Highlights
-
•
Medullary autonomic dysfunction may contribute to the cascade leading to SUDEP.
-
•
Polyglucosan bodies (PGB) identified primarily in the medullary catecholaminergic neurones has not previously been reported.
-
•
Deposition of PGB in the medulla could compromise brainstem function in the agonal peri-ictal period.
Abbreviations: MR, Median raphe; PGB, polyglucosan bodies; SUDEP, Sudden unexpected death in epilepsy; TH, Tyrosine hydroxylase; TPH, Tryptophan hydroxylase; VLM, Ventrolateral medulla
Keywords: Polyglucosan bodies, Medulla, SUDEP
Abstract
Polyglucosan bodies have been reported in the context of hypoxic-ischaemic perinatal brain injury, mainly in the pallidum but with rare reports in brainstem neurons. We report a case of a five-year-old boy with cerebral palsy and complex neurological features including epilepsy who experienced sudden nocturnal death. At post-mortem long-standing bilateral necrosis of basal ganglia and hippocampal atrophy was identified in keeping with hypoxic-ischaemic perinatal injury. In addition numerous polyglucosan bodies, which were PAS, p62 and ubiquitin positive, were noted in brainstem neurones and dendrites, primarily involving the ventrolateral and dorsomedial medulla. Immunohistochemistry confirmed relative preservation of medullary neuronal populations in the reticular formation, including catecholaminergic (tyrosine hydroxylase, TH), serotonergic (tryptophan hydroxylase) and neurokinin1 receptor/somatostatin positive neurones. The polyglucosan bodies predominated in catecholaminergic neurones which could indicate their selective vulnerability and a functional deficiency, which during a critical peri-ictal period contributed to the sudden unexpected death in epilepsy.
1. Introduction
The cause of sudden unexpected death in epilepsy (SUDEP) is mechanistically complex but one probable cause is seizure-related medullary dysfunction. Our previous post mortem studies have shown pathological alteration of specific autonomic modulatory neurones and glia in the medulla [1], [2], [3]. In the present case we report a young child with severe epilepsy following perinatal hypoxic-ischaemic injury and a sudden death in which numerous medullary polyglucosan bodies were identified at post-mortem examination. These findings resided mainly in catecholaminergic neurones. We discuss the possible functional significance of these findings.
2. Case report
Post-mortem neuropathological examination was carried out in a five-year-old male with dystonic cerebral palsy, severe developmental delay and drug-resistant epilepsy. He had been delivered by emergency caesarean following birth impaction and suffered hypoxia; he had a seizure immediately following delivery. He was diagnosed with hypoxic-ischemic encephalopathy with dystonic cerebral palsy with dyskinetic movements, dystonia and choreoathetosis and blindness. His seizures included clusters of myoclonic jerks every few minutes, tonic-clonic seizures and atypical absence episodes and had several seizure clusters a day. MRI showed abnormal signal intensity in the basal ganglia, thalamus, cortex and brainstem. Video telemetry at age 4 showed multifocal epileptiform activity with a prominent continuous source over the left frontal region. Medication at the time of death included, midazolam, melatonin, esomeprazole, clonidine, gabapentin, clobazam, chloral hydrate, topiramate, baclofen and levetiracetam. He was having a period of respite care at a hospice and had been generally well apart from a cold and mild rash noted in the previous week. He was regularly checked during the night but was found unresponsive one morning in bed and subsequent resuscitation attempts failed. A witnessed seizure immediately prior to death was not reported and there was limited information at the time of post-mortem regarding any worsening control. A full post-mortem examination did not disclose an anatomical or structural cause of death, including detailed examination of the heart and respiratory tract. Microbiology identified haemophilus influenza and Moraxella catarrhalis from the upper respiratory tract but CSF was negative following culture.
The brain was small at 1099 g (normal range for age 1292–1435 g) and the hind brain weighed 152 g and was fixed for detailed neuropathological examination with immunohistochemistry (Table 1). The meninges were translucent and the gyral pattern was normal and histological examination of the frontal, temporal and occipital cortex did not reveal meningoencephalitis and showed a normal and preserved cortical architecture with mild autolytic changes only. Hippocampi were normally rotated but showed bilateral atrophy, with neuronal loss and gliosis involving the dentate gyrus, CA1 to CA4 subfields and subiculum but no mossy fibre sprouting was shown on zinc transporter-3 immunohistochemistry. There was bilateral cystic cavitation and chronic gliosis of both putamen with focal dystrophic calcification with the caudate and globus pallidus being relatively better preserved. Focal cerebellar vermis atrophy with Purkinje cell loss and gliosis was also present but the dentate nucleus and hemispheres appeared normal.
Table 1.
Immunohistochemistry panel and antibodies used in this study.
| Immunohistochemistry marker | Clone | Dilution |
|---|---|---|
| p62* | P62-BD Transduction: 3/P62LCK Ligand | 1:100 |
| Somatostatin (SST) | Rb H-106, Santacruz Biotechnology | 1:500 |
| Neurokinin1 receptor (NK1R) | S8305, Sigma Aldrich | 1:5000 |
| Tyrosine hydroxylase (TH) | Abcam ab112 | 1: 750 |
| Tryptophan hydroxylase (TPH2) | AB121013, Abcam | 1: 500 |
| Ubiquitin* | Santa Cruz: p4D1 | 1:1200 |
| Zinc transporter 3* | Synaptic Systems: polyclonal | 1:1000 |
*These antibodies were stained using Bond-Max. (Leica Biosystems).
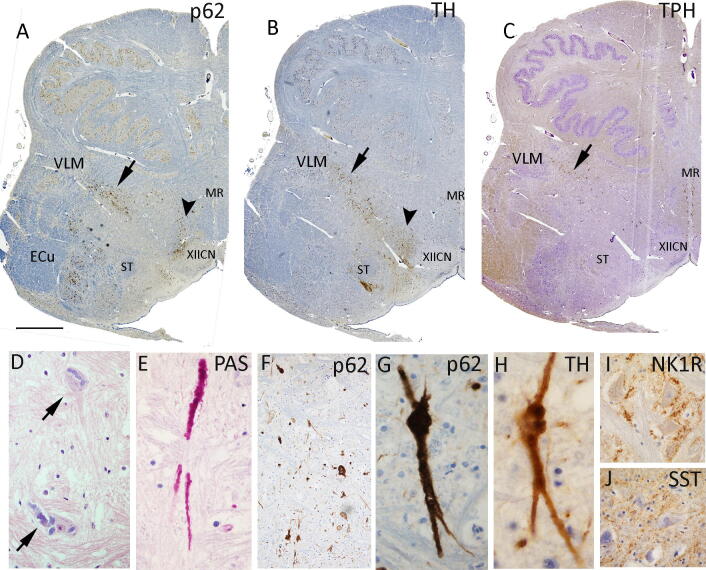
In the brainstem, anatomical structures appeared preserved, however numerous irregular and variable basophilic intraneuronal cytoplasmic inclusions were noted, PAS, ubiquitin and strongly p62 positive, distending the soma and extending into neuronal processes in keeping with polyglucosan bodies (PGB) (Fig. 1). These were more widespread in the medulla and prominent in the ventrolateral medulla extending through the intermediate reticular zone and in the dorsomedial region, with scattered inclusions in nucleus of the solitary tract and more numerous in the external cuneate nucleus. PAS-positive axonal like processes were prominent near the XII cranial nerve nucleus (CNN) but no intraneuronal inclusions were seen in the XIIth or Xth CNN, olivary, arcuate or raphe nuclei. The distribution of p62 inclusions correlated with the distribution of Tyrosine hydroxylase (TH) positive medullary catecholaminergic neurones (Fig. 1a,b) and similar irregular inclusions were frequently seen in this cell type (Fig. 1h) but were not clearly identified in Tryptophan hydroxylase (TPH2) serotonergic neurones. Neurokinin 1 receptor NK1R and somatostatin (SST) highlighted neurones in the ventrolateral medulla but also with no clear inclusions (Fig. 1i,j). There was no evidence of medullary gliosis or microgliosis. In the pons (examined at obex level 20 mm) similar PGB inclusions were noted in the Vth CNN and in the midbrain, in the inferior colliculus nuclei and occasionally in the dorsal raphe region but not in the Substantia Nigra, which appeared well preserved. Inclusions were not identified in basal ganglia, hippocampus, amygdala, cerebellum or any cortical region.
Fig. 1.
Neuropathology findings in the medulla (examined at obex 6 mm) A. p62 at low power demonstrates the distribution of neuronal inclusions in specific regions of the ventrolateral medulla (VLM, arrow) and in the dorsomedial medulla (arrowhead) in the vicinity of the XIIth cranial nerve nucleus, mainly in axons and process here. In addition, numerous inclusions were present in the external cuneate nucleus (ECu) and solitary tract (ST) nuclei. There were not identified in the median raphe (MR). B. Tyrosine hydroxylase (TH) labelling confirmed preservation and normal distribution of neurones in the VLM, dorsomedial medulla and around the solitary tract. C. Tryptophan hydroxylase (TPH) confirms neurones of normal morphology and distribution mainly in the MR and VLM. D-J. All represent high magnification images of the VLM region. D. H&E revealed numerous basophilic, irregular cytoplasmic inclusions in neuronal perikarya and dendrites. E. These irregular, globular inclusions, distending process were PAS positive in keeping with polyglucosan bodies. A high-resolution version of this slide for use with the Virtual Microscope is available as eSlide: VM06202 F. p62 labelling at lower magnification showed numerous positive structures in the VLM neurones and G. at higher magnification with structures of similar morphology to the PAS stain. H. Inclusions of similar morphology were also evident in many TH labelled neurones but not in TPH2 neurones or in pre-Botzinger neurones, characterised by I. NK1R and J. SST peripheral labelling. Bar approximately equivalent in A, B and C to 2 mm in F to 25 microns and D-J to 50 microns.
3. Discussion
Bilateral cystic gliosis of the basal ganglia and hippocampal atrophy is in keeping with perinatal hypoxic-ischaemic damage [4] resulting in the complex neurology and epilepsy in this case; the lack of a clear cause of death identified at autopsy is in keeping with a sudden and unexpected death in epilepsy (SUDEP). SUDEP deaths mainly occur nocturnally, as in this case [5] and are considered to be as frequent in childhood as adulthood, particularly in complex epilepsies [6]. Although the precise mechanisms of death are unknown, recent functional and neuroimaging studies [7] and experimental evidence [8] implicate autonomic medullary dysfunction in the peri-ictal period, even if a seizure is unwitnessed, as a likely final event. In this present case, the recent upper-respiratory tract viral infection may also have contributed to respiratory compromise and recovery following a seizure.
This pattern of basal ganglia hypoxic neonatal injury is often accompanied by extensive destruction of the brainstem reticular formation [4]. However, in this case neuronal subtypes in the medullary reticular formation appeared largely preserved, verified by immunohistochemistry, but neuronal PGB inclusions were noted with a similar anatomical distribution. PGBs, which have also historically been termed ‘Bielchowsky bodies’, differ in shape, cellular and regional distribution to Lafora disease (associated with progressive myoclonic epilepsy) [9] corpora amylacea (present in astroglia) [10] glycogen storage diseases and other rarer adult polyglucosan disorders [11], [12]. PGBs have been recognised in the context of long-standing cerebral palsy due to hypoxic-ischaemic perinatal injury, but usually involving the pallidum with rare reports of PGBs occurring in the brainstem [13] including one case report of a patient with drug-resistant epilepsy and a nocturnal sudden death [14]. The current case is however unique for the restricted location of the PGBs to the brainstem and prominent involvement of medullary catecholaminergic neurons.
PGB are sequestered aggregates of ubiquitinated and p62 enriched insoluble glucose polymers, with recent studies favouring they represent a cellular protective or clearance mechanism, activated by autophagy pathways in response to a pathogenic insult [9]; the pathways for any ongoing cellular toxicity is unclear. Interestingly, a predilection for p62 inclusions in the dendrites of brainstem catecholaminergic neurones has been noted with aging, suggesting an intrinsic vulnerability of these neurones to cellular metabolic stresses [15]. In previous post-mortem studies of the medulla in SUDEP we have shown alteration in VLM SST/NK1R neurones regionally aligning with the pre-Botzinger respiratory nucleus and its modulating serotonergic (TPH2) neurones in the raphe [1]. In addition we have reported reduced specialised medullary astroglial [2] and reduced volume in the rostral reticular formation [16]; these findings all support pre-existing acquired brainstem pathology as a risk factor for SUDEP. The VLM catecholaminergic neurones, critical to arterial BP regulation, also orchestrate a range of physiological stress responses, including breathing stimulation in hypoxia [17] and cardiorespiratory arousal during sleep [18]. TH-expressing catecholaminergic neurones represent some of the earliest brainstem neurones [19], [20] and a maturational delay has been shown in SIDS [21]. In a recent study however, we reported preservation of TH neurones in a mainly adult SUDEP series [3] but an abnormal distribution which could indicate an altered network or connectivity. Comparing to the current case, although not quantified, the TH neurones also appeared preserved. The accumulation of medullary PGBs likely reflect longstanding neuronal injury following the initial brain insult; we speculate that they may signify ongoing cellular dysfunction that has contributed to deficient cardio-respiratory regulatory responses during a critical nocturnal post-ictal period and leading to SUDEP.
Further investigation of the specific neuronal dysfunction in medullary cells harbouring PGB could provide mechanistic insights into SUDEP.
4. Conclusions
Brainstem PGBs are not commonly reported and in this case, their primary identification in medullary catecholaminergic neurons in a child with severe epilepsy and sudden death may indicate functional cellular impairment and be relevant to our understanding of SUDEP.
Ethical statement
SUDEP research is conducted under the NRES ethical approval of the UCL Epilepsy Society Brain and Tissue Bank and in this case report, specific consent for research was obtained from the next of kin.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to Irene Scheimberg, Perinatal and Paediatric pathology department, Royal London Hospital Trusts, Whitechapel Rd, Whitechapel, London E1 1FR. We are grateful for the assistance of Andrew Theodoulou in the neuropathology department for his assistance with the neuropathology techniques. UCL is part of the Center for SUDEP Research (CSR) and supported through the National Institute of Neurological Disorders And Stroke of the National Institutes of Health (Award Numbers neuropathology of SUDEP: 5U01NS090415 and SUDEP admin core grant: U01-NS090405). Epilepsy Society supports SMS, and through the Katy Baggott Foundation, supports the UCL Epilepsy Society Brain and Tissue Bank. This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme.
References
- 1.Patodia S, Somani A, O'Hare M, Venkateswaran R, Liu J, Michalak Z, et al. The ventrolateral medulla and medullary raphe in sudden unexpected death in epilepsy. Brain. 2018;141(6):1719-33. [DOI] [PMC free article] [PubMed]
- 2.Patodia S., Paradiso B., Ellis M., Somani A., Sisodiya S.M., Devinsky O., Thom M. Characterisation of medullary astrocytic populations in respiratory nuclei and alterations in sudden unexpected death in epilepsy. Epilepsy Res. 2019;157:106213. doi: 10.1016/j.eplepsyres.2019.106213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patodia S., Tan I., Ellis M., Somani A., Scheffer I.E., Sisodiya S.M., Thom M. Medullary tyrosine hydroxylase catecholaminergic neuronal populations in sudden unexpected death in epilepsy. Brain Pathol. 2021;31(1):133–143. doi: 10.1111/bpa.12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellison DP. Neuropathology: a reference text of CNS pathology. 3rd ed. / David Ellison ... [et al.]. ed. Edinburgh: Mosby/Elsevier; 2013.
- 5.Devinsky O., Hesdorffer D.C., Thurman D.J., Lhatoo S., Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. 2016;15(10):1075–1088. doi: 10.1016/S1474-4422(16)30158-2. [DOI] [PubMed] [Google Scholar]
- 6.Abdel‐Mannan O., Sutcliffe A.G. A national surveillance study of childhood epilepsy mortality in the UK and Ireland. Eur J Neurol. 2020;27(2):327–333. doi: 10.1111/ene.14081. [DOI] [PubMed] [Google Scholar]
- 7.Allen L.A., Harper R.M., Lhatoo S., Lemieux L., Diehl B. Neuroimaging of Sudden Unexpected Death in Epilepsy (SUDEP): insights from structural and resting-state functional MRI studies. Front Neurol. 2019;10:185. doi: 10.3389/fneur.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noebels Jl Md P. Brainstem spreading depolarization: rapid descent into the shadow of SUDEP. Brain. 2019;142(2):231-3. [DOI] [PMC free article] [PubMed]
- 9.Brewer M.K., Putaux J.-L., Rondon A., Uittenbogaard A., Sullivan M.A., Gentry M.S. Polyglucosan body structure in Lafora disease. Carbohydr Polym. 2020;240:116260. doi: 10.1016/j.carbpol.2020.116260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riba M., Augé E., Campo-Sabariz J., Moral-Anter D., Molina-Porcel L., Ximelis T., Ferrer R., Martín-Venegas R., Pelegrí C., Vilaplana J. Corpora amylacea act as containers that remove waste products from the brain. Proc Natl Acad Sci USA. 2019;116(51):26038–26048. doi: 10.1073/pnas.1913741116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellmann M.A., Kakhlon O.r., Landau E.H., Sadeh M., Giladi N., Schlesinger I. Frequent misdiagnosis of adult polyglucosan body disease. J Neurol. 2015;262(10):2346–2351. doi: 10.1007/s00415-015-7859-4. [DOI] [PubMed] [Google Scholar]
- 12.Sugiyama H, Hainfellner JA, Lassmann H, Indravasu S, Budka H. Uncommon types of polyglucosan bodies in the human brain: distribution and relation to disease. Acta neuropathologica. 1993;86(5):484-90. [DOI] [PubMed]
- 13.Yagishita S., Itoh Y., Nakano T., Amano N., Yokoi S., Hasegawa O., Tanaka T. Pleomorphic intra-neuronal polyglucosan bodies mainly restricted to the pallidium: a case report. Acta Neuropathol. 1983;62(1-2):159–163. doi: 10.1007/BF00684936. [DOI] [PubMed] [Google Scholar]
- 14.Wilson J.D., Horoupian D.S. Bielschowsky bodies (Lafora bodies of Bielschowsky type): report of a case associated with Rosenthal fibers in the brain stem. Acta Neuropathol. 2001;102(5):505–509. doi: 10.1007/s004010100400. [DOI] [PubMed] [Google Scholar]
- 15.Braak H., Thal D.R., Matschke J., Ghebremedhin E., Del Tredici K. Age-related appearance of dendritic inclusions in catecholaminergic brainstem neurons. Neurobiol Aging. 2013;34(1):286–297. doi: 10.1016/j.neurobiolaging.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Patodia S., Tachrount M., Somani A., Scheffer I., Yousry T., Golay X. MRI and pathology correlations in the medulla in sudden unexpected death in epilepsy (SUDEP): a postmortem study. Neuropathol Appl Neurobiol. 2021;47(1):157–170. doi: 10.1111/nan.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyenet P.G., Stornetta R.L., Bochorishvili G., DePuy S.D., Burke P.G.R., Abbott S.B.G. C1 neurons: the body's EMTs. Am J Physiol-Regul Integr Comparative Physiol. 2013;305(3):R187–R204. doi: 10.1152/ajpregu.00054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbott S.B.G., Coates M.B., Stornetta R.L., Guyenet P.G. Optogenetic stimulation of C1 and retrotrapezoid nucleus neurons causes sleep state–dependent cardiorespiratory stimulation and arousal in rats. Hypertension. 2013;61(4):835–841. doi: 10.1161/HYPERTENSIONAHA.111.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verney C. Distribution of the catecholaminergic neurons in the central nervous system of human embryos and fetuses. Microsc. Res. Tech. 1999;46(1):24–47. doi: 10.1002/(SICI)1097-0029(19990701)46:1<24::AID-JEMT3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Lorke D.E., Hang Kwong W., Yee Chan W., Yew D.T. Development of catecholaminergic neurons in the human medulla oblongata. Life Sci. 2003;73(10):1315–1331. doi: 10.1016/s0024-3205(03)00430-2. [DOI] [PubMed] [Google Scholar]
- 21.Machaalani R., Waters K.A. Neurochemical abnormalities in the brainstem of the Sudden Infant Death Syndrome (SIDS) Paediatr Respir Rev. 2014;15(4):293–300. doi: 10.1016/j.prrv.2014.09.008. [DOI] [PubMed] [Google Scholar]