Highlights
-
•
Cutaneous angiosarcoma has poor outcomes with no standardized treatment regimen.
-
•
Paclitaxel-based chemoRT (CRT) was compared to other therapies at two US institutions.
-
•
Similar oncologic outcomes and improved survival with paclitaxel CRT.
-
•
Paclitaxel CRT + surgery provided best oncologic outcomes and survival.
-
•
Paclitaxel CRT + surgery regimen now being studied in a prospective phase II trial.
Abbreviations: CRT, paclitaxel-based chemoradiation; Non-CRT, other modalities; RT, radiotherapy; LC, local control; DC, distant control; PFS, progression-free survival; OS, overall survival
Keywords: Angiosarcoma, Radiation therapy, Chemotherapy, Paclitaxel, Concurrent chemoradiation
Abstract
Introduction
We compared clinical outcomes in patients with cutaneous angiosarcoma receiving concurrent paclitaxel-based chemoradiotherapy (CRT) vs. other modalities (Non-CRT).
Materials and methods
Patients with non-metastatic cutaneous angiosarcoma diagnosed from 1998 to 2018 at two institutions were identified. In the CRT cohort, paclitaxel 80 mg/m2 weekly was given for up to 12 weeks and patients received radiotherapy (RT) during the final 6 weeks of chemotherapy. The RT dose was 50–50.4 Gy delivered in 1.8–2 Gy per fraction with an optional post-operative boost of 10–16 Gy. Kaplan-Meier and log-rank statistics were used to compare the outcomes between the two groups. P < 0.05 was considered statistically significant.
Results
Fifty-seven patients were included: 22 CRT and 35 Non-CRT. The CRT cohort had more patients > 60 years (100% vs. 60%, p < 0.001) and tumors >5 cm (68.2% vs 54.3%, p = 0.023). The median follow-up was 25.8 (1.5–155.2) months. There was no significant difference in 2-year local control (LC), distant control (DC), or progression-free survival (PFS) between the two groups. The 2-year overall survival (OS) was significantly higher for the CRT cohort (94.1% vs. 71.6%, p = 0.033). Amongst the subset of patients in the CRT cohort who received trimodality therapy, the 2-year LC, DC, PFS, and OS was 68.6%, 100%, 68.6%, and 100%, respectively.
Conclusion
The use of concurrent paclitaxel CRT demonstrates promising outcomes. Given these results, we are currently evaluating the safety and efficacy of this regimen in prospective, phase 2 trial (NCT 03921008).
1. Introduction
Cutaneous angiosarcoma is a rare and highly aggressive form of sarcoma, comprising 1–4% of all sarcomas [1], [2], [3] It most commonly arises on the scalp, head and neck, extremities, or breast [4]. Non-cutaneous forms can also arise in viscera, such as the liver, as well as elsewhere in association with lymphedema or prior radiation. Treatment for angiosarcoma typically includes surgery with or without radiation therapy (RT) and/or chemotherapy. Multimodality therapy has been associated with improved outcomes compared with surgery alone. However, even with multimodality therapy, previous studies have demonstrated very poor outcomes, with most patients developing metastatic disease within the first 2 years after diagnosis and <50% surviving >5 years [5], [6]. Given these poor outcomes, patients with angiosarcoma need novel treatment.
One potential strategy to improve outcomes in these patients could be concurrent chemotherapy and RT either as a standalone therapy or prior to surgical resection. A phase II study of weekly paclitaxel in patients with unresectable cutaneous angiosarcoma demonstrated a disease control rate of 74% [7]. A small Japanese series described use of concurrent chemoradiotherapy (CRT) (either paclitaxel or docetaxel) in 13/19 patients with node negative scalp angiosarcoma [8]. They found concurrent chemoRT followed by maintenance chemotherapy was associated with improved progression free survival suggesting a role for this treatment paradigm. At our institutions, many patients have been treated with concurrent paclitaxel-based CRT as definitive therapy or as neoadjuvant therapy. The aim of this study was to review the outcomes of patients with cutaneous angiosarcoma treated with concurrent paclitaxel CRT and compare them with patients that received other non-CRT therapies, such as surgery with chemotherapy or radiation alone.
2. Materials and methods
This study was approved by the local institutional review board. All consecutive patients with pathologic diagnosis of cutaneous angiosarcoma treated at Washington University School of Medicine and Vanderbilt University School of Medicine between the years 1998–2018 were assessed. Pathology was reviewed by pathologists with special expertise in soft tissue sarcoma at each respective institution. Patients with metastatic disease at the time of initial diagnosis, non-cutaneous angiosarcoma, received RT alone, or received chemotherapy alone, were excluded. The electronic medical records were reviewed for age, race, sex, tumor size, anatomic site, nodal stage, surgical margins, and treatment modality. Tumors were categorized as superficial or deep.
Treatment including surgery, chemotherapy, and/or RT were reviewed. Patients were divided into two groups: concurrent paclitaxel chemoradiotherapy (CRT), and those receiving other treatment modalities (Non-CRT). For all patients in the CRT cohort, concurrent paclitaxel and RT was administered as a definitive treatment or neoadjuvant treatment prior to surgical resection. Paclitaxel 80 mg/m2 weekly was administered for up to 12 cycles. For patients receiving concurrent paclitaxel and RT, was delivered after 6 weeks of induction paclitaxel. In patients who received RT, the intended RT dose was at least 50–50.4 Gy delivered in 1.8–2 Gy per fraction for both cohorts. An additional boost of 10–16 Gy was also commonly used. Further details regarding RT delivered were unavailable. Negative surgical margin was defined as no tumor cells on the inked edge. If planned surgical resection would result in gross residual disease (R2 margin status), patients were treated with nonoperative management.
Time intervals for the analysis were calculated from the date of diagnosis. Local control (LC), distant control (DC), progression-free survival (PFS), and overall survival (OS) were estimated using Kaplan Meier Methods. Progression-free survival was defined as time to any recurrence or death. Patients were censored at the time of last known live follow-up if no date of death was recorded in the medical record system. Log-rank statistics was then used to compare disease outcomes between patients receiving CRT vs. patients receiving non-CRT. In addition, comparisons between disease outcomes were performed for additional subsets of patients. A t-test or Wilcoxon rank-sum test were used to compare the baseline and treatment characteristics of the CRT and the non-CRT groups. We were unable to compute univariable or multivariable cox regression analysis due to limited number of events as well as small patient cohort. Statistical analysis was performed using SPSS Statistics (Version 26; IBM; Armonk, NY). P < 0.05 was considered statistically significant.
3. Results
A total of 57 patients were included in the final analysis: 22 patients in the CRT cohort and 35 patients in the Non-CRT cohort. Characteristics of the patient population are shown in Table 1. The median age at diagnosis was 69.2 years (range: 27–88). Ten (28.6%) patients in the non-CRT cohort had radiation-associated angiosarcomas compared to 1 (4.5%) in the CRT cohort (p < 0.001). Of these patients, 1 patient in the non-CRT cohort and 1 patient in the CRT cohort received re-irradiation. The anatomic sites of disease were scalp (n = 25, 43.9%), head and neck (n = 9, 15.8%), extremity (n = 8, 14.0%), trunk (n = 7, 12.3%), breast (n = 4, 7.0%), and other site (n = 4, 7.0%). FDG PET/CT was used for staging purposes in 6 (27.3%) patients in the CRT cohort and 5 (14.3%) patients in the non-CRT cohort. In the CRT cohort, concurrent paclitaxel and RT was administered as a definitive treatment in 13 (59.1%) patients and as neoadjuvant treatment prior to surgery in 9 (40.9%) patients. In the Non-CRT cohort, surgery + chemotherapy was used in 11 (31.4%) patients, surgery + RT was used in 10 (28.6%) patients, surgery alone was used in 6 (17.1%) patients, surgery + sequential RT + chemotherapy was used in 6 patients (17.1%), and definitive sequential RT + chemotherapy was used in 2 (5.7%) patients.
Table 1.
Baseline Patient and Treatment Characteristics.
| Non-CRT n (%) | CRT n (%) | P-value* | ||
|---|---|---|---|---|
| Age | <0.001 | |||
| <60 | 14 (40) | 0 (0) | ||
| ≥60 | 21 (60) | 22 (1 0 0) | ||
| Sex | ||||
| Male | 18 (51.4) | 15 (68.2) | 0.029 | |
| Female | 17 (48.6) | 7 (31.8) | ||
| Radiation Associated | ||||
| No | 25 (71.4) | 21 (95.5) | <0.001 | |
| Yes | 10 (28.6) | 1 (4.5) | ||
| Tumor Location | ||||
| Scalp | 12 (34.3) | 13 (59.1) | <0.001 | |
| Head and Neck | 3 (8.6) | 6 (27.3) | ||
| Trunk | 5 (14.3) | 2 (9.1) | ||
| Extremity | 8 (22.9) | 0 (0) | ||
| Breast | 4 (11.4) | 0 (0) | ||
| Other | 3 (8.6) | 1 (4.5) | ||
| Tumor Size | ||||
| ≤5 cm | 15 (42.9) | 6 (27.3) | 0.023 | |
| >5 cm | 19 (54.3) | 15 (68.2) | ||
| Unknown | 1 (2.9) | 1 (4.5) | ||
| Deep Tumor | ||||
| No | 27 (77.1) | 20 (90.1) | 0.005 | |
| Yes | 8 (22.9) | 2 (9.1) | ||
| Nodal Disease | ||||
| No | 32 (91.4) | 20 (90.1) | 0.895 | |
| Yes | 3 (8.6) | 2 (9.1) | ||
| Surgical Margin | ||||
| Negative | 21 (63.6) | 5 (55.6) | 0.331 | |
| Positive | 9 (27.3) | 4 (44.4) | ||
| Unknown | 3 (9.1) | 0 (0) | ||
| No Primary Surgery | 2 (5.7) | 13 (59.1) | ||
| Surgery | ||||
| No | 2 (5.7) | 13 (59.1) | <0.001 | |
| Yes | 33 (94.3) | 9 (40.9) | ||
| Chemotherapy | ||||
| Other Agent (s) | 5 (14.3) | 0 (0) | <0.001 | |
| Paclitaxel | 14 (40.0) | 22 (1 0 0) | ||
| No chemotherapy | 16 (45.7) | 0 (0) | ||
| Chemotherapy Cycles | Median (Range) | 4 (2–12) | 8 (3–12) | 0.503 |
| Radiation Therapy | ||||
| No | 17 (48.6) | 0 (0) | <0.001 | |
| Yes | 18 (51.4) | 22 (1 0 0) | ||
| Radiation Dose (cGy) | Median (Range) | 6600 (5000–7560) | 6000 (2700–7400) | 0.071 |
| Treatments | ||||
| Surgery Alone | 6 (17.1) | 0 (0) | 0.001 | |
| Surgery + RT | 10 (28.6) | 0 (0) | ||
| Surgery + Chemotherapy | 11 (31.4) | 0 (0) | ||
| Surgery + RT + Chemotherapy | 6 (17.1) | 0 (0) | ||
| Surgery + Concurrent RT + Chemotherapy | 0 (0) | 9 (40.9) | ||
| Definitive Concurrent RT + Chemotherapy | 0 (0) | 13 (59.1) | ||
| Definitive Sequential RT + Chemotherapy | 2 (5.7) | 0 (0) | ||
Abbreviations: RT, radiation therapy; CRT, concurrent paclitaxel chemoradiotherapy cohort; non-CRT, other treatment modalities cohort.
T-test or Wilcoxon test used for statistical analysis to compare two groups.
There were significant differences in patient characteristics between the CRT cohort and Non-CRT cohort (Table 1). All patients in the CRT cohort were ≥ 60 years compared to 21 (60%) in the non-CRT cohort (p < 0.001). Fifteen (68.2%) patients in the CRT cohort had tumors > 5 cm compared to 19 (54.3%) patients in the Non-CRT cohort (p = 0.023). Only 9 (40.9%) patients in the CRT cohort underwent surgery while 33 (94.3%) patients in the Non-CRT cohort underwent surgery (p < 0.001). Paclitaxel was delivered in all patients in the CRT cohort, however only 14 (40.0%) patients in the Non-CRT received paclitaxel, 5 (14.3%) patients received other systemic therapy agents, and 16 (45.7%) received no systemic therapy.
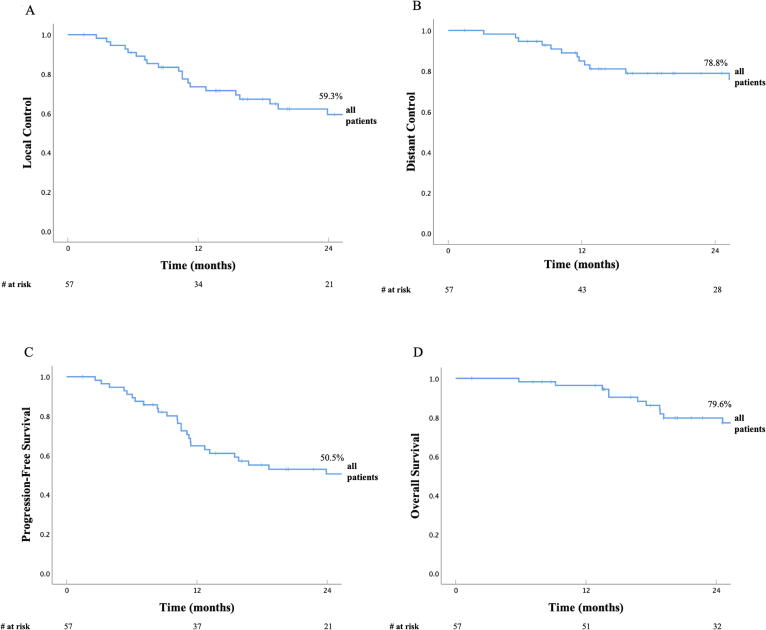
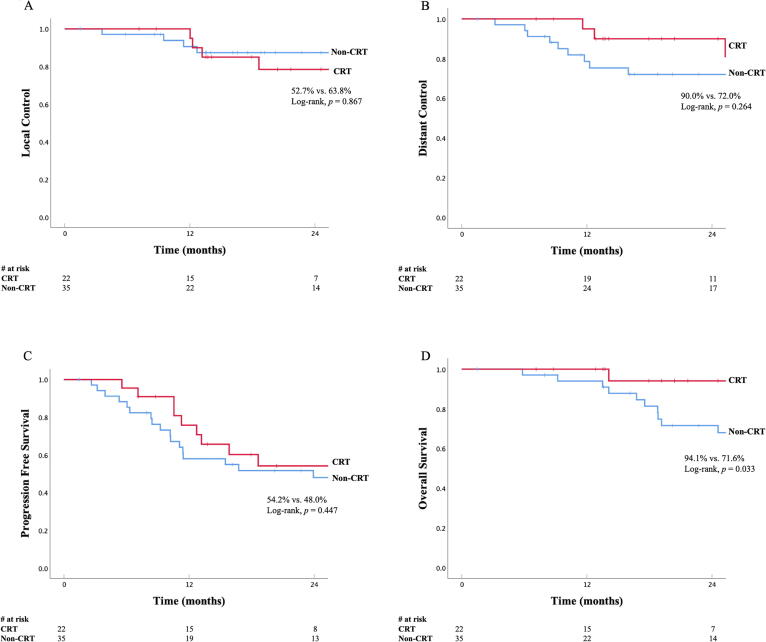
The median follow-up was 25.8 months (range: 1.5–155.2) for the whole cohort, with similar follow up for the CRT and Non-CRT cohorts (median 25.7 vs. 25.8 months). At the time of analysis, 34 patients (59.6%) had experienced a progression and 25 patients (43.9%) had died. 20 of 25 patients (80%) died from their cancer. Disease outcomes for all patients are listed in Table 2 and shown in Fig. 1. Disease outcomes for CRT cohort vs. Non-CRT cohort are also listed in Table 2 and shown in Fig. 2. There was no statistical difference in LC, DC, or PFS between the two cohorts, however OS was significantly higher in the CRT cohort. The 2-year LC in the CRT cohort was 52.7% compared to 63.8% in the Non-CRT cohort (p = 0.867, Fig. 2A). The 2-year DC in the CRT cohort was 90.0% vs. 72.0% in the Non-CRT cohort (p = 0.264, Fig. 2B). The 2-year PFS in the CRT cohort was 54.2% vs. 48.0% (p = 0.447) The 2-year OS in the CRT cohort was 94.1% vs. 71.6% in the Non-CRT cohort, respectively (p = 0.033, Fig. 2C). Disease outcomes for the subset of patients in CRT cohort who received neoadjuvant CRT followed by surgery (n = 9) are listed in Table 2. The 2-year LC, DC, PFS, and OS were 68.6%, 100%, 68.6%, and 100%, respectively. Finally, disease outcomes for patients in the CRT cohort who received definitive chemoradiation alone (n = 13) were compared to patients in the Non-CRT cohort who received surgery with sequential chemotherapy and/or RT (n = 27). The 2-year LC, DC, PFS, and OS were 48.0% vs. 64.3% (p = 0.605), 80.0% vs. 69.7% (p = 0.346), 50.0% vs. 41.5% (p = 0.414), and 87.5% vs. 73.3% (p = 0.196).
Table 2.
Kaplan-Meier disease outcomes.
| Local control | Distant control | Progression-free Survival | Overall survival | |
|---|---|---|---|---|
| All patients | ||||
| 1 year | 73.5% | 85.0% | 64.8% | 96.3% |
| 2 year | 59.3% | 78.8% | 50.5% | 79.6% |
| CRT | ||||
| 1 year | 75.8% | 95.0% | 75.8% | *100% |
| 2 year | 52.7% | 90.0% | 54.2% | *94.1% |
| Non-CRT | ||||
| 1 year | 71.9% | 78.6% | 58.0% | *94.0% |
| 2 year | 63.8% | 72.0% | 48.0% | *71.6% |
| CRT subset who received surgery | ||||
| 1 year | 85.7% | 100% | 85.7% | 100% |
| 2 year | 68.6% | 100% | 68.6% | 100% |
Abbreviations; CRT, concurrent paclitaxel chemoradiotherapy cohort; Non-CRT, other treatment modalities cohort
Disease outcomes for CRT and Non-CRT cohort compared using log rank statistical test. p = 0.033 for overall survival, all other outcomes not statistically significant.
Fig. 1.
Local Control (A), Distant Control (B), Progression-Free Survival (C) and Overall Survival (D) in all patients with cutaneous angiosarcoma.
Fig. 2.
Local Control (A), Distant Control (B), Progression-Free Survival (C) and Overall Survival (D) in patients with cutaneous angiosarcoma receiving concurrent paclitaxel-based chemoradiotherapy compared to patients receiving other treatment modalities (Surgery + RT, Surgery + chemotherapy, Surgery alone, Definitive sequential chemotherapy + RT, and Surgery + sequential chemotherapy + RT).
4. Discussion
Angiosarcoma is a rare disease for which there is no established treatment paradigm. We report herein the largest clinical study of non-metastatic cutaneous angiosarcoma treated with concurrent weekly paclitaxel and RT with or without surgery. We found that the use of concurrent paclitaxel and RT has similar 2-year LC, PFS, and DC and significantly improved 2-year OS compared to the use other treatment modalities including surgery alone, surgery + RT, surgery + chemotherapy, and surgery + sequential RT + chemotherapy, or definitive sequential RT + chemotherapy. Furthermore, we found that the subset of CRT patients who received tri-modality therapy with surgery had excellent 2-year LC, DC, PFS, and OS 68.6%, 100%, 68.6%, and 100%, respectively. These findings suggest that a treatment regimen incorporating paclitaxel-based CRT should be further evaluated in a prospective setting.
Patients in the CRT vs. non-CRT cohort were not well balanced in terms of disease or treatment characteristics. Patients in the CRT cohort were significantly older and more likely to have large (>5 cm) tumors. These factors have consistently demonstrated to portend worse disease outcomes and survival [9]. Patients in the CRT cohort also had significantly lower rates of surgical resection. Surgical resection has been noted to be an important predictor of disease outcomes and survival [10]. Despite these differences, our analysis found similar oncologic outcomes and improved survival outcomes in our CRT cohort.
All patients in the CRT cohort received systemic therapy compared to 54% in the non-CRT cohort. The CRT cohort had higher numerical rates of 2-year DC (90.0% vs. 72.0%). Given the aggressive nature of cutaneous angiosarcoma, perhaps higher rates of systemic therapy and DC led to improved OS in the CRT cohort. The CRT cohort had numerically lower rates of 2-year LC (52.7% vs. 63.8%) and significantly lower rates of surgical resection as part of definitive therapy (59.1% vs. 94.3%). Another potential explanation for the differences in survival may be that the local failures in the CRT cohort were more readily managed with salvage surgery. Unfortunately, we did not record data on salvage therapy after recurrence. Finally, potential confounders inherent to the retrospective nature of our analysis may have contributed to these results. These are discussed in further detail below.
Initial clinical trials of taxanes in patients with advanced soft tissue sarcomas found limited activity, with response rates ranging from 0 to 12.5% [11], [12]. Importantly, a patient with angiosarcoma was one of the few responders to paclitaxel. Subsequent retrospective studies suggested the efficacy of paclitaxel in angiosarcoma of the head and neck with response rates ranging from 63 to 89% [1], [13]. The activity of paclitaxel was further demonstrated in the prospective, phase II ANGIOTAX study. Patients with metastatic angiosarcoma received paclitaxel 80 mg/m2 on days 1, 8, and 15 of a 28-day cycle. The response rate was 18%, and median PFS and OS were 3.8 and 8 months, respectively. A subsequent phase II study of patients with metastatic or advanced angiosarcoma with weekly paclitaxel 90 mg/m2 demonstrated a response rate of 45%, a median PFS of 6.6 months and a median OS of 19.5 months [14]. The discrepancies in outcomes between these two studies may reconciled by the heterogeneity of the angiosarcoma subtypes included or potentially the different paclitaxel dose administered. Of note in the second paclitaxel study, radiation-associated angiosarcoma was associated with improved outcomes.
Despite multiple retrospective studies and prospective trials demonstrating efficacy of weekly paclitaxel for angiosarcoma, few studies have reported its activity solely in the non-metastatic setting. In a retrospective study of 19 patients with node negative, cutaneous angiosarcoma of the scalp, 13/19 patients received concurrent CRT with either docetaxel or paclitaxel and 11/19 patients received maintenance paclitaxel [8]. Concurrent chemoRT with maintenance chemotherapy and surgery were associated with improved PFS but not improved OS or local control. This is a small study with relatively short follow-up which may mitigate any potential OS benefit. A study by Fujisawa et al. evaluated 28 patients with localized angiosarcoma comparing chemoRT with surgery and RT. Their results showed improved outcomes in patients treated with CRT compared to patients receiving surgery + RT [9], with 5 year OS 56% vs 8% (p < 0.01), respectively. They also found significantly improved OS in patients who received maintenance chemotherapy.
Though paclitaxel and RT have been used concurrently in other cancers with excellent safety profile [15], [16], this treatment paradigm is not routine practice in the treatment of angiosarcoma. Our study herein is the largest to report its use in cutaneous angiosarcoma. The rationale for using concurrent chemotherapy and RT is to both combat early micro-metastatic disease and to provide RT sensitizing effect [17], [18], [19]. The optimal timing of concurrent paclitaxel and RT in relationship to the timing of surgery is unclear. In our study, 20 patients received either neoadjuvant CRT or definitive CRT, while only 2 patient received adjuvant CRT. Additionally, the number of paclitaxel cycles and the RT dose that is ideal for these patients needs further investigation. Interestingly, Smith et al reported excellent long-term disease control and survival in a small cohort of patients receiving hyper-fractionated and accelerated re-irradiation (HART) for management of secondary angiosarcomas after breast-conserving therapy for primary breast cancer [20]. However, patients at our institution have historically been treated with conventionally fractionated RT. Therefore, we excluded patients who received RT alone from our analysis. Our analysis demonstrates that CRT patients who undergo trimodality therapy have the most favorable oncologic and survival outcomes. Therefore, we have recently opened a prospective phase II single institutional clinical trial (NCT 03921008) to evaluate the safety and efficacy of this regimen. At our two institutions, we are fortunate to have pathologists with special expertise in soft tissue sarcoma. However, primary cutaneous angiosarcomas are rare vasoformative soft tissue sarcomas that may present a diagnostic challenge for pathologists with limited sarcoma experience. Therefore, further prospective studies aimed at improving outcomes for this aggressive cancer subtype should incorporate central pathology review to ensure appropriate diagnosis.
Given the retrospective nature of this analysis, there are several limitations in this study. Firstly, the small cohort may limit the power to detect differences in disease outcomes or other significant predictors of prognosis. Nevertheless, this remains a relatively large cohort given the rarity of this disease. Second, the CRT and Non-CRT cohorts were not well balanced in terms of baseline characteristics but attempting to correct for this through methods such as propensity score matching would have significantly reduced the number of patients in our analysis. Third, performance status of patients was not available and clearly affects the treatment selection. Fourth, we did not collect acute and late toxicity of treatment regimens which are important treatment outcomes. Finally, given that our analysis included patients treated at two institutions and the treatment of angiosarcoma is not well-protocolized, there is heterogeneity in the treatment delivered. Furthermore, specific details regarding RT delivered for each case was unavailable.
Despite these limitations, our study fills a void in the literature regarding outcomes of patients with localized cutaneous angiosarcoma who underwent concurrent chemoRT. We excluded all patients who received RT alone or chemotherapy alone, as these treatments alone are less likely to be curative for patients with such an aggressive disease. Additionally, patients in the CRT cohort receiving concurrent chemotherapy other than paclitaxel were also excluded to minimize confounding variables.
5. Conclusion
In conclusion, our study seems to suggest that concurrent paclitaxel and radiotherapy is an effective treatment for patients with cutaneous angiosarcoma and demonstrates promising outcomes. A prospective trial to study the safety, efficacy and optimal dosing is warranted and has started through a prospective phase II single institutional clinical trial (NCT 03921008).
6. Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
Dr. Davis reports grants from Karyopharm, grants from Incyte, grants from BMS, grants from Genentech, grants from TopAlliance Biosciences, grants from Actuate, outside the submitted work; Dr. Keedy reports personal fees from karyopharm, grants from tracon, from daiichi sankyo, from advenchen, from Lilly, from GSK, outside the submitted work; Dr. Oppelt reports personal fees from Bristol Myers, personal fees from Merck, personal fees from Eisai, outside the submitted work; Dr. Van Tine reports grants from Merck, grants and personal fees from Pfizer, grants from Tracon, grants, personal fees and other from GSK, personal fees and other from Polaris, personal fees from Lilly, personal fees from Caris Life Sciences, personal fees from Novartis, personal fees from CytRX, personal fees from Plexxikon, personal fees from Epizyme, personal fees from Daiihi Sankyo, personal fees from Adaptimmune, personal fees from Immune Design, personal fees from Bayer, outside the submitted work; All remaining authors have none.
References
- 1.Fata F., O'Reilly E., Ilson D., Pfister D., Leffel D., Kelsen D.P. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer. 1999;86(10):2034–2037. [PubMed] [Google Scholar]
- 2.Fayette J., Martin E., Piperno-Neumann S., Le Cesne A., Robert C., Bonvalot S. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol Off J Eur Soc Med Oncol. 2007;18(12):2030–2036. doi: 10.1093/annonc/mdm381. [DOI] [PubMed] [Google Scholar]
- 3.Penel N., Marréaud S., Robin Y.-M., Hohenberger P. Angiosarcoma: state of the art and perspectives. Crit Rev Oncol Hematol. 2011;80(2):257–263. doi: 10.1016/j.critrevonc.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Holden C.A., Spittle M.F., Jones E.W. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046–1057. doi: 10.1002/1097-0142(19870301)59:5<1046::aid-cncr2820590533>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Zagars G.K., Ballo M.T., Pisters P.W.T., Pollock R.E., Patel S.R., Benjamin R.S. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer. 2003;97(10):2530–2543. doi: 10.1002/(ISSN)1097-014210.1002/cncr.v97:1010.1002/cncr.11365. [DOI] [PubMed] [Google Scholar]
- 6.Merfeld E., Gabani P., Spraker M.B., Zoberi I., Kim H., Van Tine B. Clinical outcomes and prognostic features of angiosarcoma: significance of prior radiation therapy. Clin Oncol R Coll Radiol G B. 2019;31(4):232–241. doi: 10.1016/j.clon.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Penel N., Bui B.N., Bay J.-O., Cupissol D., Ray-Coquard I., Piperno-Neumann S. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(32):5269–5274. doi: 10.1200/JCO.2008.17.3146. [DOI] [PubMed] [Google Scholar]
- 8.Ihara H., Kaji T., Katsui K., Miyake T., Waki T., Katayama N. Single institutional experience of radiation therapy for angiosarcoma of the scalp without cervical lymph node metastases: impact of concurrent chemoradiation with maintenance chemotherapy using taxanes on patient prognosis. Mol Clin Oncol. 2019 doi: 10.3892/mco10.3892/mco.2019.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujisawa Y., Yoshino K., Kadono T., Miyagawa T., Nakamura Y., Fujimoto M. Chemoradiotherapy with taxane is superior to conventional surgery and radiotherapy in the management of cutaneous angiosarcoma: a multicentre, retrospective study. Br J Dermatol. 2014;171(6):1493–1500. doi: 10.1111/bjd.13110. [DOI] [PubMed] [Google Scholar]
- 10.Trofymenko O., Curiel-Lewandrowski C. Surgical treatment associated with improved survival in patients with cutaneous angiosarcoma. J Eur Acad Dermatol Venereol. 2018;32(1):e29–e31. doi: 10.1111/jdv.2018.32.issue-110.1111/jdv.14479. [DOI] [PubMed] [Google Scholar]
- 11.Patel S.R., Papadopoulos N.E., Plager C., Linke K.A., Moseley S.H., Spirindonidis C.H. Phase II study of paclitaxel in patients with previously treated osteosarcoma and its variants. Cancer. 1996;78:741–744. doi: 10.1002/(SICI)1097-0142(19960815)78:4<741::AID-CNCR8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 12.Balcerzak S.P., Benedetti J., Weiss G.R., Natale R.B. A phase II trial of paclitaxel in patients with advanced soft tissue sarcomas. A Southwest Oncology Group study. Cancer. 1995;76:2248–2252. doi: 10.1002/1097-0142(19951201)76:11<2248::aid-cncr2820761111>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 13.Skubitz K.M., Haddad P.A. Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer. 2005;104(2):361–366. doi: 10.1002/(ISSN)1097-014210.1002/cncr.v104:210.1002/cncr.21140. [DOI] [PubMed] [Google Scholar]
- 14.Ray-Coquard I.L., Domont J., Tresch-Bruneel E., Bompas E., Cassier P.A., Mir O. Paclitaxel Given Once Per Week With or Without Bevacizumab in Patients With Advanced Angiosarcoma: A Randomized Phase II Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(25):2797–2802. doi: 10.1200/JCO.2015.60.8505. [DOI] [PubMed] [Google Scholar]
- 15.Bradley J.D., Paulus R., Komaki R., Masters G., Blumenschein G., Schild S. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro J., van Lanschot J.J.B., Hulshof M.C.C.M., van Hagen P., van Berge Henegouwen M.I., Wijnhoven B.P.L. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 17.Tishler R.B., Geard C.R., Hall E.J., Schiff P.B. Taxol sensitizes human astrocytoma cells to radiation. Cancer Res. 1992;52:3495–3497. [PubMed] [Google Scholar]
- 18.Creane M., Seymour C.B., Colucci S., Mothersill C. Radiobiological effects of docetaxel (Taxotere): a potential radiation sensitizer. Int J Radiat Biol. 1999;75:731–737. doi: 10.1080/095530099140078. [DOI] [PubMed] [Google Scholar]
- 19.Zhang A.L., Russell P.J., Knittel T., Milross C. Paclitaxel enhanced radiation sensitization for the suppression of human prostate cancer tumor growth via a p53 independent pathway. Prostate. 2007;67(15):1630–1640. doi: 10.1002/pros.20638. [DOI] [PubMed] [Google Scholar]
- 20.Smith T.L., Morris C.G., Mendenhall N.P. Angiosarcoma after breast-conserving therapy: long-term disease control and late effects with hyperfractionated accelerated re-irradiation (HART) Acta Oncol Stockh Swed. 2014;53(2):235–241. doi: 10.3109/0284186X.2013.819117. [DOI] [PubMed] [Google Scholar]