Abstract
The somasteroids are Lower Palaeozoic star-shaped animals widely regarded as ancestors of Asterozoa, the group of echinoderms that includes brittle stars and starfish. However, the origin of asterozoans, the assembly of their distinctive body organization, and their relationships with other Cambrian and Ordovician echinoderms remain problematic owing to the difficulties of comparing the endoskeleton between disparate groups. Here, we describe the new somasteroid Cantabrigiaster fezouataensis, a primitive asterozoan from the Early Ordovician Fezouata Lagerstätte in Morocco. Cantabrigiaster shares with other somasteroids a unique endoskeletal arm organization and the presence of rod-like virgal ossicles that articulate with the ambulacrals, but differs from all other known asterozoans in the absence of adambulacral ossicles defining the arm margins, evoking parallels with non-asterozoan echinoderms. Developmentally informed Bayesian and parsimony phylogenetic analyses, which reflect the homology of the biserial ambulacral ossicles in Palaeozoic echinoderms according to the extraxial–axial theory, recover Cantabrigiaster as the earliest divergent stem-group asterozoan. Our results illuminate the ancestral morphology of Asterozoa, and clarify the affinities of problematic Ordovician Asterozoa. Bayesian inference and parsimony demonstrate that somasteroids represent a paraphyletic grade within stem- and crown-group Asterozoa, whereas stenuroids are paraphyletic within stem-group Ophiuroidea. Our results also offer potential insights on the evolutionary relationships between asterozoans, crinoids and potential Cambrian stem-group representatives.
Keywords: somasteroid, Fezouata Lagerstätte, phylogeny, stem-group Asterozoa, Crinoidea, Ordovician
1. Introduction
Asterozoans—whose most familiar members include starfish and brittle stars—are the dominant group of extant echinoderms based on their diversity, abundance and biogeographic distribution [1]. Despite their ecological success and a fossil record spanning more than 480 Myr [2–4], the origin and early evolution of asterozoans, and those of crown-group echinoderms more generally, remain uncertain given the difficulty of comparing the organization of the calcified endoskeleton in diverse Lower Palaeozoic groups, such as the edrioasteroids and blastozoans [5–13]. The extraxial–axial theory (EAT), which supports the homology of the biserial ambulacral ossicles of pentaradial and non-pentaradial echinoderms based on embryonic and ontogenetic data [14–16], has been proposed as a developmentally informed model that facilitates comparisons among groups with disparate morphologies. Although the EAT can potentially clarify the early evolution of crown-group Echinodermata, the broad implications of this hypothesis have never been examined under a comprehensive quantitative phylogenetic framework. Consequently, the main phylogenetic predictions of the EAT pertaining to the evolutionary relationships of Cambrian and Ordovician echinoderms, such as the origin of the crown group from edrioasteroid-like ancestors [14–17], although analysed with other homology schemes [9,11,18], have yet to be critically tested using EAT.
Here, we describe the new somasteroid Cantabrigiaster fezouataensis gen. et sp. nov. (figure 1) from the Early Ordovician (Tremadocian) Fezouata Shale in Zagora, central Anti-Atlas, Morocco [4] (electronic supplementary material, figure S1 and SI Text). The exceptionally preserved morphology of Cantabrigiaster reveals a unique plate organization among somasteroids, and allows us to test the phylogenetic implications of this taxon for the origin of total-group Asterozoa. Central to our phylogenetic hypothesis is the presence of an imperforate extraxial body capsule on the aboral surface of the somasteroids which is then lost in derived asterozoans so that the aboral surface is entirely composed of perforate extraxial body wall, for example, carinals in asteroids, and ventral, dorsal and lateral arm plates in ophiuroids [15].
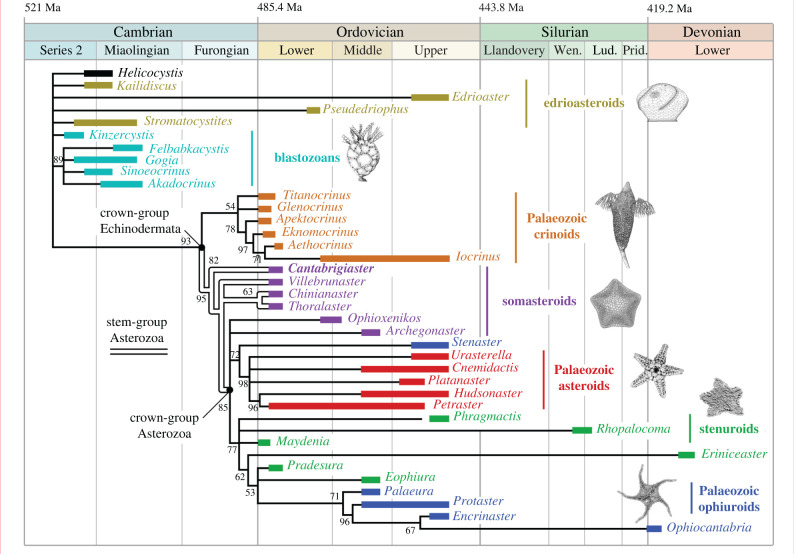
Figure 1.
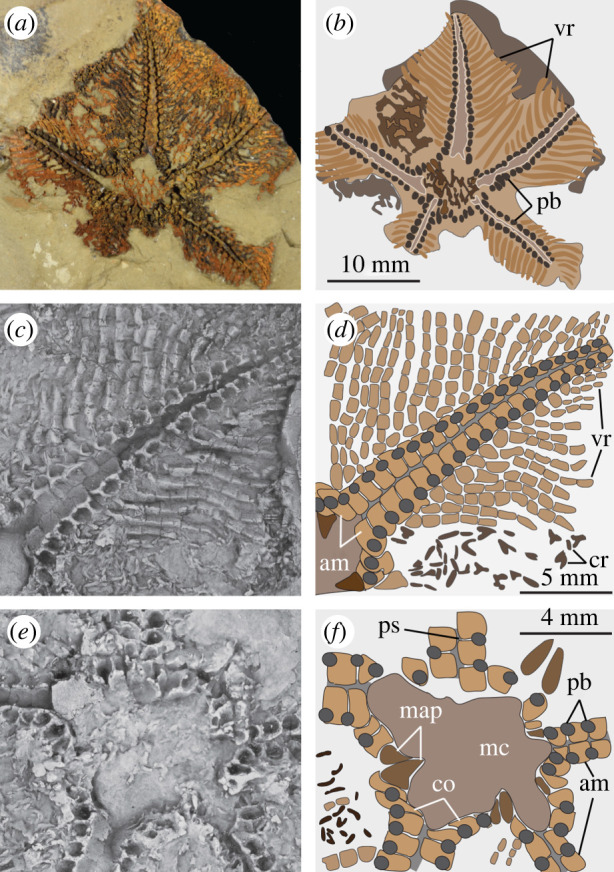
Cantabrigiaster fezouataensis from the Lower Ordovician (Tremadocian) of Morocco. Holotype UCBL-FSL 424961 (Van Roy coll.). (a) Oral view (body fossil). (b) Interpretative diagram of (a). (c) Close-up of extended arm (latex mould). (d) Interpretative diagram of (c). (e) Close-up of oral region (latex mould). (f) Interpretative diagram of (e). am, ambulacral ossicles; co, circumoral ossicles; cr, carinal region ossicles (preserved on the aboral surface); map, mouth angle plates; mc, mouth cavity; pb, podial basins; ps, podial suture; vr, virgal ossicles.
2. Material and methods
(a). Specimen analysis
The studied material is deposited in the palaeontological collections of the University of Lyon 1 (UCBL-FSL), Natural History Museum of Nantes (MHNN), National Museum, Prague (NM-P) and Yale Peabody Museum, Yale University (YPM). Latex moulds were made of all the material with the exception of that from the YPM. The material was photographed with a Nikon D5500 SLR fitted with Micro Nikkor 40 mm.
(b). Phylogenetic analysis
The character matrix for the phylogenetic analyses includes 38 taxa and 74 characters (see electronic supplementary material, datasets S1 and S2); detailed discussion of character scoring and applicability are provided in electronic supplementary material, SI Text. The Bayesian analysis was run in MrBayes 3.2 using the Monte Carlo Markov chain model for discrete morphological characters [19,20] for 10 million generations (four chains), with every 1000th sample stored (resulting in 10 000 samples), and 25% burn-in (resulting in 7500 retained samples). The parsimony analyses were run in TNT [21] under New Technology Search, using Driven Search with Sectorial Search, Ratchet, Drift and Tree fusing options activated with standard settings [22,23]. The analysis (figure 2) was set to find the minimum tree length 100 times and to collapse trees after each search. All characters were treated as unordered. For comparative purposes, analyses were performed under equal and implied weights (k = 3) to test the effect of homoplasy penalization on the position of Cantabrigiaster and the robustness of the dataset [25]. Comparisons between results of the phylogenetic analyses are presented in the electronic supplementary material. Parsimony-based analysis under Traditional Search with 10 000 replicates produced results identical to those obtained under New Technology Search.
Figure 2.
Phylogeny of total-group Echinodermata. Strict consensus topology based on the Bayesian-inference analysis of 38 taxa and 74 morphological characters informed by the EAT [14,15] (electronic supplementary material, SI Text). See electronic supplementary material, figure S6 for support values and comparison with the results of the parsimony-based phylogenetic analyses. Stratigraphic ranges of taxa based on [3,6,12,24]. The asterozoan/crinoid clade represented does not imply a sister group relationship; echinozoan/asterozoan monophyly has been established using molecular data (see electronic supplementary material, SI Text). Wen., Wenlock; Lud., Ludlow; Prid., Přídolí.
3. Systematic palaeontology
(Crown group) Echinodermata [26]
(Stem group) Asterozoa [27]
Family Somasteroidea [28]
Genus Cantabrigiaster gen. nov.
Type species fezouataensis sp. nov.
(a). Diagnosis of new genus and species
Somasteroid typified by biserial and offset ambulacrals with thin transverse bar, wide perradial groove, multiple interconnected virgal ossicles and aboral carinal region with network of spicule-like ossicles. Adambulacral ossicle series lacking along abaxial body margins (perpendiculars (virgals), structures 90° to the axial ambulacrals).
(b). Age and horizon
Primarily stylophoran-dominated beds in the upper part of the Araneograptus murrayi Zone, late Tremadocian, Z-F2 (Jbel Tizagzaouine), Z-F4 (Bou Izargane) and Z-F9 (Bou Glef), in the lower part of the Fezouata Shale Formation, Lower Ordovician, Zagora area (central Anti-Atlas), Morocco. The 70 m thick interval yields assemblages typical of the Fezouata Biota at about 260–330 m above the base of the Ordovician.
(c). Holotype material
UCBL-FSL 424961 (figure 1). Articulated specimen and latex moulds deposited at the University of Lyon 1 (UCBL-FSL) (Van Roy coll.).
(d). Referred material
Thirty-one specimens in total (see electronic supplementary material, table S1), including: specimens housed at the Yale Peabody Museum, Yale University, Hotchkiss collection (YPM IP 535545–535559, electronic supplementary material, figure S2); the collections of Vizcaïno (UCBL-FSL 424962, electronic supplementary material, figure S3f,g) and Lefebvre (UCBL-FSL 711938 and 711939, electronic supplementary material, figure S3b,d, respectively) housed at the University of Lyon 1 (reconstruction, electronic supplementary material, figure S4 based on material figured in electronic supplementary material, figures S2 and S3); and the Catto collection deposited in the Natural History Museum of Nantes (MHNN.P.045596, electronic supplementary material, figure S5e).
(e). Etymology
Genus name derived from ‘Cantabrigia’, after the cities of Cambridge in the UK and USA, which were home to the influential asterozoan workers John William Salter (University of Cambridge), Juliet Shackleton (neé Dean) (University of Cambridge) and Howard Barraclough ‘Barry’ Fell (Harvard University).
(f). Description
The arms are broad, petaloid and arranged in a pentagonal outline (figure 1a,b and electronic supplementary material, figure S2a,c,d). The aboral skeleton (carinal region) is composed of randomly scattered spicule-like ossicles arranged into an irregular network (figure 1a,b; electronic supplementary material, figures S2e,g and S3d). On the oral side, the ambulacrals consist of flattened ossicles with a subquadrate outline. These ossicles abut each other following the orientation of the perradial axis (figure 1c,d; electronic supplementary material, figure S3a,c,f,g). The perradial suture is straight, and the ambulacrals at either side are stepped out of phase by approximately half an ossicle. The abaxial organization of the ambulacrals consists of an elevated perradial ridge, less than a quarter in width relative to the ambulacral, and bears a thin transverse bar that occupies a central position, conferring a T-shape in oral view (figure 1c,d; electronic supplementary material, figure S3a,e,g). The perradial ridges of the ambulacral ossicles at either side of the perradial suture are substantially separated from each other, forming a wide oral groove (figure 1c–e; electronic supplementary material, figure S3a–c,g). The podial basins are shared equally between adjacent ambulacrals. Abaxially, the following ossicle series consist of the perpendiculars, also known as virgals in somasteroids [2,5,6]. The perpendicular series is composed of interconnected and robust rod-like virgal ossicles without spines. These ossicles follow a perpendicular orientation relative to the perradial suture (figure 1a–d; electronic supplementary material, figure S3). The virgal ossicles close to the ambulacrals are the largest, becoming smaller in length and width towards the abaxial body margins. Likewise, adjacent perpendicular series are in direct contact with each other adaxially relative to the perradial suture, whereas it is possible to observe open gaps between them towards the abaxial body margins. Proximal (relative to the mouth) perpendicular series consist of up to nine virgal ossicles, which gradually decrease in number towards the tips of the arms (figure 1a–d; electronic supplementary material, figure S4). The circumoral ossicles are enlarged relative to ambulacral ossicles, and the first podial pore is shared equally with the small and sub-triangular mouth angle plates (figure 1e,f). The madreporite is not preserved.
4. Discussion
The presence of virgal ossicles in Cantabrigiaster strongly supports its affinities with somasteroids [2,5–9]. Cantabrigiaster bears the greatest similarity to the Tremadocian taxa Chinianaster, Thoralaster and Villebrunaster (electronic supplementary material, figure S5), but is unique among somasteroids in lacking ossicles along the abaxial lateral margins of the arms (figure 1a,d). The arm construction of Cantabrigiaster consists of flattened and offset biserial ambulacrals, each of which articulates with an abaxially oriented perpendicular series composed of simple virgal ossicles (electronic supplementary material, figure S4). In addition to these features, the arms of all other somasteroids also possess a series of axially oriented ossicles along the lateral margins that vary from small and bead-like—albeit with occasional spikes—in Tremadocian taxa [2,5,6] (electronic supplementary material, figure S5a,b), to robust and block-like in the stratigraphically younger (Floian) Ophioxenikos [10] and (Darriwilian) Archegonaster [9] (electronic supplementary material, figure S5e). The absence of this key character and the results of our phylogeny (figure 2) demonstrate that Cantabrigiaster embodies the ancestral condition by virtue of lacking ossicles defining the lateral arm margins (figure 1; electronic supplementary material, figure S4), whereas other somasteroids record the first appearance of these structures along the edges of the arms, and their subsequent changes in size and shape. Based on this sequence, we propose that the origin of new axially oriented ossicle series in early asterozoans required their formation on the abaxial edges of the arms. Our hypothesis implies that the proximity of axially oriented ossicle series relative to the perradial axis reflects the order of their evolutionary appearance (figure 2; electronic supplementary material, figures S6 and S7, and SI Text); since virgals are abaxially oriented, they are not directly comparable with any of the axially oriented ossicle series observed in Palaeozoic asterozoans. In this context, Cantabrigiaster specifically lacks the adambulacral ossicle series present in more derived somasteroids, ophiuroids, asteroids and stenuroids (a group considered intermediate between somasteroids and ophiuroids/asteroids), highlighting its profound significance for understanding the evolution of the asterozoan body plan.
The EAT supports the homology of the ambulacrals across pentaradial total-group echinoderms based on their developmental origin and postembryonic ontogeny [14–17], and allows comparison of the skeletal organization of Cantabrigiaster on a broader phylogenetic scale. Outside Asterozoa, the absence of adambulacrals in Cantabrigiaster draws parallels with Tremadocian crinoids (e.g. protocrinoids, Apektocrinus, Eknomocrinus), whose arm construction incorporates flattened and offset biserial ambulacrals articulated to an abaxially oriented (perpendicular) series of simple ossicles, here expressed as the cover plates [13,16,17,24] (electronic supplementary material, figure S7 and SI Text). A similar axial skeletal organization is also observed among Cambrian forms, most notably edrioasteroids—which also possess flattened and offset biserial ambulacrals but lack feeding appendages [9,11,29]—and to a lesser extent blastozoans, which have feeding appendages formed by modified ambulacrals known as brachioles [12,30,31]. The widespread occurrence of these characters among non-asterozoan groups suggests that their presence in Cantabrigiaster is symplesiomorphic.
Our phylogenetic analysis of representative Lower Palaeozoic total-group echinoderms tests the significance of Cantabrigiaster for the origin of Asterozoa. The dataset reflects the ambulacral homology proposed by the EAT [14–17,24], the oral symmetry model proposed by Universal Element Homology [32–34] and our hypothesis for the correspondence of axially oriented ossicle series in early asterozoans (electronic supplementary material, figure S7 and SI Text). Bayesian and parsimony-based analyses recover practically identical topologies (figure 2; electronic supplementary material, figure S6), despite a loss in tree resolution in the earliest divergent representatives that can be expected from the former methodology, indicating a robust phylogenetic signal within Asterozoa [35]. Cantabrigiaster occupies the earliest diverging position within total-group Asterozoa, supporting our hypothesis that the absence of adambulacrals is an ancestral condition, rather than a case of secondary reduction. Tremadocian somasteroids are resolved as a paraphyletic grade of stem-group asterozoans (per [2,36]; contra [5]), whereas the Floian Ophioxenikos [10] and Darriwilian Archegonaster [9] consistently occupy a more derived position as members of crown-group Asterozoa. The analyses argue against the monophyly of stenuroids [6], but corroborate their close phylogenetic relationship to ophiuroids, specifically as their earliest diverging stem-group representatives [2,5,7,36]. These findings indicate that the evolution of a well-developed adambulacral ossicle series constitutes a critical step in the origin of crown-group Asterozoa, and suggest that the abaxially oriented virgals of somasteroids became independently reduced—and ultimately lost—within the stem lineages of Ophiuroidea and Asteroidea (electronic supplementary material, figure S7).
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Dr Emmanuel Robert (University of Lyon 1) is thanked for assisting access to the holotype and other figured material. Additional thanks go to Dr Martin Valent (NM-P) for access to type material of Archegonaster. Dr Fred Hotchkiss (MPRI) and the Yale Peabody Museum assisted in securing the lectotypes. We thank Bertrand Lefebvre (University of Lyon 1) and the anonymous reviewers for their help in improving previous versions of this manuscript.
Ethics
We received all the necessary permissions to use and figure the specimens from the Museum collections of the Claude Bernard University Lyon 1 and Yale University used in this study.
Data accessibility
All the dates are provided as electronic supplementary material.
Authors' contributions
A.W.H. and J.O.-H. contributed equally to the conception, design, acquisition of data, analysis and interpretation of data, discussion and drafting the article. A.W.H. photographed fossil material and prepared the specimen figures. J.O.-H. designed and ran the phylogenetic analyses, and prepared the corresponding diagrams. Both authors approved the final version of the manuscript and agree to be held accountable for the content therein.
Competing interests
We declare we have no competing interests
Funding
J.O.-H. was funded by a Herchel Smith Research Fellowship in Biological Sciences and a Bye-Fellowship at Emmanuel College, University of Cambridge, during the preparation of this manuscript. A.W.H. held a visiting fellowship at Clare Hall. National Geographic funded the collection of the holotype.
References
- 1.Brusca RC, Moore W, Shuster SM. 2016. Invertebrates, 3rd edn Sunderland, MA: Sinauer Associates. [Google Scholar]
- 2.Blake DB, Guensburg TE. 2015. The class Somasteroidea (Echinodermata, Asterozoa): morphology and occurrence. J. Paleontol. 89, 465–486. ( 10.1017/jpa.2015.22) [DOI] [Google Scholar]
- 3.Jell PA 2014. A Tremadocian asterozoan from Tasmania and a late Llandovery edrioasteroid from Victoria. Alcheringa 38, 528–540. ( 10.1080/03115518.2014.911642) [DOI] [Google Scholar]
- 4.Lefebvre B, et al. 2016. Palaeoecological aspects of the diversification of echinoderms in the Lower Ordovician of central Anti-Atlas, Morocco. Palaeogeog. Palaeoclimat. Palaeoecol. 460, 97–121. ( 10.1016/j.palaeo.2016.02.039) [DOI] [Google Scholar]
- 5.Blake DB 2013. Early asterozoan (Echinodermata) diversification: a paleontologic quandary. J. Paleontol. 87, 353–372. ( 10.1666/12-042.1) [DOI] [Google Scholar]
- 6.Shackleton JD 2005. Skeletal homologies, phylogeny and classification of the earliest asterozoan echinoderms. J. Syst. Palaeontol. 3, 29–114. ( 10.1017/S1477201905001525) [DOI] [Google Scholar]
- 7.Dean J 1999. What makes an ophiuroid? A morphological study of the problematic Ordovician stelleroid Stenaster and the palaeobiology of the earliest asteroids and ophiuroids. Zool. J. Linn. Soc. 126, 225–250. ( 10.1111/j.1096-3642.1999.tb00154.x) [DOI] [Google Scholar]
- 8.Mah CL, Blake DB. 2012. Global diversity and phylogeny of the Asteroidea (Echinodermata). PLoS ONE 7, e35644 ( 10.1371/journal.pone.0035644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith AB, Jell PA. 1990. Cambrian edrioasteroids from Australia and the origin of starfishes. Mem. Qld Mus. 28, 715–778. [Google Scholar]
- 10.Blake DB, Guensburg TE. 1993. New Lower and Middle Ordovician stelleroids (Echinodermata) and their bearing on the origins and early history of the stelleroid echinoderms. J. Paleontol. 67, 103–113. ( 10.1017/S0022336000021211) [DOI] [Google Scholar]
- 11.Paul CRC, Smith AB. 1984. The early radiation and phylogeny of echinoderms. Biol. Rev. 59, 443–481. ( 10.1111/j.1469-185X.1984.tb00411.x) [DOI] [Google Scholar]
- 12.Nardin E, Lefebvre B, Fatka O, Nohejlová M, Kašička L, Šinágl M, Szabad M. 2017. Evolutionary implications of a new transitional blastozoan echinoderm from the middle Cambrian of the Czech Republic. J. Paleontol. 91, 672–684. ( 10.1017/jpa.2016.157) [DOI] [Google Scholar]
- 13.Guensburg TE, Sprinkle J. 2001. Earliest crinoids: new evidence for the origin of the dominant Paleozoic echinoderms. Geology 29, 131–134. () [DOI] [Google Scholar]
- 14.Mooi R, David B. 2000. What a new model of skeletal homologies tells us about asteroid evolution. Am. Zool. 40, 326–339. ( 10.1093/icb/40.3.326) [DOI] [Google Scholar]
- 15.Mooi R, David B, Wray GA. 2005. Arrays in rays: terminal addition in echinoderms and its correlation with gene expression. Evol. Dev. 7, 542–555. ( 10.1111/j.1525-142X.2005.05058.x) [DOI] [PubMed] [Google Scholar]
- 16.Guensburg TE, Blake DB, Sprinkle J, Mooi R. 2015. Crinoid ancestry without blastozoans. Acta Palaeontol. Pol. 61, 253–266. ( 10.4202/app.00211.2015) [DOI] [Google Scholar]
- 17.Guensburg TE, Sprinkle J. 2009. Solving the mystery of crinoid ancestry: new fossil evidence of arm origin and development. J. Paleontol. 83, 350–364. ( 10.1666/08-090.1) [DOI] [Google Scholar]
- 18.Deline B, Thompson JR, Smith NS, Zamora S, Rahman IA, Sheffield SL, Ausich WI, Kammer TW, Sumrall CD. 2020. Evolution and development at the origin of a phylum. Curr. Biol. 30, 1672–1679. ( 10.1016/j.cub.2020.02.054) [DOI] [PubMed] [Google Scholar]
- 19.Ronquist F, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. ( 10.1093/sysbio/sys029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis PO 2001. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 50, 913–925. ( 10.1080/106351501753462876) [DOI] [PubMed] [Google Scholar]
- 21.Goloboff PA, Farris JS, Nixon KC. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786. ( 10.1111/j.1096-0031.2008.00217.x) [DOI] [Google Scholar]
- 22.Goloboff PA 1999. Analyzing large data sets in reasonable times: solutions for composite optima. Cladistics 15, 415–428. ( 10.1111/j.1096-0031.1999.tb00278.x) [DOI] [PubMed] [Google Scholar]
- 23.Nixon KC 1999. The parsimony ratchet, a new method for rapid parsimony analysis. Cladistics 15, 407–414. ( 10.1111/j.1096-0031.1999.tb00277.x) [DOI] [PubMed] [Google Scholar]
- 24.Guensburg TE 2012. Phylogenetic implications of the oldest crinoids. J. Paleontol. 86, 455–461. ( 10.1666/11-097.1) [DOI] [Google Scholar]
- 25.Smith MR, Ortega-Hernández J. 2014. Hallucigenia’s onychophoran-like claws and the case for Tactopoda. Nature 514, 363–366. ( 10.1038/nature13576) [DOI] [PubMed] [Google Scholar]
- 26.Bruguière JG 1791. L'helminthologie, ou les vers infusoires, les vers intestins, les vers mollusques, [Helminthology, or infusory worms, intestinal worms, mollusc worms]. Encyclopédie méthodique par ordre des matières [Methodical encyclopedia by order of subject matter] (eds D Diderot, J le Rond d'Alembert), vol. 5. Panckoucke. [In French.] [Google Scholar]
- 27.von Zittel KA 1895. Grundzüge der Paläontologie (Paläzoologie) [Essentials of palaeontology (palaeozoology)]. Munich, Germany: Druck und verlag von R. Oldenbourg. [In German.] [Google Scholar]
- 28.Spencer WK. 1951. Early Palaeozoic starfish. Phil. Trans. R. Soc. Lond. B 235, 87–129. ( 10.1098/rstb.1951.0001) [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y, Sumrall CD, Parsley RL, Peng J. 2010. Kailidiscus, a new plesiomorphic edrioasteroid from the basal middle Cambrian Kaili Biota of Guizhou Province, China. J. Paleontol. 84, 668–680. ( 10.1666/09-159.1) [DOI] [Google Scholar]
- 30.Clausen S, Jell PA, Legrain X, Smith AB. 2009. Pelmatozoan arms from the Middle Cambrian of Australia: bridging the gap between brachioles and brachials? Lethaia 42, 283–296. ( 10.1111/j.1502-3931.2008.00145.x) [DOI] [Google Scholar]
- 31.Parsley RL, Zhao Y. 2006. Long stalked eocrinoids in the basal Middle Cambrian Kaili Biota, Taijiang County, Guizhou Province, China. J. Paleontol. 80, 1058–1071. ( 10.1666/0022-3360(2006)80[1058:LSEITB]2.0.CO;2) [DOI] [Google Scholar]
- 32.Kammer TW, Sumrall CD, Zamora S, Ausich WI, Deline B. 2013. Oral region homologies in Paleozoic crinoids and other plesiomorphic pentaradial echinoderms. PLoS ONE 8, e77989 ( 10.1371/journal.pone.0077989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright DF, Ausich WI, Cole SR, Peter ME, Rhenberg EC. 2017. Phylogenetic taxonomy and classification of the Crinoidea (Echinodermata). J. Paleontol. 91, 829–846. ( 10.1017/jpa.2016.142) [DOI] [Google Scholar]
- 34.Ausich WI, Kammer TW, Rhenberg EC, Wright DF. 2015. Early phylogeny of crinoids within the pelmatozoan clade. Palaeontology 58, 937–952. ( 10.1111/pala.12204) [DOI] [Google Scholar]
- 35.Puttick MN, et al. 2017. Uncertain-tree: discriminating among competing approaches to the phylogenetic analysis of phenotype data. Proc. R. Soc. Lond. B 284, 20162290 ( 10.1098/rspb.2016.2290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blake DB, Zamora S, García-Alcalde JL. 2015. A new Devonian asteroid-like ophiuroid from Spain. Geol. Acta 13, 335–343. ( 10.1344/GeologicaActa2015.13.4.6) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the dates are provided as electronic supplementary material.