Abstract
Electronic cigarettes are a less harmful alternative to combustible cigarettes. We analyze data on e-cigarette choices in an online experimental market. Our data and mixed logit model capture two sources of consumer optimization errors: over-estimates of the relative risks of e-cigarettes; and present bias. Our novel data and policy analysis make three contributions. First, our predictions about e-cigarette use under counter-factual policy scenarios provide new information about current regulatory tradeoffs. Second, we provide empirical evidence about the role consumer optimization errors play in tobacco product choices. Third, we contribute to behavioral welfare analysis of policies that address individual optimization errors. Compared to standard cost-benefit analysis, our behavioral welfare economics analysis leads to much larger estimates of the costs of policies that discourage e-cigarette use or the benefits of policies that encourage e-cigarette use.
Keywords: electronic cigarettes, electronic nicotine delivery systems, tobacco control, consumer optimization, behavioral welfare analysis
1. Introduction
Electronic cigarettes and other vaping devices provide users with a vapor that contains nicotine without the combustion-generated toxicants in tobacco smoke. Vaping e-cigarettes is not harmless but poses much lower risks than smoking combustible tobacco.1 The vaping market has rapidly evolved. The first modern vaping products were introduced in U.S. markets around 2007. Annual sales grew rapidly from $500 million in 2012 to $6.6 billion in 2018 (Cowen and Company Equity Research 2019). In 2018 data for adults, 9.7 percent of current smokers, 25.2 percent of recent (past-year) former smokers, and 4.2 percent of non-recent former smokers regularly vape, compared to 1.1 percent of never smokers.2 The adult current smoking rate was 19.8% in 2007 when e-cigarettes were introduced into the United States market and has declined to 13.7% in 2018 (American Lung Association, 2020; Creamer et al. 2019).
Public policy towards vaping is also evolving. Vaping products were regulated as ordinary consumer products until 2016. In May 2016, the FDA issued a rule phasing in regulation of vaping products as tobacco products (FDA 2016). In early 2020, the FDA announced new enforcement priorities that amount to at least a temporary ban of cartridge-based flavored e-cigarettes (other than menthol) (FDA 2020a). In addition to the FDA, states, counties, and municipalities have been regulating e-cigarettes as well. For example, by January 2020, 22 states had enacted e-cigarette taxes and 15 states had enacted comprehensive indoor vaping restrictions in place for restaurants, bars, and private workplaces (Public Health Law Center 2019; CDC STATE System 2020).
The challenge for public policy is to craft e-cigarette regulations that do more good than harm. Smoking combustible cigarettes remains the leading preventable cause of death in the U.S. (U.S. Department of Health and Human Services 2014). From the perspective of public health, encouraging smokers to switch to vaping is a harm reduction strategy that could substantially reduce smoking-related mortality and morbidity (Royal College of Physicians 2016). While both cigarettes and e-cigarettes contain nicotine, the FDA does not believe nicotine itself to be dangerous for non-pregnant adults outside of causing addiction to tobacco products that contain other chemicals that are dangerous (FDA 2019b). Recently, a randomized control trial in England randomized 886 smokers into receiving either e-cigarettes or their preferred nicotine replacement product (NRP) (e.g. gum, patch, lozenge, etc.) and found that the 1-year cigarette smoking cessation rate was twice as high for the e-cigarette arm (18.0%) as the NRP arm (9.9%) (Hajek et al. 2019). Adult smokers have been found to significantly value e-cigarette products that are effective smoking cessation aids and that are healthier options (Marti et al. 2019). Harm reduction – such as providing clean needles to intravenous drug users – is an established part of public health policy, but its application to tobacco policy has been controversial.
From the perspective of welfare economics, it is important to consider the impact of the growth of the e-cigarette market and e-cigarette regulations on consumer welfare, not just public health. Behavioral welfare economics research has explored the extent to which the combustible cigarette use reflects consumer optimization mistakes, for example time-inconsistency or under-estimates of the risks of smoking (Jin et al. 2015, Cutler et al. 2016, Levy, Norton and Smith 2018). Less is known about the role of consumer optimization errors in consumer decisions about e-cigarettes. Viscusi (2016) finds that while individuals on average believe e-cigarettes are safer than cigarettes, they greatly overestimate the risk levels of e-cigarettes compared with the actual risk levels. Correcting optimization errors might encourage smokers to become vapers and increase their experienced utility and thus social welfare.
In this article we analyze stated preference data from an online discrete choice experiment (DCE) where adult smokers made hypothetical choices between disposable e-cigarettes, combustible cigarettes, and a nicotine-replacement product. DCEs are commonly used in marketing research and economics to provide predictions of consumer demand in policy-relevant scenarios that are not yet observed in actual markets (e.g., Kesternich, Heiss, McFadden, and Winter 2013). Our data and model capture two reasons that smokers might fail to make fully optimizing decisions. First, smokers might over-estimate the risks of vaping and NRPs relative to the risks of smoking combustible cigarettes (Viscusi 2016). Responses to our online survey provide individual-level measures of the perceived risks, which we include as a product attribute in a random utility model. Second, smokers might be subject to present bias and make time-inconsistent decisions about quitting (Gruber and Koscegi 2001). Responses to our online survey also provide measures of present bias. We assume that the smoker’s decision utility reflects a set of valuation weights that capture present bias (Allcott, Mullanaithan, and Taubinsky 2014). This leads to a mixed logit / random coefficients random utility model, where the valuation weights are a function of the smoker’s interest in a commitment contract to help them make time-consistent choices to quit. In our DCE the attributes of the e-cigarette were varied experimentally to match current and potential FDA regulations and state policies.
We use our novel data and estimated model of e-cigarette choices to conduct behavioral welfare analysis and policy analysis. We make three contributions. First, we provide empirical evidence about the role consumer optimization errors play in tobacco product choices. We find that risk perceptions have a significant negative impact on e-cigarette choices while present bias has a significant positive impact. Second, we address the individual optimization errors in our behavioral welfare analysis.3 We take these behavior factors into consideration when we conduct policy simulations and compute compensating variations. Third, our predictions about e-cigarette use under counter-factual policy scenarios provide new information about regulatory tradeoffs currently being considered. Unlike much of the current literature on e-cigarettes that uses geographical variation in minimum legal purchase age laws (Friedman 2015, Abouk and Adams 2017, Dave, Feng, and Pesko 2019, Pesko and Currie 2019), indoor vaping restrictions (Cooper and Pesko 2017), and taxes (Pesko, Courtemanche, and Maclean 2019, About et al. 2019, Cotti et al. 2020), our study considers several regulations that could be implemented federally by the FDA.
2. Background about Vaping and Its Regulation
For the individual user, the health risks of vaping are much lower than the health risks of smoking. The National Academies of Sciences, Engineering, and Medicine in the United States conclude that although e-cigarettes are not without risk, compared to combustible tobacco cigarettes they contain fewer toxicants (National Academies of Sciences, Engineering, and Medicine 2018). The Royal College of Physicians in England reviewed the literature and estimates that the health risks of e-cigarette for non-pregnant adults are unlikely to exceed 5% of the harm from smoking tobacco, with the potential for these risks to become even lower through product standardization and technological development (Royal College of Physicians 2016). The Royal College of Physicians also concludes that: “The main culprit is smoke and, if nicotine could be delivered effectively and acceptably to smokers without smoke, most if not all of the harm of smoking could probably be avoided.” More recently in the U.S., there was an outbreak of lung injury linked to vaping products that contain THC, the active ingredient in marijuana. The outbreak peaked in September 2019 and does not appear to involve commercially available nicotine e-cigarettes. 4 Overall, the sum of this evidence suggests nicotine e-cigarettes are safer than cigarettes for non-pregnant adults, and likely substantially safer; however, some uncertainty in the level of relative safety remains.
The 2009 Tobacco Control Act (TCA) establishes the public health standard for FDA regulation of tobacco products; in 2016 the FDA issued a regulation that deems e-cigarettes to be tobacco products under the TCA. When using its regulatory powers the FDA is required to consider the risks for the individual user and the tobacco products’ impact on public health through their impacts on cessation and initiation. Most e-cigarettes are sold to current and former smokers, many of whom report that they used e-cigarettes to quit smoking (Schoenborn and Gindi 2015, Biener and Hargraves 2014). However, if e-cigarette users continue to smoke combustible cigarettes instead of completely quitting, the availability of e-cigarettes might harm public health. Another public health concern is that e-cigarettes might act as a gateway to combustible cigarettes so their availability might increase youth smoking initiation.5 The TCA requires the FDA to make regulatory decisions about e-cigarettes that balance the lower risks to individual users with these public health concerns.
The 2016 FDA deeming regulation requires e-cigarettes to carry health warning labels and requires e-cigarette manufacturers to submit marketing applications. In 2017, the FDA extended the original two to three year phase-in period for premarket applications to August 2022, but legal decisions moved the deadline to September 2020 (FDA 2017, 2020). As noted above, in early 2020, the FDA announced new enforcement priorities that amount to at least a temporary ban of cartridge-based flavored e-cigarettes (other than menthol). In order to remain on the market, by September 2020 all manufacturers of e-cigarettes must submit an application to the FDA demonstrating that their products are appropriate for public health (FDA 2020). This regulatory pathway means that flavored e-cigarettes might return to the market, although it might require the manufacturers to take steps to prevent use by underage youth, such as reducing nicotine levels or adopting technologies such as thumb print scanners.
In addition to federal FDA regulations, e-cigarettes are also the subject of state and local policies, most notably taxes. As of January 2020, 22 states have enacted taxes on e-cigarettes at rates that are generally lower than taxes on combustible cigarettes (Public Health Law Center 2019). D.C. taxes e-cigarettes and combustible cigarettes at the same rate, currently $4.94 per pack. State Medicaid programs are required to provide insurance coverage that subsidizes the cost of approved NRPs, but to date these programs do not cover e-cigarettes.
3. Empirical Model
In this section we develop an empirical model of a consumer’s choice between three nicotine products: e-cigarettes, combustible cigarettes, and NRPs. In the standard random utility model, individual i’s utility from choosing tobacco product j, Uij, is decomposed into the observable utility component Vij that is explained by observed product attributes and an unobserved random utility component ϵij:
| (1) |
The consumer chooses the tobacco product that yields the highest utility:
| (2) |
The probability that consumer i chooses tobacco product j is thus given by:
| (3) |
The assumptions that ϵij is i.i.d. across j and follows the type I extreme value distribution leads to the logit specification:
| (4) |
The empirical model captures two sources of consumer optimization errors. First, the individual’s subjective perceived risk of each product – rather than the objective product risk – is one of the product attributes that determines Vij. Second, we introduce an individual-specific preference weight Γi into the discrete choice model. In the spirit of the reduced-form approach to behavioral welfare economics (Mullanaithan, Schwartzstein and Congdon 2012), Allcott et al. (2014) show that including the preference weight Γ is a flexible approach that captures a number of behavioral biases, including present bias and naiveté about present bias. For example, Γi < 1 captures a consumer i with present bias who under-weights the lifetime utility loss from the future health consequences of their use of a risky tobacco product.
In the empirical model we assume the observable component of utility Vij is a linear function of a vector of product attributes Xij multiplied by the preference weight Γi, so the consumer’s decision utility is given by:6
| (5) |
The preference weight Γi is a function of whether individual i is interested in a commitment contract for smoking cessation7 and an unobservable random component δi. The coefficients α1 and α2 represents the effect on the commitment contract as well as the coefficients for the constant. Following the style in Krucien et al., (2019),
| (6) |
Equations (5) and (6) lead to a mixed logit / random coefficients specification. In addition to incorporating Γ, the mixed logit specification is a highly flexible model that allows individual heterogeneity to interact with product characteristics. It also relaxes the independence of irrelevant alternatives assumption of the multinomial logit model (Train 2002). In our mixed logit model, by substituting equation (6) into (5), the decision utility function is re-written as:
| (7) |
In the mixed logit model given by equation (7), consumer optimization errors are captured by the interaction terms between Zi (the determinants of the preference weights Γi) and Xijt (the product attributes). Our empirical model tests the predictions that through Γi naiveté about present bias leads consumers to under-value product attributes such as warning labels that influence future period utility. The model also allows for optimization errors that arise because the consumer makes decisions based on his or her subjective risk (one of the product attributes) instead of the objective risk of the product choices.
Welfare Expressions
In section 6 below we use the results from our mixed logit model to predict consumer choices under policy counterfactuals and to conduct behavioral welfare economic analysis. The compensating variation (CV) in income with respect to decision utility is given by the standard expression derived by Small and Rosen (1981).8
In our behavioral welfare economic analysis, we calculate the CV for a policy change with respect to experienced utility instead of decision utility. The possibility of consumer optimization errors means that the tobacco product choices based on decision utility do not necessarily lead to the highest experienced utility. Behavioral welfare economic analysis is therefore conducted with respect to consumers’ experienced utilities (Mullainathan et al. 2012, Chetty 2015). Our analysis allows for two sources of optimization errors: consumer misinformation about tobacco product risks (one of the product attributes); and present bias captured by the preference weights Γi. Our calculations of the CVs that incorporate consumer optimization errors follow Leggett (2002) and Ketcham et al. (2019).9
4. Data
Discrete Choice Experiment
The data for our analysis are from a DCE included in an online survey conducted in December 2014 and January 2015. DCEs and other stated preference (SP) methods are used in contexts when it is difficult or impossible to examine consumers’ revealed preferences (RP) in actual markets. For example, contingent valuation surveys are widely used to collect SP data on environmental quality and other non-market goods. DCEs are often used to examine consumers’ preferences over policy-relevant product attributes that are not observed in actual markets.10 For example, in actual market conditions e-cigarettes are available in a wide range of flavors, so it is impossible to identify the impact of a flavor ban on use. Reviews of SP methods lead to a broad but not universal consensus that they provide high-quality data for policy analysis (Carson 2012, Hausman 2012, McFadden 2017). Buckell and Hess (2019) report a recent example of a DCE of e-cigarettes.
Our sample was recruited from the GfK (Knowledge Networks) online panel. KnowledgePanel recruits individuals for the panel using address-based sampling. This recruitment includes individuals using only cellphones and individuals without computer/internet access (which KnowledgePanel provides). From among this panel, we identified eligible respondents as having smoked at least 100 cigarettes in their lifetime, smoking at least one cigarette during the past 30 days, and purchasing cigarettes during the past 30 days. Our sample consisted of 1,148 current smokers. Table 1 provides descriptive statistics about the subjects, their responses to the DCE, and their use of the nicotine products over the past year. Because e- cigarette use varies with age, we intentionally over-sampled young adults aged 18 – 24. Our young adult sub-sample includes a higher fraction of females and the respondents have completed more schooling than would be expected in a nationally represented sample of smokers, perhaps because our sample was collected online.11
Table 1.
Descriptive statistics
| Mean | |
|---|---|
| DCE choices: | |
| E-cigarettes | 0. 16 |
| Cigarettes | 0. 71 |
| Nicotine replacement products (NRPs) | 0. 13 |
| Revealed preference (prior use): | |
| Also use E- cigarettes | 0.17 |
| Use cigarettes only | 0.65 |
| Also use NRPs | 0.18 |
| Selected demographics: | |
| Age 18–24 | 0.44 |
| Female | 0.55 |
| Less than high school | 0.092 |
| High school | 0.35 |
| Some college | 0.39 |
| Bachelor’s degree or higher | 0.18 |
| Respondents | 1,148 |
In the DCE subjects made hypothetical choices between purchasing a disposable e-cigarette, their usual brand of combustible cigarettes (including whether this was mentholated or not), or a NRP. To be realistic many DCEs offer subjects an “outside alternative,” which is often the option of making no purchase. All of the subjects in our DCE had smoked 100 cigarettes in their lifetime and had both smoked and purchased cigarettes during the past 30 days. In this context, the choice of purchasing their usual brand/flavor of combustible cigarettes is the outside alternative. The choice set presented in the DCE is a simplified version of the choice set smokers face each time they make an actual tobacco product purchase; multiple brands of e-cigarettes, combustible cigarettes, and NRPs are usually available at the same retail outlets and are even displayed together, e.g. at or behind the checkout counter. The DCE subjects were more familiar with their usual brand of cigarettes than with the e-cigarette and NRP alternatives, but this is also the case when smokers make actual tobacco product choices. DCEs tend to be more reliable when the subjects are familiar with the products and have experience making choices between them (McFadden 2017, p. 162).
The DCE presented subjects with different choice scenarios where the product attributes of the e-cigarette were varied to correspond to possible FDA regulations and state policies: the availability of flavors other than tobacco/menthol; health warning labels; and price. Out of the 24 possible combinations of attributes and conditions, each subject was presented with 12 choice scenarios. The design of the DCE is consistent with best practice guidelines for the number of attributes, levels, and choice scenarios (Bridges et al. 2011). The order of the choice scenarios was randomized across subjects.12 The 12 choice scenarios were chosen to maximize D-efficiency that was balanced across attribute levels, so the variations of the e-cigarette attributes are uncorrelated.13 A table with the levels and attributes, as well as an example choice scenario, is available in appendix 1. We identified 145 subjects who exhibited at least one instance of intransitive preferences; we retained these subjects to avoid introducing selection bias (e.g. Lancsar and Louviere (2008)).14
The DCE included two flavor availability conditions: the availability of a variety of flavors; or the availability of only tobacco and menthol flavors. As of early 2020 the most popular type of e-cigarettes with flavors other than menthol are temporarily banned from the U.S. market. However, manufacturers might be able to return flavored e-cigarettes to the market, so both flavor availability conditions remain policy relevant.
The DCE included four warning label conditions on the e-cigarette: no label; a proposed FDA warning label; a modified risk warning label; and a strong warning label. The FDA proposed warning label was: “WARNING: This product contains nicotine derived from tobacco. Nicotine is an addictive chemical.” This warning label, minus the part about “derived from tobacco” was adopted by the FDA action in 2016 and remains in place today. However, the FDA retains discretion to implement a stronger or modified risk warning label in the future, thus the policy significance of this choice scenario remains relevant. The modified risk warning label used in this DCE is the label proposed by Swedish Match in its modified risk tobacco product application for its smokeless tobacco products: “WARNING: No tobacco product is safe, but this product presents substantially lower risks to health than smoking cigarettes.” (FDA 2019a). The strong warning label is the label voluntarily adopted by the manufacturer of MarkTen e-cigarettes and includes a long list of counter-indications, side effects, and risks. In the DCE, the combustible cigarette option included one of the warning labels currently required on cigarettes; the cigarette warning did not vary across the choice scenarios.
The DCE included three e-cigarette price conditions: $3, $6, and $9. The $6 condition approximated the average price of an e-cigarette product equivalent to a pack of combustible cigarettes. The price of the combustible cigarette alternative was the price per pack the subject reported usually paying for their usual brand of cigarettes. The price of the NRP was $6 for a package equivalent to a pack of cigarettes. The prices of the combustible cigarette and of the NRP did not vary across the choice scenarios.
An important feature of our study is that the e-cigarette product described in the DCE was a disposable e-cigarette. More recently, pod-based e-cigarettes such as JUUL have become the leading seller in the e-cigarette industry by 2018 (King et al 2018). The refillable/pod-based e-cig is different from the disposable e-cig from the pricing perspective as an initial fixed cost is required for new entrants. However, they are similar from the regulation prospective in terms of warning and availability of flavor. Our DCE described the e-cigarette option as a disposable cigarette because that was the most common and familiar product on the market at the time (Dec. 2014 and January 2015), especially for new users (the majority of the sample). Responses to a follow-up question showed that the responses of experienced users of refillable devices were not affected by this description of the product (Pesko et al. 2016).
Measuring Optimization Errors
After the DCE, the online survey included questions to measure possible consumer optimization errors. The first set of questions measured subjects’ perceptions of the risks of the different nicotine products. 15 We followed an approach used in previous research (Viscusi 1990, 2016) and asked users: “Among 100 cigarette smokers (users of e-cigarettes), how many of them do you think will die from lung cancer, heart disease, throat cancer and all other illness because they smoke (use e-cigarettes)?” The responses provide a simple summary of the perceived lifetime risks of product use and can be compared to scientific estimates of the objective risks. The U.S. Department of Health and Human Services (2014, p. 666) estimates that the lifetime risk for a young adult smoker is 32 percent. Based on Viscusi’s (2016) estimates from dose-response relationships and the other evidence discussed above in section 2, the lifetime risks of e-cigarette use and NRP use are approximately zero.
As can be seen in Table 2, subjects in our online sample tend to substantially over-estimate the risks of e-cigarettes and NRPs. On average subjects believe that the lifetime risks are: 30 percent for e-cigarette use; 46 percent for smoking; and 28 percent for the use of NRPs. The results are comparable to previous research on the perceived risks of e-cigarette use (Viscusi 2016), smoking (Viscusi 1990), and NRPs (Shiffman et al. 2008).
Table 2.
Consumer optimization errors
| Mean | Min | Max | |
|---|---|---|---|
| # deaths out of 100 users of e-cigarettes | 30.1 | 0 | 100 |
| # deaths out of 100 users of cigarettes | 45.7 | 0 | 100 |
| # deaths out of 100 users of NRPs | 27.8 | 0.34 | 80.5 |
| β-discount (present bias) factor | 0.73 | 0.0082 | 1.24 |
| Little present bias (β-discount factor > 0.9) | 0.37 | 0 | 1 |
| Interested in using a Commitment Contract | 0.20 | 0 | 1 |
| Respondents | 1,148 | ||
Notes: # deaths out of 100 users of NRPs is derived based on self-reported # deaths out of 100 users of e-cigarettes and cigarettes and self-reported perceived harm of using e-cigarettes and NRPs (compared to smoking cigarettes).
The online survey included another set of questions to measure consumer time preference and time inconsistency. In one question subjects were told to suppose that they had won a prize of $1,000 which they could claim immediately or after a wait of one year. In another question subjects were asked about a wait of one month. Subjects were asked to state the smallest amount of money that would convince them to wait. The questions are patterned after questions included in the 2006 wave of the 1979 cohort of the National Longitudinal Survey of Youth (NLSY). If subjects are time-consistent exponential discounters their responses to the questions about waiting a year versus a month will imply the same annualized discount factor. If subjects have quasi-hyperbolic time preferences, the responses to the questions can be used to calculate the β-discount factor where β < 1 implies present bias.16
Subjects in our online survey display present bias, with an average β-discount factor of 0.73 (Table 2). The estimated β is less than 1 for 93 percent of our sample, implying that virtually all the subjects display present bias. By comparison, in their analysis of responses to the same questions included in the NLSY, Courtemanche et al. (2014) find an average β-discount factor of 0.8 with β < 1 for 85 percent of the NLSY sample. Our results also compare well to the consensus estimate from experimental research that β = 0.7 (Angeletos et al. 2001) and to results from the surveys reported in Newell and Siikamaki (2015, 2014). In an analysis of naturally occurring field data, Laibson, Repetto and Tobacman (2017) benchmark estimate of the discount factor is β = 0.5.
Behavioral economic models of hyperbolic discounting make an important distinction between naïve consumers versus sophisticated consumers who recognize their present bias (Gruber and Koszegi 2001). Sophisticated present-biased consumers should be interested in using commitment strategies to overcome their time inconsistency. To measure naïveté/sophistication, our online survey described a commitment contract for smoking cessation like those offered on the stickK webpage: “When you sign up, you lay money on the line, say $50, that you lose if your fail to meet the goal by the date that you agreed on.” Subjects responded on a Likert scale from “not at all interested” to “extremely interested.”
As shown in Table 2, 20 percent of subjects in our sample were at least somewhat interested in a commitment contract to quit smoking. By comparison, in a field experiment in the Philippines 11 percent of smokers took up an offer of a commitment contract for smoking cessation (Gine, Karlan and Zinman 2010). In further analysis, we compared the responses about β-discounting of the subjects who stated an interest in the commitment contract with those who were not interested. The average β-discount factor was 0.74 among those interested in the commitment contract compared to 0.725 among those not interested, a difference which was not statistically significant.
5. Mixed Logit Results, Counterfactuals, and Welfare Analysis
Mixed Logit Results
Table 3 presents the results of the mixed logit model given by equation (7).17 To estimate equation (7) we assume that the random coefficients δi follow the normal distribution, except for the coefficients on the price and risk variables which we assume follow the log normal distribution. We assume the independence among the random coefficients and make 500 Halton draws to construct the simulated likelihood function. The estimated parameters in Table 3 maximize the simulated likelihood function.
Table 3.
Mixed logit model of e-cigarette use
| Random coefficient | Fixed coefficient | |
|---|---|---|
| Mean | ||
| Intercept for E-cigarettes | −3.550*** | |
| (0.244) | ||
| Interaction with Γ | 2.322*** | |
| (0.476) | ||
| Intercept for NRPs | −6.911*** | |
| (0.300) | ||
| Interaction with Γ | 2.550*** | |
| (0.408) | ||
| Many flavors | 0.274** | |
| (0.102) | ||
| Interaction with Γ | −0.104 | |
| (0.189) | ||
| Warnings: FDA | −0.123 | |
| (0.125) | ||
| Interaction with Γ | −0.495* | |
| (0.237) | ||
| Warnings: Reduced risks | −0.193 | |
| (0.126) | ||
| Interaction with Γ | −0.052 | |
| (0.235) | ||
| Warnings: MarkTen | −1.604*** | |
| (0.176) | ||
| Interaction with Γ | 0.381 | |
| (0.298) | ||
| Risk of deaths (negative) | 0.970*** | |
| (0.188) | ||
| Interaction with Γ | 0.373 | |
| (1.429) | ||
| Price: $6 (negative) | 0.443** | |
| (0.137) | ||
| Interaction with Γ | −0.445 | |
| (0.261) | ||
| Price: $9 (negative) | 1.068*** | |
| (0.095) | ||
| Interaction with Γ | −0.735* | |
| (0.303) | ||
| Random coefficient Standard deviation |
||
| Intercept for E- cigarettes | 4.402*** | |
| (0.199) | ||
| Intercept for NRPs | 5.442*** | |
| (0.264) | ||
| Many flavors | 0.705*** | |
| (0.128) | ||
| Warnings: FDA | 0.125 | |
| (0.169) | ||
| Warnings: Reduced risks | 0.311 | |
| (0.188) | ||
| Warnings: MarkTen | 1.629*** | |
| (0.186) | ||
| Risk of deaths (negative) | 0.930*** | |
| (0.091) | ||
| Price: $6 (negative) | 1.580*** | |
| (0.234) | ||
| Price: $9 (negative) | 1.497*** | |
| (0.165) | ||
| Respondents x Choices | 13,760 | |
Notes: The random coefficients on the negative of price and risk variables are assumed to follow log normal distribution; other random coefficients are assumed to follow normal distributions. Halton draws are used for the simulation and independence are assumed across different random coefficients. Standard errors in parentheses.
p<0.05,
p<0.01,
p<0.001
Table 3 presents the estimated means and standard deviations of the random coefficients.18 The alternative-specific constant terms reflect the baseline utility from each tobacco product. We estimate that compared to combustible cigarettes, on average the alternatives of e-cigarettes and the NRPs provide lower baseline utilities. Flavors increase the average utility from e-cigarettes, while the strong warning label decreases the average utility from e-cigarettes. Higher prices also decrease the average utility from e-cigarettes.
The estimated standard deviations of the random coefficients show substantial heterogeneity. For example, although on average the baseline utility from e-cigarettes is negative, based on the estimated mean and standard deviation the e-cigarette-specific constant is positive for about 21 percent of the sample. Similarly, the estimates imply that while flavors increase utility on average, they decrease utility for 35 percent of the sample. This might reflect smokers who prefer e-cigarettes that are more similar to their regular combustible cigarettes.
Several statistically significant interactions with Γ are consistent with the prediction that present bias influences the utility from nicotine products. Subjects who are sophisticated about their present bias on average receive higher (less negative) baseline utility from e-cigarettes and the NRP. We also estimate that for these subjects the FDA warning has a stronger negative impact on the utility from e-cigarettes. These results are consistent with the prediction that naïve present-biased subjects place a lower value on products and product attributes related to future utility.
A common concern of using SP data for market forecasting is the existence of behavior biases or measurement errors. We use the self-reported RP data from the survey to explore the representativeness of DCE data to real market situation. We calibrate a multinomial logit model with RP data and did not find any significant shifts of coefficients or baseline market shares. This evidence suggests our SP data is relatively representative of the market situation. The details of the calibration are discussed in the second section of the Online Appendix and the results are reported in Online Appendix Table 2.
Counterfactuals
Table 4 and Online Appendix Figure 1 presents predicted choices of nicotine products under various counterfactual assumptions about product attributes and consumer optimization errors. The first set of counterfactuals predict consumer choices under policy-relevant changes in product attributes. In most of the counterfactuals relevant to the 2016 FDA deeming regulation, tobacco product choices are not predicted to change much. Under pre-2016 FDA regulation market conditions – many flavors available, no warning label, and price of $6 – our model predicts that 16 percent of consumers will choose e-cigarettes, 71 percent will choose combustible cigarettes, and 13 percent will choose the NRP. Even if the FDA review of new product applications results in an effective ban of flavors, when combined with the new FDA-required label our model predicts that the fraction of consumers who choose e-cigarettes falls only slightly from 16 to 15 percent. This suggests little welfare implications of the FDA’s recent actions to remove flavored e-cigarettes from stores accessible to minors. If the FDA were to adopt the stronger warning label, our model predicts that the fraction of consumers who choose e-cigarettes would decrease to 13 percent.
Table 4.
Counter-factuals and compensating variations (CV)
| Use | Use | Use | CV w.r.t. | decision utility | CV w.r.t. | experience utility | |
|---|---|---|---|---|---|---|---|
| e-cigarettes | cigarettes | NRPs | Per subject | Per e-cig user | Per subject | Per e-cig user | |
| Pre-2016 FDA market conditions | 16% | 71% | 13% | ||||
| Policy counter-factuals: | |||||||
| Ban flavors | 16% | 71% | 13% | 0.05 | 0.31 | 0.08 | 0.5 |
| FDA warning | 17% | 68% | 15% | 0.04 | 0.25 | 0.12 | 0.75 |
| Reduced risk warning | 17% | 68% | 15% | 0.03 | 0.19 | 0.06 | 0.38 |
| MarkTen warning | 13% | 72% | 15% | 0.15 | 0.94 | 0.26 | 1.63 |
| $3 Tax | 14% | 71% | 15% | 0.12 | 0.75 | 0.21 | 1.31 |
| $3 Subsidy | 30% | 57% | 13% | −0.38 | −2.38 | −0.61 | −3.81 |
| Ban flavors & FDA waning | 15% | 70% | 15% | 0.04 | 0.25 | 0.11 | 0.69 |
| Ban e-cigarettes | 0% | 82% | 18% | 0.49 | 3.06 | 1 | 6.25 |
| Consumer optimization error counter-factuals: | |||||||
| Correcting risk misperceptions | 22% | 60% | 18% | ||||
| Correcting naiveté about time inconsistency | 27% | 51% | 22% | ||||
| Correcting risk misperceptions & Naiveté about time inconsistency |
32% | 42% | 26% | ||||
Notes: Per e-cigarette user use the number of e-cigarette users in the baseline pre-FDA market condition.
The FDA has recently discussed the elimination of application enforcement discretion to any currently marketed e-cigarettes, which would result in a total ban (FDA 2018). A total ban of e-cigarettes is outside the range of the experimental conditions in the DCE. With that caveat in mind, our model predicts that if e-cigarettes were banned the fraction of consumers who choose combustible cigarettes would increase to 82 percent while the fraction who choose the NRP increases to 18 percent.
The counterfactual predictions suggest that policies that change e-cigarette prices will have larger effects on tobacco product choices. An e-cigarette tax that increases the price by $3 is predicted to decrease the fraction of consumers who choose e-cigarettes to 14 percent. Although policy makers are not currently considering e-cigarette subsidies, in principle they could; for example, state Medicaid coverage for smoking cessation products could be extended to e-cigarettes. An e-cigarette subsidy that decreases the price by $3 is predicted to increase the fraction who choose e-cigarettes to 30 percent. The asymmetric response to the price increase versus decrease mainly reflects subjects’ responses to the DCE experimental conditions and is not driven by functional form assumptions.
Table 4 also shows descriptive counterfactuals where there are fewer consumer optimization errors. These counterfactuals do not correspond to any plausible policy-relevant scenarios. Instead, they illustrate the magnitudes of the roles the consumer optimization errors play in tobacco product choices. Our model predicts that the fraction of consumers who choose e-cigarettes is about five percentage points higher among: consumers who have correct perceptions of e-cigarette and NRP risks; or among consumers who are not naïve about their present bias. The fraction of consumers who choose e-cigarettes is predicted to increase from the baseline of 16 percent to 32 percent among consumers who make neither optimization error, while the fraction who choose combustible cigarettes is predicted to decrease from 71 percent to 42 percent. The descriptive evidence from these counterfactuals that show large differences across consumer groups defined by their optimization errors are useful and important, in the same way descriptive evidence about gender or race differences can be.19
Welfare Analysis
The last four columns in Table 4 present the CVs in income for the policy counterfactuals, compared to the pre-2016 FDA regulation market conditions. For our standard welfare analysis, we calculate one set of CVs defined in equation (8) with respect to decision utility. For our behavioral welfare analysis, we calculate another set of CVs defined in equation (10) with respect to experienced utility. We calculate the CVs averaged over all subjects in our sample. The average CVs include the zero values for subjects who are not at the margin and choose combustible cigarettes regardless of the policy-induced changes in e-cigarette attributes. To shed light on the distribution of CVs, Table 4 also reports the CVs per e-cigarette user.
The difference between the CVs calculated with respect to decision versus experienced utility shows the importance of conducting behavioral welfare economics that accounts for optimization errors. In general, the CVs with respect to experienced utility are about twice as large (in absolute value) as the CVs with respect to decision utility, and in some cases even larger. The CV for the FDA warning with respect to experienced utility is $0.12 per subject or $0.25 per e-cigarette user, triple the size of the CV with respect to decision utility. This pattern reflects the estimated interaction with Γ, where sophisticated subjects respond more strongly to the FDA warning.
Because consumer optimization errors lead to too few smokers choosing e-cigarettes, standard estimates of the costs of FDA e-cigarette regulations will substantially under-state the behavioral welfare economic-based estimate of costs. Conversely, the standard approach to estimate the benefits of a policy like a subsidy that increases e-cigarette use will substantially under-state the behavioral economic-based benefits estimate. Back-of-the-envelope calculations help illustrate the practical importance of behavioral cost-benefit analysis. Suppose that each of the 36.5 million adult smokers in the U.S. makes a tobacco product choice once per week. In our behavioral welfare analysis we estimate that a ban of e-cigarettes (the most extreme regulatory outcome) imposes a welfare cost of $1 per smoker per choice, so the total annual costs of the ban are $1.9 billion. From a standard welfare analysis, the per smoker per choice cost is $0.49 and the total costs are only $930 million. Our estimates of the impact of e-cigarette regulations on consumer welfare contribute to recent research on the behavioral welfare economic analysis of tobacco control (Ashley, Nardinelli and Lavaty 2015, Cutler et al. 2016, Jin et al. 2015, Levy, Norton and Smith 2018).
6. Discussion
In this article we use novel data from a DCE to study consumer choices about e-cigarettes. Our policy and behavioral welfare analysis suggests that the 2016 FDA deeming regulation might be mostly harmless: the regulation’s direct requirements and the current ban on e-cigarette flavors other than menthol are not predicted to change consumer choices or welfare very much. However, potential future FDA regulations and state tax and subsidy policies could result in larger changes in the intertwined markets for e-cigarettes and combustible cigarettes. Our results suggest that because of consumer optimization errors, many smokers fail to choose e-cigarettes even though that choice would increase their experienced utility. Potential policies like strong warning labels and e-cigarette taxes that further discourage use of e-cigarettes could move consumers even further away from optimal choices and lead to potentially large consumer welfare losses.
Conversely, an e-cigarette subsidy or an information campaign to reduce optimization errors could help align actual choices with the choices that maximize experienced utility. Currently, the CDC mentions on their website that while e-cigarettes are not safe for people that do not use tobacco products currently, they “do have the potential to benefit adult smokers who are not pregnant if used as a complete substitute for regular cigarettes and other smoked tobacco products” (CDC 2019). If this messaging could be spread more broadly through an information campaign and reduce risk perception errors, it would yield large consumer welfare gains. Correcting naïveté about present bias has similar potential, but to accomplish this through an information campaign poses difficult challenges. The recent outbreak of lung injury linked to THC-containing e-cigarettes poses additional challenges to message the difference between the risks of THC- versus nicotine-containing e-cigarettes. Preliminary evidence suggests that the lung injury outbreak has increased consumer risk perceptions for all e-cigarettes, not just those containing THC (Dave et al. 2020).
The limitations of this study include the hypothetical nature of the DCE, details of the implementation and design of the DCE, and restrictive assumptions required in the econometric specification. Rapid innovations in e-cigarette markets and public policies pose a particular challenge for empirical economic research. Although we believe that the results of our analysis provide useful insights in general, it will be important to conduct new DCEs tailored to current market conditions and policy debates.
Supplementary Material
Acknowledgments
We acknowledge funding from a seed grant for collaborations between Cornell University-Ithaca and Weill Cornell Medical College faculty and from National Institutes of Health grants (CA U01-154254, 1U01CA154248-04, R01DA045016,). We thank Michael French and participants at an iHEA/AEA session, and seminar participants at Cornell’s health economics workshop for helpful comments. We have no conflicts of interest to disclose.
Appendix 1: Levels and Attributes of Discrete Choice Experiment
The following table contains the product choices, with additional information on the table following.
| “One pack of [CIG BRAND] cigarettes”] Or “One pack of [CIG BRAND] cigarettes you yourself roll”] |
Nicotine replacement product | Disposable vaping device |
| The price you usually pay: [PACK PRICE] CIG FLAVOR SURGEON GENERAL’S WARNING: Smoking Causes Lung Cancer, Heart Disease, Emphysema, And May Complicate Pregnancy |
$6 for package equivalent to a pack of cigarettes |
ECIG PRICE ECIG FLAVOR ECIG WARNING |
The following cigarette variables are entered based on information collected for each respondent earlier in the survey.
CIG BRAND: Derived from a question asking, “during the past 30 days, what brand of cigarettes did you smoke most often?”
PACK PRICE: Derived from questions asking price paid per pack or pack equivalent of the last cigarettes purchased.
CIG FLAVOR: Either “Regular” or “Mentholated”, depending on if the last brand of CIG BRAND smoked were mentholated or not.
The following e-cigarette variables are experimentally varied so that respondents are offered 1 of 24 different choices, 12 different times.
- ECIG PRICE:
- $3
- $6
- $9
- ECIG FLAVOR
- Choice of tobacco or menthol flavors
- Choice of many flavors, including tobacco, menthol, clove, spice, candy, fruit, chocolate, alcohol, and other sweets.
ECIG WARNING
NONE
WARNING: This product contains nicotine derived from tobacco. Nicotine is an addictive chemical
WARNING: No tobacco product is safe, but this product presents substantially lower risks to health than smoking cigarettes.
WARNING: This product is not a smoking cessation product and has not been tested as such. This product is intended for use by persons of legal age or older, and not by children, women who are pregnant or breast feeding, or persons with or at risk of heart disease, high blood pressure, diabetes, or taking medicine for depression or asthma. Nicotine is addictive and habit forming, and it is very toxic by inhalation, in contact with the skin, or if swallowed. Nicotine can increase your heart rate and blood pressure and cause dizziness, nausea, and stomach pain. Inhalation of this product may aggravate existing respiratory conditions. Ingestion of the non-vaporized concentrated ingredients in the cartridges can be poisonous. CA Proposition 65 Warning: This product contains nicotine, a chemical known to the State of California to cause birth defects or other reproductive harm.
The following contains an example of a choice an individual may have:
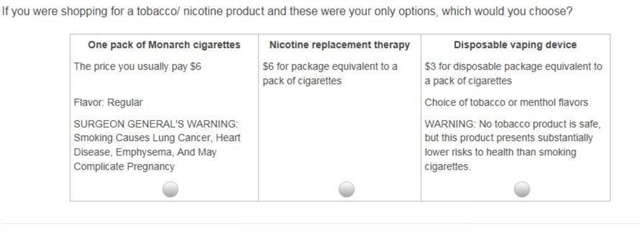
Footnotes
Section 2 below provides more discussion of the evidence on the relative risks of vaping and smoking. Unless otherwise stated we refer to the action of using e-cigarette products as vaping and the action of using any combustible tobacco product as smoking.
Authors’ calculations from the 2018 National Health Interview Survey.
Chetty (2015) provides an overview of behavioral public economics. Recent behavioral welfare economic studies examine consumer choices across various domains including prescription drug insurance (Ketcham, Kuminoff and Powers 2016), energy-efficient appliances (Allcott and Taubinsky 2015), and smoking (Jin, Kenkel, Liu and Wang 2015).
In December 2019 the CDC released four reports about the outbreak (https://www.cdc.gov/media/releases/2019/p1220-cases-EVALI.html). The findings strengthened the link between lung injury and Vitamin E acetate, which is sometimes added to vaping products that contain THC. Vitamin E acetate is not found in commercially produced nicotine e-cigarettes. The FDA does not consider THC/marijuana to be a tobacco product.
A recent meta-analysis of nine longitudinal studies suggests that e-cigarettes can serve as a gateway to later cigarette smoking among youth that initiated e-cigarette use without previous nicotine use (Soneji et al. 2017). However, this conclusion has been criticized on grounds that a gateway theory is not compatible with either (1) the decrease in smoking prevalence observed in adolescents in countries where vaping increased or (2) an increase in smoking among teenagers after age restrictions were imposed on e‐cigarette purchases (Etter J-F 2018).
The product attributes Xij depend on consumer index i because they include the individual-level subjective product risks. The vector Xij also includes alternative-specific intercept terms that capture the average utility from each product. Except for subjective product risk, the other product attributes included in Xij are alternative-specific and do not vary across individuals.
We also include the variable “Little present bias (β-discount factor > 0.9)” in a specification and report the results in Online Appendix Table 3. Because virtually all respondents are present biased so we did not include this variable in the main model.
McFadden (2017) shows that the standard parametric assumptions are not required; instead the welfare expression in equation (8) can be viewed as an approximation to the expression that follows from more basic assumptions.
For additional discussion and applications, see Allcott (2013) and Train (2015), who use a similar approach.
DCEs are related to conjoint analysis, and some researchers use the two terms interchangeably. Louviere, Flynn and Carson (2010) stress that DCEs, but not conjoint analysis, are consistent with economic demand theory because they are based on the random utility model.
DCEs are related to conjoint analysis, and some researchers use the two terms interchangeably. Louviere, Flynn and Carson (2010) stress that DCEs, but not conjoint analysis, are consistent with economic demand theory because they are based on the random utility model.
DCEs are related to conjoint analysis, and some researchers use the two terms interchangeably. Louviere, Flynn and Carson (2010) stress that DCEs, but not conjoint analysis, are consistent with economic demand theory because they are based on the random utility model.
D-efficiency is a metric based on the criterion to minimize the generalized variance of the parameter estimates. The D-efficiency of our DCE is 98 percent, relative to 100 percent that could be accomplished by asking about all 24 possible choice scenarios.
The Online Appendix provides more discussion of how we identified intransitive preferences and presents evidence that the results are robust when the subjects showing intransitive preferences are dropped from the analysis.
Although the DCE choices included warning labels, the design of the online survey should minimize their influence on subjects’ responses about product risk perceptions. At the beginning of the DCE, subjects were instructed that: “The FDA is studying whether a warning label is appropriate and what it should say…. Our survey will ask you about several possible labels. When answering the questions, you should assume that the label you are asked about in the question reflects the FDA’s labeling decision based on the best scientific evidence available.” In the DCE, over the course of 12 scenarios each subject in the DCE saw each of the four warning label conditions three times, so any influences on their risk perceptions should approximately cancel out.
See equations (8) and (9) in Courtemanche, Heutel, and McAlvanah (2014).
The Online Appendix 1 presents reduced-form results from linear probability and multinomial logit models. We also present a multinomial logit model that combines the stated preference data from the DCE and revealed preference data on subjects’ actual use of the nicotine products.
We also report the marginal effect for those coefficients in Online Appendix Table 5.
Our experimental design does not allow us to identify the causal effects of the optimization errors. An interesting direction for future work would be an experiment such as information provision to reduce subjective risk misperceptions. β-discounting and naïveté might be more-or-less unchangeable preference parameters that cannot be manipulated in an experiment or by policy. However, future work might explore nudges such as framing that could lead to fewer optimization errors even if the preference parameters remain stable.
References
- Abouk R. & Adams S. (2017). “Bans on Electronic Cigarette Sales to Minors and Smoking among High School Students.” Journal of Health Economics. 54, 17–24. [DOI] [PubMed] [Google Scholar]
- Allcott H. (2013). “The Welfare Effects of Misperceived Product Costs: Data and Calibrations from the Automobile Market.” American Economic Journal: Economic Policy, 5(3), 30–66. [Google Scholar]
- Allcott H, Mullainathan S, Taubinsky D. (2014). “Energy Policy with Externalities and Internalities.” Journal of Public Economics. 112: 72–88. [Google Scholar]
- Allcott H, Taubinsky D. (2015). “Evaluating Behaviorally-Motivated Policy: Experimental Evidence from the Lightbulb Market.” American Economic Review. 105 (8): 2501–2538. [Google Scholar]
- American Lung Association. (2020). “Overall Tobacco Trends.” Available at: https://www.lung.org/research/trends-in-lung-disease/tobacco-trends-brief/overall-tobacco-trends. (Accessed June 10, 2020).
- Angeletos G-M, Laibson A, Repetto A, Tobacman J, Weinberg S. (2001). “The Hyperbolic Consumption Model: Calibration, Simulation, and Empirical Evaluation.” Journal of Economic Perspectives. 15 (3): 47–68. [Google Scholar]
- Ashley EM, Nardinelli C. & Lavaty RA (2015). “Estimating the Benefits of Public Health Policies That Reduce Harmful Consumption.” Health Economics. 24 (5), 617–624. [DOI] [PubMed] [Google Scholar]
- Biener L, Hargraves JL (2014). “A Longitudinal Study of Electronic Cigarette Use in a Population-Based Sample of Adult Smokers: Association with Smoking Cessation and Motivation to Quit.” Nicotine & Tobacco Research. 17 (2): 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges JF, Hauber AB, Marshall D, et al. (2011). “Conjoint Analysis Applications in Health – a Checklist: A Report of the ISPOR Good Research Practices for Conjoint Analysis Task Force.” Value in Health. 14 (4): 403–413. [DOI] [PubMed] [Google Scholar]
- Buckell John, and Hess Stephane. 2019. “Stubbing Out Hypothetical Bias: Improving Tobacco Market Predictions by Combining Stated and Revealed Preference Data.” Journal of Health Economics 65 (May): 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson RT (2012). “Contingent valuation: a practical alternative when prices are not available.” Journal of Economic Perspectives. 26: 27–42. [Google Scholar]
- Centers for Disease Control and Prevention. (2019). “Electronic Cigarettes.” Available at: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/index.htm. (Accessed February 10, 2020).
- Centers for Disease Control and Prevention. (2020). “State Tobacco Activities Tracking and Evaluation (STATE) System.” Available at: https://www.cdc.gov/statesystem/factsheets/ecigarette/ECigarette.html. (Accessed February 10, 2020).
- Chetty R. (2015). “Behavioral Economics and Public Policy: A Pragmatic Perspective.” American Economic Review. 105 (5): 1–33. [Google Scholar]
- Cooper MT, and Pesko MF. (2017). “The Effect of E-Cigarette Indoor Vaping Restrictions on Adult Prenatal Smoking and Birth Outcomes.” Journal of Health Economics. 56: 178–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotti Chad D., Courtemanche Chares J., Johanna Catherine Maclean, Nesson Erik T., Pesko Michael F., Nathan Tefft (2020). “The Effects of E-cigarette Taxes on E-cigarette Prices and Tobacco Product Sales: Evidence from Retail Panel Data.” NBER Working Paper #26724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtemanche C, Heutel G, McAlvanah P. (2014). “Impatience, Incentives and Obesity.” Economic Journal. 125 (582):1–31. [Google Scholar]
- Cowen and Company Equity Research. (2019). “QUICK TAKE - Tobacco - Flavor Ban Coming, Boon for Cigarettes?” Cowen and Company Equity Research Report, September 11, 2019. (Vivien Azer: vivien.azer@cowen.com). [Google Scholar]
- Creamer MeLisa R., Wang Teresa W., Babb Stephen, Cullen Karen A., Day Hannah, Willis Gordon, Jamal Ahmed, and Neff Linda (2019). “Tobacco product use and cessation indicators among adults—United States, 2018.” Morbidity and Mortality Weekly Report 68 (45): 1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley RA, for the Health Public Policy Committee of the American College of Physicians. (2015). “Electronic Nicotine Delivery Systems: Executive Summary of a Policy Position Paper from the American College of Physicians.” Annals of Internal Medicine. 162: 583–584. [DOI] [PubMed] [Google Scholar]
- Cutler David M, Amber Jessup, Donald Kenkel, and Starr Martha A (2016). “Economic Approaches to Estimating Benefits of Regulations Affecting Addictive Goods.” American Journal of Preventive Medicine. 50 (5S1): S20–S26. [DOI] [PubMed] [Google Scholar]
- Dave D, Feng B, and Pesko MF. “The Effects of E-Cigarette Minimum Legal Sale Age Laws on Youth Substance Use.” Health Economics. 28, no. 3 (March 2019): 419–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave DM, Dench D, Kenkel DS, Mathios AD, and Wang H.. (2020). “News that Takes Your Breath Away: Risk Perceptions During an Outbreak of Vaping-related Lung Injuries.” Journal of Risk & Uncertainty, June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter J-F. (2018). “Gateway effects and electronic cigarettes.” Addiction. 1783–1776: (10)113. [DOI] [PubMed] [Google Scholar]
- Friedman AS (2015). “How Does Electronic Cigarette Access affect Adolescent Smoking?” Journal of Health Economics. 44: 300–308. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. (2016). “Deeming Tobacco Products to Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products; Final Rule.” Federal Register, 81 (90): 28974 – 29106. [PubMed] [Google Scholar]
- Food and Drug Administration. (2017). “FDA Announces Comprehensive Regulatory Plan to Shift Trajectory of Tobacco-related Disease, Death.” Press Release, July 28, 2017. Available at: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm568923.htm. [Google Scholar]
- Food and Drug Administration. (2018). “Statement from FDA Commissioner Scott Gottlieb, M.D., on proposed new steps to protect youth by preventing access to flavored tobacco products and banning menthol in cigarettes.” Statement, November 15, 2018. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm625884.htm. [Google Scholar]
- Food and Drug Administration. (2019a). “Swedish Match North America, Inc., MRTP Applications.” Press Release, February 20, 2019. Available at: https://www.fda.gov/tobaccoproducts/labeling/marketingandadvertising/ucm533454.htm. [Google Scholar]
- Food and Drug Administration. (2019b). “Nicotine: The Addictive Chemical in Tobacco Products.” Health Information, June 24, 2019. Available at: https://www.fda.gov/tobacco-products/health-information/nicotine-addictive-chemical-tobacco-products. (Accessed February 10, 2020). [Google Scholar]
- Food and Drug Administration. (2020a). “FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint.” News Release, January 2, 2020. Available at: https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children. [Google Scholar]
- Food and Drug Administraton. (2020b). “Coronavirus (COVID-19) Update: Court Grants FDA’s Request for Extension of Premarket Review Submission Deadline for Certain Tobacco Products Because of Impacts from COVID-19.” Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-court-grants-fdas-request-extension-premarket-review-submission-deadline
- Gine X, Karlan D, Zinman J. (2010). “Put Your Money Where Your Butt Is: A Commitment Contract for Smoking Cessation.” American Economic Journal: Applied Economics. 2 (4): 213–25. [Google Scholar]
- Gottlieb S. (2017). “Protecting American Families: Comprehensive Approach to Nicotine and Tobacco.” Remarks as prepared for delivery. Commission of Food and Drug Administration. Available online: https://www.fda.gov/NewsEvents/Speeches/ucm569024.htm. (Accessed February 10, 2019). [Google Scholar]
- Gruber J, Koszegi R. (2001). “Is Addiction ‘Rational’? Theory and Evidence.” Quarterly Journal of Economics. 116 (4): 1261–1303. [Google Scholar]
- Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, Li J, et al. “A Randomized Trial of E-Cigarettes Versus Nicotine-Replacement Therapy.” N Engl J Med. 380, no. 7 (February 14 2019): 629–37. [DOI] [PubMed] [Google Scholar]
- Hausman J. (2012). “Contingent valuation: from dubious to hopeless.” Journal of Economic Perspectives. 26: 43–56. [Google Scholar]
- Jin L, Kenkel D, Liu F, Wang H. (2015). “Retrospective and Prospective Benefit-Cost Analyses of U.S. Anti-Smoking Policies.” Journal of Benefit-Cost Analysis. 6 (1): 154–186. [Google Scholar]
- Kenkel D. (2016). “Healthy Innovation: Vaping, Smoking, and Public Policy.” Journal of Policy Analysis and Management. 35 (2): 473–479. [DOI] [PubMed] [Google Scholar]
- Kesternich I, Heiss F, McFadden D, Winter J. (2013). “Suit the Action to the Word, the Word to the Action: Hypothetical Choices and Real Decisions in Medicare Part D.” Journal of Health Economics. 32 (6): 1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BA, Gammon DG, Marynak KL, and Rogers T.. 2018. “Electronic Cigarette Sales in the United States, 2013–2017.” JAMA no. 320 (13):1379–1380. doi: 10.1001/jama.2018.10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krucien N, Sicsic J, & Ryan M. (2019). “For better or worse? Investigating the validity of best–worst discrete choice experiments in health.” Health Economics 28(4), 572–586. [DOI] [PubMed] [Google Scholar]
- Laibson D, Repetto A, and Tobacman J. (2007). “Estimating Discount Functions with Consumption Choices over the Lifecycle.” NBER Working Paper. [Google Scholar]
- Lancsar E, Louviere J. (2008). “Conducting discrete choice experiments to inform healthcare decision making: a user’s guide.” PharmacoEconomics. 26(8):661–77. [DOI] [PubMed] [Google Scholar]
- Leggett CG (2002). “Environmental Valuation with Imperfect Information.” Environmental and Resource Economics. 23: 343–355. [Google Scholar]
- Levy HG, Norton EC, Smith JA. (2018). “Tobacco Regulation and Cost-Benefit Analysis: How Should We Value Foregone Consumer Surplus?” Am J Health Econ. 4(1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louviere JJ, Flynn TN, Carson RT (2010). “Discrete Choice Experiments Are Not Conjoint Analysis.” Journal of Choice Modelling. 3(3): 57–72. [Google Scholar]
- Marti J, Buckell J, Maclean JC, and Sindelar J.. (2019). “To “Vape” or Smoke? Experimental Evidence on Adult Smokers.” Econ Inq. 57, no. 1 : 705–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D. (2017). “Stated Preference Methods and Their Applicability to Environmental Use and Non-use Valuations” In Contingent Valuation of Environmental Goods: A Comprehensive Critique, McFadden D. and Train K. editors. Elgar: Cheltingham. Chapter 6. [Google Scholar]
- McFadden D. (2017). “Foundations of Welfare Economics and Product Market Applications.” NBER Working Paper 23535. [Google Scholar]
- Mullainathan S, Schwartzstein J, Congdon WJ (2012). “A Reduced-form Approach to Behavioral Public Finance.” Annual Review of Economics. 4: 511–540. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. (2018). “Public health consequences of e-cigarettes.” Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Newell RG, Siikamaki J. (2015). “Individual Time Preference and Energy Efficiency.” American Economic Review: Papers & Proceedings. 105 (5): 196–200. [Google Scholar]
- Newell RG, Siikamaki J. (2014). “Nudging Energy Efficiency Behavior: The Role of Information Labels.” J Assoc Environ Reso.1(4):555–98. [Google Scholar]
- Pesko MF, Courtemanche C, Maclean JC (2019). “The Effects of Traditional Cigarette and E-Cigarette Taxes on Adult Tobacco Product Use” National Bureau of Economic Research Working Paper 26017. [Google Scholar]
- Pesko MF, Currie JM (2019). “The Effect of E-Cigarette Minimum Legal Sale Age Laws on Traditional Cigarette Use and Birth Outcomes among Pregnant Teenagers.” Journal of Health Economics. 66: 71–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesko MF, Hughes JM, Faisal FS (2016). “The Influence of Electronic Cigarette Age Purchasing Restrictions on Adolescent Tobacco and Marijuana Use.” Preventive Medicine. 87: 2017–212. [DOI] [PubMed] [Google Scholar]
- Pesko MF, Kenkel D, Wang H, Hughes JM (2016). “The Effect of Potential Electronic Nicotine Delivery System Regulations on Nicotine Product Selection.” Addiction. 111 (4): 734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Law Center. (2019). “E-Cigarette Tax—States with Laws Taxing E-Cigarettes”. Available at: https://www.publichealthlawcenter.org/sites/default/files/States-with-Laws-Taxing-ECigarettes-December152019.pdf. (Accessed February, 10, 2020.)
- Qi S. (2103). “The Impact of Advertising Regulation on Industry: The Cigarette Ban of 1971.” Rand Journal of Economics. 44 (2): 215–248, [Google Scholar]
- Royal College of Physicians. (2016). “Nicotine without smoke: Tobacco harm reduction.” London: RCP. [Google Scholar]
- Schoenborn CA, Gindi RM (2015). “Electronic Cigarette Use Among Adults: United States, 2014.” NHCS Data Brief Number 217. [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Rohay J. Gitchell JG (2008). “Perceived Safety and Efficacy of Nicotine Replacement Therapies among US Smokers and Ex-smokers: Relationship with Use and Compliance.” Addiction. 103 (8): 1371–1378. [DOI] [PubMed] [Google Scholar]
- Soneji S, Barrington-Trimis JL, Wills TA et al. (2017). “Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis.” JAMA Pediatr. 171(8):788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small KA, Rosen HS (1981). “Applied Welfare Economics with Discrete Choice Models.” Econometrica. 49 (1): 105–130. [Google Scholar]
- Train Kenneth E. (2002). Discrete Choice Models with Simulation. Cambridge: Cambridge University Press. [Google Scholar]
- Train KE (2015). “Welfare Calculations in Discrete Choice Models When Anticipated and Experienced Attributes Differ: A Guide with Examples.” Journal of Choice Modelling. 16, 15–22. [Google Scholar]
- U.S. Department of Health and Human Services. (2014). The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- U.S. Department of Health and Human Services (2016). E-Cigarette Use Among Youth and Young Adults. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- Viscusi WK (1990). “Do Smokers Underestimate Risks?” Journal of Political Economy. 98, no. 6: 1253–1269. [Google Scholar]
- Viscusi WK (2016). “Risk Beliefs and Preferences for E-Cigarettes.” American Journal of Health Economics. 2, no. 2: 213–240. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


