Abstract
Background & aims
Immune checkpoint blockade (ICB) has been approved for treatment of hepatocellular carcinoma (HCC). However, many patients with advanced HCC are non-responders to ICB monotherapy. Cytotoxic chemotherapy has been proposed to modulate the tumor microenvironment (TME) and sensitize tumors to ICB. Thus, we aimed to study the combination of cytotoxic chemotherapy and ICB in an orthotopic HCC model.
Methods
Preclinical orthotopic HCC mouse models were used to elucidate the efficacy of 5-fluorouracil (5-FU) and ICB. The mice were intrahepatically injected with RIL-175 or Hepa1-6 cells, followed by treatment with 5-FU and anti-programmed cell death ligand 1 (PD-L1) antibody. Myeloid-derived suppressor cells (MDSCs) were depleted to validate their role in attenuating sensitivity to immunotherapy. Flow cytometry-based immune profiling and immunofluorescence staining were performed in mice and patient samples, respectively.
Results
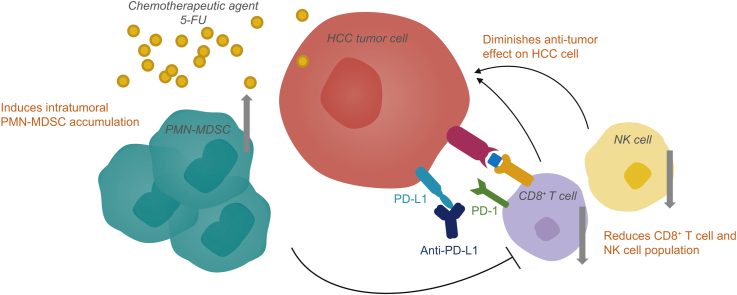
5-FU could induce intratumoral MDSC accumulation to counteract the infiltration of T lymphocytes and natural killer cells, thus abrogating the anti-tumor efficacy of PD-L1 blockade. In clinical samples, MDSCs accumulated and CD8+ T cell numbers decreased following transarterial chemoembolization.
Conclusion
5-FU can trigger the accumulation of immunosuppressive MDSCs, impairing the response to PD-L1 blockade in HCC. Our data suggest that the combination of specific chemotherapy and ICB may impair anti-tumor immune responses, warranting further study in preclinical models and consideration in clinical settings.
Lay summary
Our findings suggest that some chemotherapies may impair the anti-tumor efficacy of immunotherapy. Further studies are required to uncover the specific effects of different chemotherapies on the immunological profile of tumors. This data will be critical for the rational design of combination immunotherapy strategies for patients with hepatocellular carcinoma.
Keywords: Tumor microenvironment, Hepatocellular carcinoma, Chemotherapy, Myeloid-derived suppressor cell, Immune checkpoint blockade, Immunotherapy, TACE
Abbreviations: 5-FU, fluorouracil; HCC, hepatocellular carcinoma; ICB, immune checkpoint blockade; ICD, immunogenic cell death; NK, natural killer; MDSC(s), myeloid-derived suppressor cell(s); M-MDSC, mononuclear MDSC; PD-L1, programmed cell death ligand 1; PMN-MDSC, polymorphonuclear MDSC; TACE, transarterial chemoembolization; TME, tumor microenvironment
Graphical abstract
Highlights
-
•
Some chemotherapeutic agents could counteract the efficacy of PD-L1 blockade in HCC.
-
•
5-FU mediates MDSC accumulation in tumors which blunts the response to immunotherapy.
-
•
MDSCs are enriched in HCC patients treated with transarterial chemoembolization.
Introduction
An immunosuppressive microenvironment plays a key role in mediating immune tolerance and tumor evasion in cancer, which may limit the benefits of immune checkpoint blockade (ICB).1 Cytotoxic chemotherapy has been proposed to enhance the efficacy of ICB by exerting immunomodulatory effects on the tumor microenvironment (TME).2,3 In particular, several cytotoxic drugs, including gemcitabine, 5-fluorouracil (5-FU), doxorubicin and paclitaxel, were shown to deplete myeloid-derived suppressor cells (MDSCs) in TME.[4], [5], [6], [7] In the clinical setting, various approaches combining cytotoxic chemotherapy and ICB have been developed in a number of cancers. For example, pembrolizumab in combination with chemotherapy was approved for lung cancer treatment.8 For gastrointestinal cancers, the combination of oxaliplatin and 5-FU is currently being investigated in clinical trials (NCT03626922 and NCT02375672).
For HCC, it is evident that unique and potent hepatic-specific immune responses, including the accumulation and activation of MDSCs, are present in the liver.9,10 Therefore, the approach of combining chemotherapy and ICB used in other cancer types is also potentially applicable to HCC and similarly proposed for therapeutic development in HCC.11 In fact, clinical trials have been initiated to test ICB in combination with cytotoxic chemotherapy, administered as systemic (NCT03092895) or regional treatment (NCT03778957; NCT04340193; NCT04246177) in HCC. However, the heterogeneous hepatic TME, arising from different etiologies, could lead to different treatment outcomes than those observed in other solid tumors.12
To evaluate the therapeutic potential of ICB in combination with a chemotherapeutic agent, we first carried out orthotopic HCC mouse experiments using anti-programmed cell death ligand 1 (PD-L1) antibody and 5-FU. 5-FU was chosen in the current study because of its frequent use in regimens of systemic or transarterial treatment for HCC.
Materials and methods
Cell lines and reagents
Murine hepatoma Hepa1-6 cell line was purchased from the American Type Culture Collection and RIL-175 cell line with stable luciferase expression was established by transduction with retroviral vector carrying pBABE-luc-puro. 5-FU was purchased from Cayman Chemical Company. Anti-mouse PD-L1 antibody (10F.9G2), IgG2b control (LTF-2), anti-mouse Ly6G antibody (1A8) and IgG2a control (2A3) were obtained from Bio-X-Cell.
Cytotoxicity assay
Cells were plated in a 96-well plate and treated with 5-FU at concentrations from 10 nM to 1 mM for 24, 48, and 72 hours. Cytotoxicity was quantified by WST-1 assay (Abcam) according to the manufacturer’s protocol.
Apoptosis assay
Apoptotic events upon drug treatment were determined by Annexin-V/7-AAD labelling with subsequent flow cytometry analysis. Both early (Annexin-V+/7-AAD-) and late (Annexin-V+/7-AAD+) apoptotic events were included for cell death determination.
Mouse HCC tumor model
An orthotopic HCC tumor model was established by injecting 5x105 RIL-175 cells or 5x106 Hepa1-6 cells into the liver of C57BL/6 mice. Tumor growth was monitored by in vivo imaging every 5 days. Mice were randomized into different groups: vehicle control; 5-FU (20 mg/kg); anti-PD-L1 (10 mg/kg); 5-FU (20 mg/kg) plus anti-PD-L1 (10 mg/kg). Anti-PD-L1 and 5-FU were administrated intraperitoneally every 5 days and 3 times per week, respectively. Anti-Ly6G or IgG2a isotype was given every 7 days via i.p. injection. This model has been established by our group to study the efficacy of ICB in HCC and under the approval of the Animal Experimentation Ethics Committee (AEEC) at The Chinese University of Hong Kong.13
Flow cytometry analysis
Tumor, liver, spleen and blood of the mice were harvested at endpoint and homogenised into single cell suspensions. Cells were stained with a mixture of fluorescence conjugated antibodies as follows: myeloid markers CD11b, Gr-1, Ly6C, Ly6G; T cell markers CD3, CD4, CD8; leukocyte marker CD45. The cells were then analyzed by flow cytometry using BD Aria Fusion.
Immunofluorescence staining
Sections of formalin-fixed paraffin-embedded tumor tissues were collected from HCC patients at Prince of Wales Hospital, Hong Kong. Written consent was obtained from all patients in this study and approved by the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee. Antigen retrieval was performed with citrate buffer, followed by blocking and incubation with primary antibodies against CD11b, CD14, CD15 and CD3, CD8. After washing and incubation with fluorophore-conjugated secondary antibodies, images were captured by Axio Observer Z1 (Carl Zeiss, Germany).
Patients
The clinical part of this study came from established HCC cohorts from the Prince of Wales Hospital in Hong Kong.14,15 Approvals by ethics committee were obtained for these 2 cohorts. The inclusion and exclusion criteria for both cohorts were reported previously.14,15 To evaluate the impact of chemotherapy in this study, patients with treatment of 5-FU-based transarterial chemoembolizaion (TACE) and available baseline and post-treatment tumor tissues were identified.
Statistical analysis
An unpaired t-test was used to compare 2 groups. One-way or two-way ANOVA with Tukey’s post hoc tests were used for comparison between multiple groups. Values were presented as mean ± SD and were considered as statistically significant when ∗p <0.05; ∗∗p <0.01, ∗∗∗p <0.001 and ∗∗∗∗p <0.0001.
Results
Chemotherapy offsets the anti-tumor effect of ICB by counteracting infiltration of T cells and NK cells
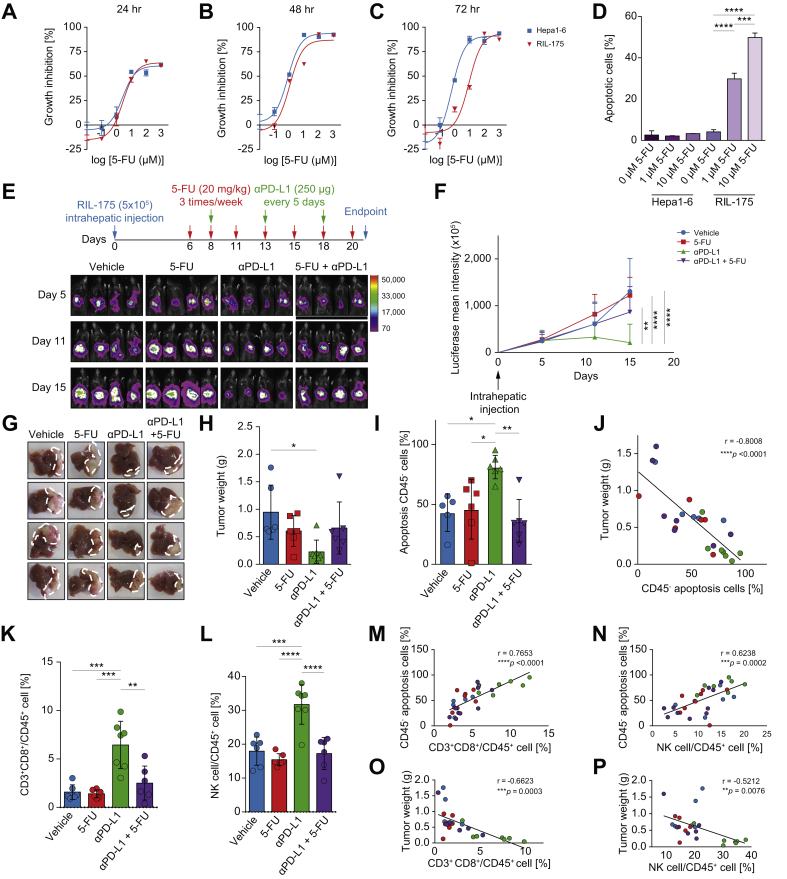
Two murine hepatoma cell lines, RIL-175 and Hepa1-6 were found to be sensitive to 5-FU treatment in vitro as indicated by the cell viability and apoptosis assay (Fig. 1A-D). The in vivo efficacy of 5-FU in combination with PD-L1 blockade was further examined using an orthotopic HCC model13,16 (Fig. 1E). Anti-PD-L1 single treatment exerted the most significant anti-tumor activity, as reflected by the retarded tumor growth rate and decreased endpoint tumor weight. On the contrary, tumors did not respond to 5-FU monotherapy, and strikingly, the significant reduction of tumor growth by PD-L1 blockade was offset in mice given combined treatment (Fig. 1F-H). Beyond tumor burden, tumor apoptotic cells were assessed based on their negative association with tumor size in our model and a similar trend was found in the combined treatment group (Fig. 1I-J). These data illustrated that 5-FU treatment abrogated the effectiveness of PD-L1 blockade in HCC.
Fig. 1.
Chemotherapy hindered anti-PD-L1 efficacy by inhibiting infiltration of immune effector cells. (A-C) Dose-response curves of 5-FU from RIL-175 and Hepa1-6 murine hepatoma cell lines. (D) Apoptotic event was determined by Annexin-V/ 7-AAD co-staining with one-way ANOVA test. (E) Orthotopic HCC model was established by intrahepatic injection of RIL-175 cells, followed by 5-FU (20 mg/kg), anti-PD-L1 (10 mg/kg) or combined treatment. Tumor growth was monitored by in vivo imaging as shown. (F) Average luciferase intensity at each time point was calculated (n >6 per group) and analysed by two-way ANOVA. (G, H) Endpoint tumor weight was measured with images displaying tumor morphology. (I) Percentage of apoptotic CD45- tumor cells under drug treatment was determined with one-way ANOVA. (J) Correlation between tumor weight and percentage of apoptotic tumor cells were denoted using Pearson correlation coefficient test. (K, L) Proportions of CD8+ T cells and NK cells in overall CD45+ leukocytes were measured in tumor. (M-P) Percentages of tumor immune effector cells were positively correlated with the percentage of apoptotic CD45- tumor cells and negatively associated with tumor weight. ∗p <0.05; ∗∗p <0.01; ∗∗∗p <0.001; ∗∗∗∗p <0.0001. 5-FU, fluorouracil; HCC, hepatocellular carcinoma; NK, natural killer; PD-L1, programmed cell death ligand 1.
We then investigated the underlying immunomodulatory mechanisms in TME. Upon co-treatment, the increase in infiltration of CD8+ T cells and natural killer (NK) cells observed with single anti-PD-L1 treatment was reversed (Fig. 1K-L). The number of intratumoral CD8+ T cells and NK cells positively correlated with apoptotic tumor cell percentage (Fig. 1M-N) and negatively correlated with tumor weight (Fig. 1O-P), illustrating that their direct killing effect on tumor cells was the prominent factor in eradicating HCC, a benefit which was abolished by 5-FU.
Recruitment of MDSCs by chemotherapy blunts the response to immunotherapy
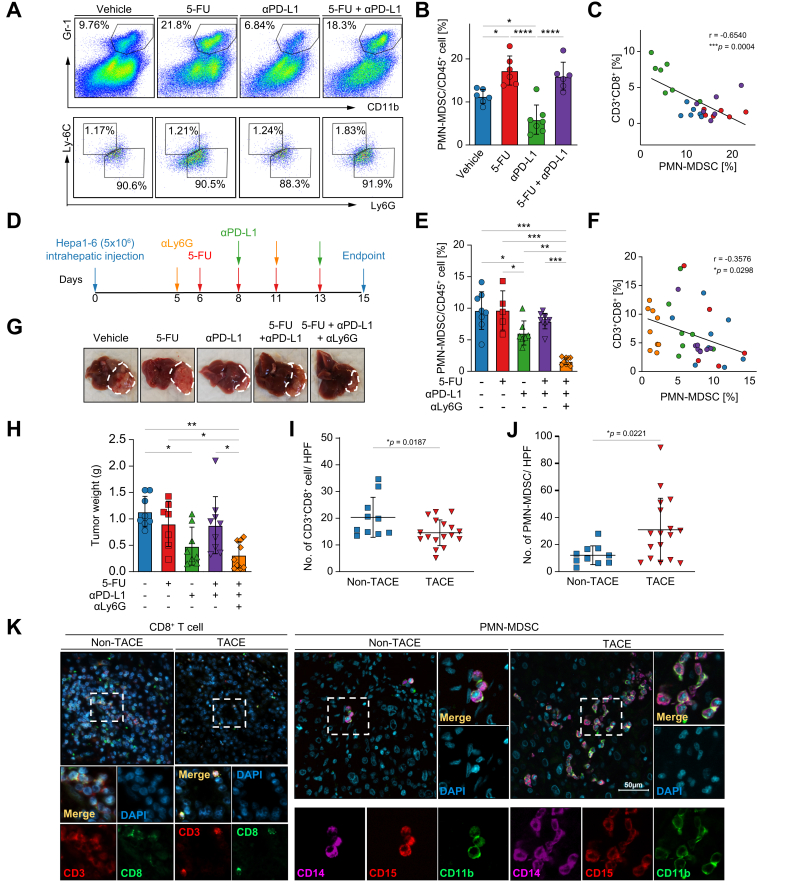
Next, we found that myeloid cells, CD11b+Gr-1+Ly6G+Ly6Cint polymorphonuclear MDSCs (PMN-MDSCs) but not mononuclear MDSCs (M-MDSCs) drastically increased in mice receiving 5-FU in both monotherapy and combined treatment (Fig. 2A-B). Given the well-known T cell suppressive activity of MDSCs, we subsequently examined the association between myeloid cells and immune effector cells and found that the abundance of CD8+ T cells was selectively influenced by PMN-MDSCs (Fig. 2C). To functionally verify the involvement of MDSCs in attenuating the response to immunotherapy, PMN-MDSCs were depleted by anti-Ly6G antibody after tumor implantation in an additional Hepa1-6 orthotopic model (Fig. 2D).17 Consistent with the RIL-175 tumor model, reduction of PMN-MDSCs by anti-PD-L1 was counteracted by 5-FU in the co-treatment group (Fig. 2E) and the proportion of PMN-MDSCs was negatively associated with CD8+ T cell numbers as well (Fig. 2F). Depleting PMN-MDSCs in the mice given combination treatment remarkably restored the anti-tumor effect of PD-L1 blockade compared to the co-treated mice without MDSC depletion (Fig. 2G-H). This result revealed that intratumoral 5-FU induced increased in PMN-MDSCs reduce the sensitivity of HCC tumors to anti-PD-L1.
Fig. 2.
5-FU induces accumulation of myeloid cells in tumor. (A) Representative flow cytometry dot plots of myeloid cells from mice tumor were displayed. (B) Proportion of PMN-MDSC among 4 groups of RIL-175 tumor-bearing mice and statistical significance was analysed by one-way ANOVA test. (C) Tumor-infiltrating PMN-MDSCs were negatively associated with intratumoral CD8+ T cells using Pearson correlation coefficient test. (D) Orthotopic mouse model using Hepa1-6 cells was established and followed by drug dosing schedule as shown. MDSC depletion was done by i.p injection of anti-Ly6G antibody. (E) Proportion of PMN-MDSCs was determined by flow cytometry analysis with one-way ANOVA test. (F) Correlation between PMN-MDSCs and CD8+ T cells in Hepa1-6 tumor site was determined by Pearson correlation coefficient test. (G, H) Representative tumor morphology is displayed and the tumor weights were measured at endpoint. (I, J) Immunofluorescence staining of CD8+ T cells and PMN-MDSCs was performed using TACE-treated (n = 17) and non-TACE-treated (n = 10) HCC patient tumor specimens followed by unpaired t test for statistical analysis. CD3+CD8+ T cells, PMN-MDSCs were quantified and averaged from 3 individual fields, represented as the number of cells per high power field. (K) Cell co-stained with CD11b and CD15 is regarded as a PMN-MDSC, Scale bar, 50 μm. ∗p <0.05; ∗∗p <0.01; ∗∗∗p <0.001; ∗∗∗∗p <0.0001. 5-FU, fluorouracil; HCC, hepatocellular carcinoma; PD-L1, programmed cell death ligand 1; PMN-MDSC, polymorphonuclear myeloid-derived suppressor cell; TACE, transarterial chemoembolization.
We validated this immunomodulation in HCC samples from patients treated with TACE. A slight decrease in CD3+CD8+ T cells was detected in tumor specimens of patients given TACE (n = 17) in comparison to those not receiving TACE (n = 10). Consistently, PMN-MDSCs were enriched in TACE-treated tumors relative to non-TACE-treated tumors (Fig. 2I-K). Taken together, chemotherapy was shown to induce accumulation of immunosuppressive MDSCs and reduce CD8+ T cells in the TME.
Discussion
In the current study, we demonstrated that 5-FU induced tumor cell apoptosis in vitro but not in an orthotopic mouse model. Such disparity implied that treatment response could be remarkably altered by immune stimulation in the TME. We showed that an elevation in immune effector cells by ICB is critical for anti-tumor immunity, yet this benefit was offset when co-treated with chemotherapy. Detailed analysis of the mechanisms underlying the adverse effects of 5-FU revealed that 5-FU increased PMN-MDSC numbers in the tumor site, leading to suppression of T cells and thereby diminishing the anti-tumor immunity elicited by PD-L1 blockade in combination treatment.
Previously, a number of chemotherapeutic agents have been reported to enhance anti-tumor immunity through elimination of immunosuppressive cells.18 Unlike our results, a study found that gemcitabine and 5-FU depleted MDSCs in EL4 tumors.5 In fact, discrepancies were identified between various studies regarding immunomodulation of 5-FU. For instance, 5-FU depletes MDSCs in the context of FOLFOX (folinic acid, 5-FU, and oxaliplatin), whereas such an effect is reversed in FOLFIRI (folinic acid, 5-FU and CPT11).3 Besides, it is notable that MDSC depletion by 5-FU in the EL4 tumor model was transient, with MDSC elevation occurring soon after tumor growth. The fact that our results were obtained at a relatively late time point reflecting advanced HCC, may explain why different immunoregulatory effects were obtained with the same chemotherapeutic agent.
Conventional chemotherapy most often causes toxic side effects, whereas low-dose metronomic chemotherapy mediates moderate antineoplastic activity.19,20 However, the capability of 5-FU to trigger immunogenic cell death (ICD) remained controversial, as it promoted non-histone chromatin-binding protein high-mobility group box 1 release yet failed to induce endoplasmic reticulum stress and subsequent chaperone calreticulin exposure.21 In our study, the dosage of 5-FU, at 20 mg/kg, was well-tolerated with no observable side effects and the equivalent dose in humans is known to be asymptomatic as well.22 In this scenario, though 5-FU was insufficient to induce significant ICD, we may exclude the possibility of ICD in causing TME alteration.
Two caveats should be considered when analyzing these results. First, only 5-FU was used in our experiments. Our findings on MDSCs may not directly apply to other chemotherapeutic agents. More studies are needed to evaluate the impact of other chemotherapeutic agents on the immune environment of HCC. Second, the sample size of the clinical cohort was relatively small due to the difficulty in identifying patients with tumor tissues obtained after TACE procedures. Based on our findings, it is possible that agents that help diminish PMN-MDSC populations in the regimen of combinational 5-FU and ICB may improve anti-tumor efficacy. For example, sunitinib or gemcitabine have been shown to counteract MDSCs.[23], [24], [25] Further studies in HCC are needed to support the feasibility of this additional combination approach.
Taken together, this study uncovered a novel immunosuppressive role of a chemotherapeutic agent in HCC which adversely effected the efficacy of a combination immunotherapy approach. In order to achieve optimal therapeutic efficacy in HCC, it is essential to clarify the precise immunomodulation caused by an individual drug candidate before testing in clinical trials. As we observed similar immune alterations in clinical samples, ICB should be used with caution in chemotherapy-pretreated patients.
Financial support
This project is supported by the Terry Fox Run Hong Kong, Health and Medical Research Fund (16170451), the University Grants Committee through General Research Fund (14108219) and Li Ka Shing Foundation.
Authors’ contributions
Study design: CHW, JYZ, ASLC and SLC; Data acquisition and analysis: TT; Clinical resources: AWHC, SLC; Writing of manuscript: TT, SLC; Critical review of manuscript: All authors.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgement
We thank Dr. Tim F. Greten (National Cancer Institute, National Institutes of Health, Bethesda, USA) and Prof. Lars Zender (University Hospital Tubingen, Germany) for providing us with RIL-175 cell line.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100224.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Pitt J.M., Vetizou M., Daillere R., Roberti M.P., Yamazaki T., Routy B. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity. 2016;44:1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Garg A.D., Dudek-Peric A.M., Romano E., Agostinis P. Immunogenic cell death. Int J Dev Biol. 2015;59:131–140. doi: 10.1387/ijdb.150061pa. [DOI] [PubMed] [Google Scholar]
- 3.Kanterman J., Sade-Feldman M., Biton M., Ish-Shalom E., Lasry A., Goldshtein A. Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Cancer Res. 2014;74:6022–6035. doi: 10.1158/0008-5472.CAN-14-0657. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson E., Wenthe J., Irenaeus S., Loskog A., Ullenhag G. Gemcitabine reduces MDSCs, tregs and TGFbeta-1 while restoring the teff/treg ratio in patients with pancreatic cancer. J Transl Med. 2016;14:282. doi: 10.1186/s12967-016-1037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent J., Mignot G., Chalmin F., Ladoire S., Bruchard M., Chevriaux A. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 6.Alizadeh D., Trad M., Hanke N.T., Larmonier C.B., Janikashvili N., Bonnotte B. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014;74:104–118. doi: 10.1158/0008-5472.CAN-13-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sevko A., Michels T., Vrohlings M., Umansky L., Beckhove P., Kato M. Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J Immunol. 2013;190:2464–2471. doi: 10.4049/jimmunol.1202781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi L., Rodriguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 9.Kalathil S., Lugade A.A., Miller A., Iyer R., Thanavala Y. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73:2435–2444. doi: 10.1158/0008-5472.CAN-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu L.C., Chang C.J., Hsu C.H. Targeting myeloid-derived suppressor cells in the treatment of hepatocellular carcinoma: current state and future perspectives. J Hepatocell Carcinoma. 2019;6:71–84. doi: 10.2147/JHC.S159693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greten T.F., Sangro B. Targets for immunotherapy of liver cancer. J Hepatol. 2017;68:157–166. doi: 10.1016/j.jhep.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J., Dang H., Wang X.W. The significance of intertumor and intratumor heterogeneity in liver cancer. Exp Mol Med. 2018;50:e416. doi: 10.1038/emm.2017.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J., Liu M., Sun H., Feng Y., Xu L., Chan A.W.H. Hepatoma-intrinsic CCRK inhibition diminishes myeloid-derived suppressor cell immunosuppression and enhances immune-checkpoint blockade efficacy. Gut. 2018;67:931–944. doi: 10.1136/gutjnl-2017-314032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan S.L., Chan A.W., Chan A.K., Jian P., Mo F., Chan C.M. Systematic evaluation of circulating inflammatory markers for hepatocellular carcinoma. Liver Int. 2017;37:280–289. doi: 10.1111/liv.13218. [DOI] [PubMed] [Google Scholar]
- 15.Chan S.L., Wong L.L., Chan K.A., Chow C., Tong J.H., Yip T.C. Development of a novel inflammation-based index for hepatocellular carcinoma. Liver Cancer. 2020;9:167–181. doi: 10.1159/000504252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M., Zhou J., Liu X., Feng Y., Yang W., Wu F. Targeting monocyte-intrinsic enhancer reprogramming improves immunotherapy efficacy in hepatocellular carcinoma. Gut. 2020;69:365–379. doi: 10.1136/gutjnl-2018-317257. [DOI] [PubMed] [Google Scholar]
- 17.Sun H., Yang W., Tian Y., Zeng X., Zhou J., Mok M.T.S. An inflammatory-CCRK circuitry drives mTORC1-dependent metabolic and immunosuppressive reprogramming in obesity-associated hepatocellular carcinoma. Nat Commun. 2018;9:5214. doi: 10.1038/s41467-018-07402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menard C., Martin F., Apetoh L., Bouyer F., Ghiringhelli F. Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol Immunother. 2008;57:1579–1587. doi: 10.1007/s00262-008-0505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nars M.S., Kaneno R. Immunomodulatory effects of low dose chemotherapy and perspectives of its combination with immunotherapy. Int J Cancer. 2013;132:2471–2478. doi: 10.1002/ijc.27801. [DOI] [PubMed] [Google Scholar]
- 20.Kerbel R.S., Kamen B.A. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 21.Bezu L., Gomes-da-Silva L.C., Dewitte H., Breckpot K., Fucikova J., Spisek R. Corrigendum: "combinatorial strategies for the induction of immunogenic cell death. Front Immunol. 2015;6:275. doi: 10.3389/fimmu.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ardalan B., Stridhar K., Reddy R., Benedetto P., Richman S., Waldman S. Phase I study of high dose 5-fluorouracil and high dose Leucovorin with low dose phosphonacetyl-L-aspartic acid in patients with advanced malignancies. Int J Radiat Oncol Biol Phys. 1992;22:511–514. doi: 10.1016/0360-3016(92)90864-e. [DOI] [PubMed] [Google Scholar]
- 23.Ko J.S., Zea A.H., Rini B.I., Ireland J.L., Elson P., Cohen P. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 24.Wu H., Tao N., Liu X., Li X., Tang J., Ma C. Polysaccharide from Lentinus edodes inhibits the immunosuppressive function of myeloid-derived suppressor cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko J.S., Rayman P., Ireland J., Swaidani S., Li G., Bunting K.D. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70:3526–3536. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.