Abstract
Introduction
The incidence of prosthetic valve dysfunction (stuck valve) is variable and is dependent on various factors. There are studies from Indian groups that have emphasized the success of thrombolysis; however, none of them reported a follow-up. This study was designed to emphasize on the follow-up of these patients at midterm.
Material and methods
The patients of prosthetic valve thrombosis between period of January 2013 and December 2017 were included in this retrospective observational study. Sixty-six patients were admitted with stuck valve during this period. Thrombolysis was preferred modality of treatment. Survivors were followed up with serial echocardiography, which included estimation of left ventricular and valve functions.
Results
Of a total of 66 patients, 59 were of stuck mitral valve and 7 stuck aortic valve. The event happened at a mean of 48.86 ± 48.80 months after index operation of valve replacement using mechanical valve prosthesis. The median age was 40.27 ± 10.8 years with 39 males and 27 females. Thrombolysis was successful in 61 patients with a mortality of 5 (7.57%). During a mean follow-up of 22.7 ± 20.9 months, 42 patients were alive with 14 (22.95%) patients dead and 5 patients lost to follow-up. The average follow-up was 18.7 ± 22.7 months before death.
Conclusion
Following good early results after thrombolysis, patients of prosthetic heart valve thrombosis experience high mortality within 2 years of follow-up. These patients require frequent follow-up to avoid early mortality.
Keywords: Prosthetic valve thrombosis, Thrombolysis, Follow-up after valve thrombosis
Introduction
Valve disease in India has been predominantly a consequence of rheumatic heart disease [1, 2]. This leaves a young population of patients, affected by rheumatic heart valve disease, with the option of replacement of heart valves [3]. Replacement of valve at a young age merits a mechanical heart valve prosthesis [4]. A bioprosthetic valve as a choice for replacement has been associated with accelerated degeneration of the valve and requirement of frequent re-replacement [5]. While replacement with prosthetic valve may not be advisable, repair of the rheumatic valve, though an option, may not last long, warranting a reoperation [6]. Following mechanical valve replacement anticoagulation with vitamin K antagonist has been recommended [7]. There have been well-researched guidelines for follow-up of valve replacement patients as well as guidelines for anticoagulation management [8, 9]. While these guidelines have been followed, yet there were instances of acute prosthetic valve thrombosis [10]. This situation, in majority of cases, has been a serious condition and leads to major hemodynamic compromise [11]. In this study, we observe the response to treatment of patients presenting with acute prosthetic valve thrombosis and its follow-up.
Material and methods
This is a retrospective, observational study of patients reporting to the department of cardiac sciences with diagnosis of acute prosthetic valve thrombosis between January 2013 and December 2018. This study was approved by the institute ethics committee. Patients reported either to the outpatient department or in emergency of the hospital.
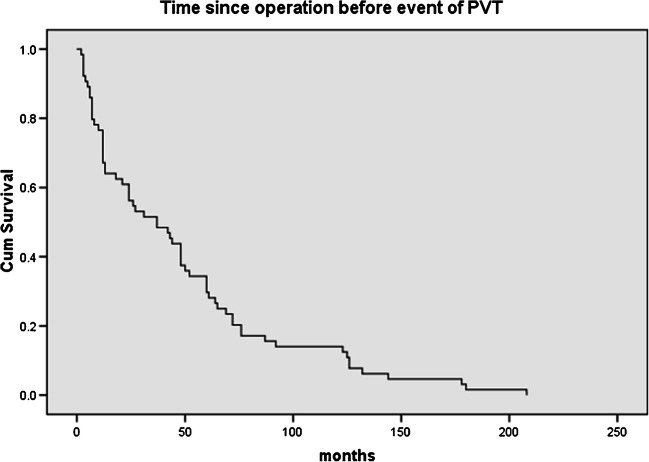
Inclusion criteria comprise all the patients reporting to hospital with acute prosthetic valve thrombosis. Exclusion criteria comprise patients who had contraindications to thrombolysis or complaint of active bleeding. The primary objective of this study was to observe the midterm outcomes after successful thrombolysis in patients of prosthetic valve thrombosis. A total of 66 patients presented with this problem and were enrolled in the study. This study enrolled patients who underwent valve replacement as long as 19 years back, from 1998. Since then, 4750 patients were operated for valve replacement in the department. However, 50% of the prosthetic valve thrombosis presented during initial 40 months from the date of index operation (Fig. 1).
Fig. 1.
Development of prosthetic valve thrombosis in months since the time of operation
The diagnosis of prosthetic valve thrombosis was confirmed by transthoracic echocardiography. Transthoracic 2D and M mode echocardiography was performed with Philips HD machine and a 3.2-MHz transducer (Philips Medical Systems, Andover, MA). The thrombus appeared as soft echo density with irregular shape and was homogenous, which was mainly around hinges or struts of the prosthetic valve. This was differentiated from pannus, which appears as circular hard and echo dense structure, mostly present in inflow or outflow areas of the valve. Movement of the prosthetic valve leaflets was confirmed, with either lack of movement of leaflets or a single leaflet in the closed or open position. The pressure half time (PHT), velocity, and rise in the gradients with acute presentation were confirmatory [12]. Fluoroscopy could not be performed as the patients presented with acute and severe discomfort. However, it is an examination with high sensitivity and specificity to diagnose this condition [13]. Thus, fluoroscopy has been used as a tool for easy and accurate diagnosis of this condition in patients who are hemodynamically stable or if the facility is available in the intensive care area.
Patients were immediately admitted and initiated on intravenous thrombolysis with loading dosage of streptokinase followed by continuous infusion in the case of first event of valve thrombosis. However, if the patient had a repeat event of thrombosis, urokinase instead of streptokinase was used intravenously. The institute’s protocol was followed for thrombolysis of prosthetic valve obstruction [14]. Streptokinase was used as intravenous infusion of 30 lac IU over 30 min, followed by 1 lac IU/h infusion for 48 h or until the response in form of reduction in mean gradient was achieved. It was given for a maximum of 48 h. Urokinase was administered intravenously 4400 IU/kg over 30 min followed by infusion of 4400 IU/kg/h for 24 h. Apart from thrombolysis, patients were stabilized by use of intravenous diuretics and use of non-invasive ventilation and intravenous inotropes, whenever required, determined by the clinical response of the patient. Following successful result of thrombolysis, patients were administered subcutaneous heparin in dose of 5000 IU four times daily with initiation oral anticoagulation with nicoumalone. Heparin was stopped after the international normalized ratio (INR) in excess of 2.0 was achieved. Follow-up echocardiography was performed periodically to document movements of leaflets and falling gradient across mitral valve. These findings were correlated with improvement in clinical condition of the patients. The response to treatment was confirmed by normalization of patient condition with reduction of mean mitral valve gradient to below 10 mmHg. If prosthetic valve thrombosis did not respond to thrombolysis, an emergency mitral valve replacement was performed.
Hospital data on acute prosthetic valve thrombosis was collected. Hospital information services were used to collect data on serial echocardiography and clinical condition on last reported date for follow-up. M mode and 2D echocardiography data, using transthoracic route, were recorded. It included left ventricular end-diastolic and end-systolic dimensions (LVEDD and LVESD) and left ventricular ejection fraction (LVEF). Peak and mean gradient across mitral and aortic valves and regurgitation across the valves and right ventricular systolic pressure (RVSP) were also recorded. Last recorded echocardiography values were used for data, if they were in agreement with the previous echocardiography values. This is an observational study focused on follow-up of patients who survived prosthetic valve thrombosis event, so the power of study was not calculated.
Statistical analysis
The statistics were computed using SPSS 17 version for windows (SPSS, Inc. Chicago, IL, USA). The categorical variables have been expressed in percentages. The continuous variables are expressed as mean ± SD. Student’s t test was performed to compare variables and a p value of < 0.05 was considered as significant. The survival analysis for various variables was performed using the Kaplan-Meier method.
Results
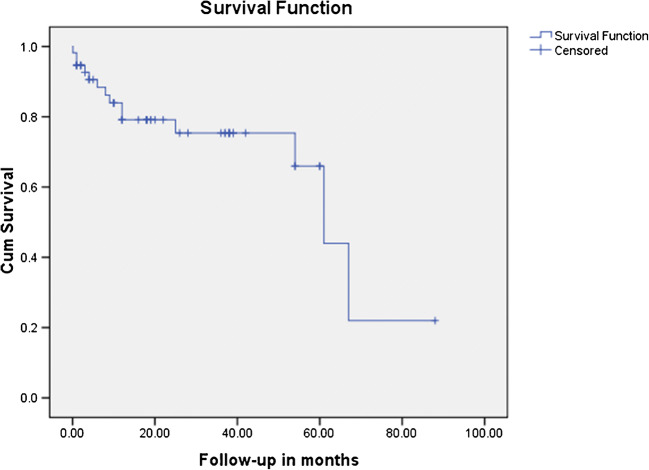
The mean age of the 66 patients presenting with the diagnosis of acute prosthetic valve thrombosis was 40.27 years ± 10.80, and of them, 39 were males and 27 females. All the patients had acute onset of symptoms and 16 presented in NYHA III and 50 patients presented in NYHA IV. Sixty patients reported in emergency and 6 patients in outpatient department. Thirty-eight patients had tachycardia with systolic blood pressure below 90 mmHg. They were admitted to cardiology intensive care unit for echocardiography followed by thrombolysis. The distribution of prosthetic valves that presented with the problem of acute thrombosis and their response to treatment is presented in Table 1. All patients with double valve replacement had thrombosis of mitral valve. The make of the valve was SJM (St. Judes Medical, MN, USA) in 45 patients in mitral and 9 patients in aortic position, ATS (ATS Medical Inc., MN, USA) in 9 in mitral and 7 in aortic position, Sorin (Sorin Group, Milano, IT) in 3 in mitral position, TTK Chitra (TTK Healthcare, Chennai, IN) in one each in aortic and in mitral position, and ON-X valve (Cryolife Inc., GA, USA) in one case in mitral position. One patient, which did not respond to thrombolysis after 24 h, was operated for mitral valve replacement after 4 days of the event (48 h following thrombolysis). The response to thrombolysis in this particular patient was partial with stabilization of hemodynamics, but with persistent symptoms. The patients presented with valve thrombosis after an average of 48.86 months ± 48.80 and the distribution of the time since operation is presented in Fig. 1. Sixty-five patients were of Rh type positive and only one was Rh negative, while all the blood groups were represented in these cases with A, B, AB, and O of 6, 13, 4, and 18 in numbers respectively. The comparison of left ventricular function and gradients across valves pre and post thrombolysis is mentioned in Table 2. In Table 2, the pre thrombolysis echocardiography values mentioned were from the echocardiography performed to reach the diagnosis of prosthetic valve thrombosis. All patients who responded did it with complete motion of both the leaflets. All the patients surviving the event were in NHYA II at the time of discharge. There were 12 patients who reported back to the hospital with repeat prosthetic valve thrombosis. They were treated with thrombolysis with urokinase. Of these, 4 patients responded to the treatment. The mean follow-up was 22.7 ± 20.9 months with a reported mortality of 22.95% (n = 14). All the mortalities (n = 14) happened in the hospital. Repeat prosthetic valve thrombosis was the reason in 8 patients, congestive heart failure in 3 patients, and sepsis in other 3 patients. A total of 61 patients had follow-up (5 patients succumbed to the primary event of valve thrombosis); of these, 5 were lost to follow-up. The mean duration before mortality during follow-up was 18.7 ± 23.7 months. Response to treatment in form of valve function at a follow-up of 22.7 ± 20.9 months is presented in Table 3. Follow-up echocardiography, done at the last follow-up, showed good function in the surviving patients (Table 4). The follow-up survival is depicted in Fig. 2.
Table 1.
Distribution of prosthetic valve dysfunction and response to treatment
| Variable | Value (%) |
|---|---|
| MVR (n) | 49 |
| AVR (n) | 7 |
| DVR (n) | 10 |
| Stuck mitral | 59 |
| Stuck aortic | 7 |
| Thrombolyis (n) | 66 |
| Redo MVR (n) | 1 |
| Discharged healthy (n) | 61 |
| Death (n) | 5 (7.57%) |
MVR mitral valve replacement, AVR aortic valve replacement, DVR double valve replacement, Redo MVR reoperation for mitral valve replacement
Table 2.
Comparison of echocardiography parameters in pre and post thrombolysis period
| Variable | Pre thrombolysis n = 61 |
Post thrombolysis n = 61 |
p value |
|---|---|---|---|
| LVEDD (mm) | 47.92 ± 13.47 | 35.97 ± 22.07 | 0.000 |
| LVESD (mm) | 30.25 ± 10.42 | 22.92 ± 14.76 | 0.001 |
| LVEF (%) | 58.8 ± 10.2 | 60.5 ± 10.8 | 0.41 |
| RVSP (mmHg) | 64.43 ± 23.33 | 41.43 ± 7.92 | 0.000 |
| MV PG (mmHg) | 25.29 ± 8.93 | 13.16 ± 7.07 | 0.000 |
| MV MG (mmHg) | 15.01 ± 7.08 | 6.01 ± 3.58 | 0.000 |
| AV PG (mmHg) | 62 ± 8.5 | 28.1 ± 14.2 | 0.000 |
| AV MG (mmHg) | 32 ± 10.2 | 10.3 ± 6.5 | 0.000 |
LVESD left ventricular systolic dimension, LVEDD left ventricular end-diastolic dimension, LVEF left ventricular ejection fraction, RVSP right ventricular systolic pressure, MV PG mitral valve peak gradient, MV MG mitral valve mean gradient, AV PG aortic valve peak gradient, AV MG aortic valve mean gradient
Table 3.
Valve function following thrombolysis at follow-up of 22.7 ± 20.9 months
| Variable | Aortic valve | Mitral valve |
|---|---|---|
| Peak gradient (mmHg) | 31.1 ± 24.2 | 15.9 ± 10.1 |
| Mean gradient (mmHg) | 12.3 ± 8.5 | 8.5 ± 7.6 |
| Trivial regurgitation | 5 | 9 |
| Mild regurgitation | 8 | 5 |
| Moderate regurgitation | 3 | 1 |
| Severe regurgitation | 1 | 0 |
Table 4.
Follow-up echocardiography
| Variable | Mean ± SD |
|---|---|
| LVEDD (mm) | 45.4 ± 5.0 |
| LVESD (mm) | 27.7 ± 4.7 |
| LVEF (%) | 55 ± 22.7 |
| RVSP (mmHg) | 43.6 ± 18.2 |
| LA size (mm) | 42.6 ± 9.9 |
LVESD left ventricular systolic dimension, LVEDD left ventricular end-diastolic dimension, LVEF left ventricular ejection fraction, RVSP right ventricular systolic pressure, LA left atrium
Fig. 2.
Survival curve of mortality after successful thrombolysis of prosthetic valve thrombosis during follow-up in months
Discussion
Incidence of prosthetic valve thrombosis has been 0.1–6% per year [15]. The most common reason for this has been inadequate anticoagulation [16]. However, proper anticoagulation may not ensure prevention from thrombosis of valve [17]. This study included patients presenting with acute symptoms due to obstructive prosthetic valve thrombosis and hemodynamic compromise; hence, thrombolysis was chosen as the initial treatment strategy [18]. In patients presenting acutely, a reliable and early diagnosis is the key to successful initiation of the treatment. Thus, a transthoracic echocardiography helps in arriving at a reliable diagnosis and it can be performed quickly. However, though a transesophageal echocardiography can delineate even a small thrombus, its use may be limited by the hemodynamic situation of the patient [19]. Next challenge in diagnosis has been to differentiate a thrombus from a pannus or pannus with formation of a thrombus. These can be differentiated by their echo density and the location on the valve [12]. Ha et al. had shown the extent of the pannus and its effect on the hemodynamic performance of the valve flow [20]. Thrombosis in the aortic position has been associated with severe symptoms and considered to be infrequent; however, there have been reports of thrombosis at aortic position [21]. In our study, seven patients presented with thrombosis after aortic valve replacement. The number of patients with aortic valve thrombosis was very small to reach to any conclusion regarding reason for thrombosis either in this group or even in all 66 patients in this study. The similar experience has been reported by Kalpana et al. [22]
Once the diagnosis of obstructive prosthetic valve thrombosis is made, the treatment can be in the form of either intravenous thrombolysis or surgical valve replacement [23]. The guidelines pitch for surgical treatment for left-sided prosthetic valve thrombosis, if the function class is low and the fibrinolytic treatment is reserved for sick patients in higher function class [24]. However, the series on successful surgical management of prosthetic valve thrombosis was encouraging, albeit in stable patients [25]. Kothari and colleagues had presented their experience with surgical treatment of prosthetic valve thrombosis with good outcomes [26]. They studied patients who were taken immediately for surgery and compared it with the group where initial thrombolysis was performed. They reported better results with the group in which initial thrombolysis was performed before taking up for surgery. In our series, one patient required operation. He was operated 48 h after failure of thrombolysis and its stoppage. The intent to wait was to reduce postoperative bleeding and complications as reported by Lee et al. [27]. It was possible as the patient was hemodynamically stable. Thrombolysis for treatment of prosthetic valve thrombosis was first reported by Luluaga and coworkers [28]. There always has been a debate over which treatment is more beneficial to the patients. Thrombolysis is quick and can be easily performed even in the setup where surgery may not be possible and thus has a better reach and easy accessibility for the patients [29]. In our series, the preferred treatment was thrombolysis, as all the patients were of obstructive type and had severe symptoms. The result was satisfactory with a mortality of 7.57%.
Short-term follow-up after successful treatment has been reported by Pradhan and colleagues; however, long-term follow-up was not reported [30]. Gupta and colleagues had presented their experience with long-term follow-up of patients managed for prosthetic valve thrombosis [31]. They reported success of more than 80% in all the function classes and an event-free survival of 61.5 and actuarial survival of 85.2% at 5 years following the event. Our study observed a mortality of 22.95% at the mean follow-up of one and a half years. The event of prosthetic valve thrombosis was followed by further repeat of similar events [32–34]. This event, thus, starts the downhill course in patients with valve replacement. The follow-up post thrombolysis also revealed increased gradient across aortic valve and appearance of moderate to severe regurgitation. The regurgitation was observed in 4 instances across aortic and 1 instance across mitral valve. This possibly happened because of underlying pannus on which thrombus would have evolved. These patients were advised reoperation for replacement of malfunctioning valves.
Limitations
This has been an observational study with focus on follow-up of patients presenting with prosthetic valve thrombosis. This study has been limited by its small numbers to comment on or evaluate the reasons for thrombosis. The other limitation was that the mean follow-up was less than 2 years. A longer follow-up is desired.
Conclusion
Thrombolysis for acute prosthetic valve thrombosis is lifesaving with good results. However, during follow-up period, the cohort experienced high mortality rate reaching 22.95% in 2 years. This prompts for regular and frequent follow-up of these patients with further investigations to watch for early signs of heart failure and evaluation for reason of thrombosis in these individuals. These patients should undergo clinical and echocardiographic evaluation 3 monthly for 6 months and then 6 monthly for 2 years, apart from the follow-up required for maintaining the adequate anticoagulation.
Funding
No funding was received.
Compliance with ethical standards:
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
The institute ethics committee granted waiver for informed consent to collect the blinded data used in this study.
Research involving human participants and/or animals:
Not applicable
Ethics Committee approval
Ethical approval was waived by the local Ethics Committee of University in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Footnotes
Discussant
Dr. Abha Chandra
Senior Professor and HOD
Department of Cardiovascular and Thoracic Surgery
Sri Venkateswara Institute of Medical Sciences
Tirupati, 517507 Andhra Pradesh
I would like to congratulate the authors for this study on valve thrombosis in the Indian subsets. It is a very important topic and because of several reasons like financial constraint, which involves omitting anticoagulation and other medicines and unable to do the PT test; diet especially in south India, where the food contains lot of tomatoes and green leafy vegetables; regular follow-ups soon after surgery, we all face this problem in day to day clinical practice. The authors have been able to lay down a protocol for the management of such patients. There are a few queries which need to be discussed:
Discussion
Q1. The diagnosis of the prosthetic valve thrombosis was confirmed by echocardiography (transthoracic) in your study. Do you think that fluoroscopy would have had a beneficial role or an added advantage in these patients?
Ans: Fluoroscopy is an accurate, fast, and most cost-effective method of diagnosing prosthetic valve thrombosis. In our study, the logistics were not feasible considering the acute presentation of the patients. We agree that it should be used more often, specially in emergency room.
Q2. An obstructed prosthetic valve could be because of the pannus growth or thrombus. How were these two differentiated? Was it possible to characterize the pannus and the thrombus by echocardiography?
Ans: The most important feature of a PVT is immobility of either one or both the leaflets. Thrombus has soft echo density with irregular shape and is homogeneous. It is located at hinges or struts. To the contrary, the pannus is echo dense, crescentic, and bright, located on the inflow or outflow areas of the valve.
Q3. Valve thrombosis in aortic position is very rare. In your study there have been seven patients with Aortic valve thrombosis. Were there any particular factors which could be elicited in these patients?
Ans: Aortic valve PVT is less common but not rare. Its incidence is 0.14% per year, but the data is extremely heterogenous [1]. The sample size was too small to comment on the interplay of factors causing this condition. However, reasons like molecular interactions between blood and artificial surface, inadequate anticoagulation or drug interactions reducing the effect of anticoagulants and clinical situations causing stasis of blood like atrial fibrillation, poor LV function etc. have been implicated for prosthetic valve thrombosis [2, 3].
Q4. It is true that thrombolysis would be effective if the patient presents to us early. What was the duration of presentation from the first onset of symptoms in patients where thrombolysis was not effective? Did any of these patients undergo thrombectomy or valve replacement?
Ans: In PVT, patients reach hospital early due to severity of symptoms. In our study, it was an average of 36 hours. Thrombolysis should be initiated immediately after the diagnosis. All but one of the patient responded to thrombolysis. He reported to the hospital after 48 hours of onset of symptoms. However, he also benefitted by stabilization of hemodynamic parameters and symptoms. He underwent a mitral valve replacement, which revealed presence of pannus tissue along with thrombus.
Q5. What was the status of the 42 patients after thrombolysis? Did they have any other event (re-thrombosis) during their follow-up?
Ans: In our study, 12 patients reported back to hospital with re-thrombosis. They were again treated with thrombolysis and 4 patients responded to treatment while 8 others succumbed in that admission. Forty-two patients are under 6 monthly clinical and echo follow-up with no further events.
Q6. Post thrombolysis, there was one patient who had severe Aortic Regurgitation and 3 patients who had moderate Aortic Regurgitation, were these patients considered for surgery, especially the one with severe AR?
Ans: Yes, all of them were advised surgery at the earliest. The aortic regurgitation in these patients is central and thus due to malfunctioning of the mechanical prosthesis. This becomes the reason for re-thrombosis in follow-up as discussed in article by Gürsoy et al. [2]. Hence, moderate regurgitation too becomes an indication of valve replacement. All these patients are awaiting surgery for want of funds.
Q7. What do you think should be the proposed PT and INR values post thrombolysis for aortic and mitral prosthetic mechanical valve in situ?
Ans: AHA guidelines (2014 with focused update of 2017) [4] mention maintenance of INR at 3 for patients with prosthetic valve and additional risk factors for thrombosis (class IB). We follow these with addition of aspirin 75 mg once daily dose.
References
1. Korteland NM, Etnel JRG, Arabkhani B, et al. Mechanical aortic valve replacement in non-elderly adults: meta-analysis and microsimulation. Eur Heart J. 2017;38: 3370–3377.
2. Gürsoy MO, Kalçık M, Yesin M, et al. A global perspective on mechanical prosthetic heart valve thrombosis: Diagnostic and therapeutic challenges. Anatol J Cardiol. 2016;16: 980–989.
3. Nunez-Gil IJ, Alkhouli M, Centola, M, Feltes G, Villablanca P, Ramakrishna H. Analysis of bioprosthetic aortic valve thrombosis— implications and management strategies. J Cardiothorac Vasc Anesth. 2019;33:2853–2860.
4. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. 2017; 10.1016/j.jacc.2017.03.011.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Manjunath CN, Srinivas P, Ravindranath KS, Dhanalakshmi C. Incidence and patterns of valvular heart disease in a tertiary care high-volume cardiac center: a single center experience. Indian Heart J. 2014;66:320–326. doi: 10.1016/j.ihj.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Negi PC, Sondhi S, Asotra S, Mahajan K, Mehta A. Current status of rheumatic heart disease in India. Indian Heart J. 2019;71:85–90. doi: 10.1016/j.ihj.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zühlke L, Engel ME, Karthikeyan G, et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study) Eur Heart J. 2015;36:1115–122a. doi: 10.1093/eurheartj/ehu449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choudhary SK, Talwar S, Airan B. Choice of prosthetic heart valve in a developing country. Heart Asia. 2016;8:65–72. doi: 10.1136/heartasia-2015-010650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tillquist MN, Maddox TM. Cardiac crossroads: deciding between mechanical or bioprosthetic heart valve replacement. Patient Prefer Adher. 2011;5:91–99. doi: 10.2147/PPA.S16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mvondo CM, Pugliese M, Giamberti A, et al. Surgery for rheumatic mitral valve disease in sub-saharan African countries: why valve repair is still the best surgical option. Pan Afr Med J. 2016;24:307. doi: 10.11604/pamj.2016.24.307.7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaneko T, Aranki SF. Anticoagulation for prosthetic valves. Thrombosis. 2013;2013:346752–346754. doi: 10.1155/2013/346752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajaj R, Karthikeyan G, Sinha N, Lokhandwala Y, Rao D, Kaushik SK, Jain SL, Narayanan S, Seth A, Satyamurthi I, Sawhney JPS, Saran RK, Sharma S, Haridas KK, Gohkroo RK, Omar AK, Dwivedi SK, Modi S, Kapur KK, Dalvi B, Bharani A, Wander GS, Venugopal K, Mahant TS. CSI consensus statement on prosthetic valve follow up. Indian Heart J. 2012;64:S3–S11. doi: 10.1016/j.ihj.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. Antithrombotic and thrombolytic therapy for valvular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e576S–e600S. doi: 10.1378/chest.11-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gürsoy MO, Kalçık M, Yesin M, Karakoyun S, Bayam E, Gündüz S, Özkan M. A global perspective on mechanical prosthetic heart valve thrombosis: diagnostic and therapeutic challenges. Anatol J Cardiol. 2016;16:980–989. doi: 10.14744/AnatolJCardiol.2016.7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma WG, Hou B, Abdurusul A, Gong DX, Tang Y, Chang Q, Xu JP, Sun HS. Dysfunction of mechanical heart valve prosthesis: experience with surgical management in 48 patients. J Thorac Dis. 2015;7:2321–2329. doi: 10.3978/j.issn.2072-1439.2015.12.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salamon J, Munoz-Mendoza J, Liebelt JJ, Taub CC. Mechanical valve obstruction: review of diagnostic and treatment strategies. World J Cardiol. 2015;7:875–881. doi: 10.4330/wjc.v7.i12.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montorsi P, De Bernardi F, Muratori M, Cavoretto D, Pepi M. Role of cine-fluoroscopy, transthoracic, and transesophageal echocardiography in patients with suspected prosthetic heart valve thrombosis. Am J Cardiol. 2000;85:58–64. doi: 10.1016/S0002-9149(99)00607-4. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Garg N, Tewari S, Kapoor A, Goel PK, Sinha N. Role of thrombolytic therapy for stuck prosthetic valves: a serial echocardiographic study. Indian Heart J. 2001;53:451–457. [PubMed] [Google Scholar]
- 15.Hermans H, Vanassche T, Herijgers P, Meuris B, Herregods MC, van de Werf F, Verhamme P. Antithrombotic therapy in patients with heart valve prostheses. Cardiol Rev. 2013;21:27–36. doi: 10.1097/CRD.0b013e3182638578. [DOI] [PubMed] [Google Scholar]
- 16.Duran NE, Biteker M, Ozkan M. Treatment alternatives in mechanical valve thrombosis. Turk Kardiyol Dern Ars. 2008;36:420–425. [PubMed] [Google Scholar]
- 17.Foley PWX, Sharma R, Kalra PR. Beware of prosthetic valve thrombosis despite therapeutic anticoagulation. Emerg Med J. 2007;24:e18. doi: 10.1136/emj.2006.042887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonou M, Lampropoulos K, Barbetseas J. Prosthetic heart valve obstruction: thrombolysis or surgical treatment? Eur Heart J Acute Cardiovasc Care. 2012;1:122–127. doi: 10.1177/2048872612451169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kannan A, Jahan K, Lotun K, Janardhanan R. Prosthetic mitral valve obstruction: role of real-time three-dimensional transesophageal echocardiography in diagnosis. BMJ Case Rep. 2015. 10.1136/bcr-2014-208243. [DOI] [PMC free article] [PubMed]
- 20.Ha H, Koo HJ, Huh HK, Kim GB, Kweon J, Kim N, Kim YH, Kang JW, Lim TH, Song JK, Lee SJ, Yang DH. Effect of pannus formation on the prosthetic heart valve: in vitro demonstration using particle image velocimetry. PLoS One. 2018;13:e0199792. doi: 10.1371/journal.pone.0199792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lengyel M, Fuster V, Keltai M, Roudaut R, Schulte HD, Seward JB, Chesebro JH, Turpie AGG. Guidelines for management of left-sided prosthetic valve thrombosis: a role for thrombolytic therapy. J Am Coll Cardiol. 1997;30:1521–1526. doi: 10.1016/S0735-1097(97)00345-8. [DOI] [PubMed] [Google Scholar]
- 22.Kalpana SR, Bharath G, Jain S, Moorthy N, Manjunath SC, Christopher R. Prosthetic valve thrombosis - association of genetic polymorphisms of VKORC1, CYP2C9 and CYP4F2 genes. Medicine (Baltimore) 2019;98:e14365. doi: 10.1097/MD.0000000000014365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roudaut R, Serri K, Lafitte S. Thrombosis of prosthetic heart valves: diagnosis and therapeutic considerations. Heart. 2007;93:137–142. doi: 10.1136/hrt.2005.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B. ACC/AHA practice guidelines for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. JACC. 2006;48:598–675. doi: 10.1016/j.jacc.2006.05.030. [DOI] [Google Scholar]
- 25.Ahn H, Kim KH, Kim KC, Kim CY. Surgical management of mechanical valve thrombosis: twenty-six years’ experience. J Korean Med Sci. 2008;23:378–382. doi: 10.3346/jkms.2008.23.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kothari J, Patel K, Brahmbhatt B, Baria K, Talsaria M, Patel S, Tailor S. Redo mitral valve replacement for prosthetic valve thrombosis: single center experience. J Clin Diagn Res. 2016;10:PC01–PC03. doi: 10.7860/JCDR/2016/20209.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KF, Mandell J, Rankin JS, Muhlbaier LH, Wechsler AS. Immediate versus delayed coronary grafting after streptokinase treatment. J Thorac Cardiovasc Surg. 1988;95:216–222. doi: 10.1016/S0022-5223(19)35781-2. [DOI] [PubMed] [Google Scholar]
- 28.Luluaga IT, Carrera D, D’Oliveira J, Cantaluppi CG, Santin H, Molteni L, Ferreira R, Zwolinski E, de Luluaga Successful thrombolytic therapy after acute tricuspid-valve obstruction. Lancet. 1971;1:1067–1068. doi: 10.1016/S0140-6736(71)91627-8. [DOI] [PubMed] [Google Scholar]
- 29.Araiza-Garaygordobil D, Aguilar-Rojas LA, Mendoza-García S, Barajas-Campos RL, Casal-Alonso S, Briseño-de-la-Cruz JL, Arias-Mendoza A. Thrombolytic treatment for acute prosthetic valve thrombosis: is it better than surgery? J Cardiol Cases. 2017;16:162–164. doi: 10.1016/j.jccase.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pradhan A, Bhandari M, Gupta V, Vishwakarma P, Sethi R, Narain VS, Chaudhary G, Chandra S, Dwivedi S. Short-term clinical follow-up after thrombolytic therapy in patients with prosthetic valve thrombosis: a single-center experience. Cardiol Res. 2019;10:345–349. doi: 10.14740/cr924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta D, Kothari SS, Bahl VK, Goswami KC, Talwar KK, Manchanda SC, Venugopal P. Thrombolytic therapy for prosthetic valve thrombosis: short- and long-term results. Am Heart J. 2000;140:906–916. doi: 10.1067/mhj.2000.111109. [DOI] [PubMed] [Google Scholar]
- 32.Reddy NK, Padmanabhan TN, Singh S, et al. Thrombolysis in left- sided prosthetic valve occlusion: immediate and follow-up results. Ann Thorac Surg. 1994;58:462–470. doi: 10.1016/0003-4975(94)92229-2. [DOI] [PubMed] [Google Scholar]
- 33.Martinell J, Jimenez A, Rabago G, Artiz V, Fraile J, Farre J. Mechanical cardiac valve thrombosis: is thrombectomy justified? Circulation. 1991;84:III70–III75. [PubMed] [Google Scholar]
- 34.Antunes MJ. Fate of thrombectomized Bjork-Shiley valves. J Thorac Cardiovasc Surg. 1986;92:965–966. doi: 10.1016/S0022-5223(19)35861-1. [DOI] [PubMed] [Google Scholar]