and ligustrazine in the treatment of stable angina. (ii) The clinical efficacy and safety of phytochemical medicines containing tanshinol and ligustrazine in the treatment of stable angina were comprehensively assessed. (iii) The efficacy of phytochemical medicines containing tanshinol and ligustrazine in the treatment of stable angina needed to be further evaluated.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
LG contributed to conception, acquisition, analysis, and interpretation; TW contributed to acquisition, analysis, and interpretation; JW contributed to interpretation; ZX contributed to acquisition and analysis; CJ contributed to acquisition and analysis; WW contributed to conception and interpretation. All authors drafted manuscript, revised manuscript, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Supplementary Materials
File name: Appendix table. Title of data: summary table of the constituents of the 28 included studies. Description of data: statements of the constituents of the included studies.
References
- 1.Al-Lamee R., Thompson D., Dehbi H.-M., et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. The Lancet. 2018;391(10115):31–40. doi: 10.1016/s0140-6736(17)32714-9. [DOI] [PubMed] [Google Scholar]
- 2.Tousoulis D. Editorial (hot topics: stable angina pectoris: novel therapeutic insights) Current Pharmaceutical Design. 2013;19(9):p. 1549. doi: 10.2174/1381612811319090001. [DOI] [PubMed] [Google Scholar]
- 3.Arnold S. V., Bhatt D. L., Barsness G. W., et al. Clinical management of stable coronary artery disease in patients with type 2 diabetes mellitus: a scientific statement from the American heart association. Circulation. 2020;141(19) doi: 10.1161/cir.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 4.Rousan T. A., Thadani U. Stable angina medical therapy management guidelines: a critical review of guidelines from the European society of cardiology and national institute for health and care excellence. European Cardiology Review. 2019;14(1):18–22. doi: 10.15420/ecr.2018.26.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrikopoulos G., Parissis J., Filippatos G., et al. Hellenic cardiovasc res S: medical management of stable angina. Hellenic Journal of Cardiology. 2014;55(4):272–280. [PubMed] [Google Scholar]
- 6.Glezer M., Vasyuk Y., Karpov Y. Efficacy of ivabradine in combination with beta-blockers versus uptitration of beta-blockers in patients with stable Angina (CONTROL-2 study) Advances in Therapy. 2018;35(3):341–352. doi: 10.1007/s12325-018-0681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rousan T. A., Mathew S. T., Thadani U. Drug therapy for stable angina pectoris. Drugs. 2017;77(3):265–284. doi: 10.1007/s40265-017-0691-7. [DOI] [PubMed] [Google Scholar]
- 8.Gould K. L., Johnson N. P. Nitroglycerine and angina. Circulation. 2017;136(1):35–38. doi: 10.1161/circulationaha.117.028791. [DOI] [PubMed] [Google Scholar]
- 9.Liao W., Ma X., Li J., et al. A review of the mechanism of action of dantonic for the treatment of chronic stable angina. Biomedicine & Pharmacotherapy. 2019;109:690–700. doi: 10.1016/j.biopha.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Shao H., Li M., Chen F., Chen L., Jiang Z., Zhao L. The efficacy of danshen injection as adjunctive therapy in treating angina pectoris: a systematic review and meta-analysis. Heart, Lung and Circulation. 2018;27(4):433–442. doi: 10.1016/j.hlc.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Yu Y. N., Hu S. Y., Li G. X., et al. Comparative effectiveness of di’ao xin xue kang capsule and compound danshen tablet in patients with symptomatic chronic stable angina. Scientific Reports. 2014;4(7058) doi: 10.1038/srep07058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L.-Y., Tang J.-Y., Liu J., et al. Dynamic changes in phenotypic groups in patients with stable angina pectoris after treatment with xinxuekang capsule: a randomized controlled trial. Current Vascular Pharmacology. 2015;13(4):492–503. doi: 10.2174/1570161112666141014151858. [DOI] [PubMed] [Google Scholar]
- 13.Gao Z., Wei B., Qian C. Puerarin injection for treatment of unstable angina pectoris: a meta-analysis and systematic review. International Journal of Clinical and Experimental Medicine. 2015;8(9):14577–14594. [PMC free article] [PubMed] [Google Scholar]
- 14.Kong D., Xia W., Zhang Z., et al. Safflower yellow injection combined with conventional therapy in treating unstable angina pectoris: a meta-analysis. Journal of Traditional Chinese Medicine. 2013;33(5):553–561. doi: 10.1016/s0254-6272(14)60021-2. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X., Wu J., Zhang B., Zhou W. Danshenchuanxiongqin injection in the treatment of unstable angina pectoris: a systematic review and meta-analysis. Journal of Traditional Chinese Medicine = Chung I Tsa Chih Ying Wen pan. 2016;36(2):144–150. doi: 10.1016/s0254-6272(16)30020-6. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Huang X., Qin F., et al. A strategy for detecting optimal ratio of cardioprotection-dependent three compounds as quality control of Guan-Xin-Er-Hao formula. Journal of Ethnopharmacology. 2011;133(2):735–742. doi: 10.1016/j.jep.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Guo M., Liu Y., Shi D. Cardiovascular actions and therapeutic potential of tetramethylpyrazine (active component isolated from rhizoma chuanxiong): roles and mechanisms. BioMed Research International. 2016;2016:9. doi: 10.1155/2016/2430329.2430329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y. S., Zhu H. F., Wang J. H., Liu Z. B., Bi J. J. Isolation and purification of salvianolic acid A and salvianolic acid B from Salvia miltiorrhiza by high-speed counter-current chromatography and comparison of their antioxidant activity. Journal of Chromatography B. 2009;877(8-9):733–737. doi: 10.1016/j.jchromb.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y., Su Y., Wang D., et al. Tanshinol attenuates the deleterious effects of oxidative stress on osteoblastic differentiation via Wnt/FoxO3a signaling. Oxidative Medicine and Cellular Longevity. 2013;2013:18. doi: 10.1155/2013/351895.351895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho J., Hong C.-Y. Salvianolic acids: small compounds with multiple mechanisms for cardiovascular protection. Journal of Biomedical Science. 2011;18(1):p. 30. doi: 10.1186/1423-0127-18-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C., Cheng G., Yang X., Li C., Shi R., Zhao N. Tanshinol suppresses endothelial cells apoptosis in mice with atherosclerosis via lncRNA TUG1 up-regulating the expression of miR-26a. American Journal of Translational Research. 2016;8(7):2981–2991. [PMC free article] [PubMed] [Google Scholar]
- 22.Ren Z., Ma J., Zhang P., et al. The effect of ligustrazine on L-type calcium current, calcium transient and contractility in rabbit ventricular myocytes. Journal of Ethnopharmacology. 2012;144(3):555–561. doi: 10.1016/j.jep.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Wu W., Yu X., Luo X.-P., Yang S.-H., Zheng D. Tetramethylpyrazine protects against scopolamine-induced memory impairments in rats by reversing the cAMP/PKA/CREB pathway. Behavioural Brain Research. 2013;253:212–216. doi: 10.1016/j.bbr.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z. G., Zhang X. L., Wang X. Y., Luo Z. R., Song J. C. Inhibition of acid sensing ion channel by ligustrazine on angina model in rat. American Journal of Translational Research. 2015;7(10):1798–1811. [PMC free article] [PubMed] [Google Scholar]
- 25.Lv X., Lu H., Cao M., Liu X., She W. Application of danshenchuanxiongqin injection. Chinese Journal of Practical Internal Medicine. 2009;29(S2):219–221. doi: 10.1111/j.1399-0004.2009.01166.x. [DOI] [Google Scholar]
- 26.Wang R., Han Q., Jia Y., Lv J. Effect of danshen chuanxiongqin injection on the myocardial damage of unstable angina patients undergoing percutaneous coronary intervention. Chinese Journal of Integrated Traditional and Western Medicine. 2011;31(7):899–902. [PubMed] [Google Scholar]
- 27.Liu Z., Wu Y., Jiang X., et al. Pharmacokinetic study of Tanshinol’s impact on ligustrazine hydrochloride from Salvia miltiorrhiza ligustrazine hydrochloride injection in rats. West China Journal of Pharmaceutical Sciences. 2017;32(2):182–185. [Google Scholar]
- 28.Ye T., Li Y., Xiong D., et al. Combination of Danshen and ligustrazine has dual anti-inflammatory effect on macrophages and endothelial cells. Journal of Ethnopharmacology. 2021;266 doi: 10.1016/j.jep.2020.113425.113425 [DOI] [PubMed] [Google Scholar]
- 29.Jia Y. L., Leung S. W., Lee M. Y., Cui G. Z., Huang X. H., Pan F. H. The efficacy of guanxinning injection in treating angina pectoris: systematic review and meta-analysis of randomized controlled trials. Evidence-Based Complementary and Alternative Medicine. 2013;2013:16. doi: 10.1155/2013/282707.282707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu G., Ding B. Meta-analysis of shenqi glucose injection in the treatment of unstable angina pectoris. Journal of Emergency in Traditional Chinese Medicine. 2016;25(2):272–275. [Google Scholar]
- 31.Fraker T. D., Fihn S. D., Gibbons R. J., et al. 2007 chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina. Circulation. 2007;116(23):2762–2772. doi: 10.1161/circulationaha.107.187930. [DOI] [PubMed] [Google Scholar]
- 32.Messerli F. H., Mancia G., Conti C. R., Pepine C. J. Guidelines on the management of stable angina pectoris: executive summary: the task force on the management of stable angina pectoris of the european society of cardiology. European Heart Journal. 2006;27(23):2902–2903. doi: 10.1093/eurheartj/ehl308. [DOI] [PubMed] [Google Scholar]
- 33.Higgins J. P. T., Altman D. G., Gotzsche P. C., et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:p. d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao Y., Wang Z. Therapeutic effect of danshen ligustrazine injection on angina pectoris. Contemporary Medical Symposium. 2017;15(16):175–176. [Google Scholar]
- 35.Chen X. Clinical analysis of 50 cases of angina pectoris treated by danshen ligustrazine injection. China Practical Medicine. 2017;12(11):145–146. [Google Scholar]
- 36.Ding X. Treatment of 30 cases of angina pectoris in the elderly by danshen ligustrazine injection. For All Health. 2016;10(35):149–150. [Google Scholar]
- 37.Han J. Effect of danshen ligustrazine injection on angina pectoris in the elderly. World Latest Medicine Information. 2016;16(79):p. 208. [Google Scholar]
- 38.He L., Li S. Study of the effect of danshen ligustrazine injection on angina pectoris. Cardiovascular Disease Journal of Integrated Traditional Chinese and Western Medicine (Electronic) 2017;5(1):74–75. [Google Scholar]
- 39.Hu L. Observation on the therapeutic effect of danshen ligustrazine injection on angina pectoris. Journal of Medical Forum. 2012;33(4):106–107. [Google Scholar]
- 40.Hua Z., Chen R., Ran C. Observation on the efficacy of danshen ligustrazine injection in the treatment of angina pectoris. Medical Aesthetics and Cosmetology. 2014;10:24–25. [Google Scholar]
- 41.Jia Z. Clinical observation of danshen ligustrazine injection in treating angina pectoris in elderly patients. Journal of Clinical Medical Literature. 2017;4(63):p. 12426. [Google Scholar]
- 42.Lan D. Clinical observation of danshen ligustrazine injection in treating angina pectoris. Contemporary Medicine. 2015;21(16):154–155. [Google Scholar]
- 43.Li J., Ceng M., Liang J. Treatment of 40 cases of stable angina pectoris in elderly patients with danshen ligustrazine injection. World Health Digest. 2012;9(51):381–382. [Google Scholar]
- 44.Li P., Li F. Clinical efficacy of danshen ligustrazine injection in the treatment of angina pectoris. Psychological Doctor. 2017;23(14):p. 69. [Google Scholar]
- 45.Li Q. To explore the clinical observation of 108 cases of angina pectoris of coronary heart disease treated by dancan chuanxiongqin zhusheye. Cardiovascular Disease Journal of Integrated Traditional Chinese and Western Medicine. 2015;3(1):79–80. [Google Scholar]
- 46.Liu L., Li Z. Clinical observation on 60 cases of angina pectoris treated by shenxiong glucose injection. Chinese Journal of Modern Drug Application. 2011;5(11):69–70. [Google Scholar]
- 47.Ma C., Qu P., Li L. Therapeutic effect of danshen ligustrazine injection on 80 patients with angina pectoris. China Health Care & Nutrition. 2013;1:p. 213. [Google Scholar]
- 48.Ma T., Yuan F., Ma F. Clinical observation on the effect of shenxiong glucose injection in treating coronary heart disease. World Chinese Medicine. 2016;11(9):1743–1745. [Google Scholar]
- 49.Ou M. Clinical observation of shenxiong glucose injection in treating angina pectoris. Modern Hospitals. 2008;8(4):46–47. [Google Scholar]
- 50.Pang G., Liu X. Therapeutic effect of shenxiong glucose injection on elderly patients with angina pectoris. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2010;19(22):2764–2765. [Google Scholar]
- 51.Sun D. Application of danshen ligustrazine injection in the treatment of angina pectoris and analysis of symptoms. Bao Jian Wen Hui. 2018;5:p. 138. [Google Scholar]
- 52.Tian H. Therapeutic effect of danshen ligustrazine injection on stable angina pectoris. Chinese Journal of Misdiagnosis. 2008;8(29):p. 7098. [Google Scholar]
- 53.Wang X., Lian H. Therapeutic observation of danshen ligustrazine injection on angina pectoris. Medical Journal of Chinese People’s Health. 2010;22(10):p. 1257. [Google Scholar]
- 54.Wang X., Wang Y. Comparison of danshen injection combined with ligustrazine and conventional drugs in treating angina pectoris. Journal of Practical Medical Techniques. 2008;15(7):870–871. [Google Scholar]
- 55.Xi H. Effect of danshen ligustrazine injection on angina pectoris and its effect on plasma endothelin and carbon monoxide levels in patients. Contemporary Medicine. 2017;23(31):84–86. [Google Scholar]
- 56.Xie D., Zhu Y. Efficacy observation of danshen injection plus ligustrazine in the treatment of angina pectoris. Journal of Practical Medical Techniques. 2006;13(11):p. 1868. [Google Scholar]
- 57.Xing B. Efficacy observation of danshen chuanxiong injection in the treatment of 100 patients with stable angina pectoris in the elderly. China Health Care & Nutrition. 2013;10:6069–6070. [Google Scholar]
- 58.Xiong B., Wang P. Clinical observation on 85 cases of angina pectoris treated by danshen ligustrazine injection. Northwest Pharmaceutical Journal. 2009;24(4):300–301. [Google Scholar]
- 59.Xu G., Qin L. Clinical research of danshen and chuanxiongqin injection on angina pectoris. Journal of Emergency in Traditional Chinese Medicine. 2012;21(4):614–615. [Google Scholar]
- 60.Yu M., Fang S. Clinical curative effect observation of salvia miltiorrhiza ligustrazine injection in treating angina pectoris of coronary heart disease. Clinical Journal of Chinese Medicine. 2016;8(6):44–45. [Google Scholar]
- 61.Zhang W. Therapeutic value of shenxiong glucose injection for angina pectoris. Guide of China Medicine. 2018;16(19):52–53. [Google Scholar]
- 62.Ma H., Shen L., Gao R., Qi W., Huo Y. Guidelines for the diagnosis and treatment of chronic stable angina. Chinese Journal of Cardiology. 2007;35(3):195–206. [Google Scholar]
- 63.Wang Z.-H., Pan J.-H., Ma X.-P., et al. Cardioprotective effect of Shenxiong glucose injection on acute myocardial infarction in rats via reduction in myocardial intracellular calcium ion overload. Tropical Journal of Pharmaceutical Research. 2017;16(5):1097–1104. doi: 10.4314/tjpr.v16i5.18. [DOI] [Google Scholar]
- 64.Huang W., Yang Y., Zeng Z., Su M., Gao Q., Zhu B. Effect of Salvia miltiorrhiza and ligustrazine injection on myocardial ischemia/reperfusion and hypoxia/reoxygenation injury. Molecular Medicine Reports. 2016;14(5):4537–4544. doi: 10.3892/mmr.2016.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cai J., Pan R., Jia X., et al. The combination of astragalus membranaceus and ligustrazine ameliorates micro-haemorrhage by maintaining blood-brain barrier integrity in cerebrally ischaemic rats. Journal of Ethnopharmacology. 2014;158:301–309. doi: 10.1016/j.jep.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 66.Liu H. B., Xu J., Peng Y., Zhou J. J., Xiao P. G. Targets of danshen’s active components for activating blood circulation activities. Acta Phys-Chim Sin. 2010;26(1):199–205. [Google Scholar]
- 67.Wu W.-y., Wang Y.-p. Pharmacological actions and therapeutic applications of Salvia miltiorrhiza depside salt and its active components. Acta Pharmacologica Sinica. 2012;33(9):1119–1130. doi: 10.1038/aps.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan H. Y., Fu F. H., Yang M. Y., Xu H., Zhang A. H., Liu K. Antiplatelet and antithrombotic activities of salvianolic acid A. Thrombosis Research. 2010;126(1):E17–E22. doi: 10.1016/j.thromres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 69.Zou J., Gao P., Hao X., Xu H., Zhan P., Liu X. Recent progress in the structural modification and pharmacological activities of ligustrazine derivatives. European Journal of Medicinal Chemistry. 2018;147:150–162. doi: 10.1016/j.ejmech.2018.01.097. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y., Zhu H., Tong J., Li Z. Ligustrazine improves blood circulation by suppressing platelet activation in a rat model of allergic asthma. Environmental Toxicology and Pharmacology. 2016;45:334–339. doi: 10.1016/j.etap.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 71.Sun Y., Li S., Quan C. Solubility of ferulic acid and tetramethylpyrazine in supercritical carbon dioxide. Journal of Chemical & Engineering Data. 2005;50(4):1125–1128. doi: 10.1021/je049715y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File name: Appendix table. Title of data: summary table of the constituents of the 28 included studies. Description of data: statements of the constituents of the included studies.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.


