Abstract
Biological mechanisms associated with response to trauma may impact risk for depression. One such mechanism is endocannabinoid signaling (eCB), a neuromodulatory system comprised of the CB1 subtype of cannabinoid receptors (CB1R), encoded by the CNR1 gene, and two primary endogenous ligands: 2-arachidonoylglycerol (2-AG) and N-arachidonylethanolamine (AEA), hydrolyzed by monoacylglycerol lipase (gene name MGLL) and fatty acid amide hydrolase (gene name FAAH). Preclinical data suggest that eCB/CB1R signaling acts as a stress buffer and its loss or suppression increases depression-like behaviors. We examined circulating concentrations of the eCBs (2-AG and AEA) days and six months after a traumatic injury as a marker of eCB/CB1R signaling and as predictors of Center for Epidemiologic Studies of Depression Scale-Revised [CESD-R] scores as a measure of depression severity six months after injury. We also explored associations of CNR1, FAAH, and MGLL genetic variance with depression severity at six months. Results from hierarchical multiple linear regressions showed that higher 2-AG serum concentrations after trauma predicted greater depression at six months (β = 0.23, p = 0.007); neither AEA after trauma, nor 2-AG and AEA at six months were significant predictors (p's > 0.305). Carriers of minor allele for the putative single nucleotide polymorphism in the CNR1 gene rs806371 (β = 0.19, p = 0.024) experienced greater depression at six months. These data suggest that the eCB signaling system is highly activated following trauma and that eCB/CB1R activity contributes to long-term depression risk.
Keywords: Trauma, Injuries, Endocannabinoids, Genotype, Depression
1. Introduction
The endocannabinoid (eCB) system is a neuromodulatory system comprised of two endogenous cannabinoid receptors, CB1R and CB2R, and their two primary endogenous ligands, 2-arachidonoylglycerol (2-AG) and N-arachidonylethanolamine (anandamide, AEA) (Hillard, 2015). The CB1R protein is encoded by the cannabinoid receptor 1 (CNR1) gene, while 2-AG and AEA are hydrolyzed by monoglyceride lipase (MGLL) and fatty acid amide hydrolase (FAAH), respectively. Brain eCB/CB1R signaling plays a prominent role in activity-dependent, retrograde regulation of synaptic activity throughout the brain (Katona and Freund, 2012) and is implicated in a number of biological systems, including the stress system. Indeed, considerable evidence shows that acute and chronic stress exposure alters eCB/CB1R signaling (Morena et al., 2016). For example, glucocorticoids elevate brain 2-AG levels and thus enhance 2AG/CB1R signaling (Hill et al., 2009), while corticotropin releasing hormone receptor activation increases AEA catabolism and thus reduces AEA/CB1R signaling (Gray et al., 2015). In both cases, the changes in eCB concentrations in the brain contribute to dampening of endocrine and neuronal responses to stress; processing of reward; formation, recall and extinction of aversive memories; and behavioral responses to fear and anxiety (see (Hill et al., 2010; Lutz et al., 2015; Morena et al., 2016) for reviews). In addition, preclinical data demonstrate that disruption of eCB/CB1R signaling causes behavioral, morphological and neuroendocrine changes that are associated with human psychopathology, including anhedonia and dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis (Hillard and Liu, 2014; Lutz et al., 2015).
In humans, eCB/CB1R signaling is also implicated in mood and depression. The most direct evidence comes from the adverse effects of rimonabant, a CB1 receptor antagonist, for the treatment of obesity. Rimonabant use produces significant increases in depressive symptoms, particularly among individuals with previous or current diagnoses of major depression (Christensen et al., 2007; Nissen et al., 2008). In accord with these data, administration of rimonabant has been shown to diminish neural responses to reward in the striatum and orbitofrontal cortex, suggesting that CB1R blockade reduces reward sensitivity, a known feature of depression (Horder et al., 2010). Patients with major depressive disorder (MDD) also have altered CB1R densities, with some studies finding lower receptor densities in the anterior cingulate cortex compared to non-depressed patients with other forms of psychopathology (e.g., schizophrenia and bipolar disorder (Choi et al., 2012; Koethe et al., 2007)). In contrast, other postmortem investigations have found elevated CB1R densities in the prefrontal cortex (PFC) (Hungund et al., 2004; Vinod et al., 2005) and ventral striatum (Vinod et al., 2010) as well as higher 2-AG concentrations (Vinod et al., 2005) in the brains of suicide victims.
Circulating eCB concentrations, hypothesized to be biomarkers of CNS eCB signaling (Hillard, 2018), are also altered in those with depression. Women with MDD have lower serum 2-AG concentrations compared to non-depressed controls (Hill et al., 2008; Hill et al., 2009) and symptoms of depressed mood are inversely correlated with 2-AG concentration (Hill et al., 2008; Stensson et al., 2018). On the other hand, a recent study found significantly higher circulating concentrations of both AEA and 2-AG in patients with MDD, although the increase in 2-AG did not survive correction for anti-depressant use (Romero-Sanchiz et al., 2019). Other studies have examined associations of acute changes in circulating eCBs with changes in mood using exercise (Heyman et al., 2012; Koltyn et al., 2014) and psychological stress (Dlugos et al., 2012) to provoke acute increases in circulating 2-AG and AEA. In healthy individuals, the increase in 2-AG – but not AEA – after an acute exercise intervention is negatively correlated with depressed mood (Brellenthin et al., 2017), which is in accord with the data from depressed individuals. Similarly, a recent study found that the changes in 2-AG and AEA concentrations evoked by exercise were positively correlated with exercise-induced reductions in depressive symptoms in individuals with MDD (Meyer et al., 2019). On the other hand, others have found that circulating AEA concentrations are positively correlated with depressive symptoms. Physically inactive, but otherwise healthy, individuals engaging in an exercise program exhibited a reduction in circulating AEA that was significantly associated with reduced symptoms of mood disturbance and anger (Belitardo de Oliveira et al., 2019), while circulating AEA concentrations in individuals with fibromyalgia were positively correlated with symptoms of depression in another study (Stensson et al., 2018).
In addition to altered CB1R density and circulating eCBs in depressed individuals, CNR1, FAAH, and MGLL genotypes have been associated with depression (Hill and Patel, 2013). Much of this research has focused on rs1049353, a snp in the coding region of the CNR1 gene. Carriers of the major G allele (AG/GG) at rs1049353 were more likely to exhibit anhedonia after childhood abuse in one study (Agrawal et al., 2012), were found to be more resistant to antidepressant treatment (Domschke et al., 2008), and display less striatal activation to happy faces, suggesting reduced reward sensitivity (Chakrabarti et al., 2006). On the other hand, other studies found that carriers of the minor A allele are more likely to suffer from recurrent major depressive episodes (Monteleone et al., 2010) and do not respond as well to antidepressants (Mitjans et al., 2012), while one study failed to find an association (Pearson et al., 2013). Thus, while these studies support the overall hypothesis that CB1R function contributes to risk for depression, the directionality of the relationship is inconsistent. Other CNR1 snps have also been associated with depression. One study found that a higher frequency of the minor G allele at rs806371 in patients with MDD compared to matched controls (Mitjans et al., 2013). In a large genome-wide association study (GWAS), rs2180619 was associated with a 4.6 times less risk for anxiety (Lazary et al., 2009), which is often comorbid with depression (Flory and Yehuda, 2015) (although see (Maple et al., 2016), which did not find an association between rs2180619 genotype and depression in a small (n = 41) sample of cannabis users). Although studied to a lesser extent FAAH and MGLL genotypes have been linked to depression (Maple et al., 2016) and substance abuse (Carey et al., 2015).
Based on this evidence, there has been substantial interest in leveraging the eCB system for the treatment of depression (Chanda, 2019). Yet, our understanding of the relationship between eCB/CB1R signaling and risk for depressive illness in humans is incomplete. In particular, the role of eCB/CB1R signaling and risk for development of depression following stress exposure has not yet been studied. Depression is one of the most common psychiatric outcomes following trauma (defined as a serious, life-threatening event such as a motor vehicle crash), affecting upwards of 9% of survivors (Bryant et al., 2010) with higher incidence (upwards of 31% when assessed six months after trauma) in survivors of traumatic injury (Shih et al., 2010). Notably, this rate is over four times the point prevalence of depression in the United States general population (7.1%) (National Survey on Drug Use and Health, 2017) and is comparable to incidence of PTSD (e.g., one study found identical rates of PTSD and depression six months after traumatic injury; (Shih et al., 2010). Nevertheless, investigations into the causes for depression after trauma remain relatively rare. It is unclear what factors predispose individuals to develop depression after trauma (Burcusa and Iacono, 2007), but peritraumatic factors associated with trauma have been linked to depression incidence (Ahl et al., 2017; Joormann et al., 2020). Given the role of eCB/CB1R in stress response and the HPA-axis, this system may also be implicated in the development of depression after trauma.
The current study investigated whether concentrations of the eCBs, 2-AG and AEA, measured in circulating blood within days of a traumatic event were significantly related to depression severity assessed at six months after the traumatic event. Based on the only other study to test the relationship between endocannabinoids and prospective illness (but not with respect to depression) (Hauer et al., 2012), we hypothesized that greater 2-AG and AEA would predict less depression severity six months after the traumatic event. We also explored the contributions of genotype at CNR1 rs806371, CNR1 rs1049353, CNR1 rs2180619, FAAH rs324420, and MGLL rs604300, hypothesizing that genetic variance in these snps would predict depression severity at six months.
2. Materials and methods
2.1. Participants
Participants (n = 278) were recruited from a Level 1 trauma center from a traumatically injured population and required hospitalization after their injuries. Initially eligible participants were identified using a trauma service census and individuals were excluded if they met any of the following exclusion criteria: 1) younger than 18 years of age, 2) traumatic brain injury resulting in greater than 30 min of peritraumatic amnesia, 3) injury that resulted in an inability to communicate, 4) Glasgow coma score of less than 13 on admission, or 5) non-English speaking.
2.2. Study procedures
The Institutional Review Board (IRB) at the Medical College of Wisconsin approved all study procedures and participants were monetarily compensated for their time. All participants completed a blood draw during their hospital stay for quantification of circulating eCBs and DNA collection. Whole blood was collected in EDTA tubes by trained phlebotomists; this is referred to as the “post-injury” sample. A number of demographic assessments were collected post-injury, including age, gender, race, ethnicity, concurrent psychiatric disorders, and current use of psychiatric medications, psychotherapy, and opiates for pain. We also assessed via self-report whether individuals used cannabis as relationships between eCBs and cannabis use has been reported previously (Morgan et al., 2013; Muhl et al., 2014; Thieme et al., 2014). Injury characteristics were assessed via the injury severity score (ISS (Tohira et al., 2012)). A number of clinical assessments were also collected post-injury. Symptoms of depression were measured using the shortened depression subscale of the Depression Anxiety and Stress Severity Scale (DASS-21) (Osman et al., 2012). Participants also completed the PTSD Symptom Checklist-Civilian Version (PCL-C (Weathers et al., 1994)) for self-reported assessment of posttraumatic stress disorder (PTSD) symptoms. When completing the PCL-C, participants were prompted to answer questions with regard to the index traumatic injury that brought them to the hospital.
Participants returned for a follow-up visit six months later for a blood draw performed by trained research nursing staff for quantification of circulating eCBs. At six months, participants completed the Clinician Administered PTSD Scale (CAPS-5 [Weathers et al., 2018];) with respect to the index trauma. While we relied on the self-reported DASS for the assessment of depression at the post-injury appointment, this was done as the recent occurrence of trauma (e.g., 3 days post) makes the clinical assessment of the development of depression at that time suspect. This is because the criteria for depression is defined as symptoms that occur ≥2 weeks in duration. In contrast, at six months, participants completed the Center for Epidemiologic Studies of Depression Scale-Revised (CESD-R (Radloff, 1977) as our main outcome measure of self-reported depression. The CESD-R has high internal consistency in community (α = .94) and clinical samples (α = .85) (Carleton et al., 2013) and is used as a screening tool for depression based on epidemiological studies (Moon et al., 2017). For the purposes of this study, a continuous sum score of depression symptom severity was utilized.
2.3. Endocannabinoid quantification
For the eCB assay, blood was collected into 2–10 ml sterile tubes (BD Vacutainer tubes) and was allowed to clot at room temperature for a least 30 and no more than 60 min. Samples were centrifuged using the Beckman Coulter Allegra 6R swinging bucket centrifuge, running at 4400 RPM and at a temperature of 4 °C for 20 min. Serum was pipetted via plastic disposable 5 ml pipettes at 1.0 ml aliquots into plastic o-ringed cryovials (Max of 4 - 1 ml samples, if available, per draw), and immediately frozen at −80 °C until transfer and analyses. 2-AG and AEA in the serum were determined in lipid extracts from serum using stable isotope dilution, liquid chromatography-mass spectrometry following previously published methods (Crombie et al., 2018).
2.4. DNA extraction and genotyping
DNA was extracted from the 1–5 ml whole blood sample using the Qiagen Gentra Puregene Blood Kit. The extracted DNA was rehydrated by adding Qiagen DNA Hydration Solution and incubating the samples in a 65 °C water bath for 1 h. DNA samples were then quantified using the Molecular Devices SpectraMax M5 spectrophotometer. Extracted DNA (10 ng) was genotyped using ThermoFisher Scientific Taqman SNP Genotyping Assays (CNR1: rs806371, rs1049353, rs2180619; FAAH: rs324420; MGLL: rs604300) and Genotyping Master Mix according to the manufacturer's instructions. Samples were analyzed on the QuantStudio 12K Flex Real-Time PCR instrument and genotypes manually called using QuantStudio 12K Flex Software v.1.2.2.
2.5. Data analysis
The Hardy-Weinberg equilibrium for genotype frequencies was assessed using chi-square tests and the R “HardyWeinberg” package (Graffelman, 2015) with R 3.6.1 (released July 2019). Changes in eCBs between post-injury and follow-up appointments were assessed using repeated samples t-test.
The relationship between eCBs and CNR1, FAAH, and MGLL genotypes and depression was tested using hierarchical linear regression (outcome = CESD-R at six months). We entered 2-AG and AEA values post-injury and all snps in step 1 as predictors-of-interest. The following post-injury covariates were added in step 2: age; gender; race; ethnicity; ISS; concurrent psychiatric medication, psychotherapy, and opiate use (in Morphine Equivalent Units [MEU]); concurrent diagnosis of psychiatric disorders; DASS-Depression scores post-injury; PCL-C scores post-injury; number of days between trauma and the post-injury appointment; time of day for the blood draw (as circulating eCBs follow a circadian rhythm [Hanlon, 2020; Hanlon et al., 2015]); and self-reported cannabis use. In step 3, we controlled for post-traumatic distress severity at six months given high concordance between the development of depression and PTSD in trauma survivors (Kessler, 1995). The CAPS-5 measures two distinct post-traumatic factors: post-traumatic distress and general post-traumatic dysphoria (Hunt et al., 2017); therefore, items assessing post-traumatic distress were summed and added to the model.
Although our prior research has found relationships between eCBs, sleep, and time since last meal (Hanlon et al., 2015; Hillard, 2018), we found that sleep and eating habits during hospitalization in this sample were disrupted and non-regular; thus, we did not add these as covariates in our analysis. We report the relationship between eCBs and these variables in Supplementary Table 1.
As values for 2-AG and AEA at the two time points (post-injury, six months) were moderately correlated (2-AG: r(118) = 0.35, p < 0.001; AEA: r(118) = 0.42, p < 0.001), we used a second hierarchical multiple linear regression to examine 2-AG and AEA values obtained at six months using a stepped approach identical to the first hierarchical linear regression. In the second regression, concurrent psychotherapy, psychiatric medication, and cannabis use reflects presence of these at the six month appointment. We again controlled for concurrent psychiatric disorders at the six month appointment with the exception of MDD, which was removed as it confounded the prediction of depression severity. Opiate use at the six month appointment was not included as it was not available. Finally, date and time of day for blood draw were recalculated for the six month appointment. In all analyses, continuous predictors, including time of day using a 24-h clock, were grand-mean centered. Gender (0 = male), race (0 = White), psychiatric medication (0 = no), psychotherapy (0 = no), concurrent psychiatric disorder (0 = no), and cannabis use (0 = no) were dummy coded. For each snp, allele frequency was dummy coded for carriers of the minor allele (0 = homozygous major). The minor alleles are: CNR1 rs806371 (G); CNR1 rs1049353 (A), CNR1 rs2180619 (A), FAAH rs32440 (A), and MGLL rs604300 (A). In reporting results, mean values are reported ± SD.
As our main interest in this analysis was eCBs post-injury and the effect of CNR1 genotypes on depression, we used a Bonferroni-correction to correct against multiple corrections for the use of five predictors (2-AG post-injury; AEA post-injury; CNR1 rs806371; CNR1 rs1049353; CNR1 rs2180619) for an α = 0.01 to consider effects significant.
3. Results
3.1. Participants
Of the 278 participants recruited, n = 172 returned for the six-month visit, reflecting a 62% retention rate comparable to other longitudinal investigations involving survivors of traumatic injury (e.g., 57% (Martin-Herz et al., 2012)). Participants lost to follow-up (n = 106) were younger (37.23 ± 14.28; t(276) = 2.53, p = 0.011), but did not differ from participants who were retained in terms of gender (χ2(1) = 0.69, p = 0.408), race/ethnicity (χ2(3) = 1.67, p = 0.644), or mechanism of injury (χ2(9) = 12.30, p = 0.197). Those retained versus lost to follow-up did not differ with regard to 2-AG (t(272) = 1.67, p = 0.096), AEA (t(272) = 1.79, p = 0.075), or genotype distributions (p's > 0.304).
The number of participants who had available genotyping and CESD-R scores at six months was n = 154 for the CNR1 rs2180619 and MGLL rs604300 snps and n = 153 for CNR1 rs806371, CNR1 rs1049353, and FAAH rs 324420. A total of n = 153 had both CESD-R scores and available eCBs post-injury and of these, n = 150 had both CESD-R scores and available eCBs at six month follow-up. Outliers in 2-AG and AEA at both visits were assessed using the outlier labeling rule of values > 2.2 SDs outside the interquartile range (Hoaglin and Iglewicz, 1987) and subsequently removed from analysis, retaining n = 142 for the post-injury eCB analysis and n = 140 for the six months eCB analysis. See Table 1 for final sample demographics.
Table 1.
Sample demographics.
| Post-injury |
Six Month |
|
|---|---|---|
| Timepoint |
Timepoint |
|
| N = 154 | N = 140 | |
| n (%) | ||
| Gender (male) | 110 (71%) | – |
| Race/Ethnicity | ||
| American Indian or Alaskan Native | 2 (1%) | – |
| Black | 67 (44%) | – |
| White | 73 (47%) | – |
| Hispanic or Latino | 13 (8%) | – |
| Mechanism of injury | – | |
| Motor vehicle crash | 49 (32%) | – |
| Fall | 28 (18%) | – |
| Gunshot wound | 25 (16%) | – |
| Stab wound | 15 (10%) | – |
| Motorcycle crash | 14 (9%) | – |
| Pedestrian accident | 9 (6%) | – |
| Crush injury | 8 (5%) | – |
| Recreational injury | 3 (2%) | – |
| Blunt assault | 3 (2%) | – |
| Psychiatric conditions | ||
| Major Depressive Disorder | 21 (14%) | 18 (13%) |
| Bipolar Disorder | 9 (6%) | 5 (4%) |
| Generalized Anxiety Disorder | 8 (5%) | 3 (2%) |
| Schizophrenia | 6 (4%) | 2 (1%) |
| Posttraumatic Stress Disorder | 4 (3%) | 7 (5%) |
| Psychiatric medications (yes) | 38 (24%) | 35 (25%) |
| Psychotherapy (yes) | 9 (6%) | 16 (11%) |
| Cannabis use (yes) | 47 (31%) | 47 (34%) |
| M (SD) | ||
| Age | 42.17 (16.37) | – |
| Injury severity score (ISS) | 10.63 (6.19) | – |
| Time since trauma | 3.04 (4.99) days | 6.33 (0.69) months |
Note. M = mean; SD = standard deviation. Concurrent psychiatric conditions are not mutually exclusive. ISS provides a clinical estimate of the severity of a traumatic injury to the body.
3.2. Depression severity post-injury and at six months
Post-injury, DASS-Depression scores ranged from 0 to 21 (3.65 ± 4.46). Using the established cut-offs, N = 110 (71%) had no depression symptoms, N = 12 (8%) had mild depression symptoms, N = 18 (12%) had moderate symptoms, N = 8 (5%) had severe symptoms, and N = 6 (4%) had extremely severe symptoms.
At six months, CESD-R scores ranged from 0 to 49 (14.94 ± 12.96). Using the established cut-off of a CESD-R score of 16 (Radloff, 1977), n = 62 (40% of the sample) met clinical criteria for MDD based on severity of their symptoms. This rate is high compared to other published work in trauma survivors (Shih et al., 2010; 29%).
3.3. Genotype distributions
The minor allele frequencies in this sample were 0.183 for CNR1 rs806371 (G), 0.167 for CNR1 rs1049353 (A), 0.470 for CNR1 rs2180619 (A), 0.324 for FAAH rs324420 (A), and 0.158 for MGLL rs604300 (A). Genotype distribution of all snps maintained the Hardy-Weinberg equilibrium (CNR1 rs806371 χ2(1) = 0.22, p = 0.636; CNR1 rs1049353 χ2(1) = 1.04, p = 0.308; CNR1 rs2180619 χ2(1) = 0.13, p = 0.714; FAAH rs324420 χ2(1) = 1.22, p = 0.270; MGLL rs604300 χ2(1) = 1.66, p = 0.197).
There were no gender differences with regard to genotypes (p's > 0.328), however presence of the minor alleles for CNR1 rs1049353 (χ2(6) = 22.30, p = 0.001), CNR1 rs2180619 (χ2(6) = 34.04, p < 0.001), FAAH rs324420 (χ2(6) = 18.39, p = 0.005), and MGLL rs604300 (χ2(3) = 21.45, p < 0.001) differed by race. Results from post-hoc tests demonstrated that Whites, compared to Blacks, were more likely to carry the minor alleles for snps CNR1 rs1049353 (χ2(2) = 15.46, p < 0.001) and MGLL rs604300 (χ2(2) = 20.12, p < 0.001). In contrast, Blacks, compared to Whites, were more likely to carry the minor alleles for snps CNR1 rs2180619 (χ2(2) = 23.86, p < 0.001) and FAAH rs324420 (χ2(2) = 16.18, p < 0.001).
3.4. 2-AG and AEA
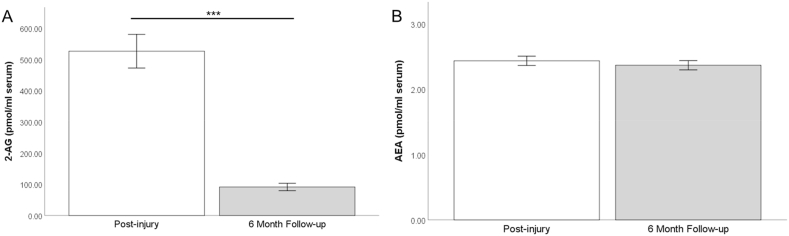
Values for 2-AG and AEA concentrations in serum are depicted in Fig. 1. Average values for 2-AG post-injury were 511.3 ng/ml (±568.2) and 2.44 ng/ml (±0.79) for AEA. At the follow-up appointment six months later, average values for 2-AG were 61.8 ng/ml (±45.6) and 2.33 ng/ml (±0.78) for AEA. Results from a repeated samples t-test demonstrated a significant decline in 2-AG from post-injury to follow-up (t(119) = 8.48, p < 0.001), but no change in AEA values between the time points (t(118) = 0.90, p = 0.371).
Fig. 1.
Values for endocannabinoids 2-arachidonylglycerol (2-AG) (Panel A) and anandamide (AEA) (Panel B) post-injury and at six months. Error bars reflect ±standard error of the mean. ***p < 0.001.
3.5. Post-injury eCBs and depression severity at six months
For all regression analyses, distribution of eCBs were assessed for normality and log-transformed to achieve normal distributions. Results of our hierarchical regression testing post-injury eCBs met all assumptions of the linear model (Table 2): errors were independent, residuals were homoscedastic and normally distributed, and there were no problems with multicollinearity among predictors. Step 1 of the model that examined eCB as predictors was significant (F(7, 139) = 2.61, p = 0.015; R2 = 0.08). Greater 2-AG post-injury was associated with greater depression severity at six months (β = 0.23, t = 2.73, p = 0.007). No other predictors were significant using the corrected α = 0.01.
Table 2.
Hierarchical linear regression testing endocannabinoids collected at post-injury.
| CESD-R |
||||||
|---|---|---|---|---|---|---|
| Variable | B | SE | β | t | p-value | |
| Step 1 | ||||||
| Intercept | 10.173 | 2.729 | 3.727 | <0.001*** | ||
| 2-AG at post-injury | 5.11 | 1.87 | 0.23 | 2.73 | 0.007** | |
| AEA at post-injury | 0.21 | 8.08 | 0.00 | 0.03 | 0.979 | |
| CNR1 rs806371 (0 = T/T) | 5.40 | 2.36 | 0.19 | 2.29 | 0.024* | |
| CNR1 rs1049353 (0 = G/G) | 1.09 | 2.43 | 0.04 | 0.45 | 0.653 | |
| CNR1 rs2180619 (0 = G/G) | 2.00 | 2.43 | 0.07 | 0.83 | 0.411 | |
| FAAH rs324420 (0 = C/C) | 3.17 | 2.22 | 0.12 | 1.43 | 0.155 | |
| MGLL rs604300 (0 = G/G) | −3.29 | 2.82 | −0.10 | −1.17 | 0.245 | |
| Step 2 | ||||||
| Intercept | 2.59 | 3.23 | 0.80 | 0.423 | ||
| 2-AG at post-injury | 5.53 | 1.71 | 0.25 | 3.24 | 0.002** | |
| AEA at post-injury | 8.11 | 7.74 | 0.09 | 1.05 | 0.297 | |
| CNR1 rs806371 (0 = T/T) | 4.92 | 2.14 | 0.18 | 2.30 | 0.024* | |
| CNR1 rs1049353 (0 = G/G) | 4.55 | 2.33 | 0.15 | 1.96 | 0.053 | |
| CNR1 rs2180619 (0 = G/G) | 4.30 | 2.20 | 0.15 | 1.96 | 0.052 | |
| FAAH rs324420 (0 = C/C) | 0.64 | 2.03 | 0.02 | 0.31 | 0.754 | |
| MGLL rs604300 (0 = G/G) | 4.44 | 2.77 | 0.13 | 1.60 | 0.112 | |
| DASS-Depression at post-injury | 0.77 | 0.29 | 0.26 | 2.72 | 0.008** | |
| PCL-C at post-injury | 0.19 | 0.08 | 0.24 | 2.45 | 0.016* | |
| Psychotherapy at post-injury (0 = no) | 1.15 | 4.45 | 0.02 | 0.26 | 0.796 | |
| Psychiatric medication at post-injury (0 = no) | 6.69 | 5.16 | 0.22 | 1.30 | 0.198 | |
| Psychiatric diagnosis at post-injury: MDD (0 = no) | −3.80 | 5.98 | −0.09 | −0.64 | 0.527 | |
| Psychiatric diagnosis at post-injury: GAD (0 = no) | −5.18 | 5.63 | −0.09 | −0.92 | 0.360 | |
| Psychiatric diagnosis at post-injury: PTSD (0 = no) | 3.46 | 7.05 | 0.04 | 0.49 | 0.625 | |
| Psychiatric diagnosis at post-injury: BD (0 = no) | −12.08 | 6.18 | −0.23 | −1.96 | 0.053 | |
| Psychiatric diagnosis at post-injury: Schizophrenia (0 = no) | −7.90 | 6.59 | −0.12 | −1.20 | 0.233 | |
| MEU | 0.00 | 0.00 | −0.03 | −0.45 | 0.656 | |
| Marijuana use at post-injury (0 = no) | 0.93 | 2.30 | 0.03 | 0.40 | 0.687 | |
| ISS | −0.12 | 0.16 | −0.06 | −0.76 | 0.450 | |
| Days since trauma at post-injury | 0.08 | 0.20 | 0.03 | 0.39 | 0.701 | |
| Time of blood draw at post-injury | 0.11 | 0.76 | 0.01 | 0.14 | 0.889 | |
| Gender (0 = male) | 1.43 | 2.55 | 0.05 | 0.56 | 0.577 | |
| Age | −0.05 | 0.07 | −0.07 | −0.73 | 0.469 | |
| Race (American Indian or Alaskan Native [0 = White]) | 10.19 | 8.89 | 0.09 | 1.15 | 0.254 | |
| Race (Black [0 = White]) | 7.81 | 2.60 | 0.30 | 3.00 | 0.003** | |
| Ethnicity (0 = Non-Hispanic or Latino) | 9.53 | 3.94 | 0.20 | 2.42 | 0.017* | |
| Step 3 | ||||||
| Intercept | 8.12 | 2.73 | 2.97 | 0.004** | ||
| 2-AG at post-injury | 2.48 | 1.44 | 0.11 | 1.72 | 0.088 | |
| AEA at post-injury | 2.28 | 6.23 | 0.02 | 0.37 | 0.715 | |
| CNR1 rs806371 (0 = T/T) | 4.49 | 1.75 | 0.16 | 2.57 | 0.012* | |
| CNR1 rs1049353 (0 = G/G) | 2.32 | 1.91 | 0.08 | 1.21 | 0.227 | |
| CNR1 rs2180619 (0 = G/G) | 2.64 | 1.80 | 0.09 | 1.47 | 0.146 | |
| FAAH rs324420 (0 = C/C) | 0.59 | 1.64 | 0.02 | 0.36 | 0.721 | |
| MGLL rs604300 (0 = G/G) | 2.61 | 2.26 | 0.08 | 1.16 | 0.250 | |
| DASS-Depression at post-injury | 0.99 | 0.20 | 0.34 | 4.97 | <0.001*** | |
| Psychotherapy at post-injury (0 = no) | 0.49 | 3.55 | 0.01 | 0.14 | 0.891 | |
| Psychiatric medication at post-injury (0 = no) | 4.47 | 4.22 | 0.15 | 1.06 | 0.292 | |
| Psychiatric diagnosis at post-injury: MDD (0 = no) | −0.36 | 4.83 | −0.01 | −0.07 | 0.941 | |
| Psychiatric diagnosis at post-injury: GAD (0 = no) | −2.63 | 4.60 | −0.04 | −0.57 | 0.569 | |
| Psychiatric diagnosis at post-injury: PTSD (0 = no) | 2.19 | 5.75 | 0.02 | 0.38 | 0.703 | |
| Psychiatric diagnosis at post-injury: BD (0 = no) | −10.00 | 5.05 | −0.19 | −1.98 | 0.050 | |
| Psychiatric diagnosis at post-injury: Schizophrenia (0 = no) | −7.48 | 5.24 | −0.12 | −1.43 | 0.156 | |
| MEU | 0.00 | 0.00 | 0.01 | 0.19 | 0.851 | |
| Marijuana use at post-injury (0 = no) | 1.88 | 1.87 | 0.07 | 1.00 | 0.318 | |
| ISS | −0.14 | 0.13 | −0.07 | −1.06 | 0.291 | |
| Days since trauma at post-injury | −0.06 | 0.17 | −0.02 | −0.34 | 0.734 | |
| Time of blood draw at post-injury | 0.00 | 0.62 | 0.00 | 0.00 | 0.999 | |
| Gender (0 = male) | −0.51 | 2.09 | −0.02 | −0.25 | 0.806 | |
| Age | 0.01 | 0.06 | 0.01 | 0.20 | 0.846 | |
| Race (American Indian or Alaskan Native [0 = White]) | 2.67 | 7.28 | 0.02 | 0.37 | 0.715 | |
| Race (Black [0 = White]) | 1.53 | 2.28 | 0.06 | 0.67 | 0.504 | |
| Ethnicity (0 = Non-Hispanic or Latino) | 5.30 | 3.26 | 0.11 | 1.62 | 0.107 | |
| CAPS Distress at six months | 0.82 | 0.10 | 0.57 | 8.10 | <0.001*** | |
Note. CESD-R = Center for Epidemiologic Studies of Depression Scale-Revised; 2-AG = 2-arachidonoyl Glycerol; AEA = Anandamide; CNR1 = Cannabinoid Receptor 1; FAAH = Fatty Acid Amide Hydrolase; MGLL = Monoglyceride Lipase; DASS = Depression Anxiety and Stress Scale; PCL-C = PTSD Checklist-Civilian Version; MDD = Major Depressive Disorder; GAD = Generalized Anxiety Disorder; PTSD = Posttraumatic Stress Disorder; BD = Bipolar Disorder; MEU = morphine equivalent units; ISS = injury severity score; CAPS = Clinician Administered PTSD Scale. ***p < 0.001, **p < 0.01, *p < 0.05.
With the inclusion of covariates in step 2, the model continued to be significant (F(26, 139) = 4.11, p < 0.001; R2 = 0.37; R2 change = 0.29) and post-injury 2-AG continued to be a significant predictor of depression at six months (β = 0.25, t = 3.24, p = 0.002).
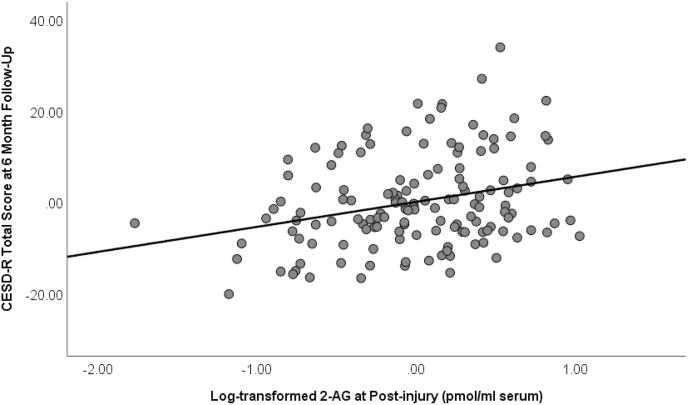
Finally, the model continued to be significant with the inclusion of distress symptoms at six months in step 3 (F(26, 139) = 8.43, p < 0.001; R2 = 0.58; R2 change = 0.21), although the effect of 2-AG was now trending (β = 0.11, t = 1.73, p = 0.088). Fig. 2 depicts partial regression plot between log 2-AG post-injury and CESD-R scores at six months, controlling for all covariates in Step 2.
Fig. 2.
Partial regression plot showing relationship between 2-arachidonylglycerol (2-AG) post-injury and depression severity at six months measured by CESD-R (Center for Epidemiologic Studies of Depression Scale-Revised). Relationship controls for all demographic covariates in Step 2 (β = 0.25, t = 3.24, p = 0.002).
3.6. Concurrent eCBs and depression severity at six months
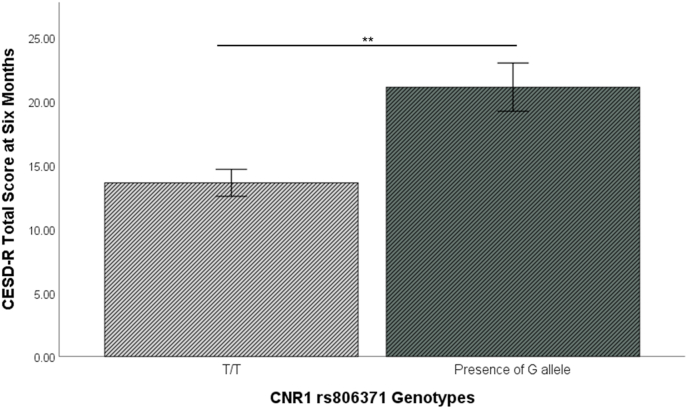
Table 3 depicts results of second hierarchical linear regression. Neither 2-AG (p = 0.306) nor AEA (p = 0.840) collected at six months were significant predictors of depression severity in step 1. However, in excluding the significant effect of 2-AG post-injury, carriers of the minor allele for CNR1 rs806371 became a significant predictor of depression symptoms in Steps 1 (β = 0.26, t = 2.89, p = 0.005) and 3 of the model (β = 0.21, t = 2.97, p = 0.004). Fig. 3 depicts greater CESD-R at six months for carriers of the minor allele for CNR1 rs806371.
Table 3.
Hierarchical linear regression testing endocannabinoids collected at six months.
| CESD-R |
||||||
|---|---|---|---|---|---|---|
| Variable | B | SE | β | t | p-value | |
| Step 1 | ||||||
| Intercept | 7.944 | 3.03 | 2.62 | 0.010* | ||
| 2-AG at six months | −4.07 | 3.95 | −0.09 | −1.03 | 0.306 | |
| AEA at six months | −1.69 | 8.38 | −0.02 | −0.20 | 0.840 | |
| CNR1 rs806371 (0 = T/T) | 7.38 | 2.56 | 0.26 | 2.89 | 0.005** | |
| CNR1 rs1049353 (0 = G/G) | 5.18 | 2.72 | 0.17 | 1.91 | 0.059 | |
| CNR1 rs2180619 (0 = G/G) | 3.58 | 2.63 | 0.12 | 1.36 | 0.175 | |
| FAAH rs324420 (0 = C/C) | 5.22 | 2.44 | 0.19 | 2.14 | 0.034* | |
| MGLL rs604300 (0 = G/G) | −4.51 | 2.99 | −0.14 | −1.51 | 0.134 | |
| Step 2 | ||||||
| Intercept | −0.77 | 3.90 | −0.20 | 0.844 | ||
| 2-AG at six months | −2.07 | 4.09 | −0.05 | −0.51 | 0.613 | |
| AEA at six months | 1.60 | 8.20 | 0.02 | 0.20 | 0.846 | |
| CNR1 rs806371 (0 = T/T) | 6.90 | 2.62 | 0.24 | 2.64 | 0.010* | |
| CNR1 rs1049353 (0 = G/G) | 6.39 | 2.75 | 0.21 | 2.32 | 0.022* | |
| CNR1 rs2180619 (0 = G/G) | 3.51 | 2.64 | 0.12 | 1.33 | 0.186 | |
| FAAH rs324420 (0 = C/C) | 3.95 | 2.42 | 0.15 | 1.63 | 0.106 | |
| MGLL rs604300 (0 = G/G) | 1.20 | 3.17 | 0.04 | 0.38 | 0.707 | |
| Psychotherapy at six months (0 = no) | 4.75 | 4.04 | 0.12 | 1.17 | 0.243 | |
| Psychiatric medication at six months (0 = no) | 6.59 | 3.59 | 0.21 | 1.84 | 0.069 | |
| Psychiatric diagnosis at six months: GAD (0 = no) | −2.69 | 7.99 | −0.03 | −0.34 | 0.737 | |
| Psychiatric diagnosis at six months: PTSD (0 = no) | 2.53 | 5.51 | 0.04 | 0.46 | 0.647 | |
| Psychiatric diagnosis at six months: BD (0 = no) | −9.55 | 7.22 | −0.13 | −1.32 | 0.189 | |
| Psychiatric diagnosis at six months: Schizophrenia (0 = no) | −11.69 | 9.91 | −0.11 | −1.18 | 0.241 | |
| Marijuana use at six months (0 = no) | 0.42 | 2.72 | 0.02 | 0.15 | 0.879 | |
| ISS | −0.15 | 0.20 | −0.07 | −0.75 | 0.456 | |
| Days since trauma at six months | 0.14 | 0.06 | 0.22 | 2.50 | 0.014* | |
| Time of blood draw at six months | −0.05 | 0.56 | −0.01 | −0.09 | 0.926 | |
| Gender (0 = male) | 2.62 | 2.77 | 0.09 | 0.95 | 0.346 | |
| Age | −0.10 | 0.09 | −0.12 | −1.08 | 0.281 | |
| Race (American Indian or Alaskan Native [0 = White]) | 9.35 | 9.92 | 0.09 | 0.94 | 0.348 | |
| Race (Black [0 = White]) | 9.91 | 3.24 | 0.37 | 3.06 | 0.003** | |
| Ethnicity (0 = Non-Hispanic or Latino) | 2.88 | 4.90 | 0.06 | 0.59 | 0.558 | |
| Step 3 | ||||||
| Intercept | 11.18 | 3.13 | 3.569 | 0.001** | ||
| 2-AG at six months | −3.56 | 2.99 | −0.08 | −1.19 | 0.238 | |
| AEA at six months | −1.97 | 6.01 | −0.02 | −0.33 | 0.743 | |
| CNR1 rs806371 (0 = T/T) | 6.06 | 1.92 | 0.21 | 3.16 | 0.002** | |
| CNR1 rs1049353 (0 = G/G) | 1.54 | 2.08 | 0.05 | 0.74 | 0.460 | |
| CNR1 rs2180619 (0 = G/G) | −0.09 | 1.97 | 0.00 | −0.05 | 0.964 | |
| FAAH rs324420 (0 = C/C) | 0.62 | 1.81 | 0.02 | 0.34 | 0.733 | |
| MGLL rs604300 (0 = G/G) | 1.22 | 2.32 | 0.04 | 0.52 | 0.602 | |
| Psychotherapy at six months (0 = no) | −0.64 | 3.01 | −0.02 | −0.21 | 0.833 | |
| Psychiatric medication at six months (0 = no) | 8.04 | 2.63 | 0.26 | 3.06 | 0.003** | |
| Psychiatric diagnosis at six months: GAD (0 = no) | −1.69 | 5.85 | −0.02 | −0.29 | 0.773 | |
| Psychiatric diagnosis at six months: PTSD (0 = no) | −6.68 | 4.15 | −0.12 | −1.61 | 0.111 | |
| Psychiatric diagnosis at six months: BD (0 = no) | −12.91 | 5.29 | −0.17 | −2.44 | 0.017 | |
| Psychiatric diagnosis at six months: Schizophrenia (0 = no) | −21.42 | 7.32 | −0.20 | −2.93 | 0.004** | |
| Marijuana use at six months (0 = no) | −0.99 | 1.99 | −0.03 | −0.50 | 0.622 | |
| ISS | −0.16 | 0.15 | −0.07 | −1.12 | 0.265 | |
| Days since trauma at six months | 0.09 | 0.04 | 0.15 | 2.25 | 0.026 | |
| Time of blood draw at six months | 0.28 | 0.41 | 0.05 | 0.69 | 0.491 | |
| Gender (0 = male) | −0.70 | 2.05 | −0.02 | −0.34 | 0.736 | |
| Age | 0.03 | 0.07 | 0.03 | 0.42 | 0.674 | |
| Race (American Indian or Alaskan Native [0 = White]) | 7.02 | 7.26 | 0.07 | 0.97 | 0.336 | |
| Race (Black [0 = White]) | 0.53 | 2.58 | 0.02 | 0.21 | 0.837 | |
| Ethnicity (0 = Non-Hispanic or Latino) | −2.19 | 3.63 | −0.05 | −0.60 | 0.548 | |
| CAPS Distress at six months | 0.48 | 0.05 | 0.76 | 9.25 | <0.001*** | |
Note. CESD-R = Center for Epidemiologic Studies of Depression Scale-Revised; 2-AG = 2-arachidonoyl Glycerol; AEA = Anandamide; CNR1 = Cannabinoid Receptor 1; FAAH = Fatty Acid Amide Hydrolase; MGLL = Monoglyceride Lipase; DASS = Depression Anxiety and Stress Scale; MDD = Major Depressive Disorder; GAD = Generalized Anxiety Disorder; PTSD = Posttraumatic Stress Disorder; BD = Bipolar Disorder; MEU = morphine equivalent units; ISS = injury severity score; CAPS = Clinician Administered PTSD Scale. ***p < 0.001, **p < 0.01, *p < 0.05.
Fig. 3.
Estimated marginal means for CESD-R at six months by CNR1 rs806371. Presence of the minor allele (G) indicates greater depression severity at six months. Effect controls for all covariates in the second full hierarchical model (β = 0.21, t = 3.16, p = 0.002). Error bars reflect ±standard error of the mean; **p < 0.01.
Given the significant effect for CNR1 rs806371, we also examined in follow-up tests whether there was a dosing effect by genotype on depression severity (G/G > G/T > T/T) using an ANCOVA, including covariates used in the regression. Pairwise comparisons showed that homozygous T/T had less depression than heterozygous G/T (p = 0.020), but that G/T did not differ from homozygous G/G (p = 0.587).
3.7. Change in eCBs and depression severity at six months
In exploratory analyses we also examined whether change in eCBs between the post-injury and the six month appointment was related to depression severity at six months. Change scores were calculated subtracting eCB values at six months from eCB values post-injury, with positive values indicating less decline over time. We found that depression severity at six months was negatively associated with change in 2-AG (r(118) = −0.30, p = 0.001), suggesting that more decline in 2-AG over time was related to more depression severity at six months. Supplemental Fig. 1 shows the relationship between change in 2-AG and depression severity at six months. In contrast, we found no relationship between change in AEA and depression symptoms at six months (r(118) = 0.04, p = 0.640).
4. Discussion
This investigation examined circulating concentrations of the eCBs, 2-AG and AEA, along with CNR1, FAAH, and MGLL polymorphisms as predictors of depression severity six months after a traumatic injury. We found that higher concentrations of 2-AG post-injury, collected an average of 3 days after injury, was a significant predictor of greater depression severity six months later. We also found that genotype CNR1 rs806371 was a significant predictor of depression severity at six months, such that individuals with one or more copies of the rare allele (G/G or G/A) experienced more depression. We did not find evidence that post-injury circulating AEA concentrations predict risk for development of depression in this study, nor that concurrent circulating concentrations of 2-AG or AEA were associated with severity of depression at six months.
The subjects in this study were exposed to a range of injury types; while injury severity was variable, all of the subjects required hospitalization. The circulating concentrations of 2-AG were very high in this population, about 100 fold higher than reported in other studies (Hillard et al., 2012). Interestingly, a study of circulating eCBs in patients undergoing cardiac surgery with cardiopulmonary bypass, a very invasive procedure, also found a large increase in circulating concentrations of 2-AG (112 ng/ml before to 321 ng/ml after the procedure) (Weis et al., 2010). Together with other studies showing more moderate increases in circulating 2-AG concentrations following less severe stress (Hillard et al., 2016), this suggests that 2-AG in the circulation is sensitive to the degree of stress or distress imposed upon the individual.
As discussed above, preclinical studies suggest that activation of the eCB system buffers the stress response, and the diathesis stress model predicts that a high 2-AG concentration at the time of acute stress/distress would reduce the impact of stress on the brain and, thus, the risk to develop chronic depression. Indeed, a far smaller study (n = 16) found that depression at six months following cardiac surgery was greater in patients with the lowest circulating 2-AG concentrations at the time of surgery (Hauer et al., 2012). By contrast, we provide evidence that higher circulating concentrations of 2-AG in the days after trauma predicts greater depressive symptoms six months later. One explanation for these results is that both a large increase in 2-AG and risk for depression are consequences of the very significant physical and psychological stress that accompanied the traumatic injuries to our sample and that 2-AG and risk for depression are not directly, causally related. A more plausible explanation based upon evidence that 2-AG/CB1R signaling is mechanistically linked to protection from stress, is that the large increase in 2-AG induced by the stress down-regulates CB1R signaling. This is consistent with evidence from preclinical studies that high brain concentrations of 2-AG are associated with reduced CB1R binding site density, likely as a result of agonist-induced desensitization and receptor down-regulation (Schlosburg et al., 2010). Thus, sustained elevated 2-AG concentrations as a consequence of the severe traumatic injury could perhaps reduce rather than enhance 2-AG/CB1R signaling. To note, the effect of 2-AG was only trending when controlling for distress-specific symptoms at six months, suggesting that while elevated 2-AG is significantly associated with depression, it is also associated with general distress symptoms of PTSD.
We did not find any relationship between concurrent measures of circulating eCBs and depression at six months. This finding is also at odds with prior studies finding diminished circulating 2-AG in individuals with established, chronic depression (Hill et al., 2008, Hill et al., 2009b). However, an earlier study found that circulating 2-AG concentrations were inversely related to the length of the depressive episode, with the data in that report showing that reductions in 2-AG were not pronounced at 26 weeks, which is the time frame of our study (Hill et al., 2008). Thus, concentrations of circulating 2-AG may be a particular feature of depression chronicity. An untested hypothesis is that circulating 2-AG concentrations would continue to fall in the individuals with depression in our study population. Encouraging evidence for this theory is the fact that we found greater decline in 2-AG over time in individuals with greater depression severity, suggesting that change in levels were meaningfully related to depression. More studies focused on the time course of changes (over days and months) in circulating eCBs after stress will help clarify these questions.
The lack of relationship between concurrent measures of circulating AEA concentrations and symptoms of depression at six months is in agreement with some (Hill et al., 2008) but not all (Hill et al., 2009a, Hill et al., 2009b) previous studies. In one study, AEA concentrations were significantly elevated in women with minor depression and significantly negatively correlated with symptoms of anxiety, rather than depression (Hill et al., 2008). In rodents, Eisenstein and colleagues found reduced 2-AG and AEA content in the brains of OBX animals, which induces a depression phenotype, but only a direct correlation between 2-AG levels and depressive behaviors (Eisenstein et al., 2010). Other work similarly shows only alterations in brain 2-AG levels in preclinical models of depression, with no evidence for altered AEA (Bortolato et al., 2007; M.N. Hill et al., 2005; Sciolino et al., 2011). Together, these data suggest that 2-AG and AEA may be differentially associated with symptoms of mental illness.
We found that genotype at CNR1 rs806371, a snp in the promoter region of CNR1, significantly contributed to risk for depression and that carriers of the minor allele (G) at rs806371 had greater depression severity at six months after injury. This finding is in accord with the results of an earlier study in more than 300 individuals with MDD in which the minor allele at this locus was significantly associated with melancholic (p = 0.04) and psychotic (p = 0.004) symptoms (Mitjans et al., 2013). Wilke and colleagues identified the same snp in a study of the impact of CNR1 genotype on cholesterol homeostasis and demonstrated that rs806371 is a functional allele within the CNR1 promoter (Feng et al., 2013). Importantly, they discovered, using electrophoretic mobility shift assays, that the presence of G, but not the major allele T, at rs806371 creates a novel regulatory binding element in the promoter. Furthermore, reporter assays demonstrated that reporter gene expression was significantly reduced when the nucleotide at rs806371 was G. Thus, these data indicate the minor allele at this site, which is associated with increased depression severity in our study, likely reduces CB1R expression. These data support the general hypothesis that CB1R signaling regulates mood and, more specifically, that reduced CB1R signaling, in this case as a result of reduced expression, contributes to increased risk for depression following trauma.
The other CNR1 alleles we studied had trending effects that did not survive correction for PTSD distress symptoms, suggesting that they do not confer risk for depression per se. In agreement with that hypothesis, earlier studies have provided strong support for the role of genotype at rs2180619 in trait anxiety (Lazary et al., 2009), fear extinction (Heitland et al., 2012), sleep quality (Maple et al., 2016), and affective working memory (Fairfield et al., 2018). All of these processes are important contributors to the symptoms of PTSD (Colvonen et al., 2019). In addition, we also did not find evidence that FAAH and MGLL snps were associated with depression, which partly contradicts prior work. For instance, Maple and colleagues found that C/C carriers of FAAH rs324420 had greater depressive symptoms in a small sample (n = 41) (Maple et al., 2016). We are presently unaware of any study linking MGLL genetic variation to depression, though its involvement in hydrolyzing 2-AG and association with comorbid conditions such as substance abuse provided impetus in this study.
In contrast, previous studies have identified associations between rs1049353 and mood, although the results have been mixed. An early imaging genetics study in less than 20 individuals found that genotype at this snp was associated with activation of striatal regions by happy faces (Chakrabarti et al., 2006), supporting the hypothesis that CNR1 genotype affects brain function. A study in 217 Whites found more carriers of the minor allele (A) at rs1049353 among MDD patients than healthy controls, suggesting that the minor allele increases risk for depression (Monteleone et al., 2010). Additionally, the major allele at this position (G) is part of a haplotype associated with protection from depression (Mitjans et al., 2013), while, in males only, GG carriers exhibited a marginally improved therapeutic response to citalopram (Mitjans et al., 2012). On the other hand, a study of more than 250 patients with MDD found that the major (G) allele was associated with increased risk for antidepressant treatment resistance and hypo-responsiveness of subcortical regions to social reward stimuli (Domschke et al., 2008). Similarly, a study that investigated two large (<1000) data sets found that carriers of the A allele were protected against the effect of childhood physical abuse to induce anhedonia as adults (Agrawal et al., 2012). However, another study asked a similar question and found no association between depression and genetic variability at rs1049535 (Pearson et al., 2013). Given that several of these effects were small in size or were obtained from small sample size studies (n = 19 to 41), it is possible that the statistical significance is spurious. It is also possible that differences in results may be attributed to specific associations between the major allele and depression symptoms (anhedonia for example [Agrawal et al., 2012]) or assessment of the presence of depression as a lifetime diagnosis, rather than current symptoms of the disorder (Pearson et al., 2013). Thus, differences in direction of effects may be driven by sub-components of depression rather than global symptoms. Finally, differences in results may be driven by racial diversity. Our study is racially diverse with approximately half (53%) of our sample classified as racial minorities. In addition, we found differences in the distribution of minor alleles at CNR1 rs1049535 and rs2180619 by race, suggesting that differences in effects between studies may be attributed to distribution of minor vs. major alleles in these samples.
The present study is not without limitations. First, although individuals that were lost to follow-up over the course of this study did not differ in post-injury eCB measures, sample size was reduced and effects of drop-out cannot be tested, particularly the question of whether these individuals experienced different levels of depression. Second, we did not measure eCB concentrations at interim months between the acute period and six months to adequately capture a slope of descending eCBs that may be meaningfully tied to depression risk. Third, a self-reported measure of depression was used and effects should be tested against clinician-assessed depression outcomes. Fourth, our prior research shows that eCBs can be influenced by exercise, although change in eCBs following exercise is attenuated in those with psychiatric illness (Crombie et al., 2018). Nevertheless, the effects of exercise was not a focus of this research and was therefore not controlled for in this analysis. Finally, there was variability in the time between blood draws and serum harvest. Although variability in these times (30–60 min) was entirely random, we cannot rule out the possibility that variation could theoretically account for relationships seen in the study.
Even with the limitations the clinical implications of this study are worth mentioning. Despite the suggestion that eCBs provide a potential novel therapeutic for psychopathology (Chanda, 2019), our data suggests that augmenting eCBs may not be clinically advised in some cases. The data here suggest that timing matters greatly if the field moves towards augmenting eCBs for the treatment of mental illness. In particular, these finding suggest that eCB/CB1R-related interventions immediately after trauma should consider the possibility that CNS eCB concentrations may be abnormally elevated by the trauma, to the point that 2-AG/CB1R signaling is at a ceiling. Therefore, interventions to increase eCB concentrations further (particularly 2-AG) could increase risk for psychopathology. Instead, this research suggests that alternative treatments, such as administration of a DAGL inhibitor shortly after trauma to inhibit 2-AG formation, may be most beneficial to reduce depression six months later. Because of these nuances, more research is needed specifically to unmask the biological processes that underlie elevated eCBs and the mechanism(s) by which elevated eCB concentrations contribute to risk for depression. An additional future direction of this research is to examine the mechanisms of the relationship between serum eCBs and brain signaling that is known to underlie the presentation of depression.
The current study is the first to link circulating concentrations of eCBs acutely after trauma to long-term depression outcomes. In previous work we found that elevated AEA (but not 2-AG) after injury was a prospective predictor of PTSD severity six months after trauma (deRoon-Cassini and Hillard, 2016) and that AEA correlated with somatic anxiety symptoms in individuals who are depressed (Hill et al., 2008). Thus, in addition, distinguishing between 2-AG (vs. AEA) as markers of the eCB system may also be important for dissociating specific risk for depression in this population.
Funding
This work was supported by the National Institute of Mental Health (NIMH R21 MH102838; deRoon-Cassini, Hillard). This funding source had no direct involvement in the design, collection, analysis, and writing of this report. Drs. Fitzgerald, Chesney, Brasel, and Larson have no financial disclosures to report.
CRediT authorship contribution statement
Jacklynn M. Fitzgerald: Formal analysis, Methodology, Visualization, writing. Samantha A. Chesney: formal analyses, review and editing. Tara Sander Lee: Data curation, Investigation, Methodology. Karen Brasel: Conceptualization, review and editing. Christine L. Larson: Supervision, review and editing. Cecilia J. Hillard: Conceptualization, Methodology, Supervision, review and editing. Terri A. deRoon-Cassini: Conceptualization, Methodology, Supervision, review and editing.
Declaration of competing interest
This work was supported by the National Institute of Mental Health (NIMH R21 MH102838; deRoon-Cassini, Hillard). This funding source had no direct involvement in the design, collection, analysis, and writing of this report. Drs. Fitzgerald, Chesney, Brasel, and Larson have no financial disclosures to report and no authors have any conflicts of interest to report.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100304.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Agrawal A., Nelson E.C., Littlefield A.K., Bucholz K.K., Degenhardt L., Henders A.K., Madden P.A.F., Martin N.G., Montgomery G.W., Pergadia M.L., Sher K.J., Heath A.C., Lynskey M.T. Cannabinoid receptor genotype moderation of the effects of childhood physical abuse on anhedonia and depression. Arch. Gen. Psychiatr. 2012;69(7) doi: 10.1001/archgenpsychiatry.2011.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahl R., Lindgren R., Cao Y., Riddez L., Mohseni S. Risk factors for depression following traumatic injury: an epidemiological study from a scandinavian trauma center. Injury. 2017;48(5):1082–1087. doi: 10.1016/j.injury.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Belitardo de Oliveira A., de Mello M.T., Tufik S., Peres M.F.P. Weight loss and improved mood after aerobic exercise training are linked to lower plasma anandamide in healthy people. Physiol. Behav. 2019;201:191–197. doi: 10.1016/j.physbeh.2018.12.018. [DOI] [PubMed] [Google Scholar]
- Bortolato M., Mangieri R.A., Fu J., Kim J.H., Arguello O., Duranti A., Tontini A., Mor M., Tarzia G., Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol. Psychiatr. 2007;62(10):1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Brellenthin A.G., Crombie K.M., Hillard C.J., Koltyn K.F. Endocannabinoid and mood responses to exercise in adults with varying activity levels. Med. Sci. Sports Exerc. 2017;49(8):1688–1696. doi: 10.1249/MSS.0000000000001276. [DOI] [PubMed] [Google Scholar]
- Bryant R.A., O'Donnell M.L., Creamer M., McFarlane A.C., Clark C.R., Silove D. The psychiatric sequelae of traumatic injury. Am. J. Psychiatr. 2010;9 doi: 10.1176/appi.ajp.2009.09050617. [DOI] [PubMed] [Google Scholar]
- Burcusa S.L., Iacono W.G. Risk for recurrence in depression. Clin. Psychol. Rev. 2007;27(8):959–985. doi: 10.1016/j.cpr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey C.E., Agrawal A., Zhang B., Conley E.D., Degenhardt L., Heath A.C., Li D., Lynskey M.T., Martin N.G., Montgomery G.W., Wang T., Bierut L.J., Hariri A.R., Nelson E.C., Bogdan R. Monoacylglycerol lipase (MGLL) polymorphism rs604300 interacts with childhood adversity to predict cannabis dependence symptoms and amygdala habituation: evidence from an endocannabinoid system-level analysis. J. Abnorm. Psychol. 2015;124(4):860–877. doi: 10.1037/abn0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton R.N., Thibodeau M.A., Teale M.J.N., Welch P.G., Abrams M.P., Robinson T., Asmundson G.J.G. The center for epidemiologic studies depression Scale: a review with a theoretical and empirical examination of item content and factor structure. PloS One. 2013;8(3) doi: 10.1371/journal.pone.0058067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti B., Kent L., Suckling J., Bullmore E., Baron-Cohen S. Variations in the human cannabinoid receptor ( CNR1 ) gene modulate striatal responses to happy faces: CNR1 gene and striatal response to happy faces. Eur. J. Neurosci. 2006;23(7):1944–1948. doi: 10.1111/j.1460-9568.2006.04697.x. [DOI] [PubMed] [Google Scholar]
- Chanda D. vol. 6. 2019. (The Endocannabinoid System_ Overview of an Emerging Multi-Faceted Therapeutic Target). [DOI] [PubMed] [Google Scholar]
- Choi K., Le T., McGuire J., Xing G., Zhang L., Li H., Parker C.C., Johnson L.R., Ursano R.J. Expression pattern of the cannabinoid receptor genes in the frontal cortex of mood disorder patients and mice selectively bred for high and low fear. J. Psychiatr. Res. 2012;46(7):882–889. doi: 10.1016/j.jpsychires.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Christensen R., Kristensen P.K., Bartels E.M., Bliddal H., Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370(9600):1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Colvonen P.J., Straus L.D., Acheson D., Gehrman P. A review of the relationship between emotional learning and memory, sleep, and PTSD. Curr. Psychiatr. Rep. 2019;21(1):2. doi: 10.1007/s11920-019-0987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombie K.M., Brellenthin A.G., Hillard C.J., Koltyn K.F. Psychobiological responses to aerobic exercise in individuals with posttraumatic stress disorder. J. Trauma Stress. 2018;31:134–145. doi: 10.1002/jts.22253. [DOI] [PubMed] [Google Scholar]
- deRoon Cassini T.A., Hillard C.J. Abstract # 1781 Relationship between cortisol, endocannabinoids, PTSD and depression immediately and six months after traumatic injury. Brain Behav. Immun. 2016;57 (Published conference abstract) [Google Scholar]
- Dlugos A., Hillard C.J., Childs E., de Wit H., Stuhr K.L. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology. 2012;37(11):2416. doi: 10.1038/npp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K., Dannlowski U., Ohrmann P., Lawford B., Bauer J., Kugel H., Heindel W., Young R., Morris P., Arolt V., Deckert J., Suslow T., Baune B.T. Cannabinoid receptor 1 (CNR1) gene: impact on antidepressant treatment response and emotion processing in Major Depression. Eur. Neuropsychopharmacol. 2008;18(10):751–759. doi: 10.1016/j.euroneuro.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Eisenstein S.A., Clapper J.R., Holmes P.V., Piomelli D., Hohmann A.G. A role for 2-arachidonoylglycerol and endocannabinoid signaling in the locomotor response to novelty induced by olfactory bulbectomy. Pharmacol. Res. 2010;61(5):419–429. doi: 10.1016/j.phrs.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfield B., Mammarella N., Franzago M., Di Domenico A., Stuppia L., Gatta V. A variant on promoter of the cannabinoid receptor 1 gene (CNR1) moderates the effect of valence on working memory. Memory. 2018;26(2):260–268. doi: 10.1080/09658211.2017.1347685. [DOI] [PubMed] [Google Scholar]
- Feng Q., Vickers K.C., Anderson M.P., Levin M.G., Chen W., Harrison D.G., Wilke R.A. A common functional promoter variant links CNR1 gene expression to HDL cholesterol level. Nat. Commun. 2013;4(1):1973. doi: 10.1038/ncomms2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory J.D., Yehuda R. Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Clin. Res. 2015;17(2):10. doi: 10.31887/DCNS.2015.17.2/jflory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graffelman J. Exploring diallelic genetic markers: the HardyWeinberg package. J. Stat. Software. 2015;64(3) doi: 10.18637/jss.v064.i03. [DOI] [Google Scholar]
- Gray J.M., Vecchiarelli H.A., Morena M., Lee T.T.Y., Hermanson D.J., Kim A.B., McLaughlin R.J., Hassan K.I., Kuhne C., Wotjak C.T., Deussing J.M., Patel S., Hill M.N. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J. Neurosci. 2015;35(9):3879–3892. doi: 10.1523/JNEUROSCI.2737-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon E.C. Impact of circadian rhythmicity and sleep restriction on circulating endocannabinoid (eCB) N-arachidonoylethanolamine (anandamide) Psychoneuroendocrinology. 2020;111:104471. doi: 10.1016/j.psyneuen.2019.104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon E.C., Tasali E., Leproult R., Stuhr K.L., Doncheck E., de Wit H., Hillard C.J., Van Cauter E. Circadian rhythm of circulating levels of the endocannabinoid 2-arachidonoylglycerol. J. Clin. Endocrinol. Metabol. 2015;100(1):220–226. doi: 10.1210/jc.2014-3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer D., Weis F., Campolongo P., Schopp M., Beiras-Fernandez A., Strewe C., Giehl M., Toth R., Kilger E., Schelling G. Glucocorticoid-endocannabinoid interaction in cardiac surgical patients: relationship to early cognitive dysfunction and late depression. Rev. Neurosci. 2012;23(5–6) doi: 10.1515/revneuro-2012-0058. [DOI] [PubMed] [Google Scholar]
- Heitland I., Klumpers F., Oosting R.S., Evers D.J.J., Leon Kenemans J., Baas J.M.P. Failure to extinguish fear and genetic variability in the human cannabinoid receptor 1. Transl. Psychiatry. 2012;2(9):e162. doi: 10.1038/tp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman E., Gamelin F.-X., Aucouturier J., Di Marzo V. The role of the endocannabinoid system in skeletal muscle and metabolic adaptations to exercise: potential implications for the treatment of obesity: exercise and the endocannabinoid system. Obes. Rev. 2012;13(12):1110–1124. doi: 10.1111/j.1467-789X.2012.01026.x. [DOI] [PubMed] [Google Scholar]
- Hill M., Miller G., Ho W.-S., Gorzalka B., Hillard C. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41(2):48–53. doi: 10.1055/s-2007-993211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., McLaughlin R.J., Bingham B., Shrestha L., Lee T.T.Y., Gray J.M., Hillard C.J., Gorzalka B.B., Viau V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc. Natl. Acad. Sci. Unit. States Am. 2010;107(20):9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill Matthew N., Patel S. Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses. Biol. Mood Anxiety Disord. 2013;3(1):19. doi: 10.1186/2045-5380-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., McLaughlin R.J., Morrish A.C., Viau V., Floresco S.B., Hillard C.J., Gorzalka B.B. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic–pituitary–adrenal Axis. Neuropsychopharmacology. 2009;34(13):2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Miller G.E., Carrier E.J., Gorzalka B.B., Hillard C.J. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009;34(8):1257–1262. doi: 10.1016/j.psyneuen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Patel S., Carrier E.J., Rademacher D.J., Ormerod B.K., Hillard C.J., Gorzalka B.B. Downregulation of endocannabinoid signaling in the Hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30(3):508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Hillard C., Liu Q. Endocannabinoid signaling in the etiology and treatment of major depressive illness. Curr. Pharmaceut. Des. 2014;20(23):3795–3811. doi: 10.2174/13816128113196660735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard Cecilia J. The endocannabinoid signaling system in the CNS. Int. Rev. Neurobiol. 2015;125:1–47. doi: 10.1016/bs.irn.2015.10.001. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard C.J. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology. 2018;43(1):155–172. doi: 10.1038/npp.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard C.J., Beatka M., Sarvaideo J. Endocannabinoid signaling and the hypothalamic-pituitary-adrenal Axis. In: Terjung R., editor. Comprehensive Physiology. John Wiley & Sons, Inc; 2016. pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard C.J., Weinlander K.M., Stuhr K.L. Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience. 2012;204:207–229. doi: 10.1016/j.neuroscience.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoaglin D.C., Iglewicz B. Fine-tuning some resistant rules for outlier labeling. J. Am. Stat. Assoc. 1987;82(400):1147–1149. [Google Scholar]
- Horder J., Harmer C.J., Cowen P.J., McCabe C. Reduced neural response to reward following 7 days treatment with the cannabinoid CB1 antagonist rimonabant in healthy volunteers. Int. J. Neuropsychopharmacol. 2010;13(8):1103–1113. doi: 10.1017/S1461145710000453. [DOI] [PubMed] [Google Scholar]
- Hungund B.L., Vinod K.Y., Kassir S.A., Basavarajappa B.S., Yalamanchili R., Cooper T.B., Mann J.J., Arango V. Upregulation of CB1 receptors and agonist-stimulated [35S]GTPγS binding in the prefrontal cortex of depressed suicide victims. Mol. Psychiatr. 2004;9(2):184–190. doi: 10.1038/sj.mp.4001376. [DOI] [PubMed] [Google Scholar]
- Hunt J.C., Chesney S.A., Jorgensen T.D., Schumann N.R., deRoon-Cassini T.A. Psychological Trauma: Theory, Research, Practice, and Policy. 2017. Exploring the gold-standard: evidence for a two-factor model of the clinician administered PTSD Scale for the DSM–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J., McLean S.A., Beaudoin F.L., An X., Stevens J.S., Zeng D., Neylan T.C., Clifford G., Linnstaedt S.D., Germine L.T., Rauch S.L., Musey P.I., Hendry P.L., Sheikh S., Jones C.W., Punches B.E., Fermann G., Hudak L.A., Mohiuddin K., Kessler R.C. Socio-demographic and trauma-related predictors of depression within eight weeks of motor vehicle collision in the AURORA study. Psychol. Med. 2020:1–14. doi: 10.1017/S0033291720003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I., Freund T.F. Multiple functions of endocannabinoid signaling in the brain. Annu. Rev. Neurosci. 2012;35(1):529–558. doi: 10.1146/annurev-neuro-062111-150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C. Posttraumatic stress disorder in the national comorbidity Survey. Arch. Gen. Psychiatr. 1995;52(12):1048. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Koethe D., Llenos I.C., Dulay J.R., Hoyer C., Torrey E.F., Leweke F.M., Weis S. Expression of CB1 cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression. J. Neural. Transm. 2007;114(8):1055–1063. doi: 10.1007/s00702-007-0660-5. [DOI] [PubMed] [Google Scholar]
- Koltyn K.F., Brellenthin A.G., Cook D.B., Sehgal N., Hillard C. Mechanisms of exercise-induced hypoalgesia. J. Pain. 2014;15(12):1294–1304. doi: 10.1016/j.jpain.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazary J., Lazary A., Gonda X., Benko A., Molnar E., Hunyady L., Juhasz G., Bagdy G. Promoter variants of the cannabinoid receptor 1 gene (CNR1) in interaction with 5-HTTLPR affect the anxious phenotype. Am. J. Med. Genet. Part B: Neuropsychiatric Genetics. 2009;150B(8):1118–1127. doi: 10.1002/ajmg.b.31024. [DOI] [PubMed] [Google Scholar]
- Lutz B., Marsicano G., Maldonado R., Hillard C.J. The endocannabinoid system in guarding against fear, anxiety and stress. Nat. Rev. Neurosci. 2015;16(12):705–718. doi: 10.1038/nrn4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple K.E., McDaniel K.A., Shollenbarger S.G., Lisdahl K.M. Dose-dependent cannabis use, depressive symptoms, and FAAH genotype predict sleep quality in emerging adults: a pilot study. Am. J. Drug Alcohol Abuse. 2016;42(4):431–440. doi: 10.3109/00952990.2016.1141913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Herz S.P., Zatzick D.F., McMahon R.J. Health-related quality of life in children and adolescents following traumatic injury: a review. Clin. Child Fam. Psychol. Rev. 2012;15(3):192–214. doi: 10.1007/s10567-012-0115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J.D., Crombie K.M., Cook D.B., Hillard C.J., Koltyn K.F. Serum endocannabinoid and mood changes after exercise in major depressive disorder. Med. Sci. Sports Exerc. 2019;51(9):1909–1917. doi: 10.1249/MSS.0000000000002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitjans M., Gastó C., Catalán R., Fañanás L., Arias B. Genetic variability in the endocannabinoid system and 12-week clinical response to citalopram treatment: the role of the CNR1, CNR2 and FAAH genes. J. Psychopharmacol. 2012;26(10):1391–1398. doi: 10.1177/0269881112454229. [DOI] [PubMed] [Google Scholar]
- Mitjans M., Serretti A., Fabbri C., Gastó C., Catalán R., Fañanás L., Arias B. Screening genetic variability at the CNR1 gene in both major depression etiology and clinical response to citalopram treatment. Psychopharmacology. 2013;227(3):509–519. doi: 10.1007/s00213-013-2995-y. [DOI] [PubMed] [Google Scholar]
- Monteleone P., Bifulco M., Maina G., Tortorella A., Gazzerro P., Proto M.C., Di Filippo C., Monteleone F., Canestrelli B., Buonerba G. Investigation of CNR1 and FAAH endocannabinoid gene polymorphisms in bipolar disorder and major depression. Pharmacol. Res. 2010;61(5):400–404. doi: 10.1016/j.phrs.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Moon J.R., Huh J., Song J., Kang I.-S., Park S.W., Chang S.-A., Yang J.-H., Jun T.-G. The Center for Epidemiologic Studies Depression Scale is an adequate screening instrument for depression and anxiety disorder in adults with congential heart disease. Health Qual. Life Outcome. 2017;15(1):176. doi: 10.1186/s12955-017-0747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M., Patel S., Bains J.S., Hill M.N. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology. 2016;41(1):80–102. doi: 10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C.J.A., Page E., Schaefer C., Chatten K., Manocha A., Gulati S., Curran H.V., Brandner B., Leweke F.M. Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. Br. J. Psychiatry. 2013;202:381–382. doi: 10.1192/bjp.bp.112.121178. [DOI] [PubMed] [Google Scholar]
- Muhl D., Kathmann M., Hoyer C., Kranaster L., Hellmich M., Gerth C.W., Faulhaber J., Schlicker E., Leweke F.M. Increased CB2 mRNA and anandamide in human blood after cessation of cannabis abuse. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014;387(7):691–695. doi: 10.1007/s00210-014-0984-2. [DOI] [PubMed] [Google Scholar]
- National Survey on Drug Use and Health . 2017. Prevalence of Major Depressive Episode Among Adults.https://www.nimh.nih.gov/health/statistics/major-depression.shtml#part_155029 [Google Scholar]
- Nissen S.E., Nicholls S.J., Wolski K., Rodés-Cabau J., Cannon C.P., Deanfield J.E., Després J.-P., Kastelein J.J.P., Steinhubl S.R., Kapadia S., Yasin M., Ruzyllo W., Gaudin C., Job B., Hu B., Bhatt D.L., Lincoff A.M., Tuzcu E.M. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. J. Am. Med. Assoc. 2008;299(13):1547. doi: 10.1001/jama.299.13.1547. [DOI] [PubMed] [Google Scholar]
- Osman A., Wong J.L., Bagge C.L., Freedenthal S., Gutierrez P.M., Lozano G. The depression anxiety stress scales-21 (DASS-21): further examination of dimensions, Scale reliability, and correlates: depression anxiety stress. J. Clin. Psychol. 2012;68(12):1322–1338. doi: 10.1002/jclp.21908. [DOI] [PubMed] [Google Scholar]
- Pearson J.F., Fergusson D.M., Horwood L.J., Miller A.L., Sullivan P.F., Youfang L.E., Kennedy M.A. Increased risk of major depression by childhood abuse is not modified by CNR1 genotype. Am. J. Med. Genet. Part B: Neuropsychiatric Genetics. 2013;162(2):224–226. doi: 10.1002/ajmg.b.32124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L.S. The CES-D Scale: a self-report depression Scale for research in the general population. Appl. Psychol. Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Romero-Sanchiz P., Nogueira-Arjona R., Pastor A., Araos P., Serrano A., Boronat A., Garcia-Marchena N., Mayoral F., Bordallo A., Alen F., Suárez J., de la Torre R., Pavón F.J., Rodríguez de Fonseca F. Plasma concentrations of oleoylethanolamide in a primary care sample of depressed patients are increased in those treated with selective serotonin reuptake inhibitor-type antidepressants. Neuropharmacology. 2019;149:212–220. doi: 10.1016/j.neuropharm.2019.02.026. [DOI] [PubMed] [Google Scholar]
- Schlosburg J.E., Blankman J.L., Long J.Z., Nomura D.K., Pan B., Kinsey S.G., Nguyen P.T., Ramesh D., Booker L., Burston J.J., Thomas E.A., Selley D.E., Sim-Selley L.J., Liu Q., Lichtman A.H., Cravatt B.F. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat. Neurosci. 2010;13(9):1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino N.R., Zhou W., Hohmann A.G. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol. Res. 2011;64(3):226–234. doi: 10.1016/j.phrs.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih R.A., Schell T.L., Hambarsoomian K., Belzberg H., Marshall G.N. Prevalence of posttraumatic stress disorder and major depression after trauma center hospitalization. J. Trauma Inj. Infect. Crit. Care. 2010;69(6):1560–1566. doi: 10.1097/TA.0b013e3181e59c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensson N., Ghafouri N., Ernberg M., Mannerkorpi K., Kosek E., Gerdle B., Ghafouri B. The relationship of endocannabinoidome lipid mediators with pain and psychological stress in women with fibromyalgia: a case-control study. J. Pain. 2018;19(11):1318–1328. doi: 10.1016/j.jpain.2018.05.008. [DOI] [PubMed] [Google Scholar]
- Thieme U., Schelling G., Hauer D., Greif R., Dame T., Laubender R.P., Bernhard W., Thieme D., Campolongo P., Theiler L. Quantification of anandamide and 2-arachidonoylglycerol plasma levels to examine potential influences of tetrahydrocannabinol application on the endocannabinoid system in humans: tetrahydrocannabinol and Endocannabinoids. Drug Test. Anal. 2014;6(1–2):17–23. doi: 10.1002/dta.1561. [DOI] [PubMed] [Google Scholar]
- Tohira H., Jacobs I., Mountain D., Gibson N., Yeo A. Systematic review of predictive performance of injury severity scoring tools. Scand. J. Trauma Resuscitation Emerg. Med. 2012;20(1):63. doi: 10.1186/1757-7241-20-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod K.Y., Arango V., Xie S., Kassir S.A., Mann J.J., Cooper T.B., Hungund B.L. Elevated levels of endocannabinoids and CB1 receptor-mediated G-protein signaling in the prefrontal cortex of alcoholic suicide victims. Biol. Psychiatr. 2005;57(5):480–486. doi: 10.1016/j.biopsych.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Vinod K.Y., Kassir S.A., Hungund B.L., Cooper T.B., Mann J.J., Arango V. Selective alterations of the CB1 receptors and the fatty acid amide hydrolase in the ventral striatum of alcoholics and suicides. J. Psychiatr. Res. 2010;44(9):591–597. doi: 10.1016/j.jpsychires.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers F.W., Bovin M.J., Lee D.J., Sloan D.M., Schnurr P.P., Kaloupek D.G., Keane T.M., Marx B.P. The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol. Assess. 2018;30(3):383–395. doi: 10.1037/pas0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers F.W., Litz B.T., Huska J.A., Keane T.M. National Center for PTSD, Behavioral Science Division; 1994. PTSD Checklist-Civilian Version. [Google Scholar]
- Weis F., Beiras-Fernandez A., Hauer D., Hornuss C., Sodian R., Kreth S., Briegel J., Schelling G. Effect of anaesthesia and cardiopulmonary bypass on blood endocannabinoid concentrations during cardiac surgery. Br. J. Anaesth. 2010;105(2):139–144. doi: 10.1093/bja/aeq117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.