Abstract
SARS‐CoV‐2, the virus responsible for the global coronavirus disease (COVID‐19) pandemic, attacks multiple organs of the human body by binding to angiotensin‐converting enzyme 2 (ACE2) to enter cells. More than 20 million people have already been infected by the virus. ACE2 is not only a functional receptor of COVID‐19 but also an important endogenous antagonist of the renin‐angiotensin system (RAS). A large number of studies have shown that ACE2 can reverse myocardial injury in various cardiovascular diseases (CVDs) as well as is exert anti‐inflammatory, antioxidant, anti‐apoptotic and anticardiomyocyte fibrosis effects by regulating transforming growth factor beta, mitogen‐activated protein kinases, calcium ions in cells and other major pathways. The ACE2/angiotensin‐(1‐7)/Mas receptor axis plays a decisive role in the cardiovascular system to combat the negative effects of the ACE/angiotensin II/angiotensin II type 1 receptor axis. However, the underlying mechanism of ACE2 in cardiac protection remains unclear. Some approaches for enhancing ACE2 expression in CVDs have been suggested, which may provide targets for the development of novel clinical therapies. In this review, we aimed to identify and summarize the role of ACE2 in CVDs.
Keywords: ACE2, cardiovascular diseases, RAS, SARS‐CoV‐2
1. INTRODUCTION
In December 2019, a novel coronavirus‐induced pneumonia called novel coronavirus disease 2019 (COVID‐19) first appeared in Wuhan, China. Due to the spread of COVID‐19, more than 200 countries around the world have been impacted. As of the end of September 2020, there have been more than 20 million confirmed cases of COVID‐19, and over 900,000 patients have died from the disease. 1 Based on epidemiological analyses, COVID‐19 patients suffer from severe multiple organ injuries, including the lungs, kidneys and liver, which are also closely related to adverse outcome in cardiovascular diseases (CVDs). 2 , 3 , 4 , 5 Due to the high homology between severe acute respiratory syndrome coronavirus (SARS‐CoV) and severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), several studies have demonstrated the mechanism by which these viruses invade the human body. SARS‐CoV‐2 invades cells by relying on the spike protein of its surface, which is similar to SARS‐CoV. The S1 subunit of the spike protein can bind to angiotensin‐converting enzyme 2 (ACE2) of the cell membrane and form a complex, which allows the virus to enter the cell by endocytosis and thereby induce cellular damage (Figure 1). 6 , 7 Because of the distribution and the function of ACE2, it must play a decisive role in COVID‐19 patients with multiple organ damage.
FIGURE 1.
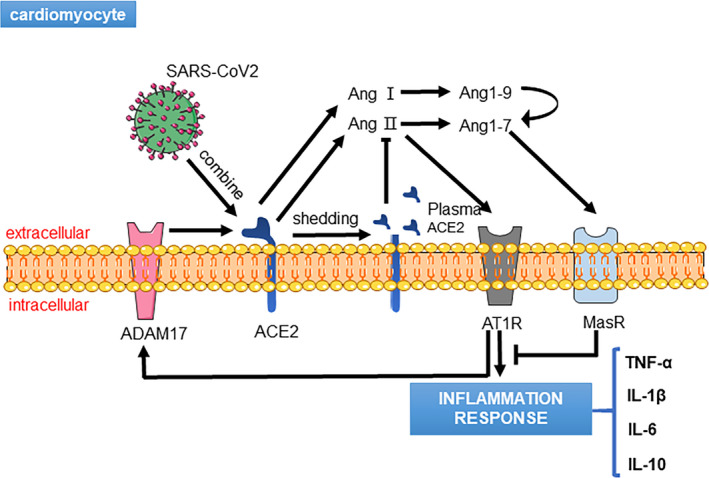
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) binds to angiotensin‐converting enzyme 2 (ACE2) and induces ACE2 shedding to produce soluble ACE2 in serum, which triggers an angiotensin II (Ang II)‐mediated inflammation response
ACE2 was first discovered in 2000 by Donoghue and Tipnis. 8 , 9 The human ACE2 gene is 40 kb long, located on chromosome Xp22 and composed of 18 exons. 8 , 9 The protein of human ACE2, which is a type I integral membrane glycoprotein with an N‐terminal signal peptide region, a hydrophobic region near the C terminus and a single active site catalytic region, is approximately 120 kD and consists of 805 amino acids. 9 ACE2 has 42% homology with the amino acid sequence of ACE and is widely expressed in the body, including the heart, vasculature, kidneys, testes, gastrointestinal tract, brain and lungs. 10 , 11 , 12 Moreover, ACE2 is not only a plasma membrane‐bound ectoenzyme; its soluble active form exists in plasma and urine, and it can be detected via routine blood tests. 13
In the heart, ACE2 is mainly expressed in cardiomyocytes, cardiac fibroblasts and coronary artery endothelial cells and also serves as an important endogenous antagonist of the renin‐angiotensin system (RAS), which mainly converts angiotensin II (Ang II) to angiotensin‐(1‐7) [Ang‐(1‐7)] and metabolizes angiotensin I (Ang I) to generate angiotensin‐(1‐9) [Ang‐(1‐9)]. Ang‐(1‐7) and Ang‐(1‐9) have been proven to have significant beneficial effects on the cardiovascular system. 9 , 14 Ang‐(1‐7) generally binds to the Mas receptor (MasR), a G protein‐coupled receptor discovered in 1986, and further regulates downstream molecular pathways, including the mitogen‐activated protein kinase (MAPK), protein kinase B (AKT) and oxidative stress‐related pathways. 15 , 16 , 17 , 18 ACE2 restrains Ang II accumulation and down‐regulates the angiotensin II type 1 receptor (AT1R), such that the ACE2/Ang‐(1‐7)/MasR axis has the opposite effect of the ACE/Ang II/AT1R axis and promotes anticardiovascular remodelling and mediate vasodilation. 19 In addition, angiotensin II type 2 receptor (AT2R), one of the core receptors of the angiotensin family, is also activated by Ang‐(1‐7). 20 ACE2/Ang‐(1‐7)/AT2R axis has the same beneficial effects as ACE2/Ang‐(1‐7)/MasR axis; however, the researches about the axis are limited. 21
Given the existing knowledge of ACE2 distribution and function in the heart, we hypothesized that ACE2 plays a crucial role in the progression of SARS‐CoV‐2‐induced myocardial injury. The present review aims to identify and summarize the functions and mechanisms of ACE2 in clinical and animal models of major CVDs.
2. COVID‐19, ACE2 AND CVDS
Due to the high expression of ACE2 in the heart and the mechanism of SARS‐CoV‐2 transfection, we speculate that the poor prognosis of COVID‐19 patients may be correlated with heart injury. At present, there are two major hypotheses about SARS‐CoV‐2‐mediated damage to the heart. On the one hand, SARS‐CoV‐2 directly causes heart injury in patients without any CVDs; on the other hand, SARS‐CoV‐2 can also contribute to the negative prognoses of patients with heart diseases. 22 , 23 In addition, some severe COVID‐19 patients display higher levels of cardiac troponin I (cTnI), N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and creatine kinase‐MB (CK‐MB), which collectively demonstrates that SARS‐CoV‐2 aggravates heart injury. 24 , 25 , 26 , 27 A meta‐analysis of cTnI in COVID‐19 patients including four studies exhibited that the standardized mean difference (SMD) of cTnI was 25.6 ng/L. 26 In addition, a multi‐centre cohort study involving 191 patients infected with SARS‐CoV‐2 showed that of the 54 deceased patients, 48% had hypertension, 24% had coronary heart disease, and approximately 23% had heart failure (HF). 28 Based on the clinical phenomena of ACE2 and heart injury, several studies have suggested that SARS‐CoV‐2 binds to ACE2 to enter cells and increases the expression levels of disintegrin and metalloproteinase 17 (ADAM17), which, in turn, induce ACE2 shedding. Thus, the function of ACE2 is compromised and simultaneously accompanied by increasing ACE and Ang II expression levels. A higher level of Ang II not only has severe negative effects on the heart, but also increases cytokine release, including interleukin (IL)‐6 and IL‐7, which activate the MAPK pathway and thereby increase ADAM17 expression to form a positive feedback loop. Thus, ACE2 appears to be a major regulating factor in COVID‐19 patients. 29 , 30 , 31 In recent years, an increasing number of studies have confirmed that neither ACE inhibitors (ACEIs) nor Ang II receptor blockers (ARBs) are associated with high mortality, which suggests that ACEIs and ARBs cannot exacerbate the prognoses of COVID‐19 patients. Meanwhile, there has been no evidence to support that evaluating ACE2 expression can improve heart injury during SARS‐CoV‐2 infection. 32 , 33 , 34 In addition, although some drugs, like remdesivir, lopinavir and dexamethasone, have been identified to improve COVID‐19 patients’ symptoms and attenuate inflammatory response, there are no specific drugs invented to treat cardiovascular injury caused by SARS‐CoV‐2. 35 , 36 , 37
Based on the aforementioned information, we believe that the most powerful approach of addressing SARS‐CoV‐2‐induced heart injury is to clarify the upstream and downstream molecular pathways of ACE2 in CVDs and find effective strategies to protect the heart.
3. ACE2 AND MYOCARDIAL INFARCTION (MI)
MI has a high incidence among patients with CVDs, and in the United States, nearly 0.8 million people are reported to suffer from MI each year. 38 In recent years, multiple studies have shown that the RAS, especially ACE2, is involved in MI‐induced myocardial remodelling. 39 In the early stage of MI, both ACE2 and ACE levels are remarkably increased in the heart, whereas in the late stage, ACE2 expression declines and is accompanied by HF, indicating the role of ACE2 against the RAS. 40 , 41 Similarly, clinical research has found that the serum ACE2 level of MI patients is significantly higher than that of healthy individuals and results in a negative prognosis. 42 , 43 Further, the results indicate that the serum level of ACE2 may be a candidate for identifying the degree of myocardial injury. Inflammatory infiltration and myocardial fibrosis are the two major factors that induce cardiac structure remodelling during MI. Following developments in basic research, ACE2 is now believed to be resistant to the negative effect of ACE on myocardial remodelling post‐MI mainly depending on the ACE2/Ang‐(1‐7)/MasR axis. In MI, loss of the ACE2 gene leads to ventricular remodelling, increased myocardial fibrosis, neutrophilic infiltration and superoxide production via up‐regulation of matrix metalloproteinase (MMP) 2, MMP9, interferon‐γ, IL‐6 and chemokines as well as regulation of phosphorylation of the extracellular regulated protein kinase (ERK) 1/2 and c‐Jun N‐terminal kinase (JNK) 1/2 signalling pathways. 44 In contrast, overexpression of ACE2 can reverse collagen deposition by inhibiting the transforming growth factor‐β (TGF‐β) pathway with decreasing collagen Ⅰ and Ⅲ, which also reduces the expression levels of inflammation‐related factors ACE and Ang Ⅱ. 45 , 46 , 47 Diminazene (DIZE), an activator of ACE2, is also used in some studies to improve myocardial function following MI. Chen et al illustrated that DIZE attenuated MI in rats via a novel signalling pathway mediated by ACE2/AT1R/MasR. 48 Given these findings, ACE2 may also be a therapeutic target for MI; however, as no effective drugs can adequately evaluate ACE2 expression in the heart, the concrete molecular mechanisms underlying ACE2 actions need to be further investigated.
4. ACE2 AND HYPERTENSION
Hypertension is one of the most common CVDs that is characterized by vascular remodelling and endothelial injury and leads to severe prognosis. RAS is a participant in the development of the disease. 49 It is known that the ACE/Ang Ⅱ/AT1R axis regulates the constriction of blood vessels, and most available drugs aim to block this pathway. The ACE2/Ang‐(1‐7)/MasR axis is a potential target for combating the negative effects of the ACE/Ang Ⅱ/AT1R axis on hypertension, and ACE2 plays a key role in this axis. ACE2 regulates blood pressure under physiological conditions, and down‐regulation of ACE2 gene leads to significant increase in the blood pressure along with an excess accumulation of Ang Ⅱ. 50 In addition, ACE2 is significantly decreased in spontaneously hypertensive rat (SHR) or Ang Ⅱ‐induced hypertension models. 51 , 52 Interestingly, ACE2 and Ang Ⅱ can regulate each other to maintain a balance. Ang Ⅱ up‐regulates AT1R and increases ADAM17 expression, which causes ACE2 shedding and diminishes the protective impact of ACE2 in hypertension whereas Ang Ⅱ can be converted into Ang‐(1‐7) by ACE2 to inhibit its own negative effect. 53 , 54 Rentzsch et al found that Ang‐(1‐7), which directly regulates blood pressure and improves endothelial function via activating the ACE2 gene, is the major downstream regulator of ACE2. 55 However, aside from Ang‐(1‐7), the downstream mechanism of ACE2 in hypertension remains unclear. Several studies have determined that overexpression of ACE2 in hypertension increases AT2R and MasR expression and inhibits AT1R expression. 56 , 57 Furthermore, they evaluated nitric oxide (NO) release and reported down‐regulation of inflammation‐related pathways mediated by IL‐1 b, IL‐6, TNF‐a and NF‐kB. 56 , 57 Activation and modification of ACE2 are essential for development of hypertension. DIZE and fibroblast growth factor 21 (FGF21) can up‐regulate ACE2 expression to improve pulmonary hypertension and decrease inflammation‐induced endothelial cell injury. 58 , 59 Zhang et al found that AMP‐activated protein kinase (AMPK) phosphorylated ACE2 Ser680 in endothelial cells can enhance the function of ACE2 depending on regulation of Ang‐(1‐7) and nitric oxide synthase. 60 From the aforementioned information, it is clear that there is limited knowledge on known signalling pathways of ACE2, and hence, further studies are warranted to analyse the potential targets in hypertension. Recently, it has been found that ACE2 overexpression also protects against neurogenic hypertension via regulation of baroreflex and autonomic function in the central nervous system (CNS). 61 Although the role of ACE2 in hypertension is clear, the underlying mechanism needs to be elucidated to identify the relevant downstream signalling pathways and clinical trials need to be designed to determine whether up‐regulating the ACE2 level can lower blood pressure in patients.
5. ACE2 AND ARRHYTHMIA
Arrhythmia is myocardial disease that is caused by electrophysiological dysfunction and is often associated with oxidative stress. 62 The RAS has been proven to be involved in the development of arrhythmia. 63 As a significant protein in the RAS, ACE2 exerts negative effects on arrhythmia during the early stage. In ACE2 transgenic hearts, the gap junction proteins connexin40 and connexin43 were significantly down‐regulated, thus prolonging PR and QRS durations and potentially inducing conduction disturbances and lethal ventricular arrhythmias. 64 However, recent studies have shown that ACE2 may be an important protective factor in lethal arrhythmia. Overexpression of ACE2 in an atrial fibrillation model activated extracellular signal‐regulated kinases and up‐regulated MAPK levels, as well as induced a decrease in the level of MAPK phosphatase 1 (MKP‐1). 65 These findings resulted from decreases in atrial fibrosis collagen protein markers and TGF‐β, which may be one of the molecular mechanisms underlying the protective effect of ACE2 in atrial fibrillation. 66 In addition, strong evidence has shown that ACE2 agonist DIZE can reverse hyperglycaemia‐induced cardiac electrical changes in ventricular repolarization, thereby shortening the QT and QTc intervals on an electrocardiogram. 67 Moreover, Ang‐(1‐7) is a crucial protein in the downstream regulation of ACE2 that also plays a role in antiarrhythmic effects via reducing action potential repolarization phases and decreasing the late sodium (Na+), L‐type Ca2+ and Na+‐Ca2+ exchanger currents, thereby further mediating the balance of intracellular Ca2+ and sarcoplasmic reticulum Ca2+. 68 , 69 Therefore, additional emphasis should be placed on the role of ACE2 in arrhythmia, and new strategies for clinical antiarrhythmic drugs should be developed.
6. ACE2 AND DIABETES RELEVANT CVDS
Metabolic dysfunction is a major consequence of diabetes and includes oxidative stress, inflammation and multiple organ injuries in the later stage, especially to the heart. The specific pathogenesis is thought to be related to the RAS. 70 Recent studies have shown that the ACE2/Ang‐(1‐7)/MasR axis plays a significant role in diabetes‐induced cardiomyopathy. The expression of ACE2 decreased in the myocardial tissue of diabetic rats; furthermore, it inhibited myocardial collagen expression as well as promoted collagen degradation by regulating the TGF‐β pathway and activating MMP2. 71 Interestingly, ACE2 also exerts this role by regulating Ang‐(1‐7) and Ang‐(1‐9) before activating angiotensin II type 2 receptor (AT2R). 72 , 73 , 74 From this point of view, TGF‐β and MMP2 may be downstream targets of AT2R. Moreover, administration of Ang‐(1‐7) significantly reduced myocardial lipid accumulation by up‐regulating the expression of myocardial triglyceride lipase, which occurred as a result of up‐regulating the level of sirtuin‐1 (SIRT1). The activation of SIRT1 further regulated transcriptional activity of FOXO1 via SIRT1‐mediated deacetylation, which was proved as one of the potential protective targets on oxidative stress and inflammatory response. 75 , 76 , 77 In addition, Ang‐(1‐7) can activate the expression of sarcoplasmic reticulum Ca2+‐ATP enzyme to improve the left ventricular systolic dysfunction and right ventricular fibrosis caused by hyperglycaemia. 73 Clinical investigations of drugs targeting the ACE2/Ang‐(1‐7)/MasR axis have proven that ARB drugs like azilsartan and statins like atorvastatin can delay the progression of diabetic cardiomyopathy by increasing the expression levels of ACE2 and Ang‐(1‐7) combined with ACEI or DIZE and neprilysin inhibition therapy to provide better heart protection. 78 , 79 , 80 , 81 Finally, from our review, there is no evidence that ACE2 has effect on regulating blood glucose to improve diabetes relevant CVDs. These data suggest that the ACE2/Ang‐(1‐7)/MasR axis may serve an important target for the treatment of diabetes‐induced myocardial injury in the future.
7. ACE2 AND OTHER CVDS‐INDUCED HF
HF is a terminal stage of heart injury caused by various factors and characterized by systolic dysfunction. Besides of the above diseases, dilated cardiomyopathy, age‐related myocardial damage and cardiac afterload pressure overload all can induce HF happening. No clinically effective treatment strategies or sensitive predictive indicators exist to provide an early warning of the disease. 82 As ACE2 was discovered, an increasing number of studies have prioritized identifying a novel therapeutic breakthrough based on the ACE2 pathways. Some reports have focused on the serum level of ACE2 in HF patients, which revealed that serum ACE2 is increased in HF and indicated that ACE2 has the potential to become a reliable marker with the same efficacy as BNP. 83 , 84 , 85 The main function of ACE2 in HF is the degradation of Ang Ⅱ whereas Ang‐(1‐7) combats oxidative stress, fibrosis and inflammation. In a pressure‐overload heart, a model for inducing dilated cardiomyopathy, Bodiga et al and Patel et al reported that loss of the ACE2 gene led to increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity and fibrosis, accompanied by up‐regulated NADPH oxidase 2 (NOX2), p47phox, MMP2 and MMP9. This in turn activated ERKs, signal transducers and activators of the transcription (STAT) and AKT pathways, thereby resulting in further cardiac dysfunction. However, these observations were significantly reversed by a supplemental Ang‐(1‐7) or AT1R blockade. 86 , 87 , 88 In age‐dependent cardiomyopathy, ACE2 deficiency not only exacerbates oxidative stress injury but also stimulates the release of inflammatory factors via activating the MAPK pathway. 88 As the absence of ACE2 causes severe myocardial damage, drugs that up‐regulate ACE2 expression may have a positive effect on improving cardiac function. Wang et al utilized common clinical Sartan drugs, including olmesartan, candesartan, telmisartan, losartan, valsartan and irbesartan, and found that only olmesartan and candesartan increased ACE2/Ang‐(1‐7)/MasR expression and resisted pressure overload‐induced pathological changes in the heart with markedly declined ACE and AT1R expression as well as inhibited ERK phosphorylation. 89 In clinical research, spironolactone, a mineralocorticoid receptor blocker, significantly inhibited oxidative stress by lowering ACE activity and increasing ACE2 expression in the macrophages of congestive HF patients. Eplerenone also appeared to attenuate NADPH oxidation, leading to the same effect as spironolactone in macrophages treated with aldosterone. 90 B38‐CAP, discovered from Paenibacillus sp, has a structure similar to ACE2 and significantly improves pressure overload‐induced HF and cardiac hypertrophy. 91 Notably, recombinant human ACE2 (rhACE2) is also widely used in basic and clinical research for converting Ang Ⅱ to Ang‐(1‐7) against dilated cardiomyopathy in HF patients. RhACE2 has also been observed to attenuate doxorubicin‐induced cardiac dysfunction by protecting cardiomyocyte autophagy. 92 , 93 However, despite the increasing number of studies that have focused on the therapeutic role of ACE2 for patients with HF, specific target drugs need to be developed.
8. CONCLUSION
A significant amount of data has shown that ACE2 plays a crucial role in countering the development and progression of CVDs. In addition, the ACE2/Ang‐(1‐7)/MasR axis can improve CVDs through vasodilation, antiventricular remodelling, anti‐inflammatory, antioxidant and antimyocardial fibrosis effects. Studies on the other signalling pathways of ACE2 are scarce, and only a handful of classic pathways have been explored in CVDs. In hypertension, the function of ACE2 is to vasodilate and reduces blood pressure primarily by antagonizing the ACE/Ang II/AT1R axis, which can also affect the NO signal pathway in the CNS. In arrhythmias, ACE2 activity can reduce the occurrence of arrhythmic events and regulate calcium‐iron discrepancies. In addition, the antidiabetic cardiomyopathy effect of ACE2 is closely related to its antifibrosis effect via regulation of the TGF‐β signalling pathway. Furthermore, ACE2 can reduce myocardial collagen deposition induced by hyperglycaemia and activate MMP2. HF is the terminal state of cardiac dysfunction resulting from various factors, and up‐regulating ACE2 can reverse HF by improving cardiac remodelling, inhibiting oxidative stress and decreasing inflammation. The specific agonists of ACE2, DIZE and rhACE2 are recognized to have good efficacy in the treatment of CVDs (Figure 2).
FIGURE 2.
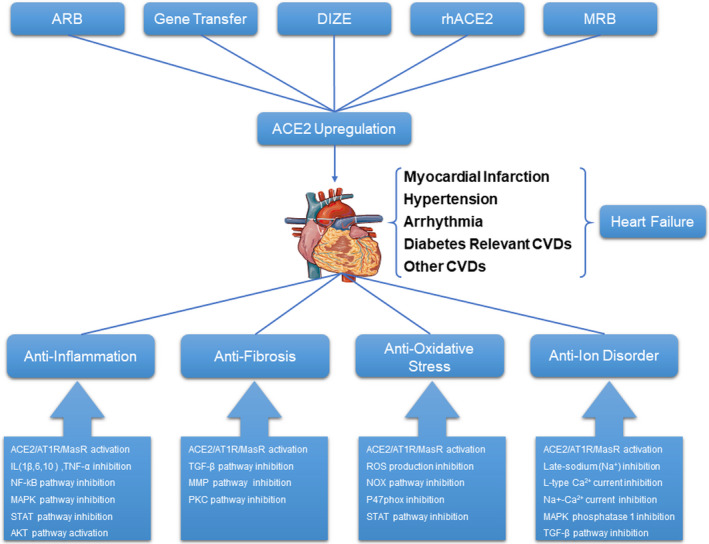
A diagram depicting the method for up‐regulating ACE2 expression and the downstream molecular mechanism of ACE2 in cardiovascular diseases (CVDs)
Nowadays, the number of COVID‐19 patients is raising rapidly, and the relevant drugs are limited. Moreover, vaccine development such as inactivated virus, adenovirus‐vectored investigational vaccine and mRNA‐based vaccine, has been in clinical trial stage, but which needs further long‐term clinical experiments. As the receptor for SARS‐CoV‐2, ACE2 has become a major concern in recent months, and related investigations have shown that it is significantly related to the virulence and severity of the virus. Due to its high expression in the heart tissue as well as clinical studies that have identified widespread myocardial injury in infected patients, understanding the pathophysiological role of ACE2 in the heart is of the utmost importance. As a result, ACE2 is regarded as a potential target for the treatment of COVID‐19 infection. In the feature, we believe the best methods defending against cardiovascular injury induced by SARS‐CoV2 infection are to focus on vaccine development and medicines targeting ACE2. Thus, identifying mechanisms to inhibit the ACE2 binding site for SARS‐CoV‐2 without harming its normal physiological function in the heart presents a new challenge for researchers in the midst of the current global pandemic.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Hanzhao Zhu: Writing‐original draft (lead); Writing‐review & editing (equal). Weixun Duan: Funding acquisition (equal); Methodology (equal); Resources (equal); Supervision (lead); Writing‐review & editing (equal). Shiqiang Yu: Funding acquisition (lead); Resources (equal); Supervision (equal). Liyun Zhang: Writing‐original draft (equal); Writing‐review & editing (equal). Yubo Ma: Writing‐original draft (equal); Writing‐review & editing (equal). Mengen Zhai: Validation (equal); Visualization (equal). Lin Xia: Methodology (equal); Validation (equal). Jincheng Liu: Conceptualization (equal).
Zhu H, Zhang L, Ma Y, et al. The role of SARS‐CoV‐2 target ACE2 in cardiovascular diseases. J Cell Mol Med. 2021;25:1342–1349. 10.1111/jcmm.16239
Hanzhao Zhu, Liyun Zhang and Yubo Ma contributed equally to this study
Funding information
This study was financially supported by the National Key Research and Development Program of China (2016YFC1301900) and the National Natural Science Foundation of China (grant nos. 81870218 and 81570230).
Contributor Information
Shiqiang Yu, Email: duanweixun@126.com, Email: yushiq@fmmu.edu.cn.
Weixun Duan, Email: duanweixun@126.com.
REFERENCES
- 1. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019
- 2. Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID‐19) pneumonia. Radiology. 2020;295(3):715‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu C, Jiang ZC, Shao CX, et al. Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: a multicenter study. Zhonghua Gan Zang Bing Za Zhi. 2020;28(2):148‐152. [DOI] [PubMed] [Google Scholar]
- 5. Inciardi RM, Lupi L, Zaccone G, et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID‐19). JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin‐converting enzyme‐related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1‐E9. [DOI] [PubMed] [Google Scholar]
- 9. Tipnis SR, Hooper NM, Hyde R, et al. A human homolog of angiotensin‐converting enzyme. Cloning and functional expression as a captopril‐insensitive carboxypeptidase. J Biol Chem. 2000;275:33238‐33243. [DOI] [PubMed] [Google Scholar]
- 10. Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532(1–2):107‐110. [DOI] [PubMed] [Google Scholar]
- 11. Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doobay MF, Talman LS, Obr TD, et al. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin–angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R373‐R381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shaltout HA, Westwood BM, Averill DB, et al. Angiotensin metabolism in renal proximal tubules, urine, and serum of sheep: evidence for ACE2‐dependent processing of angiotensin II. Am J Physiol Renal Physiol. 2007;292(1):F82‐F91. [DOI] [PubMed] [Google Scholar]
- 14. Vickers C, Hales P, Kaushik V, et al. Hydrolysis of biological peptides by human angiotensin‐converting enzyme‐related carboxypeptidase. J Biol Chem. 2002;277(17):14838‐14843. [DOI] [PubMed] [Google Scholar]
- 15. Santos Robson A S, e Silva Ana C Simoes, Maric Christine, et al. Angiotensin‐(1–7) is an endogenous ligand for the G protein‐coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100(14):8258‐8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nemoto W, Ogata Y, Nakagawasai O, et al. Angiotensin (1–7) prevents angiotensin II‐induced nociceptive behaviour via inhibition of p38 MAPK phosphorylation mediated through spinal Mas receptors in mice. Eur J Pain. 2014;18(10):1471‐1479. [DOI] [PubMed] [Google Scholar]
- 17. Rabie MA, Abd El Fattah MA, Nassar NN, et al. Angiotensin 1–7 ameliorates 6‐hydroxydopamine lesions in hemiparkinsonian rats through activation of MAS receptor/PI3K/Akt/BDNF pathway and inhibition of angiotensin II type‐1 receptor/NF‐κB axis. Biochem Pharmacol. 2018;151:126‐134. [DOI] [PubMed] [Google Scholar]
- 18. Tanno T, Tomita H, Narita I, et al. Olmesartan inhibits cardiac hypertrophy in mice overexpressing renin independently of blood pressure: Its beneficial effects on ACE2/Ang(1–7)/Mas axis and NADPH oxidase expression. J Cardiovasc Pharmacol. 2016;67(6):503‐509. [DOI] [PubMed] [Google Scholar]
- 19. Wang W, Bodiga S, Das SK, et al. Role of ACE2 in diastolic and systolic heart failure. Heart Fail Rev. 2012;17(4–5):683‐691. [DOI] [PubMed] [Google Scholar]
- 20. Kemp BA, Howell NL, Gildea JJ, et al. AT₂ receptor activation induces natriuresis and lowers blood pressure. Circ Res. 2014;115(3):388‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jang JH, Chun JN, Godo S, et al. ROS and endothelial nitric oxide synthase (eNOS)‐dependent trafficking of angiotensin II type 2 receptor begets neuronal NOS in cardiac myocytes. Basic Res Cardiol. 2015;110(3):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Inciardi RM, Adamo M, Lupi L, et al. Characteristics and outcomes of patients hospitalized for COVID‐19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41(19):1821‐1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2019;2020:e201017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 novel coronavirus‐infected pneumonia in Wuhan. China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lippi G, Lavie CJ, Sanchis‐Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID‐19): Evidence from a meta‐analysis. Prog Cardiovasc Dis. 2020;63(3):390–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li JW, Han TW, Woodward M, et al. The impact of 2019 novel coronavirus on heart injury: A Systematic review and Meta‐analysis. Prog Cardiovasc Dis. 2020;S0033–0620(20):30080‐30083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grimes JM, Grimes KV. p38 MAPK inhibition: A promising therapeutic approach for COVID‐19. J Mol Cell Cardiol. 2020;144:63‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tomasoni D, Italia L, Adamo M, et al. COVID‐19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail. 2020;22(6):957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oudit GY, Pfeffer MA. Plasma angiotensin‐converting enzyme 2: novel biomarker in heart failure with implications for COVID‐19. Eur Heart J. 2020;41(19):1818‐1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in COVID‐19. N Engl J Med. 2020;382(25):e102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33. Sama IE, Ravera A, Santema BT, et al. Circulating plasma concentrations of angiotensin‐converting enzyme 2 in men and women with heart failure and effects of renin‐angiotensin‐aldosterone inhibitors. Eur Heart J. 2020;41(19):1810‐1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Danser AHJ, Epstein M, Batlle D. Renin‐angiotensin system blockers and the COVID‐19 pandemic: At present there is no evidence to abandon renin‐angiotensin system blockers. Hypertension. 2020;75(6):1382‐1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Asselah T, Durantel D, Pasmant E, Lau G, Schinazi RF. COVID‐19: Discovery, diagnostics and drug development. J Hepatol. 2020;S0168–8278(20):33675‐33678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30:269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williamson BN, Feldmann F, Schwarz B, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS‐CoV‐2. Nature. 2020;585(7824):273‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics‐2019 update: A report from the American heart association. Circulation. 2019;139(10):e56‐e528. [DOI] [PubMed] [Google Scholar]
- 39. Wang J, He W, Guo L, et al. The ACE2‐Ang (1–7)‐Mas receptor axis attenuates cardiac remodeling and fibrosis in post‐myocardial infarction. Mol Med Rep. 2017;16(2):1973‐1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burrell LM, Risvanis J, Kubota E, et al. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J. 2005;26(4):369‐375. [DOI] [PubMed] [Google Scholar]
- 41. Ocaranza MP, Godoy I, Jalil JE, et al. Enalapril attenuates downregulation of Angiotensin‐converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension. 2006;48(4):572‐578. [DOI] [PubMed] [Google Scholar]
- 42. Ortiz‐Pérez JT, Riera M, Bosch X, et al. Role of circulating angiotensin converting enzyme 2 in left ventricular remodeling following myocardial infarction: A prospective controlled study. PLoS One. 2013;8(4):e61695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang M, Zhang W, Zhou Y, et al. Association between serum angiotensin‐converting enzyme 2 levels and postoperative myocardial infarction following coronary artery bypass grafting. Exp Ther Med. 2014;7(6):1721‐1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kassiri Z, Zhong J, Guo D, et al. Loss of angiotensin‐converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail. 2009;2(5):446‐455. [DOI] [PubMed] [Google Scholar]
- 45. Zhao YX, Yin HQ, Yu QT, et al. ACE2 overexpression ameliorates left ventricular remodeling and dysfunction in a rat model of myocardial infarction. Hum Gene Ther. 2010;21(11):1545‐1554. [DOI] [PubMed] [Google Scholar]
- 46. Der Sarkissian S, Grobe JL, Yuan L, et al. Cardiac overexpression of angiotensin converting enzyme 2 protects the heart from ischemia‐induced pathophysiology. Hypertension. 2008;51(3):712‐718. [DOI] [PubMed] [Google Scholar]
- 47. Grobe JL, Der Sarkissian S, Stewart JM, et al. ACE2 overexpression inhibits hypoxia‐induced collagen production by cardiac fibroblasts. Clin Sci (Lond). 2007;113(8):357‐364. [DOI] [PubMed] [Google Scholar]
- 48. Chen J, Cui L, Yuan J, et al. Protective effect of diminazene attenuates myocardial infarction in rats via increased inflammation and ACE2 activity. Mol Med Rep. 2017;16(4):4791‐4796. [DOI] [PubMed] [Google Scholar]
- 49. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 50. Gurley SB, Allred A, Le TH, et al. Altered blood pressure responses and normal cardiac phenotype in ACE2‐null mice. J Clin Invest. 2006;116(8):2218‐2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Crackower MA, Sarao R, Oudit GY, et al. Angiotensin‐converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822‐828. [DOI] [PubMed] [Google Scholar]
- 52. Gurley SB, Allred A, Le TH, et al. Altered blood pressure responses and normal cardiac phenotype in ACE2‐null mice. Altered blood pressure responses and normal cardiac phenotype in ACE2‐null mice. J Clin Invest. 2006;116(8):2218‐2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xia H, Sriramula S, Chhabra KH, Lazartigues E. Brain angiotensin‐converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ Res. 2013;113(9):1087‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xu J, Sriramula S, Xia H, et al. Clinical relevance and role of neuronal AT receptors in ADAM17‐mediated ACE2 shedding in neurogenic hypertension. Circ Res. 2017;121(1):43‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rentzsch B, TodiRAAS M, Iliescu R, et al. Transgenic angiotensin‐converting enzyme 2 overexpression in vessels of SHRSP rats reduces blood pressure and improves endothelial function. Hypertension. 2008;52(5):967‐973. [DOI] [PubMed] [Google Scholar]
- 56. Shenoy V, Gjymishka A, Jarajapu YP, et al. Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. Am J Respir Crit Care Med. 2013;187(6):648‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ferreira AJ, Shenoy V, Yamazato Y, et al. Evidence for angiotensin‐converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(11):1048‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Feng Y, Xia H, Cai Y, et al. Brain‐selective overexpression of human Angiotensin‐converting enzyme type 2 attenuates neurogenic hypertension. Circ Res. 2010;106(2):373‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pan X, Shao Y, Wu F, et al. FGF21 prevents angiotensin II‐induced hypertension and vascular dysfunction by activation of ACE2/Angiotensin‐(1–7) axis in mice. Cell Metab. 2018;27(6):1323‐1337.e5. [DOI] [PubMed] [Google Scholar]
- 60. Zhang J, Dong J, Martin M, et al. AMP‐activated protein kinase phosphorylation of angiotensin‐converting enzyme 2 in endothelium mitigates pulmonary hypertension. Am J Respir Crit Care Med. 2018;198(4):509‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Feng Y, Xia H, Santos RA, et al. Angiotensin‐converting enzyme 2: a new target for neurogenic hypertension. Exp Physiol. 2010;95(5):601‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Adameova A, Shah AK, Dhalla NS. Role of oxidative stress in the genesis of ventricular arrhythmias. Int J Mol Sci. 2020;21(12):E4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Calò LA, Schiavo S, Davis PA, et al. ACE2 and angiotensin 1–7 are increased in a human model of cardiovascular hyporeactivity: pathophysiological implications. J Nephrol. 2010;23(4):472‐477. [PubMed] [Google Scholar]
- 64. Donoghue M, Wakimoto H, Maguire CT, et al. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol. 2003;35(9):1043‐1053. [DOI] [PubMed] [Google Scholar]
- 65. Fan J, Zou L, Cui K, et al. Atrial overexpression of angiotensin‐converting enzyme 2 improves the canine rapid atrial pacing‐induced structural and electrical remodeling. Fan, ACE2 improves atrial substrate remodeling. Basic Res Cardiol. 2015;110(4):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Walters TE, Kalman JM, Patel SK, et al. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace. 2017;19(8):1280‐1287. [DOI] [PubMed] [Google Scholar]
- 67. Coutinho DCO, Monnerat‐Cahli G, Ferreira AJ, et al. Activation of angiotensin‐converting enzyme 2 improves cardiac electrical changes in ventricular repolarization in streptozotocin‐induced hyperglycaemic rats. Europace. 2014;16(11):1689‐1696. [DOI] [PubMed] [Google Scholar]
- 68. Joviano‐Santos JV, Santos‐Miranda A, Joca HC, et al. New insights into the elucidation of angiotensin‐(1–7) in vivo antiarrhythmic effects and its related cellular mechanisms. Exp Physiol. 2016;101(12):1506‐1516. [DOI] [PubMed] [Google Scholar]
- 69. Lu YY, Wu WS, Lin YK, et al. Angiotensin 1–7 modulates electrophysiological characteristics and calcium homoeostasis in pulmonary veins cardiomyocytes via MAS/PI3K/eNOS signalling pathway. Eur J Clin Invest. 2018;48(1):1–7. [DOI] [PubMed] [Google Scholar]
- 70. Zamora M, Villena JA. Contribution of Impaired Insulin Signaling to the Pathogenesis of Diabetic Cardiomyopathy. Int J Mol Sci. 2019;20(11):2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dong B, Yu QT, Dai HY, et al. Angiotensin‐converting enzyme‐2 overexpression improves left ventricular remodeling and function in a rat model of diabetic cardiomyopathy. J Am Coll Cardiol. 2012;59(8):739‐747. [DOI] [PubMed] [Google Scholar]
- 72. Hao P, Yang J, Liu Y, et al. Combination of angiotensin‐(1–7) with perindopril is better than single therapy in ameliorating diabetic cardiomyopathy. Sci Rep. 2015;5:8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hao PP, Yang JM, Zhang MX, et al. Angiotensin‐(1–7) treatment mitigates right ventricular fibrosis as a distinctive feature of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2015;308(9):H1007‐H1019. [DOI] [PubMed] [Google Scholar]
- 74. Zheng H, Pu SY, Fan XF, et al. Treatment with angiotensin‐(1–9) alleviates the cardiomyopathy in streptozotocin‐induced diabetic rats. Biochem Pharmacol. 2015;95(1):38‐45. [DOI] [PubMed] [Google Scholar]
- 75. Mori J, Patel VB, Abo Alrob O, et al. Angiotensin 1–7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circ Heart Fail. 2014;7(2):327‐339. [DOI] [PubMed] [Google Scholar]
- 76. Singh K, Singh T, Sharma PL. Beneficial effects of angiotensin (1–7) in diabetic rats with cardiomyopathy. Ther Adv Cardiovasc Dis. 2011;5(3):159‐167. [DOI] [PubMed] [Google Scholar]
- 77. Hariharan N, Maejima Y, Nakae J, et al. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation‐Induced Autophagy in Cardiac Myocytes. Circ Res. 2010;107(12):1470‐1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sukumaran V, Tsuchimochi H, Tatsumi E, et al. Azilsartan ameliorates diabetic cardiomyopathy in young db/db mice through the modulation of ACE‐2/ANG 1–7/Mas receptor cascade. Biochem Pharmacol. 2017;144:90‐99. [DOI] [PubMed] [Google Scholar]
- 79. Shin YH, Min JJ, Lee JH, et al. The effect of fluvastatin on cardiac fibrosis and angiotensin‐converting enzyme‐2 expression in glucose‐controlled diabetic rat hearts. Heart Vessels. 2017;32(5):618‐627. [DOI] [PubMed] [Google Scholar]
- 80. Aguilar C, Ventura F, Rodríguez‐Delfín L. Atorvastatin induced increase in homologous angiotensin I converting enzyme (ACE2) mRNA is associated to decreased fibrosis and decreased left ventricular hypertrophy in a rat model of diabetic cardiomyopathy. Rev Peru Med Exp Salud Publica. 2011;28(2):264‐272. [DOI] [PubMed] [Google Scholar]
- 81. Malek V, Sharma N, Gaikwad AB. Simultaneous inhibition of neprilysin and activation of ACE2 prevented diabetic cardiomyopathy. Pharmacol Rep. 2019;71(5):958‐967. [DOI] [PubMed] [Google Scholar]
- 82. Sliwa K. Heart failure can affect everyone: the ESC Geoffrey Rose lecture. Eur Heart J. 2020;41(12):1298‐1306. [DOI] [PubMed] [Google Scholar]
- 83. Epelman S, Tang WH, Chen SY, Van Lente F, Francis GS, Sen S. Detection of soluble angiotensin‐converting enzyme 2 in heart failure: insights into the endogenous counter‐regulatory pathway of the renin‐angiotensin‐aldosterone system. J Am Coll Cardiol. 2008;52(9):750‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Patel VB, Lezutekong JN, Chen X, Oudit GY. Recombinant Human ACE2 and the Angiotensin 1–7 Axis as Potential New Therapies for Heart Failure. Can J Cardiol. 2017;33(7):943‐946. [DOI] [PubMed] [Google Scholar]
- 85. Wang Y, Moreira Mda C, Heringer‐Walther S, et al. Plasma ACE2 activity is an independent prognostic marker in Chagas' disease and equally potent as BNP. J Card Fail. 2010;16(2):157‐163. [DOI] [PubMed] [Google Scholar]
- 86. Bodiga S, Zhong JC, Wang W, et al. Enhanced susceptibility to biomechanical stress in ACE2 null mice is prevented by loss of the p47(phox) NADPH oxidase subunit. Cardiovasc Res. 2011;91(1):151‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Patel VB, Bodiga S, Fan D, et al. Cardioprotective effects mediated by angiotensin II type 1 receptor blockade and enhancing angiotensin 1–7 in experimental heart failure in angiotensin‐converting enzyme 2‐null mice. Hypertension. 2012;59(6):1195‐1203. [DOI] [PubMed] [Google Scholar]
- 88. Oudit GY, Kassiri Z, Patel MP, et al. Angiotensin II‐mediated oxidative stress and inflammation mediate the age‐dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res. 2007;75(1):29‐39. [DOI] [PubMed] [Google Scholar]
- 89. Wang X, Ye Y, Gong H, et al. The effects of different angiotensin II type 1 receptor blockers on the regulation of the ACE‐AngII‐AT1 and ACE2‐Ang(1–7)‐Mas axes in pressure overload‐induced cardiac remodeling in male mice. J Mol Cell Cardiol. 2016;97:180‐190. [DOI] [PubMed] [Google Scholar]
- 90. Keidar S, Gamliel‐Lazarovich A, Kaplan M, et al. Mineralocorticoid receptor blocker increases angiotensin‐converting enzyme 2 activity in congestive heart failure patients. Circ Res. 2005;97(9):946‐953. [DOI] [PubMed] [Google Scholar]
- 91. Minato T, NiRAASawa S, Sato T, et al. B38‐CAP is a bacteria‐derived ACE2‐like enzyme that suppresses hypertension and cardiac dysfunction. Nat Commun. 2020;11(1):1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Basu R, Poglitsch M, Yogasundaram H, et al. Roles of Angiotensin Peptides and Recombinant Human ACE2 in Heart Failure. J Am Coll Cardiol. 2017;69(7):805‐819. [DOI] [PubMed] [Google Scholar]
- 93. Lai L, Chen J, Wang N, et al. MiRNA‐30e mediated cardioprotection of ACE2 in rats with Doxorubicin‐induced heart failure through inhibiting cardiomyocytes autophagy. Life Sci. 2017;169:69‐75. [DOI] [PubMed] [Google Scholar]


