Key Points
Question
Can artificial intelligence and machine learning, using an artificial intelligence–enhanced, 12-lead electrocardiogram (AI-ECG), distinguish patients with long QT syndrome from those who do not have the syndrome?
Findings
In a diagnostic study using a deep neural network, the AI-ECG successfully distinguished patients with long QT syndrome (n = 967) from those who were evaluated for long QT syndrome but discharged without this diagnosis (n = 1092) presenting to a specialized arrhythmia clinic. The model performed better than the corrected QT alone, even in the setting of a normal QT interval.
Meaning
The AI-ECG model evaluated was able to distinguish patients with electrocardiographically concealed long QT syndrome from those without the syndrome and could potentially provide a simple and inexpensive method for early detection of congenital long QT syndrome.
Abstract
Importance
Long QT syndrome (LQTS) is characterized by prolongation of the QT interval and is associated with an increased risk of sudden cardiac death. However, although QT interval prolongation is the hallmark feature of LQTS, approximately 40% of patients with genetically confirmed LQTS have a normal corrected QT (QTc) at rest. Distinguishing patients with LQTS from those with a normal QTc is important to correctly diagnose disease, implement simple LQTS preventive measures, and initiate prophylactic therapy if necessary.
Objective
To determine whether artificial intelligence (AI) using deep neural networks is better than the QTc alone in distinguishing patients with concealed LQTS from those with a normal QTc using a 12-lead electrocardiogram (ECG).
Design, Setting, and Participants
A diagnostic case-control study was performed using all available 12-lead ECGs from 2059 patients presenting to a specialized genetic heart rhythm clinic. Patients were included if they had a definitive clinical and/or genetic diagnosis of type 1, 2, or 3 LQTS (LQT1, 2, or 3) or were seen because of an initial suspicion for LQTS but were discharged without this diagnosis. A multilayer convolutional neural network was used to classify patients based on a 10-second, 12-lead ECG, AI-enhanced ECG (AI-ECG). The convolutional neural network was trained using 60% of the patients, validated in 10% of the patients, and tested on the remaining patients (30%). The study was conducted from January 1, 1999, to December 31, 2018.
Main Outcomes and Measures
The goal of the study was to test the ability of the convolutional neural network to distinguish patients with LQTS from those who were evaluated for LQTS but discharged without this diagnosis, especially among patients with genetically confirmed LQTS but a normal QTc value at rest (referred to as genotype positive/phenotype negative LQTS, normal QT interval LQTS, or concealed LQTS).
Results
Of the 2059 patients included, 1180 were men (57%); mean (SD) age at first ECG was 21.6 (15.6) years. All 12-lead ECGs from 967 patients with LQTS and 1092 who were evaluated for LQTS but discharged without this diagnosis were included for AI-ECG analysis. Based on the ECG-derived QTc alone, patients were classified with an area under the curve (AUC) value of 0.824 (95% CI, 0.79-0.858); using AI-ECG, the AUC was 0.900 (95% CI, 0.876-0.925). Furthermore, in the subset of patients who had a normal resting QTc (<450 milliseconds), the QTc alone distinguished those with LQTS from those without LQTS with an AUC of 0.741 (95% CI, 0.689-0.794), whereas the AI-ECG increased this discrimination to an AUC of 0.863 (95% CI, 0.824-0.903). In addition, the AI-ECG was able to distinguish the 3 main genotypic subgroups (LQT1, LQT2, and LQT3) with an AUC of 0.921 (95% CI, 0.890-0.951) for LQT1 compared with LQT2 and 3, 0.944 (95% CI, 0.918-0.970) for LQT2 compared with LQT1 and 3, and 0.863 (95% CI, 0.792-0.934) for LQT3 compared with LQT1 and 2.
Conclusions and Relevance
In this study, the AI-ECG was found to distinguish patients with electrocardiographically concealed LQTS from those discharged without a diagnosis of LQTS and provide a nearly 80% accurate pregenetic test anticipation of LQTS genotype status. This model may aid in the detection of LQTS in patients presenting to an arrhythmia clinic and, with validation, may be the stepping stone to similar tools to be developed for use in the general population.
This diagnostic study develops a model using artificial intelligence with deep neural networks based on 12-lead electrocardiograms to detect long QT syndrome in patients with electrocardiographically concealed long QT syndrome.
Introduction
Congenital long QT syndrome (LQTS) is characterized by prolongation of the QT interval on a standard 12-lead surface electrocardiogram (ECG).1 Clinically, patients are often asymptomatic but can present with syncope, seizures, or sudden cardiac death.1 Although corrected QT (QTc) values exceeding the 99th percentile in men (≥470 milliseconds) or women (≥480 milliseconds) are the thresholds for further evaluation for LQTS as an otherwise incidental finding in ECG-based screening programs, individuals with a QTc greater than or equal to 500 milliseconds are at increased risk of LQTS-associated events. Over the years, in addition to the QTc, several ECG features, such as specific T wave shapes for the LQTS genetic subtypes,2 QT prolongation on treadmill exercise testing,3 following epinephrine challenge,4 or upon brisk standing,5 as well as novel ECG- or echocardiogram-derived risk factors, such as T wave morphologic characteristics and the electromechanical window,6,7,8 have improved diagnosis and risk stratification for these patients.
However, although QT prolongation is the disease’s hallmark feature, many patients with clinically and/or genetically confirmed LQTS have an otherwise normal-looking ECG.9 In fact, approximately 40% of patients with LQTS have a normal QTc at rest (concealed LQTS).9 Especially for those patients, distinguishing the normal ECG from one in patients with LQTS is important for early diagnosis and possible treatment, particularly enabling the implementation of simple preventive measures in terms of avoiding exposure to QT–prolonging medications whenever possible. Herein, we sought to determine whether artificial intelligence (AI) using deep neural networks is better than the ECG-derived QTc alone at identifying patients with LQTS presenting to a specialized arrhythmia clinic, especially for patients with a normal QTc on their resting 12-lead ECG.
Methods
Study Cohort
For this diagnostic case-control study, we performed a retrospective review of all patients presenting to the Mayo Clinic Genetic Windland Smith Rice Genetic Heart Rhythm Clinic between January 1, 1999, and December 31, 2018. This study followed the Standards for Reporting of Diagnostic Accuracy (STARD) reporting guideline for case-control studies. This study was approved by the Mayo Clinic Institutional Review Board with waiver of consent because of minimal risk.
Patients were included if they had a definitive diagnosis of LQTS that included a positive genetic test with the identification of a LQT1-, LQT2-, or LQT3-causative variant or were evaluated because of an initial suspicion for LQTS, but subsequently were discharged without that diagnosis in the absence of sufficient evidence (including a negative LQTS genetic test) to establish this diagnosis (dismissed normal) (Figure 1), providing a carefully evaluated control group for our study. For all patients, demographic data, clinical history pertaining to LQTS, and genetic information were collected. In addition, for all patients, every ECG performed at Mayo Clinic was obtained for our AI-enhanced ECG (AI-ECG) analysis. A QTc calculated by the ECG machine’s algorithm (GE MAC 5500 HD; GE Healthcare) was obtained for each ECG and full-waveform data were obtained for model development and subsequent analyses. Each QTc was validated by a genetic cardiologist (M.J.A.) specializing in LQTS and reannotated where appropriate. To compare our neural network predictions with a clinically validated tool, we then used the Schwartz score to assign a probability of LQTS.10 A score of 3.5 is associated with a high probability of LQTS, a score between 1.5 and 3.0 indicates an intermediate probability, and a score of 1.0 or below is associated with a low probability.
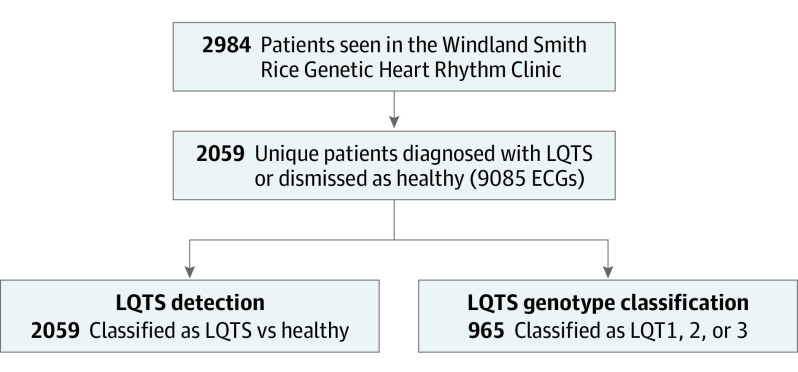
Figure 1. Study Cohort Selection.
Selection of study cohorts, subgroup assignment for long QT syndrome (LQTS) detection and LQTS genotype classification, and breakdown of training, internal validation, and testing sets. Among all patients seen in the Genetic Heart Rhythm Clinic (2984), a total of 2059 were diagnosed with LQTS or discharged without an arrhythmia diagnosis. For these patients, a total of 9085 ECGs were available. These patients were tested for models of LQTS detection or LQTS genotype (LQTS genotype classification) and both models were trained, validated, and tested on a 60%-10%-30% split.
ECG Waveform Data, Convolutional Neural Network Development, and Application
For the development of the AI-ECG, we used a convolutional neural network (CNN) model as shown in the diagram in eFigure 1 in the Supplement, which was similar in development as described before.11 The model was trained using 60% of the patients, validated in 10% of the patients, and tested on the remaining patients (30%). A detailed description of ECG waveform abstraction, CNN architecture, hyperparameters, and parameters used for training the binary and multiclass model, and details on splitting of the data set (both random and temporal) can be found in the eMethods in the Supplement.
Because the QTc is currently the most important ECG value in the diagnosis of LQTS, the goal of our study was to see whether we could develop an ECG-focused model that would be superior to this single measurement. We used the model to evaluate whether the AI-ECG could distinguish LQTS from a normal QTc (LQTS detection) and distinguish the most common genotypic subtypes of LQTS, classified as KCNQ1-encoded LQT1, KCNH2-encoded LQT2, and SCN5A-encoded LQT3 (LQTS genotype classification). To be able to classify these, we used the developed QT model as described for each one, but a softmax function for categorical classification was added as the final activation, with 2 outputs for the LQTS detection network and 3 outputs for the LQTS genotype classification network.
The QTc (defined herein as QTc alone) was used as the current standard ECG parameter for the diagnosis of LQTS to evaluate the performance of the new AI model. For subsequent analyses to test the model’s capability to detect concealed LQTS, only patients with a QTc less than 450 milliseconds were included. In addition, to account for the substantially different number of ECGs available per patient (Table) and the expected variability over time, we performed our comparisons using QTc and model output for a patient’s first ECG as well as the mean QTc and deep neural network output score from all of a patient’s ECGs.
Table. Demographic Characteristics of the Study Cohort.
| Characteristic | Mean (SD) | P value | |
|---|---|---|---|
| LQTS | Considered normal | ||
| No. | 967 | 1092 | NA |
| Total No. of ECGs | 5953 | 3132 | NA |
| No. of ECGs/pt | 6.2 (6.5) | 2.9 (4.5) | <.001 |
| Sex, No. (%) | |||
| Female | 422 (44) | 457 (42) | .42 |
| Male | 545 (56) | 635 (58) | |
| Age at first ECG, y | 21.7 (16.2) | 21.6 (15.0) | .82 |
| QTc, ms | 467 (43) | 429 (32) | <.001 |
| Concealed QTc, ms | 430 (15) | 418 (18) | <.001 |
| Schwartz scorea | 3.3 (1.7) | 1.8 (1.0) | <.001 |
Abbreviations: ECG, electrocardiogram; LQTS, long QT syndrome; ms, milliseconds; NA, not applicable; pt, patient; QTc, corrected QT.
Score of 3.5 indicates a high probability of LQTS; 1.5 to 3.0, intermediate probability; and 1.0 or below, low probability.
Statistical Analysis
Demographic and basic ECG data (continuous or categorical) were expressed as mean (SD). Differences between groups were calculated using a t test (if normally distributed) or Wilcoxon log rank test for continuous data, or a χ2 test for categorical data. All tests were unpaired, and a 2-tailed P value <.05 was considered statistically significant. All calculations were performed using JMP Pro, version 14 (SAS Institute Inc).
The model was evaluated at a single threshold selected from the internal validation data set. The selected threshold was subsequently applied to the hold-out data set and sensitivity, specificity, and F1 were calculated. The CI for the area under the curve (AUC) was determined based on the DeLong method. All analyses were computed using Python, version 3.6 (Python Software Foundation).
Results
Cohort Demographics
The process of how the cohort was obtained is detailed in Figure 1. Overall, 2984 patients were seen in the Windland Smith Rice Genetic Heart Rhythm Clinic between January 1, 1999, and December 31, 2018. One or more digitally recorded ECGs with a verified QT/QTc were available for 2059 of these patients who were either diagnosed with LQTS or who were evaluated for the suspicion of LQTS but were ultimately discharged from the clinic without this diagnosis (Table). For this study, this group of fully evaluated patients served as our control group. The 2059 patients in these 2 cohorts accounted for a total of 9085 ECGs. Of the 2059 patients, 1180 were men (57%) and 879 were women (43%); mean (SD) age at the time of the first ECG was 21.6 (15.6) years. Demographics of both cohorts are summarized in the Table, demonstrating an equal distribution of sex and mean age at first Mayo Clinic ECG for both study cohorts. In addition, as expected, the mean (SD) Schwartz scores were significantly higher for patients eventually diagnosed with LQTS compared with those considered to not have LQTS (3.3 [1.7] vs 1.8 [1.0]; P < .001) (Table). For these cohorts, we tested the model’s ability to differentiate patients with LQTS from those dismissed as normal (LQTS detection). Subsequently, to test the model’s ability to distinguish specific LQTS genetic subtypes (LQT1, 2, and 3 LQTS genotype classification) (Figure 1).
LQTS Detection
Overall, based on a patient’s first Mayo Clinic ECG—the potentially diagnostic one—patients with LQTS had a significantly higher mean (SD) QTc compared with normal (LQTS: 467 [43] milliseconds vs normal: 429 [32] milliseconds; P < .001) (Table). However, the AI-ECG outperformed the QTc determined on the ECG alone in distinguishing patients with LQTS from those who were dismissed as normal with an AUC of 0.900 (95% CI, 0.876-0.925; accuracy, 82.5%; sensitivity, 83.7%; specificity, 80.6%; positive predictive value [PPV], 83.2%; negative predictive value [NPV], 81.3%; and F1, 83.6%) compared with 0.824 for the QTc alone (95% CI, 0.79-0.858; accuracy, 76.0%; sensitivity, 74.1%; specificity, 77.7%; PPV, 79.2%; NPV, 72.4%; and F1, 76.7%) (Figure 2A). Furthermore, testing the model’s ability to distinguish persistent QT interval prolongation from a potentially incidental finding, our model slightly improved when using the mean of all available ECGs for a patient (AUC, 0.914; 95% CI, 0.892-0.937; accuracy, 84.7%; sensitivity, 83.7%; specificity, 85.4%; PPV, 86.8%; NPV, 82.1%; and F1, 85.2%) compared with an AUC of 0.847 when using the QTc alone (95% CI, 0.817-0.877; accuracy, 76.1%; sensitivity, 75.4%; specificity, 77.0%; PPV, 78.9; NPV, 73.3%; and F1, 77.1%) (Figure 2B). The CNN output modestly correlated with a patient's Schwartz score showing that a higher Schwartz score correlated with a higher neural network output value (r2 = 0.28; P < .001) (eFigure 2 in the Supplement). A similar correlation was observed when independently analyzing patients with LQTS (r2 = 0.13; P < .001).
Figure 2. Performance of a Convolutional Neural Network (CNN) in Long QT Syndrome (LQTS) Detection.
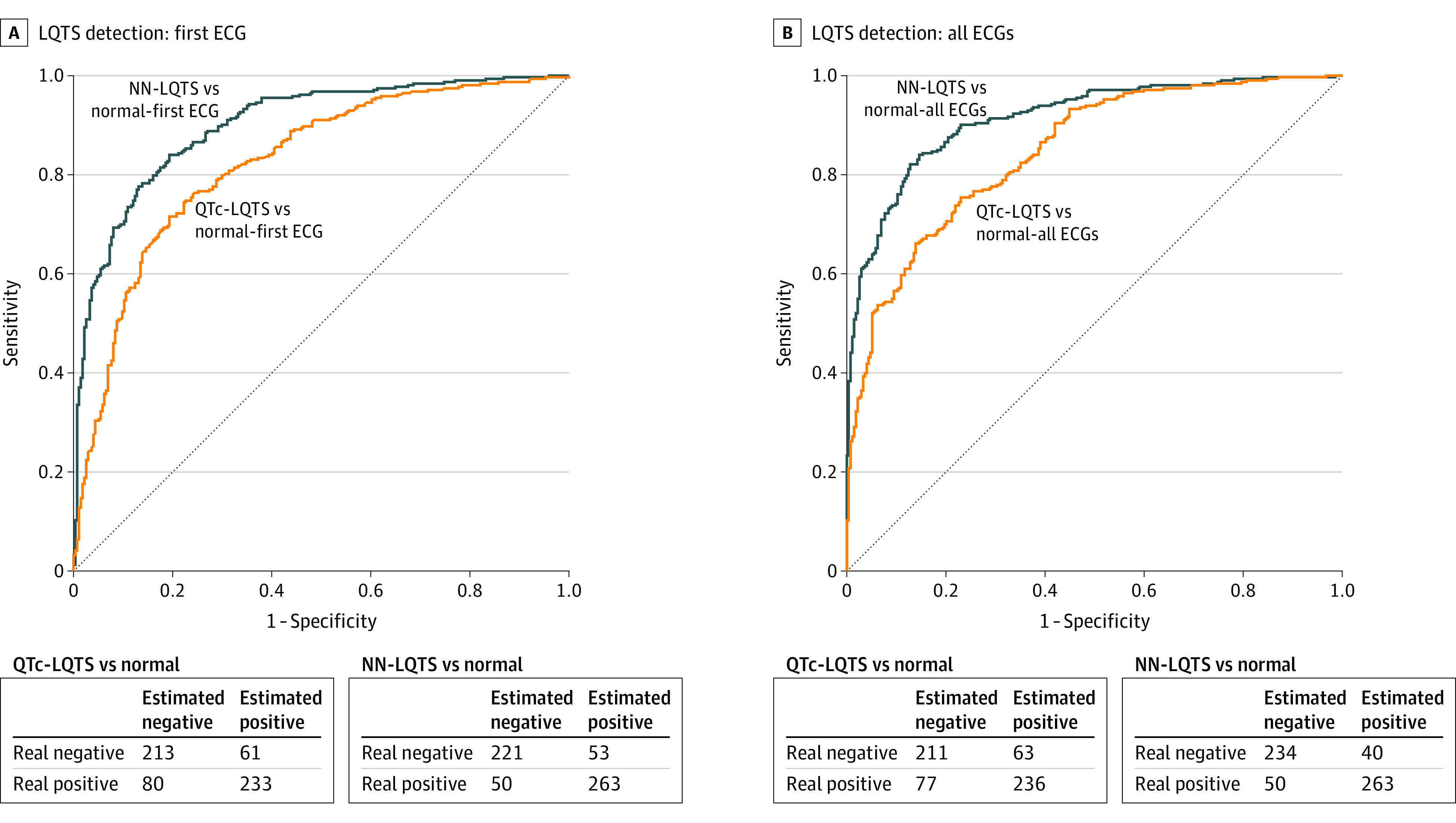
Receiver operating characteristic curves and confusion matrices for LQTS detection analyses showing results of CNN performance on the patient’s first Mayo Clinic electrocardiogram (ECG) (A) or mean of all of a patient’s ECGs (B). NN indicates neural network; QTc, corrected QT.
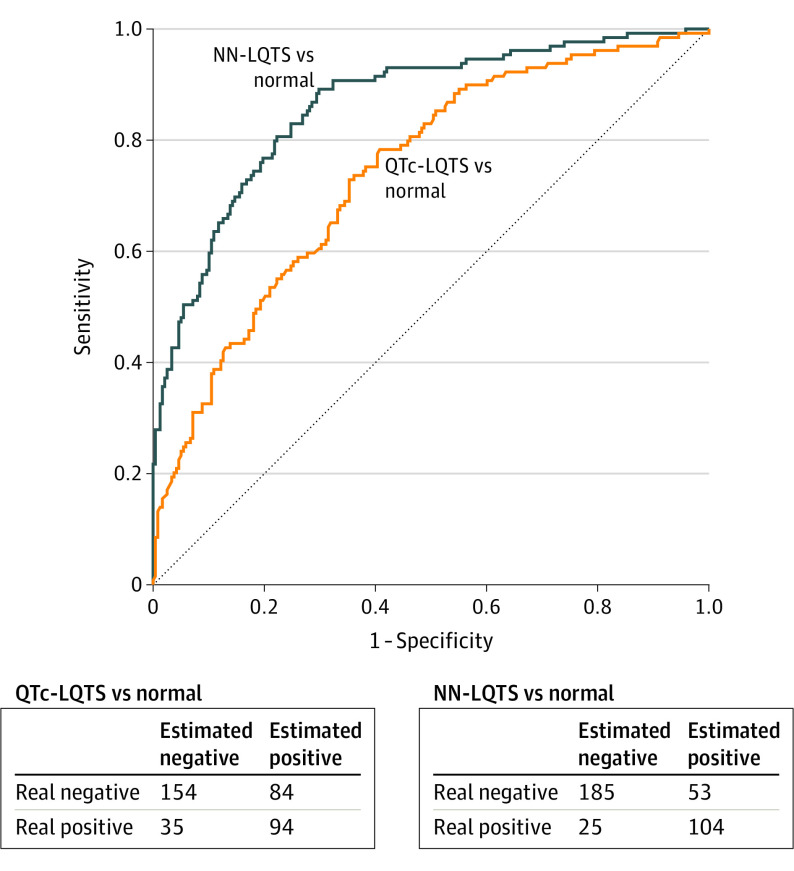
Nevertheless, approximately 40% of patients with genetically confirmed LQTS had a normal QTc on their resting 12-lead ECG. We therefore set out to subsequently test our CNN on just patients with LQTS (concealed LQTS) or individuals dismissed as normal whose QTc on a 12-lead ECG was less than 450 milliseconds. In this analysis, again, the AI-ECG successfully distinguished patients with concealed LQTS on their first Mayo Clinic 12-lead ECG (AUC, 0.863; 95% CI, 0.824-0.903; accuracy, 78.7%; sensitivity, 80.6%; specificity, 77.7%; PPV, 66.2%; NPV, 88.1%; and F1, 72.7%) compared with the QTc alone (AUC, 0.741; 95% CI, 0.689-0.794; accuracy, 67.6%; sensitivity, 72.9%; specificity, 64.7%; PPV, 52.8%; NPV, 81.5%; and F1, 61.2%) (Figure 3).
Figure 3. Performance of Convolutional Neural Network (CNN) in Concealed Long QT Syndrome (LQTS) Detection.
Receiver operating characteristics curve and confusion matrix showing performance of the CNN to distinguish patients with concealed LQTS (corrected QT [QTc] ≤450 milliseconds) from those dismissed normal. NN indicates neural network.
LQTS Genotype Classification
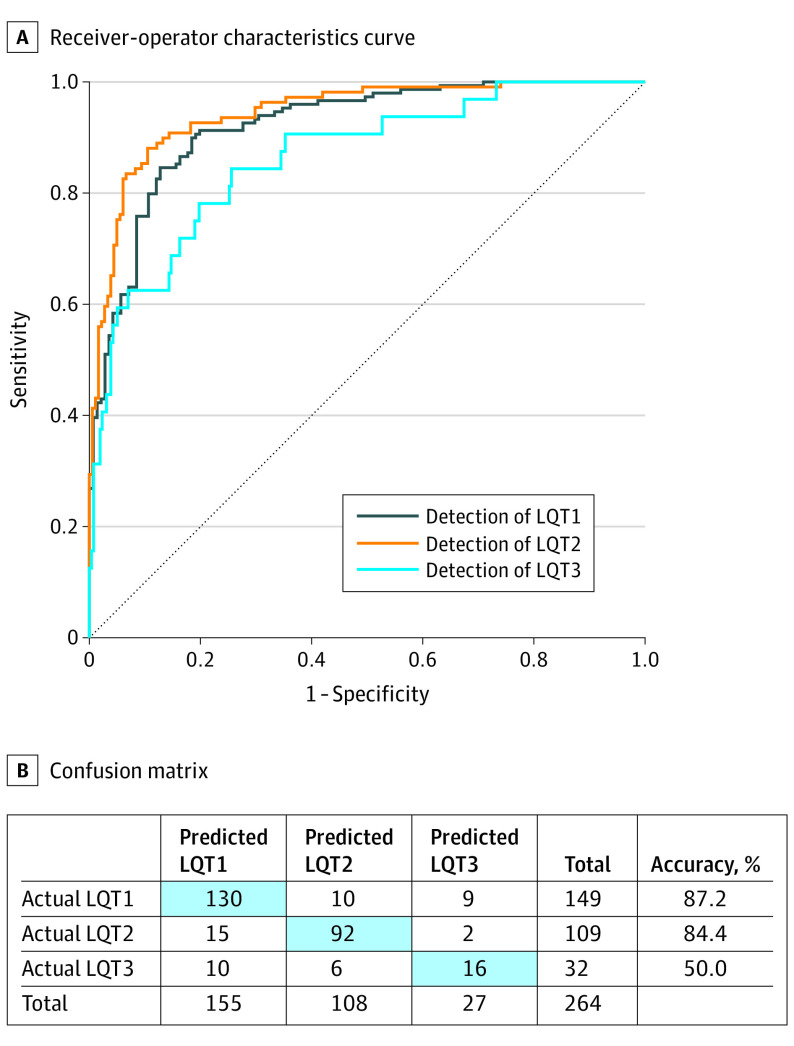
Early identification of a patient’s genetic subtype can facilitate risk stratification and disease management. We therefore tested whether the AI-ECG was able to differentiate the 3 major genetic subtypes (LQT1, 2, and 3) and demonstrated that our model was able to do so. To do this we used the same model but changed the softmax outputs from 2 to 3 parameters classifying the 3 LQTS subtypes. In the test samples for each of the genotypic subsets, the CNN was best able to distinguish LQT2 (n = 109) from LQT1 (n = 149) and LQT3 (n = 32) (AUC, 0.944; 95% CI, 0.918-0.970; accuracy, 89.0%; sensitivity, 88.1%; specificity, 89.5%; PPV, 83.5%; NPV, 92.6%; and F1, 85.7%), followed by distinguishing patients with LQT1 from those with LQT2 or LQT3 (AUC, 0.921; 95% CI, 0.890-0.951; accuracy, 85.9%; sensitivity, 84.6%; specificity, 87.2%; PPV, 87.5%; NPV, 84.2%; and F1, 86.0%) (Figure 4A). Likely owing to the small numbers, the model’s performance in the identification of patients with LQT3 was lower (AUC, 0.863; 95% CI, 0.792-0.934; accuracy, 80.0%; sensitivity; 78.1%; specificity, 80.2%; PPV, 32.9%; NPV, 96.7%; and F1, 46.3%) (Figure 4B).
Figure 4. Performance of Convolutional Neural Network (CNN) in Long QT Syndrome (LQTS) Genotype Classification.
Receiver operating characteristics curve (A) and confusion matrix (B) showing performance of the CNN to distinguish the main genetic subtypes of LQTS. LQT1 indicates type 1 LQTS; LQT2, type 2 LQTS; LQT3, type 3 LQTS.
Internal Validation Using Temporal Splitting
In the absence of an external validation cohort, we performed an internal validation of our model by using a temporal- rather than a random-splitting approach. In this newly developed temporal-splitting model, the neural network—like our original model—again outperformed use of the QTc alone in distinguishing patients with LQTS from those who were dismissed as normal. Although the AUC for the LQTS detection was slightly lower than when using the randomly split model (0.900) vs the temporally split model (0.862), the overall performance metrics for the neural network between these 2 methods were indistinguishable as evidenced by overlapping 95% CIs: AUC for random sampling, 0.900 (95% CI, 0.876-0.925; accuracy, 82.5%; sensitivity, 83.7%; specificity, 80.6%; PPV, 83.2%; NPV, 81.3%; and F1, 83.6%) vs AUC for temporal splitting, 0.862 (95% CI, 0.812-0.912; accuracy, 78.9%; sensitivity, 79.0%; specificity, 78.7%; PPV, 87.2%; NPV, 67.0%; and F1, 82.9%). Similarly, no significant differences were observed for the concealed QT detection (AUC for random splitting, 0.863; 95% CI, 0.824-0.903 vs AUC for temporal splitting, 0.785; 95% CI, 0.703-0.867) and the LQTS genotype classification model. The receiver operating characteristic curves for performance of the model following temporal splitting are shown in eFigure 3 in the Supplement, and eTable 1 in the Supplement compares the AUCs.
Because prediction of performance in rare diseases can be difficult, we also performed repeated analyses on a synthetic data set with different prevalences created by down-sampling the positive examples. As expected, the AUC, sensitivity, and specificity were not affected, but the PPV decreased with increasing prevalence (overall PPV, 86.8%; NPV, 82.4%). The PPV as a function of the prevalence is presented in eTable 2 in the Supplement.
Discussion
Characterized by QT prolongation on 12-lead ECGs, patients with LQTS can experience syncope, seizures, and sudden cardiac death as sequelae of its hallmark arrhythmia of torsades de pointes. Once the patient is fully evaluated and optimally treated, the risk of cardiac events is low; nevertheless, a small group of patients might experience potentially lethal, LQTS-triggered cardiac events.12 Although QT prolongation is LQTS’ pathognomonic feature, approximately 40% of patients with proven (genotype-positive) LQTS have a normal QT,9 highlighting a need for additional tools to identify at-risk patients. To this end, we developed and herein present a deep neural network that is able to distinguish the ECG of a patient with LQTS from the ECG of a patient who was evaluated for LQTS but discharged without this diagnosis (AUC, 0.900). Furthermore, this model was able to distinguish these 2 populations even when the QTc was normal (450 milliseconds) in both groups and differentiate the 3 most common LQTS subtypes (LQT1-3). In addition, the deep neural network correlated significantly with the patient’s clinical probability score for the diagnosis of LQTS (Schwartz score13; r2 = 0.28; P < .001).
One of the main findings of our study is that our AI model outperformed evaluation of the ECG based on the QTc alone. This finding demonstrates the network’s ability to identify additional features in an ECG waveform of a patient with LQTS, despite its generally normal appearance to the expert cardiologist.9 In addition, although previous studies have identified unique ECG features associated with LQTS or its specific genetic subtypes, these features have mostly involved specific, human-selected features of the ECG, such as shape, slope, and overall morphologic characteristics of the T wave.2,6,7,14 In a step to involve AI, Hermans and colleagues15 built upon these T wave morphologic studies by developing a machine learning, support-vector model showing that vectorcardiographic parameters from the T wave can improve diagnosis of LQTS with the capacity to distinguish patients with LQTS from genotype-negative family members with an AUC up to 0.901 based on the model used. In contrast, our AI-ECG used unsupervised feature extraction in which an agnostic approach of the complete ECG waveform across all 12 leads of the ECG, as compared with the T wave alone, was analyzed during training, with network feature selection based on minimizing an error function. This unsupervised trained model was subsequently used to distinguish between our selected (thus supervised) subgroups. Given the nature of CNNs, we were unable to determine which ECG features were important for patient classification. Nonetheless, it is not surprising that substantial diagnostic information is discarded when a complex, 3-dimensional, nonlinear biological signal (ie, the ECG) is distilled to a single number (ie, the QTc) for the diagnosis of LQTS. Thus, use of the CNN added significant diagnostic power to the ECG, permitting it to distinguish patients with LQTS from those without the disease and classify the main genotypic subtypes. This approach could significantly aid both experienced and inexperienced electrophysiologists when presented with a patient with a suspicion of LQTS in establishing this diagnosis and guiding genetic test prediction and initial treatment strategies as a bridge to genetic testing by giving an a priori designation of a possible genetic subtype. These findings could be combined with the currently used clinical score for probability of LQTS: the Schwartz score. This score was developed in 1985 by Schwartz to provide a clinical, diagnostic score to better estimate the clinical likelihood of congenital LQTS and guide treatment, family screening, and phenotype-directed genetic testing, and the test has been used and revised with updated knowledge of the disease.13,16 With a range of points assigned from 0.5 to 3 for each of the LQTS-attributable ECG findings and clinical markers, a score greater than or equal to 3.5 is associated with a high likelihood of LQTS.10,17 In this scenario, a QTc greater than or equal to 480 milliseconds already assigns 3 points, demonstrating the importance and weight of the QTc in this disease and, in reverse, downgrades its likelihood for patients with concealed LQTS. It is here that our model’s performance in patients with concealed LQTS may play an important role. In addition to functioning in providing an a priori likelihood of LQTS score based solely on the ECG, our model and deep neural network output could potentially be implemented into the Schwartz score, especially to better identify and risk stratify a patient with true LQTS with a low QTc value that would be assigned 2 points or only even 1 point based on the ECG findings alone.
Identifying At-Risk Patients
Our initial model was run on all patients with LQTS, many of whom had QT prolongation on their ECGs, permitting diagnosis by the Schwartz criteria. However, the subset of patients with a definite (genetic) diagnosis of LQTS in the setting of a normal QTc (<450 milliseconds) are at high risk of underdetection and consequent clinical events. The AI-ECG was able to distinguish patients with LQTS from those dismissed as normal with an AUC of 0.863 and overall accuracy of 78.7%. Thus, the AI-ECG may help identify healthy-appearing individuals at risk for potentially lethal, LQTS-associated arrhythmias. These individuals do not meet the QTc cutoff values recommended by international screening criteria to warrant further evaluation.18,19 Although individuals with QTc values within the normal limits are at significantly lower risk of such arrhythmias, identification of these patients is still important to, at minimum, implement the potential life-saving measure of avoiding drugs that prolong the QT interval and perhaps institute prophylactic β-blocker therapy (class IIa recommendation) and help to identify family members who might be at (possibly higher) risk. Moreover, a previous study demonstrated the use of AI-ECG to detect depressed ventricular function using smartphone-based ECG electrodes.11 If confirmed to function with fewer leads, screening for prolonged QT using this approach could be offered as a point-of-care test at pharmacies and clinics to avoid administering QT-prolonging medications to individuals with concealed LQTS with an otherwise unsuspected high risk for cardiovascular events.
Limitations
A study on a rare disease, such as LQTS, has challenges and limitations. Studies such as these demonstrate that AI can be a useful tool and, when applied to the ECG, could provide a low-cost, easily accessible replacement for more expensive tests, such as the echocardiogram or magnetic resonance imaging. However, before these kinds of AI models can be implemented into clinical practice or incorporated into mobile technology, several limitations have to be recognized and steps have to be taken to increase its validity and generalizability. First, although the AI-ECG may facilitate diagnosis, it must be recognized that this model in its current form was built on patients presenting to a dedicated LQTS specialty center with control patients evaluated under the same suspicion (ie, patients referred under the suspicion of possible LQTS, but discharged without this diagnosis). Herein, although useful for the type of patients presenting to such a clinic to determine a clear diagnosis and treatment for their disease, both the relative uniqueness of the setting as well as the selection of patients with suspicion of possible LQTS but discharged without that diagnosis may limit its generalizability.
Although we have provided an internal validation of our findings by applying an additional approach to temporal splitting of the data set, further internal validation awaits prospective enrollment of future patients seen and evaluated in our Windland Smith Rice Genetic Heart Rhythm Clinic. Second, the study lacks external validation and calibration from a cohort of similar patients evaluated at a different genetic heart rhythm clinic. Third, the performance of this neural network model compared with either healthy controls derived from an unselected cohort or the general population of ECGs is not known. To this end, to extend generalizability and potentially offer a model that could lead to a neural network that functions as a true QT screening tool, our model will need to be trained and validated in a larger, unselected population of patients to be able to develop an AI-ECG model for detection of LQTS and/or malignant QT prolongation when juxtaposed to an unselected background. However, because QT prolongation on an ECG is not unique to patients with LQTS, development of this model is dependent on recognition and differentiation of patients with other causes of acquired QT prolongation, such as medications or electrolyte abnormalities, which are seen in approximately 10% of patients who undergo ECG screening in the outpatient setting.20
Conclusions
In an AI model derived from cases and controls evaluated in a specialized genetic heart rhythm clinic, the AI-ECG was able to distinguish patients with electrocardiographically concealed LQTS from those discharged without the diagnosis and provide a 78.7% accurate pregenetic test anticipation of LQTS genotype status.
eFigure 1. Development of the CNN
eFigure 2. Correlation of Schwartz-Score (Probability of LQTS) With Neural Network Output
eFigure 3. ROC Curves for Model Using Temporal Splitting Approach
eMethods. ECG Wave Form Data, CNN Development and Application
eTable 1. Performance Comparison of Randomly and Temporally Split Models
eTable 2. Performance Comparison of Randomly and Temporally Split Models
References
- 1.Schwartz PJ, Ackerman MJ. The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. Eur Heart J. 2013;34(40):3109-3116. doi: 10.1093/eurheartj/eht089 [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Timothy KW, Vincent GM, et al. Spectrum of ST-T–wave patterns and repolarization parameters in congenital long-QT syndrome: ECG findings identify genotypes. Circulation. 2000;102(23):2849-2855. doi: 10.1161/01.CIR.102.23.2849 [DOI] [PubMed] [Google Scholar]
- 3.Sy RW, van der Werf C, Chattha IS, et al. Derivation and validation of a simple exercise-based algorithm for prediction of genetic testing in relatives of LQTS probands. Circulation. 2011;124(20):2187-2194. doi: 10.1161/CIRCULATIONAHA.111.028258 [DOI] [PubMed] [Google Scholar]
- 4.Ackerman MJ, Khositseth A, Tester DJ, Hejlik JB, Shen WK, Porter CB. Epinephrine-induced QT interval prolongation: a gene-specific paradoxical response in congenital long QT syndrome. Mayo Clin Proc. 2002;77(5):413-421. doi: 10.1016/S0025-6196(11)62209-X [DOI] [PubMed] [Google Scholar]
- 5.Viskin S, Postema PG, Bhuiyan ZA, et al. The response of the QT interval to the brief tachycardia provoked by standing: a bedside test for diagnosing long QT syndrome. J Am Coll Cardiol. 2010;55(18):1955-1961. doi: 10.1016/j.jacc.2009.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugrue A, Rohatgi RK, Noseworthy PA, et al. Architectural T-wave analysis and identification of on-therapy breakthrough arrhythmic risk in type 1 and type 2 long-QT syndrome. Circ Arrhythm Electrophysiol. 2017;10(11):e005648. doi: 10.1161/CIRCEP.117.005648 [DOI] [PubMed] [Google Scholar]
- 7.Sugrue A, Noseworthy PA, Kremen V, et al. Automated T-wave analysis can differentiate acquired QT prolongation from congenital long QT syndrome. Ann Noninvasive Electrocardiol. 2017;22(6):e12455. doi: 10.1111/anec.12455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ter Bekke RM, Haugaa KH, van den Wijngaard A, et al. Electromechanical window negativity in genotyped long-QT syndrome patients: relation to arrhythmia risk. Eur Heart J. 2015;36(3):179-186. doi: 10.1093/eurheartj/ehu370 [DOI] [PubMed] [Google Scholar]
- 9.Lane CM, Bos JM, Rohatgi RK, Ackerman MJ. Beyond the length and look of repolarization: defining the non-QTc electrocardiographic profiles of patients with congenital long QT syndrome. Heart Rhythm. 2018;15(9):1413-1419. doi: 10.1016/j.hrthm.2018.04.033 [DOI] [PubMed] [Google Scholar]
- 10.Schwartz PJ, Crotti L. QTc behavior during exercise and genetic testing for the long-QT syndrome. Circulation. 2011;124(20):2181-2184. doi: 10.1161/CIRCULATIONAHA.111.062182 [DOI] [PubMed] [Google Scholar]
- 11.Attia ZI, Kapa S, Lopez-Jimenez F, et al. Screening for cardiac contractile dysfunction using an artificial intelligence–enabled electrocardiogram. Nat Med. 2019;25(1):70-74. doi: 10.1038/s41591-018-0240-2 [DOI] [PubMed] [Google Scholar]
- 12.Rohatgi RK, Sugrue A, Bos JM, et al. Contemporary outcomes in patients with long QT syndrome. J Am Coll Cardiol. 2017;70(4):453-462. doi: 10.1016/j.jacc.2017.05.046 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz PJ. Idiopathic long QT syndrome: progress and questions. Am Heart J. 1985;109(2):399-411. doi: 10.1016/0002-8703(85)90626-X [DOI] [PubMed] [Google Scholar]
- 14.Lehmann MH, Suzuki F, Fromm BS, et al. T wave “humps” as a potential electrocardiographic marker of the long QT syndrome. J Am Coll Cardiol. 1994;24(3):746-754. doi: 10.1016/0735-1097(94)90024-8 [DOI] [PubMed] [Google Scholar]
- 15.Hermans BJM, Stoks J, Bennis FC, et al. Support vector machine-based assessment of the T-wave morphology improves long QT syndrome diagnosis. Europace. 2018;20(suppl_3):iii113-iii119. doi: 10.1093/europace/euy243 [DOI] [PubMed] [Google Scholar]
- 16.Schwartz PJ, Locati E. The idiopathic long QT syndrome: pathogenetic mechanisms and therapy. Eur Heart J. 1985;6(suppl D):103-114. doi: 10.1093/eurheartj/6.suppl_D.103 [DOI] [PubMed] [Google Scholar]
- 17.Schwartz PJ. The congenital long QT syndromes from genotype to phenotype: clinical implications. J Intern Med. 2006;259(1):39-47. doi: 10.1111/j.1365-2796.2005.01583.x [DOI] [PubMed] [Google Scholar]
- 18.Rautaharju PM, Surawicz B, Gettes LS, et al. ; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society . AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119(10):e241-e250. doi: 10.1161/CIRCULATIONAHA.108.191096 [DOI] [PubMed] [Google Scholar]
- 19.Ackerman MJ, Priori SG, Willems S, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011;8(8):1308-1339. doi: 10.1016/j.hrthm.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 20.Haugaa KH, Bos JM, Tarrell RF, Morlan BW, Caraballo PJ, Ackerman MJ. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc. 2013;88(4):315-325. doi: 10.1016/j.mayocp.2013.01.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Development of the CNN
eFigure 2. Correlation of Schwartz-Score (Probability of LQTS) With Neural Network Output
eFigure 3. ROC Curves for Model Using Temporal Splitting Approach
eMethods. ECG Wave Form Data, CNN Development and Application
eTable 1. Performance Comparison of Randomly and Temporally Split Models
eTable 2. Performance Comparison of Randomly and Temporally Split Models