Abstract
The outbreak of the SARS-CoV-2 pandemic is triggering a global health emergency alert. Until vaccination becomes available, a bundle of effective preventive measures is desperately needed. Recent research is indicating the relevance of aerosols in the spread of SARS-CoV-2. Thus, in this study commercially available antiseptic mouthwashes based on the active ingredients chlorhexidine digluconate and octenidine dihydrochloride (OCT) were investigated regarding their efficacy against SARS-CoV-2 using the European Standard 14476. Based on the requirement of EN 14476 in which reduction of at least four decimal logarithms (≥4 log10) of viral titre is requested to state efficacy, the OCT-based formulation was found to be effective within a contact time of only 15 s against SARS-CoV-2. Based on this in-vitro data the OCT mouthwash thus constitutes an interesting candidate for future clinical studies to prove its effectiveness in a potential prevention of SARS-CoV-2 transmission by aerosols.
Keywords: Coronavirus, SARS-CoV-2, Mouthwash, Octenidine dihydrochloride, Antiviral
Introduction
Coronaviruses are enveloped single-stranded RNA viruses and are characterized by club-shaped spikes on the surface of the virion, prompting the name coronavirus due to the similarity in appearance to a solar corona [1]. Until the SARS-CoV outbreak in 2002, coronaviruses were thought to only cause mild self-limiting infections in humans but were known to cause a wide variety of infections in animals [1]. Seventeen years later, in December 2019, a novel coronavirus was identified as the causative agent of severe pneumonia in a cluster of patients, designated as SARS-CoV-2 due to its relatedness to severe acute respiratory syndrome coronavirus (SARS-CoV) [2,3]. Since then SARS-CoV-2 spread around the world, thereby triggering a global health emergency alert. Thus, until vaccination becomes available a bundle of effective preventive measures is urgently needed.
In this context, recent publications suggest the use of antimicrobial mouthwashes as a preventive measure. This is based on the efficacy of antimicrobial mouthwashes to reduce the number of microorganisms in the oral cavity prompting a reduction of microorganisms in aerosols [4]. This is particularly interesting, as recent research indicates the relevance of aerosols also in the spread of SARS CoV-2 [5].
Thus, in their review summarizing data for mouthwashes with chlorhexidine digluconate (CHX), cetylpyridinium chloride, povidone–iodine, and hydrogen peroxide, Vergara-Beunaventura and Castro-Ruiz indicate an essential role of antiseptic mouthwashes to reduce SARS-CoV-2 viral load in dental practice. They underline that research on this topic is urgently needed to verify the potential of antiseptic mouth rinses as a further preventive measure [6]. The aim of our study was therefore to directly compare commercially available antiseptic mouthwash formulations. The mouthwash formulations were based on the common antiseptic active substances CHX and octenidine dihydrochloride (OCT) and were investigated regarding their efficacy against the pandemic coronavirus SARS-CoV-2.
Methods
Quantitative suspension tests according to EN 14476
Quantitative suspension tests were carried out as described in EN 14476 [7]. Briefly, efficacy against SARS-CoV-2 was studied using commercially available mouthwashes [8]. A commercially available ready-to-use formulation designated formulation A (trade name: Chlorhexamed fluid 0.1%; 100 g contains 0.1 g chlorhexidine bis-(d-gluconate); GlaxoSmithKline Consumer Health GmbH & Co. KG, Munich, Germany) was used as one test formulation. In addition, a commercially available ready-to-use formulation designated formulation B (trade name: Chlorhexamed forte alkoholfrei 0.2%; 100 g contains: 0.2 g chlorhexidine bis-(d-gluconate); GlaxoSmithKline) was used. Formulation C used in this study was also a ready-to-use preparation (trade name: Octenisept; 100 g contains: 0.1 g octenidine dihydrochloride (CAS number: 70775-75-6), 2 g phenoxyethanol; drug authorization number: 32834.00.00). Concentrations and contact times used throughout this study are indicated. In reality, organic soiling in the oral cavity can be considered quite diverse. Thus, for comparative reasons the standardized protocol of EN 14476 was chosen for this in-vitro study under conditions of low organic soiling (0.3 g/L bovine serum albumin (BSA); ‘clean conditions’) to give a first indication of the virucidal efficacy of the tested formulations against SARS-CoV-2 [7].
Data presented are based on at least two independent experiments. Validation controls as defined in EN 14476 were found to be effective in all experiments, indicating validity of presented data [7].
Results and discussion
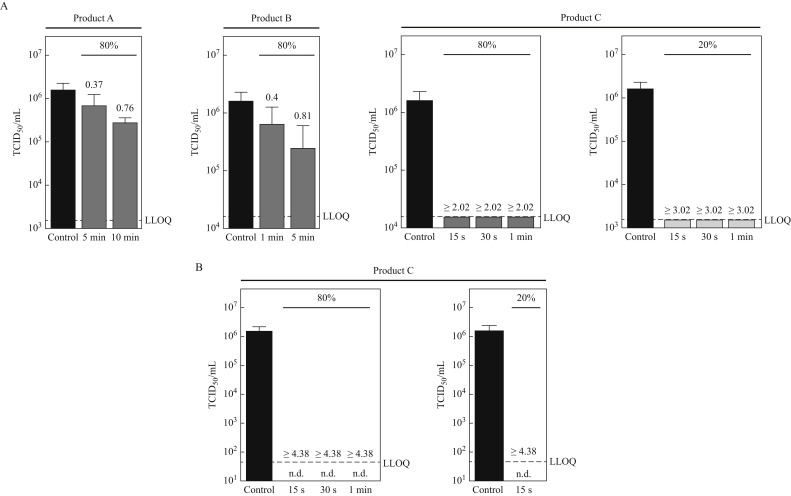
Figure 1 A shows SARS-CoV-2 reduction obtained for formulations A, B, and C using end-point titration. In these experiments the two formulations based on CHX (formulations A and B) were found to have only limited efficacy against SARS-CoV-2. Thus, at a concentration of 80% (v/v), formulation A containing 0.1% CHX reduced the virus titre even at a prolonged contact time of 10 min by <1 log10. Formulation B containing 0.2% CHX reduced SARS-CoV-2 within a contact time of 1 min as well as at a prolonged contact time of 5 min when tested at 80% (v/v) concentration also by <1 log10. No additional large volume plating (LVP) experiments were conducted for formulations A and B. For these formulations cytotoxic effects of the formulation were found to have no impact, which is indicated by the lower limit of quantification (LLOQ). This is well in line with data from screening experiments in our laboratory, where virus reduction titres were found to be not elevated due to less toxicity when both formulations were tested at a concentration of only 20% (v/v) (data not shown).
Figure 1.
Virucidal activity of oral rinses against SARS-CoV-2. SARS-CoV-2 was incubated with medium (control, black bar) or various oral rinses (products A–C) for indicated concentrations (80% and/or 20 %) and time-periods (15 s–10 min). The cytotoxic effect was monitored using non-infected cells incubated with the different products, defined as lower limit of quantification (LLOQ). Log10 reduction factors are indicated above the bars. (A) Viral titres were determined upon limited end-point titration on Vero E6 cells. Tissue culture infectious dose 50% (TCID50/mL) was calculated according to Spearman–Kärber. Due to high cytotoxic effects diminishing the measuring window for product C, large volume plating was performed to reduce cytotoxicity and evaluate the remaining titres <104 (B). No remaining cytopathic effects were observed (n.d.). Data are reported as mean values with standard deviation from at least two independent experiments. Experiments were carried out according to EN 14476 under clean conditions. n.d., not detectable.
In contrast, when looking at the data for formulation C, reduction factors were found to be 1 log10 higher (i.e. ≥3.02 log10) for the 20% (v/v) concentration of product C compared to the 80% (v/v) test concentration (i.e. ≥2.02 log10). This indicates that the measuring window for product was diminished by cytotoxicity. Therefore, additional large volume plating (LVP) experiments to obtain a wider measuring window were conducted with formulation C. Data obtained using LVP are presented in Figure 1B. LVP data indicate a reduction of SARS-CoV-2 titres by ≥4.38 log10 already within the shortest contact time of 15 s for the OCT-based mouthwash (formulation C). This was found for both concentrations tested (80% (v/v) and 20% (v/v)).
In their study on the stability of SARS-CoV-2 at different environmental conditions Chin et al. found no detectable virus when adding 15 μL viral solution (titre approximately 7–8 log10 units of tissue culture infectious dose 50% (TCID50/mL) per mL) to 135 μL CHX solution (0.05%) after 5 min contact time [9]. The detection limit for their experiments is stated to be 104 TCID50/mL. Data with a lower limit of quantification would be desirable to assess the efficacy of the rather low concentration of CHX in the study by Chin et al. [9]. In our experiments, with a lower limit of quantification, only limited efficacy of even higher concentrations of CHX was found when using the standardized EN 14476 protocol.
Data presented in this study for the two CHX-based mouthwashes (formulations A and B) are well in line with data published by Meister et al. [10]. Experiments conducted in our laboratory to directly compare the soiling conditions mimicking respiratory secretions used by Meister et al. (i.e. 100 μL mucin type I-S, 25 μL BSA fraction V, and 35 μL yeast extract) with the clean conditions (i.e. 0.03% BSA) used in this study were found to give equivalent data for all three tested formulations (data not shown) [10]. Thus, in their investigation of different mouthwashes targeting SARS-CoV-2, Meister et al. also found only a limited efficacy (i.e. <1 log10) of the two tested commercially available mouthwashes based on CHX [10]. However, looking at the data for the OCT-based mouthwashes: in the earlier study by Meister et al. only limited virucidal activity of the formulation tested (i.e. <1 log10) was found, whereas in this study the tested OCT-based formulation (C) was found to be effective against SARS-CoV-2 within 15 s (i.e. ≥4 log10). These differing data are likely to be explained by the use of two different OCT-based formulations in the two studies. In the earlier study a formulation containing OCT as the only active substance was used as compared to the OCT-based formulation (C) used in this study which contained OCT in combination with phenoxyethanol [10]. Future experiments might help to elucidate the impact of the active phenoxyethanol in more detail, e.g. by direct comparison of formulations with and without OCT in the presence or absence of phenoxyethanol. In any case, this discrepancy indicates the value of pre-evaluating each individual formulation on the basis of EN 14476 when assessing the virucidal potential against SARS-CoV-2. For this pre-evaluation the standard test surrogate virus – modified vaccinia virus strain Ankara (MVA) – to assess ‘virucidal activity against enveloped viruses’ as defined in EN 14476 has been found to be of value, as with this approach a non-pathogenic virus can be used in the laboratory to obtain reliable data regarding virucidal activity against enveloped viruses in general, including SARS-CoV-2 [7].
In conclusion, this in-vitro study demonstrated virucidal efficacy for formulation C against SARS-CoV-2, meeting the >4 log10 requirement of EN 14476 within a contact time of only 15 s [7]. These in-vitro data give a good indication of the efficacy of the tested formulations using the standardized EN 14476 protocol in the presence of low organic soiling. Clinical trial data will help to elucidate effectiveness against SARS-CoV-2 under physiological conditions, as organic soiling in the oral cavity can be considered more diverse in the field.
Thus, based on these in-vitro data, the OCT-based commercially available formulation used in this study constitutes an interesting candidate for future clinical studies to prove its effectiveness in the potential prevention of COVID-19 as a mouthwash. Clinical data will also enable recommendations to be made for use of the mouthwash in practice (clinical environment and/or general prophylaxis).
Conflict of interest statement
K.S. and L.P. are employees of Schülke & Mayr GmbH, Norderstedt, Germany.
Funding source
This study was funded by Schülke & Mayr GmbH, Norderstedt, Germany.
References
- 1.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marui V.C., Souto M.L.S., Rovai E.S., Romito G.A., Chambrone L., Pannuti C.M. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: a systematic review. J Am Dent Assoc. 2019;150:1015–1026. doi: 10.1016/j.adaj.2019.06.024. e1. [DOI] [PubMed] [Google Scholar]
- 5.Fennelly K.P. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med. 2020;8:914–924. doi: 10.1016/S2213-2600(20)30323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vergara-Buenvaventura A., Castro-Ruiz C. Use of mouthwashes against COVID-19 in dentistry. Br J Oral Maxillofac Surg. 2020;58:924–927. doi: 10.1016/j.bjoms.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Committee for Standardization . 2019. European Standard EN 14476: chemical disinfectants and antiseptics – quantitative suspension test for the evaluation of virucidal activity in the medical area – test method and requirements (Phase 2/Step 1) Brussels. [Google Scholar]
- 8.Heilingloh C.S., Aufderhorst U.W., Schipper L., Dittmer U., Witzke O., Yang D. Susceptibility of SARS-CoV-2 to UV irradiation. Am J Infect Control. 2020;48:1273–1275. doi: 10.1016/j.ajic.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1:e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meister T.L., Brüggemann Y., Todt D., Conzelmann C., Müller J.A., Groß R. Virucidal efficacy of different oral rinses against severe acute respiratory coronavirus 2. J Infect Dis. 2020;222:1289–1292. doi: 10.1093/infdis/jiaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]