Abstract
Significance: Inflammation is a critical aspect of injury repair. Nonresolving inflammation, however, is perpetuated by the local generation of extracellular matrix-derived damage-associated molecular pattern molecules (DAMPs), such as the extra domain A (EDA) isoform of fibronectin and hyaluronic acid (HA) that promote the eventual acquisition of a fibrotic response. DAMPs contribute to the inflammatory environment by engaging Toll-like, integrin, and CD44 receptors while stimulating transforming growth factor (TGF)-β signaling to activate a fibroinflammatory genomic program leading to the development of chronic disease.
Recent Advances: Signaling through TLR4, CD44, and the TGF-β pathways impact the amplitude and duration of the innate immune response to endogenous DAMPs synthesized in the context of tissue injury. New evidence indicates that crosstalk among these three networks regulates phase transitions as well as the repertoire of expressed genes in the wound healing program determining, thereby, repair outcomes. Clarifying the molecular mechanisms underlying pathway integration is necessary for the development of novel therapeutics to address the spectrum of fibroproliferative diseases that result from maladaptive tissue repair.
Critical Issues: There is an increasing appreciation for the role of DAMPs as causative factors in human fibroinflammatory disease regardless of organ site. Defining the involved intermediates essential for the development of targeted therapies is a daunting effort, however, since various classes of DAMPs activate different direct and indirect signaling pathways. Cooperation between two matrix-derived DAMPs, HA, and the EDA isoform of fibronectin, is discussed in this review as is their synergy with the TGF-β network. This information may identify nodes of signal intersection amenable to therapeutic intervention.
Future Directions: Clarifying mechanisms underlying the DAMP/growth factor signaling nexus may provide opportunities to engineer the fibroinflammatory response to injury and, thereby, wound healing outcomes. The identification of shared and unique DAMP/growth factor-activated pathways is critical to the design of optimized tissue repair therapies while preserving the host response to bacterial pathogens.
Keywords: hyaluronic acid, TGF-β, ED-fibronectin, TLR4, inflammation, fibrosis

Paula J. McKeown-Longo, PhD

Paul J. Higgins, PhD
Scope and Significance
Wound healing occurs in a continuum of overlapping phases (i.e., coagulation, inflammation, proliferation, and resolution) in which multiple cell types recognize and orchestrate the restoration of damaged tissue.1,2 The synthesis, deposition, and long-term reorganization of the trauma site stromal matrix, largely by cohorts of injury-activated epithelial cells, resident fibroblasts, and recruited vascular pericytes, provides the structural support for cell proliferation and migration within the wound field and determines repair outcomes.3,4 The remodeled extracellular matrix (ECM) also sequesters growth factors and cytokines that direct the genomic repair program while generating mechanical cues that control cell function, wound contraction, and eventual tissue restructuring.5,6
Failure to properly coordinate the repertoire of responses between cells and their ECM has pathological consequences ranging from chronic inflammation and deficient healing to exuberant repair, excessive scarring, and fibrosis.7–9 The recent adaptation of systems or network approaches provides a window into the complexity of the inflammatory and scar-forming stages following tissue injury while highlighting the underlying pathways, molecular mechanisms, and potential therapeutic targets.10 Such studies organize wound-impacted genes/proteins into functional categories or nodes providing opportunities to dissect the dynamics of nodal composition and interconnectivity to the wound repair program.
Factors released from dying or damaged cells at the site of injury (e.g., DNA, histones, high mobility group protein B1, heat shock proteins, ATP, interleukin-1 alpha) promote an innate immune response by functioning as Category IA damage-associated molecular pattern (DAMPs) molecules or Alarmins.11,12 An additional subclass (IIA) of host-derived DAMPs include fragments of ECM molecules as well as the transforming growth factor (TGF)-β1-induced matrix molecules biglycan, decorin, versican, tenascin C, hyaluronic acid (HA), and the extra domain A (EDA) isoform of fibronectin (FnEDA).13–17 Many DAMPs are endogenous toll-like receptor (TLR) agonists, or signal through other receptors that stimulate the rapid synthesis and release of proinflammatory cytokines and chemokines which, in turn, promote trafficking of immune cells (e.g., neutrophils, macrophages) to the injured tissue to initiate and sustain the process of sterile inflammation.13,18–21
While recruitment of immunocompetent cells to the wound site (in response to platelet/mast cell degranulation and locally generated DAMPs) is required for proper healing, nonresolving or chronic inflammation results in the eventual development of fibrotic disease.22–26 TLR4 is, perhaps, among the most promiscuous of the ECM DAMP-binding TLRs with regard to the diversity of ECM ligands recognized that contribute to the establishment and persistence of the inflammatory reaction to tissue injury11 and a critical contributor to the repair process. DAMP-type TLR4 ligands control the inflammatory and subsequent fibrotic responses in sterile cutaneous or ischemic wounds.27 TLR4 expression is elevated in the wound edge epithelial cohort in a mouse model of skin injury28 and is likely key to the repair process since excisional wound closure is delayed in mutant TLR4 mice.29 Recent data support the requirement for the TLR4-p38/JNK pathway in the regulation of inflammation and wound resolution as the presence of a nonfunctional receptor, or interference with TLR4 signaling, blunted both processes.28
HA and FnEDA, are prominent among the ECM DAMPs that signal through TLR4 and impact healing in their dual capacity as upregulated targets and modulators of the TGF-β response.30,31 This review focuses on the complex interactions among the DAMPs HA/FnEDA, their receptor systems, the innate immune response, and the TGF-β-signaling pathway in normal and pathological wound healing. Data suggest a model whereby the TGF-β pathway cooperates with the DAMP/TLR4 network to promote a maladaptive profibrotic response to tissue trauma.
Translational Relevance
DAMP- and growth factor-activated signaling networks intersect during tissue injury to impact the expression of a proinflammatory/profibrotic genomic program and, thereby, healing outcomes. Identification of the interacting elements, the pathways involved, and the nodes of intersection provide a roadmap of potential therapeutic targets for the treatment of fibroinflammatory disorders.
Clinical Relevance
Persistent or nonresolving inflammation due to tissue injury triggers eventual development of tissue fibrosis and organ dysfunction. There are limited treatment options for patients with fibrotic disease, which is often progressive due to the establishment of feed-forward loops. A major clinical challenge, therefore, is the design of specific therapies to attenuate the pathophysiological consequences of DAMP/growth factor collaboration while retaining the protective host response to microbial pathogens.
Discussion
HA and FnEDA: transitioning from the ECM to a DAMP
HA and HA receptors–linkages to TLR4 signaling
The role of the ECM in injury resolution is complex with distinct roles in tissue fibrosis and regeneration. Cutaneous burns in the adult, for example, heal as a scar, which is generally devoid of hair follicles and sweat glands. Fetal wounds, in contrast, undergo a repair process in which the skin architecture is regenerated with no scarring. Fetal wounds are rich in high-molecular-weight (HMW) HA, which appears to promote regenerative healing and decrease fibrosis by diminishing the inflammatory response,32 likely as a consequence of decreased levels of the proinflammatory cytokines, IL-8 and IL-6, and increases in the anti-inflammatory IL-10.33,34
In adults, cutaneous injuries heal along a continuum spanning normal healing to progression along a pathological, fibrotic pathway resulting in the development of hypertrophic scars and keloids. Hypertrophic scars are raised and stiff due to increased numbers of myofibroblast cells and changes in the deposition and organization of collagen. If cellular proliferation and inflammation persist, keloids are formed which extend beyond the original wound margins resulting in disfigurement and, in extreme cases, can lead to loss of function.33 The mechanisms regulating the pathway of tissue repair along a regenerative or scarring pathway are not well understood, but the ECM and, in particular, HA plays an important role in both tissue regeneration and pathological scarring.
HA is a nonsulfated, straight-chain glycosaminoglycan (GAG), consisting of a repeating disaccharide of glucuronic acid and N-acetyl glucosamine, and the only GAG not attached to protein.35,36 Following tissue injury and in response to wound-induced factors (e.g., TGF-β), HA is synthesized by fibroblasts, where it provides a scaffold to support cell proliferation and migration as well as promote innate immune responses. This high-negatively charged hydrophilic GAG maintains dermal hydration by regulating water balance and osmotic pressure while acting as a sieve to exclude macromolecules and prevent scarring.37,38
HA is synthesized on the inner leaflet of the plasma membrane by HA synthases (HAS) and transported into the extracellular compartment (Fig. 1). In the cutaneous matrix, HA has a relatively short half-life due to its rapid catabolism by the cell surface hyaluronidases HYAL1 and 2.39,40 HYAL2 is a lipid raft glycosylphosphatidylinositol-linked enzyme that degrades HA into small oligosaccharides for endocytosis by the raft-associated HA receptor CD44 and subsequent lysosomal degradation by HYAL1.41,42 HA processing and the regulation of HYAL activity are critical to HA function which, in turn, is dependent on HA size.43
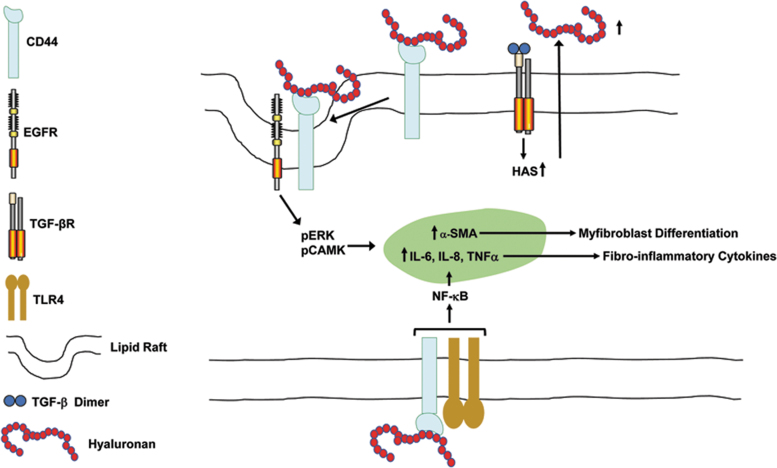
Figure 1.
Coordinate regulation of fibroinflammation by TGF-β and hyaluronan. TGF-β induces the expression of HAS resulting in the increased synthesis and release of hyaluronan. Binding of hyaluronan to CD44 results in the formation of a lipid raft-localized CD44/EGFR complex leading to the activation of ERK and CAM kinases, which regulate expression of α-SMA and myofibroblast differentiation. Hyaluronan occupancy of CD44 also promotes the TLR4-dependent induction of NF-κB-dependent proinflammatory cytokines, including IL-6, IL-8, and TNFα. α-SMA, α-smooth muscle cell actin; EGFR, epidermal growth factor receptor; HAS, hyaluronic acid synthase; TGF-β, transforming growth factor beta.
The biological roles of HA (e.g., regulation of ECM biophysical properties, mechanical signaling, tissue inflammation) are dictated by molecular weight and differential interaction with several cell surface-binding proteins, the hyaladherins, including CD44 and the receptor for hyaluronan-mediated motility (RHAMM).15,36,44,45 CD44 is the best characterized of the HA receptors and essential for cutaneous wound repair where it regulates keratinocyte adhesion, motility, proliferation, differentiation, and survival likely through an association with the actin cytoskeleton and downstream adaptor molecules.46,47
Binding of HA to CD44 activates several major signaling effectors, including MAP and CAM kinases, AKT, NF-κB, Rho GTPases, and Src47–49 (Fig. 1), as well as inducing expression of several pathophysiologically important microRNAs, including miR-21.40,50 The specific pathway engaged is linked to CD44 function as a coreceptor for TLR2, TLR4, EGFR, or c-met.15,20,44,45,51 Outcomes are dictated, however, by the nature of the formed ligand/receptor complex. HMW HA (>1,000 kDa) occupancy of CD44 downregulates inflammation and angiogenesis while promoting homeostasis, consistent with findings implicating an antiscarring role for HMW HA likely through an IL-10/HA synthase I axis.32,52
While the mechanism is unclear, HMW HA/CD44 interactions increase trafficking of TGF-β receptors to lipid rafts increasing, thereby, receptor turnover and antagonizing TGF-β1-dependent profibrotic SMAD signaling.53 The interaction of low-molecular-weight (LMW) HA (<5 kDa) with CD44, in contrast, did not exhibit similar antagonism and usually activates a TLR4-induced proinflammatory and proangiogenic program although CD44 also mediates the endocytic clearance of LMW HA, thereby dampening the immune response.54,55 Failure to remove LMW HA from the wound microenvironment (generated by hyaluronidase-mediated fragmentation of HMW HA) leads to persistent TLR-dependent NF-κB activation and the continued release of inflammatory mediators such as IL-6, IL-1β, and TNFα,56,57 while CD44 signaling dictated by HA size differentially regulates keratinocyte biological activities.47 Collectively, these findings suggest that HA-directed therapeutic modalities may have clinical applicability for the treatment of epidermal dysfunction and anomalies of cutaneous wound repair.
While HA/CD44/TLR4 inflammatory responses are regulated by NF-κB, the molecular events underlying NF-κB activation by HA are incompletely understood but may depend on both HA size and recruitment of the TLR4 coreceptor MD2 and the TLR4 adaptor myeloid differentiation factor (MyD88) and their downstream signaling intermediates to CD44/TLR complexes.51,58 The effects, however, appear cell type as well as context dependent. The downstream consequences of HA/CD44 binding is a function of HA mass, or extent of degradation, which impacts the type of receptor engaged and/or clustered and, thereby, the associated signaling pathways.59 In general, HMW HA preferentially binds CD44, smaller fragments occupy both CD44 and RHAMM, and the smallest activate TLR2 and TLR4, as well as modulate the ability of larger HA species to complex with CD44 and/or RHAMM by acting as competitive inhibitors.45,60 In the skin, however, large HA and somewhat smaller molecular mass HA fragments can bind CD44 with the pathway effectors and transcriptional read-outs dependent on the differentiation status of the involved cell types.61
The genomic program engaged appears to be dependent on the nature of the specific HA ligand/CD44 complex formed. As is the case with CD44, binding of HA to RHAMM stimulates cell migration and modulates adhesion by facilitating the formation of linkages between CD44 and/or receptor tyrosine kinases (RTKs) with the cytoskeleton, promoting Src/ERK-FAK activation of RhoA/PKCɛ/NF-κB/Stat3 or Rac1/MAPK/AP-1/p53-p63 signaling to regulate focal adhesion turnover.62,63 RHAMM-dependent motility, for example, requires association with CD44 and RTKs (e.g., EGFR1).64,65 RHAMM is upregulated by TGF-β at the site of injury, where it stimulates healing through mobilization of several pathways to coordinate inflammation and fibrogenesis.65–67 Indeed, loss of RHAMM negatively impacts both CD44 signaling and cutaneous wound repair.65,68 CD44 is also post-translationally modified and alternatively spliced, moreover, giving rise to several isoforms with distinct functions, which increases the spectrum of potential ligands and coreceptor partners.69 These diverse activities underscore the complexities involved in clarifying the role(s) of HA in wound repair,67 a challenge further complicated by the differing functions of the various isoforms of CD44 and RHAMM.40,70
Fn: the EDA isoform and TLR4 signaling
Fn is a ubiquitous, multifunctional glycoprotein found as a soluble dimer in the plasma and in a polymerized form in the ECM.71 Fn is organized into independently folded protein domains (Types I, II, and III), each with specific functional activities. ECM Fn provides both physical support and a scaffold to transduce biochemical as well as mechanical cues that dictate cell behavior.5,72,73 Remodeling of the Fn matrix in response to tissue injury or as a consequence of disease pathology involves, in large part, the synthesis of the EDA (also known as EIIIA) isoform of Fn (FnEDA). FnEDA derives from alternative transcript splicing to include an additional Type III domain, known as Extra Domain-A.74 Differential cell type-specific pathways downstream of TGF-β also regulate FnEDA splicing and, thereby, FnEDA levels.
Activation of PI3K/AKT/mTOR signaling in mouse embryonic fibroblasts, perhaps by TGF-β-induced downregulation of the phosphatase and tensin homolog on chromosome 10 (PTEN) or suppression of PTEN activity by Ser380/Thr382,383 phosphorylation,75,76 facilitates mobilization of the splicing factor SF2/ASF (also known as SRSF1), thereby increasing expression of FnEDA.77 The molecular mechanism controlling alternative mRNA splicing of the EDA exon of Fn depends on spliceosome assembly and RNA secondary structure, as well as the serine/arginine (SR)-rich family of proteins.78 Signaling from both TLR4 and the α4β1 integrin, moreover, appears required for the FnEDA-dependent expression of fibroinflammatory cytokines suggesting that regulating FnEDA splicing, and thereby EDA-initiated TLR4 activation, may represent a novel strategy for the treatment of fibrosis and disorders that derive from excessive tissue remodeling.79,80
Under normal conditions, expression of FnEDA is restricted to early development and adult wound healing.81 Wound fluid, in fact, is enriched in FnEDA as well as Fn fragments.82 The development of FnEDA-deficient mice confirmed the involvement of the EDA isoform in injury resolution, inflammation, and tissue fibrosis.83–85 While the role of FnEDA in the healing process may be multifunctional, wound site factors (e.g., TGF-β) stimulate fibroblasts to synthesize FnEDA, which is required for the conversion of fibroblasts to the contractile myofibroblastic phenotype.86,87 Dysregulated TGF-β signaling during the repair process, however, promotes high levels of FnEDA synthesis, enhanced myofibroblast persistence, and pathologic ECM accumulation substantially altering tissue mechanics leading to excessive tissue scarring and organ dysfunction.[reviewed in 85,88]
The EDA domain of Fn functions as a DAMP to activate TLR4 signaling in immune cells and human dermal fibroblasts resulting in the increased expression of several fibroinflammatory cytokines, including IL-8 and TNFα.13,89,90 In dermal fibroblasts, this response is dependent on the α4β1 integrin serving as a coreceptor.79 The molecular basis of α4β1/TLR4 activation and induction of fibroinflammatory genes by FnEDA is not well understood. There is no evidence as yet for the formation of physical complexes between α4β1 and TLR4. It is also not known whether the EDA domain binds directly to the TLR4 or if TLR4 is transactivated through integrin-initiated signals.
The EDA domain additionally mobilizes TLR4-dependent proliferative responses in keratinocytes21 and FnEDA is upregulated in the fibrotic skin of scleroderma patients, in mice with bleomycin-induced cutaneous fibrosis, as well as in keloid scars.90,91 EDA activates TLR4 signaling either as the individual type III domain or in the context of the intact molecule90,92 stimulating synthesis of collagen and α-smooth muscle cell actin (α-SMA) in skin fibroblasts.
Collectively, these findings implicate the TLR4-EDA axis in the control of a complex proinflammatory/profibrotic genomic program90 and suggests that, as the primary source of FnEDA, fibroblasts coordinate both TLR4- and α4β1-dependent autocrine loops that fuel tissue inflammation and subsequent fibrosis (Fig. 2). In the vascular system, for example, FnEDA facilitates the switch of smooth muscle cells to the synthetic phenotype characterized by increased cell proliferation and migration. This FnEDA-mediated differentiation process, which leads to vascular hyperplasia, is dependent on TLR4 and integrin receptors.93
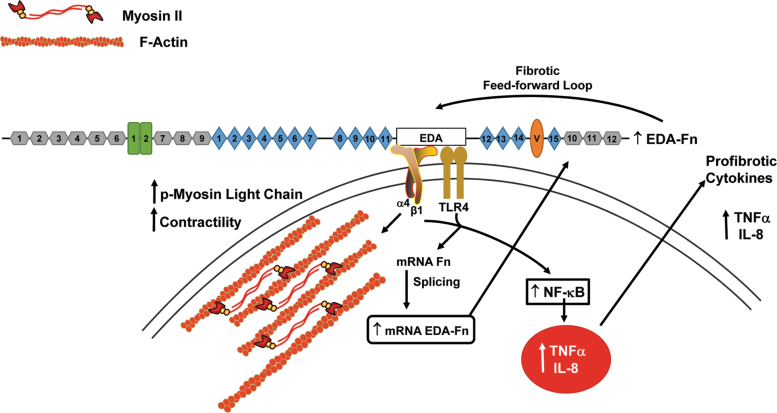
Figure 2.
The TLR4 and α4β1 integrin receptors regulate an EDA fibronectin-dependent feed-forward fibrotic loop. Binding of the EDA domain of fibronectin to the α4β1 integrin receptor on dermal fibroblasts stimulates cellular contractility by promoting the formation of actin microfilaments and the phosphorylation of myosin light chain.97 Interaction of the EDA with a functional complex of TLR4/α4β1 receptors activates NF-κB-dependent transcription of profibrotic cytokines while regulating fibronectin mRNA splicing to increase the fraction of newly synthesized fibronectin containing the EDA domain, thus creating an EDA fibronectin feed-forward loop.79 EDA, extra domain A.
Integrin/EDA cooperativity
Regulation of gene expression
Cell surface integrins are single-pass transmembrane heterodimeric receptors that couple structural and matricellular elements of the ECM to the intracellular compartment. Integrin signaling is bidirectional allowing cells to respond to changes in the stroma while regulating this process through control of integrin activation. Among the considerable repertoire of dimeric integrin receptors, only α4β1, α4β7, and α9β1 recognize the EDA domain; little is known, however, regarding the signaling pathways they impact or their role in the development of inflammation and subsequent fibrotic disease.94,95
The EDGIHEL motif in the EDA C-C′ loop is involved in the binding of α9β1 and α4β131,95 with Asp41/Gly42 specifically required for site occupancy. The α4β7-binding sequence is not yet confirmed although complex formation between integrin α4β7 and FnEDA promotes myofibroblast differentiation through the activation of FAK and ERK, the generation of increased cellular contractility and expression of α-SMA as well as collagen.94 It should be acknowledged, however, that α-SMA is an inconsistent biomarker of the contractile, collagen-expressing, fibroblastic phenotype and, therefore, may be a consequence rather than a causative factor in myofibroblast differentiation.
FnEDA occupancy of the α9β1 integrin receptor on certain cell types (e.g., colorectal, renal, pulmonary, hepatic, and epithelium) stimulates an epithelial-to-mesenchymal transition, or perhaps a more appropriately designated “plastic” response, that contributes to the myofibroblastic pool within the tumor desmoplastic tissue.96 In dermal fibroblasts, α4β1 recognition of its binding site on EDA increases actin stress fiber assembly, myosin light-chain phosphorylation, Fn synthesis, and construction of a higher-order 3-dimensional Fn matrix.97 It appears, however, that α4β1 is necessary but not sufficient in and of itself to function as a network hub in the genomic proinflammatory program. Indeed, coordinate signaling from both TLR4 and the α4β1 integrin is required for the EDA-dependent expression of fibroinflammatory cytokines as well as the increase in the proportion of newly synthesized Fn containing the EDA splice variant (Fig. 2).79 These data suggest that interactions between the EDA domain of Fn and EDA-recognizing integrins contributes to the acquisition of a fibrogenic phenotype by inducing the expression of genes that impact myofibroblast differentiation.
EDA domain in TGF-β activation
TGF-β regulates myfibroblastic conversion and wound-site ECM remodeling98 highlighting a key role for TGF-β in tissue repair and the need to define mechanisms of TGF-β activation as one interventional approach to modulate healing outcomes. The three mammalian TGF-β isoforms, however, differ in their ability to direct fibrotic vs. scarless cutaneous healing,99 suggesting that they have fundamentally distinct mechanisms of action. The TGF-β1, 2 and 3 proproteins, consisting of the dimeric growth factor and latency-associated peptide (LAP) domains, interact within the endoplasmic reticulum with the latent TGF-β-binding protein (LTBP) through disulfide bond formation between LAP and LTBP.100 Furin-directed cleavage of LAP occurs in the Golgi before extracellular secretion of this ternary large latent complex (TGF-β/LAP/LTBP), where the four LTBP isoforms possess variable affinities for elements of the ECM structural network.101
The use of genetically deficient mice and mapping of the interacting regions suggests that ECM docking of LTBP-3 and LTBP-4 occurs on fibrillin-1 microfibrils, whereas LTBP-1 interacts with the Fn network.101,102 LTBP-1 has a greater affinity for FnEDA compared with FnEDB or Fn without the EDA/B splice variants and the EDA domain facilitates the docking of LTBP-1 to the fibroblast ECM.31,101 Indeed, interference with EDA domain function attenuates both LTBP-1 binding to FnEDA and TGF-β1 activation.103 Complicating the actual identification of LTBP docking sites in the ECM, however, is the changing dynamics of binding partners (e.g., from Fn to fibrillin-1 or even fibulin) and the suggestion that heparin and heparan sulfate proteoglycans might facilitate Fn/LTBP-1 interactions, while promoting LTBP-1 multimerization and mechanical force-dependent TGF-β activation.104,105 Nevertheless, the construction of such multicomponent complexes forms the basis for TGF-β activation in the wound field. This has considerable implications since myofibroblast differentiation, a critical cell type in the wound repair program, requires a microenvironment rich in biologically active TGF-β, a progressively noncompliant stromal matrix and the expression and accumulation of FnEDA.31,103
While certain proteinases (e.g., MMP-2, MMP-9, plasmin) liberate TGF-β upon cleavage of the sensitive hinge region in LAP, other models suggest nonproteolytic mechanisms whereby multiple integrins that share the αV subunit (e.g., αVβ1, β3, β5, β6, β8), and bind the arginine–glycine–aspartic acid (RGD) motif in the N-terminal region of LAP, generate contractile forces with ECM-anchored LTBPs.86,103,104,106–108 The resulting tension induces a conformational change to the latent TGF-β1 complex releasing, and thereby activating, the TGF-β1 or TGF-β3 dimer104,109,110 without the need for participating proteases (Fig. 3). A different mode of liberation is likely involved for TGF-β2 since the TGF-β2 LAP does not possess an RGD site. Alternatively, integrins αVβ6 and αVβ3 may bind to both the latent TGF-β1 complex and proteinases, simultaneously distorting the LAP cage and providing protease access to the hinge cleavage site.
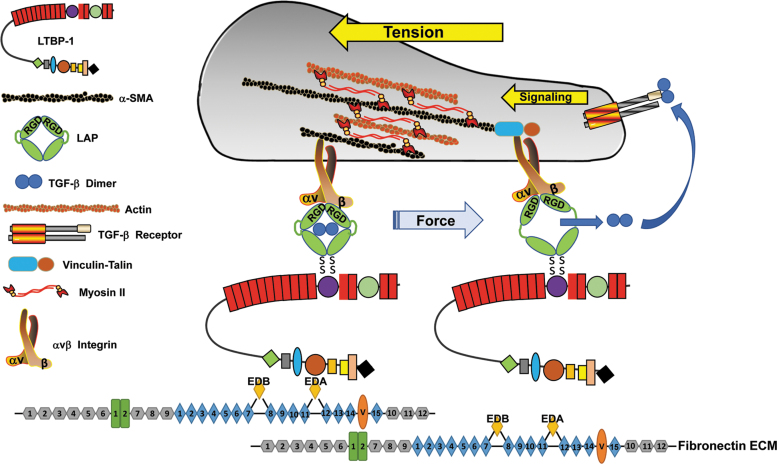
Figure 3.
A model of tension-dependent activation of TGF-β upon release from the LAP cage. The ternary large latent (LTBP/TGF-β/LAP) complex forms a bridge between an αV integrin bound to the RGD site on the latency-associated peptide and LTBP-1 tethered to the fibronectin-rich ECM. Actinomyosin-based contractility generates mechanical tension within this ternary complex inducing a conformational change in the LAP that releases the now-active TGF-β dimer.[derived from 104] Very recent findings using cryoelectron microscopy to probe LAP:TGF-β complex interactions with the αVβ8 integrin suggest, however, the existence of an alternative mechanism of TGF-β activation that does not necessitate release of dimeric TGF-β from the LAP.113 ECM, extracellular matrix; LAP, latency-associated peptide; LTBP, latent GFF-β-binding protein.
While αIIbβ3, α5β1, and α8β1 also recognize the RGD site, it appears that αV integrins are specifically poised to liberate TGF-β1 or TGF-β3.111 The role of αVβ8 in TGF-β activation, however, may be fundamentally different from other αV integrins. The αVβ8 cytoplasmic tail does not engage the actin microfilament network and, therefore, cannot generate Rho/RhoA-dependent contractile force to free the LAP-caged TGF-β dimer relying instead on proteolytic activity or alternative mechanisms of LAP-associated TGF-β activation.112,113 EDA/integrin binding may also enhance interactions between the LAP RGD motif and αV integrins suggesting that FnEDA may actually provide a platform for the generation of tractional force to promote TGF-β release.31
ECM remodeling and/or maturation during tissue repair or increased tensional stress as a consequence of accumulating FnEDA at the injury site may also contribute to a resetting of the threshold of TGB-β1 activation.31,114 These findings support a more complex mechanism for continued TGF-β1 signaling and initiation of fibrotic disease and places the TGF-β induction of FnEDA expression as a critical element in a TGF-β/FnEDA/αV integrin-positive feed-forward loop.31 Loss of elasticity and progressive ECM stiffness further stimulate expression of FnEDA while increasing the colocalization of LTBP-1 with FnEDA, integrin/LAP engagement, and cellular force generation collectively augmenting the ongoing conversion of latent to bioactive TGF-β1.22,115 This increasingly noncompliant TGF-β1-rich microenvironment promotes myofibroblastic differentiation and persistence while mobilizing the HIPPO pathway mechanosensitive effectors YAP and TAZ that, in turn, reinforce expression of genes encoding profibrotic factors.116–118 A point may be reached when progressive fibrosis becomes self-sustaining, involving both cell autonomous and ECM-driven mechanisms, resulting in the creation of a feed-forward mechanosensitive circuit and a permanent change in the mechanical properties of the supporting stroma that exacerbates disease progression.119
Integration of EDA/HA/TLR4 and TGF-β signaling
TGF-β/HA synergy
The TGF-β-directed transition of dermal fibroblasts to the myofibroblastic phenotype is required for wound contraction, collagen deposition, and scar formation,120 a process regulated by and dependent on both FnEDA and HA.20,30 TGF-β increases HA levels in the wound bed by inducing the synthesis of HAS, in large part, through the involvement of MAP kinases121 and HA appears involved in the subsequent fibrotic response.122 The endogenous synthesis and pericellular organization of polymerized HA is required for myofibroblast conversion as addition of exogenous HA does not promote differentiation.123
Induction and maintenance of the myofibroblast phenotype also requires the HA receptor, CD44. While the mechanism is unclear, TGF-β promotes complex formation between EGFR1 and CD44.124 In response to TGF-β1 stimulation, CD44 translocates into lipid rafts where it colocalizes with the epidermal growth factor receptor (EGFR) to activate a signaling cascade involving ERK1/2 and Ca2+/calmodulin kinase II (Fig. 1); both kinases are essential for myofibroblast differentiation.124 Since TGF-β1 activates Src kinase-dependent EGFRY845 phosphorylation and downstream signaling,125,126 and HA binding to CD44 similarly promotes Src activation,49 it appears that TGF-β1/HA cooperation culminates in EGFR→MAP kinase pathway activation that, in the setting of chronically elevated TGF-β1 levels, may promote maladaptive wound repair.
While HA facilitates TGF-β1-induced myofibroblast differentiation through the formation of HA/CD44 complexes promoting CD44 cellular relocation, HA/CD44 interactions may also downregulate TGF-β1signaling by trafficking the TGF-β receptor (TGF-βR) to caveolae-rich membrane rafts53 facilitating, thereby, receptor degradation.127 Additionally, the HA-dependent formation of CD44/TLR4 complexes contributes to the development of a fibrotic environment by increasing the expression of several cytokines, including TGF-β.51,128 Clearly, there are complex controls on the intensity and duration of TGF-β1 signaling at several levels in the wound repair program.
Although TGF-β1 transactivates the EGFR and, thereby, its downstream targets AKT and ERK1/2, the myofibroblast phenotype persists after TGF-β removal due to establishment of an autocrine TGF-β/HA-dependent feed-forward loop that promotes tissue fibrosis.129 Continued maintenance of the myofibroblast phenotype leads to excessive matrix deposition, ECM crosslinking, tissue stiffening, and fibrotic disease. CD44 similarly upregulates synthesis of α-SMA through an actin/MRTF pathway, which is independent of both TGF-β and HA, however, suggesting that the role of CD44 and HA in myofibroblast differentiation is quite complicated. These differential activities of CD44 are not well understood and likely depend on the specific cell types and involved tissue.
PTEN is a target of EDA/HA/TLR4 and TGF-β signaling
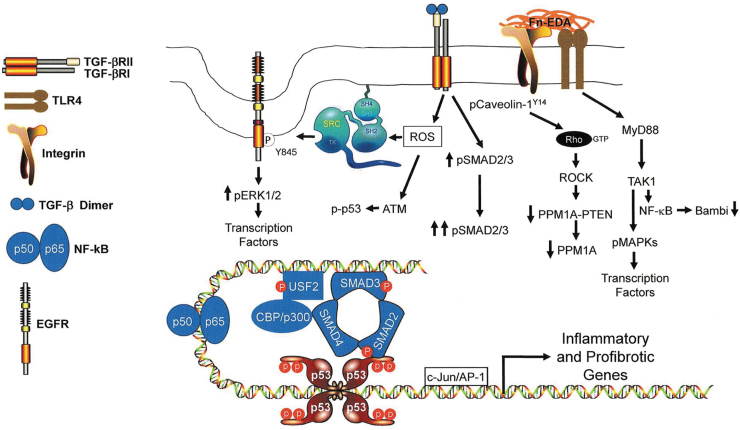
TLR4 activation by the FnEDA domain, either as the isolated type III module or in the context of the intact or fragmented FnEDA molecule, induces the expression of several proinflammatory and profibrotic genes that can impair or promote wound healing.13,14,79,89,130,131 This response involves the MyD88 adapter-like (Mal) protein/MyD88 pathway, downstream of TLR4, to activate NF-κB target genes and likely reflects the nature of the receptor complex (e.g., TLR4 vs. TLR4+coreceptors), the signaling intermediates engaged and the specific repertoire of inflammatory/profibrotic effectors expressed. TLR4 may function as a molecular “switch,” binding endogenous DAMPs (e.g., EDA) to activate a repair program while downregulating the TGF-β1 inhibitory pseudoreceptor Bambi (through the same MyD88/NF-κB pathway).132 Bambi reduction, in turn, sensitizes cells to TGF-β1 in the immediate DAMP-rich microenvironment as well as to EDA-mediated TGF-β1 upregulation promoting persistent expression of a subset of proinflammatory/profibrotic target genes, including FnEDA, creating a sustaining feed-forward TLR4→TGF-β1→ FnEDA→TLR4 loop that culminates in fibrosis and compromised tissue function (Figs. 4 and 5).
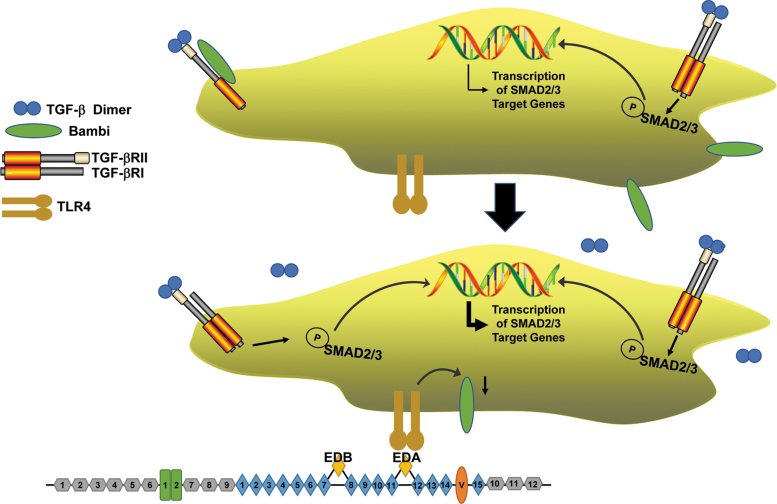
Figure 4.
TLR4 activation enhances TGF-β signaling and expression of TGF-β target genes. In unstimulated quiescent cells (top), basal production of active TGF-β engages cell surface TGF-β receptors resulting in SMAD phosphorylation and low-level transcription of SMAD-responsive genes. A fraction of TGF-βRII complexes with the TGF-β pseudoreceptor Bambi instead of TGF-βRI rendering the type II receptor signaling incompetent.(e.g., 156,157) Upon engagement of TLR4 with EDA (bottom), increased generation of active TGF-β1 coupled with Bambi downregulation sensitizes cells to TGF-β1 in the immediate microenvironment increasing TGF-βR signaling and the expression of SMAD target genes. A significant faction of profibrotic factors is induced specifically by SMAD2/3.
Figure 5.
Model illustrating TLR4-dependent signaling events that impact the expression of inflammatory and profibrotic genes. EDA stimulation of TLR4 signaling, perhaps in cooperation with the α4β1 integrin, engages the MyD88 adaptor protein pathway mobilizing TGF-β-activated kinase 1 (TAK1), also known as MAP3K7. TAK1, in turn, activates NF-κB resulting in the NF-κB-mediated downregulation of Bambi,156,157 enhancing thereby TGF-β signaling while increasing NF-κB(p50/p65)-dependent transcription of inflammatory genes. Bambi suppression, particularly in the context of increased TGF-β synthesis and/or release enhances TGF-βR-dependent SMAD2/3 phosphorylation while increasing the cellular levels of ROS. ROS signaling stimulates ATM-induced p53 phosphorylation, src kinase transactivation of the EGFR at Y845 and src-dependent phosphorylation of caveolin-1 at the Y14 site.142 EGFR-activated ERK1/2 and TAK1-stimulated MAPKs target transcription factors (e.g., NF-κB, USF, AP-1) and chromatin remodeling proteins (e.g., CB/p300) that cooperate with SMADs and p53 to influence expression of a genomic proinflammatory/profibrotic program. TGF-β1 also activates the RhoA/ROCK pathway, likely by promoting src kinase-induced caveolin-1 Y14 phosphorylation and Rho-GTP loading142 that downregulates both PTEN and PPM1A levels contributing to the persistence of SMAD2/3 phosphorylation and transcription of profibrotic genes. ROS, reactive oxygen species.
The basis for crosstalk between the TGF-β1 and TLR4 networks in cutaneous pathophysiology is not well defined although recent data suggest significant interaction between the TGF-β1/TLR4 and PTEN pathways in the control of the fibrotic phenotype. PTEN is the principle negative regulator of the PI3K/Akt pathway and a critical factor in several fibrotic disorders. PTEN expression is attenuated in various models of injury-induced tissue scarring, while PTEN deficiency drives cutaneous and renal fibrosis.76,133–135 Importantly, TLR4 signaling downregulates PTEN levels resulting in fibroblast commitment to a profibrotic phenotype136 and TGF-β1-initiated expression of fibrotic genes is enhanced by PTEN depletion.135 PTEN silencing, moreover, cooperates with TGF-β1 to further stimulate induction of the SMAD3-/p53-dependent TGF-β1 profibrotic signature genes, CCN2, SERPINE1, and FnEDA.134,135
The mechanism of PTEN reduction in the setting of fibrosis appears due to increased HA/TLR signaling and/or elevated TGF-β1 levels in the injury field.134,136 TLR4 activation (by lipopolysaccaride, LPS) stimulates miR-718 expression (in macrophages), which impacts PI3K/Akt signaling by targeting PTEN and promoting Akt phosphorylation.137 pAkt, in turn, downmodulates the expression of TLR4 and several of its signaling effectors through let-7e exerting, thereby, multilevel negative regulation to the TLR4 pathway. Whether TLR4-mobilizing DAMPs utilize the same or different intermediates is not known, but the involvement of DAMP-induced microRNAs in PTEN control is firmly established. In this regard, HA (and likely smaller MW fragments as well) bind to CD44 promoting RhoA/ROCK and NF-κB/Stat signaling while inducing expression of several microRNAs, including the PTEN suppressor miR-21, initiating acquisition of a proinflammatory program.40,61,138 TGF-β1 also stimulates miR-21 transcription which, in turn, reduces PTEN levels by direct binding to its 3′ untranslated region,139 although miR-21 has targets other than PTEN. Collectively, these data indicate that microRNAs induced upon activation of TLR4, HA/CD44, and TGF-β1 signaling attenuate PTEN levels in both dermal and nondermal cells likely impacting activation of a proinflammatory/fibrogenic program with significant implications as to tissue repair outcomes.
PTEN/PPM1A interactions: regulation of SMAD activity
Recent findings provide considerable insight into the fibroinflammatory consequences of PTEN downregulation. PTEN depletion reduces the levels of PPM1A, a C-terminal SMAD2/3 phosphatase,140 while promoting SMAD3 phosphorylation and nuclear localization, transactivation of profibrotic genes and secretion of SMAD3-dependent fibrotic factors.135,141 PPM1A overexpression attenuates fibrogenesis in murine fibroblasts treated with the TLR4-activator LPS, while persistent TGF-β1 stimulation decreases PPM1A levels through Rho/ROCK pathway activation maintaining, thereby, SMAD3-dependent transcription of profibrotic signature genes.141–143 PTEN may complex, moreover, with PPM1A in human fibroblasts,144 suggesting that PTEN is required for PPM1A stabilization and/or function. PPM1A destabilization or loss of function due to PTEN downregulation has implications with regard to TLR4 proinflammatory signaling since, in addition to targeting pSMAD2/3, PPM1A also functions as a RelA S536,276 phosphatase, thereby inhibiting NF-κB activation.145
PPM1A suppression, similar to PTEN deficiency, increases SMAD3 phosphorylation and stimulates expression of fibrotic genes while PPM1A overexpression inhibits both events.146 These findings suggest that PTEN is an upstream regulator of PPM1A in dysfunctional tissue repair and implicate PPM1A as a novel repressor of the SMAD3 fibrotic response. TGF-β1 appears to attenuate PPM1A and PTEN expression through protein ubiquitination and subsequent degradation since the proteasome inhibitor MG132 rescues PPM1A and PTEN expression, even in the presence of TGF-β1.134 While the mechanism is unclear, signaling through TLR4 or the TGF-βR as well as through HA/CD44 complexes stimulates transcription of the PTEN-targeting microRNA miR-21, and PTEN deficiency has the same outcome as PPM1A knockdown (i.e., maintenance of SMAD3 phosphorylation and induction of profibrotic genes).134,135
TGF-β1-initiated Src kinase-dependent caveolin-1 Y14 phosphorylation is a critical event in RhoA/ROCK-mediated suppression of nuclear PPM1A levels maintaining, thereby, SMAD2/3-dependent transcription of profibrotic genes.142 PTEN activity and cellular location, moreover, are also regulated by Rho kinases and ROCK can phosphorylate PTEN.147,148 One possibility is that PTEN phosphorylation dissociates PTEN-PPM1A complexes resulting in PPM1A degradation, thereby, retaining SMAD transcriptional activity.142 Thus, depending on the actual magnitude and duration of the stimulus (e.g., DAMPs and/or TGF-β1), PTEN may function as a rheostat to influence the amplitude and kinetics of the inflammatory response to tissue injury. Clarifying molecular pathways downstream of PTEN in tissue injury may lead to the identification of novel mechanistically relevant and translationally accessible targets underlying TLR4/TGF-β1 signaling to transcriptional controls on disease-causative genes.
Conclusions
The available data provide for a hypothetical model whereby cooperation among the HA/CD44, EDA/TLR4, and TGF-β1 signaling pathways converge to regulate the wound-initiated DAMP-dependent sterile fibroinflammatory response and, thereby, repair outcomes (Figs. 1 and 5). While, the DAMPs FnEDA and HA are generated at the site of injury, TGF-β1 is released by degranulated platelets, as well as produced by infiltrating immune cells and local epithelial and fibroblastic elements. FnEDA downregulates Bambi, perhaps through formation of NF-κB p50/HDAC1 complexes to repress Bambi transcription145 making cells more responsive to TGF-β1 in the wound field, resulting in the increased expression of TGF-β1 target profibrotic genes. The net outcome of this reprogramming promotes a gradual increase in tissue rigidity facilitating the tension-induced unfolding of the FnEDA molecule and exposing the EDA domain for TLR4 activation.
Although it is not clear if other endogenous DAMP-like TLR4 ligands similarly augment TGF-β1 signaling and transcription of fibroinflammatory genes, bacterial LPS also enhances cellular sensitivity to TGF-β1 through Bambi downregulation, while promoting tissue fibrosis.149 Microbial contamination, biofilm formation and prolonged inflammation are significant contributors to the pathophysiology of burn-associated hypertrophic scarring, in which dermal fibroblasts likely regulate the amplitude and duration of the LPS → TLR4-initiated inflammatory response.150 While inflammation drives wound repair and regeneration, chronic inflammation leads to the development of pathologic fibrosis,23 perhaps through induction of osteopontin expression by injury-site fibroblasts.26 In this context, osteopontin appears to inhibit the rate of cutaneous injury repair and triggers hypergranulation and subsequent fibrosis.26 Similarly, topical application of PDGF-βB to chronic ulcers accelerates healing but may also foster the development of excessive granulation tissue and scarring as part of increased osteopontin expression. Indeed, downregulation of osteopontin may be one mechanism whereby Gleevec reduces pulmonary and dermal fibrosis.26
LPS-TLR4 interactions, moreover, require a src kinase-activated EGFR to induce NF-κB-directed expression of inflammatory genes151–153 suggesting extensive crosstalk between the TLR4 and EGFR pathways. NF-κB signaling in response to EGF, moreover, requires both EGFR and TLR4 activity and TLR4-induced NF-κB mobilization following LPS stimulation is EGFR dependent.151 Src family kinases are required for NF-κB activation by EGF and LPS while the induced proinflammatory cytokine response to LPS is attenuated by the EGFR inhibitor Erlotinib.151 Similar EGFR/TLR4 crosstalk exists in response to Fn-derived DAMPs in human dermal fibroblasts resulting in TLR4 signaling. Collectively, these findings suggest that pharmacologic approaches that target the EGFR and/or src kinases may have therapeutic efficacy in regulating DAMP-initiated TGF-β1 hypersensitivity and fibroproliferative disease.131
TGF-β1 also promotes the synthesis of the EDA splice variant of Fn by increasing expression of the splicing regulatory protein SRp40 thus initiating a profibrotic feed-forward loop.154 TGF-β1-induced FnEDA production, moreover, is dependent on PI3 kinase-AKT signaling.155 Since the HA/CD44, EDA/TLR4, and TGF-β1 pathways each induce miR-21 transcription and PTEN downregulation, the subsequent increase in AKT activity would additionally reinforce FnEDA expression and progressive scar formation. Whether inhibiting the generation of FnEDA alternative splicing has therapeutic utility in the context of maladaptive wound repair is an innovative approach to healing anomalies.
Take-Home Messages
Fibrosis is a frequent pathophysiological consequence of chronic inflammation due to tissue injury.
DAMPs generated at the site of injury are TLR and CD44 agonists that stimulate the production of proinflammatory cytokines and sustain the inflammatory response.
TLR4 functions as a molecular switch, binding the EDA domain of Fn to activate transcription of NF-κB-regulated target genes while intersecting with growth factor (EGF, TGF-β)-signaling pathways.
The DAMP-rich microenvironment sensitizes cells to TGF-β1 in the immediate injury field due to TLR4-mediated downregulation of the TGF-β pseudoreceptor Bambi.
TLR4 induction of inflammatory cytokines and stimulated expression of profibrotic factors as a result of an increase in TGF-β1 signaling may create a sustained TLR4→TGF-β1→FnEDA→TLR4 feed-forward loop that culminates in excessive scarring and tissue dysfunction.
Acknowledgments and Funding Sources
Support for this work was provided by NIH grants R01-CA58626 and R21-AR0667956 (to PJM-L) and R01-GM057241 (to PJH) as well as by the Graver Family Endowed Fund, the Friedman Family Cancer Research Endowment, and the Roach Foundation (to PJH).
Abbreviations and Acronyms
- CK2
Casein kinase 2
- DAMP
damage-associated molecular pattern
- ECM
extracellular matrix
- EDA
extra domain A
- EGFR
epidermal growth factor receptor
- FnEDA
EDA isoform of fibronectin
- GAG
glycosaminoglycan
- HA
hyaluronic acid
- HAS
HA synthase
- HMW
high molecular weight
- HYAL
hyaluronidase
- IL
interleukin
- LAP
latency-associated peptide
- LMW
low-molecular-weight
- LPS
lipopolysaccharide
- LTBP
latent GFF-β-binding protein
- MAP
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- MYD88
myeloid differentiation factor 88
- PTEN
phosphatase and tensin homolog
- RGD
arginine–glycine–aspartic acid
- RHAMM
receptor for hyaluronan-mediated motility
- ROS
reactive oxygen species
- TGF-β
transforming growth factor beta
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- TRK
receptor tyrosine kinase
- α-SMA
α-smooth muscle cell actin
Author Disclosure and Ghost Writing
The authors do not have any commercial conflicts of interest. The article was written exclusively by Drs. McKeown-Longo and Higgins; no ghostwriters were involved.
About the Authors
Drs. Paula J. McKeown-Longo, PhD and Paul J. Higgins, PhD are Co-Chairs of the Department of Regenerative and Cancer Cell Biology at the Albany Medical College. Dr. McKeown-Longo is an expert in extracellular matrix biology and her work focuses on the role of fibronectin in cellular function and signaling. Research in Dr. Higgins' laboratory centers on the molecular mechanisms underlying transcription of TGF-β1 target genes and their involvement in wound healing and fibrosis.
References
- 1. Martin P Wound healing - aiming for perfect skin regeneration. Science 1997;276:75–81 [DOI] [PubMed] [Google Scholar]
- 2. Chicharro-Alcantara D, Rubsio-Zaragoza M, Damia-Gimenez E, et al. . Platelet rich plasma: New insights for cutaneous wound healing management. J Funct Biomater 2018;9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weiskirchen R, Weiskirchen S, Tacke F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol Aspects Med 2019;65:2–15 [DOI] [PubMed] [Google Scholar]
- 4. Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009;17:153–162 [DOI] [PubMed] [Google Scholar]
- 5. Hynes RO, Naba A. Overview of the matrisome - an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol 2012;4:a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong VW, Longaker MT, Gurtner GC. Soft tissue mechanotransduction in wound healing and fibrosis. Semin Cell Dev Biol 2012;23:981–986 [DOI] [PubMed] [Google Scholar]
- 7. D'Arpa P, Leung KP. Toll-like receptor signaling in burn wound healing and scarring. Adv Wound Care (New Rochelle) 2017;6:330–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev 2019;99:665–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care (New Rochelle) 2015;4:119–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arodz T, Bonchev D, Diegelmann RF. A network approach to wound healing. Adv Wound Care (New Rochelle) 2013;2:499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roh JS, Sohn DH. Damage-associated molecular patterns in inflammatory diseases. Immune Netw 2018;18:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010;10:826–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kelsh R, You R, Horzempa C, Zheng M, McKeown-Longo PJ. Regulation of the innate immune response by fibronectin: synergism between the III-1 and EDA domains. PLoS One 2014;9:e102974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McKeown-Longo PJ, Higgins PJ. Integration of canonical and noncanonical pathways in TLR4 signaling: complex regulation of the wound repair program. Adv Wound Care (New Rochelle) 2017;6:320–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muto J, Sayama K, Gallo RL, Kimata K. Emerging evidence for the essential role of hyaluronan in cutaneous biology. J Dermatol Sci 2019;94:190–195 [DOI] [PubMed] [Google Scholar]
- 16. Land WG Damage-Associated Molecular Patterns in Human Diseases. Volume 1: Injury-Induced Innate Immune Responses. London, United Kingdom:Springer, 2018 [Google Scholar]
- 17. Frevert CW, Felgenhauer J, Wygrecka M, Nastase MV, Schaefer L. Danger-associated molecular patterns derived from the extracellular matrix provide temporal control of innate immunity. J Histochem Cytochem 2018;66:213–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol 2007;23:435–461 [DOI] [PubMed] [Google Scholar]
- 19. Campo GM, Avenoso A, Campo S, D'Ascola A, Nastasi G, Calatroni A. Small hyaluronan oliosaccharides induce inflammation by engaging both toll-like-4 and CD44 receptors in human chondrocytes. Biochem Pharmacol 2010;80:480–490 [DOI] [PubMed] [Google Scholar]
- 20. Jiang D, Liang J, Fan J, et al. . Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 2005;11:1173–1179 [DOI] [PubMed] [Google Scholar]
- 21. McFadden JP, Basketter DA, Dearman RJ, Kimber IR. Extra domain A-positive fibronectin-positive feedback loops and their association with cutaneous inflammatory disease. Clin Dermatol 2011;29:257–265 [DOI] [PubMed] [Google Scholar]
- 22. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012;18:1028–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science 2017;356:1026–1030 [DOI] [PubMed] [Google Scholar]
- 24. Meyer M, Müller A-K, Yang J, Sulcová J, Werner S. The role of chronic inflammation in cutaneous fibrosis: fibroblast growth factor receptor deficiency in keratinocytes as an example. J Invest Dermatol Symp Proc 2011;15:48–52 [DOI] [PubMed] [Google Scholar]
- 25. Shaw TJ, Kishi K, Mori R. Wound-associated skin fibrosis: mechanisms and treatments based on modulating the inflammatory response. Endocr Metab Immune Disord Drug Targets 2010;10:320–330 [DOI] [PubMed] [Google Scholar]
- 26. Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med 2008;205:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brancato SK, Thomay AA, Daley JM, et al. . Toll-like receptor 4 signaling regulates the acute local inflammatory response to injury and the fibrosis/neovascularization of sterile wounds. Wound Repair Regen 2013;21:624–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen L, Guo S, Ranzer MJ, DiPietro LA. Toll-like receptor 4 has an essential role in early skin wound healing. J Invest Dermatol 2013;133:258–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suga H, Sugaya M, Fujita H, et al. . TLR4, rather thanTLR2, regulates wound healing through TGF-β and CCL5 expression. J Dermatol Sci 2014;73:117–124 [DOI] [PubMed] [Google Scholar]
- 30. Meran S, Luo DD, Simpson R, et al. . Hyaluronan facilitates transforming growth factor-β1-dependent proliferation via CD44 and epidermal growth factor receptor interaction. J Biol Chem 2011;286:17618–17630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zent J, Guo LW. Signaling mechanisms of myofibroblastic activation: outside-in and inside-out. Cell Physiol Biochem 2018;49:848–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Balaji S, King A, Marsh E, et al. . The role of interleukin-10 and hyaluronan in murine fetal fibroblast functin in vitro: implications for recapitulating fetal regenerative wound healing. PLoS One 2015;10:e124302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. desJardins-Park HE, Mascharak S, Chinta MS, Wan DC, Longaker MT. The spectrum of scarring in carniofacial wound repair. Front Physiol 2019;10:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steen EH, Wang X, Balaji S, Butte MJ, Bollyky PL, Keswani SG. The role of the anti-inflammatory cytokine interleukin-10 in tissue fibrosis. Adv Wound Care 2020;9:184–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toole BP Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 2004;4:528–539 [DOI] [PubMed] [Google Scholar]
- 36. Albeiroti S, Soroosh A, de la Motte CA. Hyaluronan's role in fibrosis: a pathogenic factor or a pasive player. Biomed Res Int 2015;2015:790203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mast BA, Flood LC, Haynes JH, et al. . Hyaluronic acid is a major component of the matrix of fetal rabbit skin and wounds: implications for healing by regeneration. Matrix 1991;11:63–68 [DOI] [PubMed] [Google Scholar]
- 38. Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 1997;242:27–33 [DOI] [PubMed] [Google Scholar]
- 39. Aya KL, Stern R. Hyaluronan in wound healing: rediscovering a major player. Wound Repair Regen 2014;22:579–593 [DOI] [PubMed] [Google Scholar]
- 40. Bourguignon V, Flamion B. Respective roles of hyaluronidases 1 and 2 in endogenous hyaluronan turnover. FASEB J 2016;30:2108–2114 [DOI] [PubMed] [Google Scholar]
- 41. Thankamony SP, Knudson W. Acylation of CD44 and its association with lipid rafts are required for receptor and hyaluronan endocytosis. J Biol Chem 2006;281:34601–34609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andre B, Duterme C, Van Moer K, Mertens-Strijthagen J, Jadot M, Flamion B. Hyal2 is a glycosylphosphatidylinositol-anchored, lipid raft-associated hyaluronidase. Biochem Biophys Res Commun 2011;411:175–179 [DOI] [PubMed] [Google Scholar]
- 43. Stern R Devising a pathway for hyaluronan catabolism: are we there yet? Glycobiology 2003;13:105R-15R [DOI] [PubMed] [Google Scholar]
- 44. Litwiniuk M, Krejner A, Grzela T. Hyaluronic acid in inflammation and tissue regeneration. Wounds 2016;28:78–88 [PubMed] [Google Scholar]
- 45. Tavianatou AG, Caon I, Franchi M, Piperigkou Z, Galesso D, Karamanos NK. Hyaluronan: molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J 2019;286:2883–2908 [DOI] [PubMed] [Google Scholar]
- 46. Stamenkovic I, Yu Q. Merlin, a “magic” linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr Protein Pept Sci 2010;11:471–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bourguignon LYW Matrix hyaluronan-activated CD44 signaling promotes keratinocyte activities and improved abnormal epidermal functions. Am J Pathol 2014;184:1912–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bourguignon LY, Xia W, Wong G. Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates beta-catenin signaling an NF-kappaB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells. J Biol Chem 2009;284:2657–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bourguignon LY, Wong G, Earle C, Krueger K, Spevak CC. Hyaluronan-CD44 interaction promotes c-Src-mediated twist signaling, microRNA-10b expression, and RhoA/RhoC up-regulation, leading to Rho-kinase-associated cytoskeleton activation and breast tumor cell invasion. J Biol Chem 2010;285:36721–36735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bourguignon LYW Matrix hyaluronan-CD44 interaction activates microRNA and LncRNA signaling associated with chemoresistance, invasion, and tumor progression. Front Oncol 2019;9:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Taylor KR, Yamasaki K, Radek KA, et al. . Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on toll-like receptor 4, CD44, and MD-2. J Biol Chem 2007;282:18265–18275 [DOI] [PubMed] [Google Scholar]
- 52. Zgheib C, Xu J, Liechty KW. Targeting inflammatory cytokines and extracellular matrix composition to promote wound regeneration. Adv Wound Care 2014;3:344–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ito T, Williams JD, Fraser DJ, Phillips AO. Hyaluronan regulates transforming growth factor-β1 receptor compartmentalization. J Biol Chem 2004;279:25326–25332 [DOI] [PubMed] [Google Scholar]
- 54. Knudson W, Chow G, Knudson CB. CD44-mediated uptake and degradation of hyaluronan. Matrix Biol 2002;21:15–23 [DOI] [PubMed] [Google Scholar]
- 55. D'Agostino A, Stellavato A, Corsuto L, et al. . Is molecular size a discriminating factor in hyaluronan interaction with human cells. Carbohydr Polym 2017;157:21–30 [DOI] [PubMed] [Google Scholar]
- 56. Misra S, Hascall VC, Markwald RR, Ghatak S. Interactions between Hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front Immunol 2015;6:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Scheibner KA, Lutz MA, Boodoo S, Fenton J, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 2006;177:1272–1281 [DOI] [PubMed] [Google Scholar]
- 58. Bourgulanon LY, Wong G, Earle CA, Xia W. Interaction of low molecular weight hyaluronan with CD44 and toll-like receptors promotes the actin filament-associated protein 110-actin binding and MyDD88-NFκB signaling leading to proinflammatory cytokine/chemokine production and breast tumor invasion. Cytoskeleton (Hoboken) 2011;68:671–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang HZ, Wang JP, Mi S, et al. . TLR4 activity is required in the resolution of pulmonary inflammation and fibrosis after acute and chronic lung injury. Am J Pathol 2012;180:275–292 [DOI] [PubMed] [Google Scholar]
- 60. Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol 2006;85:699–715 [DOI] [PubMed] [Google Scholar]
- 61. Bourguignon LY, Bikle D. Selective Haluronan-CD44 signaling promotes miRNA-21 expression and interacts with Vitamin D function during cutaneous squamous cell carcinomas progression following UV irradiation. Front Immunol 2015;6:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hall CL, Lange LA, Prober DA, Zhang S, Turley EA. pp60(c-src) is required for cell locomotion regulated by the hyaluronan receptor RHAMM. Oncogene 1996;13:2213–2224 [PubMed] [Google Scholar]
- 63. Kouvidi K, Berdiaki A, Nikitovic D, et al. . Role of receptor for hyaluronic acid-mediated motility (RHAMM) in low molecular weight hyaluronan (LMWHA)-mediated fibrosarcoma cell adhesion. J Biol Chem 2011;286:38509–38520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem 2001;276:36770–36778 [DOI] [PubMed] [Google Scholar]
- 65. Tolg C, Hamilton SR, Nakrieko KA, et al. . Rhamm-/- fibroblasts are defective in CD44-mediated ERK1,2 motogenic signaling, leading to defective skin would repair. J Cell Biol 2006;175:1017–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tolg C, Hamilton SR, Zalinska E, et al. . A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds. Am J Pathol 2012;181:1250–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tolg C, McCarthy JB, Yazdani A, Turley E. Hyaluronan and RHAMM in wound repair an the “cancerization” of stromal tissues. J Biomed Res Int 2014;2014:103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tolg C, Telmer P, Turley E. Specific sizes of hyaluronan oligosaccharides stimulate fibroblast migration and excisional wound repair. PLoS One 2014;9:e88479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Monslow J, Govindaraju P, Pure E. Hyaluronan - a functional and structure sweet spot in the tissue microenvironment. Front Immunol 2015;6:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Garantziotis S, Savani RC. Hyaluronan biology: a complex balancing act of structure, function, location and context. Matrix Biol 2019;78–79:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Maurer LM, Ma W, Mosher DF. Dynamic structure of plasma fibronectin. Crit Rev Biochem Mol Biol 2015;51:213–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bradshaw MJ, Smith ML. Multiscale relationships between fibronectin structure and functional properties. Acta Biomater 2013;10:1524–1531 [DOI] [PubMed] [Google Scholar]
- 73. Mezzenga R, Mitsi M. The molecular dance of fibronectin: conformational flexibility leads to functional versatility. Biomacromolecules 2019;20:55–72 [DOI] [PubMed] [Google Scholar]
- 74. ffrench-Constant C, van de Water L, Dvorak HF, Hynes RO. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol 1989;109:903–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tellios N, Belrose JC, Tokarewicz AC, et al. . TGF-β induces phosphorylation of phosphatase and tensin homolog: implications for fibrosis of the trabecular meshwork tissue in glaucoma. Sci Rep 2017;7:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Parapuram SK, Shi-wen X, Elliott C, et al. . Loss of PTEN expression by dermal fibroblasts causes skin fibrosis. J Invest Derrmatol 2011;131:1996–2003 [DOI] [PubMed] [Google Scholar]
- 77. White ES, Sagana RL, Booth AJ, et al. . Control of fibroblast fibronectin expression and alternative splicing via the PI3K/Akt/mTOR pathway. Exp Cell Res 2010;316:2644–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lavigueur A, La Branche H, Kornblihtt AR, Chabot B. A splicing enhancer in the human fibronectin alternatve ED1 exon interacts with SR proteins and stimulates U2 snRNP binding. Genes Dev 1993;7:2405–2417 [DOI] [PubMed] [Google Scholar]
- 79. Kelsh-Lasher RM, Ambesi A, Bertram C, McKeown-Longo PJ. Integrin α4β1 and TLR4 cooperate to induce fibrotic gene expression in response to fibronectin's EDA domain. J Invest Dermatol 2017;137:2505–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kelsh RM, McKeown-Longo PJ, Clark RAF. EDA fibronectin in keloids create a vicious cycle of fibrotic tumor formation. J Invest Dermatol 2015;135:1714–1718 [DOI] [PubMed] [Google Scholar]
- 81. Ffrench-Constant C Alternative splicing of fibronectin—many different proteins but few different functions. Exp Cell Res 1995;221:261–271 [DOI] [PubMed] [Google Scholar]
- 82. Grinnell F, Ho CH, Wysocki A. Degradation of fibronectin and vitronectin in chronic wound fluid: analysis of cell blotting, immunoblotting, and cell adhesion assays. J Invest Dermatol 1992;98:410–416 [DOI] [PubMed] [Google Scholar]
- 83. Muro AF, Chauhan AK, Gajovic S, et al. . Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol 2003;162:149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Booth AJ, Wood SC, Cornett AM, et al. . Recipient-derived EDA fibronectin promotes cardiac allograft fibrosis. J Pathol 2012;226:609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Muro AF, Moretti FA, Moore BB, et al. . An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Resp Crit Care Med 2008;177:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentation. Am J Pathol 2001;159:1009–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Serini G, Bochaton-Piallat ML, Ropraz P, et al. . The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-β1. J Cell Biol 1998;142:873–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Edsberg LE, Wyffels JT, Brogan MS, Fries KM. Analysis of the proteomic profile of chronic pressure ulcers. Wound Repair Regen 2012;20:378–401 [DOI] [PubMed] [Google Scholar]
- 89. Okamura Y, Watari M, Jerud ES, et al. . The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 2002;276:10229–10233 [DOI] [PubMed] [Google Scholar]
- 90. Bhattacharyya S, Tamaki Z, Wang W, et al. . FibronectinEDA promotes chronic cutaneous fibrosis through toll-like receptor signaling. Sci Transl Med 2014;6:232ra50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Andrews JP, Marttala J, Macarak E, Rosenbloom J, Uitto J. Keloid pathogenesis: potential role of cellular fibronectin with the EDA domain. J Invest Dermatol 2015;135:1921–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mogami H, Kishore AH, Shi H, Keller PW, Akgul Y, Word RA. Fetal fibronectin signaling induces matrix metalloproteases and cyclooxygenase-2 (COX-2) in amnion cells and preterm birth in mice. J Biol Chem 2013;288:1953–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jian M, Dhanesha N, Doddapattar P, Chorawala MR, et al. . Smooth muscle cell-specific fibronectin-EDA mediates phenotypic switching and neointimal hyperplasia. J Clin Invest 2020;130:295–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kohan M, Muro AF, White ES, Berkman N. EDA-containing cellular fibronectin induces fibroblast differentation through bindinhg of α4β7 integrin receptor and MAPK/Erk 1/2-dependent signaling. FASEB J 2010;24:4503–4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shinde AV, Bystroff C, Wang C, et al. . Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin α9β1-dependent cellular activities. J Biol Chem 2008;283:2858–2870 [DOI] [PubMed] [Google Scholar]
- 96. Ou J, Peng Y, Deng J, et al. . Endothelial cell-derived fibronectin extra domain A promotes colorectal cancer metastasis via inducing epithelial-mesenchymal transition. Carcinogenesis 2014;35:1661–1670 [DOI] [PubMed] [Google Scholar]
- 97. Shinde AV, Kelsh R, Peters JH, Sekiguchi K, van de Water L, McKeown-Longo PJ. The α4β1 integrin and the EDA domain of fibronectin regulate a profibrotic phenotype in dermal fibroblasts. Matrix Biol 2015;41:26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Finnson KW, Arany PR, Philip A. Transforming growth factor beta signaling in cutaneous wound healing: lessons learned from animal studies. Adv Wound Care (New Rochelle) 2013;2:225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lichtman MK, Oero-Vinas M, Falanga V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen 2016;24:215–222 [DOI] [PubMed] [Google Scholar]
- 100. Robertson IB, Rifkin DB. Regulation of the bioavailability of TGF-β and TGF-β-related proteins. Cold Spring Harb Perspect Biol 2016;8:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zilberberg L, Todorovic V, Dabovic B, et al. . Specificity of latent TGF-β binding protein (LTBP) incorporation into matrix: role of fibrillins and fibronectin. J Cell Physiol 2012;227:3828–3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tsuda T Extracellular interactions between fibulins and transforming growth factor (TGF)-β in physiological and pathological conditions. Int J Mol Sci 2018;19:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Klingberg F, Chau G, Walraven M, et al. . The fibronectin ED-A domain enhances recruitment of latent TGF-β binding protein-1 to the fibroblast matrix. J Cell Sci 2018;131:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hinz B The extracellular matrix and transforming growth factor-β1: tale of a strained relationship. Matrix Biol 2015;47:54–65 [DOI] [PubMed] [Google Scholar]
- 105. Troilo H, Steer R, Collins RF, Kielty CM, Baldock C. Independent multimerization of latent TGFβ binding protein-1 stabilized by cross-linking and enhanced by heparan sulfate. Sci Rep 2016;6:34347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dong X, Zhao B, Iacob RE, et al. . Force interacts with macromolecular structure in activation of TGF-β. Nature 2017;542:55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sheppard D Epithelial-mesenchymal interactions in fibrosis and repair. Transforming growth factor-β activation by epithelial cells and fibroblasts. Ann Am Thorac Soc 2015;12 (Suppl 1):S21–S23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nickel J, Ten Dijke P, Mueller TD. TGF-b family co-receptor function and signaling. Acta Biochim Biophys Sin (Shanghai) 2018;50:12–36 [DOI] [PubMed] [Google Scholar]
- 109. Buscemi L, Ramonet D, Klingberg F, et al. . The single-molecule mechanics of the latent TGF-β1 complex. Curr Biol 2011;21:2046–2054 [DOI] [PubMed] [Google Scholar]
- 110. Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep 2010;22:97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Brown NF, Marshall JF. Integrin-mediated TGFβ activation modulates the tumour microenvironment. Cancers (Basel) 2019;11:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nolte M, Margadant C. Controlling immunity and inflammation through integrin-dependent regulation of TGF-β. Trends Cell Biol 2020;30:49–59 [DOI] [PubMed] [Google Scholar]
- 113. Campbell MG, Cormier A, Ito S, et al. . Cryo-ECM reveals integrin-mediated TGF-β1 activation without release from latent TGF-β1. Cell 2020;180:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Klingberg F, Chow ML, Koehler A, et al. . Prestress in the extracellular matrix sensitizes latent TGF-β1 for activation. J Cell Biol 2014;207:283–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chang Y, Lau WL, Jo H, et al. . Pharmacologic blockade of αvβ1 integrin ameliorates renal failure and fibrosis in vivo. J Am Soc Nephrol 2017;28:1998–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jorgenson AJ, Choi KM, Sicard D, et al. . TAZ activation drives fibroblast spheroid growth, exprssion of profibrotic paracrine signals, and context-dependent ECM gene expression. Am J Physiol Cell Physiol 2017;312:C277–C285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Totaro A, Panciera T, Piccolo S. YAP/TAZ upstream signals and downstream responses. Nat Cell Biol 2018;30:888–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Anorga S, Overstreet JM, Falke LL, et al. . Deregulation of Hippo-TAZ pathway during renal injury confers a fibrotic maladaptive phenotype. FASEB J 2018;32:2644–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Herrera J, Henke CA, Bitterman PB. Extracellular matrix as a driver of progressive fibrosis. J Clin Invest 2018;128:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pakshir P, Hinz B. The big five in fibrosis: marophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol 2018;68–69:81–93 [DOI] [PubMed] [Google Scholar]
- 121. Stuhlmeier KM, Pollaschek C. Differential effect of transforming growth factor beta (TGF-beta) on the genes encoding hyaluronan synthases and utilization of the p38 MAPK pathway in TGF-beta-induced hyaluronan synthase 1 activation. J Biol Chem 2004;279:8753–8760 [DOI] [PubMed] [Google Scholar]
- 122. Guo N, Li X, Mann MM, Funderburgh ML, Du Y, Funderburgh JL. Hyaluronan synthesis mediates the fibrotic response of keratocytes to transforming growth factor β. J Biol Chem 2010;285:32012–32019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Webber J, Jenkins RH, Meran S, Phillips A, Steadman R. Modulation of TGFbeta1 dependent myofibroblast differentiation by hyaluronan. Am J Pathol 2009;175:148–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Midgley AC, Rogers M, Hallett MD, et al. . Transforming growth factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated eidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. J Biol Chem 2013;288:14824–14838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Samarakoon R, Higgins SP, Higgins CE, Higgins PJ. TGF-beta1-induced plasminoghen activator inhibitor-1 expression in vascular smooth muscle cells requires pp60(c-src)/EGFR(Y845) and Rho/ROCK signaling. J Mol Cell Cardiol 2008;44:527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Samarakoon R, Dobberfuhl AD, Cooley C, et al. . Induction of renal fibrotic genes by TGF-β1 requires EGFR activation, p53 and reactive oxygen species. Cell Signal 2013;25:2198–2209 [DOI] [PubMed] [Google Scholar]
- 127. Yakymovych I, Yakymovych M, Heldin CH. Intracellular trafficking of transforming growth factor β receptors. Acta Biochim Biophys Sin (Shanghai) 2018;50:3–11 [DOI] [PubMed] [Google Scholar]
- 128. David-Raoudi M, Tranchepain F, Deschrevel B, et al. . Differential effects of hyaluronan and its fragments on fibroblasts: relation to wound healing. Wound Repair Regen 2008;16:274–287 [DOI] [PubMed] [Google Scholar]
- 129. Webber J, Meran S, Steadman R, Phillips A. Hyaluronan orchestrates transforming growth factor-beta1-dependent maintenance of myofibroblast phenotype. J Biol Chem 2009;284:9083–9092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bhattacharyya S, Wang W, Qin W, et al. . TLR4-dependent fibroblast activation drives persistent organ fibrosis in skin and lung. J Clin Invest Insight 2018;3:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Valenty LM, Longo CM, Horzempa C, Ambesi A, McKeown-Longo PJ. TLR4 ligands selectively synergize to induce expression of IL-8. Adv Wound Care (New Rochelle) 2017;6:309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bhattacharyya S, Kelley K, Melichian D, et al. . Toll-like receptor 4 signaling augments transforming growth factor-β responses: a novel mechanism for maintaining and amplifying fibrosis in scleroderma. Am J Pathol 2013;182:192–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lan R, Geng H, Polichnowski AJ, et al. . PTEN loss defines a TGF-β-induced tubule phenotype of failed differentiation and JNK signaling during renal fibrosis. Am J Physiol Renal Physiol 2012;302:F1210–F1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tang J, Goldschmeding R, Samarakoon R, Higgins PJ. Protein phosphatase Mg2+/Mn2+ dependent-1A and PTEN deregulation in renal fibrosis: novel mechanisms and co-dependency of expression. FASEB J 2020;34:2641–2656 [DOI] [PubMed] [Google Scholar]
- 135. Samarakoon R, Helo S, Dobberfuhl AD, et al. . Loss of tumour suppressor PTEN expression in renal injury initiates SMAD3- and p53-dependent fibrotic responses. J Pathol 2015;236:421–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. He Z, Gao Y, Deng Y, et al. . Lipopolysaccharide induces lung fibroblast proliferation through Toll-like 4 signaling and the phosphoinositide3-kinase-Akt pathway. PLoS One 2012;7:e35926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kalantari P, Harandi OF, Agarwal S, et al. . miR-718 represses proinflammatory cytokine production through targetng phosphatase and tensin homolog (PTEN). J Biol Chem 2017;292:5634–5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Chen L, Bourguignon LY. Hyaluronan-CD44 interaction promotes c-Jun signaling and miRNA21 expression leading to Bcl-2 expression and chemoresistance in breast cancer cells. Mol Cancer 2014;13:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin Chim Acta 2010;411:846–852 [DOI] [PubMed] [Google Scholar]
- 140. Lin X, Duan X, Liang YY, et al. . PPM1A functions as a SMAD phosphatase to terminate TGFβ signaling. Cell 2006;125:915–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Overstreet JM, Samarakoon R, Cardona-Grau D, Goldschmeding R, Higins PJ. Tumor suppressor ataxia telangiectasia mutated functions downstream of TGF-β1 in orchestrating profibrotic responses. FASEB J 2015;29:1258–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Samarakoon R, Chitnis SS, Higgins SP, Higgins CE, Krepinsky JC, Higgins PJ. Redox-induced Src kinase and caveolin-1 signaing in TGF-β1-initiated SMAD2/3 activation and PAI-1 expression. PLoS One 2011;6:e22896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Li L, Li Q, Wei L, et al. . Chemokine (C-X-C motif) ligand 14 contributes to lipopolysaccharide-induced fibrogenesis in mouse L929 fibroblasts via modulating PPM1A. J Cell Biochem 2019;120:13372–13381 [DOI] [PubMed] [Google Scholar]
- 144. Bu S, Kapanadz B, Hsu T, Trojanowska M. Opposite effects of dihydrosphingosine 1-phosphate and sphingosine 1-phosphate on transforming growth factor-beta/Smad signaling are mediated through the PTEN/PPM1A-dependent pathway. J Biol Chem 2008;283:19593–19602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lu X, An H, Jin R, et al. . PPM1A is a RelA phosphatase with tumor suppressor-like activity. Oncogene 2014;33:2918–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Samarakoon R, Rehfuss A, Khakoo NS, et al. . Loss of expression of protein phosphatase magnesium-dependent 1A during kidney inijury promotes fibrotic maladaptive repair. FASEB J 2016;30:3308–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ross AH, Gericke A. Phosphorylation keeps PTEN phosphatase closed for business. Proc Natl Acad Sci USA 2009;106:1297–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Vazquez F, Grossman SR, Takahashi Y, Rokas MV, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J Biol Chem 2001;276:48627–48630 [DOI] [PubMed] [Google Scholar]
- 149. He Y, Ou Z, Chen X, et al. . LPS/TLR4 signaling enhances TGF-β response through downregulating BAMBI during prostatic hyperplasia. Sci Rep 2016;6:27051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wang J, Hori K, Ding J, et al. . Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J Cell Physiol 2011;226:1265–1273 [DOI] [PubMed] [Google Scholar]
- 151. De S, Zhou H, DeSantis D, Croniger CM, Li X, Stark GR. Erlotinib protects against LPS-induced endotoxicity because TLR4 needs EGFR to signal. Proc Nat Acad Sci USA 2015;112:9680–9685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Tao H, Li N, Zhang Z, et al. . Erlotinib protects LPS-induced acute lung injury in mice by inhibiting EGFR/TLR4 signaling pathway. Shock 2019;51:131–138 [DOI] [PubMed] [Google Scholar]
- 153. Qian Y, Han J, Zhou L, et al. . Inhibition of epidermal growth factor receptor (EGFR) reduces lipopolysaccaride (LPS)-induced activation and inflammatory cytokines in hepatic stellate cells in vitro. Med Sci Monit 2018;24:5533–5541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Han F, Gilbert JR, Harrison G, et al. . Transforming growth factor-beta1 regulates fibronectin isoform expression and splicing factor SRp40 expression during ATDC5 chondrogenic maturation. Exp Cell Res 2007;313:1518–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Madne TH, Dockrell MEC. TGFβ1-mediated PI3K/Akt and p38 MAP kinase dependent alternative splicing of fibronectin extra domain A in human podocyte culture. Cell Mol Biol (Noisy-le-grand) 2018;64:127–135 [PubMed] [Google Scholar]
- 156. Seki E, De Minicis S, Österreicher CH, et al. . TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat Med 2007;13:1324–1332 [DOI] [PubMed] [Google Scholar]
- 157. Liu C, Chen X, Yang L, Kisseleva T, Brenner DA, Seki E. Transcriptional repression of the transforming growth factor β (TGF-β) pseudoreceptor BMP and activin membrane-bound inhibitor (BAMBI) by nuclear factor κB (NF-κB) p50 enhances TGF-β signaling in hepatic stellate cells. J Biol Chem 2014;289:7082–7091 [DOI] [PMC free article] [PubMed] [Google Scholar]