Graphical abstract
Keywords: PAH, Benz[a]pyrene, Margin of Exposure (MOE), Monte Carlo simulation, HPLC-FLD
Highlights
-
•
B[a]A, Chry, B[b]F and B[a]P were quantified in infusions of 12 yerba mate brands with large inter-brand variability.
-
•
Infusions prepared as habitually consumed in Uruguay contained about 40 % of combined PAH in mate.
-
•
Probability of Margin of Exposure <10,000 varied in the sequence B[a]P > (B[a]P + Chry) > (B[a]A + Chry + B[b]F + B[a]P).
-
•
The probability of B[a]P Margin of Exposure <10,000 was 9 % for the infusion prepared as habitually consumed.
-
•
B[a]P amount in yerba mate infusions was the main contributor to the variability of Margin of Exposure.
Abstract
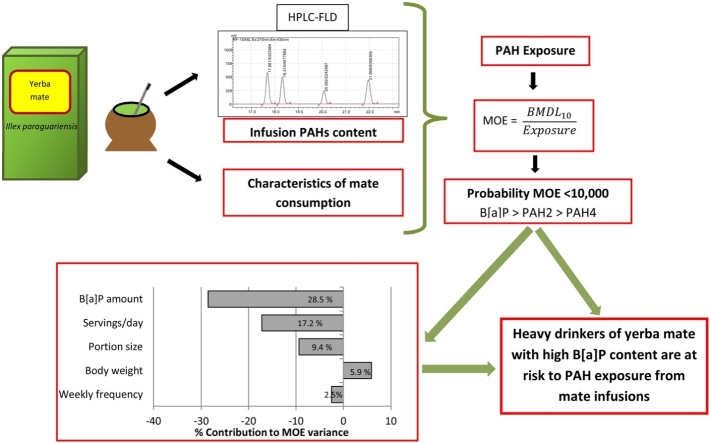
The aim of this study was to assess the risk of exposure to polycyclic aromatic hydrocarbons (PAH) from yerba mate infusions in Uruguay using the margin of exposure approach (MOE) and a probabilistic method (Monte Carlo simulation). Servings/day, portion size, weekly frequency of mate consumption and body weight were the factors considered. The amount in infusions of benz[a]pyrene (B[a]P), PAH2 (sum of chrysene and B[a]P), and PAH4 (sum of benz[a]anthracene, chrysene, benz[b]fluoranthene and B[a]P) were used as markers of PAH exposure. Total content of PAH in infusions had large inter-brand variability (48–54 %) with significant differences among brands. PAH content in infusions prepared as habitually consumed was about 40 % of total content. The probability of occurrence of MOE < 10,000 varied according to the infusion preparation and the marker of exposure used, being higher for infusions prepared for total content and when B[a]P was used as marker of exposure. When the average B[a]P amount in infusion as habitually consumed was used in the simulation model, the probability of MOE < 10,000 was 9 %. The main factors contributing to B[a]P MOE variance were B[a]P amount (28.4 %), servings/day (17.3 %), and portion size (9.6 %). Heavy drinkers of yerba mate with high B[a]P content are those at risk to PAH exposure from mate infusions.
1. Introduction
Polycyclic aromatic hydrocarbons (PAH) are a large group of lipophilic compounds consisting of two or more fused aromatic rings formed from the incomplete combustion of organic matter and some of them have been categorized as potentially carcinogenic and genotoxic in humans [1]. In the human body PAHs suffer metabolic transformations into polar products which can be excreted or transformed into active metabolites that can form covalent DNA adducts. The formation of such adducts is regarded as the first stage in chemical carcinogenesis [[1], [2], [3], [4]].
There are many routes of human exposure to PAH such as smoke (from i.e.: tobacco, wood, oil), environmental contamination, and workplace exposures of certain industries [1]. However, for individuals who are not exposed to tobacco smoke or work environments that promote the production of these compounds, diet has been defined as the greatest source of exposure, contributing to more than 90 % of total exposure to PAH [1]. Food contamination with PAH may be caused by the processing method and by the environment [5,6]. PAH contamination related to the processing method was reported in many foods such as tea [7] and yerba mate (Ilex paraguariensis) [[8], [9], [10]].
Yerba mate infusions (or “mate” for short) are habitually consumed in South America, especially in Argentina, Brazil, Paraguay and Uruguay. Uruguay has the world highest per capita consumption, with an average of 25 g of dried product/inhabitant/day [11]. Consumption of mate extracts containing products is increasing worldwide, mainly due to the high caffeine content and potential health benefits linked to polyphenolic compounds [9,[12], [13], [14]]. On the other hand, heavy mate consumption, such as occurs in Uruguay, may pose a risk of PAH exposure.
Forms of preparation of the yerba mate infusion vary between countries and this may affect PAH content [[15], [16], [17], [18]]. In Uruguay, mate is typically prepared by placing an average of 50 g of dried yerba mate product into a calabash gourd. Systematically, between 30 or 40 mL of water at 80 °C is poured over the yerba mate leaves, and the infusion is sipped through a stainless-steel straw. This procedure is repeated several times, and up to 1 L of this infusion is usually drunk [12]. There are no studies on PAH content and composition of yerba mate infusions as consumed in Uruguay. As stated by the IARC working group there are few studies reporting PAH content of mate infusions worldwide [19]. There is a need for more studies of PAH content of yerba mate infusions, with different forms of preparation and consumption, in order to assess the risk of PAH exposure from this infusion under different scenarios [19].
According to international agencies [4,20,21], risk assessment for PAH should be done using the Margin of Exposure (MOE) approach considering Benz[a]pyrene (B[a]P), PAH2 (Chrysene (Chry)+B[a]P), and PAH4 (Benz[a]anthracene (B[a]A) + Chry + Benz[b]fluoranthene (B[b]F)+B[a]P) as the most appropriate markers of exposure, and their corresponding BMDL10 (Benchmark Dose Lower Confidence Limit) [22].
BMDL10 values were derived from a carcinogenicity animal study using coal tar mixtures [23]. The magnitude of a MOE indicates the level of concern but does not quantify risk. For PAH exposure, a MOE lower than 10,000 indicates a condition of concern [20]. The use of PAH4 as a surrogate marker of overall PAH exposure is based on the assumption that all 4 PAH are present in the samples, that the sample PAH profile is similar to the mixture used in the carcinogenic study [23] and that the carcinogenicity of the total PAH increases linearly with the dose [24]. Therefore, it is important to characterize the PAH profile of the source of exposure. In addition, it is important to consider other variables affecting dietary exposure such as amount and frequency of consumption, and body weight. Probabilistic methods using Monte Carlo simulation are useful tools for the assessment of risk exposure considering multiple variables and their distributions [16,25].
The aim of the present study was to assess the content of B[a]A, Chry, B[b]F and B[a]P in infusions from different yerba mate brands available in Uruguay and to estimate the risk of PAH exposure in yerba mate consumers based on the MOE approach and the Monte Carlo method taking into account dietary exposure variables.
2. Materials and methods
2.1. Chemicals and reagents
Individual PAH standards were purchased from Supelco (Bellefonte, PA, USA): Benz[a]anthracene solution certified reference material, 200 μg/mL in methylene chloride; Chrysene solution, 100 μg/mL in methylene chloride, Benzo[b]fluoranthene analytical standard and Benzo[a]pyrene analytical standard. All the solvents used in this study were HPLC-grade. Acetonitrile (ACN) was purchased from Pharmco-Aarper Company (Brookfield, CT, USA) and hexane from Merck Chemicals (Buenos Aires, Argentina). Sodium sulfate anhydrous powder was purchased from J.T. Baker (N.J. USA) Solid phase extraction cartridges (Bond Elut Florisil, 500 mg x6 ml) were purchased from Agilent Technologies (Lake Forest, CA, USA). The water used for the preparation of the infusions was purified using a Milli Q water purification system for ultrapure water (Bedford, MA, USA).
2.2. Samples
Packages of yerba mate from 12 different commercial brands (one package for each brand: M1 to M12) were purchased in shops in the city of Montevideo, Uruguay (September 2017). All brands contained yerba mate produced and processed in Brazil. Yerba mate consumed in Uruguay is composed of olive-green colored particles made from leaves, petioles, and young stems of the Ilex paraguariensis tree, dehydrated, and crushed. 80 % of the yerba mate particles have a size between 3 and 0.3 mm and the remaining 20 % is less than 0.3 mm. The samples were stored in a dry place at room temperature until processing.
2.3. Infusion preparation
2.3.1. Infusion preparation for determination of total PAH content
Infusions were prepared by placing the yerba mate sample (50 g) in a beaker. Initially, the sample was hydrated with 200 mL of water at room temperature. Then, 800 mL ofwater at 80 °C were added and the beaker placed in a water bath at 80 °C ± 2 °C for 15 min, stirring continuously with a glass rod. Finally, a stainless-steel straw was placed in the mix, connected to a vacuum flask and by applying vacuum (20 ± 5 mmHg) the total volume of the infusion was collected (1000 mL). Aliquots of 200 mL of the infusion were transferred to individual Erlenmeyer flasks. Three independent PAH solvent extractions and HPLC analysis were performed for each brand. Total content for each yerba mate infusion was calculated as the average of the three aliquots. The reproducibility of the infusion preparation was assessed by the coefficient of variation (CV) of two independent yerba mate samples of two brands with different total PAH content. CV were 8% and 9 % for samples with total PAH content of 38 and 9 ng/g, respectively.
2.3.2. Infusion preparation for determination of PAH content as habitually consumed
To simulate the way in which the beverage is usually consumed, as sequence of partial infusions, 50 g of a yerba mate with high PAH content were placed in a beaker and a stainless steel straw was inserted in the sample previously hydrated with 60 mL of water at room temperature. Then, five successive additions of 40 mL of water at 80 °C were poured and vacuum for 15 s through the stainless-steel straw was applied after each addition. A total of 200 mL were collected (first fraction). To collect the second 200 mL fraction, the same procedure was carried out. Then, the straw was placed on the opposite side of the bohemia beaker and the operation was repeated until 3 more fractions of 200 mL were obtained. The total volume of infusion collected in the 5 sequential individual 200 mL fractions was 1000 mL. Each individual 200 mL fraction was transferred to an Erlenmeyer flask for PAH solvent extraction and HPLC analysis. These procedures were done in duplicate. PAH content of each partial infusion was determined. PAH cumulative content of sequential infusions was expressed as a percentage of total PAH content of the infusion as obtained in 2.3.1.
2.4. Extraction and cleanup
The extraction and clean up procedures were adapted from a previous study [17]. Briefly 80 mL of hexane were added to the 200 mL of yerba mate infusion and agitated continuously in a shaking water bath at 20 °C for 90 min at room temperature. Subsequently, the mix was transferred to a 500 mL separating funnel where the aqueous phase was discarded, the organic phase was collected and dried with anhydrous sodium sulfate. The extract was concentrated to 3 mL in a rotary evaporator at 40 °C and 330 mbar. Each extract was then passed through a SPE column, previously conditioned with 3 mL of hexane. The column was rinsed with 12 mL of hexane. The eluate and the rinsing were collected and evaporated to dryness at 40 °C and 260 mbar. The residue was dissolved in 3 mL of ACN in an ultrasonic bath for 1 min. The samples were stored in amber glass vials at - 18 °C until HPLC analysis.
2.5. HPLC analysis
Solvents were filtered with regenerated cellulose membrane, and standards and samples were filtered with PVDF membrane, both with a 0.45 μm pore, before HPLC analysis. The chromatographic and detection conditions are shown in Table 1. Twenty μL of each sample were injected into the HPLC system (Prominence, Shimadzu, Japan) equipped with an oven (CTO-20A), quaternary pump (LC-20A T) and a fluorescence detector (RF 10AXL). A ZORBAX Eclipse Plus PAH (RRHT) column (1.8 μm x 100 mm x 4.6 mm DI, Agilent Technologies, Lake Forest, CA, USA) was used. All results were corrected by the concentration factor due to extraction and cleanup of the infusion.
Table 1.
HPLC mobile phase gradient program and detection conditions*.
| Chromatographic Conditions | Detector Conditions |
|---|---|
| Mobile phase: Water/Acetonitrile | 0−13 min: Ex 270/Em 430nm |
| 0−2 min: 50 % water -50 % ACN | 13−19 min. Ex 270/Em 390nm |
| 2−15 min: 100 % ACN, hold 8 min. | 19−20.9 min. Ex 258/Em 430nm |
| 23−25 min: back to initial condition | 21−23 min: Ex 290/Em 430nm |
| Flow: 0.8 mL/min | 23−25 min: Ex 270/Em 430nm |
Analysis run time 25 min with 5 min post run re-equilibration time.
2.5.1. Method performance
2.5.1.1. Linearity, limit of detection, limit of quantification and precision
A stock solution containing 5 mg/L of each PAH was prepared with the standards mentioned above. Fifteen concentrations of PAH solutions prepared in ACN from the stock solution, ranging from 0.33 to 3000 ng/L, were measured in triplicate in order to obtain the calibration curves for each PAH. The following parameters were defined for the analytical method: linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy and precision, according to the established performance criteria for methods of analysis for polycyclic aromatic hydrocarbons [26]. The linearity of the method was confirmed by the coefficient (R2) of the calibration curves of each PAH. ANOVA test for the regression was performed for each PAH. The estimation of LOD was done via calibration approach and the relationship between the LOD and LOQ was calculated according the European Commission guidance [27]. Specificity of the method was confirmed by the correspondence of the retention times of the PAHs in the standard solution with the retention times of the infusion signal and by the presence of the characteristic UV spectrum of each PAH in the yerba mate infusion. Also, by verifying that the signal of each PAH increased after spiking the infusion with 100 ng/L of a 4 PAH standard solution.
2.5.1.2. Recovery
The accuracy of the method was determined by performing a recovery test. For this purpose, three infusions prepared from M2 were spiked with a 4 PAH standard mix in three levels of addition (66, 200 and 500 ng/L of each PAH). One infusion was used as blank. Recovery was calculated by dividing the measured amount of PAH by the amount of spiking (in percentage). The precision was calculated by the relative standard deviation (RSD %) of the signal area of different concentrations within the analytical range, measured in triplicate.
2.6. Risk assessment method: Margin of exposure approach
MOE was calculated dividing the BMDL10 of each marker of PAH exposure (B[a]P, PAH2 or PAH4) by the corresponding dietary exposure defined as ng PAH marker/kg body weight per day [20]. MOE models were constructed considering multiple scenarios of dietary exposure using Monte Carlo simulation with the software @Risk 7.6, Palisade Corporation. This program performs risk analysis and presents the results of a model and the probability of its occurrence. Factors included in the models were: portion size of yerba mate, servings per day, weekly frequency of mate consumption, body weight and PAH (B[a]P, PAH2 or PAH4) content in yerba mate infusions. Portion size and number of servings per day were estimated based on popular habits of mate consumption in Uruguay. Portion size was set as a normal distribution with a median of 50 g (amount for a regular calabash gourd) and a standard deviation of 12.5 g. Truncation limits were set at a minimum 12.5 g and a maximum of 100 g in order to consider real situations. Servings per day referred to the number of portions consumed per day, set as a binomial distribution with a minimum of 1, a maximum of 3 and a probability of 0.5. Weekly frequency of mate consumption was taken from the database of a National Survey [28]. The data were adjusted using @Risk as a binomial distribution (minimum of 1, maximum of 7 and probability of 0.9) as the best fit according to the Akaike information criterion (AIC). Body weight of yerba mate consumers was also taken from the aforementioned database [28] and adjusted using @Risk as a Pearson V distribution according to the AIC. Body weight distribution had a median of 74.1 kg and a standard deviation of 16.4 kg. PAH contents used were those obtained from the analysis of the yerba mate infusions expressed as ng (B[a]P, PAH2 or PAH4) per gram of yerba mate. Values were adjusted to a normal distribution, for individual brands truncation limits were set at ± 3 standard deviation and for the average of the twelve brands were set as the lowest and highest PAH value obtained. Models were performed for total content and content as habitually consumed of B[a]P, PAH2 and PAH4 in each yerba mate brand and in the average of the twelve brands. In each model, the Monte Carlo simulation was run with 100,000 iterations and results were expressed as the percent probability of occurrence of MOE lower than 10,000. Sensitivity analyses were performed in order to determine the contribution of each factor to the MOE variance.
2.7. Statistical analysis
ANOVA analysis followed by Tukey HSD test was performed to compare B[a]A, Chry, B[b]F, B[a]P, PAH2 and PAH4 content among brands, and by LSD Fisher to compare PAH content in cumulative volumes of partial sequential infusions. Significance was set at p < 0.05.
3. Results
3.1. Method validation and performance
All the analytical parameters complied with the criteria required for the methods of analysis of PAH of the European Union [26]: calibration curves for the 4 PAH showed good linearity within the analytical range (R2> 0.999) relative standard deviation (RSD) for the repeatability test was less than 2 %, and mean percent recoveries for the 4 PAH were between 50 and 120 % (Table 2). In addition, ANOVA test confirmed the strong linear correlation (p < 0.0001) and a non-significant lack of fit (p > 0.132) for each PAH analyzed.
Table 2.
Performance characteristics of the analytical PAH method.
| PAH1 | Analytical range2 (ng/L) | Linearity R² | LOD (ng/L) | LOQ (ng/L) | RSD3 (%) | Recovery4 (%) |
|---|---|---|---|---|---|---|
| B[a]A | 11.7 -1500 | 0.9998 | 3.5 | 11.7 | 1.52 | 72 |
| Chry | 28.6 - 3000 | 0.9997 | 8.6 | 28.6 | 1.11 | 93 |
| B[b]F | 26.7 - 2640 | 0.9997 | 8.0 | 26.7 | 1.33 | 83 |
| B[a]P | 7.14 - 3003 | 0.9999 | 2.1 | 7.14 | 1.15 | 84 |
LOD, limit of detection; LOQ, limit of quantification; RSD, repeatability test.
B[a]A, benz[a]anthracene; chry, chrysene; B[b]F, benz[b]fluoranthene; B[a]P,benz[a]pyrene.
Values referred to the infusion.
Mean values within the analytical range, analyzed in triplicate.
Mean recoveries of three different concentration levels in triplicate.
3.2. PAH in yerba mate infusions
3.2.1. Total content
The 4 PAH studied were detected and quantified in the 12 yerba mate infusions analyzed (Table 3). Total content in 1 L prepared with 50 g of yerba mate varied between 143.1 to 653.4 ng for B[a]P, from 280.8 to 1319.6 ng for PAH2, and from 436.8 to 2045 ng for PAH4. Contribution of B[a]P to PAH2 and PAH4 content ranged from 46 to 64 % and from 28 to 40 %, respectively. The coefficient of variation among brands for B[a]A was 50 %, for Chry was 54 %, for B[b]F was 50 %, and for B[a]P was 48 %. Significant differences among brands were found for each PAH analyzed (p < 0.05). For B[a]P, M2 and M8 had the highest content, and M11 and M12 the lowest content. For PAH2 and PAH4, M1, M2 and M8 had the highest content, and M7, M10, M11 and M12 the lowest content.
Table 3.
Total PAH content in infusions of different brands of yerba mate.
| Yerba mate brands | PAH (ng/L)1 |
|||||
|---|---|---|---|---|---|---|
| B[a]A | Chry | B[b]F | B[a]P | PAH2 | PAH4 | |
| M1 | 350.7 ± 36d | 702.4 ± 74c | 390.3 ± 61c | 592.6 ± 86c,d | 1294.9 ± 149d | 2035.8 ± 246d |
| M2 | 307.6 ± 24c,d | 663.4 ± 51c | 430.8 ± 47c | 630.1 ± 67d | 1293.5 ± 118d | 2031.9 ± 189d |
| M3 | 314.2 ± 17c,d | 634.3 ± 29c | 417.7 ± 20c | 601.4 ± 27c,d | 1235.7 ± 57c,d | 1967.6 ± 95c,d |
| M4 | 157.5 ± 16b | 304.4 ± 27a, b | 218.3 ± 17a,b | 323.3 ± 30a,b | 627.8 ± 57a,b | 1003.6 ± 90a,b |
| M5 | 142.8 ± 15b | 227.4 ± 15a | 228.9 ± 33a,b | 407.0 ± 53b,c | 634.5 ± 68a,b | 1006.1 ± 116a,b |
| M6 | 256.8 ± 56c | 463.3 ± 96b | 301.9 ± 78b,c | 410.6 ± 74b,c | 873.9 ± 169b,c | 1432.6 ± 304b,c |
| M7 | 102.1 ± 16a,b | 224.9 ± 37a | 150.4 ± 30a | 239.4 ± 51a,b | 464.3 ± 88a | 716.9 ± 134a |
| M8 | 308.3 ± 52c,d | 666.2 ± 117c | 417.4 ± 102c | 653.4 ± 147d | 1319.6 ± 264d | 2045.3 ± 418d |
| M9 | 152.7 ± 43b | 295.9 ± 74a | 207.1 ± 5a,b | 317.3 ± 88a,b | 613.2 ± 162a,b | 972.9 ± 263a,b |
| M10 | 97.7 ± 4.0a,b | 218.7 ± 14a | 149.8 ± 5a | 234.9 ± 6a,b | 453.6 ± 20a | 701.0 ± 29a |
| M11 | 76.8 ± 7a,b | 184.8 ± 6a | 116.8 ± 27a | 174.8 ± 21a | 359.7 ± 27a | 553.4 ± 62a |
| M12 | 57.5 ± 6a | 137.8 ± 15a | 98.5 ± 12a | 143.1 ± 20a | 280.8 ± 36a | 436.8 ± 54a |
| Average of all brands | 193.7 ± 106 (50 %) | 393.6 ± 216 (54 %) | 260.6 ± 129 (50 %) | 393.9 ± 185 (48 %) | 787.6 ± 398 | 1242.0 ± 627 |
M1 to M12= different commercial brands.
B[a]A, benz[a]anthracene; Chry, chrysene; B[b]F, benz[b]fluoranthene; B[a]P.benz[a]pyrene.
PAH2 = Σ B[a]P, Chry; PAH4 = Σ B[a]P, Chry, B[a]A, B[b]F.
Content in infusion prepared with 50 g of yerba mate (mean ± standard deviation, n = 3).a, b, c, d Different letters in each column indicate significant differences between brands, Tukey HSD (p < 0.05). % between brackets indicates the coefficient of variation calculated as the percentage of the ratio of the standard deviation to the mean.
3.2.2. Content as habitually consumed
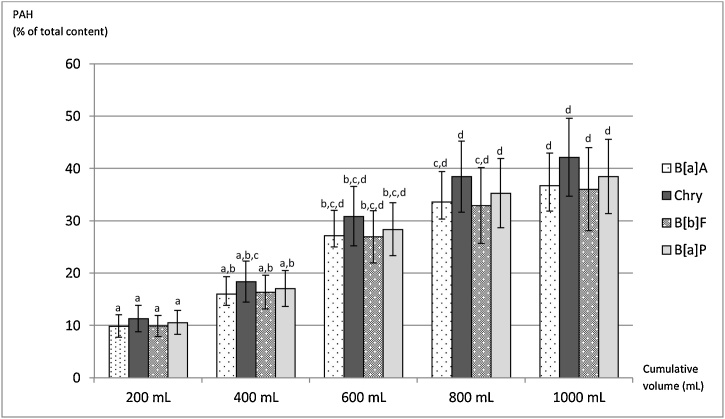
PAH content of sequential cumulative partial infusions prepared as habitually consumed is presented in the Supplementary File (Fig. 1). PAH cumulative content of the sequential partial infusions expressed as percentage of the total PAH content of the infusion is shown in Fig. 1. In 1 L of the infusion prepared as habitually consumed, the percentages of B[a]A, Chry, B[b]F and B[a]P relative to the total content were 37, 42, 36 and 38 %, respectively (Fig. 1). For PAH2 and PAH4 relative contents were 40 and 39 %, respectively. There was no significant difference among the percentages of B[a]A, Chry, B[b]F and B[a]P extracted in each cumulative volume of partial infusion (p > 0.05) (Fig. 1).
Fig. 1.
PAH content in cumulative partial sequential infusions prepared as habitually consumed1, expressed as % of total content.
1Five sequential partial infusions (200 mL each), obtained from 50 g of yerba mate M2 to a final cumulative total volume of 1000 mL. PAH content in each cumulative sequential infusion was expressed as a percentage of total content (Table 3). The bars represent mean ± SE of two independent analyses.Different letters indicate a significant difference (two-way ANOVA followed by LSD Fisher, p < 0.05).
3.2.3. Risk assessment
In the conditions tested, estimated mean intake (5th- 95th percentiles) (ng/day) of mate infusion as habitually consumed was 244 (58–574) for B[a]P, 522 (123–1233) for PAH2, and 800 (188–1902) for PAH4. When adjusted for body weight, mean exposure (5th- 95th percentiles) (ng/kg bw/day) was 3.5 (0.8–8.5) for B[a]P, 7.4 (1.6–18) for PAH2, and 11.3 (2.5–28) for PAH4 (Supplementary File, Figs. 2–7).
The probability of occurrence of MOE lower than 10,000 differed according to the PAH marker of exposure used irrespective of the infusion preparation (total content or habitually consumed) and the yerba mate brand (Table 4). The probability of occurrence of MOE lower than 10,000 was in all cases higher when B[a]P was used as a marker than when PAH2 and PAH4 were used, in the sequence B[a]P > PAH2 > PAH4. The probability of occurrence of B[a]P, PAH2 and PAH4 MOE lower than 10,000 increased with the PAH content, consequently related to the yerba mate brand and the form of preparation.
Table 4.
Probability of occurrence of MOE lower than 10,000 for B[a]P, PAH2 and PAH4 total content and content in infusions as habitually consumed.
| Yerba mate brands and infusion preparation | % Probability of occurrence of a MOE < 10,0001 |
||
|---|---|---|---|
| % for B[a]P | % for PAH2 | % for PAH4 | |
| M1 | |||
| Total content | 77.7 | 72.0 | 57.2 |
| Habitually consumed content | 20.6 | 17.6 | 6.9 |
| M2 | |||
| total content | 81.2 | 72.1 | 57.2 |
| Habitually consumed content | 23.8 | 18.3 | 8.3 |
| M3 | |||
| Total content | 79.3 | 69.8 | 55.5 |
| Habitually consumed content | 21.1 | 15.0 | 5.7 |
| M4 | |||
| Total content | 42.0 | 28.3 | 16.1 |
| Habitually consumed content | 1.8 | 0.6 | 0.1 |
| M5 | |||
| Total content | 55.1 | 28.7 | 16.0 |
| Habitually consumed content | 5.5 | 0.6 | 0.1 |
| M6 | |||
| Total content | 55.4 | 47.3 | 35.4 |
| Habitually consumed content | 6.3 | 4.5 | 1.6 |
| M7 | |||
| Total content | 23.9 | 12.9 | 4.9 |
| Habitually consumed content | 0.4 | 0.1 | 0.0 |
| M8 | |||
| Total content | 80.9 | 71.9 | 56.8 |
| Habitually consumed content | 25.8 | 18.8 | 7.8 |
| M9 | |||
| total content | 38.6 | 26.6 | 15.6 |
| Habitually consumed content | 2.4 | 0.9 | 0.2 |
| M10 | |||
| Total content | 22.6 | 11.3 | 3.7 |
| Habitually consumed content | 0.1 | 0.0 | 0.0 |
| M11 | |||
| Total content | 9.1 | 4.4 | 1.1 |
| Habitually consumed content | 0.0 | 0.0 | 0.0 |
| M12 | |||
| Total content | 4.2 | 1.2 | 0.2 |
| Habitually consumed content | 0.0 | 0.0 | 0.0 |
| Average of all brands. | |||
| Total content | 52.1 | 42.4 | 30.1 |
| Habitually consumed content | 9.0 | 6.3 | 2.3 |
Estimated using Monte Carlo simulation with 100,000 iterations (@Risk, Palisade Corporation). The adjusted variables for dietary exposure for each PAH marker of exposure were B[a]P, PAH2 and PAH4 content, frequency of yerba mate infusion consumption portion size and body weight.
For the brands with the highest total content (M2 and M8) the percent probability of B[a]P MOE < 10,000 was 81.2 and 80.9 %, and for the brands with the lowest total content (M11 and M12), the probability was 9.1 and 4.2 %. When considering the infusion as habitually consumed in all brands, the probability of occurrence of a B[a]PMOE < 10,000 ranged from 25.8 % to 0.0 % (Table 4). For the brands M1, M2, M3 and M8, this probability was higher than 20 % whereas for the other brands the probability was lower than 6.5 %.
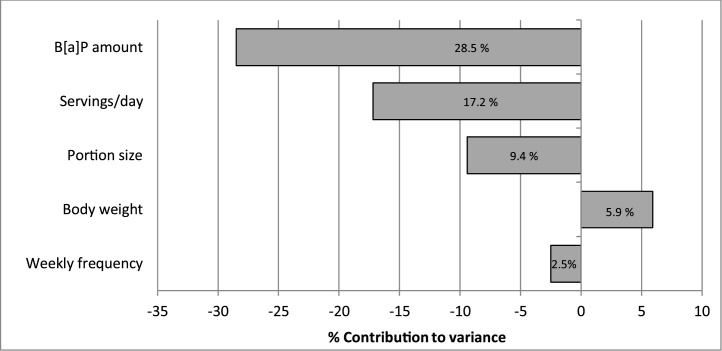
B[a]P MOE was modeled using a probabilistic method considering as factors servings per day, portion size, body weight, weekly frequency and B[a]P amount. In this model, B[a]P was set as the average content of the 12 yerba mate brand infusions as habitually consumed (Fig. 2). Factors with the largest contribution to the variance of B[a]P MOE were B[a]P amount (28,5%), servings per day (17,2 %) and portion size (9,4 %). The increase in all factors except for body weight was associated with reduced B[a]P MOE.
Fig. 2.
Contribution of characteristics of the population and intake habits to the variance of B[a]P MOE in infusions as habitually consumed1.
1 Servings/day was set as a binomial distribution with a minimum of 1, a maximum of 3 and a probability of 0.5. Portion size was set as a normal distribution with a median of 50 g and a standard deviation of 12.5 g. Truncation limits were set at a minimum 12.5 g and a maximum of 100 g. Body weight was set as a Pearson V distribution with a median of 74.1 kg and a standard deviation of 16.4 kg. Weekly frequency was set as a minimum of 1, a maximum of 7 times per week and probability of 0.9. B[a]P amount was set as a normal distribution of the average content of the 12 yerba mate brands infusion corrected by 38 % according to the partial infusion test.
4. Discussion
In this study, a probabilistic risk assessment of PAH exposure from consumption of yerba mate infusions using the MOE approach is reported. PAH exposure was assessed based on the analysis of PAH content of mate infusions as consumed in Uruguay and population intake habits. Results of the study indicated that B[a]A, Chry, B[b]F and B[a]P total content in yerba mate infusions varied among brands, and infusion prepared as habitually consumed had about 40 % of total PAH content. Risk assessment results differed according to the PAH marker of exposure used, being higher for B[a]P MOE. B[a]P amount, servings per day and portion size were the main contributors to the variability of the B[a]P MOE.
Total PAH content was measured in infusions prepared to ensure a high transference of PAH from the dry yerba mate leaves to the water. Total PAH content varied substantially among brands, possibly due to differences in the industrial drying process of yerba mate leaves which generate different PAH contamination [8]. Substantial variation among brands in the PAH content of yerba mate infusions were previously reported [17]. The PAH content of yerba mate infusions were reported in previous studies [15,17,18]. Mean total PAH content of infusions in our study are similar to those reported by Kamanagar [15] but higher than those reported by Zuin [18] and Thea [17]. Different PAH contents in yerba mate infusions of different studies may be due to difference in PAH content in dried yerba mate and in the procedures used for infusions preparation, such as dry leaves/water ratio, water temperature and/or length of time of the infusion.
The infusion prepared as habitually consumed had a lower PAH content than the infusion prepared for measuring total content. This was due to incomplete PAH transference in each of the five sequential partial infusions, adding up about 40 % of the total content at the end of the process. B[a]A Chry, B[b]F and B[a]P are similarly poor water soluble (0.002−0.012 mg/L, 25◦C) [29] and this is consistent with no differences among PAHs in the percent transferred to the partial infusions.
In our study, estimated mean PAH exposures (ng/kg bw/d) from mate infusion as habitually consumed (3.5 for B[a]P; 7.4 for PAH2 and 11.3 for PAH4) were similar to those estimated for very heavy mate drinking (4.3 for B[a]P and 10.8 for PAH4) [16] based on PAH content of mate infusions lower than from those our study [17,18]. Also, it was about the same magnitude as estimated from the whole diet for the European population (3.9 for B[a]P; 10.7 for PAH2 and 19.5 for PAH4) [20], especially for B[a]P exposure. This is of concern since our estimate was based on only one food item and therefore does not account for other PAH dietary sources.
PAH total content and content of the infusion as habitually consumed were used to estimate the risk of PAH exposure for all brands based on the MOE approach using different markers (B[a]P, PAH2 and PAH4). Irrespective of the marker used the probability of MOE lower than 10,000 was consistently higher when estimation was done using total PAH content than when using PAH content in infusions as habitually consumed, as expected. In all simulations the probability of MOE lower than 10,000 was always greater when B[a]P was used as a marker of exposure than when the PAH2 and PAH4 markers were used. Although these results appear surprising, they could be explained based on the assumptions for the surrogate marker of exposure approach [24]. The PAH profile in the infusions analyzed in the present study was different from that of the carcinogenicity study [23] used to derive the critical toxicological parameter BMDL10 [20,24]. In our study, the percent contribution of B[a]P to PAH2 and PAH4 content of the infusions (46–64 % and from 28 to 40 %, respectively) was higher than that of the mixture used in the carcinogenicity study (43–48 % and from 21 to 23 %, respectively) [23]. It appears that when B[a]P contribution to the PAH profile is higher in the food or diet source than the one used to derive the BMDL10, the use of PAH2 and PAH4 as surrogate markers will underestimate the risk of exposure from that dietary source. Therefore, in the risk of exposure analysis from yerba mate infusion in our study the worst-case scenario was chosen. B[a]P was used as the most conservative marker as being suggested [24], although it may overestimate the risk of exposure.
In infusions as habitually consumed, increase in B[a]P content, servings per day, portion size and weekly frequency was associated with reduction in the B[a]P MOE. In contrast, increase in body weight was associated with increase in the B[a]P MOE. These are expected results since these factors modify exposure to B[a]P. B[a]P content was the main contributor (28.5 %) to the variance of the B[a]P MOE, due to the large variability of B[a]P content among brands. Of the twelve brands analyzed, concern of PAH exposure was low or negligible for eight brands (less than 6.5 % probability of a B[a]P MOE lower than 10,000), and high for the other four brands (20.5–25.8 % probability of a B[a]P MOE lower than 10,000).The lower contribution of the consumption factors servings per day (17.2 %), portion size (9.4 %) and weekly frequency (2.5 %) to the B[a]P MOE variance was due to the similar consumption habits of the Uruguayan consumers resulting in small variability of these factors [12,28]. Under these scenarios, the predominant factor modifying B[a]P MOE is the B[a]P content of the infusions.
A limitation of this study was the non-representative samples analyzed which may not capture the variability in PAH content due to variation in external factors such as processing, seasonality and environmental contamination [8,10]. However, even though different lots of the same brand of yerba mate were not analyzed, 12 different brands of yerba mate were analyzed, representing almost all the brands consumed in Uruguay. On the other hand, the study has strengths. The analytical method used to quantify PAH in yerba mate infusion was robust and with low detection limits allowing quantification of the four PAH in all the samples. In addition, PAH content in infusions prepared as habitually consumed were determined for the first time in Uruguay. The Monte Carlo simulation approach contributed to minimize uncertainties by using data from a national survey and the PAH content determined in this study.
5. Conclusions
The probability of concern of PAH exposure (MOE < 10,000) from yerba mate infusions varied considerably according to the form of infusion preparation and the marker of exposure used, being consistently higher for infusions prepared for total content and when B[a]P was used as a marker of exposure. Within the framework of the simulations performed, the main factors contributing to the B[a]P MOE variance were B[a]P amount, servings per day and portion size. The probability of B[a]P MOE < 10,000 was 9 % for the infusion prepared as habitually consumed, suggesting a condition of concern for heavy mate consumers in Uruguay. More studies are needed to characterize factors affecting the variability of PAH content in yerba mate in order to reduce the uncertainty of the risk assessment.
Funding
This research was supported by Programa de Apoyo a la Investigación, Comisión Honoraria de Lucha Contra el Cáncer-Fundación Manuel Pérez, Uruguay. C.M received a fellowship from Programa de Investigación Biomédica (Pro.In.Bio), Facultad de Medicina, UdelaR, Uruguay.
CRediT authorship contribution statement
Carolina Menoni: Investigation, Formal analysis, Writing - original draft, Writing - review & editing. Carmen Marino Donangelo: Conceptualization, Resources, Supervision, Writing - original draft, Writing - review & editing. Caterina Rufo: Conceptualization, Resources, Supervision, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank Dr Carmen Rossini and Dr Andrés Gonzalez (Facultad de Química, UdelaR, Uruguay) for access to the HPLC system, and María José Rodriguez (Área Programática Enfermedades No Transmisibles DIGESA – MSP) for providing the database of the Ministerio de Salud Pública Uruguay, 2da Encuesta Nacional de Factores de Riesgo de Enfermedades No Transmisibles, 2013.
Edited by Dr. A.M. Tsatsaka
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2021.01.017.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.IARC Monographs . 2010. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Google Scholar]
- 2.EFSA Opinion of the scientific committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic (Request No EFSA-Q-2004-020) EFSA J. 2005;282:1–31. doi: 10.1021/bk-2010-1048. [DOI] [Google Scholar]
- 3.Penning T.M. 1st ed. Humana Press; 2011. Chemical Carcinogenesis. [DOI] [Google Scholar]
- 4.SCF Opinion of the scientific committee on food on the risks to human health of polycyclic aromatic hydrocarbons in food. Management. 2002 [Google Scholar]
- 5.Crépineau C., Rychen G., Feidt C., Le Roux Y., Lichtfouse E., Laurent F. Contamination of pastures by polycyclic aromatic hydrocarbons (PAHs) in the vicinity of a highway. J. Agric. Food Chem. 2003;51:4841–4845. doi: 10.1021/jf0210371. [DOI] [PubMed] [Google Scholar]
- 6.Wiekstrijm K., Pyysalo H., Plaami-heikkilii S., Tuominen J. Polycyclic aromatic compounds (PAC) in leaf lettuce. Zeitschrift für Leb. und Forsch. 1986;183:182–185. doi: 10.1007/bf01027443. [DOI] [PubMed] [Google Scholar]
- 7.Pincemaille J., Schummer C., Heinen E., Moris G. Determination of polycyclic aromatic hydrocarbons in smoked and non-smoked black teas and tea infusions. Food Chem. 2014;145:807–813. doi: 10.1016/j.foodchem.2013.08.121. [DOI] [PubMed] [Google Scholar]
- 8.Golozar A., Fagundes R.B., Etemadi A., Schantz M.M., Kamangar F., Abnet C.C., Dawsey S.M. Significant variation in the concentration of carcinogenic polycyclic aromatic hydrocarbons in yerba mat?? Samples by brand, batch, and processing method. Environ. Sci. Technol. 2012;46:13488–13493. doi: 10.1021/es303494s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heck C.I., de Mejia E.G. Yerba Mate Tea (Ilex paraguariensis): a comprehensive review on chemistry, health implications, and technological considerations. J. Food Sci. 2007;72:R138–51. doi: 10.1111/j.1750-3841.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 10.Vieira M.A., Maraschin M., Rovaris A.A., Amboni R.D.D.M.C., Pagliosa C.M., Xavier J.J.M., Amante E.R. Occurrence of polycyclic aromatic hydrocarbons throughout the processing stages of erva-mate (Ilex paraguariensis) Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010;27:776–782. doi: 10.1080/19440041003587310. [DOI] [PubMed] [Google Scholar]
- 11.INE . 2006. Encuesta Nacional de Gastos e Ingresos de los Hogares. Los alimentos y bebidas en los hogares. [Google Scholar]
- 12.Bracesco N., Sanchez A.G., Contreras V., Menini T., Gugliucci A. Recent advances on Ilex paraguariensis research: minireview. J. Ethnopharmacol. 2011;136:378–384. doi: 10.1016/j.jep.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez-Mares M.V., Kobayashi H., de Mejia E.G. Inhibitory effect of Camellia sinensis, Ilex paraguariensis and Ardisia compressa tea extracts on the proliferation of human head and neck squamous carcinoma cells. Toxicol. Rep. 2016;3:269–278. doi: 10.1016/j.toxrep.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkler G., Damaty M.El, Schröder C., Lachenmeier D.W. Mate – a “new” caffeine-containing ingredient for the food and beverage industry. Ernahrungs Umschau. 2014;61:160–163. doi: 10.4455/eu.2014.027. [DOI] [Google Scholar]
- 15.Kamangar F., Schantz M.M., Abnet C.C., Fagundes R.B., Dawsey S.M. High levels of carcinogenic polycyclic aromatic hydrocarbons in mate drinks. Cancer Epidemiol. Biomarkers Prev. 2008;17:1262–1268. doi: 10.1158/1055-9965.EPI-08-0025. [DOI] [PubMed] [Google Scholar]
- 16.Okaru A.O., Rullmann A., Farah A., De Mejia E.G., Stern M.C., Lachenmeier D.W. 2018. Comparative Oesophageal Cancer Risk Assessment of Hot Beverage Consumption (coffee, Mate and Tea): the Margin of Exposure of PAH vs Very Hot Temperatures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thea A.E., Ferreira D., Brumovsky L.A., Schmalko M.E. Polycyclic aromatic hydrocarbons (PAHs) in yerba maté (Ilex paraguariensis St. Hil) traditional infusions (mate and tereré) Food Control. 2016;60:215–220. doi: 10.1016/j.foodcont.2015.07.046. [DOI] [Google Scholar]
- 18.Zuin V.G., Montero L., Bauer C., Popp P. Stir bar sorptive extraction and high-performance liquid chromatography–fluorescence detection for the determination of polycyclic aromatic hydrocarbons in Mate teas. J. Chromatogr. A. 2005;1091:2–10. doi: 10.1016/j.chroma.2005.07.057. [DOI] [PubMed] [Google Scholar]
- 19.IARC Monographs . 2018. Drinking Coffee, Mate, and Very Hot Beverages. [PubMed] [Google Scholar]
- 20.EFSA Polycyclic aromatic hydrocarbons in food1 scientific opinion of the panel on contaminants in the food chain (question N° EFSA-Q-2007-136) EFSA J. 2008;724:1–114. [Google Scholar]
- 21.US EPA . 2008. Polycyclic Aromatic Hydrocarbons (PAHs) [Google Scholar]
- 22.Crump K.S. A new method for determining allowable daily intakes. Fondam. Appl. Toxicol. 1984;871:854–871. doi: 10.1016/0272-0590(84)90107-6. [DOI] [PubMed] [Google Scholar]
- 23.Culp S.J., Gaylor D.W., Sheldon W.G., Goldstein L.S., Beland F.a. A comparison of the tumors induced by coal tar and benzo[a]pyrene in a 2-year bioassay. Carcinogenesis. 1998;19:117–124. doi: 10.1093/carcin/19.1.117. [DOI] [PubMed] [Google Scholar]
- 24.Public Health England . 2017. Risk Assessment Approaches for Polycyclic Aromatic Hydrocarbons (PAHs). Contam. L. Inf. Sheet; pp. 1–19. [Google Scholar]
- 25.Fakhri Y., Mousavi Khaneghah A., Ghasemzadeh-Mohammadi V., Khorshidian N., Yousefi M., Hosseini H., Shemshadi G. Polycyclic aromatic hydrocarbons (PAHs) content of edible vegetable oils in Iran: a risk assessment study. Food Chem. Toxicol. 2018;118:480–489. doi: 10.1016/j.fct.2018.05.063. [DOI] [PubMed] [Google Scholar]
- 26.EC Commission Regulation (EU) No 836/2011. Off. J. Eur. Union L. 2011;215:9–16. [Google Scholar]
- 27.Wenzl T., Haedrich J., Schaechtele A., Robouch P., Stroka J. 2016. Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food. [DOI] [Google Scholar]
- 28.Ministerio de Salud Pública Uruguay . 2013. 2da Encuesta Nacional de Factores de Riesgo de Enfermedades No Transmisibles.https://www.who.int/ncds/surveillance/steps/2DA_ENCUESTA_NACIONAL_final_WEB22.pdf [Google Scholar]
- 29.Karcher W., Ewald M., Jacob J. Vol. 2. 1987. (Spectral Atlas of Polycyclic Aromatic Compounds Including Data on Physico-Chemical Properties, Occurrence and Biological). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.