Abstract
Context
Nutritional interventions stimulate muscle protein synthesis in older adults. To optimize muscle mass preservation and gains, several factors, including type, dose, frequency, timing, duration, and adherence have to be considered.
Objective
This systematic review and meta-analysis aimed to summarize these factors influencing the efficacy of nutritional interventions on muscle mass in older adults.
Data Sources
A systematic search was performed using the electronic databases MEDLINE, Embase, CINAHL, Cochrane Central Register of Controlled Trials, and SPORTDiscus from inception date to November 22, 2017, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Inclusion criteria included randomized controlled trials, mean or median age ≥65 years, and reporting muscle mass at baseline and postintervention. Exclusion criteria included genetically inherited diseases, anabolic drugs or hormone therapies, neuromuscular electrical stimulation, chronic kidney disease, kidney failure, neuromuscular disorders, and cancer.
Data Extraction
Extracted data included study characteristics (ie, population, sample size, age, sex), muscle mass measurements (ie, method, measure, unit), effect of the intervention vs the control group, and nutritional intervention factors (ie, type, composition, dose, duration, frequency, timing, and adherence).
Data Analysis
Standardized mean differences and 95%CIs were calculated from baseline to postintervention. A meta-analysis was performed using a random-effects model and grouped by the type of intervention.
Conclusions
Twenty-nine studies were included, encompassing 2255 participants (mean age, 78.1 years; SD, 2.22). Amino acids, creatine, β-hydroxy-β-methylbutyrate, and protein with amino acids supplementation significantly improved muscle mass. No effect was found for protein supplementation alone, protein and other components, and polyunsaturated fatty acids. High interstudy variability was observed regarding the dose, duration, and frequency, coupled with inconsistency in reporting timing and adherence. Overall, several nutritional interventions could be effective to improve muscle mass measures in older adults. Because of the substantial variability of the intervention factors among studies, the optimum profile is yet to be established.
Systematic Review Registration
PROSPERO registration no. CRD42018111306.
Keywords: aged, muscle mass, nutrition therapy, sarcopenia
INTRODUCTION
Advancing age is associated with a progressive loss of muscle mass, strength, and physical performance, which, when below a certain threshold, is defined as sarcopenia.1–3 Sarcopenia is prevalent in up to 50% of community-dwelling adults older than 80 years, according to the European Working Group on Sarcopenia in Older People 2010 definition4; contributes to increased risk of falls and fractures5; and exacerbates the debilitating effects of chronic diseases.6 Muscle mass declines 3% to 8% per decade after the age of 30 years and continues to decrease at a faster rate after the age of 60 years,7 greatly increasing the risk for development of sarcopenia. The variation in the rates of muscle mass decline among adults is dependent on modifiable lifestyle factors such as nutrition and physical activity.8–10 Thus, interventions targeting these factors are thought to play an important role in the prevention and management of sarcopenia.11
Poor caloric and protein intake impairs muscle protein synthesis and leads to skeletal muscle atrophy, causing impairment of physical performance over time.12 Nutritional supplementation, such as protein, creatine (CR), and essential amino acids or their metabolites, such as β-hydroxy-β-methylbutyrate (HMB), stimulate muscle protein synthesis in older adults.13–16 To optimize muscle mass preservation and gains, several factors, including type, dose, frequency, timing, duration, and treatment adherence have to be considered.17
The aim for this systematic review and meta-analysis was to summarize the aforementioned factors influencing the efficacy of nutritional interventions (ie, provision of nutrients separately from the diet18) on muscle mass measures in older adults. The systematic review and meta-analysis were performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.19
METHODS
Literature search
Participants, Interventions, Comparisons, Outcomes, and Study Design criteria were used to define the research question (Table 1). A systematic search was performed using 5 different electronic databases (namely, MEDLINE, Embase, CINAHL, Cochrane Central Register of Controlled Trials, and SPORTDiscus) to identify randomized controlled trials (RCTs) evaluating the effect of nutritional interventions on muscle mass measures of older adults. The systematic search was constructed by a senior liaison librarian (research and expert searching) from a biomedical university library. The search was performed from the inception date of each database to November 22, 2017. The systematic review was registered with the PROSPERO International Prospective Register of Systematic Reviews (registration no. CRD42018111306). The combination of Medical Subject Headings terms and keywords included muscle mass, fat-free mass, lean mass, nutrition, diet, and elderly. The complete search strategy is listed in Table S1 in the Supporting Information online.
Table 1.
Participants, Interventions, Comparisons, Outcomes, and Study Design (PICOS) criteria
| Parameter | Criteria |
|---|---|
| Participants | Older adults with a mean or median age of ≥ 65 years |
| Interventions | Nutritional interventions defined as the provision of nutrients separately from the diet |
| Comparisons | Control group defined as a placebo product, involving no additional nutritional supplementation or as the same supplementation as the intervention group without the ingredient of interest |
| Outcomes | Muscle mass measures, at least 1 muscle mass measurement (ie, lean mass, appendicular lean mass, skeletal muscle mass, or fat-free mass) reported both at baseline and postintervention |
| Study design | Randomized controlled trials |
Article selection
After the search, all studies obtained were assessed for eligibility by 2 independent assessors by reviewing titles and abstracts, followed by a full-text review. Any disagreements were settled through a discussion with a third assessor. The inclusion criteria required RCTs to be published in English, include human participants with a mean or median age of 65 years or older, and report at least 1 muscle mass measurement (ie, lean mass, appendicular lean mass, skeletal muscle mass, or fat-free mass) both at baseline and postintervention. Nutritional interventions included were defined as the provision of nutrients separately from the diet.18 The control group was required to consist of a placebo product, involve no additional nutritional supplementation, or include the same supplementation as the intervention group without the ingredient of interest. Studies involving nutritional counseling or education as the control group were also included but only if this was the intervention group. Studies including an exercise intervention were included if they had a separate intervention arm receiving only the nutritional intervention and a control group meeting the aforementioned criteria. The exclusion criteria consisted of any animal or in vitro studies, any population with genetically inherited diseases (eg, muscular dystrophies or inflammatory myopathies),20 studies involving the use of anabolic drugs or hormone therapies or neuromuscular electrical stimulation, populations with chronic kidney disease or kidney failure, and any population with diseases known to significantly affect muscle mass (eg, neuromuscular disorders,21 cancer,22 or HIV/AIDS).23
Data extraction
Data from the included studies were extracted independently by 2 assessors and cross-checked to settle any discrepancies with a third assessor. Any data not reported in table format were extracted from the text or figures. The following variables were extracted: author, year of publication, study population, type and composition of the intervention, sample size in the intervention and control group, mean or median age of the participants in years in each group, and the percentage of women in each group. The extracted sample size was the number of participants included in the analyses of the study (excluding participants who dropped out or were lost to follow-up). The following details were extracted for the nutritional intervention: type of intervention, dose (grams), duration (weeks), frequency (times per day), timing of administration, and adherence (percentage). Data extracted in relation to muscle mass measures encompassed the following: instrument or method used to measure muscle mass (eg, bioelectrical impedance analysis), the measure of muscle mass (ie, lean mass, fat free mass), units to express muscle mass (ie, kilograms, percentage, kilogram per square meter, cubic meter), the effect expressed as the mean difference in muscle mass measures from baseline to end of intervention, and the statistical significance.
Data synthesis
Studies were divided into groups according to the type of intervention, classified as amino acids (AAs; essential or nonessential), CR (including creatine monohydrate), HMB (or calcium HMB), polyunsaturated fatty acids (PUFAs), and protein supplementation. Studies with protein supplementation were further divided into 3 groups: protein supplementation alone, protein with AAs, or protein in combination with other supplements (namely, CR, HMB, and PUFAs). If the ingredient breakdown of a supplement was not provided, an online search of the specific product was performed to ensure the supplement was categorized correctly. Although all proteins are composed of AAs,24 only those protein interventions for which the specific AA composition of the protein was specified were grouped into the protein plus AA group.
A heat map was generated, grouped by type of intervention, to visualize a potential pattern for the dose, duration, frequency, timing, and adherence in relation to the effect size of the intervention in each study. Colors were assigned on the basis of what was hypothesized to be more effective for that particular factor: longer durations, larger doses, greater frequencies, and better adherence were expected to be more effective at increasing muscle mass measures.17 The colors were presented gradually relative to each other on a scale of red (less effective) to yellow to green (more effective). The dose was colored per group of type of intervention; the dose for all protein studies (ie, protein, protein plus AA, protein plus other) was colored as 1 group (relative to each other). The timing of the intervention administration was not colored as part of the heat map, because it was not possible to compare each variant of timing relative to each other. P values were colored as follows: green for P < 0.05, yellow for P values between ≥ 0.05 and < 0.10 (indicating a trend), and red for P ≥ 0.10.
Quality assessment
The quality assessment of studies was performed independently by 2 assessors and discrepancies were discussed with a third assessor using the Cochrane Risk of Bias Tool.25 This tool classifies studies as “low risk,” “high risk,” or “unclear risk” in regard to 7 possible sources of bias: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other sources of bias. Studies were graded as of high, moderate, or low quality in accordance with the following criteria: (1) high quality if all domains were met (all sources of bias are low risk) or 1 domain was of unclear risk; (2) moderate quality if 1 domain was not met (high risk) and 1 was of unclear risk, or alternatively, if 2 were of unclear risk; and (3) low quality if ≥ 3 domains were of unclear risk or ≥ 2 were not met (high risk).25
Meta-analysis
Muscle mass measures were extracted as mean and SD and/or change in (Δ) muscle mass and SD, or Δ muscle mass and 95%CI for the intervention and control groups. If muscle mass was measured at several time points, only baseline and postintervention measures were extracted. If the sampling distribution was provided as the SEM, this was converted to SD for analysis by multiplying SEM by the square root of the sample size.26 If data were reported for separate groups (eg, men and women), a combined mean for the 2 groups was obtained by calculating the weighted mean. Standardized mean differences (SMDs) were used to allow for comparison of effect sizes between studies27 and were expressed as SMD and 95%CIs. SMDs represented the net difference in muscle mass measures from baseline to the end of the intervention between the intervention and control groups.
A forest plot was generated for visualization of the meta-analysis results and grouped by the type of intervention. Meta-analyses were performed when ≥ 2 studies could be pooled. If studies did not report either sample size, baseline and postintervention values of muscle mass measures (or Δ) in terms of mean and SD, SEM, 95%CI, or exact P value, these studies were excluded from the meta-analysis, because the SMD could not be calculated.
A random-effect model was used because demographics and health status of participants differed across studies; therefore, the presence of heterogeneity was assumed.28 Heterogeneity was assessed using the I2 test, considering low heterogeneity present when I2 ≤ 25%, moderate heterogeneity when I2 > 25% and ≤ 50%; and high heterogeneity was considered present when I2 > 50%.29 For all statistical procedures, P < 0.05 was considered statistically significant. All analyses were performed using Comprehensive Meta-Analysis, version 3.3 (Biostat Inc., Englewood, NJ).
RESULTS
Search results
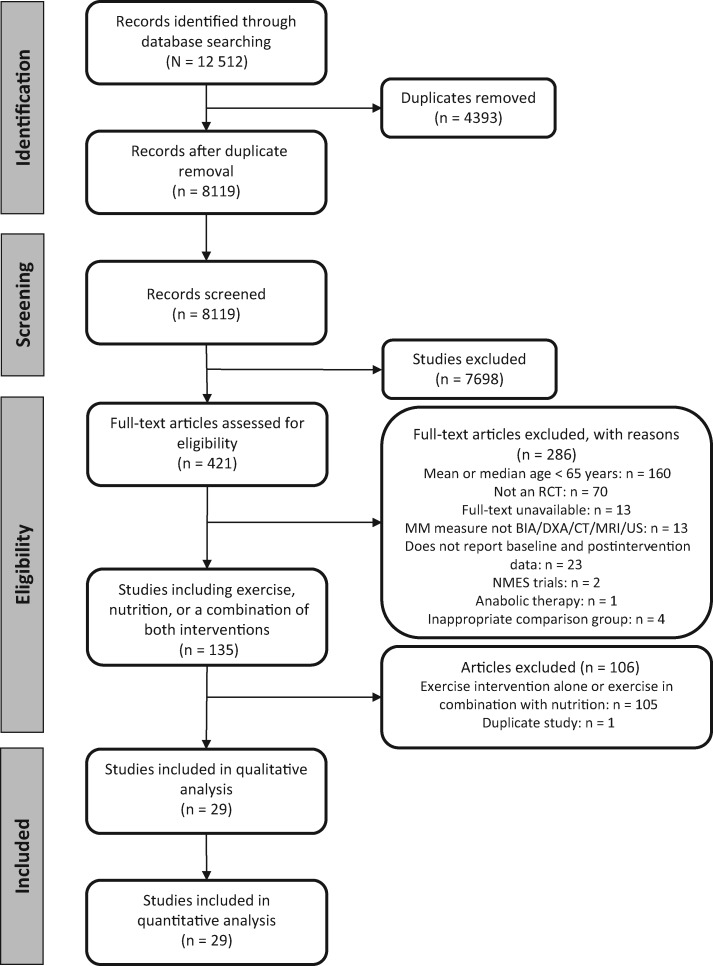
Figure 1 shows the study selection process. A total of 12 512 studies were identified through the database search. After removing duplicates, 8119 studies were screened for title and abstract and 421 studies were eligible for full-text screening. In total, 29 studies (reporting on 29 different studies) were included in the systematic review and meta-analysis.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart for the study selection process. Abbreviations: BIA, bioelectrical impedance analysis; CT, computed tomography; DXA, dual-energy X-ray absorptiometry; MM, muscle mass; MRI, magnetic resonance imaging; NMES, neuromuscular electrical stimulation; RCT, randomized controlled trial, US, ultrasound.
Article characteristics
Table 2 lists the characteristics of the included participants, the measurements of muscle mass, and the effect of the interventions. In total, 2255 participants were included; the range of participants per study was 18 to 380 and the overall median was 54 participants per study (interquartile range, 30–80). The weighted mean age was 78.1 years (SD, 2.22) and the proportion of women was 55.1%. Participants in studies that reported CR supplementation were all men. Most of the studies were performed in community-based populations (n = 20), 2 studies involved a combined population of community-dwelling and institutionalized older adults, 5 studies involved hospitalized patients, and 2 studies included geriatric outpatients. Muscle mass measures were mainly assessed using dual-energy X-ray absorptiometry (n = 16 studies) and bioelectrical impedance analysis (n = 8 studies).
Table 2.
Study characteristics of included studies, muscle mass measurements, and the effect of the intervention group compared with the control group
| Author (year) | Population | Intervention group |
Control group |
Muscle mass |
Effect |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | No.a | Age (years) ± SD | Female, No. (%) | No.a | Age (years) ± SD | Female, No. (%) | Method | Measure | Unit | MDb | P | ||
| AAs | |||||||||||||
| Dal Negro et al (2010)30 | Outpatients with COPD and sarcopenia | AAs | 16 | 75 ± 7 | 2 (12.5) | 16 | 75 ± 7 | 5 (31.3) | BIA | LMBI | kg/m2 | 1.360 | 0.09 |
| Dal Negro et al (2012)31 | Outpatients with COPD | AAs | 44 | 75 ± 5 | 12 (27.3) | 44 | 73 ± 8 | 15 (34.1) | BIA | LMBI | kg/m2 | 1.360 | 0.1 |
| Leenders et al (2011)32 | Community-dwelling with T2D | AAs | 29 | 71 ±1 | 0 | 28 | 71 ± 1 | 0 | DXA | LM | kg | 0.100 | NS |
| Malaguarnera et al (2007)33 | Community-dwelling, centenarians | AAs | 32 | 101 ± 1.3 | 22 (68.8) | 34 | 101 ± 1.4 | 23 (67.6) | BIA | FFM | kg | 3.000 | < 0.01 |
| Verhoeven et al (2009)34 | Community-dwelling, healthy | AAs | 15 | 71 ± 4c,d | 0 | 14 | 71 ± 4c,d | 0 | DXA | LM | kg | 0.000 | NS |
| Creatine | |||||||||||||
| Gotshalk et al. (2002)35 | Community-dwelling, healthy | CR | 10 | 65.4 ± 1.5 | 0 | 8 | 65.7 ± 2.0 | 0 | Hydrodensitometry | FFM | kg | 2.220 | < 0.05 |
| Marinari et al (2013)36 | Community-dwelling patients with COPD with CRF | CR | 30 | 73.2 ± 8.7 | NA | 25 | 73.9 ± 7.7 | NA | BIA | FFMI | kg/m2 | 4.300 | < 0.001 |
| Rawson et al (1999)37 | Community-dwelling, healthy | CR | 10 | 66.7 ± 1.9e | 0 | 10 | 66.9 ± 2.2e | 0 | Hydrodensitometry | FFM | kg | 0.500 | NS |
| HMB | |||||||||||||
| Baier et al(2009)38 | Community-dwelling and institutionalized | HMB | 40 | 75.4 ± 1.54 | 19 (47.5) | 37 | 76.2 ± 1.6 | 20 (54.0) | DXA | LM | kg | 0.380 | 0.05 |
| Deutz et al(2013)39 | Community-dwelling, healthy | HMB | 10 | 67.4 ± 1.4 | 8 (72.7) | 8 | 67.1 ± 1.7 | 7 (87.5) | DXA | LM | kg | 1.870 | 0.02 |
| Flakoll et al(2004)40 | Community-dwelling and institutionalized | HMB | 27 | 77.7 ± 1.5 | 27 (100) | 23 | 75.7 ± 1.6 | 23 (100) | BIA | FFM | kg | 0.700 | 0.08 |
| Protein | |||||||||||||
| Aleman-Mateo et al (2012)41 | Community-dwelling, healthy with sarcopenia | Protein | 20 | 75.4 ± 5.0 | 12 (60) | 20 | 76.7 ± 5.8 | 11 (55) | DXA | ALM | kg | 0.100 | 0.54 |
| Aleman-Mateo et al (2014)42 | Community-dwelling, healthy | Protein | 50 | 70.8 ± 7.6 | 25 (50) | 50 | 69.6 ± 6.4 | 25 (50) | DXA | ALM | kg | –0.200 | 0.009 |
| Bos et al(2000)43 | Hospitalized malnourished patients | Protein | 17 | 80 ± 7 | 10 (58.8) | 6 | 76 ± 6 | 3 (50) | DXA | ALM | kg | 0.500 | NS |
| Flodin et al(2015)44 | Hospitalized patients with hip fracture | Protein | 26 | 81 ± 8 | 19 (73) | 28 | 80 ± 9 | 18 (64) | DXA | ALM | kg | –0.380 | NS |
| Ha et al(2010)f,45 | Hospitalized patients with acute stroke at nutritional risk | Protein | 58 | 78.5 ± 7.4 | 33 (57) | 66 | 79.7 ± 6.8 | 31 (47) | BIS | LTM | kg | 0.099 | NS |
| Kerstetter et al (2015)46 | Community-dwelling | Protein | 106 | 69.9 ± 6.1 | 89 (84.0) | 102 | 70.5 ± 6.4 | 89 (87.3) | DXA | LM | kg | 0.500 | NS |
| Lauque et al(2004)47 | Hospitalized patients with AD, at risk of malnutrition | Protein | 37 | 79.5 ± 6.0 | NA | 43 | 78.1 ± 4.8 | NA | DXA | AFFM | kg | 0.030 | NS |
| Tieland et al (2012)48 | Community-dwelling with prefrailty or frailty | Protein | 34 | 78 ± 1 | 20 (58.8) | 31 | 81 ± 1 | 16 (51.6) | DXA | LM | kg | 0.100 | NS |
| Zhu et al(2015)49 | Community-dwelling, healthy, postmenopausal | Protein | 93 | 74.2 ± 2.8 | 93 (100) | 88 | 74.3 ± 2.6 | 88 (100) | DXA | ALM | kg | –0.060 | NS |
| Protein with AAs | |||||||||||||
| Bauer et al(2015)50 | Community-dwelling with sarcopenia | Protein + AAs | 184 | 77.3 ± 6.7 | 120 (65.2) | 196 | 78.1 ± 7.0 | 129 (65.8) | DXA | ALM | kg | 0.170 | 0.045 |
| Bonnefoy et al (2010)51 | Hospitalized acute patients with malnutrition and catabolic state | Protein + AAs | 15 | 82.5 ± 8.2 | 9 (61.5) | 15 | 79.4 ± 6.7 | 9 (57.1) | Deuterium dilution | FFM | kg | –0.700 | NS |
| Chanet et al(2017)52 | Community-dwelling, healthy | Protein + AAs | 12 | 70.3 ± 4.3 | 0 | 12 | 70.8 ± 3.5 | 0 | DXA | ALM | kg | 0.370 | 0.035 |
| Kemmler et al (2017)53 | Community-dwelling with sarcopenic obesity | Protein + AAs | 33 | 78.1±5.1 | 0 | 34 | 76.9 ± 5.1 | 0 | BIA | SMI | kg/m2 | 0.016 | 0.009 |
| Protein and other | |||||||||||||
| Bell et al(2017)54 | Community-dwelling, healthy | Protein + other | 25 | 71 ± 1 | 0 | 24 | 74 ± 1 | 0 | DXA | tLM | kg | 0.100 | < 0.05 |
| Cramer et al(2016)55 | Community-dwelling with sarcopenia and malnutrition | Protein + other | 101 | Median, 77 (IQR, 71–81) | 63 (62) | 83 | Median, 77 (IQR, 71–81) | 51 (62) | DXA | legMM | kg | 0.140 | NS |
| PUFAs | |||||||||||||
| Krzyminska-Siemaszko et al (2015)56 | Community-dwelling at risk of or with low muscle mass | PUFAs | 30 | 75.0 ± 8.23 | 19 (63) | 20 | 74.9 ± 7.49 | 14 (70) | BIA | SMM | kg | 0.020 | 0.99 |
| Logan et al(2015)57 | Community-dwelling, healthy | PUFAs | 12 | 66 ±1a | 12 (100) | 12 | 66 ± 1a | 12 (100) | BIA | LM | kg | 1.200 | NS |
| Smith et al(2015)58 | Community-dwelling, healthy | PUFAs | 29 | 68 ± 5 | 19 (66) | 15 | 69 ± 7 | 10 (67) | MRI | TMV | cm3 | 3.600 | < 0.05 |
Sample sizes are presented after participant drop out, as the sample used in analysis.
Mean difference defined as the mean change of muscle mass in the intervention group minus the mean change of muscle mass in the control group.
Age was only presented for the total sample and not reported for the intervention and control groups separately.
Presented as mean with SEM.
Presented as mean with SE. Age in years is presented as mean ± SD unless indicated otherwise.
Male and female subgroups were pooled and a mean change value for both groups was obtained. Sample sizes for each group were combined.
Abbreviations : AA, amino acid; AD, Alzheimer’s disease; AFFM, appendicular fat-free mass; ALM, appendicular lean mass; BIA, bioelectrical impedance analysis; BIS, bioimpedance spectroscopy; COPD, chronic obstructive pulmonary disease; CR, creatine; CRF, chronic respiratory failure; CV, cardiovascular; DXA, dual-energy X-ray absorptiometry; FFM, fat-free mass; FFMI, fat-free mass index; HMB, β-hydroxy-β-methylbutyrate; IQR, interquartile range; legMM, leg muscle mass; LM, lean mass; LMBI, lean body mass index; LTM, lean tissue mass; MD, mean differences; MRI, magnetic resonance imaging; NA, not available; NS, not significant; PUFA, polyunsaturated fatty acid; SMI, skeletal mass index; SMM, skeletal muscle mass; tLM, trunk lean mass; TMV, thigh muscle volume; T2D, type II diabetes.
Quality assessment
Figure S2 in the Supporting Information online provides a summary of the methodological quality of the studies. Nine studies were graded as being of high quality, 6 as of moderate quality, and 14 as being of low quality. Overall, more than half of the studies were classified as having unclear risk of bias regarding selection bias (ie, random sequence generation and allocation concealment (both n = 15 of 29). In 11 of the 29 studies, it was unclear whether blinding of the outcome assessment was performed (detection bias).
Nutritional intervention factors
Table 3 lists detailed information regarding the composition, dose, duration, frequency, timing, and adherence of the nutritional interventions and the control groups.
Table 3.
Nutritional intervention factors, muscle mass measurements, and the effect of the intervention group compared with the control group
| Author (year) | Nutritional intervention factors |
Control group |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Composition | Form | Total dose (g/d) | Per serving (g/d) | Duration (wk) | Freq (times/d) | Timing | Adh (%) | Composition | |
| AAs | ||||||||||
| Dal Negro et al (2010)30 | AAs | EAAs | Sachet | 8.00 | 4.00 | 12 | 2 | NA | NA | Placebo |
| Dal Negro et al (2012)31 | AAs | EAAs | Sachet | 8.00 | 4.00 | 12 | 2 | 10 am and 5 pm | NA | Placebo: isocaloric |
| Leenders et al (2011)32 | AAs | Leucine | Capsule | 7.50 | 2.50 | 24 | 3 | Breakfast, lunch, dinner | NA | Placebo: wheat-flour capsules |
| Malaguarnera et al (2007)33 | AAs | l-carnitine | Vial | 2.00 | 2.00 | 24 | 1 | NA | 80–120 | Placebo |
| Verhoeven et al (2009)34 | AAs | Leucine | Capsule | 7.50 | 2.50 | 12 | 3 | Breakfast, lunch, dinner | NA | Placebo: wheat-flour capsules |
| Creatine | ||||||||||
| Gotshalk et al (2002)35 | CR | Creatine monohydrate 0.3 g/kg body mass | Capsule | Individual | Individual | 1 | 3 | Breakfast, lunch, dinner | NA | Placebo: powdered cellulose capsules |
| Marinari et al (2013)36 | CR | CR | NA | 0.34 | 0.17 | 8 | 2 | NA | NA | Placebo (bags) |
| Rawson et al (1999)37 | CR | Creatine monohydrate | Tablet | 20.0 and 4.00a | 4.50 | 4.29 | 4 and 1a | NA | NA | Placebo |
| HMB | ||||||||||
| Baier et al (2009)38 | HMB | CaHMB | Sachet + water | 2.00–3.00b | 2.00–3.00b | 52 | 1 | Breakfast | 95c | Isonitrogenous and isocaloric drink |
| Deutz et al (2013)39 | HMB | CaHMB | Sachet + liquid | 3.00 | 1.50 | 2.14 | 2 | Morning, evening | NA | Placebo (sachets) |
| Flakoll et al (2004)40 | HMB | CaHMB | Drink (8 oz) | 2.00 | 2.00 | 12 | 1 | Breakfast | 100c | Placebo: isocaloric drink or isocaloric isonitrogenous mixture |
| Protein | ||||||||||
| Aleman-Mateo et al (2012)41 | Protein | Ricotta (210 g) | Food product | 15.70 | 5.23 | 12 | 3 | Breakfast, lunch, dinner | NA | Habitual diet |
| Aleman-Mateo et al (2014)42 | Protein | Ricotta (210 g) | Food product | 18.12 | 6.04 | 12 | 3 | Breakfast, lunch, dinner | NA | Habitual diet |
| Bos et al (2000)43 | Protein | Oral high-protein formula (protein: Ca caseinates) | Drink (400 mL) | 30.00 | 30.00 | 1.43 | 1 | NA | NA | No supplementation |
| Flodin et al (2015)44 | Protein | Protein and energy supplement, risedronate 1 weekly, Ca and vitamin D 800 IU (2 daily doses for 12 mo) | Drink (200 mL) | 40.00 | 20.00 | 48 | 2 | NA | NA | Risedronate 1 weekly, and calcium and vitamin D (2 daily doses for 12 mo) |
| Ha et al (2010)45f | Protein | Energy- and protein enriched meals, sip feedings, or enteral tube feeding | Solid or liquid | Individual | Individual | 1 | NA | NA | NA | Usual care |
| Kerstetter et al (2015)46 | Protein | Whey protein isolate | Powder | 40.00 | 40.00 | 72 | 1 | NA | NA | Isocaloric supplementation (powder) |
| Lauque et al (2004)47 | Protein | ONS (soup, dessert, and drink) protein enriched | Solid or liquid | Individual | Individual | 12 | NA | NA | NA | Usual care |
| Tieland et al (2012)48 | Protein | Milk-protein concentrate (MPC80) | Drink (250 mL) | 30.00 | 15.00 | 24 | 2 | After breakfast, after lunch | 92 | Placebo: no protein |
| Zhu et al (2015)49 | Protein | Skim milk–based high-protein supplement (skim milk plus whey protein isolate) | Powder + water (250 mL) | 30.00 | 30.00 | 104 | 1 | Breakfast | 87.1 | Placebo: skim milk based supplement |
| Protein with AAs | ||||||||||
| Bauer et al (2015)50 | Protein + AAs | Whey protein, leucine, vitamin D 800 IU | Powder + water (100–150 mL) | 41.40 (P)5.60 (AA) | 20.70 (P)2.80 (AA) | 13 | 2 | Breakfast, lunch | 93 | Placebo: isocaloric |
| Bonnefoy et al (2010)51 | Protein + AAs | Protein noncaloric supplementation enriched with BCAAs (l-leucine, l-isoleucine, l-valine), 3–5 sachets (15–25 g) | Sachets | 14.70 (P)6.98 (AA) | 7.35 (P)3.49 (AA) | 2 | 2 | Lunch, dinner | NA | Usual and balanced diet |
| Chanet et al (2017)52 | Protein + AAs | Whey protein, leucine, including protein-bound and free l-leucine | Drink (200 mL) | 20.00 (P)3.00 (AA) | 20.00 (P)3.00 (AA) | 6 | 1 | Breakfast | 99 | Noncaloric, flavored, watery placebo (drink, 200 mL) |
| Kemmler et al (2017)53 | Protein + AAs | Whey protein, high l-leucine, EAA, vitamin D supplement (800 IU) | Powder + water | Individualized to achieve 1.7–1.8 g/kg body mass | Individual | 16 | NA | No specific time | NA | No supplementation |
| Protein and other | ||||||||||
| Bell et al (2017)54 | Protein + other | Whey protein, CR, PUFAs (EPA and DHA) | Sachet + water (425 mL)Oil liquid | 60.00 (P)5.00 (CR)300 (PUFA) | 30.00 (P)2.50 (C)300 (PUFA) | 6 | 2 (P + CR)1 (PUFA) | Breakfast, 1 h before bed | 87 ± 2 | Placebo (sachet). Safflower oil (measured out) |
| Cramer et al (2016)55 | Protein + other | Isocaloric high protein supplement with CaHMB and vitamin D 499 IU | Drink (220 mL) | 40.00 (P)3.00 (HMB) | 20.00 (P)1.50 (HMB) | 24 | 2 | Between regular meals | 86 | Isocaloric supplement (drink, 220 mL) |
| PUFAs | ||||||||||
| Krzyminska-Siemaszko et al (2015)56 | PUFAs | PUFA (EPA, DHA, other omega-3 fatty acids) | Capsule | 1.30 | 0.65 | 12 | 2 | During or immediately after meals | NA | Vitamin E solution |
| Logan et al (2015)57 | PUFAs | Fish oil (EPA and DHA) | Capsule | 5.00 | 1.00–2.00d | 12 | 3 | Breakfast, lunch, dinner | NA | Olive oil (3 capsules) |
| Smith et al (2015)58 | PUFAs | PUFA (EPA and DHA) | Pill | 4.00 | 2.00 | 24 | 2 | Breakfast, dinner | 93.6 ± 7.4 | Corn oil (4 capsules) |
20 g in the first 10 days, after 4 g in the next 20 days.
2 g if participant weighed ≤68 kg or 3 g if participant weighed >68 kg.
Subject reported.
One capsule for breakfast, 2 capsules for lunch, and 2 capsules for dinner.
Abbreviations: AA, amino acid; Adh, adherence; Ca, calcium; CR, creatine; DHA, docosahexaenoic acid; EAA, essential amino acid; EPA, eicosapentanoic acid; Freq: frequency; HMB, β-hydroxy-β-methylbutyrate; NA, not available; ONS, oral nutritional supplement; P, protein; PUFA, poly-unsaturated fatty acid.
Type of intervention
Five studies involved AA supplementation,30–34 3 studies used CR,35–37 3 studies included HMB supplementation,38–40 15 studies included protein supplementation,41–55 and 3 included PUFA supplementation56–58 (Tables 2 and 3). Supplements were multinutrient (n = 25) or single-nutrient (n = 4). Table S2 in the Supporting Information online lists the composition of the nutritional supplements and control products.
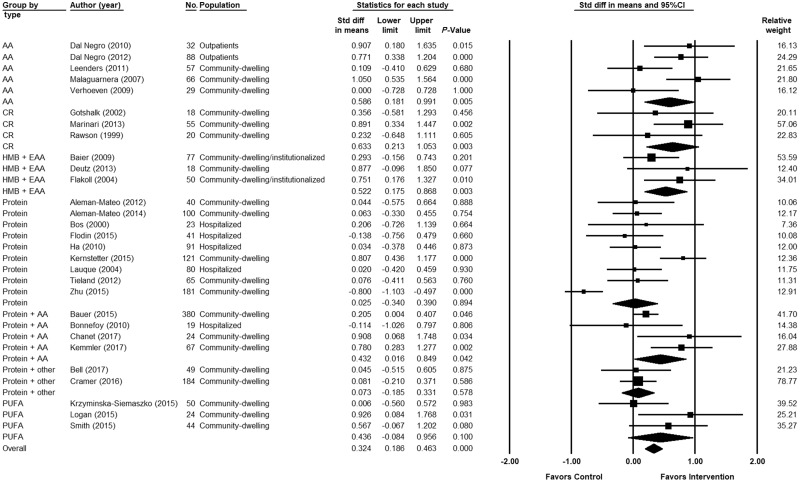
Figure 2 shows the meta-analysis of the pooled effect sizes. Nutritional interventions showed an overall positive effect in muscle mass measures (SMD, 0.324; 95%CI, 0.186–0.463; P ≤ 0.001; I2, 72.5%). Four of the 7 types of interventions showed a significant positive effect on muscle mass measures: AA (SMD, 0.586; 95%CI, 0.181–0.991; P = 0.005; I2: 60.9%), CR (SMD, 0.633; 95%CI, 0.213–1.053; P = 0.003; I2, 0%), HMB (SMD, 0.522; 95%CI, 0.175–0.868; P = 0.003; I2, 5.40%), and protein plus AA (SMD, 0.432; 95%CI, 0.016–0.849; P = 0.042; I2, 58.5%). CR showed the greatest significant improvement in muscle mass measures. No significant differences were found between the intervention and control groups for protein, protein plus other, and PUFAs. Per subgroup of the type of intervention, significant positive effects on muscle mass measures were found in 3 of 5 studies for AA30,31,33; 1 of 3 studies for CR36; 1 of 3 studies for HMB40; 1 of 9 studies for protein46; 3 of 4 studies for protein plus AA50,52,53; none for protein plus other; and 1 of 3 studies for PUFAs.57
Figure 2.
Forest plot showing the effect of nutritional interventions on muscle mass in older adults, grouped by the type of intervention. Heterogeneity (reported as I2 value [%]): amino acids (AAs): 60.9%; creatine (CR), 0%; &bgr;-hydroxy-&bgr;-methylbutyrate (HMB), 5.40%; protein, 82.4%; protein plus AA, 58.5%; protein plus other, 0%; polyunsaturated fatty acids (PUFAs), 44.9%; overall I2, 72.5%. Abbreviations: EAA, essential amino acid; Std diff, standardized difference.
Low heterogeneity was present for CR, HMB, and protein plus other, and high heterogeneity for AA, protein, protein plus AA, and PUFAs.
The baseline protein intake was reported in 5 of 8 studies for the protein subgroup and in all studies for protein plus AA and protein plus other (Table S3 in the Supporting Information online). Baseline protein intake was ≥ 1.0 g/kg body weight, as recommended for healthy older adults,59,60 except in 3 studies.43,51,55
Dose, duration, frequency, timing, and adherence
All studies reported the dose and duration of the intervention (Table 3). The frequency of the intervention was reported in 26 of the 29 studies and the timing was specified in 20 of the 29 studies. Ten studies reported supplement adherence for the intervention or control groups.
Table 4 reports data from the heat map for the visualization of patterns regarding the efficacy of the factors dose, duration, frequency, timing, and adherence. Higher doses, longer durations, greater frequencies, and better adherence did not appear to be clustered together, yielding more positive results. In 4 studies, the dose was individualized to the participant. Seventeen studies reported administering the nutritional supplementation around meals (ie, before or after meals), 2 studies administered supplementation throughout the day not related to meals, and 1 study reported having no specific time to administer the nutritional supplementation. No clear pattern could be observed with regard to the effect of the timing of the intervention on muscle mass measures. Those studies that did report treatment adherence, with the exception of 1,49 all had positive effects on muscle mass measures.
Table 4.
Data from heat map of the impact of the nutritional intervention factors on muscle mass, grouped by the type of intervention
| Author (year) | Type | Dose (g/d) | Duration(wk) | Frequency (times/d) | Timing | Adherence (%) | Effect (SMD) | P |
|---|---|---|---|---|---|---|---|---|
| AAs | ||||||||
| Dal Negro et al (2010)30 | AAs | 8 | 12 | 2 | NA | NA | 0.907 | 0.015 |
| Dal Negro et al (2012)31 | AAs | 8 | 12 | 2 | 10 am and 5 pm | NA | 0.771 | 0.000 |
| Leenders et al (2011)32 | AAs | 7.5 | 24 | 3 | Breakfast, lunch, dinner | NA | 0.109 | 0.680 |
| Malaguarnera et al (2007)33 | AAs | 2 | 24 | 1 | NA | 80–120 | 1.050 | 0.000 |
| Verhoeven et al (2009)34 | AAs | 7.5 | 12 | 3 | Breakfast, lunch, dinner | NA | 0.000 | 1.000 |
| Creatine | ||||||||
| Gotshalk et al (2002)35 | CR | Individual | 1 | 3 | Breakfast, lunch, dinner | NA | 0.356 | 0.456 |
| Marinari et al (2013)36 | CR | 0.34 | 8 | 2 | NA | NA | 0.891 | 0.002 |
| Rawson et al (1999)37 | CR | 20 | 4.29 | 4 | NA | NA | 0.232 | 0.605 |
| HMB | ||||||||
| Baier et al (2009)38 | HMB | 2.5 | 52 | 1 | Breakfast | 95 | 0.293 | 0.201 |
| Deutz et al (2013)39 | HMB | 3 | 2.14 | 2 | Morning, evening | NA | 0.877 | 0.077 |
| Flakoll et al (2004)40 | HMB | 2 | 12 | 1 | Breakfast | 100 | 0.751 | 0.010 |
| Protein supplementation | ||||||||
| Aleman-Mateo et al (2012)41 | Protein | 15.7 | 12 | 3 | Breakfast, lunch, dinner | NA | 0.044 | 0.888 |
| Aleman-Mateo et al (2014)42 | Protein | 18.12 | 12 | 3 | Breakfast, lunch, dinner | NA | 0.063 | 0.754 |
| Bos et al (2000)43 | Protein | 30 | 1.43 | 1 | NA | NA | 0.206 | 0.664 |
| Flodin et al (2015)44 | Protein | 40 | 48 | 2 | NA | NA | −0.138 | 0.660 |
| Ha et al (2010)45 | Protein | Individual | 1 | NA | NA | NA | 0.034 | 0.873 |
| Kerstetter et al (2015)46 | Protein | 40 | 72 | 1 | NA | NA | 0.807 | 0.000 |
| Lauque et al (2004)47 | Protein | Individual | 12 | NA | NA | NA | 0.020 | 0.930 |
| Tieland et al (2012)48 | Protein | 30 | 24 | 2 | After breakfast and lunch | 92 | 0.076 | 0.760 |
| Zhu et al (2015)49 | Protein | 30 | 104 | 1 | Breakfast | 87.1 | −0.800 | 0.000 |
| Protein + AAs | ||||||||
| Bauer et al (2015)50 | Protein + AA | 41.4 | 13 | 2 | Breakfast, lunch | 93 | 0.205 | 0.046 |
| Bonnefoy et al (2010)51 | Protein + AA | 14.7 | 2 | 2 | Lunch, dinner | NA | −0.114 | 0.806 |
| Chanet et al (2017)52 | Protein + AA | 20 | 6 | 1 | Breakfast | 99 | 0.908 | 0.034 |
| Kemmler et al (2017)53 | Protein + AA | Individual | 16 | NA | NA | NA | 0.780 | 0.002 |
| Protein + other | ||||||||
| Bell et al (2017)54 | Protein + other | 60 | 6 | 2 | Breakfast, bedtime | 87 | 0.045 | 0.875 |
| Cramer et al (2016)55 | Protein + other | 40 | 24 | 2 | Between meals | 86 | 0.081 | 0.586 |
| PUFAs | ||||||||
| Krzyminska-Siemaszko et al (2015)56 | PUFAs | 1.3 | 12 | 2 | During/after meals | NA | 0.006 | 0.983 |
| Logan et al (2015)57 | PUFAs | 5 | 12 | 3 | Breakfast, lunch, dinner | NA | 0.926 | 0.031 |
| Smith et al (2015)58 | PUFAs | 3.36 | 24 | 2 | Breakfast, dinner | 93.6 | 0.567 | 0.080 |
Colours: red (less effective) – yellow – green (more effective). The dose was coloured per group of type of intervention; the dose for all protein articles (protein, protein + AA, protein + other) was coloured as one group (relative to each other). The timing of the intervention administration was not coloured, as it was not possible to compare each variant of timing relative to each other. P values were coloured as follow: green for P values < 0.05, yellow for P values between ≥ 0.05 and < 0.10 (indicating a trend), red for P values ≥ 0.10. Abbreviations: AA, amino acid; CR, creatine, HMB, β-hydroxy-β-methylbutyric acid; NA: not available; PUFA, poly-unsaturated fatty acids; SMD, standardized mean difference.
DISCUSSION
Nutritional interventions showed an overall significant positive effect on muscle mass measures in older adults. When grouped for the type of intervention, the interventions with AAs, CR, HMB, and protein plus AAs showed significant positive effects on muscle mass measures. However, few studies were included per type of intervention and only a few individual studies showed a significant positive effect on muscle mass. Because of the high variability in the composition, dose, duration, frequency, and timing of the intervention, coupled with insufficient reporting of treatment adherence, no conclusion can be drawn on the most effective combination of factors of a nutritional intervention on increasing muscle mass measures. High heterogeneity was present among all types of intervention except for CR, HMB, and protein plus other, which could be attributed to methodological differences, including not only the factors of interest but also the different instruments of measuring muscle mass.
Amino acids
Although limited to a few studies, AAs were among the most effective nutritional interventions for increasing muscle mass measures in community-dwelling older adults and outpatients. Another review, although limited to essential amino acids only, also found this nutritional strategy to be an effective supplement in improving muscle mass in older adults with acute or chronic conditions.61 Furthermore, supplementation of branch-chained amino acids was found to increase muscle mass in hospitalized older patients in acute and rehabilitation wards.62 AAs (essential and nonessential) act as primary stimuli for muscle protein anabolism by initiating messenger RNA translation through the activation of the mechanistic target of rapamycin complex 1, a protein complex that controls the metabolic response to nutrients and proteins.63,64
Creatine
The results point to the significant positive effects of CR supplementation on muscle mass measures; however these were limited to 3 studies and all were conducted with community-dwelling men only. The effects of CR have been frequently explored in the context of resistance exercise training. Two previous systematic reviews reported positive effects of CR on muscle mass combined with an exercise intervention, including populations aged ≥ 50 years65 and ≥ 60 years.11 It has been suggested that CR supplementation alone is limited in its effect on satellite cell mitotic activity and that CR supplementation needs to be combined with exercise to promote muscular hypertrophy.66 However, the underlying mechanisms of CR remain unknown,67 highlighting the need for additional investigations.
β-Hydroxy-β-methylbutyric acid
The results demonstrate a significant increase in muscle mass measures with HMB supplementation in community-dwelling and institutionalized older adults. These findings in relation to HMB, a key metabolite of the AA leucine, are in line with those of a previous meta-analysis.68 HMB is increasingly receiving attention for its ability to inhibit protein breakdown in skeletal muscle and its upregulation of protein synthesis through the activation of the mechanistic target of rapamycin.69,70 The HMB supplements used in the studies in the present review38–40,71 mainly consisted of HMB (or calcium HMB) in combination with the essential amino acids arginine and lysine, suggesting that perhaps this combination could be optimal for building and maintaining muscle mass. A recent study also showed the positive effect of HMB combined with arginine and glutamine on muscle mass.72
Protein supplementation
Although the protein plus AA group yielded a significant positive effect in community-dwelling older adults, protein alone and protein plus other did not show significant results on muscle mass measures, in concordance with other studies.8,73 Another meta-analysis showed that protein supplementation did not increase muscle mass in community-dwelling older adults with sufficient baseline protein intakes.73 Recently, literature on protein supplementation has also shown no significant positive effect on muscle mass in older adults74–76 or a positive effect on muscle mass, depending on the muscle mass measure.76 It has also been suggested that protein supplementation combined with resistance exercise training could be more effective on muscle mass, strength, and physical performance than protein supplementation alone. Simultaneously, protein supplementation could augment the effects of resistance exercise training compared with exercise alone; however, results are contradictory.8,73,77,78
All protein interventions contained a certain dose of AAs; however, the protein plus AA group had a greater improvement in muscle mass measures compared with studies in which participants received protein and protein plus other interventions. These results, therefore, revealed that all types of interventions containing AAs (where the quantity was specified in the studies) had promising effects on muscle mass measures, suggesting AAs are a key ingredient to ensuring the efficacy of a nutritional intervention in increasing muscle mass.
Polyunsaturated fatty acids
PUFA supplementation was not beneficial for increasing muscle mass measures in community-dwelling older adults. A study examining the effect of n-3 PUFA therapy on muscle transcriptome of older individuals found this nutritional supplement had a very small effect in augmenting muscle mass.79 However, another study that included a resistance exercise program concluded that the anti-inflammatory properties of PUFAs significantly affected skeletal muscle function in older adults, leading to an increased anabolic response to exercise.33 This amplified effect of PUFA supplementation, when combined with an exercise intervention, was also supported in a recent narrative review that highlighted the potential beneficial effects of PUFA supplementation on muscle mass in older adults.80 A recent systematic review supports the inconsistency in findings across studies, highlighting the need for more trial data.8
Dose, duration, frequency, timing, and adherence
The high variability among studies regarding the dose, duration, frequency, timing, and adherence challenges any conclusions that can be drawn regarding the most effective combination of these factors. To overcome the anabolic resistance at older age,81 10–15 g of AA (containing ≥ 3 g of leucine) has been proposed as the optimum dose for older individuals.82 However, even lower doses of AAs, depending on the type of AAs, might be more effective; for example, the administration of only 2 g of l-carnitine (an essential metabolite) resulted in the greatest increase in muscle mass in 1 study.33 The optimal dose of protein intake has been proposed to be 1.0–1.2 g/kg body weight per day for community-dwelling older adults, but higher doses might be needed for hospitalized or institutionalized older adults.59,60 These recommendations also allude to the need for additional trials with respect to the timing and pattern of distribution of the intervention. For instance, although 1 study found that the supplementation of protein all in 1 meal was more effective than its distribution across 4 meals,83 other studies showed that an even protein distribution (25–30 g/meal [ie, breakfast, lunch, dinner]) throughout the day elicits a greater anabolic response.84,85
In addition, many studies have been conducted in conjunction with an exercise program,86 and many of the existing recommendations on the optimal frequency and timing of supplements are tailored to athletes and physically active adults,87 making it difficult to generalize the findings across older populations. The observed variability regarding the optimal duration of a nutritional intervention is in line with another review, which found no clear indications regarding the optimal duration to maximize muscle growth.70 In fact, although 6 months has been suggested as the minimum period to elicit measurable alterations in muscle,88 it remains unknown whether nutritional interventions stimulate muscle changes linearly with time or if a ceiling effect is observed before any more increments in muscle mass can take place.
Treatment adherence is a critical factor for the efficacy of an intervention,89 particularly when nutritional supplements and alterations to dietary patterns are known to be difficult to adhere to.90 Only one-third of studies reported treatment adherence, and all of these, with the exception of 1, reported a positive effect on muscle mass measures. This reiterates the association between ensuring sufficient adherence and the success of an intervention. Furthermore, adequate reporting of treatment adherence is required, as well as of the other intervention factors, among RCTs.91
Nutritional interventions and muscle strength and physical performance
The results showed that AAs, CR, HMB, and protein plus AA interventions had a positive effect on muscle mass measures, one of the diagnostic measures of sarcopenia according to the European Working Group on Sarcopenia in Older People definition.4,92 Current definitions of sarcopenia also include muscle strength and physical performance as diagnostic measures. A recent meta-analysis showed that multinutrient supplements had a positive effect on physical performance (chair-stand test) and muscle strength (handgrip strength), whereas proteins, as a single-nutrient supplement, only showed a positive effect on muscle strength.93 In this meta-analysis, multinutrient supplements were defined as any supplement consisting of multiple nutritional components,93 thus, different types of interventions were grouped (eg, supplements with whey protein, vitamin D, and/or leucine, supplements with essential amino acids, and multivitamins supplements). This approach does not enable identification of which type of intervention was most effective and, therefore, these results are still inconclusive about which type of intervention is effective on physical performance and muscle strength.
Strengths and limitations
The strength of this review is its broad inclusion criteria not being limited to any particular population or nutritional intervention, making it possible to compare various nutritional interventions. Half of the reviewed studies were assigned an unclear or high risk of bias regarding their blinding, implying a certain degree of performance bias in the studies, which could have affected the results. The effectiveness of nutritional interventions differs across various health care settings.94 That most of the studies included community-dwelling older adults could have affected the results positively, because community-based older populations tend to have a more adequate nutritional status than their hospitalized counterparts.95 The effect of nutritional interventions was studied on muscle mass measures in older adults, not taking into account muscle strength and physical performance as outcome parameters.
Recommendations for future research
Current RCTs with nutritional interventions are mainly performed in community-dwelling or healthy older populations, and there is a lack of RCTs in clinical populations such as hospitalized or institutionalized older adults. Therefore, RCTs in these clinically relevant populations is needed, because nutritional interventions could improve outcomes. Furthermore, future studies should take into account the protein-energy intake as part of the diet, and vitamin D levels96 to ensure this intake is adequate. It could be hypothesized that if the protein-energy intake is inadequate, an additional nutritional supplement alone might be less effective. However, there is a lack of evidence to support this hypothesis and, therefore, future research should assess the protein-energy intake at baseline and follow-up throughout the nutritional intervention to ensure the protein-energy intake remains adequate. The recently published international clinical practice guideline for sarcopenia also supports this hypothesis.97 Nutritional research should also explore the differences in effectiveness between multinutrient and single-nutrient supplements, as well as dietary patterns, specific foods, or food fortification. In general, there is a need for RCTs with larger sample sizes to increase statistical power, and RCTs should aim to reduce selection bias, detection bias, and attrition, and increase adherence. Finally, authors should adhere to the Consolidated Standards of Reporting Trials statement98 for reporting RCTs and the Template for Intervention Description and Replication checklist.99
CONCLUSION
The findings highlight the potential role of nutrition as a strategy for the prevention and treatment of sarcopenia in older age. Pooled summary effects indicated that AAs, CR, HMB, and protein plus AAs are effective interventions for increasing muscle mass measures in older adults. A few studies were included per type of intervention and a few individual studies showed a significant positive effect on muscle mass. Because of the interstudy variability of the included studies in this review with regard to the dose, duration, frequency, and timing of the intervention, the optimal profile of a nutritional intervention is yet to be elucidated. Appropriate adherence to treatment was associated with positive effects on muscle mass measures, and efforts should be made to ensure adherence is assessed and reported in RCTs. Studies are needed to bridge the gap in knowledge regarding the optimization of nutritional interventions, whereby more-homogenous methods should be followed to enable a comparison of factors among studies. High-quality investigations should also aim to define the optimal profile of exercise interventions as well as in combination with nutritional interventions.
Supplementary Material
Acknowledgments
The authors thank Jimmy Ky and Anthony A. Kamleh for their contribution in the screening of eligible studies and data extraction. The authors also thank Patrick Condron (senior liasion librarian, Brownless Biomedical Library, Faculty of Medicine, Dentistry & Health Sciences, the University of Melbourne), who greatly assisted with the construction of the search strategy.
Author contributions. Study design: all authors. Data extraction: A.M.-C., E.M.R., B.M.T.G. Data analysis: A.M.-C., E.M.R. Data interpretation: all authors. A.M.-C. and E.M.R. wrote the original draft of the manuscript. Manuscript review and editing: all authors. All authors reviewed the manuscript and provided final approval of the version to be published.
Funding. This work was supported by European Union’s Horizon 2020 research and innovation program (grants no. 689238 [2015] and 675003 [2015]). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of interest. The authors declare no conflict of interest.
Supporting Information
The following Supporting Information is available through the online version of this study at the publisher’s website.
Figure S1 Risk of bias of the included studies. (A) Summary of each risk-of-bias item presented as a percentage across all included studies, indicated by low risk of bias (green), unclear risk of bias (yellow), or high risk of bias (red). (B) Summary of each risk-of-bias item for each included study.
Table S1 Search strategy
Table S2 Composition of the nutritional supplements and placebos
Table S3 Baseline protein intake for the intervention and control groups
References
- 1. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 2. Reijnierse EM, Trappenburg MC, Leter MJ, et al. The impact of different diagnostic criteria on the prevalence of sarcopenia in healthy elderly participants and geriatric outpatients. Gerontology. 2015;61:491–496. [DOI] [PubMed] [Google Scholar]
- 3. Bijlsma AY, Meskers CG, Ling CH, et al. Defining sarcopenia: the impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age (Dordr). 2013;35:871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yeung SSY, Reijnierse EM, Pham VK, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10:485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li CI, Li TC, Lin WY, et al. Combined association of chronic disease and low skeletal muscle mass with physical performance in older adults in the Sarcopenia and Translational Aging Research in Taiwan (START) study. BMC Geriatr. 2015;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Volpi E, Nazemi R, Fujita S.. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care. 2004;7:405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robinson SM, Reginster JY, Rizzoli R, et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin Nutr. 2018;37:1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marzetti E, Calvani R, Tosato M. et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin Exp Res. 2017;29:35–42. [DOI] [PubMed] [Google Scholar]
- 10. Reijnierse EM, Trappenburg MC, Leter MJ, et al. The association between parameters of malnutrition and diagnostic measures of sarcopenia in geriatric outpatients. PLoS One. 2015;10:E0135933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beaudart C, Dawson A, Shaw SC, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int. 2017;28:1817–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thalacker-Mercer AE, Fleet JC, Craig BA, et al. Inadequate protein intake affects skeletal muscle transcript profiles in older humans. Am J Clin Nutr. 2007;85:1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morley JE, Argiles JM, Evans WJ, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010;11:391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoshimura Y, Wakabayashi H, Yamada M, et al. Interventions for treating sarcopenia: a systematic review and meta-analysis of randomized controlled studies. J Am Med Dir Assoc. 2017;18:553.e1–553.e16. [DOI] [PubMed] [Google Scholar]
- 15. Woo J. Nutritional interventions in sarcopenia: where do we stand? Curr Opin Clin Nutr Metab Care. 2018;21:19–23. [DOI] [PubMed] [Google Scholar]
- 16. Park Y, Choi JE, Hwang HS.. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2018;108:1026–1033. [DOI] [PubMed] [Google Scholar]
- 17. Witard OC, Wardle SL, Macnaughton LS, et al. Protein considerations for optimising skeletal muscle mass in healthy young and older adults. Nutrients. 2016;8:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Nutrition interventions [e-Library of Evidence for Nutrition Actions (eLENA)]. https://www.who.int/elena/intervention/en/. Accessed July 20, 2019.
- 19. Moher D, Liberati A, Tetzlaff J, et al.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:E1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cardamone M, Darras BT, Ryan MM.. Inherited myopathies and muscular dystrophies. Semin Neurol. 2008;28:250–259. [DOI] [PubMed] [Google Scholar]
- 21. Laing NG. Genetics of neuromuscular disorders. Crit Rev Clin Lab Sci. 2012;49:33–48. [DOI] [PubMed] [Google Scholar]
- 22. Barreto R, Mandili G, Witzmann FA, et al. Cancer and chemotherapy contribute to muscle loss by activating common signaling pathways. Front Physiol. 2016;7:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pinto Neto LF, Sales MC, Scaramussa ES, et al. Human immunodeficiency virus infection and its association with sarcopenia. Braz J Infect Dis. 2016;20:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Block RJ, Bolling D.. The amino acid composition of proteins and foods. Science. 1946;103:431–432. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fay DS, Gerow K.. A biologist’s guide to statistical thinking and analysis. WormBook. 2013;1–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takeshima N, Sozu T, Tajika A, et al. Which is more generalizable, powerful and interpretable in meta-analyses, mean difference or standardized mean difference? BMC Med Res Methodol. 2014;14:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Method. 2010;1:97–111. [DOI] [PubMed] [Google Scholar]
- 29. Ioannidis JP, Patsopoulos NA, Evangelou E.. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335:914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dal Negro RW, Aquilani R, Bertacco S, et al. Comprehensive effects of supplemented essential amino acids in patients with severe COPD and sarcopenia. Monaldi Arch Chest Dis. 2016;73:25–33. [DOI] [PubMed] [Google Scholar]
- 31. Dal Negro RW, Testa A, Aquilani R, et al. Essential amino acid supplementation in patients with severe COPD: a step towards home rehabilitation. Monaldi Arch Chest Dis. 2015;77:67–75. [DOI] [PubMed] [Google Scholar]
- 32. Leenders M, Verdijk LB, van der Hoeven L, et al. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J Nutr. 2011;141:1070–1076. [DOI] [PubMed] [Google Scholar]
- 33. Malaguarnera M, Cammalleri L, Gargante MP, et al. l-Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trial. Am J Clin Nutr. 2007;86:1738–1744. [DOI] [PubMed] [Google Scholar]
- 34. Verhoeven S, Vanschoonbeek K, Verdijk LB, et al. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr. 2009;89:1468–1475. [DOI] [PubMed] [Google Scholar]
- 35. Gotshalk LA, Volek JS, Staron RS, et al. Creatine supplementation improves muscular performance in older men. Med Sci Sports Exerc. 2002;34:537–543. [DOI] [PubMed] [Google Scholar]
- 36. Marinari S, Manigrasso MR, De Benedetto F.. Effects of nutraceutical diet integration, with coenzyme Q10 (Q-Ter multicomposite) and creatine, on dyspnea, exercise tolerance, and quality of life in COPD patients with chronic respiratory failure. Multidiscip Respir Med. 2013;8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rawson ES, Wehnert ML, Clarkson PM.. Effects of 30 days of creatine ingestion in older men. Eur J Appl Physiol. 1999;80:139–144. [DOI] [PubMed] [Google Scholar]
- 38. Baier S, Johannsen D, Abumrad N, et al. Year-long changes in protein metabolism in elderly men and women supplemented with a nutrition cocktail of beta-hydroxy-beta-methylbutyrate (HMB), l-arginine, and l-lysine. JPEN J Parenter Enteral Nutr. 2009;33:71–82. [DOI] [PubMed] [Google Scholar]
- 39. Deutz NE, Pereira SL, Hays NP, et al. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr. 2013;32:704–712. [DOI] [PubMed] [Google Scholar]
- 40. Flakoll P, Sharp R, Baier S, et al. Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition. 2004;20:445–451. [DOI] [PubMed] [Google Scholar]
- 41. Aleman-Mateo H, Macias L, Esparza-Romero J, et al. Physiological effects beyond the significant gain in muscle mass in sarcopenic elderly men: evidence from a randomized clinical trial using a protein-rich food. Clin Interv Aging. 2012;7:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aleman-Mateo H, Carreon VR, Macias L, et al. Nutrient-rich dairy proteins improve appendicular skeletal muscle mass and physical performance, and attenuate the loss of muscle strength in older men and women subjects: a single-blind randomized clinical trial. Clin Interv Aging. 2014;9:1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bos C, Benamouzig R, Bruhat A, et al. Short-term protein and energy supplementation activates nitrogen kinetics and accretion in poorly nourished elderly subjects. Am J Clin Nutr. 2000;71:1129–1137. [DOI] [PubMed] [Google Scholar]
- 44. Flodin L, Cederholm T, Saaf M, et al. Effects of protein-rich nutritional supplementation and bisphosphonates on body composition, handgrip strength and health-related quality of life after hip fracture: a 12-month randomized controlled study. BMC Geriatr. 2015;15:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ha L, Hauge T, Iversen PO.. Body composition in older acute stroke patients after treatment with individualized, nutritional supplementation while in hospital. BMC Geriatr. 2010;10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kerstetter JE, Bihuniak JD, Brindisi J, et al. The effect of a whey protein supplement on bone mass in older Caucasian adults. J Clin Endocrinol Metab. 2015;100:2214–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lauque S, Arnaud-Battandier F, Gillette S, et al. Improvement of weight and fat-free mass with oral nutritional supplementation in patients with Alzheimer’s disease at risk of malnutrition: a prospective randomized study. J Am Geriatr Soc. 2004;52:1702–1707. [DOI] [PubMed] [Google Scholar]
- 48. Tieland M, van de Rest O, Dirks ML, et al. Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13:720–726. [DOI] [PubMed] [Google Scholar]
- 49. Zhu K, Kerr DA, Meng X, et al. Two-year whey protein supplementation did not enhance muscle mass and physical function in well-nourished healthy older postmenopausal women. J Nutr. 2015;145:2520–2526. [DOI] [PubMed] [Google Scholar]
- 50. Bauer JM, Verlaan S, Bautmans I, et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2015;16:740–747. [DOI] [PubMed] [Google Scholar]
- 51. Bonnefoy M, Laville M, Ecochard R, et al. Effects of branched amino acids supplementation in malnourished elderly with catabolic status. J Nutr Health Aging. 2010;14:579–584. [DOI] [PubMed] [Google Scholar]
- 52. Chanet A, Verlaan S, Salles J, et al. Supplementing breakfast with a vitamin D and leucine-enriched whey protein medical nutrition drink enhances postprandial muscle protein synthesis and muscle mass in healthy older men. J Nutr. 2017;147:2262–2271. [DOI] [PubMed] [Google Scholar]
- 53. Kemmler W, Weissenfels A, Teschler M, et al. Whole-body electromyostimulation and protein supplementation favorably affect sarcopenic obesity in community-dwelling older men at risk: The randomized controlled FranSO study. Clin Interv Aging. 2017;12:1503–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bell KE, Snijders T, Zulyniak M, et al. A whey protein-based multi-ingredient nutritional supplement stimulates gains in lean body mass and strength in healthy older men: a randomized controlled trial. PLoS One. 2017;12:E0181387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cramer JT, Cruz-Jentoft AJ, Landi F, et al. Impacts of high-protein oral nutritional supplements among malnourished men and women with sarcopenia: a multicenter, randomized, double-blinded, controlled trial. J Am Med Dir Assoc. 2016;17:1044–1055. [DOI] [PubMed] [Google Scholar]
- 56. Krzymińska-Siemaszko R, Czepulis N, Lewandowicz M, et al. The effect of a 12-week omega-3 supplementation on body composition, muscle strength and physical performance in elderly individuals with decreased muscle mass. Int J Environ Res Public Health. 2015;12:10558–10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Logan SL, Spriet LL.. Omega-3 fatty acid supplementation for 12 weeks increases resting and exercise metabolic rate in healthy community-dwelling older females. PLoS One. 2015;10:E0144828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Smith GI, Julliand S, Reeds DN, et al. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr. 2015;102:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14:542–559. [DOI] [PubMed] [Google Scholar]
- 60. Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cheng H, Kong J, Underwood C, et al. Systematic review and meta-analysis of the effect of protein and amino acid supplements in older adults with acute or chronic conditions. Br J Nutr. 2018;119:527–542. [DOI] [PubMed] [Google Scholar]
- 62. Moriwaki M, Wakabayashi H, Sakata K, et al. The effect of branched chain amino acids-enriched nutritional supplements on activities of daily living and muscle mass in inpatients with gait impairments: a randomized controlled trial. J Nutr Health Aging. 2019;23:348–353. [DOI] [PubMed] [Google Scholar]
- 63. Fujita S, Volpi E.. Amino acids and muscle loss with aging. J Nutr. 2006;136:277S–280S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Weinert DJ. Nutrition and muscle protein synthesis: a descriptive review. J Can Chiropr Assoc. 2009;53:186–193. [PMC free article] [PubMed] [Google Scholar]
- 65. Chilibeck PD, Kaviani M, Candow DG, et al. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: a meta-analysis. Open Access J Sports Med. 2017;8:213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dangott B, Schultz E, Mozdziak PE.. Dietary creatine monohydrate supplementation increases satellite cell mitotic activity during compensatory hypertrophy. Int J Sports Med. 2000;21:13–16. [DOI] [PubMed] [Google Scholar]
- 67. Farshidfar F, Pinder MA, Myrie SB.. Creatine supplementation and skeletal muscle metabolism for building muscle mass- review of the potential mechanisms of action. Curr Protein Pept Sci. 2017;18:1273–1287. [DOI] [PubMed] [Google Scholar]
- 68. Wu H, Xia Y, Jiang J, et al. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. 2015;61:168–175. [DOI] [PubMed] [Google Scholar]
- 69. Wilkinson DJ, Hossain T, Hill DS, et al. Effects of leucine and its metabolite beta-hydroxy-beta-methylbutyrate on human skeletal muscle protein metabolism. J Physiol. 2013;591:2911–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Calvani R, Miccheli A, Landi F, et al. Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J Frailty Aging. 2013;2:38–53. [PMC free article] [PubMed] [Google Scholar]
- 71. Fuller JC Jr., Baier S, Flakoll P, et al. Vitamin D status affects strength gains in older adults supplemented with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and lysine: a cohort study. JPEN J Parenter Enteral Nutr. 2011;35:757–762. [DOI] [PubMed] [Google Scholar]
- 72. Ellis AC, Hunter GR, Goss AM, et al. Oral supplementation with beta-hydroxy-beta-methylbutyrate, arginine, and glutamine improves lean body mass in healthy older adults. J Diet suppl. 2019;16:281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ten Haaf DSM, Nuijten MAH, Maessen MFH, et al. Effects of protein supplementation on lean body mass, muscle strength, and physical performance in nonfrail community-dwelling older adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018;108:1043–1059. [DOI] [PubMed] [Google Scholar]
- 74. Ottestad I, Lovstad AT, Gjevestad GO, et al. Intake of a protein-enriched milk and effects on muscle mass and strength. A 12-week randomized placebo controlled trial among community-dwelling older adults. J Nutr Health Aging. 2017;21:1160–1169. [DOI] [PubMed] [Google Scholar]
- 75. Yamada M, Kimura Y, Ishiyama D, et al. Synergistic effect of bodyweight resistance exercise and protein supplementation on skeletal muscle in sarcopenic or dynapenic older adults. Geriatr Gerontol Int. 2019;19:429–437. [DOI] [PubMed] [Google Scholar]
- 76. Bo Y, Liu C, Ji Z, et al. A high whey protein, vitamin D and E supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: a double-blind randomized controlled trial. Clin Nutr. 2019;38:159–164. [DOI] [PubMed] [Google Scholar]
- 77. Liao CD, Tsauo JY, Wu YT, et al. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta-analysis. Am J Clin Nutr. 2017;106:1078–1091. [DOI] [PubMed] [Google Scholar]
- 78. Thomas DK, Quinn MA, Saunders DH, et al. Protein supplementation does not significantly augment the effects of resistance exercise training in older adults: a systematic review. J Am Med Dir Assoc. 2016;17:959 e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yoshino J, Smith GI, Kelly SC, et al. Effect of dietary n-3 PUFA supplementation on the muscle transcriptome in older adults. Physiol Rep. 2016;4:E12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dupont J, Dedeyne L, Dalle S, et al. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin Exp Res. 2019;31:825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Burd NA, Gorissen SH, van Loon LJ.. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev. 2013;41:169–173. [DOI] [PubMed] [Google Scholar]
- 82. Katsanos CS, Kobayashi H, Sheffield-Moore M, et al. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–7. [DOI] [PubMed] [Google Scholar]
- 83. Arnal MA, Mosoni L, Boirie Y, et al. Protein pulse feeding improves protein retention in elderly women. Am J Clin Nutr. 1999;69:1202–1208. [DOI] [PubMed] [Google Scholar]
- 84. Paddon-Jones D, Rasmussen BB.. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Symons TB, Sheffield-Moore M, Wolfe RR, et al. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc. 2009;109:1582–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schoenfeld BJ, Aragon AA, Krieger JW.. The effect of protein timing on muscle strength and hypertrophy: a meta-analysis. J Int Soc Sports Nutr. 2013;10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kerksick CM, Wilborn CD, Roberts MD, et al. ISSN exercise & sports nutrition review update: research & recommendations. J Int Soc Sports Nutr. 2018;15:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cesari M, Fielding RA, Pahor M, et al. Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle. 2012;3:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Czobor P, Skolnick P.. The secrets of a successful clinical trial: compliance, compliance, and compliance. Mol Interv. 2011;11:107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hubbard GP, Elia M, Holdoway A, et al. A systematic review of compliance to oral nutritional supplements. Clin Nutr. 2012;31:293–312. [DOI] [PubMed] [Google Scholar]
- 91. Liljeberg E, Andersson A, Lovestam E, et al. Incomplete descriptions of oral nutritional supplement interventions in reports of randomised controlled trials. Clin Nutr. 2018;37:61–71. [DOI] [PubMed] [Google Scholar]
- 92. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Veronese N, Stubbs B, Punzi L, et al. Effect of nutritional supplementations on physical performance and muscle strength parameters in older people: a systematic review and meta-analysis. Ageing Res Rev. 2019;51:48–54. [DOI] [PubMed] [Google Scholar]
- 94. Reinders I, Volkert D, de Groot L, et al. Effectiveness of nutritional interventions in older adults at risk of malnutrition across different health care settings: pooled analyses of individual participant data from nine randomized controlled trials. Clin Nutr. 2019;38:1797–1806. [DOI] [PubMed] [Google Scholar]
- 95. Vandewoude MF, Alish CJ, Sauer AC, et al. Malnutrition-sarcopenia syndrome: is this the future of nutrition screening and assessment for older adults? J Aging Res. 2012;2012:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Verlaan S, Maier AB, Bauer JM, et al. Sufficient levels of 25-hydroxyvitamin D and protein intake required to increase muscle mass in sarcopenic older adults - The PROVIDE study. Clin Nutr. 2018;37:551–557. [DOI] [PubMed] [Google Scholar]
- 97. Dent E, Morley JE, Cruz-Jentoft AJ, et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22:1148–1161. [DOI] [PubMed] [Google Scholar]
- 98. Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.