Abstract
Clinical reports of neurological manifestations associated with severe coronavirus disease 2019 (COVID-19), such as acute ischemic stroke (AIS), encephalopathy, seizures, headaches, acute necrotizing encephalitis, cerebral microbleeds, posterior reversible leukoencephalopathy syndrome, hemophagocytic lymphohistiocytosis, peripheral neuropathy, cranial nerve palsies, transverse myelitis, and demyelinating disorders, are increasing rapidly. However, there are comparatively few studies investigating the potential impact of immunological responses secondary to hypoxia, oxidative stress, and excessive platelet-induced aggregation on the brain. This scoping review has focused on the pathophysiological mechanisms associated with peripheral and consequential neural (central) inflammation leading to COVID-19-related ischemic strokes. It also highlights the common biological processes shared between AIS and COVID-19 infection and the importance of the recognition that severe respiratory dysfunction and neurological impairments associated with COVID and chronic inflammation [post-COVID-19 neurological syndrome (PCNS)] may significantly impact recovery and ability to benefit from neurorehabilitation. This study provides a comprehensive review of the pathobiology of COVID-19 and ischemic stroke. It also affirms that the immunological contribution to the pathophysiology of COVID-19 is predictive of the neurological sequelae particularly ischemic stroke, which makes it the expectation rather than the exception. This work is of fundamental significance to the neurorehabilitation community given the increasing number of COVID-related ischemic strokes, the current limited knowledge regarding the risk of reinfection, and recent reports of a PCNS. It further highlights the need for global collaboration and research into new pathobiology-based neurorehabilitation treatment strategies and more integrated evidence-based care.
Keywords: COVID-19, stroke, ACE2, cytokines, pathogen associated molecular pattern, post Covid19 neurology syndrome, PCNS
Introduction
Since the first case of coronavirus disease 2019 (COVID-19) infection was reported in Wuhan, China in December 2019, a significant number of thrombotic complications affecting the venous and arterial systems have been published in the literature (1–3), with the World Stroke Organization now recognizing that acute ischemic stroke (AIS) increases the severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) viral infection by 2.5-fold (4, 5). Similar associations between systemic infections, inflammation, and AIS are longstanding (6). However, to date, few reports are reviewing the molecular bases of the peripheral and central mechanisms induced by SARS-CoV2 infection and potential neurological manifestations with focused attention on AIS.
Neurological manifestations of COVID-19 infection were first described by Mao et al. who observed six cases of acute cerebrovascular disease (2). Subsequently, 19 cases of COVID-19-related strokes particularly affecting the young and involving medium to large arteries were reported from a tertiary center in New York (3), highlighting the need for better understanding of the potential mechanisms linking COVID-19 and AIS.
Stroke has been associated with other earlier coronavirus infections. In 2002, AIS was first detailed by Umapathi et al., in 4 of 206 patients who presented with large vessel occlusion associated with SARS-CoV infection in Singapore in 2002 (7). Two other patients with AIS were also described in Middle East respiratory syndrome corona virus (MERS-CoV)-infected patients in Saudi Arabia during the 2015 epidemic (8).
SARS-CoV2 is known to initially bind to the angiotensin-converting enzyme 2 (ACE2) receptors of epithelial and endothelial cells where an immediate immunological activation occurs that can, in severe cases, eventually lead to hypercoagulability or thrombophilia and increased tendency of clots forming in the blood and potentially AIS (9). However, ŧhere is limited information on the physiological abnormalities and mechanisms linking COVID-19 and AIS, although a number of mechanisms have been proposed. The major mechanisms that have been proposed to date include systemic innate immunity-mediated hyperinflammation, neurovascular endothelial dysfunction, endotheliitis, central nervous system renin–angiotensin–aldosterone system (RAAS) dysregulation, oxidative stress, and excessive platelet aggregation (10–12). Thus, this scoping review aimed to elucidate potential pathophysiological mechanisms predisposing patients with COVID-19 to a higher risk for neurovascular events.
Methodology
The authors of this scoping review used the Arksey and O'Malley methodology to identify and extract useful literature (13). The steps undertaken include (1) research questions identification; (2) relevant literature identification; (3) screening and selection of relevant literature; (4) data charting; and (5) analyzing, summarizing, and reporting the results.
Identification and Development of the Research Question
This focused on the general research question: “Are there mechanisms associated with COVID-19 infection that are likely to predispose patients to ischemic stroke?”
The following are the specific areas of interest:
a. Can current understanding of the immunological mechanisms associated with the inflammatory responses to severe COVID-19 potentially predispose a patient to AIS?
b. Are there other potential pathophysiological mechanisms predisposing COVID-19 patients to upregulate procoagulable mechanisms leading to thrombi formation and potentially AIS?
Relevant Literature Identification
A literature review of articles regarding cases and mechanisms underlying the co-occurrence of COVID-19-and AIS, published in English between January 2000 and August 12th, was carried out up to August 15th, 2020. Only studies (qualitative and quantitative studies, systematic reviews, metanalysis, case reports, and case series) that directly or indirectly link pathophysiological mechanisms to ischemic stroke and COVID-19 infection have been included in the final review (see Table 1 detailing the studies).
Table 1.
Summary of the main studies on the mechanisms of COVID-19-related strokes.
| Publication date | Author | Type of study | Objectives | Conclusion |
|---|---|---|---|---|
| July 18, 2020 | Kempuraj et al. (14) | Review article | To understand the relationship between COVID-19 infection and the neuroinflammatory responses in the brain, especially concerning psychological stress, mast cell activation, and cytokine storm–associated responses. | COVID-19 19 can worsen neuroinflammation thru activation of mast cells, neurons, astrocytes, microglia, endothelial cells, and increase inflammatory cytokine and chemokine levels in the CNS |
| June 26, 2020 | van den Berg et al. (15) | Review article | To review the role of NLRP3 inflammasome dysregulation in the severe COVID-1919 infection. | NLRP3 inflammasome and its associated pathways plays an important role in severe COVID-19 19 infection especially in patients with impaired immune function and predisposes patients with pre-existing suboptimal immune responses to poor outcomes. |
| June 19, 2020 | Rodrigues-Diez et al. (16) | Review article | To discuss the potential benefits of statins in COVID-1919 infection. | Statins could reduce viral replication by autophagy activation and exert anti-inflammatory properties particularly on NF-κB and NLRP3 inflammasomes. It can also modulate coagulation responses. |
| April 14, 2020 | Zuo et al. (17) | Prospective Cohort study | To assess the role of neutrophil extracellular traps (NETs) in COVID-19 19 infection and identify the relationship to severity of illness. | High levels of NETS in patients with COVID-19 19 may contribute to cytokine release and respiratory failure. |
| June 2, 2020 | Tomar et al. (17) | Review article | To identify the role of enhanced neutrophil infiltration and the release of NETs, complement activation and vascular thrombosis during necroinflammation in COVID-19 | NET formation induces production of proinflammatory cytokines leading to tissue inflammation responsible for cytokine storm and sepsis. |
| June 29, 2020 | Middleton et al. (18) | Prospective Cohort study | To assess the role of neutrophil extracellular traps (NETs) in COVID-19 19 infection and identify the relationship to severity and progression of illness. | Neutrophils of COVID-19 19 patients displayed excessive NET at baseline and was blocked by neonatal NET-Inhibitory factor. Levels also correlate with intubation and death. |
| May 2010 | Hermus et al. (19) | Review article | To identify the biomarkers associated with carotid plaque formation | Various biomarkers linked to inflammation, lipid accumulation, thrombosis and angiogenesis have been related to plaque formation and vulnerability. |
| July 2020 | Mohamud et al. (20) | Case series | To describe six cases of COVID-19 positive patients with associated intraluminal carotid artery thrombus. | Inflammation related to COVID-19 19 may lead to plaque rupture of previously known vulnerable atheroma leading to thrombosis an ischemic stroke. |
| June 27, 2020 | Ueland et al. (21) | Letter to the editor/prospective Cohort study | To identify relationship of plasma markers reflecting inflammation and fibrosis and respiratory failure in hospitalized COVID-19 patients. | Methyl-metalloproteinases (MMP) may be an early indicator of respiratory failure in COVID-19 patients and underscore the role ECM remodeling and fibrosis in this disorder. |
| June 5, 2020 | Solun et al. (22) | Review article | To review the role of matrix MMP and the kinin-kallikrein system (KKS) in the pathomechanism associated with COVID-19 19 related acute lung injury. | Overexpression of MMP results in acute lung injury and remodeling among COVID-19 19 patients and the use of its inhibitor may be a potential therapeutic strategy. |
| June 29, 2020 | Schönrich et al. (23) | Review article | To review the role of overproduction of reactive oxygen species (ROS) in local and systemic tissue damage associated with COVID-19 19 infection. | ROS increases the formation of NETs and suppresses the adaptive immune system. |
| May 15, 2020 | Wright et al. (24) | Prospective Cohort | To determine the correlation between thromboelastography measurements of coagulation and thromboembolic events in COVID-19 19 patients. | Failure of clot lysis at 30 min on thromboelastography is predictive of thromboembolic events in critically ill COVID-19 19 patients. |
| July 17, 2020 | Kunutsor et al. (25) | Meta-analysis | To identify the association of CRP and VTE risk. | Elevated CRP is associated with greater VTE risk, consistent with a linear dose–response relationship. |
| July 9, 2020 | Zhang et al. (26) | Cross-sectional study | To determine the coagulation profiles of routine hemostasis tests, natural anticoagulants, coagulant factors and antiphospholipid antibodies in critically ill COVID-19 patients. | The low activities of natural anticoagulants, elevated factor VIII level and the presence of antiphospholipid antibodies, together, may contribute to the etiopathology of coagulopathy in COVID-19 patients. |
| June 11, 2020 | DiNicolantonio et al. (27) | Review article | To identify the role of endothelial tissue expression of tissue factor in COVID-19 infection | SARS-CoV-2 infection of endothelial cells evokes the expression of TF which is contingent on endosomal NADPH oxidase activation. |
| June 9, 2002 | Bautista-Vargas et al. (28) | Review article | To identify the role of tissue factor (TF) in the hypercoagulability associated with COVID-19 19 infection. | TF may be a critical mediator associated with the development of thrombotic phenomena in COVID-19. |
| September 2015 | Saha et al. (29) | Review article | To identify the mechanisms associated with tissue factor production and atherothrombosis. | TF is essential in the initiation of the extrinsic pathway of the coagulation cascade and appears to be a critical determinant of atherosclerotic plaque thrombogenicity. |
| December 5, 2002 | Akerström et al. (30) | Experimental study | To identify the role of nitric oxide in SARS-CoV infection. | SARS-CoV infection inhibits viral replication by affecting RNA replication and reduction in expressed spiked protein production. |
| July 2020 | Cheng et al (31) | Review article | To identify the correlation between angiotensin-converting enzyme 2 (ACE2) and severe risk factors for coronavirus disease 2019 | ACE2 is an essential part of the RAS, and it has extensive vascular and organ protection functions in hypertension, diabetes, cardiovascular disease, and ARDS |
| May 7, 2020 | Hess et al. (32) | Review article | To identify the role of ACE in COVID-19 related strokes. | Binding to and depletion of ACE2 may tip the RAS balance in favor of the ACE-1-angiotensin II-AT1 axis and contribute to endothelial dysfunction, organ damage, and stroke. |
| August 22, 2008 | McColl et al. (6) | Review article | To explore the impact of systemic infections and inflammation on stroke susceptibility | The role of systemic infections and inflammation in stroke (onset of the stroke as well as post stroke outcome) |
| July 2, 2020 | Ellul et al. (33) | Review article | To identify the clinical manifestation of neurological disorders caused by COVID-19 | The wide assortment of neurological disorders affecting 901 patients by May 2020 (at the time of the review) |
| August 7, 2019 | Albensi B. C. (34) | Review article | To explore the importance and science around NF-?B activity with a view to correlate this with COVID-19 and immune involvement | NF-?B is an important transcription factor with critical roles in mitochondrial function, apoptosis, mechanisms of disease in COVID-19 and brain involvement as well |
| September 3, 2020 | Wijeratne et al. (35) | Review article | To identify the role of inflammation affecting vascular systems as well as the importance of NLR as a potential biomarker in this context | NLR is a useful and easily available biomarker in atheromatous vascular disease supporting the same role in COVID-19 and large vessel disease |
| June 2020 | Boldrini et al. (36) | Special article | To identify the impact of COVId-19 outbreak on rehabilitation services in Italy | Increasing pressure from the acute services to transfer the patients to rehabilitation services while the challenges to provide rehabilitation services in the outpatient as well as home settings due the restrictions |
| June 25, 2020 | Varatharaj et al. (37) | Research paper (nationwide, cross speciality surveillance study) | To identify the epidemiology of acute neurological and psychiatric complications of COVID-19 as reported by the national registry in UK. | One hundred and Twenty five patients with complete data set noted 57 had an ischemic stroke (74%), nine had intracerebral hemorrhage (12%) and one with (1%) CNS vasculitis. Thirty nine patients (31%) had altered mental status while 69% had a neuropsychiatric syndrome. |
| July 20, 2020 | Spence et al. (11) | Review | To identify the mechanisms of stroke in COVID-19 | Vasculitis, hypercoagulability, endothelialinjury, microvascular thrombosis, cytokine storm, systemic hypoxia, fresh DVT, cardiac alterations |
| September 10, 2020 | Wijeratne et al. (38) | Case Report and review | 1.To correlate the CRP, D-dimer levels, NLR ratio changes with variety of clinical events at the intensive care unit and neurorehabilitation ward. 2. To explore the utility of bedside tablet use such as MRF visual fields and Lee-Rayan eye-hand coordination test during neurorehabilitation | The in-depth case study illustrates the significant of close monitoring of simple blood tests such as CRP, NLR with predicable ability of clinical worsening through fast recognition of pattern changes. |
| June 26, 2020 | South et al. (39) | Review | To identify the risk of stroke in the context of preceding infection, including COVID-19 | SARS-CoV-2 global pandemic is not the first viral infection to be linked with stroke. The current pandemic is an ideal opportunity to acquire valuable insight toward the relationship between stroke and infections |
| September 2020 | Sivan et al. (40) | Short communication (research paper) | To explore the authors, work on development of an integrated rehabilitation pathway with systematic, efficient tele-rehabilitation model | COVID-19 Yorkshire Rehabilitation Screen(C19-YRS) (previously developed telephone screening tool) can be incorporated toward an efficient, systematic, telerehabilitation pathway |
Screening and Selection of Relevant Literature
In the first step, MEDLINE, Cochrane, and Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases were searched to identify useful keywords. Subsequently, the identified keywords were used to search the same databases for relevant studies. The literature was first screened at the title and the abstract level; then, full-text articles were assessed for eligibility. The manual bibliographic search of identified studies was also done in the last step of the literature search. The following keywords were used: COVID-19, coronavirus, mechanism, inflammation, thrombosis, embolism, endotheliitis, arteritis, neuroinflammation, and ACE2 receptors. Two researchers (CS and TW) reviewed relevant articles independently. Any disagreement for inclusion was resolved by a third author (LK).
Data Charting and Analysis
The included studies were charted, and the following parameters were taken into account: publication year, type of study, aims and objectives of the study, and study findings and conclusion. Included studies were analyzed extensively and are listed in Table 1.
Results and Analysis
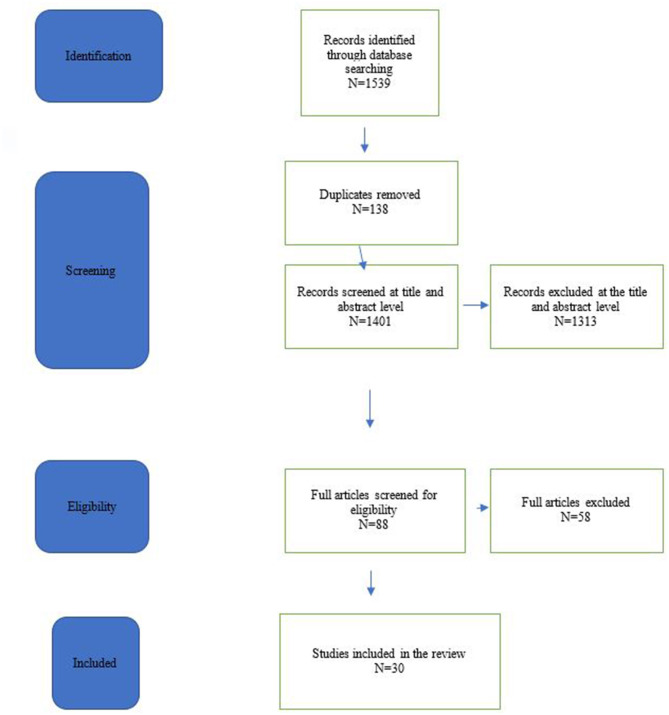
A total of 1,539 studies were identified in the electronic search. After removal of duplicates and screening at the title level, 230 articles were further reviewed at the abstract level, yielding 88 articles requiring assessment. Fifty-eight further articles were excluded, as these papers were not addressing the predefined research questions. A thorough review by two authors (CS and TW) deemed these papers to contain secondary information with repetition rather than an original contribution to the research questions. A total of 30 articles were included in the study. The details of the said studies are outlined in Table 1. The search process is summarized in a Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) in Figure 1.
Figure 1.
Prisma chart.
At the time of this submission (as of September 5th, 2020, WHO situation report), the global number of confirmed SARS-CoV2 cases were 26,171,112 with 865,154 confirmed deaths with a mortality rate of ~3.3%. It is also important to note that only a small proportion of COVID-19-infected individuals progress to severe disease, and of these, a smaller number experience stroke and/or death. The most likely predisposing factors to serious COVID-19 disease states are age, sex, and immune system inability to deal with environmental infection together with genetic factors, and associated cardiovascular risk comorbidities such as hypertension, diabetes, obesity, neurological disorders, and medication interactions. Indeed, it appears that exaggerated immune responses to infection and chronic inflammation may be the key factor in the pathogenesis of severe COVID-19 and associated cerebrovascular complications.
Inflammatory Mechanisms of COVID-19
Initial Inflammatory Responses (Key References Are in Table 1; at the End of the Paper)
It is currently accepted that the SARS-CoV2 virus, like other coronaviruses, attacks host cells by binding to ACE2 (12, 41–43). Initial virus recognition occurs via the epithelial cells of the olfactory and respiratory tract of the infected person (39, 43) and would be expected to immediately activate the innate immune system of individual cells and the associated vascular supply (44). The cells within the respiratory epithelia are assumed to release the first set of cytokines such as tumor necrosis factor (TNF) and interleukin-6 (IL-6) (45) that then activate further proinflammatory macrophages and monocytes to accumulate in the alveoli.
The recruited monocytes and macrophages are a further rich source of cytokines and monocyte chemoattractant protein-1 with an additional army of immune-related cells coming to play a major component (45), as described in Tables 2, 3. At this stage, the majority of individuals will be asymptomatic, while some may experience flu-like symptoms, sore throat, myalgia, diarrhea, fever and headache, etc.
Table 2.
Pathophysiology of COVID-19 and acute ischemic stroke.
| Pathophysiology of COVID-19 and Acute Ischemic Stroke—Chronological Order |
|---|
| 1. Binding of SARS-CoV-2 to ACE-2 on the surface of alveolar pneumocytes (ACE2 is the receptor for host cell entry of SARS-CoV-2. ACE-2 is widely expressed on the alveolar epithelial cells, endothelial cells, enterocytes of the small intestine and arterial smooth muscle cells) (46) |
| 2. Viral infection activates intracellular pattern recognition receptors (PRRs) in the host. PRRs sense pathogen associated molecular patterns (PAMPs) [not this is very similar to pathophysiology of AIS where acute ischemia induced damage associated molecular patterns (DAMPs) perform exactly the same task] (10, 47–49) |
| 3. PAMPs sets off cytolytic immune responses with type one interferon and natural killer cells (NK) with aim of removing the pathogens (50) |
| 4. Endothelial activation secondary to direct viral activation |
| 5. Innate immune response with the recruitment of macrophages, neutrophils, and monocytes to the alveoli. |
| 6. Further innate immune reaction with T helper cells 1&17 T cells and ongoing recruitment of monocytes, macrophages and neutrophils (51) |
| 7. Endothelial activation secondary to direct viral action |
| 8. Endothelial activation secondary to persistent inflammation |
| 9. Activation of cell adhesion molecules and further recruitment of T cells, monocytes and macrophages |
| 10. Release of tissues factors from the activated endothelium |
| 11. Recruitment of intravascular neutrophils |
| 12. Release of neutrophil extracellular traps (NET) from the activated endothelium |
| 13. Release of von Willebrand factor (vWF) and micro thrombosis (52) |
| 14. Maladapted innate immune response and procoagulant activity lead to systemic cytokine rise |
| 15. Increased platelet aggregation and thrombo-embolism |
| 16. Activation of cell adhesion molecules and ongoing recruitment of monocytes, macrophages and neutrophils within the cerebral blood vessels |
| 17. Endothelial activation in cerebral blood vessels |
| 18. Endothelial dysfunction in the brain |
| 19. Local immune and inflammatory activity in the brain |
| 20. Disruption of the blood-brain barrier |
| 21. Activation of microglia in the brain |
| 22. Further recruitment of resident immune cells in the brain |
| 23. Increased inflammatory activity in the brain |
| 24. Micro-thrombosis, thrombo-embolism in the brain |
| 25. Hypoperfusion of cerebral tissues |
| 26. Acute Ischemic stroke |
Attempts have been made to assimilate all available current evidence to formulate this table. However, we are aware the pandemic is still evolving, and additional molecular mechanisms and pathways are likely to be discovered as time goes by.
Table 3.
Summary of Pathophysiological mechanisms of COVDI-19 related Acute Ischemic Strokes.
| Inflammatory mediators of ischemic stroke | The role of cytokines | • First released by the respiratory epithelium (45) • Further released by T lymphocytes activates other cellular mediators mediating secondary hemophagocytic lymphohistiocytosis (58, 59) |
| NLRP3 inflammasomes | • Activated by viroporin protein 3a (63–65) • Drives production of inflammatory cytokines, pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) (63) • May induce plaque instability by overdriving response of cellular mediators such as macrophages, neutrophils, lymphocytes and vascular smooth muscle cell (70–72) |
|
| Inflammation-induced plaque vulnerability | • Predominance of T cells, macrophages and neutrophils populating an atheromatous plaque leading to plaque rupture (73) • Characterized by elevation of proteolyic biomarkers such as metalloproteinases (MMPs) and cathepsin cysteine proteases (CCP's) (19, 21, 22, 75) |
|
| Oxidized Low-Density Lipoproteins (oxLDL) | • A marker of increased oxidative stress (23) • COVID-19-related disruption of the receptor-mediated uptake of oxLDL (78) |
|
| Neutrophil extracellular traps (NETs) | • Networks of chromatin, proteins and oxidant enzymes that protrude from membranes of activated neutrophil and mediate infection containment (17) • Also a marker of inflammation-related thrombosis (17, 25, 35, 101–106) |
|
| Coagulatory disfunction | D-dimer | • Mediates immunologic defense systems resulting in thrombi formation (107) • Increased level is a biomarker of fibrinolytic shutdown leading to massive fibrin formation resulting in thrombosis (108). |
| Natural anticoagulants and antiphospholipid antibodies | • COVID-19 induced decrease in the amounts of physiological anticoagulants and increased levels of coagulant factors and antiphospholipid antibodies (26, 109, 110) | |
| Endotheliopathy | Endothelium-driven activation of the extrinsic coagulation system | • Imbalance in the ACE2 and angiotension II (AT-II) receptors leads to the upregulation of tissue factor (27, 28) • Tissue factor interacts with Factor VII to activate the extrinsic coagulation system (27, 28) |
| Endothelium-driven nitrous oxide deficiency | • COVI-19 related endotheliopathy results in the suppression of nitric oxide synthase (NOS), resulting in nitric oxide deficiency (30) • Results in loss of vasodilatory effect and promotes adhesion of platelets and leukocytes to the vessel wall (111) |
|
| Renin Angiotensin Aldosterone System (RAAS) and ACE-2 deficiency | Alterations in the balance between the classical RAAS and the ACE2 pathways | • Promotes organ damaging effects of the classical RAAS pathway (112) • Promotes overactivity of the sympathetic nervous system resulting in the exacerbation of traditional stroke risk factors (113) |
The intrinsic pathogenicity of severe coronavirus infection in vulnerable people ensures that the acute severe inflammatory response to the COVID-induced respiratory distress results in decreased levels of circulating lymphocytes, secondary hemophagocytic lymphohistiocytosis (HLH) (46–50) shifts immune defenses toward natural killer (NK) and circulating macrophages (macrophage activation syndrome), and increased neutrophils. An early and important mediator to this phenomenon is the significant elevation of proinflammatory cytokines that fuel various processes in cerebrovascular ischemia (Tables 2, 3). Indeed, sepsis-induced stimulation of the immune system leads to a clonal expansion of antigen-specific T lymphocytes, which result in the further release of proinflammatory cytokines and activation of cells such as macrophages and cytotoxic T lymphocytes, which are otherwise known as the HLH (51, 52), and are thought to be caused by a dysfunction of the normal regulatory mechanisms (52). As a result of the build-up of proinflammatory immune responses, an imbalance favoring the mass increase in neutrophil to leukocyte ratio, and inflammatory cytokines such as TNF, interferon-γ, IL-1, IL-6, IL-18, and IL-33 propels global inflammatory activity (52–55), which is known to lead to multiorgan dysfunction among critically ill patients (45).
Nod-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasomes play an important role in innate immunity and are directly activated by the virus itself via the small viral protein, viroporin protein 3a, that is known to modify cellular membranes and facilitate virus release from host-infected cells (56–58). This multiprotein complex in the cytosol drives a cascade of reactions resulting in the formation of proinflammatory cytokines such as interleukin-1β (IL-1β), interleukin-18 (IL-18), pathogen-associated molecular patterns (PAMPs), and damage-associated molecular patterns (DAMPs), which are usually tightly regulated in patients with normal immune systems (56). Animal studies related to SARS-CoV infection have demonstrated that the overactivation of the NLRP3 inflammasome contributes to the significantly increased virulence and the high incidence of acute lung injury (59, 60). This is also one of the rationales behind the Greek Study in the Effects of Colchicine in COVID-19 Complications Prevention (GRECCO) trial, which is investigating the utility of colchicine, as a non-specific inhibitor of the NLRP3 inflammasome, in the treatment of COVID-19 infection (61, 62). By inhibiting this pathway along with nuclear factor kappa B (NF-KB) inhibition of the innate immune response (63), statins have also been shown to be potentially beneficial for patients with COVID19 infection via intervention through this pathway (64).
In patients with stroke, it has been shown that the NLRP3 inflammasome plays a significant role in cerebral atherogenesis by similar activation of the immune system and increase in macrophages, neutrophils, lymphocytes, and vascular smooth muscle cell, which also play an important role in plaque instability (65–67). The neuronal cell death, which can be attributed to the NLRP3 inflammasome activation, has also been shown to be reversible with immunoglobulin in 3-month-old mice stroke models (65). However, in patients with impaired immune responses, such as the elderly, there is an unprecedented activation of the NLRP3 inflammasome coupled with mitochondrial dysfunction and increased proportions of the mitochondrial reactive oxygen species, which further worsen tissue damage and initiate cell death (66). In patients with obesity and diabetes in which NLRP3 inflammasomes are already basally activated, and the immune responses are suboptimal, viral-induced activation of the former may worsen a preexisting chronic inflammatory state (67). Hence, this factor may be related to the poor outcomes of elderly patients with COVID-19 and stroke and with traditional cardiovascular risk factors such as obesity and diabetes (10).
Inflammation-Induced Plaque Progression and Vulnerability
COVID-19 inflammation induces a prothrombotic milieu among patients who are at risk of vascular events and those with the pre-existing atheromatous disease. Among patients with coronary disease, evidence suggests that virally induced inflammatory infiltrates such as T cells, macrophages, and neutrophils populate the atheromatous plaque leading to a cascade of events including vascular permeability, endothelial disruption, and exposure of prothrombotic elements such as collagen, tissue factor, and platelet adhesion molecules, which all play a role in thrombogenesis (68). Furthermore, it is known that carotid artery plaques with features of a thin fibrous cap, large core lipid, intraplaque bleeding, and the abundance of monocyte-derived macrophages and activated smooth muscle cells cause instability and vulnerability to plaque rupture (69). The presence of proteolytic enzymes such as metalloproteinases (MMPs) and cathepsin cysteine proteases (CCPs), which are secreted by activated macrophages, promote degradation of the extracellular matrix and plaque fragility and potentially result in thromboembolism (16, 34) while making acute ischemic stroke a likely event in the potential trajectories of patients with systemic COVID-19 infection.
Indeed Mohamud et al. have described five cases of acute ischemic stroke associated with an intraluminal carotid artery thrombus with concomitant COVID-19 symptoms 0–14 days before the onset of stroke (70). A proposed mechanism of this co-occurrence is inflammation-related plaque rupture as manifested by the elevated levels of inflammatory biomarkers such as lactate dehydrogenase, C-reactive protein, ferritin, D-dimer, and interleukin (70). Another study reported a case of symptomatic intracranial stenosis in a confirmed COVID-19 patient in a hyperinflammatory state, as evidenced by elevated acute inflammatory biomarkers (71). A similar hyperimmune trend was described in another patient with symptomatic posterior circulation stenosis and concomitant stroke (72). While these biomarkers are not specific for plaque rupture, there is evidence to suggest tha tplaque-rupture-specific MMPs implicate temporal association with the onset of acute respiratory failure among COVID-19 patients (73). Moreover, it has been proposed that aprotinin, a protease inhibitor that inhibits MMPs, may provide benefit for patients with COVID-19-related acute respiratory distress syndrome in experimental studies (74). However, at least in severe cases, the use of aprotinin to inhibit clot breakdown may need to be cautioned if the likelihood of COVID-19 inducing a proinflammatory hypercoagulation response to the SARS-CoV2 virus is considered.
Another putative mechanism that can lead to plaque progression in patients with COVID-19 infection is the disruption of the receptor-mediated uptake of oxidized low-density lipoproteins (oxLDL) by the monocyte-derived macrophages with exposure to proinflammatory stimuli (19). Indeed, animal and human studies indicate that increased expression of lectin-like oxidized lipoprotein 1 receptor (LOX-1) is a risk factor for stroke and promotes restenosis among patients with preexisting cardiovascular disease (75). It is also evident that COVID-19 infection results in a disproportionate increase in reactive oxygen species, which translate to lipid oxidation and increased oxLDL levels (20). Thus, this is presumably another one of the reasons why elderly patients with preexisting cardiovascular risk have a higher propensity of developing severe COVID-19 illness (76) and AIS.
The Role of Peripheral Biomarkers to COVID-19 That Are Also Characteristic of Ischemic Stroke
The pathognomonic features of sepsis-induced inflammation among patients with coronavirus infection is manifested peripherally as neutrophilia in combination with marked lymphopenia (77). This is characterized by the marked increase in various inflammatory biomarkers such as the neutrophil to lymphocyte ratio (NLR), C-reactive protein, and serum ferritin (21–23, 78–84). In a prospective cohort study involving patients with concomitant COVID-19 and ischemic stroke, 9 of the 10 patients have elevated NLR (85). Similarly, the majority of AIS patients presented recently (86, 87) and in various COVID-19 and AIS case series has reported increased NLR values (72, 88–91). A significant degree of lymphopenia coupled with viral-induced neutrophilia and the migration of the neutrophils to the ischemic core may explain the elevated NLR among patients with ischemic stroke (92).
CRP is another inflammatory biomarker that is disproportionately increased among patients with coronavirus infection and AIS. The largest cohort study involving 32 ischemic stroke patients with concomitant COVID-19 infection reported that elevated CRP occurred in more than 90% of the patients (93, 94). All of the six ischemic stroke patients reported by Beyroutti et al. and three of the four patients observed by Tunc et al. showed elevated CRP levels (72, 89). AIS patients described in various case reports and case series with COVID-19 infection have also been reported to have raised CRP levels (16, 38, 91, 95–98) While CRP is a marker of inflammation, a meta-analysis likewise confirms its role in thromboembolic events (98). On the other hand, serum ferritin, an acute phase reactant and an inflammatory biomarker has also been reported to be elevated among patients with COVID-19 and AIS (85, 88, 89, 99, 100). Apart from its role in systemic inflammation, serum ferritin likewise predicts the degree of neural damage among patients with AIS as characterized by its correlation with markers of blood–brain barrier damage such as glutamate, interleukin-6, matrix metalloproteinase-9, and cellular fibronectin (35, 82).
Neutrophil Increase, Lymphocyte Decrease, and Neutrophil Extracellular Traps in Thrombin in General and Previously Associated With Acute Ischemic Stroke
As alluded to above, COVID-19 infection is associated with reduced leukocyte number and increased neutrophil to leukocyte ratio and so makes the recent evidence of dysregulated neutrophil extracellular traps (NETs) predictable (101–103). NETs are neutrophil-produced extracellular networks of chromatin, proteins, and oxidant enzymes that protrude from membranes of activated neutrophil and mediate infection containment (101). NETs also play a role in thrombus formation. Evidence indicates that, in patients with COVID-19 infection, there is an upsurge of NET production that further propagates inflammation and thrombosis (101–103). The NET formation is characterized by elevated levels of cell-free DNA, myeloperoxidase-DNA (MPO-DNA), and citrullinated histone H3 (Cit-H3) (25, 101). A study of normal subjects compared with patients with COVID-19 infection has shown that the latter have higher levels of cell-free DNA, MPO-DNA, and Cit-H3 with both MPO-DNA and Cit-H3 being significantly elevated in mechanically ventilated patients (101). Pulmonary autopsies of patients with confirmed COVID-19 patients also confirm the presence of NET-containing microthrombi with neutrophil–platelet infiltration (103, 104).
The evidence of the role of NETs in AIS is well-described (17, 18, 105, 106, 114). Laridan et al. examined specimens of thrombi of patients undergoing endovascular thrombectomy and found that Cit-H3, the hallmark for NETs, was observed in the majority of the samples (17). Interestingly, patients with cardioembolic etiology have been shown to have a higher burden of NETs (102). Another study revealed that the presence of NETs may convey reperfusion resistance to mechanical and systemic revascularization procedures (17). Whether the role of neutrophils and NET in coronavirus-related stroke is secondary to micro- or macrothrombosis needs further elucidation.
Viral-Induced Coagulation Dysfunction
Another important mechanism resulting in life-threatening systemic thromboembolic events among patients with coronavirus infection is the disruption of the coagulation pathways. Pro- and hypercoagulation affecting macro- and microvascular systems have also been implicated in various vascular events such as ischemic strokes. The International Society of Thrombosis and Hemostasis (ISTH) recognizes this unique phenomenon of sepsis-induced coagulopathy and proposed monitoring of platelet, D-dimer, PT, and fibrinogen among COVID-19 patients needing admission (115).
Fibrinolytic Shutdown
The imbalance between coagulation and fibrinolysis favoring the former manifests hematologically as an increase in D-dimer levels (115, 116). This is an immunological defense for the body to contain the viral infection, which results in thrombi formation (116). Furthermore, this implies a shutdown in the fibrinolytic system to clear the necrotic debris leading to massive fibrin formation, which also orchestrates coagulopathy (117). This shutdown has been further analyzed among critically ill COVID-19 patients where it has been demonstrated that, apart from the elevation D-dimer levels, more than 50% of the patients failed to lyse clots on thromboelastography (118–120) and hence increasing the likelihood of AIS as a further manifestation of COVID-19.
The tendency for 5% (this is still a gross underestimation, as we may never know the exact number strokes among deaths and even the mild strokes that might not present to the hospitals, as almost 40% of the stroke patients are not attending the hospitals during the pandemic time) of patients with COVID-19-related strokes to show spectacularly elevated D-dimer levels has been reported in several studies (91, 121, 122). Among the 32 cases reported by Yaghi et al., almost 50% showed D-dimer levels elevated to more than five times the normal range (94). Five of the six cases described by Beyrouti et al. also demonstrated D-dimer levels elevated more than 5–150 times above the normal limit (72). Avula et al., Lodigiani et al., Oxley et al., Berekashvili et al., and Wijeratne et al. also observed similar trends in the patients they described (3, 85, 88, 91, 123).
Venous thrombosis has also been described in a number of cases of COVID-19 infection resulting in fatal outcomes (107). Unsurprisingly, D-dimer levels were disproportionately elevated among patients with cerebral venous thrombosis (107, 108, 124). Another biomarker of fibrinolytic shutdown is the accumulation of fibrinogen and fibrin degradation products (119), and again, such retrospective analysis comparing COVID-19 and non-COVID-19 patients revealed higher fibrinogen levels in the former (123). Similarly, patients described in the literature with stroke and active COVID-19 infection have elevated serum fibrinogen levels (3, 72, 85).
The abnormalities of the coagulation biomarkers associated with COVID infection have been used to justify the use of systemic anticoagulation even in the acute stroke period. However, the outcomes for COVID patients are inconsistent (89, 123). Two of the five patients described by Lodigiani et al. who received Nadroparin died, and two of the four patients in Tunc's case series remain bedridden despite heparinization at the time of the publication (89, 123). Among the 32 COVID-19-related AIS described by Yaghi et al., 25 of whom received anticoagulation, more than 80% died or remained critically ill at the time of the publication (94). While the reason for poor neurological outcomes among these patients is multifactorial, a potential contributor is heparin resistance, especially among critically ill COVID-19 patients (24, 125).
For the small number of cases of COVID-19 patients who have also suffered AIS and received reperfusion therapy, consisting of intravenous thrombolysis or endovascular thrombectomy or a combination of both, the outcomes have not been particularly positive. In a case series regarding four patients with COVID-19-related AIS who also received systemic thrombolysis, all died (126). Similarly, in COVID-19-infected stroke patients reported in a healthcare system in New York who received alteplase and mechanical thrombectomy, the neurological outcomes were also poor (94). Currently, there is inadequate data to determine whether patients with COVID-19-related strokes may have resistance to tPA; however, it is known that the activation of the renin–angiotensin–aldosterone system results in the formation of plasminogen activator inhibitor (PAI-1), an inhibitor of tPA (127). Thus, it remains to be investigated whether these poor outcomes are related to counteractive mechanisms of PAI-I, increased thrombus burden, or resistance to tPA (128).
The Role of Natural Anticoagulants and Antiphospholipid Antibodies
Hypercoagulability is another sequela of COVID-19, especially in critically ill patients. One of the putative mechanisms, to which this is attributed, is the depletion of physiological anticoagulants and increased levels of coagulant factors and antiphospholipid antibodies (129–131). In a cross-sectional study of COVID-19-positive critically ill patients in China, it has been shown that the plasma levels of natural anticoagulants such as protein C, protein S, and antithrombin were below physiologically normal levels (127). The same study revealed that more than 50% of the patients developed antiphospholipid antibodies (127) that have been suggested to play a role in the pathogenesis of COVID-19-related strokes. Zhang et al. described three patients with multiple cerebral infarctions who tested positive for anticardiolipin immunoglobulin A (IgA) antibodies as well as anti-β2-glycoprotein I IgA and IgG antibodies (130). On the other hand, five of the six COVID-19-associated stroke patients described by Beyroutti et al. did not show antiphospholipid antibodies, but all of them were positive for lupus anticoagulant (LA) (72). Two other severe stroke patients with poor neurological outcome and concomitant COVID-19 infection have also been reported as testing negative for both antiphospholipid antibodies and LA (99, 100). Thus, while there is inconsistency in the trend of these procoagulant factors in ischemic stroke patients, Zhang has proposed a possible secondary antiphospholipid antibody syndrome especially in patients who have thrombotic manifestations and positive serum antibodies (130). If this was the case, then the initiation of therapeutic anticoagulation would be required for these patients (130). However, it has not translated to screening of all COVID-19 patients, as a transient increase in these parameters is expected in viral infections (129).
The Role of the Endothelium
A component of Virchow's triad, endothelial dysfunction is another major contributor to SARS-CoV-associated thrombosis. The virus has been shown to demonstrate a predilection to invade vascular endothelial cells by attaching to the ACE2 protein, which facilitates subsequent invasion (9, 132, 133).
Endothelium-Driven Activation of the Extrinsic Coagulation System
COVID-19 promotes an imbalance between ACE2 and angiotensin II (AT-II) receptors leading to the upregulation of the latter, which is responsible for the tonic production of platelet tissue factor (TF or factor111) in platelets and macrophages, and further interaction with factor VII to activate the extrinsic coagulation system (134, 135). TF is essential for fibrin formation at the site of vascular injury and may also be an important factor contributing to thrombogenesis, especially with the induction of inflammatory cells originating from the subendothelium (26).
Increased expression of TF has previously also been shown to be associated with thrombus formation in patients with ischemic stroke (109, 110). The EPICOR study in 2015 revealed that patients with elevated TF doubled the risk of stroke (109). Experimental studies also demonstrated that the inhibition of TNF-α-induced TF significantly reduces atheromatous plaque formation (30). Whether inflammation-induced tissue factor formation has a role in intracranial thrombosis in COVID patients particularly remains speculative.
Endothelium-Driven Nitrous Oxide Deficiency
Healthy endothelium is necessary to produce nitric oxide (NO), which is known as a vasodilator and plays a role in preventing thrombosis (27). SARS-CoV2-related endotheliopathy results in the suppression of nitric oxide synthase (NOS), resulting in nitric oxide deficiency (133). In vitro studies performed in 2009 on SARS-CoV also revealed that nitrous oxide inhibited viral entry replication by decreasing spike (S) protein expression and via its effects on the cysteine proteases encoded in the SARS-CoV (133).
Endothelium-derived nitrous oxide affects the cerebral circulation by its tonic vasodilatory effect and the prevention of adhesion of platelets and leukocytes to the vessel wall, both of which have an obligatory role in stroke pathogenesis (28). A study comparing stroke patients and healthy controls revealed that the levels of asymmetrical dimethylarginine (ADMA), a NOS inhibitor, is increased in the former, making it a risk factor for stroke (29). To date, few studies, if any, are looking into the potential role of NO in the treatment and prevention of COVID-19-related strokes; however, there is evidence to suggest that NO may also have a potential benefit for pulmonary-related COVID-19 complications (136).
The Role of the Renin–Angiotensin–Aldosterone System and Ace2 Deficiency
A unique characteristic of the SARS-CoV2 infection is its avid interaction with the ACE2 receptors, which affect the RAAS to a significant degree (137). It is known that viral-induced ACE2 deficiency results in significant alterations in the balance between the classical RAAS and the counterregulatory ACE2, which is essential for maintaining homeostatic mechanisms in the body (137). In particular, ACE2, which is ubiquitous in the brain, heart, and the vascular systems protects patients from the organ-damaging effects of the classical RAAS (138). This COVID-19-related disruption of the RAAS axes is likely to contribute to stroke pathogenesis, as it promotes inflammation, vasoconstriction, and end-organ damage (139, 140).
Various COVID-19 registries have shown that there is an increased fatality rate among patients with preexisting cardiovascular diseases such as hypertension and diabetes (140). Similarly, a majority of patients with AIS and COVID-19 report hypertension as a classical risk factor (111). The contribution of hypertension in the outcomes of patients with COVID-19 infection is likely explained in various mechanisms. Still, overactivity of the classical RAAS along with viral-induced endothelial vasoconstriction may be factors adding to the neurological exacerbation of COVID infection (140). Worse neurological outcomes among patients with COVID and ischemic strokes may be attributable to the unimpeded activity of the classical RAAS pathway in the brain, which has also been implicated in higher brain functions such as memory and cognition (141). Furthermore, upregulation of the classical RAAS pathway is likely to result in the overactivity of the sympathetic nervous system, which may exacerbate preexisting traditional risk factors for stroke such as hypertension, diabetes, and cardiac dysrhythmias (142).
Covid, Neurological Manifestations and Implications for Neurorehabilitation
Neurorehabilitation for patients recovering from severe COVID and AIS is set to be extra challenging in times of pandemic with its requirements for social distancing and, in many cases, need for online delivery in the immediate post-COVID stages. Currently, there are little reliable data about how well individuals with severe respiratory system and lung damage will recover or whether scarring will make such patients vulnerable to other viral infections (31). Furthermore, questions remain regarding whether the hypoxia engendered during the acute hospitalization phase will have lasting effects on the brain and nerve tissue especially on high metabolic demand areas including the sympathetic system and the fast visual attention and eye movements pathways (112) and long motor pathways.
Helms et al. first described the occurrence of dysexecutive syndrome in 36% of patients of critically ill COVID survivors in a French cohort characterized by lack of attention and disorganized movement with commands (143). Various case reports likewise described cases of patients who had severe impairments in executive function in the postacute stage who had significant recovery after aggressive neurorehabilitation (37, 144). In the same manner, varying degrees of depression and anxiety have also been identified after the acute stage of infection among COVID survivors (36, 145, 146). Interestingly, a meta-analysis of observational studies also notes that, among patients with previous SARS and MERS-CoV infection, neuropsychiatric manifestations such as depression, anxiety, irritability, memory impairment, sleep deprivation, and posttraumatic disorder were strikingly prevalent (147). One would also expect that psychological anxiety and the physiologically high immune responses (psychoneuroimmunological outcomes) would also lead to various psychiatric sequelae that have adverse effects on mental health of patients (32, 148). The neurological sequelae of SARS-CoV2 infection have gained recent publicity in social and print media (Facebook “Long haul COVID support groups”). Symptoms include persistent “brain fog,” fatigue, breathlessness, anxiety, depressive mood, and motor weakness, affecting subsets of young, middle aged, and older patients including a significant proportion who showed only mild symptoms, during the infected stage (40, 149). We have suggested that these symptoms comprise aspects of PCNS and hypothesized that such a syndrome is due to persistent inflammation following COVID-19 infection (113, 150). These complications are undeniably posing significant challenges ahead for the neurorehabilitation fraternity.
Mathew et al. identified a subgroup of patients with T-cell activation characteristic of acute viral response plasmablast responses reaching over 30% of the circulating cells with three different immunotypes associated with poor clinical trajectories supporting the current understanding of the pathobiology (151).
Fridman et al. found the frequency of stroke to be high among patients with severe COVID-19 with a very high inpatient mortality across all age groups demonstrating further evidence to the shared pathobiology as one go through the cluster analysis of this paper (143).
Overall, our review of the pathobiological basis of COVID-19 demonstrates that it is a multisystem illness with high likelihood of long-term physical, cognitive, psychological, and social sequalae in those who survive the illness. The scale of the burden of the disease and impact is enormous globally.
Furthermore, there is also no doubt that the current pandemic is overwhelming the neurorehabilitation sector globally. While it is too early to comment about the potential pandemic of long-term neurological issues for the millions of infected patients worldwide, the current review predicts the high likelihood of this possibility. Similarly, Boldrini et al. has noted that the two main factors already impacting the neurorehabilitation sector during the first phase of the pandemic (144) are the following:
(i) increased pressure from the acute care services to transfer the patients to rehabilitation medicine services;
(ii) increased difficulties without patient rehabilitation activities and home-based rehabilitation due to the restrictions enforced by the local and national authorities (144).
Indeed, the challenges described by Boldrini et al. above make the situation even more complex. It is critical to create an efficient healthcare delivery system tailored to each individual, each community, and each country. It is equally important to understand the biological underpinning behind the illness and post-COVID-19 complications. Tailor-made, individualized, targeted neurorehabilitation program should be developed with the available resources (e.g., telemedicine can be tested with easily available smart phone-based deliverables such as WhatsApp, Viber, Facetime, and Google Talk where professional personnel are not always available and sophisticated technology are not around). Family members can be a really useful resource in these challenging circumstances (36, 37, 145, 146).
To this end, we recommend considerations of Mantovani et al. (152) who recently collected and reviewed relevant evidence-based recommendations on the efficacy of cognitive rehabilitation, based on a comprehensive evaluation of 491 papers presenting the latest evidence-based practice for neurorehabiliation of traumatic brain injury (TBI) patients whose pathobiology bears many similarities to that presented in this review in the context of COVID-19 and systemic involvement (147, 152, 153).
Mantovani et al. as well offer a road map and a comprehensive perspective on the role of tele-neurorehabilitation and virtual rehabilitation to gain adequate cognitive stimulation in the era of physical distancing and lockdowns related to COVID-19 pandemic, reminding us of the potential opportunities globally (152). For further details, see Mantovani et al. and the 29 recommendations on evidence-based cognitive rehabilitation in the context of stroke and TBI nominated by the Cognitive Rehabilitation Task Force (CRTF), which suggests that a more comprehensive biological understanding of the various systems affected by TBI should lead to more collaborative translational research utilizing artificial intelligence and novel technology to find better individually designed precision solutions (e.g., video-consults, tablet-based measurements, and use of wearable devices). Lastly, the integration of services, including private and public partnerships such as university–public hospital partnerships and partnerships with community organizations and volunteers, are likely to create excellent opportunities for the future (112, 154, 155) and better evidence-based neurorehabilitation pathways.
In support of this more generalized theoretical approach, our group has demonstrated here the expected neurological impact of shared pathobiology between AIS and COVID-19 here, and the debilitating condition that characterizes PCNS (150) manifests as chronic fatigue, impaired thinking, depression, anxiety, breathing difficulties, and muscle weakness (40, 40, 149, 150). Thus, we suggest that treatment with known Food and Drug Administration (FDA)-approved immunomodulatory hormones such as melatonin (14, 15, 33, 156–162) and nutraceuticals such as curcumin (163) might be expected to enhance the likelihood of PCNS patients benefitting from any neurorehabilitation interventions as a matter of priority.
Conclusion
Our review has shown here that severe COVID-19 infection has the potential to lead to the disruption of most physiological systems and results in various multisystemic thrombotic phenomena including acute ischemic stroke. While inflammation orchestrates its pathogenesis, the further perturbation of the coagulation system resulting in fibrinolytic shutdown likewise contributes to neurological manifestation. Furthermore, the predilection of the virus to attach to ACE2 receptors in various cells, including the vascular endothelial system, may also disrupt the renin–angiotensin system, which further contributes to stroke pathogenesis. Clearly, there is a gap in the understanding of this phenomenon, and large-scale human and animal studies are necessary, as this co-occurrence results in deleterious outcomes.
The impact of COVID-19 in the human brain is as such that millions of patients are likely to experience problems with normal functioning requiring far more psychoneuroimmunological evidence-based understanding of neurorehabilitation services that are able to overcome incipient recovery problems.
Data Availability Statement
The original contributions generated in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
TW conceived the idea. TW and CS performed the literature search independently. TW, CS, SC, and LK contributed to the manuscript writing with critical revisions. All authors read the final manuscript and agreed.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Al-Ani F, Chehade S, Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res. (2020) 192:152–60. 10.1016/j.thromres.2020.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. (2020) 77:683–90. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of covid-19 in the Young. N Engl J Med. (2020) 382:e60. 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markus HS, Brainin M. COVID-19 and stroke-a global world stroke organization perspective. Int J Stroke. (2020) 15:361–4. 10.1177/1747493020923472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal G, Lippi G, Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with Coronavirus Disease 2019 (COVID-19): a pooled analysis of published literature. Int J Stroke. (2020) 15:385–9. 10.1177/1747493020921664 [DOI] [PubMed] [Google Scholar]
- 6.McColl BW, Allan SM, Rothwell NJ. Systemic infection, inflammation and acute ischemic stroke. Neuroscience. (2009) 158:1049–61. 10.1016/j.neuroscience.2008.08.019 [DOI] [PubMed] [Google Scholar]
- 7.Umapathi T, Kor AC, Venketasubramanian N, Lim CC, Pang BC, Yeo TT, et al. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS). J Neurol. (2004) 251:1227–31. 10.1007/s00415-004-0519-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JE, Heo JH, Kim HO, Song SH, Park SS, Park TH, et al. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol. (2017) 13:227–33. 10.3988/jcn.2017.13.3.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. (2020) 24:422. 10.1186/s13054-020-03120-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Najjar S, Najjar A, Chong DJ, Pramanik BK, Kirsch C, Kuzniecky RI, et al. Central nervous system complications associated with SARS-CoV-2 infection: integrative concepts of pathophysiology and case reports. J Neuroinflammation. (2020) 17:231. 10.1186/s12974-020-01896-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spence JD, de Freitas GR, Pettigrew LC, Ay H, Liebeskind DS, Kase CS, et al. Mechanisms of Stroke in COVID-19. Cerebrovasc Dis. (2020) 49:451–8. 10.1159/000509581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. (2020) 26:1017–32. 10.1038/s41591-020-0968-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 14.Anderson G, Reiter RJ. Melatonin: roles in influenza, Covid-19, and other viral infections. Rev Med Virol. (2020) 30:e2109. 10.1002/rmv.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrillo-Vico A, Lardone PJ, Alvarez-Sánchez N, Rodríguez-Rodríguez A, Guerrero JM. Melatonin: buffering the immune system. Int J Mol Sci. (2013) 14:8638–83. 10.3390/ijms14048638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camaré C, Pucelle M, Nègre-Salvayre A, Salvayre R. Angiogenesis in the atherosclerotic plaque. Redox Biol. (2017) 12:18–34. 10.1016/j.redox.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laridan E, Denorme F, Desender L, François O, Andersson T, Deckmyn H, et al. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. (2017) 82:223–32. 10.1002/ana.24993 [DOI] [PubMed] [Google Scholar]
- 18.Lim HH, Jeong IH, An GD, Woo KS, Kim KH, Kim JM, et al. Evaluation of neutrophil extracellular traps as the circulating marker for patients with acute coronary syndrome and acute ischemic stroke. J Clin Lab Anal. (2020) 34:e23190. 10.1002/jcla.23190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. (2013) 2013:152786. 10.1155/2013/152786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schonrich G, Raftery MJ, Samstag Y. Devilishly radical NETwork in COVID-19: oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv Biol Regul. (2020) 77:100741 10.1016/j.jbior.2020.100741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HHX, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. (2020) 81:e6–12. 10.1016/j.jinf.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. (2020) 55:102763. 10.1016/j.ebiom.2020.102763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Accinelli RA, Zhang Xu CM, Ju Wang JD, Yachachin-Chavez JM, Caceres-Pizarro JA, Tafur-Bances KB, et al. COVID-19: la pandemia por el nuevo virus SARS-CoV-2. Rev Peru Med Exp Salud Publica. (2020) 37:302–11. 10.17843/rpmesp.2020.372.5411 [DOI] [PubMed] [Google Scholar]
- 24.White D, MacDonald S, Bull T, Hayman M, de Monteverde-Robb R, Sapsford D, et al. Heparin resistance in COVID-19 patients in the intensive care unit. J Thromb Thrombolysis. (2020) 50:287–91. 10.1007/s11239-020-02145-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirose T, Hamaguchi S, Matsumoto N, Irisawa T, Seki M, Tasaki O, et al. Presence of neutrophil extracellular traps and citrullinated histone H3 in the bloodstream of critically ill patients. PLoS ONE. (2014) 9:e111755. 10.1371/journal.pone.0111755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saha D, Saha S, Sergeeva EG, Ionova ZI, Gorbach AV. Tissue factor and atherothrombosis. Curr Pharm Des. (2015) 21:1152–7. 10.2174/1381612820666141013154946 [DOI] [PubMed] [Google Scholar]
- 27.Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. (2012) 10:4–18. 10.2174/157016112798829760 [DOI] [PubMed] [Google Scholar]
- 28.Cosentino F, Rubattu S, Savoia C, Venturelli V, Pagannonne E, Volpe M. Endothelial dysfunction and stroke. J Cardiovasc Pharmacol. (2001) 38(Suppl. 2):S75–8. 10.1097/00005344-200111002-00018 [DOI] [PubMed] [Google Scholar]
- 29.Ercan M, Mungan S, Güzel I, Celik HT, Bal C, Abusoglu S, et al. Serum asymmetric dimethylarginine and nitric oxide levels in Turkish patients with acute ischemic stroke. Adv Clin Exp Med. (2019) 28:693–8. 10.17219/acem/78360 [DOI] [PubMed] [Google Scholar]
- 30.Hao E, Pang G, Du Z, Lai Y-H, Chen J-R, Xie J, et al. Peach kernel oil downregulates expression of tissue factor and reduces atherosclerosis in ApoE knockout mice. Int J Mol Sci. (2019) 20:405. 10.3390/ijms20020405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee C-H, Giuliani F. The role of inflammation in depression and fatigue. Front Immunol. (2019) 10:1696. 10.3389/fimmu.2019.01696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slavich GM, Auerbach RP. Stress and its Sequelae: Depression, Suicide, Inflammation, and Physical Illness. In: Hooley JM, Butcher JN, editor. Washington DC: American Psychological Association; (2018). [Google Scholar]
- 33.Chitimus DM, Popescu MR, Voiculescu SE, Panaitescu AM, Pavel B, Zagrean L, et al. Melatonin's impact on antioxidative and anti-inflammatory reprogramming in homeostasis and disease. Biomolecules. (2020) 10:1211. 10.3390/biom10091211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hermus L, Lefrandt JD, Tio RA, Breek JC, Zeebregts CJ. Carotid plaque formation and serum biomarkers. Atherosclerosis. (2010) 213:21–9. 10.1016/j.atherosclerosis.2010.05.013 [DOI] [PubMed] [Google Scholar]
- 35.Millán M, Sobrino T, Arenillas JF, Rodríguez-Yáñez M, García M, Nombela F, et al. Biological signatures of brain damage associated with high serum ferritin levels in patients with acute ischemic stroke and thrombolytic treatment. Dis Markers. (2008) 25:181–8. 10.1155/2008/380356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bermúdez i Badia S, Cameirão MS. The neurorehabilitation training toolkit (NTT): a novel worldwide accessible motor training approach for at-home rehabilitation after stroke. Stroke Res Treat. (2012) 2012:802157. 10.1155/2012/802157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foster AM, Armstrong J, Buckley A, Sherry J, Young T, Foliaki S, et al. Encouraging family engagement in the rehabilitation process: a rehabilitation provider's development of support strategies for family members of people with traumatic brain injury. Disabil Rehabili. (2012) 34:1855–62. 10.3109/09638288.2012.670028 [DOI] [PubMed] [Google Scholar]
- 38.González-Pinto T, Luna-Rodríguez A, Moreno-Estébanez A, Agirre-Beitia G, Rodríguez-Antigüedad A, Ruiz-Lopez M. Emergency room neurology in times of COVID-19: malignant ischaemic stroke and SARS-CoV-2 infection. Eur J Neurol. (2020) 27:e35–6. 10.1111/ene.14286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.South K, McCulloch L, McColl BW, Elkind MS, Allan SM, Smith CJ. Preceding infection and risk of stroke: an old concept revived by the COVID-19 pandemic. Int J Stroke. 2020. 15:722–32. 10.1177/1747493020943815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goërtz YMJ, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FVC, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? Spruit ERJ Open Res. (2020) 6:00542–2020. 10.1183/23120541.00542-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adamczyk-Sowa M, Niedziela N, Kubicka-Baczyk K, Wierzbicki K, Jaroszewicz J, Sowa P. Neurological symptoms as a clinical manifestation of COVID-19: implications for internists. Pol Arch Intern Med. (2020). 10.20452/pamw.15575. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42.Mankad K, Perry MD, Mirsky DM, Rossi A. COVID-19: a primer for Neuroradiologists. Neuroradiology. (2020) 62:647–8. 10.1007/s00234-020-02437-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. (2020) 579:270–3. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang M, Li CK, Li K, Hon KLE, Ng MHL, Chan PKS, et al. Hematological findings in SARS patients and possible mechanisms (review). Int J Mol Med. (2004) 14:311–5. 10.3892/ijmm.14.2.311 [DOI] [PubMed] [Google Scholar]
- 45.Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. (2020) 39:2085–94. 10.1007/s10067-020-05190-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agbuduwe C, Basu S. Haematological manifestations of COVID-19: from cytopenia to coagulopathy. Eur J Haematol. (2020) 105:540–6. 10.1111/ejh.13491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.An PJ, Zhu YZ, Yang LP. Biochemical indicators of coronavirus disease 2019 exacerbation and the clinical implications. Pharmacol Res. (2020) 159:104946. 10.1016/j.phrs.2020.104946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bindoli S, Felicetti M, Sfriso P, Doria A. The amount of cytokine-release defines different shades of Sars-Cov2 infection. Exp Biol Med (Maywood). (2020) 245:970–6. 10.1177/1535370220928964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Misra DP, Agarwal V, Gasparyan AY, Zimba O. Rheumatologists' perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin Rheumatol. (2020) 39:2055–62. 10.1007/s10067-020-05073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soy M, Atagündüz P, Atagündüz I, Sucak GT. Hemophagocytic lymphohistiocytosis: a review inspired by the COVID-19 pandemic. Rheumatol Int. (2020) 6:1–12. 10.1007/s00296-020-04636-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crayne CB, Albeituni S, Nichols KE, Cron RQ. The immunology of macrophage activation syndrome. Front Immunol. (2019) 10:119. 10.3389/fimmu.2019.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raschke RA, Garcia-Orr R. Hemophagocytic lymphohistiocytosis: a potentially underrecognized association with systemic inflammatory response syndrome, severe sepsis, and septic shock in adults. CHEST. (2011) 140:933–8. 10.1378/chest.11-0619 [DOI] [PubMed] [Google Scholar]
- 53.Filipovich AH. Hemophagocytic lymphohistiocytosis and other hemophagocytic disorders. Immunol Allergy Clin. (2008) 28:293–313. 10.1016/j.iac.2008.01.010 [DOI] [PubMed] [Google Scholar]
- 54.Emmenegger U, Schaer DJ, Larroche C, Neftel KA. Haemophagocytic syndromes in adults: current concepts and challenges ahead. Swiss Med Wkly. (2005) 135:299–314. [DOI] [PubMed] [Google Scholar]
- 55.Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/ systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. (2009) 10:387–92. 10.1097/PCC.0b013e3181a1ae08 [DOI] [PubMed] [Google Scholar]
- 56.Chen I-Y, Moriyama M, Chang M-F, Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front Microbiol. (2019) 10:50. 10.3389/fmicb.2019.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tong Y, Ding Z-H, Zhan F-X, Cai L, Yin X, Ling J-L, et al. The NLRP3 inflammasome and stroke. Int J Clin Exp Med. (2015) 8:4787–94. [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. (2019) 10:128. 10.1038/s41419-019-1413-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shah A. Novel coronavirus-induced NLRP3 inflammasome activation: a potential drug target in the treatment of COVID-19. Front Immunol. (2020) 11:1021. 10.3389/fimmu.2020.01021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freeman TL, Swartz TH. Targeting the NLRP3 inflammasome in severe COVID-19. Front Immunol. (2020) 11:1518. 10.3389/fimmu.2020.01518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. (2018) 15:203–14. 10.1038/nrcardio.2017.161 [DOI] [PubMed] [Google Scholar]
- 62.Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, Gargalianos P, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open. (2020) 3:e2013136. 10.1001/jamanetworkopen.2020.13136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Albensi BC. What is nuclear factor kappa B (NF-κB) doing in and to the mitochondrion? Front Cell Dev Biol. (2019) 7:154. 10.3389/fcell.2019.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodrigues-Diez RR, Tejera-Muñoz A, Marquez-Exposito L, Rayego-Mateos S, Santos Sanchez L, Marchant V, et al. Statins: could an old friend help in the fight against COVID-19? Br J Pharmacol. (2020) 177:4873–86. 10.1111/bph.15166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fann DY, Lee SY, Manzanero S, Tang SC, Gelderblom M, Chunduri P, et al. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. (2013) 4:e790. 10.1038/cddis.2013.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lara PC, Macías-Verde D, Burgos-Burgos J. Age-induced NLRP3 inflammasome over-activation increases lethality of SARS-CoV-2 pneumonia in elderly patients. Aging Dis. (2020) 11:756–62. 10.14336/AD.2020.0601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bertocchi I, Foglietta F, Collotta D, Eva C, Brancaleone V, Thiemermann C, et al. The hidden role of NLRP3 inflammasome in obesity-related COVID-19 exacerbations: lessons for drug repurposing. Br J Pharmacol. (2020) 177:4921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pereira MdP, Lima EG, Serrano Junior CV. Viral infections and atherothrombosis: another caution in the wake of COVID-19? Revista da Associação Médica Brasileira. (2020) 66:366–9. 10.1590/1806-9282.66.3.366 [DOI] [PubMed] [Google Scholar]
- 69.Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med. (2015) 278:483–93. 10.1111/joim.12406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mohamud AY, Griffith B, Rehman M, Miller D, Chebl A, Patel SC, et al. Intraluminal carotid artery thrombus in COVID-19: another danger of cytokine storm? Am J Neuroradiol. (2020) 41:1677–82. 10.3174/ajnr.A6674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Co COC, Yu JRT, Laxamana LC, David-Ona DIA. Intravenous thrombolysis for stroke in a COVID-19 positive filipino patient, a case report. J Clin Neurosci. (2020) 77:234–6. 10.1016/j.jocn.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. (2020) 91:889–91. 10.1136/jnnp-2020-323586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ueland T, Holter JC, Holten AR, Müller KE, Lind A, Bekken GK, et al. Distinct and early increase in circulating MMP-9 in COVID-19 patients with respiratory failure. J Infect. (2020) 81:e41–3. 10.1016/j.jinf.2020.06.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Solun B, Shoenfeld Y. Inhibition of metalloproteinases in therapy for severe lung injury due to COVID-19. Med Drug Discov. (2020) 7:100052. 10.1016/j.medidd.2020.100052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sawamura T, Fujita Y, Horiuchi S, Kakino A. LOX-1 in ischemic stroke. J Atheroscler Thromb. (2017) 24:566–8. 10.5551/jat.ED071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Erol A. Role of oxidized LDL-induced “trained macrophages” in the pathogenesis of COVID-19 and benefits of pioglitazone: a hypothesis. Diabetes Metab Syndr. (2020) 14:713–4. 10.1016/j.dsx.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Deng Y, et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int J Infect Dis. (2020) 96:131–5. 10.1016/j.ijid.2020.04.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xia X, Wen M, Zhan S, He J, Chen W. An increased neutrophil/lymphocyte ratio is an early warning signal of severe COVID-19. Nan Fang Yi Ke Da Xue Xue Bao. (2020) 40:333–6. 10.12122/j.issn.1673-4254.2020.03.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Y-P, Li G-M, He J, Liu Y, Li M, Zhang R, et al. Combined use of the neutrophil-to-lymphocyte ratio and CRP to predict 7-day disease severity in 84 hospitalized patients with COVID-19 pneumonia: a retrospective cohort study. Ann Transl Med. (2020) 8:635. 10.21037/atm-20-2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang C, Deng R, Gou L, Fu Z, Zhang X, Shao F, et al. Preliminary study to identify severe from moderate cases of COVID-19 using combined hematology parameters. Ann Transl Med. (2020) 8:593. 10.21037/atm-20-3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shang W, Dong J, Ren Y, Tian M, Li W, Hu J, et al. The value of clinical parameters in predicting the severity of COVID-19. J Med Virol. (2020) 92:2188–92. 10.1002/jmv.26031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang ZL, Hou YL, Li DT, Li FZ. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand J Clin Lab Invest. (2020) 80:441–7. 10.1080/00365513.2020.1768587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, von Bergwelt-Baildon M, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. (2020) 146:128–36.e4. 10.1016/j.jaci.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. (2020) 19:102568. 10.1016/j.autrev.2020.102568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berekashvili K, Dmytriw AA, Vulkanov V, Agarwal S, Khaneja A, Turkel-Parella D, et al. Etiologic subtypes of ischemic stroke in SARS-COV-2 virus patients. medRxiv. (2020) 10.1101/2020.05.03.20077206. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang J, Ren Q, Song Y, He M, Zeng Y, Liu Z, et al. Prognostic role of neutrophil-lymphocyte ratio in patients with acute ischemic stroke. Medicine. (2017) 96:e8624. 10.1097/MD.0000000000008624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nguyen VA, Crewther SG, Howells DW, Wijeratne T, Ma H, Hankey GJ, et al. Acute routine leukocyte and neutrophil counts are predictive of poststroke recovery at 3 and 12 months poststroke: an exploratory study. Neurorehabil Neural Repair. (2020) 34:844–55. 10.1177/1545968320948607 [DOI] [PubMed] [Google Scholar]
- 88.Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S, et al. COVID-19 presenting as stroke. Brain Behav Immun. (2020) 87:115–9. 10.1016/j.bbi.2020.04.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.TunÇ A, ÜnlÜbaS Y, Alemdar M, AkyÜz E. Coexistence of COVID-19 and acute ischemic stroke report of four cases. J Clin Neurosci. (2020) 77:227–9. 10.1016/j.jocn.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tissa Wijeratne CS, Karimi L, Crewther SG. Acute ischemic stroke in COVID-19: a case-based systematic review. Front Neurol. (2020) 11:1031. 10.3389/fneur.2020.01031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wijeratne T, Sales CA, Crewther SG, Nguyen V, Karimi L. First Australian case of good recovery of a COVID-19 patient with severe neurological symptoms post prolonged hospitalization. Cureus. (2020) 12:10366–77. 10.7759/cureus.10366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. (2013) 13:159–75. 10.1038/nri3399 [DOI] [PubMed] [Google Scholar]
- 93.Agarwal S, Scher E, Rossan-Raghunath N, Marolia D, Butnar M, Torres J, et al. Acute stroke care in a New York City comprehensive stroke center during the COVID-19 pandemic. J Stroke Cerebrovasc Dis. (2020) 29:105068. 10.1016/j.jstrokecerebrovasdis.2020.105068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yaghi S, Ishida K, Torres J, Grory BM, Raz E, Humbert K, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. (2020) 51:2002–11. 10.1161/STROKEAHA.120.030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wijeratne T, Menon R, Sales C, Karimi L, Crewther S. Carotid artery stenosis and inflammatory biomarkers: the role of inflammation-induced immunological responses affecting the vascular systems. Ann Transl Med. (2020) 8:1276. 10.21037/atm-20-4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morassi M, Bagatto D, Cobelli M, D'Agostini S, Gigli GL, Bnà C, et al. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. (2020) 267:2185–92. 10.1007/s00415-020-09885-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Valderrama EV, Humbert K, Lord A, Frontera J, Yaghi S. Severe acute respiratory syndrome coronavirus 2 infection and ischemic stroke. Stroke. (2020) 51:e124–7. 10.1161/STROKEAHA.120.030153 [DOI] [PubMed] [Google Scholar]
- 98.Kunutsor SK, Seidu S, Blom AW, Khunti K, Laukkanen JA. Serum C-reactive protein increases the risk of venous thromboembolism: a prospective study and meta-analysis of published prospective evidence. Eur J Epidemiol. (2017) 32:657–67. 10.1007/s10654-017-0277-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deliwala S, Abdulhamid S, Abusalih MF, Al-Qasmi MM, Bachuwa G. Encephalopathy as the sentinel sign of a cortical stroke in a patient infected with coronavirus disease-19 (COVID-19). Cureus. (2020) 12:e8121. 10.7759/cureus.8121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gunasekaran K, Amoah K, Rajasurya V, Buscher MG. Stroke in a young COVID-19 patient. QJM Int J Med. (2020) 113:573–4. 10.1093/qjmed/hcaa177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil extracellular traps (NETs) as markers of disease severity in COVID-19. medRxiv. (2020) 10.1101/2020.04.09.20059626. [Epub ahead of print]. [DOI] [Google Scholar]
- 102.Tomar B, Anders H-J, Desai J, Mulay SR. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells. (2020) 9:1383. 10.3390/cells9061383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. (2020) 136:1169–79. 10.1182/blood.2020007008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. (2009) 5:e1000639. 10.1371/journal.ppat.1000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou P, Li T, Jin J, Liu Y, Li B, Sun Q, et al. Interactions between neutrophil extracellular traps and activated platelets enhance procoagulant activity in acute stroke patients with ICA occlusion. EBioMedicine. (2020) 53:102671. 10.1016/j.ebiom.2020.102671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kang L, Yu H, Yang X, Zhu Y, Bai X, Wang R, et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun. (2020) 11:2488. 10.1038/s41467-020-16191-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cavalcanti DD, Raz E, Shapiro M, Dehkharghani S, Yaghi S, Lillemoe K, et al. Cerebral venous thrombosis associated with COVID-19. Am J Neuroradiol. (2020) 41:1370–6. 10.3174/ajnr.A6644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Garaci F, Di Giuliano F, Picchi E, Da Ros V, Floris R. Venous cerebral thrombosis in COVID-19 patient. J Neurol Sci. (2020) 414:116871. 10.1016/j.jns.2020.116871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iacoviello L, Castelnuovo AD, Curtis Ad, Agnoli C, Frasca G, Mattiello A, et al. Circulating tissue factor levels and risk of stroke. Stroke. (2015) 46:1501–7. 10.1161/STROKEAHA.115.008678 [DOI] [PubMed] [Google Scholar]
- 110.Previtali E, Bucciarelli P, Passamonti SM, Martinelli I. Risk factors for venous and arterial thrombosis. Blood Transfus. (2011) 9:120–38. 10.2450/2010.0066-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tan Y-K, Goh C, Leow AST, Tambyah PA, Ang A, Yap E-S, et al. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J Thromb Thrombolysis. (2020) 50:587–95. 10.1007/s11239-020-02228-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wijesundera C, Vingrys AJ, Wijeratne T, Crewther SG. Acquired visual deficits independent of lesion site in acute stroke. Front Neurol. (2020) 11:705. 10.3389/fneur.2020.00705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tissa Wijeratne SGC, Wijeratne T. What is Post Covid 19 Neurological Syndrome? University of Melbourne (2020). Available online at: https://pursuit.unimelb.edu.au/articles/what-is-post-covid-19-neurological-syndrome?fbclid=IwAR1H5RVh7Fmr9orgJYst2c-NSdhjFz1Ybxmu-hEhTP4M5AUSo_hbXVjo-1w (accessed January 8, 2020).
- 114.Peña-Martínez C, Durán-Laforet V, García-Culebras A, Ostos F, Hernández-Jiménez M, Bravo-Ferrer I, et al. Pharmacological modulation of neutrophil extracellular traps reverses thrombotic stroke tPA (tissue-type plasminogen activator) resistance. Stroke. (2019) 50:3228–37. 10.1161/STROKEAHA.119.026848 [DOI] [PubMed] [Google Scholar]
- 115.Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. (2020) 18:1023–6. 10.1111/jth.14810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thachil J. All those D-dimers in COVID-19. J Thromb Haemost. (2020) 18:2075–6. 10.1111/jth.14939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Medcalf RL, Keragala CB, Myles PS. Fibrinolysis and COVID-19: a plasmin paradox. J Thromb Haemost. (2020) 18:2118–22. 10.1111/jth.14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Creel-Bulos C, Auld SC, Caridi-Scheible M, Barker N, Friend S, Gaddh M, et al. Fibrinolysis shutdown and thrombosis in a COVID-19 ICU. Shock. (2020). 10.1097/SHK.0000000000001635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wright FL, Vogler TO, Moore EE, Moore HB, Wohlauer MV, Urban S, et al. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. (2020) 231:193–203.e1. 10.1016/j.jamcollsurg.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shaydakov ME, Sigmon DF, Blebea J. Thromboelastography (TEG). In: StatPearls. Treasure Island, FL: StatPearls Publishing (2020). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK537061/ (accessed April 24, 2020).
- 121.Requena M, Olivé-Gadea M, Muchada M, García-Tornel Á, Deck M, Juega J, et al. COVID-19 and stroke: incidence and etiological description in a high-volume center. J Stroke Cerebrovasc Dis. (2020) 29:105225. 10.1016/j.jstrokecerebrovasdis.2020.105225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yao Y, Cao J, Wang Q, Shi Q, Liu K, Luo Z, et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care. (2020) 8:49. 10.1186/s40560-020-00466-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. (2020) 191:9–14. 10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klein DE, Libman R, Kirsch C, Arora R. Cerebral venous thrombosis: a typical presentation of COVID-19 in the young. J Stroke Cerebrovasc Dis. (2020) 29:104989. 10.1016/j.jstrokecerebrovasdis.2020.104989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Beun R, Kusadasi N, Sikma M, Westerink J, Huisman A. Thromboembolic events and apparent heparin resistance in patients infected with SARS-CoV-2. Int J Lab Hematol. (2020) 42(Suppl. 1):19–20. 10.1111/ijlh.13230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sangalli D, Polonia V, Colombo D, Mantero V, Filizzolo M, Scaccabarozzi C, et al. A single-centre experience of intravenous thrombolysis for stroke in COVID-19 patients. Neurol Sci. (2020) 41:2325–9. 10.1007/s10072-020-04591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ahmed S, Zimba O, Gasparyan AY. Thrombosis in coronavirus disease 2019 (COVID-19) through the prism of Virchow's triad. Clin Rheumatol. (2020) 39:2529–43. 10.1007/s10067-020-05275-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Setia G, Tyler J, Kwan A, Faguet J, Sharma S, Singh S, et al. High thrombus burden despite thrombolytic therapy in ST-elevation myocardial infarction in a patient with COVID-19. Rev Cardiovasc Med. (2020) 21:289–95. 10.31083/j.rcm.2020.02.92 [DOI] [PubMed] [Google Scholar]
- 129.Zhang Y, Cao W, Jiang W, Xiao M, Li Y, Tang N, et al. Profile of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients. J Thromb Thrombolysis. (2020) 50:1–7. 10.1007/s11239-020-02182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N Engl J Med. (2020) 382:e38. 10.1056/NEJMc2007575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aubignat M, Godefroy O. COVID-19 and ischemic stroke: should we systematically look for lupus anticoagulant and antiphospholipid antibodies? Rev Neurol (Paris). (2020) 176:505–6. 10.1016/j.neurol.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. (2020) 41:3038–44. 10.1093/eurheartj/ehaa623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Akerström S, Gunalan V, Keng CT, Tan Y-J, Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology. (2009) 395:1–9. 10.1016/j.virol.2009.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.DiNicolantonio JJ, McCarty M. Thrombotic complications of COVID-19 may reflect an upregulation of endothelial tissue factor expression that is contingent on activation of endosomal NADPH oxidase. Open Heart. (2020) 7:e001337. 10.1136/openhrt-2020-001337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bautista-Vargas M, Bonilla-Abadía F, Cañas CA. Potential role for tissue factor in the pathogenesis of hypercoagulability associated with in COVID-19. J Thromb Thrombolysis. (2020) 50:1–5. 10.1007/s11239-020-02172-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Martel J, Ko Y-F, Young JD, Ojcius DM. Could nasal nitric oxide help to mitigate the severity of COVID-19? Microbes Infect. (2020) 22:168–71. 10.1016/j.micinf.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. (2020) 92:726–30. 10.1002/jmv.25785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gan R, Rosoman NP, Henshaw DJE, Noble EP, Georgius P, Sommerfeld N. COVID-19 as a viral functional ACE2 deficiency disorder with ACE2 related multi-organ disease. Med Hypotheses. (2020) 144:110024. 10.1016/j.mehy.2020.110024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hess DC, Eldahshan W, Rutkowski E. COVID-19-related stroke. Transl Stroke Res. (2020) 11:322–5. 10.1007/s12975-020-00818-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huang S, Wang J, Liu F, Liu J, Cao G, Yang C, et al. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens Res. (2020) 43:824–31. 10.1038/s41440-020-0485-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gironacci MM, Vicario A, Cerezo G, Silva MG. The depressor axis of the renin-angiotensin system and brain disorders: a translational approach. Clin Sci (Lond). (2018) 132:1021–38. 10.1042/CS20180189 [DOI] [PubMed] [Google Scholar]
- 142.Tsuda K. Renin-Angiotensin system and sympathetic neurotransmitter release in the central nervous system of hypertension. Int J Hypertens. (2012) 2012:474870. 10.1155/2012/474870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fridman S, Bullrich MB, Jimenez-Ruiz A, Costantini P, Shah P, Just C, et al. Stroke risk, phenotypes, and death in COVID-19: systematic review and newly reported cases. Neurology. (2020) 95:e3373–85. 10.1212/WNL.0000000000010851 [DOI] [PubMed] [Google Scholar]
- 144.Boldrini P, Bernetti A, Fiore P. Impact of COVID-19 outbreak on rehabilitation services and physical and rehabilitation medicine physicians' activities in Italy. An official document of the Italian PRM Society (SIMFER). Eur J Phys Rehabil Med. (2020) 56:316–8. 10.23736/S1973-9087.20.06256-5 [DOI] [PubMed] [Google Scholar]
- 145.Giansanti D. WhatsApp in mHealth: an overview on the potentialities and the opportunities in medical imaging. Mhealth. (2020) 6:19. 10.21037/mhealth.2019.11.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Davis TL, DiClemente R, Prietula M. Taking mHealth forward: examining the core characteristics. JMIR Mhealth Uhealth. (2016)4:e97. 10.2196/mhealth.5659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cicerone KD, Goldin Y, Ganci K, Rosenbaum A, Wethe JV, Langenbahn DM, et al. Evidence-based cognitive rehabilitation: systematic review of the literature from 2009 through 2014. Arch Phys Med Rehabil. (2019) 100:1515–33. 10.1016/j.apmr.2019.02.011 [DOI] [PubMed] [Google Scholar]
- 148.Slavich GM. Psychoneuroimmunology of stress and mental health. In: Harkness KL, Hayden EP, editors. The Oxford Handbook of Stress and Mental Health. Oxford: Oxford University Press; (2020). [Google Scholar]
- 149.Vaes AW, Machado FVC, Meys R, Delbressine JM, Goertz YMJ, Van Herck M, et al. Care dependency in non-hospitalized patients with COVID-19. J Clin Med. (2020) 9. 10.3390/jcm9092946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wijeratne T, Crewther S. Post-COVID 19 neurological syndrome (PCNS); a novel syndrome with challenges for the global neurology community. J Neurol Sci. (2020) 419:117179. 10.1016/j.jns.2020.117179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. (2020) 369:eabc8511. 10.1126/science.abc8511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mantovani E, Zucchella C, Bottiroli S, Federico A, Giugno R, Sandrini G, et al. Telemedicine and virtual reality for cognitive rehabilitation: a roadmap for the COVID-19 pandemic. Front Neurol. (2020) 11:926. 10.3389/fneur.2020.00926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cicerone KD, Dahlberg C, Kalmar K, Langenbahn DM, Malec JF, Bergquist TF, et al. Evidence-based cognitive rehabilitation: recommendations for clinical practice. Arch Phys Med Rehabil. (2000) 81:1596–615. 10.1053/apmr.2000.19240 [DOI] [PubMed] [Google Scholar]
- 154.Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton IJ. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. (2020) 52:jrm00089. 10.2340/16501977-2727 [DOI] [PubMed] [Google Scholar]
- 155.Wade DT. Rehabilitation after COVID-19: an evidence-based approach. Clin Med (Lond). (2020) 20:359–65. 10.7861/clinmed.2020-0353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Garcia IG, Rodriguez-Rubio M, Mariblanca AR, de Soto LM, Garcia LD, Villatoro JM, et al. A randomized multicenter clinical trial to evaluate the efficacy of melatonin in the prophylaxis of SARS-CoV-2 infection in high-risk contacts (MeCOVID Trial): a structured summary of a study protocol for a randomised controlled trial. Trials. (2020) 21:466. 10.1186/s13063-020-04436-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Galano A, Tan DX, Reiter RJ. Melatonin: a versatile protector against oxidative DNA damage. Molecules. (2018) 23. 10.3390/molecules23030530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jean-Louis G, von Gizycki H, Zizi F. Melatonin effects on sleep, mood, and cognition in elderly with mild cognitive impairment. J Pineal Res. (1998) 25:177–83. 10.1111/j.1600-079X.1998.tb00557.x [DOI] [PubMed] [Google Scholar]
- 159.Kleszczynski K, Slominski AT, Steinbrink K, Reiter RJ. Clinical trials for use of melatonin to fight against COVID-19 are urgently needed. Nutrients. (2020) 12:2561. 10.3390/nu12092561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Raza H, John A, Brown EM, Benedict S, Kambal A. Alterations in mitochondrial respiratory functions, redox metabolism and apoptosis by oxidant 4-hydroxynonenal and antioxidants curcumin and melatonin in PC12 cells. Toxicol Appl Pharmacol. (2008) 226:161–8. 10.1016/j.taap.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 161.Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Jou MJ, Acuna-Castroviejo D. Melatonin mitigates mitochondrial meltdown: interactions with SIRT3. Int J Mol Sci. (2018) 19:2439. 10.3390/ijms19082439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sadanandan N, Cozene B, Cho J, Park YJ, Saft M, Gonzales-Portillo B, et al. Melatonin-A potent therapeutic for stroke and stroke-related dementia. Antioxidants (Basel). (2020) 9:672. 10.3390/antiox9080672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Manoharan Y, Haridas V, Vasanthakumar KC, Muthu S, Thavoorullah FF, Shetty P. Curcumin: a wonder drug as a preventive measure for COVID19 management. Indian J Clin Biochem. (2020) 35:373–5. 10.1007/s12291-020-00902-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions generated in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.