Highlights
-
•
Improved synthesis of designer lipids using acidolysis reaction between palm olein and caprylic acid.
-
•
Intensification based on the sonication with study into effect of different parameters.
-
•
Study on Reusability of Novozyme 435 in order to make process of intensification more economical.
-
•
Palm olein designer lipid demonstrated excellent oxidative stability.
Keywords: Designer lipids, Medium chain fatty acid, Ultrasound, Intensification, Long chain triglycerides
Abstract
The present study deals with intensified synthesis of designer lipids with application of ultrasound based on biocatalyzed reaction between long chain triglyceride and medium chain fatty acid. The effects of various reaction conditions like molar ratio of reactant, reaction temperature, and enzyme loading along with the effect of ultrasound parameters such as duty cycle and irradiation time on the rate of formation of designer lipids has been investigated. The ultrasound assisted process was also compared with the traditional process so as to clearly bring out the intensification effects. During the study, it was clearly demonstrated that the optimum reaction conditions for maximum yield of designer lipids as 92% was molar ratio of medium chain fatty acid to long chain triglyceride as 4:1, reaction temperature of 40 °C, enzyme loading of 3%, duty cycle of 70%, 240 W as power dissipation and 360 min as reaction time. The recyclability study of enzyme showed its effectiveness up to 10 cycles. The synthesized designer lipid showed higher oxidative stability for 35 days and also showed Newtonian behaviour with eye appealing colour. The current study demonstrates development of an eco-friendly technique for intensified synthesis of designer lipids having numerous nutraceutical benefits.
1. Introduction:
Designer lipids are commonly referred to as structured lipids or tailor made lipids synthesized by rearranging or altering the position of fatty acid to increase the nutraceutical value [1]. Due to rapid globalization and change in life style of people, there is increase in risk of cardiovascular diseases and obesity across the world due to intake of high fat foods [2]. The modification of fatty acids (addition or removal) from glycerol backbone can be performed as per need making lipid molecule more health friendly. The desired fatty acid can be incorporated at specific position on glycerol backbone to treat certain illness or syndrome [3]. Due to various health benefits of designer lipids, there is an ever increasing need for the synthesis and as the conventional methods are not sufficient, there is also a need to develop a greener and intensified method for synthesis of designer lipids.
The designer lipids can be synthesized by a chemical or an enzymatic process based on esterification, interesterification, acidolysis, and alcoholysis. Chemical synthesis of designer lipid is economical as compared to enzymatic process but due to the high temperature applied in the chemical process, there is formation of undesired by-products and random distribution of fatty acid on the glycerol backbone [4]. Enzymatic process gives pure product and is an eco-friendly process carried out at low temperatures. Amongst the different processes for synthesis of designer lipids [5], [6], acidolysis is the frequently used process involving medium chain fatty acids and long chain triglycerides as the reactants and lipase as the catalyst. Lipase show more affinity towards medium chain fatty acid than long chain fatty acids [7]. Medium chain fatty acids include fatty acid with carbon chain length from C6 – C12, namely caproic acid, caprylic acid, capric acid and lauric acid. When these medium chain fatty acids are esterified with the glycerol backbone, they form medium chain triglyceride [8]. Due to short chain length and different properties, medium chain triglycerides are believed to be chief source of energy and mainly used as infant food, additives for weight loss, in various clinical nutrition and nutraceutical applications [9]. Medium chain triglycerides are metabolized in human body, easily absorbed and as it goes to liver, it also provides immediate energy (8.4 Kcal/g) and also not stored in body as a fat [10]. Natural source of medium chain triglycerides is coconut oil and bovine milk [11] though it can be also synthesized by esterification reaction [12]. When incorporated into designer lipids the medium chain fatty acids provides neutraceutical advantages due to its shorter chain length and different pattern of metabolism [13]. Medium chain triglycerides are also used in food preparation in blends with other oil to improve oxidative stability of deep fried foods [14]. Medium chain triglycerides offer various health benefits but they lack presence of unsaturated fatty acids. Human body requires unsaturated fatty acids for various cellular functions and normal growth of body. Combining medium chain fatty acid with unsaturated fatty acid would be a great combination for development of designer lipid and hence the present work is based on synthesis of such designer lipids from palm olein and caprylic acid.
Palm olein is widely used oil across the world for various food and pharmaceutical applications due to its unique properties and fatty acid composition. Cost wise also palm olein is cheaper as compared to other oils and it is available in ample quantum and throughout the year. It contains 43.9% oleic acid which has single unsaturation, 40.8% palmitic acid which is saturated fatty acid and 13% linoleic acid which has two unsaturation [15]. Presence of monounsaturated and poly unsaturated fatty acid in palm olein makes it healthy and nutrition rich oil and also offers improved physical properties like cloud point and pour point whereas presence of saturated fatty acid improves its stability towards oxidation and rancidity. Due to its unique fatty acid composition and presence of fat soluble vitamin and various phenolic components, it offers significant benefits against obesity, cancer, cardiovascular diseases, diabetes etc. [16]. Considering the numerous advantages offered by palm olein, it has been considered in the present work for synthesis of designer lipids.
The conventional methods for synthesis of designer lipids offers disadvantages in terms of higher reaction times, lower yields and use of stringent conditions prompting the need for application of intensification approaches. Sonication is used extensively for intensification of organic synthesis based on the significant offered effects by ultrasound induced cavitation. In ultrasound induced cavitation, there is formation phase, growth phase and collapse phase of bubbles or cavities in liquid, ultimately releasing significant energy on collapse [17]. We now present literature analysis to highlight the utility of the current work. More et al. [18] used sonication for intensified synthesis of designer lipids from various edible oil sources and caprylic acid. Intensified yield of 84.5% was reported using ultrasound at 60% duty cycle in 9.6 h. Deshmane et al [19] investigated synthesis of medium chain triglycerides using ultrasound based on sulphuric acid as a catalyst. Around 98.5% conversion of fatty acids was reported in 360 min of reaction time. Mohod et al. [20] studied use of ultrasound for intensification of chemically catalysed esterification reaction between fatty acid (lauric acid) and glycerol at larger capacity of 4L using direct and indirect cavitation. Higher conversion of 77.5% using direct mode of operation for cavitation was reported in 270 min. Some intensification studies using sulphuric acid (H2SO4) as an acid catalyst are also reported giving higher conversion in less time [20]. For example, More et al. [21] reported higher conversion (%) of esterification reaction for the synthesis of triglycerides of caprylic acid using acid catalyst like sulphuric acid, hydrochloric acid and p-toluenesulphonic acid. Higher conversion of 96.6% was also reported using sulphuric acid in 540 min of reaction time. The chemical catalysts like sulphuric acid and hydrochloric acid are strong acids which are critical to handle especially at larger scale. Also, with an increase in temperature, the acid catalyst initiates charring or blackening of product which again requires additional step of bleaching to remove colour from product. As an alternative to chemical catalyst, use of enzymatic catalyst is preferable.
More et al. [18] investigated synthesis of structured lipids catalysed by Novozyme 435 and another form of lipase using various intensification approaches and reported that Novozyme 435 showed higher catalytic activity (84.4% yield in 360 min of reaction time) as compared to other form of lipase. Novozyme 435 is also used as a biocatalyst for synthesis of designer lipids. Novozyme 435 is sn-1, 3 specific enzyme having affinity towards medium chain triglycerides. In palm olein, sn-1 and sn-3 are esterified with saturated palmitic acid and sn-2 is esterified with mono and poly unsaturated oleic and linoleic fatty acid [22]. The present work concentrates on intensified synthesis of designer lipids using palm olein and caprylic acid catalysed by Novozyme 435. Designer lipids containing medium chain fatty acid and monounsaturated fatty acids will give benefits of both fatty acids to human body. The study also focuses on use of sonication as an intensification technique and understanding influence of ultrasound parameters and reaction parameters on yield (%) of designer lipid. The recyclability of Novozyme 435 has also been studied with an intention of designing profitable process for commercial utilization. The ultrasound assisted process is also compared with the traditional synthesis of designer lipids to bring out the advantages of using sonication for intensification. The literature analysis revealed that there is no study on ultrasound assisted intensified synthesis of designer lipids using medium chain triglyceride and palm olein clearly demonstrating the novelty of the work.
2. Materials and methods
2.1. Materials
Palm olein was purchased from local market in Mumbai, India. Caprylic acid was procured from Ami Chemicals Pvt. Ltd. Mumbai, India and Novozyme 435 was obtained as a gift sample from Novozymes Pvt. Ltd. Bangalore, India.
2.2. Reaction scheme
The acidolysis reaction for intensified synthesis of designer lipid carried out between palm olein and caprylic acid catalysed by novozyme 435 can be represented as follows:
2.3. Experimental methodology
2.3.1. Traditional process of synthesis
A round bottom flask was used for traditional process of synthesis of designer lipid. The known quantity of caprylic acid and palm olein along with novozyme 435 was added in the round bottom flask, which was then kept in rotary shaker operated at 120 rpm (Royal scientific RSW) and maintained at constant temperature. Both the reactant quantity was varied from 1 ml to 30 ml with enzyme quantity from 0.1 g to 7g. At regular intervals, 0.5 ml sample was withdrawn for analysis to check the progress of reaction.
2.3.2. Ultrasound assisted synthesis of designer lipids
Ultrasonic horn was used for generating cavitation effect in the reaction mixture. The operating frequency for ultrasonic horn was fixed at 20 kHz with rated power of 240 W. The ultrasonic horn tip diameter was 20 mm. The actual calorimetric efficiency was 7% with actual power supplied in the system as 16.8 W. The low transfer efficiency can be attributed to the very low transfer area available in the case of ultrasonic horn as well as high viscosity of the reaction mixture used in the study. The reaction mixture containing caprylic acid, palm olein and Novozyme 435 was taken in a 100 ml reaction flask. The flask was kept on magnetic stirrer for proper agitation and ultrasonic horn was immersed in the reaction mixture such that the tip of horn was 15 mm below the top surface of reaction mixture. The ultrasound duty cycle and irradiation time was varied from 50% to 90% and up to 7 h respectively. The sample was removed at fixed interval to check the progress of reaction. The reactions were carried out three times to examine the reproducibility of the results obtained in the study. The data plotted in figure and discussed in the text is average of 3 readings. Typically the observed variations in the data was ± 2% of the reported data as also represented in the figures in the form of error bars.
2.4. Purification of product
Immediately after 360 min of reaction time, the Novozyme 435 was separated from reaction mixture simply by filtration using whatmann filter paper 1 with pore size of 11 µm. The separated Novozyme 435 was rinsed with an n-hexane and then dried and it was then reused to check recyclability of Novozyme 435. The filtrate was given an alkali wash in order to remove free fatty acid and then it was allowed to pass through a packed bed of silica to obtain desired purity of designer lipid.
2.5. Analytical methods
2.5.1. Rheological property
Viscosity of designer lipid was measured to ensure flowability of designer lipid. Viscosity was measured using Brookfield viscometer with LV2 Spindle.
2.5.2. Colour analysis
Colour of fat / oil is the main attraction of any consumer. Colour is also an indicator of quality of oil / fat. Colour of oil was evaluated using AOCS (1989). Lovibond Tintometer was used for colour analysis of oil. Tintometer used for colour analysis uses series of gradients like red, yellow, blue colour glasses whereas the sample is taken in a glass tube. The tube is inserted in the comparator and compared with a yellow coloured glass discs (taken in the present work) until the nearest possible match is found. 0 refers to very light colour and 100 refers to very dark yellow colour.
During the analysis, all the oil samples were heated at 45 °C and mixed well. 12 ml of oil sample was poured in sample vial and sample vial was fixed at sample holding cell. The same process was used for all the oil samples during the reaction. Designer lipid formed was also analysed using yellow scale. Typically with the attachment of caprylic acid at sn 1, 3 position, the colour of reaction mixture changes to yellow colour with progress of reaction.
2.5.3. Acid value
The presence of free fatty acid is determined according to American Oil Chemist Society Official Method Te 1a-64. 1 g of sample from reaction mixture was taken in a conical bottom glass flask and 20 ml of neutral alcohol was added followed by phenolphthalein indicator. The solution in conical bottom flask was titrated against 0.01 N KOH. The acid value was calculated using following equation:
Acid value was measured as mg KOH / g of sample. Acid value gives an idea about the unreacted free fatty acid still present in the reaction mixture.
2.5.4. Peroxide value
Peroxide value of sample is an indicator of oxidative damage to lipid molecule. Peroxide value was estimated as per standard protocol given by the American Oil Chemist Society. 5 g of oil sample was taken in a conical flask and mixed with 3:2 acetic acid: chloroform solution (50 ml) along with 0.5 ml of saturated potassium iodide. The resulting mixture was kept in dark for 1 min for liberation of iodine. After 1 min, the solution was mixed with 30 ml distilled water and this mixture was then titrated with 0.01 N sodium thiosulphate with starch indicator. The following equation was used to calculate the peroxide value of the sample.
2.5.5. Calculation of yield (%)
The yield (%) of the designer lipid is calculated on the basis of theoretical and actual quantum of the designer lipid obtained in the reaction. The theoretical yield is the maximum amount of product that could be formed from the given amount of reactant. Actual yield is the amount of product which is actually formed in the reaction in any specific set of reaction. For example, when 10 g of palm olein with average molecular weight 854.3 g/mol was taken for reaction along with 6.74 g of caprylic acid with molecular weight of 144.21 g/mol, the theoretical expected yield is 7.37 g for the palm olein designer lipid (629.92 g/mol as the molecular weight). The actual quantum of the product obtained in the experiment was 6.8 gm giving a yield of 92%.
2.5.6. HPLC analysis
HPLC instrument obtained Shimadzu was used for confirmatory analysis of formation of the designer lipids. The technical details of HPLC system are given below:
Column: - HiQSil C18 with internal diameter of 4.6 mm and height of 250 mm.
Detector: - RID 6A refractive index.
Other accessories: LC-AS pump and CR 4A Integrator.
During the analysis, the sample was dissolved in the mobile phase consisting of mixture of acetonitrile and acetic acid (90:10 by volume) and injected at steady flow rate of 1 ml/min.
3. Results and discussion
3.1. Effect of molar ratio on yield of reaction
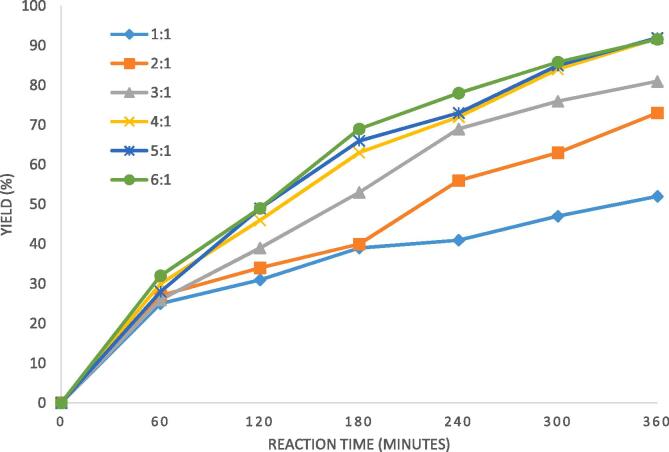
The effect of molar ratio of caprylic acid: palm olein on the yield of reaction was studied using different molar ratio as 1:1, 2:1, 3:1, 4:1, 5:1 and 6:1 with reaction temperature maintained constant as 40 °C and Novozyme 435 loading as 3%. The obtained results are represented in Fig. 1 where it is seen that the obtained yields at the final equilibrium were higher at higher molar ratio and the effect was significant for an optimum change in the molar ratio. Presence of excess free fatty acid in the reaction mixture drives the reaction in forward direction to a greater extent and gives maximum yield in given time of reaction. It was also seen that the increase in molar ratio increased yields significantly only up to a certain point. The optimum molar ratio which gave maximum yield was 4:1 (caprylic acid: palm olein) and further increase in molar ratio had an insignificant effect on the yield of reaction. Increase in molar ratio beyond 4:1 will increase the free fatty acids beyond ideal requirement in the reaction mixture which are not utilized positively and also creates difficulty in separating unreacted fatty acids from product in the subsequent processing. Taking this aspect into consideration, the optimum molar ratio was established as 4:1 and this molar ratio was considered for further investigations. Rupani et al. [23] also studied the effect of molar ratio on the yield of transesterification reaction catalysed by lipase and reported maximum yield with molar ratio of α-linolenic acid and olive oil as 4:1. Silroy et al. [24] also investigated effect of molar ratio of rice bran oil and capric acid on the yield of acidolysis reaction for synthesis of structured lipids catalysed by sn-1,3 specific biocatalyst at 45 °C. It was reported that maximum yield of 30.8% was seen at optimum molar ratio of 3:1 (capric acid: vegetable oil). Shieh et al. [25] reported highest yield of 51% at molar ratio of capric acid to triglyceride of oleic acid as 4:1 using immobilized lipases in a solvent based reaction based on hexane as a solvent. It can be clearly seen that though the trends of optimum molar ratio is similar, there is a quantitative difference in the optimum value as well as the maximum yield clearly establishing the utility of the detailed investigation presented in the current work.
Fig. 1.
Effect of molar ratio of caprylic acid: palm olein on the yield of palm olein designer lipid under conditions of reaction temperature as 40 °C, Novozyme 435 loading of 3%, ultrasonic duty cycle of 70% and irradiation time of 360 min.
3.2. Effect of reaction temperature
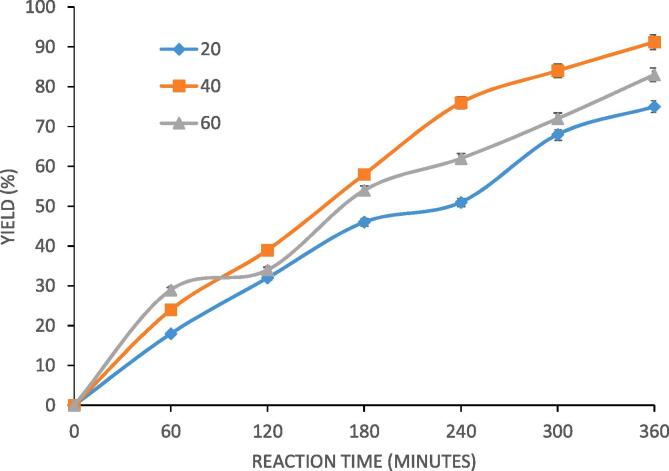
The effect of temperature on yield was studied by changing reaction temperature from 20 °C to 60 °C at constant conditions of the molar ratio and Novozyme 435 loading as 4:1 and 3% respectively. The result is represented in Fig. 2 where it can be seen that with an increase in temperature of the reaction mixture, the rate of reaction increases and the maximum yield is obtained at 40 °C. Beyond 40 °C, there is no observable increase in the reaction rate with an increase in the reaction temperature. A positive change in the temperature increases the rate of formation of product based on intrinsic kinetics but at the same time it has adverse effect on the activity of Novozyme 435 as well as the cavitational activity. The ability of Novozyme 435 to catalyse the reaction typically decreases beyond the optimum temperature of operation and this coupled with dominantly reduced cavitational activity results in an insignificant change in the yield of palm olein designer lipid. The elevated temperature beyond the optimum results in breaking of weak hydrogen bonds of protein present in enzymes which affects the catalytic activity of Novozyme 435. A too much increase in temperature results in too many cavitation events leading to cushioned collapse as well as formation of vaporous cavities collapsing with lower intensity, both contributing to reduced cavitational effects. These counteracting effects of increased temperatures explains the existence of optimum temperature. Zou et al. [26] investigated the effect of temperature on the acidolysis reaction for synthesis of designer lipids using BCF oil. It was reported that maximum yield of structured lipids as 43.8% was obtained at 50 °C. Cao et al. [27] studied interesterification reaction of triacetin catalysed by Novozyme 435 elucidating the effect of temperature on the lipid yield and reported that the maximum yield of designer lipid was 52.1% at optimum temperature of 50 °C. Kim et al. [28] also demonstrated the effect of temperature on the acidolysis reaction for synthesis of designer lipids from corn oil and caprylic acid. Investigations revealed that 55 °C is the optimum temperature for obtaining maximum yield of designer lipids from corn oil. It is again important to say that optimum temperature is indeed dependent on the specific system in question. Considering the outcome of effect of temperature on yield in the present work, the ideal condition of temperature was established as 40 °C for maximum yield. The established temperature of 40 °C was considered subsequently for studying effect of other reaction parameters on the progress of the reaction.
Fig. 2.
Effect of reaction temperature on the yield of palm olein designer lipid under conditions of molar ratio as 4:1 (caprylic acid: palm olein), 3% loading of Novozyme 435, 70% duty cycle and 360 min as irradiation time.
3.3. Effect of Novozyme 435 loading
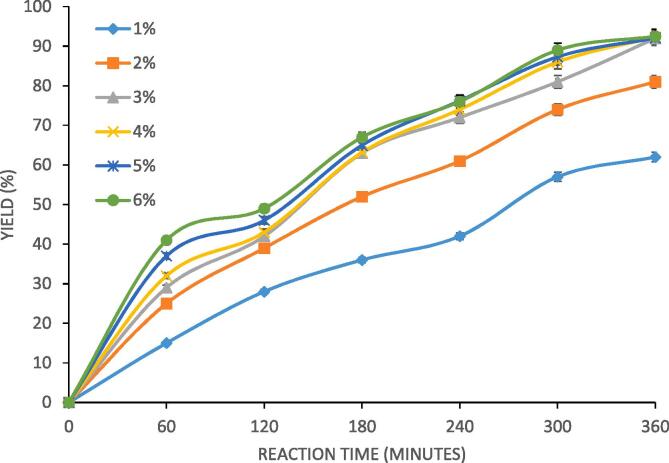
Novozyme 435 loading was varied from 1% to 6% to study its effect on the progress of reaction under constant reaction temperature and molar ratio as 40 °C and 4:1 respectively. The results represented in Fig. 3 explain that Novozyme 435 loading of 1% showed lower yield and the yield of acidolysis reaction increases with an increase in the Novozyme 435 loading up to 3%. Further loading of novozyme 435 at 4% − 6% resulted in no significant effect on the yield of designer lipid. Increase in the rate of reaction initially with an increase in Novozyme 435 loading can be credited to the fact that an increase in the loading of the enzyme offers more active sites for substrate which drives reaction in the forward direction at a faster rate. Novozyme 435 loading beyond 3% did not increase yield of reaction because of shift in the controlling mechanism for the reaction and availability of enough active sites for the reaction at 3% loading. Use of Novozyme 435 loading higher than 3% does not give any benefit in terms of reduction in activation energy leading almost similar extents of reaction. Considering the fact that Novozyme 435 loading of 3% gave excellent results and the high cost of catalyst, the optimum loading was fixed as 3%. Ab et al. [29] studied effect of enzyme loading on the rate of formation of structured lipids and reported 6% as an optimum loading of lipase for interesterification of palm stearin and coconut oil performed under conditions of reaction temperature as 60 °C and reaction time of 360 min. Lee et al [5] investigated the synthesis of structured lipid using acidolysis reaction between groundnut oil and medium chain fatty acid (C8) in the presence of immobilized lipase as the catalyst. It was elucidated that maximum yield of structured lipids as 57.7% were obtained at lipase loading of 4% with the total reaction time of 24 h. The optimum enzyme loading is dependent on the reaction considered for synthesis of designer lipids directing the novelty of the current work. The ideal Novozyme 435 loading established in the work for any specific system helps in reduction in the total processing cost since enzyme cost is typically higher compared with the chemical catalysts.
Fig. 3.
Effect of Novozyme 435 loading on the yield of palm olein designer lipid under conditions of reaction temperature as 40 °C, molar ratio of 4:1 (caprylic acid: palm olein), 70% duty cycle and 360 min as irradiation time.
3.4. Effect of ultrasound parameters
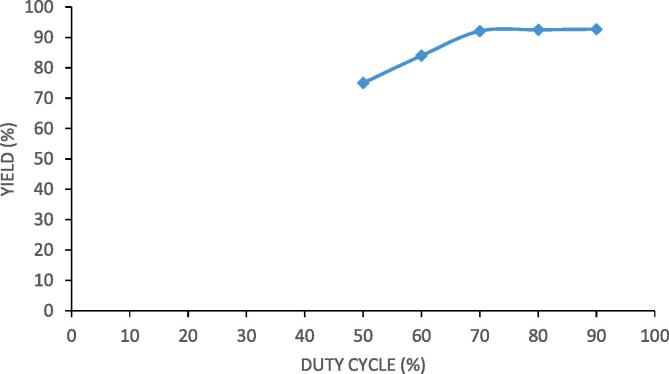
Ultrasound parameters play an important role in deciding the extent of intensification for the synthesis of designer lipids. The parameters which were studied in present research were duty cycle and irradiation time. Duty cycle is principal parameter which not only affects yield of reaction but also affects the economy of the ultrasound assisted intensification process. Duty cycle means on and off time during irradiation and the duty cycle was varied from 50% (5 s on and 5 s off) to 90% (9 s on and 1 s off) in the present work. The effect of duty cycle on the yield is shown in Fig. 4 where it is seen that with an increase in the on time of irradiation, the process becomes more intensified. Increase in duty cycle increases exposure of reaction mixture to the cavitation effect leading to better progress of the reaction. The maximum increase in the yield was obtained at duty cycle of 70% and only marginal increase in yield was seen with subsequent increase to 80% and 90%. Subjecting reaction mixture to cavitation for longer period may not be economical just for a marginal increase in the yield and hence it is very important to establish the trends for duty cycle. At very high duty cycle of 80% and 90%, Novozyme 435 is exposed to the cavitation effect for longer duration and longer exposure of Novozyme 435 to cavitation effects may result in the damage to enzyme thereby effecting the enzymatic activity. Longer exposure also affects the recyclability of Novozyme 435. Considering all these aspects, duty cycle of 70% was established as the best duty cycle for the intensified synthesis of palm olein designer lipid.
Fig. 4.
Effect of ultrasonic duty cycle on the yield of palm olein designer lipids under conditions of molar ratio as 4:1 (caprylic acid: palm olein), reaction temperature of 40 °C, Novozyme 435 loading as 3% and 360 min as irradiation time.
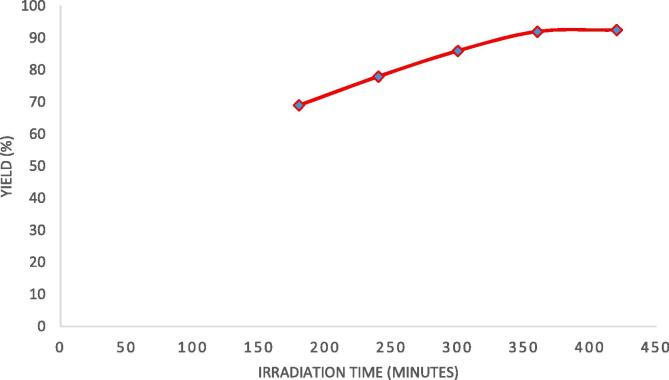
Similar to the duty cycle, irradiation time is equally important in determining yield of reaction. The irradiation time was varied from 3 h to 7 h and the optimum time for obtaining significant intensification of the synthesis was 6 h as per the results shown in Fig. 5. It was observed that as the irradiation time increased the reaction mixture was exposed to ultrasonic effect for more time thereby driving the reaction in forward direction due to higher cavitation effect.
Fig. 5.
Effect of ultrasonic irradiation time on the yield of designer lipid under conditions of molar ratio as 4:1 (caprylic acid: palm olein), reaction temperature as 40 °C, 70% duty cycle and Novozyme 435 loading of 3%.
Credence to the importance of the presented results can be obtained using the comparison with the literature. Khan et al. [30] reported maximum yield of 96.6% in 50 min of synthesis at duty cycle of 70% for the system of synthesis of n-butyl palmitate by esterification reaction catalysed using immobilized lipase. Similar results for the effect of ultrasound duty cycle on the yield of structured lipids were also reported by Liu et al. [31]. 30% as duty cycle was demonstrated as optimum for enzymatic synthesis of designer lipids using oleic acid as fatty acid and triglycerides of palmitic acid. The optimum conditions resulted in maximum yield of 51.8% in 240 min of reaction. More et al. [32] studied the effect of ultrasonic treatment on the intensified synthesis of tricaprylin by esterification reaction between caprylic acid and glycerol catalysed using lipase. It was reported that 70% duty cycle was optimum for maximum conversion of 94.8% in 420 min of reaction time. In present study, 70% duty cycle was established as optimum for intensified synthesis of palm olein designer lipids without any adverse effect on reusability and enzymatic activity of the Novozyme 435. The quantitative differences in the optimum duty cycle and the time of reaction established based on the literature comparison clearly depict the importance of the presented detailed study of duty cycle and irradiation time in the current system.
3.5. Reusability of Novozyme 435
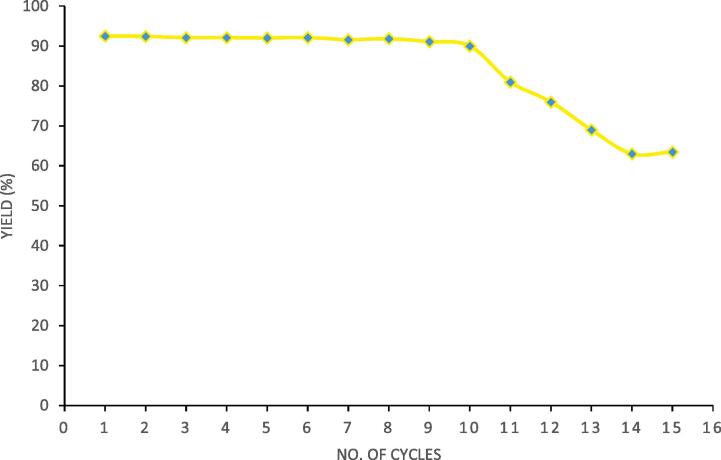
Recyclability of biocatalyst is important to make process economical and eco-friendly for possible application in industrial synthesis of designer lipids. The recyclability of Novozyme 435 enzyme was studied based on using Novozyme 435 for 15 cycles of esterification reaction between caprylic acid and palm olein. The obtained results are represented in Fig. 6. From the Fig. 6, it is clear that there is marginal change in yield of the esterification reaction for 10 cycles and beyond 10 cycles there is significant decrease in the enzymatic activity of Novozyme 435 as represented by lower yields in the reaction. The obtained results explain that application of ultrasound for intensified synthesis did not affect enzymatic activity of Novozyme 435 significantly for 10 cycles and hence there can be effective recycle for 10 cycles. The reasons for the decreased yields after usage for 10 cycles can be based on the inhibitions due to the chemical reactions and/or damage to the enzyme due to cavitational effects. It is interesting to compare the results with the literature. Liu et al. [31] also reported recyclability of immobilized lipase for 10 cycles for the case of ultrasound assisted synthesis of designer lipids from triglyceride of palmitic acid and oleic acid. More et al. [32] also reported recyclability of Novozyme 435 up to 10 cycles for the case of ultrasound assisted production of triglycerides of caprylic acid. More et al. [18] reported recyclability of Novozyme 435 for synthesis of structured lipids up to 15 cycles with optimized sonication duty cycle of 60% giving 84.5% yield of structured lipids. It is thus important to note that the effective reuse of the enzymes depends on the specific system, more importantly, on the interactions of enzyme with the substrate as well as the cavitational intensity generated based on the use of ultrasound. The negative effects of ultrasound can be minimized by optimizing the ultrasonic parameters, mainly the power dissipation and duty cycle. The results demonstrated in the current work clearly confirmed the effective reuse of the catalyst proving the advantage for effective application at commercial levels.
Fig. 6.
Study on reusability of Novozyme 435 over 15 cycles of acidolysis reaction under optimized reaction and ultrasound parameters (molar ratio of 4:1 (caprylic acid: palm olein), reaction temperature of 40 °C, Novozyme 435 loading of 3%, 70% ultrasound duty cycle and 360 min as irradiation time).
3.6. Comparison of ultrasound assisted synthesis and traditional synthesis of designer lipids
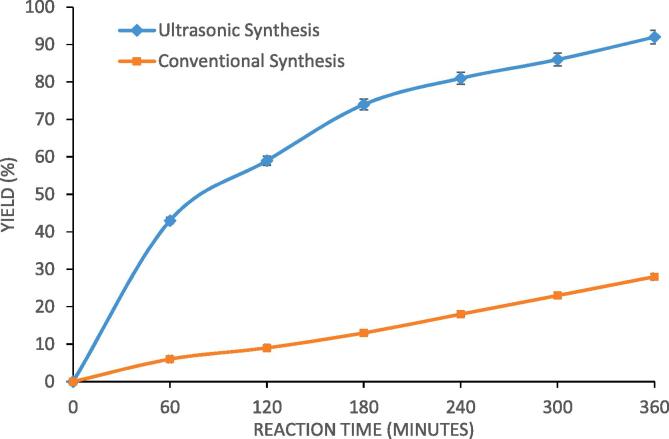
The traditional or conventional synthesis of designer lipid is compared with the ultrasonic process for synthesis of designer lipids so as to establish the process intensification benefits. The obtained results are represented in Fig. 7. The ultrasonic process with molar ratio of caprylic acid: palm olein as 4:1, reaction temperature of 40 °C and Novozyme 435 loading of 3% gave maximum yield of 92% in 360 min of reaction time whereas the conventional synthesis with same reaction parameters without ultrasound resulted in yield of only 28% in 360 min. The conventional process took 28 h for reaching the maximum yield of 90%. In ultrasound assisted process, the passage of ultrasound through the reaction mixture gives rise to cavitational effects based on the introduced pressure fluctuations by ultrasound. The generation of turbulence and liquid circulation eliminates the mass transfer resistances commonly associated with enzymatic reactions. The high degree of turbulence enhances the rate of mass transfer which results in intensification [17]. The significant intensification seen in the present work along with effective recycle of the biocatalyst clearly establish that the ultrasonic synthesis of designer lipids is an intensified synthesis process using recyclable Novozyme 435 and has significant commercial interest. More et al. [18] also investigated synthesis of structured lipids using sonication and Novozyme 435 as the lipase form and reported that the sonication approach gave high yield of 84.5% in 360 min as compared to conventional approach which gave yield of 77.1% after 24 h of reaction time. It can be thus said that though the trends are similar, the quantitative differences in the intensification confirm the importance of the present work dealing with designer palm olein based lipids.
Fig. 7.
Comparison between conventional synthesis and ultrasonic assisted synthesis of palm olein designer lipids (molar ratio of 4:1 (caprylic acid: palm olein), reaction temperature of 40 °C, Novozyme 435 loading of 3%, 70% ultrasound duty cycle and 360 min as irradiation time).
3.7. Rheological properties
The most important rheological property is the viscosity, which is measure of resistance to flow. Palm olein and caprylic acid are both Newtonian fluids. In the synthesis of designer lipids, there is change in fatty acid composition of resulting designer lipid, which may increase the viscosity of products and Newtonian fluids may change to non-Newtonian characteristics, which may affect the heat transfer rate during the reaction. The relationship between shear stress and rate of shear is given by Newton’s law of viscosity as per the following equation
Where,
τ = Shear stress (mPa)
μ = Viscosity
du/dy = Shear rate (s−1)
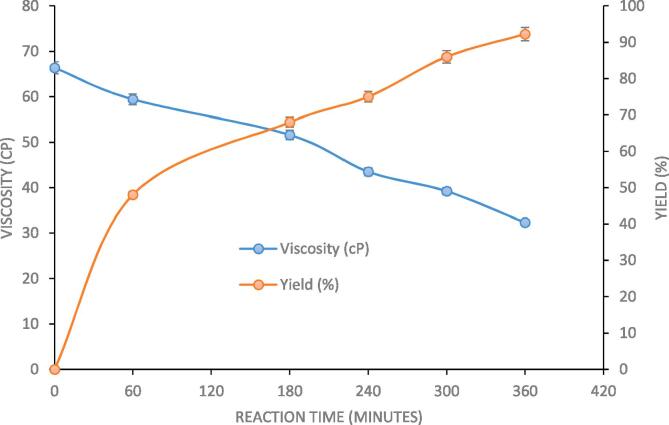
The change in viscosity of reaction mixture with progress of reaction and increase in the yield (%) is shown in Fig. 8. The reaction mixture was taken in beaker and spindle was dipped in the reaction mixture and allowed to rotate and reading was displayed on the screen of viscometer which was then recorded. It is seen that the viscosity of the designer lipid decreases as the reaction progresses, due to the fact that the sn-1,3 positions are occupied by caprylic acid due to action of sn-1,3 specific Novozyme 435. Since caprylic acid have shorter chain length and hence lower molecular weight as compared to fatty acid present in Palm olein, the product has lower viscosity. Decrease in viscosity can be hence considered as an indicator of progress of reaction and intensive formation of palm olein designer lipids.
Fig. 8.
Reduction in viscosity of palm olein with progress of reaction (molar ratio of 4:1 (caprylic acid: palm olein), reaction temperature of 40 °C, Novozyme 435 loading of 3%, 70% ultrasound duty cycle and 360 min as irradiation time).
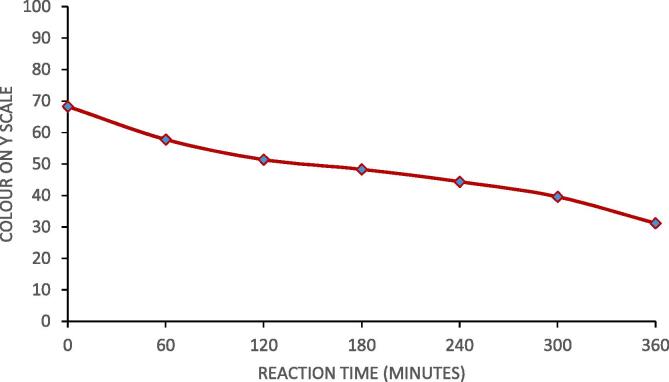
3.8. Colour of palm olein designer lipids
Colour is the first point of attraction towards any food and the colour should be eye appealing. The colour of palm olein was dark yellow whereas colour of caprylic acid was colourless. The change in colour was measured on yellow scale. The change in colour with progress of reaction is shown in Fig. 9. With progress of reaction, the dark colour of reaction mixture fades and becomes light yellow. Colour of designer lipid can be easily noticed by naked eyes also. The change in colour of reaction mixture with progress of reaction was due to change in fatty acid composition of palm olein and attachment of caprylic acid on sn-1,3 position of palm olein. The change in colour was again an indicator of progress of reaction and formation of palm olein designer lipid.
Fig. 9.
Change in colour of palm olein with progress of reaction (molar ratio of 4:1 (caprylic acid: palm olein), reaction temperature of 40 °C, Novozyme 435 loading of 3%, 70% ultrasound duty cycle and 360 min as irradiation time).
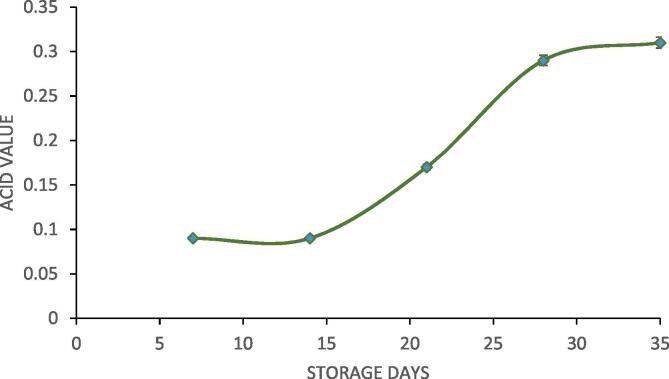
3.9. Change in acid value
In the present work, acid value of designer lipid (the final product) was also monitored in order to study the storage stability of designer lipid. Acid value is an indicator of presence of free fatty acids. The fatty acids are released from the attachments on the glycerol backbone due to action of heat, water etc. and formation of free fatty acid makes oil unsuitable for human consumption. Ideally acid value of oil should be less than 1. The storage stability of palm olein designer lipid was checked by monitoring the change in acid value over a period of 35 days. The acid value was checked after every 7 days for 35 days and the obtained results are represented in Fig. 10 where it can be concluded that the acid value was very low on 7 day of storage time and it remained unchanged up to 14 day. Subsequently, there was very slight increase in the acid value of designer lipid and even after 35 days of storage the acid value of palm olein designer lipid was below 1 making it suitable for human consumption even after 35 days. The results confirmed excellent shelf life for the synthesized designer lipids in the present work.
Fig. 10.
Stability study in terms of formation of free fatty acids in the palm olein designer lipid.
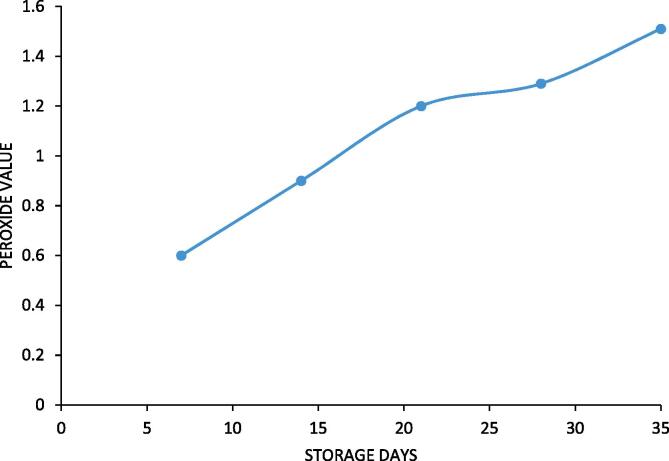
3.10. Peroxide value
Peroxide value is an indicator of oxidative damage to the designer lipid. The oxidative stability of palm olein designer lipid was also studied for a storage time of 35 days. The palm olein designer lipid was checked for its oxidative stability by storing it in a dark place at room temperature. The analysis of peroxide value was performed after every 7 days for a period of 35 days. The change in peroxide value is represented in Fig. 11. The peroxide value of synthesized palm olein designer lipid analysed fresh was 0.6 Meq/kg. The peroxide value remained same exactly for first 7 days and after that also there was small increase in the peroxide value. The peroxide value even after 35 days of storage was 1.51 Meq/kg, which was within the acceptable limits. The critical value of 10Meq/kg and above indicates oxidative damage to oil making it unfit for human consumption and clearly this limit was not reached in the current work. The palm olein designer lipid was thus demonstrated stable to oxidative damage when stored at room temperature for 35 days. The stability is mainly attributed to presence of saturated caprylic acid at sn-1,3 position. Saturated fatty acid are stable and resistant to oxidative damage due to presence of single bond. The marginal change in peroxide value obtained can be attributed to the presence of monounsaturated oleic acid which is susceptible to oxidation. Overall it can be said that the developed palm olein designer lipid was stable to oxidation for 35 days at room temperature.
Fig. 11.
Stability Study of palm olein designer lipid in terms of oxidative deterioration.
3.11. HPLC analysis
The formation of designer lipid was confirmed with the High Performance Liquid Chromatography analysis. The HPLC Chromatogram is shown in Fig. 12. The designer lipid contains caprylic acid at position 1 and 3 and oleic acid at position 2 esterified to glycerol backbone. The HPLC peak was obtained at 6.7 min retention time in the present work. More et al. [33] also reported HPLC retention time of Moringa oil designer lipid as 6.7 min. Moringa oil designer lipid also contains caprylic acid at sn-1,3 position and oleic acid at 2 position similar to the designer lipid obtained in the current work. The HPLC analysis indeed confirmed the formation of palm olein designer lipids.
Fig. 12.
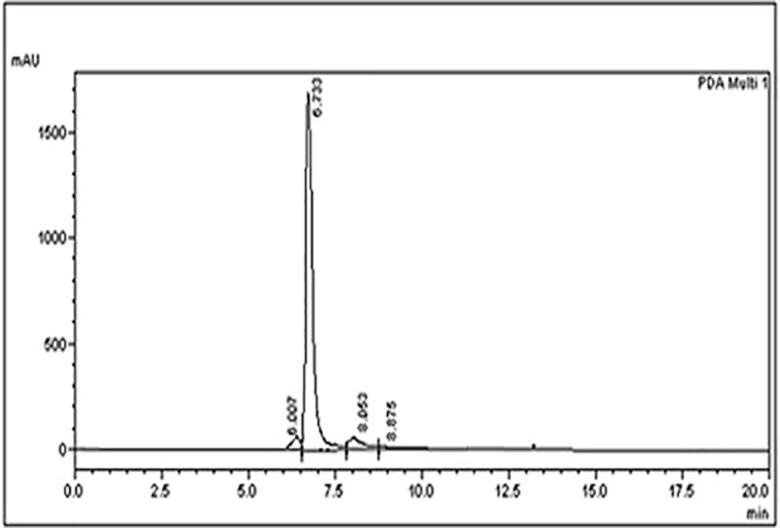
HPLC chromatogram to establish the presence of caprylic acid in designer lipid.
4. Conclusions
The present work focused on intensified synthesis of palm olein designer lipid from palm olein and caprylic acid using Novozyme 435 as the catalyst. The work demonstrated that with the use of ultrasonic irradiation, the yield of reaction increased to 92% in much shorter reaction time of 360 min compared to conventional synthesis where only 28% yield was obtained at 360 min and 90% yield obtained after 28 h. Effect of different operating conditions revealed that excellent results were obtained at 70% duty cycle and 360 min irradiation time. The Novozyme 435 showed same enzymatic activity for 10 cycles and hence it can be successfully reused for 10 cycles of reaction without affecting yield. The decrease in viscosity and formation of light yellow colour of reaction mixture confirmed the formation of palm olein designer lipid. The oxidative stability of formed palm olein designer lipid was also demonstrated for 35 days at room temperature. Overall the work clearly demonstrated an intensified synthesis process for obtaining palm olein designer lipid having significant commercial importance based on established optimum parameters and the recyclability of the biocatalyst.
CRediT authorship contribution statement
Harsh B. Jadhav: Methodology, Investigation, Writing - original draft. Parag R. Gogate: Supervision, Investigation, Writing - review & editing. Jyotsna T. Waghmare: Supervision, Writing - review & editing. Uday S. Annapure: Supervision, Writing - review & editing, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Harsh B. Jadhav is thankful to Department of Science and Technology, Government of India for providing DST-Inspire fellowship for doctoral research.
Contributor Information
Parag R. Gogate, Email: pr.gogate@ictmumbai.edu.in.
Jyotsna T. Waghmare, Email: jt.waghmare@ictmumbai.edu.in.
Uday S. Annapure, Email: us.annapure@ictmumbai.edu.in.
References
- 1.Abed S.M., Ali A.H., Noman A., SobiaNiazi, Al-fargaAmmar, Bakry A.M. Structured lipids : Enzymatic synthesis, health benefits and nutraceutical characteristics - A review. International Jornal of Research in Agricultiure Sciences. 2016;3:206–215. [Google Scholar]
- 2.Gul H., Kart F.M., Gul M., Akpinar M.G. Bakery Products Consumption and Consumers’ Awareness in Urban Areas of Isparta City, Turkey. Scientific Papers: Management, Economic Engineering in Agriculture & Rural Development. 2017;17:137–145. https://ezproxy.lib.uconn.edu/login?url=https://search.ebscohost.com/login.aspx?direct=true&db=aph&AN=124329838&site=ehost-live [Google Scholar]
- 3.Stein J. Chemically defined structured lipids: Current status and future directions in gastrointestinal diseases. Int. J. Colorectal Dis. 1999;14(2):79–85. doi: 10.1007/s003840050190. [DOI] [PubMed] [Google Scholar]
- 4.Hamam F. Specialty Lipids in Health and Disease. Food and Nutrition Sciences. 2013;04(09):63–70. doi: 10.4236/fns.2013.49A1011. [DOI] [Google Scholar]
- 5.Lee K., Akoh C.C. Structured lipids: Synthesis and applications. Food Reviews International. 1998;14(1):17–34. doi: 10.1080/87559129809541148. [DOI] [Google Scholar]
- 6.Kapoor M., Gupta M.N. Lipase promiscuity and its biochemical applications. Process Biochem. 2012;47(4):555–569. doi: 10.1016/j.procbio.2012.01.011. [DOI] [Google Scholar]
- 7.Christensen M.S., Høy C.E., Becker C.C., Redgrave T.G. Intestinal absorption and lymphatic transport of eicosapentaenoic (EPA), docosahexaenoic (DHA), and decanoic acids: Dependence on intramolecular triacylglycerol structure. Am. J. Clin. Nutr. 1995;61:56–61. doi: 10.1093/ajcn/61.1.56. [DOI] [PubMed] [Google Scholar]
- 8.Babayan V.K. Medium-chain triglycerides-their composition, preparation, and application. Journal of the American Oil Chemists’ Society. 1968;45(1):23–25. doi: 10.1007/BF02679040. [DOI] [PubMed] [Google Scholar]
- 9.Marten B., Pfeuffer M., Schrezenmeir Jürgen. Medium-chain triglycerides. Int. Dairy J. 2006;16(11):1374–1382. doi: 10.1016/j.idairyj.2006.06.015. [DOI] [Google Scholar]
- 10.You Y.-Q., Ling P.-R., Qu J.Z., Bistrian B.R. Effects of medium-chain triglycerides, long-chain triglycerides, or 2-monododecanoin on fatty acid composition in the portal vein, intestinal lymph, and systemic circulation in rats. Journal of Parenteral and Enteral Nutrition. 2008;32(2):169–175. doi: 10.1177/0148607108314758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babayan V.K. Medium chain triglycerides and structured lipids. Lipids. 1987;22(6):417–420. doi: 10.1007/BF02537271. [DOI] [PubMed] [Google Scholar]
- 12.Jadhav H., Annapure U. Studies on Chemical esterification process for synthesis of medium chain triglyceride using Amberlyst and Ultrasonic pre-treatment. Science and Engineering Journal. 2020;24:255–267. [Google Scholar]
- 13.Jennings B.H., Akoh C.C. Characterization of a rice bran oil structured lipid. J. Agric. Food. Chem. 2009;57(8):3346–3350. doi: 10.1021/jf803825m. [DOI] [PubMed] [Google Scholar]
- 14.Jadhav H., Annapure U., Waghmare J. Study on Oxidative Stability of deep fat fried food in Canola oil blended with Medium Chain Triglyceride. Science and Engineering Journal. 2020;24:304–313. [Google Scholar]
- 15.Derawi D., Abdullah B.M., Zaman Huri H., Yusop R.M., Salimon J., Hairunisa N., Salih N. Palm olein as renewable raw materials for industrial and pharmaceutical products applications: Chemical characterization and physicochemical properties studies. Adv. Mater. Sci. Eng. 2014;2014:1–5. doi: 10.1155/2014/134063. [DOI] [Google Scholar]
- 16.Absalome M.Aké., Massara C.-C., Alexandre A.Aké., Gervais K., Chantal G.-A., Ferdinand D., Rhedoor A.J., Coulibaly I., George T.G., Brigitte T., Marion M., Jean-Paul C. Biochemical properties, nutritional values, health benefits and sustainability of palm oil. Biochimie. 2020;178:81–95. doi: 10.1016/j.biochi.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Chatel G., Colmenares J.C. Sonochemistry: from Basic Principles to Innovative Applications. Top. Curr. Chem. 2017;375:1–4. doi: 10.1007/s41061-016-0096-1. [DOI] [PubMed] [Google Scholar]
- 18.More S.B., Gogate P.R., Waghmare J.S., Naik S.N. Intensified synthesis of structured triacylglycerols from fish, flaxseed and rice bran oil using supercritical CO2 or ultrasound. Chemical Engineering and Processing - Process Intensification. 2019;144:107650. doi: 10.1016/j.cep.2019.107650. [DOI] [Google Scholar]
- 19.Deshmane V.G., Gogate P.R., Pandit A.B. Process intensification of synthesis process for medium chain glycerides using cavitation. Chem. Eng. J. 2008;145(2):351–354. doi: 10.1016/j.cej.2008.08.012. [DOI] [Google Scholar]
- 20.Mohod A.V., Gogate P.R. Intensified synthesis of medium chain triglycerides using ultrasonic reactors at a capacity of 4L. Ultrason. Sonochem. 2018;42:347–355. doi: 10.1016/j.ultsonch.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 21.More S.B., Gogate P.R., Waghmare J.S. Intensification of acid catalyzed synthesis of tricaprylin using ultrasound pretreatment. Chem. Eng. Process. Process Intensif. 2017;120:317–329. doi: 10.1016/j.cep.2017.07.027. [DOI] [Google Scholar]
- 22.Mancini A., Imperlini E., Nigro E., Montagnese C., Daniele A., Orrù S., Buono P. Biological and nutritional properties of palm oil and palmitic acid: Effects on health. Molecules. 2015;20:17339–17361. doi: 10.3390/molecules200917339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rupani B. Enrichment of Olive Oil with Alpha Linolenic Acid Catalyzed by Lipase Mediated Trans-Esterification. Iranica Journal of Energy and Environment. 2014;5:18–25. doi: 10.5829/idosi.ijee.2014.05.01.04. [DOI] [Google Scholar]
- 24.SilRoy S., Ghosh M. Enzymatic Synthesis of Capric Acid-Rich Structured Lipids (MUM type) Using Candida antarctica Lipase. Journal of Oleo Sciences. 2011;60(6):275–280. doi: 10.5650/jos.60.275. [DOI] [PubMed] [Google Scholar]
- 25.Shieh C.-J., Akoh C.C., Koehler P.E. Four-Factor Response Surface Optimization of the Enzymatic Modification of Triolein to Structured Lipids. Journal of American Oil Chemist Society. 1995;72(6):619–623. [Google Scholar]
- 26.Zou X., Jin Q., Guo Z., Huang J., Xu X., Wang X. Preparation of 1, 3-dioleoyl-2-palmitoylglycerol-rich structured lipids from basa cat fi sh oil : Combination of fractionation and enzymatic acidolysis. Eur. J. Lipid Sci. Technol. 2016;118:708–715. doi: 10.1002/ejlt.201500226. [DOI] [Google Scholar]
- 27.Cao Y., Qi S., Zhang Y., Wang X., Yang B., Wang Y. Synthesis of Structured Lipids by Lipase-Catalyzed Interesterification of Triacetin with Camellia Oil Methyl Esters and Preliminary Evaluation of their Plasma Lipid-Lowering Effect in Mice. Journal of Molecules. 2013;18:3733–3744. doi: 10.3390/molecules18043733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim I.-H., Ko S.-N., Lee S.-M., Chung S.-H., Kim H., Lee K.-T., Ha T.-Y. Production of structured lipids by lipase-catalyzed acidolysis in supercritical carbon dioxide: Effect on acyl migration. JAOCS, Journal of the American Oil Chemists’ Society. 2004;81(6):537–541. doi: 10.1007/s11746-006-0937-0. [DOI] [Google Scholar]
- 29.Ab K. for the Production of Margarine Fats in a 1 Kg Scale Stirred Tank Reactor. Eur. J. Lipid Sci. Technol. 2000;102:411–418. [Google Scholar]
- 30.Khan N.R., Gawas S.D., Rathod V.K. Enzyme-catalysed production of n-butyl palmitate using ultrasound-assisted esterification of palmitic acid in a solvent-free system. Bioprocess Biosyst. Eng. 2018;41(11):1621–1634. doi: 10.1007/s00449-018-1988-y. [DOI] [PubMed] [Google Scholar]
- 31.Liu S.L., Dong X.Y., Wei F., Wang X., Lv X., Zhong J., Wu L., Quek S.Y., Chen H. Ultrasonic pretreatment in lipase-catalyzed synthesis of structured lipids with high 1,3-dioleoyl-2-palmitoylglycerol content. Ultrason. Sonochem. 2015;23:100–108. doi: 10.1016/j.ultsonch.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 32.More S.B., Waghmare J.T., Gogate P.R. Ultrasound pretreatment as a novel approach for intensification of lipase catalyzed esterification of tricaprylin. Ultrason. Sonochem. 2017;36:253–261. doi: 10.1016/j.ultsonch.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 33.More S., Gogate P., Waghmare J., Naik S.N. Intensified synthesis of structured lipids from oleic acid rich moringa oil in the presence of supercritical CO2. Food Bioprod. Process. 2018;112:86–95. doi: 10.1016/j.fbp.2018.09.004. [DOI] [Google Scholar]