Abstract
The aim of this study was to investigate retinal and peripheral microvascular function in asymptomatic individuals that fall into different BP groups when using either the ESC/ESH or the ACC/AHA guidelines. Retinal and peripheral microvascular function was assessed in 358 participants by means of dynamic retinal vessel analysis and digital thermal monitoring, respectively. Blood pressure and lipid panel were also evaluated. Retinal vascular function measured in all groups belonging to the ACC/ASH classifications were within the normal values for age-matched normal population. Individuals classed as grade 1 hypertension according to the ESC/ESH guidelines, however, exhibited a significantly decreased artery baseline (p = 0.0004) and MC (p = 0.040), higher slopeAD (p = 0.0018) and decreased vein MC (p = 0.0446) compared to age matched normal individuals. In addition, they also had significant lower artery baseline, artery BDF, MD and MC than individuals classed as stage 1 hypertension based on the ACC/ASH guidelines (p = 0.00022, p = 0.0179, p = 0.0409 and p = 0.0329 respectively). Peripheral vascular reactivity (aTR) was lower in ESC /ESH grade I compared to those graded ACC/ASH stage I hypertension (p = 0.0122). The conclusion of this study is that microvascular dysfunctions is present at multiple levels only in individuals with ESC/ESH grade 1 hypertension. This observation could be important when deciding personalised care in individuals with early hypertensive changes.
Subject terms: Cardiology, Diseases, Medical research, Risk factors
Introduction
One of the main differences between the latest guidelines published in 2018 by the European Society of Cardiology/European Society of Hypertension (ESC/ESH) and those published in 2017 by The American College of Cardiology/American Heart Association (ACC/AHA) is the cut-off for what is considered elevated blood pressure (BP) and the first stage of hypertension (HTN) diagnosis1. As it can be expected, such difference is bound to have a high impact on clinical diagnosis and management of HTN. By using the ACC/AHA guidelines, the number of patients diagnosed with having this disease increased significantly and so did the pressure on the health system and economy. In addition, such change in practice has also a significant impact on the patients’ physical and psychological wellbeing1. Nevertheless, it would seem sensible that, in individuals with higher risk for cardiovascular disease (CVD), early diagnosis and interventions are applied at lower BP values. Indeed, both ECC/ESH guidelines and ACC/AHA have recognised this fact and recommended considering treating patients with high CVD risk at a BP threshold lower than their current cut-off for grade/stage 1 HTN2. In this way, positive progress is made towards considering individual over population factors when considering someone of being at risk for CVD.
But what is the real measure of a high CVD risk? Today, the gold standard for absolute CVD risk is based on the Framingham 10-year CVD risk score (FRS)3. Other risk scores, such as the Prospective Cardiovascular Mὒnster (PROCAM) and the European Society of Cardiology Systematic Coronary Risk Evaluation (SCORE)4,5 are also being used for the same purpose. These calculations are based, however, only on few non-modifiable as well as some modifiable risk factors, thus limiting their ability to accurately predict the risk for CVD in all individuals. Indeed, it has been shown that they could either over‐ or underestimate actual risk for CVD in a large number cases4,6,7. Consequently, it has been suggested that other factors, such as obesity, psychosocial stress and lifestyle, to name just a few, should also be included for more precise estimations8. In addition, vascular endothelial function, a parameter often overlooked, is essential not only for investigations into the pathophysiology of CVD but also for better CVD risk stratification9–11. Assessing vascular endothelial function is usually performed using techniques such as ultrasound flow‐mediated dilation (FMD), pulse wave analysis (PWA), plethysmography and iontophoresis12. These tests can, however, be complex and time‐consuming, and are performed only in highly specialized services, this contributing to the lack of inclusion of this parameter in the more largely circulated risk scores. Nevertheless, parameters such as retinal microvascular function has been found to show a good association not only with various circulatory markers for CVD13,14, but also with other modifiable and non-modifiable risk factors for this disease such as obesity15, family history16 and age17,18. Retinal microvascular function assessments provide instant integrated and dynamic data analysis that is specific to each individual. In addition, it also correlates with peripheral markers for endothelial dysfunction. Indeed, we have recently shown that signs of abnormal vascular function are similarly present and detectable in various microvascular beds, despite existing differences in their anatomical and physiological properties19. This observation is important and helps clinician to detect signs of microvascular dysfunction regardless of the method they can access to. By having a choice, practitioners can make better decisions whether to treat or not selected patients with lower BP values but with additional CVD risk, thus making decisions based on individual, rather that population risk factors, an important step towards personalised management of HTN. Nevertheless, in individuals without higher risk, the decision to treat might not be always justified. In order to shed a possible light on the validity of lowering the cut-off for the diagnosis of early hypertension in individuals without additional CVD risk, this study aimed to analyse the retinal and peripheral microvascular function in subjects that fall into different BP groups when using either the ESC/ESH or the ACC/AHA guidelines2,20.
Methods
Study participants
Healthy individuals over the age of 30 were recruited through advertisements at the Vascular Research Laboratory, Aston University (Birmingham, UK). Ethical approval was sought from the relevant local ethics committee (Aston University's Ethics Committee), and written informed consent was received from all participants prior to study enrolment. The study was designed and conducted in accordance with the tenets of the Declaration of Helsinki, and all study‐related procedures adhered to institutional guidelines14,17.
Study exclusion criteria were defined as the positive diagnosis of hypertension, CVD, cerebrovascular disease, peripheral vascular disease, dyslipidaemia, diabetes, as well as other metabolic disorders or chronic diseases that required treatment. Individuals using any vasoactive medications were also excluded from the study. Potential participants were also screened for ocular diseases and were excluded from the study if they had a refractive error of more than ± 3DS and more than ± 1DC equivalent, intra‐ocular pressure (IOP) greater than 21 mmHg, cataract or any other media opacities, as well as history of intra‐ocular surgery or any form of retinal or neuro‐ophthalmic disease affecting the ocular vascular system14,18.
General investigations
Participants who met the inclusion criteria were invited to a 2-h visit. Initially, they were requested to complete a general health history questionnaire, also detailing daily diet, physical activity and alcohol consumption. All study‐related measurements were performed between 8 and 11 am following a 12‐hr overnight fast, which included refraining from alcohol and caffeine.
Standard anthropometric measures of height and weight were recorded to determine body mass index (BMI = weight/height2)14.
Blood pressure assessment and patients grouping
Measurements of BP were performed on two separate occasions, one in-clinic and one out-of-clinic2,20. During the in-clinic visit, BP was measured in a quiet environment, with the patient seated for 5 min, 3 times at 1–2 min intervals using an automatic BP monitor (UA‐767; A & D Instruments Ltd, UK) and an adequate cuff size. The BP values were confirmed by a second measurement using a computer‐operated ambulatory BP and electrocardiogram (ECG) monitor (Cardiotens‐01, Meditech Ltd, Budapest, Hungary). All subjects maintained their normal activity and were carefully instructed to complete a diary each time their activities changed, or when any chronic medication was taken. The 24‐h BP were later downloaded and analysed using the ‘Medibase’ software program (Meditech). SBP as well as diastolic DBP measurements were calculated for the day-time (6 am to 10 pm) and night-time (10 pm to 6 am) intervals. At least 80% of the programmed recordings were required for a diurnal curve to be considered in the present analysis14,19.
Using the day-time SBP and DBP values, study participants were stratified into three subcategories: “normal”, “high normal” and ‘’Grade I’’ as recommended by the 2018 ESC/ESH Guidelines. They were then also further classified into three other subcategories: “normal”, “elevated” and “stage I” as recommended by the 2017 ACC/AHA guidelines. Subjects classed as “optimal” according to the 2018 ESC/ESH guidelines were excluded and their values were included in our pool of “normal” data14.
Dynamic retinal vessel analysis
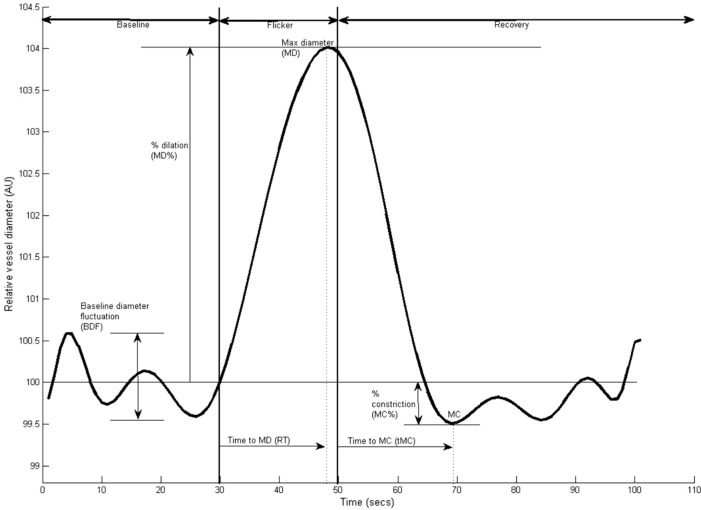
Retinal vessel reactivity was assessed using the dynamic retinal vessel analyser (DVA, IMEDOS GmbH, Jena, Germany) in accordance with an established protocol21. All measurements were performed in a temperature-controlled environment (22 °C) following pupil dilation with 1% Tropicamide (Chauvin Pharmaceuticals Ltd, UK) and were taken from the inferior temporal vessel branches approximately one and a half disc diameters from the optic nerve head of one unselected eye. Using a validated in-house algorithm, the following vessel reactivity and time-course parameters were determined for each flicker cycle and then averaged over the 3 cycles, with the artery and vein regarded separately as follows: the average baseline diameter and range of maximum and minimum baseline vessel diameters (baseline diameter fluctuation, BDF); the maximum vessel dilation diameter during flicker stimulation expressed as a percentage change relative to baseline diameter (MD%) and the time taken in seconds to reach the maximum diameter (tMD); the maximum vessel constriction diameter during the postflicker recovery period expressed as a percentage change relative to baseline diameter (MC%) and the time taken in seconds to reach the maximum vessel constriction diameter (tMC); the overall dilation amplitude (DA) calculated as the difference between MD and MC; and the baseline-corrected flicker response (BCFR) used to describe the overall dilation amplitude after normalizing for fluctuations in baseline diameters (DA-BDF). In addition, the arterial (A) and venous (V) dilation slopes (SlopeAD/VD = (MD − baseline diameter) / tMD) and constriction slopes (SlopeAC/VC = (MC − MD) / tMC) were also calculated (Fig. 1)16. The sensitivity and reproducibility of DVA assessments in healthy subjects has been reported previously and the normal expected retinal responses to flicker-light stimulation have been reported to be around 6.9 ± 2.8% in arteries and 6.5 ± 2.8% in veins22,23.
Figure 1.
Graphical presentation of the dynamic vessel response profile displaying the parameters calculated and used in analysis. (DA) calculated as (MD-MC). (MD%) calculated as the percent increase from baseline to MD. (MC%) calculated as the percent constriction below baseline following MD.
Digital thermal monitoring (DTM)
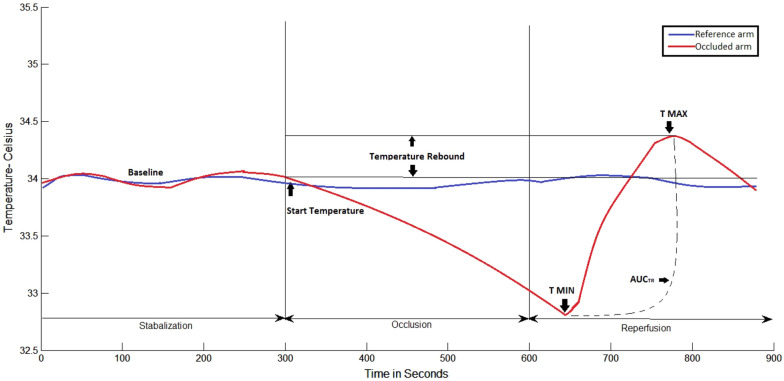
The peripheral microvascular reactivity at the level of the fingertips was assessed using VENDYS 5000 BCE DTM system (Endothelix, Inc, Houston, TX, USA) according to an established protocol24. Figure 2 shows a representative example of a temperature–time trace and the primary DTM-derived measures, related to thermal debt and recovery that were recorded and calculated.
Figure 2 .
Graphical representation of the Digital Thermal Monitor software analysis. T MAX, maximum temperature; TMIN, minimum temperature; AUCTR, Area under the curve temperature.
Temperature rebound (TR): maximum temperature − start temperature (just before cuff inflation); adjusted temperature rebound (aTR): temperature rebound/start temperature; area under the curve temperature rebound (AUCTR): area under the curve between maximum temperature and minimum temperature. The post-occlusive adjusted temperature rebound aTR determined by the software algorithm is directly associated with the extent of the subjects vascular reactivity25. An aTR below 1 is considered poor cardiovascular reactivity, whereas an aTR between 1 and 2 is considered intermediate vascular reactivity and an aTR of > 2 is considered healthy vascular reactivity.
Statistical analysis
All data are reported as mean (SD) unless otherwise indicated. The Shapiro–Wilk test was used to determine the distribution of the data. Univariate associations were determined using Pearson's (normally distributed data) or Spearman's method (non‐normally distributed data), and forward stepwise regression analyses were performed to test the influences of systemic and circulating markers on the measured variables. Differences between groups were subsequently assessed using one‐way analysis of variance (anova) or analysis of covariance (ancova). p values of < 0.05 were considered significant. All analyses were performed using Statistica software.
Results
A total number of 372 participants were initially screened for study inclusion of which 14 individuals were excluded based on the quality of retinal vascular image analysis or to the fact that they did not tolerate the ambulatory BP measurements. The remaining 358 participants were included in the final analysis and classified into 3 study groups using the current ESC/ESH Guidelines (normal BP:66 individuals,; high normal BP: 40 individuals; and grade 1 hypertension: 53 individuals). A further three groups were decided based on the ACC/AHA guidelines: (normal BP: 83 individuals; Elevated BP: 46 individuals; and stage 1 hypertension: 70 individuals).
General characteristics of the study population are presented in Table 1. There were no significant differences in age, gender, BMI, HR, IOP, glucose, TG, cholesterol, LDL-c, HDL-c and body composition between all the study groups (all p > 0.05). There was a significant difference between groups in SBP and DBP (p < 0.001). In addition, there was also significant differences between the peripheral vascular reactivity (aTR) between ESC /ESH grade I compared to ACC/ASH stage I hypertension, with individuals graded as ESC /ESH grade I having lower aTR than those graded ACC/ASH stage I hypertension (p = 0.0122).
Table 1.
General characteristics of the study population.
|
ACC/AHA Normal < 120& < 80 |
ESC/ESH Normal SBP:120–129 DBP:80–84 |
p value |
ACC/AHA Elevated SBP: 120–129 DBP: < 80 |
ESC/ESH High Normal SBP:130–139 DBP:85–89 |
p value |
ACC/AHA Stage I SBP:130–139 DBP:80–89 |
ESC/ESH Grade I SBP:140–159 DBP:90–99 |
p value | |
|---|---|---|---|---|---|---|---|---|---|
| Number | 83 | 66 | – | 46 | 40 | – | 70 | 53 | – |
| Gender | 30 M:53F | 34 M:32F | – | 29 M:17F | 22:18F | – | 44 M:37F | 32 M:21F | – |
| Age (years) | 40.1.8 (1.35) | 43.91 (1.70) | 0.0859 | 42.83 (2.31) | 45.80 (2.53) | 0.3897 | 44.88 (3.4) | 48.77 (2.7) | 0.1063 |
| SBP(mmHg) | 109.43 7.08) | 122.52 (4.98) | 0.0000* | 124.17 (6.88) | 131.03 (6.74) | 0.0002* | 134.61 (8.72) | 142.00 (7.52) | 0.0000* |
| DBP(mmHg) | 68.33 (6.15) | 76.15 (5.92) | 0.0000* | 72.76 (4.98) | 83.93 (5.37) | 0.0000* | 83.95 (4.71) | 89.47 (6.54) | 0.0000* |
| HR (bpm) | 68.055 (9.28) | 65.06 (10.15) | 0.0659 | 63.31 (9.38) | 67.37 (10.85) | 0.1085 | 68.37 (10.87) | 69.59 (10.93) | 0.5467 |
| IOP | 13.30 (2.34) | 13.41 (2.64) | 0.8139 | 13.25 (2.59) | 13.43 (2.25) | 0.7123 | 14.33 (2.63) | 15.29 (3.01) | 0.0970 |
| BMI (kg/m2) | 24.69 (0.36) | 25.66 (0.45) | 0.0934 | 26.73 (0.71) | 25.70 (0.78) | 0.3175 | 26.49 (4.20) | 28.09 (4.40) | 0.0680 |
| Body fat % | 28.00 (1.68) | 23.76 (1.76) | 0.0864 | 23.02 (1.90) | 28.31 (2.06) | 0.0670 | 27.65 (2.43) | 31.93 (3.97) | 0.3651 |
| Body water % | 52.51 (1.65) | 51.56 (1.73) | 0.6907 | 54.25 (2.30) | 48.84 (2.48) | 0.1180 | 46.80 (2.96) | 49.44 (4.84) | 0.6445 |
| Muscle mass % | 47.45 (2.59) | 50.89 (2.72) | 0.1673 | 60.94 (3.13) | 55.68 (3.38) | 0.2599 | 52.75 (4.29) | 43.30 (6.10) | 0.2583 |
| Glucose (mmol/L) | 4.87 (0.55) | 4.93 (0.86) | 0.6178 | 5.07 (0.82) | 5.03 (0.60) | 0.8433 | 4.93 (0.92) | 5.15 (1.31) | 0.3356 |
| TG (mmol/L) | 1.139 (0.56) | 1.19 (0.61) | 0.6345 | 1.15 (0.48) | 1.25 (0.46) | 0.4397 | 1.263 (0.62) | 1.44 (0.80) | 0.2210 |
| T.Chol (mmol/L) | 4.40 (0.78) | 4.70 (1.02) | 0.5642 | 4.88 (1.07) | 4.55 (0.91) | 0.2040 | 4.50 (0.89) | 4.72 (0.86) | 0.2516 |
| HDL-C (mmol/L) | 1.33 (0.41) | 1.37 (0.39) | 0.5212 | 1.35 (0.38) | 1.23 (0.58) | 0.3288 | 1.33 (0.56) | 1.21 (0.44) | 0.2848 |
| LDL-C (mmol/L) | 2.57 (0.91) | 2.78 (0.12) | 0.1639 | 2.10 (1.09) | 2.82 (1.05) | 0.5199 | 2.59 (0.94) | 2.90 (0.93) | 0.1131 |
| TG/HDL | 1.00 (0.81) | 1.01 (0.81) | 0.9561 | 0.98 (0.63) | 1.35 (0.91) | 0.0683 | 1.21 (0.92) | 1.36 (0.94) | 0.4460 |
| aTR | 1.60 (0.13) | 1.93 (0.11) | 0.0365* | 1.70 (0.11) | 1.63 (0.11) | 0.7790 | 1.80 (0.12) | 1.20 (0.20) | 0.0122* |
Abbreviations: SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; IOP: intraocular pressure; BMI: body mass index; TG: triglycerides; T. Chol: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein; aTR: adjusted temperature rebound *Significant p values are indicated where p < 0.05 was considered significant.
Comparisons of group differences in measured retinal arterial and venous DVA parameters after flicker stimulation are presented in Tables 2 and 3. All values reported are based on data averaged across the three flicker cycles with arteries and veins considered separately. Values were further compared to the average normal values for the groups’ corresponding age range.
Table 2.
Summary of retinal arterial vascular function parameters.
| Normal Average |
ACC/AHA Normal < 120& < 80 |
ESC/ESH Normal SBP:120–129 DBP:80–84 |
ANOVA/ANCOA p value |
ACC/AHA Elevated SBP:120–129 DBP: < 80 |
ESC/ESH High Normal SBP:130–139 DBP:85–89 |
ANOVA/ANOVA p value |
ACC/AHA Stage I SBP:130–139 DBP:80–89 |
ESC/ESH Grade I SBP:140–159 DBP:90–99 |
ANOVA/ANCOVA p value |
|---|---|---|---|---|---|---|---|---|---|
| Artery baseline 99.99 (0.002) | 99.98 (0.12) | 99.88 (0.77) | 0.1979 | 99.83 (0.95) | 99.97 (0.92) | 0.4346 | 99.99 (0.62) | 97.93 (0.27) | 0.0002* |
|
Artery-BDF 5.66 (2.63) |
5.64 (2.650) | 6.14 (3.46) | 0.3461 | 6.28 (2.87) | 5.83 (2.74) | 0.5250 | 5.98 (3.42) | 5.02 (2.41) | 0.0179* |
|
Artery-MD 103.67 (2.20) |
103.57 (2.20) | 104.07 (2.24) | 0.2000 | 104.44 (2.2) | 104.19 (2.74) | 0.5780 | 104.14 (2.43) | 102.75 (4.26) | 0.0409* |
|
Artery-tMD 22.14 (10.09) |
21.88 (9.63) | 20.87 (6.27) | 0.5047 | 20.84 (5.83) | 20.27 (6.44) | 0.7120 | 19.86 (6.74) | 20.10 (7.11) | 0.9493 |
|
Artery-MDp 3.67 (2.21) |
3.59 (2.20) | 4.20 (2.53) | 0.1400 | 4.64 (2.63) | 4.48 (2.75) | 0.7901 | 4.15 (2.44) | 4.01 (2.55) | 0.1020 |
|
Artery-MC 97.51 (1.89) |
97.73 (0.19) | 97.64 (0.25) | 0.7691 | 97.83 (1.82) | 97.53 (2.15) | 0.5410 | 97.58 (1.80) | 96.08 (4.97) | 0.0329* |
|
Artery-tMC 27.05 (8.31) |
27.47 (7.36) | 27.88 (6.98) | 0.7455 | 28.09 (6.60) | 28.05 (6.44) | 0.9810 | 27.49 (6.70) | 28.85 (7.96) | 0.3641 |
|
Artery-MCp − 2.49 (1.89) |
− 2.22 (1.84) | − 2.056 (1.87) | 0.6124 | − 1.99 (1.95) | − 2.14 (2.14) | 0.3810 | − 2.41 (1.80) | − 1.94 (1.72) | 0.2002 |
|
Artery-DA 6.16 (2.95) |
5.77 (2.87) | 6.17 (2.96) | 0.5191 | 6.61 (2.95) | 7.30 (3.63) | 0.4310 | 6.57 (3.02) | 6.71 (2.91) | 0.8096 |
|
Artery-BCFR 0.56 (2.73) |
0.30 (2.57) | 0.26 (2.97) | 0.9244 | 0.43 (2.74) | 0.44 (3.02) | 0.1681 | 0.67 (3.07) | 0.89 (3.12) | 0.7326 |
|
Artery-SlopeAD 0.21 (0.13) |
0.21 (0.16) | 0.25 (0.15) | 0.1347 | 0.27 (0.16) | 0.26 (0.32) | 0.1902 | 0.28 (0.20) | 0.35 (0.30) | 0.2335 |
|
Artery-SlopeAC − 0.36 (0.30) |
− 0.36 (0.45) | − 0.30 (0.22) | 0.3600 | − 0.31 (0.22) | − 0.32 (0.18) | 0.9603 | − 0.29 (0.20) | − 0.27 (0.51) | 0.2826 |
Abbreviations: ANOVA, analysis of variance; ANCOVA, analysis of covariance; Baseline, baseline diameter; BDF, baseline diameter fluctuation; BCFR, Baseline corrected flicker response; tMD, time to reach MD; MDp, percent dilation; tMC, time to reach MC; MC (%), percent constriction; DA, dilation amplitude (difference between MD and MC during flicker) SlopeAD, slope of arterial dilation; SlopeAC, slope of arterial constriction. *Significant p values are indicated where p < 0.05 was considered significant.
Table 3.
Summary of retinal arterial vascular function parameters.
| Normal Average |
ACC Normal < 120& < 80 |
ESC Normal SBP:120–129 DBP:80–84 |
ANOVA/ANCOVA p value |
ACC Elevated SBP:120–129 DBP: < 80 |
ESC High Normal SBP:130–139 DBP:85–89 |
ANOVA/ANCOVA p value |
ACC Stage I SBP:130–139 DBP:80–89 |
ESC Grade I SBP:140–159 DBP:90–99 |
ANOVA/ANCOVA p value |
|---|---|---|---|---|---|---|---|---|---|
|
Vein-Baseline 99.98 (0.15) |
99.96 (0.20) | 99.90 (0.78) | 0.3996 | 99.83 (0.97) | 99.99 (0.002) | 0.3733 | 99.99 (0.0025) | 99.91 (0.0022) | 0.4251 |
|
Vein-BDF 4.39 (2.04) |
4.427 (2.22) | 3.94 (2.02) | 0.2059 | 4.09 (2.22) | 5.149 (3.48) | 0.1583 | 4.70 (2.74) | 4.92 (2.48) | 0.7667 |
|
Vein-MD 104.34 (2.26) |
104.19 (2.16) | 104.06 (2.24) | 0.7239 | 104.01 (2.19) | 105.31 (2.83) | 0.5790 | 105.10 (2.73) | 104.61 (2.245) | 0.6224 |
|
Vein-tMD 22.55 (6.93) |
22.37 (6.90) | 21.53 (7.40) | 0.5058 | 22.68 (8.62) | 21.25 (6.46) | 0.5343 | 19.82 (5.45) | 20.43 (5.81) | 0.4213 |
|
Vein-MDp 4.36 (2.26) |
4.24 (2.15) | 4.19 (2.61) | 0.9114 | 4.21 (2.75) | 5.310 (2.93) | 0.1326 | 5.10 (2.73) | 4.612 (2.24) | 0.5127 |
|
Vein MC 98.71 (1.27) |
98.87 (1.14) | 99.20 (1.12) | 0.0957 | 98.36 (1.02) | 98.70 (1.51) | 0.2408 | 98.46 (1.123) | 97.13 (12.5) | 0.0383* |
|
Vein-tMC 31.68 (5.51) |
31.23 (5.62) | 30.15 (6.38) | 0.2990 | 31.26 (6.61) | 30.54 (6.34) | 0.6757 | 30.37 (5.76) | 31.0384 (5.20) | 0.6642 |
|
Vein-MCp − 1.27 (1.28) |
− 1.09 (1.18) | − 0.70 (1.55) | 0.0744 | − 0.85 (0.70) | − 1.29 (0.81) | 0.1973 | − 1.17 (1.25) | − 1.06925902 | 0.5289 |
|
Vein-DA 5.63 (2.88) |
5.325 (2.69) | 4.85 (2.81) | 0.3302 | 4.86 (1.69) | 6.60 (3.20) | 0.0869 | 6.27 (3.16) | 5.6811 (2.60) | 0.4637 |
|
Vein-BCFR 1.23 (2.19) |
0.94 (2.37) | 0.98 (2.86) | 0.9383 | 0.66 (2.79) | 1.56 (2.84) | 0.2202 | 1.52 (2.49) | 0.76220 (1.97) | 0.2591 |
|
Vein-SlopeAD 0.23 (0.13) |
0.23 (0.14) | 0.24 (0.16) | 0.5595 | 0.24 (0.175) | 0.50 (1.109) | 0.1881 | 0.39 (0.74) | 0.2697 (0.13) | 0.4007 |
|
Vein-SlopeVC − 0.24 (0.15) |
− 0.23 (0.14) | − 0.20 (0.13) | 0.2551 | − 0.20 (0.13) | − 0.27 (0.16) | 0.1062 | − 0.25 (0.17) | − 0.2318 (0.16) | 0.7306 |
Abbreviations ANOVA, analysis of variance; ANCOVA, analysis of covariance; Baseline, baseline diameter; BDF, baseline diameter fluctuation; BCFR, Baseline corrected flicker response; tMD, time to reach MD; MD (%), percent dilation; tMC, time to reach MC; MC (%), percent constriction; DA, dilation amplitude (difference between MD and MC during flicker) SlopeAD, slope of venous dilation; SlopeVC, slope of venous constriction. *Significant p values are indicated where p < 0.05 was considered significant.
Retinal vascular response
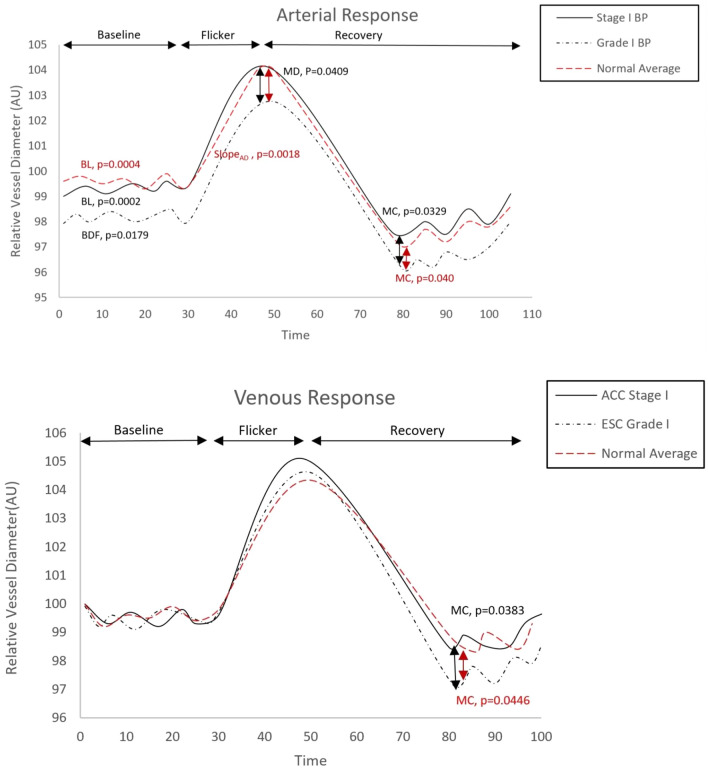
After controlling all the influential covariates identified in multivariate analysis, there were no statistically significant differences in baseline diameter, BDF, BCFR, tMD and tMC, slopeAD and slopeAC between the ACC/AHA and ESC/ESH normal BP groups (p > 0.05, Table 2). Similarly, there were no significant group differences found between ACC/AHA elevated and ESC/ESH high normal BP groups (p > 0.05, Table 2). However, individuals classed as grade 1 hypertension according to the ESC/ESH guidelines had significant lower artery baseline, artery BDF, MD and MC than individuals classed as stage 1 hypertension based on the ACC/ASH guidelines (p = 0.0002, p = 0.0179, p = 0.0409 and p = 0.0329 respectively). In addition, while the retinal vascular reactivity parameters measured in individuals classed as stage 1 hypertension according to ACC/ASH guidelines were still within the normal limits for their age, the ESC/ESH grade I hypertensive group exhibited a significantly decreased artery baseline (p = 0.0004) and MC (p = 0.040) as well as higher slopeAD (p = 0.0018) compared to age matched population average (Table 2; Fig. 3). Similarly, vein MC was significantly decreased in ESC /ESH grade I compared to ACC/ASH stage I hypertension (p = 0.0383). In addition, vein MC was significantly decreased only in the ESC/ESH grade I hypertension compared to age matched population average (p = 0.0446) (Table 3; Fig. 3).
Figure 3.
Comparison of retinal arterial and venous response profile across groups. AU, arbitrary units; BDF, baseline diameter fluctuation calculated as the maximum range in vessel diameter during first 30 s of baseline readings; MD%, calculated as the percentage change in vessel diameter from baseline to maximum following onset of flicker; tMD, time to reach maximum diameter during flicker; tMC, time to reach maximum constriction post flicker; slopeAC & SlopeVC, calculated as (MC-MD)/(tMC).
No significant group differences in any of the other measured arterial DVA parameters were identified (all p > 0.05).
Discussion
The present study looked at the peripheral and retinal microvascular function in various BP groups divided according to either the ESC/ESH or the ACC/AHA guidelines. Our report shows, for the first time, that independent of all systemic influences, retinal microvascular dysfunction firstly manifest itself only in individuals classed as ESC/ESH grade 1 hypertension. In contrast, subjects classed as ACC/AHA stage 1 hypertension exhibited normal retinal microvascular behaviour after provocation using flickering light. In addition, ESC/ESH grade 1 hypertension individuals also exhibited lower peripheral vascular reactivity than those graded as ACC/AHA stage 1 hypertension.
It is well known that microvascular dysfunction is a parameter highly predictive of cardiovascular events26. Due to this quality, its early identification is extremely important, especially in individuals at risk for CVD, including those with borderline BP values or classed as grade/stage 1 hypertension. Indeed, similar to what is known for so long in the literature about the effects of systemic hypertension on microvasculature27 in the present study we have shown that individuals classed as ESC/ESH grade 1 hypertension demonstrated abnormal peripheral and retinal microvascular function. Indeed, at the retinal vascular level, both vasodilation and vasoconstriction of the retinal arteries and veins were affected. Nevertheless, as all patients with essential hypertension are known to demonstrate microvascular endothelial dysfunction from early stages28, it is puzzling how individuals classed as ACC/AHA stage 1 hypertension failed to show such abnormalities. This is an important observation that requires further careful consideration since changes in diagnostic cut-off for systemic hypertension towards lower BP values in individuals without additional risk factors has a very high impact not only on the clinical practice and economy but also on each individual well-being1.
Beside its direct effect on the vascular functionality, hypertension is also known to cause an acceleration of the ageing process of the vascular function29. We have previously demonstrated that, in ageing, normal individuals there is a decline in retinal vasoregulative capacity in both dilatory and constrictory phases that was independent of any systemic influences17 and we have linked this abnormal response to the possibility of an age-related increase in oxidative stress19. Nevertheless, the subjects included in the current paper were much younger that those included in our ageing studies. It is possible that, the abnormal microvascular function observed in individuals classed as ESC/ESH grade 1 hypertension was indeed due to a reduced nitric oxide (NO) availability through excess inhibition by reactive oxygen species (ROS)30; however, in this case, the main culprit for ROS excess was not ageing but, possibly, an increased BP. Indeed, it has been shown that, in hypertensive patients, NO availability is reduced early in the course of the disease29. Although we did not measure either the level of NO or antioxidative markers in our current cohort of patients, in the light of our previous discovery showing that retinal microvascular dysfunction occurs in parallel with changes observed in the level of oxidative stress both in normal19 and individuals with abnormal BP levels15, it is logic to presume that similar abnormalities occurred in our current cohort of individuals with ESC/ESH grade 1 hypertension, thus contributing to the abnormal retinal microvascular function measured in these individuals.
Our ESC/ESH grade 1 hypertension also exhibited abnormal retinal venous response to flicker. In previous studies, we have already documented that individuals with other categories of cardiovascular risk also exhibit abnormal retinal venous responses to flicker-induced provocation14,16,31,32. Structural retinal venular dilation has previously implicated as a strong prediction of adverse CVD events33,34. Nevertheless, the abnormal functional retinal venous responses, as measured by DVA, are still to be confirmed as signs of endothelial dysfunction in their own right or as epiphenomena of retinal arterial dysfunction14,17. In this particular context, our observed retinal venular dysfunctionality could reflect a compensatory adaptation following sustained arterial dilation during flicker. Further investigation is required to understand the relevance of this kind of response; however, it could be hypothesized that a change in venous calibre associated with either structural or endothelial irregularities could also be used as a marker for cardiovascular risk in individuals with early hypertension14,16,17.
In addition to a general retinal microvascular dysfunction, our cohort of individuals with ESC/ESH grade 1 hypertension also exhibited abnormal peripheral microvascular reactivity as assessed by DTM, showing that abnormal microvascular function is a general process in these individuals and can be detected al multiple levels despite differences in vascular physiology and methodologies.
This study found microvascular dysfunctions to be present at multiple levels only in individuals with ESC/ESH grade 1 hypertension and not in those classed as stage 1 hypertension according to the latest ACC/AHA guidelines. The implications of these observations could be important, especially when clinicians decide their intervention based only on controversial, borderline BP values measured in individuals without other CVD risk.
Author contributions
H.S. study design, data collection and analysis and manuscript writing-up and corrections. D.G. project initiation, principal investigator, study design, overall project supervision, data analysis, manuscript writing-up and corrections. All authors read and approved the final version of the manuscript.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goel H, Tayel H, Nadar SK. Aiming higher in hopes to achieve lower: the European Society of Cardiology/European Society of Hypertension versus the American College of Cardiology/American Heart Association guidelines for diagnosis and management of hypertension. J. Hum. Hypertens. 2019;33:635–638. doi: 10.1038/s41371-019-0227-6. [DOI] [PubMed] [Google Scholar]
- 2.Williams B, et al. 2018 practice guidelines for the management of arterial hypertension of the European society of cardiology and the European society of hypertension ESC/ESH task force for the management of arterial hypertension. J. Hypertens. 2018;36:2284–2309. doi: 10.1097/HJH.0000000000001961. [DOI] [PubMed] [Google Scholar]
- 3.Wannamethee, S. G., Shaper, A. G., Lennon, L. & Morris, R. W. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Archives Intern Med.165(22), 2644–2650 (2005). [DOI] [PubMed]
- 4.Cohn JN. Identifying the risk and preventing the consequences of cardiovascular disease. Hear. Lung Circ. 2013;22:512–516. doi: 10.1016/j.hlc.2013.03.083. [DOI] [PubMed] [Google Scholar]
- 5.Koenig W. Cardiovascular biomarkers: Added value with an integrated approach? Circulation. 2007;116:3–5. doi: 10.1161/CIRCULATIONAHA.107.707984. [DOI] [PubMed] [Google Scholar]
- 6.Vasan RS. Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 7.Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler. Thromb. Vasc. Biol. 2007;27:15–26. doi: 10.1161/01.ATV.0000251503.35795.4f. [DOI] [PubMed] [Google Scholar]
- 8.Cochrane, T. et al. Contribution of individual risk factor changes to reductions in population absolute cardiovascular risk. Biomed Res. Int.2014, 1–6 (2014). [DOI] [PMC free article] [PubMed]
- 9.Mitchell GF, et al. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: The Framingham Heart Study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 10.Anderson TJ, et al. Microvascular function predicts cardiovascular events in primary prevention: Long-term results from the firefighters and their endothelium (FATE) study. Circulation. 2011;123:163–169. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 11.Cooper LL, et al. Microvascular function contributes to the relation between aortic stiffness and cardiovascular events. Circ. Cardiovasc. Imaging. 2016;9:1–8. doi: 10.1161/CIRCIMAGING.116.004979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanch N, Clifton PM, Keogh JB. A systematic review of vascular and endothelial function: Effects of fruit, vegetable and potassium intake. Nutr. Metab. Cardiovasc. Dis. 2015;25:253–266. doi: 10.1016/j.numecd.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Patel SR, et al. Abnormal retinal vascular function and lipid levels in a sample of healthy UK South Asians. Br. J. Ophthalmol. 2011 doi: 10.1136/bjo.2010.201665. [DOI] [PubMed] [Google Scholar]
- 14.Shokr H, Dias IHK, Gherghel D. Microvascular function and oxidative stress in adult individuals with early onset of cardiovascular disease. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-60766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel SR, Bellary S, Karimzad S, Gherghel D. Overweight status is associated with extensive signs of microvascular dysfunction and cardiovascular risk. Sci. Rep. 2016;6:1–8. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seshadri S, Karimzad SE, Shokr H, Gherghel D. Retinal vascular function in asymptomatic individuals with a positive family history of cardiovascular disease. Acta Ophthalmol. 2018;96:e956–e962. doi: 10.1111/aos.13783. [DOI] [PubMed] [Google Scholar]
- 17.Seshadri S, Ekart A, Gherghel D. Ageing effect on flicker-induced diameter changes in retinal microvessels of healthy individuals. Acta Ophthalmol. 2016 doi: 10.1111/aos.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seshadri S, et al. Systemic circulatory influences on retinal microvascular function in middle-age individuals with low to moderate cardiovascular risk. Acta Ophthalmol. 2015;93:e266–e274. doi: 10.1111/aos.12594. [DOI] [PubMed] [Google Scholar]
- 19.Karimzad, S. E., Shokr, H. & Gherghel, D. Retinal and peripheral vascular function in healthy individuals with low cardiovascular risk. Microvasc. Res.126, 1–7 (2019). [DOI] [PubMed]
- 20.Whelton, P. K. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults a report of the American College of Cardiology/American Heart Association Task Force on Clinical pr. Hypertension71 (2018). [DOI] [PubMed]
- 21.Nagel E, Vilser W, Lanzl I. Age, blood pressure, and vessel diameter as factors influencing the arterial retinal flicker response. Investig. Ophthalmol. Vis. Sci. 2004;45:1486–1492. doi: 10.1167/iovs.03-0667. [DOI] [PubMed] [Google Scholar]
- 22.Pache M, Nagel E, Flammer J. Reproducibility of measurements with the retinal vessel analyzer under optimal conditions. Klin. Monbl. Augenheilkd. 2002 doi: 10.1055/s-2002-33589. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen TT, et al. Reproducibility of the retinal vascular response to flicker light in Asians. Curr. Eye Res. 2009 doi: 10.3109/02713680903353764. [DOI] [PubMed] [Google Scholar]
- 24.Schier R, et al. Evaluation of digital thermal monitoring as a tool to assess perioperative vascular reactivity. J. Atheroscler. Thromb. 2013;20:277–286. doi: 10.5551/jat.15255. [DOI] [PubMed] [Google Scholar]
- 25.Lin HF, et al. Impact of blood pressure cuff inflation rates on flow-mediated dilatation and contralateral arm response. J. Hum. Hypertens. 2012;26:35–40. doi: 10.1038/jhh.2010.128. [DOI] [PubMed] [Google Scholar]
- 26.Touyz RM. Vascular remodeling, retinal arteries, and hypertension. Hypertension. 2007;50:603–604. doi: 10.1161/HYPERTENSIONAHA.107.095638. [DOI] [PubMed] [Google Scholar]
- 27.Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HAJ. Microcirculation in hypertension: A new target for treatment? Circulation. 2001;104:735–740. doi: 10.1161/hc3101.091158. [DOI] [PubMed] [Google Scholar]
- 28.Virdis A, Taddei S. Endothelial dysfunction in resistance arteries of hypertensive humans: Old and new conspirators. J. Cardiovasc. Pharmacol. 2016;67(6):451–457. doi: 10.1097/FJC.0000000000000362. [DOI] [PubMed] [Google Scholar]
- 29.Bruno RM, Masi S, Taddei M, Taddei S, Virdis A. Essential hypertension and functional microvascular ageing. High Blood Press. Cardiovasc. Prev. 2018;25:35–40. doi: 10.1007/s40292-017-0245-9. [DOI] [PubMed] [Google Scholar]
- 30.Nasri H, Baradaran A, Rafieian-Kopaei M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. Sci: J. Res. Med; 2014. [PMC free article] [PubMed] [Google Scholar]
- 31.Mroczkowska S, et al. Primary open-angle glaucoma vs normal-tension glaucoma: The vascular perspective. Arch. Ophthalmol. 2013;131:36–43. doi: 10.1001/2013.jamaophthalmol.1. [DOI] [PubMed] [Google Scholar]
- 32.Qin L, et al. Patients with early age-related macular degeneration exhibit signs of macro- and micro-vascular disease and abnormal blood glutathione levels. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014;252:23–30. doi: 10.1007/s00417-013-2418-0. [DOI] [PubMed] [Google Scholar]
- 33.Wong T, Mitchell P. The eye in hypertension. Lancet. 2007 doi: 10.1016/S0140-6736(07)60198-6. [DOI] [PubMed] [Google Scholar]
- 34.Tien YW, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: The Multi-Ethnic Study of Atherosclerosis (MESA) Investig. Ophthalmol. Vis. Sci. 2006;47:2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.