Abstract
The interaction between gut microbiota and the host has gained widespread concern. Gut microbiota not only provides nutrients from the ingested food but also generates bioactive metabolites and signalling molecules to impact host physiology, especially in chronic kidney disease (CKD). The development of CKD, accompanied by changed diet and medication, alters the gut flora and causes the effect in distant organs, leading to clinical complications. Vascular calcification (VC) is an actively regulated process and a high prevalence of VC in CKD has also been linked to an imbalance in gut microbiota and altered metabolites. In this review, we focused on gut microbiota‐derived metabolites involved in VC in CKD and explained how these metabolites influence the calcification process. Correcting the imbalance of gut microbiota and regulating microbiota‐derived metabolites by dietary modification and probiotics are new targets for the improvement of the gut‐kidney axis, which indicate innovative treatment options of VC in CKD.
Keywords: chronic kidney disease, gut microbiota‐derived metabolites, vascular calcification
1. INTRODUCTION
Chronic kidney disease (CKD) is a global public health problem. The Global Burden of Disease study estimated that nearly 697 million persons worldwide had reduced the estimated glomerular filtration rate (eGFR) in 2016. It had increased by 70% since 1990. 1 Cardiovascular disease (CVD) is the leading cause of early death in the setting of CKD. 2 In addition, vascular calcification (VC) is a risk factor for major adverse cardiovascular events (MACEs), especially in patients with CKD. 3 , 4
Gut microbiota is a broader ecological community that influences the body's normal physiological function and disease susceptibility through the interaction between metabolism and host. 5 Simenhoff et al, through endoscopy, first demonstrated in the 1970s that gut microbiota was significantly altered in both CKD and non‐CKD patients. In CKD patients, aerobic and anaerobic bacteria were intensely colonized in the duodenum and jejunum. 6 In addition, decreased Lactobacillus and increased Enterobacteriaceae had been observed. 7 Moreover, faecal analysis of dialysis patients revealed a decreased level of short‐chain fatty acid butyrate. 8 Once gut flora destroys the intestinal epithelial barrier and releases such detrimental metabolites into circulation, inflammation, oxidative stress and direct active pathways induced by the metabolites eventually lead to the development and progression of VC. 9 Accumulating evidence demonstrated that gut microbiota might play an essential role in VC in CKD patients. 10 , 11 Therefore, this review intended to provide an overview of gut microbiota‐derived metabolites on the facilitation of VC and to propose new thoughts based on the interference of gut microbiota‐derived metabolites to retard VC in CKD patients.
2. VC IN CKD
The vascular wall is composed of three differently structured layers from the periphery to the lumen of the vessel. Blood vessels contain two primary cell types, endothelial cells (ECs) and vascular smooth muscle cells (VSMCs), which exert essential functions to maintain vascular homeostasis. Being different from ECs, VSMCs are not terminally differentiated and preserve their plasticity. VC can develop in the intimal and medial layers of arteries. Alternatively, medial calcification is characterized by VSMCs' transformation into osteoblast‐like cells and is more common in CKD patients. 12 Overproduction of reactive oxygen species (ROS) in VSMCs is related to vascular dysfunction, which is a risk factor for CKD patients. VC is an active biological process associated with hydroxyapatite crystallisation in the vascular wall. The decline of inhibitors such as Matrix Gla Protein (MGP), Gla‐rich protein (GRP), osteoprotegerin (OPG), bone morphogenetic protein 7 (BMP‐7) and the increase of calcification inducers lead to more extensive VC in the CKD population. 13 , 14 Furthermore, uremic toxins, calcium and phosphate metabolism dysfunction may directly influence VSMCs' physiological function, leading to irregular senescence, proliferation and migration of VSMC, ultimately leading to VC.
3. THE GUT MICROBIOTA IN CKD
The human gastrointestinal tract is inhabited with 100 trillion different microbes, including bacteria (Lactobacillus), viruses (primarily phage), fungi, archaea and so on. 15 There are two characteristics of gut microbiota in healthy adults; one is taxonomical diversity, such as Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria and Verrucomicrobia all be seen in the healthy gut. 16 The other is functional diversity, such as enhancing the host against enteral pathogens, modulating systemic immunity. In the body, approximately 70% of the immune cells reside in the gut, cutting down bacterial dissemination and producing vitamins and essential metabolites that are not synthesized by the host. 17 A study amongst end‐stage renal disease (ESRD) patients found high phyla Firmicutes, Proteobacteria and Actinobacteria, and a decrease in Lactobacilli, Roseburia and Phytoalexin. 18
Currently, several studies proposed the role of the gut‐kidney axis (Figure 1). For example, excessive protein intake in the diet can cause intra‐glomerular hypertension, which leads to kidney ultrafiltration and glomerular damage. Long‐term high protein intake may lead to CKD. In CKD patients, a reduction in renal filtering capacity results in the deposition and accumulation of waste products in the blood, which eventually develops into uremia. Uremia can lead to malnutrition, which leads to an imbalance of intestinal flora and abnormal metabolites, increased intestinal permeability. Finally, kidney damage is aggravated again. 19 In another study with CKD patients, it was demonstrated that the reduction infiltration capacity of the kidneys led to the deposition and accumulation of toxic waste products in the blood. 7 The genome of the gut microbiome contains 3.3 million genes. It is 150 times the human genome. Recently, a study has shown that dual‐omics (metagenomic and metabolomics) data can reveal the connections between gut microbes and circulating metabolites perturbed in CKD. In the early stage of CKD, microbial genes to secondary bile acid biosynthesis were differentially abundant. In the advanced stage that lipid metabolism and lipopolysaccharide biosynthesis were enriched. However, the research lacks a replication cohort. 20
FIGURE 1.
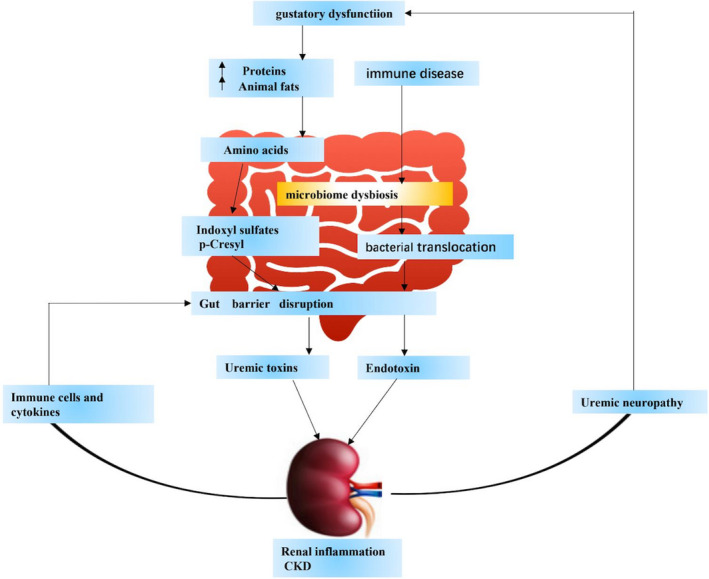
The kidney plays an important role in nutritional homeostasis. Base on Chronic kidney disease causes kidney damage, Increased susceptibility to malnutrition injury. In CKD, a reduction in renal filtering capacity results in the deposition and accumulation of waste products in the blood, which eventually develops into uremia. In addition, complications of uremia include uremic neuropathy, which can contribute to gustatory dysfunction leads to an imbalanced diet. Dysbiosis caused by an imbalanced diet (for example, a diet high in protein and animal fat) leads to excessive production and accumulation of p‐cresol and indoxyl sulfate in the gut. This accumulation destroys the intestinal barrier, thereby increasing the permeability of the intestine. Therefore, it can cause kidney damage (like inflammation of the kidneys). Metabolite causes activation of immune cells and factors, and continuous destruction of the intestinal barrier. This process into a vicious circle
4. INFLUENCE OF DIET ON GUT MICROBIOTA
Diet strongly affects human health. Most of the beneficial effects are obtained by modulating gut microbiome composition. According to the dominant bacterial system type, the gut microbiota of adults can be divided into two main types. Both intestinal types are closely related to long‐term diet. The main bacterial population of intestinal type 1 is Bacteroides, which mainly metabolizes proteins, while gut type 2 is mainly glycolytic chlorella. 21 Previously, animal studies reported that intake of a high‐unsaturated fat‐rich diet would increase Actinobacteria (Lactobacillus and Streptococcus) and Verrucomicrobia. Probiotics intake, such as cultured milk products and yogurt, is a source of ingestible microorganisms. 22 Diet containing high fat can increase Lipopolysaccharides (LPS) translocation. 23 Common food like fruits, vegetables and tea are all rich in polyphenols. 24 Probiotics and Polyphenols both enhance Bifidobacterium and Lactic acid‐producing bacteria and reduce enteropathogenic bacteria. 25 The renal diet (low potassium, low phosphorus), which is lacking in plant fibre, can lead to the overgrowth of bacteria with harmful metabolites like uremic toxins. 26 A study reported that older adults with CKD had a higher taste sensation for phosphate‐containing salts. Hyperphosphatemia can accelerate VSMCs transdifferentiating and directly participate in the deposition of the calcium‐containing osteoid matrix in vascular media. Therefore, CKD with gustatory dysfunction can develop metabolism perturbation and contribute to uremic neuropathy. If the gustatory function in CKD patients can be preserved, food intake can be improved and reduce the production of uremic solutes, leading to a lower risk of VC. 27 Therefore, diet can adjust the type of gut microbiota and metabolites. In CKD patients, more attention should be paid to the impact of diet in VC.
5. THE RELATIONSHIP BETWEEN GUT MICROBIOTA‐DERIVED METABOLITES AND VC IN CKD
Altered gut microbiota and metabolites in CKD are believed to be involved in the VC process. 28 LPS and bacterial DNA could directly induce inflammation and immune response, leading to end‐organ damage. 29 Gut microbiota‐derived metabolites such as uremic toxins, trimethylamine N‐oxide (TMAO), bile acid are all associated with VC in CKD by regulating vascular phenotype, oxidative stress and epigenetics. 30 , 31 The following is a detailed introduction of gut microbiota‐derived metabolites on vascular calcification (Figure 2).
FIGURE 2.
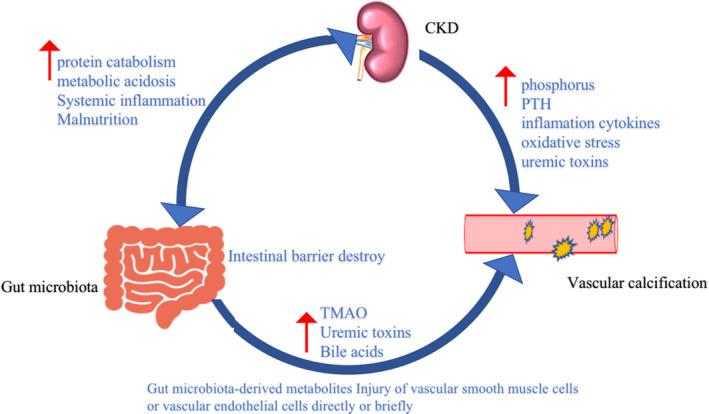
In chronic kidney disease (CKD) patients, decreased renal function leads to decreased glomerular filtration rate (GFR), increased proteinuria and uremic toxins, and damage to glomeruli and tubulointerstitial. Also cause metabolic acidosis, accelerated protein catabolism, resulting in malnourished patients. Malnutrition also leads to an imbalance of intestinal homeostasis, which is characterized by increased mucosal inflammation, increased intestinal permeability and abnormally increased gut microbiota‐derived metabolites like (p‐cresol, indoxyl sulfate and Trimethylamine N‐oxide (TMAO)). It can directly or indirectly affect vascular smooth muscle cells or vascular endothelial cells and induce vascular calcification. And high phosphorus caused by chronic kidney disease, parathyroid hormone (PTH), inflammatory cytokines, oxidative stress and uremic toxins can induce vascular calcification
5.1. Uremic toxins
Gut microbiota‐derived metabolites of amino acids are uremic toxins, including indole‐3 acetic acid, indoxyl sulfate (IS) and p‐cresyl sulfate (PCS), which translocate into the bloodstream and cause extensive oxidative stress‐induced damage to the kidneys. Uremic toxins can lead to endothelial dysfunction, vascular senescence, vascular inflammation in CKD patients. It is supporting the link between uremic toxins and vascular dysfunction. 32 Silvia D et al demonstrated that uremic toxins impaired the autophagic flux leading to endothelial dysfunction. 33 Generally, gut bacteria metabolize tryptophan into indole, further exchanged into indoxyl sulfate in the liver after intestinal absorption. 34 Gut bacteria also metabolize aromatic amino acids into tyrosine phenylalanine and p‐cresol, which are bio‐transformed by sulfotransferase into PCS in the liver. 35 , 36 In CKD patients, the production of uremic toxins (such as IS, PCS) by bacteria increases.
IS, a critical protein‐bound uremic toxin, can be described as a significant risk factor of VC in CKD patients. 37 Research has shown that uremic toxins (mainly Pi) are responsible for the high prevalence of VC in the CKD population. 38 In a healthy human beings, IS concentration ranges from 10 to 130 mg/day. But excessive IS induces the production of free radicals in both renal cells and VSMC through oxidative stress and inflammation to cause tissue injury. 39 In CKD patients, clinical evidence has shown that IS plasma levels are associated with pulse wave velocity, ankle‐brachial index, which are markers of arteriosclerosis and aortic calcification. IS can induce vascular inflammation through the delta‐like (DII) 4‐Notch signalling pathway. 40 Moreover, IS can induce the proliferation, osteogenic differentiation and senescence of VSMC by regulating the Mitogen‐activated protein kinase (MAPK) pathway, p21‐p27‐p53 pathway and PI3K/Akt/NF‐κB pathway. 41 IS promotes VSMC calcification through the secretion of IL‐8 by endothelial cells in the presence of inorganic phosphate. In addition, it enhances the cytosine‐guanine CpG hypermethylation of klotho and epigenetic modification of klotho to promote the process of VC in CKD. It was shown to up‐regulate the expression of intercellular cell adhesion molecule‐1 (ICAM‐1) and monocyte chemotactic protein (MCP‐1) in a vascular endothelial cell through ROS‐induced activation of nuclear factor‐κβ (NF‐κβ). 42 Also, IS induced methyltransferase‐like (METTL14)‐dependent N6‐methyladenosine (m6A) to regulate VC. 43
PCS is a prototype protein‐bound molecule. 44 PCS's concentration ranges between 2.8 ± 1.7 mg/L and 6.6 ± 3.7 mg/L in healthy human plasma. However, PCS is significantly increased in end‐stage renal disease (ESRD) patients (21.8 ± 12.4 mg/L and 106.9 ± 44.6 mg/L). 45 PCS induced inflammatory factors that triggered monocyte‐endothelial cell interaction and incriminated oxidative stress in human VSMCs. 46 In addition, the effect of PCS on renal injury has been reported. Sun et al demonstrated that PCS was activated by the renal renin‐angiotensin‐aldosterone system and then induced epithelial‐mesenchymal transition, contributing to kidney injury. 47
Moreover, a study has demonstrated that IS and PCS both could potentially induce endothelial dysfunction and distinct calcification in the arteries of CKD rats. 31 On the one hand, IS generates stress in ECs and induces premature senescence via increasing macro‐vesicles (MVs) release. On the other hand, PCS also promotes EMV release, which causes the dysregulation of vascular homeostasis. At the beginning of the development of VC, MVs are involved in the inflammatory response of VSMCs. The MVs might be useful to develop as biomarkers and therapeutic tools for preventing CKD at risk of developing VC. 48 , 49 Further in vivo experiment is still needed to illustrate its role. AST‐120 is an oral carbon adsorbent. It can absorb IS and PCS precursors, reducing serum IS and PCS levels in CKD patients, which may be beneficial to reduce VC. 50 Clinical trials demonstrated that symbiotic (pre‐and probiotic) therapy decreased serum PCS and modified stool microbiome. 51 Thus, it could be a novel therapy for the management of VC in CKD patients.
Recent studies have shown that microRNAs (miRs) as epigenetic regulators are involved in uremic toxins‐induced vascular calcification. MicroRNAs are small non‐coding RNAs and regulate the protein expression without affecting the gene sequence. Besides, microRNAs are potential biomarkers of VC induced by uremic toxins, such as miR‐223, miR‐125 and miR‐143. 52 , 53 In cultured endothelial cells (ECs), IS up‐regulated miR‐92a to activate the inflammasome in ECs and enhanced vascular inflammation. 54 IS also down‐regulated miR‐29b and activated Wnt/β‐catenin signalling to induce vascular activation. 55 In conclusion, IS and PCS are considered harmful vascular toxins and promote VC in CKD patients. MVs and miRNAs might be therapeutic targets to prevent vascular disease in CKD (Figure 3).
FIGURE 3.
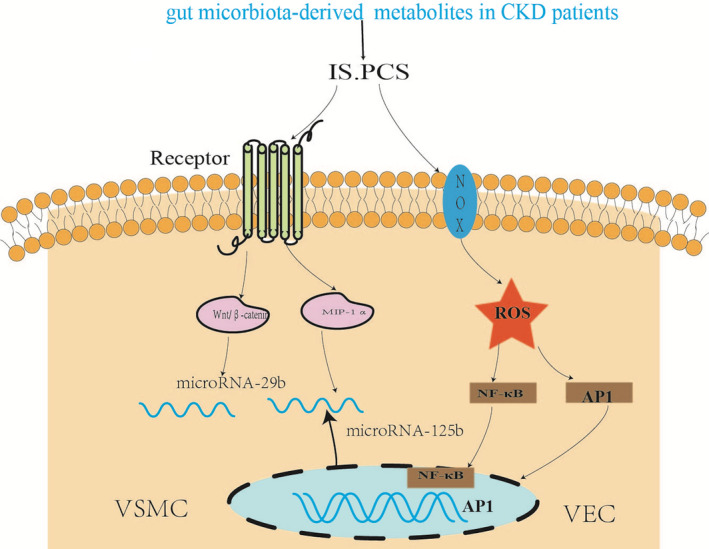
In patients with chronic kidney disease (CKD), the effect of gut microbiota‐derived metabolites indoxyl sulfate (IS) and p‐cresyl sulfate (PCS) on vascular smooth muscle cell (VSMC). IS down‐regulated miR‐29b and activated Wnt/β‐catenin signalling to induce vascular activation. IS induced reactive oxygen species (ROS) also promote the activation of Nuclear factor‐κB (NF‐κB) and activating protein 1 (AP‐1) pathways, increase inflammation and damage the endothelial cell
5.2. Trimethylamine N‐oxide (TMAO)
Trimethylamine N‐oxide is a product of the gut microbiome. Choline and phosphatidylcholine are catalysed into trimethylamine (TMA) in intestinal microbiota (such as phosphatidylcholine, betaine and I‐carnitine), which is further oxidized as TMAO in the human liver. 56
In vitro experiments showed that TMAO promoted VC only in a calcifying medium, explaining that high calcium and phosphate were critical for TMAO‐induced VC. In vivo study demonstrated that TMAO promoted VC in CKD rats with high calcium/ phosphorus (Ca/P) diet. 57 Besides, TMAO was shown to increase the expression of pro‐inflammatory genes such as interleukin‐18 (IL‐18), interleukin‐6 (IL‐6) and interleukin‐1β (IL‐1β) adhesion molecules and chemokines. 58 Besides, TMAO increased the expression of pro‐inflammatory genes such as IL‐18, IL‐6 and IL‐1β adhesion molecules and chemokines. 59 In human umbilical vein endothelial cells (HUVEC) and aortas from ApoE knockout mouse, TMAO also induced oxidative stress and nucleotide‐blinding domain, leucine‐rich‐containing family, pyrin domain‐containing‐3 (NLRP3) activation signals, releasing of inflammatory cytokines. 59 , 60 , 61 TMAO activated endothelial cell mitogen‐activated protein kinase (MAPK) and VSMC through NF‐κβ pathway, leading to inflammatory gene expression and augmenting Ca2+ release from intracellular stores (Figure 4).
FIGURE 4.
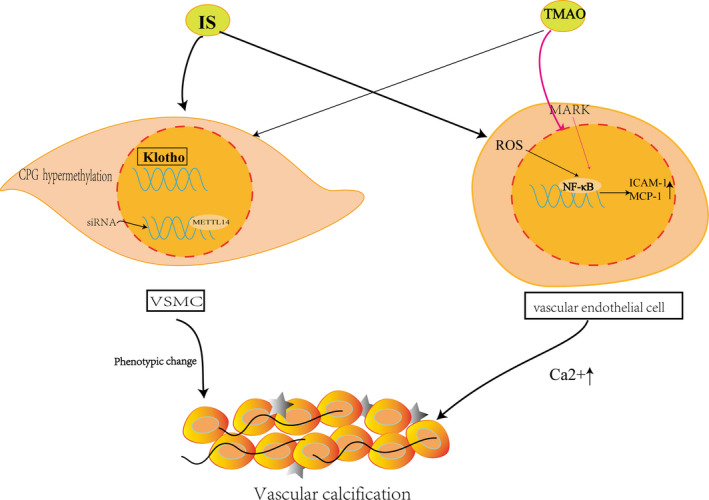
IS enhances the CpG hypermethylation of Klotho and epigenetic modification of klotho to promote the process of VC in CKD and induced methyltransferase‐like (METTL14) ‐dependent N6‐methyladenosine (m6A) regulated vascular calcification in VSMC.TMAO activates endothelial cell mitogen‐activated protein kinase (MAPK) and vascular smooth muscle cell (VSMC) through nuclear factor‐κB (NF‐κB) pathway, leading to inflammatory gene expression and augmenting Ca2+ release from intracellular stores
In summary, the elevated TMAO level in CKD is a considerable promoter of VC. Several approaches are being explored to reduce TMAO levels, like oral broad‐spectrum antibiotics or promoting the growth of bacteria that use TMAO as matrix and baicalin. 61 , 62 , 64
Recently, the Pretest‐posttest study showed that a diet supplemented with β‐glucan was potentially efficient in lowering serum concentrations of TMAO in patients with CKD. 30 But whether these methods are able to prevent VC still needs further research.
5.3. Bile acids
Bile acids are amphipathic molecules that have two types. One is primary bile acids and another is secondary bile acids. Primary bile acids produced by cholesterol in the liver are processed into secondary bile acids by gut microbiota. Bile acid metabolism affects host metabolism through the regulation of the cholesterol cholestero7‐α hydroxylase (CYP7A1) and G protein‐coupled receptor (TGR5, GPBAR1). 65 , 66 In CKD patients, circulate bile acids present a high level. When bile acids are perturbed, primary bile acid is decreased, and secondary bile acid, deoxycholic acid (DCA) are increased.
DCA is directly toxic to VSMCs. A clinical trial proved that high DCA is an independent risk factor for VC in CKD patients. 67 DCA can induce mineralisation and osteogenic differentiation of VSMCs by regulating endoplasmic reticulum (ER) stress. Emerging data implicate the role of ER stress as a new mechanism for VC. 68 Primary bile acids (BAs) increase the colon Retinoid‐related orphan receptor) ROR acids + Regulatory T (Treg) cells improve host susceptibility. Tregs can modulate both innate and adaptive immune responses to suppress VC in CKD. 69 Researchers have discovered that the genome‐wide biliary network interaction between intestinal bacteria and the host can control the host's immune homeostasis. 70 Besides, Bas and their nuclear receptor, such as farnesoid X receptor (FXR), are found in macrophages and vasculature. Importantly, activation of FXR was shown to reduce VC in the CKD model. 71
5.4. Lipopolysaccharide (LPS)
LPS, also called endotoxin, is a specific type of gut microbiota‐derived metabolite. It is a component of the outer membrane of gram‐negative bacteria. If the intestinal barrier is impaired by bacterial translocation and gut microbiota disorder, LPS can enter the circulation through the gut wall and induce a systemic inflammatory response. 72 , 73
Evidence suggested that pro‐inflammatory cytokines such as IL‐6 induced VSMC mineralisation and osteogenic transition, playing a pivotal role in the progression of VC. 74 The research demonstrated that IL‐18 also contributed to VC in pro‐inflammatory conditions. 75 Inflammatory cytokines increased the expression of bone morphogenetic protein 2 (BMP2) and reduced MGP expression, further promoting VC formation in VSMCs. 76 Inflammatory responses are essential regulators in the development of VC in CKD patients. A study found in ESRD patients that great phyla Firmicutes, Proteobacteria and Actinobacteria have decreased in Lactobacilli, Roseburia and Phytoalexin. Furthermore, intestinal microbiome dysbiosis also led to bacterial translocation that colon wall inflammation follows with the destruction of the enteric epithelial barrier, which leads to LPS and translocation of bacterial DNA into the bloodstream, triggering a state of persistent systemic inflammation in CKD patients. 18 , 77 Recently, having evidence that LPS and translocation of bacterial DNA can promote Pi‐induced calcification and osteoblastic differentiation in human aortic smooth muscle cells (HASMCs) through TLR9/NF‐κβ/BMP‐2signalling.Furthermore, Toll‐like receptor 4 (TLR4) and Toll‐like receptor 9 is un‐regulated in VSMCs aggravated inorganic Pi induced VC in CKD patients. Sanchis P et al demonstrated that level of inflammatory markers correlated with VC, while blocking ataxia‐telangiectasia mutated (ATM)‐mediated DNA damage signalling reduced inflammation and calcification in CKD patients. 78 , 79 , 80
5.5. Short‐chain fatty acids (SCFAs)
Short‐chain fatty acids (SCFAs) are another significant gut microbiota‐derived metabolite. SCFAs are produced through the fermentation of dietary fibres by anaerobic gut bacteria in the caecum and proximal colon. 81 SCFAs are mainly composed of acetate, propionate and butyrate. Butyrate is primarily metabolized by the colonic commensal bacteria, which regulates cell growth and differentiation. 82 SCFAs can be used to maintain the gut barrier and inhibit pathogenic microbe proliferation in acidic PH conditions. 83 It is also mediated by G protein‐coupled receptors GRP419 (FFAR3) and GRP43 (FFAR2), predominantly expressed in the immune cell. SCFAs also can contribute to improving vascular phenotypes. 84 , 85
6. POTENTIAL THERAPEUTIC STRATEGIES AGAINST VC
The intervention of gut microbiota‐derived metabolites is a potential strategy to reduce calcification in CKD patients. Resveratrol is a dietary polyphenol compound. It has anti‐inflammatory, antioxidative properties. Recently, studies also showed that resveratrol is a phytoalexin and scavenger for many free radicals. Resveratrol can decreased plasma TMAO and regulated sirtuin‐1 (Sirt‐1) and nuclear factor‐E2‐associated factor 2 (Nrf2) signalling pathway to ameliorate VC vascular calcification. 86 Besides, Resveratrol can reverse the effect of IS. Also, resveratrol was shown to inhibit IS‐activated aryl hydrocarbon receptor (AHR) and regulate VE‐cadherin and permeability‐induced increase in Src activation. 87 Resveratrol could protect rat VMSCs against oxidative injury in VC. A range of studies has highlighted that interfering with some intestinal flora metabolites is beneficial for vascular suppression (Table 1). 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 S‐equol is produced naturally in the gut by the bacterial biotransformation of daidzein, a soy isoflavone. A number of studies suggested that S‐equol can inhibit vascular remodelling in the protective vascular system. S‐equol can protect vasculature against cardiovascular diseases. Thus, S‐equol should have an important role in the field of vascular research. 96 It is thus proposed that the use of oral iron supplements might improve the gut microbiome. However, iron supplements can lead to a decreased abundance of Lactobacillus and Bifidobacterium species. 97 Recently, many ongoing experiments have been focusing on the diet being the most significant modifiable factor capable of changing gut microbiota. 98 The development of diet (such as probiotics) as a primary management option to regulate the intestinal flora is pivotal to retard the development and progression of VC.
TABLE 1.
Change in gut microbiota‐derived metabolites to treat VC
| Gut microbiota | Function | Reference number | |
|---|---|---|---|
| Anthocyanins(blackberries) | LPS | Inhibitory gram‐negative bacteria | 90.91 |
| PCA | Produced by intestinal explanation | Reduce miRNA‐10b expression (miRNA‐10b directly harmful regulates human) | 92 |
| Probiotics |
SCFAs Bifidobacterium |
Increase SCFAs, Bifidobacterium, lactic acid bacteria |
93.94 |
| Probiotics | Roseburia intestinalis | Anti‐inflammatory response by regulating Treg cells. | 95 |
| Antibiotics | TMAO | Reduce TMAO | 62 |
| Buckwheat honey | S. aureus and E. coli | Inhibitory activity | 96 |
| Gallic acid | Harmful bacteria | Clostridium histolyticum | 97 |
Abbreviations: E. coli, Escherichia coli; LPS, Lipopolysaccharide; PCA, Protocatechuic acid; SCFAs, Short‐chain fatty acids; TMAO, Trimethylamine N‐oxide; VC, vascular calcification.
7. CONCLUSION
Gut microbiota is closely related to human health. The gut microbiota and metabolites have significant effects on CKD patients especially with the complication of VC. In this review, we focused on intestinal microbiota‐derived metabolites such as uremic toxins, TMAO and SCFAs in VC. The multi‐factorial mechanism of VC suggests that the intervention of gut microbiota‐derived metabolites could be one of the important strategies to inhibit VC in CKD. There are existing methods like dietary or pharmacological intervention affecting genomics, intestinal absorption, gut‐kidney axis to reduce the production of harmful substances in the intestine and might further to improve VC. Thus, considering the significance of gut microbiota, future research should further explore the direct relationship between gut microbiota‐derived metabolites and VC.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Li Yin: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Methodology (equal); Writing‐original draft (lead); Writing‐review & editing (equal). xiao xue Li: Formal analysis (equal); Resources (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Sounak Ghosh: Writing‐review & editing (lead). Changming Xie: Resources (equal). Jie Chen: Supervision (equal); Visualization (equal). Hui Huang: Conceptualization (lead); Formal analysis (lead); Methodology (lead); Resources (lead); Supervision (lead); Writing‐original draft (equal); Writing‐review & editing (lead).
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (8201101103, 81870506, 81670676 and 81422011), Project of Traditional Chinese Medicine in Guangdong Province (20201062), Basic Research Project of Shenzhen Science and Technology Innovation Committee (JCYJ20180306174648342 and JCYJ20190808102005602), Shenzhen Futian District Public Health Research Project (FTWS2019003) and Shenzhen Key Medical Discipline Construction Fund (SZXK002) to HH In addition, this work was supported by the National Natural Science Foundation of China 82073408 to JC
Yin L, Li X, Ghosh S, Xie C, Chen J, Huang H. Role of gut microbiota‐derived metabolites on vascular calcification in CKD. J Cell Mol Med.2021;25:1332–1341. 10.1111/jcmm.16230
REFERENCES
- 1. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haarhaus M, Brandenburg V, Kalantar‐Zadeh K, Stenvinkel P, Magnusson P. Alkaline phosphatase: a novel treatment target for cardiovascular disease in CKD. Nat Rev Nephrol. 2017;13:429‐442. [DOI] [PubMed] [Google Scholar]
- 3. Strauss HW, Nakahara T, Narula N, Narula J. Vascular calcification: the evolving relationship of vascular calcification to major acute coronary events. J Nucl Med. 2019;60:1207‐1212. [DOI] [PubMed] [Google Scholar]
- 4. Viegas C, Araujo N, Marreiros C, Simes D. The interplay between mineral metabolism, vascular calcification and inflammation in Chronic Kidney Disease (CKD): challenging old concepts with new facts. Aging. 2019;11:4274‐4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heintz‐Buschart A, Wilmes P. Human gut microbiome: function matters. Trends Microbiol. 2018;26:563‐574. [DOI] [PubMed] [Google Scholar]
- 6. Simenhoff ML, Saukkonen JJ, Burke JF, Wesson LG, Schaedler RW, Gordon SJ. Bacterial populations of the small intestine in uremia. Nephron. 1978;22:63‐68. [DOI] [PubMed] [Google Scholar]
- 7. Yang T, Richards EM, Pepine CJ, Raizada MK. The gut microbiota and the brain‐gut‐kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018;14:442‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease‐ and uricase‐containing, indole‐ and p‐cresol‐forming and contraction of short‐chain fatty acid‐producing intestinal microbiota in ESRD. Am J Nephrol. 2014;39:230‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lau WL, Savoj J, Nakata MB, Vaziri ND. Altered microbiome in chronic kidney disease: systemic effects of gut‐derived uremic toxins. Clin Sci. 2018;132:509‐522. [DOI] [PubMed] [Google Scholar]
- 10. Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242‐249. [DOI] [PubMed] [Google Scholar]
- 11. Durack J, Lynch SV. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med. 2019;216:20‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ryu J, Kwon D‐H, Choe N, et al. Characterization of circular RNAs in vascular smooth muscle cells with vascular calcification. Mol Ther Nucleic Acids. 2020;19:31‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zununi Vahed S, Mostafavi S, Hosseiniyan Khatibi SM, Shoja MM, Ardalan M. Vascular calcification: an important understanding in nephrology. Vasc Health Risk Manag. 2020;16:167‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Silva AP, Viegas CS, Mendes F, et al. Gla‐rich protein (GRP) as an early and novel marker of vascular calcification and kidney dysfunction in diabetic patients with CKD: a pilot cross‐sectional study. J Clin Med. 2020;9:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med. 2018;24:392‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science (New York, NY). 2005;308:1635‐1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wampach L, Heintz‐Buschart A, Hogan A, et al. Colonization and succession within the human gut microbiome by archaea, bacteria, and microeukaryotes during the first year of life. Front Microbiol. 2017;8:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lau WL, Kalantar‐Zadeh K, Vaziri ND. The gut as a source of inflammation in chronic kidney disease. Nephron. 2015;130:92‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ko G‐J, Rhee CM, Kalantar‐Zadeh K, Joshi S. The Effects of high‐protein diets on kidney health and longevity. J Am Soc Nephrol. 2020;31:1667‐1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu IW, Gao S‐S, Chou H‐C, et al. Integrative metagenomic and metabolomic analyses reveal severity‐specific signatures of gut microbiota in chronic kidney disease. Theranostics. 2020;10:5398‐5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu GD, Chen J, Hoffmann C, et al. Linking long‐term dietary patterns with gut microbial enterotypes. Science (New York, NY). 2011;334:105‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang X, Yang Y, Huycke MM. Risks associated with enterococci as probiotics. Food Res Int. 2020;129:108788. [DOI] [PubMed] [Google Scholar]
- 23. Jamar G, Ribeiro DA, Pisani LP. High‐fat or high‐sugar diets as trigger inflammation in the microbiota‐gut‐brain axis. Crit Rev Food Sci Nutr. 2020;8:1‐19. [DOI] [PubMed] [Google Scholar]
- 24. Pérez‐Jiménez J, Neveu V, Vos F, Scalbert A. Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol‐Explorer database. Eur J Clin Nutr. 2010;64(Suppl 3):S112‐S120. [DOI] [PubMed] [Google Scholar]
- 25. Singh RK, Chang H‐W, Yan D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crespo‐Salgado J, Vehaskari VM, Stewart T, et al. Intestinal microbiota in pediatric patients with end stage renal disease: a Midwest Pediatric Nephrology Consortium study. Microbiome. 2016;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen SI, Chiang CL, Chao CT, Chiang CK, Huang JW. Gustatory function and the uremic toxin, phosphate, are modulators of the risk of vascular calcification among patients with chronic kidney disease: a pilot study. Toxins (Basel). 2020;12:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen Y‐Y, Chen D‐Q, Chen L, et al. Microbiome‐metabolome reveals the contribution of gut‐kidney axis on kidney disease. J Transl Med. 2019;17:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ascher S, Reinhardt C. The gut microbiota: an emerging risk factor for cardiovascular and cerebrovascular disease. Eur J Immunol. 2018;48:564‐575. [DOI] [PubMed] [Google Scholar]
- 30. Hill E, Sapa H, Negrea L, et al. Effect of Oat β‐Glucan supplementation on chronic kidney disease: a feasibility study. J Ren Nutr. 2020;30:208‐215. [DOI] [PubMed] [Google Scholar]
- 31. Opdebeeck B, Maudsley S, Azmi A, et al. Indoxyl sulfate and p‐Cresyl sulfate promote vascular calcification and associate with glucose intolerance. J Am Soc Nephrol. 2019;30:751‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Six I, Flissi N, Lenglet G, et al. Uremic toxins and vascular dysfunction. Toxins (Basel). 2020;12:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodrigues SD, Santos SS, Meireles T, et al. Uremic toxins promote accumulation of oxidized protein and increased sensitivity to hydrogen peroxide in endothelial cells by impairing the autophagic flux. Biochem Biophys Res Commun. 2020;523:123‐129. [DOI] [PubMed] [Google Scholar]
- 34. Devlin AS, Marcobal A, Dodd D, et al. Modulation of a circulating uremic solute via rational genetic manipulation of the gut microbiota. Cell Host Microbe. 2016;20:709‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paroni R, Casati S, Dei Cas M, Bignotto M, Rubino FM, Ciuffreda P. Unambiguous characterization of ‐cresyl sulfate, a protein‐bound uremic toxin, as biomarker of heart and kidney disease. Molecules. 2019;24:3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jovanovich A, Isakova T, Stubbs J. Microbiome and cardiovascular disease in CKD. Clin J Am Soc Nephrol. 2018;13:1598‐1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lano G, Burtey S, Sallee M. Indoxyl sulfate, a uremic endotheliotoxin. Toxins (Basel). 2020;12:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hénaut L, Mary A, Chillon J‐M, Kamel S, Massy ZA. The impact of uremic toxins on vascular smooth muscle cell function. Toxins (Basel). 2018;10:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chitalia VC, Shivanna S, Martorell J, et al. Uremic serum and solutes increase post‐vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation. 2013;127:365‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nakano T, Katsuki S, Chen M, et al. Uremic toxin indoxyl sulfate promotes proinflammatory macrophage activation via the interplay of OATP2B1 and Dll4‐Notch signaling. Circulation. 2019;139:78‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. He X, Jiang H, Gao F, Liang S, Wei M, Chen L. Indoxyl sulfate‐induced calcification of vascular smooth muscle cells via the PI3K/Akt/NF‐κB signaling pathway. Microsc Res Tech. 2019;82:2000‐2006. [DOI] [PubMed] [Google Scholar]
- 42. Tumur Z, Shimizu H, Enomoto A, Miyazaki H, Niwa T. Indoxyl sulfate upregulates expression of ICAM‐1 and MCP‐1 by oxidative stress‐induced NF‐kappaB activation. Am J Nephrol. 2010;31:435‐441. [DOI] [PubMed] [Google Scholar]
- 43. Chen J, Ning Y, Zhang H, et al. METTL14‐dependent m6A regulates vascular calcification induced by indoxyl sulfate. Life Sci. 2019;239:117034. [DOI] [PubMed] [Google Scholar]
- 44. Gryp T, Vanholder R, Vaneechoutte M, Glorieux G. p‐Cresyl Sulfate. Toxins (Basel). 2017;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boelaert J, Lynen F, Glorieux G, et al. A novel UPLC‐MS‐MS method for simultaneous determination of seven uremic retention toxins with cardiovascular relevance in chronic kidney disease patients. Anal Bioanal Chem. 2013;405:1937‐1947. [DOI] [PubMed] [Google Scholar]
- 46. Jing YJ, Ni JW, Ding FH, et al. p‐Cresyl sulfate is associated with carotid arteriosclerosis in hemodialysis patients and promotes atherogenesis in apoE‐/‐ mice. Kidney Int. 2016;89:439‐449. [DOI] [PubMed] [Google Scholar]
- 47. Sun C‐Y, Chang S‐C, Wu M‐S. Uremic toxins induce kidney fibrosis by activating intrarenal renin‐angiotensin‐aldosterone system associated epithelial‐to‐mesenchymal transition. PLoS ONE. 2012;7:e34026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alique M, Bodega G, Corchete E, et al. Microvesicles from indoxyl sulfate‐treated endothelial cells induce vascular calcification in vitro. Comput Struct Biotechnol J. 2020;18:953‐966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guerrero F, Carmona A, Obrero T, et al. Role of endothelial microvesicles released by p‐cresol on endothelial dysfunction. Sci Rep. 2020;10:10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rayego‐Mateos S, Valdivielso JM. New therapeutic targets in chronic kidney disease progression and renal fibrosis. Expert Opin Ther Targets. 2020;24:655‐670. [DOI] [PubMed] [Google Scholar]
- 51. Rossi M, Johnson DW, Morrison M, et al. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial. Clin J Am Soc Nephrol. 2016;11:223‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Opdebeeck B, D'Haese PC, Verhulst A. Molecular and cellular mechanisms that induce arterial calcification by indoxyl sulfate and P‐Cresyl sulfate. Toxins (Basel). 2020;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Massy ZA, Metzinger‐Le Meuth V, Metzinger L. MicroRNAs are associated with uremic toxicity, cardiovascular calcification, and disease. Contrib Nephrol. 2017;189:160‐168. [DOI] [PubMed] [Google Scholar]
- 54. Shang F, Wang S‐C, Hsu C‐Y, et al. MicroRNA‐92a mediates endothelial dysfunction in CKD. J Am Soc Nephrol. 2017;28:3251‐3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang H, Chen J, Shen Z, et al. Indoxyl sulfate accelerates vascular smooth muscle cell calcification via microRNA‐29b dependent regulation of Wnt/β‐catenin signaling. Toxicol Lett. 2018;284:29‐36. [DOI] [PubMed] [Google Scholar]
- 56. Hoyles L, Jiménez‐Pranteda ML, Chilloux J, et al. Metabolic retroconversion of trimethylamine N‐oxide and the gut microbiota. Microbiome. 2018;6:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang X, Li Y, Yang P, et al. Trimethylamine‐N‐Oxide promotes vascular calcification through activation of NLRP3 (Nucleotide‐Binding Domain, Leucine‐Rich‐Containing Family, Pyrin Domain‐Containing‐3) inflammasome and NF‐κB (Nuclear Factor κB) signals. Arterioscler Thromb Vasc Biol. 2020;40:751‐765. [DOI] [PubMed] [Google Scholar]
- 58. Benz K, Hilgers K‐F, Daniel C, Amann K. Vascular calcification in chronic kidney disease: the role of inflammation. Int J Nephrol. 2018;2018:4310379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. López‐Mejías R, González‐Gay MA. IL‐6: linking chronic inflammation and vascular calcification. Nat Rev Rheumatol. 2019;15:457‐459. [DOI] [PubMed] [Google Scholar]
- 60. Sun X, Jiao X, Ma Y, et al. Trimethylamine N‐oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS‐TXNIP‐NLRP3 inflammasome. Biochem Biophys Res Commun. 2016;481:63‐70. [DOI] [PubMed] [Google Scholar]
- 61. Chen M‐L, Zhu X‐H, Ran L, Lang H‐D, Yi L, Mi M‐T. Trimethylamine‐N‐oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3‐SOD2‐mtROS signaling pathway. J Am Heart Assoc. 2017;6(9):e006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tang WHW, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA, Solas M. Implication of trimethylamine N‐oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients. 2018;10:1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu J, Zhang T, Wang Y, et al. Baicalin ameliorates neuropathology in repeated cerebral ischemia‐reperfusion injury model mice by remodeling the gut microbiota. Aging. 2020;12:3791‐3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pathak P, Xie C, Nichols RG, et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G‐protein bile acid receptor‐1 signaling to improve metabolism. Hepatology. 2018;68:1574‐1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shapiro H, Kolodziejczyk AA, Halstuch D, Elinav E. Bile acids in glucose metabolism in health and disease. J Exp Med. 2018;215:383‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gai Z, Chu L, Hiller C, et al. Effect of chronic renal failure on the hepatic, intestinal, and renal expression of bile acid transporters. Am J Physiol Renal Physiol. 2014;306:F130‐F137. [DOI] [PubMed] [Google Scholar]
- 68. Panda DK, Bai X, Sabbagh Y, et al. Defective interplay between mTORC1 activity and endoplasmic reticulum stress‐unfolded protein response in uremic vascular calcification. Am J Physiol Renal Physiol. 2018;314:F1046‐F1061. [DOI] [PubMed] [Google Scholar]
- 69. Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. 2017;13:368‐380. [DOI] [PubMed] [Google Scholar]
- 70. Song X, Sun X, Oh SF, et al. Microbial bile acid metabolites modulate gut RORgamma(+) regulatory T cell homeostasis. Nature. 2020;577:410‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Miyazaki‐Anzai S, Levi M, Kratzer A, Ting TC, Lewis LB, Miyazaki M. Farnesoid X receptor activation prevents the development of vascular calcification in ApoE‐/‐ mice with chronic kidney disease. Circ Res. 2010;106:1807‐1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692‐703. [DOI] [PubMed] [Google Scholar]
- 73. de Punder K, Pruimboom L. Stress induces endotoxemia and low‐grade inflammation by increasing barrier permeability. Front Immunol. 2015;6:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hénaut L, Massy ZA. New insights into the key role of interleukin 6 in vascular calcification of chronic kidney disease. Nephrol Dial Transplant. 2018;33:543‐548. [DOI] [PubMed] [Google Scholar]
- 75. Zhang K, Zhang Y, Feng W, et al. Interleukin‐18 enhances vascular calcification and osteogenic differentiation of vascular smooth muscle cells through TRPM7 activation. Arterioscler Thromb Vasc Biol. 2017;37:1933‐1943. [DOI] [PubMed] [Google Scholar]
- 76. Wu M, Rementer C, Giachelli CM. Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int. 2013;93:365‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vaziri ND, Wong J, Pahl M, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308‐315. [DOI] [PubMed] [Google Scholar]
- 78. Brandsma E, Kloosterhuis NJ, Koster M, et al. A proinflammatory gut microbiota increases systemic inflammation and accelerates atherosclerosis. Circ Res. 2019;124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhao Y, Cai Y, Cui LY, et al. Suppression of gut bacterial translocation ameliorates vascular calcification through inhibiting toll‐like receptor 9‐mediated BMP‐2 expression. Oxid Med Cell Longev. 2019;2019:3415682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. González‐Guerrero C, Cannata‐Ortiz P, Guerri C, Egido J, Ortiz A, Ramos AM. TLR4‐mediated inflammation is a key pathogenic event leading to kidney damage and fibrosis in cyclosporine nephrotoxicity. Arch Toxicol. 2017;91:1925‐1939. [DOI] [PubMed] [Google Scholar]
- 81. Koh A, De Vadder F, Kovatcheva‐Datchary P, Backhed F. From dietary fiber to host physiology: short‐chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332‐1345. [DOI] [PubMed] [Google Scholar]
- 82. Ahmad MS, Krishnan S, Ramakrishna BS, Mathan M, Pulimood AB, Murthy SN. Butyrate and glucose metabolism by colonocytes in experimental colitis in mice. Gut. 2000;46:493‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Saad MJ, Santos A, Prada PO. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology (Bethesda, Md). 2016;31:283‐293. [DOI] [PubMed] [Google Scholar]
- 84. Bartolomaeus H, Balogh A, Yakoub M, et al. Short‐chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139:1407‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yu X, Wu Z, Song Z, et al. Single‐anastomosis duodenal jejunal bypass improve glucose metabolism by regulating gut microbiota and short‐chain fatty acids in Goto‐Kakisaki rats. Front Microbiol. 2020;11:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang P, Li Y, Du Y, Li G, Wang L, Zhou F. Resveratrol ameliorated vascular calcification by regulating Sirt‐1 and Nrf2. Transpl Proc. 2016;48:3378‐3386. [DOI] [PubMed] [Google Scholar]
- 87. Assefa EG, Yan Q, Gezahegn SB, et al. Role of resveratrol on indoxyl sulfate‐induced endothelial hyperpermeability via aryl hydrocarbon receptor (AHR)/Src‐dependent pathway. Oxid Med Cell Longev. 2019;2019:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. 2017;61:1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kimble R, Keane KM, Lodge JK, Howatson G. Dietary intake of anthocyanins and risk of cardiovascular disease: A systematic review and meta‐analysis of prospective cohort studies. Crit Rev Food Sci Nutr. 2019;59:3032‐3043. [DOI] [PubMed] [Google Scholar]
- 90. Wang D, Xia M, Yan X, et al. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA‐10b. Circ Res. 2012;111:967‐981. [DOI] [PubMed] [Google Scholar]
- 91. Tsai Y‐L, Lin T‐L, Chang C‐J, et al. Probiotics, prebiotics and amelioration of diseases. J Biomed Sci. 2019;26:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. da Silva TF, Casarotti SN, de Oliveira GLV, Penna ALB. The impact of probiotics, prebiotics, and synbiotics on the biochemical, clinical, and immunological markers, as well as on the gut microbiota of obese hosts. Crit Rev Food Sci Nutr. 2020;61(2):337‐355. [DOI] [PubMed] [Google Scholar]
- 93. Shen Z, Zhu C, Quan Y, et al. Insights into Roseburia intestinalis which alleviates experimental colitis pathology by inducing anti‐inflammatory responses. J Gastroenterol Hepatol. 2018;33:1751‐1760. [DOI] [PubMed] [Google Scholar]
- 94. Jiang L, Xie M, Chen G, Qiao J, Zhang H, Zeng X. Phenolics and carbohydrates in buckwheat honey regulate the human intestinal microbiota. Evid Based Complement Alternat Med. 2020;2020:6432942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hidalgo M, Oruna‐Concha MJ, Kolida S, et al. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J Agric Food Chem. 2012;60:3882‐3890. [DOI] [PubMed] [Google Scholar]
- 96. Matsumoto T, Kojima M, Takayanagi K, Taguchi K, Kobayashi T. Role of S‐Equol, indoxyl sulfate, and trimethylamine N‐oxide on vascular function. Am J Hypertens. 2020;33(9):793‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jaeggi T, Kortman GAM, Moretti D, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. 2015;64:731‐742. [DOI] [PubMed] [Google Scholar]
- 98. Wang Z, Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein & Cell. 2018;9:416‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]


