Abstract
Purpose:
Pre-exposure prophylaxis (PrEP)—an effective and safe intervention to prevent HIV transmission—was recently approved by the Food and Drug Administration for use by adolescents. Informed by studies of sexual behavior and PrEP adherence, retention, and promotion, we model the potential impact of PrEP use among at-risk adolescent sexual minority males.
Methods:
We simulate an HIV epidemic among men who have sex with men (MSM) aged 13–39. We assume adult MSM ages 19–39 have had PrEP available for 3 years with 20% coverage among eligible MSM based on the Centers for Disease Control and Prevention guidelines. PrEP interventions for ages 16–18 are then simulated using adherence and retention profiles drawn from the ATN113 and Enhancing Preexposure Prophylaxis in Community studies across a range of uptake parameters (10%–100%). Partnerships across age groups were modeled using parameterizations from the RADAR study. We compare the percent of incident infections averted (impact), person-years on PrEP per infection averted (efficiency), and changes in prevalence over 10 years.
Results:
As compared to no PrEP use, baseline PrEP adherence and retention among adolescent sexual minority males drawn from the ATN113 and Enhancing Preexposure Prophylaxis in Community studies averted from 2.8% to 41.0% of HIV infections depending on the fraction of eligible adolescent sexual minority males that initiated PrEP at their annual health-care visit. Improved adherence and retention achieved with an array of focused interventions from real-world settings increased the percent of infections averted by as much as 26%–70%.
Conclusions:
Empirically demonstrated improvements in the PrEP continuum of care in response to existing interventions can substantially reduce incident HIV infections among adolescent sexual minority males.
Keywords: Adolescent sexual minority, HIV, Pre-exposure prophylaxis, Agent-based network model
Adolescent sexual minority males (ASMM), defined here as sexual minority males aged 13–18 years, are a high-risk population for acquiring HIV. In 2017, youth aged 13–24 years made up 21% (8,164) of the 38,739 new HIV diagnoses in the U.S., 81% of which were in the male-to-male sexual contact risk category [1]. HIV incidence among 16- to 17-year-old Chicago ASMM has been estimated at 5.2/100 person-years [2], and a three-city study reported incidence of 3.4/100 person-years among ASMM [3]. ASMM are also less likely to be tested for HIV compared to adult men who have sex with men (MSM) [4,5].
PrEP is an effective and safe intervention to prevent HIV [6,7], approved by the Food and Drug Administration (FDA) in 2018 for use by adolescents weighing at least 77 pounds. PrEP could significantly contribute to reducing the HIV burden among ASMM [8], but little is known about how effective a PrEP program is likely to be for this population [9]. Previous modeling studies have demonstrated that PrEP use by ASMM can be highly impactful in reducing new infections [10,11] and potentially cost-effective in some subpopulations in high-incidence settings [12]. However, these studies were limited by a scarcity of available data on the PrEP continuum of care (CoC) for ASMM.
Researchers have proposed different conceptualizations of the PrEPCoC [13,14], but at their core each comprises three essential steps: uptake, adherence and retention (continued participation in the PrEP program, regardless of adherence). Using empirical estimates of the components of the PrEPCoC will allow us to determine how changes at different loci along the CoC might affect epidemic outcomes.
Now that PrEP has been available for many years (at least among adults), several studies have evaluated PrEP programs and interventions and provided estimates for several aspects of the PrEPCoC missing from previous models. Specifically, two of those studies—the Enhancing Preexposure Prophylaxis in Community (EPIC) study [15] and Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) 113 [16]—provided estimates for adherence and retention.
EPIC evaluated PrEPmate, anmHealth (mobile health) intervention for PrEP adherence among younger MSM (ages 18–29) that included both texting and interactive online content (Table 1) [15]. EPIC provided baseline adherence and retention measures in a real-world setting for young MSM and potential intervention effect sizes. Adherence at 36 weeks was 57% versus 72% for control and intervention, respectively, a statistically significant 26% difference. Retention at 36 weeks was 57% and 80%, a significant 40% increase.
Table 1.
Scenario sets to evaluate the impact of pre-exposure prophylaxis (PrEP) use by adolescent sexual minority males using empirical adherence and retention parameters
| Scenario seta | Data source | Study arm | Adherence (% of PrEP users adhering at each level) |
Retention |
|---|---|---|---|---|
| 1 | EPIC: N = 121; MSM (ages 18–29); recruited through the CORE center, Chicago Il.; Primary outcomes: retention - attendance at 4, 12, 24, and 36 weeks; adherence - TFV-DP levels | Standard care | 57% – High (≥4 days; >719 fmol/punch plasma tenofovir) 14.3% – Moderate (2–3 days)b 14.3% – Low (<2 days)b 14.3% – None (BLQc)b |
57% at 36 weeks |
| Intervention | 72% – High (≥4 days; > 719 fmol/punch plasma tenofovir) 9.3% – Moderate (2–3 days)b 9.3% – Low (<2 days)b 9.3% – None (BLQc)b |
80% at 36 weeks | ||
| 2 | ATN113: N = 78; ASMM (ages 15–17); recruited across the ATN cities; Primary outcomes: retentions - attendance at 4, 8, 12, 24, and 36 weeks; adherence - TFV-DP levels | Standard care (last 36 weeks of ATN113) | 27.5% – High (≥4 days; >719 fmol/punch plasma tenofovir) 9.3% – Moderate (2–3 days) 26.3% – Low (<2 days) 36.9% – None (BLQc) |
50% at 48 weeks |
| Intervention (first 12 weeks of ATN113) | 55.8% – High (≥4 days; >719 fmol/punch plasma Tenofovir) 16.9% – Moderate (2–3 days) 22.5% – Low (<2 days) 4.8% – None (BLQc) |
|||
| 3 | EPIC and ATN113 | Standard care (entire ATN113 study period) | 41.6% – High (≥4 days; >719 fmol/punch plasma tenofovir) 13.1% – Moderate (2–3 days) 24.4% – Low (<2 days) 20.9% – None (BLQc) |
50% at 48 weeks |
| Intervention (relative change in EPIC applied to standard care) | 52.4% – High (≥4 days; >719 fmol/punch plasma tenofovir) 10.5% – Moderate (2–3 days) 20.0% – Low (<2 days) 17.1% – None (BLQc) |
70% at 48 weeks |
ASMM = adolescent sexual minority males; MSM = men who have sex with men.
Estimates of sexual mixing by age were derived from RADAR, a Chicago longitudinal cohort study of AMSM and young adult MSM.
EPIC only reported high adherence so the remaining fraction was distributed equally across the remaining categories.
BLQ = below the limit of quantitation.
There are few data specific to PrEP use by ASMM, because FDA approval for adolescent use was not given until 2018. However, the ATN113 safety and feasibility trial focused specifically on U.S. ASMM aged 15–17 years (Table 1) [16]. ATN113 did not evaluate an intervention, but it does provide measures of PrEP adherence (intracellular tenofovir diphosphate (TFV-DP) and emtricitabine triphosphate concentrations in red blood cells) and retention for ASMM aged 15–17 years. ATN113 was conducted in two phases, providing a natural experiment: follow-up initially occurred every 4 weeks, with high adherence (≥4 pills/week) averaging 55.8% (range across visits 52.4%–60%), and then dropped to every 12 weeks, with a corresponding drop in high adherence to 27.5% (range 22.7%–31.5%). The roughly 50% difference in average adherence between the two phases coupled with the remarkably consistent level of high adherence within each phase, indicated by the ranges, suggest that the two distinct levels may, in part, result from follow-up frequency. This provides an estimate for how much follow-up frequency affects adherence among ASMM.
In this analysis, we leverage these recently available data sources to address existing limitations in adolescent PrEP models with the goal of providing more grounded estimates of PrEP impact that can inform public health planning.
This modeling study was determined not to involve human subjects; institution review board approval was not required.
Methods
Model
We used a previously described stochastic dynamic network model comprising ~54,000 adolescent and adult MSM ages 13–39 [17]. Similar to previous analyses [17], we modeled sexual relationship formation and dissolution; sexual behavior within partnerships; HIV testing; initiation, adherence and discontinuation of both ART treatment and PrEP; transmission; intrahost viral dynamics, including viral suppression but excluding drug resistance; and demographic change. The model was implemented using the EpiModel platform [18].
Unique to this model, partnerships between ASMM and adult MSM were modeled using parameters calculated from self-reports of partner age in the RADAR study, a longitudinal cohort study of MSM ages 16–29 [19]. We found that 53.8% of partners reported by 16- to 18-year-old ASMM were aged 19+. The mean absolute difference in the cube root of the ages of the respondents and their partners was .144. The cube root parameter was selected for the model because it allows for a rapid increase in mean partner age differences as adolescents mature to early adulthood. Figure 1 shows the kernel density estimation of age mixing based on our baseline simulation results using this parametrization. The high-density region on the right shows that among the ASMM the majority of those in partnerships were ages 17–19, and the high-density ridge sloping upward from left to right indicates that the partners of ASMM tended to be a similar age or slightly older, with most <2 years older.
Figure 1.
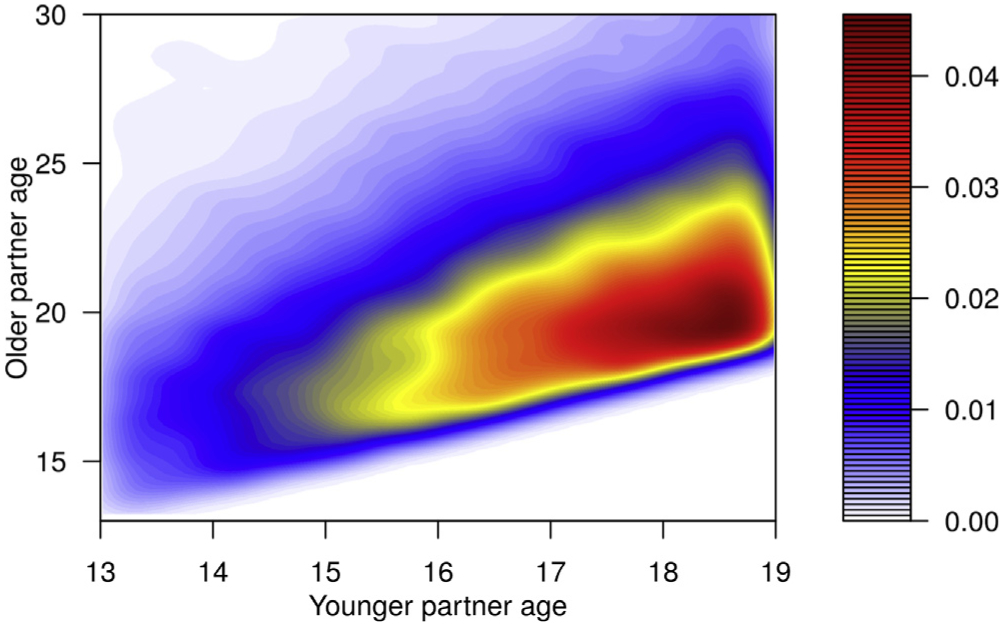
Density heat map of partnerships involving adolescent sexual minority males by age. The density heat map shows the distribution of partnerships that include an adolescent sexual minority male based on the ages of the two individuals in the partnership. The age of the younger partner, who is always an adolescent sexual minority male, is shown on the x axis, and the age of the older partner, who could be another adolescent or an adult, is shown on the y axis. The density of partnerships at any age by age intersection on the map is indicated by the color with density increasing from white to red.
We calibrated our model using approximate Bayesian computation to estimate values for three calibration parameters: (1) the daily frequency of one-time partnerships among adult MSM (between .0083 and .012 depending on an individual’s number of other partnerships); (2) the frequency of anal intercourse (AI) within partnerships involving at least one ASMM (.72 acts/week); (3) the baseline probability of transmission given condomless AI (receptive-.0117, insertive-.0045). The low frequency of AI combined with the short mean duration of ASMM partnerships—20 days—resulted in a mean of just 2.16 acts of AI within each ASMM partnership. The model was calibrated to yield simulated epidemics that matched two epidemic targets: (1) 7% HIV prevalence among sexually active 18-year-old ASMM [20]; and (2) 28.3% prevalence among adult MSM [21].
PrEP adherence was modeled using four different adherence levels, corresponding to no measurable adherence, low (<2 days/week), moderate (2–3 days/week), and high (≥4 days/week). The per-act transmission probability was reduced by 0%, 31%, 81%, and 95%, respectively, based on derivations from Grant et al. [22]. Adherence in the ATN113 study was reported for all four adherence levels described previously, but EPIC only reported high adherence, so we assumed a uniform distribution across the remaining categories when using parameters from the latter. Retention was modeled as a constant rate of discontinuation, calculated to match the proportion retained at specific time-points reported by each study. There are no data currently available on PrEP uptake among ASMM given the recency of FDA approval, so we adopted a conservative approach—each ASMM was assigned a date for an annual doctor visit where they were assessed for the modeled eligibility criteria: age ≥ 16 years and ever having had condomless AI. The probability of initiating PrEP when offered was varied across five levels (.1, .25, .50, .75, and 1.0).
All simulations included background PrEP use by adult MSM starting 3 years before PrEP’s availability for ASMM. Data from the two most recent MSM cycles of National HIV Behavioral Surveillance (2014 and 2017) indicate that PrEP use among eligible MSM has increased (6%–35%) over that period [23]. For simplicity, we approximated the average and set coverage, defined here as the proportion of MSM eligible to be on PrEP based on Centers for Disease Control and Prevention guidelines [24], who are actively taking PrEP, to 20%.
Scenarios
Our analysis included three scenario sets shown in Table 1. Scenario set 1 modeled transmission given PrEP adherence and retention levels reported in EPIC. Scenario set 2 modeled transmission given PrEP adherence levels reported during the first 12 weeks versus last 36 weeks of ATN113. That is, we treat the more frequent patient-doctor interactions during the first 12 weeks as if they were an intervention, with the less frequent interactions during later weeks as the control. Disentangling the impact of follow-up frequency on retention was more challenging, since all participants in this study experienced both frequencies as they transitioned from the first 12 weeks to the last 36 weeks. Thus, for both groups, we used the study’s overall retention rate. Scenario set 3 uses data from both EPIC and ATN113. For both adherence and retention, we calculated an average over the entire ATN113 study period. Because this study included only ASMM, we use it here to reflect baseline, or control levels of PrEP use by ASMM. For the intervention arm, we applied the relative improvement in both measures observed in EPIC as an indicator of potential improvements to each of these aspects of the PrEPCoC with a targeted intervention similar to the PrEPMate intervention tested in EPIC. Even though each of these scenarios use a different set of PrEP intervention parameters, the scenario sets are not intended to represent different interventions for evaluation or comparisons between different interventions. Rather, they are intended to be viewed collectively as an indicator of the potential impact of PrEP programs on the HIV epidemic among ASMM.
Each scenario was run 100 times for 10 years. Key outcomes reported are the mean reduction in prevalence among 18-year-old ASMM after 10 years, the number of infections averted (NIA) among ASMM per 100K person years at risk, the percentage of infections averted (PIA) among ASMM, and the number-needed-to-treat (NNT) to avert a single infection among ASMM. We use prevalence among 18-year-old ASMM rather than prevalence among all ASMM for consistency with previous studies and because it is an approximate measure of cumulative incidence, given few deaths among youth. We report mean outcomes across the 100 simulations and 95% simulation intervals (SI; the middle 95% of simulated outcomes). Negative values in the SI do not indicate an increase in incidence; rather they indicate that in some simulations there were by chance more incident infections than there were in the baseline simulations on average.
Results
For scenario set 1, (Table 2), we modeled PrEP adherence and retention based on estimates from EPIC. In this set of analyses, the control group indicates expected baseline PrEP use by ASMM in a real-world setting and outcomes are compared to no PrEP use by ASMM. The intervention captures potential improvements in adherence and retention that could be achieved with a focused mHealth intervention. In the control condition, PrEP prevented 4.0% (SI: −9.2, 16.3), 21.3% (SI: 8.6, 30.3), and 41.0% (SI: 32.7, 50.1) of infections with 10%, 50%, and 100% uptake, respectively. In the intervention, PrEP prevented 7.9% (SI: −5.2, 19.5), 31.8% (SI: 22.1, 41.7), and 50.5% (SI: 42.3, 56.9) of infections with 10%, 50%, and 100% uptake, respectively.
Table 2.
Scenario set 1: simulated HIV epidemic outcomes assuming pre-exposure prophylaxis (PrEP) adherence and retention profiles among adolescent sexual minority males (ASMM) from EPIC
| Scenarios | Uptake | Incidence (95% SI) | NIA/100K person-years at risk (95% SI) |
PIA (95% SI) | PrEP coverage among all sexually active ASMM (95% SI) |
NNT (95% SI) | Prevalence among 18-year-old ASMM after 10 years (95% SI) |
|---|---|---|---|---|---|---|---|
| No PrEP | NA | 1,191 (1,090, 1,276) | NA | NA | NA | 5.8 (4.3, 7.6) | |
| Control | .1 | 1,139 (1,042, 1,238) | 77 (−155, 318) | 4.0 (−9.2, 16.3) | 4.3 (4.2, 4.5) | 37 (−641, 232) | 5.3 (4.1, 6.4) |
| Intervention | 1,092 (996, 1,176) | 149 (−87, 376) | 7.9 (−5.2, 19.5) | 6.1 (5.8, 6.3) | 71 (−1,139, 341) | 5.0 (4.0, 6.2) | |
| Control | .25 | 1,046 (960, 1,144) | 219 (−13, 443) | 11.9 (−.8, 22.6) | 10.5 (10.3, 10.7) | 68 (38, 350) | 4.8 (3.8, 5.9) |
| Intervention | 977 (890, 1,080) | 322 (49, 563) | 17.6 (2.9, 29.8) | 14.5 (14.1, 14.8) | 95 (54, 208) | 4.5 (3.5, 5.5) | |
| Control | .5 | 934 (856, 1,016) | 388 (146, 580) | 21.3 (8.6, 30.3) | 19.9 (19.7, 20.2) | 76 (55, 172) | 4.3 (3.4, 5.2) |
| Intervention | 809 (736, 881) | 576 (369, 804) | 31.8 (22.1,41.7) | 26.5 (26.1, 26.7) | 96 (71, 138) | 3.8 (2.7, 4.7) | |
| Control | .75 | 828 (772, 893) | 548 (300, 771) | 30.1 (18.0, 39.7) | 28.3 (28.0, 28.5) | 78 (58, 122) | 3.8 (2.8, 4.7) |
| Intervention | 688 (626, 763) | 755 (504, 953) | 42.0 (30.6, 49.6) | 36.2 (35.9, 36.5) | 96 (79, 132) | 3.1 (2.1, 4.1) | |
| Control | 1 | 699 (613, 773) | 741 (542, 959) | 41.0 (32.7, 50.1) | 35.7 (35.5, 36.0) | 72 (56, 91) | 3.0 (2.1, 4.2) |
| Intervention | 587 (532, 643) | 907 (700, 1,087) | 50.5 (42.3, 56.9) | 44.1 (43.8, 44.3) | 94 (81, 116) | 2.6 (1.8, 3.5) |
NIA = number of infections averted; NNT = number needed to treat to avert a single infection; PIA = percent of infections averted; SI = simulation interval.
The NIA increased linearly with uptake at both the control and intervention levels of adherence and retention (Figure 2), but the difference in the NIA between the control and intervention condition increased with uptake at a rate greater than the sum of the two independent effects, suggesting a possible interaction effect. However, the variance in the outcome estimates makes it difficult to draw any definitive conclusions. In the control, the NIA was 77 (SI: −155, 318), 388 (SI: 146, 580), and 741 (SI: 542, 959) with 10%, 50% and 100% uptake, respectively. In the intervention, the NIA was 149 (SI: −87, 376), 576 (SI: 369, 804), and 907 (SI: 700, 1,087) with 10%, 50% and 100% uptake, respectively.
Figure 2.
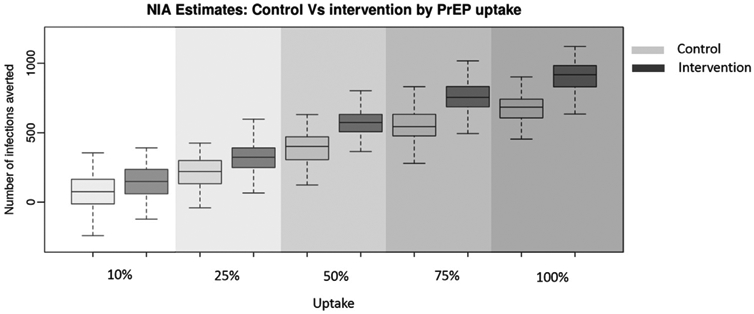
The number of infections averted among adolescent sexual minority males with pre-exposure prophylaxis (PrEP) adherence and retention based on the control and intervention arms of EPIC across five levels of PrEP uptake. Boxplots show the median (center line), interquartile range (outer box) and 95% credible interval (whiskers) for the number of infections averted under the control and intervention conditions across five levels of pre-exposure prophylaxis uptake.
Coverage in the control was 4.3% (SI: 4.2, 4.5) with 10% uptake and 35.7% (SI: 35.5, 36.0) with 100% uptake. In the intervention, coverage was 6.1% (SI: 5.8, 6.3) with 10% uptake and 44.1% (SI: 43.8, 44.3) with 100% uptake. The NNT in the control increased along with uptake from 37 (SI: −641, 232) at 10% uptake to 72 (SI: 56, 91) at 100% uptake, but the uncertainty based on the SI declined dramatically. Overall prevalence among 18-year-old ASMM declined from 5.8% (SI: 4.3, 7.6) without PrEP to 2.6% (SI: 1.8, 3.5) in the intervention with 100% uptake.
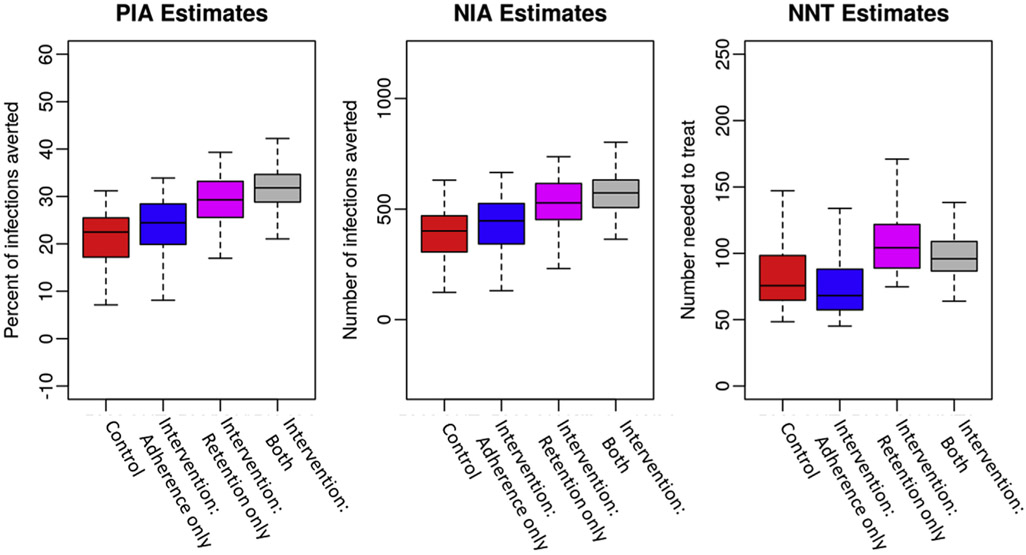
In addition to looking at the extent of improved epidemic outcomes for ASMM based on observed changes in adherence and retention in EPIC, we were also able to assess each of these improvements in isolation. Figure 3 shows the PIA, NIA, and NNT for the control, the intervention change in adherence only, in retention only, and the combined intervention effects. In this example, overall uptake was 50%. An increase in retention had a larger independent effect on both the PIA and NIA than improved adherence. However, improved retention alone increased the NNT compared to all other conditions, indicating it was less efficient because overall person-time on PrEP was increasing with less efficacious adherence.
Figure 3.
The percent and number of infections averted and the number needed to treat among adolescent sexual minority males with pre-exposure prophylaxis (PrEP) adherence and retention based on EPIC and uptake at 50%. Boxplots show the median (center line), interquartile range (outer box) and 95% credible interval (whiskers) for four scenarios. The four scenarios are the two by two interactions of pre-exposure prophylaxis adherence and retention observed in the control and intervention arms of the EPIC study. NIA, number of infections averted; PIA, percent of infections averted; NNT, number needed to treat to avert a single infection.
Table 3 shows results for scenario set 2, the ATN113 adherence “natural experiment” modeling the potential impact of an intervention focused on more frequent patient contacts. In the control PrEP prevented 2.8% (SI: −11.0, 15.8), 13.4% (SI: −.4, 23.1), and 24.6% (SI: 13.3, 33.8) of infections with 10%, 50%, and 100% uptake, respectively. In the intervention PrEP prevented 3.8% (SI: −11.4, 15.4), 23.7% (SI: 12.2, 34.8), and 39.6% (SI: 29.8, 48.2) of infections with 10%, 50%, and 100% uptake, respectively.
Table 3.
Scenario set 2: simulated HIV epidemic outcomes assuming pre-exposure prophylaxis (PrEP) adherence and retention profiles among adolescent sexual minority males (ASMM) from the ATN113 trial with retention averaged across the study duration
| Scenarios | Uptake | Incidence (95% SI) | NIA/100K person-years at risk (95% SI) |
PIA (95% SI) | PrEP coverage among all sexually active ASMM (95% SI) |
NNT (95% SI) | Prevalence among 18-year-old ASMM after 10 years (95% SI) |
|---|---|---|---|---|---|---|---|
| No PrEP | NA | 1,191 (1,090, 1,276) | NA | NA | NA | 5.8 (4.3, 7.6) | |
| Control | .1 | 1,153 (1,055, 1,252) | 57 (−183, 307) | 2.8 (−11.0, 15.8) | 4.5 (4.4, 4.7) | 40.2 (−267.2, 603.5) | 5.4 (4.2, 6.8) |
| Intervention | 1,141 (1,061, 1,220) | 74 (−189, 297) | 3.8 (−11.4, 15.4) | 4.5 (4.4, 4.7) | 48.8 (−410.3, 351.8) | 5.3 (4.2, 6.6) | |
| Control | .25 | 1,098 (999, 1,198) | 139 (−100, 382) | 7.4 (−5.8, 19.7) | 11.0 (10.8, 11.2) | 96.7 (−307.4, 610.8) | 5.1 (4.0, 6.3) |
| Intervention | 1,032 (951, 1,110) | 240 (−27, 460) | 13.0 (−1.6, 24.1) | 11.0 (10.9,11.2) | 69.9 (−250.4, 387.7) | 4.7 (3.7, 5.7) | |
| Control | .5 | 1,028 (928, 1,103) | 246 (−6, 437) | 13.4 (−.4, 23.1) | 20.7 (20.5, 21.0) | 128.8 (73.8, 446.8) | 4.8 (3.6, 5.9) |
| Intervention | 905 (825, 1,000) | 431 (198, 670) | 23.7 (12.2, 34.8) | 20.8 (20.6, 21.0) | 75.9 (52.1, 130.6) | 4.2 (2.9, 5.5) | |
| Control | .75 | 955 (858, 1,037) | 356.(100, 606) | 19.5 (6.0, 31.3) | 29.3 (29.1, 29.6) | 131 (77.1, 295.6) | 4.3 (3.0, 5.7) |
| Intervention | 800 (727, 877) | 590 (395,81) | 32.6 (23.9, 41.7) | 29.4 (29.2, 29.7) | 75.6 (59, 103.3) | 3.5 (2.6, 4.4) | |
| Control | 1 | 895 (819, 984) | 447 (222, 640) | 24.6 (13.3, 33.8) | 36.8 (36.5, 37.1) | 126.8 (92.6, 217.8) | 4.1 (3.1, 5.3) |
| Intervention | 716 (643, 789) | 715 (501, 911) | 39.6 (29.8, 48.2) | 36.9 (36.7, 37.2) | 78.1 (63.2, 104) | 3.2 (2.3, 4.1) |
NIA = number of infections averted; NNT = number needed to treat to avert a single infection; PIA = percent of infections averted; SI = simulation interval.
In the control, the NIA was 57 (SI: −183, 307), 246 (SI: −6, 437), and 447 (SI: 222, 640) with 10%, 50%, and 100% uptake, respectively. In the intervention the NIA was 74 (SI: −189, 297), 431 (SI: 198, 670), and 715 (SI: 501, 911) with 10%, 50%, and 100% uptake, respectively.
Coverage was the same between control and intervention conditions because it is a function of uptake and retention, which were equal in both conditions in this scenario. Prevalence overall declined from 5.8 (SI: 4.3, 7.6) with no PrEP to 3.2% (SI: 2.3, 4.1) with the intervention and 100% uptake.
In scenario set 3, we used average adherence and retention from ATN113 for the control, then increased high adherence by 26% and retention by 40%, the relative improvements seen in EPIC, to reflect expected improvements from a comprehensive mHealth program (Table 4). In the control, PrEP prevented 3.3% (SI: −10.2, 13.5), 18.6% (SI: 3.2, 29.2), and 32.3% (SI: 21.7, 40.3) of infections with 10%, 50%, and 100% uptake, respectively. In the intervention PrEP prevented 5.7% (SI: −8.5, 18.8), 25.4% (SI: 13.5, 35.4), and 40.8% (SI: 32.2, 48.4) of infections with 10%, 50%, and 100% uptake, respectively.
Table 4.
Scenario set 3: simulated HIV epidemic outcomes among adolescent sexual minority males (ASMM) assuming pre-exposure prophylaxis (PrEP) adherence and retention averaged across the ATN113 study and relative improvements based on changes reported in EPIC
| Scenarios | Uptake | Incidence (95% SI) | NIA/100K person-years at risk (95% SI) |
PIA (95% SI) | PrEP coverage among all sexually active ASMM (95% SI) |
NNT (95% SI) | Prevalence among 18-year-old ASMM after 10 years (95% SI) |
|---|---|---|---|---|---|---|---|
| No PrEP | NA | 1,191 (1,090, 1,276) | NA | NA | NA | 5.8 (4.3, 7.6) | |
| Control | .1 | 1,148 (1,073, 1,253) | 64 (−172, 263) | 3.3 (−10.2, 13.5) | 4.5 (4.4, 4.7) | 52 (−339, 537) | 5.2 (4, 6.3) |
| Intervention | 1,118 (1,011, 1,215) | 110 (−142, 369) | 5.7 (−8.5, 18.8) | 5.8 (5.6, 6.0) | 111 (−453, 826) | 5.2 (3.9, 6.5) | |
| Control | .25 | 1,079 (982, 1,175) | 169 (−134, 426) | 9.0 (−8.1, 22.2) | 11.0 (10.8, 11.2) | 93 (−181, 587) | 5.2 (4, 6.4) |
| Intervention | 1,024 (932, 1,116) | 252 (20, 500) | 13.7 (1.1, 25.9) | 13.8 (13.6, 14.0) | 109 (56,310) | 4.7 (3.6, 5.7) | |
| Control | .5 | 965 (896, 1,047) | 341 (52, 571) | 18.6 (3.2, 29.2) | 20.8 (20.5, 21.0) | 95 (61, 248) | 4.4 (3.2, 5.6) |
| Intervention | 885 (814, 959) | 461 (221, 688) | 25.4 (13.5, 35.4) | 25.4 (25.1, 25.7) | 112 (75, 178) | 3.9 (3, 5.1) | |
| Control | .75 | 883 (798, 966) | 464 (240, 656) | 25.6 (14.5, 34.9) | 29.3 (29.1, 29.6) | 97 (73, 150) | 4.0 (2.9, 5.4) |
| Intervention | 781 (681, 862) | 619 (405, 830) | 34.2 (23.7, 44.0) | 35.0 (34.7, 35.3) | 71 (56, 103) | 3.4 (2.4, 4.5) | |
| Control | 1 | 803 (736, 890) | 584 (355, 776) | 32.3 (21.7, 40.3) | 36.9 (36.6, 37.2) | 94 (75, 145) | 3.6 (2.6, 4.7) |
| Intervention | 702 (636, 761) | 736 (523, 924) | 40.8 (32.2, 48.4) | 42.8 (42.5, 43.1) | 68 (54,91) | 3.1 (2.1, 4.1) |
NIA = number of infections averted; NNT = number needed to treat to avert a single infection; PIA = percent of infections averted; SI = simulation interval.
The NIA in the control was 64 (SI: −172, 263), 341 (SI: 52, 571), and 584 (SI: 355, 776) with 10%, 50%, and 100% uptake, respectively. In the intervention the NIA was 110 (SI: −142, 369), 461 (SI: 221, 688), and 736 (SI: 523, 924) with 10%, 50%, and 100% uptake, respectively.
Coverage in the control was 4.5% (SI: 4.4, 4.7) with 10% uptake and 36.9% (SI: 36.6, 37.2) with 100% uptake. In the intervention, coverage was 5.8% (SI: 5.6, 6.0) with 10% uptake and 42.8% (SI: 42.5, 43.1) with 100% uptake. Prevalence overall declined from 5.8 (SI: 4.3, 7.6) with no PrEP to 3.1% (SI: 2.1, 4.1) with the intervention and 100% uptake.
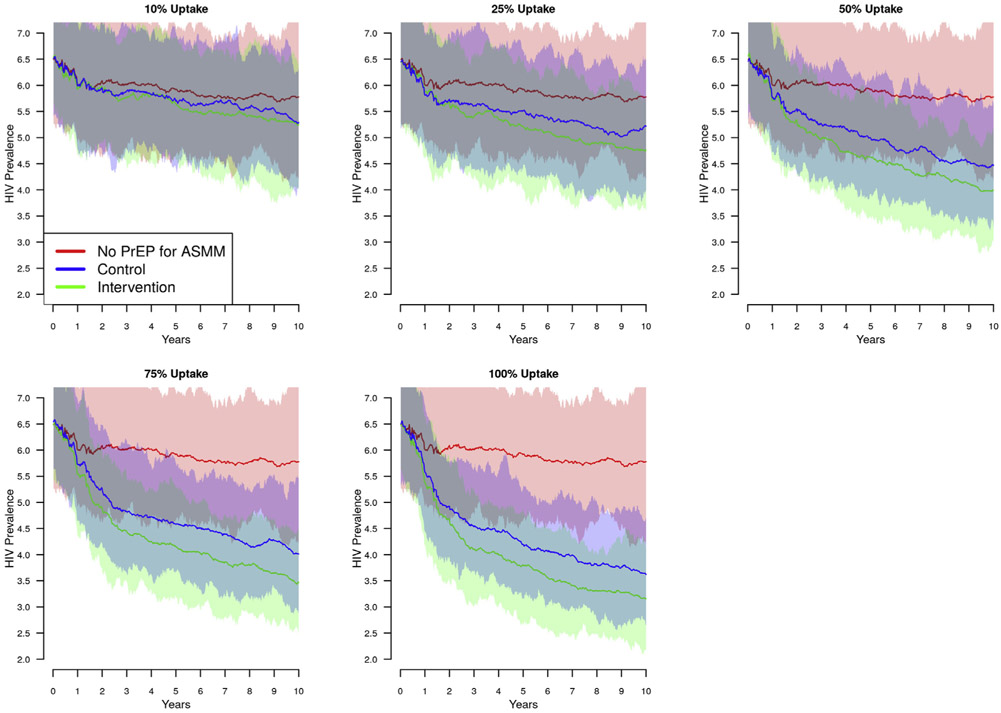
In all scenario sets, prevalence declined continuously over the 10-year simulation period as a function of PrEP use among both adult MSM and ASMM. Figure 4 shows the trajectories for prevalence among 18-year-old ASMM for scenario set 3. In each plot, the red line indicates prevalence in the absence of PrEP for ASMM; declines are due solely to the 20% PrEP coverage among adult MSM. The blue is the control condition, or what we might expect from a basic PrEP program implemented for ASMM. The green line is the outcome based on additional improvements in PrEP adherence and retention. At each uptake level, PrEP use among ASMM accelerates the prevalence decline. While the intervention condition also accelerates the decline, the absolute difference between the intervention and control conditions stabilizes over time.
Figure 4.
HIV prevalence among 18-year-old adolescent sexual minority males (ASMM) over 10 years of pre-exposure prophylaxis (PrEP) use with adherence and retention levels reported in the ATN113 trial and increased levels based on differences in the intervention and control arm of EPIC (scenario set 3). PrEP indications for this scenario are age 16–18 and having initiated anal intercourse; uptake ranged from 10% to 100%; adherence profile and retention is drawn from the ATN113 trial (see text). The difference in adherence and retention is drawn from the relative differences between the control and intervention arms of the EPIC study. Dark lines = means of 100 simulations from a given set of parameters; lighter areas = 95% credible interval across 100 simulations. (a) HIV prevalence among 18-year-old ASMM in the absence (red), presence (blue), and intervention base improvements in PrEP over the 10 years after rollout. Time scale on the x-axis reflects time relative to PrEP rollout; PrEP was available for adults for 3 years prior to rollout for adolescents. The decline in prevalence among 18-year-old adolescent sexual minority males in the absence of PrEP use by adolescents, shown in red, reflects the trickle-down effect of PrEP use among adults.
Discussion
This study models the potential impact of PrEP use by ASMM on the HIV epidemic in this population. PrEP adherence and retention estimates were drawn from empirical studies, and the observed PrEP adherence and retention, while far from perfect, were sufficient to significantly reduce the number of new infections among ASMM. Our key findings address two questions: What is the impact of observed PrEP use on epidemic outcomes among ASMM, and how much can outcomes be improved by empirically tested interventions aimed to improve adherence and retention?
In scenario set 2, we used our most conservative PrEPCoC estimates, but also the only ones drawn directly from ASMM, so they may be our best indication of behavior in this population. Under these conservative conditions, PrEP use averted 2.8%–24.6% of incident infections depending on uptake. There are currently no available data on PrEP uptake among ASMM, so it is unknown where in this range outcomes are likely to fall. As expected, the highest—and fairly optimistic—level of uptake, 100%, resulted in the largest PIA. However, in the simulation uptake only applies to sexually active ≥ age 16 ASMM when they are offered PrEP just once per year. Consequently, overall coverage is still quite low. A targeted intervention that included outreach and active enrollment of ASMM could provide additional opportunities to start PrEP and thereby achieve coverage within the target population similar to coverage simulated with 100% uptake.
There is reason to believe that recruitment will be challenging, thereby limiting uptake, but there is also reason to be optimistic [25]. On one hand, health-care providers are at times hesitant to prescribe medication to prevent HIV infection, due to concerns about side effects [6,26,27]. In addition, the physicians who are best trained and most willing to prescribe PrEP tend to be HIV specialists, while those who regularly care for HIV-negative patients (e.g. primary care or general practitioners) are often not trained to provide PrEP [28,29]. Some adolescent health providers are willing to provide PrEP but access to these providers is limited [30]. Most young men also have infrequent interactions with health-care providers and have never had an HIV test [4]. On the other hand, there are countervailing forces that could bode well for uptake among ASMM. PrEP is becoming normative, as its use expands among adult MSM. Ongoing educational campaigns and outreach programs focused on both potential clients and providers are working to increase knowledge and reduce barriers, such as stigma and misinformation about side effects and drug interactions. The expected subsequent decline in social barriers may extend down to ASMM, facilitating rapid adoption. Many of the financial barriers to PrEP access are also being addressed through programs like Ready, Set, PrEP, a new program led by Health and Human Services to provide PrEP medications at no cost [31]. PrEP delivery is also rapidly evolving with long-acting injectable PrEP currently in clinical trials, which could increase both adherence and retention.
In both scenario sets 1 and 2, the changes in adherence and retention increased the PIA between 23% and 97% depending on the scenario and uptake. This level of improvement was in response to a youth-tailored, bidirectional text-messaging intervention [15], suggesting that similar approaches could yield similar results among ASMM.
In scenario 2, the differences between the level of adherence in the control and intervention arms reflected adherence observed during the phase of ATN113 when follow-up was conducted every 4 weeks versus adherence attained when follow-ups occurred every 12 weeks. The higher adherence increased the PIA by 36%–77% depending on uptake. As caveats, ATN113 also included a comprehensive prevention and counseling protocol, and our adaptation of the design into a natural experiment assumes that high adherence could be maintained over longer periods of time with frequent follow-up, which may be optimistic. The ATN 113 study itself was also small (N = 78) with only 47 participants completing the study, and the researchers that conducted the study did not report specific findings examining the relationship between adherence to follow-up frequency. Thus, our findings suggest the potential importance of frequent follow-up with this population, but they are not conclusive, and this is an area that will require additional research. In addition, given the success of a text-messaging intervention in EPIC, follow-up may not need to be in person.
Research is currently underway to more thoroughly assess and improve the PrEP continuum among younger cohorts specifically. For example, the P3 (Prepared, Protected, emPowered) intervention is a smartphone app for HIV-uninfected young MSM that utilizes social networking and game-based mechanics to improve PrEP adherence [32]. The P3 study is specifically powered to detect a difference in adherence of ≥20.9%, which is similar to the 26% improvement found in EPIC. Findings from this randomized control trial are expected in 2021, and our model may be further updated at that time. However, the recent FDA approval for ASMM, combined with the rapid expansion of PrEP year-over-year among MSM overall, suggests that population-level predictions of PrEP impact among ASMM are needed now. Assessments based on current knowledge, even if imperfect, are needed to guide public health efforts as jurisdictions prepare to invest in programs supporting each stage of the PrEP continuum for ASMM.
Our study had several limitations. Although we were able to parametrize our model with considerably more data than previously, there remain large gaps, especially in terms of predictions about possible levels of uptake among ASMM. Overall, younger individuals seem to initiate PrEP at lower rates than older persons [33]. While EPIC focused on young adult MSM aged 18–29 years, this is still not a perfect indication of initiation for 16- to 17-year-old ASMM, who inhabit a much different social, legal, and developmental context. We are hopeful that future studies include adolescents [34-36]. In terms of adherence and discontinuation rates, ATN113 provides valuable initial estimates for ASMM specifically; however, it reflects a highly specialized trial context that may not reflect behavior in real-world settings with a wider cross-section of ASMM. We also only used PrEP adherence and retention data from two studies. However, we reviewed the reported outcomes from numerous studies and found that others produced similar estimates of both PrEP uptake and adherence [37-39].
As public health agencies craft prevention and treatment policies, they will inevitably be required to make tradeoffs between prevention modalities covered and populations served as well as between efforts supporting PrEP recruitment, adherence, and retention. Our findings suggest that PrEP use among ASMM can significantly reduce HIV incidence despite suboptimal uptake, adherence, and retention. In addition, empirically demonstrated improvements in the PrEPCoC in response to existing interventions are sufficient to substantially improve the percent of infections averted within this highly affected population.
IMPLICATIONS AND CONTRIBUTION.
Modeling based on current PrEP adherence and retention data suggests that continued expansion of PrEP programs for adolescents can substantially reduce HIV incidence among adolescent sexual minority males. Prevention may be further improved with the adoption of existing, empirically tested, interventions to facilitate engagement along the PrEP continuum of care.
Acknowledgments
Funding Sources
This research was funded by the US Centers for Disease Control and Prevention National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (Epidemiologic and Economic Modeling Agreement number 6NU38PS004646), the National Institutes of Health (grant number R01HD068395, R21HD075662, R24HD042828), and the Emory Center for AIDS Research (grant number P30AI050409). Data collection and analysis of the RADAR cohort study was supported by the National Institute on Drug Abuse (grant number U01DA036939).
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institute on Drug Abuse.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- [1].Cenetrs for Disease Control and Prevention. HIV and youth. 2019. Available at: https://www.cdc.gov/hiv/group/age/youth/index.html. Accessed October 9, 2019.
- [2].Garofalo R, Hotton AL, Kuhns LM, et al. Incidence of HIV infection and sexually transmitted infections and related risk factors among very young men who have sex with men. J Acquir Immune Defic Syndr 2016;72:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Balaji AB, An Q, Smith JC, et al. High human immunodeficiency virus incidence and prevalence and associated factors among adolescent sexual minority males-3 cities, 2015. Clin Infect Dis 2018;66:936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Phillips G 2nd, Ybarra ML, Prescott TL, et al. Low rates of human immunodeficiency virus testing among adolescent gay, bisexual, and queer men. J Adolesc Health 2015;57:407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pierce JD, Ylitalo KR, Lanning BA, Limbers CC. Sex education and HIV testing among young men who have sex with men: Findings from the 2006-2010 and 2011-2015 National Survey of Family Growth. J Acquir Immune Defic Syndr 2018;79:179–85. [DOI] [PubMed] [Google Scholar]
- [6].Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363: 2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu AY, Cohen SE, Vittinghoff E, et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med 2016;176:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tanner MR, Miele P, Carter W, et al. Preexposure prophylaxis for prevention of HIV acquisition among adolescents: Clinical considerations, 2020. MMWR Recomm Rep 2020;69:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Item of Interest: FDA approves PrEP therapy for adolescents at risk of HIV [press release]. 2018. Available at: https://www.nichd.nih.gov/newsroom/releases/051618-PrEP. Accessed May 16, 2018.
- [10].Goodreau SM, Hamilton DT, Jenness SM, et al. Targeting human immunodeficiency virus pre-exposure prophylaxis to adolescent sexual minority males in higher prevalence areas of the United States: A modeling study. J Adolesc Health 2018;62:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hamilton DT, Rosenberg ES, Jenness S, et al. An agent-based network model of HIV transmission: The impact of age bounded analysis and age mixing assumptions on the evaluation of HIV preexposure prophylaxis. Paper presented at: International Sunbelt Social Network Conference; June 18 to June 23, 2019; Université du Québec à Montréal (UQAM), Montréal, Québec, Canada. [Google Scholar]
- [12].Wang LY, Hamilton DT, Rosenberg ES, et al. Cost-effectiveness of preexposure prophylaxis among adolecent sexual minority males. J Adolesc Health 2020;66:100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kelley CF, Kahle E, Siegler A, et al. Applying a PrEPcontinuum of care for men who have sex with men in Atlanta, Georgia. Clin Infect Dis 2015;61: 1590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nunn AS, Brinkley-Rubinstein L, Oldenburg CE, et al. Defining the HIV preexposure prophylaxis care continuum. AIDS 2017;31:731–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu AY, Vittinghoff E, vonFelten P, et al. Randomized controlled trial of a mobile health intervention to promote retention and adherence to pre-exposure prophylaxis Among young people at risk for human immunodeficiency virus: The EPIC study. Clin Infect Dis 2019;68:2010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hosek SG, Landovitz RJ, Kapogiannis B, et al. Safety and feasibility of antiretroviral preexposure prophylaxis for adolescent men who have sex with men aged 15 to 17 years in the United States. JAMA Pediatr 2017;171: 1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hamilton DT, Rosenberg ES, Jenness SM, et al. Modeling the joint effects of adolescent and adult PrEP for sexual minority males in the United States. PLoS One 2019;14:e0217315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jenness SM, Goodreau SM, Morris M. EpiModel: An R package for mathematical modeling of infectious disease over networks. J Stat Softw 2018;84: 1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mustanski B, Morgan E, D’Aquila R, et al. Individual and network factors associated with racial disparities in HIV among young men who have sex with men: Results from the RADAR cohort study. J Acquir Immune Defic Syndr 2019;80:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sullivan PS, Rosenberg ES, Sanchez TH, et al. Explaining racial disparities in HIV incidence in black and white men who have sex with men in Atlanta, GA: A prospective observational cohort study. Ann Epidemiol 2015;25: 445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sullivan PS, Peterson J, Rosenberg ES, et al. Understanding racial HIV/STI disparities in black and white men who have sex with men: A multilevel approach. PLoS One 2014;9:e90514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: A cohort study. Lancet Infect Dis 2014;14: 820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Finlayson T, Cha S, Denson D, et al. Changes in HIV PrEP awareness and use among men who have sex with men, 2014 vs 2017. Paper presented at: CROI; March 4–7, 2019; Seattle, Washington. [Google Scholar]
- [24].Centers for Disease Control Prevention: US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: A clinical practice guideline. Available at: https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed August 5, 2020.
- [25].Huebner DM, Mustanski B. Navigating the long road forward for maximizing PrEPimpact among adolescent men who have sex with men. Arch Sex Behav 2020;49:211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012;367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Parisi D, Warren B, Leung SJ, et al. A multicomponent approach to evaluating a pre-exposure prophylaxis (PrEP) implementation program in five agencies in New York. J Assoc Nurses AIDS Care 2018;29:10–9. [DOI] [PubMed] [Google Scholar]
- [28].Krakower D, Ware N, Mitty JA, et al. HIV providers’ perceived barriers and facilitators to implementing pre-exposure prophylaxis in care settings: A qualitative study. AIDS Behav 2014;18:1712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Silapaswan A, Krakower D, Mayer KH. Pre-exposure prophylaxis: A narrative review of provider behavior and interventions to increase PrE-Pimplementation in primary care. J Gen Intern Med 2017;32:192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hart-Cooper GD, Allen I, Irwin CE Jr, Scott H. Adolescent health providers’ willingness to prescribe pre-exposure prophylaxis (PrEP) to youth at risk of HIV infection in the United States. J Adolesc Health 2018;63:242–4. [DOI] [PubMed] [Google Scholar]
- [31].U.S. Department of Health and Human Services. Available at: https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/prep-program. Accessed April 24, 2020.
- [32].LeGrand S, Knudtson K, Benkeser D, et al. Testing the efficacy of a social networking gamification app to improve pre-exposure prophylaxis adherence (P3: prepared, protected, emPowered): Protocol for a randomized controlled trial. JMIR Res Protoc 2018;7:e10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mera R, Magnuson D, Hawkins T, et al. Changes in Truvada (TVD) for HIV pre-exposure prophylaxis (PrEP) utilization in the United States: (2012-2016). Paper presented at: 19th IAS Conference on HIV Science; July 23-26, 2017; Paris, France. [Google Scholar]
- [34].Hume M, Lewis LL, Nelson RM. Meeting the goal of concurrent adolescent and adult licensure of HIV prevention and treatment strategies. J Med Ethics 2017;43:857–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nelson RM, Lewis LL, Struble K, Wood SF. Ethical and regulatory considerations for the inclusion of adolescents in HIV biomedical prevention research. J Acquir Immune Defic Syndr 2010;54:S18–24. [DOI] [PubMed] [Google Scholar]
- [36].Shaddy RE, Denne SC, Committee on Drugs and Committee on Pediatric Research. Clinical report–guidelines for the ethical conduct of studies to evaluate drugs in pediatric populations. Pediatrics 2010;125:850–60. [DOI] [PubMed] [Google Scholar]
- [37].Mayer KH, Safren SA, Elsesser SA, et al. Optimizing pre-exposure antiretroviral prophylaxis adherence in men who have sex with men: Results of a pilot randomized controlled trial of “Life-Steps for PrEP”. AIDS Behav 2017;21:1350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Daughtridge GW, Conyngham SC, Ramirez N, Koenig HC. I am men’s health: Generating adherence to HIV pre-exposure prophylaxis (PrEP) in young men of color who have sex with men. J Int Assoc Provid AIDS Care 2015;14:103–7. [DOI] [PubMed] [Google Scholar]
- [39].Marcus JL, Hurley LB, Hare CB, et al. Preexposure prophylaxis for HIV prevention in a large integrated health care system: Adherence, renal safety, and discontinuation. J Acquir Immune Defic Syndr 2016;73:540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]