We found that inhibition of PLCγ1 phosphorylation by U73122 improved cartilage matrix synthesis in IL‐1β‐treated rat chondrocytes. However, it also increased cell apoptosis and necroptosis, which reduced the effect on cartilage matrix synthesis. Inhibition of programmed cell death by simultaneous treatment with apoptosis and necroptosis inhibitors increased chondrocyte proliferation and further enhanced cartilage matrix synthesis.
Keywords: apoptosis, necroptosis, osteoarthritis, PLCγ1, U73122
Abstract
Osteoarthritis (OA) is an age‐related, chronic degenerative disease. With the increasing median age of the population, this disease has become an important public health problem. New, disease‐modifying therapies are needed. A potential novel molecular target is phospholipase Cγ1 (PLCγ1), a critical enzyme with important functions including calcium signaling regulation and cell proliferation. In rat chondrocytes treated with IL‐1β (20 ng·mL−1 for 36 h), inhibition of PLCγ1 with U73122 (2 μm for 12 h) increased levels and expression of the cartilage matrix components Collagen2 and Aggrecan. This beneficial effect of PLCγ1 inhibition was counteracted by increased chondrocyte apoptosis and necroptosis, increased cell death, and increase levels of ROS, all potentially negative for OA. Combined treatment of IL‐1β + U73122‐treated chondrocytes with inhibitors of apoptosis (Z‐VAD, 10 μm) and necroptosis (Nec‐1, 30 μm) enhanced the increases in levels and expression of Collagen2 and Aggrecan, and prevented the increases in cell death and ROS levels. These results suggest that PLCγ1 inhibition may be a viable approach for an OA therapy, if combined with targeted inhibition of chondrocyte apoptosis and necroptosis.
Abbreviations
- Nec‐1
necrostatin‐1
- OA
osteoarthritis
- PLCγ1
phospholipase Cγ1
- ROS
reactive oxide species
- RT‐PCR/qPCR
real‐time quantitative polymerase chain reaction
- Z‐VAD
Z‐VAD‐FMK
Osteoarthritis (OA) is a chronic disease that is manifest mainly by cartilage damage and degeneration. It is caused by many factors, but the number one risk factor is age. Worldwide, more than 10% of men and 18% of women over the age of 60 suffer from osteoarthritis [1]. With the increasing median age of the population, the number of OA patients is rising. OA represents a huge medical burden to patients, to their families, and to society [2].
Originally, OA was thought to be caused simply by mechanical wear of articular cartilage [3], but a deeper understanding of the disease shows it to involve lesions of all the joint components and even systemic responses [4]. Despite this, regulation of and changes in the articular cartilage are still the main focus for OA research [5]. Articular cartilage is mainly made up of chondrocytes and a 3D cartilage matrix that is synthesized by the chondrocytes [6]. The cartilage matrix is composed of type II collagen (Collagen2), hyaluronic acid, and proteoglycans or Aggrecans [7, 8]. Changes in the activity of the chondrocytes, or in the levels of the Collagen2 and Aggrecans can lead to changes in the structure and health of the cartilage. These changes may be used as criteria to help quantify OA severity [9].
Treatment of OA includes both surgical and nonsurgical (including pharmaceutical) approaches [10]. With developments in artificial joint replacement, surgical treatment has become the most effective treatment for advanced OA [11, 12]. In comparison, development of nonsurgical and pharmaceutical treatments is relatively limited. Nonsurgical treatments are not as effective as surgical. Pharmaceutical treatments are limited mainly to analgesics, with no effective disease‐modifying drugs currently available [10]. However, artificial joint replacement is not the perfect solution for OA and is associated with problems including joint prosthesis aging and loosening [13, 14]. It is important therefore to continue to search for effective disease‐modifying pharmaceutical treatments for OA focusing on new molecular targets, with the goal of slowing disease progression and preventing or delaying the need for joint replacement. Our focus is on approaches to improve chondrocyte function and survival and thus preserve cartilage matrix synthesis.
Phospholipase Cγ1 (PLCγ1) is a critical enzyme on the cytoplasmic membrane that is involved in transmembrane calcium flux. It can be activated by receptor tyrosine kinase, and its phosphorylation site Tyr783 is the site for the PLCγ1 main function, hydrolysis of PIP2 into DAG and IP3 [17], which regulate Ca2+ flow [18]. Several extracellular factors work via PLCγ1 to regulate cell proliferation, differentiation, migration, apoptosis, and autophagy [15, 16]. U73122 is a commonly used PLCγ1 inhibitor and can effectively inhibit IP3 synthesis and calcium release [19]. A previous study showed that U73122 intra‐articular injection in rats reduced OA damage [20]. However, there are also studies showing that U73122 increases cell apoptosis [21, 22].
Apoptosis was the first described form of programmed cell death. It can involve caspase activation and is accompanied by pyknosis, karyolysis, and apoptotic body formation [23, 24]. Necroptosis is another form of programmed cell death and is different from classic necrosis. In necroptosis, receptor‐interacting protein 1/3 (RIP1/3) activates a series of death signal systems, which can destroy organelles such as mitochondria and lead to cell death [25, 26]. Previous studies have shown that both apoptosis and necroptosis in chondrocytes are OA risk factors [27, 28, 29, 30].
In order to explore new ideas for pharmaceutical treatment of OA, we used Il‐1β‐activated chondrocytes to mimic the OA state and investigated the effects of inhibiting PLCγ1 (with U73122) [20], apoptosis (with caspase inhibitor Z‐VAD‐FMK or Z‐VAD), and necroptosis (with RIP1 inhibitor necrostatin‐1 or Nec‐1), alone and in combination, on production of the cartilage matrix components Collagen2 and Aggrecan.
Materials and methods
Antibodies and reagents
Antibodies used in this study were purchased from the following companies: antibodies against Collagen2 (1 : 1000), Bcl‐2 (1 : 1000), Bax (S757) (1 : 1000), P53 (1 : 1000), RIP1 (1 : 1000), and RIP3 (1 : 1000) were purchased from Abcam Inc. (Cambridge, MA, USA); antibodies targeting PLCγ1 (1 : 1000), p‐PLCγ1 (Y783) (1 : 1000), and caspase3 (1 : 1000) were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA); and antibodies against Aggrecan (1 : 1000) and β‐actin (1 : 40 000) were purchased from Sigma‐Aldrich in China (Shanghai, China), respectively. Inhibitors used in this study (U73122, Z‐VAD, and Nec‐1) were purchased from MedChemExpress (Monmouth Junction, NJ, USA). Cytokine used in this study such as recombinant rat IL‐1β was purchased from PeproTech (Rocky Hill, NJ, USA). Other reagents were of the highest grade commercially available.
Isolation, culture, and treatment of rat chondrocytes
All the operations were approved by the committee on the Ethics of Animal Experiments of Xiamen University (ID no. 20170301).
Chondrocytes were isolated from knee cartilage of neonatal Sprague Dawley rats (within 1–2 days after birth) by mechanical and collagenase digestions [31, 32]. Primary chondrocytes were cultured in DMEM/F12 with 10% fetal bovine serum and penicillin (100 U·mL−1)/streptomycin (0.1 mg·mL−1). At 80% confluence, the cells were then plated in 60‐mm cell culture dishes at 1 : 3 or 1 : 4 at 37 °C, 95% humidity, and 5% CO2, and F2 generation cells were used for the experiments. IL‐1β (10, 20, and 40 ng·mL−1) when studied alone was added to the culture for 36 h to activate the cells [32]. The inhibitors U73122 (1, 2, 4, and 6 μm), Z‐VAD (10 μm), and Nec‐1 (30 μm) were added to the IL‐1β‐stimulated chondrocyte cell culture for 12 h. Those inhibitors were added for the last 12 h of the 36 h of IL‐1β. When studied together, inhibitors were added at 10‐min intervals.
Western blotting analysis
Protein extracts were subjected to SDS‐PAGE (8–15%) and transferred to a PVDF membrane (GE Healthcare, Hertfordshire, UK) as described before [33, 34]. Briefly, the PVDF membrane was clipped and incubated with the above‐mentioned primary antibodies at 4 °C overnight, followed by complete elution of the primary antibodies and the addition of the corresponding secondary antibodies at room temperature for 1 h. An enhanced chemiluminescence (ECL) detection kit was used to detect antibody reactivity (Pierce, Rockford, IL, USA).
Cell viability assay
For the cell viability assay, 1 × 103 cells were cultured in 96‐well plates and were treated with IL‐1β and the inhibitors as described. At the end of the treatment period, 3‐(4,‐dimethylthiazol‐2‐y)‐2, 5‐diphenyl‐tetrazolium bromide (MTT reagent) was added to the cultures as per the manufacturer’s instruction and as described in a previous study [35]. After 4 h, DMSO was added to stop the reaction and solubilize the formazan. The optical density was measured at 490 nm with GloMax 20/20 luminometer (Promega, Madison, WI, USA).
Real‐time quantitative polymerase chain reaction (RT‐PCR/qPCR)
Total RNA was extracted from the chondrocytes using TRIzol (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized with 1 μg of total RNA at 37 °C for 15 min using a PrimeScript RT Master Mix Kit (Takara, Dalian, China). Real‐time PCR was then performed using a Roche LightCycler 96 (Roche, Basel, Switzerland) with a SYBR Premix Ex Taq II Kit (Takara). The results were normalized to GAPDH and analyzed using sds software v2.1 as previously described [33, 34]. The following primers were used in quantitative PCR for measuring gene expression relative to GAPDH.
Primer sequences used in this study were as follows:
-
Collagen2:
Forward 5‐TCCTAAGGGTGCCAATGGTGA‐3,
Reverse 5‐GGACCAACTTTGCCTTGAGGAC‐3;
-
Aggrecan:
Forward 5‐TCCGCTGGTCTGATGGACAC‐3,
Reverse 5‐CCAGATCATCACTACGCAGTCCTC‐3;
-
GAPDH:
Forward 5‐CAAGTTCAACGGCACAGTCAAG‐3,
Reverse 5‐ACATACTCAGCACCAGCATCAC‐3.
Apoptosis and necroptosis analysis
The detection of apoptotic cells was performed using a Beckman CytoFlex (Beckman, Brea, CA, USA) with the Annexin V‐FITC/PI detection kit (Beyotime Biotechnology, Shanghai, China), as per the manufacturer's instructions. The results were analyzed using cytexpert 1.2.11.0 and flowjo 10 as previously described. The percentage of cell death (including dying and dead cells) was calculated from the total number of viable apoptotic cells and nonviable apoptotic cells (from Annexin V‐FITC with and without PI staining) [36]. Protein levels of apoptosis and necroptosis indices Bcl‐2, Bax, P53, pro/cleaved‐caspase3, RIP1, and RIP3 were analyzed by Western blotting.
Reactive oxide species (ROS) analysis
ROS were analyzed with the ROS detection kit (Beyotime Biotechnology), as per the manufacturer's instructions. The results were analyzed using cytexpert 1.2.11.0 and flowjo 10 as previously described [37].
Statistical analysis
Data are expressed as the mean ± 95% confidence interval (CI) of three independent experiments for each experiment. One‐way analysis of variance (ANOVA) with the Dunnett test was used to compare the control group with treatment groups by graphpad prism 5 software (GraphPad Software, San Diego, CA, USA). Differences at a value of P < 0.05 were regarded as statistically significant.
Results
Effects of PLCγ1 inhibitor U73122 on Collagen2 and Aggrecan levels in IL‐1β‐treated rat chondrocytes
To mimic the OA state, rat chondrocytes were treated with different concentrations of IL‐1β (10, 20, and 40 ng·mL−1) for 36 h. Collagen2 and Aggrecan levels decreased significantly at IL‐1β 20 and 40 ng·mL−1 (Fig. 1A). To determine effects of PLCγ1 inhibition, chondrocytes treated with IL‐1β (20 ng·mL−1) were treated with the PLCγ1 inhibitor U73122 at 1, 2, 4, and 6 μm for 12 h. Compared with the IL‐1β‐treated control group, U73122 at 2 μm significantly decreased the phosphorylation of PLCγ1 (Figs. 1B and S1). This was accompanied by significantly higher Collagen2 and Aggrecan protein levels (Figs. 1B and S1) and mRNA levels (Fig. 1C).
Fig. 1.
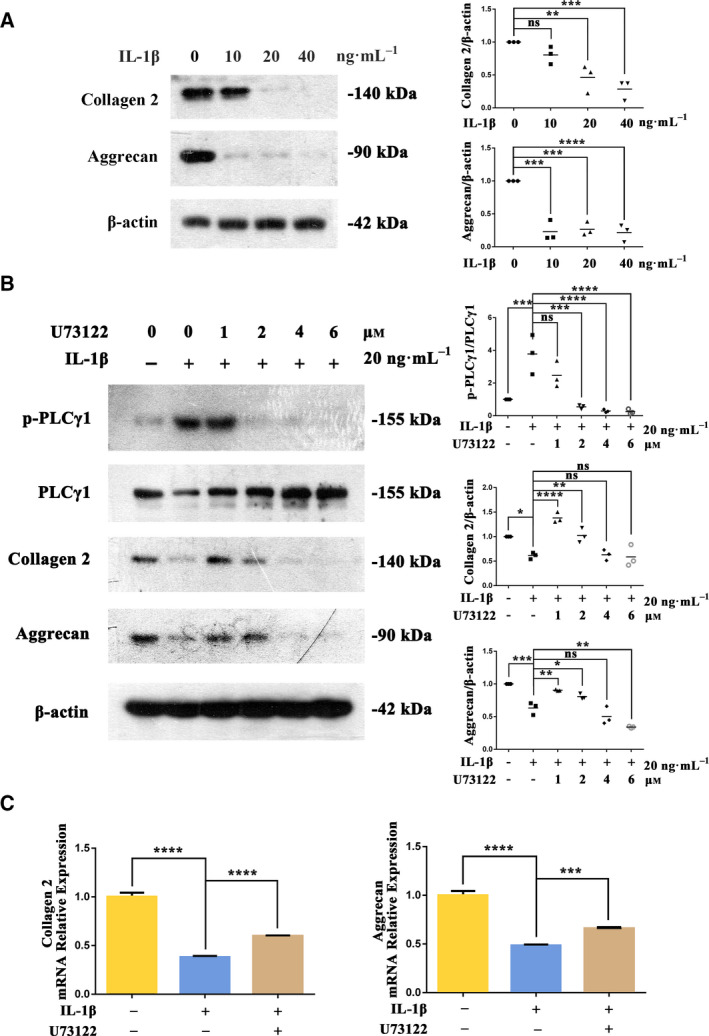
Inhibition of PLCγ1 with U73122 increased Collagen2 and Aggrecan levels in IL‐1β‐treated rat chondrocytes. Rat chondrocytes were treated with IL‐1β at 10, 20, and 40 ng·mL−1 for 36 h. Protein levels of Collagen2 and Aggrecan were analyzed by Western blotting (A). Chondrocytes pretreated with IL‐1β (20 ng·mL−1 for 36 h) were treated with the PLCγ1 inhibitor U73122 at 1, 2, 4, and 6 μm for 12 h. Total and phosphorylated protein levels of PLCγ1, Collagen2, and Aggrecan were analyzed by Western blotting (B). In addition, chondrocytes pretreated with IL‐1β (20 ng·mL−1 for 36 h) were treated with U73122 (2 μm for 12 h), and mRNA levels of Collagen2 and Aggrecan were analyzed by RT‐PCR (C). β‐Actin was used as the control for Western blotting, and GAPDH was used as the control for RT‐PCR. Values are means and standard deviations, the error bars represent SD. One‐way ANOVA with the Dunnett test was used to calculate P values. These results are representative of at least three independent experiments in each experiment. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Higher concentrations of U73122 (4 and 6 μm) also significantly reduced PLCγ1 phosphorylation but did not increase Collagen2 and Aggrecan levels compared with the IL‐1β‐treated control group (the Aggrecan level at 6 μm was significantly decreased). Interestingly, compared with the 1 μm group, U73122 at 2 μm had a significantly greater decrease in PLCγ1 phosphorylation but a smaller increase in Collagen2 and Aggrecan levels.
Effects of U73122 alone and combined with Z‐VAD and Nec‐1 on chondrocyte apoptosis and necroptosis
The MTT assay showed that IL‐1β (20 ng·mL−1) did not affect chondrocyte proliferation with or without PLCγ1 inhibition with U73122 (2 μm) (Fig. 2A). However, compared with the IL‐1β‐treated group the percent dead cells increased significantly in the IL‐1β + U73122‐treated group (Annexin V‐FITC/PI assay; Fig. 2B). ROS levels significantly increased in the IL‐1β‐treated group vs untreated control group (Fig. 2C), and levels were further increased significantly in the IL‐1β + U73122 group.
Fig. 2.
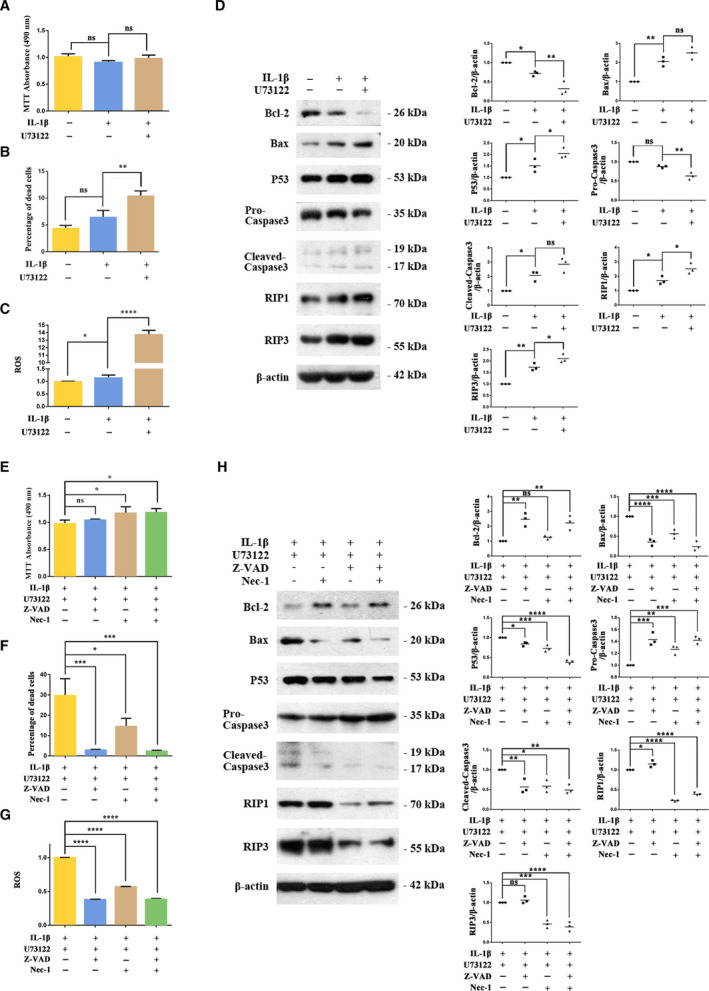
U73122‐induced apoptosis and necroptosis in IL‐1β‐treated rat chondrocytes, and the effect of combined treatment with the apoptosis and necroptosis inhibitors Z‐VAD and Nec‐1, respectively. Rat chondrocytes pretreated with IL‐1β (20 ng·mL−1 for 36 h) were treated with U73122 (2 μm for 12 h). Cell proliferation (A), percentage of dead cells (B), and ROS level (C) were measured. Protein levels of apoptosis and necroptosis indexes Bcl‐2, Bax, P53, pro/cleaved‐caspase3, RIP1, and RIP3 were analyzed by Western blotting (D). Chondrocytes pretreated by IL‐1β (20 ng·mL−1 for 36 h) and U73122 (2 μm for 12 h) were treated with Z‐VAD (10 μm for 12 h) or/and Nec‐1 (30 μm for 12 h). Cell proliferation (E), percentage of dead cells (F), and ROS level (G) were measured, and protein levels of apoptosis and necroptosis indexes were analyzed by Western blotting (H). The results of ROS were normalized, means of the first group in Fig. 2C,G were used as the control for ROS. β‐Actin was used as the control for Western blotting. Values are means and standard deviations, the error bars represent SD. One‐way ANOVA with the Dunnett test was used to calculate P values. These results are representative of at least three independent experiments in each experiment. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Changes in markers of apoptosis and necroptosis were consistent with IL‐1β‐induced chondrocyte apoptosis and necroptosis that were further increased by PLCγ1 inhibition with U73122 (Fig. 2D). Bcl‐2 level decreased in the IL‐1β group (vs untreated group) and further decreased significantly in the IL‐1β + U73122 group (vs IL‐1β‐treated group). Bax and p53 levels increased in the IL‐1β group (vs untreated group) and further increased significantly in the IL‐1β + U73122 group (vs IL‐1β‐treated group). In the IL‐1β group, there was a small but not significant decrease in the pro‐caspase3 level and a significant increase in the cleaved‐caspase3 level (vs untreated group). In the IL‐1β + U73122 group, the pro‐caspase3 level was further decreased and the cleaved‐caspase3 level showed a further small but not significant increase (vs IL‐1β‐treated group). Levels of the necroptosis markers RIP1 and RIP3 increased in the IL‐1β group (vs untreated group) and were further increased significantly in the IL‐1β + U73122 group (vs IL‐1β‐treated group).
We hypothesized that PLCγ1 inhibition with U73122 could increase Collagen2 and Aggrecan levels, but the effect was offset by increased chondrocyte apoptosis and necroptosis, especially at higher PLCγ1 inhibition. To try to separate these effects, in IL‐1β + U73122‐treated chondrocytes, we inhibited apoptosis (with Z‐VAD) and necroptosis (with Nec‐1) alone and combined. Compared with the IL‐1β + U73122‐treated group, combined treatment with Z‐VAD (10 μm) and Nec‐1 (30 μm) significantly increased chondrocyte proliferation (Fig. 2E), significantly decreased the percentage of dead cells, and significantly decreased ROS levels (Fig. 2F,G). With Z‐VAD treatment alone, changes in markers were consistent with inhibition of apoptosis (Fig. 2H). Compared with the IL‐1β + U73122‐treated control group, the Bcl‐2 level was increased, Bax and P53 levels were decreased, pro‐caspase3 level was increased, and cleaved‐caspase3 level was decreased. There were small significant increases in RIP1 and RIP3 with Z‐VAD treatment alone compared with the control group. With Nec‐1 treatment alone, changes in markers were consistent with inhibition of necroptosis (Fig. 2H). Compared with the IL‐1β + U73122‐treated control group, RIP1 and RIP3 levels were significantly reduced. Treatment with Nec‐1 alone also appeared to reduce apoptosis with decreases in Bax, P53, and cleaved‐caspase3 (vs IL‐1β + U73122‐treated group), but these effects were less than with Z‐VAD treatment. The largest changes in markers of apoptosis and necroptosis were observed in the combined Z‐VAD and Nec‐1‐treated group.
U73122 combined with apoptosis and necroptosis inhibitors increased Collagen2 and Aggrecan levels in IL‐1β‐treated rat chondrocytes
Treatment with Z‐VAD or Nec‐1 alone had no significant effect on Collagen2 or Aggrecan levels compared with the IL‐1β + U73122‐treated control group (Fig. 3A). Both Z‐VAD and Nec‐1 produced small decreases in Collagen2 mRNA level but had no effect on Aggrecan mRNA level. (Fig. 3B). Combined treatment with Z‐VAD and Nec‐1 significantly increased protein and mRNA levels of Collagen2 and Aggrecan compared with the IL‐1β + U73122‐treated control group.
Fig. 3.
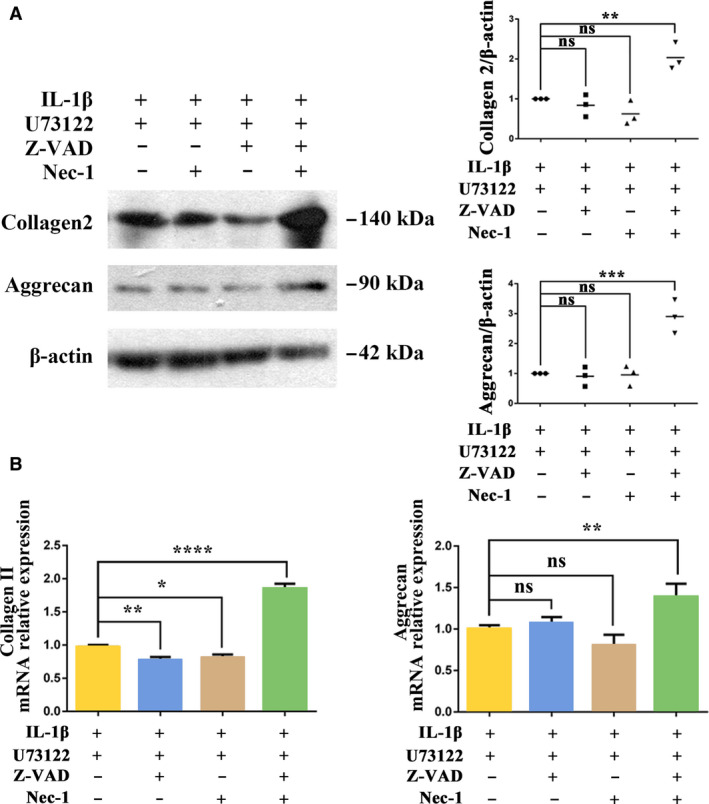
U73122 combined with apoptosis and necroptosis inhibitors increased Collagen2 and Aggrecan levels in IL‐1β‐treated rat chondrocytes. Chondrocytes pretreated by IL‐1β (20 ng·mL−1 for 36 h) and U73122 (2 μm for 12 h) were treated with Z‐VAD (10 μm for 12 h) or/and Nec‐1 (30 μm for 12 h). Protein (A) and mRNA (B) levels of Collagen2 and Aggrecan were analyzed by Western blotting and RT‐PCR, respectively. β‐Actin was used as the control for Western blotting, and GAPDH was used as the control for RT‐PCR. Values are means and standard deviations, the error bars represent SD. One‐way ANOVA with the Dunnett test was used to calculate P values. These results are representative of at least three independent experiments in each experiment. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
As one of the most important members of the phospholipase family, the role of PLCγ1 in osteoarthritis, especially in chondrocytes, has become a research focus [38, 39]. In a previous study in a Sprague Dawley rat model of OA, we showed that intra‐articular injection of the PLCγ1 inhibitor U73122 reduced cartilage damage [20]. This suggested that targeted inhibition of PLCγ1 might be a potential therapy for OA. In this current study in IL‐1β‐treated rat chondrocytes, we found that a low concentration (2 μm) of the PLCγ1 inhibitor U73122 decreased PLCγ1 phosphorylation and improved cartilage matrix synthesis, with increased levels of Collagen2 and Aggrecan. However, higher concentrations of U73122 reduced levels of Collagen2 and Aggrecan. These observations are consistent with our previous results [40] and with those of Gao [41], who found U73122 10 μm reduced Collagen2 and Aggrecan mRNA levels in rat nucleus pulposus cells. Our subsequent experiments showed this decreased matrix synthesis to result from increased apoptosis and necroptosis of chondrocytes at the higher concentrations of U73122 and higher level of PLCγ1 inhibition.
The conclusion from these observations is that PLCγ1 inhibition and cartilage matrix synthesis is not a simple linear relationship, and there might be a crossover point between them such that a successful therapy for OA would provide some but not complete PLCγ1 inhibition.
Increased chondrocyte death and ROS levels are risk factors for OA [42], and the results of our experiments are consistent with these factors being associated with reduced cartilage matrix synthesis. Increases in cell death and ROS levels are closely related to programmed cell death (PCD), especially apoptosis and necroptosis, and apoptosis and necrosis inhibitors can reduce both factors [43]. Our current results are also consistent with this. A study by Yuan et al. [21] showed that inhibition of PLCγ1 phosphorylation with U73122 10 μm increased apoptosis in pheochromocytoma 12 (PC12) cells induced by hydrogen peroxide. A study by Jiang et al. [22] also showed that knockdown of PLCγ1 can increase apoptosis of vascular smooth muscle cells. The increased level of chondrocyte apoptosis after PLCγ1 inhibition with U73122 in our studies was similar to that observed by Xiao et al. [39]. In addition, chondrocyte apoptosis is a risk factor for the initiation and development of OA, and inhibition of chondrocyte apoptosis can relieve OA [27, 28]. Our current study results are consistent with these data.
Although necroptosis is currently a focus for research in programmed cell death, the direct relationship between PLCγ1 and necroptosis is still not clear. However, it is clear that PLCγ1 is an important regulator of Ca2+, and Chang et al. have shown that Ca2+ regulates RIP3 through CaMKII, thus affecting the necroptosis level in rat ventricular cardiomyocytes [44, 45]. We therefore had reason to believe there was a relationship between PLCγ1 and necroptosis in chondrocytes. This was confirmed, and our results showed that chondrocytes necroptosis increased with PLCγ1 inhibition with U73122 treatment.
It is worth noting that in this study, Z‐VAD and Nec‐1 treatment alone did reduce chondrocyte apoptosis and necroptosis, respectively, but neither improved cartilage matrix synthesis. Previous reports show that apoptosis and necroptosis share the same signal pathway in the early stage, and it is difficult to completely inhibit programmed cell death by inhibiting just one process [46]. In our study, combined inhibition of apoptosis and necroptosis with Z‐VAD and Nec‐1 combined produced greater inhibition of cell death and increased cartilage matrix synthesis.
The observation of enhanced cartilage matrix synthesis at a low concentration of U73122 but reduced cartilage matrix synthesis at a higher concentration is not rare in pharmaceutical research and clinical drug usage [47, 48, 49]. For example, except for leukemia [50], tretinoin is also used in photodamaged skin and cosmetology [51]. However, because of its strong inhibition of keratinization a large overdose can damage the skin, causing skin erythema and ulceration [52, 53]. Although U73122 at 2 μm improved cartilage matrix synthesis, this concentration did increase chondrocyte apoptosis and necroptosis levels, which would partially counteract the effect on matrix synthesis. The full and potentially curative effect on matrix synthesis was apparent when the combined treatment with Z‐VAC and Nec‐1 was used to inhibit the U73122‐induced increases in apoptosis and necroptosis, respectively. This is similar to the situation of reducing or alleviating the skin erythema and ulceration caused by tretinoin treatment by reducing drug dose or by combining with nonsteroidal drugs [52, 53]. Another example of ‘cocktail therapy’ is the treatment of AIDS, where use of multiple drugs can reduce drug resistance and side effects, and improve the overall therapeutic effect through the synergistic effects of the multiple drugs [54].
In summary, inhibition of PLCγ1 phosphorylation by U73122 improved cartilage matrix synthesis in IL‐1β‐treated rat chondrocytes. However, it also increased cell apoptosis and necroptosis, which reduced the effect on cartilage matrix synthesis. Inhibition of the U73122‐induced programmed cell death by simultaneous treatment with the apoptosis inhibitor Z‐VAD and the necroptosis inhibitor Nec‐1 increased chondrocyte proliferation and further enhanced cartilage matrix synthesis. Therefore, we propose PLCγ1 as a new molecular target as a potential disease‐modifying therapy for OA (Fig. 4).
Fig. 4.
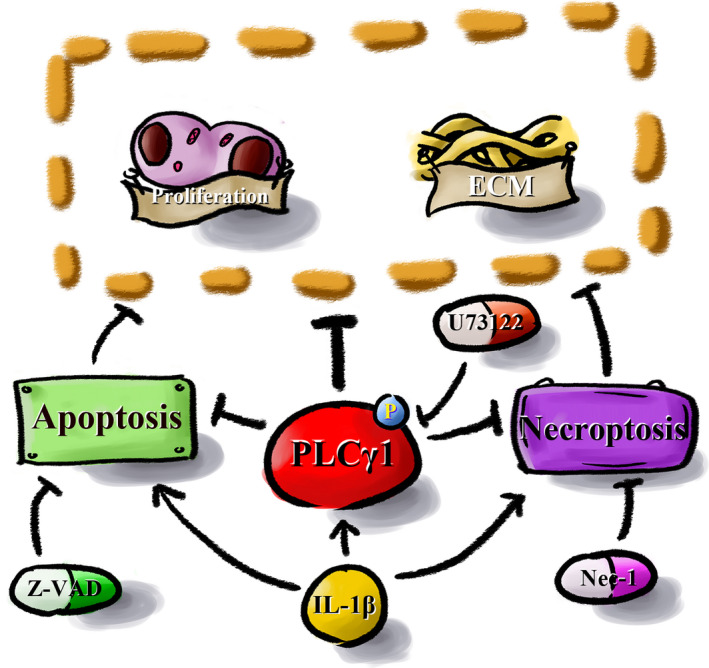
Molecular mechanism of PLCγ1 inhibition combined with inhibition of apoptosis and necroptosis increases cartilage matrix synthesis in IL‐1β‐treated rat chondrocytes. Inhibition of PLCγ1 phosphorylation by U73122 improved cartilage matrix synthesis in IL‐1β‐treated rat chondrocytes. However, it also increased cell apoptosis and necroptosis, which reduced the effect on cartilage matrix synthesis. Inhibition of programmed cell death by simultaneous treatment with the apoptosis and the necroptosis inhibitor increased chondrocyte proliferation and further enhanced cartilage matrix synthesis.
Conflicts of interest
The authors declare no conflict of interest.
Author contributions
XC and CX conceived the study and designed the experiments. XC, RC, and YX contributed to the data collection. XC and RC performed the data analysis and interpreted the results. XC wrote the manuscript. CX contributed to the critical revision of the article. All authors read and approved the final manuscript.
Supporting information
Fig. S1. U73343 can’t inhibit PLCγ1 and also can’t increase Collagen2 and Aggrecan levels in IL‐1β‐treated rat chondrocytes. Rat chondrocytes pretreated with IL‐1β (20 ng/ml for 36 hours) were treated with U73122 or U73343 (2 μM for 12 hours).
Acknowledgements
We would like to thank Prof. Bing Zhang for scientific idea support. We would like to express our gratitude to EditSprings (https://www.editsprings.com/) for the expert linguistic services provided.
Data accessibility
The data will be available from the corresponding author upon reasonable request.
References
- 1. Malfait AM (2016) Osteoarthritis year in review 2015: biology. Osteoarthr Cartil 24, 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Busija L, Bridgett L, Williams SRM, Osborne RH, Buchbinder R, March L and Fransen M (2010) Osteoarthritis. Best Pract Res Clin Rheumatol 24, 757–768. [DOI] [PubMed] [Google Scholar]
- 3. Barnett R (2018) Osteoarthritis. Lancet 391, 1985. [DOI] [PubMed] [Google Scholar]
- 4. Glyn‐Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H and Carr AJ (2015) Osteoarthritis. Lancet 386, 376–387. [DOI] [PubMed] [Google Scholar]
- 5. Accadbled F, Vial J and Sales de Gauzy J (2018) Osteochondritis dissecans of the knee. Orthop Traumatol Surg Res 104, S97–S105. [DOI] [PubMed] [Google Scholar]
- 6. Krishnan Y and Grodzinsky AJ (2018) Cartilage diseases. Matrix Biol 71–72, 51–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luo Y, Sinkeviciute D, He Y, Karsdal M, Henrotin Y, Mobasheri A, Önnerfjord P and Bay‐Jensen A (2017) The minor collagens in articular cartilage. Protein Cell 8, 560–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rojas FP, Batista MA, Lindburg CA, Dean D, Grodzinsky AJ, Ortiz C and Han L (2014) Molecular adhesion between cartilage extracellular matrix macromolecules. Biomacromol 15, 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schneevoigt J, Fabian C, Leovsky C, Seeger J and Bahramsoltani M (2017) In vitro expression of the extracellular matrix components aggrecan, collagen types I and II by articular cartilage‐derived chondrocytes. Anatomia Histologia Embryologia 46, 43–50. [DOI] [PubMed] [Google Scholar]
- 10. Taruc‐Uy RL and Lynch SA (2013) Diagnosis and treatment of osteoarthritis. Prim Care 40, 821–836. [DOI] [PubMed] [Google Scholar]
- 11. Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma‐Zeinstra SMA, Kraus VB, Lohmander LS, Abbott JH, Bhandari M et al (2019) OARSI guidelines for the non‐surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil 27, 1578–1589. [DOI] [PubMed] [Google Scholar]
- 12. Rodríguez‐Merchán EC (2019) The stiff total knee arthroplasty: causes, treatment modalities and results. EFORT Open Rev 4, 602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Otto‐Lambertz C, Yagdiran A, Wallscheid F, Eysel P and Jung N (2017) Periprosthetic infection in joint replacement. Dtsch Arztebl Int 114, 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benkovich V, Klassov Y, Mazilis B and Bloom S (2020) Periprosthetic fractures of the knee: a comprehensive review. Eur J Orthop Surg Traumatol 30, 387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang YR, Kang D‐S, Lee C, Seok H, Follo MY, Cocco L and Suh P‐G (2016) Primary phospholipase C and brain disorders. Adv Biol Regul 61, 80–85. [DOI] [PubMed] [Google Scholar]
- 16. Yang YR, Follo MY, Cocco L and Suh PG (2013) The physiological roles of primary phospholipase C. Adv Biol Regul 53, 232–241. [DOI] [PubMed] [Google Scholar]
- 17. Kang DS, Yang YR, Lee C, Kim S, Ryu SH and Suh PG (2016) Roles of phosphoinositide‐specific phospholipase Cγ1 in brain development. Adv Biol Regul 60, 167–173. [DOI] [PubMed] [Google Scholar]
- 18. Kunze K, Spieker T, Gamerdinger U, Nau K, Berger J, Dreyer T, Sindermann JR, Hoffmeier A, Gattenlohner S and Brauninger A (2014) A recurrent activating PLCG1 mutation in cardiac angiosarcomas increases apoptosis resistance and invasiveness of endothelial cells. Cancer Res 74, 6173–6183. [DOI] [PubMed] [Google Scholar]
- 19. Bleasdale JE, Bundy GL, Bunting S, Fitzpatrick FA, Huff RM, Sun FF and Pike JE (1989) Inhibition of phospholipase C dependent processes by U‐73, 122. Adv Prostaglandin Thromboxane Leukot Res 19, 590–593. [PubMed] [Google Scholar]
- 20. Cai H, Qu N, Chen X, Zhou Y, Zheng X, Zhang B and Xia C (2017) The inhibition of PLCγ1 protects chondrocytes against osteoarthritis, implicating its binding to Akt. Oncotarget 9, 4461–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hwang HS and Kim HA (2015) Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci 16, 26035–26054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yuan W, Guo J, Li X, Zou Z, Chen G, Sun J, Wang T and Lu D (2009) Hydrogen peroxide induces the activation of the phospholipase C‐gamma1 survival pathway in PC12 cells: protective role in apoptosis. Acta Biochim Biophys Sin (Shanghai) 41, 625–630. [DOI] [PubMed] [Google Scholar]
- 23. Choi A‐Y, Choi JH, Hwang K‐Y, Jeong YJ, Choe W, Yoon K‐S, Ha J, Kim SS, Youn JH, Yeo E‐J et al (2019) Licochalcone A induces apoptosis through endoplasmic reticulum stress via a phospholipase Cγ1‐, Ca (2+)‐, and reactive oxygen species‐dependent pathway in HepG2 human hepatocellular carcinoma cells [published correction appears in Apoptosis. 2019 Feb; 24(1–2):200–203]. Apoptosis 19, 682–697. [Google Scholar]
- 24. Pistritto G, Trisciuoglio D, Ceci C, Garufi A and D'Orazi G (2016) Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY) 8, 603–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krysko O, Aaes TL, Kagan VE, D'Herde K, Bachert C, Leybaert L, Vandenabeele P and Krysko DV (2017) Necroptotic cell death in anti‐cancer therapy. Immunol Rev 280, 207–219. [DOI] [PubMed] [Google Scholar]
- 26. Petrie EJ, Czabotar PE and Murphy JM (2019) The structural basis of necroptotic cell death signaling. Trends Biochem Sci 44, 53–63. [DOI] [PubMed] [Google Scholar]
- 27. Wang BW, Jiang Y, Yao ZL, Chen PS, Yu B and Wang SN (2019) Aucubin protects chondrocytes against IL‐1β‐induced apoptosis in vitro and inhibits osteoarthritis in mice model. Drug Des Devel Ther 13, 3529–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Igaki T (2009) Correcting developmental errors by apoptosis: lessons from Drosophila JNK signaling. Apoptosis 14, 1021–1028. [DOI] [PubMed] [Google Scholar]
- 29. Liang S, Lv Z‐t, Zhang J‐m, Wang Y‐t, Dong Y‐h, Wang Z‐g, Chen K, Cheng P, Yang Q, Guo F‐j et al (2018) Necrostatin‐1 attenuates trauma‐induced mouse osteoarthritis and IL‐1β induced apoptosis via HMGB1/TLR4/SDF‐1 in primary mouse chondrocytes. Front Pharmacol 9, 1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Riegger J and Brenner RE (2019) Evidence of necroptosis in osteoarthritic disease: investigation of blunt mechanical impact as possible trigger in regulated necrosis. Cell Death Dis 10, 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang J, Xia C, Zheng X, Yi T, Wang X, Song G and Zhang B (2011) 17β‐Estradiol promotes cell proliferation in rat osteoarthritis model chondrocytes via PI3K/Akt pathway. Cell Mol Biol Lett 16, 564–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou PH, Liu SQ and Peng H (2008) The effect of hyaluronic acid on IL‐1beta‐induced chondrocyte apoptosis in a rat model of osteoarthritis. J Orthop Res 26, 1643–1648. [DOI] [PubMed] [Google Scholar]
- 33. Zhang B, Wang F, Dai L, Cai H, Zhan Y, Gang S, Hu T, Xia C and Zhang B (2016) Lentivirus‐mediated PLCγ1 gene short‐hairpin RNA suppresses tumor growth and metastasis of human gastric adenocarcinoma. Oncotarget 7, 8043–8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dai L, Chen X, Lu X, Wang F, Zhan Y, Song G, Hu T, Xia C and Zhang B (2017) Phosphoinositide‐specific phospholipase Cγ1 inhibition induces autophagy in human colon cancer and hepatocellular carcinoma cells. Sci Rep 7, 13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang X, Chattopadhyay A, Ji QS, Owen JD, Ruest PJ, Carpenter G and Hanks SK (1999) Focal adhesion kinase promotes phospholipase C‐gamma1 activity. Proc Natl Acad Sci USA 96, 9021–9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu X, Qin D, Cui Y, Chen L, Li H, Chen Z, Gao L, Li Y and Liu J (2010) The effect of calcium phosphate nanoparticles on hormone production and apoptosis in human granulosa cells. Reprod Biol Endocrinol 8, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guan L, Han B, Li Z, Hua F, Huang F, Wei W, Yang Y and Xu C (2009) Sodium selenite induces apoptosis by ROS‐mediated endoplasmic reticulum stress and mitochondrial dysfunction in human acute promyelocytic leukemia NB4 cells. Apoptosis 14, 218–225. [DOI] [PubMed] [Google Scholar]
- 38. Ren K, Ma Y, Huang Y, Liang W, Liu F, Wang Q, Cui W, Liu Z, Yin G and Fan W (2011) Periodic mechanical stress activates MEK1/2‐ERK1/2 mitogenic signals in rat chondrocytes through Src and PLCγ1. Braz J Med Biol Res 44, 1231–1242. [DOI] [PubMed] [Google Scholar]
- 39. Xiao J, Chen X, Xu L, Zhang Y, Yin Q and Wang F (2014) PDGF regulates chondrocyte proliferation through activation of the GIT1‐ and PLCγ1‐mediated ERK1/2 signaling pathway. Mol Med Rep 10, 2409–2414. [DOI] [PubMed] [Google Scholar]
- 40. Zeng G, Cui X, Liu Z, Zhao H, Zheng X, Zhang B and Xia C (2014) Disruption of phosphoinositide‐specific phospholipases Cγ1 contributes to extracellular matrix synthesis of human osteoarthritis chondrocytes. Int J Mol Sci 15, 13236–13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gao G, He J, Nong L, Xie H, Huang Y, Xu N and Zhou D (2016) Periodic mechanical stress induces the extracellular matrix expression and migration of rat nucleus pulposus cells by upregulating the expression of integrin α1 and phosphorylation of downstream phospholipase Cγ1. Mol Med Rep 14, 2457–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bolduc JA, Collins JA and Loeser RF (2019) Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med 132, 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou B, Zhang J‐y, Liu X‐s, Chen H‐z, Ai Y‐l, Cheng K, Sun R‐y, Zhou D, Han J and Wu Q (2018) Tom20 senses iron‐activated ROS signaling to promote melanoma cell pyroptosis. Cell Res 28, 1171–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Livezey M, Huang R, Hergenrother PJ and Shapiro DJ (2018) Strong and sustained activation of the anticipatory unfolded protein response induces necrotic cell death. Cell Death Differ 25, 1796–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chang L, Wang Z, Ma F, Tran B, Zhong R, Xiong Y, Dai T, Wu J, Xin X, Guo W et al (2019) ZYZ‐803 mitigates endoplasmic reticulum stress‐related necroptosis after acute myocardial infarction through downregulating the RIP3‐CaMKII signaling pathway. Oxid Med Cell Longev 2019, 6173685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hanson B (2016) Necroptosis: a new way of dying? Cancer Biol Ther 17, 899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carey CM (2014) Tooth whitening: what we now know. J Evid Based Dent Pract 14(Suppl), 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yoo JK, Seikaly H and Calhoun KH (1997) Extended use of topical nasal decongestants. Laryngoscope 107, 40–43. [DOI] [PubMed] [Google Scholar]
- 49. Guerrini R, Belmonte A and Genton P (1998) Antiepileptic drug‐induced worsening of seizures in children. Epilepsia 39(Suppl 3), S2–S10. [DOI] [PubMed] [Google Scholar]
- 50. Osman AEG, Anderson J, Churpek JE, Christ TN, Curran E, Godley LA, Liu H, Thirman MJ, Odenike T, Stock W et al (2018) Treatment of acute promyelocytic leukemia in adults. J Oncol Pract 14, 649–657. [DOI] [PubMed] [Google Scholar]
- 51. Noble S and Wagstaff AJ (1995) A review of its pharmacological properties and clinical efficacy in the topical treatment of photodamaged skin. Drugs Aging 6, 479–496. [DOI] [PubMed] [Google Scholar]
- 52. Barth JH, Macdonald‐Hull SP, Mark J, Jones RG and Cunliffe WJ (1993) Isotretinoin therapy for acne vulgaris: a re‐evaluation of the need for measurements of plasma lipids and liver function tests. Br J Dermatol 129, 704–707. [DOI] [PubMed] [Google Scholar]
- 53. Cunliffe WJ, Poncet M, Loesche C and Verschoore M (1998) A comparison of the efficacy and tolerability of adapalene 0.1% gel versus tretinoin 0.025% gel in patients with acne vulgaris: a meta‐analysis of five randomized trials. Br J Dermatol 139(Suppl 52), 48–56. [DOI] [PubMed] [Google Scholar]
- 54. Voshavar C (2019) Protease inhibitors for the treatment of HIV/AIDS: recent advances and future challenges. Curr Top Med Chem 19, 1571–1598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. U73343 can’t inhibit PLCγ1 and also can’t increase Collagen2 and Aggrecan levels in IL‐1β‐treated rat chondrocytes. Rat chondrocytes pretreated with IL‐1β (20 ng/ml for 36 hours) were treated with U73122 or U73343 (2 μM for 12 hours).
Data Availability Statement
The data will be available from the corresponding author upon reasonable request.


