Abstract
Tissue-resident macrophages are highly specialized to their tissue-specific microenvironments, activated by various inflammatory signals and modulated by genetic and environmental factors. Osteoclasts and microglia are distinct tissue-resident cells of the macrophage lineage in bone and brain that are responsible for pathological changes in osteoporosis and Alzheimer’s disease (AD), respectively. Osteoporosis is more frequently observed in individuals with AD compared to the prevalence in general population. Diagnosis of AD is often delayed until underlying pathophysiological changes progress and cause irreversible damages in structure and function of brain. As such earlier diagnosis and intervention of individuals at higher risk would be indispensable to modify clinical courses. Pleiotropy is the phenomenon that a genetic variant affects multiple traits and the genetic correlation between two traits could suggest a shared molecular mechanism. In this review, we discuss that the Pyk2-mediated actin polymerization pathway in osteoclasts and microglia in bone and brain, respectively, is the horizontal pleiotropic mediator of shared risk factors for osteoporosis and AD.
Subject terms: Bone, Neurophysiology
Introduction
Bone undergoes constant remodeling to maintain bone homeostasis between bone formation and resorption by osteoblasts and osteoclasts, respectively.1 Bone remodeling is a process of bone resorption followed by replacement of new bone formation, which is a tightly balanced work referred to as coupling.2 The osteoclast is a large multinucleated cell derived from monocyte/macrophage lineage of pluripotential hematopoietic stem cells (HSCs). Osteoclasts degrade the bone matrix with hydrogen ions and catalytic enzymes, whereas osteoblasts—mononuclear cells derived from mesenchymal cells—secrete organic matrix molecules that contribute to the formation of new bone. Osteoclasts undergo differentiation and fusion resulting in multinucleated cells, in the presence of macrophage colony-stimulating factor (M-CSF, also known as CSF1) and the key osteoclastogenic cytokine, receptor activator of NF-kB ligand (RANKL), characterized by expression of osteoclast markers, such as tartrate-resistant acid phosphatase,3 matrix metalloproteinase (MMP-9),4 cathepsin K5, and vacuolar [H+]-ATPase.6 Bone resorption is a necessary process for bone growth, tooth eruption, fracture healing and maintaining appropriate level of calcium in blood. Under pathological conditions such as estrogen deficiency or inflammatory conditions like rheumatoid arthritis, abnormal osteoclast proliferation, and differentiation accelerate bone resorption that results in osteolysis.1
Osteoporosis is a systemic disorder characterized by abnormally increased osteoclasts activity, therefore leading to bone fragility and an increased risk of fracture, and current therapies are targeting inhibition of osteoclast differentiation and function.7,8 This imbalance between resorption and formation is induced by diverse risk factors such as alteration in hormone expression, nutrition, mobility, and senescence. Additionally, some rare genetic disorders show decreased bone resorption that leads to osteopetrosis.9,10
Microglia are innate immune cells in the central nervous system (CNS) that account for 10%–15% of all cells in the human brain. Microglia are specialized brain-resident macrophages whose functions in the brain include phagocytosis and provision of trophic support.11,12 Besides, these cells are active regulators of synapse formation, plasticity, and elimination.13 Microglia engulf C1q—the initiating protein of classical complement pathway—tagged synapses in developing brain12 and seem to be responsible for synaptic loss in neurodegenerative disorders. As such dysregulation of microglia has been found in diverse neuropsychiatric, neurodegenerative, and neuroinflammatory diseases.14
Alzheimer’s disease (AD) is the most common neurodegenerative disorder that is likely affecting over 40 million patients worldwide. AD is also the most common form of dementia that accounts for more than 60% of sporadic cases. Our understanding of AD pathobiology has been greatly improved over the past two decades; however, no disease-modifying treatment is available for individuals diagnosed with AD.15 Age is the primary risk factor for AD and an accumulation of misfolded/aggregated proteins such as senile plaques and neurofibrillary tangles (NFTs) cause pathological changes in brain.16 The amyloid β peptides (Aβ) are natural cleavage products of the Aβ precursor protein (APP) by β- and γ-secretases and aggregate to form oligomers and fibrils. Of Aβ peptides, Aβ42 (i.e., 42 amino acids long cleavage product of APP) is aggregation-prone and more immunogenic compared to the other isoforms such as Aβ40. Brain is the most studied organ for APP expression and function; however, the other splicing isoforms of APP are expressed in many other tissues such as skin, heart, muscle, adipose tissue, liver, spleen, skin, and intestine.17 Soluble Aβ can be transported across the blood–brain barrier, from blood to brain via the receptor for advanced glycation end-products (RAGE), and from brain to blood via low-density lipoprotein receptor-related protein 1 (LRP1). Interestingly, Aβ42 can enhance osteoclast differentiation and activation.18 Aβ deposition is less correlated with cognitive decline compared to accumulation of NFTs due to hyperphosphorylated forms of tau. Multiple evidence support that Aβ accumulation precedes and drives tau aggregation. Nonetheless, Aβ seems to be responsible for synaptic failure and mitochondrial dysfunction that are observed in multiple brain regions in patients with AD. The innate immune system responds to Aβ deposition. In early stage of disease, microglia engulf Aβ through phagocytosis and astrocytes use receptor-mediated internalization to clear Aβ.19,20 As the disease progresses, activated microglia release chemokines and cytokines to further facilitate inflammatory reaction that damages neural tissues in later stage of AD.15 Moreover, genes with common and rare risk alleles for AD (e.g., TREM221) are preferentially or exclusively expressed in microglia. These findings strongly support the role of microglia in the pathogenesis of AD according to the disease stages of AD.22 To date, clinical trials targeting Aβ failed to demonstrate clinical efficacy. The Aβ deposition likely begins as early as 20 years before cognitive impairment.15 Therefore, early identification of high-risk groups for AD using clinical and molecular biomarkers is highly required.
Pleiotropic effect of DNA variants associated with bone and brain disorders
Two tissue-resident cells of myeloid origin—i.e., osteoclasts and microglia—contribute the pathogenesis of osteoporosis and AD, respectively.23,24 These cell types are specialized to their microenvironment for its main functional role in each tissue, and seem to be disconnected, physically and functionally; however, a rare genetic disorder affecting brain and bone suggests the pleiotropy of a causal gene. Nasu-Hakola disease (NHD) is an autosomal recessive disorder caused by rare genetic variants in either triggering receptor expressed on myeloid cells 2 (TREM2) or DNAX Adaptor Protein 12 kD (DAP12) genes. NHD is characterized by recurrent bone fractures and progressive presenile dementia.25 Osseous symptoms due to osteoporotic lesions start typically in the age of 20–30 years followed by neurologic symptoms such as dementia that are observed in the age of 40–50 years. DAP12 encodes the tyrosine kinase binding adaptor protein (TYROBP) and TREM2 encodes the triggering receptor expressed on myeloid cells 2 (TREM2). These genes are the components of a signaling complex involved in the regulation of immune responses, the differentiation of osteoclasts, and in the phagocytic activity of microglia.23 The exact mechanism of pathogenesis for NHD is unknown.
Low bone mineral density (BMD) is associated with cognitive decline and a higher risk of AD, and the risk of fracture is increased in patients with AD. As such epidemiological studies suggest associations between AD and osteoporosis26–28; however, there is no consistent evidence that osteoporosis is a risk factor of AD at the population-scale. As often observed in individuals with NHD, BMD change may precede cognitive decline in AD due to shared biological pathways that are affected by common genetic and/or environmental risk factors.29 Here we describe converging molecular pathways between osteoclasts and microglia that may explain, in part, horizontal pleiotropy of shared genetic risk factor between osteoporosis and AD. Firstly, we review the developmental origins of osteoclasts and microglia. Secondly, the signaling pathways—i.e., TREM2/DAP12, CSF1/CSF1R and CCR5 pathways—that are key regulators of both cell types are summarized. Thirdly, a converging pathway, PYK2 pathway, involving actin/microtubule formation for cytoskeletal rearrangement is highlighted. Finally, we discuss the genetic correlation between osteoporosis and AD.
Developmental perspective: osteoclasts and microglia
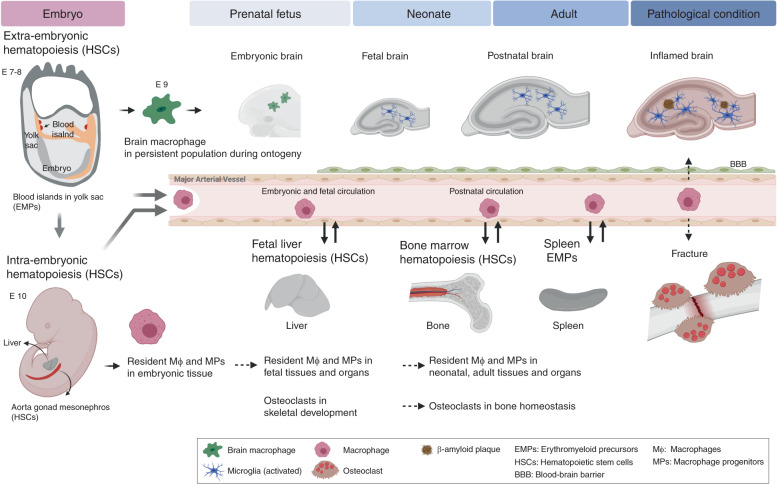
Osteoclasts and microglia are tissue-resident and -specific cells of monocyte/macrophage lineage in skeletal and neural systems, respectively.30,31 During ontogeny, the initial origin of macrophages is the blood island in yolk sac (YS), in which extraembryonic hematopoiesis takes place at mouse embryonic days E7–8, and 2–3 weeks of human gestation (Fig. 1). These YS-derived cells can produce macrophages, erythrocytes and lymphocytes.32,33 The YS-derived macrophages are distributed in later stages of whole embryonic body and primitive organs, in which hematopoiesis is succeeded to intraembryonic tissues which encompasses the aorta, gonads, and mesonephros (AGM) where HSCs are initially generated.30,31,34
Fig. 1.
Developmental overview and homeostasis regulations of osteoclasts and microglia. Primitive macrophages exit blood islands of the yolk sac (YS) with the initiation of circulation and colonized the neuroepithelium from E8.5 to give rise to microglia. In a steady state, YS-derived macrophages are differentiated into microglia which, in turn, self-renew throughout ontogeny until adulthood. Under pathological conditions, circulating progenitors or resident macrophages that are allowed to reside in brain contribute to newly differentiated microglia. The migration of definitive brain macrophage is initiated from the HSCs of the embryonic aorta-gonad-mesonephros (AGM). Subsequently, HSCs expand in the fetal liver, which is the main source for tissue-specific macrophages. Osteoclasts originated from resident embryonic-myeloid progenitors (EMPs) lineage, which are long-lived and can participate in postnatal bone maintenance. Adult bone homeostasis is maintained by osteoclast fusion that are derived from EMPs and HSCs supplied by bone marrow and possibly by spleen. In injured skeletal tissues, for instance due to bone fracture, circulating monocytes and macrophages from spleen migrate into damaged tissue and contribute to tissue repairing
In adulthood, there are mainly three origins of intraembryonic hematopoiesis: embryonic vessels, fetal liver, and bone marrow. The migration of YS-derived hematopoietic precursors rapidly initiates the intraembryonic hematopoiesis from major arterial vessels in mouse embryos beginning at E10.5 in mice (around 3 weeks of human gestation). This intraembryonic hematopoiesis is definitive to give rise to HSCs with multi-lineage potential. Subsequently HSCs expand in the fetal liver; main site of fetal hematopoiesis that peaks at E16.5 (around 4 weeks human gestation) before transitioning to the bone marrow that becomes the main site of hematopoiesis in adult life. Therefore, prior to the intraembryonic hematopoiesis, embryonic macrophages throughout whole body including brain-specific microglia are originated from extraembryonic YS.30,31 In the next step, the fetal liver gives rise to fetal monocytes, which subsequently differentiate and replace fetal and prenatal macrophages except microglia in CNS. Although the bone marrow hematopoiesis is established since perinatal stages, and becomes main source of macrophages in adulthood, it is not still clear the contributions of the embryonic YS- and fetal liver-derived macrophages to the adult tissue-resident macrophages.
The developmental origin of microglia is the blood island developed in embryonic YS at E7-8 in mouse. The YS-derived brain macrophages colonize to the neuroepithelium around E9, and then differentiate into microglia that is maintained throughout ontogeny although brain under pathological conditions like inflammation allow circulating progenitors reside in brain and contribute to newly differentiated microglia35,36 (Fig. 1). In bone and mineral homeostasis, it has been dogmatically understood that bone marrow-derived monocytes can be a major population of osteoclast precursors. In fact, osteopetrotic phenotype has been partially treated by bone marrow transplantation in humans and mice, suggesting partial requirement of bone marrow as a source of osteoclasts.37 Contribution of circulating osteoclastic precursors has been also reported.38 During perinatal skeletal development, osteoclasts and chondroclasts (cartilage resorbing cells) participate in bone modeling, endochondral bone formation, and tooth eruption, all of which are essential process for pre- and postnatal skeletal development. Recent findings showed that embryonic erythromyeloid precursors (EMPs) derived from YS and fetal liver contributed to osteoclasts participated in early skeletogenesis.37,39 Adult bone homeostasis is maintained by iterative fusion of osteoclasts derived from the fetal EMPs and HSCs supplied by bone marrow.39 Of note, YS-derived osteoclasts can be colonized in the adult spleen and contribute to bone repair after injury.39
In terms of differentiation potential hierarchy, dendritic cells (DCs), monocytes and macrophages are understood to share the monocyte-macrophage DC progenitor (MDP) derived from HSCs. More recent studies identified a downstream progenitor cell population from the MDP, namely common monocyte progenitor (cMoP), whose differentiation potential is restricted to monocytes and macrophages.40 The cMoP is also identified in human umbilical cord blood and in bone marrow. Although it remains elusive what is the exact population of circulating progenitors that contribute to tissue-specific macrophages both in injured bone and brain, these populations likely share common regulatory pathways in their cellular differentiation.
Common signaling pathways between osteoclasts and microglia
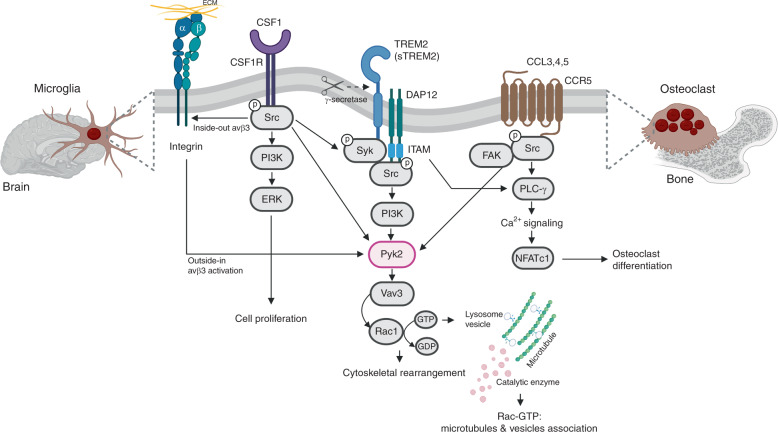
Osteoclasts and microglia diverge during development and differentiation according to their tissue microenvironments. Nonetheless, they share key signaling pathways of which three receptor signaling pathways—i.e., TREM2/DAP12, CSF1, and CCR5—that converge to regulate actin-microtubule dynamics and cytoskeleton organization through Pyk2 signaling pathway (Fig. 2).
Fig. 2.
TREM2/DAP12-, CSF1/CSF1R-, and CCR5 pathways in osteoclasts and microglia. Once ligands bind to TREM2, two tyrosine residues in the immunoreceptor tyrosine-based activation motif (ITAM) of DAP12 are phosphorylated, which in turn recruits Syk kinase to activate downstream molecules such as Src tyrosine kinase and phosphatidylinositol 3-kinase (PI3K). The soluble form of TREM2 (sTREM2) is generated by ɣ-secretase, which activates PI3K, extracellular signal-regulated protein kinase (ERK), and NF-kB. Src, the main effector of CSF1R, is a tyrosine kinase that phosphorylates the ITAM tyrosine residues. CCL3/4/5 binding to CCR5 activates G protein and multiple downstream signals such as Src, PLC-ɣ and PI3K. PI3K triggers the tyrosine phosphorylation of focal adhesion complex components such as Pyk2, paxillin, Crk, and p130Cas, leading to interaction with Vav. Pyk2-Vav interaction may control Rho family GTPase activation, thereby driving localized actin polymerization that stabilizes contacts with matrix and/or promote migration
TREM2/DAP12 signaling pathway
TREM2 is a transmembrane glycoprotein of about 40 kD with a single extracellular immunoglobulin-like domain, a transmembrane domain and a short cytoplasmic tail without signaling motif.41 The cytoplasmic domain of TREM2 does not have signaling motifs, therefore its transmembrane domain interacts with the adaptor protein DAP12. DAP12 is an adaptor protein that contains immunoreceptor tyrosine-based activation motif (ITAMs) in their cytoplasmic domain, which function as docking sites for protein ligand.42 The ITAMs of DAP12 are phosphorylated with TREM2 ligand binding, and subsequently recruit spleen tyrosine kinase (SYK) that initiates activation of various signaling cascades such as phosphoinositide 3-kinase (PI3K), VAV (Vav guanine nucleotide exchange factor), and mitogen-activated protein kinase (MAPK) activation and calcium mobilization.42 The ectodomain of TREM2 undergoes sequential cleavages by α-secretase and metalloprotease domain-containing protein 17 (ADAM17), which produces soluble TREM2 (sTREM2).43,44 DAP12 is released once the remaining C-terminal portion of TREM2 is cleaved by γ-secretase. The ITAM is rapidly phosphorylated Src tyrosine kinase, thus allowing a docking site for the SH2 domains of other kinases, such as Syk.42 TREM2 is expressed by osteoclasts and microglia in vivo as well as monocyte-derived DCs and bone marrow-derived macrophages for both human and mouse.45
TREM2/DAP12 mediates osteoclasts differentiation and function
Osteoclastogenesis and bone remodeling are controlled by TREM2/DAP12.46,47 The mechanisms underlying bone phenotype in NHD are not fully understood yet while mutations in TREM2/DAP12 are likely to disable osteoclasts to differentiate and migrate. TREM2, together with DAP12, recruits the protein tyrosine kinase Syk. Phosphorylation of Src, PI3K, and PLC-ɣ leads to Ca2+ mobilization and activation of the transcription factors—NFAT, NF-kB, and AP1—for osteoclast differentiation.48,49 ITAM-harboring adaptors FcRγ and DAP12 are essential for osteoclast differentiation through activation of Ca2+ oscillations and NFATc1 production in RANKL-induced osteoclastogenesis.49 Consistent with these findings, blockade of TREM2 inhibited bone resorption and TREM2 stimulation enhanced migration of mature osteoclasts that were generated from bone marrow macrophages or RAW264.7 cells.50 Additionally, human studies showed that loss-of-function variants in TREM2 or DAP12 result in a defective osteoclasts differentiation in response to RANKL, which cause the reduction of bone resorption ability in vitro.51,52 F-actin was significantly decreased in TREM2-deficient osteoclasts compared to wildtype.51,52 Proper polarization of F-actin is required for promoting cell fusion as well as transforming osteoclast precursors into multinucleated osteoclasts. Although the bone resorption capability of TREM2-/DAP12-deficient osteoclasts was impaired in vitro, the bone of NHD patients is osteoporotic due to the loss of trabeculae bone. Given these pathologic discrepancies between genetically deficient/cultured osteoclasts in vitro and local osteolytic phenotype in NHD patients, the role of TREM2/DAP12 in regulating osteoclastogenesis could be context specific. One possibility is that enhanced bone loss in vivo can be explained by systemic or local factors (endocrine, paracrine, etc.) affecting the differentiation or activation of osteoclasts in situ. A similar osteoporotic phenotype in NHD observed in Trem2-deficient mice.53 Alternatively, TREM2/β-catenin pathway regulated bone mass by modulating the rate of osteoclastogenesis.53 Deletion of either TREM2 or β-catenin inhibits the CSF-1-induced proliferation of osteoclast precursors but accelerates their differentiation into mature osteoclasts, which ultimately causes osteoporotic phenotype like NHD. Understanding the role of osteoclasts in the bone phenotype in NHD is yet to be elucidated while TREM2/DAP12 pathway is an attractive target for treatment of osteoporosis.
TREM2/DAP12 mediates microglia function
In brain, TREM2 and DAP12 are mainly expressed in microglia, but not in astrocytes.54 TREM2 is actively transcribed in microglia at homeostasis. Immunogenic molecules such as lipopolysaccharides and interferon-γ suppress TREM2 expression.55
Damaged neurons increase the expression of endogenous TREM2 ligands, which induces phagocytic activity by microglia.56 In AD brain, TREM2 is detected in neuronal and microglial cells in the vicinity of Aβ-containing plaques that leads to phagocytosis of neuronal cells with Aβ-containing plaques,57 which most possible is sTREM2 attached on neuronal cells. The functional deficit of TREM2, DAP12 or both leads to a failure of Aβ engulfment. As such Trem2 deficiency augments Aβ accumulation is due to dysfunctional responses of microglia: failing to form cluster around Aβ and becoming apoptotic rather than undergoing activation and proliferation.58 Disease-associated microglia (DAM) are localized with Aβ-containing plaques in 5XFAD mice that express human APP and PSEN1 transgenes with five known AD-linked mutations. The microglia from AD mice showed up-regulation of Apoe, Tyrobp, and Trem2 and downregulation of homeostatic genes such as P2ry12 and Cx3cr1.59 Once microglia are exposed to Aβ containing plaques, TREM2-APOE pathway is activated to transform a subset of homeostatic microglia to DAM.60 In summary, TREM2/DAP12 pathway is required for the recruitment and phagocytic activity of microglia in AD brain.
CSF1 receptor signaling pathway
Colony-stimulating factor-1 (CSF1) is an essential factor that stimulates the proliferation and differentiation of macrophages from its progenitors. The expression level of CSF1 is low in HSCs and high in monocytes, tissue macrophages, osteoclast, myeloid DCs61, and microglia.62 CSF1 receptor (CSF1R, also known as c-FMS) has five immunoglobulin domains, a transmembrane domain, an intracellular juxtamembrane domain, and an intracellular tyrosine kinase domain.63 CSF1 binding initially leads to rapid dimerization of the CSF1R, and autophosphorylation of specific tyrosine residues and then transphosphorylation of several other proteins including SRC, PLC-γ, PI3K, AKT, and ERK in cytoplasmic tail of CSF1R.64 CSF1 stimulation enhanced the actin cytoskeleton reorganization and adhesion formation through integrin signal activation in macrophages.65
CSF1 stimulates the osteoclast proliferation and adhesion
Osteoclastogenesis depends on CSF1 and RANKL that are produced by osteoblasts and osteocytes. In the bone marrow microenvironment, CSF1 primarily promotes the proliferation and survival of osteoclast precursors. Csf1op/op mice that express nonfunctional CSF1 lack osteoclasts and develop severe osteopetrosis. The administration of soluble CSF1 to Csf1op/op mice rescues osteoclast formation ability and results in osteoporosis instead of osteopetrosis.66 The functional relationship between Csf1 and its receptor was established in mice lacking c-Fms (or Csf1r), which also have osteopetrosis. These mice had decreased number of macrophages in bone marrow and presented severe osteopetrosis owing to the lack of osteoclasts.67,68 Dimerization of CSF1R which initiated by CSF1 binding activate its tyrosine kinase domain followed by phosphorylation of six tyrosine residues in the cytoplasmic domain. These residues facilitate as high-affinity binding sites for Src homology region 2 (SH2) domains within cytoplasmic region. In turn, phosphor-Y599/c-Src recruits PI3K to activate the Akt69 pathway and also recruit c-Cbl E3 ubiquitin ligase complex70 that also regulates the proliferation of osteoclast precursors.71 Moreover, integrin signaling in osteoclast associated with Src is mediated by ITAM proteins including DAP12 and FcRγ, thus organizing the osteoclast cytoskeleton.72
CSF1 controls microglia homeostasis in brain
Signaling through the CSF1R is required for microglia homeostasis during development and in mature brain.73 Microglia are depleted in the brain of Csf1r-deficient mice from birth and at all stages of postnatal development.74,75 However, the brain phenotype caused by the loss of Csf1 signaling seems to be mild such as sensory deficit and electrophysiological abnormalities in neuronal cells.76 The discovery of interleukin 34 (IL-34) as a ligand for CSF1R explained, in part, the discrepancy between phenotypes of Csf1 knock-out and Csf1r knock-out mice.77 Dominant-negative mutations in the tyrosine kinase domain of CSF1R causes hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS) that is characterized by a broader range of neurological symptoms including progressive cognitive decline, motor disturbances and seizures.78 Also, CSF1 deficiency resulted in severe cerebellar phenotype such as defects in motor function and social behavior.79 The CSF1 receptor antagonist JNJ-40346527 is in phase I clinical trial for individuals with mild cognitive impairment (ClinicalTrial.gov NCT04121208).80 Importantly, this trial is designed to identify biomarkers of CSF1 signaling pathway such as IL-34 and CSF1 that may be changed in response to CSF1 receptor antagonist.
CCR5 signaling pathway
CCR5 is belonging to the G-protein coupled receptor (GPCR) superfamily. Members of Rho family of GTPases (Cdc42, Rac1, Rac2, and RhoA etc.) are important components of signal transduction for the actin cytoskeletal rearrangement in chemokine-mediated cellular events. CCR5 regulates chemotaxis through interactions with MIP-1alpha (CCL3), MIP-1beta (CCL4), and RANTES (CCL5).81 Binding of CCL5 to CCR5 initiates conformational changes in G-proteins, in turn, activates multiple signaling cascades such as RAC1 and PAK2 in a Gi- and PI3K, protein kinase C, MAPK, Pyk2, and arrestin pathways as well as calcium influx.81–83 These signaling pathways stimulate various cellular functions including cytoskeleton rearrangement, and chemotaxis.
CCR5 regulates osteoclast function
Epidemiological studies suggest that CCR5-Δ32 variant is associated with a reduced incidence and severity of bone destruction disease such as rheumatoid arthritis.84 Inhibition of Ccr5 in mice decreased osteoclast formation suggesting beneficial skeletal effects of the functional loss of Ccr5.85,86 Blocking of CCR5 using antibodies impaired human osteoclast function in vitro.87 Ccr5 deficient (Ccr5−/−) mice were less susceptibility to osteoporotic stimulation via the administration of RANKL, which induces osteoporosis. In vitro, Ccr5–/– osteoclasts were showed defective actin ring formation. Also, Ccr5−/− mice showed disorganized cellular motility, which was associated with reduction in the RANKL-induced phosphorylation of Src, Pyk2, and subsequent downstream signals. In patients with HIV-1 infection, maraviroc (a CCR5 antagonist) treatment was associated with reduced bone loss at the hip and lumbar spine compared to tenofovir disoproxil fumarate (TDF)-containing antiretroviral therapy (ART).88 These data suggest that CCR5 has a critical role in bone disorders through the functional regulation of osteoclasts.
Microglia migration and recruitment via CCR5 in AD
CCR5 is a co-receptor for HIV-I entry into microglia that are the primary targets of HIV infection in the CNS.89 HIV-infected patients develop a progressive dementia with motor and behavioral impairment, which affected 20%–30% of patients with AIDS until the introduction of combined ART in the mid-1990s.90 Of note, the inhibition of CCR5 signaling could be associated with the enhancement of learning and memory by elevating MAPK and CREB levels in hippocampus and cortical circuits.91 Treatment with maraviroc improved the neurocognitive test performance in patients with moderate cognitive impairment,92,93 which was presumably due to reduced immune activation and inflammation.
CCL5 is a potent mediator of microglia recruitment to the site of CNS inflammation. The reorganization of actin cytoskeleton and migration of microglia were promoted in response to CCL5 in adult rat microglia and a human microglial cell line.94 Immunohistochemical study demonstrated that CCR3 and CCR5 were present on microglia of normal and AD brains and upregulated in reactive microglia observed in AD.95 Moreover, an increased CCL5 level in microglia in the vicinity of Aβ containing plaques seems to reduce Aβ deposition.96
PYK2-mediated actin cytoskeleton rearrangement
Pyk2 is a non-receptor tyrosine kinase and a member of focal adhesion kinase (FAK) family.97 Pyk2 is 65% homologous to FAK and shares a common domain structure—an N-terminal FERM domain, a protein tyrosine kinase (PTK) domain, three proline-rich regions, and a focal adhesion targeting (FAT) domain at the C-terminus—and has a SH2- and SH3-domain binding site. Although FAK is ubiquitously expressed in diverse cell types, the expression of Pyk2 is restricted to the CNS and in hematopoietic cells.98 Pyk2 is activated by various extracellular signals including cytokines, intracellular Ca2+ concentration, and integrin-mediated cell adhesion.98,99 FAT domain of Pyk2 is thought to interact with a paxillin and Pyk2-FAT and paxillin complex organize focal adhesion complexes and cytoskeletal rearrangement.100 Pyk2−/− mice are viable and fertile, without disability in development or behavior.101 However, macrophages isolated from Pyk2–/– mice were impaired to migrate in response to chemokine stimulation.101
The actin cytoskeleton plays essential roles for diverse cellular processes such as cell migration, axonal growth, phagocytosis and many other aspects of normal cell physiology.102 In immune cells, the ability to rapidly change shape in response to various stresses is critical for phagocytosis. Moreover, the cytoskeleton brings surface receptors and their substrates together to regulate signal transduction. Here we highlight that Pyk2 is a tethering mediator for actin reorganization of microglia and osteoclasts and that Pyk2 downstream pathway could be a converging point of cell receptor signaling pathways described above in the context of driving pathophysiological changes in osteoporosis and AD.
Pyk2 regulates osteoclastic bone resorption
Osteoclasts derived from hematopoietic precursor cells of the phagocyte lineage and differentiate into giant multinucleated cells by the fusion of osteoclast precursors.1,103 Mature osteoclasts have highly specialized morphological structures such as actin rings, sealing zone, and ruffled borders that construct an efficient machinery for dissolving hydroxyapatite and degrading bone matrix. Adhesion to bone matrix initiates osteoclast activation.104 Bone resorption is activated, the actin cytoskeletal reorganization is then regulated by a signaling network that includes integrins, the assembly and disassembly of focal adhesion proteins (paxillin, vinculin, and talin), c-Src105,106 and Pyk2107 in osteoclasts. Pyk2 is a main adherent tyrosine kinase in osteoclasts and regulates osteoclastic actin cytoskeletal organization in podosome for bone resorption.108 Once attached to the bone matrix, Pyk2 localizes to cytoskeletal proteins and colocalizes with the F-actin of podosomes.109 Pyk2 also colocalizes with vinculin in actin rings of osteoclasts when cultured on glass. Impaired bone resorption was observed in Pyk2-deficient osteoclasts due to their defection of podosome formation at the cell periphery.110 Moreover, Pyk2-deficient osteoclasts had significant reduction of microtubule acetylation and stability. As C-terminal domain of Pyk2 has paxillin-binding sites, Pyk2–paxillin complex is tightly associated with the recruitment of cytoskeletal proteins and the integrin activation in osteoclasts.107 Binding of Csf1 to its receptor, Csf1r-αVβ3 integrin association regulates the podosomal actin ring of osteoclast during adhesion by the pathway involving Pyk2, p130Cas and c-Cbl that known as downstream regulators of integrin-mediated signaling.111 Moreover, Dap12 activates Syk, and Pyk2, which promote phosphorylation and nuclear translocation of β-catenin.53,112 Together, multiple studies support that Pyk2 is required for normal cytoskeletal organization in osteoclasts for bone resorption.
Dysregulation of microglia function through Pyk2 signaling pathway in AD brain
Microglia are highly dynamic cells that undergo rapid cellular remodeling during membrane extension, migration, and phagocytosis.113 These processes are orchestrated by changes in the organization of the actin cytoskeleton and focal adhesions. Upon brain injuries or under pathological conditions, microglia undergo morphological transformation from “inactive” to “active” state. In response to inflammatory signals, CCL5 can elicit a change in the organization of F-actin cytoskeleton in rat microglia and human fetal microglial cell line that drives chemotaxis.114,115 Binding of a chemokine ligand to its seven-transmembrane domain receptor initiates the release of intracellular second messengers via G-protein complexes. This, in turn, causes downstream effects such as the reorganization of the cytoskeleton, formation of focal adhesion, and pseudopod extension, that are required for cell locomotion.94 Pyk2 is closely related to p125 FAK—coupling several receptors including integrin and chemokine receptors—for which a variety of downstream effectors—e.g., small G proteins—are involved in actin reorganization events, membrane ruffling and motility.116 For instance, tyrosine-phosphorylated Pyk2 was rapidly upregulated in activated microglia after focal cerebral ischemia and epilepsy in rat model.117 Several studies demonstrated that Aβ binding to CD36 on microglia initiated signal transduction and activation. Fyn (a Src family kinase) is activated by CD36 after binding to Aβ. In turn, Fyn phosphorylates p130Cas.113 Then, p130Cas is associated with the Pyk2 and paxillin for regulating the microglial cytoskeletal reorganization.96 Therefore, these downstream cascade of CD36 highlight the importance of microglial migration via actin polymerization in AD.118
Pyk2-mediated actin cytoskeleton reorganization in other immune cells
Cytotoxic T lymphocytes (CTL) are antigen (Ag)-specific cytotoxic cells, migrate to the infection area, and adhere to infected cells. Once T cells are stimulated with various ligands via T-cell receptor and integrins, and downstream Pyk2 is then activated. Pyk2 inhibition in CTL caused reduced cell motility and chemotactic difference.119 In a natural killer cell line (i.e., NK-92), the inhibition of Pyk2 activity decreased the integrin-regulated adhesion and defected clustering with a target cell.120 Activated eosinophils are recruited into infection cell and participate in inflammatory processes, such as allergic reactions.121 Blockade of Pyk2 using dominant-negative C-terminal Pyk2 fused to a TAT protein transduction domain (TAT-Pyk2-CT) inhibited the migration of eosinophils in a murine model of asthma.122 Pyk2 is activated by β2-integrin binding and is a required signal for eosinophil mobility and subsequent chemotaxsis.123 Similar to integrin ligation, neutrophil-like cells (HL-60) by silencing of Pyk2 expression were also attenuated cell migration.124 In DCs, interaction of gp120 with CCR5 initiated that the signal cascade of Pyk2 phosphorylation, which in turn activates p38 MAPK. p38 MAPK activates LSP1 (leukocyte-specific protein 1, F-actin-binding phospho-protein expressed in all human leukocytes), which then associates with actin, leading to consequent dendritic migration and chemotaxis.125 Taken together, these studies support an evidence of the Pyk2 function to actin cytoskeleton rearrangement, demonstrating that it contributes to the promotion of phagocytosis and migration in various cell type.
PTK2B encompasses risk alleles for osteoporosis and AD
Pleiotropy is the phenomenon of a single gene affecting multiple traits. In the case of NHD, rare loss-of-function variants in TREM2 or DAP12 result in osteoporosis and presenile dementia. Interestingly, osseous symptoms precede neurological manifestation. For this phenomenon, vertical pleiotropy can be the case where TREM2/DAP12 variants cause osteoporosis in the age of 20–30 years, which in turn causes presenile dementia in later life. Yet there is no evidence that osteoporosis causes AD. An alternative explanation can be the genetic variants in TREM2 or DAP12 are associated with two traits independently—i.e., horizontal pleiotropy—that are mediated by a common protein or pathway. The genetic variants in protein tyrosine kinase 2β (PTK2B) are significantly associated with AD, body mass index, BMD, and Takayasu arteritis suggesting that Pyk2 pathway could be a converging pathway of the genetic correlation between osteoporosis and AD (Fig. 3, Table 1).
Fig. 3.
Horizontal pleiotropy of common genetic factors. Pyk2 signal could be a converging pathway of the genetic correlation between osteoporosis and Alzheimer’s disease (AD). Genetic variants in PTK2B are significantly associated with AD, body mass index, and bone mineral density suggesting that the two traits—osteoporosis and AD—could be linked by horizontal pleiotropy
Table 1.
Risk alleles in three signaling pathways and PTK2B associated with bone and brain phenotypes from GWAS catalog
| Common pathway | Symbol | GWAS catalog | Trait(s) | PMID | rsID | Gene name | |||
|---|---|---|---|---|---|---|---|---|---|
| GWAS:BMD | GWAS:AD | Bone related | Brain related | Bone | Brain | ||||
| TREM2/DAP12 | TREM2 | No | Yes | Alzheimers disease (late onset) | PMID: 23150908 | rs75932628-T | Triggering receptor expressed on myeloid cells 2 | ||
| TYROBP | No | No | Transmembrane immune signaling adaptor | ||||||
| SYK | No | No | Spleen associated tyrosine kinase | ||||||
| PI3K | No | No | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha | ||||||
| SRC | Yes | No | Rheumatoid arthritis | PMID: 30891314 | SRC proto-oncogene, non-recpetor Tyrosine kinase | ||||
| mTOR | Yes | No | Heel bone mineral density | PMID: 28869591, PMID: 30048462 | rs75077113-A | Mechanistic target of rapamycin kinase | |||
| RAC1 | No | No | Post-traumatic stress disorder | PMID: 31358989 | rs33986000-? | Rac family small GTPase 1 | |||
| VAV3 | Yes | No | Heel bone mineral density | Anxiety disorder/bipolar disorder/PHF-tau measurement | PMID: 30595370 | PMID: 31043756, PMID: 32450446, PMID: 26989097 | rs10881475-?, rs436129-G, rs1777451-?, rs10881475-? | Vav guanine nucleotide exchange factor 3 | |
| PTPN6 | No | Yes | Schizophrenia | PMID: 27846195 | rs7963446-? | Protein tyrosine phosphatase non-receptor type 6 | |||
| CSF1/CSF1R | CSF1 | Yes | No | Bone density/heel bone mineral density | PMID: 29304378, PMID: 30595370 | rs7548588-T, rs7364724-A | Colony stimulating factor 1 | ||
| CSF1R | No | No | Feeling emotionally hurt measurement | PMID: 29500382 | rs2027798-T | Colony stimulating factor 1 receptor | |||
| CCR5 | CCR5 | No | No | C-C motif chemokine receptor 5 | |||||
| CREB1 | No | No | cAMP responsive element binding protein 1 | ||||||
| PTK2 (FAK) | No | No | Cognitive function/neurociticism /brain volume | PMID: 32439900, PMID: 29500382, PMID: 31676860 | rs10106406-C, rs6997840-T, rs1326108-? | Protein tyrosine kinase 2 | |||
| CDC42 | Yes | No | Heel bone mineral density | Intelligence/cognitive function/Schizophrenia | PMID: 30048462 | PMID: 29844566, PMID: 31374203 | rs10917152-T, rs5772984-?, rs2143103-A, rs2143103-? | Cell division cycle 42 | |
| RHOA | Yes | No | Vitamin D measurement | Intelligence/congnitive function | PMID: 32059762 | PMID: 29942086, PMID:30038396 | rs7623659-T | Ras homolog family member A | |
| ACTR2 | Yes | No | Heel bone mineral density | PMID: 30598549, PMID: 30048462 | rs36010930-T, rs4358110-? | Actin related protein 2 | |||
| Pyk2 | PTK2B | Yes | Yes | Bone mineral density/Takayasu arteritis/bone mineral content | Alzheimer’s disease (late onset) | PMID: 31790847, PMID: 25604533 | PMID: 24162737, PMID: 30617256, PMID: 29777097, PMID: 31473137 | rs28834970-C, rs2271920-A, rs7000615-C, rs2322599-G, rs7005183-? | Protein tyrosine kinase 2 beta |
Autosomal dominant AD is caused by one of the mutations in APP, PSEN1, or PSEN2. Rare genetic variants in these genes have high impact; however, such causal variants are not found in the majority of individuals with late-onset AD. A large-scale meta-analysis of 74 046 individuals highlighted novel susceptibly loci for AD.126 In addition to the APOE locus, this study discovered 11 novel loci that were significantly associated with AD. Among these, rs28834970 in the PTK2B gene is associated with the increased risk of AD (OR 1.10, 95% CIs 1.08–1.13, corrected P value 7.4 × 10−24). The PTK2B gene encodes Pyk2 that is a key regulator of converging pathways between osteoclasts and microglia as described above. The association has been replicated in later studies.127 Moreover, an unbiased association study using all UK Biobank traits discovered the association between another variant in PTK2B gene (rs7000615) and BMD (P value 7 × 10−8).128 The heritability of BMD was reported as 0.50–0.85 based on twin and family studies.129
The genetic correlation between osteoporosis and AD shall be polygenic. Using published GWAS results, we checked if there exists shared genetic risk between osteoporosis/BMD and AD by calculating polygenic risk scores (PRSs) for the two traits in population data and testing correlation between the PRSs from two traits. To calculate PRS for osteoporosis and AD, we used the GWAS summary statistics published by UK Biobank (http://www.nealelab.is/uk-biobank) for phenotype codes “20002_1309” (Non-cancer illness code, self-reported: osteoporosis) and “AD”. For 2 504 individuals from the phase 3 release of the 1 000 Genomes project, we calculated PRSs for osteoporosis and AD using the summary statistics. We did not observe significant correlation between the PRSs for osteoporosis and AD across individuals (r2 = −0.023, P value of 0.605 8 for Europeans). Therefore, the genetic risks due to common variants for two conditions are likely independent to each other. Alternatively, some risk loci could increase the risk for one condition but decrease the risk for the other and inter-individual variation in genetic susceptibility to environmental risk factors (i.e., gene–environment interactions) exists. Finally, two conditions may not share the majority of the genetic risks due to common variants except for those in PTK2B.
Concluding remarks
Discovering diagnostic and treatment biomarkers for neurodevelopmental and neurodegenerative disorders is challenging, in part, due to limited accessibility to directly affected tissue and cell types in human. Early diagnosis of neurodegenerative disorders could change clinical course and outcome; however, clinical suspicion is often delayed until overt signs and symptoms are severe enough to get attention. Aβ deposition starts 15–20 years before cognitive symptom appears, which suggests time window for effective disease-modifying treatment for AD shall be much earlier than current practice and clinical trials.15 Clinical manifestation of distant organs such as BMD changes precedes later-onset AD in some cases. In NHD, mutations in TREM2/DAP12 cause bone disorder followed by brain phenotype. There are shared pathobiological mechanisms involving several molecular pathways between bone and brain although the origin of pleiotropic effects is yet to be elucidated. Here we reviewed the signaling pathways important for osteoclasts and microglia, and highlighted the convergence of these pathways to the regulation of actin cytoskeleton remodeling via Pyk2 pathway. As genetic variants in PTK2B increase the risk of osteoporosis and AD, Pyk2 pathway may be eligible for horizontal pleiotropy and suggest novel diagnostic and treatment biomarkers for AD. With the availability of medical big data from electronic health records, longitudinal analysis of diverse clinical phenotype for each individual can reveal association between diseases affecting distant organs. This type of analysis is complementary to the framework of case-control comparisons including GWAS although statistical approach and power analysis need to be established for longitudinal medical data. For individuals with osteoporosis, preemptive genotyping for TREM2/DAP12, CSF1, CCR5, and Pyk2 signaling pathways could suggest relative risks for AD compared to population norm with a matched genetic background. As early detection and pharmacological intervention are the only effective treatment for AD, careful evaluation and follow-up for individuals with osteoporosis can modify clinical outcome at least in a subgroup.
Acknowledgements
S.W.K. was supported by the grants from the National Institution of Health (R01MH107205, R24OD024622, and U01TR002623). J.W.L. was supported by Grant-in-Aids for Scientific Research from the Japan Society for the Promotion of Science (19K10044).
Competing interests
The authors declare no competing interests.
References
- 1.Vaananen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J. Cell Sci. 2000;113:377–381. doi: 10.1242/jcs.113.3.377. [DOI] [PubMed] [Google Scholar]
- 2.Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol. Med. 2005;11:76–81. doi: 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Hayman AR, et al. Mice lacking tartrate-resistant acid phosphatase (Acp 5) have disrupted endochondral ossification and mild osteopetrosis. Development. 1996;122:3151–3162. doi: 10.1242/dev.122.10.3151. [DOI] [PubMed] [Google Scholar]
- 4.Vu TH, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/S0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelb BD, Shi GP, Chapman HA, Desnick RJ. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science. 1996;273:1236–1238. doi: 10.1126/science.273.5279.1236. [DOI] [PubMed] [Google Scholar]
- 6.Frattini A, et al. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat. Genet. 2000;25:343–346. doi: 10.1038/77131. [DOI] [PubMed] [Google Scholar]
- 7.Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N. Engl. J. Med. 2016;374:254–262. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 8.Orwoll E, et al. Alendronate for the treatment of osteoporosis in men. N. Engl. J. Med. 2000;343:604–610. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 9.El-Sobky TA, Elsobky E, Sadek I, Elsayed SM, Khattab MF. A case of infantile osteopetrosis: The radioclinical features with literature update. Bone Rep. 2016;4:11–16. doi: 10.1016/j.bonr.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orchard PJ, et al. Hematopoietic stem cell transplantation for infantile osteopetrosis. Blood. 2015;126:270–276. doi: 10.1182/blood-2015-01-625541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safaiyan S, et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 2016;19:995–998. doi: 10.1038/nn.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 13.Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat. Med. 2017;23:1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 15.Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179:312–339. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Querfurth HW, LaFerla FM. Alzheimer’s disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 17.Puig KL, Combs CK. Expression and function of APP and its metabolites outside the central nervous system. Exp. Gerontol. 2013;48:608–611. doi: 10.1016/j.exger.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, Liu B, Zhang L, Rong L. Amyloid beta peptide is elevated in osteoporotic bone tissues and enhances osteoclast function. Bone. 2014;61:164–175. doi: 10.1016/j.bone.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Bedner P, et al. Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain. 2015;138:1208–1222. doi: 10.1093/brain/awv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verkhratsky A, Steardo L, Parpura V, Montana V. Translational potential of astrocytes in brain disorders. Prog. Neurobiol. 2016;144:188–205. doi: 10.1016/j.pneurobio.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colonna M, Wang Y. TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat. Rev. Neurosci. 2016;17:201–207. doi: 10.1038/nrn.2016.7. [DOI] [PubMed] [Google Scholar]
- 22.Cuello AC. Early and late CNS inflammation in Alzheimer’s disease: two extremes of a continuum? Trends Pharmacol. Sci. 2017;38:956–966. doi: 10.1016/j.tips.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Ulland TK, Colonna M. TREM2—a key player in microglial biology and Alzheimer disease. Nat. Rev. Neurol. 2018;14:667–675. doi: 10.1038/s41582-018-0072-1. [DOI] [PubMed] [Google Scholar]
- 24.Tsukasaki M, Takayanagi H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 2019;19:626–642. doi: 10.1038/s41577-019-0178-8. [DOI] [PubMed] [Google Scholar]
- 25.Bianchin MM, Martin KC, de Souza AC, de Oliveira MA, Rieder CR. Nasu-Hakola disease and primary microglial dysfunction. Nat. Rev. Neurol. 2010;6:523. doi: 10.1038/nrneurol.2010.17-c1. [DOI] [PubMed] [Google Scholar]
- 26.Amouzougan A, et al. High prevalence of dementia in women with osteoporosis. Jt. Bone Spine. 2017;84:611–614. doi: 10.1016/j.jbspin.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Chang KH, et al. Increased risk of dementia in patients with osteoporosis: a population-based retrospective cohort analysis. Age. 2014;36:967–975. doi: 10.1007/s11357-013-9608-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou R, Deng J, Zhang M, Zhou HD, Wang YJ. Association between bone mineral density and the risk of Alzheimer’s disease. J. Alzheimers Dis. 2011;24:101–108. doi: 10.3233/JAD-2010-101467. [DOI] [PubMed] [Google Scholar]
- 29.Loskutova N, Honea RA, Vidoni ED, Brooks WM, Burns JM. Bone density and brain atrophy in early Alzheimer’s disease. J. Alzheimers Dis. 2009;18:777–785. doi: 10.3233/JAD-2009-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat. Rev. Immunol. 2015;15:731–744. doi: 10.1038/nri3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gentek R, et al. Epidermal gammadelta T cells originate from yolk sac hematopoiesis and clonally self-renew in the adult. J. Exp. Med. 2018;215:2994–3005. doi: 10.1084/jem.20181206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoder MC, Hiatt K, Mukherjee P. In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proc. Natl Acad. Sci. USA. 1997;94:6776–6780. doi: 10.1073/pnas.94.13.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev. Immunol. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- 35.Hammond TR, Robinton D, Stevens B. Microglia and the brain: complementary partners in development and disease. Annu. Rev. Cell Dev. Biol. 2018;34:523–544. doi: 10.1146/annurev-cellbio-100616-060509. [DOI] [PubMed] [Google Scholar]
- 36.Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev. Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacome-Galarza CE, et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature. 2019;568:541–545. doi: 10.1038/s41586-019-1105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizoguchi T, et al. Identification of cell cycle-arrested quiescent osteoclast precursors in vivo. J. Cell Biol. 2009;184:541–554. doi: 10.1083/jcb.200806139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yahara Y, et al. Erythromyeloid progenitors give rise to a population of osteoclasts that contribute to bone homeostasis and repair. Nat. Cell Biol. 2020;22:49–59. doi: 10.1038/s41556-019-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hettinger J, et al. Origin of monocytes and macrophages in a committed progenitor. Nat. Immunol. 2013;14:821–830. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- 41.Colonna M. TREMs in the immune system and beyond. Nat. Rev. Immunol. 2003;3:445–453. doi: 10.1038/nri1106. [DOI] [PubMed] [Google Scholar]
- 42.Lowell, C. A. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signaling cross talk. Cold Spring Harb. Perspect. Biol. 3, a002352 (2011). [DOI] [PMC free article] [PubMed]
- 43.Kleinberger G, et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci. Transl. Med. 2014;6:243ra86. doi: 10.1126/scitranslmed.3009093. [DOI] [PubMed] [Google Scholar]
- 44.Wunderlich P, et al. Sequential proteolytic processing of the triggering receptor expressed on myeloid cells-2 (TREM2) protein by ectodomain shedding and gamma-secretase-dependent intramembranous cleavage. J. Biol. Chem. 2013;288:33027–33036. doi: 10.1074/jbc.M113.517540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouchon A, Hernandez-Munain C, Cella M, Colonna M. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J. Exp. Med. 2001;194:1111–1122. doi: 10.1084/jem.194.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamerman JA, Ni M, Killebrew JR, Chu CL, Lowell CA. The expanding roles of ITAM adapters FcRgamma and DAP12 in myeloid cells. Immunol. Rev. 2009;232:42–58. doi: 10.1111/j.1600-065X.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ivashkiv LB. Cross-regulation of signaling by ITAM-associated receptors. Nat. Immunol. 2009;10:340–347. doi: 10.1038/ni.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McVicar DW, Trinchieri G. CSF-1R, DAP12 and beta-catenin: a menage a trois. Nat. Immunol. 2009;10:681–683. doi: 10.1038/ni0709-681. [DOI] [PubMed] [Google Scholar]
- 49.Koga T, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 50.Humphrey MB, et al. TREM2, a DAP12-associated receptor, regulates osteoclast differentiation and function. J. Bone Min. Res. 2006;21:237–245. doi: 10.1359/JBMR.051016. [DOI] [PubMed] [Google Scholar]
- 51.Paloneva J, et al. DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. J. Exp. Med. 2003;198:669–675. doi: 10.1084/jem.20030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cella M, et al. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J. Exp. Med. 2003;198:645–651. doi: 10.1084/jem.20022220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Otero K, et al. TREM2 and beta-catenin regulate bone homeostasis by controlling the rate of osteoclastogenesis. J. Immunol. 2012;188:2612–2621. doi: 10.4049/jimmunol.1102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sessa G, et al. Distribution and signaling of TREM2/DAP12, the receptor system mutated in human polycystic lipomembraneous osteodysplasia with sclerosing leukoencephalopathy dementia. Eur. J. Neurosci. 2004;20:2617–2628. doi: 10.1111/j.1460-9568.2004.03729.x. [DOI] [PubMed] [Google Scholar]
- 55.Schmid CD, et al. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J. Neurochem. 2002;83:1309–1320. doi: 10.1046/j.1471-4159.2002.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsieh CL, et al. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J. Neurochem. 2009;109:1144–1156. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lue LF, Schmitz C, Walker DG. What happens to microglial TREM2 in Alzheimer’s disease: Immunoregulatory turned into immunopathogenic? Neuroscience. 2015;302:138–150. doi: 10.1016/j.neuroscience.2014.09.050. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015;160:1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keren-Shaul H, et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169:1276–1290.e17. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 60.Krasemann S, et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47:566–581.e9. doi: 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacDonald KP, et al. The colony-stimulating factor 1 receptor is expressed on dendritic cells during differentiation and regulates their expansion. J. Immunol. 2005;175:1399–1405. doi: 10.4049/jimmunol.175.3.1399. [DOI] [PubMed] [Google Scholar]
- 62.Nandi S, et al. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev. Biol. 2012;367:100–113. doi: 10.1016/j.ydbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coussens L, et al. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature. 1986;320:277–280. doi: 10.1038/320277a0. [DOI] [PubMed] [Google Scholar]
- 64.Aoki N, Ito K, Ito M. Isolation and characterization of mouse high-glycine/tyrosine proteins. J. Biol. Chem. 1997;272:30512–30518. doi: 10.1074/jbc.272.48.30512. [DOI] [PubMed] [Google Scholar]
- 65.Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 66.Abboud SL, Woodruff K, Liu C, Shen V, Ghosh-Choudhury N. Rescue of the osteopetrotic defect in op/op mice by osteoblast-specific targeting of soluble colony-stimulating factor-1. Endocrinology. 2002;143:1942–1949. doi: 10.1210/endo.143.5.8775. [DOI] [PubMed] [Google Scholar]
- 67.Dai XM, Zong XH, Sylvestre V, Stanley ER. Incomplete restoration of colony-stimulating factor 1 (CSF-1) function in CSF-1-deficient Csf1op/Csf1op mice by transgenic expression of cell surface CSF-1. Blood. 2004;103:1114–1123. doi: 10.1182/blood-2003-08-2739. [DOI] [PubMed] [Google Scholar]
- 68.Wiktor-Jedrzejczak W, et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl Acad. Sci. USA. 1990;87:4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee AW, States DJ. Both src-dependent and -independent mechanisms mediate phosphatidylinositol 3-kinase regulation of colony-stimulating factor 1-activated mitogen-activated protein kinases in myeloid progenitors. Mol. Cell Biol. 2000;20:6779–6798. doi: 10.1128/MCB.20.18.6779-6798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiong Y, et al. A CSF-1 receptor phosphotyrosine 559 signaling pathway regulates receptor ubiquitination and tyrosine phosphorylation. J. Biol. Chem. 2011;286:952–960. doi: 10.1074/jbc.M110.166702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Long CL, Berry WL, Zhao Y, Sun XH, Humphrey MB. E proteins regulate osteoclast maturation and survival. J. Bone Min. Res. 2012;27:2476–2489. doi: 10.1002/jbmr.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zou W, et al. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J. Cell Biol. 2007;176:877–888. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stanley, E. R. & Chitu, V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb. Perspect. Biol. 6, a021857 (2014). [DOI] [PMC free article] [PubMed]
- 74.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Michaelson MD, et al. CSF-1 deficiency in mice results in abnormal brain development. Development. 1996;122:2661–2672. doi: 10.1242/dev.122.9.2661. [DOI] [PubMed] [Google Scholar]
- 77.Lin H, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–811. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- 78.Rademakers R, et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat. Genet. 2011;44:200–205. doi: 10.1038/ng.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kana V, et al. CSF-1 controls cerebellar microglia and is required for motor function and social interaction. J. Exp. Med. 2019;216:2265–2281. doi: 10.1084/jem.20182037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mancuso R, et al. CSF1R inhibitor JNJ-40346527 attenuates microglial proliferation and neurodegeneration in P301S mice. Brain. 2019;142:3243–3264. doi: 10.1093/brain/awz241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oppermann M. Chemokine receptor CCR5: insights into structure, function, and regulation. Cell. Signal. 2004;16:1201–1210. doi: 10.1016/j.cellsig.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 82.Weiss-Haljiti C, et al. Involvement of phosphoinositide 3-kinase gamma, Rac, and PAK signaling in chemokine-induced macrophage migration. J. Biol. Chem. 2004;279:43273–43284. doi: 10.1074/jbc.M402924200. [DOI] [PubMed] [Google Scholar]
- 83.Del Corno M, et al. HIV-1 gp120 and chemokine activation of Pyk2 and mitogen-activated protein kinases in primary macrophages mediated by calcium-dependent, pertussis toxin-insensitive chemokine receptor signaling. Blood. 2001;98:2909–2916. doi: 10.1182/blood.V98.10.2909. [DOI] [PubMed] [Google Scholar]
- 84.Prahalad S, et al. Association of two functional polymorphisms in the CCR5 gene with juvenile rheumatoid arthritis. Genes Immun. 2006;7:468–475. doi: 10.1038/sj.gene.6364317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han JH, et al. Macrophage inflammatory protein-1alpha is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor kappaB ligand. Blood. 2001;97:3349–3353. doi: 10.1182/blood.V97.11.3349. [DOI] [PubMed] [Google Scholar]
- 86.Oba Y, et al. MIP-1alpha utilizes both CCR1 and CCR5 to induce osteoclast formation and increase adhesion of myeloma cells to marrow stromal cells. Exp. Hematol. 2005;33:272–278. doi: 10.1016/j.exphem.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 87.Lee JW, et al. The HIV co-receptor CCR5 regulates osteoclast function. Nat. Commun. 2017;8:2226. doi: 10.1038/s41467-017-02368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taiwo BO, et al. Less bone loss with maraviroc—versus tenofovir-containing antiretroviral therapy in the AIDS Clinical Trials Group A5303 Study. Clin. Infect. Dis. 2015;61:1179–1188. doi: 10.1093/cid/civ455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.He J, et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 90.Spudich S, Gonzalez-Scarano F. HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harb. Perspect. Med. 2012;2:a007120. doi: 10.1101/cshperspect.a007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou, M. et al. CCR5 is a suppressor for cortical plasticity and hippocampal learning and memory. Elife5, e20985 (2016). [DOI] [PMC free article] [PubMed]
- 92.Joy MT, et al. CCR5 is a therapeutic target for recovery after stroke and traumatic brain injury. Cell. 2019;176:1143–1157.e13. doi: 10.1016/j.cell.2019.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ndhlovu LC, et al. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND) J. Neurovirol. 2014;20:571–582. doi: 10.1007/s13365-014-0279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cross AK, Woodroofe MN. Chemokine modulation of matrix metalloproteinase and TIMP production in adult rat brain microglia and a human microglial cell line in vitro. Glia. 1999;28:183–189. doi: 10.1002/(SICI)1098-1136(199912)28:3<183::AID-GLIA2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 95.Xia MQ, Qin SX, Wu LJ, Mackay CR, Hyman BT. Immunohistochemical study of the beta-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer’s disease brains. Am. J. Pathol. 1998;153:31–37. doi: 10.1016/S0002-9440(10)65542-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee SH, Hollingsworth R, Kwon HY, Lee N, Chung CY. beta-arrestin 2-dependent activation of ERK1/2 is required for ADP-induced paxillin phosphorylation at Ser(83) and microglia chemotaxis. Glia. 2012;60:1366–1377. doi: 10.1002/glia.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sasaki H, et al. Cloning and characterization of cell adhesion kinase beta, a novel protein-tyrosine kinase of the focal adhesion kinase subfamily. J. Biol. Chem. 1995;270:21206–21219. doi: 10.1074/jbc.270.36.21206. [DOI] [PubMed] [Google Scholar]
- 98.Lev S, et al. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 99.Faccio R, Novack DV, Zallone A, Ross FP, Teitelbaum SL. Dynamic changes in the osteoclast cytoskeleton in response to growth factors and cell attachment are controlled by beta3 integrin. J. Cell Biol. 2003;162:499–509. doi: 10.1083/jcb.200212082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Turner CE. Paxillin. Int J. Biochem. Cell Biol. 1998;30:955–959. doi: 10.1016/S1357-2725(98)00062-4. [DOI] [PubMed] [Google Scholar]
- 101.Okigaki M, et al. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc. Natl Acad. Sci. USA. 2003;100:10740–10745. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Suarez C, Kovar DR. Internetwork competition for monomers governs actin cytoskeleton organization. Nat. Rev. Mol. Cell Biol. 2016;17:799–810. doi: 10.1038/nrm.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Suda T, Nakamura I, Jimi E, Takahashi N. Regulation of osteoclast function. J. Bone Min. Res. 1997;12:869–879. doi: 10.1359/jbmr.1997.12.6.869. [DOI] [PubMed] [Google Scholar]
- 104.Henriksen K, Bollerslev J, Everts V, Karsdal MA. Osteoclast activity and subtypes as a function of physiology and pathology–implications for future treatments of osteoporosis. Endocr. Rev. 2011;32:31–63. doi: 10.1210/er.2010-0006. [DOI] [PubMed] [Google Scholar]
- 105.Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J. Clin. Investig. 1992;90:1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schwartzberg PL, et al. Rescue of osteoclast function by transgenic expression of kinase-deficient Src in src-/- mutant mice. Genes Dev. 1997;11:2835–2844. doi: 10.1101/gad.11.21.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pfaff M, Jurdic P. Podosomes in osteoclast-like cells: structural analysis and cooperative roles of paxillin, proline-rich tyrosine kinase 2 (Pyk2) and integrin alphaVbeta3. J. Cell Sci. 2001;114:2775–2786. doi: 10.1242/jcs.114.15.2775. [DOI] [PubMed] [Google Scholar]
- 108.Zhao H, et al. Osteoprotegerin disrupts peripheral adhesive structures of osteoclasts by modulating Pyk2 and Src activities. Cell Adh. Migr. 2016;10:299–309. doi: 10.1080/19336918.2015.1129480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kwon JO, Jin WJ, Kim B, Kim HH, Lee ZH. Myristoleic acid inhibits osteoclast formation and bone resorption by suppressing the RANKL activation of Src and Pyk2. Eur. J. Pharmacol. 2015;768:189–198. doi: 10.1016/j.ejphar.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 110.Gil-Henn H, et al. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(-/-) mice. J. Cell Biol. 2007;178:1053–1064. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Elsegood CL, et al. M-CSF induces the stable interaction of cFms with alphaVbeta3 integrin in osteoclasts. Int J. Biochem. Cell Biol. 2006;38:1518–1529. doi: 10.1016/j.biocel.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 112.Otero K, et al. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta-catenin. Nat. Immunol. 2009;10:734–743. doi: 10.1038/ni.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stuart LM, et al. CD36 signals to the actin cytoskeleton and regulates microglial migration via a p130Cas complex. J. Biol. Chem. 2007;282:27392–27401. doi: 10.1074/jbc.M702887200. [DOI] [PubMed] [Google Scholar]
- 114.Ganju RK, et al. Beta-chemokine receptor CCR5 signals via the novel tyrosine kinase RAFTK. Blood. 1998;91:791–797. doi: 10.1182/blood.V91.3.791. [DOI] [PubMed] [Google Scholar]
- 115.Janabi N, Peudenier S, Heron B, Ng KH, Tardieu M. Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neurosci. Lett. 1995;195:105–108. doi: 10.1016/0304-3940(94)11792-H. [DOI] [PubMed] [Google Scholar]
- 116.Premack BA, Schall TJ. Chemokine receptors: gateways to inflammation and infection. Nat. Med. 1996;2:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 117.Tian D, Litvak V, Lev S. Cerebral ischemia and seizures induce tyrosine phosphorylation of PYK2 in neurons and microglial cells. J. Neurosci. 2000;20:6478–6487. doi: 10.1523/JNEUROSCI.20-17-06478.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.El Khoury JB, et al. CD36 mediates the innate host response to beta-amyloid. J. Exp. Med. 2003;197:1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheung SM, Ostergaard HL. Pyk2 controls integrin-dependent CTL migration through regulation of de-adhesion. J. Immunol. 2016;197:1945–1956. doi: 10.4049/jimmunol.1501505. [DOI] [PubMed] [Google Scholar]
- 120.Steblyanko M, Anikeeva N, Campbell KS, Keen JH, Sykulev Y. Integrins Influence the Size and Dynamics of Signaling Microclusters in a Pyk2-dependent Manner. J. Biol. Chem. 2015;290:11833–11842. doi: 10.1074/jbc.M114.614719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J. Allergy Clin. Immunol. 2007;119:1303–1310, quiz 1311–1312. doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 122.Duan Y, et al. Inhibition of Pyk2 blocks airway inflammation and hyperresponsiveness in a mouse model of asthma. Am. J. Respir. Cell Mol. Biol. 2010;42:491–497. doi: 10.1165/rcmb.2008-0469OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu X, et al. Proline-rich tyrosine kinase 2 regulates spreading and migration of eosinophils after beta2-integrin adhesion. Am. J. Respir. Cell Mol. Biol. 2008;39:263–269. doi: 10.1165/rcmb.2008-0047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang L, Learoyd J, Duan Y, Leff AR, Zhu X. Hematopoietic Pyk2 regulates migration of differentiated HL-60 cells. J. Inflamm. 2010;7:26. doi: 10.1186/1476-9255-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anand AR, et al. HIV-1 gp120-induced migration of dendritic cells is regulated by a novel kinase cascade involving Pyk2, p38 MAP kinase, and LSP1. Blood. 2009;114:3588–3600. doi: 10.1182/blood-2009-02-206342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lambert JC, et al. Meta-analysis of 74 046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jansen IE, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 2019;51:404–413. doi: 10.1038/s41588-018-0311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hou R, et al. Genetic variants affecting bone mineral density and bone mineral content at multiple skeletal sites in Hispanic children. Bone. 2020;132:115175. doi: 10.1016/j.bone.2019.115175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peacock M, Turner CH, Econs MJ, Foroud T. Genetics of osteoporosis. Endocr. Rev. 2002;23:303–326. doi: 10.1210/edrv.23.3.0464. [DOI] [PubMed] [Google Scholar]