Abstract
Background
Recent studies have demonstrated a complex interplay between comorbid cardiovascular disease, COVID-19 pathophysiology, and poor clinical outcomes. Coronary artery calcification (CAC) may therefore aid in risk stratification of COVID-19 patients.
Methods
Non-contrast chest CT studies on 180 COVID-19 patients ≥ age 21 admitted from March 1, 2020 to April 27, 2020 were retrospectively reviewed by two radiologists to determine CAC scores. Following feature selection, multivariable logistic regression was utilized to evaluate the relationship between CAC scores and patient outcomes.
Results
The presence of any identified CAC was associated with intubation (AOR: 3.6, CI: 1.4–9.6) and mortality (AOR: 3.2, CI: 1.4–7.9). Severe CAC was independently associated with intubation (AOR: 4.0, CI: 1.3–13) and mortality (AOR: 5.1, CI: 1.9–15). A greater CAC score (UOR: 1.2, CI: 1.02–1.3) and number of vessels with calcium (UOR: 1.3, CI: 1.02–1.6) was associated with mortality. Visualized coronary stent or coronary artery bypass graft surgery (CABG) had no statistically significant association with intubation (AOR: 1.9, CI: 0.4–7.7) or death (AOR: 3.4, CI: 1.0–12).
Conclusion
COVID-19 patients with any CAC were more likely to require intubation and die than those without CAC. Increasing CAC and number of affected arteries was associated with mortality. Severe CAC was associated with higher intubation risk. Prior CABG or stenting had no association with elevated intubation or death.
Abbreviations: COVID-19, coronavirus disease 2019; CAC, coronary artery calcium; NCCT, non-contrast computed tomography; ECG, electrocardiogram; SAPT, single antiplatelet therapy; DAPT, dual antiplatelet therapy; OAC, oral anticoagulant; AKI, acute kidney injury; VTE, venous thromboembolism; COPD, chronic obstructive pulmonary disease; UOR, unadjusted odds ratio; AOR, adjusted odds ratio
Keywords: COVID-19, Coronary artery disease (CAD), Coronary artery calcification (CAC), Computed tomography (CT)
1. Introduction
As the global coronavirus disease (COVID-19) pandemic continues, risk stratification for infected patients has become increasingly important to decrease morbidity and mortality. Recent studies have demonstrated a complex interplay between cardiovascular disease, COVID-19 pathophysiology, and poor clinical outcomes [[1], 2., [3], [4], [5], [6]]. Imaging biomarkers such as coronary artery calcium (CAC) score have an established role in long-term cardiovascular event risk stratification but might also provide important prognostic information and pathophysiologic insights in patients acutely ill with COVID-19.
CAC score is an independent predictor of cardiovascular events [7,8]. Visual CAC scoring on non-electrocardiogram (ECG)-gated, non-contrast chest CT studies (NCCT) is a well-established, efficient, and reproducible surrogate for quantitative analysis, demonstrating strong association with the widely utilized Agatston score, without the need for an additional dedicated ECG-gated cardiac CT study or computational software [[9], [10], [11]]. NCCTs are sometimes obtained in COVID-19 patients to assess the extent of pulmonary disease, monitor disease progression, and to investigate the presence of disease complications [12]. NCCTs have also been widely used in some countries for rapid detection of suspected COVID-19 and is also often performed as a diagnostic supplement to PCR testing in other countries such as the United States [13,14]. Mining of these studies for imaging biomarkers may provide further insight into the disease process from both pathophysiologic and clinical perspectives, performed in the context of a pandemic that has necessitated efficient use of often limited healthcare resources.
In this study, we examine the association between visual CAC scoring on NCCTs in COVID-19 patients with adverse clinical outcomes. In addition, we perform a comparative analysis of CAC severity with respect to adverse outcomes to a variety of clinical, imaging, and laboratory factors. We hypothesize that increasing severity and extent of CAC are independent predictors of poor outcomes in COVID-19.
2. Materials and methods
This is an IRB-approved retrospective cohort study of 180 patients ≥ age 21 admitted from March 1, 2020 to April 27, 2020 within a large urban multicenter system positive for COVID-19 by reverse transcription polymerase chain reaction and underwent NCCT.
2.1. Inclusion criteria for patients
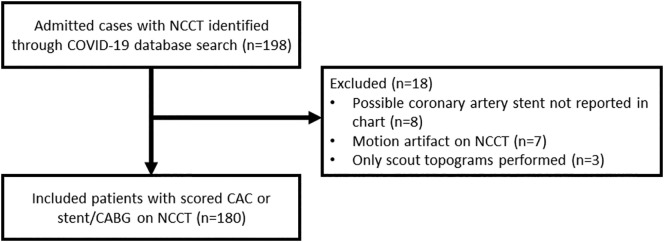
Using an institutional COVID-19 dataset, 198 admitted patients ≥ the age 21 with NCCT within a 3-month period prior to and after admissions were identified. Patients with prior coronary artery bypass graft (CABG) surgery or coronary arterial stenting, determined by radiologist suspicion with electronic medical record (EMR) confirmation, were excluded from CAC scoring but were included in outcomes analysis. NCCTs on which at least one radiologist suspected coronary stenting that could not be confirmed or repudiated by EMR were excluded (n = 8). NCCTs deemed too limited by motion artifact for adequate CAC assessment by at least one radiologist were excluded (n = 7). After exclusions, 180 patients were included for outcomes analysis (Fig. 1 ).
Fig. 1.
Study flow diagram.
This diagram demonstrates how we arrived at the total number of patients included in the outcomes analysis for our retrospective cohort study.
2.2. Clinical data collection
Patient information was collected from the EMR. Demographic variables included age, gender, self-reported race and ethnicity. Clinical variables included past medical history (as shown in Table 1 ), body mass index (BMI), smoking history, echocardiography results, laboratory variables including troponins, and selected outpatient medications (statins, antiplatelet regimen, anticoagulant regimen). Antithrombotic therapy was classified as single antiplatelet (SAPT), dual antiplatelet (DAPT), or oral anticoagulant (OAC) [14]. The statin regimen was categorized as combined low/moderate or high intensity following available ACC guidelines [16., [17], [18]]. The highest available D-dimer level during admission was recorded (D-dimer level > 1 μg/mL was considered elevated) [19,20]. Echocardiography results were obtained from EMR. End results of every admission (discharge or death) were recorded for every encounter.
Table 1.
Admitted patient demographics and past medical histories in relation to intubation and death
| Variables | Outcomes |
||||||
|---|---|---|---|---|---|---|---|
| Admitted patients (n = 180) | Not intubated (n = 126) | Intubated (n = 54) | P-value | No death (n = 121) | Death (n = 59) | P-value | |
| Age median [IQR] | 68 [59, 80] | 74 [60, 83] | 63 [56, 70] | 0.001 | 68 [58, 80] | 71 [61, 83] | 0.13 |
| Sex (% female) | 82 (46) | 58 (46) | 24 (44) | 0.97 | 55 (46) | 27 (46) | 1 |
| Race (%) | 0.2 | 0.5 | |||||
| White | 57 (32) | 45 (36) | 12 (22) | 37 (31) | 20 (34) | ||
| Black | 70 (39) | 46 (37) | 24 (44) | 45 (37) | 25 (42) | ||
| Other/Unknown | 53 (29) | 35 (28) | 18 (33) | 39 (32) | 14 (24) | ||
| Ethnicity (%) | 0.59 | 0.31 | |||||
| Non-Hispanic | 122 (68) | 84 (67) | 38 (71) | 78 (65) | 44 (75) | ||
| Hispanic | 20 (11) | 16 (13) | 4 (7.4) | 16 (13) | 4 (6.8) | ||
| Unknown | 38 (21) | 26 (21) | 12 (22) | 27 (22) | 11 (19) | ||
| Smoking history (%) | 0.13 | 0.26 | |||||
| Never | 96 (53) | 67 (53) | 29 (54) | 64 (53) | 32 (54) | ||
| Former/Current | 54 (30) | 42 (33) | 12 (22) | 40 (33) | 14 (24) | ||
| Unknown | 30 (17) | 17 (14) | 13 (24) | 17 (14) | 13 (22) | ||
| BMI median (kg/m2) [IQR] | 28 [24,34] | 26 [23,32] | 30 [26, 38] | 0.003 | 27 [23, 32] | 30 [25, 38] | 0.01 |
| BMI (kg/m2) (cutoffs) | 0.08 | 0.08 | |||||
| Normal (<25) | 59 (35) | 48 (41) | 11 (22) | 44 (39) | 15 (27) | ||
| Overweight (26–30) | 42 (25) | 28 (24) | 14 (28) | 30 (27) | 12 (21) | ||
| Obese (31–40) | 46 (27) | 30 (25) | 16 (31) | 29 (26) | 17 (30) | ||
| Morbidly Obese (>40) | 22 (13) | 12 (10) | 10 (20) | 10 (8.8) | 12 (21) | ||
| Comorbidities (%) | |||||||
| Asthma | 7 (3.9) | 6 (4.8) | 1 (1.9) | 0.61 | 6 (5.0) | 1 (1.7) | 0.51 |
| COPD | 8 (4.4) | 8 (6.3) | 0 (0.0) | 0.13 | 5 (4.1) | 3 (5.1) | 1 |
| HTN | 60 (33) | 38 (30) | 22 (41) | 0.23 | 38 (31) | 22 (37) | 0.54 |
| DM | 42 (23) | 30 (24) | 12 (22) | 0.97 | 28 (23) | 14 (24) | 1 |
| Cancer | 27 (15) | 22 (18) | 5 (9.3) | 0.24 | 19 (16) | 8 (14) | 0.88 |
| CKD | 30 (17) | 17 (14) | 13 (24) | 0.13 | 14 (12) | 16 (27) | 0.02 |
| HF | 22 (12) | 12 (9.5) | 10 (19) | 0.15 | 13 (11) | 9 (15) | 0.53 |
| CAD | 25 (14) | 18 (14) | 7 (13) | 1 | 14 (12) | 11 (19) | 0.29 |
| AFIB | 7 (3.9) | 7 (5.6) | 0 (0) | 0.18 | 4 (3.3) | 3 (5.1) | 0.87 |
| Antiplatelet/Anticoagulation regiment (%) | 0.29 | 0.09 | |||||
| None | 95 (53) | 61 (48) | 34 (63) | 65 (54) | 30 (51) | ||
| SAPT | 49 (27) | 36 (29) | 13 (24) | 28 (23) | 21 (36) | ||
| DAPT | 14 (7.8) | 11 (8) | 3 (5.6) | 13 (11) | 1 (1.7) | ||
| OAC | 22 (12) | 18 (14) | 4 (7.4) | 15 (12) | 7 (12) | ||
| Statin categories (%) | 0.62 | 0.61 | |||||
| None | 94 (52) | 63 (50.0) | 31 (57 | 63 (52) | 31 (53) | ||
| Low/moderate intensity | 55 (31) | 41 (32.5) | 14 (26) | 35 (29) | 20 (34) | ||
| High intensity | 31 (17) | 22 (17.5) | 9 (17) | 23 (19) | 8 (14) | ||
| CAC score (%) | 0.33 | 0.03 | |||||
| Absent | 51 (28) | 38 (30) | 13 (24) | 43 (36) | 8 (14) | ||
| Mild | 42 (23) | 27 (21) | 15 (28) | 27 (22) | 15 (25) | ||
| Moderate | 23 (13) | 13 (10) | 10 (19) | 15 (12) | 8 (14) | ||
| Severe | 42 (23) | 30 (24) | 12 (22) | 23 (19) | 19 (32) | ||
| Stent/CABG | 22 (12) | 18 (14) | 4 (7.4) | 13 (11) | 9 (15) | ||
Categorical variables are expressed as counts and percentages. Continuous variables are expressed as medians with interquartile ranges (IQR). Significant p-values (≤0.05) are bolded. BMI = body mass index; COPD = chronic obstructive pulmonary disease; HTN = hypertension; DM = diabetes mellitus; CKD = chronic kidney disease; HF = heart failure; CAD = coronary artery disease; AFIB = atrial fibrillation; SAPT = single antiplatelet therapy; DAPT = dual antiplatelet therapy; OAC = oral anticoagulant; CAC = coronary artery calcium; CABG = coronary artery bypass grafting;
2.3. Imaging data collection
All NCCTs contained 1-mm-thick slices. They were obtained from five sites in New York City. In Brooklyn, 108 patients were scanned on Aquillon PRIME (Toshiba, Tokyo, Japan). In Manhattan, 39 patients were scanned on Somatom Definition AS+ (Siemens, Munich, Germany), 14 patients were scanned on iCT 256 (Philips, Amsterdam, The Netherlands), and 4 patients were scanned on Revolution EVO (General Electric, Chicago, USA). In Queens, 15 patients were scanned on Revolution HD (General Electric, Chicago, USA).
2.4. Imaging analysis
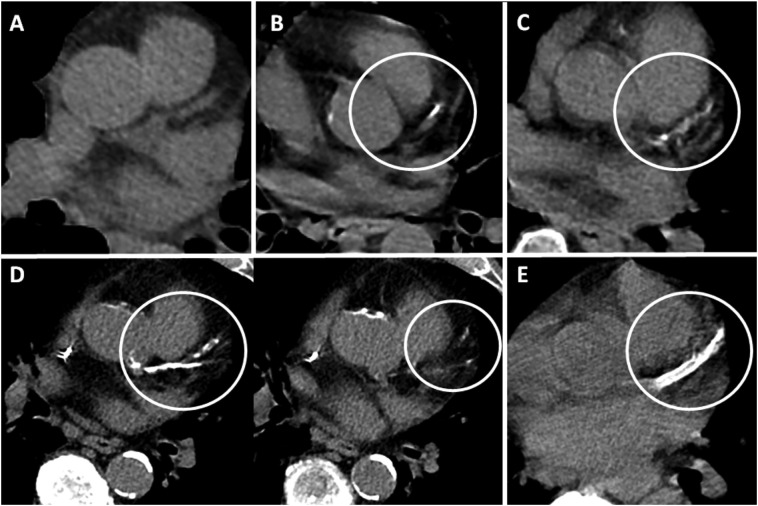
Two fellowship-trained cardiothoracic radiologists (Y.S.G. and J.C.; total experience of 12 years) with extensive experience in CAC assessment independently scored each NCCT on soft tissue window of a standard PACS workstation, blinded to patient histories other than COVID-19 positivity. Visual assessment of CAC was performed according to an established ordinal scoring method [21]. Each of the four main coronary arteries was identified (left main, left anterior descending, left circumflex, and right coronary arteries). Calcium extent was scored as 0, 1, 2, or 3 (Fig. 2 ). A score of 1 is defined as involvement of less than one third of the vessel length; 2 as involvement of one third to two thirds of the vessel length; 3 as greater than two thirds of the vessel length. These scores were summed to obtain a total ordinal score of 0–12 for each scan. The total scores were categorized as absent CAC for score 0, mild for score 1–3, moderate for a score 4–5, and severe for score ≥ 6.
Fig. 2.
Coronary artery calcium scoring.
Examples of coronary artery calcium (CAC) scoring on non-ECG gated non-contrast CT performed in axial plane. (A) Ordinal score of 0. No CAC within the partially imaged left anterior descending artery (LAD) (B) Ordinal score of 1. CAC involves less than one third of the LAD vessel length (circled). (C) Ordinal score of 2. CAC involves one third to two thirds of the LAD vessel length (circled). (D) Ordinal score of 3. CAC involves greater than two thirds of the length of the LAD and branch vessels (circled) on sequential images. (E) Stent within the LAD (circled) precludes CAC assessment.
When patients had changes indicative of prior coronary artery bypass graft (CABG) surgery or stent placement, confirmed on EMR, CAC scoring was not performed.
2.5. Statistical analysis
Weighted Cohen's kappa coefficient was used to assess radiologists' agreement in scoring. For individual vessels, stent and CABG cases were excluded in this calculation, due to these being unscorable. The mean of the total scores was calculated and categorized based on above described parameters. In order to ensure usability of as many records as possible in multivariable analysis, missing BMI (11/180, 6.1%) were imputed using predictive mean matching using models that included the outcomes of interest (intubation status, death), demographic information (age, ethnicity, and race), clinical variables (smoking status and comorbidities listed in Table 1), and the CAC category. These values were then utilized in the multivariable model through multiple imputation according to Rubin's rules [22]. Bivariate analysis of continuous variables (BMI and age) was performed using the Kruskal-Wallis H Test. Bivariate analysis of categorical variables including race, gender, smoking history, and comorbidities was performed using chi-squared test. These comparisons were performed in order to ascertain statistically significant differences in relation to the primary outcomes of interest (intubation and death). Univariable logistic regression was utilized to estimate the relative effect of variables by calculating unadjusted odds ratios (UOR) for categorical covariates in relation to the outcomes of interest. Random forest was used for feature selection utilizing the Boruta approach [23]. This approach was chosen because it is an all-relevant variable selection method as opposed to a minimal optimal method while providing valuable information concerning the relative importance of features. Feature selection was applied separately to each individual adverse outcome. A multivariable logistic regression model adjusting for selected features was performed for the outcomes of intubation, death, and elevated D-dimer. Two such models were created, one involving the categorical variable of CAC category and one involving the binary variable of presence of any calcification. Adjusted odds ratios (AOR) are presented from these models. A p-value of less than 0.05 (two-tailed) was considered statistically significant. All analysis was completed using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Population characteristics, comorbidities, and medications
A total of 180 patients were included in the analysis (median age 68 [interquartile range (IQR) 59–80]; 46% female). Fifty-four out of 180 (30%) patients reported being current or former smokers, and 68/169 (40%) were obese or morbidly obese (as defined by BMI > 30). The most common comorbidities were hypertension (60/180; 33%) and diabetes mellitus type II (42/180; 23%). Admitted patient demographics and past medical histories in relation to intubation and expiration are displayed in Table 1. Encounter location is described in Supplementary Table 1.
Fourteen patients out of 180 (8%) were found to be on DAPT, 49/180 (27%) were on SAPT, 22/180 (12%) on OAC, and 95/180 (53%) were on no antithrombotic regimen. Thirty-one out of 180 patients (17%) were on high intensity statin therapy prior to admission, 55/180 (31%) were on low/moderate intensity statin therapy, and 94/180 (52%) were not on any statin regimen. Of the patients with stents and/or CABG, 20/22 (91%) were on a statin regimen, and 21/22 (95%) were on an antithrombotic regimen. Of the patients with CAD, 9/25 (36%) were found to have stents and/or CABG. Admitted patient CAC scores, echocardiography findings, and laboratory parameters in relation to intubation and expiration are shown in Table 2. Additional data relating lab and echocardiography findings in relation to the outcomes of interest are provided in Supplemental Table 1.
Table 2.
Distribution of coronary artery calcium (CAC) score by coronary artery
| CAC distribution | Left main | Left anterior descending | Left circumflex | Right coronary |
|---|---|---|---|---|
| Reader 1 | 31.0* (49) | 60.8 (96) | 32.5 (51) | 42.4 (67) |
| Reader 2 | 44.3 (70) | 62.0 (98) | 44.3 (70) | 45.6 (72) |
| Kappa | 0.61 | 0.79 | 0.68 | 0.80 |
Data is provided in percentages, with number of patients in parenthesis.
3.2. Coronary artery calcium scores
One-hundred-eighty NCCT scored by the two radiologists had almost perfect concordance based on category (Kappa 0.84). Concordance scores for individual vessels were: left main coronary artery (Kappa 0.61), left anterior descending artery (Kappa 0.79), left circumflex coronary artery (Kappa 0.68), and right coronary artery (Kappa 0.80). Concordance of number of calcified vessels, calculated by summing the number vessels with greater than 0 CAC score, was substantial (Kappa 0.82) as was the total summed score (Kappa 0.73).
3.3. Clinical outcomes
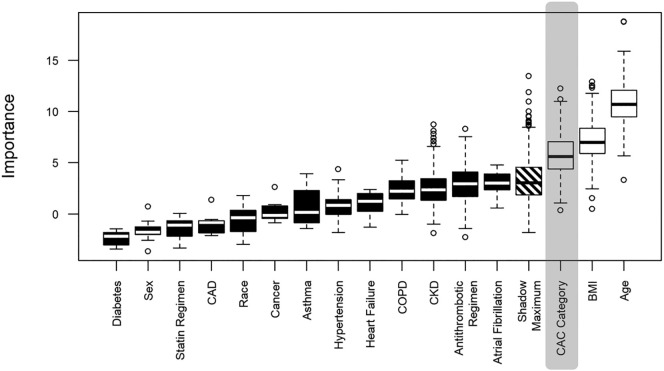
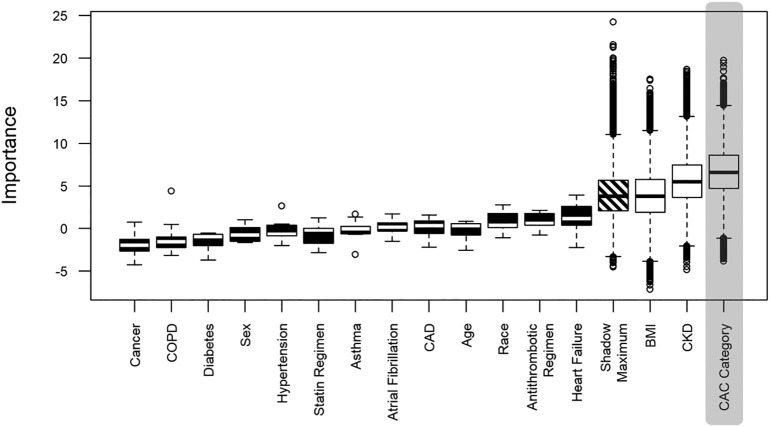
Fifty-four patients (30%) were intubated during their admission, 59 (33%) died, and 95 (86%) had elevated D-dimer (as defined by >1 μg/L). Results of feature selection for the multivariable analysis involving CAC categories showing the relative calculated importance of different features is displayed in Fig. 3, Fig. 4 . Every category of CAC score was associated with increased odds of intubation in reference to absence of CAC (mild - AOR: 3.3, CI: 1.1–9.8, moderate - AOR: 6.5, CI 1.9–24, severe - AOR 4.0, CI 1.3–13). A severe CAC score was associated with increased odds of mortality (AOR: 5.1, CI: 1.9–15). Additionally, in the model utilizing CAC category, morbid obesity was associated with increased risk of both intubation (AOR: 3.3, CI: 1.1–10) and mortality (AOR: 5.4, CI: 1.8–17). No statistically significant association was found between the category of patients with stent and/or CABG and the outcomes of intubation (AOR: 1.9, CI: 0.4–7.7) or mortality (AOR: 3.4, CI: 1.0–12). For complete univariable and multivariable logistic regres sion analysis results, see Table 3 . Increasing CAC score was found to be associated with mortality (UOR: 1.2, CI: 1.0–1.3), but not intubation (UOR: 1.0, CI: 0.9–1.1). Increasing number of vessels with calcification was associated with mortality (UOR: 1.3, CI: 1.02–1.6), but not intubation (UOR: 1.0, CI: 0.8–1.2). There was no association between statin regimen and antithrombotic regimen and the outcomes of interest (Table 3).
Fig. 3.
Coronary artery calcification and intubation.
Boxplot showing distribution of attribute importance relative to the outcome of intubation over runs. Black and white boxplots represent Z scores of rejected and confirmed attributes respectively. Confirmed attributes were included in multivariable analysis. CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; CKD = chronic kidney disease; CAC = coronary artery calcification.
Fig. 4.
Coronary artery calcification and death.
Boxplot showing distribution of attribute importance relative to the outcome of death over runs. Black and white boxplots represent Z scores of rejected and confirmed attributes respectively. Confirmed and tentative attributes were included in multivariable analysis. CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; CKD = chronic kidney disease; CAC = coronary artery calcification.
Table 3.
Risk of intubation anddeath in admitted patients with COVID-19. Model covariates for the adjusted model were chosen through feature selection
| Variable | Intubation |
Death |
||||
|---|---|---|---|---|---|---|
| Unadjusted odds ratio | Adjusted odds ratio for CAC category | Adjusted odds ratio for any calcification | Unadjusted odds ratio | Adjusted odds ratio for CAC category | Adjusted odds ratio for any calcification | |
| Age | 0.97 (0.95–0.99) | 0.95 (0.93–0.98) | 0.96 (0.93–0.99) | 1.0 (1.0–1.04) | – | – |
| Race | ||||||
| White | Reference | – | – | Reference | Reference | – |
| Black | 2.0 (0.89–4.5) | – | – | 1.0 (0.49–2.2) | – | – |
| Other/unknown | 1.9 (0.83–4.6) | – | – | 0.66 (0.29–1.5) | – | – |
| Sex | ||||||
| Male | Reference | – | – | Reference | Reference | – |
| Female | 0.94 (0.49–1.8) | – | – | 1.0 (0.54–1.9) | – | – |
| BMI cutoffs (kg/m2) | ||||||
| Normal (<25) | Reference | Reference | Reference | Reference | Reference | – |
| Overweight (26–30) | 2.4 (0.97–5.9) | 2.5 (0.98–6.8) | 2.5 (0.98–6.8) | 1.5 (0.61–3.4) | 2.4 (0.9–6.3) | – |
| Obese (31–40) | 2.5 (1.1–6.2) | 2.0 (0.8–5.3) | 1.9 (0.8–5.0) | 1.9 (0.82–4.3) | 2.4 (1.0–6.1) | – |
| Morbidly obese (>40) | 4.3 (1.5–13) | 3.3 (1.1–10) | 4.0 (1.3–13) | 3.5 (1.3–9.7) | 5.4 (1.8–16.9) | – |
| Comorbidities | ||||||
| COPD | 0 | – | – | 1.2 (0.25–5.3) | – | – |
| HTN | 1.6 (0.82–3.1) | – | – | 1.3 (0.67–2.5) | – | – |
| DM | 0.91 (0.41–1.9) | – | – | 1.0 (0.49–2.1) | – | – |
| AFIB | 0 | – | – | 1.6 (0.30–7.3) | – | – |
| Cancer | 0.48 (0.15–1.3) | – | – | 0.84 (0.33–2.0) | – | – |
| CKD | 2.0 (0.90–4.6) | – | – | 2.8 (1.3–6.4) | 2.5 (1.1–6.9) | 2.5 (1.1–5.6) |
| HF | 2.2 (0.85–5.4) | – | – | 1.5 (0.58–3.7) | – | – |
| CAD | 0.9 (0.3–2.2) | – | – | 1.8 (0.7–4.1) | – | – |
| Presence of calcification | ||||||
| No calcification | Reference | – | Reference | Reference | – | Reference |
| Any calcification | 1.4 (0.67–2.9) | – | 3.6 (1.4–9.6) | 3.5 (1.60–8.6) | – | 3.2 (1.4–7.9) |
| Number of vessels involved | 1.0 (0.8–1.2) | – | – | 1.3 (1.02–1.6) | – | – |
| CAC score categories | ||||||
| Absent | Reference | Reference | – | Reference | Reference | – |
| Mild | 1.6 (0.67–4.0) | 3.3 (1.1–9.8) | – | 3.0 (1.1–8.3) | 3.0 (1.1–8.9) | – |
| Moderate | 2.2 (0.79–6.4) | 6.5 (1.9–24) | – | 2.9 (0.91–9.2) | 3.2 (0.97–11) | – |
| Severe | 1.2 (0.46–3.0) | 4.0 (1.3–13) | – | 4.4 (1.7–12.3) | 5.1 (1.9–15) | – |
| Stent/CABG | 0.65 (0.16–2.1) | 1.9 (0.4–7.7) | – | 3.7 (1.2–12) | 3.4 (1.0–12) | – |
| CAC continuous score | 1.0 (0.90–1.1) | – | – | 1.2 (1.02–1.3) | – | – |
| Antithrombotic regimen | ||||||
| None | Reference | – | – | Reference | – | – |
| SAPT | 0.65 (0.30 to 1.4) | – | – | 1.6 (0.79–3.3) | – | – |
| DAPT | 0.49 (0.11–1.7) | – | – | 0.17 (0.01–0.90) | – | – |
| OAC | 0.40 (0.11–1.2) | – | – | 1.01 (0.35–2.7) | – | – |
| Statin regimen | ||||||
| None | Reference | – | – | Reference | – | – |
| Low/moderate | 0.69 (0.32–1.4) | – | – | 1.2 (0.57–2.3) | – | – |
| High | 0.83 (0.33–2.0) | – | – | 0.71 (0.27–1.7) | – | – |
Coronary artery calcium score is expressed by categories and as a continuous score. Data in parenthesis are 95% confidence intervals. BMI = body mass index; COPD = chronic obstructive pulmonary disease; HTN = hypertension; DM = diabetes mellitus; CKD = chronic kidney disease; HF = heart failure; CAD = coronary artery disease; AFIB = atrial fibrillation CAC = coronary artery calcium; CABG = coronary artery bypass grafting. Mild and moderate groups for CAC were combined for the analysis relating to elevated D-dimer as an outcome due to low counts in the moderate group.
3.4. Secondary outcomes
Both mild/moderate (AOR: 5.7, CI: 1.1–40) and severe (AOR: 11, CI: 1.4–124) CAC score categories were found to be associated with an elevated D-dimer. No association was found between CAC categories and score in relation to acute kidney injury (AKI), elevated troponin, and elevated liver function tests. Patients on higher intensity statins had a higher CAC score (none - median 0.75 [IQR 0.00, 3.50], low/moderate - median 3.50 [IQR 1.38, 5.75], high - median 5.50 [IQR 2.75, 8.00], p < 0.001). Elevated D-dimer was not associated with presence or absence of stents or history of CABG (AOR: 5.0, CI: 0.4–78).
4. Discussion
In this retrospective analysis, we studied the relationship between the presence and extent of CAC with adverse outcomes in COVID-19 patients. Our results indicate that COVID-19 patients with any degree of CAC were more likely to require intubation or expire than those without CAC. Higher CAC score was associated with increased mortality. Patients with severe CAC as opposed to patients with lesser degrees of CAC had a higher risk of intubation compared to patients without CAC. These findings are concordant with a study by Dillinger et al., in which the presence and extent of CAC was associated with worse prognosis in hospitalized patients with COVID-19 [24].
In our cohort, the presence and severity of CAC as independent predictors of mortality and intubation were superior to several reported risk factors including sex, diabetes, hypertension, atrial fibrillation, heart failure, and chronic obstructive pulmonary disease [20,25,26]. Number of vessels with CAC and visual score was associated with poor outcomes. This association might reflect the effects of a severe inflammatory cascade resulting in destabilization of pre-existing cardiovascular disease and subsequent mixed shock, as suggested by recent literature [27,28]. It may also point to underlying chronic inflammation that predisposes to atherosclerotic disease and to acute proinflammatory states such as the cytokine storm.
Although studies have found that COVID-19 patients with CAD are more likely to have myocardial injury, we did not find a similar association in patients with a pre-admission diagnosis of CAD (25/180), which may be attributable to under-diagnosis given the large group of patients with visually moderate or severe CAC (65/180) [1,29]. We did not find a significant association between CAC and troponin elevation, although this may be secondary to relatively small sample size. The association between CAC and mortality in our study in the absence of clinical evidence of myocardial injury suggests the predictive value of CAC may not be limited to the cardiac domain, reflecting host propensity for widespread proinflammatory response and endothelial dysfunction. In autopsy studies of COVID-19 patients, endothelial injury, perivascular inflammation, and/or microthrombosis have recently been implicated as dominant findings in multiple organs, including the lungs, brain, kidneys, skin, and gastrointestinal tract [[30], [31], [32]].
We found no statistically significant association between CABG or stent placement and intubation or death in our cohort. Recent literature suggests that hospitalized COVID-19 patients on anticoagulation have reduced mortality risk while those on statins may benefit [29,33].We hypothesized that the CABG/stent patients may have benefitted from such medications as they are more likely to be on them. However, upon analysis of our entire cohort, we found these therapies did not confer an advantage. One possibility is that CABG/stented patients benefited from surgical/procedural management of obstructive CAD in this regard. In keeping with prior studies, our data demonstrates a positive association of intensity of statin regimen with CAC scores [34,35].
Our results indicate a significant role for assessing CAC severity and extent in COVID-19 patients. Visual CAC scoring demonstrated almost perfect interobserver agreement in our study (Kappa = 0.84), which is in keeping with existing literature (kappa was as high as 0.95 in the previously referenced study by Azour et al.) [21]. As such, visual CAC scoring is a reliable method that can facilitate prognostication in COVID-19 patients. Additionally, our study supports the feasibility of CAC assessment on almost all COVID-19 patients who undergo non-ECG-gated NCCTs around the time of illness.
NCCTs are sometimes performed on hospitalized COVID-19 patients for various reasons; therefore, visual CAC scoring does not require additional imaging assessment but can provide useful prognostic data [36]. Although CAC is an established imaging marker for predicting cardiovascular event risk, CAC reporting incidence may be as low as 1% [36,37]. CAC on NCCT may be the first evidence of undiagnosed CAD and may be a more objective and dependable means of rapid risk stratification in COVID-19 patients, unaffected by confounding variables such as recall bias that may occur during history taking. We recommend radiologists report CAC in all COVID-19 patients to best guide clinical management.
Limitations of this study include its retrospective nature, which may introduce observer bias in CAC assessment. Our cohort was limited to patients who had a NCCT, which may impart selection bias and may not represent the full array of associations of CAC with COVID-19 outcomes. Furthermore, as described previously, patients with NCCT within a 3-month period prior to and after admission were included, which may have resulted in higher relative inclusion of patients who survived. Future investigation may incorporate a cohort with more diverse imaging to determine an association of CAC with other adverse events, including VTE, stroke, and bowel ischemia.
5. Conclusion
Visual CAC scoring on routine NCCTs in hospitalized COVID-19 patients is a rapid and reproducible imaging biomarker that may provide an independent assessment of risk of intubation and death, with those having severe CAC at higher risk. Patients treated with CABG/stents did not incur a similar risk, possibly reflecting a combination of treated CAD and medical therapy. Assessing CAC severity on NCCTs imparts valuable information regarding risk prognostication and may guide management in COVID-19 patients.
The following are the supplementary data related to this article.
Admitted patient echocardiography findings, and laboratory parameters in relation to intubation and expiration.
Funding sources/disclosures
Each author has no grants, disclosures, or other assistance.
Consent
IRB approval was obtained for this study.
Acknowledgments
The authors thank Andrew Pagano MD, Sharon Steinberger MD, and Lena Marra for their guidance and commitment to further understanding of the COVID-19 infection during these unprecedented times.
References
- 1.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lala A, Johnson KW, Russak AJ, Paranjpe I, Zhao S, Solani S, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection n.d. doi: 10.1101/2020.04.20.20072702. [DOI] [PMC free article] [PubMed]
- 3.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santoso A., Pranata R., Wibowo A., Al-Farabi M.J., Huang I., Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matos P., Paparo F., Mussetto I., Baciagalupo L., Veneziano A., Bernardi A.P. Evaluation of novel coronavirus disease (COVID-19) using quantitative lung CT and clinical data: prediction of short-term outcome. European Radiology Experimental. 2020;4:39. doi: 10.1186/s41747-020-00167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenland P., Bonow R.O., Brundage B.H., Budoff M.J., Eisenberg M.J., Grundy S.M., et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation clinical expert consensus task force (ACCF/AHA writing committee to update the 2000 expert consensus document on electron beam computed tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Hecht H.S., Cronin P., Blaha M.J., Budoff M.J., Kazerooni E.A., Narula J., et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of thoracic radiology. J Cardiovasc Comput Tomogr. 2017;11:74–84. doi: 10.1016/j.jcct.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Einstein A.J., Johnson L.L., Bokhari S., Son J., Thompson R.C., Bateman T.M., et al. Agreement of visual estimation of coronary artery calcium from low-dose CT attenuation correction scans in hybrid PET/CT and SPECT/CT with standard Agatston score. J Am Coll Cardiol. 2010;56:1914–1921. doi: 10.1016/j.jacc.2010.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiles C., Duan F., Gladish G.W., Ravenel J.G., Baginski S.G., Snyder B.S., et al. Association of Coronary Artery Calcification and Mortality in the National Lung Screening Trial: a comparison of three scoring methods. Radiology. 2015;276:82–90. doi: 10.1148/radiol.15142062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shemesh J., Henschke C.I., Shaham D., Yip R., Farooqi A.O., Cham M.D., et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541–548. doi: 10.1148/radiol.10100383. [DOI] [PubMed] [Google Scholar]
- 12.Pan F., Ye T., Sun P., Gui S., Liang B., Li L., et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caruso D., Zerunian M., Polici M., Pucciarelli F., Polidori T., Rucci C., et al. Chest CT features of COVID-19 in Rome, Italy. Radiology. 2020;296(2):E79–E85. doi: 10.1148/radiol.2020201237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang W., Sirajuddin A., Zhang X., Liu G., Teng Z., Zhao S., et al. The role of imaging in 2019 novel coronavirus pneumonia (COVID-19) Eur Radiol. 2020 doi: 10.1007/s00330-020-06827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2019;73:e285–350. [DOI] [PubMed]
- 17.Mora S., Caulfield M.P., Wohlgemuth J., Chen Z., Superko H.R., Rowland C.M., et al. Atherogenic lipoprotein subfractions determined by ion mobility and first cardiovascular events after random allocation to high-intensity statin or placebo: the justification for the use of statins in prevention: an intervention trial evaluating Rosuvastatin (JUPITER) trial. Circulation. 2015;132:2220–2229. doi: 10.1161/CIRCULATIONAHA.115.016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z., Edwards D., Massou E., Saunders C.L., Brayne C., Mant J. Statin use and high-dose statin use after ischemic stroke in the UK: a retrospective cohort study. Clin Epidemiol. 2019;11:495–508. doi: 10.2147/CLEP.S201983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.COVID-19 and D-dimer - Hematology.org n.d. https://www.hematology.org:443/covid-19/covid-19-and-d-dimer (accessed October 4, 2020).
- 20.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azour L., Kadoch M.A., Ward T.J., Eber C.D., Jacobi A.H. Estimation of cardiovascular risk on routine chest CT: ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J Cardiovasc Comput Tomogr. 2017;11:8–15. doi: 10.1016/j.jcct.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Rubin D.B., editor. Multiple imputation for nonresponse in surveys. John Wiley & Sons, Inc; Hoboken, NJ, USA: 1987. [Google Scholar]
- 23.Kursa M.B., Jankowski A., Rudnicki W.R. Boruta – a system for feature selection. Fund Inform. 2010;101:271–285. [Google Scholar]
- 24.Dillinger J.G., Benmessaoud F.A., Pezel T., Voicu S., Sideris G., Chergui N., et al. Coronary artery calcification and complications in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13:2468–2470. doi: 10.1016/j.jcmg.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inciardi R.M., Adamo M., Lupi L., Cani D.S., Di Pasquale M., Tomasoni D., et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in northern Italy. Eur Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;e35:8. [PMC free article] [PubMed] [Google Scholar]
- 27.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 28.Fried J.A., Ramasubbu K., Bhatt R., Topkara V.K., Clerkin K.J., Horn E., et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paranjpe I., Fuster V., Lala A., Russak A., Glicksberg B.S., Levin M.A., et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bermejo-Martin J.F., Almansa R., Torres A., González-Rivera M., Kelvin D.J. COVID-19 as a cardiovascular disease: the potential role of chronic endothelial dysfunction. Cardiovasc Res. 2020 doi: 10.1093/cvr/cvaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhayana R., Som A., Li M.D., Carey D.E., Anderson M.A., Blake M.A., et al. Abdominal imaging findings in COVID-19: preliminary observations. Radiology. 2020;297(1):E207–E215. doi: 10.1148/radiol.2020201908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The General Hospital Corporation. Rationale for consideration of statins for COVID-19 patients n.d. https://www.massgeneral.org/assets/MGH/pdf/news/coronavirus/rationale-for-consideration-of-statins-for-COVID-19-patient.pdf (accessed June 4, 2020).
- 34.Dykun I., Lehmann N., Kälsch H., Möhlenkamp S., Moebus S., Budde T., et al. Statin medication enhances progression of coronary artery calcification: the Heinz Nixdorf recall study. J Am Coll Cardiol. 2016;68:2123–2125. doi: 10.1016/j.jacc.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 35.Puri R., Nicholls S.J., Shao M., Kataoka Y., Uno K., Kapadia S.R., et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273–1282. doi: 10.1016/j.jacc.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 36.Mendoza D.P., Kako B., Digumarthy S.R., Shepard J.-A.O., Little B.P. Impact of significant coronary artery calcification reported on low-dose computed tomography lung cancer screening. J Thorac Imaging. 2020;35:129–135. doi: 10.1097/RTI.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 37.Johnson C., Khalilzadeh O., Novelline R.A., Choy G. Coronary artery calcification is often not reported in pulmonary CT angiography in patients with suspected pulmonary embolism: an opportunity to improve diagnosis of acute coronary syndrome. Am J Roentgenol. 2014;202:725–729. doi: 10.2214/ajr.13.11326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Admitted patient echocardiography findings, and laboratory parameters in relation to intubation and expiration.