Abstract
Background:
The immunoinhibitory receptor Siglec-8 on the surface of human eosinophils and mast cells binds to sialic acid-containing ligands in the local milieu resulting in eosinophil apoptosis, inhibition of mast cell degranulation and suppression of inflammation. Siglec-8 ligands were found on postmortem human trachea and bronchi and on upper airways in two compartments, cartilage and submucosal glands, but were surprisingly absent from the epithelium. We hypothesized that Siglec-8 ligands in submucosal glands and ducts are normally transported to the airway mucus layer, which was lost during tissue preparation.
Objective:
Identify the major Siglec-8 sialoglycan ligand on the mucus layer of human airways.
Methods:
Human upper airway mucus layer proteins were recovered during presurgical nasal lavage of sinus clinic patients. Proteins were resolved by gel electrophoresis, blotted, and Siglec-8 ligands detected. Ligands were purified by size exclusion and affinity chromatography, identified by proteomic mass spectrometry, and validated by electrophoretic and histochemical co-localization. The affinity of Siglec-8 binding to purified human airway ligand was determined by glycan binding inhibition.
Results:
A Siglec-8-ligand of ~1,000 kDa was found in all patient nasal lavage samples. Purification and identification revealed DMBT1 (GP340, SALSA), a large glycoprotein with multiple O-glycosylation repeats. Immunoblotting, immunohistochemistry and enzyme treatments confirmed that Siglec-8 ligand on the human airway mucus layer is an isoform of DMBT1 carrying O-linked sialylated keratan sulfate chains (DMBT1S8). Quantitative inhibition revealed DMBT1S8 has picomolar affinity for Siglec-8.
Conclusion:
A distinct DMBT1 isoform, DMBT1S8, is the major high-avidity ligand for Siglec-8 on human airways.
Keywords: Siglec-8, upper airway, mucus layer, nasal lavage, DMBT1, GP340, SALSA, sialic acid, keratan sulfate, submucosal gland
Capsule Summary:
An isoform of the glycoprotein DMBT1 that is post-translationally decorated with sialylated keratan sulfate is the major ligand on the human airway mucus layer for Siglec-8, an anti-inflammatory receptor on eosinophils and mast cells.
INTRODUCTION
Under optimal conditions, airway inflammation is tuned to maximize clearance of pathogens and limit host tissue damage.1 Among the leukocyte cell surface receptors that down-regulate and resolve ongoing inflammatory responses are Siglecs, sialic acid binding immunoglobulin-like lectins, a family of 14 human proteins, most of which are expressed on overlapping subsets of immune cells and most of which carry intracellular immunoreceptor tyrosine-based inhibitory motifs.2 Among these, Siglec-8, which is expressed selectively on human eosinophils, mast cells, and basophils,3, 4 can significantly down-regulate allergic inflammation and is under investigation as a therapeutic target in eosinophil- and mast cell-mediated diseases.5, 6 Siglecs bind to sialic acid-containing glycans (sialoglycans) on cell surfaces or secreted into the local cellular milieu of inflammatory target tissues. Molecular recognition between Siglec-8 and its endogenous sialoglycan ligands induces immune inhibition.2, 7
Siglec-8 is highly specific in its structural specificity for sialoglycan binding, requiring the precise juxtaposition of a sialic acid and a sulfate carried on the same sub-terminal galactose.8 Although this binding specificity is well established by structural analysis of Siglec-8 bound to a synthetic glycan, the site affinity (KD ≥ 0.2 mM) is far too low to be functional. Finding a high-affinity endogenous ligand can provide the context in which the binding determinant engages Sigelc-8 in vivo.
Siglec-8 ligands are found on large human airways (trachea, bronchus) of postmortem human lung,9 and on upper airways.10 On airway tissue sections, Siglec-8 ligands were found in two compartments, cartilage and submucosal glands, but were surprisingly absent from the epithelium. Since Siglec-8 ligands appeared in submucosal gland cells (primarily serous cells) and ducts, we hypothesized that a secreted form of the Siglec-8 ligand is transported to the airway mucus layer, which was lost during tissue preparation. To experimentally address this hypothesis, the human airway mucus layer from patient upper airways was collected during clinical preparation for sinus surgery and tested for the presence of Siglec-8 ligands. This revealed a single previously unidentified major high-affinity Siglec-8 ligand on human airways described here.
METHODS
Human tissues and fluids
Postmortem unfixed human trachea and bronchus were obtained from four organ donors via the National Disease Research Interchange (Philadelphia, PA) within 24 h of removal. Donor airway disease status, cross referenced to the data in each figure, are listed in Table E1. Trachea and bronchus were dissected, transferred to ice cold RPMI-1640 containing antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin), and further processed. One trachea was opened longitudinally and the mucosa/submucosa were blunt dissected from the underlying cartilage and adventitia. A portion of each tissue was flash frozen for subsequent biochemical studies, and a portion was fixed in neutral 4% paraformaldehyde in Dulbecco’s phosphate-buffered saline (PBS) at 4°C for 16 h, embedded in paraffin, sectioned to 5 μm and captured on glass slides for histochemical studies.
Nasal lavage aspirates were collected from 27 patients at the time of sinus surgery as part of a prospective cross-sectional study approved by the Johns Hopkins University School of Medicine Institutional Review Board. The decision to undergo surgery and all clinical interventions were obtained during the course of clinical care and not part of the study protocol. Patient airway disease annotations, correlated with the data in each figure, are listed in Table E1. Nasal lavage was collected according to previously described methods.11 Lavage was completed with sterile saline solution warmed to 37°C. With the patient’s head in the recumbent position, 10 ml of saline was instilled into each nasal cavity with a syringe while simultaneously suctioning the irrigant using a straight 8–0 suction and mucous collection trap. The lavage aspirates were centrifuged at 3,000 × g for 15 min and frozen at −80°C.
Human airway lectin (Siglec-8) overlay histochemistry and immunohistochemistry
Siglec-8 ligands were detected on airway sections by overlay with an expressed chimera consisting of the entire Siglec-8 extracellular domain in frame with the human Fc domain of IgG1 prepared as described.9, 10 Briefly, airway tissue sections were deparaffinized, heated in 10 mM sodium citrate (pH 6.0) for antigen retrieval, incubated at ambient temperature in endogenous enzyme Blocking Reagent (Dako North America, Carpinteria, CA) for 10 min, and then in Fc Receptor Blocker (Innovex Biosciences, Richmond, CA) for 30 min. Siglec-8-Fc (20 μg/ml) was pre-conjugated to secondary antibody (2 μg/ml of AP-conjugated goat anti-human IgG Fc-specific, 109-055-008, Jackson Immunoresearch, West Grove, PA) in PBS containing 0.1% Triton X-100 (PBST) supplement with 10 mg/ml bovine serum albumin (BSA) for 30 min. Blocked slides were washed in PBST and overlaid with the precomplexed Siglec-8-Fc solution for 2 h. Slides were washed with PBST and equilibrated in 100 mM Tris-HCl pH 8.3 containing 0.1% Tween-20 before detection of bound lectin with Vector Red AP substrate (Vector Laboratories, Burlingame, CA). Slides were counterstained with Hematoxylin QS (Vector Laboratories), dehydrated, mounted in Krystalon (MilliporeSigma, Burlington, MA) and imaged using a Nikon Eclipse 90i microscope (Nikon Instruments, Melville, NY). As indicated, prior to Siglec-8-Fc histochemistry, selected tissue sections were incubated for 2.5 h at 37°C with PBS (control) or PBS containing 100 mU/mL V. cholerae sialidase.12
Fluorescent aggrecan immunohistochemistry was performed after enzymatic pre-treatment of deparaffinized blocked tissue sections with 0.25 U/ml of chondroitinase ABC (P. vulgaris, Amsbio, Cambridge, MA) in 100 mM Tris-HCl, 100 mM sodium acetate, pH 8.0 overnight at 37°C. Slides were washed and overlaid with 10 μg/ml rabbit polyclonal anti-aggrecan (G3, Thermo Fisher Scientific, Waltham, MA) in PBST with 10 mg/ml BSA overnight at 4°C followed by washing and overlaying with 5 μg/ml Alexa Fluor 488 goat anti-rabbit IgG (111-546-144, Jackson ImmunoResearch, West Grove, PA) in the same buffer for 2 h at ambient temperature. After washing and mounting, images were collected as above.
For double label immunofluorescence staining with anti-DMBT1 and Siglec-8-Fc, the deparaffinized, blocked tissue section was overlaid with anti-DMBT1 antibody (HYB 213-06-02, Thermo Fisher) at 1:1000 in PBST supplemented with 10 mg/ml bovine serum albumin (BSA). The slide was incubated for 2 h at room temperature. During the incubation, Siglec-8-Fc was preconjugated with Alexa Fluor 594 labeled goat anti-human Fc (109-585-098, Jackson ImmunoResearch) as follows. To Siglec-8-Fc (20 μg/ml) in PBST/BSA was added secondary antibody at 7 μg/ml. After 30 min on ice anti-DMBT1 (1:1000) was added. The solution on the slide was removed and replaced with the precomplexed Siglec-8-Fc/anti-DMBT1 mixture. After overnight incubation at 4°C, the slide was washed with PBST and the tissue section overlaid with Alexa Fluor 488 labeled donkey anti-mouse IgG (A32766, Thermo Fisher) in the same buffer for 1 h and washed in PSBT for DMBT1 detection. Tissue was exposed to DAPI stain (Vector Laboratories) at 1:1000 dilution, then the slides were washed, dried, mounted, and imaged as above.
Molecular analyses of human fluids and tissues
Nasal lavage samples were thawed and centrifuged in aliquots at 21,000 × g for 20 min. For most samples the clear supernatants were stored at −20°C and the small pellets discarded. In some samples, centrifugation resulted in clear supernatant above a highly viscous pellet. In those cases, the clear supernatant was collected and to the viscous pellet was added an equal volume of 1 mg/ml DNAse I (MilliporeSigma) and 1:100 dilution each of protease inhibitor cocktail (P8340, MilliporeSigma) and penicillin-streptomycin solution (15140163, ThermoFisher). After incubation overnight at 4°C with end-over-end mixing, two volumes of guanidinium hydrochloride (GuHCl) extraction buffer (6 M GuHCl, 20 mM sodium phosphate pH 6.5, 5 mM EDTA, 1:100 protease inhibitor cocktail, and 100 mM dithiothreitol) were added and the mixture incubated overnight as above. Samples were then centrifuged (3,000 × g, 1 h) and the clear supernatant collected. An aliquot was dialyzed against urea buffer (1 M urea, 20 mM sodium phosphate pH 7.4) for electrophoresis and the remaining extract was stored at −20°C.
Prior to electrophoresis, as indicated, some nasal lavage samples were treated with hydrolytic enzymes to identify functional binding determinants. To equal aliquots were added buffer alone (control), 7 mU/ml keratanase I (Pseudomonas sp., Amsbio), 7 mU/ml keratanase II (B. circulans expressed in E. coli) prepared as described,13 0.25 U/ml chondroitinase ABC, 0.25 U/ml heparinase (H3917, MilliporeSigma) or 76 mU/ml sialidase (V. cholerae expressed in E. coli as described).12 Samples were incubated at 37°C for 20 h (except sialidase, 1.5 h) prior to electrophoresis.
Siglec ligands were extracted from human postmortem tracheal tissues as described previously.14 Briefly, tissues were pulverized under liquid nitrogen, weighed, and incubated with 10 ml/g of GuHCl extraction buffer. Extracts were clarified by centrifugation, and aliquots dialyzed against urea buffer for analysis.
For electrophoresis and blotting, nasal lavage supernatants were mixed 1:1 with NuPAGE LDS Sample Buffer (Thermo Fisher). Equal sample volumes were resolved on custom prepared composite acrylamide (2%) and agarose (1.5%) gels for 2 h at 100 V, conditions that resolve proteins in the 200–4,000 kDa molecular weight range.10, 15 A custom molecular weight marker was prepared by mixing 1 mg/ml human IgM (ThermoFisher) with 2.5 mM bis(sulfosuccinimidyl)suberate (ThermoFisher) in PBS for 20 min, followed by addition of 90 mM Tris-HCl. The marker was stained using Visio real-time stain (Advansta, San Jose, CA) resulting in bands visible under white light or 800 nm IR at ~950 kDa (pentamer) and occasionally 1.9 MDa (decamer). Resolved proteins were transferred to polyvinylidene difluoride membranes via iBlot dry transfer (ThermoFisher). Membranes were blocked with 5% nonfat dry milk in PBST for 30 min. During blocking, Siglec-8-Fc was precomplexed with secondary antibody in PBST containing 20 μg/ml of Siglec-8-Fc and 14 μg/ml horseradish peroxidase-conjugated goat anti-human IgG Fc-specific (A0170, MilliporeSigma). After incubation on ice for 30 min, the solution was diluted 40-fold with PBST. Blots were overlaid with precomplexed Siglec-8-Fc solution, incubated 16 h at 4°C, washed with PBST, and developed using enhanced chemiluminescence (ECL Prime, GE Life Sciences, Marlborough, MA). Highly sulfated keratan sulfate chains were detected on blots using mouse monoclonal antibody 5D4 (270427-CS, Amsbio) at 1:200 in PBST detected with peroxidase-conjugated horse anti-mouse IgG (7076, Cell Signaling Technology, Danvers, MA) at 1:2000 and detected by ECL. Images were captured using a Syngene PXi (Syngene, Frederick, MD) and band intensities quantified using ImageJ.16
For near-infrared fluorescent double-labeling blocked blots were incubated at ambient temperature with rabbit polyclonal anti-DMBT1 (ARP42352_P050, Aviva Systems Biology, San Diego, CA, 1:1000) in PBST containing 1% nonfat dry milk for 2 h, after which precomplexed Siglec-8-Fc was added (1 μg/ml Siglec-8-Fc in PBST incubated for 30 min at 0°C with 0.7 μg/ml unconjugated goat anti-human IgG, Fc-specific (I2136, Millipore Sigma)). After further incubation for 16 h at 4°C, the blot was washed 3 times with PBST, then overlaid with PBST containing IRDye 680RD donkey anti-rabbit IgG (926–68071, LI-COR Biosciences, Lincoln, NE, 1:2000) to detect anti-DMBT1 and IRDye 800CW donkey anti-goat IgG (926–32214, LI-COR, 1:4000) to detect Siglec-8-Fc precomplex. After 1 h at room temperature blots were washed with PBST and scanned with an Odyssey CLx infrared imager (LI-COR).
Siglec-8 ligand purification
Screened nasal lavage samples with high Siglec-8 binding intensity (by lectin overlay blotting) were combined, 2 volumes of GuHCl extraction buffer added, and the mixture was incubated at 4°C overnight with mixing. The solution was then buffer exchanged with size exclusion buffer (4 M GuHCl, 20 mM sodium phosphate pH 7.0) by ultrafiltration (UFC910024, MilliporeSigma). The sample was concentrated ~10-fold and loaded onto a HiPrep 26/60 Sephacryl S-500 HR size exclusion column (GE Healthcare) and eluted using an ÄKTA chromatography system (GE Healthcare) at a flow rate of 1.0 ml/min collecting 2-ml fractions. A small aliquot of each fraction was dialyzed against urea buffer (Slide-A-Lyzer, ThermoFisher), electrophoresed, blotted and Siglec-8 ligands detected by lectin overlay. Fractions containing Siglec-8 ligand were combined, buffer exchanged to urea buffer, and concentrated by ultrafiltration.
Concentrated combined size exclusion fractions were precleared by mixing overnight at 4°C with 200 μl of Protein G magnetic beads (28967070, GE Healthcare) loaded with 250 μg of human IgG-Fc (AG714, MilliporeSigma). The beads were removed and the cleared supernatant was mixed as above with Protein G magnetic beads loaded with 250 μg of Siglec-8-Fc to capture Siglec-8 ligands. Unbound material in the supernatant was collected, and the beads washed multiply (0.5 ml each) with wash buffer (1 M urea, 150 mM NaCl, 20 mM sodium phosphate, pH 7.4), then ligand eluted by consecutive incubations (0.25 ml each) with the same buffer containing 1M NaCl in wash buffer. For binding inhibition assays (see below), the purified ligand was buffer exchanged to PBS by ultrafiltration as above.
Proteomic Mass Spectrometry
Purified Siglec-8 ligand from human nasal lavage was subjected to proteomic mass spectrometry as detailed previously.14 Briefly, the sample was desalted by ultrafiltration, reduced with dithiothreitol and carbamidomethylated with iodoacetamide prior to digestion with Lys-C and trypsin. The resulting peptides were bulk purified using C18 Tips (Thermo Fisher) then subjected to liquid chromatography-mass spectrometry using an Orbitrap Fusion Lumos tribrid mass spectrometer (ThermoFisher) equipped with UltiMate3000 RSLCnano liquid chromatograph using a C18 analytical column. Peptides were fragmented using higher energy collisional dissociation, electron transfer dissociation, and collision-induced dissociation. Full scan mass spectra were acquired in the positive ion mode over the range m/z = 400 to 1600 using the Orbitrap mass analyzer in profile format with a mass resolution setting of 60,000. MS2 scans were collected in the quadrupole or ion trap for the most intense ions. Data were processed using Proteome Discoverer (version 2.4, Thermo Fisher).
Inhibition Assay
A synthetic glycolipid bearing the Siglec-8 binding determinant (Neu5Acα2–3[6S]Galβ1–4GlcNAc) and a control glycolipid with an isomer that doesn’t bind Siglec-8 (Neu5Acα2–3Galβ1–4[6S]GlcNAc) were synthesized as described.9 The glycolipids were adsorbed to 96-well polystyrene microwells at 25 pmol/well as a monolayer with phosphatidylcholine (25 pmol/well) and cholesterol (100 pmol/well).9 After adsorption, plates were washed and blocked with 1 mg/ml BSA in PBS for 45 min. Siglec-8-Fc (40 μg/ml) was premixed with alkaline phosphatase-labeled anti-human Fc antibody (109-055-008, Jackson ImmunoResearch, 20 μg/ml) for 30 min at 37°C, diluted 10-fold in 2 mg/ml BSA in PBS, then diluted 1:1 with PBS or purified Siglec-8 ligands at different concentrations in PBS as potential inhibitors. After further incubation (37°C, 30 min) 50 μl were added to glycolipid-adsorbed wells. After incubation for 90 min at ambient temperature, the wells were washed and Siglec-8-Fc detected as phosphatase activity using p-nitrophenylphosphate.
To test whether inhibition was via sialoglycan binding, aliquots of purified Siglec-8 ligand were pretreated with or without beads carrying covalently immobilized sialidase (GAL-132100, Axxora, Farmingdale, NY). Ligand was mixed with 50 mU/ml of sialidase beads at 37°C for 1 h, the beads removed by centrifugation (3,000 × g, 3 min) and the supernatant retrieved.
RESULTS
A previously unidentified Siglec-8 ligand is synthesized by human airway submucosal gland cells and secreted onto airways
Siglec-8 ligands are found on large human airways (trachea, bronchus) of postmortem human lung,9 and on upper airways.10 As published previously,9 on postmortem human trachea and bronchus sections, Siglec-8 ligands were found in two compartments, cartilage and submucosal glands, but were surprisingly absent from the epithelium (and see Fig 1 and Fig E1). The specificity of Siglec-8 ligand detection by Siglec-8-Fc overlay histochemistry was determined by pretreatment with sialidase, which completely eliminated binding (Fig E1). Airway from donors with (Fig 1) and without (Fig E1) airway disease displayed the same selective expression of Siglec-8 ligands in submucosal glands and ducts. Likewise, submucosal gland acini are the site of Siglec-8 ligand expression on fixed human upper airway (nasal turbinate) sections.10
FIG 1.
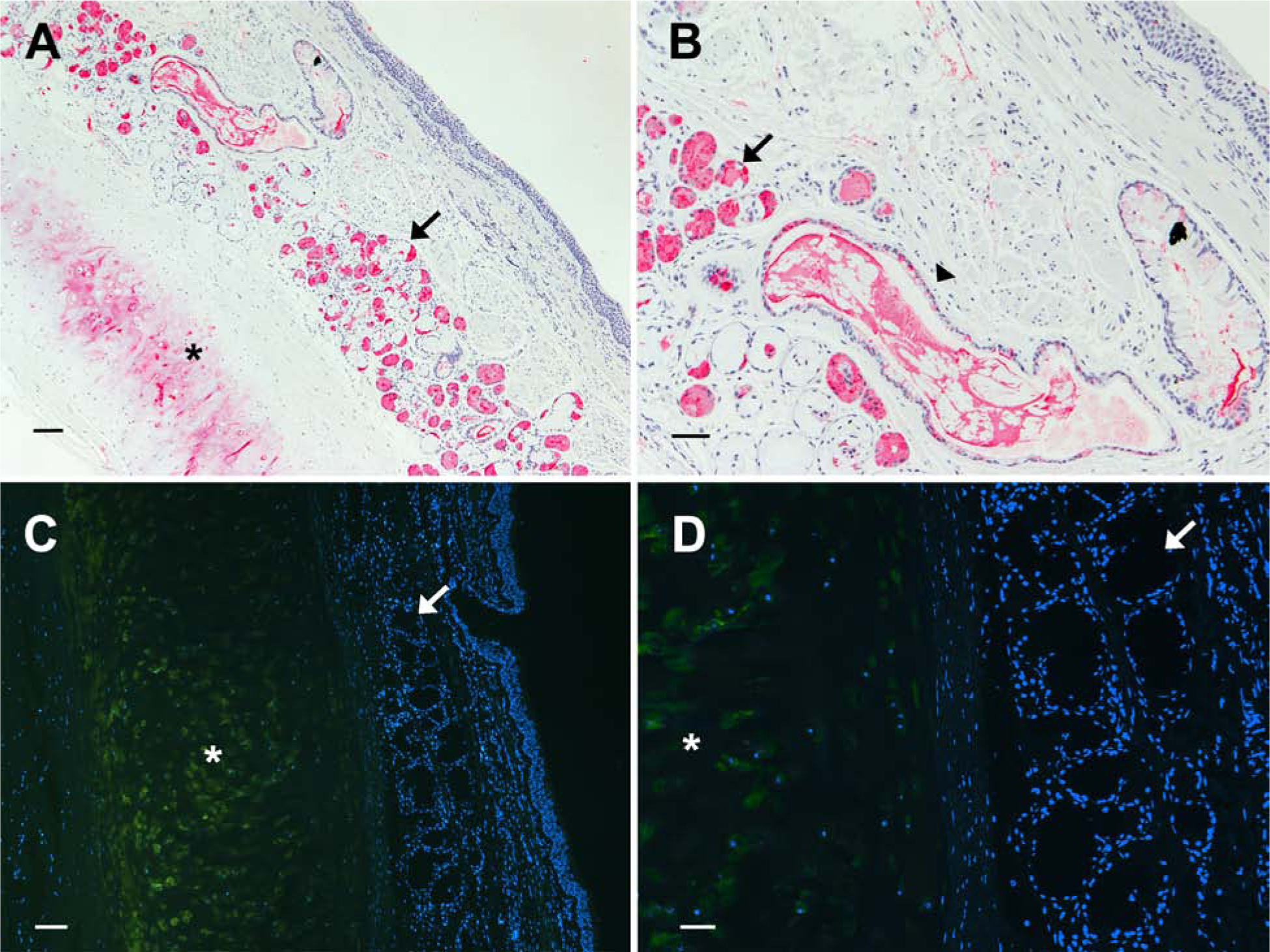
Siglec-8 ligand is abundant in human postmortem submucosal glands and ducts, and is distinct from the cartilage ligand aggrecanS8. Bronchial tissue sections from an asthmatic donor were stained for Siglec-8 ligand by Siglec-8-Fc overlay histochemistry (A, B). Consistent with prior reports, airway cartilage (*) and submucosal glands (arrow) were labeled. A higher power image (B) shows abundant Siglec-8 ligand remaining in a structure consistent with transport of secreted material to the airway, although Siglec-8 ligand is absent from the airway epithelium on postmortem tissue sections.9 Fluorescent immunohistochemistry of human trachea from an asthmatic donor (C, D) reveals aggrecan in the cartilage (*) but absent from the submucosal glands (arrow). Scale bars: 100 μm (A,C) and 50 μm (B, D).
Since Siglec-8 ligands appeared in submucosal gland cells (primarily serous cells) and were seen in some submucosal ducts, we hypothesized that a secreted form of the Siglec-8 ligand is transported to the airway mucus layer, which was lost during tissue preparation. Evidence to support this hypothesis came from occasional bronchus sections (Fig 1, B, arrowhead) in which elongated structures full of densely stained Siglec-8 ligand were seen, presumably in route to the airway mucus layer. Since we previously purified Siglec-8 ligand from postmortem human trachea and identified the sialoglycan carrier as an isoform (glycoform) of the extracellular matrix protein aggrecan (aggrecanS8), we analyzed whether aggrecan in any isoform was produced in submucosal glands. Fluorescent immunohistochemistry indicated that submucosal gland acini are devoid of aggrecan (Fig 1, C & D). Together, these data support the hypothesis that human airway submucosal glands synthesize and secrete a unique Siglec-8 ligand that is transported to the airway mucus layer to moderate allergic inflammation.
A distinct Siglec-8 ligand on the human airway mucus layer is recovered by saline lavage
To test the hypothesis that a novel soluble Siglec-8 ligand is secreted onto the airway, but lost during tissue processing, human airway mucus layer proteins were collected from patients by routine saline lavage prior to sinus surgery. After centrifugation, most samples contained a small pellet of debris (which was discarded) and a clear supernatant. Electrophoresis of aliquots of clarified nasal lavage on agarose-acrylamide composite gels to separate large molecular weight proteins, followed by blotting and overlay with Siglec-8-Fc to reveal Siglec-8 ligands, indicated one major species at ~1,000 kDa (1 MDa) molecular weight in all samples (Fig 2, A). Less intense binding to larger molecular weight species (2–4 MDa) was variably detected. Whereas most lavage samples were a single aqueous phase (donor 65, Fig. 2, B) upon centrifugation, some samples (donors 64 & 66, Fig. 2, B) separated into a clear aqueous supernatant above a viscous pellet. Solubilization of the pellet revealed the same major molecular species. Testing of a second saline installation from one patient (donor 64, Fig. 2, B) revealed that the initial nasal lavage efficiently removed essentially all of the Siglec-8 ligand from the airway, consistent with its absence from the epithelium of washed tissue sections. Since we had previously found that all Siglec-8 ligands revealed by airway tissue section overlay, on both cartilage and submucosal glands, were carried on keratan sulfate glycan chains,14 nasal lavage samples were resolved and blotted in duplicate and tested for the presence of Siglec-8 ligands and keratan sulfate, the later using an antibody to highly sulfated KS. The major KS proteoglycans in human lavage overlapped but were not congruent with Siglec-8 ligand (Fig 2, B), consistent with the conclusion that not all KS proteoglycans are Siglec-8 ligands. Nevertheless, quantitative assessment revealed a high correlation (ρ = 0.86) between the presence of Siglec-8 ligand and highly sulfated KS among >20 independent human nasal lavage samples (Fig 2, C).
FIG 2.
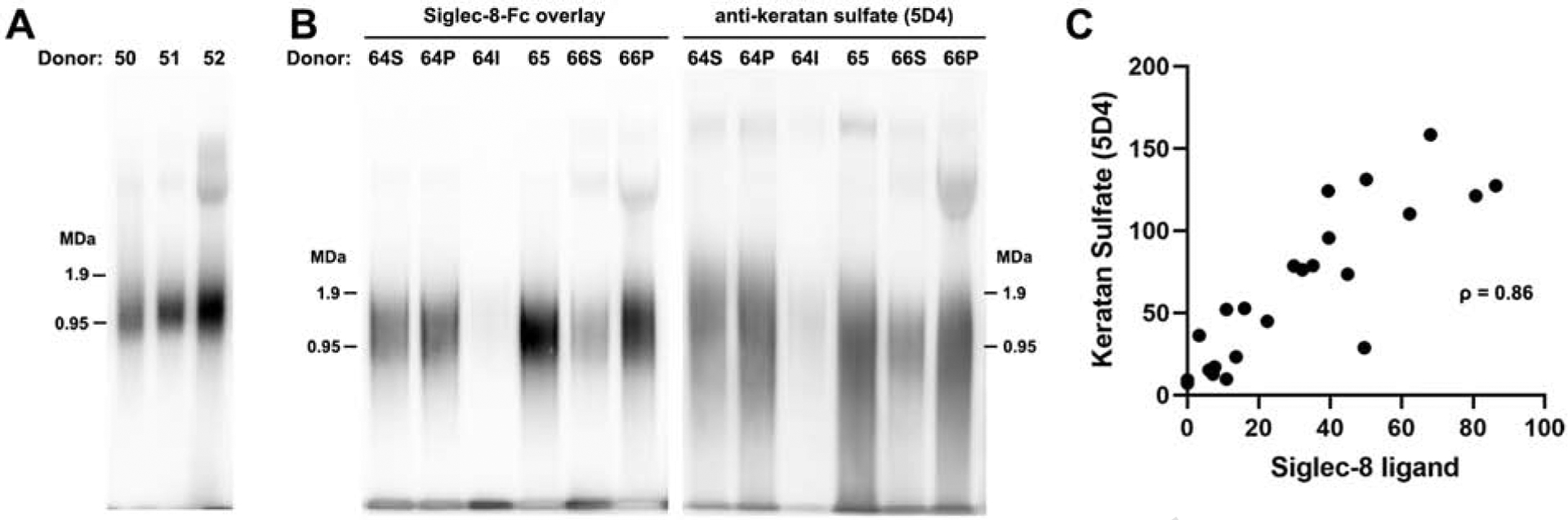
The major Siglec-8 ligand on the human airway is ~1 MDa and co-migrates with keratan sulfate proteoglycans. A, Nasal lavage proteins were resolved by composite agarose-acrylamide electrophoresis, blotted, and Siglec-8 ligands detected by overlay with Siglec-8-Fc. Three patient lavage samples were compared at equal volumes loaded. B, Nasal lavage proteins from three patients were resolved and blotted as in Panel A. As with most samples, donor 65 lavage was a single aqueous phase upon centrifugation, whereas two other samples (donors 64 & 66) separated into a clear solution (64S, 66S) and a viscous pellet that was extracted with GuHCl (64P, 66P). One of these patients was re-instilled and the second lavage solution analyzed as a separate sample (64I). Replicate blots were probed with Siglec-8-Fc and anti-keratan sulfate antibody as indicated. C, Staining intensities of lavage samples from 23 donors were resolved, blotted for Siglec-8-Fc binding and anti-keratan sulfate immunostaining, and staining intensities determined by image analysis as indicated on the axes. See Table E1 for donor airway disease status.
The nasal lavage Siglec-8 ligand is a sialylated keratan sulfate proteoglycan secreted from submucosal glands
Based on prior findings,9, 10, 14 we hypothesized that the human airway lavage ligand is composed of sialylated keratan sulfate chains carried on a large polypeptide backbone. To test this hypothesis, samples were treated in the absence or presence of sialidase, keratanase I or keratanase II prior to electrophoresis and blotting (Fig 3). Treatment with each enzyme reduced or eliminated Siglec-8 binding, confirming sialylated KS chains as the glycan element required for Siglec-8 binding. The difference between the two keratanases may reflect their differential sensitivity to sulfation and/or fucosylation on extended KS chains.17
FIG 3.
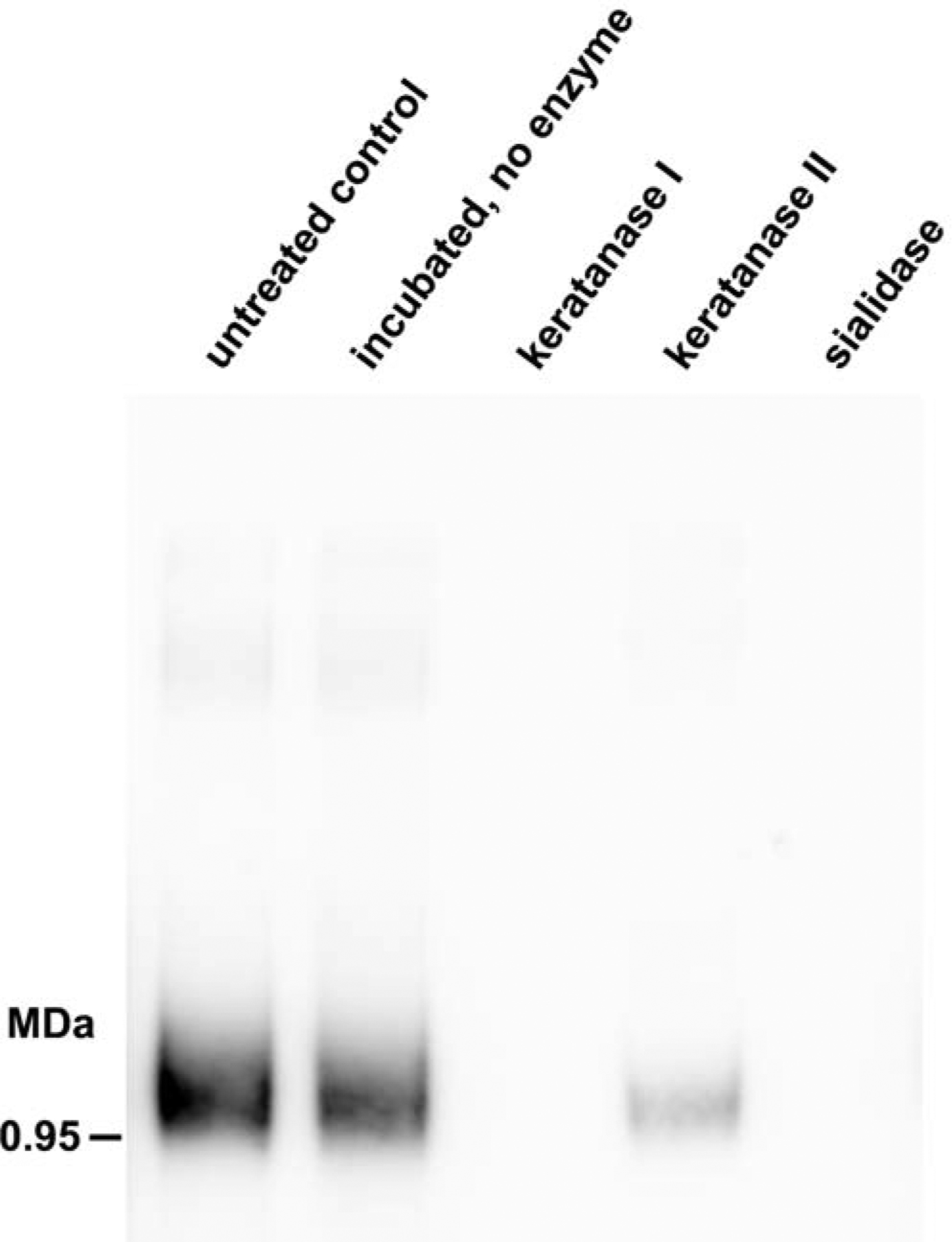
Enzymatic characterization of nasal lavage Siglec-8 ligand. Aliquots of nasal lavage supernatant were incubated with PBS (incubated, no enzyme) or with the indicated glycolytic enzymes (sialidase, keratanase I, or keratanase II). Equal amounts of each reaction were resolved by electrophoresis, blotted and Siglec-8-Fc binding determined by lectin overlay.
To test for the presence of other proteoglycan chains on the ligand, a lavage sample was treated with heparinase or chondroitinase, neither of which altered Siglec-8 binding or electrophoretic mobility (Fig E2). We conclude that a distinct sialylated KS proteoglycan is secreted onto the human airway where it engages Siglec-8 on immune cells.
Tissue overlay with Siglec-8-Fc (Fig 1, A) suggested that the source of the airway lavage ligand is the submucosal gland. To test this, the mucosa/submucosa were blunt dissected from the underlying cartilage/adventitia from postmortem human trachea, resulting in reasonable separation of the tissue compartments (Fig E3, A & B). Extraction of the cartilage/adventitia separately from the mucosa/submucosa revealed distinct molecular species in the two compartments (Fig E3, C). Whereas the cartilage-containing tissue yielded three molecular weight Siglec-8 binding species that we previously identified as isoforms of aggrecan,14 the submucosa-containing tissue revealed two species, one at ~1 MDa and another at much higher molecular weight. Consistent with immunohistochemical data in Fig. 1, the mucosa/submucosa extract was essentially devoid of aggrecan (Fig E3, D). The ~1 MDa mucosa/submucosa extract species migrated similarly to the airway lavage ligands (Fig E3, E), consistent with submucosal glands as the source of the Siglec-8 ligand recovered by airway lavage.
Purification and identification of the human airway Siglec-8 ligand
Nasal lavage samples from multiple donors were combined, concentrated, and subjected to size exclusion chromatography in GuHCl solution to ensure separation of large molecular weight proteoglycans (Fig 4, A & B). Aliquots of each fraction were resolved by electrophoresis, blotted, and probed with Siglec-8-Fc, revealing that the ligand eluted as a single peak (Fig 4, A) ahead of (larger than) the major lavage proteins (Fig 4, B). Fractions containing the ligand were combined, concentrated and incubated with magnetic beads carrying Siglec-8-Fc, resulting in capture of a portion of the total Siglec-8 ligand (Fig 4, C). After washing, bound ligand was efficiently eluted with high salt and subjected to proteomic mass spectrometry.
FIG 4.

Purification of Siglec-8 ligand from human nasal lavage. A, Combined nasal lavage samples in GuHCl solution were concentrated and resolved by size exclusion chromatography. Aliquots of fractions were resolved on composite agarose-acrylamide gels, blotted, and Siglec-8 ligand detected by Siglec-8-Fc lectin overlay. The two gels with consecutive fractions containing Siglec-8 ligand are shown. Fractions combined for further purification are designated by a horizontal line. B, The protein elution profile (A280) from two consecutive size exclusion chromatographic runs are overlaid. Fractions containing Siglec-8 ligand that were combined for further purification are designated by a horizontal line. C, Combined and concentrated size exclusion fractions with Siglec-8 ligand were loaded onto a Siglec-8-Fc affinity resin which was washed with buffer and then Siglec-8 ligand eluted using the same solution with increased salt. Comparison of the “load” and “unbound” lanes reveal that only a portion of the Siglec-8 ligand was bound. Subsequent reloading of the unbound fraction resulted in eventual capture and elution of all Siglec-8 ligand in the sample (data not shown).
Proteomic analysis revealed that the top polypeptide identified in the affinity-purified Siglec-8 ligand was DMBT1 (deleted in malignant brain tumors 1). This protein, also known as salivary scavenger and agglutinin (SALSA), salivary agglutinin (SAG), GP340, and muclin, is a well-known secreted protein involved in inflammation and immunity.18, 19 It is very large (2,413 amino acids) and carries an abundance of sialylated O-linked glycans.20 Proteomic mass spectrometry of the purified Siglec-8 ligand found six unique high confidence peptides attributed to DMBT1 (Table E2). Because DMBT1 contains repeat sequences, the six peptides cover 22% of the entire polypeptide, distributed over the N-terminal three quarters of the large protein (Fig 5). The only other proteins confidently identified in the screen (those with more than one attributed peptide) were contaminants of affinity purification and handling (Siglec-8, human Fc, keratin), making DMBT1 the most likely candidate as Siglec-8 ligand (Table E3).
FIG 5.

DMBT1 protein map. The amino acid sequence of DMBT1 (UniProt Q9UGM3.2) is presented below a linear map of the 2413-amino acid polypeptide. Peptide sequences that were detected by proteomic mass spectrometry are highlighted in green. Interspersed sequences predicted to be O-glycosylated are highlighted in blue.
It should be emphasized that DMBT1 itself is not the Siglec-8 ligand. Instead, an isoform (glycoform) of DMBT1 that carries the precise sialoglycan structure required for Siglec-8 binding appears to the be major Siglec-8 ligand on human airways. Repeated affinity clearance of Siglec-8 ligand from partially purified (size-excluded) nasal lavage sample eventually cleared all of the Siglec-8 binding material but left the majority of the DMBT1 (data not shown). We conclude that most DMBT1 isoforms are not post-translationally glycosylated to carry Siglec-8-binding glycans. Consistent with proposed nomenclature for functional glycoforms,21 we designate the Siglec-8 binding isoform as DMBT1S8 to distinguish it from other isoforms of DMBT1 that lack the Siglec-8 binding determinant.
Primary sequence analysis of DMBT1 reveals repeat sequences rich in prolines, serines and threonines that are predicted to be rich in O-glycosylation using the predictive tool NetOGlyc4.0 (Fig 5).22 These regions are dispersed among but not overlapping the peptides discovered by proteomic mass spectrometry. These data are consistent with O-glycosylation in these regions resulting in loss of bioinformatic detection of the predicted naked peptide masses. Additional data supporting O-linked sialylated keratan sulfate chains as Siglec-8 ligands on DMBT1 was obtained by treating the purified ligand with PNGaseF to remove N-linked glycans. No decrease in Siglec-8 binding was detected (data not shown). There is no comparable enzyme to remove O-linked glycans, and chemical de-O-glycosylation resulted in partial protein degradation precluding more definitive glycan linkage characterization.
Validation of DMBT1S8 as the major Siglec-8 ligand from human airways was via electrophoretic co-migration (Fig 6). Double-labeled staining of resolved unprocessed nasal lavage proteins with Siglec-8-Fc and anti-DMBT1 revealed nearly precise co-migration of the major molecular species at ~1 MDa. Notably, the minor (and variable) high molecular weight Siglec-8 ligand failed to stain for DMBT1. The ratio of Siglec-8-Fc binding (Siglec-8 ligand quantification) and α-DMBT1 antibody binding across a larger number of patient nasal lavage samples varied, indicating differential regulation of DMBT1S8 expression (data not shown).
FIG 6.
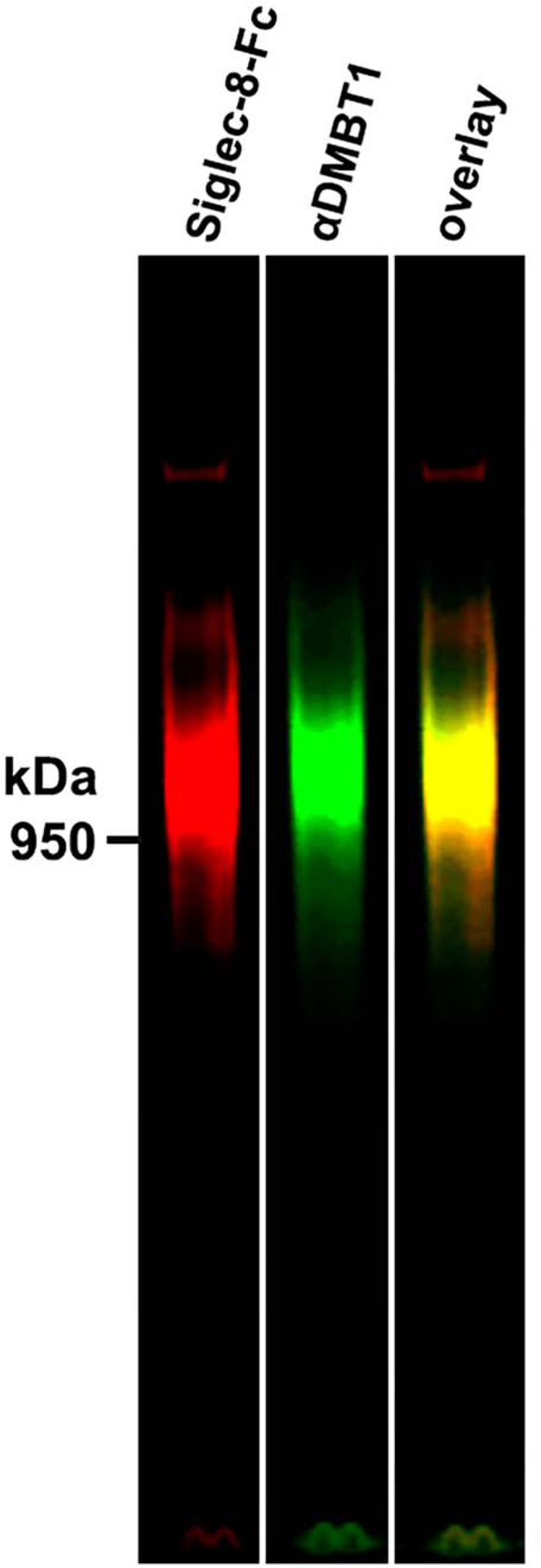
Double label co-migration of Siglec-8 ligand (via Siglec-8-Fc lectin overlay) and DMBT1 (via immunoblot). Nasal lavage rich in Siglec-8 ligand was resolved and double labeled with Siglec-8-Fc and anti-DMBT1 antibody using near IR tagged secondary antibodies.
Further evidence consistent with posttranslational modification of DMBT1 with Siglec-8 binding glycans in airway submucosal glands came from double-label lectin- (Siglec-8-Fc) and immuno- (anti-DMBT1) histochemistry (Fig 7). Consistent with prior experiments (and Fig 1, A), postmortem human tracheal tissue staining with Siglec-8-Fc revealed Siglec-8 ligand in submucosal glands and cartilage (Fig 7, A). Notably, anti-DMBT1 failed to stain cartilage, but stained the same submucosal acini as Siglec-8-Fc (Fig 7, B–F). These data are consistent with the recent report of DMBT1 on human airway submucosal glands and ducts but not respiratory epithelium,23 as previously reported for Siglec-8 ligands.9 Likewise, single-cell transcriptomics revealed that DMBT1 gene expression in human lungs and airways is remarkably restricted to submucosal gland cells.24 These data, along with the data in Fig 1, indicate that while an isoform of aggrecan in cartilage carries Siglec-8 ligands,14 the major Siglec-8 ligand in submucosal glands and secreted onto the human airway is DMBT1S8.
FIG 7.
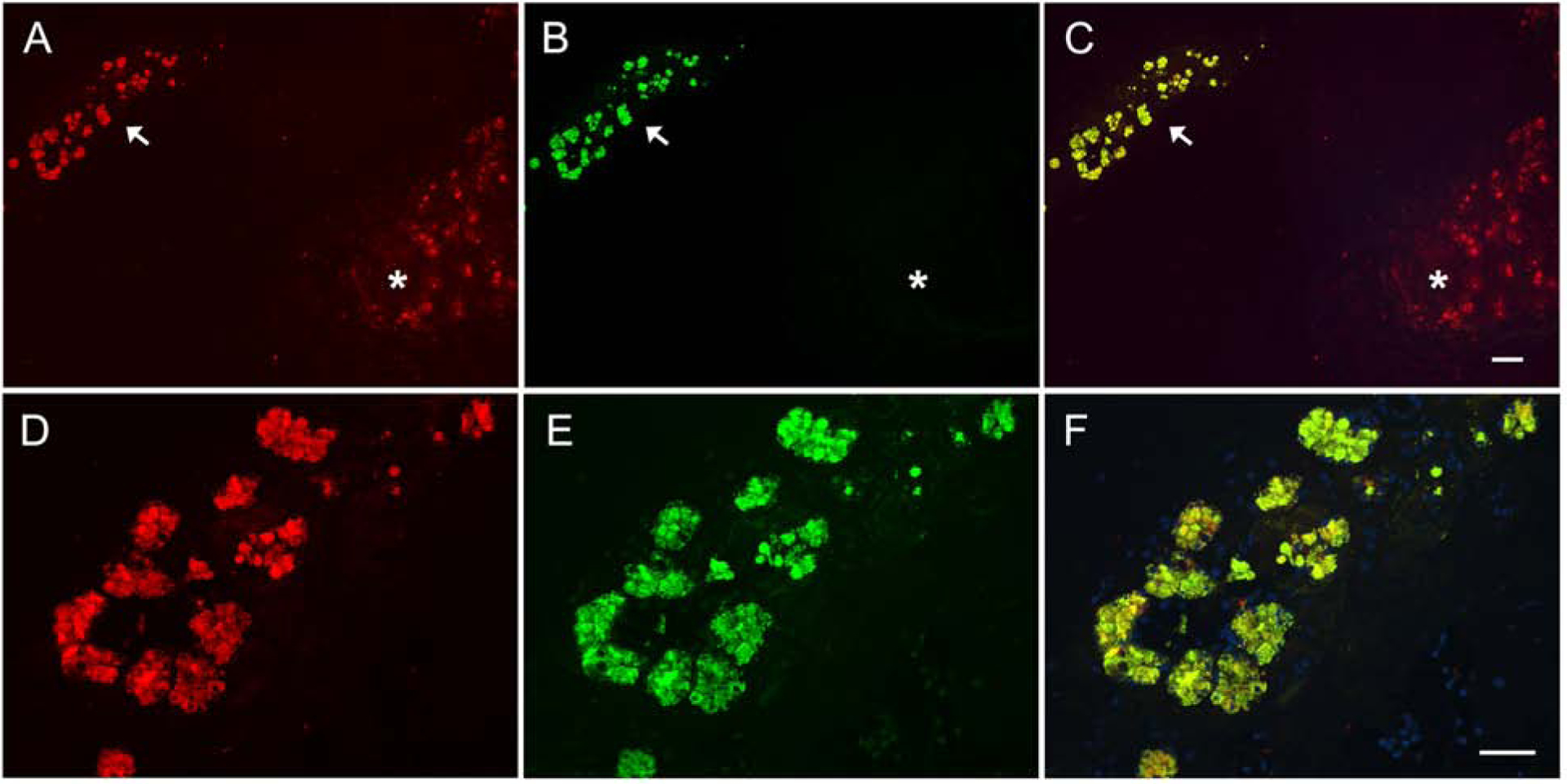
Postmortem human trachea sections double-stained for Siglec-8 ligand and DMBT1. A section of trachea containing submucosal glands (arrow) and cartilage (*) was prepared from donor without airway disease. The section was co-stained for (A, D) Siglec-8 ligands using Siglec-8-Fc lectin overlay (Alexa Fluor 594, red) and (B, E) DMBT1 using anti-DMBT1 antibody (Alex Fluor 488, green). Overlay of the color images (C, F) indicate co-localization in the submucosal glands and absence of DMBT1 in the cartilage. Scale bars: A-C, 200 μm; D-F, 100 μm.
DMBT1S8 is a high-affinity Siglec-8 ligand
We hypothesized that DMBT1S8 is a high-affinity Siglec-8 ligand because we were unable to detect it with sensitive protein stains on electrophoretic blots despite high-intensity Siglec-8-Fc binding to the same blots. To directly measure DMBT1S8 affinity for Siglec-8, we used a competitive binding inhibition assay (Fig 8). The protein concentration of a DMBT1S8 preparation was estimated using anti-DMBT1 immunoblot intensity compared to a preparation of DMBT1 isoforms of similar size, isolated during purification, that did not bind to the Siglec-8 affinity beads or Siglec-8-Fc but were sufficiently abundant to determine protein concentration by protein staining (data not shown). Siglec-8-Fc was mixed with a series of concentrations of DMBT1S8, and the mixture was added to microwells adsorbed with a synthetic Siglec-8-binding glycan.9 After incubation, the inhibitory effect of DMBT1S8 on Siglec-8-Fc binding was quantified.
FIG 8.
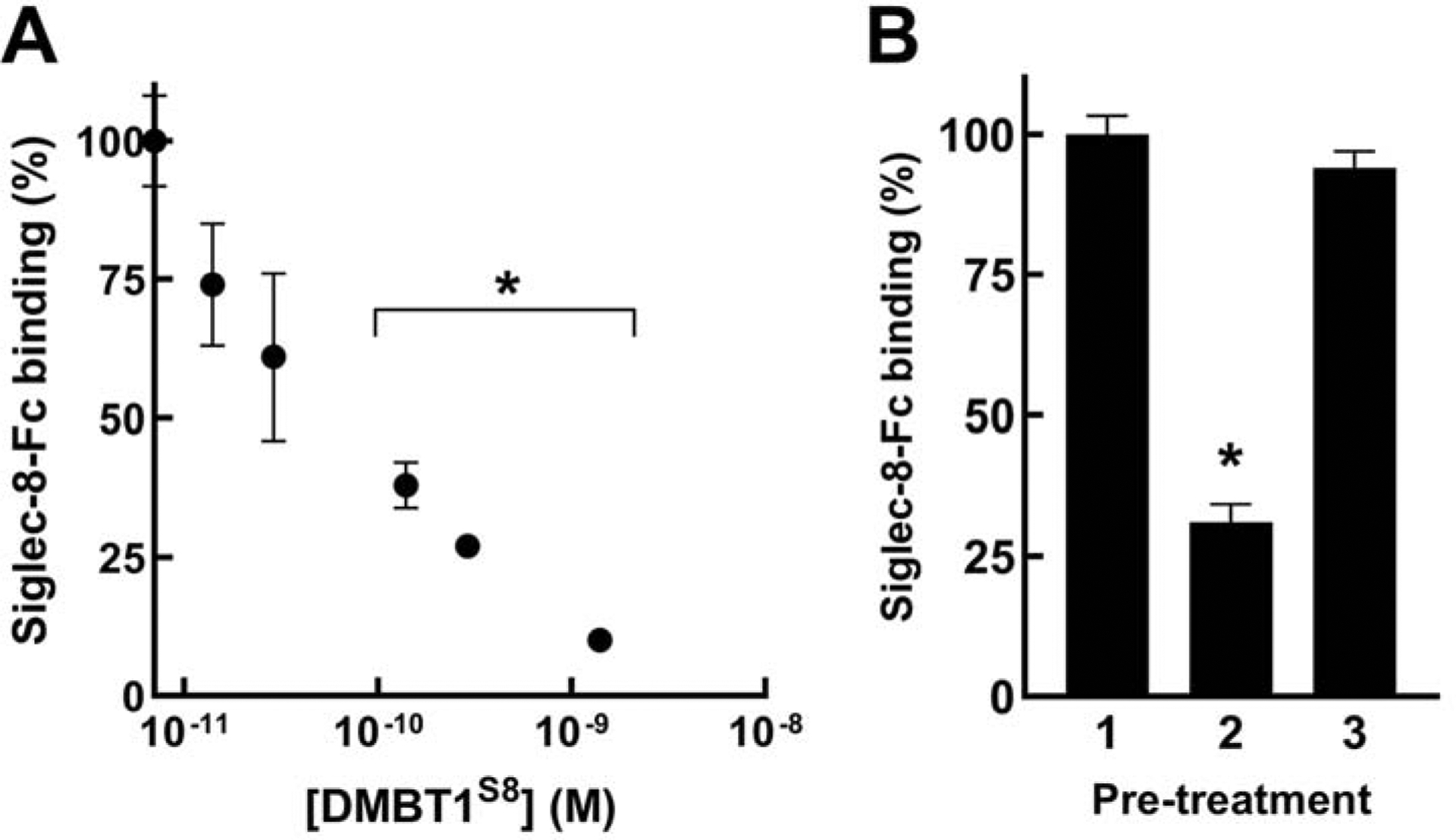
DMBT1S8 is a high affinity Siglec-8 ligand. A, Siglec-8-Fc precomplexed with secondary antibody was preincubated in PBS alone or PBS containing the indicated molar concentrations of purified DMBT1S8. The mixtures were pipetted into microwells pre-adsorbed with a synthetic glycolipid bearing the Siglec-8 ligand Neu5Acα2–3[6S]Galβ1–4GlcNAc. As a control Siglec-8-Fc preincubated in PBS was pipetted into microwells pre-adsorbed with control synthetic glycolipid that does not bind Siglec-8 (Neu5Acα2–3Galβ1–4[6S]GlcNAc, data not shown). After incubation, bound Siglec-8-Fc was determined. In five experiments the signal:background averaged >20-fold. Binding data were background-subtracted and binding relative to the PBS alone condition are plotted as the mean percent ± SEM for 3–16 replicate wells. The SEM for PBS alone wells is shown as error bars from 100% on the Y-axis. *, p < 0.005 compared to PBS alone. B, An experiment was performed as in (A) with DMBT1S8 (140 pM) preincubated with (or without) sialidase-derivatized beads. Lane 1, no DMBT1S8 added; Lane 2, DMBT1S8 preincubated with PBS; Lane 3, DMBT1S8 preincubated with sialidase beads. Background subtracted binding is presented relative to no inhibitor added as mean percent ± SEM. Signal:noise, >60. *, p < 10−5 compared to no inhibitor or preincubation with sialidase.
Inhibition was concentration dependent with a half-maximal inhibitory concentration of <100 pM (Fig 8, A). To establish that the inhibition was due to ligand binding via the Siglec-8 sialoglycan binding site, a portion of the DMBT1S8 was pre-treated with covalently immobilized sialidase, which completely reversed its inhibition (Fig 8, B). We conclude that DMBT1S8 is secreted at low concentration onto the airway mucus layer, and has high affinity for engaging Siglec-8.
DISCUSSION
The Siglec family of immune cell surface receptors and the extracellular sialoglycans to which they bind provide receptor-ligand interactions that modulate immune responses.2 Although much has been learned about Siglecs and their potential as drug targets,5, 25 less is known about their endogenous sialoglycan ligands. Nature typically builds glycans into multivalent arrays that enhance site affinity by clustering glycans on protein carriers that engage receptors with high avidity to initiate physiological outcomes.7, 26 Isolation and characterization of Siglec ligands promises to provide structural information about the glycan determinants and their molecular context that will reveal natural pathways of immune regulation and inform therapeutic development. Siglec-8 is expressed selectively on eosinophils and mast cells on human inflamed airways, where crosslinking induces eosinophil apoptosis and inhibits mast cells,6 providing motivation to identify endogenous human Siglec-8 ligands.
Our data indicate that on the surface of human airways there is a single major endogenous Siglec-8 ligand, ~1 MDa, recovered by saline lavage. Our finding that the protein carrier is DMBT1 is not particularly surprising, since it was originally isolated (as gp-340) from human bronchoalveolar lavage.27 DMBT1 is a common secreted protein with multiple domains for O-glycosylation. When a particular cell type, in this case submucosal gland cells, expresses the suite of glycosyltransferases and sulfotransferases required to synthesize the Siglec-8 binding determinant on keratan sulfate chains, that particular sialoglycan structure is built on nascent DMBT1 to convert it to DMBT1S8. DBMT1 is like a canvas on which Siglec-8 binding determinants are painted. In nasal lavage most DMBT1, a multifunctional protein, does not carry the Siglec-8 binding determinant, is likely decorated with other glycans, and supports other functions on the airway surface.18, 19 DMBT1 is expressed on many human tissues,23 most abundantly on intestine and salivary gland, where it may not carry Siglec-8 ligands (preliminary data not shown).
The conclusion that submucosal gland cells are programed to synthesize and secrete DMBT1S8 is supported by the finding that extracts of dissected tracheal mucosa/submucosa tissue contain a molecule with the properties of that in nasal lavage. We hypothesize that DMBT1S8 is secreted onto the airway mucus layer as a mechanism to engage activated eosinophils, which are uniquely sensitive to Siglec-8 crosslinking,7 and resolve ongoing eosinophilic inflammation. Whether DMBT1 conversion to DMBT1S8 is regulated in response to eosinophilic or mast cell mediated inflammation is worthy of investigation. Knowledge of the genes responsible for building DMBT1S8 and their regulation may provide useful information about resolution pathways of eosinophilic and mast cell inflammation in health and disease.
The observation that chondrocytes in cartilage express the enzymes required to convert aggrecan to aggrecanS8 raises the question of why there is an immune modulatory glycan decorating a subpopulation of this major cartilage proteoglycan. Whether the presence of the Siglec-8-binding structural determinant on aggrecan plays another role in cartilage, or is involved in modulating inflammation, has yet to be determined.
The remarkably high apparent affinity of DMBT1S8 for Siglec-8, <10−10 M, is notable, in that the site affinity for the minimal glycan binding determinant, Neu5Acα2–3[6S]Galβ1–4GlcNAc, is >10−4 M. Whether DMBT1S8 carries additional binding motifs that engage Siglec-8 to enhance affinity, or expresses precisely spaced multiple binding determinants that account for the higher avidity, has yet to be determined. With purified DMBT1S8 from the human airway mucus layer in hand, structure-function studies to address these questions can begin.
Supplementary Material
Key Messages:
An isoform of the secreted protein DMBT1 that binds the immunoinhibitory eosinophil and mast cell surface receptor Siglec-8 (DMBT1S8) is produced in human airway submucosal glands and secreted onto the airway mucus layer.
DMBT1S8 has picomolar avidity for Siglec-8.
We hypothesize that DMBT1S8, the major Siglec-8 ligand on human airways, is an endogenous regulator of eosinophilic and mast cell airway inflammation.
Funding:
Supported by National Institutes of Health grants U19-AI136443 (RLS), K12-HL141952 (AGG, JK), T32-GM0087623 (TAL), T32-GM080189 (RNP and HT), the Flight Attendent Medical Research Institute, and a research gift from the Carl and Kara Pittinger Family (HL, JK).
We thank Corwin Nycholat for providing important reagents.
Abbreviations used:
- BSA
bovine serum albumin
- GuHCl
guanidinium hydrochloride
- KS
keratan sulfate
- PBS
Dulbecco’s phosphate-buffered saline
- PBST
PBS supplemented with 0.1% Triton X-100
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures:
Jean Kim has consulted for ALK and Genentech. Other authors declare no conflicts of interest.
REFERENCES
- 1.Barnig C, Frossard N, Levy BD. Towards targeting resolution pathways of airway inflammation in asthma. Pharmacol Ther 2018; 186:98–113. [DOI] [PubMed] [Google Scholar]
- 2.Duan S, Paulson JC. Siglecs as Immune Cell Checkpoints in Disease. Annu Rev Immunol 2020. [DOI] [PubMed] [Google Scholar]
- 3.Kikly KK, Bochner BS, Freeman SD, Tan KB, Gallagher KT, D’alessio KJ, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J. Allergy Clin. Immunol 2000; 105:1093–100. [DOI] [PubMed] [Google Scholar]
- 4.Johansson MW, Kelly EA, Nguyen CL, Jarjour NN, Bochner BS. Characterization of Siglec-8 Expression on Lavage Cells after Segmental Lung Allergen Challenge. Int Arch Allergy Immunol 2018; 177:16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Sullivan JA, Chang AT, Youngblood BA, Bochner BS. Eosinophil and mast cell Siglecs: From biology to drug target. J Leukoc Biol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerr SC, Gonzalez JR, Schanin J, Peters MC, Lambrecht BN, Brock EC, et al. An Anti-Siglec-8 Antibody Depletes Sputum Eosinophils from Asthmatic Subjects and Inhibits Lung Mast Cells. Clin Exp Allergy 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll DJ, O’Sullivan JA, Nix DB, Cao Y, Tiemeyer M, Bochner BS. Sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8) is an activating receptor mediating beta2-integrin-dependent function in human eosinophils. J Allergy Clin Immunol 2017; 141:2196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Propster JM, Yang F, Rabbani S, Ernst B, Allain FH, Schubert M. Structural basis for sulfation-dependent self-glycan recognition by the human immune-inhibitory receptor Siglec-8. Proc Natl Acad Sci U S A 2016; 113:E4170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu H, Gonzalez-Gil A, Wei Y, Fernandes SM, Porell RN, Vajn K, et al. Siglec-8 and Siglec-9 binding specificities and endogenous airway ligand distributions and properties. Glycobiology 2017; 27:657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia Y, Yu H, Fernandes SM, Wei Y, Gonzalez-Gil A, Motari MG, et al. Expression of ligands for Siglec-8 and Siglec-9 in human airways and airway cells. J. Allergy Clin. Immunol 2015; 135:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HS, Myers A, Kim J. Vascular endothelial growth factor drives autocrine epithelial cell proliferation and survival in chronic rhinosinusitis with nasal polyposis. Am J Respir Crit Care Med 2009; 180:1056–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moustafa I, Connaris H, Taylor M, Zaitsev V, Wilson JC, Kiefel MJ, et al. Sialic acid recognition by Vibrio cholerae neuraminidase. J. Biol. Chem 2004; 279:40819–26. [DOI] [PubMed] [Google Scholar]
- 13.Steward M, Berezovskaya Y, Zhou H, Shediac R, Sun C, Miller N, et al. Recombinant, truncated B. circulans keratanase-II: Description and characterisation of a novel enzyme for use in measuring urinary keratan sulphate levels via LC-MS/MS in Morquio A syndrome. Clin Biochem 2015; 48:796–802. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Gil A, Porell RN, Fernandes SM, Wei Y, Yu H, Carroll DJ, et al. Sialylated keratan sulfate proteoglycans are Siglec-8 ligands in human airways. Glycobiology 2018; 28:786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz BL, Packer NH, Karlsson NG. Small-scale analysis of O-linked oligosaccharides from glycoproteins and mucins separated by gel electrophoresis. Anal Chem 2002; 74:6088–97. [DOI] [PubMed] [Google Scholar]
- 16.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, He W, Jiang P, Yu Y, Lin L, Sun X, et al. Construction and functional characterization of truncated versions of recombinant keratanase II from Bacillus circulans. Glycoconj J 2017; 34:643–9. [DOI] [PubMed] [Google Scholar]
- 18.Madsen J, Mollenhauer J, Holmskov U. Review: Gp-340/DMBT1 in mucosal innate immunity. Innate Immun 2010; 16:160–7. [DOI] [PubMed] [Google Scholar]
- 19.Reichhardt MP, Holmskov U, Meri S. SALSA-A dance on a slippery floor with changing partners. Mol Immunol 2017; 89:100–10. [DOI] [PubMed] [Google Scholar]
- 20.Schulz BL, Oxley D, Packer NH, Karlsson NG. Identification of two highly sialylated human tear-fluid DMBT1 isoforms: the major high-molecular-mass glycoproteins in human tears. Biochem J 2002; 366:511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor ME, Drickamer K, Schnaar RL, Etzler ME, Varki A. Discovery and Classification of Glycan-Binding Proteins. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al. , editors. Essentials of Glycobiology, Third Edition. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2017. p. 361–72. [Google Scholar]
- 22.Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J 2013; 32:1478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bathum Nexoe A, Pedersen AA, von Huth S, Detlefsen S, Hansen PL, Holmskov U. Immunohistochemical Localization of Deleted in Malignant Brain Tumors 1 in Normal Human Tissues. J Histochem Cytochem 2020; 68:377–87. [DOI] [PubMed] [Google Scholar]
- 24.Vieira Braga FA, Kar G, Berg M, Carpaij OA, Polanski K, Simon LM, et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat Med 2019; 25:1153–63. [DOI] [PubMed] [Google Scholar]
- 25.Laubli H, Varki A. Sialic acid-binding immunoglobulin-like lectins (Siglecs) detect self-associated molecular patterns to regulate immune responses. Cell Mol Life Sci 2020; 77:593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meiers J, Siebs E, Zahorska E, Titz A. Lectin antagonists in infection, immunity, and inflammation. Curr Opin Chem Biol 2019; 53:51–67. [DOI] [PubMed] [Google Scholar]
- 27.Holmskov U, Lawson P, Teisner B, Tornoe I, Willis AC, Morgan C, et al. Isolation and characterization of a new member of the scavenger receptor superfamily, glycoprotein-340 (gp-340), as a lung surfactant protein-D binding molecule. J Biol Chem 1997; 272:13743–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


